Abstract
Objectives
The aim of this study was to optimize the diagnosis of feline panleukopenia virus (FPV) infection in a shelter setting by: (1) comparing the results of the canine parvovirus IDEXX SNAP Parvo (SNAP) point-of-care ELISA with a commercial FPV quantitative real-time PCR (qPCR) test; (2) assessing whether vomit and anal/rectal swabs could be used for early diagnosis; and (3) clarifying the interpretation of weak-positive SNAP test results.
Methods
The study included shelter cats and kittens with incomplete or unknown vaccination history that had clinical signs suspicious for feline panleukopenia and fecal SNAP and PCR tests performed within 24 h of onset. Feces, anal/rectal swabs and vomit were tested using SNAP and PCR, with fecal PCR utilized as the reference standard.
Results
One hundred and forty-five cats were included. Seventeen were diagnosed with FPV infection and 62 were negative; 66 could not be individually designated because they were co-housed. Sensitivity was as follows: fecal SNAP 55% (n = 102; 95% confidence interval [CI] 32–77); swab SNAP 30% (n = 55; 95% CI 7–65); swab PCR 77% (n = 55; 95% CI 46–95); and vomit PCR 100% (n = 17; 95% CI 16–100). Specificity was high (96–100%) for all sample and test types. For PCR-positive fecal samples, true-positive SNAP tests (including weak positives) had significantly higher DNA viral copy numbers than false-negative SNAP tests (P = 0.0031).
Conclusions and relevance
The SNAP ELISA should be viewed as an initial diagnostic test to rule in feline panleukopenia. Positive fecal SNAP test results, including weak positives, are highly likely to be true positives in clinically affected animals. Negative results in clinically affected animals are unreliable and should be followed up with PCR testing.
Keywords: Panleukopenia, parvovirus, diagnostics, point-of-care, polymerase chain reaction, real-time PCR, vomit, feces
Introduction
Feline panleukopenia virus (FPV), or feline parvovirus, is a highly contagious pathogen that can cause severe illness in cats, particularly kittens, with a mortality rate of 50–90%.1–4 Feline panleukopenia (FP) infection is important in animal shelters, which frequently house large numbers of un- or under-vaccinated kittens.3,5 Outbreaks can result in high mortality, euthanasia and shelter closures. Rapid and accurate diagnosis is therefore essential, but this remains a challenge.
Transmission of FPV is primarily via the fecal–oral route, but large quantities of virus are shed in the saliva, urine, feces and vomit of infected cats.1,6,7 Clinical signs of FP can be non-specific, particularly early in the disease and in severely affected kittens.1,3,5,6,8 The peracute presentation includes septic shock and sudden death. Acute early signs include lethargy, pyrexia, anorexia and vomiting. Diarrhea is not a reliable clinical sign,3,9 and is less common in neonates. 10 Hemorrhagic enteritis, a classic presentation of canine parvovirus (CPV) enteritis, is an uncommon presenting sign in FP. 3
Feces is the recommended diagnostic sample type for FPV 11 and is most commonly used in shelters, but other sample types could be considered. Anal or rectal swabs would allow the individual source animal in a group to be identified and are also a potential sample source for early diagnosis. Anecdotally in our shelter, vomit is another potentially useful alternative sample type for early diagnosis; this sample type has not been validated for available tests.
PCR is a sensitive testing modality for parvoviruses and has been used as the reference standard for other diagnostic tests for FP.12–14 Quantitative real-time PCR (qPCR) offers the additional advantage of being able to quantify viral load. However, PCR is expensive and technically difficult, with a delay of 1–3 days for results from a reference laboratory. Rapid, inexpensive point-of-care (POC) tests are therefore preferred by shelters.
FPV is closely related to CPV, and CPV can cause FP syndrome, although this is uncommon.1,15 In the absence of a feline-specific POC test, CPV fecal antigen ELISA kits are used to diagnose FPV infection. (The authors are aware of one POC test for FPV; this test had sensitivity of 88% compared with PCR. 14 Little additional information could be found and the product is not available in Canada.) These tests are able to detect CPV-2a-c and FPV antigen. 1 However, CPV tests have not been validated for FPV by the manufacturers. 11 The IDEXX SNAP Parvo (SNAP) POC test for CPV had high specificity for FPV,13,16 while sensitivity was low in one study 16 and high in another. 13 Four other POC tests had low-to-moderate sensitivity (50–80%) and good-to-excellent specificity (94.2–100%). 16
The SNAP test is commonly used in North American animal shelters. It relies on a color change to indicate the presence of viral antigen. Interpretation of weak-positive results can be difficult. 17 For samples from dogs with clinical signs of parvoviral enteritis, the manufacturer recommends interpreting a faint positive color change as positive. 11 It is not known whether this recommendation should be applied to feline samples.
The objectives of this study were to optimize the diagnosis of FPV infection in a shelter setting by: (1) comparing the results of a CPV POC test with an FPV qPCR test; (2) assessing whether vomit and anal/rectal swabs could be used for early diagnosis; and (3) clarifying the interpretation of weak-positive POC test results.
Materials and methods
Institutional review and approval
The study was approved by the chief executive officer of the shelter.
Setting
The study was performed at Toronto Humane Society, a private, limited-admission shelter in Ontario, Canada. The shelter admits animals through owner relinquishment, stray intake and rescue transport, and has a full-service veterinary hospital. The shelter’s capacity for cats is approximately 200. Panleukopenia is seen with some regularity, although case numbers are low. It is more likely to occur during the spring and summer when large numbers of juveniles are admitted. Shelter-acquired infections are unusual. Affected cats are regularly transferred from other shelters that lack the resources to treat the disease.
At intake, cats ⩾4 weeks of age were vaccinated with a modified live subcutaneous feline viral rhinotracheitis, feline calicivirus and FPV (FVRCP) vaccine. Using recommendations for animal shelters,18–20 kittens were revaccinated every 2–3 weeks, and adult cats were revaccinated once after 2–4 weeks. Further standardized intake procedures were physical examination by a veterinarian or registered veterinary technician; treatment with selamectin, pyrantel (all) and ponazuril (kittens); Wood’s lamp and retroviral screening; and rabies vaccination for cats ⩾12 weeks of age. Pyrantel was repeated after 2 weeks and continued every 2–3 weeks for kittens. Additional diagnostics and treatment were provided as needed.
Study design and case selection
This was a prospective, observational field study, carried out from May to November 2019. Cats known or assessed by veterinary personnel to be <20 weeks of age were defined as kittens. Cats were housed singly, in family groups or, in the case of healthy singleton kittens, co-housed with age- and weight-matched kittens when possible.
Criteria were set to identify cats with clinical signs suspicious for early FPV infection. These criteria were based on the protocol for this shelter. All cats that met the criteria were enrolled in the study: (1) death within 12 h of admission, with few or no clinical signs; (2) dehydration, obtundation to coma, hypothermia or hypoglycemia; (3) adult cats: anorexia/hyporexia and lethargy in association with vomiting and/or diarrhea; (4) kittens: anorexia/hyporexia, lethargy and weight loss; or diarrhea with or without additional signs; or vomiting with anorexia/hyporexia or pyrexia.
Diarrhea was defined as stool score of 5–7 using the Purina Veterinary Diets Fecal Scoring Chart (http://vhc.missouri.edu/wp-content/uploads/2020/07/Nestle-Purina-Fecal-Scoring-System.pdf).
Adult cats with up-to-date FVRCP vaccines at admission were excluded. Strays with unknown histories were presumed to be unvaccinated. If vomiting or diarrhea occurred in adult cats ⩾10 days following vaccination upon intake to the shelter, these cats were considered likely to be protected from FPV and were also excluded.
Case definition and PCR test
The case definition for FPV infection was ‘compatible clinical signs, as described above, and a positive FPV PCR test on a fecal sample within 24 h of onset’.
The reference laboratory (IDEXX Reference Laboratories, Markham, Canada) defined a positive PCR result as cycle threshold (Ct) value of ⩽26, which corresponded to ⩾1.59 × 106 (1,588,799) viral DNA copies per gram (EA Chan, IDEXX Reference Laboratories, personal communication).
The qPCR targets the FP VP2 gene EU252145, 21 and is able to detect both FPV and CPV. The analytic sensitivity of the assay is 10 DNA copies/reaction. The positive cut-off value for the PCR test is a technical cut-off, meaning that viral DNA is still detectable between Ct 26 and 40 but is not considered clinically significant in this range. The technical cut-off is included in the assay design to distinguish positives consistent with clinical infection from the low positive background level of FPV commonly detectable in healthy animals post-vaccination with a modified-live vaccine (or in subclinically infected, healthy shedders) (MA Seguin, IDEXX Reference Laboratories, personal communication).
Sample collection
Anal or rectal swabs and, where available, feces and vomit, were collected within 24 h of identification of a suspected case of FP. Swabs were moistened with sterile saline prior to collection and were collected from the rectum unless abundant fecal material was present on the anus or perineum. Feces and vomit samples from co-housed cats were labelled as grouped samples if the individual source could not be identified.
Sample handling and testing
Samples were processed immediately and tested using the IDEXX SNAP Parvo (SNAP) test and the Diarrhea RealPCR Panel (Comprehensive). Each stool sample was scored using the Purina Fecal Scoring Chart. SNAP tests were performed by a trained research assistant, according to the manufacturer’s instructions. All results were photographed to provide a permanent record and for verification of results when needed. Positive SNAP results were reported as ‘weak positive’ or ‘positive’ based on the subjective intensity of the color change, compared with the positive control. PCR tests were performed by the reference laboratory. DNA viral copy numbers were generated from Ct values by the laboratory, using a proprietary calculation.
Data analysis
For co-housed kittens, fecal and vomit samples could be identified individually in some cases but not others. Only individually identified samples were used to compare test results of vomit vs feces. For the comparison between feces and swabs, fecal samples that were not individually identified were included as follows: if swabs had been tested for each member of the group and all results were the same (ie, all positive/weak positive or all negative), the fecal result was analyzed against the swab result. If any individual swab result for a group disagreed with the rest, the group was excluded from this analysis.
The fecal PCR test was used as the reference standard. Sensitivity and specificity were calculated. For fecal samples with positive PCR results, the Mann–Whitney test was used to compare DNA viral copy numbers between SNAP-positive (true-positive) and SNAP-negative (false-negative) results. DNA viral copy numbers for co-housed and individually housed cats were compared prior to this analysis, using the Mann–Whitney test, to determine whether the viral loads differed by housing arrangement. Statistical analysis was conducted in GraphPad Prism 8.4.3. Significance was set at P <0.05.
Results
During the study period, 1340 individual cats were admitted to the shelter, of which 198 showed clinical signs consistent with the case criteria. Seventeen of these were excluded based on vaccination history and 36 owing to missing data, leaving a study population of 145 cats. Seventy-eight were individually housed (10 adults and 68 kittens) and 67 were housed in groups (24 groups containing 67 kittens). Seventeen (two adults and 15 kittens) met the case definition for FPV infection, while 62 (eight adults and 54 kittens) tested negative on PCR. Sixty-six animals could not be designated negative or positive for the purposes of the study, because the samples were not individually identified.
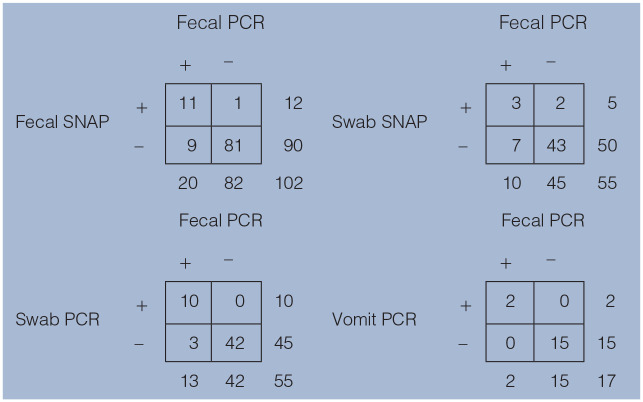
The study population produced 102 fecal samples (78 individuals and 24 groups), 17 individually identified vomit samples and 55 individual swabs. All swab SNAP results that were in agreement for an entire group were negative. Twenty of 102 fecal samples (19.6%) were positive on the PCR test (Table 1). The sensitivity and specificity for the different test and sample types are shown in Table 2.
Table 1.
Point-of-care and PCR tests, using fecal, swab and vomit samples from shelter cats with suspected feline panleukopenia virus infection
|
Fecal PCR was used as the reference standard
SNAP = IDEXX CPV SNAP Parvo Test; PCR = feline panleukopenia real-time PCR test; swab = anal or rectal swab
Table 2.
Sensitivity and specificity for the different test and sample types
| SNAP (fecal; n = 102) | SNAP (swab; n = 55) | PCR (swab; n = 55) | PCR (vomit; n = 17) | |
|---|---|---|---|---|
| Sensitivity (95% CI) | 55 (32–77) | 30 (7–65) | 77 (46–95) | 100 (16–100) |
| Specificity (95% CI) | 99 (93–100) | 96 (85–99) | 100 (92–100) | 100 (78–100) |
CI = confidence interval
Individual DNA viral copy numbers for PCR-positive fecal samples (n = 20) are shown in Table 3. The median copy number for the 20 PCR-positive fecal samples was 3.85 × 108 copies/g (range 2.2 × 106 to 1.06 × 1010). For all 44 fecal samples with detectable DNA, including those that fell below the technical laboratory cut-off, the median was 1.58 × 105 and the lowest value was 2.0 × 10² (201 DNA copies/g).
Table 3.
Cycle threshold (Ct) values, DNA viral copy numbers and SNAP test results in fecal samples from clinically affected shelter cats with a positive feline panleukopenia PCR test (Ct value ⩽26 or ⩾1.59 × 106 DNA viral copies/g feces)
| Sample number | Ct value | DNA viral copies/g feces | SNAP result |
|---|---|---|---|
| 62 | 13.25 | 1.06 × 1010 | P |
| 35 | 14.08 | 5.99 × 109 | WP |
| 26 | 14.70 | 3.90 × 109 | P |
| 60 | 15.00 | 3.17 × 109 | P |
| 4 | 15.76 | 1.88 × 109 | WP |
| 33 | 16.05 | 1.54 × 109 | N |
| 36 | 16.62 | 1.04 × 109 | WP |
| 27 | 17.17 | 7.08 × 108 | WP |
| 94 | 17.38 | 6.13 × 108 | WP |
| 97 | 17.61 | 5.23 × 108 | WP |
| 38 | 18.69 | 2.48 × 108 | N |
| 13 | 19.53 | 1.39 × 108 | N |
| 84 | 19.79 | 1.16 × 108 | N |
| 100 | 20.10 | 9.36 × 107 | WP |
| 59 | 20.12 | 9.23 × 107 | P |
| 34 | 21.89 | 2.72 × 107 | N |
| 25 | 23.07 | 1.20 × 107 | N |
| 101 | 23.46 | 9.18 × 106 | N |
| 50 | 25.29 | 2.59 × 106 | N |
| 12 | 25.55 | 2.17 × 106 | N |
PCR = feline panleukopenia real-time PCR test; SNAP = IDEXX CPV SNAP Parvo Test; N = negative; P = positive; WP = weak positive
There was no significant difference between fecal DNA viral copy numbers for co-housed (median 0.0; interquartile range [IQR] 0.0–1.22 × 105) and singly housed cats (median 1.04 ×102; IQR 0.0–1.69 × 109; two-tailed P = 0.533). For PCR-positive fecal samples, there was a significant difference (two-tailed P = 0.0031) between DNA viral copy numbers for false-negative (n = 9) and true-positive (n = 11) SNAP test results, with higher median copy numbers for true positive results (Figure 1). Median DNA viral copy numbers for false-negative results were 2.72 × 107 (IQR 2.17 × 106 to 1.54 × 109) and for true positives 1.04 × 109 (IQR 9.23 × 107 to 1.06 × 10¹0). All true-positive SNAP tests had DNA viral copy numbers ⩾9.23 × 107. The single false-positive fecal SNAP result, a weak positive, had an associated DNA viral copy number of 8.02 × 105. A subsequent stool sample from this cat tested PCR positive beyond the 24 h limit for early diagnosis.
Figure 1.
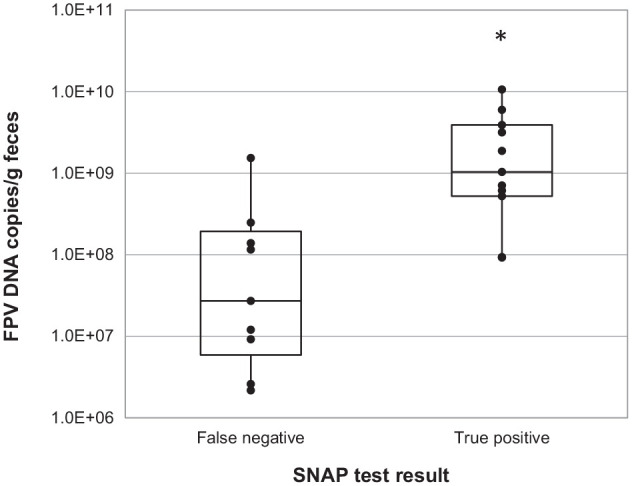
Feline panleukopenia DNA viral copy numbers for 20 PCR-positive fecal samples from clinically affected shelter cats. The asterisk indicates a statistically significant difference between DNA copy numbers for false-negative and true-positive SNAP test results. FPV = feline panleukopenia virus; PCR = feline panleukopenia-specific real-time PCR test; SNAP = IDEXX CPV SNAP Parvo Test
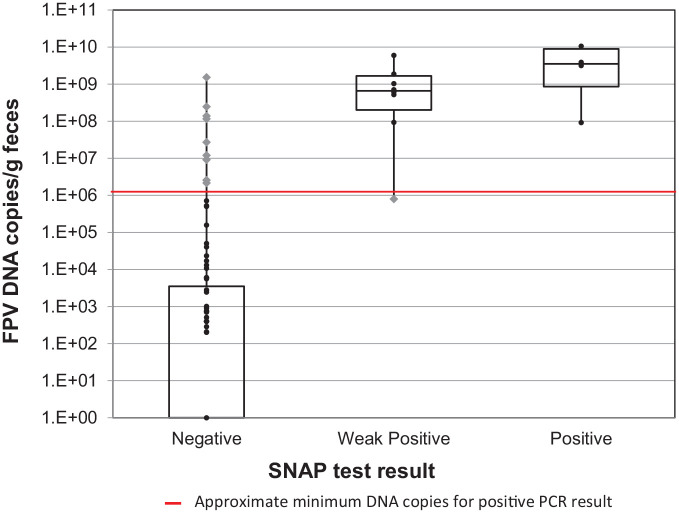
DNA viral copy numbers for fecal samples that were negative, weak positive and positive on the SNAP test are shown in Figure 2 and Table 4. The number of weak-positive and positive samples was too small to allow for statistical comparison. However, the IQRs for weak positive and positive SNAP results overlapped with one another, but neither overlapped with the IQR for negative SNAP results (Figure 2).
Figure 2.
Feline panleukopenia DNA viral copy numbers for fecal samples with negative, weak-positive and positive point-of-care test results. Box plots show median and interquartile range. FPV = feline panleukopenia virus; SNAP = IDEXX CPV SNAP Parvo Test; PCR = feline panleukopenia-specific real-time PCR test; black circle = agreement between SNAP and PCR; gray diamond = discordant results
Table 4.
Feline panleukopenia virus (FPV) DNA viral copy numbers for fecal samples from clinically affected shelter cats with negative, weak positive and positive SNAP test results
| FPV PCR DNA viral copy number | ||||
|---|---|---|---|---|
| SNAP result | n | Median | IQR | Range |
| Negative | 90 | 0.0 | 0.0–3.51 × 10³ | 0.0–1.53 × 109 |
| Weak positive | 8 | 6.60 × 108 | 2.01 × 108 to 1.67 × 109 | 8.02 × 105 to 5.99 × 109 |
| Positive | 4 | 3.54 × 109 | 8.62 × 108 to 8.94 × 109 | 9.23 × 107 to 1.06 × 10¹0 |
PCR = feline panleukopenia real-time PCR test; SNAP = IDEXX CPV SNAP Parvo Test; IQR = interquartile range
Discussion
The specificity of the SNAP test in this study was high for all sample types. The current study therefore confirms previous findings that a positive SNAP result is reliable for diagnosing FP in clinically affected cats.13,16 Interpretation of weak-positive SNAP test results can be challenging. In our experience, in some cases one observer was able to see the faint color change, while another was not, and the significance of a weak-positive result for a feline sample has not previously been clarified. Although subgroups were small and statistical comparison was not possible, DNA viral copy numbers overlapped for samples with weak-positive and positive SNAP results. The implication of this finding, in combination with the high specificity of the SNAP test, is that any positive result in a cat or kitten with clinical signs of FP is highly likely to be a true positive, regardless of the intensity of the color change. This is consistent with recommendations for canine samples. 11
The sensitivity of the fecal SNAP test was low (55%) in this study, with the upper limit of the confidence interval (CI) being only 77%. These findings are similar to those of a previous study that used electron microscopy as the reference method and reported sensitivity of 60%, 16 but they differ from the only other published study that compared PCR and the SNAP test for FPV detection, in which sensitivity was 94.7%. 13 It is unlikely that the variability of sensitivity in the SNAP studies can be attributed to the CPV-specific nature of the test, given the conclusive demonstration that FPV can be detected in most infected cats in some circumstances. 13 The sensitivity of the SNAP test for CPV in dogs can also be highly variable, having been reported as 18.4%, 22 56.2% 17 and 81.8% 23 in studies that used PCR as the reference standard. CPV and FPV are genetically similar, and differ by only six amino acid residues. 24 Although these differences occur in important epitopes of VP2 (the major capsid protein of the viruses), this did not prevent detection of FPV. 13 Virus mutation also did not account for the low sensitivity of the SNAP test in one canine study. 25 Evolutionarily, FPV is an extremely stable virus and very little nucleotide variation was found in FPV isolates. 13
Instead, differing viral loads may account for apparently contradictory sensitivity results in different studies. Average viral loads may differ between study populations owing to differences in vaccination protocols, test timing and/or population susceptibility factors. Maternal antibodies in young kittens and a higher likelihood of previous vaccination or exposure in adults may affect viral loads in study populations with differing age structures. One of the previous study populations was composed of privately owned cats with unreported vaccination histories and age ranges. 16 The other differed from the current study in that 41% of cats were privately owned and 40% were older than 6 months. 13 The timing of testing, based on differing criteria or other factors, may also affect results. Even short delays in testing may result in lower viral loads in the sample collected. 25 Conversely, to account for low viral loads early in the disease, a repeat antigen test is recommended after 1–2 days in dogs with clinical signs and an initial negative test. 26
qPCR offers the ability to quantify viral load and provides insights into the performance of the POC test. As in the current study, there was quite substantial overlap in DNA viral copy numbers for samples from dogs with true-positive SNAP results compared with false negatives. 25 This was thought to be attributable, at least in part, to antigen–antibody binding in the intestine. 25 While this would interfere with detection of antigen by the SNAP test, it would not affect DNA detection by PCR.
While not assessed as a study objective, the positive cutoff value for the PCR test warrants comment. The use of this cutoff was described in a previous canine study. 27 A cutoff is required because of the exquisite sensitivity of the qPCR (10 DNA copies/reaction), which detects subclinical parvovirus shedding in healthy animals, particularly following vaccination with modified live virus.28–32 Conventional PCR testing does not distinguish between the vaccine and field virus unless sequencing is performed. 28 While the vaccine and field virus cannot be reliably distinguished based on viral load alone, vaccine virus shedding is typically associated with low viral loads.28–31 In shelter cats and kittens, PCR diagnosis of FPV relies on the cut-off to avoid false-positive post-vaccine results. It is important to note, however, that vaccine virus loads can sometimes exceed this cut-off.28,30,32 The only way to reliably distinguish the two is by Sanger sequencing, which is unavailable in a clinical setting. In clinical cases, history and clinical signs must be carefully assessed when interpreting PCR results, as the cutoff will not always differentiate true infection from post-vaccinal shedding or a carrier state.
It has been suggested that the less-sensitive SNAP test has a shorter window of detection in dogs than the PCR test, owing to the typically short period during which large quantities of CPV are shed. 25 This window can be very short (less than a day). 25 In the current study, sensitivity was low despite attempts at early diagnosis of infection. However, as this was a field study, some animals might have begun to develop clinical signs prior to entering the shelter.
The sample sizes for swabs and vomit were small, leading to wide CIs. The low sensitivity of the swab sample type for both SNAP and PCR, compared with the fecal sample type, was unexpected. Although the upper limit of the CI was high (95%), the lower limit was only 46%. Anal, rather than rectal, swabs were used for kittens with an obviously soiled perineum; this was done to minimize discomfort, but the amount of fecal material on some swabs may have been insufficient. The vomit samples had perfect agreement with PCR. However, only 17 samples were tested; therefore, no definite conclusions can be drawn. Vomit was unexpectedly difficult to collect as it dried quickly, was ingested or was cleaned by shelter staff. Another difficulty was the inability to separate kittens for the purpose of individual sample collection, owing to high numbers in the shelter during the spring and summer.
Conclusions
The SNAP test had high specificity and low sensitivity for FPV in this study. The low sensitivity is suspected to be due to relatively low viral loads in many animals.
POC ELISAs should be viewed as initial diagnostic tests to rule in FP. Positive fecal SNAP test results, including weak positives, are highly likely to be true positives in clinically affected animals. Negative results in clinically affected animals are unreliable and should be followed up with PCR testing.
Vomit and anal/rectal swabs could have limited use for early diagnosis if stool cannot be obtained; larger sample sizes are required to further assess the utility of these sample types.
Acknowledgments
With grateful thanks to Toronto Humane Society leadership and staff, and to Dr Roxanne Chan, Dr Christian Leutenegger and Gina Lockwood for their invaluable advice and assistance. The authors gratefully acknowledge EveryCat Health Foundation (formerly the Winn Feline Foundation), PetSmart Charities and IDEXX Laboratories for their generous support.
Footnotes
Accepted: 23 February 2021
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: EveryCat Health Foundation, in collaboration with PetSmart Charities, provided funding for grants with special emphasis in feline shelter medicine (Grant W19-003). IDEXX Laboratories Canada provided complimentary PCR testing.
Ethical approval: This work involved the use of non-experimental animals only (including owned or unowned animals and data from prospective or retrospective studies). Established internationally recognized high standards (‘best practice’) of individual veterinary clinical patient care were followed. Ethical approval from a committee was therefore not specifically required for publication in JFMS.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Linda S Jacobson  https://orcid.org/0000-0001-9704-8737
https://orcid.org/0000-0001-9704-8737
References
- 1. Barrs VR. Feline panleukopenia: a re-emergent disease. Vet Clin North Am Small Anim Pract 2019; 49: 651–670. [DOI] [PubMed] [Google Scholar]
- 2. Kruse BD, Unterer S, Horlacher K, et al. Prognostic factors in cats with feline panleukopenia. J Vet Intern Med 2010; 24: 1271–1276. [DOI] [PubMed] [Google Scholar]
- 3. Litster A, Benjanirut C. Case series of feline panleukopenia virus in an animal shelter. J Feline Med Surg 2014; 16: 346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Porporato F, Horzinek MC, Hofmann-Lehmann R, et al. Survival estimates and outcome predictors for shelter cats with feline panleukopenia virus infection. J Am Vet Med Assoc 2018; 253: 188–195. [DOI] [PubMed] [Google Scholar]
- 5. Greene CE. Feline enteric viral infections. In: Greene C. (ed). Infectious diseases of the dog and cat. St Louis, MO: Elsevier Saunders, 2012, pp 80–91. [Google Scholar]
- 6. Hartmann K. ABCD guidelines: feline panleukopenia. http://www.abcdcatsvets.org/abcd-guidelines-on-feline-panleukopenia-2012-edition/ (2017, accessed May 4, 2020).
- 7. Sykes JE. Feline panleukopenia virus infection and other viral enteritides. In: Sykes J. (ed). Canine and feline infectious diseases. Philadelphia, PA: Elsevier Saunders, 2013, pp 187–194. [Google Scholar]
- 8. Lawrence JS, Syverton JT, Shaw JS, et al. Infectious feline agranulocytosis. Am J Pathol 1940; 16: 333–354. [PMC free article] [PubMed] [Google Scholar]
- 9. Lawrence JS, Syverton JT. Spontaneous agranulocytosis in the cat. Proc Soc Exp Biol Med 1938; 38: 914–918. [Google Scholar]
- 10. Csiza CK, De Lahunta A, Scott FW, et al. Pathogenesis of feline panleukopenia virus in susceptible newborn kittens I. Clinical signs, hematology, serology, and virology. Infect Immun 1971; 3: 833–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. IDEXX Reference Laboratories. Frequently asked questions about SNAP Parvo Test. https://idexxcom-live-b02da1e51e754c9cb292133b-9c56c33.aldryn-media.com/filer_public/94/bb/94bb23b2-7d3a-4d40-a119-1b82cc0f145e/snap-parvo-test-faqs.pdf (2017, accessed February 23, 2021).
- 12. Faz M, Martínez S, Quijano-Hernández I, et al. Reliability of clinical diagnosis and laboratory testing techniques currently used for identification of canine parvovirus enteritis in clinical settings. J Vet Med Sci 2017; 79: 213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abd-Eldaim M, Beall MJ, Kennedy MA. Detection of feline panleukopenia virus using a commercial ELISA for canine parvovirus. Vet Ther 2009; 10: E1–E6. [PubMed] [Google Scholar]
- 14. Awad RA, Khalil WKB, Attallah AG. Epidemiology and diagnosis of feline panleukopenia virus in Egypt: clinical and molecular diagnosis in cats. Vet World 2018; 11: 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brindhalakshmi B, Mukhopadhyay HK, Antony PX, et al. Isolation and molecular characterization of canine and feline parvovirus strains – an updated review. J Dairy Vet Anim Res 2016; 3: 164–169. [Google Scholar]
- 16. Neuerer FF, Horlacher K, Truyen U, et al. Comparison of different in-house test systems to detect parvovirus in faeces of cats. J Feline Med Surg 2008; 10: 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desario C, Decaro N, Campolo M, et al. Canine parvovirus infection: which diagnostic test for virus? J Virol Methods 2005; 126: 179–185. [DOI] [PubMed] [Google Scholar]
- 18. DiGangi BA, Levy JK, Reese MJ, et al. Effects of maternally-derived antibodies on serologic responses to vaccination in kittens. J Feline Med Surg 2012; 14: 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hosie MJ, Addie DD, Boucraut-Baralon C, et al. Matrix vaccination guidelines. J Feline Med Surg 2015; 17: 583–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scherk MA, Ford RB, Gaskell RM, et al. 2013 AAFP feline vaccination advisory panel report. J Feline Med Surg 2013; 15: 785–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andersen LA, Levy JK, McManus CM, et al. Prevalence of enteropathogens in cats with and without diarrhea in four different management models for unowned cats in the southeast United States. Vet J 2018; 236: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmitz S, Coenen C, König M, et al. Comparison of three rapid commercial canine parvovirus antigen detection tests with electron microscopy and polymerase chain reaction. J Vet Diagnostic Investig 2009; 21: 344–345. [DOI] [PubMed] [Google Scholar]
- 23. Markovich JE, Stucker KM, Carr AH, et al. Effects of canine parvovirus strain variations on diagnostic test results and clinical management of enteritis in dogs. J Am Vet Med Assoc 2012; 241: 66–72. [DOI] [PubMed] [Google Scholar]
- 24. Hueffer K, Parker JSL, Weichert WS, et al. The natural host range shift and subsequent evolution of canine parvovirus resulted from virus-specific binding to the canine transferrin receptor. J Virol 2003; 77: 1718–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Proksch AL, Unterer S, Speck S, et al. Influence of clinical and laboratory variables on faecal antigen ELISA results in dogs with canine parvovirus infection. Vet J 2015; 204: 304–308. [DOI] [PubMed] [Google Scholar]
- 26. IDEXX Reference Laboratories. SNAP Parvo Test kit insert. https://idexxcom-live-b02da1e51e754c9cb292133b-9c56c33.aldryn-media.com/filer_public/53/40/5340b1ed-2788-4d6f-b1fb-e7fb07e9e988/snap-parvo-pkg-insert-en.pdf (accessed February 23, 2021).
- 27. Gizzi ABDR, Oliveira ST, Leutenegger CM, et al. Presence of infectious agents and co-infections in diarrheic dogs determined with a real-time polymerase chain reaction-based panel. BMC Vet Res 2014; 10. DOI: 10.1186/1746-6148-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meggiolaro MN, Ly A, Rysnik-Steck B, et al. MT-PCR panel detection of canine parvovirus (CPV-2): vaccine and wild-type CPV-2 can be difficult to differentiate in canine diagnostic fecal samples. Mol Cell Probes 2017; 33: 20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jas D, Aeberlé C, Lacombe V, et al. Onset of immunity in kittens after vaccination with a non-adjuvanted vaccine against feline panleucopenia, feline calicivirus and feline herpesvirus. Vet J 2009; 182: 86–93. [DOI] [PubMed] [Google Scholar]
- 30. Decaro N, Crescenzo G, Desario C, et al. Long-term viremia and fecal shedding in pups after modified-live canine parvovirus vaccination. Vaccine 2014; 32: 3850–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Freisl M, Speck S, Truyen U, et al. Faecal shedding of canine parvovirus after modified-live vaccination in healthy adult dogs. Vet J 2017; 219: 15–21. [DOI] [PubMed] [Google Scholar]
- 32. Bergmann M, Schwertler S, Speck S, et al. Faecal shedding of parvovirus deoxyribonucleic acid following modified live feline panleucopenia virus vaccination in healthy cats. Vet Rec 2019; 185: 83. DOI: 10.1136/vr.104661. [DOI] [PubMed] [Google Scholar]