Abstract
Objective:
Examine the relationship between prescription opioid analgesic use during pregnancy and preterm birth or term low birthweight.
Design, setting, and participants:
We analyzed data from the National Birth Defects Prevention Study, a US multisite, population-based study, for births from 1997 to 2011. We defined exposure as self-reported prescription opioid use between one month before conception and the end of pregnancy, and we dichotomized opioid use duration by ≤7 days and >7 days.
Main outcome measures:
We examined the association between opioid use and preterm birth (defined as gestational age <37 weeks) and term low birthweight (defined as <2500 g at gestational age ≥37 weeks).
Results:
Among 10,491 singleton mother/infant pairs, 470 (4.5 percent) reported opioid use. Among women reporting opioid use, 236 (50 percent) used opioids for > 7 days: codeine (170, 36 percent) and hydrocodone (163, 35 percent) were the most commonly reported opioids. Opioid use was associated with slightly increased risk for preterm birth [adjusted odds ratio, 1.4; 95 percent confidence interval, 1.0, 1.9], particularly with hydrocodone [1.6: 1.0, 2.6], meperidine [2.5; 1.2, 5.2], or morphine [3.0; 1.5, 6.1] use for any duration: however, opioid use was not significantly associated with term low birthweight.
Conclusions:
Preterm birth occurred more frequently among infants of women reporting prescription opioid use during pregnancy. However, we could not determine if these risks relate to the drug or to indications for use. Patients who use opioids during pregnancy should be counseled by their practitioners about this and other potential risks associated with opioid use in pregnancy.
Keywords: analgesics, opioid, infant, low birthweight, pregnancy, premature birth
INTRODUCTION
Opioids are often used for the treatment of severe acute pain;1 their use for chronic pain has recently declined following the Centers for Disease Control and Prevention’s (CDC) 20f6 prescribing guidelines.1-5 Opioid prescriptions had steadily increased during the f990s and 2000s, and use was common among women of reproductive age.6-9 Annually, from 2008 to 2012, approximately one-third of reproductive-aged women filled an opioid prescription, regardless of health insurance type.10 Opioid use was also frequent among pregnant women.11 On average, 14 percent of privately insured women and 22 percent of Medicaid-enrolled women used opioids during pregnancy.12,13 Among Medicaid-enrolled pregnant women, this represents an increase from 19 percent (2000) to 23 percent (2007).13
Previous studies have assessed the impact of prescription opioid analgesic use during pregnancy on the development of neonatal abstinence syndrome and birth defects.9,11,13-18 However, there is some evidence that opioid use in pregnancy might also be related to preterm birth (gestational age < 37 weeks) and low birthweight (<2500 g).9,11,13,14 One retrospective cohort study using several Swedish registers concluded that observed associations between prescription opioid analgesic use during pregnancy and risks for preterm birth and small for gestational age were predominantly due to unmeasured confounding factors.19
Women with opioid use disorder more commonly have adverse pregnancy outcomes, which include poor fetal growth and preterm birth.20 In a comprehensive review of prescription opioid use during pregnancy and birth outcomes published in 2015, Yazdy et al.21 summarized that study results for birthweight and preterm birth were inconclusive, indicating that further studies—ideally those able to examine individual opioid medications—were needed to assess whether prescription opioid use during pregnancy poses increased risk for either low birthweight or preterm birth.
In the United States, the annual rates of preterm birth and low birthweight are 10 percent and 8 percent, respectively.22 Preterm birth and low birthweight are of public health concern as they are associated with numerous negative infant outcomes, most importantly neonatal death.23-27
The objectives of this study were to (1) determine the proportion and duration of self-reported opioid analgesic use between one month before conception and the end of pregnancy among mothers of liveborn, singleton infants without any major birth defect and (2) examine the relationship between maternal opioid analgesic use and (a) preterm birth or (b) low birthweight among term births.
METHODS
The National Birth Defects Prevention Study (NBDPS) is a population-based, case-control study that included infants with or without major structural birth defects from ten centers across the United States.28 Mothers were asked about all medications they took, regardless of whether they were prescription or nonprescription (illicit or otherwise), for treatment of diabetes, high blood pressure, seizures, respiratory illnesses, infections, fevers, chronic diseases, injuries, and surgeries from three months before conception through the end of pregnancy and were encouraged to report any additional medications. NBDPS methods are described in detail elsewhere.28,29 Informed consent was obtained from all participants, and all protocols, contact materials, and interview content were approved by the Centers for Disease Control and Prevention Institutional Review Board (IRB) and the local IRB(s) for each Center. Previous publications using these study data have examined maternal opioid use in relation to other birth outcomes, such as birth defects.16,30
Data from NBDPS controls (infants without a major birth defect) born between October 1, 1997, and December 31, 2011, were utilized for this analysis. Control infants had been randomly selected for inclusion in NBDPS using birth certificates or hospital data, depending on the study site. The data included in this report represent those most recently available from this study. For the current study, the participation rate among controls was 65 percent, and the mean time from delivery to interview for controls was nine months (range: six months-two years). Mothers were interviewed with a computer-assisted telephone interview in English or Spanish. The interviews consisted of various sections focusing on maternal health factors, pregnancy history, dietary and drug exposures, and sociodemographic characteristics. For each section of the interview, all reported medications were compiled and coded using the Slone Drug Dictionary, which links the medication products to their active ingredients (licensed by the NBDPS from Boston University’s Slone Epidemiology Center).31 Mother/infant pairs were excluded if the infant had a birth defect (cases); the birth was plural; opioid use data, birthweight, or gestational age was missing; implausible birthweight (<10 grams) or gestational age (≤20 weeks); self-reported heroin or opioid abuse in pregnancy; or data for any of the confounders examined were missing.
The outcomes of interest were preterm birth, regardless of whether preterm birth was medically indicated or spontaneous, and term (gestational age between 37 and 45 weeks) low birthweight. Exposed women were defined as those self-reporting use of any of the following medications for any medical indication and in any dose between one month before conception and the end of pregnancy: buprenorphine, butorphanol, codeine, fentanyl, hydrocodone, hydromorphone, meperidine, methadone, morphine, nalbuphine, oxycodone, pentazocine, propoxyphene, or tramadol. Total opioid use duration was analyzed as any duration, as well as dichotomized into seven days or less use versus more than seven days of use (based on median use duration), and compared to no opioid exposure between one month before conception and the end of pregnancy. Additionally, the relationship between specific opioids and the outcomes of interest was examined, where the sample size permitted.
Descriptive statistical analyses were performed to obtain the median and interquartile range for the duration of opioid use (days) and the mean values and the corresponding standard deviations for gestational age, birthweight (among term births), and maternal age at delivery. Analyses were also conducted to obtain descriptive statistics for potential covariates including maternal age at delivery, race/ethnicity, education, pre-pregnancy body mass index (BMI), smoking between one month before conception and the third month of pregnancy, alcohol consumption between one month before conception and the third month of pregnancy, total household income, infant sex, and study location. These a priori covariates were selected based on the preterm birth and low birthweight literature.32-34 A stepwise selection of all possible subsets removed total household income and infant sex from the regression. Two-sample t-tests were used to assess associations with continuous variables, and chi-square and Fisher’s exact tests were used to assess associations with categorical variables. Additionally, specific opioids for which five or more mother/infant pairs with preterm birth were exposed were examined in relation to exposure duration. Logistic regression was used to obtain the crude and adjusted odds ratios (ORs) and 95 percent confidence intervals (CI) for preterm birth and term low birthweight by opioid exposure overall, by opioid exposure dichotomized by duration of use, and between preterm birth and specific opioid exposures.
We examined the interview section in which opioid use was reported. No further details about conditions that necessitated opioid use during pregnancy were available in the database. Because opioid use during delivery could bias the observed associations (because the infants would only have been exposed in the last hours of pregnancy rather than during the developmental stages), a sensitivity analysis was performed where mothers presumably exposed only during delivery were excluded. These included mothers who reported opioid use for seven days or less during the ninth month of pregnancy and who reported their opioid use in either the procedure or pregnancy complication sections of the computer-assisted telephone interview or reported their opioid use in the additional unspecified reporting section of the interview. Additionally, we conducted a sensitivity analysis of the association between opioid use and preterm birth and term low birthweight stratified by trimester of opioid use. A p value of <0.05 was considered significant. All analyses were performed using SAS 9.4.
RESULTS
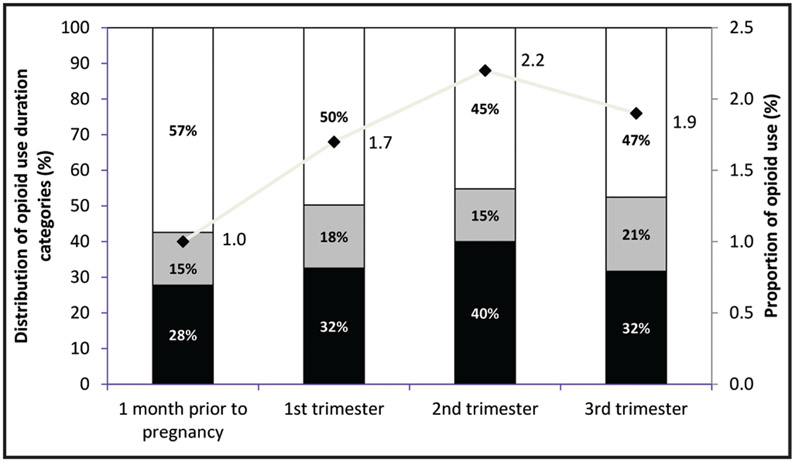
Among the 10,491 singleton mother/infant pairs meeting inclusion criteria (Figure 1), 470 (4.5 percent) mothers reported opioid use at any time between one month before conception and the end of pregnancy. The proportion of opioid use was the lowest one month prior to pregnancy (1.0 percent, 95 percent CI 0.8 percent, 1.2 percent; n = 108) and during the first trimester (1.7 percent, 95 percent CI 1.4 percent, 1.9 percent; n = 175), while the highest proportion of use occurred during the second trimester (2.2 percent, 95 percent CI 1.9 percent, 2.5 percent, n = 230) and dropped slightly in the third trimester (1.9 percent, 95 percent CI 1.7 percent, 2.2 percent; n = 202) (Figure 2). Among exposed women, 50 percent (n = 236) reported opioid use for more than seven days (Table 1). The overall median opioid use duration was eight days.
Figure 1.
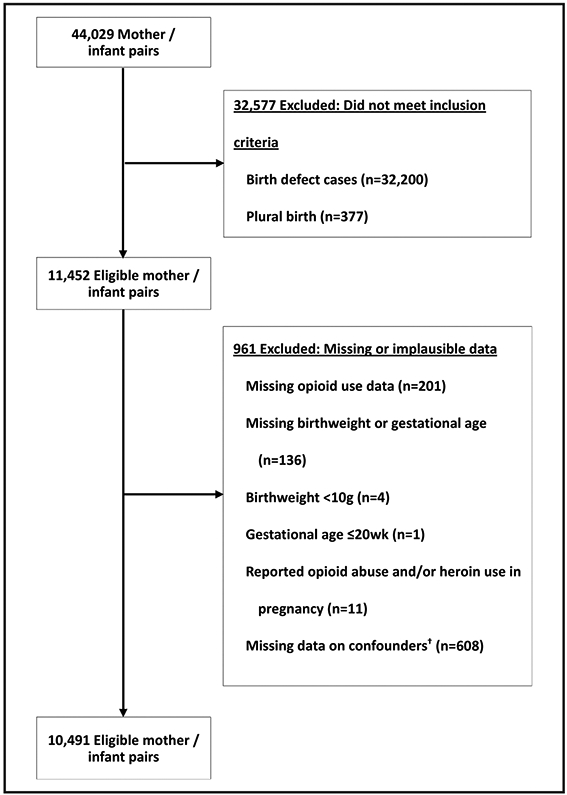
Derivation of the study sample, National Birth Defects Prevention Study, 1997-2011. Note. †Missing any values for maternal age at delivery, race/ethnicity, education, pre-pregnancy body mass index, maternal smoking between one month before conception and the third month of pregnancy, or alcohol consumption between one month before conception and the third month of pregnancy.
Figure 2.
Proportion and duration of opioid use by trimester, National Birth Defects Prevention Study (controls), 1997-2011. Groups are not mutually exclusive. Legend: Black, ≤ 7 days of treatment; gray, 8-30 days of treatment; white: > 30 days of treatment; line: proportion of opioid use.
Table 1.
Demographic characteristics by opioid use between one month before conception and the end of pregnancy, National Birth Defects Prevention Study (controls), 1997-2011
| Total (n = 10,491) |
No opioid use (n = 10,021) |
Opioid use duration | ||||||
|---|---|---|---|---|---|---|---|---|
| Any (n = 470) | p† | ≤7 days (n = 234) |
p† | >7 days (n = 236) |
p† | |||
| Median opioid use duration (day) | NA | NA | 8 (2-45) | NA | 2 (1-4) | NA | 45 (21-153) | NA |
| Gestational age (week) | 38.7 ± 1.9 | 38.7 ± 1.9 | 38.5 ± 1.9 | 0.013 | 38.6 ± 1.6 | 0.679 | 38.3 ± 2.2 | 0.005 |
| <37 (preterm) | 838 (8.0) | 787 (7.9) | 51 (10.9) | 0.019 | 24 (10.3) | 0.178 | 27 (11.4) | 0.044 |
| ≥37 (term) | 9,653 (92.0) | 9,234 (92.1) | 419 (89.1) | 210 (897) | 209 (88.6) | |||
| Birthweight among term births (g)‡ | 3430.9 ± 460.1 | 3431.4 ± 459.6 | 3420.9 ± 469.6 | 0.647 | 3459.6 ± 464.7 | 0.380 | 3382.0 ± 472.5 | 0.125 |
| <2500 (low) | 172 (1.8) | 167 (1.8) | 5 (1.2) | 0.352 | 1 (0.5) | 0.189 | 4 (1.9) | 0.792 |
| ≥2500 (normal) | 9,481 (98.2) | 9,067 (98.2) | 414 (98.8) | 209 (99.5) | 205 (98.1) | |||
| Maternal age at delivery (years) | 27.7 ± 6.1 | 27.8 ± 6.1 | 27.3 ± 5.5 | 0.079 | 27.2 ± 57 | 0.141 | 27.4 ± 5.4 | 0.354 |
| <20 | 1,034 (9.9) | 1,003 (10.0) | 31 (6.6) | 0.0002 | 15 (6.4) | 0.035 | 16 (6.8) | 0.003 |
| 20-34 | 7,977 (76.0) | 7,582 (75.7) | 395 (84.0) | 194 (82.9) | 201 (85.2) | |||
| ≥35 | 1,480 (14.1) | 1,436 (14.3) | 44 (9.4) | 25 (10.7) | 19 (8.0) | |||
| Maternal race/ethnicity | <0.0001 | <0.0001 | <0.0001 | |||||
| Non-Hispanic White | 6,313 (60.2) | 5,954 (59.4) | 359 (76.4) | 180 (76.9) | 179 (75.9) | |||
| Non-Hispanic Black | 1,199 (11.4) | 1,151 (11.5) | 48 (10.2) | 21 (9.0) | 27 (11.4) | |||
| Hispanic | 2,270 (21.6) | 2,228 (22.2) | 42 (8.9) | 21 (9.0) | 21 (8.9) | |||
| Other/mixed | 709 (6.8) | 688 (6.9) | 21 (4.5) | 12 (5.1) | 9 (3.8) | |||
| Maternal education | <0.0001 | 0.0006 | 0.018 | |||||
| <High school | 1,527 (14.6) | 1,489 (14.9) | 38 (8.1) | 16 (6.8) | 22 (9.3) | |||
| ≥High school | 8,964 (85.4) | 8,532 (85.1) | 432 (91.9) | 218 (93.2) | 214 (90.7) | |||
| Maternal pre-pregnancy BMI (kg/m2) | 0.040 | 0.112 | 0.175 | |||||
| Not obese (<30) | 8,568 (81.7) | 8,201 (81.8) | 367 (78.1) | 182 (77.8) | 185 (78.4) | |||
| Obese (≥30) | 1,923 (18.3) | 1,820 (18.2) | 103 (21.9) | 52 (22.2) | 51 (21.6) | |||
| Maternal smoking§ | <0.0001 | <0.0001 | <0.0001 | |||||
| Yes | 1,945 (18.5) | 1,766 (17.6) | 179 (38.1) | 79 (33.8) | 100 (42.4) | |||
| No | 8,546 (81.5) | 8,255 (82.4) | 291 (61.9) | 155 (66.2) | 136 (57.6) | |||
| Maternal alcohol consumption§ | 0.020 | 0.321 | 0.019 | |||||
| Yes | 4,039 (38.5) | 3,834 (38.3) | 205 (43.6) | 97 (41.5) | 108 (45.8) | |||
| No | 6,452 (61.5) | 6,187 (61.7) | 265 (56.4) | 137 (58.5) | 128 (54.2) | |||
| Maternal residence | <0.0001 | 0.005 | <0.0001 | |||||
| Arkansas | 1,351 (12.9) | 1,251 (12.5) | 100 (21.3) | 43 (18.4) | 57 (24.2) | |||
| California | 1,087 (10.4) | 1,047 (10.4) | 40 (8.5) | 22 (9.4) | 18 (7.6) | |||
| Georgia | 1,121 (10.7) | 1,080 (10.8) | 41 (8.7) | 23 (9.8) | 18 (7.6) | |||
| Iowa | 1,207 (11.5) | 1,140 (11.4) | 67 (14.2) | 40 (17.1) | 27 (11.4) | |||
| Massachusetts | 1,295 (12.3) | 1,236 (12.3) | 59 (12.5) | 30 (12.8) | 29 (12.3) | |||
| New Jersey | 523 (5.0) | 510 (5.1) | 13 (2.8) | 7 (3.0) | 6 (2.6) | |||
| New York | 914 (8.7) | 884 (8.8) | 30 (6.4) | 12 (5.1) | 18 (7.6) | |||
| North Carolina | 884 (8.4) | 847 (8.5) | 37 (7.9) | 20 (8.6) | 17 (7.2) | |||
| Texas | 1,157 (11.0) | 1,121 (11.2) | 36 (7.7) | 15 (6.4) | 21 (8.9) | |||
| Utah | 952 (9.1) | 905 (9.0) | 47 (10.0) | 22 (9.4) | 25 (10.6) | |||
| Estimated date of delivery year | 0.221 | 0.372 | 0.378 | |||||
| 1997-2001 | 3,128 (29.8) | 3,004 (30.0) | 124 (26.4) | 63 (26.9) | 61 (25.9) | |||
| 2002-2006 | 3,822 (36.4) | 3,638 (36.3) | 184 (39.1) | 95 (40.6) | 89 (37.7) | |||
| 2007-2011 | 3,541 (33.8) | 3,379 (33.7) | 162 (34.5) | 76 (32.5) | 86 (36.4) | |||
NA, not applicable; BMI, body mass index.
Data are median (25th percentile-75th percentile), mean ± standard deviation, or n (percent).
Compared to no opioid use, p values were calculated using two-sample t-tests for continuous variables and chi-square and Fisher’s exact tests for categorical variables.
Total term births: n = 9,653; No opioid use term births: n = 9,234; Any opioid use term births: n = 414; ≤ 7 days opioid use term births: n = 210; > 7 days of opioid use term births: n = 209.
Reported use between one month before conception and the third month of pregnancy.
Preterm birth was more common among women who reported opioid use than among women with no opioid exposure (10.9 percent compared with 7.9 percent, p = 0.019). Births that were very preterm (gestational age < 32 weeks) made up 9.8 percent of preterm births among women who reported opioid use compared to 13.0 percent of preterm births among women with no reported opioid exposure (p = 0.923), while late preterm births (gestational age between 34 and 36 weeks) accounted for 82.4 percent of preterm births among women who used opioids compared to 74.5 percent among those with no use (p = 0.006; data not shown). Birthweight among term births did not differ significantly by opioid exposure status (Table 1). Women who used opioids were more likely to be younger, white, more educated, obese, and smoke and/or drink alcohol periconceptionally, and opioid use varied by study location. Among exposed women, those reporting opioid use for more than seven days more often smoked and/or drank alcohol periconceptionally than women who reported opioid use for seven days or less (42.2 percent and 45.8 percent compared with 33.8 percent and 41.5 percent, respectively).
The majority of exposed women reported codeine (36.2 percent) or hydrocodone (34.7 percent) use, followed by oxycodone (l6.0 percent), morphine (11.1 percent), or meperidine (10.6 percent) (Table 2). No women reported use of fentanyl or pentazocine. One in five opioid users (19 percent) reported using more than one opioid.
Table 2.
Opioid exposure type and duration between one month before conception and the end of pregnancy, National Birth Defects Prevention Study (controls), 1997-2011
| Opioid name | Opioid use duration† | ||
|---|---|---|---|
| Any N (percent) | ≤7 days N (percent) | >7 days N (percent) | |
| Any opioid | 470 (100) | 234 (49.8) | 236 (50.2) |
| Specific opioids‡ | |||
| Buprenorphine | 2 (0.4) | 0 | 2 (0.9) |
| Butorphanol | 7 (1.5) | 2 (0.9) | 5 (2.1) |
| Codeine | 170 (36.2) | 82 (35.0) | 88 (37.3) |
| Hydrocodone | 163 (34.7) | 71 (30.3) | 92 (39.0) |
| Hydromorphone | 7 (1.5) | 1 (0.4) | 6 (2.5) |
| Meperidine | 50 (10.6) | 26 (11.1) | 24 (10.2) |
| Methadone | 7 (1.5) | 0 | 7 (3.0) |
| Morphine | 52 (11.1) | 28 (12.0) | 24 (10.2) |
| Nalbuphine | 1 (0.2) | 0 | 1 (0.4) |
| Oxycodone | 75 (16.0) | 38 (16.2) | 37 (15.7) |
| Propoxyphene | 33 (7.0) | 8 (3.4) | 25 (10.6) |
| Tramadol | 8 (1.7) | 2 (0.9) | 6 (2.5) |
Exposures for individual medications do not add to the total opioid exposed count of 470 because 87 (19 percent) women reported using more than one opioid medication.
There was no reported use of fentanyl and pentazocine.
When adjusted for maternal age at delivery, race/ethnicity, education, pre-pregnancy BMI, alcohol use, smoking, and study location, any opioid use was significantly associated (p = 0.039) with an increase in odds for preterm birth (adjusted OR, 1.4; 95 percent CI, 1.0, 1.9). When dichotomizing opioid use duration, opioid use was no longer significantly associated with preterm birth (Table 3). However, the change in estimate was only slight (seven days or less: adjusted OR, 1.3; 95 percent CI, 0.9, 2.0; more than seven days: adjusted OR, 1.4; 95 percent CI, 1.0, 2.2). The opioid use of any duration was not significantly associated with an increase in odds for term low birthweight.
Table 3.
Associations between opioid use from one month before conception and the end of pregnancy and preterm birth or term low birthweight, National Birth Defects Prevention Study (controls), 1997-2011
| Unexposed N (percent) | Exposed N (percent) | Crude OR (95 percent CI) |
Adjusted† OR (95 percent CI) |
|
|---|---|---|---|---|
| Preterm birth | 787 (7.9) | |||
| Any duration | 51 (10.9) | 1.4 [1.1, 1.9]* | 1.4 [1.0, 1.9]* | |
| ≤7 days | 24 (10.3) | 1.3 10.9, 2.1] | 1.3 [0.9, 2.0] | |
| >7 days | 27 (11.4) | 1.5 [1.0, 2.3]* | 1.4 [1.0, 2.2] | |
| Term low birthweight | 167 (1.8) | |||
| Any duration | 5 (1.2) | 0.7 [0.3, 1.6] | 0.7 [0.3, 1.7] | |
| ≤7 Days | 1 (0.5) | Not calculated | Not calculated | |
| >7 Days | 4 (1.9) | 1.1 [0.4, 2.9] | 1.0 [0.4, 2.7] |
OR, odds ratio; CI, confidence interval.
Adjusted for maternal age at delivery, race/ethnicity, education, prepregnancy body mass index, maternal smoking between one month before conception and the third month of pregnancy, alcohol consumption between one month before conception and the third month of pregnancy, and study location.
p < 0.05.
Among specific opioids, only codeine, hydrocodone, meperidine, morphine, and oxycodone had more than five exposed preterm births and were further examined. Hydrocodone (adjusted OR, 1.6; 95 percent CI, 1.0, 2.6), meperidine (adjusted OR, 2.5; 95 percent CI, 1.2, 5.2), and morphine (adjusted OR, 3.0; 95 percent CI, 1.5, 6.1) use for any duration were associated with preterm birth (Table 4). When excluding women who reported the use of meperidine and morphine for more than seven days (the strongest associations with preterm birth, n = 42), the association between opioid use and preterm birth was no longer statistically significant (adjusted OR, 1.2; 95 percent CI, 0.9, 1.7). There were not enough exposed term births with low birthweight to examine specific opioids and low birthweight.
Table 4.
Associations between specific opioid use from one month before conception and the end of pregnancy and preterm birth, National Birth Defects Prevention Study (controls), 1997-2011
| Preterm/ Term N (percent) |
Crude OR (95 percent CI) |
Adjusted† OR (95 percent CI) |
|
|---|---|---|---|
| Codeine | |||
| Any duration | 19/151 (11.2) | 1.5 (0.9, 2.4) | 1.4 (0.8, 2.2) |
| Hydrocodone | |||
| Any duration | 20/143 (12.3) | 1.6 (1.0, 2.6)* | 1.6 (1.0, 2.6) |
| Meperidine | |||
| Any duration | 9/41 (18.0) | 2.6 (1.2, 5.3)* | 2.5 (1.2, 5.2)* |
| Morphine | |||
| Any duration | 10/42 (19.2) | 2.8 (1.4, 5.6)** | 3.0 (1.5, 6.1)** |
| Oxycodone | |||
| Any duration | 6/69 (8.0) | 1.0 (0.4, 2.4) | 1.1 (0.5, 2.5) |
OR, odds ratio; CI, confidence interval.
Adjusted for maternal age at delivery, race/ethnicity, education, prepregnancy body mass index, maternal smoking between one month before conception and the third month of pregnancy, alcohol consumption between one month before conception and the third month of pregnancy, and study location.
p < 0.05.
p < 0.01.
Opioid use was reported in a number of different questionnaire sections. The most commonly reported sections were procedures (unspecified; 30.3 percent among opioid use for seven days or less and 14.8 percent among opioid use for more than seven days) and infections (22.7 percent and 20.3 percent), followed by other diseases/disorders (10.3 percent and 17.0 percent), injuries (13.7 percent and 12.7 percent), and pregnancy complications (0 percent and 0.4 percent). Reasons for opioid use were unspecified (medication catch all section) in 26.1 percent among opioid use for seven days or less and 45.8 percent among use for more than seven days. The sensitivity analysis excluding women potentially exposed only during delivery revealed that eight mothers reported opioid use for seven days or less during the ninth month of pregnancy for reasons that may have been related to delivery: five for a procedure and three for unspecified reasons. After excluding these eight mothers, there were no meaningful changes in the associations between opioid use and preterm birth or term low birthweight (data not shown).
In the sensitivity analysis of trimester of use, the risk for preterm birth was no longer significant when opioid use was reported during the first trimester (adjusted OR, 1.2; 95 percent CI, 0.7, 2.0) or the third trimester (adjusted OR, 1.2; 95 percent CI, 0.7, 1.9) but was significant when opioid use was reported during the second trimester (adjusted OR, 2.0; 95 percent CI, 1.4, 2.9). However, the study size was small in analyses of specific trimester of use, and when examining second trimester use, the majority (53.5 percent) also used opioids in other trimesters.
DISCUSSION
Our study suggests that women who use prescription opioids during pregnancy, particularly hydrocodone, meperidine, or morphine for any duration, were more likely to have medically indicated and/or spontaneous preterm birth. This is important as approximately 600,000 to 900,000 pregnant women in the United States use opioids every year.12,13,35
Previous studies have found similar associations between opioid use during pregnancy and increased risk for preterm birth. Källén et al.36 found similar risks for preterm birth (OR, 1.1; 95 percent CI, 1.0, 1.2), but their study was restricted to opioid use during the second or third trimester, while we examined use throughout pregnancy. Nørgaard et al.,37 Cleary et al.,38 and Greig et al.39 found higher risks for preterm birth (OR, 2.8; 95 percent CI, 2.3, 3.4; OR, 2.5; 95 percent CI, 2.0, 3.1; and risk ratio, 2.5; 95 percent CI, 1.7, 3.9, respectively); however, these studies focused on women who were abusing opioids or were on methadone maintenance treatment during pregnancy. Creanga et al.11 found the highest risk previously reported for preterm birth (OR, 3.0; 95 percent CI, 2.3, 3.1), but they did not separate opioid use from that of other illicit substances, and Sharpe and Kuschel40 found very high frequencies of preterm birth among pregnant women who used opioids for pain management but only among a sample of 19 women. Similar to our study, Patrick et al.14 focused on opioid use for pain in pregnancy and found that 11.6 percent of those exposed had preterm births, analogous to our finding of 11.0 percent. Nezvalová-Henriksen et al.41 found no significant association between opioids and preterm birth; however, their study was restricted to codeine, and in our analysis of specific opioids, codeine was also not significantly associated with preterm birth. One other study where the majority of opioids used were acetaminophen with oxycodone, codeine, or hydrocodone found no significant association between opioid use and preterm birth.42 One retrospective cohort study using several Swedish registers concluded that an observed association between prescription opioid analgesic use during pregnancy and risk for preterm birth was predominantly due to unmeasured confounding factors.19
While some previous literature has suggested a link between prenatal opioid use and impaired fetal growth, this analysis did not observe an increased risk for term low birthweight, nor did we see a significant difference in mean birthweight; however, we only had five exposed term low birthweight infants and were thus not powered to detect odds ratios between 0.1 and 2 in this instance. Nørgaard et al.37 found a prevalence ratio of 4.3 (95 percent CI, 3.0, 6.1) for term low birthweight, and Greig et al.39 found a risk ratio of 2.2 (95 percent CI, 1.3, 3.7), but as mentioned above, their studies focused on opioid abuse. Cleary et al.38 found increased risk for small for gestational age (OR, 2.2; 95 percent CI, 1.9, 2.6), but they did not examine low birthweight separately. Creanga et al.11 and Patrick et al.14 found increased risk for low birthweight but did not restrict their analysis to infants achieving term birth. Findings by Nezvalová-Henriksen et al.41 and Källén et al.36 were similar to ours, with no significant associations found. The retrospective cohort study by Sujan et al.19 using several Swedish registers concluded that an observed association between prescription opioid analgesic use during pregnancy and risk for small for gestational age was predominantly due to unmeasured confounding factors.
While the current study utilizes the largest US population-based study of birth defects and includes important information such as trimester of exposure and rates of opioid use, this study is subject to several limitations. First, while study controls in the NBDPS were generally representative [absolute differences in sociodemographic and other characteristics between controls and populations from which controls were selected were ≤1.3 percent43] of populations in their respective study sites, they were more often non-Hispanic white and obtained higher education, and their infants were more likely to have birthweights ≥2,500 g and were more likely to be born at ≥37 weeks. Therefore, rates of preterm birth and term low birthweight in the United States were somewhat higher than among the unexposed in this study (9.6 percent vs. 8.0 percent and 2.7 percent vs. 1.8 percent, respectively).35 Second, we were not able to distinguish between spontaneous and induced preterm birth. Medically induced preterm birth, which accounts for 25 percent of all preterm births, results from conditions such as maternal hypertension, abruptio placentae, intrauterine growth restriction, or fetal distress.44 The need for opioids prior to delivery could be related to a condition that resulted in induced preterm birth, biasing our results. Third, exposure underreporting is possible due to illicit opioid use, social desirability bias, and retrospective self-report. In this study, < 5 percent of pregnant women reported opioid use, whereas other estimates range from 14 percent-22 percent.12,13 However, those estimates used dispensing and prescription data to indirectly measure opioid use. Regardless, nondifferential underreporting of opioid use in this study could produce misclassification that would reduce an effect of opioid use. If women with preterm births were less likely than women with term births to underreport, then the observed effect could be biased upward. However, we believe that this is unlikely because of the survey methodology. Fourth, we were not able to assess the use of methadone or buprenorphine for the indication of treatment of opioid use disorder. We excluded women who self-reported opioid abuse to try to address this issue. Fifth, there were small numbers in analyses of specific opioid use and sensitivity analyses by trimester of use, leading to little statistical power to discern individual associations; however, when rerunning analyses using exact logistic regression for sparse data, no changes in statistical significance were found. Sixth, the NBDPS did not collect dosage, so we were unable to assess dose-response relationships. Seventh, there is a potential for confounding by indication for some underlying condition(s). Notwithstanding, this study gives a baseline for future studies to examine indication. Pregnant women with severe illnesses may need opioid medication, and that underlying condition could pose a risk for adverse pregnancy outcomes. Eighth, the exclusion of 8 percent of eligible controls due to missing or implausible values may bias findings; however, the distribution of all demographic factors was similar between those included and excluded from analyses, eg, similar maternal age, race/ethnicity, and education, thus reducing the likelihood of such bias. Finally, we could not rule out all sources of confounding.
Preterm birth occurred more frequently among infants of women reporting prescription opioid use during pregnancy. However, we could not determine if these risks relate to the drug or to indications for use. The finding that use of specific opioids for more than seven days may have a stronger association with preterm birth suggests that the clinical significance of these issues needs to be explored further. Patients who use opioids during pregnancy should be counseled by their practitioners about this and other potential risks associated with opioid use in pregnancy.
ACKNOWLEDGMENTS
This project was supported in part by an appointment to the Research Participation Program at the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention (CDC), administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and CDC. The authors thank the participating families, scientists, and staff from all of the NBDPS sites. The authors wish to thank Dr. Margaret Honein for her contributions to this project.
Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
Julia D. Interrante, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, Georgia; Oak Ridge Institute for Science and Education, Oak Ridge, Tennessee..
Stacey L. P. Scroggs, New Mexico State University, Las Cruces, New Mexico..
Carol J. Hogue, Rollins School of Public Health, Emory University, Atlanta, Georgia..
Jan M. Friedman, Department of Medical Genetics and Genomics, University of British Columbia, Vancouver, British Columbia, Canada..
Jennita Reefhuis, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, Georgia..
Michael W. Jann, University of North Texas Health Science Center, Fort Worth, Texas..
Cheryl S. Broussard, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, Georgia..
REFERENCES
- 1.ACPA resource guide to pain medication and treatments. American Chronic Pain Association, 2019. Available at https://www.theacpa.org/wp-content/uploads/2019/02/ACPA_Resource_Guide_2019.pdf. Accessed February 16, 2021. [Google Scholar]
- 2.Dowell D, Haegerich TM, Chou R: CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016; 65: 1–49. [DOI] [PubMed] [Google Scholar]
- 3.Dowell D, Haegerich T, Chou R: No shortcuts to safer opioid prescribing. N Engl J Med. 2019; 380: 2285–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohnert ASB, Guy GP Jr, Losby JL: Opioid prescribing in the United States before and after the Centers for Disease Control and Prevention’s 2016 opioid guideline. Ann Intern Med. 2018; 169: 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Center for Injury Prevention and Control: Opioid overdose: US prescribing rate maps. Centers for Disease Control and Prevention, 2017. Available at https://www.cdc.gov/drugoverdose/maps/rxrate-maps.html. Accessed February 16, 2021.
- 6.Manchikanti L, Singh A: Therapeutic opioids: A ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008; 11: S63–S88. [PubMed] [Google Scholar]
- 7.Joranso DE, Ryan KM, Gilson AM: Trends in medical use and abuse of opioid analgesics. JAMA. 2000; 283: 1710–1714. [DOI] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics: Health, United States, 2006. Hyattsvile, MD: Department of Health and Human Services, 2007. [Google Scholar]
- 9.Kellogg A, Rose CH, Harms RH: Current trends in narcotic use in pregnancy and neonatal outcomes. Am J Obstetr Gynecol. 2011; 204: 259.e1–259.e4. [DOI] [PubMed] [Google Scholar]
- 10.Ailes EC, Dawson AL, Lind JN, et al. : Opioid prescription claims among women of reproductive age—United States, 2008-2012. MMWR. 2015; 64: 37–41. [PMC free article] [PubMed] [Google Scholar]
- 11.Creanga AA, Sabel JC, Ko JY, et al. : Maternal drug use and its effect on neonates: A population-based study in Washington State. Obstetr Gynecol. 2012; 119: 924–933. [DOI] [PubMed] [Google Scholar]
- 12.Bateman BT, Hernandez-Diaz S, Rathmell JP, et al. : Patterns of opioid utilization in pregnancy in a large cohort of commercial insurance beneficiaries in the United States. Anesthesiology. 2014; 120: 1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai RJ, Hernandez-Diaz S, Bateman BT, et al. : Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstetr Gynecol. 2014; 123: 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patrick SW, Dudley J, Martin PR, et al. : Prescription opioid epidemic and infant outcomes. Pediatrics. 2015; 135: 842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desai RJ, Huybrechts KF, Hernandez-Diaz S, et al. : Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study. BMJ. 2015; 350: h2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broussard CS, Rasmussen SA, Reefhuis J, et al. : Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstetr Gynecol. 2011; 204: 314.e1–314.e11. [DOI] [PubMed] [Google Scholar]
- 17.Yazdy MM, Mitchell AA, Tinker SC, et al. : Periconceptional use of opioids and the risk of neural tube defects. Obstetr Gynecol. 2013; 122: 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilbourne P, Wallerstedt C, Dorato V, et al. : Clinical management of methadone dependence during pregnancy. J Perinatal Neonatal Nurs. 2001; 14: 26–45. [DOI] [PubMed] [Google Scholar]
- 19.Sujan AC, Quinn PD, Rickert ME, et al. : Maternal prescribed opioid analgesic use during pregnancy and associations with adverse birth outcomes: A population-based study. PLoS Med. 2019; 16(12): e1002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy UM, Davis JM, Ren Z, et al. : Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes: Executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American College of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstet Gynecol. 2017; 130(1):10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yazdy MM, Desai RJ, Brogly SB: Prescription opioids in pregnancy and birth outcomes: A review of the literature. J Pediatr Genet. 2015; 4: 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin JA, Hamilton BE, Osterman MJK, et al. : Births: Final data for 2017. Natl Vital Stat Rep. 2018; 67(8): 1–50. [PubMed] [Google Scholar]
- 23.Martin JA, Kirmeyer S, Osterman M, et al. : Born a bit too early: Recent trends in late preterm births. NCHS Data Brief, No. 24. Hyattsville, MD: National Center for Health Statistics, 2009: 1–8. [PubMed] [Google Scholar]
- 24.Mathews TJ, MacDorman MF: Infant mortality statistics from the 2007 period linked birth/infant death data set. Natl Vital Stat Rep. 2011; 59: 1–30. [PubMed] [Google Scholar]
- 25.Arnon S, Dolfin T, Litmanovitz I, et al. : Preterm labour at 34—36 weeks of gestation: Should it be arrested? Paediatr Perinatal Epidemiol. 2001;15: 252–256. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandrappa A, Jain L: Health issues of the late preterm infant. Pediatr Clin North Am. 2009; 56: 565–577. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox AJ: Outcomes. In: Fertility and Pregnancy: An Epidemiologic Perspective. New York: Oxford University Press, 2010: 123–229. [Google Scholar]
- 28.Yoon PW, Rasmussen SA, Lynberg MC, et al. : The National Birth Defects Prevention Study. Public Health Rep. 2001; 116: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reefhuis J, Gilboa SM, Anderka M, et al. : The National Birth Defects Prevention Study: A review of the methods. Birth Defects Res Part A. 2015; 103: 656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Interrante JD, Ailes EC, Lind JN, et al. : Risk comparison for prenatal use of analgesics and selected birth defects, National Birth Defects Prevention Study 1997-2011. Ann Epidemiol. 2017; 27(10): 645–653.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelley K, Kelly T, Kaufman D, et al. : The Slone drug dictionary: A research-driven pharmacoepidemiology tool. Pharmacoepidemiol Drug Saf. 2003; 12: S168–S169. [Google Scholar]
- 32.Kramer MS: The epidemiology of adverse pregnancy outcomes: An overview. J Nutr. 2003; 133(5): 1592S–1596S. [DOI] [PubMed] [Google Scholar]
- 33.Goldenberg RL, Culhane JF, Iams JD: Epidemiology and causes of preterm birth. Lancet. 2008; 371(9606): 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shapiro-Mendoza CK, Lackritz EM: Epidemiology of late and moderate preterm birth. Semin Fetal Neonatal Med. 2012; 17(3): 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton BE, Martin JA, Osterman MJ, et al. : Births: Final data for 2014. Natl Vital Stat Rep. 2015; 64: 1–64. [PubMed] [Google Scholar]
- 36.Källén B, Borg N, Reis M: The use of central nervous system active drugs during pregnancy. Pharmaceuticals. 2013; 6: 1221–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nørgaard M, Nielsson MS, Heide-Jørgensen U: Birth and neonatal outcomes following opioid use in pregnancy: A Danish population-based study. Subst Abuse: Res Treat. 2015; 9: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cleary BJ, Donnelly JM, Strawbridge JD, et al. : Methadone and perinatal outcomes: A retrospective cohort study. Am J Obstetr Gynecol. 2011; 204: 139.e1–139.e9. [DOI] [PubMed] [Google Scholar]
- 39.Greig E, Ash A, Douiri A: Maternal and neonatal outcomes following methadone substitution during pregnancy. Arch Gynecol Obstetr. 2012; 286: 843–851. [DOI] [PubMed] [Google Scholar]
- 40.Sharpe C, Kuschel C: Outcomes of infants born to mothers receiving methadone for pain management in pregnancy. Arch Disease Childhood. 2004; 89: F33–F36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nezvalová-Henriksen K, Spigset O, Nordeng H: Effects of codeine on pregnancy outcome: Results from a large population-based cohort study. Eur J Clin Pharmacol. 67: 1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith MV, Costello D, Yonkers KA: Clinical correlates of prescription opioid analgesic use in pregnancy. Matern Child Health J. 2015; 19(3): 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cogswell ME, Bitsko RH, Anderka M et al. : Control selection and participation in an ongoing, population-based, case-control study of birth defects: the National Birth Defects Prevention Study. Am J Epidemiol. 2009; 170: 975–985. [DOI] [PubMed] [Google Scholar]
- 44.Moutquin JM: Classification and heterogeneity of preterm birth. Br J Obstetr Gynaecol. 2003; 110 Suppl 20: 30–33. [DOI] [PubMed] [Google Scholar]