Key Points
Question
Are patients with psoriasis at an increased risk for incident venous thromboembolism or peripheral vascular disease?
Findings
In this systematic review and meta-analysis including 13 cohort studies with 12 435 982 participants, patients with psoriasis had a 1.26-fold risk for incident venous thromboembolism and a 1.27-fold risk for incident peripheral vascular disease compared with those without psoriasis.
Meaning
This systematic review and meta-analysis suggests that there is an association of psoriasis with venous thromboembolism and peripheral vascular disease; further research is needed to understand how best to mitigate the potential increased risk of venous thromboembolism and peripheral vascular disease among patients with psoriasis and psoriatic arthritis.
Abstract
Importance
Psoriasis, venous thromboembolism (VTE), and peripheral vascular disease (PVD) share similar mechanisms involving chronic inflammation. However, the associations between psoriasis and VTE or PVD are unclear.
Objective
To determine the association of psoriasis with incident VTE and PVD.
Data Sources
MEDLINE, Embase, Cochrane Library, Web of Science, and Cumulative Index to Nursing and Allied Health Literature were systematically searched for relevant publications from their respective inception through May 21, 2021. No restrictions on language or geographic locations were imposed.
Study Selection
Two authors independently selected cohort studies that investigated the risk for incident VTE or PVD in patients with psoriasis. Any discrepancy was resolved through discussion with 2 senior authors until reaching consensus. Only 13 initially identified studies met the selection criteria for qualitative review, and only 9 of these for quantitative analysis.
Data Extraction and Synthesis
The Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline was followed. Two authors independently extracted data and assessed the risk of bias of included studies by using the Newcastle-Ottawa Scale. Disagreements were resolved by discussion with 2 other authors. A random-effects model meta-analysis was conducted to calculate the pooled hazard ratios (HRs) with the corresponding confidence intervals for incident VTE and PVD. Subgroup analyses based on arthritis status, psoriasis severity, sex, and geographic location were also performed.
Main Outcomes and Measures
Hazard ratios for incident VTE and PVD associated with psoriasis.
Results
A total of 13 cohort studies with 12 435 982 participants were included. The meta-analysis demonstrated a significantly increased risk for incident VTE (pooled HR, 1.26; 95% CI, 1.08-1.48) and PVD (pooled HR, 1.27; 95% CI, 1.16-1.40) among patients with psoriasis. Subgroup analyses illustrated increased risk for incident VTE among participants with psoriatic arthritis (pooled HR, 1.24; 95% CI, 1.01-1.53), women (pooled HR, 1.89; 95% CI, 1.36-2.61), and those in Asia (pooled HR, 2.02; 95% CI, 1.42-2.88) and Europe (pooled HR, 1.28; 95% CI, 1.06-1.53).
Conclusions and Relevance
This systematic review and meta-analysis found an increased risk for incident VTE and PVD among patients with psoriatic disease. Typical presentations of VTE or PVD should not be overlooked in patients with psoriasis. Risk factors, such as obesity, physical inactivity, smoking, and varicose veins, should be identified and treated in patients with psoriasis, and medications like hormone-related therapies should be prescribed with caution.
This systematic review and meta-analysis of 13 studies examines the association of psoriasis with incident venous thromboembolism and peripheral vascular disease.
Introduction
Psoriasis is an inflammatory dermatosis involving 1% to 2% of the global population.1,2,3 Patients with psoriasis have been found to be up to 50% more likely to develop cardiovascular comorbidities.4,5 Activated immune responses in psoriasis may contribute to vascular inflammation and subsequent atherosclerosis.6,7
Venous thromboembolism (VTE) and peripheral vascular disease (PVD) are vascular diseases that are associated with serious systemic effects.8,9 Chronic inflammation is considered the pivotal role in the pathophysiology of VTE and PVD.10,11 As psoriasis shares similar inflammatory pathomechanisms with VTE and PVD, previous meta-analyses in 2013 and 2014 discussed the potential associations of psoriasis with VTE and PVD.12,13,14,15 Nevertheless, the number of included studies in these meta-analyses was relatively small, with a resultant lack of statistical power to draw solid conclusions.16 Moreover, the pooled results in these meta-analyses were mainly based on cross-sectional studies, which failed to prove a temporal association.13,14,15,17 Since then, nearly 10 additional studies investigating the associations of psoriasis with VTE and PVD have been published, with inconsistent results reported. Therefore, a comprehensive systematic review is warranted to provide better understanding and compile current evidence.
Regarding the current psoriasis guidelines, little is known about whether psoriasis might be associated with VTE or PVD apart from most cardiovascular comorbidities of psoriasis.18 Thus, we aimed to examine the associations of psoriasis with incident VTE and PVD by evaluating the evidence to date.
Methods
This systematic review and meta-analysis was conducted according to the recommendations of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline group19 and reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting 2020 guideline.20 The institutional review board of the Chang Gung Medical Foundation exempted this study from approval (202001932B1). The protocol was prespecified and registered with PROSPERO (CRD42021254889).
Data Sources and Literature Search
We searched MEDLINE, Embase, Cochrane Library, Web of Science, and the Cumulative Index to Nursing and Allied Health Literature for publications that evaluated the associations of psoriasis with VTE or PVD from their respective inception to May 21, 2021. An information specialist (L.C.) assisted in devising the search strategy (eTable 1 in the Supplement). No restrictions on language or geographic locations were imposed. The bibliographies of included studies and relevant reviews were also manually screened for additional candidate studies.
Study Selection and Outcomes
To compare the risk for incident VTE and PVD over time and reduce recall bias, we only included cohort studies.16,17 The inclusion criteria were: (1) cohort studies that examined the associations of psoriasis with VTE or PVD; (2) an exposure group comprising patients with psoriasis and a nonexposure control group comprising people without psoriasis; and (3) reporting the risk estimates of VTE and/or PVD. We only enrolled studies with confirmed diagnoses of psoriasis, VTE, and PVD by clinical criteria or validated diagnostic codes. Peripheral vascular disease was defined clinically either by ankle-brachial index values or using previously validated diagnostic codes. Cross-sectional studies, case-control studies, case reports, editorials, review articles, and nonhuman studies were excluded. Two authors (T.C. and L.L.) independently selected studies by scanning the titles and abstracts of search results. The full text of potential studies was examined for eligibility. Any discrepancy was resolved through discussion with 2 senior authors (H.H. and C.C.) until consensus was reached.
Data Extraction and Risk of Bias Assessment
Two authors (T.C. and L.L.) independently collected the following data: first author, year of publication, country, database, study period, patient characteristics (sample size, mean age, and sex), definition of psoriasis, and outcomes of interest (VTE and PVD). In case of any misclassification regarding the author-reported study design in observational studies, the Design Algorithm for Medical Literature was applied to reclassify the eligible studies.21,22 We also extracted the adjusted risk estimates, including hazard ratios (HRs), risk ratios (RRs), and standardized incidence ratios (SIRs), with 95% CIs. The estimates with the most appropriate adjustment for confounders, such as age, sex, and comorbidities, were used for data synthesis.
The Newcastle-Ottawa Scale was applied to assess the risk of bias of included studies by 2 authors independently (T.C. and L.L.).23 Disagreements in the extracted data and risk of bias assessment were resolved by discussion with other authors (H.H. and J.W.).
Data Synthesis and Statistical Analysis
For studies that lacked sufficient data for meta-analysis, we contacted the corresponding authors to request information. If the information was still unavailable, we calculated the crude risk estimates with 95% CIs by using MedCalc (https://www.medcalc.org/calc/relative_risk.php). For studies that merely reported adjusted risk estimates of subgroups, we also contacted the corresponding authors for the overall effects. If the data could still not be retrieved, we estimated the effects in each subgroup by using the methods proposed by Hamling et al.24,25 Then, we combined the subgroups to yield the overall adjusted risk estimates. The statistical process is reported in the eMethods in the Supplement. We performed a sensitivity analysis by excluding studies with only crude risk estimates available.
We used Review Manager, version 5.4.1 (The Cochrane Collaboration), and Stata, version 17 (StataCorp), to conduct meta-analyses. A P value of <.05 was defined as statistically significant. To analyze the risk over the entire study period, only HRs were used for the meta-analysis.16,17 The SIRs were treated as HRs.26 The pooled HRs and corresponding CIs were synthesized by using the random-effects model based on the assumption of considerable clinical heterogeneity.27
Between-study heterogeneity was quantified using the I2 statistic, with an I2 of greater than 50% indicating at least moderate heterogeneity.28 To determine whether certain study-level factors affected the pooled results, we performed predefined subgroup analyses based on arthritis status, psoriasis severity, sex, and geographic location. To test the robustness of our primary analyses, we conducted a sensitivity analysis by omitting 1 study at a time (leave-one-out sensitivity analysis) and by excluding studies with a high risk of bias. We planned to draw funnel plots to evaluate publication bias if there were 10 or more studies that assessed an outcome.27
Results
Study Selection
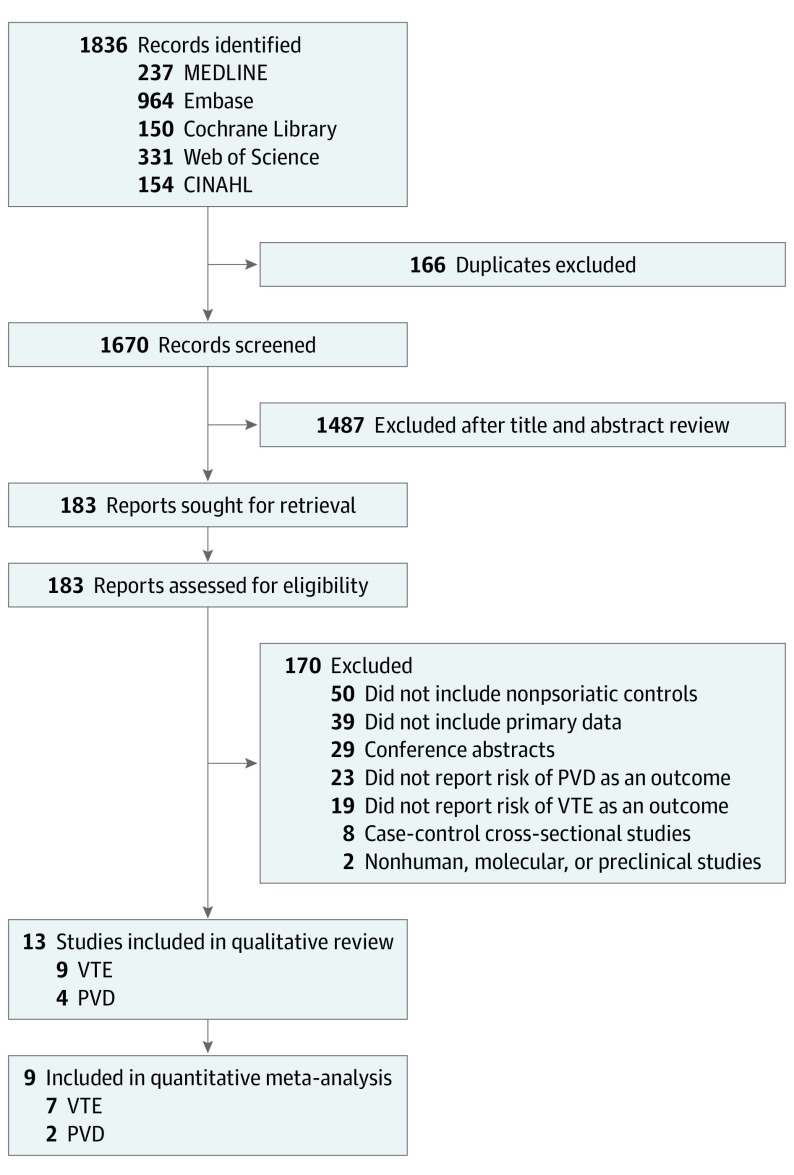
The PRISMA study flow diagram is illustrated in Figure 1. A total of 1836 records were retrieved from the search. After screening the titles and abstracts, the full text of 183 citations was assessed for eligibility. Eventually, 13 articles were eligible for the qualitative review. Among them, 9 studies (69.2%) reported the risk estimates of incident VTE,29,30,31,32,33,34,35,36,37 while 4 studies (30.8%) reported the risk estimates of incident PVD.38,39,40,41 Nine studies (69.2%) reporting HRs or SIRs were included in the quantitative meta-analysis.30,31,32,33,34,36,37,40,41
Figure 1. PRISMA Flow Diagram.
CINAHL indicates Cumulative Index to Nursing and Allied Health Literature; PVD, peripheral vascular disease; VTE, venous thromboembolism.
Characteristics of Included Studies
Demographic data and relevant outcomes are summarized in Table 1.29,30,31,32,33,34,35,36,37,38,39,40,41 A total of 13 cohort studies with 12 435 982 participants were included. Eight studies (61.5%) were conducted in Europe, with 4 in America and 1 in Asia. The definition of psoriasis was mostly based on International Classification of Diseases (ICD) Seventh, Eighth, Ninth, and Tenth Revision codes and Read codes, which have been validated in previous studies.42,43 The adjusted covariates of each study were listed in eTable 2 in the Supplement.
Table 1. Characteristics of Included Studies.
| Source | Data source | No. of cases/controls | Study period | Age, mean (SD), y | Female sex, % | Definition of psoriasis | Risk of VTE or PVD, HR (95% CI) |
|---|---|---|---|---|---|---|---|
| Ahlehoff et al,29 2011; Denmark | National Prescription Registry, National Causes of Death Register, and Central Population Register | 38 664/4 126 075 | 1997-2006 | 47.8 (16) | 49.9 | Psoriasis criteria ICD-10 codes L40 or M070–M073 | RR, 1.33 (1.21-1.47) for VTE |
| Bengtsson et al,30 2017; Sweden | Swedish National Patient Register and Swedish Population Register | 16 063/266 435 | 2006-2012 | 53.2 (13.8) | 55.1 | ICD-8 code 696.0, ICD-9 code 696.0, or ICD-10 codes L40 or M070–M073 | 1.46 (1.29-1.65) for VTE |
| Charlton et al,38 2018; UK | Clinical Practice Research Datalink | 33 915/27 132 | 1998-2014 | Median (IQR), 49 (39-59) | 50.9 | Read code | RR, 1.40 (1.02-1.92) for PVD |
| Chung et al,31 2017; Taiwan | National Health Insurance Research Database | 8945/8945 | 2000-2011 | 54.8 (17.1) | 26.6 | ICD-9 code 696 | 2.02 (1.42-2.88) for VTE |
| Dowlatshahi et al,39 2013; the Netherlands | Rotterdam Study | 262/8009 | 1990-2011 | 64.3 (6.8) | 56.1 | General practitioner code S91 | RR, 1.04 (0.73-1.47) for PVD |
| Galloway,32 2020; UK | Royal College of General Practitioners Research and Surveillance Centre database | 6297/213 512 | 1999-2018 | 49.2 (13.8) | 50.9 | Read code | 1.20 (0.96-1.52) for VTE |
| Kaine et al,40 2018; US | Truven Health Analytics MarketScan Commercial Claims and Medicare supplemental databases | 14 898/35 037 | 2008-2015 | 53.4 (12.4) | 55.4 | ICD-9 code 696.0 | 1.25 (1.09-1.44) for PVD |
| Kaye et al,41 2008; US | General Practice Research Database | 44 164/219 784 | 1991-2005 | NS | 52.2 | Oxford Medical Information System and Read codes | 1.29 (1.13-1.47) for PVD |
| Lutsey et al,33 2012; US | Iowa Women's Health Study | 859/37 749 | 1991-2004 | 67.8 (3) | 100 | ICD-9 code 696.1 | 1.39 (1.00-1.93) for VTE |
| Ogdie et al,34 2018; UK | The Health Improvement Network | 194 288/1 225 571 | 1994-2014 | 46.7 (17.5) | 51.1 | Read code | 1.08 (1.03-1.12) for VTE |
| Ramagopalan et al,35 2011; UK | English national hospital episode statistics | 85 358/3 621 957 | 1999-2008 | NS | 48.0 | ICD-9 codes 696.0, 696.1, 696.8, or 696.9 | RR, 1.66 (1.57-1.75) for VTE |
| Schneeweiss et al,36 2021; US | Optum deidentified Clinformatics Data Mart Database | 96 138/1 570 387 | 2004-2019 | 49.8 (15.9) | 50.5 | ICD-9 code 696.1 or ICD-10 code L40 | 0.86 (0.75-0.99) for VTE |
| Zoller et al,37 2012; Sweden | MigMed2 | 25 869/509 669 | 1964-2008 | NS | 47.2 | ICD-7 code 706, ICD-8 code 696, ICD-9 code 696, or ICD-10 code L40 | SIR, 1.40 (1.31-1.50) for VTE |
Abbreviations: HR, hazard ratio; ICD, International Classification of Diseases; NS, not specified; PVD, peripheral vascular disease; RR, risk ratio; SIR, standardized incidence ratio; VTE, venous thromboembolism.
Risk of Bias Assessment
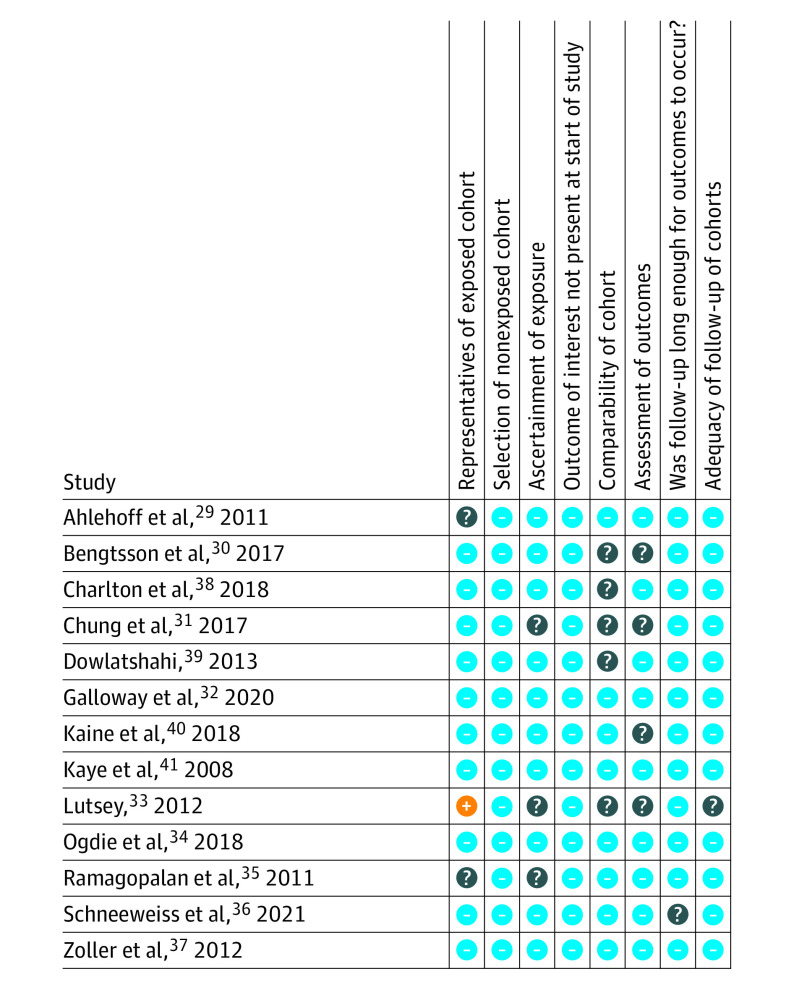
The risk of bias assessment is summarized in Figure 2. Lutsey et al33 was considered as having high risk of bias for representativeness because it only enrolled women who were living in Iowa. Five studies were considered to have an unclear risk for the comparability of cohorts because they did not adjust for one of the major confounders, namely age, sex, or comorbidities.30,31,33,38,39 As to the ascertainment of exposures and outcomes, 5 studies (38.5%) were rated as having unclear risk because the validation of diagnostic codes was not mentioned.30,31,33,35,40
Figure 2. Summary of Risk of Bias Assessment.
The blue minus circle denotes low risk of bias, question mark circle unclear risk of bias, and orange plus sign circle high risk of bias.
Risk for Incident VTE
Nine studies with 12 052 781 participants examined the association of psoriasis with incident VTE.29,30,31,32,33,34,35,36,37 As demonstrated in Figure 3, patients with psoriasis had an increased risk for incident VTE (pooled HR, 1.26; 95% CI, 1.08-1.48; I2 = 93%) compared with those without psoriasis. The studies by Ahlehoff et al29 and Ramagopalan et al35 also reported an elevated risk of incident VTE in patients with psoriasis (Table 1).
Figure 3. Association of Psoriasis With Venous Thromboembolism (VTE) and Peripheral Vascular Disease (PVD).
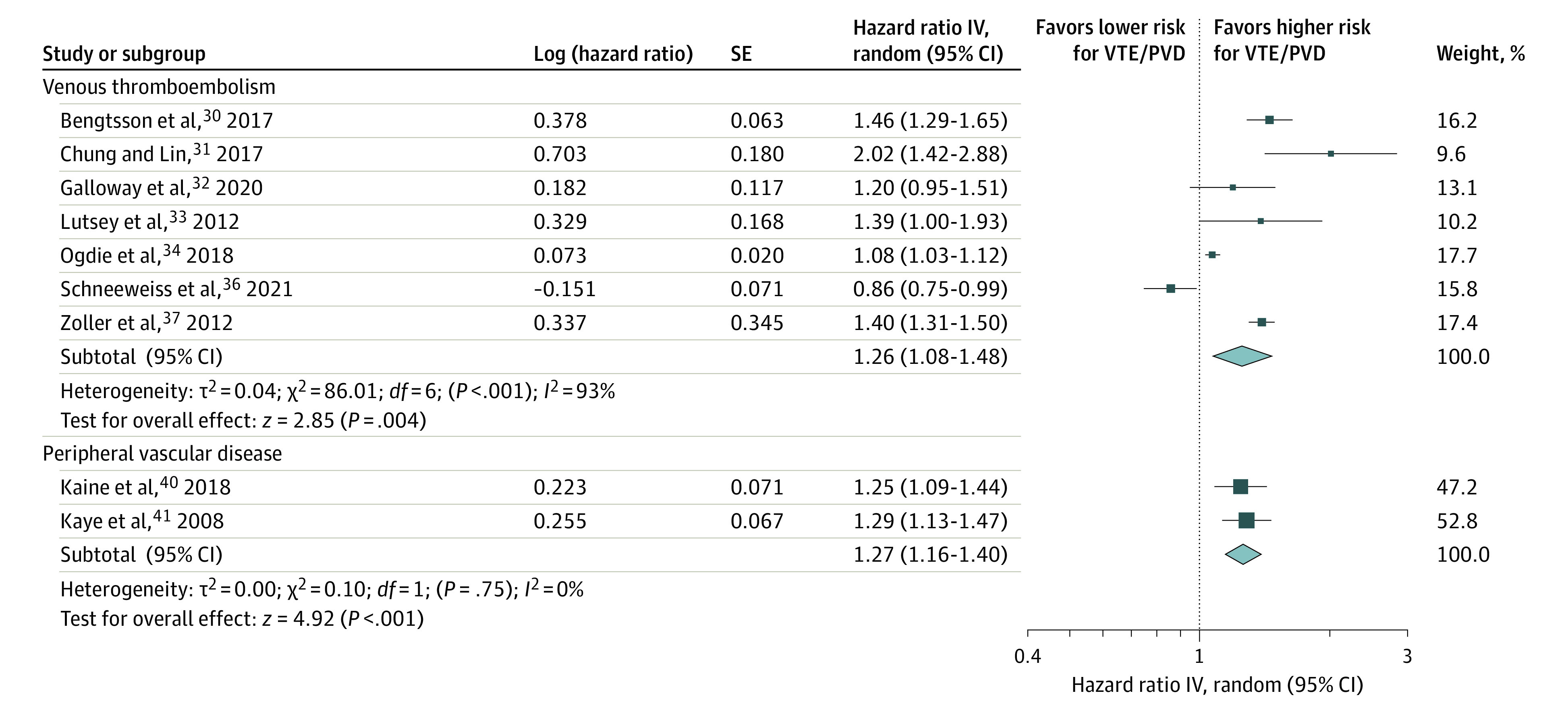
The meta-analysis illustrated a significant association of psoriasis with VTE (hazard ratio, 1.26; 95% CI, 1.08-1.48) and PVD (hazard ratio, 1.27; 95% CI, 1.16-1.40). IV indicates inverse variance.
The subgroup analyses regarding VTE are summarized in Table 2, and the detailed forest plots according to each subgroup were presented in eFigures 1 to 3 in the Supplement. When stratified by the arthritis status of study participants, the association remained significant in studies on patients with psoriatic arthritis (pooled HR, 1.24; 95% CI, 1.01-1.53; eFigure 1 in the Supplement). By contrast, the risk for incident VTE did not significantly increase among patients with psoriasis alone (pooled HR, 0.97; 95% CI, 0.78-1.21; eFigure 1 in the Supplement).
Table 2. Association of Psoriasis With Venous Thromboembolism According to Subgroups.
| Subgroups | Venous thromboembolism | Subgroup differences | ||||
|---|---|---|---|---|---|---|
| No. of studies | Pooled HR (95% CI) | P value | I2 (%) | P value | I2 (%) | |
| Arthritis status | ||||||
| Psoriasis alone | 2 | 0.97 (0.78-1.21) | .79 | 89 | .11 | 61.2 |
| Psoriatic arthritis | 3 | 1.24 (1.01-1.53)a | .04 | 82 | ||
| Psoriasis severity | ||||||
| Mild psoriasis | 1 | 1.07 (1.03-1.12)a | .002 | NA | .45 | 0 |
| Severe psoriasis | 1 | 1.13 (0.99-1.30) | .08 | NA | ||
| Sex | ||||||
| Men | 2 | 1.40 (0.90-2.19) | .13 | 93 | .29 | 9.2 |
| Women | 3 | 1.89 (1.36-2.61)a | <.001 | 84 | ||
| Geographic location | ||||||
| US | 2 | 1.07 (0.67-1.70) | .78 | 86 | .04 | 68.3 |
| Asia | 1 | 2.02 (1.42-2.88)a | <.001 | NA | ||
| Europe | 4 | 1.28 (1.06-1.53)a | .01 | 95 | ||
Abbreviations: HR, hazard ratio; NA, not applicable.
P < .05.
The association remained significant in studies on women (pooled HR, 1.89; 95% CI, 1.36-2.61; eFigure 2 in the Supplement). However, the risk for incident VTE did not significantly increase among men (pooled HR, 1.40; 95% CI, 0.90-2.19; eFigure 2 in the Supplement).
The subgroup analysis based on geographic regions demonstrated various risk estimates across different continents. Studies conducted in Asia and Europe showed an increased risk for incident VTE among patients with psoriasis, whereas studies conducted in the US did not show similar findings (eFigure 3 in the Supplement). Subgroup analyses indicated that geographic location might be a potential study-level factor that affected the pooled risk estimates (Table 2).
Risk for Incident PVD
Four studies (30.8%) with 383 201 participants provided data on the association of psoriasis with incident PVD.38,39,40,41 As shown in Figure 3, the meta-analysis found that patients with psoriasis were at a higher risk for incident PVD (pooled HR, 1.27; 95% CI, 1.16-1.40; I2 = 0%) than those without psoriasis. Charlton et al38 also reported an association between psoriasis and incident PVD (RR, 1.40; 95% CI, 1.02-1.92). However, Dowlatshahi et al39 did not detect a similar association (Table 1).
Sensitivity Analyses
The results of sensitivity analyses gained by excluding studies with only calculated risk estimates available and by excluding studies rated with a high risk of bias were similar to the primary analyses (eTable 3 in the Supplement). Moreover, the leave-one out sensitivity analyses in both outcomes yielded similar results (eFigures 4 and 5 in the Supplement).
Discussion
This systematic review and meta-analysis found that patients with psoriasis had a 1.26-fold risk for new-onset VTE and a 1.27-fold risk for new-onset PVD compared with those without psoriasis. The association of psoriasis with incident VTE remained significant among patients with concomitant psoriatic arthritis.
There are several possible explanations for these findings. First, increased levels of inflammatory and prothrombotic markers have been found among patients with psoriasis.44,45 Inflammation mediated via T-helper 17 cells and their cytokine interleukin 17 are involved in the pathogenesis of psoriasis and cardiovascular diseases.46,47 Second, recent studies suggest that platelets are activated in psoriasis and may induce an endothelial proinflammatory response.48,49 Platelet aggregation and vascular inflammation may promote coagulation,50 followed by development of thromboembolic events. Compared with psoriasis alone, the further increased vascular inflammation in psoriatic arthritis could explain the higher risk for incident VTE found in this population. A trend of higher VTE risk among patients with severe psoriasis has been observed compared with those with mild psoriasis by Ahlehoff et al29 and Ogdie et al.34 However, the data presented at this time were not sufficient for meta-analysis to reveal a dose-response relationship between psoriasis severity and risks of VTE. Third, excessive feed-forward amplification of inflammation in psoriasis has also been suggested as being associated with vascular dysfunction and endothelial damage, facilitating the development of atherosclerosis.51,52 The increased risk for PVD among patients with psoriasis may be explained by the promoted atherosclerosis in peripheral vessels. Fourth, oxidative stress in psoriasis may also contribute to thrombosis and atherosclerosis in the vessels.53 The role of oxidative stress in the pathogenesis of psoriasis has been illustrated in animal models.54,55 Clinical evidence has also shown similarities in psoriasis and cardiovascular comorbidities regarding redox imbalance.56,57
The increased risk for incident VTE among patients with psoriasis supports the findings of a previous meta-analysis in 2014.12 However, the evidence from that meta-analysis was based on 4 Western studies, and the findings among the included studies were consistent. We believe that our analysis provides better generalizability because we included more studies, especially a Taiwanese study31 and several additional Western studies.30,32,34,36 Moreover, our subgroup analysis demonstrated that the risk for VTE among patients with psoriasis differed among various geographic locations, which may be associated with different genetic and environmental predispositions for psoriasis in various populations.58,59 The increased risk of VTE in studies from Asia and Europe may be associated with the inclusion of mainly hospitalized patients with risk factors, such as decreased physical activity and prolonged length of hospital stay. Further multiethnic research may help to elucidate the underlying mechanisms.
Previous meta-analyses have investigated the association between psoriasis and PVD.13,14,15 Miller et al13 indicated that patients with psoriasis were at an increased risk for PVD (pooled OR, 1.5; 95% CI, 1.2-1.8) according to 4 studies (2 cross-sectional and 2 case-control studies). Gupta et al14 reported a 1.6% pooled prevalence of PVD in patients with psoriatic arthritis. Horreau et al15 highlighted that PVD was not consistently defined in studies, although it was associated with peripheral artery disease in most situations.
The between-study heterogeneity regarding VTE was high. Potential effect modifiers were identified to explain the heterogeneity, such as the geographic location. The subgroup differences were significant. Additionally, the heterogeneity might be associated with the heterogenous adjusted covariates among individual studies (eTable 2 in the Supplement), which was inevitable when pooling various observational studies.60 A pooling of crude risk estimates was not performed because it did not consider confounding factors and might have led to greater biases.60 A more in-depth analysis could be performed if individual patient-level data were available.
Exposure to hormonal therapies, such as combined oral contraceptives and hormone replacement therapy, may potentially affect the risk for VTE.61,62 After adjustment for hormonal therapy use, Lutsey et al33 and Ogdie et al34 found an increased risk for VTE in participants with psoriasis. Galloway et al32 reported a nonsignificant increased risk of VTE among patients with psoriasis compared with nonpsoriatic controls. By contrast, Schneeweiss et al36 revealed a reduced risk of VTE in patients with psoriasis after propensity score matching of hormone-related therapies. This inconsistency might be associated with the highly heterogeneous formulations in hormonal therapy across countries and the different hormone-related preparations examined in these studies. The prevalence of hormonal therapy use in the psoriatic population ranged from around 3% to more than 20%. Because of the limitation of retrieving hormonal prescription data from register-based studies, the underlying mechanisms and the role of hormonal therapies on VTE risk in individuals with psoriasis is yet to be confirmed.
As the applications of biologic therapy in psoriasis increase, the therapeutic association with the risk of cardiovascular events has been debated. The benefits of biologics and small molecule inhibitors in reducing vascular inflammation have been elucidated in recent animal and clinical studies.63,64,65 By contrast, biologics used in psoriasis treatment come with a warning for serious adverse events like VTE.66,67 However, there was insufficient evidence to support either an increased or decreased risk of biologic-associated VTE or PVD based on currently available data. In this study, the effects of antipsoriatic treatments, such as methotrexate and biologics, were not elucidated. Considering the antiinflammatory effects of biologics and small molecule inhibitors, more studies are warranted to assess their associations with incident VTE and PVD.
Strengths and Limitations
The main strength of this systematic review and meta-analysis is the inclusion of multiple population-based studies. From a clinical viewpoint, this study prompts clinicians to pay attention to the risk of VTE and PVD in patients with psoriasis based on real-world evidence. Risk prediction models, like the Wells score or ankle-brachial index screening, could be considered in the current psoriasis guidelines for preventing VTE and PVD.
This study has a few limitations. First, the diagnosis of psoriasis, VTE, or PVD was mainly based on diagnostic codes. Although the codes have been validated in some databases,42,43 misclassification bias might still exist. However, sensitivity analyses using different definitions of diagnostic codes produced similar results (eTable 3 in the Supplement). Second, there was only 1 Asian study31 that investigated the association of psoriasis and VTE. The uneven distribution in the subgroup regarding geography may limit the external validity. Third, the confounding effect of cigarette smoking, an important risk factor for VTE and PVD, was only adjusted in 4 of the included studies.32,33,34,38 Although the prevalence of smoking is increased in patients with psoriasis compared with the general population,68,69 to our knowledge, there is a lack of studies that evaluate the risk of VTE or PVD in psoriasis that compare the different status of cigarette consumption. Moreover, the recall bias of smoking status in observational studies should also be considered. Further well-designed studies are warranted. Fourth, the number of studies included in each subgroup was small. As Schneeweiss et al36 was the only study that reported a decreased risk of VTE, the negative results in the subgroup analyses might be dependent on this study. The follow-up time of this study (median, 1.9 years) might not be enough for the occurrence of VTE events because systemic chronic inflammation necessitates time to cause vascular injuries. In addition, the small number of studies prevented the application of funnel plots in detecting publication bias.
Conclusions
To our knowledge, there are no reviews that examine the associations of psoriasis with incident VTE and PVD; this systematic review and meta-analysis was conducted to address this knowledge gap. The results of this study suggests that psoriasis is associated with increased risk for incident VTE and PVD, especially in patients with psoriatic arthritis, women, and patients located in Asia and Europe. Physicians and patients with psoriasis should be aware of the risk of VTE and PVD. Typical presentations (eg, unexplained dyspnea, chest pain, and painful edematous swelling, as well as pulselessness and sensorimotor abnormalities of leg) should not be ignored by physicians who are treating patients with psoriasis. Risk factors, such as obesity, physical inactivity, smoking, and varicose veins, should be identified and treated in patients with psoriasis, and medications like hormone-related therapies should be prescribed cautiously.
eMethods. Approximation of Adjusted Risk Estimates
eTable 1. Search Strategies Modified for (a) Medline, (b) Embase, (c) Cochrane Library, (d) Web of science, and (e) CINAHL
eTable 2. Adjusted Covariates of Included Studies
eTable 3. Sensitivity Analyses
eFigure 1. Subgroup Analysis of Venous Thromboembolism According to Arthritis Status
eFigure 2. Subgroup Analysis of Venous Thromboembolism According to Gender.
eFigure 3. Subgroup Analysis of Venous Thromboembolism According to Geographic Location
eFigure 4. Leave-one Out Sensitivity Analysis of Venous Thromboembolism
eFigure 5. Leave-one Out Sensitivity Analysis of Peripheral Vascular Disease
References
- 1.Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512-516. doi: 10.1016/j.jaad.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 2.Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM; Global Psoriasis Atlas . National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590. doi: 10.1136/bmj.m1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082. doi: 10.1038/nrdp.2016.82 [DOI] [PubMed] [Google Scholar]
- 4.Garshick MS, Ward NL, Krueger JG, Berger JS. Cardiovascular risk in patients with psoriasis: JACC review topic of the week. J Am Coll Cardiol. 2021;77(13):1670-1680. doi: 10.1016/j.jacc.2021.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samarasekera EJ, Neilson JM, Warren RB, Parnham J, Smith CH. Incidence of cardiovascular disease in individuals with psoriasis: a systematic review and meta-analysis. J Invest Dermatol. 2013;133(10):2340-2346. doi: 10.1038/jid.2013.149 [DOI] [PubMed] [Google Scholar]
- 6.Verma D, Fekri SZ, Sigurdardottir G, Bivik Eding C, Sandin C, Enerbäck C. Enhanced inflammasome activity in patients with psoriasis promotes systemic inflammation. J Invest Dermatol. 2021;141(3):586-595.e5. doi: 10.1016/j.jid.2020.07.012 [DOI] [PubMed] [Google Scholar]
- 7.Teague HL, Varghese NJ, Tsoi LC, et al. Neutrophil subsets, platelets, and vascular disease in psoriasis. JACC Basic Transl Sci. 2019;4(1):1-14. doi: 10.1016/j.jacbts.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Søgaard KK, Schmidt M, Pedersen L, Horváth-Puhó E, Sørensen HT. 30-Year mortality after venous thromboembolism: a population-based cohort study. Circulation. 2014;130(10):829-836. doi: 10.1161/CIRCULATIONAHA.114.009107 [DOI] [PubMed] [Google Scholar]
- 9.Agnelli G, Belch JJF, Baumgartner I, Giovas P, Hoffmann U. Morbidity and mortality associated with atherosclerotic peripheral artery disease: a systematic review. Atherosclerosis. 2020;293:94-100. doi: 10.1016/j.atherosclerosis.2019.09.012 [DOI] [PubMed] [Google Scholar]
- 10.Chang SL, Huang YL, Lee MC, et al. Association of varicose veins with incident venous thromboembolism and peripheral artery disease. JAMA. 2018;319(8):807-817. doi: 10.1001/jama.2018.0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riva N, Donadini MP, Ageno W. Epidemiology and pathophysiology of venous thromboembolism: similarities with atherothrombosis and the role of inflammation. Thromb Haemost. 2015;113(6):1176-1183. doi: 10.1160/TH14-06-0563 [DOI] [PubMed] [Google Scholar]
- 12.Ungprasert P, Sanguankeo A, Upala S, Suksaranjit P. Psoriasis and risk of venous thromboembolism: a systematic review and meta-analysis. QJM. 2014;107(10):793-797. doi: 10.1093/qjmed/hcu073 [DOI] [PubMed] [Google Scholar]
- 13.Miller IM, Ellervik C, Yazdanyar S, Jemec GB. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69(6):1014-1024. doi: 10.1016/j.jaad.2013.06.053 [DOI] [PubMed] [Google Scholar]
- 14.Gupta S, Syrimi Z, Hughes DM, Zhao SS. Comorbidities in psoriatic arthritis: a systematic review and meta-analysis. Rheumatol Int. 2021;41(2):275-284. doi: 10.1007/s00296-020-04775-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horreau C, Pouplard C, Brenaut E, et al. Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):12-29. doi: 10.1111/jdv.12163 [DOI] [PubMed] [Google Scholar]
- 16.Roberts MR, Ashrafzadeh S, Asgari MM. Research techniques made simple: interpreting measures of association in clinical research. J Invest Dermatol. 2019;139(3):502-511.e1. doi: 10.1016/j.jid.2018.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverberg JI. Study designs in dermatology: a review for the clinical dermatologist. J Am Acad Dermatol. 2015;73(5):721-731. doi: 10.1016/j.jaad.2014.08.029 [DOI] [PubMed] [Google Scholar]
- 18.Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073-1113. doi: 10.1016/j.jaad.2018.11.058 [DOI] [PubMed] [Google Scholar]
- 19.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting: Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(n71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo HJ, Kim SY, Lee YJ, et al. A newly developed tool for classifying study designs in systematic reviews of interventions and exposures showed substantial reliability and validity. J Clin Epidemiol. 2016;70:200-205. doi: 10.1016/j.jclinepi.2015.09.013 [DOI] [PubMed] [Google Scholar]
- 22.Ohn J, Eun SJ, Kim DY, Park HS, Cho S, Yoon HS. Misclassification of study designs in the dermatology literature. J Am Acad Dermatol. 2018;79(2):315-319. doi: 10.1016/j.jaad.2017.10.049 [DOI] [PubMed] [Google Scholar]
- 23.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. Accessed June 19, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 24.Hamling J, Lee P, Weitkunat R, Ambühl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27(7):954-970. doi: 10.1002/sim.3013 [DOI] [PubMed] [Google Scholar]
- 25.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66-73. doi: 10.1093/aje/kwr265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernán MA. The hazards of hazard ratios. Epidemiology. 2010;21(1):13-15. doi: 10.1097/EDE.0b013e3181c1ea43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ. Cochrane handbook for systematic reviews of interventions version 6.2. Accessed May 15, 2021. http://www.training.cochrane.org/handbook.
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahlehoff O, Gislason GH, Lindhardsen J, et al. Psoriasis carries an increased risk of venous thromboembolism: a Danish nationwide cohort study. PLoS One. 2011;6(3):e18125. doi: 10.1371/journal.pone.0018125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bengtsson K, Forsblad-d’Elia H, Lie E, et al. Are ankylosing spondylitis, psoriatic arthritis and undifferentiated spondyloarthritis associated with an increased risk of cardiovascular events? a prospective nationwide population-based cohort study. Arthritis Res Ther. 2017;19(1):102. doi: 10.1186/s13075-017-1315-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung WS, Lin CL. Increased risks of venous thromboembolism in patients with psoriasis: a nationwide cohort study. Thromb Haemost. 2017;117(8):1637-1643. doi: 10.1160/TH17-01-0039 [DOI] [PubMed] [Google Scholar]
- 32.Galloway J, Barrett K, Irving P, et al. Risk of venous thromboembolism in immune-mediated inflammatory diseases: a UK matched cohort study. RMD Open. 2020;6(3):e001392. doi: 10.1136/rmdopen-2020-001392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutsey PL, Prizment AE, Folsom AR. Psoriasis is associated with a greater risk of incident venous thromboembolism: the Iowa Women’s Health Study. J Thromb Haemost. 2012;10(4):708-711. doi: 10.1111/j.1538-7836.2012.04646.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogdie A, Kay McGill N, Shin DB, et al. Risk of venous thromboembolism in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a general population-based cohort study. Eur Heart J. 2018;39(39):3608-3614. doi: 10.1093/eurheartj/ehx145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramagopalan SV, Wotton CJ, Handel AE, Yeates D, Goldacre MJ. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: record-linkage study. BMC Med. 2011;9:1. doi: 10.1186/1741-7015-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneeweiss MC, Kim SC, Wyss R, et al. Incidence of venous thromboembolism in patients with dermatologist-diagnosed chronic inflammatory skin diseases. JAMA Dermatol. 2021;157(7):805-816. doi: 10.1001/jamadermatol.2021.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zöller B, Li X, Sundquist J, Sundquist K. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow-up study from Sweden. Lancet. 2012;379(9812):244-249. doi: 10.1016/S0140-6736(11)61306-8 [DOI] [PubMed] [Google Scholar]
- 38.Charlton R, Green A, Shaddick G, et al. ; PROMPT study group . Risk of type 2 diabetes and cardiovascular disease in an incident cohort of people with psoriatic arthritis: a population-based cohort study. Rheumatology (Oxford). 2019;58(1):144-148. doi: 10.1093/rheumatology/key286 [DOI] [PubMed] [Google Scholar]
- 39.Dowlatshahi EA, Kavousi M, Nijsten T, et al. Psoriasis is not associated with atherosclerosis and incident cardiovascular events: the Rotterdam Study. J Invest Dermatol. 2013;133(10):2347-2354. doi: 10.1038/jid.2013.131 [DOI] [PubMed] [Google Scholar]
- 40.Kaine J, Song X, Kim G, Hur P, Palmer JB. Higher incidence rates of comorbidities in patients with psoriatic arthritis compared with the general population using U.S. administrative claims data. J Manag Care Spec Pharm. 2019;25(1):122-132. doi: 10.18553/jmcp.2018.17421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159(4):895-902. doi: 10.1111/j.1365-2133.2008.08707.x [DOI] [PubMed] [Google Scholar]
- 42.Asgari MM, Wu JJ, Gelfand JM, et al. Validity of diagnostic codes and prevalence of psoriasis and psoriatic arthritis in a managed care population, 1996-2009. Pharmacoepidemiol Drug Saf. 2013;22(8):842-849. doi: 10.1002/pds.3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seminara NM, Abuabara K, Shin DB, et al. Validity of The Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol. 2011;164(3):602-609. doi: 10.1111/j.1365-2133.2010.10134.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sokolova MV, Simon D, Nas K, et al. A set of serum markers detecting systemic inflammation in psoriatic skin, entheseal, and joint disease in the absence of C-reactive protein and its link to clinical disease manifestations. Arthritis Res Ther. 2020;22(1):26. doi: 10.1186/s13075-020-2111-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolliker Frers RA, Cosentino V, Tau J, et al. Immune-mediated inflammation promotes subclinical atherosclerosis in recent-onset psoriatic arthritis patients without conventional cardiovascular risk factors. Front Immunol. 2018;9:139. doi: 10.3389/fimmu.2018.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lockshin B, Balagula Y, Merola JF. Interleukin 17, inflammation, and cardiovascular risk in patients with psoriasis. J Am Acad Dermatol. 2018;79(2):345-352. doi: 10.1016/j.jaad.2018.02.040 [DOI] [PubMed] [Google Scholar]
- 47.Fu Y, Lee CH, Chi CC. Association of psoriasis with colorectal cancer: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;S0190-9622(20)32643-8. doi: 10.1016/j.jaad.2020.09.050 [DOI] [PubMed] [Google Scholar]
- 48.Garshick MS, Tawil M, Barrett TJ, et al. Activated platelets induce endothelial cell inflammatory response in psoriasis via COX-1. Arterioscler Thromb Vasc Biol. 2020;40(5):1340-1351. doi: 10.1161/ATVBAHA.119.314008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zwain A, Aldiwani M, Taqi H. The association between psoriasis and cardiovascular diseases. Eur Cardiol. 2021;16:e19. doi: 10.15420/ecr.2020.15.R2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan Z, Wang L, Jiang H, Lin Y, Wang Z. Platelet dysfunction and its role in the pathogenesis of psoriasis. Dermatology. 2021;237(1):56-65. doi: 10.1159/000505536 [DOI] [PubMed] [Google Scholar]
- 51.Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945-1960. doi: 10.1001/jama.2020.4006 [DOI] [PubMed] [Google Scholar]
- 52.Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021;397(10281):1301-1315. doi: 10.1016/S0140-6736(20)32549-6 [DOI] [PubMed] [Google Scholar]
- 53.Pleńkowska J, Gabig-Cimińska M, Mozolewski P. Oxidative stress as an important contributor to the pathogenesis of psoriasis. Int J Mol Sci. 2020;21(17):E6206. doi: 10.3390/ijms21176206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baek JO, Byamba D, Wu WH, Kim TG, Lee MG. Assessment of an imiquimod-induced psoriatic mouse model in relation to oxidative stress. Arch Dermatol Res. 2012;304(9):699-706. doi: 10.1007/s00403-012-1272-y [DOI] [PubMed] [Google Scholar]
- 55.Schüler R, Brand A, Klebow S, et al. Antagonization of IL-17A attenuates skin inflammation and vascular dysfunction in mouse models of psoriasis. J Invest Dermatol. 2019;139(3):638-647. doi: 10.1016/j.jid.2018.09.021 [DOI] [PubMed] [Google Scholar]
- 56.Ikonomidis I, Makavos G, Papadavid E, et al. Similarities in coronary function and myocardial deformation between psoriasis and coronary artery disease: the role of oxidative stress and inflammation. Can J Cardiol. 2015;31(3):287-295. doi: 10.1016/j.cjca.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 57.Ger TY, Fu Y, Chi CC. Bidirectional association between psoriasis and obstructive sleep apnea: a systematic review and meta-analysis. Sci Rep. 2020;10(1):5931. doi: 10.1038/s41598-020-62834-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogawa K, Okada Y. The current landscape of psoriasis genetics in 2020. J Dermatol Sci. 2020;99(1):2-8. doi: 10.1016/j.jdermsci.2020.05.008 [DOI] [PubMed] [Google Scholar]
- 59.Zeng J, Luo S, Huang Y, Lu Q. Critical role of environmental factors in the pathogenesis of psoriasis. J Dermatol. 2017;44(8):863-872. doi: 10.1111/1346-8138.13806 [DOI] [PubMed] [Google Scholar]
- 60.Dekkers OM, Vandenbroucke JP, Cevallos M, Renehan AG, Altman DG, Egger M. COSMOS-E: guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 2019;16(2):e1002742. doi: 10.1371/journal.pmed.1002742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vinogradova Y, Coupland C, Hippisley-Cox J. Use of combined oral contraceptives and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2015;350:h2135. doi: 10.1136/bmj.h2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.García-Martínez P, Collado-Díaz V, Mateu-Puchades A, et al. Differential effects of biologics on psoriasis-related vascular inflammation and risk of thrombosis. J Invest Dermatol. 2020;140(11):2294-2298.e6. doi: 10.1016/j.jid.2020.02.039 [DOI] [PubMed] [Google Scholar]
- 64.von Stebut E, Reich K, Thaçi D, et al. Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. J Invest Dermatol. 2019;139(5):1054-1062. doi: 10.1016/j.jid.2018.10.042 [DOI] [PubMed] [Google Scholar]
- 65.Lee MP, Desai RJ, Jin Y, Brill G, Ogdie A, Kim SC. Association of ustekinumab vs TNF inhibitor therapy with risk of atrial fibrillation and cardiovascular events in patients with psoriasis or psoriatic arthritis. JAMA Dermatol. 2019;155(6):700-707. doi: 10.1001/jamadermatol.2019.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vajravelu R, Kaelber D. Venous thromboembolism risk and anti-tumor necrosis alpha agents in inflammatory bowel disease and other chronic inflammatory diseases. Am J Gastroenterol. 2012;107:S612. doi: 10.14309/00000434-201210001-01525 [DOI] [Google Scholar]
- 67.Sandborn WJ, Feagan BG, Danese S, et al. Safety of ustekinumab in inflammatory bowel disease: pooled safety analysis of results from phase 2/3 studies. Inflamm Bowel Dis. 2021;27(7):994-1007. doi: 10.1093/ibd/izaa236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Temiz SA, Özer İ, Ataseven A, Dursun R, Uyar M. The effect of smoking on the psoriasis: is it related to nail involvement? Dermatol Ther. 2020;33(6):e13960. doi: 10.1111/dth.13960 [DOI] [PubMed] [Google Scholar]
- 69.Armstrong AW, Harskamp CT, Dhillon JS, Armstrong EJ. Psoriasis and smoking: a systematic review and meta-analysis. Br J Dermatol. 2014;170(2):304-314. doi: 10.1111/bjd.12670 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Approximation of Adjusted Risk Estimates
eTable 1. Search Strategies Modified for (a) Medline, (b) Embase, (c) Cochrane Library, (d) Web of science, and (e) CINAHL
eTable 2. Adjusted Covariates of Included Studies
eTable 3. Sensitivity Analyses
eFigure 1. Subgroup Analysis of Venous Thromboembolism According to Arthritis Status
eFigure 2. Subgroup Analysis of Venous Thromboembolism According to Gender.
eFigure 3. Subgroup Analysis of Venous Thromboembolism According to Geographic Location
eFigure 4. Leave-one Out Sensitivity Analysis of Venous Thromboembolism
eFigure 5. Leave-one Out Sensitivity Analysis of Peripheral Vascular Disease