Abstract
Tendon rupture can occur at any age and is commonly treated nonoperatively, yet can result in persisting symptoms. Thus, a need exists to improve nonoperative treatments of injured tendons. Photobiomodulation (PBM) therapy has shown promise in the clinic and is hypothesized to stimulate mitochondrial-related metabolism and improve healing. However, the effect of PBM therapy on mitochondrial function during tendon maturation and healing are unknown, and its effect on tendon structure and function remain unclear. In this study, near-infrared light (980:810 nm blend, 2.5 J/cm2) was applied at low (30 mW/cm2) or high (300 mW/cm2) irradiance to unilateral Achilles tendons of CD-1 mice during postnatal growth (maturation) as well as adult mice with bilateral Achilles tenotomy (healing). The chronic effect of PBM therapy on tendon structure and function was determined using histology and mechanics, and the acute effect of PBM therapy on mitochondrial-related gene expression was assessed. During maturation and healing, collagen alignment, cell number, and nuclear shape were unaffected by chronic PBM therapy. We found a sex-dependent effect of PBM therapy during healing on mechanical outcomes (eg, increased stiffness and Young's modulus for PBM-treated females, and increased strain at ultimate stress for PBM-treated males). Mitochondria-related gene expression was marginally influenced by PBM therapy for both maturation and healing studies. This study was the first to implement PBM therapy during both growth and healing of the murine tendon. PBM therapy resulted in marginal and sex-dependent effects on the murine tendon. Clinical significance: PBM may be beneficial for tendon healing because functional remodeling improves without adverse effects.
Keywords: Achilles tendon, healing, low-level laser light therapy, maturation, mitochondria, mouse, photobiomodulation
1 |. INTRODUCTION
Injury of the Achilles tendon is one of the most common tendon injuries that affects adolescent and adult populations.1–3 Achilles rupture and tendinopathy are often debilitating, resulting in limited mobility, impaired joint function, and elevated risk of long-term disability and pain if left untreated.1,4,5 Unfortunately, tendon injuries are difficult to treat.6,7 Following surgical repair, the risk of rerupture of the Achilles tendon is greater than 15% and is elevated in patients under the age of 30.8–10 Similarly, nonoperative therapies, such as nonsteroidal antiinflammatory drugs, platelet-rich plasma injections, and physical therapy regimens, often leave patients with persisting symptoms, reduced function, and high rerupture rates.11 Failure of the Achilles tendon to adequately heal often results from poor cell-mediated reintegration, remodeling, and regeneration during the healing process.12 Therefore, developing and improving rehabilitative tools that can regulate cell behavior during tendon healing are needed to accelerate tendon reintegration following injury and reduce the risk of rerupture following repair.
Photobiomodulation (PBM) therapy is a clinically available tool for noninvasive treatment of musculoskeletal injuries. PBM therapy can reduce rehabilitation time and improve treatment outcomes when implemented in physical therapy and outpatient clinics, specifically for Achilles tendinopathy.13–18 PBM therapy is the application of near-infrared light to increase cellular metabolism, including adenosine triphosphate (ATP)19 and collagen production.20,21 PBM use in muscle can activate mitochondria,22,23 yet it is unclear if the cellular mechanism of PBM is the same during tendon maturation and healing. Increased mitochondrial signaling can influence many downstream targets that control metabolic activity as well as apoptosis, cell proliferation, and collagen synthesis.24 In addition, tissue remodeling and pain may be regulated by the metabolic activity of tissues.25 While promising results have been shown for PBM treatment of adult tendon injuries in the clinic,18 with small animal models,26–31 and in tandem with other therapies,18,32–34 the effect of PBM therapy on the maturation of tendon has not yet been explored. This is important, because PBM therapy of adolescent tendon injuries may influence the metabolic and remodeling processes of the tendon during growth, and, therefore, influence the long-term health of the tendon. Additionally, while PBM therapy is used to reduce pain and increase return-to function for many patients with tendinopathy15 and animal models of tendinopathy,26–31 little work has systematically investigated the therapeutic potential and functional outcomes of PBM therapy on adult tendon healing.35 Here, we investigated how PBM therapy modulates mitochondrial metabolism in tendon and determined if and how PBM therapy can regulate the organization, structure, and function of the tendon during maturation and healing.
The purpose of this study was to investigate how PBM therapy affects the maturation of tendon in vivo, as well as how PBM therapy modulates the structural and biomechanical characteristics of the healing tendon. Specifically, we (a) assessed the effect of low-, high-, and no-irradiance PBM therapy during tendon maturation and (b) evaluated the efficacy of high vs no irradiance of PBM therapy during adult tendon healing. Using a paired study design (Figure 1), we hypothesized that PBM therapy at low or high power would acutely increase gene expression related to mitochondria metabolism during tendon maturation and healing, not damage the structural and mechanical properties of the maturing tendon, and improve the structural and mechanical properties of the healing tendon.
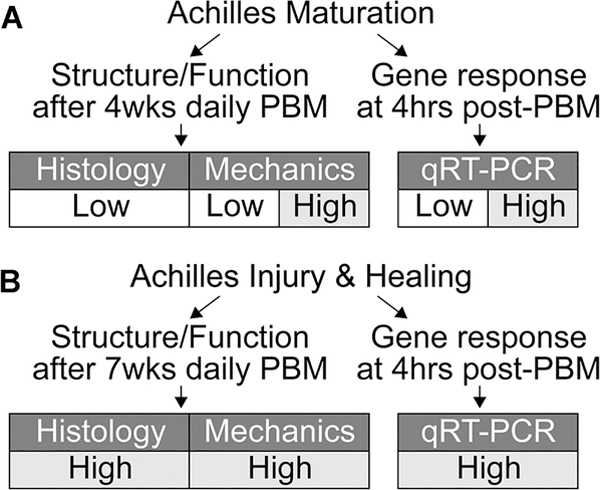
FIGURE 1.
Study design. The effects of photobiomodulation (PBM) on the structure, function, and gene expression of the mouse Achilles tendon during (A) maturation and (B) injury and healing were observed in this study. qRT-PCR, quantitative real-time polymerase chain reaction
2 |. MATERIALS AND METHODS
2.1 |. Study design
A total of 54 CD-1 mice (Envigo) were used for all experiments in accordance with approval from the University of Delaware Institutional Animal Care and Use Committee. We designed a two-part study to establish the effect of PBM on Achilles tendon maturation as well as adult healing (Figure 1). Continuous-wave of concurrently delivered 980 nm and 810 nm near-infrared light (80:20 power ratio, 1.5 cm2 cross-sectional area (CSA), hexagon, LightForce FXi Laser; LiteCure) was applied to Achilles tendons unilaterally and the paired contralateral Achilles tendon (untreated) was used as controls for all experiments. For each dose of light, the exposure time was controlled to deliver 2.5 J/cm2 at either 30 or 300 mW/cm2 (Figure 1). The effect of irradiance was tested in the maturation study, and only high irradiance was used for the healing study because we did not identify differences between low and high irradiance in the maturation study for gene expression or mechanics (Figure 1; Figures S3 and S4). The dose parameters were chosen based on previous research and the recommended guidelines from Tumilty et al14,15 who observed positive clinical outcomes for the treatment of Achilles tendinopathy, with 30 mW/cm2 within the clinical range and 300 mW/cm2 outside of this range and a log increase from 30 mW/cm2. For all studies, the PBM-dosed tendon (left or right Achilles) was assigned using controlled randomization.
2.2 |. Bilateral tenotomy of the mouse Achilles tendon
Mice (N = 17) were anesthetized using 3% isoflurane carried by oxygen and buprenorphine (0.01 mg/kg) was administered subcutaneously as analgesia. Hair was removed from the skin for subcutaneous exposure of both Achilles tendons using a topical cream. A skin incision was made medially to expose the Achilles tendon. Bilateral Achilles tendons were transected at the midsubstance using #11 scalpel blades (with no repair). Skin incisions were closed using 5–0 degradable suture (Vicryl, Ethicon Inc, Somerville, NJ). Mice were given bupivacaine hydrochloride (0.01 mg/kg) for nerve block. PBM dosing began at 1-week following Achilles tenotomy to allow the skin sutures to degrade and skin incisions to close.
2.3 |. PBM therapy for Mice
Mice were anesthetized via 3% isoflurane carried by oxygen for the duration of PBM dosing, and the anesthesia time was controlled for all experiments (~10 min/dose). Mice were weighed during the dosing periods for the maturation study to make sure that repetitive anesthesia or PBM therapy did not affect their growth (Figure S1). Prior to PBM dosing, the hair above the Achilles tendon and calf muscles was removed as needed using a topical cream because hair absorbs a significant amount of light (Figure S5).36 Mice were then placed in the prone position on a custom-made jig that holds the light probe perpendicular and juxtaposed to the dorsal Achilles tendon in a hands-free manner to reduce variability between doses (Thor Labs, Newton, NJ). Briefly, the dorsal foot was taped down in plantar flexion to keep the Achilles tendon stable during dosing, then the light probe was lowered perpendicular to and directly on top of the dorsal side of the Achilles tendon. The PBM dose was applied, the contralateral limb was manipulated in the same manner but without PBM dosing, and then mice were monitored until awake and alert.
To determine the maturation-dependent effect of PBM irradiance on Achilles tendon structure and function, unilateral Achilles tendons of 2-week old mice (N = 37 total) were dosed with low irradiance (n = 13, seven females and six males) or high irradiance (n = 8, four females and four males; functional outcomes only) daily for 4 weeks (Figure 1A). The acute effect of PBM and irradiance during maturation was measured at 4-hours posttreatment of PBM on unilateral Achilles tendons of 2-week old mice that were dosed once with low irradiance (n = 6, three females and three males; n = 4, two females and two males at 24-hours post-PBM) or once with high irradiance (n = 6, three females and three males; Figure 1A and Figure S4). On the basis of prior research that shows acute changes in gene expression due to PBM and our finding of no differences between 4 and 24-hours post-PBM, we chose to analyze all gene expression at 4-hours post-PBM.16,37
To determine the healing-dependent effect of PBM on Achilles tendons, adult mice (N = 17 total) that underwent bilateral Achilles tenotomy and 1 week of rest were dosed unilaterally with high irradiance daily for either 7 weeks (8 weeks total; structure/function assays; Figure 1B; n = 13, six females and seven males) or for 3 days and then sacrificed 4 hours after the third dose (differential gene expression; n = 4 females, Figure 1B). Mice were euthanized with carbon dioxide asphyxiation and thoracotomy.
2.4 |. Tendon histology
After euthanasia, hindlimbs were scanned using a dental X-ray prior to fixation to compare bone and joint morphology following PBM during maturation (Figure S2; Nomad Pro, Aribex). Achilles's tendons were dissected and fixed for 24 to 48 hours in 4% paraformaldehyde. Tendons were processed for paraffin sectioning, sectioned in the sagittal plane, then stained using Picrosirius Red (PSR) for collagen organization, 4',6-diamidino-2-phenylindole (DAPI, NucBlue Fixed, Life Technologies) for cell count and nuclear aspect ratio, and Hematoxylin & Eosin (H&E) for qualitative assessment of tissue morphology (eg, fibrosis, vessel formation and fat infiltration). Stained sections were imaged using an epifluorescent/brightfield microscope (Axio Observer Z1; Carl Zeiss, Thornwood, NY). Sections stained with PSR were imaged in the same orientation using circular polarized light microscopy, and the alignment of collagen fibrils was semiquantitatively scored by four blinded individuals (JMS) using the following scoring rubric: 5 = extremely organized and yellow, 4 = predominantly organized and orange, 3 = mixed organization and green, 2 = predominantly random and red/green, and 1 = extremely random and red/black. Sections stained with DAPI were imaged at six nonoverlapping locations (0.011 mm2/image, ×40) along the length of the tendon that served as technical replicates. Cell count and nuclear aspect ratio were quantified by a custom MATLAB code for each technical replicate and then averaged per sample (R2016b; MathWorks, Natick, MA).
2.5 |. Tendon uniaxial tensile tests
After euthanasia, mice for biomechanical testing were immediately frozen at −20°C. Prior to mechanical tests, mice were thawed and Achilles tendons were dissected from the proximal and distal tibias. Achilles-calcaneus attachments were left intact, and the gastrocnemius muscle was carefully removed using a #11 scalpel blade. Uniaxial tensile tests were performed (Instron 5943, Norwood, MA) in a phosphate-buffered saline bath at room temperature using custom fixtures to ensure uniaxial loading. Mechanical tests included a 0.01 N preload, 10 preconditioning cycles from 0.02 to 0.04 N at 0.02 mm/s, and ramp to failure at 0.02 mm/s. CSA and grip-to-grip gauge lengths were measured after the preload, and CSAs were assumed to be ellipsoidal. Load and displacement data were recorded (1 kHz acquisition rate), and data were converted to stress and strain based on the CSA and gauge length. Structural and mechanical properties (stiffness, ultimate load, Young's modulus, ultimate stress, strain at ultimate stress, and area under the curve [AUC] at ultimate stress) were calculated using a custom MATLAB code. Stiffness and Young's modulus were calculated as the slope of the linear region from the load/displacement and stress/strain curves, respectively. Ultimate load, ultimate stress, and strain at ultimate stress were calculated as the maximum load/stress/strain from their respective curves. AUC was calculated by taking the integral of the stress/strain curve to the ultimate stress.
2.6 |. Mitochondria gene expression in tendon
To investigate the cellular mechanism of PBM, Achilles tendons were dissected following euthanasia (4 hours after last PBM dose) in RNase-free conditions. Tendons were frozen in liquid nitrogen and stored at −80°C. Tissues were pulverized for 30 seconds in 2 mL tubes with steel balls in liquid nitrogen-cooled blocks (MM400; Retsch, Verder Scientific). RNA was extracted using a commercially available kit (PureLink RNA Mini Kit; Invitrogen). Total RNA was reverse transcribed using Superscript III VILO (Invitrogen). Quantitative real-time polymerase chain reaction (PCR) was performed using RT2 Profiler PCR Array for Mouse Mitochondria (330231; Qiagen) with PowerUp SYBR Green (Applied Biosystems) on a LightCycler 96 System (Roche). ΔCT (CT target gene – CT reference gene) values were calculated for each gene using Gusb as the reference gene (most stable reference gene across all conditions with 30.9 ± 1.1 for maturation and 26.5 ± 0.4 for healing Ct values). The reference gene was selected as the gene that changed the least due to PBM treatment from a panel of five reference genes (Actb, B2m, Gapdh, Gusb, and Hsp90ab1). ΔCT values for each target gene were transformed to and then averaged for control and PBM-treated tendons. Fold change was calculated by dividing the average of PBM treated by the average of control for each gene.
2.7 |. Statistical analysis
All statistical comparisons were made using Prism (v8.2.0; GraphPad, LaJolla, CA). Paired t test was used to compare the ΔCT of each gene when comparing tendons treated with PBM during maturation (low), maturation (high), and healing to their paired control tendons. For mechanical outcomes, repeated-measure (control vs PBM tendon) two-way analysis of variances with Sidak's correction for multiple comparisons (sex differences) were used. Wilcoxon matched-pair tests were used to compare semiquantitative scores of collagen alignment. Paired t tests were used to compare quantitative measures of cell number and nuclear aspect ratio. The fold change and P value for each gene were log2 and −log10 transformed, respectively, to visualize results in volcano plots. For genes that were statistically different between control and PBM, the mean ± standard deviation was plotted as . Differential gene expressions with a fold change of less than −1.5 or greater than 1.5 were plotted for paired comparisons. Mechanical properties, collagen alignment score, cell number, and nuclear aspect ratio were plotted as mean ± standard deviation.
3 |. RESULTS
3.1 |. Daily PBM does not affect tendon structure during postnatal growth or healing
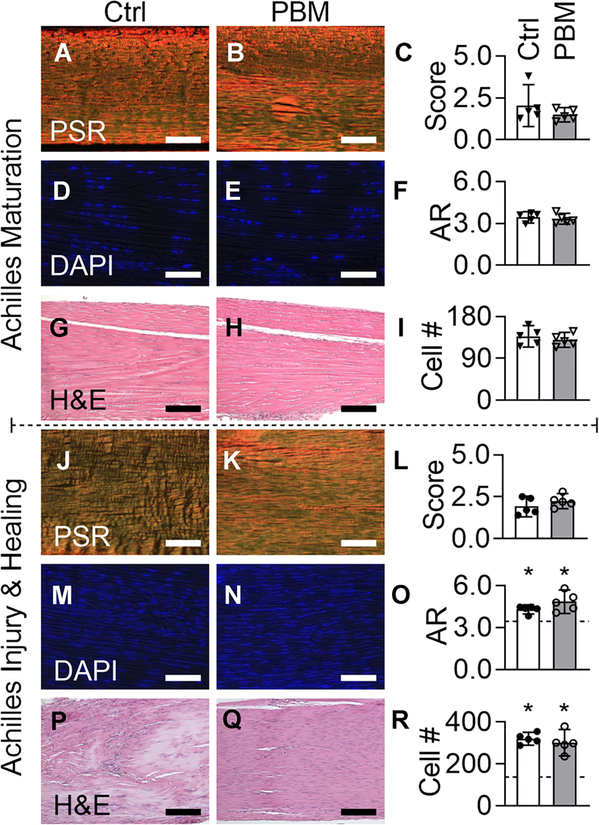
During maturation, daily doses of PBM therapy did not affect the structural or cellular morphology of the growing mouse Achilles tendon (Figure 2). Specifically, collagen alignment was not affected by daily PBM during maturation (Figure 2A–C). Additionally, there were no differences in the collagen alignment of transected tendons between PBM treated and untreated tendons following 8 weeks of healing (Figure 2J–L). Nuclear shape and cell numbers were not affected by PBM during maturation (Figure 2D–F) and healing (Figure 2M–O). The number of cells and aspect ratio were significantly increased during healing compared to maturation controls (Figure 2O). From H&E-stained sections, no differences were observed between control and PBM groups for maturation (Figure 2G,H) and healing (Figure 2P–R). No differences were observed in proliferation (Figure S6), fibrosis, fat infiltration, and vessel formation between control and PBM groups for healing (Figure 2P–R). The tendon stumps, the distal and proximal ends of the Achilles tendon that were separated by tenotomy, were observed in control and PBM groups. Daily PBM and repeated anesthesia needed for each dose of PBM therapy did not affect the gross morphology or mouse growth, respectively (Figures S1 and S2).
FIGURE 2.
During maturation and adult injury and healing, daily doses of PBM therapy did not affect the structural or cellular properties of the mouse Achilles tendon. (A-C, maturation; J-L, healing) Collagen alignment, (D-F, maturation; M-O, healing) nuclear aspect ratio (AR) and (I, maturation; R, healing) cell number, and (G and H, maturation; P and Q healing) fibrosis were observed for control (Ctrl, white bars) and PBM (gray bars) treated tendons using Picrosirius Red (PSR), DAPI, and Hematoxylin and Eosin (H&E) stained sections, respectively. O and R, Dashed line is the mean of maturation controls and asterisks represent a significant difference from maturation controls (P < .05). Scale bar = 75 μm for panels A and B, G and H, P and Q, and 40 μm for panels D and E and J-N. Maturation and healing data points are downward arrowheads and circles, respectively. Data are presented as mean ± standard deviation. Ctrl, control; DAPI, 4',6-diamidino-2-phenylindole; PBM, photobiomodulation
3.2 |. Daily PBM does not influence the mechanical properties of tendon maturation and improves tendon healing in a sex-dependent manner
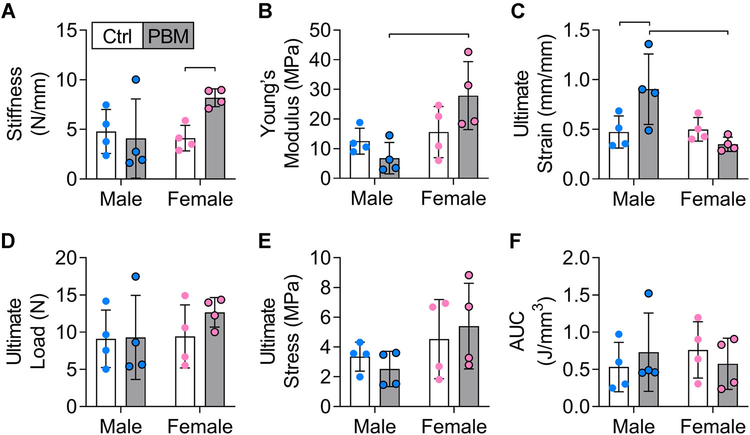
During maturation, daily doses of PBM therapy at both low and high irradiance for 4 weeks had no significant effect on the mechanical properties of the mouse Achilles tendon (Figure S3). No significant differences were observed between the low and high PBM groups or compared to their paired controls (Figure S3). Additionally, no sex-dependent significant differences were observed during maturation (Figure S3).
Contrastingly, during healing, daily doses of PBM therapy for 7 weeks had sex-dependent effects on the mechanical and structural properties of mouse Achilles tendons (Figure 3). Achilles's tendons were stiffer for females in the PBM group compared to sex-matched untreated controls (Figure 3A), and Young's modulus was significantly higher in the female PBM group compared to the male PBM group (Figure 3B). Ultimate strain in the male PBM group was significantly higher compared to the male control group and the female PBM group (Figure 3C). No differences were observed for ultimate load, ultimate stress, and AUC at ultimate stress between paired control and PBM-treated tendons. There were no significant differences in mechanical properties during healing when mice were not analyzed by gender (ie, when mice were analyzed only by control and PBM groups).
FIGURE 3.
During healing, daily doses of PBM therapy for 7 weeks had mild sex-dependent effects on the mechanical properties of the mouse Achilles tendon. (A) Stiffness, (B) Young's modulus, (C) ultimate strain, (D) ultimate load, (E) ultimate stress, and (F) area under the curve (AUC) for control (Ctrl, white bars) and PBM (gray bars) treated tendons of males and females. Data are presented as mean ± standard deviation. Bars represent significant differences (P < .05). Male and female data points are blue and pink, respectively. PBM, photobiomodulation
3.3 |. Acute PBM marginally alters mitochondrial gene expression
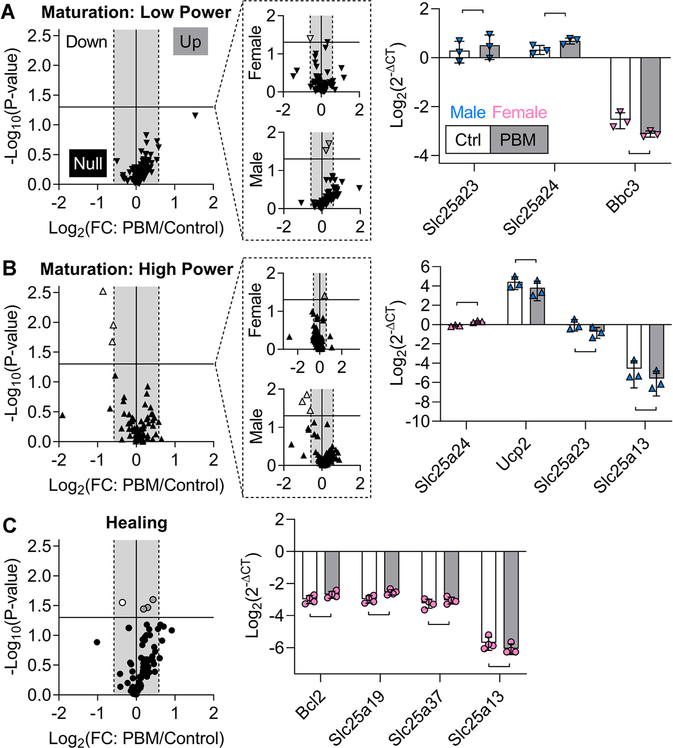
During both maturation and healing, several genes related to mitochondrial signaling were differentially expressed following PBM treatment (Table 1). During maturation, a single dose of low irradiance PBM treatment downregulated Bbc3 in females and upregulated Slc25a23 and Slc25a24 in males compared to paired controls (Figure 4A). During maturation, a single dose of high irradiance PBM in male tendons led to downregulation of three mitochondria-related genes (Ucp2, Slc25a23, and Slc25a13) compared to paired controls, and this effect was sex-specific (Figure 4B). In females during maturation, high-irradiance PBM treatment led to significant upregulation of Slc25a24 compared to paired controls (Figure 4B). At 24 hours after a single dose of low irradiance PBM, only Slc25a21 was downregulated by PBM compared to paired controls during maturation (Figure S4). During maturation, there were no differences between 4 and 24 hours after a single dose of low-irradiance PBM, and Slc25a13 was upregulated in the low group compared to the high group at 4 hours (Figure S4). During healing, 3 consecutive days of high-irradiance PBM statistically resulted in the significant upregulation of three genes (Bcl2, Slc25a19, and Slc25a37) and downregulation of Slc25a13 compared to paired controls (Figure 4C).
TABLE 1.
Genes affected by PBM during tendon maturation and healing (descriptions from UniProt38)
| Sex | Gene | P value | PBM/Ctrl | Function |
|---|---|---|---|---|
| Maturation: Low power | ||||
| M | Slc25a23 | .034 | 1.16 | Mediates the transport of Mg-ATP in exchange for phosphate, catalyzing uptake, or efflux of adenine across the inner membrane. Regulates calcium uptake via interaction with MCU and MICU1. |
| M | Slc25a24 | .018 | 1.29 | Protects cells against oxidative stress-induced cell death by promoting the formation of calcium-phosphate precipitates in the mitochondrial matrix, and thereby buffering calcium levels in the matrix. |
| F | Bbc3 | .036 | 0.67 | Essential mediator of p53/TP53-dependent and p53/TP53-independent apoptosis. Functions by promoting partial unfolding of BCL2L1 and dissociation of BCL2L1 from p53/TP53. Regulates ER stress-induced neuronal apoptosis. |
| Maturation: High power | ||||
| F | Slc25a24 | .039 | 1.339 | Described above. |
| M | Ucp2 | .036 | 0.653 | Mitochondrial transporter protein that creates proton leaks across the inner mitochondrial membrane, thus uncoupling oxidative phosphorylation from ATP synthesis. |
| M | Slc25a23 | .014 | 0.578 | Described above. |
| M | Slc25a13 | .021 | 0.489 | Catalyzes the calcium-dependent exchange of cytoplasmic glutamate with mitochondrial aspartate across the inner membrane. May have a function in the urea cycle. |
| Healing | ||||
| F | Bcl2 | .034 | 1.228 | Suppresses apoptosis in a variety of cell systems by controlling the mitochondrial membrane permeability. Inhibits caspase activity either by preventing the release of cytochrome c from the mitochondria and/or by binding to the apoptosis-activating factor. |
| F | Slc25a19 | .025 | 1.351 | Mitochondrial transporter mediating uptake of thiamine pyrophosphate into mitochondria. |
| F | Slc25a37 | .036 | 1.14 | Mitochondrial iron transporter that plays an essential role in heme biosynthesis. The iron delivered into the mitochondria may be delivered to ferrochelatase to catalyze Fe2+ incorporation into protoporphyrin IX to make heme. |
| F | Slc25a13 | .028 | 0.78 | Described above. |
Abbreviations: ATP, adenosine triphosphate; Ctrl, control; ER, estrogen receptor; PBM, photobiomodulation.
FIGURE 4.
During maturation and healing, mitochondria-related genes were marginally affected by PBM. A-C, Volcano plots of the acute response from mitochondria-related genes after PBM during maturation (A, low and B, high) and healing (C). Volcano plots separated by sex are shown in dashed outcall boxes. Horizontal line: P = .05, gray box: −1.5 to 1.5-fold change. Maturation (low), maturation (high), and healing data points are downward arrowheads, upward arrowheads, and circles, respectively. Data points for downregulated genes are white, upregulated genes are gray, and nonregulated genes are black. For bar graphs, the control (Ctrl) and PBM groups are white and gray bars, and male and female data points are blue and pink, respectively. A, During maturation, low-irradiance PBM significantly downregulated Bbc3 in females and upregulated Slc25a23 and Slc25a24 in males. B, During maturation, high-irradiance PBM downregulated three mitochondria-related genes (Ucp2, Slc25a23, and Slc25a13), and this effect was due to males, not females; only Slc25a24 was significantly upregulated in females. C, During healing, high-irradiance PBM of females statistically upregulated three genes (Bcl2, Slc25a19, and Slc25a37) and downregulated one gene (Slc25a13). Data are presented as mean ± standard deviation. Bars represent significant differences (P < .05). PBM, photobiomodulation
4 |. DISCUSSION
This study investigated the role of PBM therapy on the structure and function of the murine Achilles tendon, as well as mechanistically identified that PBM therapy marginally affects mitochondria gene expression, during maturation and healing. We have demonstrated that PBM therapy during tendon maturation does not alter the structural and mechanical properties, regardless of the power level, suggesting that it is a safe therapy for growing tendon. We showed that daily PBM therapy during tendon healing has sex-dependent effects in the mechanical properties of tendon. Mechanistically, we found that PBM therapy marginally influences gene expression related to mitochondrial metabolism.
The mechanism of action of PBM therapy on cells remains unclear and may not be ubiquitous in all cell types. We hypothesized that the initial response of tendon cells to PBM therapy would lead to elevated mitochondria metabolism through the acceptance of photons via photoacceptors like cytochrome c oxidase.14,39 In turn, the accepted photons would lead to increased availability of electrons for the reduction of oxygen, thereby increasing the rate of oxidative phosphorylation and ATP synthesis.14,39 Our gene expression experiments did not support this hypothesis.
Previous research has shown that PBM therapy can increase cell proliferation, inflammation, extracellular matrix remodeling, and collagen synthesis.26–31 However, ours is the first work to systematically investigate the role of PBM therapy on tendon structure, function, and mitochondrial activity. In the present study, we did not find an overwhelming effect of PBM therapy, with the parameters and times that we used, on tendon-specific mitochondrial metabolism during growth or following injury. These findings may suggest that tendon-specific mitochondria are not sufficiently influenced by PBM therapy. Alternatively, it is possible that the parameters we used did not alter mitochondria metabolism. It is possible that other parameters and altered dosing of PBM therapy may lead to altered mitochondrial-related gene expression.40 For example, a single dose of PBM therapy with different parameters than this study (ie, 810 nm, 1 J, and 3.57 W/cm2) at 1 hour after collagenase-induced Achilles tendinitis in rats, as opposed to delayed PBM therapy at 1 week after Achilles tenotomy, reduced inflammatory and protease genes at 1-hour post-PBM and improved mechanical properties at 7-days post-PBM.41 Potentially, the cell populations immediately following Achilles tendon injury, including neutrophils and macrophages, are more susceptible to PBM therapy.38,42 Although a reduction in inflammatory genes via immediate PBM therapy following Achilles tenotomy may have led to a more pronounced effect in this study, delayed treatment following injury more closely mimics the human scenario of PBM therapy for Achilles tendon rupture and avoids the confounding effects of inflammation on the mitochondrial response to PBM therapy. At the parameters and time points that we assessed, we did identify marginal statistically significant differences in expression of several mitochondria-related genes induced by PBM therapy during tendon maturation and healing (Table 1).43 The majority of differentially expressed genes that were regulated by PBM therapy was calcium-dependent small molecule transporters that shuttle molecules into mitochondria through the inner membrane, which suggests that calcium signaling may be affected by PBM therapy.44,45
During maturation, the sex-dependent effects of PBM therapy (ie, differential gene expression in males compared to females) are important to consider when establishing the mechanism of action of PBM therapy or other treatment modalities that target mitochondria. Both low- and high-irradiance PBM therapy regulated Slc25a23 and Slc25a24, and these genes may be targets of PBM therapy in this scenario of tendon maturation.44,45 The differences we found between irradiance groups (low vs high) suggest that the irradiance used may alter the mechanism of action of PBM therapy. For example, we found that low irradiance led to upregulated Slc25a23 and Slc25a24 and downregulated Bbc3, whereas high irradiance led to downregulated Ucp2, Slc25a23, and Slc25a13 and upregulated Slc25a24. Although we identified similar genes that were differentially expressed due to low- and high-irradiance PBM therapy, these changes did not lead to changes in the structure or mechanical properties of the tendon during maturation, as hypothesized. It is possible that an earlier start to treatment (eg, at 1 week old) or longer treatment period similar to our healing study (eg, 8 weeks instead of 4 weeks) may have led to more substantial effects to the mouse Achilles tendon during maturation.
During healing, we observed increased mechanical properties as well as differentially expressed genes in females, but we did not observe changes in the tendon structure. The genes that we found to be differentially expressed following PBM therapy in the healing context may have enhanced the functional remodeling of the ruptured tendon. We found that, in females, high-irradiance PBM therapy during both maturation and tendon healing led to downregulated Slc25a13. Slc25a13 encodes instructions to make the protein CITRIN, which transports cytoplasmic glutamate for mitochondrial aspartate across the inner membrane, a key feature of the citric acid cycle. Downregulation of Slc25a13 indicates that the citric acid cycle would rely more on pyruvate than glutamate for the production of amino acids and ATP, but it is unclear how this would benefit the healing of the tendon. Additionally, during healing, PBM therapy increased expression of Bcl2, which is known to inhibit apoptosis by regulating cytochrome c release from mitochondria or by interacting with Apaf1.46 PBM therapy during healing also upregulated Slc25a19 and Slc25a37, which, respectively, function to transport thiamine pyrophosphate,47 a critical coenzyme in the citric acid cycle, and iron, a necessity for the synthesis of heme,48 into mitochondria through the inner membrane. Yet, it is unclear if the differential expression of these genes led to changes in mechanical outcomes (eg, stiffness) of healing tendons.
4.1 |. Clinical implications
In the clinic, PBM therapy alone or in conjunction with traditional interventions, such as physical therapy, has shown promising results for the treatment of tendon injuries in both human and veterinary medicine.18,49 Our results corroborate that PBM therapy may be beneficial for tendon healing and that PBM therapy does not alter Achilles tendon maturation. Our findings support that PBM therapy is a safe treatment modality for growing tissues and that PBM may improve the mechanical properties of the healing adult tendon. Recently, PBM therapy was shown to not alter the morphology or mechanics of human tendon,50 and our results support these findings. Several studies in humans have shown that repetitive PBM therapy improves the healing process, function, and pain levels of injured tendons.15,18,25 These studies highlight the importance of using defined parameters and dosing that warrant beneficial effects on healing. PBM therapy in the clinic and in this study may be beneficial for tendon healing because the functional remodeling of the tendon during healing improves without adverse effects.
4.2 |. Limitations
The sex-dependent effect of PBM on tendon mechanical properties during healing was likely underpowered, thus future studies are required to further investigate the sex-dependent effect of PBM treatment. On the basis of our results, we estimate a sample size for detecting sex differences of ~12 animals per group. The effect of PBM therapy on gene expression may be dependent on the energy density applied, yet we only used one energy density for all studies, thus other energy densities may have a greater effect on gene expression. Additionally, during maturation, more doses may have amplified or altered gene expression further, yet we quantified the effect of repeated doses in the healing study and did not find an amplified effect. Furthermore, fewer doses might have altered gene expression differently during healing. Gene expression is highly time-dependent, thus we also analyzed gene expression at 24 hours after PBM therapy but did not find meaningful differences compared to 4 hours. A second limitation of this study is that we did not identify structural changes in the tendon that may have led to improvements in mechanical properties. Future work may use more quantitative measures of tendon structure, such as second-harmonic generation imaging, as well as study the direct role of mitochondria in the healing response of tendon.
5 |. CONCLUSION
This was the first study to systematically assess the paired effect of PBM therapy on the cellular, structural, and mechanical properties of the tendon during maturation and healing. This study shows that the mechanism of action of PBM therapy may not be via mitochondria that PBM therapy does not damage the maturation of tendon and that PBM therapy mildly improves the healing of ruptured tendon in a sex-dependent manner.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Elahe Ganji for preliminary help with Achilles' tensile tests. This study was supported by Delaware Bioscience Center for Advanced Technology Applied Research Collaborations, Eunice Kennedy Shriver National Institute of Child Health & Human Development (R03HD094594) and LiteCure donation of IR laser.
Funding information
Delaware Bioscience Center for Advanced Technology Applied Research Collaborations; Eunice Kennedy Shriver National Institute of Child Health & Human Development, Grant/Award Number: R03HD094594
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Huttunen TT, Kannus P, Rolf C, Felländer-Tsai L, Mattila VM. Acute Achilles tendon ruptures: incidence of injury and surgery in Sweden between 2001 and 2012. Am J Sports Med. 2014;42:2419–2423. [DOI] [PubMed] [Google Scholar]
- 2.Lantto I, Heikkinen J, Flinkkilä T, Ohtonen P, Leppilahti J. Epidemiology of Achilles tendon ruptures: increasing incidence over a 33-year period. Scand J Med Sci Sports. 2015;25:e133–e138. [DOI] [PubMed] [Google Scholar]
- 3.Cassel M, Baur H, Hirschmüller A, Carlsohn A, Fröhlich K, Mayer F. Prevalence of Achilles and patellar tendinopathy and their association to intratendinous changes in adolescent athletes. Scand J Med Sci Sports. 2015;25:e310–e318. [DOI] [PubMed] [Google Scholar]
- 4.Zellers JA, Christensen M, Kjaer IL, Rathleff MS, Silbernagel KG. Defining components of early functional rehabilitation for acute Achilles tendon rupture: A systematic review. Orthop J Sports Med. 2019;7, 10.1177/2325967119884071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman BR, Gordon JA, Bhatt PR, et al. Nonsurgical treatment and early return to activity leads to improved Achilles tendon fatigue mechanics and functional outcomes during early healing in an animal model. J Orthop Res. 2016;34:2172–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barfod KW, Sveen TM, Ganestam A, Ebskov LB, Troelsen A. Severe functional debilitations after complications associated with acute Achilles tendon rupture with 9 years of follow-up. J Foot Ankle Surg. 2017;56:440–444. 10.1053/j.jfas.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 7.Holm C, Kjaer M, Eliasson P. Achilles tendon rupture—treatment and complications: a systematic review. Scand J Med Sci Sports. 2015;25: e1–e10. [DOI] [PubMed] [Google Scholar]
- 8.Del Buono A, Volpin A, Maffulli N. Minimally invasive versus open surgery for acute Achilles tendon rupture: a systematic review. Br Med Bull. 2014;109:45–54. [DOI] [PubMed] [Google Scholar]
- 9.Olsson N, Silbernagel KG, Eriksson BI, et al. Stable surgical repair with accelerated rehabilitation versus nonsurgical treatment for acute Achilles tendon ruptures: a randomized controlled study. Am J Sports Med. 2013;41:2867–2876. [DOI] [PubMed] [Google Scholar]
- 10.Olsson N, Petzold M, Brorsson A, Karlsson J, Eriksson BI, Grävare Silbernagel K. Predictors of Clinical outcome after acute Achilles tendon ruptures. Am J Sports Med. 2014;42:1448–1455. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro E, Grande D, Drakos M. Biologics in Achilles tendon healing and repair: a review. Curr Rev Musculoskelet Med. 2015;8:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howell K, Chien C, Bell R, et al. Novel model of tendon regeneration reveals distinct cell mechanisms underlying regenerative and fibrotic tendon healing. Sci Rep. 2017;7:45238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atik OS. Photobiomodulation for Achilles tendinopathy. Photomed Laser Surg. 2017;36:1–2. [DOI] [PubMed] [Google Scholar]
- 14.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40:516–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tumilty S, Munn J, McDonough S, Hurley DA, Basford JR, Baxter GD. Low level laser treatment of tendinopathy: a systematic review with meta-analysis. Photomed Laser Surg. 2009;28:3–16. [DOI] [PubMed] [Google Scholar]
- 16.Bjordal JM, Johnson MI, Iversen V, Aimbire F, Lopes-Martins RAB. Low-level laser therapy in acute pain: a systematic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trials. Photomed. Laser Ther. 2006;24:158–168. [DOI] [PubMed] [Google Scholar]
- 17.Stergioulas A, Stergioula M, Aarskog R, Lopes-Martins RAB, Bjordal JM. Effects of Low-level laser therapy and eccentric exercises in the treatment of recreational athletes with chronic Achilles tendinopathy. Am J Sports Med. 2008;36:881–887. [DOI] [PubMed] [Google Scholar]
- 18.Tumilty S, Mani R, Baxter GD. Photobiomodulation and eccentric exercise for Achilles tendinopathy: a randomized controlled trial. Lasers Med Sci. 2016;31:127–135. [DOI] [PubMed] [Google Scholar]
- 19.Keshri GK, Gupta A, Yadav A, Sharma SK, Singh SB. Photobiomodulation with pulsed and continuous wave near-infrared laser (810 nm, Al-Ga-As) augments dermal wound healing in immunosuppressed rats. PLoS One. 2016;11:e0166705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy GK, Stehno-Bittel L, Enwemeka CS. Laser photostimulation of collagen production in healing rabbit Achilles tendons. Lasers Surg Med. 1998;22:281–287. [DOI] [PubMed] [Google Scholar]
- 21.Pereira AN, Eduardo Cde P, Matson E, Marques MM. Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg Med. 2002;31:263–267. [DOI] [PubMed] [Google Scholar]
- 22.de Oliveira HA, Antonio EL, Silva FA, et al. Protective effects of photobiomodulation against resistance exercise-induced muscle damage and inflammation in rats. J Sports Sci. 2018;36:2349–2357. [DOI] [PubMed] [Google Scholar]
- 23.Hayworth CR, Rojas JC, Padilla E, Holmes GM, Sheridan EC, Gonzalez-Lima F. In vivo low-level light therapy increases cytochrome oxidase in skeletal muscle. Photochem Photobiol. 2010; 86:673–680. [DOI] [PubMed] [Google Scholar]
- 24.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. [DOI] [PubMed] [Google Scholar]
- 25.Poorpezeshk N, Ghoreishi SK, Bayat M, Pouriran R, Yavari M. Early low-level laser therapy improves the passive range of motion and decreases pain in patients with flexor tendon injury. Photomed Laser Surg. 2018;36:530–535. [DOI] [PubMed] [Google Scholar]
- 26.Gomes CAFP Dibai-Filho AV, Pallotta RC, et al. Effects of low-level laser therapy on the modulation of tissue temperature and hyperalgesia following a partial Achilles tendon injury in rats. J Cosmet Laser Ther. 2017;19:391–396. [DOI] [PubMed] [Google Scholar]
- 27.Da Ré Guerra F, Vieira CP, Marques PP, Oliveira LP, Pimentel ER. Low level laser therapy accelerates the extracellular matrix reorganization of inflamed tendon. Tissue Cell. 2017;49:483–488. [DOI] [PubMed] [Google Scholar]
- 28.Iacopetti I, Perazzi A, Maniero V, et al. Effect of MLS® laser therapy with different dose regimes for the treatment of experimentally induced tendinopathy in sheep: pilot study. Photomed Laser Surg. 2015;33:154–163. [DOI] [PubMed] [Google Scholar]
- 29.de Jesus JF, Spadacci-Morena DD, Rabelo NDA, Pinfildi CE, Fukuda TY, Plapler H. Low-level laser therapy on tissue repair of partially injured Achilles tendon in rats. Photomed Laser Surg. 2014;32:345–350. [DOI] [PubMed] [Google Scholar]
- 30.Casalechi HL, de Farias Marques AC, da Silva EAP, et al. Analysis of the effect of phototherapy in model with traumatic Achilles tendon injury in rats. Lasers Med Sci. 2014;29:1075–1081. [DOI] [PubMed] [Google Scholar]
- 31.Neves MAI, Pinfildi CE, Wood VT, et al. Different power settings of LLLT on the repair of the calcaneal tendon. Photomed Laser Surg. 2011;29:663–668. [DOI] [PubMed] [Google Scholar]
- 32.Wood VT, Pinfildi CE, Neves MAI, Parizoto NA, Hochman B, Ferreira LM. Collagen changes and realignment induced by low-level laser therapy and low-intensity ultrasound in the calcaneal tendon. Lasers Surg Med. 2010;42:559–565. [DOI] [PubMed] [Google Scholar]
- 33.de Carvalho PK, Silveira L, Barbosa D, Munin E, Salgado MAC, Villaverde AB. Analysis of experimental tendinitis in rats treated with laser and platelet-rich plasma therapies by Raman spectroscopy and histometry. Lasers Med Sci. 2016;31:19–26. [DOI] [PubMed] [Google Scholar]
- 34.Haslerud S, Lopes-Martins RAB, Frigo L, et al. Low-level laser therapy and cryotherapy as mono- and adjunctive therapies for Achilles tendinopathy in rats. Photomed Laser Surg. 2016;35: 32–42. [DOI] [PubMed] [Google Scholar]
- 35.Carrinho PM, Renno ACM, Koeke P, Salate ACB, Parizotto NA, Vidal BC. Comparative study using 685-nm and 830-nm lasers in the tissue repair of tenotomized tendons in the mouse. Photomed Laser Surg. 2006;24:754–758. [DOI] [PubMed] [Google Scholar]
- 36.Duesterdieck-Zellmer KF, Larson MK, Plant TK, Sundholm-Tepper A, Payton ME. Ex vivo penetration of low-level laser light through equine skin and flexor tendons. Am J Vet Res. 2016;77:991–999. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Song S, Fong CC, Tsang CH, Yang Z, Yang M. cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. Soc Inv Derm. 2003;120:849–857. [DOI] [PubMed] [Google Scholar]
- 38.Naterstad IF, Rossi RP, Marcos RL, et al. Comparison of photobiomodulation and antiinflammatory drugs on tissue repair on collagenase-induced Achilles tendon inflammation in rats. Photomed Laser Surg. 2017;36:137–145. [DOI] [PubMed] [Google Scholar]
- 39.de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016;22:348–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fillipin LI, Mauriz JL, Vedovelli K, et al. Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med. 2005;37:293–300. [DOI] [PubMed] [Google Scholar]
- 41.Marcos RL, Leal-Junior ECP, Arnold G, et al. Low-level laser therapy in collagenase-induced Achilles tendinitis in rats: analyses of biochemical and biomechanical aspects. J Orthop Res. 2012;30: 1945–1951. [DOI] [PubMed] [Google Scholar]
- 42.Marsolais D, Cǒté CH, Frenette J. Neutrophils and macrophages accumulate sequentially following Achilles tendon injury. J Orthop Res. 2001;19:1203–1209. [DOI] [PubMed] [Google Scholar]
- 43.UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffman NE, Chandramoorthy HC, Shanmughapriya S, et al. SLC25A23 augments mitochondrial Ca2+ uptake, interacts with MCU, and induces oxidative stress-mediated cell death. Mol Biol Cell. 2014;25:936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ehmke N, Graul-Neumann L, Smorag L, et al. De novo mutations in SLC25A24 cause a craniosynostosis syndrome with hypertrichosis, progeroid appearance, and mitochondrial dysfunction. Am J Hum Genet. 2017;101:833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruey J-M, Bruey-Sedano N, Luciano F, et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129:45–56. [DOI] [PubMed] [Google Scholar]
- 47.Kang J, Samuels DC. The evidence that the DNC (SLC25A19) is not the mitochondrial deoxyribonucleotide carrier. Mitochondrion. 2008;8:103–108. [DOI] [PubMed] [Google Scholar]
- 48.Shaw GC, Cope JJ, Li L, et al. Mitoferrin is essential for erythroid iron assimilation. Nature. 2006;440:96–100. [DOI] [PubMed] [Google Scholar]
- 49.Pryor B, Millis DL. Therapeutic laser in veterinary medicine. Vet Clin North Am Small Anim Pract. 2015;45:45–56. [DOI] [PubMed] [Google Scholar]
- 50.Corrigan P, Cortes DH, Silbernagel KG. Immediate effect of photobiomodulation therapy on Achilles tendon morphology and mechanical properties: an exploratory study. Transl. Sports Med. 2019;2:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.