Abstract
Immune-related neuromuscular adverse events are rare, but potentially life-threatening side-effects of immune checkpoint inhibitors (ICIs). They usually arise within the first 3 months after initiation of ICIs. Subacute symptom onset with more rapid progression than in idiopathic autoimmune neuromuscular diseases is typical. Prompt clinical diagnosis and treatment is essential for a favourable outcome. The importance of careful medical history and a well-established clinical diagnosis is emphasised rather than antibody detection or radiologic visualisation. Muscle weakness as a leading symptom can give rise to the suspicion of either neuropathy or myositis-myasthenia complex and differentiation may be complicated by their overlap. It is of utmost importance to recognise immune-related myositis and monitor for myocardial as well as bulbar involvement that may rapidly lead to cardiac or respiratory failure, persisting disability or even a fatal outcome.
Symptoms typically improve with ICI discontinuation and early administration of glucocorticoids (prednisolone 1-2 mg/kg/day) in patients markedly affected. Severe and persisting symptoms including myocardial or bulbar affection can require therapy escalation to steroid-sparing agents. In patients with mild symptoms, not influencing functional abilities, careful clinical monitoring while staying on ICI therapy may be sufficient.
Re-challenging with ICIs may be considered in selected cases, based on the initial severity of immune-related adverse events (irAEs) and clinical disease course. Depending on the individual irAE characteristics, the decision should be preferably discussed in an interdisciplinary irAE expert team with an experienced neurologist, rheumatologist and/or cardiologist and take the patient's preferences into account. The yet unmet need of systematic data on treatment, follow-up results and options of re-challenge of ICI treatment in neuromuscular toxicity has to be particularly considered in the shared decision-making process.
Key words: neuromuscular toxicity, immune checkpoint inhibitors, neuropathy, myositis, myasthenia gravis
Highlights
-
•
Immune-related neuromuscular adverse events are rare, but potentially life-threatening side-effects of immunotherapy.
-
•
Muscle weakness as the leading symptom can give rise to the suspicion of either neuropathy or myositis-myasthenia complex.
-
•
Symptoms typically improve with ICI discontinuation and early administration of glucocorticoids.
-
•
Severe persisting symptoms including myocardial or bulbar affection can require therapy escalation to steroid-sparing agents.
Introduction
Immune checkpoint inhibitors (ICIs) have been increasingly used in the treatment of various tumour types and lead to favourable outcomes even in advanced-stage cancer patients. ICIs are capable of hijacking immune checkpoints and evade immune attack, they unleash the immune system against cancer and inevitably induce specific off-target toxicities.1,2 Therefore, ICI treatment has been associated with a fairly broad spectrum of immune-related adverse events (irAEs) including the central and peripheral nervous systems as well as the musculoskeletal system. The estimated incidence of neurological irAEs is reported to be around 1%-5%3,4 in which neuromuscular disorders account for >50% of neurological irAEs. These include primarily myositis, myasthenia gravis (MG) and demyelinating polyradiculoneuropathy as well as overlaps.5 Based on cumulative experience of ∼1000 cases in the literature and our own experience, clinical findings often differ from those of typical neuromuscular disorders occurring outside ICI treatment.4 A majority of neuromuscular irAEs are of lower grade (e.g. transient mild neuropathies) and can be managed effectively by withholding therapy and administering glucocorticoids. In several cases, however, neuromuscular toxicities may be very aggressive in symptom development leading to long-term sequelae and the occasional fatality.2 Therefore, prompt recognition of symptoms and appropriate management are absolutely mandatory.6 Patients with a history of autoimmune disease are at a higher risk for worsening of their autoimmune disease while on ICI therapy. Controlled data on treatment of these irAEs are still missing. The treatment of irAEs does not differ between targeting mechanisms of the used ICIs. Herein, we discuss specific characteristics of neuromuscular irAEs, namely myositis, MG, overlap syndromes and peripheral neuropathy, and present a comprehensible symptom table (Table 1), and a diagnostic and therapeutic approach (Figure 1).
Table 1.
Clinical findings in neuromuscular immune-related adverse events
| Clinical findings in NMD induced by ICIs | Myasthenia gravis |
Myositis |
Guillain-Barré syndrome |
|---|---|---|---|
| Frequency of symptoms | |||
| Ocular weakness (ptosis/double vision) | +++ | +++ | + In cranial nerve affection |
| Facial weakness | ++ | ++ | + |
| Bulbar symptoms (dysarthria/dysphagia) | +++ | ++ 50% of the cases | +/++ |
| Extremity weakness | + Symmetrical proximal |
++ Symmetrical proximal |
++ Mostly symmetrical proximal or distal |
| Dropped head | ++ | +++ 70% of the cases | (+) |
| Limb girdle weakness | +/++ | ++ | + |
| Pain | (+) | +++ 70% of the cases | Possible in sensory involvement |
| Respiratory failure | Frequent due to diaphragm involvement or aspiration | ||
| Reflexes | Normal | May be reduced according to paresis | Reduced or absent |
| Cardiac pathology |
Rare in isolated MG, 10% overlapping myositis-myocarditis |
++ 25%-35% myocarditis, arrhythmia |
Possible with autonomic involvement |
| Additional findings | |||
|---|---|---|---|
| Laboratory findings: CK | Normal, elevated in myositis overlap | Markedly (fivefold to 10-fold) elevated (including troponin) up to 100×, rarely normala | Normal |
| Cerebrospinal fluid | Normal | Normal | Cytoalbuminary dissociation |
| Antibody status | May be positive for AChR, often negative or very low titres | Negative | Negative |
| Red flags | Fluctuation of symptoms, no pain | At onset: 20% fever, pain | Concurrent sensory impairment/paraesthesia, autonomic involvement: consider leptomeningeal carcinomatosis as a differential diagnosis |
| Indicative neurophysiology | Pathologic decrement in affected muscle group, but may also be absent | Pathologic fibrillation, myogenic muscle potentials,b in doubt consider EMG in paraspinal muscles also | Demyelination (missing or prolonged F-wave latency, temporal dispersion, possible conduction block) |
| Optional additional investigation in doubtful cases | Response to pyridostigmine or ice pack on eyes | Only necessary in very rare cases: imaging muscle abnormalities (MRI, PET)c | MRI spinal cord or brainstem |
| Therapy response | Usually rapid and effective if treated early with -glucocorticoids, in myositis correlating with fast CK decline; careful consideration of therapy escalation if unresponsive to glucocorticoids (IVIG/plasma exchange, see Figure 1) | ||
| Outcome | Usually favourable, however fatal outcome is possible, mortality primarily defined by cardiac and pulmonary complication | ||
(+) rare; + sometimes; ++ often; +++ very often.
AChR, acetylcholine receptor; CK, creatine kinase; EMG, electromyography; ICIs, immune checkpoint inhibitors; IVIG, intravenous immunoglobulins; MG, myasthenia gravis; MRI, magnetic resonance imaging; NMD, neuromuscular disease; PET, positron emission tomography.
Normal in case of oculobulbar predominance (resembles MG).
Early recruiting, short-duration, low-amplitude motor unit potentials.
May show muscular T2 gadolinium enhancement or increased [18F]2-fluoro-2-deoxy-D-glucose uptake.
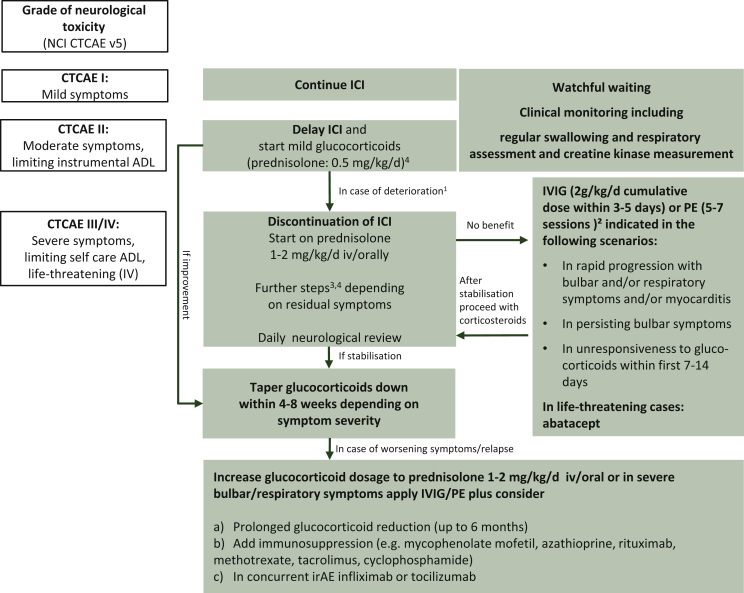
Figure 1.
Suggested therapeutic management algorithm according to severity of neuromuscular irAE, (all recommendations are based on level of evidence V in accordance with the ESMO guidelines2).
Re-challenge of ICI only if: symptoms remitted or at least stabilized, free or on low-level glucocorticoids for 4-8 weeks; convincing initial response to therapy, preceding CTCAE I/II irAE; follow more closely the more severe initial irAEs were.
ADL, activities of daily living; CTCAE, Common Terminology Criteria for Adverse Events; irAE, immune-related adverse event; ICI, immune checkpoint inhibitor; IVIG, intravenous immunoglobulins; NCI, National Cancer Institute; PE, plasma exchange.
1Timely consultation of a neurologist.
2In life-threatening symptoms, PE might be the favourable option; consider contraindications: renal failure, hypercoagulable states, sepsis, haemodynamic instability.
3Glucocorticoids are usually not recommended for idiopathic Guillain-Barré syndrome, however in mild ICI-related forms, a trial is reasonable (methylprednisolone 2-4 mg/kg/day), followed by slow glucocorticoid taper.
4Pulse corticosteroid dosing (methylprednisolone 1 g/day for 5 days) may also be considered for G3-4 along with IVIG or plasmapheresis.
5In all scenarios pyridostigmine starting from 3 × 30 mg orally up to 600 mg daily may be used in case of myasthenic symptoms. A dose of 30 mg oral pyridostigmine corresponds to 1 mg pyridostigmine intravenously or 0.75 mg neostigmine intramuscularly. In case of intubation pyridostigmine may be discontinued/on hold.
Myositis
Myositis associated with ICIs (IrMyositis) is the neuromuscular toxicity of most concern in ICI use representing de novo presentation in 99.6%. Based on Vigibase (WHO international pharmacovigilance database, http://www.vigiaccess.org/) 465 out of 54 416 (0.86%) patients with ICI-induced myositis have been reported from 2008 to 2019.7 As reported, myositis incidence is higher in patients treated with ICI combination (79/5023; 1.57%) and higher in anti-programmed cell death protein 1/programmed death-ligand 1 monotherapy (355/39 768; 0.89%) versus anti-CTLA4 monotherapy (31/9625; 0.32%).7 IrMyositis has been qualified as a new entity of myositis and highly differs from characteristics of paraneoplastic myositis.1,5,8 Symptoms typically occur during the first 2-3 months of ICI treatment and rapidly progress to maximal severity within 1-30 days.1,8 The clinical presentation includes myalgia with axial, limb-girdle, bulbar and oculomotor weakness (Table 1). The pathomechanism of rhabdomyolysis leads to predominantly strong creatine kinase (CK) increase, pathologic spontaneous activity in electromyography (EMG) of affected muscles along with a myogenic recruitment pattern of muscle fibres.
In our experience, the typical clinical picture including subacute muscle weakness, CK elevation and typical EMG findings generally allows the diagnosis of myositis usually without the need for muscle histology. If done, skeletal muscle biopsy represents corresponding necrotic changes with some superimposed macrophages.5,8 The pattern of necrosis is reported to be sometimes only focal and does not necessarily always lead to EMG pathology and markedly increased CK (defined in Table 1). In doubtful cases, muscle magnetic resonance imaging may show hyperintense T2 lesions with contrast media enhancement. Myositis-associated antibodies are usually missing.
Neither normal or slightly increased CK nor absence of EMG changes, however, safely rule out an irMyositis diagnosis, but favour differential diagnoses like myasthenic syndrome or a polymyalgia rheumatica-like irAE.
At the peak of irMyositis, some patients develop diffuse muscle weakness leading to severe bulbar symptoms (dysphagia, dysarthria) and respiratory failure requiring mechanical ventilation in up to 20%.1,5 Collectively, irMyositis can be a fatal complication of ICIs not only due to bulbar muscle affection, but also to secondary myocardial inflammation (see overlap syndromes).9
More than 80% of patients with irMyositis experience a favourable clinical outcome after ICI discontinuation and immunomodulatory treatment within several months.1,5 Glucocorticoids represent the first therapeutic choice (Figure 1).1,2,5,8,10 Additional treatment options such as intravenous immunoglobulins (IVIG) and/or plasma exchange are necessary in ∼40% of patients.1,2,5,10 Very often, CK normalizes within 8 days (range 1-35 days) and stays on a third of the preceding level in others.5 In patients only mildly affected, improvement is often noted spontaneously within days after ICI discontinuation.1,8 Mostly, ICI discontinuation is reported to be permanent.
Follow-up data on clinical outcome are rare. In a series of 24 patients, approximately one-third of patients were able to stop immunomodulatory treatment after a median of 5 months (range 1-24 months); the others required ongoing immunosuppression primarily based on prednisolone lower than 20 mg daily.5 Generally, in cases of therapy resistance, higher-dosed long-term glucocorticoid usage (>10 mg prednisolone daily) or unsuccessful steroid tapering, additional immunosuppressants like azathioprine or mycophenolate mofetil, should be considered as steroid-sparing agents.2,10,11 The onset of therapeutic response, however, may take several weeks. Rituximab is another steroid-sparing option working effectively within 3 months (Figure 1).2,11,12 In life-threatening cases, the recombinant fusion protein abatacept may be considered as salvage therapy.12 In the myositis series mentioned previously, re-challenge of ICIs led 3 out of 22 patients to a severe irAE (1 patient was on prednisolone <20 mg/d, 2 patients had no immunosuppressive treatment).5
MG
With an incidence of <1%, ICI-associated MG (irMG) is a rare, but an increasingly recognized and feared complication of ICI therapy. It may, however, be underrecognised due to milder cases with non-specific symptoms such as generalized weakness and fatigue.13 Mostly, irMG occurs de novo.3 Whereas about two-thirds of patients with irMG are positive for anti-acetylcholine receptor antibodies, the concentration of antibody titre in irMG tends to be lower compared with patients with idiopathic MG.3
Typical symptoms of irMG include exercise-dependent fluctuation of weakness of proximal extremities or bulbar muscle groups in addition to ocular symptoms like ptosis and diplopia. In contrast to preceding symptoms of irMyositis, pain and CK elevation are unusual in MG.
Rapid progression of disease, including bulbar and respiratory symptoms requiring respiratory support, were observed in up to 50% of patients.4,14 In a retrospective cohort of 65 irMG patients (including 20% with pre-existing MG), 45% of patients developed respiratory failure. Myasthenic symptoms developed after a median of 4 weeks (1-16 weeks) after ICI initiation; the median time from symptom onset to respiratory failure requiring intubation was only 7 days.13
General treatment recommendations are limited, as case series usually describe only more severe or fulminant cases.1,2,14 Along with ICI discontinuation, glucocorticoids are the first-line treatment together with pyridostigmine as shown in Figure 1.1,2,10,15 In analogy to irMyositis, severe initial presentation including respiratory and bulbar symptoms often requires the immediate use of IVIG and/or plasma exchange.2,10 Early recognition of irMG is of utmost importance as a fatal outcome in 20% of cases is reported and is primarily associated with respiratory complications or fatal myocarditis overlap.1,4,13
Rarely, symptoms resolve completely and patients are often maintained on prolonged steroid taper for months.1,13,14 Importantly, remission without longstanding use of immunosuppression has been noted only in a few patients with mild symptoms limited to ocular or facial muscles.1
There are also, however, a certain number of patients (∼20%) with mild and limited ocular symptoms like fluctuating ptosis and double vision.14, 15, 16 In these patients, we recommend initiation of mild prednisolone 0.25-0.5 mg/kg/day with slow tapering after symptom remission. Continuous clinical monitoring of possible progression is necessary. Only in very limited cases are watchful waiting or pyridostigmine monotherapy justifiable.
Myasthenia-myositis-myocarditis overlap
Particularly, an ICI-induced overlap of myositis and myasthenia has been reported in up to one-third of both entities.1,3,13 As both may involve weakness of ocular, facial and bulbar muscles, and proximal tetraparesis, it is essential to recognize clinical hints for potential myositis (CK elevation, pain). This should be clinically highly concerning because myocarditis has been diagnosed in ∼30% of irMyositis1,5 and 8% of irMG patients.3 A complicating factor in this regard can be the asymptomatic or non-specific symptom presentation or an abrupt arrhythmia leading to death.1 The mortality rate in irMyositis without myocarditis is ∼15% versus approximately 25% in myositis-myocarditis case series.1,15 In idiopathic disease, coincidence of MG, myositis and myocardial involvement is extremely rare and only seen in patients with thymoma.14
Finally, immediate immunomodulating therapy including interdisciplinary decision making is required in myasthenia and myositis as well. Based on our experience, pyridostigmine leading to improved neuromuscular transmission may show some additional effect in both.
Peripheral neuropathy
ICI-associated neuropathies (IrNeuropathies) are the most common neuromuscular complications with an incidence of 1.16% according to Vigibase and a frequency of 1%-3% in case series.4,7,15, 16, 17 Neuropathy grading varies depending on the assessment tool used and clinical severity.18 As the peripheral sensorimotor pattern of these irAEs often presents only transiently, there is a tendency of underestimation.
IrNeuropathies are mostly demyelinating and may present as an acute polyradiculoneuritis (Guillain-Barré syndrome, irGBS) with an incidence of ∼0.2%-0.4%. Chronic demyelinating neuropathy is less common and casually appears in older women, presenting with paraesthesia and weakness.3 Usually, irGBS occurs at the start or even several months after the initiation of therapy (median of 3-3.5 ICI doses applied).1,15 Clinical findings resemble classical ascending GBS symptoms, including bilateral proximal weakness, ataxia, distal sensory and autonomic disturbances, as well as cranial nerve involvement. In case only ventral roots are affected, symptoms may be limited to (asymmetric) weakness only. Corresponding swelling of nerve roots impairs cerebrospinal fluid flow leading to cytoalbuminary dissociation. Antiganglioside antibodies are negative. Prompt recognition of symptoms is essential to prevent respiratory insufficiency due to cervical root affection. In contrast to non-ICI associated GBS, glucocorticoids have been associated with a favourable outcome in irGBS and are therefore recommended as first-line treatment (Figure 1).1 IVIG are used as an additional or alternative treatment if steroids are not possible. In patients with severe or rapidly progressing clinical symptoms, including bulbar or respiratory muscles, plasma exchange should be promptly initiated.2,3,10 Most peripheral neuropathies, however, improve rapidly with ICI discontinuation, glucocorticoids and/or IVIG.17
Further, immune-related axonal sensory-motor neuropathies and even pure sensory neuropathies are also described. It is difficult to attribute axonal changes only to ICIs, however, as many patients had undergone cytotoxic therapy before.17 In general, immune-related axonal neuropathies tend to be benign (Common Terminology Criteria for Adverse Events 1-2) usually remitting without intervention. Nevertheless, they may present as painful, long-term, persisting permanent burning due to small fibre damage, requiring membrane stabilizers such as pregabalin, amitriptyline or duloxetine, or due to rare peripheral nervous system vasculitis.17,18
Based on our experience, a few irNeuropathy patients present with painful new-onset carpal tunnel syndrome due to immune-related hypothyreosis. In these patients, night splints for the wrist and special support bandages are more adequate than glucocorticoids.
More rarely, irNeuropathies present as isolated cranial neuropathies affecting the optic or abducens nerve, facial palsy, trigeminal neuralgia, plexopathy or casually as autonomic neuropathy, resulting in orthostasis, anhidrosis, gastrointestinal dysmotility and/or urinary retention.1,16 Presentation may be isolated or as an overlap with meningeal inflammation.
Conclusion
Immune-related neuromuscular toxicity varies in clinical phenotype and is often transient and mild. Rapid progression of muscle weakness involving respiratory, bulbar and/or cardiac functions should, however, raise concern for severe and potentially fatal immune-related neuromuscular events. In these cases, immediate discontinuation of ICI therapy is required followed by vigilant intensive care unit treatment including a multidisciplinary irAE expert team particularly consisting of neurologists with neuromuscular expertise. The clinical management of severe neuromuscular toxicity usually involves glucocorticoids, IVIG and/or plasma exchange. Steroid-sparing agents may be needed for long-term treatment. Re-challenging with ICIs may be discussed particularly in mild irAEs, however, the lack of systematic controlled data on treatment and outcome in neuromuscular toxicity has to be considered in the shared decision-making process.
Acknowledgments
Funding
None declared.
Disclosure
BJ: advisory board and/or honoraria for presentations for Alexion Pharmaceuticals, Temmler (Hormosan Pharma), Novartis, Biomarin, Merz Pharmaceuticals, Ipsen Pharma and Allergan. KB: Consultancy and/or speaker fees and/or travel reimbursements: Abbvie, Bristol Myers Squibb (BMS), Janssen, Merck Sharp & Dohme (MSD), Mundipharma, Novartis, Pfizer, Roche, UCB. Scientific support: AbbVie, United States, Novartis, Switzerland. JH: advisory board and/or honoraria for presentations Almirall, BMS, Immunocore, MSD, Novartis Pharma, Pierre Fabre Pharmaceuticals, Inc., Roche Germany, Sanofi-Aventis, Sun Pharmaceutical Industries Inc. WW has declared no conflicts of interest. KJ: advisory board and/or honoraria for MSD, Merck, Amgen, Hexal, Riemser, Helsinn, Kreussler, Voluntis, Pfizer, Pomme-med, PharmaMar, art tempi and Onko Solutions.
References
- 1.Psimaras D., Velasco R., Birzu C., et al. Immune checkpoint inhibitors-induced neuromuscular toxicity: from pathogenesis to treatment. J Peripher Nerv Syst. 2019;24(suppl 2):S74–S85. doi: 10.1111/jns.12339. [DOI] [PubMed] [Google Scholar]
- 2.Haanen J., Carbonnel F., Robert C., et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 3.Haugh A.M., Probasco J.C., Johnson D.B. Neurologic complications of immune checkpoint inhibitors. Expert Opin Drug Saf. 2020;19:479–488. doi: 10.1080/14740338.2020.1738382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson D.B., Manouchehri A., Haugh A.M., et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J Immunother Cancer. 2019;7:134. doi: 10.1186/s40425-019-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shelly S., Triplett J.D., Pinto M.V., et al. Immune checkpoint inhibitor-associated myopathy: a clinicoseropathologically distinct myopathy. Brain Commun. 2020;2:fcaa181. doi: 10.1093/braincomms/fcaa181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan K., Aapro M., Kaasa S., et al. European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Ann Oncol. 2018;29:36–43. doi: 10.1093/annonc/mdx757. [DOI] [PubMed] [Google Scholar]
- 7.Allenbach Y., Anquetil C., Manouchehri A., et al. Immune checkpoint inhibitor-induced myositis, the earliest and most lethal complication among rheumatic and musculoskeletal toxicities. Autoimmun Rev. 2020;19:102586. doi: 10.1016/j.autrev.2020.102586. [DOI] [PubMed] [Google Scholar]
- 8.Touat M., Maisonobe T., Knauss S., et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. 2018;91:e985–e994. doi: 10.1212/WNL.0000000000006124. [DOI] [PubMed] [Google Scholar]
- 9.Wang D.Y., Salem J.E., Cohen J.V., et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer J.R., Lacchetti C., Schneider B.J., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benesova K., Lorenz H.M., Leipe J., Jordan K. How I treat cancer: treatment of rheumatological side effects of immunotherapy. ESMO Open. 2019;4:e000529. doi: 10.1136/esmoopen-2019-000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kostine M., Finckh A., Bingham C.O., 3rd, et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann Rheum Dis. 2020;80:36–48. doi: 10.1136/annrheumdis-2020-217139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safa H., Johnson D.H., Trinh V.A., et al. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J Immunother Cancer. 2019;7:319. doi: 10.1186/s40425-019-0774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki S., Ishikawa N., Konoeda F., et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology. 2017;89:1127–1134. doi: 10.1212/WNL.0000000000004359. [DOI] [PubMed] [Google Scholar]
- 15.Kao J.C., Brickshawana A., Liewluck T. neuromuscular complications of programmed cell death-1 (PD-1) inhibitors. Curr Neurol Neurosci Rep. 2018;18:63. doi: 10.1007/s11910-018-0878-7. [DOI] [PubMed] [Google Scholar]
- 16.Dalakas M.C. Neurological complications of immune checkpoint inhibitors: what happens when you 'take the brakes off' the immune system. Ther Adv Neurol Disord. 2018;11 doi: 10.1177/1756286418799864. 1756286418799864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubey D., David W.S., Amato A.A., et al. Varied phenotypes and management of immune checkpoint inhibitor-associated neuropathies. Neurology. 2019;93:e1093–e1103. doi: 10.1212/WNL.0000000000008091. [DOI] [PubMed] [Google Scholar]
- 18.Jordan B., Margulies A., Cardoso F., et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann Oncol. 2020;31:1306–1319. doi: 10.1016/j.annonc.2020.07.003. [DOI] [PubMed] [Google Scholar]