Abstract
Despite global therapeutic advancements, tropical parasitic infections like trypanosomiasis and leishmaniasis continue to be a major health concern in developing countries. These two tropical infectious diseases lead to enormous economic loss, significant disability, and morbidity, accounting for over one million deaths per year worldwide. The causative parasites, which shuttle between an insect vector and a mammalian host, thrive either in the bloodstream or in the intramacrophage environments. Essentially, the parasites live in an environment of oxidative stress and therefore require metabolic pathways to counterbalance the host immune response and survive the adverse conditions. Apart from the trypanothione pathway elucidated in the parasites, there exists a tocopherol pathway that functions to scavenge the reactive chemical species. This pathway, unique to photosynthetic organisms, is essential for the parasite’s survival, though the enzymes involved remain largely uncharacterized. Consequently, an understanding of the origin of the pathway and where and how the interconnected tocopherol pathway functions may result in the identification of promising and potential therapeutic interventions to combat these deadly diseases. Recent works underline the presence of the tocopherol pathway in trypanosomatids and hypothesize that trypanosomatids may be tocopherol prototrophs. This review focuses on the biosynthesis of tocopherols in Trypanosoma and Leishmania in light of the current evidence.
1. Tocopherol Biosynthesis Pathway: An Essential Pathway for Parasite Survival
Trypanosoma spp. and Leishmania spp. are important parasites causing trypanosomiasis and leishmaniasis, respectively, in humans and other animals. They are phylogenetically related protozoan parasites of the Class Kinetoplastida and have similar structural and biochemical features. These parasites are called trypanosomatid parasites, and the diseases are called kinetoplastid diseases, a subset of neglected tropical diseases. Trypanosoma spp. and Leishmania spp. possess a unique digenetic life cycle to adapt to the frequent transmission between mammalian hosts by insect vectors.1 Trypanosomatids survive extreme oxidative environments to complete each phase of their life cycle. Each distinct morphological stage in the digenetic life cycle is capable of acclimatizing to the adverse conditions associated with their shuttling hosts. Both parasites need to overcome immune responses mounted by the host and the unfavorable conditions inside the vectors. These adverse environments usually include the presence and production of reactive oxygen species/reactive nitrogen species (ROS/RNS) such as a hydroxyl radical (OH•), hydrogen peroxide (H2O2), a superoxide anion radical (O2•), nitric oxide (NO), and peroxynitrite (ONOO•) by macrophages. Through time, these parasites endured the highly oxidative environs and survived to become fatal for the human population worldwide. It is compelling to understand how these parasites endured these harsh conditions despite being sensitive to ROS/RNS generated by the host immune system. The answer lies in the unique redox metabolism pathway adopted by trypanosomatids that helps maintain their redox homeostasis. The mammalian host cells carry out the radical scavenging function by the glutathione-based redox pathway; in contrast, the trypanosomatids rely on the biosynthesis of trypanothione (glutathione + spermidine) for this function. The classical reviews by Krauth-Siegel and colleagues reveal how these parasites adopted the trypanothione-based thiol metabolism for redox control and discuss the potential use of active enzymes in the pathway as effective drug targets.2 The observed divergence in the redox metabolism of the trypanosomatids might be an outcome of time constraints and the evolution shared with humans for millions of years.
Notably, in addition to the redox pathway, a tocopherol biosynthesis pathway exists in the trypanosomatids that produce α-tocopherol and homologues to protect them from oxidative stress. Tocopherols act as antioxidants and free radical scavengers in various organisms, including Plasmodium spp.3 The tocopherols are lipid-based compounds synthesized only by plants and other photosynthetic microorganisms. These compounds possess antioxidant properties and play critical roles in protecting the cells from adverse oxidative environments. There are four naturally occurring forms of tocopherols: α-, β-, γ-, and δ-tocopherol, with the only difference in the numbering and positioning of methyl substituents on the aromatic ring. In plants, the predominant form is the α-tocopherol, which is contained in the plastids for scavenging reactive oxygen species and lipid peroxy radicals. However, the presence of tocopherols in trypanosomatids is a peculiar observation considering the genetic divergence of trypanosomatids from plants. A possible explanation can be drawn from the study by Leander in 2004, who argued the existence of photosynthetic ancestors for trypanosomatids. The study was also intriguing because the author drew a relation between the presence of phototrophic genes and trypanosomatids from ultrastructure evidence.4 Another plausible explanation was given by Opperdoes and Michels, who argued that trypanosomatids could have acquired a large number of genes from plants and bacteria through lateral gene transfer during the process of evolution.5 These two probable hypotheses reinforce the presence of the tocopherol pathway in trypanosomatids and that trypanosomatids may be tocopherol prototrophs. Considering the available evidence, in this review, we focus on the biosynthesis of tocopherols in Trypanosoma spp. and Leishmania spp.
2. Substrates and Enzymes Involved in Tocopherol and Related Pathways
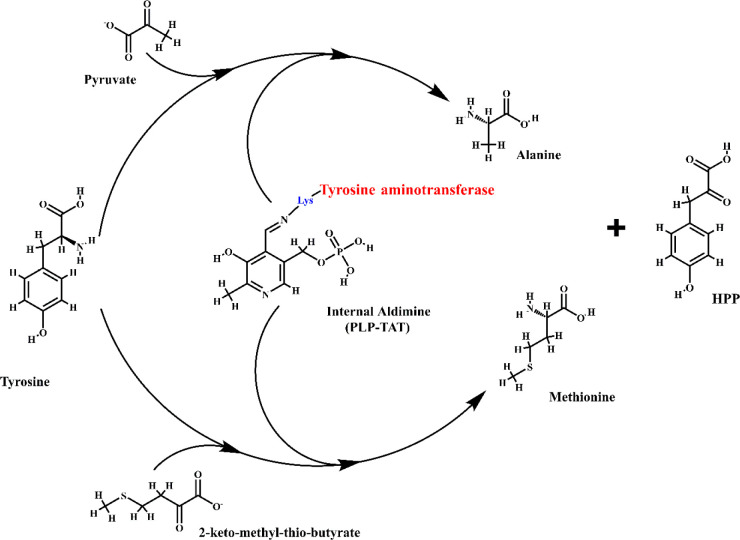
The degradation of tyrosine is the first step in the tocopherol pathway. l-Tyrosine obtained by hydroxylation of phenylalanine is initially transformed into 4-hydroxyphenylpyruvate (HPP), and this reaction is catalyzed by tyrosine aminotransferase with the help of cofactor pyridoxal-l-phosphate (Figure 1). l-Tyrosine degradation is essential in blood-feeding arthropods.6 The degradation involves the transfer of amino groups from tyrosine to ketoacids, resulting in the synthesis of respective amino acids like alanine, methionine, and valine. Alanine is involved in converting NADP+ to NADPH, while methionine converts to S-adenosyl methionine (SAM) and participates in redox metabolism. Valine, on the other hand, undergoes catalysis and engages in the TCA cycle. HPP is formed as a byproduct of tyrosine in the reaction whose presence has been attributed to pathogenicity in trypanosomatids. Sawalhy et al. detected high levels of 4-hydroxyphenylpyruvate in the urine of mice infected with T. brucei, and this effect was reversed when suramin (a drug that inhibits the oxidation of reduced NADH) was administered.7 The enzyme tyrosine aminotransferase (EC 2.6.1.5; TATase) has been well-characterized in both Trypanosoma spp.8 as well as Leishmania spp.9,10 HPP is subsequently catalyzed by 4-hydroxyphenylpyruvate dioxygenase (HPPD, EC 1.13.11.27) and converted to homogentisate (HGA). HPDD belongs to the α-ketoacid-dependent oxygenases and is an exception in its class as it does not utilize α-ketoglutarate as a substrate. Instead, it incorporates both atoms of the dioxygen and results in the formation of the aromatic product, HGA (Figure 2). HPDD remains unidentified in g spp. and Leishmania spp. but is well-characterized in plants. Interestingly, in T. cruzi, HPP is also known to be catalyzed by an l-α-hydroxyacid dehydrogenase, and its presence in Leishmania spp. has been established.9 Franke et al. suggested that l-α-hydroxyacid dehydrogenase is an isoform of malate dehydrogenase though the enzyme does not possess any malate dehydrogenase activity. However, unlike HPPD, which produces homogentisate, this reaction by l-α-hydroxyacid dehydrogenase produces p-hydroxyphenyl lactate (HPL). Specific inhibition of HPPD in plants has been carried out by natural and artificially synthesized compounds as effective herbicides.11 With the help of plant HPPD inhibitors (usnic acid and nitisinone), the presence of the tocopherol pathway in L. amazonenesis was recognized.12 Balanco et al. used radio-labeled [1-(n)3-H]-phytol to study the pathway, and the treatment with the inhibitors showed potent inhibition of tocopherol synthesis.12 Notably, the radiolabeled isoprenoid precursors were incorporated into the observed tocopherol products when the parasites were grown in a tocopherol-free medium. The study essentially confirmed the presence of a tocopherol pathway in Leishmania. However, further studies are required to identify the other substrates and enzymes in this pathway.12 Another study involving the treatment of T. cruzi with usnic acid also resulted in the death of the parasite.13 Though there was no mention of the presence of the tocopherol pathway in the study, usnic acid being an inhibitor of HPPD indicates the same.13
Figure 1.
Tyrosine aminotransferase (TAT) enzyme mechanism. Tyrosine aminotransferase is unique in trypanosomatids as they utilize pyruvate as a cosubstrate rather than α-ketoglutarate as observed in mammals. The transamination mechanism proceeds in two steps wherein tyrosine act as the amino donor and pyruvate or 2-ketomethylthiobutyrate as the amino acceptor. An internal aldimine (PLP-TAT) is formed between the active site lysine (in blue) of TAT (in red) and the carbonyl group of pyridoxal-l-phosphate (PLP). The internal aldimine then facilitates the transfer of the amino group. The end products formed from pyruvate/2-ketomethylthiobutyrate are alanine/methionine, respectively, along with 4-hydroxyphenylpyruvate (HPP) formed from tyrosine, which is utilized in the next step of the tocopherol pathway.
Figure 2.
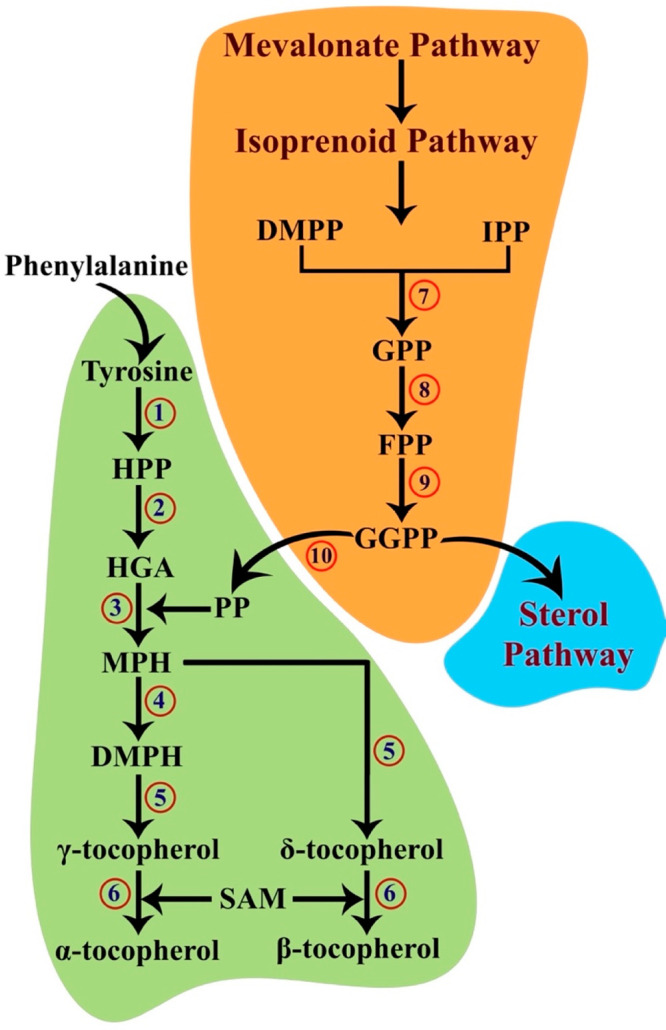
Hypothesized tocopherol biosynthesis pathway. Tyrosine produced as a result of phenylalanine hydroxylase is the first substrate in the tocopherol pathway. The produced tyrosine immediately transfers an amino group to a ketoacid and transmutes into 4-hydroxyphenylpyruvate (HPP). HPP is converted in the presence of oxygen and Fe2+ into homogentisate (HGA). The consequent reaction requires phytyl phosphate (PP) produced by a parallel mevalonate–isoprenoid pathway. The downstream isoprenoid focuses on the production of sterols and contains isopentyl diphosphate (IPP), dimethylallyl diphosphate (DMPP), geranyl diphosphate (GPP), farnesyl diphosphate (FPP), and geranylgeranyl diphosphate (GGPP). PP is produced when GGPP is reduced. The phytyl group of PP is transferred to HGA, giving rise to 2-methyl-6-phytylhydroquinone (MPH), and a methyl group is then transferred to MPH to result in 2,3-dimethyl-5-phytylhydroquinone (DMPH). DMPH and MPH are then subjected to cyclization to produce the γ- and δ-homologues of tocopherols. The α- and β-homologues are formed by transferring a methyl group from S-adenosyl-l-methionine to the formerly synthesized homologues: 1, tyrosine aminotransferase; 2, 4-hydroxyphenylpyruvate dioxygenase; 3, homogentisate phytyl transferase; 4, 2-methyl-6-phytylhydroquinone methyltransferase; 5, tocopherol cyclase; 6, γ-tocopherol methyltransferase; 7, geranyl/farnesyl diphosphate synthase; 8, geranyl/farnesyl diphosphate synthase; 9, geranylgeranyl diphosphate synthase; 10, geranylgeranyl diphosphate reductase.
Before venturing further to this pathway, it is worthwhile to understand the source of the second substrate, phytyl pyrophosphate (PP), for the subsequent reaction. PP is formed by the reduction of geranylgeranyl diphosphate (GGPP) by the enzyme geranylgeranyl diphosphate reductase (EC 1.3.1.83) (GPPR) (Figure 2). GGPP is produced by geranylgeranyl diphosphate synthase (GGPPS) of the isoprenoid pathway, which is a successive pathway to the mevalonate pathway. GGPP produced at the end of the isoprenoid pathway further proceeds to the sterol biosynthesis pathway to form ergosterol. It is at this juncture that the fate of GGPP bifurcates, and GGPPR reduces GGPP to PP. It should be mentioned that GPPR remains uncharacterized in both the parasites; however, as evident from the radiolabeled phytol study by Balanco et al., Leishmania does utilize PP.12 Subsequently, homogentisate phytyl transferase (HPT) (EC 2.5.1.116) catalyzes the transfer of the phytyl group from PP to HGA to form 2-methyl-6-phytylhydroquinone (MPH). 2-Methyl-6-phytylhydroquinone methyltransferase (MPHMT) (EC 2.1.1.295) further catalyzes the conversion of MPH to 2,3-dimethyl-5-phytylhydroquinone (DMPH). Later, tocopherol cyclase (TC) (EC 5.5.1.24) converts both MPH and DMPH to δ-tocopherol and γ-tocopherol, respectively. Ultimately, both δ-tocopherol and γ-tocopherol undergo a transfer of a methyl group by the enzyme γ-tocopherol methyltransferase (TMT) (EC 2.1.1.95) to form β-tocopherol and α-tocopherol, respectively (Figure 2). The methyl group is contributed by S-adenosyl-l-methionine (SAM) formed by the transfer of the adenosyl group of ATP to methionine by S-adenosylmethionine synthetase (MAT) (EC 2.5.1.6). SAM is available for uptake through membrane SAM transporters as well as synthesized de novo in trypanosomatids. Curiously, the first enzyme in this pathway, TAT, also catalyzes methionine formation by employing 2-ketomethylthiobutyrate as a cosubstrate (Figure 1).10 Unfortunately, the enzymes HPT, MPHMR, TC, and TMT are unidentified in the trypanosomatids but are well-characterized in plants.
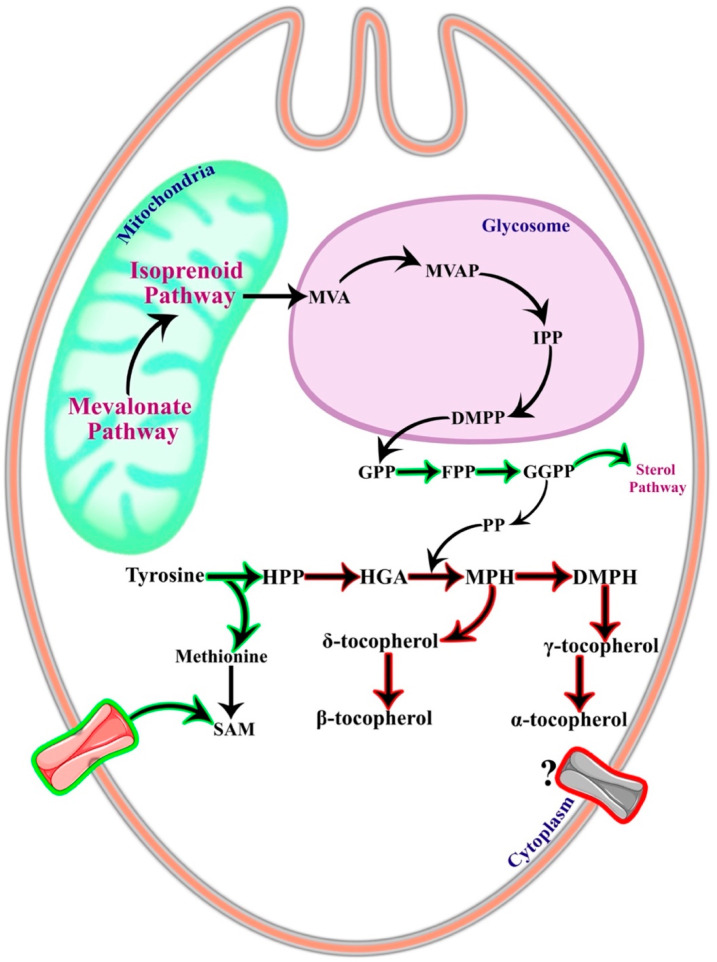
3. Subcellular Localization of the Tocopherol Pathway
As discussed previously, the presence of the tocopherol pathway is only partially elucidated in the Trypanosoma spp. and Leishmania spp. parasites, and therefore, the exact localization of the pathway enzymes remains elusive. However, it is possible to derive a presumptive location of this pathway from the available studies on localization. The catabolism of tyrosine to HPP occurs in the cytoplasm as the enzyme tyrosine aminotransferase is localized in the cytoplasm in L. infantum(9) and T. cruzi.14 The cosubstrate catalyzed by TAT, i.e., pyruvate, is also present in the cytoplasm of trypanosomatids since pyruvate is formed due to glycolysis. TAT, being a broad aminotransferase, also catalyzes the formation of methionine in the parasites, which is an essential amino acid required for the synthesis of SAM by MAT (Figure 3). Since TAT is located in the cytoplasm, methionine should also be present in the cytoplasmic pool. As discussed earlier, trypanosomatids are partially auxotrophic for SAM, and SAM transporters are localized on the membrane of the parasites. Collectively, from these data, it can be hypothesized that the last step of the tocopherol pathway should also proceed in the cytoplasm (Figure 3).
Figure 3.
Localization of the tocopherol pathway. The tocopherol pathway localization is based on the localization of the enzyme and substrates characterized until now. All known and characterized reactions are represented in the green outlined black arrows, and unknown or uncharacterized enzymes are marked in the red outlined black arrows. The aromatic amino acid pool is present in the cytoplasm of the parasites. Tyrosine aminotransferase that catalyzes tyrosine conversion to 4-hydroxypheylpyruvate and forms methionine is known to be localized in the cytoplasm. S-Adenosyl-l-methionine (SAM) transporter (pink transporter in green outline) has been known to uptake SAM into the cytoplasm, and SAM is utilized in the final step of the tocopherol pathway as a cosubstrate. The products and enzymes of the downstream isoprenoid pathway geranyl diphosphate (GPP), farnesyl diphosphate (FPP), and geranylgeranyl diphosphate (GGPP) are restricted to the cytoplasm in the trypanosomatids. From these known localized substrates and enzymes, it can be hypothesized that the tocopherol pathway functions in the cytoplasm. There might also be a tocopherol transporter (?) on the parasite cell wall that might be regulated at different parasite stages to balance the tocopherol pool in the cytoplasm. MVA, mevalonate; MVAP, mevalonate phosphate; IPP, isopentyl diphosphate; DMPP, dimethylallyl diphosphate; HPP, 4-hydroxyphenylpyruvate; HGA, homogentisate; PP, phytyl phosphate; MPH, 2-methyl-6-phytylhydroquinone; DMPH, 2,3-dimethyl-5-phytylhydroquinone.
Another evidence corroborating the cytoplasmic presence is the GPP from the isoprenoid pathway that gets reduced to PP for prenylation in the tocopherol pathway. GPP is formed by the condensation of isopentyl diphosphate (IPP) and dimethylallyl diphosphate (DMPP), and, subsequently, the addition of another IPP to geranyl diphosphate (GPP) forms farnesyl diphosphate (FPP) (Figure 3). Farnesyl diphosphate synthase (FPPS) (EC 2.5.1.10), the enzyme responsible for the catalysis of the later reaction, is localized in the cytoplasm in both parasites.15 This is different from Toxoplasma gondii, where FPPS partially appears in mitochondria. The presence of FPPS in the cytoplasm also suggests the presence of GPP in the cytoplasm, thereby allowing it to participate in the tocopherol pathway (Figure 3). The other enzymes of this pathway are still uncharacterized, and therefore, it is difficult to conclude if any part of the pathway occurs inside an organelle, in particular.
It will be meaningful to briefly compare the localization of the tocopherol pathway in plants considering Leander’s hypothesis of a photosynthetic ancestor of trypanosomatids.4 Plants are regularly prone to ROS stress, and chloroplast is the major ROS source in plants. In Arabidopsis, the enzymes TAT and HPPD are localized in the cytoplasm,16 whereas in maize, tomato, and cotton, HPPD is localized in the chloroplast. In soybean, HPPD is localized in both compartments. It is anticipated that plants in which the HGA biosynthesis is localized in the cytosol must have transporters to import HGA back into the chloroplast. This is because the rest of the enzymes, including tocopherol cyclase, are present inside the chloroplast in all plants. In contrast to plants, trypanosomatids are more prone to oxidative stress and do not possess any organelles resembling plastids. Therefore, the compartmentalization of the tocopherol pathway in parasites seems very unlikely.
4. Inhibitors of Tocopherol Pathway Enzymes
Research targeting inhibitors of tocopherol pathway enzymes is limited due to the fact that most of the enzymes are uncharacterized in the trypanosomatids. However, few inhibitors have been discovered and designed for similar enzymes characterized in other organisms. The in vitro and in vivo activity of these inhibitors have been tested. Essentially important among them is usnic acid, a compound extracted from lichens and a known inhibitor of HPDD.17 The treatment of usnic acid displayed deleterious effects on the survival of L. amazonenesis,12L. braziliensis, and L. infantum.18 The effects of usnic acid on T. cruzi were similar, with the trypomastigote form experiencing intense cytoplasm vacuolation and enlarged flagellar pockets on usnic acid addition.13 Treatment of the amastigote form also resulted in cytoplasm vacuolation and disorganization of kinetoplast and mitochondria in the parasite.13 Nitisinone (NTBC) is yet another widely used synthetic herbicidal drug that inhibits HPPD with an additional therapeutic value.19 The effect of NTBC on L. amazonensis was significant, though weak.12 Interestingly, a recent study carried out focused on the repurposing of NTBC to control the transmission of African trypanosomiasis; however, the NTBC acted and killed the tsetse flies and not the parasites directly.20
Studies for inhibition of the isoprenoid pathway have also been carried out.21,22 Though not directly involved, these inhibitors affect the tocopherol pathway through PP production reaction. Bisphosphonates are one such class of synthetic drugs developed in the 19th century to treat bone metabolic disorders. Bisphosphonates are structurally similar to pyrophosphate and hence target all pyrophosphate synthases, including FPPS and geranylgeranyl diphosphate synthase (GGPPS).23 The pyrophosphate synthases (FPPS and GGPPS) are involved in phytyl pyrophosphate production, as shown in Figure 2. These bisphosphonates are capable of inhibiting FPPS in T. brucei rhodiense.21 These compounds also inhibit the growth of T. brucei, T. cruzi, L. tarentolae,24 and L. donovani.25 The treatment of pamidronate (a bisphosphonate drug) on L. mexicana amazonensis was found effective by Rodriguez et al.26 Detailed reviews on the use of bisphosphonate-based compounds as antiparasitic drugs can be found elsewhere.27 Notably, supplementation of vitamin E promoted the multiplication of L. donovani in experimental hamsters. However, inhibitors targeting other enzymes of this pathway have not been studied and remain a potential research area.
5. Tocopherol Pathway in Other Parasites
Apart from the possible occurrence of the tocopherol pathway in Leishmania spp. and Trypanosoma spp., this pathway is also elucidated in other parasites. Rodrigo et al. confirmed the biosynthesis of vitamin E (tocopherols) in intraerythrocytic stages of P. falciparum.28 They supplemented radiolabeled GGPP and FPP to the parasite and discovered the presence of α-tocopherol and γ-tocopherol by RP-HPLC and GC-MS. The addition of usnic acid also inhibited the growth of the parasite.28 Another study showed that the α-tocopherol transfer protein (TTP) knockout mice were resistant to P. berghei NK65 infection.29 Notably, it is proved that the addition of tocopherol decreases the ROS levels to the basal level, even in the presence of usnic acid, confirming the protection of malarial parasites by tocopherols from increased oxidative stress.17 A combination of probucol and dihydroartemisinin displayed beneficial effects with more pronounced effects than artemisinin or probucol administration individually.3 Such studies on TTP knockout should also be performed in trypanosomatids to determine the possible presence of a TTP-like protein (? in Figure 3). Such an experiment will highlight the transport of α-tocopherol to the area of infection and proliferation. Yet another study by Imlay et al. established the isoprenoid metabolism in apicomplexan parasites, and further studies by Kim et al. suggested the involvement of tocopherol in the mitochondrial oxidation in the Toxoplasma gondii.30
6. Conclusion and Future Perspectives
Recent studies have corroborated the presence of a tocopherol pathway in the trypanosomatids; however, the complete pathway remains to be explored. The initial enzyme in the pathway, TAT, has been well-characterized in Leishmania and Trypanosoma and indicated properties of a promising drug target. TAT has low sequence similarity with its human homologue, further establishing its strong candidature.9,10 It will be worthy of characterizing the downstream enzymes of the tocopherol pathway and understanding their potential as drug targets since the enzymes would be of either prokaryotic or photosynthetic origin.4,5 Though not directly involved in the tocopherol pathway, the enzymes of the mevalonate, isoprenoid, and sterol biosynthesis pathways contribute to the production of PP and thereby tocopherols. The inhibition of these enzymes can be a potential possibility considering the nonavailability of characterized enzymes in the tocopherol pathway. This may expedite the process of drug design and discovery. Other than the enzymes of the tocopherol pathway, it principally remains elusive if α-tocopherol transfer protein in hosts affects parasite proliferation and survival and whether the parasites are entirely or partially tocopherol prototrophs. Interpreting these mechanisms will pave the way for a profound understanding of the parasite functioning as well as for developing combinatorial therapy against these parasites.3
It is debatable whether a complete tocopherol pathway or a TTP-like protein exists in these parasites, but the enzymes of the pathway are similar to that of other organisms with identical pathways. The presence of different forms of tocopherols in the parasites is now well-established through the study involving HPPD inhibitor, usnic acid, and radiolabeled PP.12 However, a detailed and complete understanding remains to be explored. Therefore, uncovering the tocopherol pathway will help to understand and answer various questions put forth in this review. Unraveling this pathway will provide insights into the biochemistry of the parasite, metabolic pathways, and ROS scavenging mechanisms and may help design novel therapeutic targets to thwart leishmaniasis and trypanosomiasis.
Acknowledgments
This work is supported by a research grant from the Science and Engineering Research Board, DST, Government of India (Grant No. EEQ/2018/000484). S.S. acknowledges a research fellowship from NIT Warangal.
Biographies

Santanu Sasidharan received his Bachelors in Biotechnology from Bannari Amman Institute of Technology, Sathyamangalam, India. He is currently pursuing his doctoral degree at the National Institute of Technology-Warangal, India. His area of expertise lies in protein characterization, molecular parasitology, and computational biology.

Timir Tripathi is a Senior Assistant Professor of Biochemistry at North-Eastern Hill University, India. He holds a Ph.D. from the Central Drug Research Institute, India. He was a visiting faculty at ICGEB, New Delhi, India (2011), and Khon Kaen University, Thailand (2015). He is interested in studying protein–substrate interaction and dynamics and understanding the roles of noncatalytic domains in regulating the catalytic activity of proteins. His recent projects include studying the conformational dynamics, self-assembly, and stabilization of the complexes formed by intrinsically disordered neuropathological protein aggregates. He has received various awards, including the Prof. B.K. Bachhawat Memorial Young Scientist Lecture Award (2020) by the National Academy of Sciences, India, ISCB-Young Scientist Award (2019), ICMR-Shakuntala Amir Chand Prize (2018), BRSI-Malviya Memorial Award (2017), DST Fasttrack Young Scientist Award (2012), DBT Overseas Associateship Award (2012), Dr. D.M. Bose Award (2008), etc. He is an elected member of the National Academy of Sciences, India, and the Royal Society of Biology, UK. He has published over 90 research papers, reviews, editorials, commentaries, and viewpoint articles in highly reputed international journals, has edited two books, and published several book chapters. He currently serves on the editorial boards of the International Journal of Biological Macromolecules, Acta Tropica, Scientific Reports, Frontiers in Molecular Biosciences, and PLoS One.

Prakash Saudagar is an active researcher and a sterling classroom teacher, currently working as Assistant Professor at NIT Warangal, India. He obtained his Ph.D. from the Indian Institute of Technology, Guwahati, India, in 2013. His research has immensely contributed to explored potential of drug target proteins and inhibitors. He has a strong command over computational techniques and in vitro techniques in studying drug target protein. He has publications in reputed journals such as the International Journal of Biological Molecules, FEBS, FEBS OpenBio, PLOS one, Scientific Reports, Biological Chemistry, Parasitology International, Molecular Simulation, etc., and book chapters in Elsevier and Springer to his name. He has been PI/Co-PI in research grants from SERB and DST. He has been awarded the B.S. Narasinga Rao award SBC, India (2011); Young Faculty Award, VIF India (2016); Young Scientist Award, Telangana Academy of Science (2018); and Best presentation award, ICIDN, Nepal (2015). He is an associate fellow of the Telangana Academy of Science (2018) and a life member of The Indian Science Congress and Society of Biological Chemists. He has many interdisciplinary collaborating partners in prestigious institutions both in India and abroad.
The authors declare no competing financial interest.
References
- Sasidharan S.; Saudagar P. Leishmaniasis: where are we and where are we heading?. Parasitol. Res. 2021, 120, 1541–1554. 10.1007/s00436-021-07139-2. [DOI] [PubMed] [Google Scholar]
- Krauth-Siegel R. L.; Comini M. A. Redox control in trypanosomatids, parasitic protozoa with trypanothione-based thiol metabolism. Biochim. Biophys. Acta, Gen. Subj. 2008, 1780 (11), 1236–1248. 10.1016/j.bbagen.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Suzuki H.; Kume A.; Herbas M. S. Potential of Vitamin E Deficiency, Induced by Inhibition of α-Tocopherol Efflux, in Murine Malaria Infection. Int. J. Mol. Sci. 2019, 20 (1), 64. 10.3390/ijms20010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leander B. S. Did trypanosomatid parasites have photosynthetic ancestors?. Trends Microbiol. 2004, 12 (6), 251–258. 10.1016/j.tim.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R.; Michels P. A. Horizontal gene transfer in trypanosomatids. Trends Parasitol. 2007, 23 (10), 470–476. 10.1016/j.pt.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Sterkel M.; Perdomo H. D.; Guizzo M. G.; Barletta A. B. F.; Nunes R. D.; Dias F. A.; Sorgine M. H.; Oliveira P. L. Tyrosine detoxification is an essential trait in the life history of blood-feeding arthropods. Curr. Biol. 2016, 26 (16), 2188–2193. 10.1016/j.cub.2016.06.025. [DOI] [PubMed] [Google Scholar]
- El Sawalhy A.; Seed J. R.; Hall J. E.; El Attar H. Increased excretion of aromatic amino acid catabolites in animals infected with Trypanosoma brucei evansi. J. Parasitol. 1998, 84, 469–473. 10.2307/3284707. [DOI] [PubMed] [Google Scholar]
- Marciano D.; Maugeri D. A.; Cazzulo J. J.; Nowicki C. Functional characterization of stage-specific aminotransferases from trypanosomatids. Mol. Biochem. Parasitol. 2009, 166 (2), 172–82. 10.1016/j.molbiopara.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Moreno M. A.; Alonso A.; Alcolea P. J.; Abramov A.; de Lacoba M. G.; Abendroth J.; Zhang S.; Edwards T.; Lorimer D.; Myler P. J.; Larraga V. Tyrosine aminotransferase from Leishmania infantum: A new drug target candidate. Int. J. Parasitol.: Drugs Drug Resist. 2014, 4 (3), 347–54. 10.1016/j.ijpddr.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasidharan S.; Saudagar P. Biochemical and structural characterization of tyrosine aminotransferase suggests broad substrate specificity and a two-state folding mechanism in Leishmania donovani. FEBS Open Bio 2019, 9 (10), 1769–1783. 10.1002/2211-5463.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.-L.; Lin H.-Y.; Ruan X.; Yang S.-G.; Hao G.-F.; Yang W.-C.; Yang G.-F. Synthesis and bioevaluation of pyrazole-benzimidazolone hybrids as novel human 4-Hydroxyphenylpyruvate dioxygenase inhibitors. Eur. J. Med. Chem. 2015, 92, 427–438. 10.1016/j.ejmech.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Balanco J. M. F.; Sussmann R. A.; Verdaguer I. B.; Gabriel H. B.; Kimura E. A.; Katzin A. M. Tocopherol biosynthesis in Leishmania (L.) amazonensis promastigotes. FEBS Open Bio 2019, 9 (4), 743–754. 10.1002/2211-5463.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carvalho E.; Andrade P.; Silva N.; Pereira E.; Figueiredo R. Effect of usnic acid from the lichen Cladonia substellata on Trypanosoma cruzi in vitro: an ultrastructural study. Micron 2005, 36 (2), 155–161. 10.1016/j.micron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Nowicki C.; Montemartini M.; Duschak V.; Santome J. A.; Cazzulo J. J. Presence and subcellular localization of tyrosine aminotransferase and p-hydroxyphenyllactate dehydrogenase in epimastigotes of Trypanosoma cruzi. FEMS Microbiol. Lett. 1992, 92, 119–124. 10.1111/j.1574-6968.1992.tb05245.x. [DOI] [PubMed] [Google Scholar]
- Ferella M.; Li Z.-H.; Andersson B.; Docampo R. Farnesyl diphosphate synthase localizes to the cytoplasm of Trypanosoma cruzi and T. brucei. Exp. Parasitol. 2008, 119 (2), 308–312. 10.1016/j.exppara.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Toda K.; Maeda H. A. Biochemical properties and subcellular localization of tyrosine aminotransferases in Arabidopsis thaliana. Phytochemistry 2016, 132, 16–25. 10.1016/j.phytochem.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Sussmann R. A.; Fotoran W. L.; Kimura E. A.; Katzin A. M. Plasmodium falciparum uses vitamin E to avoid oxidative stress. Parasites Vectors 2017, 10 (1), 461. 10.1186/s13071-017-2402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeda-Hirschmann G.; Tapia A.; Lima B.; Pertino M.; Sortino M.; Zacchino S.; Arias A. R. d.; Feresin G. E. A new antifungal and antiprotozoal depside from the Andean lichen Protousnea poeppigii. Phytother. Res. 2008, 22 (3), 349–355. 10.1002/ptr.2321. [DOI] [PubMed] [Google Scholar]
- Zeybek C. A.; Zubarioglu T. Nitisinone: a review. Orphan Drugs: Res. Rev. 2017, 7, 25–35. 10.2147/ODRR.S92995. [DOI] [Google Scholar]
- Sterkel M.; Haines L. R.; Casas-Sanchez A.; Owino Adung’a V.; Vionette-Amaral R. J.; Quek S.; Rose C.; Silva dos Santos M.; Garcia Escude N.; Ismail H. M. Repurposing the orphan drug nitisinone to control the transmission of African trypanosomiasis. PLoS Biol. 2021, 19 (1), e3000796. 10.1371/journal.pbio.3000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R.; Chen C. K. M.; Guo R. T.; Wang A. H. J.; Oldfield E. Structures of a potent phenylalkyl bisphosphonate inhibitor bound to farnesyl and geranylgeranyl diphosphate synthases. Proteins: Struct., Funct., Genet. 2008, 73 (2), 431–439. 10.1002/prot.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudock M. P.; Zhang Y.; Guo R.-T.; Cao R.; No J. H.; Liang P.-H.; Ko T.-P.; Chang T.-H.; Chang S.-c.; Song Y.; et al. Inhibition of geranylgeranyl diphosphate synthase by bisphosphonates: a crystallographic and computational investigation. J. Med. Chem. 2008, 51 (18), 5594–5607. 10.1021/jm800325y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari S.; Kim Y. S.; Aripirala S.; Murphy M.; Amzel L. M.; Gabelli S. B. Identifying structural determinants of product specificity in Leishmania major farnesyl diphosphate synthase. Biochemistry 2020, 59, 2751–2759. 10.1021/acs.biochem.0c00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A. T.; McLauchlan C. C.; Dolbecq A.; Mialane P.; Jones M. A. Studies of the effectiveness of bisphosphonate and vanadium-bisphosphonate compounds in vitro against axenic Leishmania tarentolae. Oxid. Med. Cell. Longevity 2016, 2016, 1. 10.1155/2016/9025627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. B.; Grimley J. S.; Lewis J. C.; Heath H. T.; Bailey B. N.; Kendrick H.; Yardley V.; Caldera A.; Lira R.; Urbina J. A.; et al. Bisphosphonates Inhibit the Growth of Trypanosoma b rucei, Trypanosoma c ruzi, Leishmania d onovani, Toxoplasma g ondii, and Plasmodium f alciparum: A Potential Route to Chemotherapy. J. Med. Chem. 2001, 44 (6), 909–916. 10.1021/jm0002578. [DOI] [PubMed] [Google Scholar]
- Rodriguez N.; Bailey B. N.; Martin M. B.; Oldfield E.; Urbina J. A.; Docampo R. Radical cure of experimental cutaneous leishmaniasis by the bisphosphonate pamidronate. J. Infect. Dis. 2002, 186 (1), 138–140. 10.1086/341074. [DOI] [PubMed] [Google Scholar]
- Branco Santos J. C.; de Melo J. A.; Maheshwari S.; de Medeiros W. M. T. Q.; de Freitas Oliveira J. W.; Moreno C. J.; Mario Amzel L.; Gabelli S. B.; Sousa Silva M. Bisphosphonate-Based Molecules as Potential New Antiparasitic Drugs. Molecules 2020, 25 (11), 2602. 10.3390/molecules25112602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussmann R. A.; Angeli C. B.; Peres V. J.; Kimura E. A.; Katzin A. M. Intraerythrocytic stages of Plasmodium falciparum biosynthesize vitamin E. FEBS Lett. 2011, 585 (24), 3985–3991. 10.1016/j.febslet.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Herbas M. S.; Ueta Y. Y.; Ichikawa C.; Chiba M.; Ishibashi K.; Shichiri M.; Fukumoto S.; Yokoyama N.; Takeya M.; Xuan X.; et al. Alpha-tocopherol transfer protein disruption confers resistance to malarial infection in mice. Malar. J. 2010, 9 (1), 101. 10.1186/1475-2875-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-R.; Kim J.-S.; Yun J.-S.; Kim S.; Kim S. Y.; Jang K.; Yang C.-S. Toxoplasma gondii GRA8 induces ATP5A1–SIRT3-mediated mitochondrial metabolic resuscitation: a potential therapy for sepsis. Exp. Mol. Med. 2018, 50 (3), e464–e464. 10.1038/emm.2017.308. [DOI] [PMC free article] [PubMed] [Google Scholar]