Abstract
Calcific uremic arteriolopathy, commonly referred to as “calciphylaxis,” is a rare life-threatening condition observed in patients with chronic kidney disease and end-stage renal disease on dialysis. This results in necrotic, ischemic, tender dermal lesions anywhere in the body, but mainly on the abdominal wall and lower extremities, where subcutaneous tissue is abundant. Histologically, it is defined by calcification in dermal capillaries, arterioles, and subcutaneous adipose tissues. It can occur in all advanced stages of chronic kidney disease as well as end-stage renal disease patients on hemodialysis or peritoneal dialysis. Our case highlights a successful case of calciphylaxis in a young female patient who underwent parathyroidectomy and intensification of peritoneal dialysis regimen along with the infusion of sodium thiosulphate injection resulting in complete resolution of the lesion in 3 months. With limited evidence of treatment options and increased frequency of this condition in a dialysis patient, our case highlights the key aspects of calciphylaxis management in a young end-stage renal disease patient who didn’t need a change of dialysis modality. We also review the risk factors and current practiced management options of this condition in our article.
Keywords: calciphylaxis, peritoneal dialysis, parathyroidectomy, sodium thiosulphate, end stage renal disease
Introduction
Calcific uremic arteriolopathy, commonly referred to as “calciphylaxis,” is a rare life-threatening condition observed in patients with chronic kidney disease and end-stage renal disease on dialysis. This results in necrotic, ischemic, tender dermal lesions anywhere in the body, but a mainly abdominal wall and lower extremities. Histologically, it is defined by calcification in dermal capillaries, arterioles, and subcutaneous adipose tissues. This condition can occur in all advanced stages of chronic kidney diseases (non-uremic calciphylaxis) as well as in end-stage renal disease (ESRD) patients on hemodialysis patients(HD) or peritoneal dialysis (PD).1,2 It is also reported in renal transplant recipients. 2 The United States Renal Data System suggests the mortality of long-term hemodialysis with calcific uremic arteriolopathy is 3-times higher compared to dialysis patients without this condition. 3 Some studies have even shown a 6-month survival rate of patients with this condition to be only about 50%. 4
Case Report
A 34-year-old Hispanic female was admitted with the chief complaint of a painful nonhealing abdominal wound for 2 weeks duration. The patient initially developed few scabs in the infra umbilical area which led to frequent itching and irritation. She used plain cotton gauze with vaseline ointment to treat them by herself. The scabs later turned into ulcers. Her past medical history was significant for ESRD, and she has been on continuous ambulatory peritoneal dialysis (CAPD) for 5 years, gastroesophageal reflux disease, obesity, anemia of chronic kidney disease (CKD), secondary hyperparathyroidism of renal origin, and coccidioidomycosis infection. Her chronic renal disease was from amphotericin B drug toxicity secondary to previous disseminated coccidioidomycosis treatment. The patient had a history of uncontrolled hyperparathyroidism with an intact parathyroid hormone level as high as 2400 picograms/milliliter (pg/mL) and an elevated phosphorus level of 10 mg/dL as an outpatient. These were values 6 months before the current hospitalization. This was difficult to control mainly because of financial constraints in getting the medications and nonadherence to prescribed phosphate binders. Home medications include sevelamer carbonate 3200 milligrams (mg) 3 times a day with meals. The patient lived at her parent’s home and has stopped working because of multiple medical issues. The patient denied any history of smoking, alcohol, or illicit drug use. Her peritoneal dialysis prescription was 4 daytime exchanges of 2-L peritoneal dialysate fluid, each bag composed of 2.5% dextrose and each exchange for 4 hours daily. Her weekly Kt/V was 2.1, with no residual kidney function.
Vital signs on presentation were, temperature of 98.6 Fahrenheit (F), pulse rate of 84 beats per minute (bpm), respiratory rate of 18 breaths per minute, blood pressure 117/59 mm Hg, and oxygen saturation of 99% on room air. Physical examination showed a morbidly obese female in mild distress due to abdominal pain. Abdominal examination revealed multiple tender indurated ulcers with surrounding erythema and black eschar in the lower abdomen section (Figures 1 and 2) along with skin ulceration with exposed subcutaneous tissue and granulation in lateral walls (Figures 3 and 4). The rest of the physical examination was non-significant.
Figure 1.
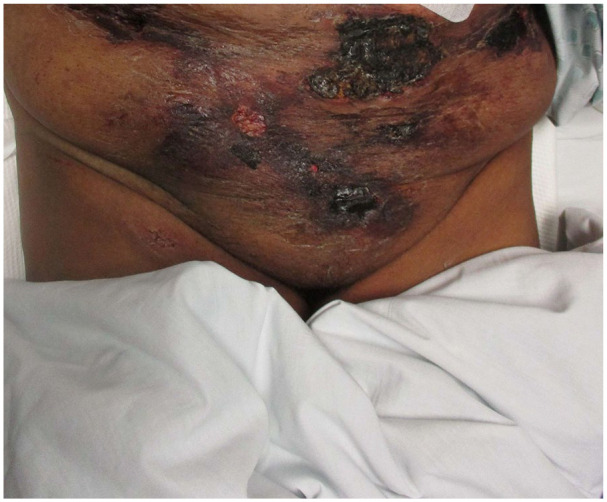
Abdominal lesion.
Figure 2.
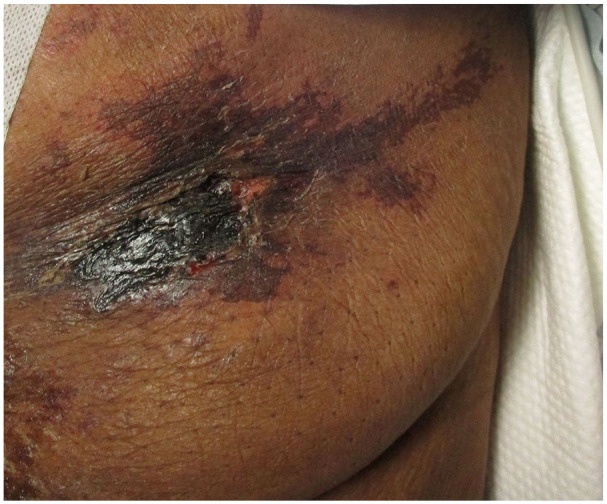
Abdominal lesion close up.
Figure 3.
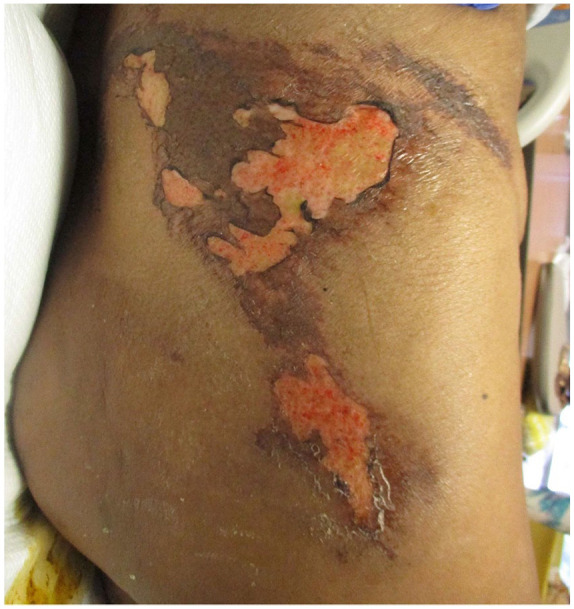
Lateral wall lesion.
Figure 4.
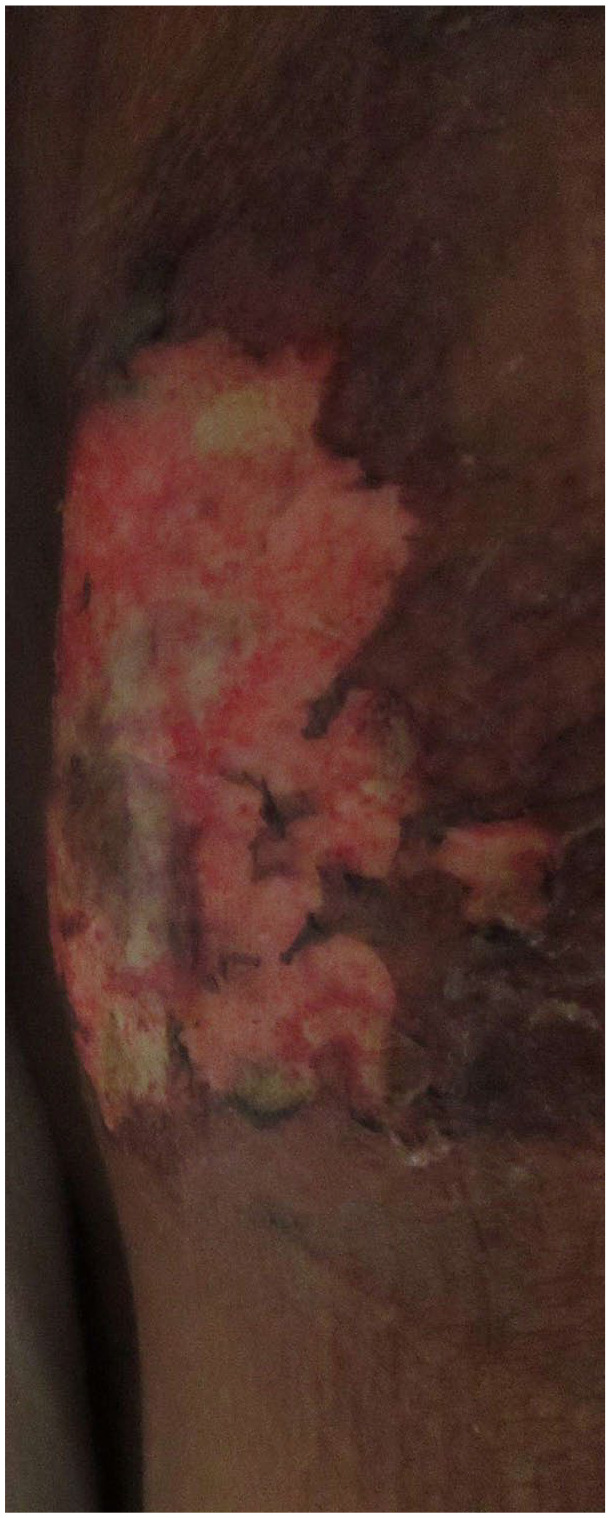
Lateral wall lesion close up.
Laboratory data revealed elevated white blood count (WBC) 21,000/mm3, hemoglobin 8.5 g/dL, platelets 526,000/mm3, sodium 131 mmol/L, potassium 3.4 mmol/L, blood urea nitrogen 59 mg/dL, creatinine 13.26 mg/dL, albumin 3.3 g/dL, calcium 8.6 mg/dL, phosphorus 8.3 mg/dL, parathyroid hormone 712 pg./mL, C reactive protein 129.8 mg/L, CAPD fluid was not positive for an infection, and blood cultures were negative for any infection. Computerized tomography (CT) abdomen and pelvis with and without contrast revealed anterior abdominal wall subcutaneous calcification. Parathyroid imaging by 29.2 mCi Technetium 99m Sestamibi scan revealed no abnormal radiotracer accumulation to suggest parathyroid adenoma.
To the patient was initially treated with empiric antibiotics until the infection was ruled out and was also started on sodium thiosulphate 25 g in sterile water intravenous infusion over 30 minutes, 3 times a week for 3 months. Due to unavailability of dermatologist and skilled surgeons, skin biopsy was not possible. The peritoneal dialysis regimen was intensified with the patient switching to continuous cycler peritoneal dialysis and doing 6 exchanges throughout the night with 2.5-L peritoneal dialysate fluid, each bag composed of 2.5 % dextrose and each exchange for 2.5 hours. The patient underwent 4-gland parathyroidectomy with implantation of half of the right superior parathyroid gland on the right forearm. The patient also received aggressive wound care daily. The calciphylaxis lesions healed completely in 3 months with intense peritoneal dialysis, sodium thiosulfate infusion for 3 months, and parathyroidectomy. The dialysis modality was never changed to hemodialysis during the management of calciphylaxis lesions.
Discussion
Calcific uremic arteriolopathy is becoming more prevalent in the current dialysis population. However, the literature on this condition is still limited, especially in the peritoneal dialysis population, and management options are suboptimal with no specific Food and Drug Administration (FDA)-approved therapy. Initially, this nomenclature was inducted into modern medicine in 1961 by H. Seyle, based on his experience of promoting vascular calcification in rodents. 5
Due to limited experience and infrequent reporting, the risk factors are still anecdotal without definitive large-scale study. Some postulated risk factors for the development of calciphylaxis in HD patients include diabetes, warfarin therapy, and irregularities in mineral bone factors like phosphate dysregulation. Data on the incidence and risk factors for the development of calciphylaxis in PD patients are limited, but a study done by Zhang et al with 63 adult PD patients identified several potential risk factors among the 7 patients that developed calciphylaxis. The PD patients that developed calciphylaxis were more likely to be female, previously have been on HD, and have higher frequencies of obesity. Warfarin therapy was among the highest risk for calciphylaxis among PD patients. 1 In advanced CKD cases, approximately 1% to 4% of patients manifest calciphylaxis with a 5% manifestation rate in patients with ESRD. 6 In a study, by Zacharias and Fine, on a pool of patients with 78 % peritoneal dialysis cohort, diabetes mellitus, peritoneal dialysis, high serum phosphorus, calcium-based phosphate binders, and vitamin D supplements were considered risk factors. 7 Increased serum phosphorus, liver disease, obesity, increased calcium-phosphate product, systemic corticosteroid use, and increased serum albumin were postulated to be a risk factor in 49 dialysis patients in a study conducted by Weenig et al. 8 Certain autoimmune conditions, namely antiphospholipid antibody syndrome, rheumatoid arthritis, temporal arteritis, and systemic lupus erythematosus (SLE), have been suggested as risk factors for the development of calciphylaxis.9-12 Recurrent skin trauma has also been described as a risk factor as well as longer dialysis vintage.12,13 Also, it is more common in the 5th decade of life, and female Caucasian population.6-8 Obesity is a unique risk factor contributing to the lesions developing in areas like breasts, trunks, and thighs.14,15
Summarization of the risk factors is described in Table 1.
Table 1.
Risk factors for calciphylaxis.
| Risk factors for calciphylaxis |
|---|
| ■ Diabetes mellitus ■ Hypoalbuminemia ■ Medications like calcium-based binders and vitamin D analogs, nutritional vitamin D, warfarin use, and systemic glucocorticoids ■ Female sex ■ Recurrent skin trauma ■ Hyperphosphatemia ■ Obesity (body mass index [BMI] >30) ■ More years on dialysis (dialysis vintages) ■ Autoimmune and inflammatory diseases ■ Hypercoagulable states, such as protein C and S deficiency and antiphospholipid syndrome |
Pathogenesis and Diagnosis
Dermatological diseases in CKD and ESRD are morphologically quite variable. 16 Calciphylaxis is commonly identified in patients by painful ischemic purpura and necrotic skin ulceration. Calciphylaxis lesions are tender regions that may eventually progress to stellate-shaped ulcers with black eschar as per the highly cited study by Nikko et al. 17 Poor healing of skin lesions along with blistering are common signs. 16 Palpation of firm calcified hypodermic tissue can indicate calciphylaxis prevalence in both dialysis patients and patients possessing other potential risk factors. Dermatological differential diagnoses to consider are various vasculitis lesions like IgA nephropathy, necrotizing vasculitis, nephrogenic systemic fibrosis from Gadolinium-enhanced dye in magnetic resonance imaging, warfarin-induced dermal necrosis, purpura fulminans, and atherosclerotic vascular disease.9,10
A skin biopsy is required for a definitive diagnosis, but this poses a risk of inducing necrosis or propagating new lesions.9,10 It is preferable is biopsy is performed only by experienced surgeons or dermatologists to avoid complications. Also, the area of biopsy is important as a sample from the necrotic areas and central part may not yield accurate diagnostic features.9,10 Histologic findings of a skin biopsy were revealed in a case study done by Nikko et al, the biopsy was performed on a female patient with chronic renal failure who had developed calciphylaxis revealing small arteriole calcification in the deep dermis, a mixture of neutrophils and lymphocytes, and a degeneration of collagen bundles. 17 Other histological features that have been identified include micro thrombosis and fibrointimal hyperplasia of subcutaneous arteries. 18 Von Kossa or Alizarin red stains can also help detect regions of micro-calcification and other tools such as X-rays and nuclear bone scans have assisted with the diagnosis as well. 19 The pathogenesis of calciphylaxis is still poorly understood, but the likely events are activation of nuclear factor NFkB, arteriolar calcification, thrombosis, and skin ischemia. 20
Treatment
Most of the data on treatment originates from case reports and retrospective cohort studies as there is not a lot of information available from large-scale studies of this disease. Multidisciplinary input from the specialties of nephrology, dermatology, and dermatopathology is crucial and a management plan should be created as soon as calciphylaxis is suspected. There are still no controlled trials or guidelines published for the management of calciphylaxis and management is based on multiple case series and retrospective studies only. A 3-pronged approach through mitigation of risk factors, care of the wound, and modification of calcification through therapeutics seem to have the best outcome in most of the reported cases. Table 2 summarizes the treatment options which should be considered.
Table 2.
Management Strategies.
| a) Modification of precipitating factors | Normalize Parathyroid Hormone levels (through Cinacalcet/parathyroidectomy) |
| Avoid vitamin D analogs | |
| Avoid calcium based phosphate binders | |
| Avoid high Calcium bath | |
| Optimize dialysis prescription | |
| Avoid vitamin K antagonists | |
| Optimize nutrition status | |
| b) Wound care measures | Surgical cleaning and dressing |
| Analgesics | |
| Antibiotics | |
| Hyperbaric oxygen therapy | |
| c) Calcification reversal/halting | Sodium thiosulphate |
| Bisphosphonates | |
| Experimental modalities | |
| SNF 472- currently in clinical trial 21 |
Prevention of risk factors should always be a cornerstone in the management of calciphylaxis. While the KDIGO 2009 guideline says the parathyroid levels should be aimed to be maintained at a normal range of 150 to 300 pg/ml, 22 the normal levels may vary based on individual patient’s bone physiology and thus strict target chasing is not recommended. Most standard dialysis centers in United States like DaVita or Fresenius utilize a target of up to 600 pg/ml.
Floege et al through the EVOLVE trial have postulated that management of secondary hyperparathyroidism with cinacalcet can lower the incidence of calciphylaxis. 23 Subtotal parathyroidectomy, vs patients without parathyroidectomy, has shown significantly better survival at both 6 months (90% versus 42%) and 5 years (53% versus 11%) in patients with severe secondary hyperparathyroidism. 24 An increase in the frequency and/or duration of dialysis has been shown to result in partial or complete resolution in skin lesions in a retrospective study of 24 patients with calciphylaxis. 25 This also helps in the clearance of uremic toxins and phosphate which might be contributing to it. Avoidance of hypercalcemia and hyperparathyroidism should be crucial factors to prevent the disease process. Discontinuation of calcium-based binders and vitamin D analogs always helps to mitigate the risk involved. Non-calcium-based binders like sevelamer or lanthanum should be used in these conditions to help in preventing calcium excess. Nutrition assessments should be a cornerstone of management and always aims should be made to prevent protein-calorie malnutrition which might interfere with wound healing. As vitamin K antagonists, especially warfarin, are known risk factors to precipitate the disease,9,10 attempts should always be made to discontinue warfarin and switch to subcutaneous heparin or newer oral anticoagulants like apixaban, rivaroxaban, although, efficacy proving studies of these agents in dialysis patients are lacking. This approach should be taken on a case-by-case basis depending on the urgency and need to maintain anticoagulation.
Wound management is an important area of treatment with the main purpose of keeping the wound free of necrosed devitalized tissue and thus mitigate wound infection. The involvement of a dedicated wound care team makes a huge difference in inpatient care. Retrospective studies demonstrated that the 1-year survival of patients with wound debridement was 60% at 6 months and 70% at 1 year, while in compared to 27% at 6 months and 50% after a year, respectively in patients who didn’t undergo debridement. 26 However, these studies are limited by selection bias and thus may not reflect complete improvement. When the wound is not infected, with dry eschar and limited tissue distribution, chemical debridement is preferential compared to surgical debridement. 27 Sterile maggots of Lucia sericata has been reported to be a successful adjunct therapy in few calciphylaxis lesion, especially with extensive necrosis and panniculitis. 28 The digestive enzymes by these larvae stimulate wound healing by selectively dissolve necrotic tissue and disinfect the wound.
Pain management is an important aspect in these patients. Ischemic pain with a component of neuropathic element contributes to the pain symptoms and attempts should be made to alleviate it with help of a pain management team. Antibiotics should be considered when there is suspicion of associated wound infection. Given the propensity of gram-positive organisms and anaerobes to involve skin more, empirical antibiotics should be selected accordingly. Case series have been reported where analgesics like ketamine, opioids, and gabapentin/ tricyclic antidepressants have helped. 29 Given the detrimental side effects of these individual agents, careful monitoring is always recommended. Hyperbaric oxygen therapy can help in recalcitrant calciphylaxis wounds through the proliferation of collagen tissue, angiogenesis, and fibroblasts stimulation. 30
The final approach of therapy should include measures to inhibit calcification. The most popular medication utilized worldwide is intravenous sodium thiosulphate (STS), which is not approved for use by FDA. 31 It is thought to act by forming soluble complexes with various minerals and metals as well as through its antioxidant, vasodilator effects, and as an inhibitor of calcification.9,10 The commonly used dose is 25 gm intravenously in 100 cc of isotonic normal saline through the last half hour of hemodialysis, thrice weekly. 31 Peritoneal dialysis patients also get it 3 times weekly, over 60 minutes each interval. Non-dialysis patients with advanced CKD, STS can be given up to 4 times weekly as tolerated. 32 Intralesional STS has been tried in patients unable to tolerate through IV. 33 Due to the risk of chemical peritonitis, intralesional STS is not recommended. 34 No optimum duration of therapy is there, depending on the positive or negative response to the drug in the first 2 to 4 weeks, duration is modified.
In a retrospective study by Torregrosa et al, there were some benefits in 11 patients treated with bisphosphonates followed over 8 years. 35 Through binding hydroxyapatite crystals, bisphosphonates theoretically can show benefit in calciphylaxis treatment. However, given its serious toxicity itself in ESRD patients, the risk and benefits need to be carefully considered. Other modalities that have been mentioned in the literature are low-density lipoprotein apheresis, 36 vitamin K supplementation, 37 daily infusion of tissue plasminogen activator 38 but they should be attempted only on an individual case by case basis when other options have been exhausted. An exciting new intravenous formulation of Myo-inositol hexaphosphate, coined as SNF 472, has shown promising results in phase 2 trials and a large, randomized trial is being planned. 21
A study was done in 2019 by Udomkarnjananun et al reviewed a combination of case series, case reports, and cohort studies of calciphylaxis treatments with a total of 856 patients. The most utilized treatment modality was sodium thiosulfate infusion in 50.3% of patients, followed by surgical parathyroidectomy, cinacalcet, hyperbaric oxygen therapy, and bisphosphonates. 39 The review of these treatment options concluded that there was no significant clinical benefit found in any of the top 5 treatments for calciphylaxis in patients with CKD. Thus, more controlled trials are required to examine the effectiveness of these therapies. With a 6-month mortality rate of 30% and a 50% mortality rate at 1 year, a more comprehensive study must be done to better understand the pathogenetic mechanisms and the development of new treatment and prevention methods. 40
Conclusion
Calciphylaxis is still a challenge faced by nephrologists in treating patients on dialysis, especially those with a longer dialysis vintage. As more ESRD patients are encouraged toward performing home dialysis, this condition will be frequently encountered in home dialysis patients and thus better understanding of it is of paramount importance, among both nephrologists and primary care physicians. Due to limited therapy options and reported high morbidity and mortality associated with this condition, more extensive studies and case reports need to be conducted and published, to facilitate better insight and improved management of this debilitating condition.
Footnotes
Data Availability: PubMed, Goggle Scholar databases. The authors declare that data supporting the findings of this article are available within the article
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethics approval for reporting individual cases
Informed Consent: Written informed consent was obtained from the patient for her anonymous information to be published in this article
ORCID iDs: Sasmit Roy  https://orcid.org/0000-0002-2509-3915
https://orcid.org/0000-0002-2509-3915
Sohil Narasimha Reddy  https://orcid.org/0000-0002-3746-6424
https://orcid.org/0000-0002-3746-6424
Ebad Ur Rahman  https://orcid.org/0000-0002-4743-5680
https://orcid.org/0000-0002-4743-5680
Sreedhar Adapa  https://orcid.org/0000-0001-5608-5654
https://orcid.org/0000-0001-5608-5654
References
- 1. Zhang Y, Corapi KM, Luongo M, Thadhani R, Nigwekar SU. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brandenburg VM, Kramann R, Rothe H, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nationwide registry. Nephrol Dial Transplant. 2017;32(1):126-132. doi: 10.1093/ndt/gfv438. [DOI] [PubMed] [Google Scholar]
- 3. Nigwekar SU, Solid CA, Ankers E, et al. Quantifying a rare disease in administrative data: the example of calciphylaxis. J Gen Intern Med. 2014;29(suppl 3):S724-S731. doi: 10.1007/s11606-014-2910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91(10):1384-1394. doi: 10.1016/j.mayocp.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 5. Selye H, Gentile G, Prioreschi P. Cutaneous molt induced by calciphylaxis in the rat. Science. 1961;134(3493):1876-1877. doi: 10.1126/science.134.3493.1876. [DOI] [PubMed] [Google Scholar]
- 6. New N, Mohandas J, John GT, et al. Calcific uremic arteriolopathy in peritoneal dialysis populations. Int J Nephrol. 2011;2011:982854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61(6):2210-2217. doi: 10.1046/j.1523-1755.2002.00375.x. [DOI] [PubMed] [Google Scholar]
- 8. Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56(4):569-579. doi: 10.1016/j.jaad.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 9. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mandelbrot DA, Santos PW, Burt RK, et al. Resolution of SLE-related soft-tissue calcification following haematopoietic stem cell transplantation. Nephrol Dial Transplant. 2008;23:2679-2684. [DOI] [PubMed] [Google Scholar]
- 11. Slough S, Servilla KS, Harford AM, Konstantinov KN, Harris A, Tzamaloukas AH. Association between calciphylaxis and inflammation in two patients on chronic dialysis. Adv Perit Dial. 2006;22:171-174. [PubMed] [Google Scholar]
- 12. Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27(11):3421-3429. doi: 10.1681/ASN.2015091065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Angelis M, Wong LL, Myers SA, Wong LM. Calciphylaxis in patients on hemodialysis: a prevalence study. Surgery. 1997;122(6):1083-1089; discussion 1089-1090. doi: 10.1016/s0039-6060(97)90212-9. [DOI] [PubMed] [Google Scholar]
- 14. Bleyer AJ, Choi M, Igwemezie B, de la Torre E, White WL. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32(3):376-383. doi:10.1053/ajkd. 1998.v32.pm9740152. [DOI] [PubMed] [Google Scholar]
- 15. Adapa S, Naramala S, Gayam V, et al. Calciphylaxis in a patient on home hemodialysis. J Investig Med High Impact Case Rep. 2020;8:2324709620922718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brewster UC. Dermatological disease in patients with CKD. Am J Kidney Dis. 2008;51(2):331-44. doi: 10.1053/j.ajkd.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 17. Nikko AP, Dunningan M, Cockerell CJ. Calciphylaxis with histologic changes of pseudoxanthoma elasticum. Am J Dermatopathol. 1996;18(4):396-399. [DOI] [PubMed] [Google Scholar]
- 18. Mochel MC, Arakaki RY, Wang G, Kroshinsky D, Hoang M. Cutaneous calciphylaxis: a retrospective histopathologic evaluation. Am J Dermatopathol. 2013;35(5):582-6. doi: 10.1097/DAD.0b013e31827c7f5d. [DOI] [PubMed] [Google Scholar]
- 19. Au S, Crawford RI. Three-dimensional analysis of a calciphylaxis plaque: clues to pathogenesis. J Am Acad Dermatol. 2002;47(1):53-57. [DOI] [PubMed] [Google Scholar]
- 20. Bhambri A, Del Rosso JQ. Calciphylaxis: a review. J Clin Aesthet Dermatol. 2008;1(2):38. [PMC free article] [PubMed] [Google Scholar]
- 21. Brandenburg VM, Sinha S, Torregrosa JV, et al. Improvement in wound healing, pain, and quality of life after 12 weeks of SNF472 treatment: a phase 2 open-label study of patients with calciphylaxis. J Nephrol. 2019;32(5):811-821. doi: 10.1007/s40620-019-00631-0. [DOI] [PubMed] [Google Scholar]
- 22. Barreto FC, Barreto DV, Moysés RM, et al. KDOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int. 2008;73(6):771-777. doi: 10.1038/sj.ki.5002769. [DOI] [PubMed] [Google Scholar]
- 23. Floege J, Kubo Y, Floege A, Chertow GM, Parfrey PS. The effect of cinacalcet on calcific uremic arteriolopathy events in patients receiving hemodialysis: the EVOLVE trial. Clin J Am Soc Nephrol. 2015;10(5):800-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016; 91:1384-1394. [DOI] [PubMed] [Google Scholar]
- 25. Harris C, Kiaii M, Lau W, Farah M. Multi-intervention management of calcific uremic arteriolopathy in 24 patients. Clin Kidney J. 2018. Oct;11(5):704-709. doi: 10.1093/ckj/sfy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weening RH, Sewell LD, Davis MDP, McCarthy JT, Pittelkow MR. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56(4):569-579. [DOI] [PubMed] [Google Scholar]
- 27. Sato T, Ichioka S. How should we manage multiple skin ulcers associated with calciphylaxis? J Dermatol. 2012;39(11):966-968. doi:10.1111/j.1346-8138.2012.01510. x. [DOI] [PubMed] [Google Scholar]
- 28. Picazo M, Bover J, de la Fuente J, Sans R, Cuxart M, Matas M. Sterile maggots like coadjuvanted at the local treatment in a patient with proximal calciphylaxis. Nefrologia. 2005;25:559-562. [PubMed] [Google Scholar]
- 29. Polizzotto MN, Bryan T, Ashby MA, Martin P. Symptomatic management of calciphylaxis: a case series and review of the literature. J Pain Symptom Manage. 2006;32:186-190. [DOI] [PubMed] [Google Scholar]
- 30. Basile C, Montanaro A, Masi M, Pati G, De Maio P, Gismondi A. Hyperbaric oxygen therapy for calcific uremic arteriolopathy: a case series. J Nephrol. 2002;15:676-680. [PubMed] [Google Scholar]
- 31. Nigwekar SU, Brunelli SM, Meade D, Wang W, Hymes J, Lacson E., Jr. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nigwekar S, Thadani R. Calciphylaxis. In Melin JA. ed., UpToDate. https://www.uptodate.com/contents/calciphylaxis-calcific-uremic-arteriolopathy. Published 2021. Accessed November 10, 2021
- 33. Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149(8):946-949. doi: 10.1001/jamadermatol.2013.4565. [DOI] [PubMed] [Google Scholar]
- 34. Gupta DR, Sangha H, Khanna R. Chemical peritonitis after intraperitoneal sodium thiosulfate. Perit Dial Int. 2012;32(2):220-222. doi: 10.3747/pdi.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Torregrosa JV, Sánchez-Escuredo A, Barros X, Blasco M, Campistol JM. Clinical management of calcific uremic arteriolopathy before and after therapeutic inclusion of bisphosphonates. Clin Nephrol. 2015;83:231-234. [DOI] [PubMed] [Google Scholar]
- 36. Iwagami M, Mochida Y, Ishioka K, et al. LDL-apheresis dramatically improves generalized calciphylaxis in a patient undergoing hemodialysis. Clin Nephrol. 2014;81(3):198-202. doi: 10.5414/CN107482. [DOI] [PubMed] [Google Scholar]
- 37. Brandenburg VM, Kramann R, Rothe H, et al. Calcific uremic arteriolopathy (calciphylaxis): data from a large nationwide registry. Nephrol Dial Transplant. 2017;32(1):126-132. doi: 10.1093/ndt/gfv438. [DOI] [PubMed] [Google Scholar]
- 38. el-Azhary RA, Arthur AK, Davis MD, et al. Retrospective analysis of tissue plasminogen activator as an adjuvant treatment for calciphylaxis. JAMA Dermatol. 2013;149(1):63-67. doi: 10.1001/2013.jamadermatol.5. [DOI] [PubMed] [Google Scholar]
- 39. Udomkarnjananun S, Kongnatthasate K, Praditpornsilpa K, et al. Treatment of calciphylaxis in CKD: a systematic review and meta-analysis. Kidney Int Rep. 2019;4(2):231-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nigwekar SU. Calciphylaxis. Curr Opin Nephrol Hypertens. 2017;26(4):276-281. [DOI] [PMC free article] [PubMed] [Google Scholar]


