Abstract
The high expense of chemical coagulant-treated water forces most people in rural regions to rely on easily available sources, which are usually of poor quality, and expose them to waterborne infections. According to this statement, the purpose of this study was to confirm the efficiency of extracting powder Moringa oleifera seeds, which are widely available in rural regions. The experiment was done based on a random design load of 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6 g/500 ml of powder extracted from Moringa seeds. Chemical oxygen demand (COD), color, and turbidity were determined for both acidic and basic characteristics of wastewater. The optimum dosage of Moringa oleifera was 0.4 g/500 ml in both characteristics of wastewater in the case of color and turbidity. Moringa oleifera maximum reduction in turbidity, color, and COD in acidic wastewater was 98 %, 90.76 %, and 65.8 % respectively; while, the maximum reduction of turbidity, color, and COD in basic wastewater were 99.5 %, 97.7 %, and 65.82 % respectively. The study was demonstrated that, the application of RSM for seeking optimum conditions in the coagulation process for the treatment of wastewater. Moringa seed powder works best with a 7–9 pH range. The study also investigated that, best adsorption equilibrium was observed when using 0.1 g of Moringa oleifera seed powder. All the results showed that Moringa oleifera seeds were very effective for the removal of impurities.
Keywords: COD, Color, Domestic wastewater, Moringa oleifera, RSM, Turbidity
COD; Color; Domestic Wastewater; Moringa oleifera; RSM; Turbidity.
1. Introduction
Water is an important natural resource for human life (Ugwu et al., 2017). Increased population, economic development, and industrialization have resulted in not only increased freshwater consumption but also significant mismanagement of this natural resource. The researchers show that progress in the population's way of life in terms of production and consumption has resulted in an exponential increase in household waste (Chaouki et al., 2021). Effluents from various sources reduce the consistency of natural and surface water by flowing and discharging into the rivers because of the harmful and undesirable chemical compounds that are found in them and are therefore a significant cause of water contamination. The most harmful of these sewage materials in the oxidizing process, possessing water degradation, oxygen, resulting in increased chemical oxygen demand in one of the water contents (Narmatha et al., 2017). Abiyu et al. (2018), discussed, the lack of safe drinking water is a major source of death and disease around the world. Today, environmental engineers' primary concern is how to reduce the cost of coagulants and flocculants and improve the characteristics of the generated sludge for safe use (Abdelaal, 2004).
Given its simplicity and cost-effectiveness, the commonly utilized coagulation and flocculation process is a crucial stage in water and wastewater treatment. Researchers have discovered that physicochemical processes such as coagulation and flocculation can minimize pollution and provide clean water for reuse (Manhokwe and Zvidzai, 2019). Coagulation-flocculation is commonly included either as a pretreatment phase or as a post-treatment step, depending on the nature of the sample being treated (e.g., various types of water or wastewater) and the overall treatment plan used. The entire coagulation-flocculation treatment cycle can be separated into two different treatments, to be performed consecutively. The first, so-called coagulation, is the mechanism by which a given colloidal suspension or solution is destabilized. The second sub-process, called flocculation, refers to the incorporation of destabilizing particles to come together, make contact and thus form large agglomerates, which can normally be more easily separated by settling gravity. Coagulation is frequently over in a matter of seconds (e.g., 10 s), but flocculation takes 20–45 min. The resulting destabilized particles are sufficiently stuck and settle in the following treatment unit (Narmatha et al., 2017). The most important ingredient in water purification is a natural coagulant, such as Moringa oleifera (MO) seed has been used to treat wastewater because it is not harmful to humans and has no notable drawbacks (Alo et al., 2012; Bina et al., 2010; Eman et al., 2014; Shan et al., 2017).
The advantages of natural coagulants that they are environmentally sustainable, environmentally friendly, inexpensive, and a simple process for developing countries. The performance is independent of raw water pH safe for human health, and antibiotic impact on various bacteria and fungi. The alkalinity of wastewater can be significantly reduced, compared to chemical coagulants. Natural coagulants are cost-effective. According to Vigneshwaran et al. (2020), the widespread use of aluminum-based chemical coagulants causes a variety of neurological problems, whereas bio-coagulants have natural properties that make them toxic to aquatic life. Different researchers studied natural coagulant such as microbial polysaccharides (Saleem and Bachmann, 2019), bio-wastes (Atchudan et al., 2020), gelatin, cellulose-based materials (Leite et al., 2017), chitosan (Agbovi and Wilson, 2019), Moringa oleifera (Rocha et al., 2020), Moringa oil (Chitra and Muruganandam, 2019). Natural coagulants are commonly used in less developed populations as a point-of-use product, as they are fairly cost-effective compared to chemical coagulants.
Moringa oleifera is an exceptional source of nutritional components and an excellent source of nutritional and natural energy boosters and bioactive compounds (Leone et al., 2015). Furthermore, the major variations in Moringa oleifera's polyphenol content from different regions suggest that Moringa oleifera's genetic diversity was relatively high, likely due to differences in cultivation conditions, climate, or soil environment resulting in the accumulation of various polyphenols and also resistance to drought (Rani et al., 2018). During experimentation optimization of the parameter very essential, because it saves experiment time and chemical cost. Response surface methodology (RSM) is can do such things. RSM is a mixture of mathematical and statistical techniques for experiment design, model construction, achieving ideal conditions for good reactions with a small number of planned tests, and the effects of various factors and their interactions (Srinu Naik and Pydi Setty, 2014; Subramonian et al., 2015; Wang et al., 2014). Indeed, a variety of specialized techniques are used to eliminate turbidity, color, and chemical oxygen demand, pollutants that may pose health risks, from wastewater (Abiyu et al., 2018).
The study's needs are: aluminum causes chronic effects. Long-term aluminum exposure can cause brain and bone effects. Aluminum, once allowed to penetrate the bloodstream, is a neurotoxic agent. The sludge generated after treatment is voluminous and not biodegradable and therefore poses problems with disposal, which leads to increased treatment costs. The cost of these chemicals for imported chemicals has increased at an alarming rate for foreign exchange problems in developing countries. Most of the study was focused on the use of Moringa oleifera to remove turbidity from wastewater. In addition, determining the efficiency of natural coagulants at different wastewater characteristics is very crucial. Therefore, this experiment was designed to remove pollutants from wastewater using natural coagulants at different pH ranges. The specific work of this study was evaluating the performance of Moringa seed powder for removing pollutants (color, turbidity, and COD) from wastewater. The study also sought to explore protocols for assessing the potency of main water treatment by evaluating the ability of its seed powder to remove turbidity, color, and COD. It also allows estimation of the optimum amount of Moringa seed extract that should be used minimized residues in treated water. The study also used methods from the response surface to optimize the operating parameters.
2. Materials and methods
2.1. Materials
Wastewater was collected from Jimma university institute of technology Oromia regional state, Ethiopia. While collecting wastewater grab sampling was used and the wastewater was stored at 4 °C to keep wastewater quality as much as possible. There were different materials used for the investigation such as pan, sieve, mortar, oven dray, paper, oven, beaker, test jar, COD digester, pH meter, turbidity meter, and spectrophotometer.
2.2. Methods
An experimental investigation of wastewater using Moringa Oleifera Seeds was conducted using test jar apparatus, where Powder of Moringa Oleifera was added to the prepared beaker by adjusting the quantity of dosage, pH, stirring time, and rotating speed of the test jar as shown in Figure 3, Figure 2. The removal percentage of color, COD, and turbidity were determined based on the operating parameters. The color removal percentage of wastewater was determined using a UV/Vis spectrophotometer (Jasco, V-570) at a wavelength of 450 nm maximum absorbance, and turbidity was determined by pH meter [Model: HANNA]. On the other hand, COD was determined using a closed reflux method based on the APHA guidelines where closed reflux methods use modest amounts of reagents and produce small amounts of hazardous waste. Closed reflux methods also use beakers and culture tubes with premeasured reagents, after which samples are inserted in the tube, and COD is evaluated through titration.
Figure 3.
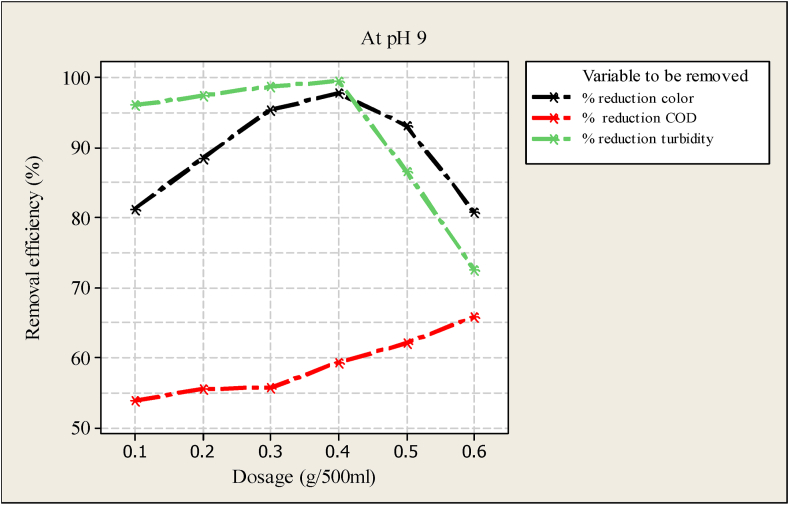
Optimal dosage of Moringa seed powder basic characteristics.
Figure 2.
A conventional jar test apparatus.
2.2.1. Coagulant preparation procedure
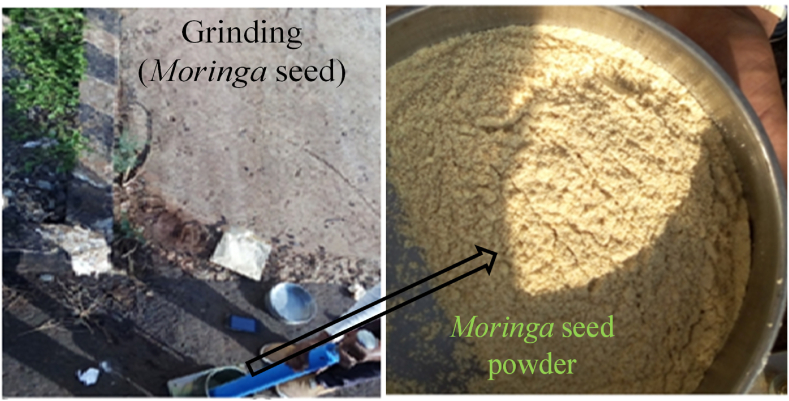
Moringa seeds are unfortunately not used by the population in Ethiopia, given their natural coagulant properties, which could be used very easily for sanitation and health at the household level. The average weight per seed is 0.3 g and, to purify one polluted liter, one seed is needed. Moringa seed powder preparation procedure (pan, sieve, mortar, oven dray, paper) Collect Moringa seeds from the available area; remove the hulls and wings from the kernels; place the crushed seeds in the oven-dry using a pan at a temperature of 105 °C for 7 h; grade the dried seeds using mortar and sieve within 710 sizes, and finally take Moringa seed powder as shown in Figure 1.
Figure 1.
Grinding Moringa seed and powder form.
2.2.2. Experimental procedure
In the coagulation and flocculation processes, the jar test is the most extensively used experimental instrument. To coagulate the wastewater, a conventional jar test device was utilized using a natural coagulant.
The study was conducted by taking acidic and basic characteristics of wastewater. The optimum dosage of coagulant was evaluated by varying the dosage of 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6 g/500 ml at pH of 9 and 3 as shown in Figure 2.
2.2.3. Analysis
Analysis of wastewater based on the laboratory investigation was done for wastewater parameters like turbidity, color, and COD using empirical formulas indicated in Equations ((1)–(5)) under different operating parameters. In addition to this statistical and mathematical data were analyzed with Response surface methodology (Design of Expert 11) and Minitab version 16 statistical software.
Turbidity Testing Procedure: First a water sample was added to the beaker; then the turbidity meter was calibrated and inserted into the sample then the value was recorded. The general formula to calculate the percentage removal of turbidity:
| (1) |
where; TURi: turbidity of raw sample and TURf: turbidity after treatment.
COD Tasting Procedure: 2.5 ml of sample was taken into the kit; 1.5 ml of potassium dichromate and 3.5 ml of sulfuric acid solution was added, then the kit was put on the COD reactor for 2 h at 150 °C. After that, the kit was transferred into the desiccator for 15 min; then dropped the value of the kit was into the cylindrical flask. Three droplets of ferrous indicator and FAS were added until the color changed to brown. Then the number of a droplet of FAS was taken. Finally, the COD was calculated using the general formula. The general formula used to calculate the percentage removal of COD was:
| (2) |
where; A: Blank sample; B: FAS consumed by the sample, N: normality of FAS and V: volume of sample. Chemical Oxygen Demand percentage removal can be simply determined using the mentioned formula below (Asaithambi et al., 2016)
| (3) |
where, CODo and CODt are the chemical oxygen demand at time = 0 (initial) and at t (reaction time t) respectively.
Color Tasting Procedure: First the spectrophotometer was adjusted; the beaker and the spectrophotometer were calibrated; then the sample was added into the beaker and put the beaker that is filled by the sample into the spectrophotometer; then the scan was touched and the value was recorded from the diagram; finally, the value was calculated by using Eq. (4). The general formula to calculate the percentage removal of color was:
| (4) |
where; Rwi; raw water sample and Wof observed treated water sample.
Adsorption equilibrium: adsorption is defined as the accumulation of substance or material at the interface between a solid face and a solution. The adsorbing surface is the adsorbent, and the material concentrated or adsorbed at the surface is the adsorbant. In this study, the adsorbent was the amount of Moringa oleifera seed powder and the adsorbant was the pollutants from wastewater (color, turbidity, and COD). Adsorption of equilibrium was determined for each executed experiment for dosage of Moringa oleifera powder as indicated in Eq. (5).
| (5) |
where, Co and Ce (mgL−1) are initial and equilibrium or final concentrations of pollutants, qe is equilibrium adsorption amount (mg/g), V is the volume of wastewater (L) and W is mass of Moringa oleifera powder.
3. Results and discussion
In this study, the use of natural coagulants in domestic wastewater treatments was investigated in both acidic and basic wastewater characteristics. As observed from the analyzed results, a natural coagulant is effective for removing the physicochemical water quality parameters. Figure 3, Figure 4 show the results obtained from the application of Moringa powder to both types of wastewater.
Figure 4.
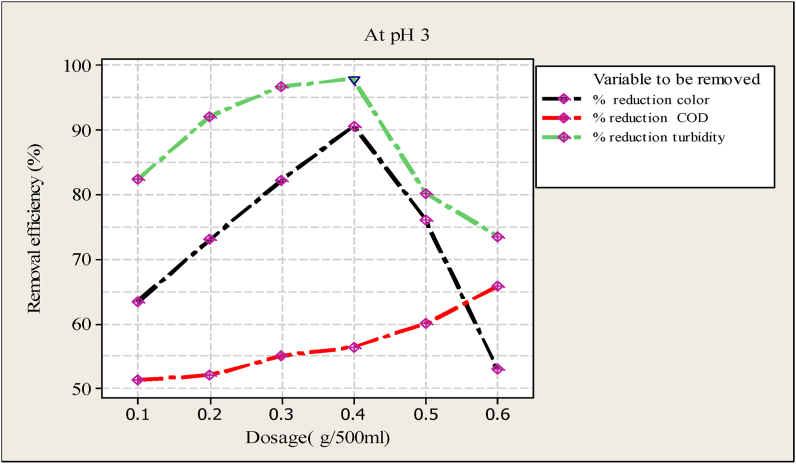
Optimal dosage of Moringa seed powder acidic characteristics.
3.1. Process analysis
For analysis of the data, Minitab version 16 statistical software was used. Figure 3 demonstrated the result obtained from the treatment of the basic characteristics of wastewater. The figure specifies the effect of varying the dosage on the removal of the parameters. The dosage was started to add to the sample water from 0.1 g/500 l to 0.6 g/500 ml. As observed from Figure 3 the removal efficiency was increased to 0.4 g/500 ml dosage and the maximum reduction in turbidity and color was obtained at this point. After this point the graph becomes bending down; this is due to the dosage being more than the impurity in the sample water. The experiment was done using a similar dosage of Moringa seed powder to remove COD. As indicated in Figure 3, a higher dosage of Moringa powder is needed to get a maximum reduction of COD and the optimum dosage of Moringa seed powder. This is because the use of Moringa as a coagulant is disadvantageous. After all, organic matter from the seed is released into the treatment system of wastewater, which often leads to a higher demand for chemical oxygen (COD) (Baptista et al., 2017; Eman et al., 2014); however, it is possible to remove COD using a natural coagulant as indicated in Figure 3, Figure 4. The result in Figure 3 shows that using Moringa powder reveals a reverse effect on water clarity, where it decreases the intensity by increasing the dosage of Moringa powder. The decrease may be due to the presence of more positive ions.
Figure 4 verified the results obtained from the treatment of acidic characteristics of wastewater. The figure stipulates the effect of varying the dosage on the removal of the parameters. A similar experiment was done as basic characteristics by taking more acidic characteristics of sample wastewater to observe the effectiveness of Moringa seed powder for removing pollutants. The experiment was started using a similar dosage of Moringa seed powder from 0.1 g/500 l to 0.6 g/500 ml. As perceived from Figure 4 the removal of the pollutant was increased to 0.4 g/500 ml dosage and the maximum reduction of turbidity and color was attained at this point. After the optimum point, the graph becomes bending down; this is due to the dosage being more than the impurity in the sample water and a more positive ion was developed. This means that when the coagulant (Moringa seed powder) was added to the sample followed by rapid string the resulting cationic protein from Moringa seed powder was distributed to all parts of the liquid and interacting with the negatively charged particles that caused disturbed turbidity. When cation was added more than the anion, the cation by itself increases the turbidity of the water, because there is not enough negatively charged particle to interact. The experiment was done using a similar dosage of Moringa seed powder to remove COD. As indicated in Figure 4, a more dosage of Moringa seed powder is needed to get a maximum reduction of COD and the optimum dosage of Moringa seed powder. When the experiment was carried out at 0.6 g of morning seed powder, the removal of color dropped to 53 percent, as indicated in Figure 4. The removal of color, COD, and turbidity using Moringa seed powder was more effective in more basic than acidic characteristics of wastewater.
3.2. Adsorption equilibrium
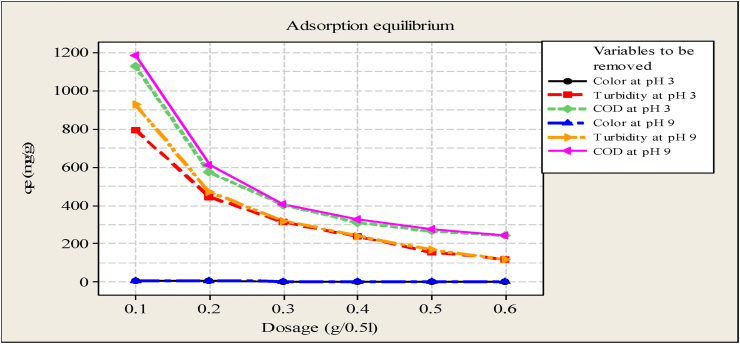
Figure 5 indicated that, determination of adsorption equilibrium for each experiment at the different dosages of Moringa oleifera seed powder and pH values of 3 and 9. The result for color, turbidity, and COD uptake using various amounts of Moringa seed powder (0.1–0.6 g/500 ml) was shown in Figure 5. It can be observed that the uptake of color, turbidity and COD increase at the initial stage (at 0.1 g). This was because the availability of the active site and surface area of the adsorbent was high. As observed from Figure 5, the equilibrium color, turbidity, and COD uptake capacity (qe) were found to decrease with an increased dosage of the adsorbent and best when using 0.1 g of Moringa oleifera seed powder. The splitting effect of the concentration gradient between the adsorbant and adsorbent caused the color, turbidity, and COD absorption value of Moringa seed powder to decrease. Furthermore, the availability of the active site and surface area of adsorbent were fully occupied due to the limited capacity of Moringa seed powder adsorbent to adsorb high amounts of color, turbidity, and COD. The removal of color, turbidity, and COD were higher at pH 9 than pH 3 because the surface of the coagulant was negatively charged. The decrease in adsorption capacity at low pH values would be expected because the acidic medium would lead to an increase in hydrogen ion concentration, which would then neutralize the negatively charged coagulant surface, reducing the adsorption of positively charged ions due to a reduction in the force of attraction between adsorbant and adsorbent.
Figure 5.
Effects of adsorbent dose on the removal of color, turbidity, and COD.
3.3. Process comparison
Moringa oleifera Seeds were found to be particularly successful at removing color, turbidity, and COD from water samples with diverse characteristics. However, as compared to acidic water, it is more effective in terms of basic qualities. In deeded similar concentrations of Moringa oleifera seeds (0.4 mg/500 ml), 99.99 percent turbidity, 95.34 percent color, and 59.99 percent COD were removed in basic water characteristics, while 95.99 percent turbidity, 90 percent color, and 55.99 percent COD were removed in acidic water characteristics. According to other studies, MO seed flour is effective at removing turbidity, which has been connected to the removal of helminth eggs in turbid wastewater (Sengupta et al., 2012). These findings were in line with previous research findings, which revealed that treating water samples with MO seed cake reduced turbidity (Ghebremichael et al., 2005; Nand, 2012; Shan et al., 2017; Sotheeswaran et al., 2011). According to Govindan (2018), color and turbidity removal effectiveness of up to 98.4 percent and 84.3 percent respectively was achieved employing 70 mg/L coagulant extract of Moringa Stenopetala. Garde et al. (2017) discovered that utilizing 0–0.4 g/L of coagulant dose resulted in 1–25 percent COD removal. By adding 20–80 mg/l of coagulant dose David et al. (2016), were able to achieve 97 percent color, 90 percent COD, and 99 percent turbidity removal. According to Scholes et al. (2020), a 78.6 percent reduction in chemical oxygen demand (COD) in treated water was observed.
3.4. Process optimization
The analysis of variance (ANOVA) was used to analyze the interaction between the proses variable and the response. The model F-value of 4.90 for color, 23.01 for turbidity, and 129.37 for COD imply the model was significant. There was only a 3.02 % for color, 0.03 % for turbidity, and 0.01 % for the COD likelihood that a large F-value could be caused by noise. Model terms with P-values of less than 0.0500 were considered significant. A, A2, and B2 were crucial model terms in this example. The model terms are not important if the value is bigger than 0.1000. Model reduction may improve the model if there are many inconsequential model terms (not including those required to support hierarchy). As Table 1 shows that, the model was significant at 95 % confidence level by the F-test with all p-values of regression <0.05. In addition, the model does not exhibit lack of-fit (P > 0.05). The lack of fit test measures the failure of the model to represent data in an experimental domain at points that are not included in the regression. If a model is significant meaning that, the model contains one or more important terms, and the model does not suffer from lack of fit. If the experimental environment is quite noisy, or some important variables are out of the experiment, it is possible that the portion of the variability in the data not explained by the model also called the residual could be large. Table 1 depicted that, the P-values of color, turbidity, and COD were less than 0.05, indicating that the factor affecting the response variables.
Table 1.
ANOVA for Quadratic model.
| Source | Sum of Squares | df | Mean Square | F-value | P-value | Remark | Response |
|---|---|---|---|---|---|---|---|
| Model | 5684.32 | 5 | 1136.86 | 4.90 | 0.0302 | Significant | Color |
| A-PH | 1856.55 | 1 | 1856.55 | 8.00 | 0.0255 | ||
| B-Dosage | 550.84 | 1 | 550.84 | 2.37 | 0.1673 | ||
| AB | 669.70 | 1 | 669.70 | 2.89 | 0.1331 | ||
| A2 | 1485.28 | 1 | 1485.28 | 6.40 | 0.0392 | ||
| B2 | 2598.83 | 1 | 2598.83 | 11.20 | 0.0123 | ||
| Residual | 1624.05 | 7 | 232.01 | ||||
| Cor Total | 7308.37 | 12 | |||||
| Model | 1348.29 | 5 | 269.66 | 23.01 | 0.0003 | Significant | Turbidity |
| A-PH | 62.02 | 1 | 62.02 | 5.29 | 0.0550 | ||
| B-Dosage | 371.51 | 1 | 371.51 | 31.70 | 0.0008 | ||
| AB | 35.72 | 1 | 35.72 | 3.05 | 0.1243 | ||
| A2 | 132.85 | 1 | 132.85 | 11.33 | 0.0120 | ||
| B2 | 559.29 | 1 | 559.29 | 47.72 | 0.0002 | ||
| Residual | 82.05 | 7 | 11.72 | ||||
| Cor Total | 1430.33 | 12 | |||||
| Model | 298.36 | 5 | 59.67 | 129.37 | <0.0001 | Significant | COD |
| A-PH | 11.04 | 1 | 11.04 | 23.93 | 0.0018 | ||
| B-Dosage | 235.02 | 1 | 235.02 | 509.52 | <0.0001 | ||
| AB | 1.48 | 1 | 1.48 | 3.22 | 0.1160 | ||
| A2 | 18.20 | 1 | 18.20 | 39.45 | 0.0004 | ||
| B2 | 14.29 | 1 | 14.29 | 30.98 | 0.0008 | ||
| Residual | 3.23 | 7 | 0.4613 | ||||
| Cor Total | 301.59 | 12 |
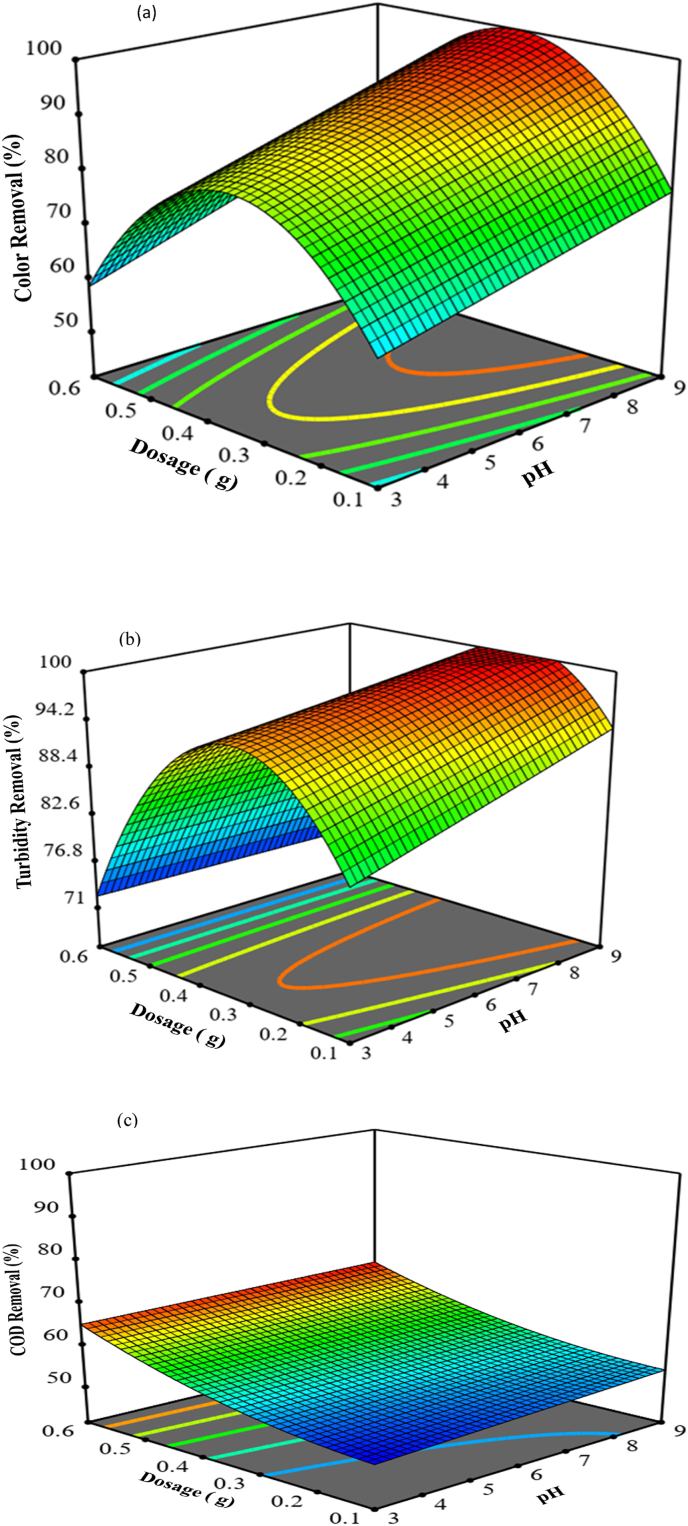
Optimization of the treatment process using Moringa oleifera seed was conducted using RSM to minimize the operating cost and time by reducing the coagulant dosages. During optimization, the highest removal efficiency of color, turbidity, and COD from wastewater was achieved. Figures 6a, b, and c showed that 3D response surface plots of the quadratic models concerning color, turbidity, and COD removal by a factor of pH and dosage similar results to (Trinh and Kang, 2011). As illustrated in figure a, increasing color removal was observed with increasing both Moringa powder and pH, and higher removal efficiency of 95 % was obtained at 0.467 g/500 ml of Moringa powder and 8.78 pH. However, like in Figure 3, Figure 4 , an increase in both Moringa powder and pH beyond the optimum range resulted in decreasing removal efficiency of color. In Figure 6b, similar results were observed as in Figure 6a; but, the higher removal efficiency of 98.5 % of turbidity was obtained at 0.467 g/500 ml Moringa powder and 8.78 pH. However, analogous trained was occurred after the optimum region like in Figure 6a. As the doses increased higher than 0.467 g/500 ml, the removal efficiency began to decrease at all coagulation pH, similar to (Trinh and Kang, 2011). This indicates that there was an overdose in the reaction solution. This created increasing color and turbidity of the water. As figure c illustrated, the increasing COD removal efficiency was achieved by increasing both Moringa powder and pH. But, as shown in Figure 6c, the optimum removal efficiency was not obtained. This implies that for the removal of COD, more Moringa powder was required other than color and turbidity.
Figure 6.
Design expert; surface plots; (a), (b), and (c) removal efficiency of color, turbidity, and COD respectively.
3.5. Factors affecting the operating parameters
The methodology has shown that the optimal coagulant dose was achieved efficiently and directly by reducing the turbidity, color, and COD by considering several inputs or process parameters of coagulation processes. The input was as follows: initial turbidity, color, COD, natural coagulant dose, process temperature (T), time, and pH. These are highly useful when determining operational factors such as coagulant selection and dose, optimization pH, and mixing process optimization and duration. During coagulation testing, the coagulant is dosed into water samples, and the resulting solutions are mixed to destabilize and incorporate contaminants into the aggregates (flocs) (Naceradska et al., 2019). After flocculation, a 15-minute stirring phase was entered at 200 r/min and the flocs were broken. Following the breakage, a slow stir was reintroduced at 40 r/min and allowed to be aggregated for another 15 min. For the removal of turbidity, the dosage for maximum reduction is the same. As Okey-Onyesolu et al. (2020) indicated, the set-up time of 49.25 min, which is a little longer, was the best time to remove the color.
3.5.1. Effects of dosage
The effects of Moringa seed amount on the removal of pollutants (color, turbidity, and COD) from wastewater were presented in Figure 3, Figure 4 A steep increase in removal efficiency of color at pH 9 and 3 with the dosage of from 0.1- 0.3 g/500 ml (Figure 3, Figure 4). Some gentle increment was observed in the removal efficiency of turbidity and COD with similar dosage and pH values (Figure 3, Figure 4). After 0.4 g/500 ml dosage of Moringa seed powder, the removal efficiency of color and turbidity was reduced, implying that more cation was present in the wastewater sample than anion. However, the COD removal efficiency was linearly increased up to the maximum dosage of Moringa seed powder (0.6 g/500 ml). This implied that more dosage was needed to attain the maximum removal efficiency of COD (Figure 3, Figure 4).
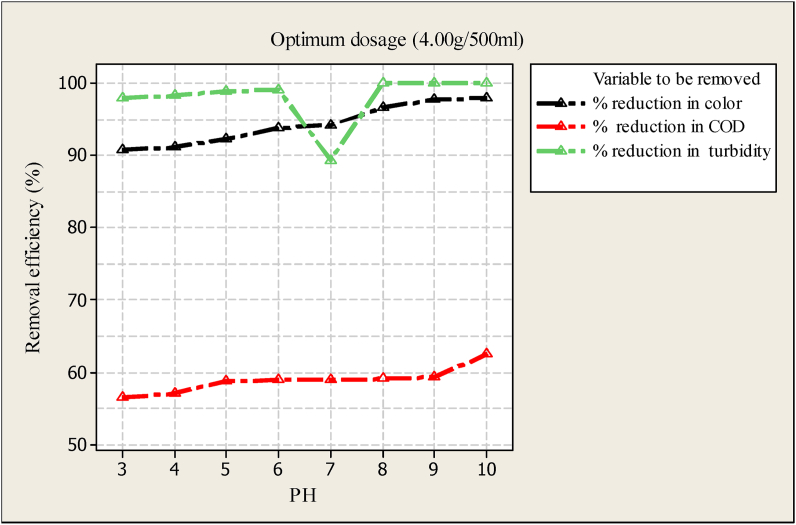
3.5.2. Effects of pH
As observed from Figure 7, pH has a significant impact on the removal of color, COD, and turbidity. At a similar value of Morniga seed powder (4 mg/L) and pH of 3, COD was reduced by 56.34 %, color was reduced to 91.56 % and turbidity was reduced to 98.02 %. As the pH value increases, the removal efficiency increases, and increasing up to 9 pH values. Furthermore, as the water becomes stronger with a base pH greater than 9, the removal efficiency becomes reduced. This may be attributed to the presence of more hydrogen ions at lower pH, resulting in increased uptake of wastewater components by coagulant surface. On the other hand, the presence of hydroxyl ions at higher pH may result in suppression of adsorption for all the wastewater components onto Moringa seed powder. This indicated that Moringa seed powder was effective in removing color, COD, and turbidity with a pH range of 7–9. It looks there is no significant difference in removal efficiency of both color and turbidity in the PH range of 7–9. However, when the pH was more than 9 the removal efficiency of Moringa seed powder affects significantly.
Figure 7.
Effect of pH for removal of color; COD and turbidity.
4. Conclusion
The Moringa is a multipurpose tree with a significant economic and societal value that is grown in almost every developing country. The southern part of Ethiopia is the major one in the country. It's viewed as a sustainable, effective, and appropriate water treatment technology. In addition to treating wastewater, its nutritional and medicinal application are very effective. The study proved that the use of Moringa seed powder in the reduction of color, turbidity, and COD is highly effective. The maximum removal of color and turbidity was achieved at 0.4 g per 500 ml of wastewater sample, but further increasing the dosage of Moringa seed powder above 0.4 gms led to a decrease in turbidity and color due to floc restabilization. In the case of the COD, further experimentation is needed by increasing the dosage of Moringa seed powder. The study also showed that Moringa seed powder has high removal efficiency in turbidity and color rather than COD. The application of RSM to optimize the coagulation process for wastewater treatment using Moringa powder was investigated. As the results showed that RSM was an effective method to optimize experimental parameters in the treatment of wastewater. The removal efficiency of Moringa seed powder depends on the pH value. The adsorption equilibrium of color, turbidity, and COD uptake capacity (qe) was found to decrease with an increased dosage of the adsorbent and best when using 0.1 g of Moringa oleifera seed powder The use of Moringa seed powder in water treatment as a coagulant could be applied both at household and industrial scale. For developing countries, Moringa oleifera should be used without any transformation to avoid extra expense which is not always granted for these countries. The benefits endowed with this tree need to be explored in all implications especially its economic importance going by profitability index.
Thus, due to the diverse use of this tree, the Ethiopian government, in particular, takes initiative to plant more and more trees in all areas and for increased economic efficiency, processing, packing, and preservation should be adopted and supported. More research should be undertaken using another part of this tree for different application areas. It is also suggested that increasing the dosage of Moringa and other natural coagulants, as well as experimenting with different ratios, be investigated to isolate various contaminants from water and wastewater.
Declarations
Author contribution statement
Wendesen Mekonin Desta & Million Ebba Bote: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors gratefully acknowledge Jimma University, Jimma Institute of Technology, Department of Water Supply and Environmental Engineering, Environmental Engineering Laboratory for supporting us in all cases.
References
- Abdelaal A.M. Eighth International Water Technology Conference. Vol. 8. 2004. Using a natural coagulant for treating wastewater; pp. 781–792. (August) [Google Scholar]
- Abiyu A., Yan D., Girma A., Song X., Wang H. Wastewater treatment potential of Moringa stenopetala over Moringa olifera as a natural coagulant, antimicrobial agent and heavy metal removals. Cogent Environ. Sci. 2018;4(1) [Google Scholar]
- Agbovi H.K., Wilson L.D. Optimisation of orthophosphate and turbidity removal using an amphoteric chitosan-based flocculant-ferric chloride coagulant system. Environ. Chem. 2019;16(8):599–612. [Google Scholar]
- Alo M.N., Anyim C., Elom M. Coagulation and antimicrobial activities of Moringa oleifera seed storage at 3°C temperature in turbid water. Adv. Appl. Sci. Res. 2012;3(2):887–894. http://www.pelagiaresearchlibrary.com/advances-in-applied-science/vol3-iss2/AASR-2012-3-2-887-894.pdf [Google Scholar]
- Asaithambi P., Aziz A.R.A., Daud W.M.A.B.W. Integrated ozone—electrocoagulation process for the removal of pollutant from industrial effluent: optimization through response surface methodology. Chem. Eng. Process: Process Intens. 2016;105:92–102. [Google Scholar]
- Atchudan R., Edison T.N.J.I., Perumal S., Muthuchamy N., Lee Y.R. Hydrophilic nitrogen-doped carbon dots from biowaste using dwarf banana peel for environmental and biological applications. Fuel. 2020;275(April):117821. [Google Scholar]
- Baptista A.T.A., Silva M.O., Gomes R.G., Bergamasco R., Vieira M.F., Vieira A.M.S. Protein fractionation of seeds of Moringa oleifera lam and its application in superficial water treatment. Separ. Purif. Technol. 2017;180:114–124. [Google Scholar]
- Bina B., Mehdinejad M.H., Dalhammer G., Rajarao G., Nikaeen M., Movahedian Attar H. Effectiveness of Moringa oleifera Coagulant Protein as natural coagulant aid in removal of turbidity and bacteria from turbid waters. World Acad. Sci. Eng. Tech. 2010;43(7):618–620. [Google Scholar]
- Chaouki Z., Hadri M., Nawdali M., Benzina M., Zaitan H. Treatment of a landfill leachate from Casablanca city by a coagulation-flocculation and adsorption process using a palm bark powder (PBP) Sci. Afr. 2021;12 e00721. [Google Scholar]
- Chitra D., Muruganandam L. Performance of natural coagulants on greywater treatment. Recent Innov. Chem. Eng. Formerly Recent Patents Chem. Eng. 2019;13(1):81–92. [Google Scholar]
- David C., Narlawar R., Arivazhagan M. Performance evaluation of Moringa oleifera seed extract (MOSE) in conjunction with chemical coagulants for treating distillery spent wash. Indian Chem. Eng. 2016;58(3):189–200. [Google Scholar]
- Eman N.A., Tan C.S., Makky E.A. Impact of Moringa oleifera cake residue application on wastewater treatment: a case study. J. Water Resour. Protect. 2014;6(7):677–687. [Google Scholar]
- Garde W.K., Buchberger S.G., Wendell D., Kupferle M.J. Application of Moringa oleifera seed extract to treat coffee fermentation wastewater. J. Hazard Mater. 2017;329:102–109. doi: 10.1016/j.jhazmat.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Ghebremichael K.A., Gunaratna K.R., Henriksson H., Brumer H., Dalhammar G. A simple purification and activity assay of the coagulant protein from Moringa oleifera seed. Water Res. 2005;39(11):2338–2344. doi: 10.1016/j.watres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Govindan N. Use of Moringa (Moringa stenopetala) seed extract for removal of some anionic dyes (direct and reactive dyes) in textile wastewater. Curr. Trends Fashion Tech. Textile Eng. 2018;4(4) [Google Scholar]
- Leite A.L.M.P., Zanon C.D., Menegalli F.C. Isolation and characterization of cellulose nanofibers from cassava root bagasse and peelings. Carbohydr. Polym. 2017;157:962–970. doi: 10.1016/j.carbpol.2016.10.048. [DOI] [PubMed] [Google Scholar]
- Leone A., Spada A., Battezzati A., Schiraldi A., Aristil J., Bertoli S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: an overview. Int. J. Mol. Sci. 2015;16(6):12791–12835. doi: 10.3390/ijms160612791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhokwe S., Zvidzai C. Post-treatment of yeast processing effluent from a bioreactor using aluminium chlorohydrate polydadmac as a coagulant. Sci. Afr. 2019;6:4–11. [Google Scholar]
- Naceradska J., Pivokonska L., Pivokonsky M. On the importance of pH value in coagulation. J. Water Supply Res. Technol. - Aqua. 2019;68(3):222–230. [Google Scholar]
- Nand V. Water purification using Moringa oleifera and other locally available seeds in Fiji for heavy metal removal. Int. J. Appl. Sci. Tech. 2012;2(5):125–129. [Google Scholar]
- Narmatha M., Sangavi S.K., Sripavithra G. Effluent treatment of sago wastewater by using natural coagulants. Imperial J. Interdiscipl. Res. 2017;3(9) 2454–1362. [Google Scholar]
- Okey-Onyesolu C.F., Chukwuma E.C., Okoye C.C., Onukwuli O.D. Response Surface Methodology optimization of chito-protein synthesized from crab shell in treatment of abattoir wastewater. Heliyon. 2020;6(10) doi: 10.1016/j.heliyon.2020.e05186. e05186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani N.Z.A., Husain K., Kumolosasi E. Moringa genus: a review of phytochemistry and pharmacology. Front. Pharmacol. 2018;9(FEB):1–26. doi: 10.3389/fphar.2018.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha V.V.F., dos Santos I.F.S., Silva A.M.L., Sant’Anna D.O., Junho A.L., Barros R.M. Clarification of high-turbidity waters: a comparison of Moringa oleifera and virgin and recovered aluminum sulfate-based coagulants. Environ. Dev. Sustain. 2020;22(5):4551–4562. [Google Scholar]
- Saleem M., Bachmann R.T. A contemporary review on plant-based coagulants for applications in water treatment. J. Ind. Eng. Chem. 2019;72:281–297. [Google Scholar]
- Scholes A., Adenike K., Aderonke O. Heliyon Ef fi cacy of a natural coagulant protein from Moringa oleifera ( Lam ) seeds in treatment of Opa reservoir water, Ile-Ife, Nigeria. Heliyon. 2020;6(December 2019):e03335. doi: 10.1016/j.heliyon.2020.e03335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta M.E., Keraita B., Olsen A., Boateng O.K., Thamsborg S.M., Pálsdóttir G.R., Dalsgaard A. Use of Moringa oleifera seed extracts to reduce helminth egg numbers and turbidity in irrigation water. Water Res. 2012;46(11):3646–3656. doi: 10.1016/j.watres.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Shan T.C., Matar M. Al, Makky E.A., Ali E.N. The use of Moringa oleifera seed as a natural coagulant for wastewater treatment and heavy metals removal. Appl. Water Sci. 2017;7(3):1369–1376. [Google Scholar]
- Sotheeswaran S., Nand V., Matakite M., Kanayathu K. Vol. 2015. 2011. Moringa oleifera and Other Local seeds in Water Purification in Developing countries. March. [Google Scholar]
- Srinu Naik S., Pydi Setty Y. Optimization of parameters using response surface methodology and genetic algorithm for biological denitrification of wastewater. Int. J. Environ. Sci. Technol. 2014;11(3):823–830. [Google Scholar]
- Subramonian W., Wu T.Y., Chai S.P. An application of response surface methodology for optimizing coagulation process of raw industrial effluent using Cassia obtusifolia seed gum together with alum. Ind. Crop. Prod. 2015;70:107–115. [Google Scholar]
- Trinh T.K., Kang L.S. Response surface methodological approach to optimize the coagulation-flocculation process in drinking water treatment. Chem. Eng. Res. Des. 2011;89(7):1126–1135. [Google Scholar]
- Ugwu S.N., Umuokoro A.F., Echiegu E.A., Ugwuishiwu B.O., Enweremadu C.C. Comparative study of the use of natural and artificial coagulants for the treatment of sullage (domestic wastewater) Cogent Eng. 2017;4(1):1–13. [Google Scholar]
- Vigneshwaran S., Karthikeyan P., Sirajudheen P., Meenakshi S. Optimization of sustainable chitosan/Moringa. oleifera as coagulant aid for the treatment of synthetic turbid water – a systemic study. Environ. Chem. Ecotoxicol. 2020;2:132–140. [Google Scholar]
- Wang Y., Chen K., Mo L., Li J., Xu J. Optimization of coagulation-flocculation process for papermaking-reconstituted tobacco slice wastewater treatment using response surface methodology. J. Ind. Eng. Chem. 2014;20(2):391–396. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.