Abstract
Butyrylcholinesterase (BChE) is a nonspecific cholinesterase enzyme that hydrolyzes choline-based esters. BChE plays a critical role in maintaining normal cholinergic function like acetylcholinesterase (AChE) through hydrolyzing acetylcholine (ACh). Selective BChE inhibition has been regarded as a viable therapeutic approach in Alzheimer’s disease. As of now, a limited number of selective BChE inhibitors are available. To identify BChE inhibitors rapidly and efficiently, we have screened 8998 compounds from several annotated libraries against an enzyme-based BChE inhibition assay in a quantitative high-throughput screening (qHTS) format. From the primary screening, we identified a group of 125 compounds that were further confirmed to inhibit BChE activity, including previously reported BChE inhibitors (e.g., bambuterol and rivastigmine) and potential novel BChE inhibitors (e.g., pancuronium bromide and NNC 756), representing diverse structural classes. These BChE inhibitors were also tested for their selectivity by comparing their IC50 values in BChE and AChE inhibition assays. The binding modes of these compounds were further studied using molecular docking analyses to identify the differences between the interactions of these BChE inhibitors within the active sites of AChE and BChE. Our qHTS approach allowed us to establish a robust and reliable process to screen large compound collections for potential BChE inhibitors.
Keywords: butyrylcholinesterase, BChE inhibitors, colorimetric BChE assay, qHTS, molecular docking
Introduction
Butyrylcholinesterase (BChE; EC 3.1.1.8) hydrolyzes acetylcholine (ACh) and shares 54% of its amino acid sequence with acetylcholinesterase (AChE; EC 3.1.1.7). 1 Serum BChE can detoxify xenobiotics (e.g., organophosphates, carbamate pesticides, cocaine), activate drugs such as bambuterol and heroin,2–5 and hydrolyze a peptide hormone (ghrelin) involved in hunger, feeding, and stress, as demonstrated in mice. 6 Currently, several cholinesterase inhibitors including donepezil, galantamine, and physostigmine have been used for treating neurodegenerative disorders. 7 However, few highly selective and potent BChE inhibitors have been reported.
Under physiological conditions, AChE plays a more dominant role than BChE in cholinergic neurotransmission by regulating ACh levels. However, in patients with advanced Alzheimer’s disease (AD), AChE levels decline to 55%–67%, while BChE increases to 120% of normal levels. 8 Although BChE is largely of glial origin and AChE is largely of neuronal origin, BChE has been shown to compensate for the deficit in AChE by hydrolyzing ACh in the presence of a cholinesterase inhibitor. Selective BChE inhibition reduced β-amyloid peptide levels in mice brain and human neuroblastoma cells without decreasing cell viability. Furthermore, selective BChE inhibition augmented maze performance in aged rats and long-term potentiation in rat brains. 9 BChE was also reported to attenuate formation of amyloid fibril in vitro. 10 Moreover, high BChE levels were found to be associated with neuropathologic hallmarks of AD such as neuritic plaques and neurofibrillary tangles. 11 Therefore, the discovery of highly potent and selective BChE inhibitors warrants drug development for the potential to treat AD.
Some selective BChE inhibitors, selective AChE inhibitors, or dual AChE and BChE inhibitors have been reported previously. 12 For example, bambuterol is a well-known selective BChE inhibitor that can distinguish BChE from AChE. 13 Using hierarchical virtual screening and biochemical assays, a piperidin-3-ylmethanamine-based selective BChE inhibitor was identified, showing reversible binding inhibition with a potent low nanomolar IC50. 14 Another study utilized this compound as the starting point and synthesized a series of potent and selective BChE inhibitors. 15 Testosterone and 10 of its metabolites were identified as selective inhibitors of BChE. 16 Some BChE inhibitors, such as metoclopramide, ranitidine, and tiapride, have been shown to have a protective role against inhibition by potent organophosphorus compounds.17,18 Selective BChE inhibitors have the advantage of preserving long-term stable cognition and behavior in patients with advanced AD. 19 However, none of the current selective BChE inhibitors have been approved for treating AD, so there is a great need to discover more selective BChE inhibitors for potential clinical use.
To identify compounds that inhibit BChE activity, we used an enzyme-based BChE inhibition assay in a quantitative high-throughput screening (qHTS) format to screen 8998 compounds from the following compound libraries: Library of Pharmacologically Active Compounds (LOPAC), National Center for Advancing Translational Sciences (NCATS) Pharmacologically Active Chemical Toolbox (NPACT), and NCATS Pharmaceutical Collection (NPC). 20 Based on the potency and efficacy of compounds from the primary screening, we identified and selected 125 BChE inhibitors to further study their inhibitory effectiveness on BChE compared with AChE. These BChE inhibitors represent several structural classes. In addition, molecular docking analysis was used to study the binding mode of the most potent BChE inhibitors. Identification of BChE inhibitors from structurally diverse chemical libraries provides an efficient way to prioritize compounds for pharmacological applications.
Methods
Reagents and Compound Libraries
Amplite Colorimetric BChE and AChE assay kits were purchased from AAT Bioquest, Inc. (Sunnyvale, CA). Ethopropazine, physostigmine, BW284c51, and DMSO were purchased from Sigma-Aldrich Co. (St. Louis, MO). Chlorpyrifos-oxon was purchased from Chem Service, Inc. (West Chester, PA). Purified recombinant human BChE protein was from R&D Systems, Inc. (Minneapolis, MN). Purified recombinant AChE protein was from Sigma-Aldrich Co. LOPAC 1280 was purchased from Sigma-Aldrich Co. The NPC library contains 2816 compounds that are approved or investigational drugs. 20 The NPACT library contains 4902 structurally diverse compounds with known target or mechanism annotations.
BChE Inhibition Assays
Recombinant human BChE (50 mU/mL, 6.54 nM for colorimetric readout; 10 mU/mL, 1.31 nM for fluorescence readout) in 50 mM phosphate-buffered saline (PBS) at pH 7.4 was dispensed (4 µL/well) into black clear-bottom 1536-well plates (Greiner Bio-One North America, Monroe, NC). BChE in the presence of 0.05% Triton was used in a parallel colorimetric assay to rule out compound aggregation. Ethopropazine and physostigmine, known BChE inhibitors, were used as positive controls. Controls and test compounds (at eight different final concentrations from 0.37 nM to 28.75 μM) were transferred into the assay plates (23 nL/well) using a Wako Pintool station (Wako Automation, San Diego, CA). After a 30 min incubation period at room temperature, 4 µL of colorimetric detection cocktail solution (DTNB, butyrylcholine) or 4 µL of fluorometric detection cocktail solution (Thiolite Green, butyrylcholine) was added to each well using a BioRaptr Flying Reagent Dispenser (FRD) (Beckman Coulter, Brea, CA). The final DMSO concentration in the assay well was 0.29%. Assay plates were incubated at room temperature for another 10 min before measuring absorbance readout (405 nm) or measuring fluorescence readout (excitation = 480, emission =540) using an Envision plate reader (PerkinElmer, Shelton, CT).
AChE Inhibition Assay
The AChE inhibition assay was described in our previous study. 21 Briefly, recombinant human AChE (50 mU/mL) was dispensed (4 µL/well) into black clear-bottom 1536-well plates. Chlorpyrifos-oxon and BW284C51, known nonselective and selective AChE inhibitors, respectively, were used as positive controls. Twenty-three nanoliters of test compounds with concentrations ranging from 0.37 nM to 28.75 μM and controls were transferred into the assay plates using a Wako Pintool. After a 30 min incubation period at room temperature, 4 µL of colorimetric detection cocktail solution (DTNB, acetylthiocholine) was added to each well using a BioRaptr FRD (Beckman Coulter). The final DMSO concentration in the assay well was 0.29%. Assay plates were incubated at room temperature for another 10 min, followed by measuring the absorbance readout (405 nm) using an Envision plate reader (PerkinElmer).
Chemical Structure Data and Clustering
Chemical structures were converted to ToxPrint chemotype fingerprints, which were composed of 729 defined chemical features encoded by the XML-based Chemical Subgraphs and Reactions Markup Language (CSRML). These features were generated within the publicly available ChemoTyper application (https://chemotyper.org/) using the latest ToxPrint feature set (V2.0_r711; https://toxprint.org/) developed by Altamira (Columbus, OH) and Molecular Networks (Erlangen, Germany) under contract from the U.S. Food and Drug Administration (FDA). 22 The structure for each compound was represented as a bit vector where the presence or absence of the feature was recorded in a binary system as a 1 or 0, respectively. 22 Hierarchical clustering was performed using Euclidean distance as the similarity metric and the complete linkage method based on ToxPrint fingerprints. Graphing was performed in R software version 3.6.3 with the “ggplot2” package.
Molecular Docking
The structures of BChE and AChE were retrieved from the Protein Data Bank (PDB), and molecular structures of the inhibitors were downloaded from PubChem in sdf format. The current docking study used the published crystal structures of human cholinesterase in complex with inhibitors, including BChE-tacrine (PDB ID: 4BDS) and AChE-huprine W (PDB ID: 4BDT). 23 First, the ligands and proteins were prepared using AutoDock Tools, where heteroatoms from the protein are removed and polar hydrogen atoms and Kollman charges are added and saved in pdbqt format. The AutoDock Tools were used to create a grid box (size_x = 40; size_y = 40; size_z = 40) to incorporate the entire active site for each protein structure of BChE (with coordinates of center_x = 136.26; center_y = 115.98; center_z = 42.30) and AChE (with coordinates of center_x = −1.18; center_y = −36.63; center_z = −51.58). The potent compounds (IC50 < 1.0 µM) from the experimental confirmation were further selected for docking analysis. The molecular docking was performed by Autodock Vina, an open-source docking program. 24 The protein–ligand docked complexes were visualized using the PyMOL tool.
QHTS Data Analysis
Compound concentration–response data analysis was performed as previously described.25,26 First, raw plate reads for each titration point were normalized relative to the positive control compound (ethopropazine for the assay; –100%) and DMSO-only wells (0%) as follows: % Activity = (Vcompound-VDMSO)/(VDMSO-Vpos) × 100, where Vcompound represents the compound well values, Vpos represents the positive control well median values, and VDMSO represents the DMSO-only well median values. An in-house pattern correction algorithm was applied in data set correction using the DMSO-only compound plates at the beginning and end of the compound plate stack. 27 Fitting the concentration–response curves of each compound to a four-parameter Hill equation provided the half maximum inhibition values (IC50) and maximum response values (efficacy) for each compound. 28 Compounds received a curve class designation (classes 1–4) according to the type of concentration–response curve observed. 26 In the present study, antagonists were defined as compounds that inhibited BChE activity. Compounds exhibiting class –1.1, –1.2, –2.1, or –2.2 (efficacy < –50%) curves were considered active. Compounds exhibiting class 4 curves were considered inactive, and compounds with all other curve classes were defined as inconclusive. Only compounds that passed the chemical quality control test for identity (confirmed by molecular weight) and purity (>75%) were selected for confirmation and follow-up studies. Further data analysis was performed (e.g., t test) and depicted using GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA).
Results
Screening Performance for BChE Inhibition Assay
Enzyme-based, human BChE inhibition assay with colorimetric readout was first optimized by testing various enzyme concentrations ranging from 0.98 to 1000 mU/mL and different reaction time points from 6 to 30 min ( Suppl. Fig. S1A ). The 50 mU/mL BChE concentration was selected based on the linear portion of the curve with a good signal-to-background (S/B) window ( Suppl. Fig. S1B ). After optimization, this BChE inhibition assay was used to screen LOPAC, NPACT, and NPC libraries for potential BChE inhibitors. Ethopropazine hydrochloride, a selective BChE inhibitor, and physostigmine, a dual BChE and AChE inhibitor, were used as positive controls in the screening. Both ethopropazine hydrochloride and physostigmine inhibited BChE activity in a concentration-dependent manner with IC50 values of 1.70 ± 0.53 µM and 34.4 ± 14.7 nM, respectively. Screening performance parameters were determined in an enzyme-based screening assay with an S/B ratio of 5.89 ± 1.81, coefficient of variation (CV) of 5.75 ± 3.05, and Z factor (Z′) of 0.76 ± 0.15. The minimum significant ratio (MSRs) were also calculated based on the Assay Guidance Manual, 29 which were 2.7 and 3.3 for ethopropazine hydrochloride and physostigmine, respectively. Overall, these values demonstrate good assay performance.
Identification of Drug-Like Molecules That Inhibit BChE
From primary screening against three libraries (LOPAC, NPC, NPACT) containing 8998 small-molecule compounds, including many drugs and natural products, 315 (3.5%) compounds were identified to inhibit BChE activity with IC50 values less than 10 µM and efficacy more than 50%. One hundred twenty-eight compounds were selected for further follow-up studies based on efficacy (>50%) and IC50 (≤5 µM). Of the 128 compounds, 125 compounds were confirmed to be active, yielding a 98% confirmation rate. The top 20 potent compounds with IC50 values less than 1 µM are listed in Table 1 . Many known BChE inhibitors, including bambuterol hydrochloride, tacrine, PE154, ethyl 4-nitrophenyl ethylphosphonate, and physostigmine, were also identified as BChE inhibitors from the current study. A group of potential novel BChE inhibitors, such as diethylumbelliferyl phosphate, fenoverine, LX-7101, and NDT 9513727, were identified as well, with IC50 values of 0.01 μM, 0.09 μM, 0.22 μM, and 0.21 μM, respectively ( Table 1 ).
Table 1.
Top 20 Potent BChE Inhibitors.
| Compound Name | CAS No. | AChE, IC50 in µM (% Efficacy) | BChE, IC50 in µM (% Efficacy) |
|---|---|---|---|
| 9-Aminoacridine monohydrochloride monohydrate a | 52417-22-8 | 1.43 ± 0.18 (–95.4 ± 9.6) | 0.16 ± 0.05 (–101 ± 4.43) |
| Alverine citrate | 5560-59-8 | Inactive | 0.38 ± 0.09 (–95 ± 0.93) |
| Bentamapimod | 848344-36-5 | Inactive | 0.33 ± 0.02 (–98.8 ± 2.41) |
| Bambuterol hydrochloride a | 81732-46-9 | Inactive | 0.03 ± 0.01 (–91.4 ± 1.91) |
| BTZO 1 | 99420-15-2 | Inactive | 0.49 ± 0.25 (–94.6 ± 2.01) |
| Carvedilol metabolite 4-hydroxyphenyl carvedilol | 142227-49-4 | Inactive | 0.97 ± 0.11 (–100 ± 2.04) |
| Diethylumbelliferyl phosphate a | 897-83-6 | 0.42 ± 0.03 (–98.6 ± 5.66) | 0.01 ± 0.01 (–89.0 ± 1.08) |
| Eluxadoline | 864821-90-9 | Inactive | 0.34 ± 0.04 (–100 ± 4.44) |
| Ethyl 4-nitrophenyl ethylphosphonate a | 546-71-4 | 0.06 ± 0.03 (–94.6 ± 4.39) | 0.08 ± 0.03 (–95.1 ± 4.28) |
| Fenoverine | 37561-27-6 | Inactive | 0.09 ± 0.01 (–97.3 ± 2.29) |
| Haloxon a | 321-55-1 | 0.32 ± 0.02 (–101 ± 4.43) | 0.03 ± 0.01 (–91.2 ± 2.06) |
| LX-7101 | 1192189-69-7 | 7.71 ± 1.49 (-92.4 ± 7.28) | 0.22 ± 0 (–96.6 ± 6.87) |
| NDT 9513727 | 439571-48-9 | Inactive | 0.21 ± 0.01 (–101 ± 2.16) |
| Neostigmine bromide a | 114-80-7 | 0.12 ± 0.03 (–94.6 ± 12.9) | 0.9 ± 0.11 (–102 ± 4.4) |
| NNC 756 | 131796-63-9 | Inactive | 0.84 ± 0.11 (–98.2 ± 6.31) |
| Orlistat | 96829-58-2 | 1.01 ± 0.07 (–80.5 ± 29.9) | 0.12 ± 0.03 (–92.9 ± 11.5) |
| PE154 a | 1192750-33-6 | 0.02 ± 0.01 (–89.6 ± 0.63) | 0.39 ± 0.04 (–99.3 ± 5.35) |
| Physostigmine a | 57-47-6 | 0.18 ± 0.05 (–102.6 ± 4.47) | 0.09 ± 0.01 (–98.4 ± 4.12) |
| Tacrine hydrochloride a | 321-64-2 | 1.6 ± 0.11 (–97.5 ± 4.75) | 0.09 ± 0.01 (–93.4 ± 12.1) |
| Turofexorate isopropyl | 629664-81-9 | Inactive | 0.29 ± 0.07 (–98.5 ± 8.66) |
These compounds are known BChE inhibitors. Each value of potency (IC50 in µM) and efficacy (% of positive control, expressed in parentheses) is the mean ± SD of the results from three experiments.
To further confirm these compounds for their BChE inhibition, we used an orthogonal BChE inhibition assay with a fluorescent readout to test these 125 BChE inhibitors and found a 97.6% (122/125) confirmation rate for BChE inhibitors between the fluorescence readout assay and colorimetric readout assay. Three compounds, ACon1002016, LUF6000, and WIN 51708 hydrate, did not show inhibition against BChE in the fluorescent assay ( Suppl. Table S1 ).
To rule out compound aggregation issues, we tested these 125 BChE inhibitors in the assay buffer containing 0.05% Triton-100 and found a 93% concordance rate with the initial assay without Triton addition ( Suppl. Table S1 ).
Structure–Activity Relationship Analysis of Compounds That Inhibit BChE
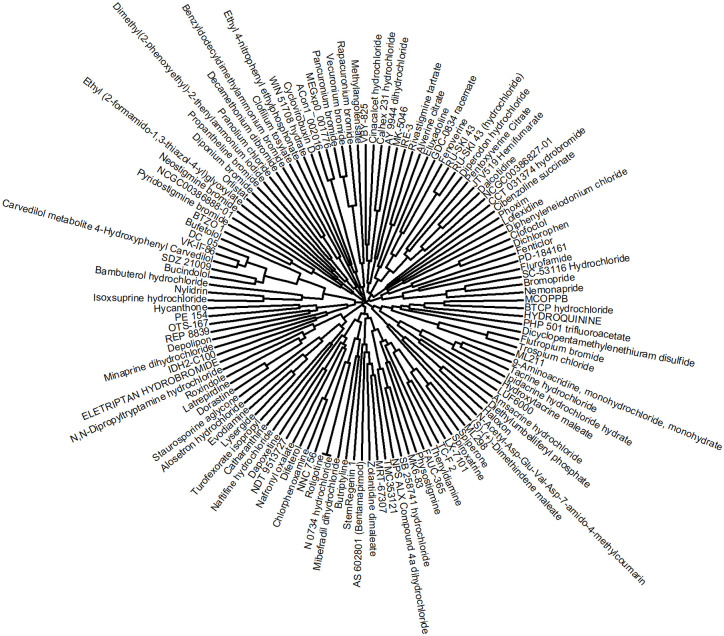
The 125 potential BChE inhibitors were grouped into 40 clusters of different sizes (ranging from one to nine compounds) based on their structural similarity ( Fig. 1 ). Of the 40 clusters, the largest cluster contains nine compounds (bufetolol, DC_05, VK-II-86, carvedilol metabolite 4-hydroxyphenyl carvedilol, SDZ 21009, bucindolol, bambuterol hydrochloride, nylidrin, and isoxsuprine hydrochloride) ( Suppl. Table S1 ). Three of the compounds in this cluster are among the top 20 most potent inhibitors, including carvedilol metabolite 4-hydroxyphenyl carvedilol and bambuterol hydrochloride ( Table 1 ). The second largest cluster contains the following six compounds: tacrine hydrochloride, hydroxytacrine maleate, 9-aminoacridine monohydrochloride monohydrate, amsacrine hydrochloride, ipidacrine hydrochloride hydrate, and LUF6000, most of which are known potent BChE inhibitors. Two clusters with five compounds each—zolantidine dimaleate, stemRegenin 1, MRT-67307, TMC353121, and bentamapimod in one cluster, and nafronyl oxalate, naftifine hydrochloride, difeterol, dapoxetine, and NDT 9513727 in the other—are potential novel inhibitors. Furthermore, seven clusters were singletons containing one compound each: neostigmine bromide, phoxim, dicyclopentame thylenethiuram disulfide, ethyl 4-nitrophenyl ethylphosphonate, cibenzoline succinate, eluxadoline, and CCT 031374 hydrobromide (Fig. 1 and Suppl. Table S1).
Figure 1.
Hierarchical clustering results of the 125 follow-up compounds. The clustering was performed using Euclidean distance with the complete linkage method based on ToxPrint fingerprints generated within the publicly available ChemoTyper application.
Comparison of Compound Potency in BChE and AChE Inhibition
To investigate the target selectivity of the BChE inhibitor identified from the current study, an AChE inhibition assay was used to evaluate the compounds. Of the 125 compounds tested, 100 only inhibited BChE and were inactive against AChE, while the remaining 25 compounds exhibited inhibitory activity toward both BChE and AChE. Bambuterol hydrochloride, a known BChE inhibitor, was confirmed in the current study ( Fig. 2A ). Many other compounds, such as tacrine, haloxon, physostigmine, PE154, and neostigmine, were known dual BChE and AChE inhibitors ( Table 1 ). The IC50 (BChE/AChE) ratios were 17 and 2 for tacrine and physostigmine, respectively ( Fig. 2B,C ). We also identified several potential novel dual BChE and AChE inhibitors, such as orlistat, zolantidine, and roxindole ( Table 1 ). Diethylumbelliferyl phosphate exhibited higher potency in BChE inhibition ( Fig. 2D ) with an IC50 (BChE/AChE) ratio of 42. Moreover, several potential novel BChE inhibitors did not show AChE inhibition at the highest test concentration, including bentamapimod ( Fig. 2E ), fenoverine ( Fig. 2F ), turofexorate isopropyl ( Fig. 2G ), and NDT 9513727 ( Fig. 2H ).
Figure 2.
Concentration–response curves of representative compounds in BChE and AChE assays. (A) Bambuterol hydrochloride, (B) tacrine hydrochloride, (C) physostigmine, (D) diethylumbelliferyl phosphate, (E) bentamapimod, (F) fenoverine, (G) turofexorate isopropyl, and (H) NDT 9513727. Each value represents the mean ± SD of three independent experiments.
Molecular Docking
To gain insights on the specific binding of inhibitors with BChE or AChE, molecular docking analysis was performed with 20 selected active compounds (IC50 < 1.0 µM), and all of which yielded binding affinities less than –6.4 kcal/mol and are given in Supplemental Table S2 . The well-defined binding sites have been indicated in the published crystal structures of both BChE and AChE and were reported to conserve key interactions, namely, Pi-Pi with Trp82/Trp86 and hydrogen bonding with His438/His447 in BChE/AChE, respectively. 23 In addition to the conserved residues in both proteins, some other common interactions were reported, including Asp70/Asp74, Ser198/Ser203, Ala328/Tyr337, Trp430/Trp439, Met434/Met443, and Met437/Pro446 of BChE/AChE, respectively. All these conserved and common amino acids were considered key residues for the active sites of our docking study. All the compounds interacted with at least one of the key residues present in the active site of BChE ( Suppl. Fig. S2A ). For example, bentamapimod and turofexorate isopropyl interacted with key residues, including Ser198 and His438 present in the active site of BChE (Fig. 3A,B). In the AChE docking analysis, bentamapimod and turofexorate isopropyl interacted with Tyr124 and Tyr72, respectively, which are not the key residues of the AChE active site ( Fig. 3C,D ). For AChE, 10 compounds docked within the active site of the protein and interacted with at least one or more key residues, whereas the remaining 10 showed no interaction with key residues present in the active site ( Suppl. Fig. S2B ). These compounds showed no interactions with the key residues present in the active site including bentamapimod, eluxadoline, ethyl 4-nitrophenyl ethylphosphonate, fenoverine, haloxon, LX-7101, NDT 9513727, NNC 756, PE154, and turofexorate isopropyl. Among these 10 compounds, only 3, including ethyl 4-nitrophenyl ethylphosphonate, haloxon, and PE154, were shown to be active in the experimental confirmation against both BChE and AChE ( Suppl. Table S2 ). The molecular docking analysis also revealed that the remaining seven inhibitors that were inactive or less potent against AChE did not interact with any of the key residues, nor the residues associated with a group of aromatic amino acids specific to AChE, which include Trp236, Phe295, Tyr337, Phe338, and Trp439 ( Suppl. Fig. S2B ).
Figure 3.
Molecular docking analyses for selected BChE inhibitors. Both (A) bentamapimod and (B) turofexorate isopropyl interacted with key residues in the BChE active site, but (C) bentamapimod and (D) turofexorate isopropyl did not bind to key residues in the AChE active site. The magenta dotted lines are the H-bond interactions with the amino acid residues (cyan lines with colored atoms) of the protein (gray cartoon), and the inhibitors shown as green sticks with colored atoms. The structures of protein–ligand docked complexes were analyzed using the PyMOL visualization tool.
Discussion
In this study, we performed a qHTS BChE inhibition assay using recombinant human BChE to screen a large set of known drugs and bioactive compounds for potential BChE inhibitors. The identified BChE inhibitors were structurally diverse, spanning several different chemotypes. In addition, an enzyme-based assay using recombinant human AChE was used to study the selectivity of the compounds that demonstrated BChE inhibitory activity. The binding mode and selectivity of these potential inhibitors were further examined through molecular docking. This high-throughput screening approach combined with molecular docking analyses provided an efficient way to identify potential novel BChE inhibitors from large chemical libraries.
Many BChE inhibitors have been identified from the primary screening. Among the 125 confirmed BChE inhibitors from the current study, 23 compounds demonstrated highly potent BChE inhibition with IC50 values <1 μM. These compounds included known dual BChE and AChE inhibitors that have been approved for treating AD, such as donepezil and rivastigmine. 12 In our study, we also identified a group of potentially novel selective BChE inhibitors, which are drugs used clinically for various indications. For example, fenoverine, an antispasmodic drug for smooth muscle relaxation, 30 was identified as a potent selective BChE inhibitor. Alverine citrate, a drug used for functional gastrointestinal disorders, 31 was shown to selectively inhibit BChE. Bentamapimod, a c-Jun NH2-terminal kinase inhibitor and a potential anticancer stem cell drug,32,33 was also identified to be a novel selective BChE inhibitor. NDT 9513727, a human C5a receptor inverse agonist, 34 showed selective BChE inhibition. Therefore, screening known drug and bioactive compound libraries for novel BChE inhibitors may be valuable for repurposing existing drugs for potential treatment of AD, entailing further evaluation in more physiologically relevant conditions and potential drug combination studies. 35
The 125 confirmed BChE inhibitors were grouped into several structural clusters with different chemotypes. One cluster contains acridine derivatives, such as tacrine hydrochloride, hydroxytacrine maleate, and 9-aminoacridine monohydrochloride monohydrate, which have been shown to be effective inhibitors of BChE and AChE in the pharmacotherapy of AD. 36 Bambuterol hydrochloride, a known selective BChE inhibitor, 13 is a carbamate ester. Most carbamates are inhibitors of BChE and AChE. 37 The other six chemicals clustered with bambuterol hydrochloride include bufetolol, DC_05, VK-II-86, carvedilol metabolite 4-hydroxyphenyl carvedilol, SDZ 21009, bucindolol, nylidrin, and isoxsuprine hydrochloride. A group of purine derivatives including zolantidine dimaleate, stemRegenin 1, MRT-67307, TMC353121, and bentamapimod were identified as novel BChE inhibitory compounds. Purine derivatives are reported to be potent inhibitors of cholinesterases. 38 In addition, a novel cluster containing nafronyl oxalate, naftifine hydrochloride, difeterol, dapoxetine, and NDT 9513727 was shown to inhibit BChE.
Many BChE inhibitors identified from the current study are also known AChE inhibitors. Among these 125 confirmed BChE inhibitors, 100 compounds were selective BChE inhibitors and 25 compounds were dual AChE and BChE inhibitors. Some compounds, such as donepezil, rivastigmine, and tacrine, were known nonselective cholinesterase inhibitors, 12 which our current study confirmed. Other dual inhibitors, such as ethyl 4-nitrophenyl ethylphosphonate, haloxon, and neostigmine bromide, were also confirmed. We also identified several novel nonselective cholinesterase inhibitors such as LX-7101 and orlistat. LX-7101 is a dual LIM-kinase and ROCK inhibitor that can be used for treating ocular hypertension and associated glaucoma. 39 Orlistat is a natural inhibitor of pancreatic lipases used for obesity treatment. 40
Molecular docking is a useful tool for exploring molecular interactions between proteins and ligands. To identify the differences between the interactions of the BChE inhibitors with the key residues present in the active sites of BChE and AChE, molecular docking analysis was performed with selected potent compounds. All the compounds docked well within the binding pocket of BChE. For AChE, some compounds, such as bentamapimod and turofexorate isopropyl, showed no interactions with the key residues that are present in the active site ( Fig. 3 ). The docking results were consistent with our experimental data, so it can be hypothesized that these two compounds were selective for BChE. In comparison to AChE, BChE has a broader variety of small molecules that interact with the enzyme. 41 This can be explained by the acyl-binding pocket difference between the two enzymes, which accommodates the acyl moiety. AChE contains two bulky amino acids (Phe), which are replaced with two smaller amino acids (Val and Leu) in BChE, resulting in a larger and more accessible active site. This enzyme structural difference enables BChE to accommodate chemically diverse molecules.42,43
In summary, we utilized a qHTS BChE inhibition assay to screen nearly 9000 bioactive compounds and natural products for their ability to inhibit BChE. Many identified selective BChE inhibitors are clinically used drugs with pharmaceutical applications. Several potentially novel BChE inhibitors identified from the current study may have repurposing value to treat neurological diseases such as AD. These potentially novel BChE inhibitors would require further testing with in vivo models to gain an enhanced understanding of their pharmacokinetic properties in a biological setting. For example, these novel BChE inhibitors can be further tested for the cognitive performance of elderly rats in a 14-unit T-maze paradigm. 9 Moreover, anticholinergic activity and β-amyloid peptide levels can also be evaluated using animals to explore the roles of these BChE inhibitors in treating AD. The use of the qHTS BChE inhibition assay in combination with molecular docking allowed us to identify and evaluate compounds for their inhibitory effects on BChE. The large data set generated from this study provides valuable training data for developing computational models that can be used to predict novel BChE inhibitors.
Supplemental Material
Supplemental material, sj-pdf-1-jbx-10.1177_24725552211030897 for Identification of Compounds for Butyrylcholinesterase Inhibition by Shuaizhang Li, Andrew Li, Jameson Travers, Tuan Xu, Srilatha Sakamuru, Carleen Klumpp-Thomas, Ruili Huang and Menghang Xia in SLAS Discovery
Acknowledgments
We thank Zina Itkin and Paul Shinn for compound management.
The views expressed in this paper are those of the authors and do not necessarily reflect the statements, opinions, views, conclusions, or policies of the National Center for Advancing Translational Sciences, National Institutes of Health. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Footnotes
Supplemental material is available online with this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by the Intramural Research Program of the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health.
ORCID iDs: Tuan Xu  https://orcid.org/0000-0001-6430-3500
https://orcid.org/0000-0001-6430-3500
Menghang Xia  https://orcid.org/0000-0001-7285-8469
https://orcid.org/0000-0001-7285-8469
References
- 1. Taylor P., Radic Z. The Cholinesterases—From Genes to Proteins. Annu. Rev. Pharmacol. 1994, 34, 281–320. [DOI] [PubMed] [Google Scholar]
- 2. Chatonnet A., Lockridge O. Comparison of Butyrylcholinesterase and Acetylcholinesterase. Biochem. J. 1989, 260, 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li B., Sedlacek M., Manoharan I., et al. Butyrylcholinesterase, Paraoxonase, and Albumin Esterase, but Not Carboxylesterase, Are Present in Human Plasma. Biochem. Pharmacol. 2005, 70, 1673–1684. [DOI] [PubMed] [Google Scholar]
- 4. Zhan C. G., Zheng F., Landry D. W. Fundamental Reaction Mechanism for Cocaine Hydrolysis in Human Butyrylcholinesterase. J. Am. Chem. Soc. 2003, 125, 2462–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lockridge O. Genetic Variants of Human Serum Cholinesterase Influence Metabolism of the Muscle Relaxant Succinylcholine. Pharmacol. Ther. 1990, 47, 35–60. [DOI] [PubMed] [Google Scholar]
- 6. Chen V. P., Gao Y., Geng L., et al. Plasma Butyrylcholinesterase Regulates Ghrelin to Control Aggression. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 2251–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Darvesh S., Walsh R., Kumar R., et al. Inhibition of Human Cholinesterases by Drugs Used to Treat Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2003, 17, 117–126. [DOI] [PubMed] [Google Scholar]
- 8. Li Q., Yang H. Y., Chen Y., et al. Recent Progress in the Identification of Selective Butyrylcholinesterase Inhibitors for Alzheimer’s Disease. Eur. J. Med. Chem. 2017, 132, 294–309. [DOI] [PubMed] [Google Scholar]
- 9. Greig N. H., Utsuki T., Ingram D. K., et al. Selective Butyrylcholinesterase Inhibition Elevates Brain Acetylcholine, Augments Learning and Lowers Alzheimer β-Amyloid Peptide in Rodent. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 17213–17218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diamant S., Podoly E., Friedler A., et al. Butyrylcholinesterase Attenuates Amyloid Fibril Formation In Vitro. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 8628–8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guillozet A. L., Smiley J. F., Mash D. C., et al. Butyrylcholinesterase in the Life Cycle of Amyloid Plaques. Ann. Neurol. 1997, 42, 909–918. [DOI] [PubMed] [Google Scholar]
- 12. Giacobini E. Selective Inhibitors of Butyrylcholinesterase: A Valid Alternative for Therapy of Alzheimer’s Disease? Drugs Aging 2001, 18, 891–898. [DOI] [PubMed] [Google Scholar]
- 13. Kovarik Z., Radic Z., Grgas B., et al. Amino Acid Residues Involved in the Interaction of Acetylcholinesterase and Butyrylcholinesterase with the Carbamates Ro 02-0683 and Bambuterol, and with Terbutaline. Biochim. Biophys. Acta 1999, 1433, 261–271. [DOI] [PubMed] [Google Scholar]
- 14. Brus B., Kosak U., Turk S., et al. Discovery, Biological Evaluation, and Crystal Structure of a Novel Nanomolar Selective Butyrylcholinesterase Inhibitor. J. Med. Chem. 2014, 57, 8167–8179. [DOI] [PubMed] [Google Scholar]
- 15. Kosak U., Brus B., Knez D., et al. Development of an In-Vivo Active Reversible Butyrylcholinesterase Inhibitor. Sci. Rep. 2016, 6, 39495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al-Aboudi A., Odeh H., Khalid A., et al. Butyrylcholinesterase Inhibitory Activity of Testosterone and Some of Its Metabolites. J. Enzyme Inhib. Med. Chem. 2009, 24, 553–558. [DOI] [PubMed] [Google Scholar]
- 17. Stojan J., Golicnik M., Froment M. T., et al. Concentration-Dependent Reversible Activation-Inhibition of Human Butyrylcholinesterase by Tetraethylammonium Ion. Eur. J. Biochem. 2002, 269, 1154–1161. [DOI] [PubMed] [Google Scholar]
- 18. Masson P., Froment M. T., Gillon E., et al. Kinetic Analysis of Effector Modulation of Butyrylcholinesterase-Catalysed Hydrolysis of Acetanilides and Homologous Esters. FEBS J. 2008, 275, 2617–2631. [DOI] [PubMed] [Google Scholar]
- 19. Giacobini E. Cholinesterase Inhibitors Stabilize Alzheimer’s Disease. Ann. N.Y. Acad. Sci. 2000, 920, 321–327. [DOI] [PubMed] [Google Scholar]
- 20. Huang R., Southall N., Wang Y., et al. The NCGC Pharmaceutical Collection: A Comprehensive Resource of Clinically Approved Drugs Enabling Repurposing and Chemical Genomics. Sci. Transl. Med. 2011, 3, 80ps16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li S., Zhao J., Huang R., et al. Use of High-Throughput Enzyme-Based Assay with Xenobiotic Metabolic Capability to Evaluate the Inhibition of Acetylcholinesterase Activity by Organophosphorous Pesticides. Toxicol. In Vitro 2019, 56, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang C., Tarkhov A., Marusczyk J., et al. New Publicly Available Chemical Query Language, CSRML, to Support Chemotype Representations for Application to Data Mining and Modeling. J. Chem. Inf. Model. 2015, 55, 510–528. [DOI] [PubMed] [Google Scholar]
- 23. Nachon F., Carletti E., Ronco C., et al. Crystal Structures of Human Cholinesterases in Complex with Huprine W and Tacrine: Elements of Specificity for Anti-Alzheimer’s Drugs Targeting Acetyl- and Butyryl-Cholinesterase. Biochem. J. 2013, 453, 393–399. [DOI] [PubMed] [Google Scholar]
- 24. Trott O., Olson A. J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang R. A Quantitative High-Throughput Screening Data Analysis Pipeline for Activity Profiling. Methods Mol. Biol. 2016, 1473, 111–122. [DOI] [PubMed] [Google Scholar]
- 26. Inglese J., Auld D. S., Jadhav A., et al. Quantitative High-Throughput Screening: A Titration-Based Approach That Efficiently Identifies Biological Activities in Large Chemical Libraries. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 11473–11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y., Huang R. Correction of Microplate Data from High-Throughput Screening. Methods Mol. Biol. 2016, 1473, 123–134. [DOI] [PubMed] [Google Scholar]
- 28. Wang Y., Jadhav A., Southal N., et al. A Grid Algorithm for High Throughput Fitting of Dose-Response Curve Data. Curr. Chem. Genomics 2010, 4, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haas J. V., Eastwood B. J., Iversen P. W., et al. Minimum Significant Ratio—A Statistic to Assess Assay Variability. In Assay Guidance Manual; Markossian S., Sittampalam G. S., Grossman A., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, 2004. [PubMed] [Google Scholar]
- 30. Camarri E. Fenoverine: Smooth Muscle Synchronizer for the Management of Gastro-Intestinal Conditions. II. A Trimebutine-Controlled, Double-Blind, Crossover Clinical Evaluation. Curr. Med. Res. Opin. 1986, 10, 52–57. [DOI] [PubMed] [Google Scholar]
- 31. Wittmann T., Paradowski L., Ducrotte P., et al. Clinical Trial: The Efficacy of Alverine Citrate/Simeticone Combination on Abdominal Pain/Discomfort in Irritable Bowel Syndrome—A Randomized, Double-Blind, Placebo-Controlled Study. Aliment. Pharmacol. Ther. 2010, 31, 615–624. [DOI] [PubMed] [Google Scholar]
- 32. Messoussi A., Feneyrolles C., Bros A., et al. Recent Progress in the Design, Study, and Development of c-Jun N-Terminal Kinase Inhibitors as Anticancer Agents. Chem. Biol. 2014, 21, 1433–1443. [DOI] [PubMed] [Google Scholar]
- 33. Kuramoto K., Yamamoto M., Suzuki S., et al. AS602801, an Anti-Cancer Stem Cell Drug Candidate, Suppresses Gap-Junction Communication between Lung Cancer Stem Cells and Astrocytes. Anticancer Res. 2018, 38, 5093–5099. [DOI] [PubMed] [Google Scholar]
- 34. Brodbeck R. M., Cortright D. N., Kieltyka A. P., et al. Identification and Characterization of NDT 9513727 [N,N-Bis(1,3-benzodioxol-5-ylmethyl)-1-butyl-2,4-diphenyl-1H-imidazole-5-methanamine], a Novel, Orally Bioavailable C5a Receptor Inverse Agonist. J. Pharmacol. Exp. Ther. 2008, 327, 898–909. [DOI] [PubMed] [Google Scholar]
- 35. Shinn P., Chen L., Ferrer M., et al. High-Throughput Screening for Drug Combinations. In Bioinformatics and Drug Discovery; Springer: Berlin, 2019; pp 11–35. [DOI] [PubMed] [Google Scholar]
- 36. Makhaeva G. F., Lushchekina S. V., Boltneva N. P., et al. 9-Substituted Acridine Derivatives as Acetylcholinesterase and Butyrylcholinesterase Inhibitors Possessing Antioxidant Activity for Alzheimer’s Disease Treatment. Bioorg. Med. Chem. 2017, 25, 5981–5994. [DOI] [PubMed] [Google Scholar]
- 37. Darvesh S., Darvesh K. V., McDonald R. S., et al. Carbamates with Differential Mechanism of Inhibition toward Acetylcholinesterase and Butyrylcholinesterase. J. Med. Chem. 2008, 51, 4200–4212. [DOI] [PubMed] [Google Scholar]
- 38. Rodriguez-Franco M. I., Fernandez-Bachiller M. I., Perez C., et al. Design and Synthesis of N-Benzylpiperidine-Purine Derivatives as New Dual Inhibitors of Acetyl- and Butyrylcholinesterase. Bioorg. Med. Chem. 2005, 13, 6795–6802. [DOI] [PubMed] [Google Scholar]
- 39. Harrison B. A., Almstead Z. Y., Burgoon H., et al. Discovery and Development of LX7101, a Dual LIM-Kinase and ROCK Inhibitor for the Treatment of Glaucoma. ACS Med. Chem. Lett. 2015, 6, 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heck A. M., Yanovski J. A., Calis K. A. Orlistat, a New Lipase Inhibitor for the Management of Obesity. Pharmacotherapy 2000, 20, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosenberry T. L., Brazzolotto X., Macdonald I. R., et al. Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study. Molecules 2017, 22, 2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sussman J. L., Harel M., Frolow F., et al. Atomic Structure of Acetylcholinesterase from Torpedo californica: A Prototypic Acetylcholine-Binding Protein. Science 1991, 253, 872–879. [DOI] [PubMed] [Google Scholar]
- 43. Vellom D. C., Radic Z., Li Y., et al. Amino Acid Residues Controlling Acetylcholinesterase and Butyrylcholinesterase Specificity. Biochemistry 1993, 32, 12–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jbx-10.1177_24725552211030897 for Identification of Compounds for Butyrylcholinesterase Inhibition by Shuaizhang Li, Andrew Li, Jameson Travers, Tuan Xu, Srilatha Sakamuru, Carleen Klumpp-Thomas, Ruili Huang and Menghang Xia in SLAS Discovery