Key Points
Question
What is the drug survival of biologic therapies in patients with hidradenitis suppurativa (HS) that are used in a real-world setting?
Findings
In this nationwide cohort study of 241 Danish patients with HS, drug survival was comparable between adalimumab, infliximab, and ustekinumab, but significantly lower for etanercept. There were no differences in drug survival among biologic therapy–naive and nonnaive patients.
Meaning
The data in this study suggest that the drug survival of biologics in HS is more reduced than for other chronic inflammatory diseases, thus highlighting the need for further novel targeted therapies for HS.
Abstract
Importance
Biologics are important in treating patients with hidradenitis suppurativa (HS). However, to our knowledge, data on their real-life performance and treatment patterns in HS are limited.
Objective
To examine the drug survival of biologic therapies for HS in a real-world setting.
Design, Setting, and Participants
This cohort study included all patients with HS between January 1, 2005, and December 31, 2018, who were treated with biologics at the 5 academic hospital clinics where all biologic treatment for HS is conducted in Denmark. Biologics included adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, infliximab, secukinumab, and ustekinumab. Data were analyzed between June 1, 2021, and June 20, 2021.
Main Outcomes and Measures
Drug survival was depicted through Kaplan-Meier curves, and Cox regression models were used to calculate adjusted (age, sex, previous number of biologic treatment series) hazard ratios (aHRs) with 95% CIs for the risk of treatment discontinuation. Switching patterns were visualized through a Sankey diagram.
Results
The study comprised 241 patients (176 women [61.8%]; total of 386 treatment series) with a mean (SD) age of 41.8 (12.6) years at initiation of first biologic therapy. There were a total of 256 (189 [73.8%] biologic naive), 66 (32 [48.5%] biologic naive), 23 (9 [39.1%] biologic naive), and 22 (9 [40.9%] biologic naive) treatment series with adalimumab, infliximab, etanercept, and ustekinumab, respectively. The median time to discontinuation was 36.0 (IQR, 21.9-63.0), 28.7 (IQR, 15.1-62.9), 26.0 (IQR, 16.9-155.9), and 17.9 weeks (IQR, 12.9-41.0) for adalimumab, infliximab, ustekinumab and etanercept, respectively. The risk of drug discontinuation was significantly higher for etanercept compared with adalimumab (aHR, 1.81; 95% CI, 1.16-2.82), infliximab (aHR, 1.77; 95% CI, 1.03-3.05), and ustekinumab (aHR, 2.49; 95% CI, 1.12-5.52), whereas no difference was observed when comparing these 3 therapies with each other. We found no significant differences in drug survival for biologic-naive vs nonnaive treatment series. Increasing C-reactive protein levels (aHR, 1.01; 95% CI, 1.00-1.03) and concomitant antibiotic treatment (aHR, 2.82; 95% CI, 1.36-5.86) were associated with the risk of discontinuing infliximab therapy. Men (aHR, 0.69; 95% CI, 0.51-0.91) had a reduced risk of discontinuing use of adalimumab.
Conclusions and Relevance
In this cohort study, drug survival was comparable between adalimumab, infliximab, and ustekinumab but significantly lower for etanercept. There were no differences in drug survival among biologic-naive and nonnaive patients.
This cohort study examines the drug survival of biologic therapies for Danish patients with hidradenitis suppurativa.
Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory disease that is characterized by recurrent nodules, tunnels, and excessive scarring involving predominately intertriginous regions.1 Interleukin-17A and tumor necrosis factor levels are increased in serum and HS lesions and are associated with HS severity.2,3
Hidradenitis suppurativa is a heterogeneous disease with multiple phenotypes and is notoriously a challenge to treat. However, with the introduction of biologics, such as tumor necrosis factor–α inhibitors, disease control has become possible in some patients. In the trials PIONEER I and II,4,5 41.8% and 58.9% of adalimumab-treated patients with HS, respectively, achieved at least a 50% reduction in inflammatory nodule and abscess count compared with baseline after 12 weeks of therapy. Although biologics appear to play an increasing role in treating severe HS, data on the real-life treatment patterns and performance of biologic therapies in HS are scarce.
Because biologics may lose their efficacy over time and be discontinued because of several reasons, drug survival (the probability of continuing to receive therapy) is a well-established proxy measure of real-life drug performance. Thus, knowledge of the real-world efficacy of biologics in HS may aid clinicians in achieving a better understanding of the potential treatment outcomes.
Considering that, to our knowledge, adalimumab is the only US Food and Drug Administration–approved biologic for HS, we hypothesized that this therapy would exhibit a longer drug survival time compared with other off-label biologics. Therefore, we examined the real-world drug survival and switching patterns of biologics in a nationwide cohort of patients with HS in Denmark.
Methods
A full description of the data sources, study design, and statistical methods are available in the Methods in the Supplement. The study was approved by the Danish Data Protection Agency and registered at the Capital Region’s inventory (Videnscenter for Dataanmeldelser, reference number VD-2018-286). The study constituted the necessary legal requirements, and therefore, informed consent was waived. Briefly, the Civil Registration System enables unambiguous individual-level linkage across more than 150 nationwide registries in Denmark. The National Patient Registry contains information about medical diagnosis, comorbidities, surgical procedures, and treatment with biologic therapies of all patients treated for HS in Denmark. Prescription data from all community pharmacies are recorded in the National Prescription Registry, and laboratory measurements are recorded in the Registry of Laboratory Results for Research.
Study Design
We identified all Danish patients from 2005 through 2018 who were treated for HS with biologic therapies. We generated treatment series (ie, sequences of continuous treatment with the same drug), and follow-up started on the date of initiation of a treatment series and ended on the date of discontinuation of therapy, switching of therapy, or on December 31, 2018, whichever came first.
Statistical Analysis
We used a Cox regression to create adjusted (age, sex, and number of previous therapies) hazard ratios (aHRs). All biologics were included in a Sankey diagram, whereas only adalimumab, infliximab, etanercept, and ustekinumab were included in the remaining analyses, as these were the only biologics with a reasonably large number of treatment series. All analyses were performed using SAS, version 9.4 (SAS Institute), and Stata, version 15.0 (StataCorp). P < .05 was considered statistically significant, and results were reported with 95% CIs when applicable.
Results
The study comprised 241 patients with a mean (SD) age of 41.8 (12.6) years at initiation of first biologic therapy (eTable 1 in the Supplement). There were 256 adalimumab treatment series, of which 189 (73.8%) were biologic naive (bio-naive). Sixty-six infliximab series (27.4%; 32 [48.5%] bio-naive), 23 (9.5%; 9 [39.1%] bio-naive) etanercept, and 22 (9.1%; 9 [40.9%] bio-naive) ustekinumab treatment series were identified (Table).
Table. Characteristics of Patients Receiving at Least 1 Treatment Series With Adalimumab, Etanercept, Infliximab, or Ustekinumab.
| Characteristic | No. (%)a | |||
|---|---|---|---|---|
| Adalimumab | Etanercept | Infliximab | Ustekinumab | |
| Patients, No. | 202 | 17 | 58 | 20 |
| Age, mean (SD), y | 41.9 (12.6) | 38.4 (10.2) | 45.3 (13.5) | 42.9 (14.5) |
| Sex | ||||
| Women | 120 (59.4) | 13 (76.5) | 31 (53.5) | 12 (60.0) |
| Men | 82 (40.6) | 4 (23.5) | 27 (46.5) | 8 (40.0) |
| BMI, mean (SD) | 32.3 (8.3) | 28.4 (5.2) | 31.8 (8.7) | 30.2 (11.6) |
| Body weight, mean (SD), kg | 103.1 (28.9) | 101 (27.1) | 103.0 (30.6) | 123.2 (45.3) |
| Treatment series | ||||
| All, No. series (%) | 256 (100) | 23 (100) | 66 (100) | 22 (100) |
| Bio-naive, No. series (%) | 189 (73.8) | 9 (39.1) | 32 (48.5) | 9 (40.9) |
| Nonnaive, No. series (%) | 67 (26.2) | 14 (60.9) | 34 (51.5) | 13 (59.1) |
| Previous antibiotics | 191 (95.0) | 17 (100) | 55 (94.8) | 16 (80.0) |
| Doxycycline | 68 (33.7) | 5 (29.4) | 21 (36.2) | 5 (25.0) |
| Lymecycline | 72 (35.6) | 7 (41.2) | 18 (31.0) | 8 (40.0) |
| Tetracycline | 152 (75.3) | 16 (94.1) | 47 (81.0) | 13 (65.0) |
| Rifampicin/clindamycin | 136 (67.3) | 9 (52.9) | 39 (67.2) | 9 (45.0) |
| Previous HS surgery | 171 (84.7) | 15 (88.2) | 54 (93.1) | 18 (90.0) |
| Comorbidity | ||||
| Inflammatory bowel disease | 25 (12.4) | 3 (16.7) | 12 (20.7) | 4 (20.0) |
| Inflammatory rheumatic disease | 30 (14.9) | 3 (16.7) | 10 (17.2) | <3 (NS) |
| Ischemic heart disease | 17 (8.4) | 0 | 7 (12.1) | <3 (NS) |
| Liver disease | 9 (4.5) | 0 | 5 (8.6) | <3 (NS) |
| Kidney disease | 9 (4.5) | 0 | 4 (6.9) | <3 (NS) |
| Diabetes | 30 (14.9) | <3 (NS) | 12 (20.7) | 4 (20.0) |
| History of depression | 12 (5.9) | <3 (NS) | 6 (10.3) | <3 (NS) |
| CRP values, median (IQR) | 9 (3-22) | 8 (5-9) | 14 (5-29) | 13 (9-42) |
Abbreviations: bio-naive; biologic-naive; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CRP, C-reactive protein; HS, hidradenitis suppurativa; NS, not shown because of data security requirements.
Patients may have received several different therapies over time. Therefore, the same patient may be represented in several groups (eg, adalimumab and etanercept) in the table.
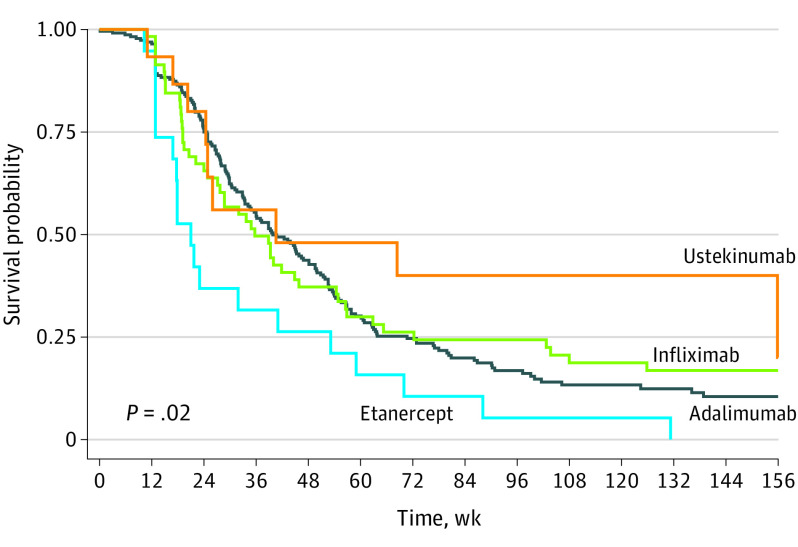
The median survival time for adalimumab was 36.0 (IQR, 21.9-63.0) weeks. Stratified analyses yielded median survival times of 39.6 (IQR, 22.9-63.4) and 28.9 (IQR, 13.3-60.1) weeks, for bio-naive and nonnaive patients, respectively (P = .25) (eTable 2 in the Supplement; Figure 1). For infliximab, median time to discontinuation was 28.7 (IQR, 15.1-62.9) weeks, with a median of 33.7 (IQR, 12.9-65.3) and 27.1 (IQR, 18.6-56.9) weeks for bio-naive and nonnaive patients, respectively (P = .63) (eTable 2 and eFigure 1 in the Supplement). Time to etanercept discontinuation was lower (median, 17.9 weeks; IQR, 12.9-41.0 weeks). In this instance, the median survival time for bio-naive and nonnaive patients was virtually identical (bio-naive, 17.9 weeks; IQR, 12.9-23.0 weeks; nonnaive, 17.9 weeks; IQR, 12.9-41.0 weeks; P = .89). The median survival time for ustekinumab was 26.0 (IQR, 16.9-155.9) weeks, while the median survival time was 40.6 (IQR not applicable) and 26.0 (IQR, 20.3-155.9) weeks for bio-naive and nonnaive patients (P = .23). Etanercept treatment conferred a significantly higher discontinuation risk compared with adalimumab (aHR, 1.81; 95% CI, 1.16-2.82), infliximab (aHR, 1.77; 95% CI, 1.03-3.05), and ustekinumab (aHR, 2.49; 95% CI, 1.12-5.52).
Figure 1. Overall Drug Survival Through 156 Weeks.
Female sex was associated with reduced adalimumab survival (eTable 3 in the Supplement). Furthermore, analysis showed that increasing C-reactive protein (CRP) levels (aHR, 1.01; 95% CI, 1.00-1.03) and concomitant antibiotic treatment (aHR, 2.82; 95% CI, 1.36-5.86) were associated with reduced drug survival for infliximab (eTable 3 in the Supplement). Absolute CRP values are shown in the Table.
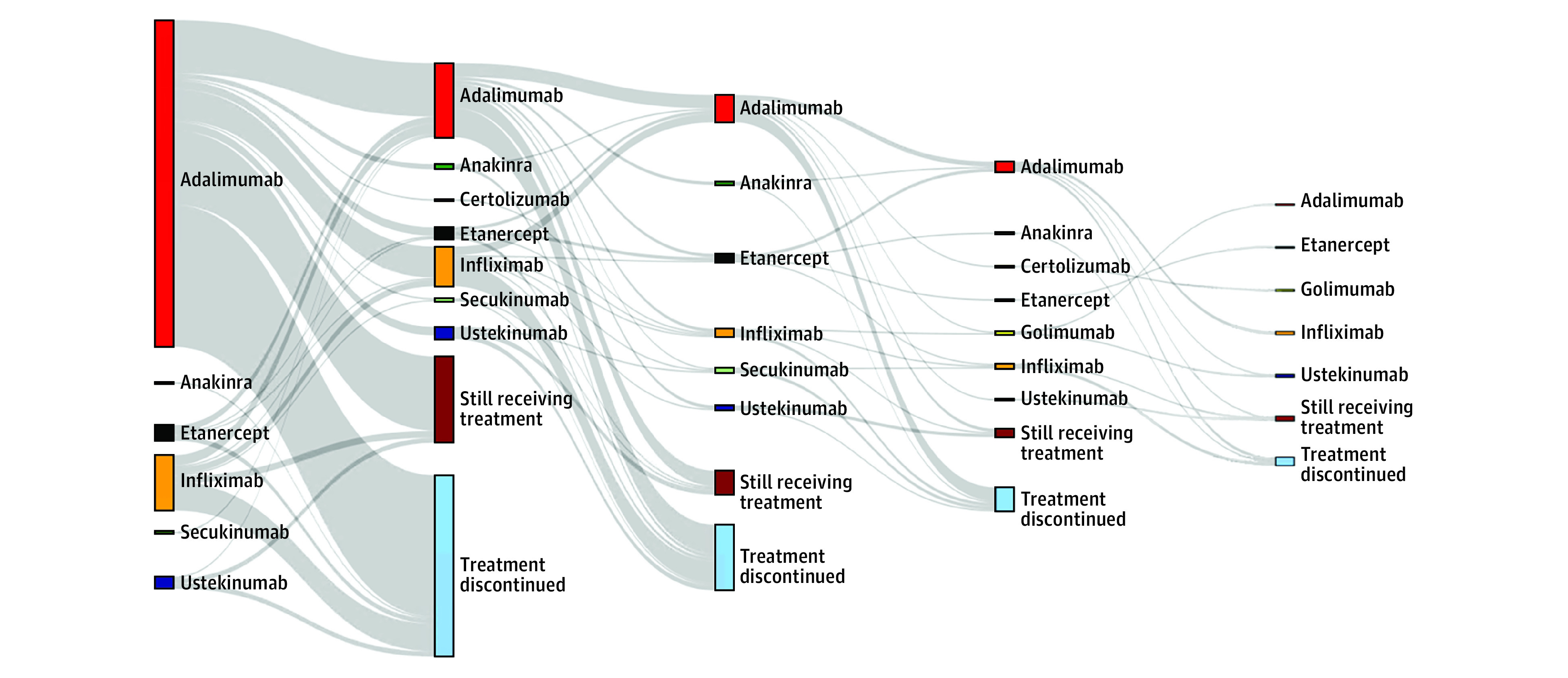
Among patients who were initiating first-time biologic therapy, most discontinued treatment without receiving another biologic treatment thereafter (Figure 2). Collectively, patients most often switched to receiving treatment with adalimumab in a second series, followed by infliximab and etanercept. Furthermore, we observed that patients often were treated with several courses of adalimumab (ie, readministering therapy in a new treatment series after a longer drug-free period). More infrequently used biologics (eg, certolizumab pegol, anakinra, golimumab, and secukinumab) were most often used for patients who had previously been treated with adalimumab, infliximab, etanercept, or ustekinumab in 1 or more treatment series (Figure 2).
Figure 2. Visualization of Switching Patterns in Patients With Hidradenitis Suppurativa Who Were Treated With Biologics.
Discussion
In this nationwide cohort study of 241 patients with HS, drug survival was comparable between adalimumab, infliximab, and ustekinumab and was similar among bio-naive and nonnaive patients. Etanercept was associated with a significantly increased risk of discontinuation and had considerably shorter drug survival compared with the other biologics. Although etanercept is not recommended for patients with HS,6 the study data suggest that dermatologists appear to choose the drug nevertheless. Moreover, the average time to discontinuation of etanercept use may also add valuable information for clinicians and patients.
To our knowledge, only 2 studies have previously reported HS drug survival.7,8 A Dutch dual-center study of 104 patients who were treated with adalimumab and 44 patients treated with infliximab suggested that increasing age, longer disease duration, higher body mass index (calculated as weight in kilograms divided by height in meters squared), and more severe disease was associated with longer adalimumab survival.7 These study results differ from those of the present study, in which female sex was the only predictor of adalimumab discontinuation. Moreover, this study found that for infliximab, HS surgery during therapy was associated with better drug survival. In contrast, concomitant antibiotics and increasing CRP levels were the only significant predictors for poor drug survival in the present study. We found that for each point increase in CRP, the risk of infliximab discontinuation increased with 1%. This may suggest that CRP represents a biomarker of more severe HS or flares of other inflammatory comorbidities.9
We found a conspicuously lower drug survival for biologics in patients with HS than seen for patients with psoriasis, rheumatological diseases, or inflammatory bowel disease.10,11,12 The higher inflammatory load in HS, as well as potential cooccurrence of secondary infections of anaerobic Gram-negative bacteria during HS flares, could13,14 partially explain this lower efficacy in HS.
Limitations
This study was limited by few patients being treated with etanercept and ustekinumab. However, the nationwide cohort, in which all biologics-treated patients with HS were included, the relatively long follow-up, and the detailed description of switching patterns may help to improve the current understanding of the role of biologics in treating HS. Lastly, the study was limited by the lack of data on dermal tunnels or family history of HS.
Conclusions
In this cohort study, drug survival was comparable between adalimumab, infliximab, and ustekinumab, but significantly lower for etanercept. Drug survival in patients with HS appears to be considerably lower than seen for other inflammatory diseases, such as psoriasis, thus suggesting the complex role of proinflammatory cytokines in the pathogenesis of HS. These findings highlight the need for further development of novel targeted therapies for treating HS.
eMethods.
eTable 1. Characteristics of HS patients, prescribing hospitals and treatment series
eTable 2. Follow-up time, failure rates and median time to discontinuation in HS patients treated with biologics
eTable 3. Univariate Cox regression analysis for adalimumab and infliximab
eFigure. Drug-specific persistence in bio-naïve and non-naïve patient
eReferences
References
- 1.Jemec GBE. Clinical practice: hidradenitis suppurativa. N Engl J Med. 2012;366(2):158-164. doi: 10.1056/NEJMcp1014163 [DOI] [PubMed] [Google Scholar]
- 2.Matusiak Ł, Szczęch J, Bieniek A, Nowicka-Suszko D, Szepietowski JC. Increased interleukin (IL)-17 serum levels in patients with hidradenitis suppurativa: implications for treatment with anti-IL-17 agents. J Am Acad Dermatol. 2017;76(4):670-675. doi: 10.1016/j.jaad.2016.10.042 [DOI] [PubMed] [Google Scholar]
- 3.van der Zee HH, de Ruiter L, van den Broecke DG, Dik WA, Laman JD, Prens EP. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164(6):1292-1298. doi: 10.1111/j.1365-2133.2011.10254.x [DOI] [PubMed] [Google Scholar]
- 4.Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375(5):422-434. doi: 10.1056/NEJMoa1504370 [DOI] [PubMed] [Google Scholar]
- 5.Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157(12):846-855. doi: 10.7326/0003-4819-157-12-201212180-00004 [DOI] [PubMed] [Google Scholar]
- 6.Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization—systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019;33(1):19-31. doi: 10.1111/jdv.15233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prens LM, Bouwman K, Aarts P, et al. Adalimumab and infliximab survival in patients with hidradenitis suppurativa: a daily practice cohort study. Br J Dermatol. 2021;185(1):177-184. doi: 10.1111/bjd.19863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomsen SF, Sand FL. Adherence to TNF-alpha inhibitors in patients with hidradenitis suppurativa. J Dermatolog Treat. 2015;26(1):97-98. doi: 10.3109/09546634.2013.879092 [DOI] [PubMed] [Google Scholar]
- 9.Miller IM, Ellervik C, Vinding GR, et al. Association of metabolic syndrome and hidradenitis suppurativa. JAMA Dermatol. 2014;150(12):1273-1280. doi: 10.1001/jamadermatol.2014.1165 [DOI] [PubMed] [Google Scholar]
- 10.Egeberg A, Ottosen MB, Gniadecki R, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178(2):509-519. doi: 10.1111/bjd.16102 [DOI] [PubMed] [Google Scholar]
- 11.Gil-Candel M, Gascón-Cánovas JJ, Urbieta-Sanz E, Rentero-Redondo L, Onteniente-Candela M, Iniesta-Navalón C. Comparison of drug survival between infliximab and adalimumab in inflammatory bowel disease. Int J Clin Pharm. 2020;42(2):500-507. doi: 10.1007/s11096-020-00978-6 [DOI] [PubMed] [Google Scholar]
- 12.Flouri ID, Markatseli TE, Boki KA, et al. Comparative analysis and predictors of 10-year tumor necrosis factor inhibitors drug survival in patients with spondyloarthritis: First-year response predicts long term drug persistence. J Rheumatol. 2018;45(6):785-794. doi: 10.3899/jrheum.170477 [DOI] [PubMed] [Google Scholar]
- 13.Ring HC, Thorsen J, Saunte DM, et al. The follicular skin microbiome in patients with hidradenitis suppurativa and healthy controls. JAMA Dermatol. 2017;153(9):897-905. doi: 10.1001/jamadermatol.2017.0904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ring HC, Sigsgaard V, Thorsen J, et al. The microbiome of tunnels in hidradenitis suppurativa patients. J Eur Acad Dermatol Venereol. 2019;33(9):1775-1780. doi: 10.1111/jdv.15597 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Characteristics of HS patients, prescribing hospitals and treatment series
eTable 2. Follow-up time, failure rates and median time to discontinuation in HS patients treated with biologics
eTable 3. Univariate Cox regression analysis for adalimumab and infliximab
eFigure. Drug-specific persistence in bio-naïve and non-naïve patient
eReferences