Abstract
Using a yeast two-hybrid system, we isolated a novel human centrosomal protein, CPAP (centrosomal P4.1-associated protein), which specifically interacts with the head domain of the 135-kDa protein 4.1R isoform (4.1R-135). Sequence analysis revealed that the carboxyl terminus of CPAP has 31.3% amino acid identity with human Tcp-10 (a t-complex responder gene product). Interestingly, most of the sequence identity is restricted to two conserved regions. One carries a leucine zipper, which may form a series of heptad repeats involved in coiled-coil formation; the other contains unusual glycine repeats with unknown function. Immunofluorescence analysis revealed that CPAP and γ-tubulin are localized within the centrosome throughout the cell cycle. CPAP cosediments with γ-tubulin in sucrose gradients and coimmunoprecipitates with γ-tubulin, indicating that CPAP is a part of the γ-tubulin complex. Furthermore, functional analysis revealed that CPAP is localized within the center of microtubule asters and may participate in microtubule nucleation. The formation of microtubule asters was significantly inhibited by anti-CPAP antibody. Together, these observations indicate that CPAP may play an important role in cell division and centrosome function.
The erythrocyte protein 4.1 (4.1R), originally identified as an 80-kDa protein (4.1R-80) in human erythrocytes, plays a crucial role in the maintenance of red cell morphology and mechanical integrity through its binding of glycophorin C and band 3 proteins with the spectrin/actin-based cytoskeletal network. The importance of 4.1R-80 to the structural integrity of red blood cells is underscored by the abnormal erythrocyte phenotype seen in 4.1R-deficient patients (6) and in 4.1R-null mice (36). Deficiency of 4.1R-80 in red blood cells leads to assembly of an unstable cytoskeleton structure that manifests as hereditary elliptocytosis (HE). The red blood cells from HE patients are prone to fragmentation, resulting in different degrees of hemolytic anemia.
The major functions of 4.1R-80 in red blood cells have been well characterized. Limited chymotryptic digestion of erythroid 4.1R-80 generates four structural domains (30, 16, 10, and 22 to 24 kDa). The 30-kDa domain mediates the binding of 4.1R-80 to the plasma membrane via glycophorin C (25) and band 3 (20), whereas the 10-kDa domain contains the binding site for the spectrin and actin complex (7). Molecular characterization of 4.1R size variants in HE patients always involves rearrangements in the sequences encoding the spectrin-actin binding domain (4). This finding suggests that a particular 4.1R isoform retains a proper spectrin-actin binding domain and is functionally important for maintaining the correct morphology of mature red cells.
The initial picture of 4.1R-80 has subsequently become complicated by the identification of multiple 4.1R isoforms generated by extensive alternative RNA splicing in erythroid (3, 41) and nonerythroid (17, 42) cells. We previously reported that the 4.1R gene comprises at least 23 exons, including 13 constitutive exons and 10 alternative exons (17). Interestingly, different alternatively spliced 4.1R isoforms might possess different functions. For example, one 4.1R isoform (4.1R-80) inserts an exon 16-encoded peptide which is necessary for formation of the ternary complex with spectrin and actin in red blood cells (11, 15), while selective use of exon 2′, which carries an upstream translation initiation codon, may generate a 135-kDa 4.1R isoform (4.1R-135) that is predominantly expressed in nonerythroid cells (42).
Immunoreactive epitopes of 4.1R have been identified in cell-cell contact regions, stress fibers, nuclei, and centrosomes (1, 8, 21–23, 43). However, the functions of these 4.1R isoforms in nonerythroid cells are yet to be characterized. We previously isolated a lymphoid 4.1R isoform (4.1R-135), which was generated by an alternative mRNA splicing mechanism, that resulted in the addition of a 209-amino-acid head domain (HD) to the N terminus of erythroid 4.1R-80 (42). 4.1R-135 was reported to interact with the nuclear mitotic apparatus protein in the interphase nucleus and form a complex with components of the mitotic apparatus, indicating that 4.1R-135 may play a role in organizing the nuclear architecture and mitotic spindle poles (26).
To investigate the possible functions of 4.1R-135, we searched for proteins that interact with the head domain of 4.1R-135. Using a yeast two-hybrid system, we isolated a human cDNA clone encoding a novel centrosomal P4.1-associated protein (CPAP) that specifically binds to the head domain (residues 1 to 209) of 4.1R-135. Our results demonstrate that CPAP not only interacts with 4.1R-135 but also associates with the γ-tubulin complex. The so-called γ-tubulin ring complex (γ-TuRC), isolated from mitotic Xenopus egg extracts, possesses the ability to nucleate microtubules in vitro (47). γ-TuRC contains several proteins which may constitute a ring structure with a left-handed helical shape. In this report, the possible functions of 4.1R-135 and CPAP are discussed.
MATERIALS AND METHODS
Yeast two-hybrid screen.
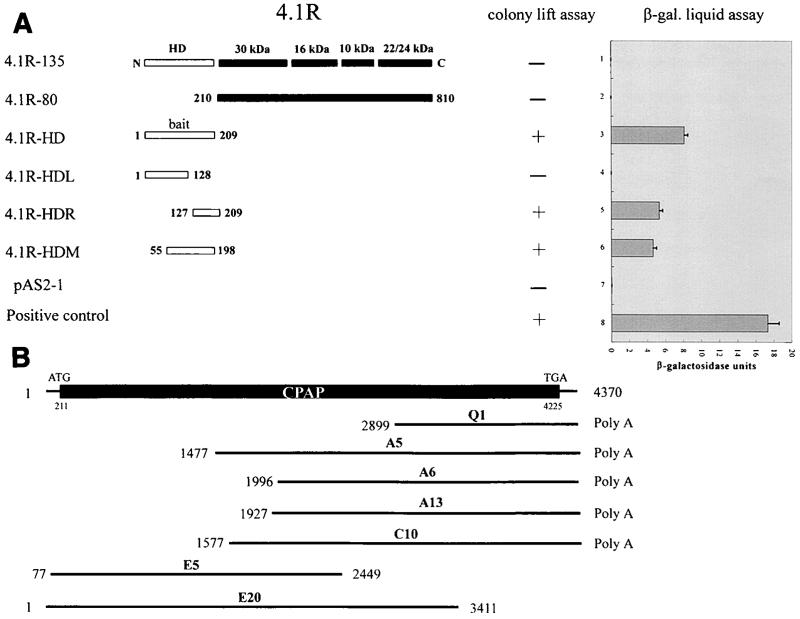
The yeast Matchmaker two-hybrid system (Clontech, Palo Alto, Calif.) was used to screen for proteins that interact with 4.1R-135. The head domain (HD; residues 1 to 209) of 4.1R-135 (4.1R-HD) was fused to the GAL4 DNA-binding domain (GAL4-DB) in vector pAS2-1 (Clontech). This construct was used as bait to screen a human lymphocyte cDNA library fused to a GAL4 activation domain (GAL4-AD) in the pACT2 vector (Clontech). The two types of plasmids were then cotransformed into Saccharomyces cerevisiae Y190, and the transformants were selected on SD minimal medium as previously described (40). Positive colonies were further tested for β-galactosidase activity using a colony-lift assay and liquid assay as described by the manufacturer (Clontech). To narrow down the head domain region of 4.1R (4.1R-HD) that binds to CPAP, constructs containing various portions of 4.1R-HD were fused to GAL4-DB of the pAS2-1 vector (Fig. 1A). The C terminus of CPAP (residues 897 to 1338) was subcloned into the pACT2 vector. Yeast cells (Y187) were simultaneously transformed with the above two constructs and assayed for β-galactosidase activity using a colony-lift assay or liquid assay as described above.
FIG. 1.
4.1R interacts with CPAP in a yeast two-hybrid system. The CPAP (Q1) clone was first isolated by a yeast two-hybrid screen from a human lymphocyte cDNA library using the head domain (residues 1 to 209) of 4.1R-135 as bait (4.1R-HD). (A) Mapping the region of 4.1R-135 that interacts with CPAP. The constructs containing various portions of 4.1R-135 fused in-frame to the Gal4 DNA-binding domain were cotransformed with a Q1-ACT2 clone that expressed CPAP (residues 897 to 1338) fused to the activation domain of Gal4. The expression (+) or nonexpression (−) of the lacZ reporter gene using a colony-lift assay is shown. The column on the right represents the liquid assay for β-galactosidase (β-gal) activity using ONPG as a substrate. (B) Schematic drawing of the overlapping CPAP cDNA clones that span the entire coding region of CPAP.
Isolation of CPAP cDNA clones and Northern blot analysis.
The initial CPAP cDNA clone (Q1) isolated by yeast two-hybrid screen was used as a probe to screen a human testis cDNA library (Clontech). Several overlapping cDNA clones that cover the entire coding region of CPAP were obtained (Fig. 1B). The conditions for screening and DNA sequencing were described previously (46). All DNA sequencing data were compiled and analyzed using the GCG software programs of the Wisconsin Sequence Analysis Package.
For RNA analysis, a blot filter (Clontech) with 2 μg of polyadenylated RNA from multiple human tissues was hybridized with a 32P-labeled CPAP cDNA probe (nucleotides [nt] 2899 to 3423) as previously described (46). The same probe was stripped and reprobed with β-actin cDNA to quantify RNA loading.
Antibody production.
Polyclonal antibodies against CPAP and the head domain of 4.1R-135 (anti-HD-4.1R) were raised in rabbits. The cDNAs encoding the C-terminal region of CPAP (cCPAP; residues 1070 to 1338) and the head domain (HD; residues 55 to 198) of 4.1R-135 were fused in frame to glutathione-S-transferase (GST) in pGEX-2T and to a His-tagged pQE32 expression vector, respectively. Overexpression and affinity purification of GST-cCPAP and His-tagged HD recombinant proteins were performed as previously described (40). Aliquots of GST-cCPAP and His-tagged HD recombinant proteins were mixed with complete Freund's adjuvant and separately injected into New Zealand White rabbits. After 4 weeks, the rabbits were boosted with GST-cCPAP or His-tagged HD mixed with incomplete adjuvant. The sera were affinity purified using GST-cCPAP or His-tagged HD immobilized on polyvinylidene difluoride (PVDF) membranes. Polyclonal antibody against GST-cCPAP was also raised in mice as previously described (16).
The immunization and generation of monoclonal antibody (MAb) anti-N-4.1R against the head domain (residues 55 to 198) of 4.1R-135 were performed as previously described (44). Polyclonal anti-C-4.1R antibodies against the C-terminal 22- to 24-kDa domain of 4.1R were raised in rabbits as previously described (16). The anti-Flag (M5), anti-γ-tubulin (GTU88), and anti-α-tubulin (N356) MAbs were purchased from Kodak (Eastman Kodak Company, New Haven, Conn.), Sigma (Saint Louis, Mo.), and Amersham (Amersham Pharmacia Biotech, Piscataway, N.J.), respectively.
Cell culture, transfection, and Western blot analysis.
SiHa (a human cervical carcinoma cell line) cells were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum. Molt4 cells (a human leukemia cell line) were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum. The cDNA encoding the full-length human CPAP was subcloned in-frame into a cytomegalovirus promoter-driven FLAG epitope-tagged expression vector. SiHa cells (5 × 106) were transiently transfected with 10 μg of FLAG-tagged CPAP plasmid as previously described (40). For Western blot analysis, the cell extracts prepared from the indicated cells or tissues were separated by sodium dodecyl sulfate–7.5% polyacrylamide gel electrophoresis (SDS-PAGE), blotted onto a PVDF membrane, and probed with the antibodies indicated in Fig. 5 as previously described (40). The immunoreactive proteins were visualized using an enhanced chemiluminescence detection system (Pierce, Rockford, Ill.).
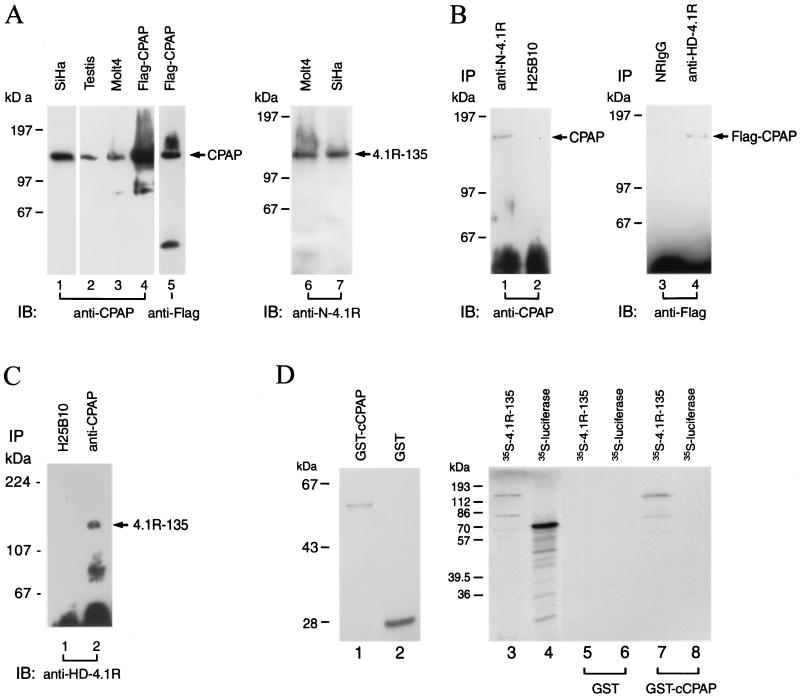
FIG. 5.
Direct association of 4.1R-135 with CPAP in vivo and in vitro. (A) Characterization of anti-CPAP and anti-N-4.1R antibodies. The production of antibodies against the C terminus of CPAP (anti-CPAP, a polyclonal antibody) and the N-terminal head domain of 4.1R-135 (anti-N-4.1R MAb) is described in the text. SiHa cells were transiently transfected with a FLAG-tagged CPAP plasmid. The cell lysates (∼50 μg) prepared from mouse testis, untransfected cells (SiHa and Molt4), and transfected SiHa cells, as indicated, were separated by SDS-PAGE and immunoblotted with anti-CPAP (lanes 1 to 4), anti-FLAG (lane 5), or anti-N-4.1R (lanes 6 and 7) antibodies. (B) Direct association of 4.1R-135 with CPAP in vivo. The cell lysates prepared from SiHa cells were immunoprecipitated (IP) with anti-N-4.1R MAb (lane 1) or a nonrelevant MAb (H25B10, lane 2). The immunoprecipitated protein complexes were then analyzed by immunoblotting (IB) with anti-CPAP antibody. Furthermore, the cell lysates prepared from FLAG-tagged CPAP-transfected cells were immunoprecipitated with NRIgG (lane 3) or anti-HD-4.1R (a polyclonal antibody against the head domain of 4.1R-135; lane 4) and reprobed with anti-FLAG MAb. (C) Reverse immunoprecipitation. The cell lysates prepared from SiHa cells were immunoprecipitated with H25B10 (lane 1) or mouse anti-CPAP (lane 2) antibody and analyzed by immunoblotting with rabbit anti-HD-4.1R antibody. (D) Direct association of 4.1R-135 and CPAP in vitro. The cDNA encoding the C-terminal domain of CPAP (cCPAP) was subcloned into the pGEX-2T vector and expressed as GST-cCPAP fusion protein in E. coli. Purified GST-cCPAP (lane 1; 2 μg) and GST (lane 2; 10 μg) were visualized by Coomassie blue staining. [35S]methionine-labeled 4.1R-135 (lane 3) and [35S]methionine-labeled luciferase (lane 4) were incubated with affinity-purified GST (lanes 5 and 6; 10 μg) or GST-cCPAP (lanes 7 and 8; 10 μg). The bound complexes were analyzed by SDS-PAGE and autoradiography. Radiolabeled 4.1R-135 bound to GST-cCPAP fusion protein (lane 7) but not GST alone (lane 5). [35S]methionine-labeled luciferase and GST were used as negative controls.
Immunoprecipitation analysis.
Coimmunoprecipitation of endogenous 4.1R-135 and CPAP in vivo was performed in SiHa cells (see Fig. 5B, lanes 1 and 2). Cells were lysed in EBC buffer (50 mM Tris-HCl [pH 8.0], 120 mM NaCl, 0.5% NP-40, and 1 mM phenylmethylsulfonyl fluoride [PMSF] plus aprotinin and leupeptin [1 μg/ml each]), and the soluble supernatant was collected by centrifugation at 16,000 × g for 5 min at 4°C. The supernatant was precleared by protein G-Sepharose beads, immunoprecipitated with anti-N-4.1R MAb or a nonrelevant MAb for 2 h at 4°C, and incubated with protein G-Sepharose beads for an additional 1 h. Immunoprecipitates were then washed three times with EBC buffer and twice with phosphate-buffered saline (PBS). The samples were resuspended in 10 μl of SDS sample buffer (50 mM Tris-HCl [pH 6.8], 2% SDS, 5% 2-mercaptoethanol, 0.1% bromophenol blue, and 10% glycerol) and heated at 98°C for 5 min. The samples were then centrifuged, and the supernatants were separated by SDS–7.5% PAGE. After transfer to a PVDF membrane, immunoreactive proteins were detected by rabbit anti-CPAP polyclonal antibody. For coimmunoprecipitation (see Fig. 5B, lanes 3 and 4), cell lysates prepared from FLAG-tagged CPAP-transfected SiHa cells were immunoprecipitated with normal rabbit immunoglobulin G (NRIgG) or anti-HD-4.1R antibody, a rabbit polyclonal antibody against the head domain (residues 55 to 198) of 4.1R-135. Immunoprecipitates were then immunoprobed with anti-FLAG MAb under the conditions described above. For reverse immunoprecipitation (see Fig. 5C), the cell lysates were immunoprecipitated with mouse anti-CPAP and analyzed by immunoblotting with rabbit anti-HD-4.1R antibody.
Similarly, coimmunoprecipitation of endogenous CPAP and γ-tubulin (see Fig. 7D) was performed using the conditions described above. Cell lysates prepared from SiHa cells were first immunoprecipitated with NRIgG or rabbit anti-CPAP antibody. Immunoprecipitates were then resuspended in SDS nonreducing buffer (without 2-mercaptoethanol) and immunoprobed with anti-γ-tubulin MAb GTU88. The specific association between CPAP and γ-tubulin in vivo was further confirmed by reverse immunoprecipitation. The cell lysates were first immunoprecipitated with anti-γ-tubulin MAb and then immunoprobed with rabbit anti-CPAP antibody.
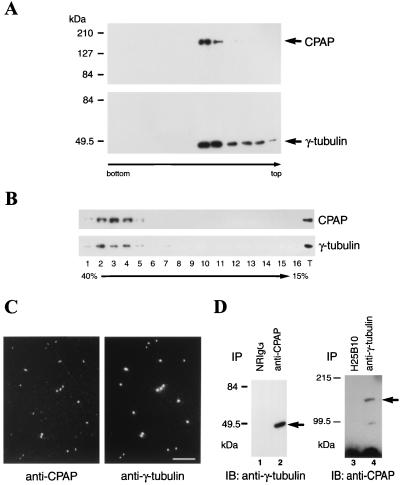
FIG. 7.
Cytosolic and centrosomal forms of CPAP are associated with γ-tubulin. (A) CPAP and γ-tubulin are present in the centrosomes. The centrosome fractions of Molt4 cells were prepared by discontinuous sucrose density gradient (70, 50, and 40% sucrose solutions). Gradient fractions were immunoblotted with either anti-CPAP (upper) or anti-γ-tubulin (lower) antibodies. (B) CPAP and γ-tubulin cosedimented in the same cytosolic fractions. The cytosolic fractions prepared from Molt4 cells were loaded on a 15 to 40% sucrose linear gradient as described in the text. After centrifugation, the fractions were immunoprobed with anti-CPAP and anti-γ-tubulin antibodies. The lane marked T represents a positive control by immunoblotting of the Molt4 cell cytosolic extracts with anti-CPAP and anti-γ-tubulin antibodies. (C) Colocalization of CPAP and γ-tubulin in the isolated centrosomes. The centrosome fractions in panel A were spun onto acid-treated coverslips for immunofluorescence analysis. The coverslips were then double stained with anti-CPAP (left) and anti-γ-tubulin (right) antibodies. Bar, 10 μm. (D) Coimmunoprecipitation of CPAP and γ-tubulin in vivo. The cell extracts of SiHa cells were immunoprecipitated (IP) with NRIgG (lane 1) or anti-CPAP (lane 2) antibody. The bound protein complexes were analyzed by immunoblotting (IB) with anti-γ-tubulin antibody. To further confirm the association between CPAP and γ-tubulin, the cell lysates were first immunoprecipitated with anti-γ-tubulin MAb (lane 4) or a control hybridoma (H25B10, lane 3). The immunoprecipitated complexes were then reprobed with anti-CPAP antibody. Both results showed that CPAP and γ-tubulin associate in vivo.
In vitro binding assay.
The cDNA that encodes the C-terminal domain of CPAP (cCPAP; residues 1070 to 1338) was constructed in-frame in the pGEX-2T vector. The recombinant GST-cCPAP fusion proteins were expressed in Escherichia coli and purified on glutathione-agarose beads as previously described (40). The cDNA encoding 4.1R-135 was constructed in a pSG5 vector. The luciferase cDNA was purchased from Promega (Madison, Wis.). Synthetic sense-capped mRNAs were generated from the T7 promoter within the vectors. The sense mRNAs for 4.1R-135 and luciferase were translated in a coupled in vitro transcription-translation system (TNT rabbit reticulocyte lysate; Promega) in the presence of [35S]methionine to radiolabel newly synthesized proteins.
Equal portions of the labeled proteins were incubated with affinity-purified GST or GST-cCPAP fusion proteins as described (16). After incubation, the immobilized complexes were washed four times with bead binding buffer, and the bound protein complexes were analyzed on an SDS–12% PAGE gel and visualized by autoradiography (16).
Fluorescence and confocal microscopy.
Confocal fluorescence microscopy was performed as previously described (44). SiHa cells were grown on coverslips and fixed in methanol-acetone (1:1, vol/vol) at −20°C for 10 min. The fixed cells were probed with affinity-purified anti-CPAP antibody and anti-γ-tubulin MAb (see Fig. 6A) or probed with anti-C-4.1R antibody and anti-γ-tubulin MAb (Fig. 6B). The bound antibodies were detected with Alexa 488, a green fluorophore-conjugated goat anti-rabbit IgG, and Cy5, a cyanine-conjugated goat anti-mouse IgG (Molecular Probes, Molecular Research Center, Inc., Cincinnati, Ohio). DNA was stained with propidium iodide (Sigma, Saint Louis, Mo.). Coverslips were mounted and observed using a laser scanning confocal system (MRC 1000; Bio-Rad Laboratories).
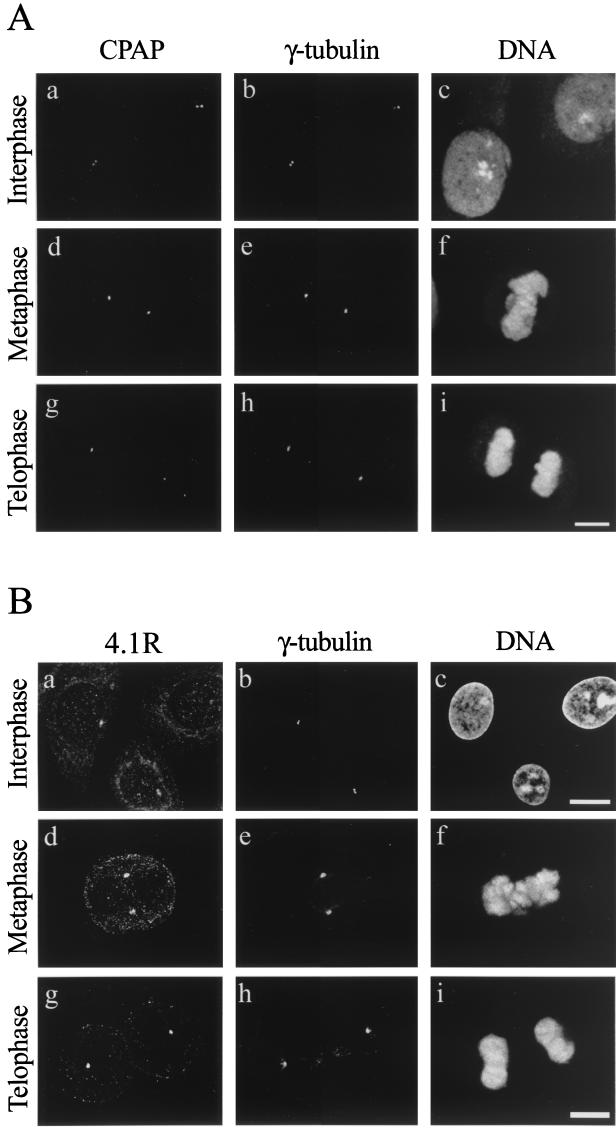
FIG. 6.
(A) Confocal microscopy analysis of the subcellular localization of human CPAP and γ-tubulin in various cell dividing stages. SiHa cells were triply stained with affinity-purified anti-CPAP antibody, a γ-tubulin MAb, and a DNA-specific dye, propidium iodide. (B) Triple immunostaining of SiHa cells with anti-C-4.1R and γ-tubulin antibodies. DNA was stained by propidium iodide. Each image represents a confocal optic section, and only the sections that revealed the centrosomal localization were selected and composed in panel B. By adjusting the focus, 4.1R was seen in the nucleus as well as in the cytosol (which are located at different focal planes). Bars, 10 μm.
The effects of microtubules in the centrosomal association of CPAP were analyzed using nocodazole or cold treatment conditions to disturb the microtubule network (see Fig. 9). The conditions for treatment of SiHa cells with nocodazole (10 μM at 37°C for 4 h [18]) or taxol (5 μM at 37°C for 4 h [14]) were as previously described. Cold treatment was performed by placing the cells on ice for 60 min. After cold treatment, the cold medium was replaced with warm medium (30°C), and cells were incubated at 30°C for an additional 2 or 10 min. Cells were then fixed and stained with anti-CPAP antibody and anti-α-tubulin MAb. The bound anti-CPAP and anti-α-tubulin antibodies were detected with Alexa 488 and Alexa 594 (a Texas Red-conjugated goat anti-mouse IgG), respectively.
FIG. 9.
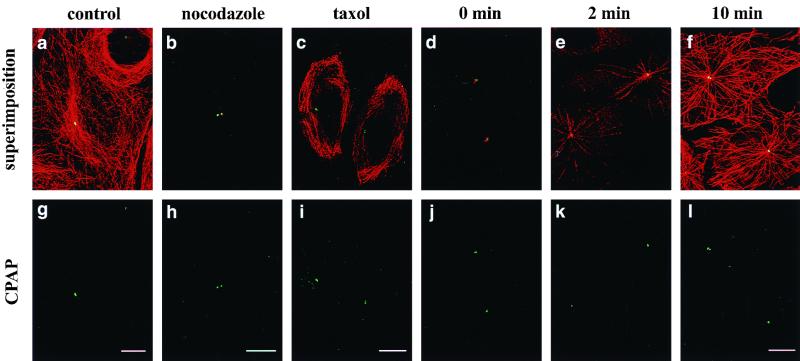
Association of CPAP with the centrosome is independent of the presence of microtubules. SiHa cells growing on coverslips were untreated (a and g), treated with nocodazole (b and h) or taxol (c and i), or incubated for 60 min on ice (d and j). Cells were then fixed and doubly stained with anti-CPAP (green) and anti-α-tubulin MAb (red). After cold treatment, the cold medium was replaced with warm medium (30°C), and the cells were incubated for another 2 min (e and k) or 10 min (f and l). Microtubules recovered from cold treatment were analyzed by immunofluorescence assay using anti-α-tubulin antibody. CPAP is strongly associated with the centrosome despite cold, nocodazole, or taxol treatment. Bars, 10 μm.
Isolation and analysis of centrosomes.
Isolation of human centrosomes from Molt4 cells was carried out following protocols previously described (29). Briefly, nocodazole-treated Molt4 cells (∼109) were used for centrosome isolation. About ∼150 mg of protein was applied to a discontinuous sucrose gradient using the procedure described previously (29). Gradient fractions were immunoblotted with anti-CPAP or anti-γ-tubulin (GTU88) antibodies (Fig. 7A). For immunofluorescence analysis of centrosomes (Fig. 7C), the centrosome-containing fractions were spun onto acid-treated coverslips and probed with anti-CPAP and anti-γ-tubulin (GTU88) antibodies using protocols described previously (29).
Preparation of cytosolic cell extracts.
The cytosolic extracts were prepared as described by Tassin et al. (45). Briefly, nocodazole-treated Molt4 cells were disrupted by a homogenizer in cold KHM buffer (50 mM HEPES [pH 7.4], 78 mM KCl, 1 mM MgCl2, 1 mM dithiothreitol, and 1 mM PMSF, plus aprotinin and leupeptin [1 μg/ml each]). The homogenate was centrifuged at 150,000 × g in an ultracentrifuge (Beckman Instruments, Fullerton, Calif.) with a swinging SW50.1 rotor for 30 min at 4°C. The supernatant, representing the cytosolic fraction, was then applied to a 15 to 40% sucrose linear gradient and centrifuged using a swinging bucket rotor (model SW41) at 100,000 × g for 16 h. To improve the resolution, the γ-tubulin-enriched fractions were loaded on a second sucrose gradient (15 to 40%) as described (45). The gradient was centrifuged at 100,000 × g for 16 h. After centrifugation, the fractions collected from the gradient were probed with anti-CPAP and anti-γ-tubulin antibodies (Fig. 7B).
Microtubule nucleation test.
The microtubule nucleation activities of isolated centrosomes were analyzed according to Mitchison and Kirchner (27). The centrosome-containing fractions (∼5 μl) were incubated in 60 μl of RG1 solution (80 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid), pH 6.8], 1 mM MgCl2, 1 mM EGTA, and 1 mM GTP) containing 125 μg of bovine brain tubulin (Molecular Probes) for 8 min at 37°C. Microtubules were fixed by adding glutaraldehyde (1%), sedimented onto acid-treated coverslips, and then subjected to immunofluorescence analysis using affinity-purified anti-CPAP polyclonal antibody and anti-α-tubulin MAb N356 (Amersham, Piscataway, N.J.) as described above.
To test the effect of anti-CPAP antibody on microtubule nucleation (Fig. 8), the centrosome-containing fractions were preincubated with affinity-purified anti-CPAP antibody for 30 min at 4°C. Anti-γ-tubulin antibody and NRIgG were used as a positive and negative control, respectively, for assaying microtubule nucleation. Microtubules were allowed to regrow for 4 min at 37°C. Centrosomes were detected with anti-CPAP antibody, and microtubules were visualized using anti-α-tubulin MAb.
FIG. 8.
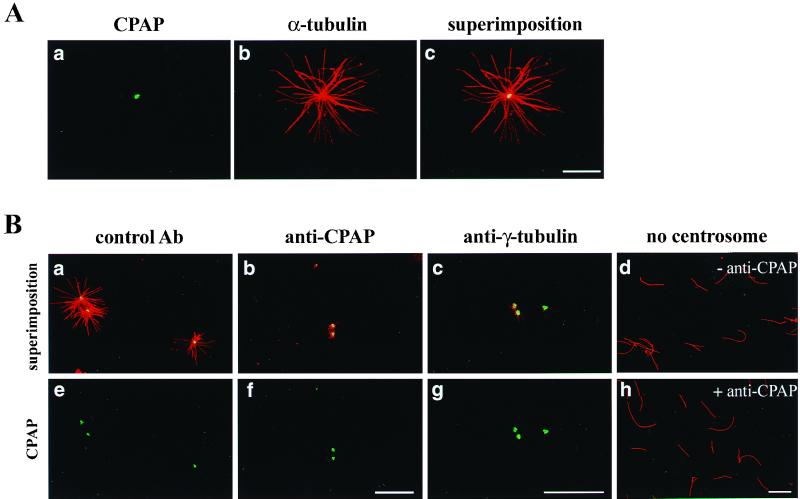
(A) In vitro microtubule nucleation assay. The isolated centrosomes (see Fig. 7C) were analyzed for their ability to form microtubule asters as described in the text. In vitro-nucleated microtubules were spun down, fixed, and then double stained with anti-CPAP (a) and anti-α-tubulin (b) antibodies. Superimposed photographs a and b are shown in c. Bar, 10 μm. (B) Inhibition of microtubule nucleation by anti-CPAP and anti-γ-tubulin antibodies. The isolated centrosomes (Fig. 7C) were preincubated with control antibody (a and e), affinity-purified anti-CPAP antibody (b and f), or anti-γ-tubulin antibody (c and g) for 30 min at 4°C, and then tubulin was added to start in vitro microtubule nucleation. The microtubule asters were doubly stained with affinity-purified anti-CPAP antibody (green) and anti-α-tubulin MAb (red). The two staining patterns were superimposed and are shown in a, b, and c. An in vitro microtubule-nucleating assay in the absence of isolated centrosomes with (h) or without (d) the addition of anti-CPAP antibody was used as a negative control. The microtubules were stained by anti-α-tubulin antibody (red). Bars, 10 μm.
RESULTS
Screening for proteins that interact with the head domain of 4.1R (4.1R-HD).
We previously showed that the 30-kDa domain of 4.1R-80 interacts with pICln, a protein involved in cellular volume regulation, in a yeast two-hybrid screen (40). In the present study, we set out to identify proteins that interact with the head domain (residues 1 to 209) of the 135-kDa 4.1R isoform (4.1R-HD). The 4.1R-HD cDNA, fused in-frame to the Gal4 DNA-binding domain (Gal4-BD), was used as bait (Fig. 1A) to screen a human lymphocyte cDNA library previously ligated to a Gal4 activation domain (Gal4-AD). A total of 3 × 106 colonies were screened, and five positive clones were obtained. Sequence analyses revealed that four of these five clones represented the same cDNA encoding a portion of CPAP, while the other was confirmed later to be a false-positive clone. The largest positive clone (Q1) identified here is 1,472 bp in length and contains an open reading frame encoding the carboxyl terminus of CPAP (Fig. 1B).
To determine the region of 4.1R-HD that specifically interacts with CPAP, constructs containing different portions of 4.1R in the pAS2-1 vector and CPAP (Q1) in the pACT2 vector were cotransformed into yeast cells and subjected to a colony-lift assay. As shown in Fig. 1A, CPAP interacted with 4.1R-HD (residues 1 to 209), 4.1R-HDR (residues 127 to 209), and 4.1R-HDM (residues 55 to 198), but not with 4.1R-HDL, 4.1R-80, 4.1R-135, or the pAS2-1 vector alone. These results were further evident with the more sensitive liquid assay for β-galactosidase activity using ONPG (o-nitrophenyl-β-d-galactopyranoside) as the substrate. The negative interaction between 4.1R-135 and CPAP could be due to the steric hindrance caused by the large size (135 kDa) of 4.1R-135, rendering it unable to interact with CPAP in a yeast two-hybrid screen. Indeed, our coimmunoprecipitation experiment demonstrated that the intact 4.1R-135 (135 kDa) did interact with CPAP in vivo (see Fig. 5B and 5C). We concluded from these results that the 71 amino acids derived from the head domain (residues 127 to 198) of 4.1R-135 contain the binding site for CPAP.
Primary structure of CPAP and Northern blot analysis.
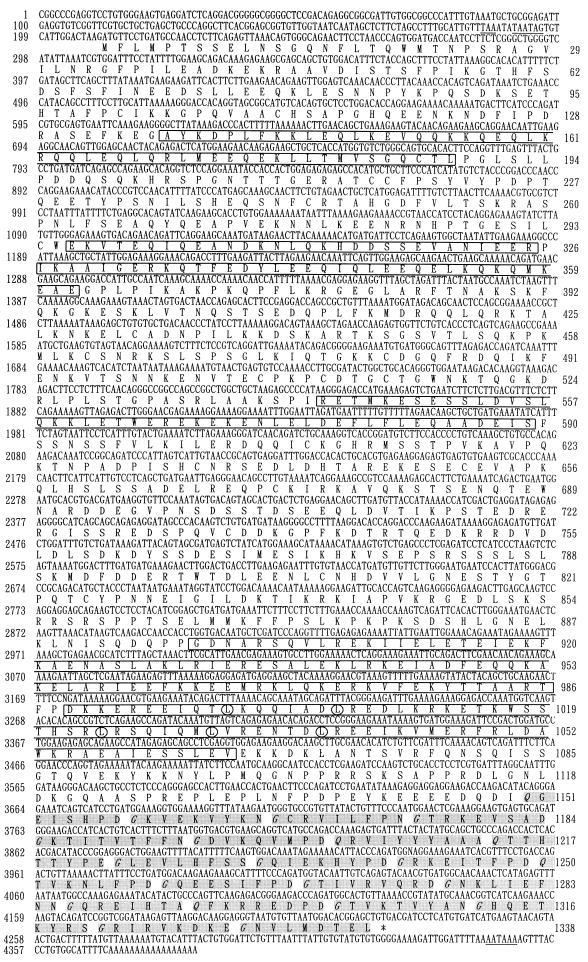
To obtain cDNA clones that cover the entire coding region of CPAP, the Q1 cDNA was used as a probe to screen a human testis cDNA library. Of 106 phages screened, several positive clones were identified (Fig. 1B). Sequence analysis revealed that these positive clones are overlapping cDNA clones which share portions of the identical sequence of CPAP. The nucleotide and deduced amino acid sequences of CPAP were thus assembled from these overlapping cDNA clones and are depicted in Fig. 2. Analysis of the CPAP cDNA revealed a nucleotide sequence of 4,370 bases that contains a single open reading frame (ORF) of 1,338 amino acids, with a predicted molecular mass of 153,012 Da and a pI of 6.23. This ORF starts at position 211 with an in-frame ATG and ends with a translation stop codon, TGA, located at nt 4225. The first ATG codon is preceded by several in-frame translation terminators. A potential polyadenylation signal, AATAAA, is present 21 nt upstream of the poly(A) sequence (Fig. 2).
FIG. 2.
Nucleotide and deduced amino acid sequences of human CPAP. The upstream in-frame stop codons and a potential polyadenylation signal (AATAAA) are underlined. Conserved leucine (L) residues in a leucine zipper motif are circled. The G repeats are highlighted in gray. Conserved glycine (G) and glutamine (Q) residues in G repeats are italicized. The predicted coiled-coil domains are boxed. The sequence data are available from GenBank under accession number AF139625.
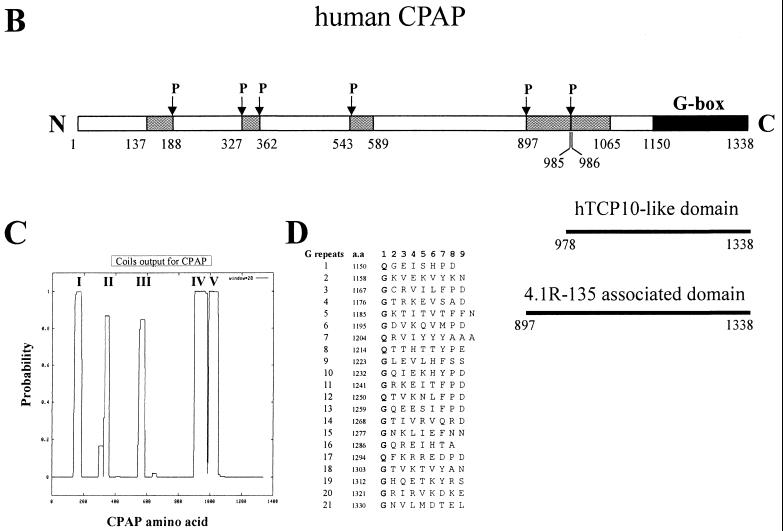
A search of GenBank showed that the carboxyl terminus of CPAP (residues 978 to 1338) has 31.3% identity (76.6% homology) with human Tcp10 (residues 29 to 392), a human t-complex responder gene product (19) (Fig. 3A). Interestingly, most of the sequence identity is restricted to the two conserved regions of Tcp10. The first conserved region contains a leucine zipper, which may form a series of heptad repeats and is involved in coiled-coil formation (Fig. 3A). The second conserved region contains 21 short nonamer motifs with an average occurrence of nine residues in the C terminus of CPAP (Fig. 3A and D). Interestingly, approximately 76% of the amino acids in the first position of this nonamer motif are glycine (G), while the other 24% are glutamine (Q) (Fig. 3D). We thus refer to these nonamer repeats as glycine repeats (G repeats or G-box). In addition to these repeats, other hydrophobic heptad repeats are spread into the CPAP protein. These heptad repeats are predicted to fold into five coiled-coil configurations (Fig. 3C), which are punctuated by a series of helix-disrupting proline residues (Fig. 3B). The CPAP protein sequence also possesses a number of other interesting sequence motifs. For example, there are four potential phosphorylation sites for cyclic AMP-dependent protein kinase at positions 257 to 260, 334 to 337, 439 to 442, and 1012 to 1015; 23 sites for protein kinase C; and 35 sites for casein kinase II.
FIG. 3.
(A) Alignment of amino acid sequences of human CPAP (this report) and human Tcp10 (19) (GenBank accession number U03399). Amino acid identity is indicated by vertical bars, and conserved changes are indicated by colons. Conserved leucine (L) residues in a leucine zipper motif are boxed. Conserved glycine (G) and glutamine (Q) residues in G repeats are highlighted in gray. The positions of G repeats are underlined. (B) Schematic representation of the structural domain of human CPAP. The regions of CPAP predicted to form five α-helical coiled-coil structures are indicated as boxes. P, proline. The proline residue usually disrupts the coiled-coil secondary structure. (C) The probability of five α-helical coiled-coil structures calculated for CPAP (24). (D) The G repeats of CPAP. The amino acid (a.a.) sequence of the G repeats at the C terminus of CPAP (residues 1150 to 1338) is presented in single-letter code.
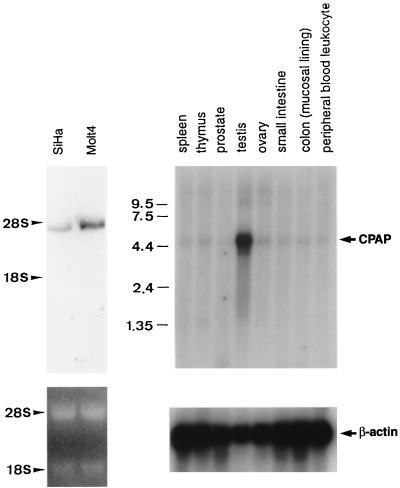
To examine the expression pattern of CPAP, blotted filters with mRNAs from various human tissues and cell lines (SiHa and Molt4) were probed with the CPAP cDNA (nt 2899 to 3423). Northern blot analysis revealed a major 4.5-kb transcript with different degrees of intensity present in all tissues and cell lines (SiHa and Molt4) examined here. The CPAP transcript was predominantly expressed in human testis (Fig. 4).
FIG. 4.
Expression of CPAP mRNA. (Right) Blotted filters (Clontech) with 2 μg of polyadenylated RNA from various human tissues were hybridized with a 32P-labeled human CPAP cDNA probe (nt 2899 to 3423). The same blot was stripped and reprobed with β-actin to quantify RNA loading. (Left) Total RNA (20 μg) extracted from SiHa and Molt4 cells was blotted on a nylon membrane and hybridized with a 32P-labeled CPAP cDNA probe described above. Ethidium bromide staining of the 18S and 28S rRNA was used as a quantitative control. The exposure time for the blots that hybridized with the CPAP cDNA probe was 18 h. Sizes are shown in kilobases.
CPAP and 4.1R-135 associate in vivo and in vitro.
Polyclonal antibody (anti-CPAP) against the C terminus (residues 1070 to 1338) of CPAP and MAb (anti-N-4.1R) against the N-terminal head domain (residues 55 to 198) of the 135-kDa 4.1R isoform were raised to characterize the interaction between CPAP and 4.1R-135 (see Materials and Methods). To test the specificity of the anti-CPAP antibody, cell extracts prepared from SiHa, Molt4, testis (mouse), and SiHa cells transfected with a FLAG-tagged CPAP plasmid were subjected to immunoblot analysis using affinity-purified anti-CPAP or anti-FLAG antibodies. As shown in Fig. 5A, the anti-CPAP antibody recognized a single high-molecular-mass 153-kDa polypeptide in mouse testis (lane 2), as well as in whole-cell extracts of SiHa (lane 1) and Molt4 (lane 3) cells. In transfected SiHa cells, the FLAG-tagged CPAP protein was detected by both anti-CPAP (lane 4) and anti-FLAG (lane 5) antibodies. Similarly, the specificity of anti-N-4.1R antibody was demonstrated by Western blot analysis. A major immunoreactive band corresponding to the 135-kDa 4.1R isoform was detected in the lysates from Molt4 (lane 6) and SiHa (lane 7) cells.
To examine the direct association between CPAP and 4.1R-135 in vivo, we performed a coimmunoprecipitation assay. The cell lysates prepared from SiHa cells were first immunoprecipitated with either anti-N-4.1R MAb or a control hybridoma (H25B10). The precipitated protein complexes were then immunoblotted with an anti-CPAP polyclonal antibody. As shown in Fig. 5B, no endogenous CPAP was detected when control hybridoma was used for the immunoprecipitation experiment (lane 2), while CPAP was coprecipitated with anti-N-4.1R MAb and detected by anti-CPAP antibody (lane 1). The specific interaction between CPAP and 4.1R-135 was further confirmed when we transiently transfected FLAG-tagged CPAP into SiHa cells (Fig. 5B, lanes 3 and 4). The cell extracts prepared from transfected cells were first immunoprecipitated with a polyclonal antibody (anti-HD-4.1R) against the head domain (residues 55 to 198) of 4.1R-135, and the membrane blots were probed with an MAb against the FLAG peptide. Our results showed that FLAG-tagged CPAP formed a complex with endogenous 4.1R-135 (Fig. 5B, lane 4; anti-HD-4.1R). However, no such complex was detected in the presence of NRIgG (Fig. 5B, lane 3). The in vivo association of CPAP and 4.1R-135 was further confirmed by reverse immunoprecipitation (Fig. 5C). The cell lysates were first immunoprecipitated with either control antibody (5C, lane 1; H25B10) or mouse anti-CPAP antibody (5C, lane 2), followed by immunoblotting of coprecipitated proteins with rabbit anti-HD-4.1R antibody. As shown in Fig. 5C, CPAP was pulled down by anti-CPAP antibody (lane 2), but not by a control antibody (lane 1). The low-molecular-weight band is likely a degradation product of 4.1R-135. Taken together, these results demonstrate a direct association between CPAP and 4.1R-135 in vivo.
The direct interaction between 4.1R-135 and CPAP was further analyzed using an in vitro binding assay. The cDNA encoding the C-terminal domain of CPAP (cCPAP; residues 1070 to 1338) was subcloned into the pGEX-2T vector. Both GST (Fig. 5D, lane 2) and GST-cCPAP (Fig. 5D, lane 1) fusion proteins were expressed and purified with glutathione-agarose beads. Affinity-purified GST and GST-cCPAP were incubated with [35S]methionine-labeled 4.1R-135 or luciferase, and the bound complexes were analyzed by SDS-PAGE. As shown in Fig. 5D, GST-cCPAP specifically interacted with labeled 4.1R-135 (Fig. 5D, lane 7) but not with a nonrelevant luciferase protein (Fig. 5D, lane 8). In contrast, the control GST fusion protein failed to bind to either labeled 4.1R-135 (Fig. 5D, lane 5) or labeled luciferase (Fig. 5D, lane 6). Consistent with the yeast two-hybrid assays and coimmunoprecipitation experiments, these results indicated that 4.1R-135 specifically interacts with CPAP.
Intracellular localization of CPAP.
To study the intracellular localization of CPAP, SiHa cells were triply stained with affinity-purified anti-CPAP antibody (Fig. 6A, left panel), MAb to γ-tubulin (Fig. 6A, middle panel), and a DNA dye (propidium iodide) (Fig. 6A, right panel). Both CPAP and γ-tubulin were prominently labeled as a pair of small round dots in interphase cells (a and b). When the cells entered metaphase, CPAP (d) and γ-tubulin (e) concentrated in the center of the mitotic spindle poles. As the cells progressed through metaphase to telophase, the prominently stained round dots remained in the centrosome (g and h). These immunofluorescence results indicated that CPAP was colocalized with γ-tubulin in all of the stages described above. Because γ-tubulin is a well-known centrosomal protein (38), we concluded that CPAP and γ-tubulin were colocalized within the centrosome throughout the cell cycle.
It was recently reported that some 4.1R epitopes were detectable in the pericentriolar material (PCM) region of the centrosome (21). Consistent with this finding, our immunofluorescence analysis revealed that γ-tubulin and some 4.1R epitopes localize predominantly to similar areas of the PCM throughout the cell cycle (Fig. 6B).
CPAP is associated with γ-tubulin in vivo in both the centrosomal and cytosolic fractions.
To determine whether CPAP can interact with essential centrosomal proteins, particularly with those responsible for the microtubule-nucleating activity in the centrosome, e.g., γ-tubulin (31), centrosome fractions were prepared from Molt4 cells using sucrose density gradient centrifugation as described in Materials and Methods. Immunoblotting analysis revealed that both CPAP and γ-tubulin cosedimented with the centrosomal fractions (Fig. 7A). The isolated centrosomes were further analyzed by immunofluorescence experiments using antibodies against CPAP as well as against γ-tubulin. As shown in Fig. 7C, both proteins colocalized to the isolated centrosome. These results indicate that CPAP, like γ-tubulin, is an intrinsic centrosomal component.
Previous studies showed that a major portion of γ-tubulin is soluble and exists in the form of the cytosolic γ-tubulin complex in animal cells (13, 28, 39). We examined whether CPAP and γ-tubulin are components of the cytosolic γ-tubulin complex. The cytosolic extracts of human Molt4 cells were fractionated on 15 to 40% sucrose gradients and probed with antibodies against CPAP and γ-tubulin as described in Materials and Methods. As shown in Fig. 7B, both CPAP and γ-tubulin sedimented with the same velocity in the sucrose gradients.
The physical association between CPAP and γ-tubulin was further analyzed by a coimmunoprecipitation assay. The cell extracts prepared from SiHa (Fig. 7D) and Molt4 (data not shown) cells were first immunoprecipitated with either NRIgG or a polyclonal antibody against CPAP, followed by immunoblotting of coprecipitated proteins with anti-γ-tubulin MAb. As shown in Fig. 7D, γ-tubulin was detected when anti-CPAP antibody was used for the immunoprecipitation experiment (lane 2). However, no endogenous γ-tubulin was detected when NRIgG was used as a control (lane 1). Similarly, CPAP was coprecipitated with anti-γ-tubulin MAb and immunoreacted with anti-CPAP antibody (lane 4). A low-molecular-weight band which was not detected by a control hybridoma (H25B10, lane 3) is likely a degradation product of CPAP. Taken together, these results indicated that endogenous CPAP is associated with γ-tubulin in vivo.
CPAP is localized within the center of the microtubule asters and may be involved in microtubule nucleation.
To examine the localization of CPAP in microtubule asters assembled in vitro, the asters were monitored by double immunofluorescence with anti-CPAP (Fig. 8A-a, green) antibody and anti-α-tubulin MAb (Fig. 8A-b, red). As shown by superimposed photography, CPAP was localized at the central part of the microtubule aster (Fig. 8A-c). We next evaluated the possible role of CPAP in microtubule-nucleating activity. The centrosome fractions isolated from Molt4 cells were incubated with affinity-purified anti-CPAP antibody and then assayed for aster formation. As shown in Fig. 8B, most centrosomes nucleated microtubule asters in the presence of control antibody (a and e). In contrast, the number and the length of nucleating microtubules were significantly diminished when the centrosomes were incubated with anti-CPAP (Fig. 8B-b and f) or anti-γ-tubulin (Fig. 8B-c and g) antibodies. Table 1 shows a quantitative analysis of the effect of anti-CPAP and anti-γ-tubulin antibodies on microtubule nucleation. Both the number and the length of the microtubules were significantly affected by coincubation with anti-CPAP or anti-γ-tubulin antibodies (Table 1). However, the polymerization of microtubules in the absence of centrosome fractions was not inhibited by the addition of anti-CPAP antibody (Fig. 8B-d and h). These results suggest that CPAP is a centrosomal protein that may play a positive role in microtubule nucleation.
TABLE 1.
Effect of anti-CPAP and anti-γ-tubulin antibodies on microtubule nucleation in vitroa
| Antibody | Mean microtubule length (μm) ± SD | No. of microtubules/ centrosomeb
|
||
|---|---|---|---|---|
| <5 | 5–15 | >15 | ||
| NRIgG | 9.0 ± 1.6 | 3 | 10 | 90 |
| Anti-CPAP | 2.7 ± 0.5 | 37 | 82 | 5 |
| Anti-γ-tubulin | 3.5 ± 0.5 | 7 | 72 | 24 |
The in vitro microtubule nucleation test from three independent experiments was performed as described in the text. Photographs were taken, and the length and number of microtubules were calculated for ∼100 asters.
Data indicate the number of centrosomas with <5, 5 to 15, or >15 microtubules per centrosome.
To determine whether the centrosomal association of CPAP was dependent on the polymerization of microtubules, we treated cells under conditions that perturb the microtubule network. In nocodazole- or cold-treated SiHa cells (4°C, 60 min), interphase microtubules were depolymerized (Fig. 9b and Fig. 9d). However, compared to untreated cells (Fig. 9a and g), no significant decrease in the concentration of CPAP was observed in nocodazole- (Fig. 9h) or cold-treated (Fig. 9j) cells. To ensure that CPAP is indeed located at the centrosome where microtubule growth is initiated, the cold-treated cells were returned to 30°C for 2 (Fig. 9e and k) and 10 (Fig. 9f and l) min. As shown in Fig. 9, microtubule growth started at the centrosome where CPAP is located. Similar results were observed when the SiHa cells were treated with taxol, a microtubule-stabilizing agent. In these cells, CPAP was still strongly associated with the centrosome but not with the microtubule bundles (Fig. 9c and i). From these results, we concluded that CPAP is a bona fide core component of the centrosome and that its association with the centrosome is independent of the presence of microtubules.
DISCUSSION
We (17, 41, 42) and others (3, 5) previously reported that 4.1R is composed of multiple isoforms that vary in size and subcellular localization and exhibit tissue- and development-specific expression patterns. Many of these isoforms are present in nonerythroid cells (17, 42) or in erythroid cells at different developmental stages (5, 41). Although the functional significance of the 4.1R-80 isoform in red blood cells has been well characterized, the role of 4.1R-135 in nonerythroid cells (42) remains largely unknown.
4.1R-135 in the centrosome.
In most cells, the centrosome is composed of a pair of centrioles surrounded by an electron-dense fibrogranular area called the PCM. In metazoa, the primary microtubule-organizing center (MTOC) is the centrosome. However, the PCM is the primary site that nucleates microtubules from soluble tubulin subunits, which results in a radial array centered at the centrosome (34). Recently, a new species of tubulin, γ-tubulin, was identified as a universal MTOC component that plays an important role in microtubule nucleation (31). Information regarding the composition and function of the centrosome is just beginning to emerge.
In the present study, using a yeast two-hybrid screen, we isolated a novel centrosomal protein (termed CPAP) that specifically binds to the unique head domain (residues 1 to 209) of 4.1R-135. CPAP is a bona fide centrosomal protein that colocalizes with γ-tubulin in isolated centrosomes (Fig. 6A and 7). The interaction between CPAP and 4.1R-135 is specific and was demonstrated by (i) yeast two-hybrid screening (Fig. 1), (ii) an in vitro binding assay (Fig. 5D), and (iii) cross coimmunoprecipitation studies in vivo (Fig. 5B and C). Recently, it was reported that some 4.1R isoforms are present within the centrosome (21; this report, Fig. 6B). However, no endogenous 4.1R-135 was detected in isolated centrosomes by our Western blot and immunofluorescence analyses (data not shown). We may have failed to detect 4.1R in the isolated centrosomes because we used a very low-ionic-strength condition to isolate the centrosomes (29). Under such conditions, the centrosomes are separated from the nucleus by disassembly of the microtubule and microfilament cytoskeletal networks (29). Thus, 4.1R-135 may be located at the centrosomal region close to an undefined centrosome-cytoskeletal network junction, but is absent in the isolated centrosomes. The functional role of 4.1R-135 in centrosomes is not clear at present. It is possible that the 4.1R-135 protein serves as an adapter that anchors the CPAP/γ-tubulin complex to the centrosome. A clear picture of this model should be revealed in future studies using immunoelectron microscopy.
Structural and functional domains of CPAP.
CPAP is predicted to possess two structurally distinct domains. One contains five short coiled-coil segments (Fig. 3C) with similarity to heptad repeat regions. The other contains unusual glycine repeats (G-box) with unknown function (Fig. 3D). Interestingly, the carboxyl terminus of CPAP (residues 978 to 1338), which includes the fifth coiled-coil structure and the G box, revealed 31.3% amino acid identity (76.6% homology) with human Tcp10 (residues 29 to 392). Tcp10 is a t-complex responder (Tcr) gene that may play a role in the transmission ratio distortion phenotype (37). The molecular mechanism that regulates this phenotype remains largely unknown.
Cohen and Parry (2) first described the features of heptad repeats. They found that the first and the fourth residue in each heptad repeat are predominantly hydrophobic. These hydrophobic residues form a stripe that winds around the α-helix, which is capable of interacting with the hydrophobic residues of a second molecule to form a coiled-coil structure (2). Thus the heptad repeats of CPAP are predicted to form a dimer with themselves or with other molecules that carry similar repeats. CPAP (this report) and Tcp10 (35) were predominantly expressed in testis and both carry heptad repeats; it will be interesting to examine in future studies the interaction between CPAP and Tcp10 proteins and the possible effects resulting from this interaction.
Furthermore, our current results show that the interaction between 4.1R-135 and CPAP occurs through the head domain (residues 127 to 198) of 4.1R-135 and the C terminus (residues 897 to 1338) of CPAP (Fig. 1A and 3B). The C-terminal domain of CPAP contains the fourth and fifth coiled-coil motifs and the G repeats (Fig. 3B). Since the head domain of 4.1R-135 does not contain typical heptad repeats, it is possible that the G repeats of CPAP may serve as a protein interaction domain that participates in the interaction with 4.1R-135.
Possible roles for CPAP.
It is well known that γ-tubulin is a universal component of MTOCs that function in microtubule nucleation. Two possible models for the nucleation of microtubules by γ-TuRC have been proposed. The first model, proposed by Oakley (32) and extended by Zheng et al. (47), implied that the nontubulin proteins in γ-TuRC provide a scaffold to which the γ-tubulins bind. The α- and β-tubulins interact with γ-tubulin, providing a seed for the assembly of microtubules. Erickson and Stoffler (12) proposed an alternative model. In this model, γ-tubulin forms a curved protofilament (γ-tubulin spiral) that serves as a stable seed for nucleation of an α/β protofilament. Generally, most information about γ-tubulin comes from studies of the cytoplasmic γ-tubulin complex, yet the major site for γ-tubulin action is thought to be the centrosome. The biological significance of the centrosomal and cytoplasmic forms of γ-tubulin remains largely unknown.
In the present study, we found that CPAP is tightly associated with the centrosome, but not with the microtubule bundles (Fig. 9), when cells were treated with cold temperature or with microtubule-perturbing drugs (taxol and nocodazole). Using these criteria (33), CPAP should be considered a bona fide component of the core centrosome. Furthermore, previous reports have shown that a large pool of γ-tubulin is present in a soluble form in Xenopus egg extracts (13, 39) and in the cytosolic fractions of mammalian cells (28). To examine whether CPAP is also associated with the cytosolic γ-tubulin complex. Molt4 cell lysates were separated into Triton X-100-soluble and -insoluble fractions and tested for the presence of CPAP and γ-tubulin. Our results showed that both CPAP and γ-tubulin have a similar soluble-insoluble distribution, with >70% of the total protein being soluble (data not shown). The insoluble part is presumably associated with the centrosome. We then analyzed the cytosolic fractions of Molt4 cells using sucrose gradient sedimentation. Our results showed that CPAP colocalized with γ-tubulin in the same sucrose gradient fractions (Fig. 7B) and coimmunoprecipitated with γ-tubulin in vivo (Fig. 7D), suggesting that CPAP is a part of the γ-tubulin complex. In addition, our functional analysis showed that CPAP was localized within the center of microtubule asters (Fig. 8A) and that the formation of microtubule asters was significantly inhibited by anti-CPAP antibody (Fig. 8B, Table 1). Taken together, these results indicate that CPAP is a part of the γ-tubulin complex and that it may participate in microtubule nucleation.
Zheng et al. (47) characterized the so-called γ-TuRC from a mitotic Xenopus egg extract and found that γ-TuRC is composed of at least seven different proteins. This complex has an open ring structure that might act as an active microtubule-nucleating unit and caps the minus ends of microtubules in vitro. Recently, several proteins, including pericentrin (10), GAPCenA (9), and two human homologues (hGCP2 and hGCP3) of the yeast spindle pole body proteins Spc97p and Spc98p (30, 45) were reported to form complexes with cytosolic γ-tubulin. Among these, hGCP2 and hGCP3 were reported to be components of both the centrosomal and the cytosolic γ-tubulin complexes in mammalian cells (30, 45). The relationship between CPAP and these proteins associated with the γ-tubulin complex is not clear. Although we have not yet examined the precise composition of the CPAP/γ-tubulin complex, it is possible that CPAP and other centrosomal proteins may constitute a scaffold that tethers the CPAP/γ-tubulin complex at the centrosome.
In summary, in the present study we isolated and characterized a novel centrosomal protein, CPAP, which specifically interacts with the head domain of a nonerythroid 4.1R-135 isoform. CPAP is a novel centrosomal protein that is associated with the γ-tubulin complex. We propose a model whereby CPAP not only may constitute the scaffold of the centrosomal γ-tubulin complex, but also may serve as an attachment site for 4.1R-135. Answers to many of the important questions regarding the structural and functional role of 4.1R-135 and CPAP in our proposed model will require immunoelectron microscopic analysis and in vitro reconstruction of all or part of the complex. With the identification of CPAP as a conserved component of the γ-tubulin complex, we anticipate that study of CPAP and its associated proteins will help more fully elucidate centrosome functions.
ACKNOWLEDGMENTS
We are grateful for Szecheng J. Lo for his thoughtful comments and suggestions and Kuan-Yu Chu for his expertise in confocal microscopy.
We thank the Foundation of Biomedical Sciences (ROC) for providing a travel award to Liang-Yi Hung. This work was supported by a grant (NSC89-2320-B001-003) from the National Science Council and an institutional grant from Academia Sinica (ROC).
REFERENCES
- 1.Cohen C M, Foley S F, Korsgen C. A protein immunologically related to erythrocyte band 4.1 is found on stress fibers of non-erythroid cells. Nature. 1982;299:648–650. doi: 10.1038/299648a0. [DOI] [PubMed] [Google Scholar]
- 2.Cohen C, Parry D A D. Alpha-helical coiled-coils a widespread motif in proteins. Trends Biochem Sci. 1986;11:245–248. [Google Scholar]
- 3.Conboy J G, Chan J, Mohandas N, Kan Y W. Multiple protein 4.1 isoforms produced by alternative splicing in human erythroid cells. Proc Natl Acad Sci USA. 1988;85:9062–9065. doi: 10.1073/pnas.85.23.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conboy J G, Marchesi S, Kim R, Agre P, Kan Y W, Mohandas N. Molecular analysis of insertion/deletion mutations in protein 4.1 in elliptocytosis. II. Determination of molecular genetic origins of rearrangements. J Clin Investig. 1990;86:524–530. doi: 10.1172/JCI114739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conboy J G, Chan J Y, Chasis J A, Kan Y W, Mohandas N. Tissue- and development-specific alternative RNA splicing regulates expression of multiple isoforms of erythroid membrane protein 4.1. J Biol Chem. 1991;266:8273–8280. [PubMed] [Google Scholar]
- 6.Conboy J G. Structure, function, and molecular genetics of erythroid membrane skeletal protein 4.1 in normal and abnormal red blood cells. Semin Hematol. 1993;30:58–73. [PubMed] [Google Scholar]
- 7.Correas I, Leto T L, Speicher D W, Marchesi V T. Identification of the functional site of erythrocyte protein 4.1 involved in spectrin-actin associations. J Biol Chem. 1986;261:3310–3315. [PubMed] [Google Scholar]
- 8.Correas I. Characterization of isoforms of protein 4.1 present in the nucleus. Biochem J. 1991;279:581–585. doi: 10.1042/bj2790581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuif M-H, Possmayer F, Zander H, Bordes N, Jollivet F, Couedel-Courteille A, Janoueix-Lerosey I, Langsley G, Bornens M, Goud B. Characterization of GAPCenA, a GTPase activating protein for Rab6, part of which associates with the centrosome. EMBO J. 1999;18:1772–1782. doi: 10.1093/emboj/18.7.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dictenberg J B, Zimmerman W, Sparks C A, Young A, Vidair C, Zheng Y, Carrington W, Fay F S, Doxsey S J. Pericentrin and γ-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J Cell Biol. 1998;141:163–174. doi: 10.1083/jcb.141.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Discher D, Parra M, Conboy J G, Mohandas N. Mechanochemistry of the alternatively spliced spectrin-actin binding domain in membrane skeletal protein 4.1. J Biol Chem. 1993;268:7186–7195. [PubMed] [Google Scholar]
- 12.Erickson H P, Stoffler D. Protofilaments and rings, two conformations of the tubulin family conserved from bacterial FtsZ to alpha/beta and gamma tubulin. J Cell Biol. 1996;135:5–8. doi: 10.1083/jcb.135.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felix M A, Antony C, Wright M, Maro B. Centrosome assembly in vitro: role of gamma-tubulin recruitment in Xenopus sperm aster formation. J Cell Biol. 1994;124:19–31. doi: 10.1083/jcb.124.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fry A M, Meraldi P, Nigg E A. A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. EMBO J. 1998;17:470–481. doi: 10.1093/emboj/17.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horne W C, Huang S C, Becker P S, Tang T K, Benz E J., Jr Tissue-specific alternative splicing of protein 4.1 inserts an exon necessary for formation of the ternary complex with erythrocyte spectrin and F-actin. Blood. 1993;82:2558–2563. [PubMed] [Google Scholar]
- 16.Hou C L, Tang C J C, Roffler S R, Tang T K. Protein 4.1R binding to eIF3-p44 suggests an interaction between the cytoskeletal network and the translation apparatus. Blood. 2000;96:747–753. [PubMed] [Google Scholar]
- 17.Huang J P, Tang C J C, Kou G H, Marchesi V T, Benz E J, Jr, Tang T K. Genomic structure of the locus encoding protein 4.1: structural basis for complex combinational pattern of tissue-specific alternative RNA splicing. J Biol Chem. 1993;268:3758–3766. [PubMed] [Google Scholar]
- 18.Infante C, Ramos-Morales F, Fedriani C, Bornens M, Rios R M. GMAP-210, a cis-Golgi network-associated protein, is a minus end microtubule-binding protein. J Cell Biol. 1999;145:83–98. doi: 10.1083/jcb.145.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islam S D, Pilder S H, Decker C L, Cebra-Thomas J A, Silver L M. The human homolog of a candidate mouse t complex responder gene: conserved motifs and evolution with punctuated equilibria. Hum Mol Genet. 1993;2:2075–2079. doi: 10.1093/hmg/2.12.2075. [DOI] [PubMed] [Google Scholar]
- 20.Jons T, Drenckhahn D. Identification of the binding interface involved in linkage of cytoskeletal protein 4.1 to the erythrocyte anion exchanger. EMBO J. 1992;11:2863–2867. doi: 10.1002/j.1460-2075.1992.tb05354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krauss S W, Chasis J A, Rogers C, Mohandas N, Krockmalnic G, Penman S. Structure of protein 4.1 is located in mammalian centrosomes. Proc Natl Acad Sci USA. 1997;94:7297–7302. doi: 10.1073/pnas.94.14.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krauss S W, Larabell C A, Lockett S, Gascard P, Penman S, Mohandas N, Chasis J A. Structural protein 4.1 in the nucleus of human cells: dynamic rearrangements during cell division. J Cell Biol. 1997;137:275–289. doi: 10.1083/jcb.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leto T L, Pratt B M, Madri J A. Mechanisms of cytoskeleton regulation: modulation of aortic endothelial cell protein band 4.1 by the extracellular matrix. J Cell Physiol. 1986;127:423–431. doi: 10.1002/jcp.1041270311. [DOI] [PubMed] [Google Scholar]
- 24.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 25.Marfatia S M, Lue R A, Branton D, Chishti A H. In vitro binding studies suggest a membrane-associated complex between erythroid p55, protein 4.1, and glycophorin C. J Biol Chem. 1994;269:8631–8634. [PubMed] [Google Scholar]
- 26.Mattagajasingh S N, Huang S C, Hartenstein J S, Snyder M, Marchesi V T, Benz E J., Jr A nonerythroid isoform of protein 4.1R interacts with the nuclear mitotic apparatus (NuMA) protein. J Cell Biol. 1999;145:29–43. doi: 10.1083/jcb.145.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchison T, Kirschner M. Microtubule assembly nucleated by isolated centrosomes. Nature. 1984;312:232–237. doi: 10.1038/312232a0. [DOI] [PubMed] [Google Scholar]
- 28.Moudjou M, Bordes N, Paintrand M, Bornens M. Gamma-tubulin in mammalian cells: the centrosomal and the cytosolic forms. J Cell Sci. 1996;109:875–887. doi: 10.1242/jcs.109.4.875. [DOI] [PubMed] [Google Scholar]
- 29.Moudjou M, Bornens M. Method of centrosome isolation from cultured animal cells. In: Celis J E, editor. Cell biology: a laboratory handbook. 2nd ed. Vol. 2. London, England: Academic Press; 1998. pp. 111–119. [Google Scholar]
- 30.Murphy S M, Urbani L, Stearns T. The mammalian γ-tubulin complex contains homologues of the yeast spindle pole body components Spc97p and Spc98p. J Cell Biol. 1998;141:663–674. doi: 10.1083/jcb.141.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oakley B R, Oakley C E, Yoon Y, Jung M K. γ-Tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans. Cell. 1990;61:1289–1301. doi: 10.1016/0092-8674(90)90693-9. [DOI] [PubMed] [Google Scholar]
- 32.Oakley B R. γ-Tubulin: the microtubule organizer? Trends Cell Biol. 1992;2:1–5. doi: 10.1016/0962-8924(92)90125-7. [DOI] [PubMed] [Google Scholar]
- 33.Oegema K, Whitfield W G, Alberts B. The cell cycle-dependent localization of the CP190 centrosomal protein is determined by the coordinate action of two separable domains. J Cell Biol. 1995;131:1261–1273. doi: 10.1083/jcb.131.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira G, Schiebel E. Centrosome-microtubule nucleation. J Cell Sci. 1997;110:295–300. doi: 10.1242/jcs.110.3.295. [DOI] [PubMed] [Google Scholar]
- 35.Schimenti J, Cebra-Thomas J A, Decker C L, Islam S D, Pilder S H, Silver L M. A candidate gene family for the mouse t complex responder (Tcr) locus responsible for haploid effects on sperm function. Cell. 1988;55:71–78. doi: 10.1016/0092-8674(88)90010-4. [DOI] [PubMed] [Google Scholar]
- 36.Shi Z T, Afzal V, Coller B, Patel D, Chasis J A, Parra M, Lee G, Paszty C, Stevens M, Walensky L, Peters L L, Mohandas N, Rubin E, Conboy J G. Protein 4.1R-deficient mice are viable but have erythroid membrane skeleton abnormalities. J Clin Investig. 1999;103:331–340. doi: 10.1172/JCI3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silver L M. Mouse t haplotypes. Annu Rev Genet. 1985;19:179–208. doi: 10.1146/annurev.ge.19.120185.001143. [DOI] [PubMed] [Google Scholar]
- 38.Stearns T, Evans L, Kirschner M. Gamma-tubulin is a highly conserved component of the centrosome. Cell. 1991;65:825–836. doi: 10.1016/0092-8674(91)90390-k. [DOI] [PubMed] [Google Scholar]
- 39.Stearns T, Kirschner M. In vitro reconstitution of centrosome assembly and function: the central role of gamma-tubulin. Cell. 1994;76:623–637. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- 40.Tang C J C, Tang T K. The 30-kDa domain of protein 4.1 mediates its binding to the carboxyl terminus of pICln, a protein involved in cellular volume regulation. Blood. 1998;92:1442–1447. [PubMed] [Google Scholar]
- 41.Tang T K, Leto T L, Correas I, Alonso M A, Marchesi V T, Benz E J., Jr Selective expression of an erythroid-specific isoform of protein 4.1. Proc Natl Acad Sci USA. 1988;85:3713–3717. doi: 10.1073/pnas.85.11.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang T K, Qin Z, Leto T, Marchesi V T, Benz E J., Jr Heterogeneity of mRNA and protein products arising from the protein 4.1 gene in erythroid and nonerythroid tissues. J Cell Biol. 1990;110:617–624. doi: 10.1083/jcb.110.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang T K, Mazzucco C E, Benz E J, Jr, Marchesi V T. Molecular cloning and nuclear localization of lymphoid membrane skeletal protein 4.1. In: Wang E, Wang J L, Chien S, Cheung W-Y, Wu C W, editors. Biochemical and structural dynamics of the cell nucleus. San Diego, Calif: Academic Press; 1990. pp. 43–57. [Google Scholar]
- 44.Tang T K, Tang C J C, Chao Y J, Wu C W. Nuclear mitotic apparatus protein (NuMA): spindle association, nuclear targeting and differential subcellular localization of various NuMA isoforms. J Cell Sci. 1994;107:1389–1402. doi: 10.1242/jcs.107.6.1389. [DOI] [PubMed] [Google Scholar]
- 45.Tassin A-M, Celati C, Moudjou M, Bornens M. Characterization of the human homologue of the yeast Spc98p and its association with γ-tubulin. J Cell Biol. 1998;141:689–701. doi: 10.1083/jcb.141.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tseng T C, Chen S H, Hsu Y P, Tang T K. Protein kinase profile of sperm and eggs: cloning and characterization of two novel testis-specific protein kinases (AIE1, AIE2) related to yeast and fly chromosome segregation regulators. DNA Cell Biol. 1998;17:823–833. doi: 10.1089/dna.1998.17.823. [DOI] [PubMed] [Google Scholar]
- 47.Zheng Y, Wong M L, Alberts B, Mitchison T. Nucleation of microtubule assembly by a gamma-tubulin-containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]