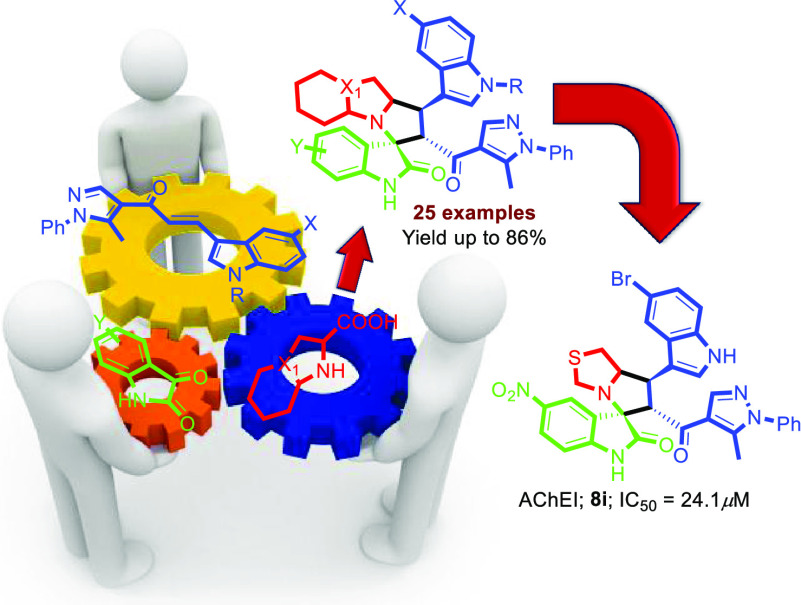
Abstract
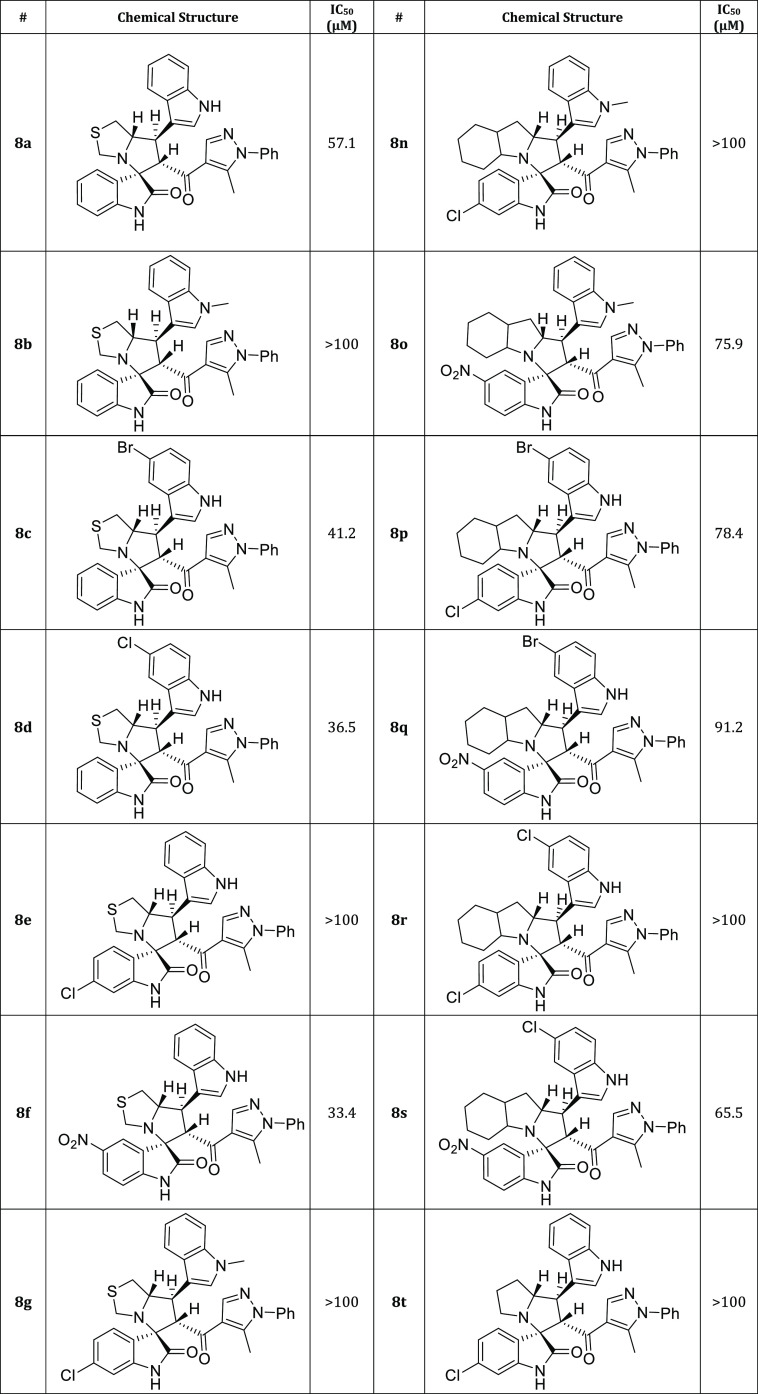
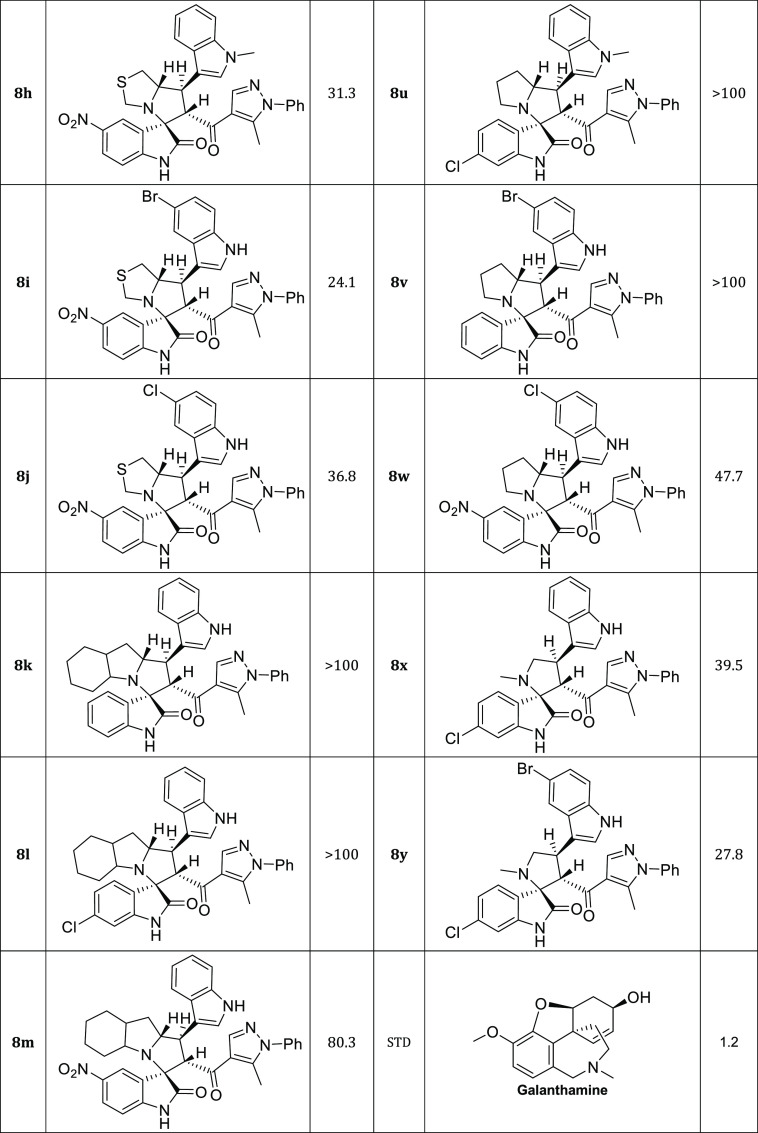
Twenty-five new hits of spirooxindole analogs 8a–y engrafted with indole and pyrazole scaffolds were designed and constructed via a [3+2]cycloaddition (32CA) reaction starting from three components: new chalcone-based indole and pyrazole scaffolds 5a–d, substituted isatins 6a–c, and secondary amines 7a–d. The potency of the compounds were assessed in modulating cholinesterase (AChE) activity using Ellman’s method. Compounds 8i and 8y showed the strongest acetylcholine esterase inhibition (AChEI) with IC50 values of 24.1 and 27.8 μM, respectively. Molecular docking was used to study their interaction with the active site of hAChE.
Introduction
Neurodegeneration is a key aspect of a large number of diseases that come under the umbrella of neurodegenerative disease. Of these different disorders, the most notable are Parkinson’s disease, Huntington’s disease, and Alzheimer’s disease (AD). Alzheimer’s disease (AD) symptoms are memory loss, impairment of cognitive functions, and dementia. AD involves two major neuropathological hallmarks causing neuronal dysfunctions and cell death: the presence of extracellular amyloid β-peptide (Aβ) deposits (senile plaques) and aggregates of the hyperphosphorylated tau protein (neurofibrillary tangles)1 along with the tau hyperphosphorylation are the most proposed pathogenetic mechanisms,2 and mitochondrial cascade hypothesis has attracted much interest recently.3 Other much debatable AD hypotheses are the tau hypothesis,4 cholesterol hypothesis,5 inflammatory hypothesis,6 oxidative stress hypothesis,7 metal hypothesis,8 vascular hypothesis,9 and cell cycle hypothesis.10 Up to date, rivastigmine, galantamine, and donepezil represent the only ChE inhibitors approved for AD treatment, differing in chemical structures and pharmacologic and pharmacokinetic profiles. To design and discover a new agent that might work as ChE inhibitors is a challenge.
Heterocycles having azoles as a core structure have been discovered for several applications.11 Pyrazoles are one of the important heterocycles, which exhibited significant properties in material sciences,12 agriculture development,13 medicine,14 and pharmacological applications.15 Among pharmacological applications are antibiotic,16 sensors,17 pesticide,18 antibacterial,19 and antifungal activities.20 On the other hand, several molecules engrafted with the pyrazole scaffold have shown high efficacy toward antiviral,21 antitumor,22 anti-inflammatory,23 antioxidant,24 and antidepressant activities.25 Indeed, many drugs incorporated the pyrazole moiety employed for the treatment of metabolic disorder diseases such as Alzheimer’s,26 Parkinson’s,27 and neuroprotective,28 which makes this pharmacophore very attractive for drug discovery. One representative example of advanced glycation inhibitors reported by Han et al. is that this agent is based on the pyrazole-5-carboxamide as a core structure.29 However, Turkan et al. have reported substituted pyrazole derivatives, which have been discovered as potent cholinesterase inhibitors.30
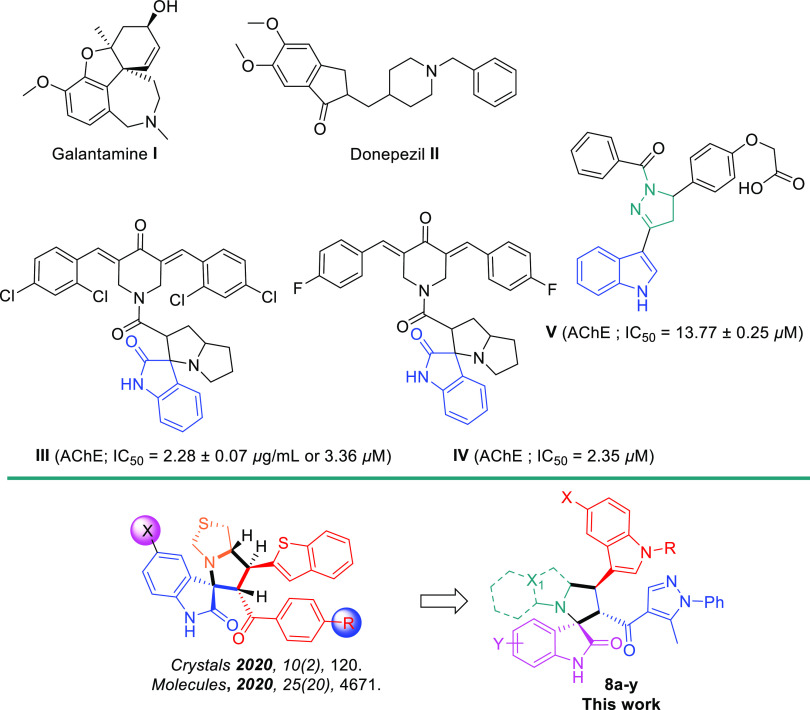
Spirooxindoles exhibit a broad range of biological effects and are well-tolerated in biomedical applications.31 Their applications use AChEs.32 Kia et al. reported representative examples, including spirooxindoles engrafted with piperidine and pyrrolizine scaffolds, which are found to be beneficial for AChE (compound III; IC50 = 3.36 μM or 2.28 ± 0.07 μg/mL) (Figure 1),32d and another representative example based on mono- and bis-spiro-pyrrolidines, where the hit IV shows high efficacy against AChE with an IC50 value of 2.35 μM (Figure 1).32f Chigurupati et al. reported indolopyrazolines with the high biochemical application against AChE inhibition (V; IC50 = 13.77 ± 0.25 lM), respectively.33 Extending our recent efforts on the development of cholinesterase inhibition, Barakat et al. reported a new series of spirooxindoles engrafted with the benzo[b]thiophene scaffold, which were found to exhibit moderate potential against AD (Figure 1).32h,34 The above reports inspired the investigation of several pharmacophores inside the rigid spirooxindole privileged structure, such as indole and pyrazole scaffolds, which might act as better AChE inhibitors.
Figure 1.
Significant acetylcholinesterase (AChE) inhibitory activity of representative spirooxindole analogues.
Several progresses have been devoted toward the synthesis of spirooxindoles recently.35 In between these approaches, the [3 + 2] cycloaddition reaction protocol was efficient and promising to afford the spirooxindole privileged structures with several stereogenic centers.36
In this paper, we described the [3 + 2] cycloaddition reaction approach for the synthesis of new spirooxindoles based on a new chalcone engrafted with indole and pyrazole motifs. Many substituted isatins and amino acids were also investigated. The biochemical potential of AChE was also studied.
Results and Discussion
Synthesis of Spirooxindole Analogs 8a–y Engrafted with Indole and Pyrazole Scaffolds
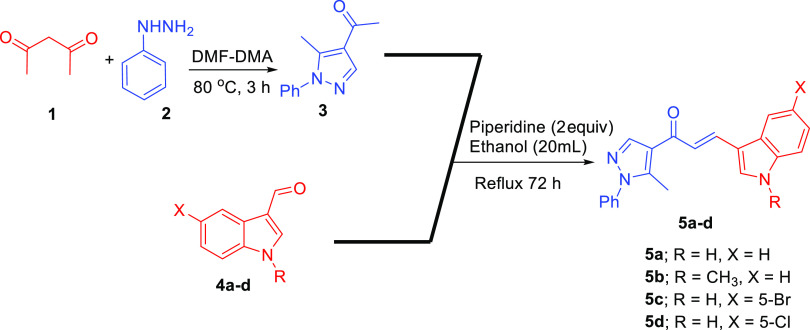
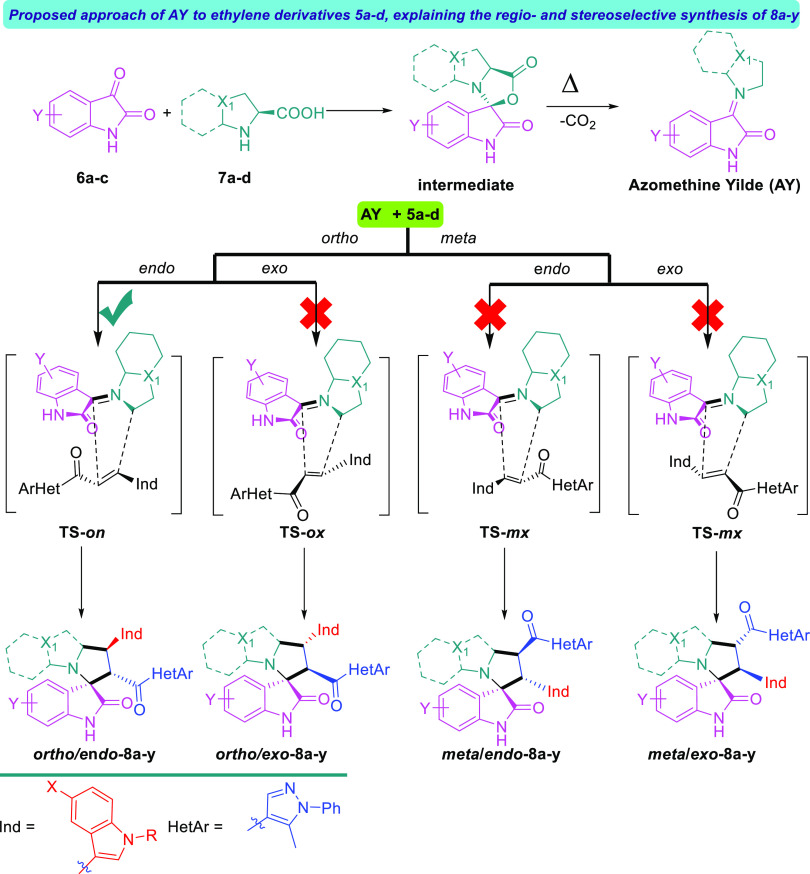
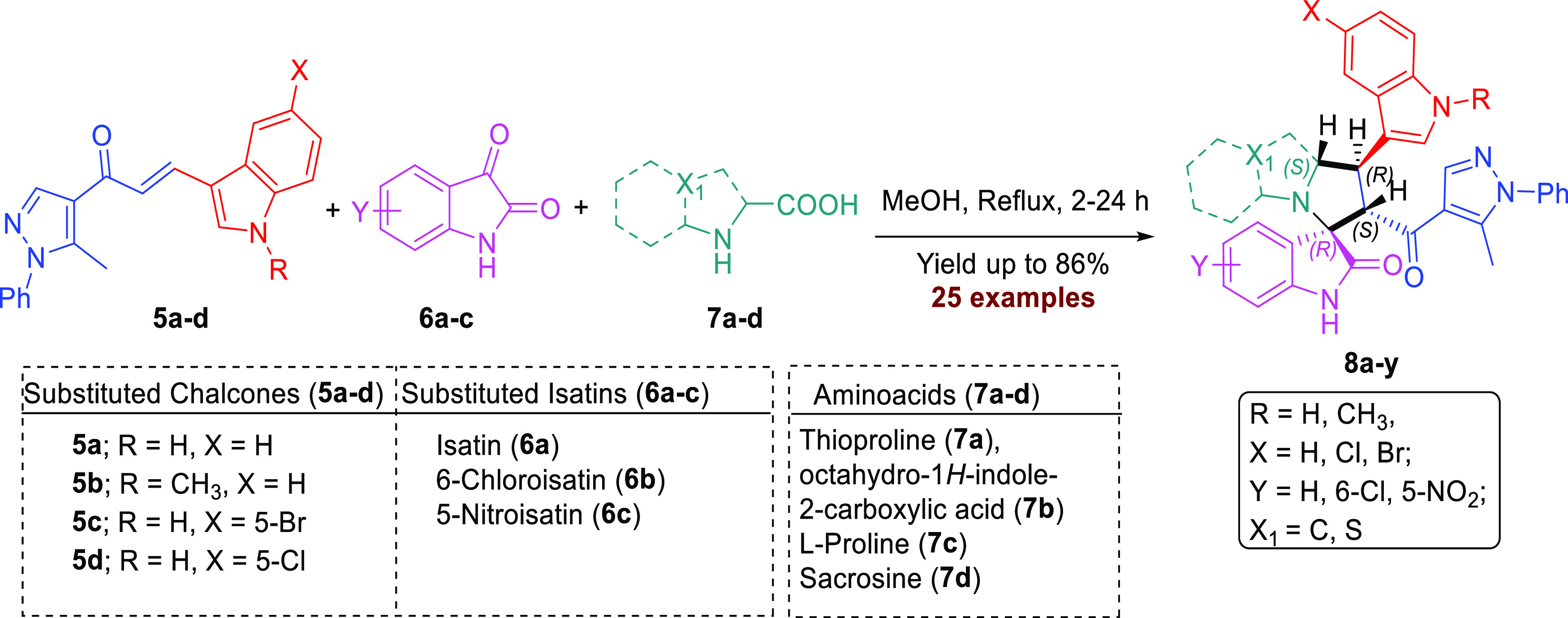
The indole and pyrazole scaffolds as interesting pharmacophores are combined into the spirooxindole analogs for exploring the acetylcholinesterase (AChE) inhibitory activity. Initially, we synthesized the new chalcones 5a–d by aldol condensation of acetyl-pyrazole and substituted indole-3-carbaldehyde in basic condition under reflux for 72 h. The new chalcone-based indole and pyrazole scaffolds, which act as a synthon for the [3 + 2] cycloaddition (32CA) reaction, are depicted in Scheme 1. Subsequently, the new series of spirooxindole analogues tethered indole and pyrazole scaffolds were constructed via a one-pot multicomponent reaction approach37 in MeOH under reflux conditions for 2–24 h (Scheme 2). Twenty-five examples were achieved by variation of many substituted isatins 6a–c with different electronic effects (isatin 6a; 6-chloroisatin 6b; 5-nitroisatin 6c) with four different amino acids 7a–d (thioproline 7a, octahydro-1H-indole-2-carboxylic acid 7b; l-proline 7c; sacrosine 7d). The spirooxindole analogues engrafted with indole and pyrazole scaffolds were isolated in a single regio- and diastereoselective isomer in acceptable to excellent chemical yield up to 86%. The proposed mechanism, as shown in Scheme 3, proceeded via an ortho/endo 32CA approach.38 The optical rotation of the synthesized compounds was measured, and the regio- and diastereoselectivity of the cycloadducts were confirmed. Single-crystal X-ray diffraction analysis of compound 8c confirmed that our hypothesis belongs to the final stereoselectivity of the cycloadducts of the final compounds. The absolute configurations of products 8a–y were assigned based on the obtained x-ray diffraction analysis as follows for the 4 stereogenic centers as R, S, R, S.
Scheme 1. Synthesis of the Chalcone Engrafted with Indole and Pyrazole Scaffolds 5a–d.
Scheme 2. Synthesis of the Spirooxindole Analogues Engrafted with Indole and Pyrazole Scaffolds 8a–y.
Scheme 3. Proposed Approach for the [3 + 2] Cycloaddition Reaction, Explaining the Regio- and Diastereoselective Synthesis.
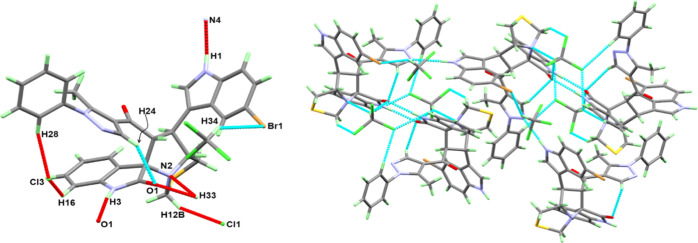
Crystal Structure Description
The X-ray structure of 8c showing atom numbering and thermal ellipsoids drawn at a 30% probability level is shown in Figure 2. The structure agreed very well with the spectral analyses and revealed the presence of asymmetric centers at C9, C21, C10, and C13. The crystal data and structure refinement details are depicted in Table 1. The compound crystallized in the triclinic system and the P1̿ space group with unit cell parameters of a = 12.71740(10) Å, b = 15.72830(10) Å, c = 19.3853(2) Å, and β = 105.9640(10)°. The unit cell volume is 3727.97(6) Å3 with Z = 4. The asymmetric unit comprised one molecule and two chloroform molecules as the crystal solvent. The selected bond distances and angles are listed in Table S1 (Supporting Information).
Figure 2.
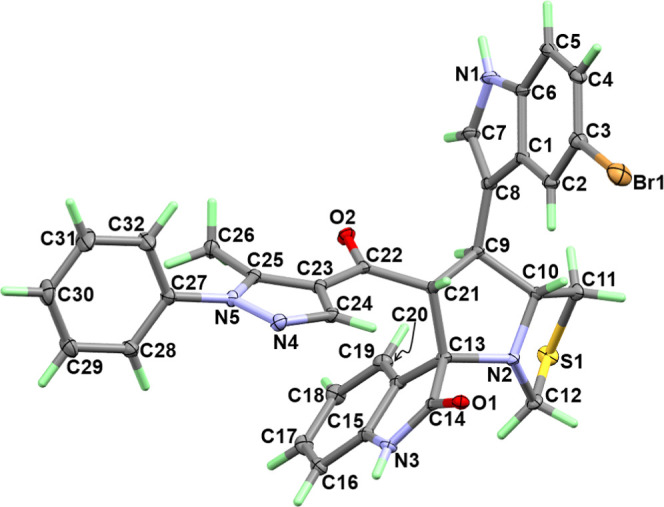
Atom numbering and thermal ellipsoids at a 30% probability level for 8c.
Table 1. Crystal Data.
| 8c | |
|---|---|
| CCDC | 2105913 |
| empirical formula | C34H28BrCl6N5O2S |
| fw | 863.28 |
| temp (K) | 120(2) |
| λ (Å) | 1.54184 |
| cryst syst | monoclinic |
| space group | P21/n |
| a (Å) | 12.71740(10) |
| b (Å) | 15.72830(10) |
| c (Å) | 19.3853(2) |
| β (deg) | 105.9640(10) |
| V (Å3) | 3727.97(6) |
| Z | 4 |
| ρcalc (Mg/m3) | 1.538 |
| μ (Mo Kα) (mm–1) | 6.305 |
| no. reflns. | 38891 |
| unique reflns. | 7824 |
| completeness to θ = 67.684° | 100.0% |
| GOOF (F2) | 1.024 |
| Rint | 0.0352 |
| R1a (I ≥ 2σ) | 0.0354 |
| wR2b (I ≥ 2σ) | 0.0861 |
R1 = Σ||Fo| – |Fc||/Σ|Fo|.
wR2 = [Σ[w(Fo2 – Fc2)2]/ Σ[w(Fo2)2]]1/2.
The molecular packing in 8c is controlled by strong N–H...O and N–H...N hydrogen bonds as well as weak C––H...X interactions (X=Cl, Br, N, or O). The corresponding hydrogen bond parameters are listed in Table S2 (Supporting Information) and are shown in the left part of Figure 3. The hydrogen bond network is shown in the right part of the same figure.
Figure 3.
Hydrogen bond contacts (left) and packing of molecular units via hydrogen bonding interactions (right).
Acetylcholine Esterase Inhibitory Activity
The ability of the synthesized compounds to inhibit acetylcholine esterase (AChE) was evaluated using Ellman’s method.39 Compounds 8i and 8y showed the strongest acetylcholine esterase inhibition (AChEI) with IC50 values of 24.1 and 27.8 μM, respectively.
Four Compounds; 8c, 8d, 8f, 8h, 8j, 8w, and 8x showed moderate activity inhibitory activity (with IC50 ≤ 50 μM). Compounds 8m, 8o, 8p, 8q, and 8s had weak activity (with IC50 values 65–90 μM), while compounds 8b, 8e, 8q, 8k, 8e, 9r, 8r, 8t, 8u, and 8v were in active with IC50 > 100 μM (Table 2).
Table 2. AChE Inhibitory Activity of the Synthesized Spirooxindole Analogues Engrafted with Indole and Pyrazole Scaffolds 8a–y.
Molecular Docking Study
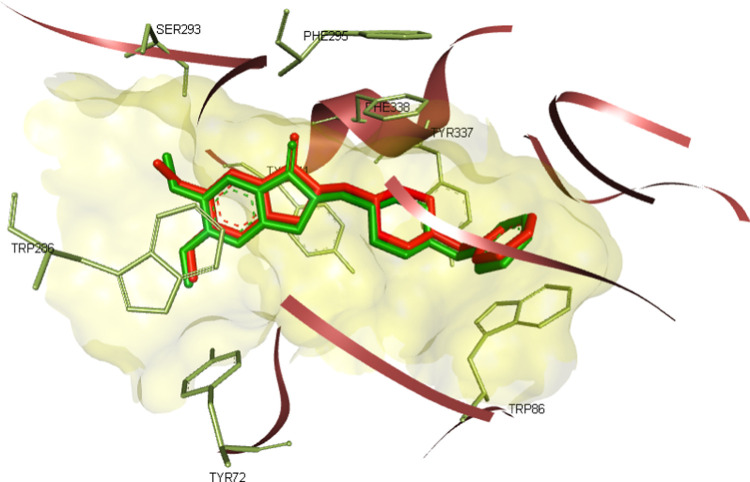
Molecular docking has been used extensively to identify and explain the molecular mechanism of several lead compounds in drug discovery.40 The software validation revealed that it was able to reproduce the experimental pose with root-mean-square deviation (RMSD) equal to 0.39 (Figure 4). Since in vitro enzyme inhibition showed that compounds 8i, 8h, and 8y are the most active among the synthesized compounds with moderate inhibition activity, we used molecular docking to gain insights into the molecular interaction of the compound with the active site of hACHE.
Figure 4.
Donepezil (Red) docked in the active site of ACHE (4EY7) and overlaid with cocrystallized ligand (Green) RMSD = 0.39.
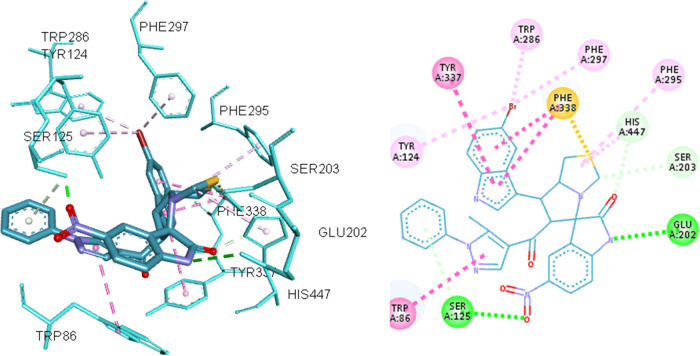
In the context of the total energy required for binding, the cocrystallized ligand achieved much better binding affinity than the 3 compounds, yet their binding energy was found to be reasonable with respect to their moderate activity in the enzyme inhibition assay, as presented in Table 3. Post docking analysis showed that compound 8i has different binding modes rather than compounds 8h and 8y, as the first one tends to bind in the middle of the gorge of hACHE. On the other hand, compounds 8h and 8y showed most of their interactions with amino acids located at the entrance of the gorge. For example, compound 8i was able to interact with amino acids in diverse sites in the enzyme such as TRP-86 and TYR-337 in the anionic site, Ser-203 and HIS-447 in the catalytic site, PHE-295 and PHE-297 in the acyl binding site, and formed 2 hydrogen bonds with SER-125 and GLU-202, as depicted in Figure 5, these interactions are well reported to be important for achieving good inhibitory activity.41
Table 3. Binding Energy of Compound Docked in the Binding Site of the hACHE Active Sitea.
| compound | total energy | VDW | H-bond | |
|---|---|---|---|---|
| 1. | 8i | –127.335 | –121.335 | –6 |
| 2. | 8y | –126.589 | –118.253 | –8.336 |
| 3. | 8h | –123.962 | –118.983 | –4.979 |
| 4. | cocrystallized ligand | –149.55 | –139.4 | –10.15 |
VDW = Van der Waals force and H-Bond = Hydrogen bond.
Figure 5.
8i docked in the active site of ACHE (4EY7) and corresponding two-dimensional (2D) presentation. The H-bond is represented by green dotted lines, hydrophobic interactions are represented by magenta dotted lines, and Pi-sulfur interaction is represented by orange dotted lines.
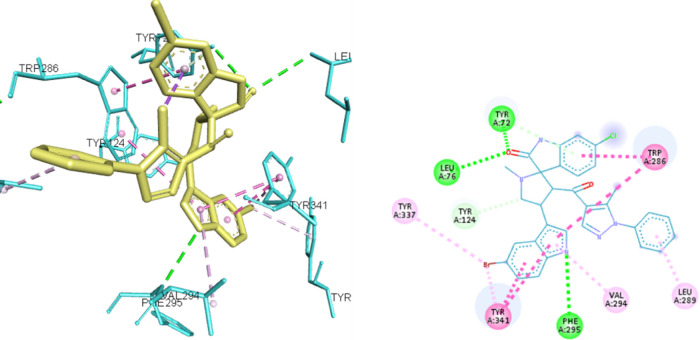
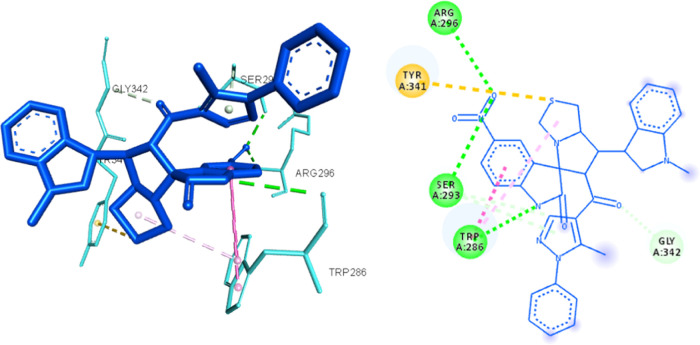
On the other hand, compound 8y showed interactions only with TYR-337 and PHE-295 from the anionic and acyl binding sites, respectively, but with less hydrophobic interaction than 8i. Nevertheless, they were able to access the peripheral anionic site by interacting with amino acids, such as TYR-124, TRY-341, and TRP-286, as shown in Figures 6 and 7, which might explain their ability to achieve the moderate inhibitory effect as observed in the enzyme inhibition assay.
Figure 6.
8y docked in the active site of ACHE (4EY7) and corresponding 2D presentation. The H-bond is represented by green dotted lines, hydrophobic interactions are represented by magenta dotted lines, and Pi-sulfur interaction is represented by orange dotted lines.
Figure 7.
8h docked in the active site of ACHE (4EY7) and corresponding 2D presentation. The H-bond is represented by green dotted lines, hydrophobic interactions are represented by magenta dotted lines, and Pi-sulfur interaction is represented by orange dotted lines.
Conclusions
We summarized in this paper that we had synthesized 25 hits based on a spirooxindole scaffold engrafted with two other pharmacophores, including indole and pyrazole moieties. The results of the AChE assay show that compounds 8i and 8y show the strongest acetylcholine esterase inhibition (AChEI) with IC50 values of 24.1 and 27.8 μM, respectively. The AChE activity exhibited promising results, which make them candidates for further research.
Experimental Section
General information
Phenylhydrazine, acetylacetone, N,N-dimethylformamide-dimethyl acetal (DMF-DMA), Piperidine, and NaOH were purchased from Aldrich and used as received. All of the indole derivatives were purchased from Aldrich and used as is. All bases were used as received (in air) or dried under vacuum at 100 °C (under an inert atmosphere). All solvents were used as received when experiments were conducted in air. Flash chromatography was performed on 100–200 mesh silica gel. 1H- and 13C-nuclear magnetic resonance (NMR) spectra were recorded on JEOL-700 MHz spectrometers at ambient temperature in CDCl3 & DMSO-d6, which were purchased from Sigma-Aldrich. Chemical shifts (ppm) are referenced to the residual solvent peak. Coupling constants, J, are given in hertz. Abbreviations used in the designation of the signals: s = singlet, d = doublet, dd = doublet of doublets, ddd = doublet of doublet of doublets, dt = doublet of triplets, t = triplet, td = triplet of doublets, and m = multiplet. All melting points were measured on a Gallenkamp melting point apparatus in open glass capillaries and are uncorrected. IR Spectra were measured as KBr pellets on a Nicolet 6700 FT-IR spectrophotometer. Specific rotations were recorded in ‘A KRÜSS Optronic GmbH P8000 polarimeter.
Synthesis of 1-(5-Methyl-1-phenyl-1H-pyrazol-4-yl)ethanone (3)
N,N-Dimethylformamide-dimethyl acetal (DMF-DMA) (17.85 g, 0.15 mol) was added to acetylacetone 1 (0.1 mol) and stirred for 10 min at ambient temperature, followed by the addition of phenylhydrazine derivative 2 (0.1 mol), and the reaction mixture was heated at 70–80 °C for 24 h. The completion of the reaction was monitored by thin-layer chromatography (TLC) (20% EA/n-hexane). The reaction mixture was then allowed to cool and kept in a fridge for 24 h and a white solid precipitated out, which was isolated by simple filtration and washed with diethyl ether to afford pure white product 3 (5 g, 25 mmol, 25% yield). The MLs part was concentrated and purified using a column to afford another 6 g of pure white product 3 (30 mmol, 30%). The overall yield of acetyl-pyrazole-3 (11.0 g, 55%).42
m.p.: 88–90 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.99 (s, 1H, pyrazole-H), 7.52–7.46 (m, 2H, Ar-H), 7.44 (d, J = 6.8 Hz, 1H, Ar-H), 7.41–7.36 (m, 2H, Ar-H), 2.56 (s, 3H, COCH3), 2.47 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ (ppm) = 193.56 (CO), 143.05, 141.97, 138.58, 129.36, 128.89, 125.61, 121.16, 28.75 (COCH3), 12.44 (CH3); IR (KBr, cm–1): 3060, 3001, 1660, 1597, 1545, 1502, 1457, 1399, 1382, 1363, 1276, 1238, 1196, 1171, 1011, 937, 884, 866, 769, 718, 690, 662, 637, 558; [anal. calcd. for C12H12N2O: C, 71.98; H, 6.04; N, 13.99; found: C, 72.07; H, 6.01; N, 13.94]; LC/MS (ESI, m/z): found 201 [M + H]+; exact mass 200.09 for C12H12N2O. All of the analytical data are in accordance with the reported literature.42
Synthesis of Chalcones (5a–d) from Acetyl-pyrazole (3) and Substituted Indole-3-Carboxyaldehyde (4a–d) (GP1)
Compound 3 (1 g, 5 mmol) and substituted indole-3-carboxyaldehyde 4a–d (5 mmol) were dissolved in ethanol (20 mL) in a 100 mL round bottom flask. Piperidine (950 mg, 2 equiv) was added to the reaction mixture and refluxed at 80 °C 48–72 h. The completion of the reaction was monitored by TLC (30% EA/n-hexane). Then, the solid precipitated out, which was isolated by simple filtration and washed with ethanol to afford pure white/yellow product 5a–d (70–80% yield).
Synthesis of (E)-3-(1H-Indol-3-yl)-1-(5-methyl-1-phenyl-1H-pyrazol-4-yl)prop-2-en-1-one (5a)
Following the general procedure (GP1), acetyl-pyrazole 3 (1.0 g, 5.0 mmol) and indole-3-carboxyaldehyde 4a (0.87 g, 6.0 mmol) produce pyrazolenone-5a (yield 0.9 g, 55%); m.p.: 222–224; 1H NMR (400 MHz, DMSO-d6): δ (ppm) = δ 11.85 (s, 1H, NH), 8.59 (s, 1H, Ar-H), 8.17–8.11 (m, 1H, Ar-H), 8.08 (s, 1H, Ar-H), 8.00 (d, J = 15.4 Hz, 1H, CH=CH), 7.58 (d, J = 4.3 Hz, 4H, Ar-H), 7.54–7.41 (m, 3H, Ar-H & CH=CH), 7.29–7.17 (m, 2H, Ar-H), 2.62 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ (ppm) = δ 184.19 (CO), 142.69, 141.53, 138.53, 137.47, 136.61, 132.48, 129.30, 128.56, 125.41, 125.20, 122.58, 121.61, 121.00, 120.51, 118.15, 112.55, 112.33, 12.20 (CH3); IR (KBr, cm–1) νmax = 3158, 3106, 3047, 2877, 1649, 1638, 1581, 1521, 1502, 1453, 1384, 1347, 1279, 1255, 1229, 1217, 1190, 1140, 1115, 1072, 1064, 1036, 1034, 1004, 974, 945, 893, 879, 852, 834, 762, 734, 714, 696, 656, 641, 603, 593, 558, 507; LC/MS (ESI, m/z): 328.2 [M + H]+, exact mass 327.14 for C21H17N3O.
Synthesis of (E)-1-(5-Methyl-1-phenyl-1H-pyrazol-4-yl)-3-(1-methyl-1H-indol-3-yl)prop-2-en-1-one (5b)
Following the general procedure (GP1), acetyl-pyrazole 3 (1.0 g, 5.0 mmol) and indole-1-methyl-3-carboxyaldehyde 4b (0.95 g, 6.0 mmol) produce pyrazolenone-5b (yield 1.0 g, 56%); m.p.: 182–183; 1H NMR (400 MHz, DMSO-d6): δ (ppm) = δ 8.58 (s, 1H, Ar-H), 8.15 (d, J = 7.8 Hz, 1H, Ar-H), 8.04 (s, 1H, Ar-H), 7.95 (d, J = 15.5 Hz, 1H, CH=CH), 7.58 (d, J = 4.4 Hz, 3H, Ar-H), 7.57–7.49 (m, 3H, Ar-H), 7.46 (d, J = 15.5 Hz, 1H, CH=CH), 7.33–7.25 (m, 2H, Ar-H), 3.85 (s, 3H, CH3), 2.62 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ (ppm) = δ 184.09 (CO), 142.71, 141.53, 141.49, 138.51, 137.93, 135.98, 135.89, 129.30, 128.56, 125.63, 125.40, 122.66, 121.59, 121.26, 120.67, 120.61, 118.17, 118.10, 111.56, 110.75, 110.66, 32.97 (CH3), 12.15 (CH3); IR (KBr, cm–1) νmax = 3108, 3045, 1643, 1569, 1524, 1501, 1472, 1464, 1386, 1373, 1341, 1281, 1259, 1219, 1187, 1178, 1157, 1132, 1075, 1039, 1003, 937, 855, 841, 821, 771, 751, 699, 681, 654, 540; LC/MS (ESI, m/z): found 342.2 [M + H]+, exact mass 341.15 for C22H19N3O.
Synthesis of (E)-3-(5-Bromo-1H-indol-3-yl)-1-(5-methyl-1-phenyl-1H-pyrazol-4-yl)prop-2-en-1-one (5c)
Following the general procedure (GP1), acetyl-pyrazole 3 (1.0 g, 5.0 mmol) and 5-bromoindole-3-carboxyaldehyde 4c (1.34 g, 6.0 mmol) produce pyrazolenone-5c (yield 0.85 g, 42%); m.p.: 214–215; 1H NMR (400 MHz, DMSO-d6): δ (ppm) = δ 12.02 (s, 1H, NH), 8.63 (s, 1H, Ar-H), 8.26 (d, J = 1.9 Hz, 1H, Ar-H), 8.14 (s, 1H, Ar-H), 7.95 (d, J = 15.5 Hz, 1H, CH=CH), 7.60–7.55 (m, 4H, Ar-H + CH=CH), 7.53–7.49 (m, 1H, Ar-H), 7.46 (d, J = 3.9 Hz, 1H, Ar-H), 7.45 (d, J = 3.1 Hz, 1H, Ar-H), 7.35 (dd, J = 8.6, 1.9 Hz, 1H, Ar-H), 2.61 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ (ppm) = δ 184.22 (CO), 142.77, 141.76, 138.50, 136.07, 135.67, 132.99, 129.30, 128.56, 127.02, 125.39, 125.18, 122.39, 121.52, 119.03, 114.25, 113.74, 112.17, 12.19 (CH3); IR (KBr, cm–1) νmax = 3153, 3077, 3030, 2934, 2900, 1643, 1559, 1500, 1454, 1433, 1394, 1370, 1299, 1273, 1243, 1223, 1182, 1136, 1095, 1038, 1022, 1007, 956, 938, 881, 851, 824, 789, 758, 689, 660, 636, 609, 554; LC/MS (ESI, m/z): found 406.6 [M(79Br) + H]+, 408.1 [M(81Br) + H]+; exact mass 405.05 for C21H16BrN3O.
Synthesis of (E)-3-(5-Chloro-1H-indol-3-yl)-1-(5-methyl-1-phenyl-1H-pyrazol-4-yl) prop-2-en-1-one (5d)
Following the general procedure (GP1), acetyl-pyrazole 3 (1.0 g, 5.0 mmol) and 5-chloroindole-3-carboxyaldehyde 4d (1.07 g, 6.0 mmol) produce pyrazolenone-5d (yield 0.9 g, 50%); m.p.: 237–238; 1H NMR (400 MHz, DMSO-d6): δ (ppm) = δ 11.95 (bs, 1H, NH), 8.65 (s, 1H, Ar-H), 8.20–8.05 (m, 2H, Ar-H), 7.95 (d, J = 15.4 Hz, 1H, CH=CH), 7.57 (d, J = 4.4 Hz, 4H, Ar-H), 7.50 (dd, J = 8.7, 4.6 Hz, 2H, Ar-H), 7.46 (d, J = 15.7 Hz, 1H, CH=CH), 7.23 (dd, J = 8.2, 2.1 Hz, 1H, Ar-H), 2.61 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ (ppm) = δ 184.24 (CO), 142.78, 141.80, 138.52, 135.86, 135.76, 133.34, 129.30, 128.56, 126.35, 125.73, 125.40, 122.60, 121.53, 119.55, 118.92, 113.81, 112.30, 12.19 (CH3); IR (KBr, cm–1) νmax = 3107, 2896, 1648, 1637, 1576, 1518, 1500, 1453, 1395, 1383, 1373, 1314, 1251, 1212, 1142, 1123, 1072, 1047, 1005, 973, 944, 897, 863, 837, 794, 765, 740, 697, 651, 643, 609, 594, 575; LC/MS (ESI, m/z): found 362.1 [M(35Cl) + H]+, 364.1 [M(37Cl) + H]+; exact mass 361.09 for C21H16ClN3O.
Synthesis of Spirooxindole Derivatives 8a–y
General Procedure (GP2)
Chalcones 5a–d (0.25 mmol), isatin derivatives 6a–c (0.25 mmol), and amino acids 7a–d (1.5 equiv, 0.37 mmol) were dissolved in methanol (20 mL), and the reaction mixture was refluxed for 2–4 h. Finally, the products were isolated by flash column chromatography, using 1–3% MeOH/DCM to afford pyrazole spirooxindole 8a–y.
(3R,6′S,7′R,7a’S)-7′-(1H-Indol-3-yl)-6′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-1′,6′,7′,7a’-tetrahydro-3′H-spiro[indoline-3,5′-pyrrolo[1,2-c]thiazol]-2-one (8a)
Following the general procedure (GP2), chalcone 5a (82 mg, 0.25 mmol), isatin 6a (37 mg, 0.25 mmol), and thioproline 7a (50 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield light yellow solid compound 8a; yield (85 mg, 62%); m.p.: 138–139 °C; [α]D25 = −16.19° (c 0.13, MeOH); 1H NMR (700 MHz, CDCl3) δ (ppm) = 8.59 (s, 1H, NH), 8.33 (s, 1H, NH), 8.10 (d, J = 7.1 Hz, 1H, Ar-H), 7.92 (s, 1H, Ar-H), 7.72 (d, J = 7.6 Hz, 1H, Ar-H), 7.40–7.33 (m, 4H, Ar-H), 7.28 (d, J = 2.5 Hz, 1H, Ar-H), 7.20–7.17 (m, 2H, Ar-H), 7.17–7.14 (m, 3H, Ar-H), 7.04–7.01 (m, 1H, Ar-H), 6.64 (d, J = 7.7 Hz, 1H, Ar-H), 4.79 (d, J = 11.8 Hz, 1H, CHCO), 4.66–4.61 (m, 1H, NCH), 4.25 (dd, J = 11.8, 9.7 Hz, 1H, NCHCH), 3.94 (d, J = 10.5 Hz, 1H, NCH2), 3.61 (d, J = 10.5 Hz, 1H, NCH2), 3.11–3.03 (m, 2H, SCH2), 1.92 (s, 3H, CH3); 13C NMR (176 MHz, CDCl3) δ (ppm) = 191.09 (CO), 180.75 (CO), 143.50, 141.28, 141.03, 138.31, 136.74, 129.76, 129.29, 129.15, 128.88, 126.46, 125.47, 124.08, 123.02, 122.28, 122.27, 121.00, 119.93, 119.84, 113.65, 111.58, 109.65, 75.12, 73.75, 63.86, 55.16, 43.58, 37.41, 11.75 (CH3); IR (KBr, cm–1) νmax = 3288, 3058, 2921, 1733, 1722, 1717, 1699, 1694, 1682, 1674, 1668, 1661, 1652, 1619, 1615, 1597, 1538, 1532, 1504, 1470, 1456, 1393, 1337, 1222, 1119, 935, 875, 807, 749, 695; [anal. calcd. for C32H27N5O2S: C, 70.44; H, 4.99; N, 12.83; found: C, 70.31; H, 5.05; N, 12.91]; LC/MS (ESI, m/z): found 546.4 [M + H]+, exact mass 545.19 for C32H27N5O2S.
(3R,6′S,7′R,7a’S)-6′-(5-Methyl-1-phenyl-1H-pyrazole-4-carbonyl)-7′-(1-methyl-1H-indol-3-yl)-1′,6′,7′,7a’-tetrahydro-3′H-spiro[indoline-3,5′-pyrrolo[1,2-c]thiazol]-2-one (8b)
Following the general procedure (GP2), chalcone 5b (85 mg, 0.25 mmol), isatin 6a (37 mg, 0.25 mmol), and thioproline 7a (50 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield light yellow solid compound 8b; yield (101 mg, 72%); m.p.: 186–187 °C; [α]D25 = −12.53° (c 0.13, MeOH); 1H NMR (700 MHz, CDCl3) δ (ppm) = 8.80 (s, 1H, NH), 8.09 (d, J = 7.24 Hz, 1H, Ar-H), 7.92 (s, 1H, Ar-H), 7.72 (d, J = 7.63 Hz, 1H, Ar-H), 7.39–7.35 (m, 3H, Ar-H), 7.29 (ddd, J = 8.1, 1.4, 0.7 Hz, 1H, Ar-H), 7.25 (dd, J = 6.9, 1.3 Hz, 1H, Ar-H), 7.21 (dd, J = 5.0, 1.4 Hz, 1H, Ar-H), 7.19 (s, 1H, Ar-H), 7.19–7.16 (m, 2H, Ar-H), 7.16–7.12 (m, 2H, Ar-H), 7.03 (td, J = 7.6, 1.1 Hz, 1H, Ar-H), 6.65 (d, J = 7.73 Hz, 1H, Ar-H), 4.77 (d, J = 11.8 Hz, 1H, CHCO), 4.67–4.61 (m, 1H, NCH), 4.22 (dd, J = 11.8, 9.8 Hz, 1H, NCHCH), 3.94 (d, J = 10.6 Hz, 1H, NCH2), 3.74 (s, 3H, CH3), 3.61 (d, J = 10.5 Hz, 1H, NCH2), 3.07 (d, J = 4.3 Hz, 2H, SCH2), 1.92 (s, 3H, CH3); 13C NMR (176 MHz, CDCl3) δ (ppm) = 191.09 (CO), 180.96 (CO), 143.43, 141.25, 141.21, 138.27, 137.40, 129.70, 129.23, 129.03, 128.81, 127.60, 126.88, 125.45, 124.02, 122.13, 121.80, 120.97, 119.90, 119.38, 112.03, 109.75, 109.58, 75.20, 73.86, 63.89, 55.20, 43.45, 37.35, 32.80 (CH3), 11.67 (CH3); IR (KBr, cm–1) νmax = 3237, 2923, 1738, 1733, 1722, 1699, 1694, 1682, 1674, 1668, 1661, 1652, 1645, 1634, 1622, 1615, 1597, 1557, 1538, 1532, 1505, 1470, 1456, 1398, 1329, 1229, 1180, 1156, 1115, 1069, 1013, 934, 801, 741, 695; [anal. calcd. for C33H29N5O2S: C, 70.82; H, 5.22; N, 12.51; found: C, 71.01; H, 5.15; N, 12.39]; LC/MS (ESI, m/z): found 560.4 [M + H]+, exact mass 559.14 for C33H29N5O2S.
(3R,6′S,7′R,7a’S)-7′-(5-Bromo-1H-indol-3-yl)-6′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-1′,6′,7′,7a’-tetrahydro-3′H-spiro[indoline-3,5′-pyrrolo[1,2-c]thiazol]-2-one (8c)
Following the general procedure (GP2), chalcone 5c (102 mg, 0.25 mmol), isatin 6a (37 mg, 0.25 mmol), and thioproline 7a (50 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield light yellow solid compound 8c; yield (59 mg, 38%); m.p.: 180–181 °C; [α]D25 = −12.53° (c 0.11, MeOH); 1H NMR (700 MHz, CDCl3) δ (ppm) = 9.05 (s, 1H, NH), 8.66 (s, 1H, NH), 8.18 (s, 1H, Ar-H), 7.98 (s, 1H, Ar-H), 7.69 (d, J = 7.5 Hz, 1H, Ar-H), 7.38–7.35 (m, 4H, Ar-H), 7.20–7.18 (m, 1H, Ar-H), 7.15–7.13 (m, 2H, Ar-H), 7.03 (d, J = 7.6 Hz, 1H, Ar-H), 6.62 (d, J = 7.7 Hz, 1H, Ar-H), 4.63 (d, J = 11.6 Hz, 1H, CHCO), 4.50–4.49 (ddd, J = 9.4, 6.2, 2.5 Hz, 1H, NCH), 4.21 (m, 1H, NCHCH), 3.92 (d, J = 10.4 Hz, 1H, NCH2), 3.60 (d, J = 10.4 Hz, 1H, NCH2), 3.08–3.04 (m, 1H, SCH2), 3.03–3.98 (m, 1H, SCH2), 1.91 (s, 3H, CH3); 13C NMR (176 MHz, CDCl3) δ (ppm) = 191.02 (CO), 180.91 (CO), 143.59, 141.14, 138.23, 135.18, 129.84, 129.33, 128.96, 128.58, 127.75, 127.12, 125.47, 125.19, 124.00, 123.78, 122.33, 122.04, 120.91, 113.74, 113.18, 113.03, 109.80, 75.06, 74.25, 65.41, 54.90, 42.63, 37.28, 11.70 (CH3); IR (KBr, cm–1) νmax = 3273, 2924, 1721, 1668, 1617, 1597, 1537, 1503, 1470, 1455, 1396, 1331, 1283, 1223, 1179, 1099, 934, 884, 807, 795, 762, 752, 694, 656, 604; [anal. calcd. for C32H26BrN5O2S: C, 61.54; H, 4.20; N, 11.21; found: C, 61.43; H, 4.35; N, 11.27]; LC/MS (ESI, m/z): found 624.7 [M(79Br) + H]+, 626.1 [M(81Br) + H]+; exact mass 623.10 for C32H26BrN5O2S.
(3R,6′S,7′R,7a’S)-7′-(5-Chloro-1H-indol-3-yl)-6′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-1′,6′,7′,7a’-tetrahydro-3′H-spiro[indoline-3,5′-pyrrolo[1,2-c]thiazol]-2-one (8d)
Following the general procedure (GP2), chalcone 5d (91 mg, 0.25 mmol), isatin 6a (37 mg, 0.25 mmol), and thioproline 7a (50 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield light yellow solid compound 8d; yield (76 mg, 52%); m.p.: 210–212 °C; [α]D25 = −12.03° (c 0.11, MeOH); 1H NMR (700 MHz, CDCl3) δ (ppm) = 8.61 (s, 1H, NH), 8.38 (s, 1H, NH), 8.00 (s, 1H, Ar-H), 7.86 (s, 1H, Ar-H), 7.64 (d, J = 7.6 Hz, 1H, Ar-H), 7.33 (dd, J = 10.7, 7.0 Hz, 3H, Ar-H), 7.23–7.18 (m, 2H, Ar-H), 7.09 (dd, J = 15.4, 8.3 Hz, 4H, Ar-H), 7.00–6.96 (m, 1H, Ar-H), 6.60 (d, J = 7.8 Hz, 1H, Ar-H), 4.58 (d, J = 11.6 Hz, 1H, CHCO), 4.50–4.42 (m, 1H, NCH), 4.16 (t, J = 10.6 Hz, 1H, NCHCH), 3.88 (d, J = 10.4 Hz, 1H, NCH2), 3.55 (d, J = 10.4 Hz, 1H, NCH2), 3.06–2.94 (m, 2H, SCH2), 1.86 (s, 3H, CH3); 13C NMR (176 MHz, CDCl3) δ (ppm) = 190.95 (CO), 180.78 (CO), 143.60, 141.17, 141.01, 138.27, 134.95, 129.88, 129.35, 129.06, 128.97, 127.87, 125.71, 125.50, 124.00, 122.75, 122.40, 120.91, 119.13, 113.90, 112.54, 109.76, 74.12, 64.53, 54.95, 42.77, 37.30, 11.72 (CH3); IR (KBr, cm–1) νmax = 3284, 2926, 1720, 1668, 1652, 1616, 1597, 1538, 1504, 1470, 1397, 1329, 1283, 1268, 1223, 1180, 1103, 934, 893, 806, 796, 763, 752, 694, 683, 658, 605; [anal. calcd. for C32H26ClN5O2S: C, 66.26; H, 4.52; N, 12.07; found: C, 66.14; H, 4.63; N, 12.15]; LC/MS (ESI, m/z): found 580.6 [M(35Cl) + H]+, 582.3 [M(37Cl) + H]+; exact mass 579.15 for C32H26ClN5O2S.
(3R,6′S,7′R,7a’S)-6-Chloro-7′-(1H-indol-3-yl)-6′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-3′,6′,7′,7a’-tetrahydro-1′H-spiro[indoline-3,5′-pyrrolo[1,2-c]thiazol]-2-one (8e)
Following the general procedure (GP2), chalcone 5a (82 mg, 0.25 mmol), 6-chloroisatin 6b (46 mg, 0.25 mmol), and thioproline 7a (50 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield light yellow solid compound 8e; yield (44 mg, 30%); m.p.: 188–190 °C; [α]D25 = −18.78° (c 0.11, MeOH); 1H NMR (700 MHz, DMSO-d6): δ (ppm) = 11.00 (s, 1H, NH), 10.68 (s, 1H, NH), 7.87 (d, J = 7.8 Hz, 1H, Ar-H), 7.84 (d, J = 2.5 Hz, 1H, Ar-H), 7.54 (d, J = 8.2 Hz, 1H, Ar-H), 7.51–7.45 (m, 4H, Ar-H), 7.38–7.32 (m, 3H, Ar-H), 7.11–7.06 (m, 2H, Ar-H), 7.04 (d, J = 8.2 Hz, 1H, Ar-H), 6.67 (s, 1H, Ar-H), 4.73 (d, J = 11.8 Hz, 1H, CHCO), 4.33–4.29 (m, 1H, NCH), 4.13–4.09 (m, 1H, NCHCH), 3.78 (d, J = 10.3 Hz, 1H, NCH2(a)), 3.43 (d, J = 8.2 Hz, 1H, NCH2(b)), 3.07–2.99 (m, 2H, SCH2), 1.92 (s, 3H, CH3); 13C NMR (176 MHz, DMSO-d6): δ (ppm) =190.34 (CO), 178.87 (CO), 143.68, 142.64, 140.80, 137.91, 136.59, 133.90, 129.93, 129.31, 128.87, 128.07, 126.65, 126.45, 126.21, 125.26, 123.43, 122.45, 121.19, 120.54, 118.77, 112.02, 109.44, 73.87, 73.77, 62.86, 54.93, 53.92, 43.19, 36.70, 11.22 (CH3); IR (KBr, cm–1) νmax = 3295, 2928, 1723, 1717, 1699, 1668, 1645, 1615, 1598, 1538, 1533, 1504, 1483, 1456, 1398, 1379, 1338, 1325, 1282, 1244, 1224, 1183, 1125, 1096, 1072, 926, 852, 811, 764, 743, 694, 658, 594, 529; [anal. calcd. for C32H26ClN5O2S: C, 66.26; H, 4.52; N, 12.07; found: C, 66.12; H, 4.67; N, 12.22]; LC/MS (ESI, m/z): found 580.5 [M(35Cl) + H]+, 582.3 [M(37Cl) + H]+; exact mass 579.15 for C32H26ClN5O2S.
(3R,6′S,7′R,7a’S)-7′-(1H-Indol-3-yl)-6′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-5-nitro-1′,6′,7′,7a’-tetrahydro-3′H-spiro[indoline-3,5′-pyrrolo[1,2-c]thiazol]-2-one (8f)
Following the general procedure (GP2), chalcone 5a (82 mg, 0.25 mmol), 5-nitroisatin 6c (48 mg, 0.25 mmol), and thioproline 7a (50 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield yellow solid compound 8f; yield (49 mg, 33%); m.p.: 213–214 °C; [α]D25 = −36.24° (c 0.15, MeOH); 1H NMR (700 MHz, DMSO-d6) δ (ppm) = 11.26 (s, 1H, NH), 11.04 (s, 1H, NH), 8.46 (s, 1H, Ar-H), 8.17 (d, J = 8.2 Hz, 1H, Ar-H), 7.89–7.84 (m, 2H, Ar-H), 7.55 (s, 1H, Ar-H), 7.50–7.47 (m, 2H, Ar-H), 7.46 (d, J = 7.5 Hz, 1H, Ar-H), 7.36 (d, J = 6.9 Hz, 1H, Ar-H), 7.32–7.30 (m, 2H, Ar-H), 7.09–7.10 (m, 2H, Ar-H), 6.88 (d, J = 8.6 Hz, 1H, Ar-H), 4.79 (d, J = 12.0 Hz, 1H, CHCO), 4.41–4.36 (m, 1H, NCH), 4.14–4.09 (m, 1H, NCHCH), 3.80 (d, J = 10.8 Hz, 1H, NCH2(a)), 3.50 (d, J = 10.8 Hz, 1H, NCH2(b)), 3.08 (t, J = 3.6 Hz, 2H, SCH2), 1.86 (s, 3H, CH3); 13C NMR (176 MHz, DMSO-d6) δ (ppm) = 190.24 (CO), 179.44 (CO), 148.63, 142.79, 141.34, 140.76, 137.81, 136.64, 129.34, 128.93, 128.08, 126.92, 126.46, 126.07, 125.26, 124.34, 123.83, 121.25, 120.47, 118.89, 118.54, 111.42, 109.90, 74.15, 73.58, 62.93, 54.48, 43.59, 36.77, 11.25 (CH3); IR (KBr, cm–1) νmax = 3252, 2924, 2853, 1736, 1729, 1665, 1652, 1626, 1598, 1526, 1504, 1478, 1455, 1398, 1338, 1300, 1248, 1223, 1198, 1180, 1124, 1098, 1069, 932, 907, 828, 807, 763, 743, 694, 557; [anal. calcd. for C32H26N6O4S: C, 65.07; H, 4.44; N, 14.23; found: C, 64.91; H, 4.56; N, 14.04]; LC/MS (ESI, m/z): found 591.7 [M + H], exact mass 590.17 for C32H26N6O4S.
(3R,6′S,7′R,7a’S)-6-Chloro-6′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-7′-(1-methyl-1H-indol-3-yl)-1′,6′,7′,7a’-tetrahydro-3′H-spiro[indoline-3,5′-pyrrolo[1,2-c]thiazol]-2-one (8g)
Following the general procedure (GP2), chalcone 5b (85 mg, 0.25 mmol), 6-chloroisatin 6b (46 mg, 0.25 mmol), and thioproline 7a (50 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield light yellow solid compound 8g; yield (55 mg, 37%); m.p.: 164–165 °C; [α]D25 = −41.94° (c 0.12, MeOH); 1H NMR (700 MHz, CDCl3) δ (ppm) = 9.23 (s, 1H, NH), 8.07 (d, J = 7.9 Hz, 1H, Ar-H), 7.94 (s, 1H, Ar-H), 7.64 (d, J = 8.1 Hz, 1H, Ar-H), 7.40 (d, J = 6.2 Hz, 2H, Ar-H), 7.36 (s, 1H, Ar-H), 7.29 (d, J = 8.2 Hz, 1H, Ar-H), 7.25–7.22 (m, 1H, Ar-H), 7.21–7.18 (m, 2H, Ar-H), 7.16 (d, J = 8.1 Hz, 2H, Ar-H), 7.00 (d, J = 8.1 Hz, 1H, Ar-H), 6.51 (s, 1H, Ar-H), 4.75 (d, J = 11.8 Hz, 1H, CHCO), 4.63–4.60 (m, 1H, NCH), 4.21–4.15 (m, 1H, NCHCH), 3.93 (d, J = 10.6 Hz, 1H, NCH2(a)), 3.74 (s, 3H, NCH3), 3.56 (d, J = 10.7 Hz, 1H, NCH2(b)), 3.09–3.02 (m, 2H, SCH2), 1.99 (s, 3H, CH3); 13C NMR (176 MHz, CDCl3) δ (ppm) = 190.90 (CO), 180.77 (CO), 143.66, 141.19, 138.14, 137.42, 135.36, 130.05, 129.39, 129.11, 128.65, 127.59, 127.10, 126.86, 125.53, 122.49, 121.90, 120.90, 119.84, 119.50, 111.84, 110.17, 109.63, 74.78, 73.80, 63.95, 55.17, 43.58, 37.44, 32.85 (NCH3), 11.71 (CH3); IR (KBr, cm–1) νmax = 3234, 2922, 2849, 1733, 1717, 1699, 1667, 1652, 1612, 1598, 1544, 1537, 1532, 1503, 1483, 1454, 1401, 1377, 1327, 1261, 1223, 1182, 157, 1123, 1071, 1012, 925, 808, 765, 738, 694, 612, 529; [anal. calcd. for C33H28ClN5O2S: C, 66.71; H, 4.75; N, 11.79; found: C, 66.85; H, 4.62; N, 12.03]; LC/MS (ESI, m/z): found 594.5 [M(35Cl) + H]+, 596.0 [M(37Cl) + H]+; exact mass 593.17 for C33H28ClN5O2S.
(3R,6′S,7′R,7a’S)-6′-(5-Methyl-1-phenyl-1H-pyrazole-4-carbonyl)-7′-(1-methyl-1H-indol-3-yl)-5-nitro-3′,6′,7′,7a’-tetrahydro-1′H-spiro[indoline-3,5′-pyrrolo[1,2-c]thiazol]-2-one (8h)
Following the general procedure (GP2), chalcone 5b (85 mg, 0.25 mmol), 5-nitroisatin 6c (48 mg, 0.25 mmol), and thioproline 7a (50 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 16 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield yellow solid compound 8h; yield (41 mg, 27%); m.p.: 172–173 °C; [α]D25 = −22.87° (c 0.13, MeOH); 1H NMR (700 MHz, CDCl3) δ (ppm) = 9.78 (s, 1H, NH), 8.73 (s, 1H, Ar-H), 8.14 (dd, J = 27.8, 8.4 Hz, 2H, Ar-H), 7.99 (s, 1H, Ar-H), 7.47–7.41 (m, 3H, Ar-H), 7.40 (d, J = 4.3 Hz, 1H, Ar-H), 7.35 (d, J = 8.2 Hz, 1H, Ar-H), 7.30 (d, J = 8.2 Hz, 1H, Ar-H), 7.23–7.19 (m, 1H, Ar-H), 7.16 (dd, J = 6.7, 3.1 Hz, 2H, Ar-H), 6.69 (d, J = 9.2 Hz, 1H, Ar-H), 4.89 (d, J = 11.5 Hz, 1H CHCO), 4.73 (m, 1H NCH), 4.30 (t, J = 10.7 Hz, 1H, NCHCH), 3.99 (d, J = 10.4 Hz, 1H, NCH2(a)), 3.79 (s, 3H, CH3), 3.51 (d, J = 10.5 Hz, 1H, NCH2(b)), 3.16 (d, J = 4.4 Hz, 2H, SCH2), 2.01 (s, 3H, CH3); 13C NMR (176 MHz, CDCl3) δ (ppm) = 190.04 (CO), 180.99 (CO), 146.95, 143.91, 142.89, 141.14, 137.92, 137.52, 129.47, 129.26, 128.66, 128.04, 127.74, 127.12, 126.55, 125.38, 125.06, 124.92, 121.96, 120.90, 119.75, 111.02, 109.77, 73.37, 63.71, 55.11, 44.29, 37.47, 32.86 (CH3), 11.86 (CH3); IR (KBr, cm–1) νmax = 3208, 2926, 2859, 1736, 1716, 1699, 1682, 1678, 1668, 1652, 1622, 1615, 1598, 1524, 1504, 1475, 1455, 1404, 1337, 1221, 1177, 1123, 1103, 932, 833, 805, 765, 741, 694, 556; [anal. calcd. for C33H28N6O4S: C, 65.55; H, 4.67; N, 13.90; found: C, 65.67; H, 4.81; N, 14.04]; LC/MS (ESI, m/z): found 605.6 [M + H], exact mass 604.19 for C33H28N6O4S.
(3R,6′S,7′R,7a′S)-7′-(5-Bromo-1H-indol-3-yl)-6′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-5-nitro-3′,6′,7′,7a’-tetrahydro-1′H-spiro[indoline-3,5′-pyrrolo[1,2-c]thiazol]-2-one (8i)
Following the general procedure (GP2), chalcone 5c (102 mg, 0.25 mmol), 5-nitroisatin 6c (48 mg, 0.25 mmol), and thioproline 7a (50 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 16 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield yellow solid compound 8i; yield (82 mg, 49%); m.p.: 250–251 °C; [α]D25 = −126.29° (c 0.19, MeOH); 1H NMR (700 MHz, DMSO-d6) δ (ppm) = 11.30 (d, J = 2.6 Hz, 1H, NH), 11.25 (s, 1H, NH), 8.45 (d, J = 2.4 Hz, 1H, Ar-H), 8.17 (dd, J = 8.6, 2.4 Hz, 1H, Ar-H), 7.98 (d, J = 1.9 Hz, 1H, Ar-H), 7.84 (s, 1H, Ar-H), 7.67 (d, J = 2.6 Hz, 1H, Ar-H), 7.51–7.48 (m, 2H, Ar-H), 7.48–7.45 (m, 1H, Ar-H), 7.35–7.31 (m, 3H, Ar-H), 7.21 (dd, J = 8.5, 1.9 Hz, 1H, Ar-H), 6.87 (d, J = 8.6 Hz, 1H, Ar-H), 4.67 (d, J = 11.9 Hz, 1H, CHCO), 4.37–4.33 (m, 1H, NCH), 4.10 (dd, J = 11.8, 9.5 Hz, 1H, NCHCH), 3.79 (d, J = 10.7 Hz, 1H, NCH2(a)), 3.50 (d, J = 10.6 Hz, 1H, NCH2(b)), 3.09–3.04 (m, 2H, SCH2), 1.86 (s, 3H, CH3); 13C NMR (176 MHz, DMSO-d6) δ (ppm) = 190.22 (CO), 179.25 (CO), 148.59, 142.75, 141.34, 140.74, 137.77, 135.16, 129.31, 128.90, 128.07, 126.90, 125.42, 125.21, 124.29, 123.85, 123.69, 120.73, 120.38, 113.84, 111.50, 111.42, 109.86, 73.95, 73.48, 63.46, 54.14, 42.83, 36.56, 11.21 (CH3); IR (KBr, cm–1) νmax = 3383, 3101, 2855, 1747, 1729, 1649, 1622, 1598, 1530, 1504, 1463, 1454, 1412, 1337, 1290, 1253, 1222, 1199, 1173, 1097, 933, 880, 830, 799, 753, 693, 607; [anal. calcd. for C32H25BrN6O4S: C, 57.40; H, 3.76; N, 12.55; found: C, 57.36; H, 3.84; N, 12.59]; LC/MS (ESI, m/z): found 669.6 [M(79Br) + H]+, 671.5 [M(81Br) + H]+; exact mass 668.08 for C32H25BrN6O4S.
(3R,6′S,7′R,7a’S)-7′-(5-Chloro-1H-indol-3-yl)-6′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-5-nitro-3′,6′,7′,7a’-tetrahydro-1′H-spiro[indoline-3,5′-pyrrolo[1,2-c]thiazol]-2-one (8j)
Following the general procedure (GP2), chalcone 5d (91 mg, 0.25 mmol), 5-nitroisatin 6c (48 mg, 0.25 mmol), and thioproline 7a (50 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield yellow solid compound 8j; yield (53 mg, 40%); m.p.: 196–197 °C; [α]D25 = −53.73° (c 0.10, MeOH); 1H NMR (700 MHz, DMSO-d6) δ (ppm) = 11.30 (s, 1H, NH), 11.27 (s, 1H, NH), 8.46 (s, 1H, Ar-H), 8.18 (d, J = 8.6 Hz, 1H, Ar-H), 7.85 (s, 1H, Ar-H), 7.69 (s, 1H, Ar-H), 7.53–7.46 (m, 2H, Ar-H), 7.48 (d, J = 5.5 Hz, 1H, Ar-H), 7.39 (d, J = 8.6 Hz, 1H, Ar-H), 7.34–7.31 (m, 3H, Ar-H), 7.12 (d, J = 8.6 Hz, 1H, Ar-H), 6.88 (d, J = 8.7 Hz, 1H, Ar-H), 4.68 (d, J = 11.7 Hz, 1H, CHCO), 4.39–4.35 (m, 1H, NCH), 4.12 (t, J = 12.1 Hz, 1H, NCHCH), 3.80 (d, J = 10.9 Hz, 1H, NCH2(a)), 3.51 (d, J = 10.9 Hz, 1H, NCH2(b)), 3.11–3.05 (m, 2H, SCH2), 1.87 (s, 3H, CH3); 13C NMR (176 MHz, DMSO-d6) δ (ppm) = 190.27 (CO), 179.33 (CO), 148.63, 142.79, 141.39, 140.77, 137.80, 134.98, 129.35, 128.94, 127.38, 126.94, 126.66, 126.45, 125.62, 124.33, 123.50, 121.22, 120.41, 117.74, 113.42, 111.62, 109.90, 74.00, 73.49, 62.93, 54.94, 42.87, 36.62, 11.23 (CH3); IR (KBr, cm–1) νmax = 3344, 3270, 2932, 2861, 1725, 1657, 1623, 1598, 1530, 1503, 1477, 1454, 1339, 1296, 1224, 1180, 1101, 931, 892, 797, 764, 693, 614, 553; [Anal. Calcd. for C32H25ClN6O4S: C, 61.49; H, 4.03; N, 13.44; found: C, 61.35; H, 3.91; N, 13.62]; LC/MS (ESI, m/z): found 625.8 [M (35Cl) + H]+, 627.2 [M (37Cl) + H]+; exact mass 624.13 for C32H25ClN6O4S.
(1′R,2′S,3R,9a’R)-1′-(1H-Indol-3-yl)-2′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-1′,2′,4a’,5′,6′,7′,8′,8a’,9′,9a’-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (8k)
Following the general procedure (GP2), chalcone 5a (82 mg, 0.25 mmol), isatin 6a (37 mg, 0.25 mmol), and octahydro-1H-indole-2-carboxylic acid 7b (64 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield light yellow solid compound 8k; yield (125 mg, 86%); m.p.: 200–201 °C; [α]D25 = −18.76° (c 0.12, MeOH); 1H NMR (700 MHz, CDCl3) δ (ppm) = 8.33 (s, 1H, NH), 8.04 (d, J = 1.2 Hz, 1H, Ar-H), 8.04–8.02 (m, 1H, Ar-H), 7.95 (s, 1H, NH), 7.42 (d, J = 7.4 Hz, 1H, Ar-H), 7.40–7.34 (m, 3H, Ar-H), 7.33–7.29 (m, 1H, Ar-H), 7.20–7.14 (m, 5H, Ar-H), 7.13–7.09 (m, 1H, Ar-H), 7.06–7.02 (m, 1H, Ar-H), 6.57 (d, J = 7.7 Hz, 1H, Ar-H), 4.96 (d, J = 11.9 Hz, 1H, CHCO), 4.57–4.52 (m, 1H, NCH), 4.20 (dd, J = 12.0, 10.0 Hz, 1H, NCHCH), 3.27 (q, J = 3.8 Hz, 1H, NCH), 2.19–2.14 (m, 1H, NCHCH), 1.93 (s, 3H, CH3), 1.85–1.78 (m, 2H, CH2), 1.59–1.44 (m, 4H, CH2), 1.20–1.15 (m, 1H, CH2), 1.07–1.01 (m, 1H, CH2), 1.01–0.95 (m, 2H, CH2); 13C NMR (176 MHz, CDCl3) δ (ppm) = 191.46 (CO), 181.76 (CO), 143.42, 141.62, 140.65, 138.43, 136.60, 129.25, 128.94, 128.74, 128.53, 127.06, 125.41, 125.10, 121.99, 121.78, 121.74, 121.26, 120.04, 119.48, 114.40, 111.35, 109.61, 72.74, 70.23, 66.94, 57.75, 45.57, 41.98, 38.40, 28.59, 27.78, 24.86, 20.13, 11.88 (CH3); IR (KBr, cm–1) νmax = 3291, 2926, 2854, 1716, 1699, 1694, 1683, 1668, 1660, 1652, 1617, 1597, 1557, 1538, 1505, 1475, 1456, 1398, 1373, 1330, 1221, 1179, 1099, 1011, 934, 870, 790, 741, 694; [anal. calcd. for C37H35N5O2: C, 76.40; H, 6.06; N, 12.04; found: C, 76.28; H, 6.14; N, 12.11]; LC/MS (ESI, m/z): found 582.5 [M + H]+, exact mass 581.28 for C37H35N5O2.
(1′R,2′S,3R,9a’R)-6-Chloro-1′-(1H-indol-3-yl)-2′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-1′,2′,4a’,5′,6′,7′,8′,8a’,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (8l)
Following the general procedure (GP2), chalcone 5a (82 mg, 0.25 mmol), 6-chloroisatin 6b (46 mg, 0.25 mmol), and octahydro-1H-indole-2-carboxylic acid 7b (64 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield light yellow solid compound 8l; yield (131 mg, 85%); m.p.: 205–206 °C; [α]D25 = −20.56° (c 0.11, MeOH); 1H NMR (700 MHz, CDCl3) δ (ppm) = 8.55 (s, 1H, NH), 8.32 (s, 1H, NH), 8.10 (d, J = 2.8 Hz, 1H, Ar-H), 8.00 (d, J = 7.6 Hz, 1H, Ar-H), 7.42–7.38 (m, 3H, Ar-H), 7.32 (d, J = 8.4 Hz, 2H, Ar-H), 7.19–7.11 (m, 5H, Ar-H), 7.01 (d, J = 8.0 Hz, 1H, Ar-H), 6.41 (d, J = 2.3 Hz, 1H, Ar-H), 4.96 (d, J = 12.0 Hz, 1H, CHCO), 4.56–4.52 (m, 1H, NCH), 4.15 (dd, J = 12.0, 10.0 Hz, 1H, NCHCH), 3.22 (q, J = 3.8 Hz, 1H, NCH), 2.19–2.14 (m, 1H, NCHCH), 2.00 (s, 3H, CH3), 1.82–1.76 (m, 2H, CH2), 1.59–1.55 (m, 1H, CH2), 1.54–1.46 (m, 3H, CH2), 1.20–1.16 (m, 1H, CH2), 1.07–0.96 (m, 3H, CH2); 13C NMR (176 MHz, CDCl3): δ (ppm) = 191.36 (CO), 181.77 (CO), 143.61, 142.07, 141.60, 138.28, 136.59, 134.55, 129.39, 129.03, 128.68, 126.99, 125.46, 123.59, 122.06, 121.81, 121.56, 121.16, 119.95, 119.55, 114.15, 111.40, 110.13, 72.39, 70.19, 67.06, 57.81, 45.69, 41.98, 38.35, 28.65, 27.77, 24.81, 20.14, 11.86 (CH3); IR (KBr, cm–1) νmax = 3298, 2926, 2851, 1725, 1668, 1652, 1614, 1598, 1538, 1532, 1504, 1484, 1455, 1447, 1398, 1373, 1323, 1284, 1243, 1222, 1179, 1130, 1072, 1011, 937, 924, 869, 795, 764, 741, 694, 660, 597, 584, 524; [anal. calcd. for C37H34ClN5O2: C, 72.12; H, 5.56; N, 11.37; found: C, 71.96; H, 5.63; N, 11.28]; LC/MS (ESI, m/z): found 616.6 [M(35Cl) + H]+, 618.3 [M(37Cl) + H]+; exact mass 615.24 for C37H34ClN5O2.
(1′R,2′S,3R,9a’R)-1′-(1H-Indol-3-yl)-2′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-5-nitro-1′,2′,4a’,5′,6′,7′,8′,8a’,9′,9a’-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (8m)
Following the general procedure (GP2), chalcone 5a (82 mg, 0.25 mmol), 5-nitroisatin 6c (48 mg, 0.25 mmol), and octahydro-1H-indole-2-carboxylic acid 7b (64 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 4 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield yellow solid compound 8m; yield (103 mg, 66%); m.p.: 210–211 °C; [α]D25 = −15.63° (c 0.13, MeOH); 1H NMR (700 MHz, CDCl3) δ (ppm) = 9.19 (s, 1H, NH), 8.27–8.22 (m, 2H, Ar-H), 8.11 (s, 1H, NH), 8.06 (d, J = 8.6 Hz, 1H, Ar-H), 8.02 (d, J = 7.9 Hz, 1H, Ar-H), 7.42–7.37 (m, 3H, Ar-H), 7.34 (d, J = 7.0 Hz, 1H, Ar-H), 7.20 (s, 1H, Ar-H), 7.18–7.14 (m, 2H, Ar-H), 7.10 (d, J = 7.5 Hz, 2H, Ar-H), 6.42 (d, J = 8.7 Hz, 1H, Ar-H), 5.03 (d, J = 12.0 Hz, 1H, CHCO), 4.63–4.58 (m, 1H, NCH), 4.21 (t, J = 11.0 Hz, 1H, NCHCH), 3.24 (q, J = 3.7 Hz, 1H, NCH), 2.23–2.18 (m, 1H, NCHCH), 1.95 (s, 3H, CH3), 1.90–1.86 (m, 1H, CH2), 1.84–1.81 (m, 1H, CH2), 1.61–1.56 (m, 1H, CH2), 1.53–1.46 (m, 3H, CH2), 1.22–1.17 (m, 1H, CH2), 1.08–0.98 (m, 2H, CH2), 0.93–0.89 (m, 1H, CH2); 13C NMR (176 MHz, CDCl3): δ (ppm) = 190.53 (CO), 182.11 (CO), 146.67, 143.82, 142.71, 141.51, 138.12, 136.64, 129.48, 129.20, 127.14, 126.80, 126.02, 125.38, 123.96, 122.21, 121.96, 121.13, 120.02, 119.71, 113.58, 111.43, 109.26, 72.52, 70.05, 67.02, 57.95, 46.02, 42.11, 38.27, 28.56, 27.72, 24.77, 20.05, 11.95 (CH3); IR (KBr, cm–1) νmax = 3341, 2926, 2854, 1733, 1668, 1653, 1623, 1598, 1533, 1503, 1477, 1455, 1397, 1338, 1221, 1178, 1125, 1096, 1072, 1011, 930, 833, 764, 742, 695, 660, 555; [anal. calcd. for C37H34N6O4: C, 70.91; H, 5.47; N, 13.41; found: C, 71.06; H, 5.39; N, 13.49]; LC/MS (ESI, m/z): found 627.5 [M + H]+; exact mass 626.26 for C37H34N6O4.
(1′R,2′S,3R,9a’R)-6-Chloro-2′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-1′-(1-methyl-1H-indol-3-yl)-1′,2′,4a’,5′,6′,7′,8′,8a’,9′,9a’-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (8n)
Following the general procedure (GP2), chalcone 5b (85 mg, 0.25 mmol), 6-chloroisatin 6b (46 mg, 0.25 mmol), and octahydro-1H-indole-2-carboxylic acid 7b (64 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield light yellow solid compound 8n; yield (134 mg, 85%); m.p.: 172–173 °C; [α]D25 = −19.29° (c 0.10, MeOH); 1H NMR (700 MHz, CDCl3) δ (ppm) = 8.06 (s, 1H, NH), 8.01 (d, J = 7.9 Hz, 1H, Ar-H), 7.44–7.40 (m, 3H, Ar-H), 7.31 (d, J = 8.0 Hz, 1H, Ar-H), 7.27 (s, 1H, Ar-H), 7.23–7.20 (m, 1H, Ar-H), 7.17 (d, J = 7.7 Hz, 3H, Ar-H), 7.10 (s, 1H, Ar-H), 7.02 (d, J = 8.0 Hz, 1H, Ar-H), 6.43 (s, 1H, Ar-H), 4.93 (d, J = 12.0 Hz, 1H, CHCO), 4.56–4.52 (m, 1H, NCH), 4.16–4.11 (m, 1H, NCHCH), 3.73 (s, 3H, NCH3), 3.23 (q, J = 3.8 Hz, 1H, NCH), 2.20–2.15 (m, 1H, NCHCH), 2.02 (s, 3H, CH3), 1.84–1.79 (m, 2H, CH2), 1.60–1.56 (m, 1H, CH2), 1.55–1.48 (m, 3H, CH2), 1.21–1.17 (m, 1H, CH2), 1.08–0.96 (m, 3H, CH2); 13C NMR (176 MHz, CDCl3): δ (ppm) = 191.30 (CO), 181.64 (CO), 143.59, 142.02, 141.57, 138.33, 137.27, 134.52, 129.38, 128.99, 127.44, 127.12, 126.40, 125.46, 123.59, 121.66, 121.56, 121.16, 120.13, 119.09, 112.69, 110.05, 109.36, 72.29, 70.32, 67.20, 57.79, 45.60, 42.00, 38.38, 32.83 (NCH3), 28.64, 27.78, 24.84, 20.11, 11.88 (CH3); IR (KBr, cm–1) νmax = 3057, 2926, 2849, 1733, 1668, 1652, 1615, 1538, 1532, 1504, 1484, 1455, 1404, 1373, 1328, 1282, 1224, 1179, 1130, 1071, 1012, 935, 924, 869, 796, 764, 740, 694; [anal. calcd. for C38H36ClN5O2: C, 72.43; H, 5.76; N, 11.11; found: C, 72.52; H, 5.71; N, 11.03]; LC/MS (ESI, m/z): found 630.6 [M(35Cl) + H]+, 632.3 [M(37Cl) + H]+; exact mass 629.26 for C38H36ClN5O2.
(1′R,2′S,3R,9a’R)-2′-(5-Methyl-1-phenyl-1H-pyrazole-4-carbonyl)-1′-(1-methyl-1H-indol-3-yl)-5-nitro-1′,2′,4a’,5′,6′,7′,8′,8a’,9′,9a’-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (8o)
Following the general procedure (GP2), chalcone 5b (85 mg, 0.25 mmol), 5-nitroisatin 6c (48 mg, 0.25 mmol), and octahydro-1H-indole-2-carboxylic acid 7b (64 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 4 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield yellow solid compound 8o; yield (119 mg, 74%); m.p.: 185–186 °C; [α]D25 = −32.74° (c 0.14, MeOH); 1H NMR (700 MHz, CDCl3) δ (ppm) = 11.03 (s, 1H, NH), 8.16 (d, J = 7.6 Hz, 2H, Ar-H), 7.98 (s, 1H, Ar-H), 7.86 (d, J = 7.9 Hz, 1H, Ar-H), 7.52–7.50 (m, 2H, Ar-H), 7.49–7.45 (m, 2H, Ar-H), 7.38–7.33 (m, 3H, Ar-H), 7.16–7.13 (m, 1H, Ar-H), 7.10–7.07 (m, 1H, Ar-H), 6.85 (d, J = 9.1 Hz, 1H, Ar-H), 4.92 (d, J = 12.1 Hz, 1H, CHCO), 4.25–4.12 (m, 1H, NCH), 4.13 (dd, J = 12.1, 9.9 Hz, 1H, NCHCH), 3.71 (s, 3H, NCH3), 3.22 (q, J = 3.5 Hz, 1H, NCH), 2.19–2.13 (m, 1H, NCHCH), 2.05–1.99 (m, 1H, CH2), 1.81 (s, 3H, CH3), 1.65–1.61 (m, 1H, CH2), 1.55–1.50 (m, 1H, CH2), 1.48–1.43 (m, 1H, CH2), 1.39–1.27 (m, 2H, CH2), 1.15–1.10 (m, 1H, CH2), 1.04–0.97 (m, 1H, CH2), 0.96–0.90 (m, 1H, CH2), 0.80–0.74 (m, 1H, CH2); 13C NMR (176 MHz, CDCl3): δ (ppm) = 190.37 (CO), 181.03 (CO), 148.21, 142.53, 141.42, 141.08, 137.89, 136.71, 129.31, 128.82, 128.03, 127.10, 126.54, 126.41, 125.20, 125.07, 123.11, 121.17, 120.49, 119.19, 118.56, 111.66, 109.68, 71.51, 70.53, 66.16, 56.87, 44.37, 41.52, 37.53, 32.33 (NCH3), 27.69, 27.27, 24.37, 19.27, 11.08 (CH3); IR (KBr, cm–1) νmax = 3056, 2927, 2854, 1738, 1733, 1716, 1699, 1694, 1682, 1674, 1668, 1661, 1652, 1645, 1634, 1622, 1615, 1598, 1557, 1538, 1524, 1519, 1505, 1475, 1464, 1456, 1436, 1427, 1398, 1373, 1338, 1223, 1178, 1127, 1072, 1011, 930, 833, 795; [anal. calcd. for C38H36N6O4: C, 71.23; H, 5.66; N, 13.12; found: C, 71.31; H, 5.74; N, 13.27]; LC/MS (ESI, m/z): found 641.5 [M + H]+; exact mass 640.26 for C38H36N6O4.
(1′R,2′S,3R,9a’R)-1′-(5-Bromo-1H-indol-3-yl)-6-chloro-2′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-1′,2′,4a’,5′,6′,7′,8′,8a’,9′,9a’-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (8p)
Following the general procedure (GP2), chalcone 5c (102 mg, 0.25 mmol), 6-chloroisatin 6b (46 mg, 0.25 mmol), and octahydro-1H-indole-2-carboxylic acid 7b (64 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield light yellow solid compound 8p; yield (155 mg, 89%); m.p.: 199–200 °C; [α]D25 = −26.47° (c 0.12, MeOH); 1H NMR (700 MHz, CDCl3) δ (ppm) = 8.72 (s, 1H, NH), 8.63 (s, 1H, NH), 8.12 (s, 1H, Ar-H), 8.05 (s, 1H, Ar-H), 7.43–7.39 (m, 3H, Ar-H), 7.31 (d, J = 8.0 Hz, 1H, Ar-H), 7.23 (d, J = 1.5 Hz, 2H, Ar-H), 7.15–7.12 (m, 2H, Ar-H), 7.11 (d, J = 2.5 Hz, 1H, Ar-H), 7.02 (dd, J = 8.0, 1.9 Hz, 1H, Ar-H), 6.35 (s, 1H, Ar-H), 4.79 (d, J = 12.0 Hz, 1H, CHCO), 4.38–4.33 (m, 1H, NCH), 4.09 (dd, J = 12.1, 10.1 Hz, 1H, NCHCH), 3.21 (q, J = 3.7 Hz, 1H, NCH), 2.22–2.17 (m, 1H, NCHCH), 1.98 (s, 3H, CH3), 1.85–1.78 (m, 2H, CH2), 1.60–1.56 (m, 1H, CH2), 1.54–1.45 (m, 3H, CH2), 1.22–1.17 (m, 1H, CH2), 1.09–0.97 (m, 3H, CH2); 13C NMR (176 MHz, CDCl3): δ (ppm) = 191.05 (CO), 181.83 (CO), 143.75, 142.03, 141.48, 138.22, 135.08, 134.65, 129.46, 129.36, 129.17, 129.15, 128.70, 127.14, 125.45, 125.03, 123.42, 122.38, 122.12, 121.67, 121.05, 114.32, 112.87, 110.15, 72.37, 70.86, 67.65, 57.77, 44.84, 41.96, 38.35, 28.57, 27.77, 24.77, 20.11, 11.80 (CH3); IR (KBr, cm–1) νmax = 3273, 2926, 2853, 1733, 1699, 1674, 1652, 1615, 1557, 1538, 1532, 1504, 1483, 1456, 1398, 1373, 1322, 1278, 1220, 1179, 1130, 1102, 1072, 936, 932, 884, 869, 797, 765, 693, 670, 595, 525; [anal. calcd. for C37H33BrClN5O2: C, 63.94; H, 4.79; N, 10.08; found: C, 64.09; H, 4.86; N, 10.13]; LC/MS (ESI, m/z): found 695.0 [M(35Cl/79Br) + H]+, 696.3 [M(37Cl/81Br) + H]+, 698.1 [M(37Cl + 81Br) + H]+; exact mass 693.15 for C37H33BrClN5O2.
(1′R,2′S,3R,9a’R)-1′-(5-Bromo-1H-indol-3-yl)-6-chloro-2′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-1′,2′,4a’,5′,6′,7′,8′,8a’,9′,9a’-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (8q)
Following the general procedure (GP2), chalcone 5c (102 mg, 0.25 mmol), 5-nitroisatin 6c (48 mg, 0.25 mmol), and octahydro-1H-indole-2-carboxylic acid 7b (64 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 4 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield yellow solid compound 8q; yield (120 mg, 68%); m.p.: 190–199 °C; [α]D25 = −167.95° (c 0.10, MeOH); 1H NMR (700 MHz, DMSO-d6): δ (ppm) = 11.17 (d, J = 2.7 Hz, 1H, NH), 11.01 (s, 1H, NH), 8.26 (d, J = 2.3 Hz, 1H, Ar-H), 8.15 (dd, J = 8.6, 2.4 Hz, 1H, Ar-H), 8.04 (s, 1H, Ar-H), 8.03 (d, J = 1.9 Hz, 1H, Ar-H), 7.57 (d, J = 2.6 Hz, 1H, Ar-H), 7.53–7.50 (m, 2H, Ar-H), 7.48–7.45 (m, 1H, Ar-H), 7.36 (d, J = 7.1 Hz, 2H, Ar-H), 7.29 (d, J = 8.6 Hz, 1H, Ar-H), 7.18 (dd, J = 8.5, 1.9 Hz, 1H, Ar-H), 6.84 (d, J = 8.6 Hz, 1H, Ar-H), 4.88 (d, J = 12.0 Hz, 1H, CHCO), 4.17–4.11 (m, 2H, NCH + NCHCH), 3.23 (q, J = 3.4 Hz, 1H, NCH), 2.18–2.10 (m, 2H NCHCH2), 1.81 (s, 3H, CH3), 1.63–1.58 (m, 1H, CH2), 1.54–150 (m, 1H, CH2), 1.49–1.45 (m, 1H, CH2), 1.38–1.28 (m, 2H, CH2), 1.15–1.10 (m, 1H, CH2), 1.03–0.97 (m, 1H, CH2), 0.96–0.90 (m, 1H, CH2), 0.77–0.72 (m, 1H, CH2); 13C NMR (176 MHz, DMSO-d6): δ (ppm) = 190.50 (CO), 180.99 (CO), 148.17, 142.49, 141.54, 141.20, 137.91, 134.91, 129.31, 128.92, 128.81, 128.03, 126.51, 126.40, 125.20, 125.06, 123.62, 123.52, 123.33, 121.21, 120.55, 113.43, 112.57, 111.14, 109.61, 71.50, 70.72, 66.35, 56.77, 43.80, 41.56, 37.06, 27.65, 27.29, 24.42, 19.25, 11.08 (CH3); IR (KBr, cm–1) νmax = 3363, 2930, 2854, 1734, 1762, 1621, 1599, 1528, 1503, 1478, 1454, 1398, 1337, 1221, 1178, 1126, 1098, 1081, 928, 884, 866, 831, 795, 765, 753, 693, 659, 598, 553; [anal. calcd. for C37H33BrN6O4: C, 63.94; H, 4.79; N, 10.08; found: C, 64.09; H, 4.86; N, 10.13]; LC/MS (ESI, m/z): found 705.8 [M(79Br) + H]+, 707.5 [M(81Br) + H]+, exact mass 704.17 for C37H33BrN6O4.
(1′R,2′S,3R,9a’R)-6-Chloro-1′-(5-chloro-1H-indol-3-yl)-2′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-1′,2′,4a’,5′,6′,7′,8′,8a’,9′,9a’-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (8r)
Following the general procedure (GP2), chalcone 5d (91 mg, 0.25 mmol), 6-chloroisatin 6b (46 mg, 0.25 mmol), and octahydro-1H-indole-2-carboxylic acid 7b (64 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield light yellow solid compound 8r; yield (148 mg, 91%); m.p.: 168–169 °C; [α]D25 = −121.51° (c 0.12, MeOH); 1H NMR (700 MHz, CDCl3) δ (ppm) = 8.77 (d, J = 11.7 Hz, 1H, NH), 8.63 (s, 1H, NH), 8.13 (d, J = 2.0 Hz, 1H, Ar-H), 7.91 (d, J = 2.0 Hz, 1H, Ar-H), 7.45–7.39 (m, 3H, Ar-H), 7.31 (d, J = 8.0 Hz, 1H, Ar-H), 7.28–7.26 (m, 1H, Ar-H), 7.15–7.12 (m, 3H, Ar-H), 7.11 (dd, J = 8.6, 2.0 Hz, 1H, Ar-H), 7.02 (dd, J = 8.0, 1.9 Hz, 1H, Ar-H), 6.35 (s, 1H, Ar-H), 4.81 (d, J = 12.0 Hz, 1H, CHCO), 4.39–4.36 (m, 1H, NCH), 4.10 (dd, J = 11.9, 10.0 Hz, 1H, NCHCH), 3.22 (q, J = 3.7 Hz, 1H, NCH), 2.19–2.14 (m, 1H, NCHCH), 1.99 (s, 3H), 1.83–1.76 (m, 2H, CH2), 1.58–1.53 (m, 1H, CH2), 1.52–1.44 (m, 3H, CH2), 1.19–1.15 (m, 1H, CH2), 1.07–0.95 (m, 3H, CH2); 13C NMR (176 MHz, CDCl3): δ (ppm) = 191.10 (CO), 181.85 (CO), 143.75, 142.06, 141.49, 138.22, 134.82, 134.65, 129.46, 129.16, 128.69, 128.47, 127.14, 125.45, 123.43, 122.60, 122.47, 121.65, 121.05, 119.07, 114.34, 112.48, 110.16, 72.39, 70.79, 67.65, 57.78, 44.90, 41.96, 38.35, 28.58, 27.77, 24.77, 20.12, 11.79 (CH3); IR (KBr, cm–1) νmax = 3287,2925, 2854, 1723, 1661, 1652, 1615, 1538, 1533, 1504, 1484, 1398, 1373, 1320, 1277, 1221, 1178, 1131, 1104, 1072, 736, 926, 893, 869, 796, 763, 694, 606, 525; [anal. calcd. for C37H33Cl2N5O2: C, 68.31; H, 5.11; N, 10.76; found: C, 68.25; H, 5.07; N, 10.84]; LC/MS (ESI, m/z): found 650.8 [M(35Cl) + H]+, 652.0 [M(37Cl) + H]+, 654.0 [M(37Cl + 37Cl) + H]+; exact mass 649.2 for C37H33Cl2N5O2.
(1′R,2′S,3R,9a’R)-1′-(5-Chloro-1H-indol-3-yl)-2′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-5-nitro-1′,2′,4a’,5′,6′,7′,8′,8a’,9′,9a’-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (8s)
Following the general procedure (GP2), chalcone 5d (91 mg, 0.25 mmol), 5-nitroisatin 6c (48 mg, 0.25 mmol), and octahydro-1H-indole-2-carboxylic acid 7b (64 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 4 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield yellow solid compound 8s; yield (132 mg, 80%); m.p.: 172–173 °C; [α]D25 = −11.15° (c 0.10, MeOH); 1H NMR (700 MHz, DMSO-d6): δ (ppm) = 11.15 (d, J = 2.6 Hz, 1H, NH), 11.01 (s, 1H, NH), 8.25 (d, J = 2.3 Hz, 1H, Ar-H), 8.15 (dd, J = 8.6, 2.3 Hz, 1H, Ar-H), 8.03 (s, 1H, Ar-H), 7.89 (d, J = 2.1 Hz, 1H, Ar-H), 7.58 (d, J = 2.5 Hz, 1H, Ar-H), 7.53–7.49 (m, 2H, Ar-H), 7.49–7.45 (m, 1H, Ar-H), 7.38–7.35 (m, 2H, Ar-H), 7.33 (d, J = 8.6 Hz, 1H, Ar-H), 7.07 (dd, J = 8.6, 2.0 Hz, 1H, Ar-H), 6.84 (d, J = 8.6 Hz, 1H, Ar-H), 4.88 (d, J = 12.0 Hz, 1H, CHCO), 4.15 (m, 2H, NCH + NCHCH), 3.23 (q, J = 3.4 Hz, 1H, NCH), 2.19–2.09 (m, 2H, CH2), 1.81 (s, 3H, CH3), 1.62–1.59 (m, 1H, CH2), 1.55–1.50 (m, 1H, CH2), 1.49–1.44 (m, 1H, CH2), 1.39–1.27 (m, 2H, CH2), 1.14–1.10 (m, 1H, CH2), 1.03–0.97 (m, 1H, CH2), 0.97–0.91 (m, 1H, CH2), 0.77–0.72 (m, 1H, CH2); 13C NMR (176 MHz, DMSO-d6): δ (ppm) = 190.50 (CO), 180.99 (CO), 148.17, 142.49, 141.53, 141.20, 137.91, 134.69, 129.31, 128.81, 128.18, 128.03, 126.51, 126.40, 125.20, 123.81, 123.15, 121.00, 120.54, 118.20, 112.96, 112.64, 109.61, 71.51, 70.67, 66.34, 56.78, 43.83, 41.56, 37.09, 27.65, 27.29, 24.42, 19.25, 11.07 (CH3); IR (KBr, cm–1) νmax = 3349, 2928, 2853, 1732, 1661, 1623, 1598, 1537, 1525, 1504, 1455, 1398, 1337, 1220, 1178, 1128, 1099, 1078, 931, 892, 867, 833, 798, 766, 755, 694, 552; [anal. calcd. for C37H33ClN6O4: C, 67.22; H, 5.03; N, 12.71; found: C, 67.34; H, 12.63; N, 12.56]; LC/MS (ESI, m/z): found 661.6 [M(35Cl) + H]+, 663.3 [M(37Cl) + H]+, exact mass 660.23 for C37H33ClN6O4.
(1′R,2′S,3R,7a’R)-6-Chloro-1′-(1H-indol-3-yl)-2′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-1′,2′,5′,6′,7′,7a’-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one (8t)
Following the general procedure (GP2), chalcone 5a (82 mg, 0.25 mmol), 6-chloroisatin 6b (46 mg, 0.25 mmol), and l-proline 7c (43 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield light yellow solid compound 8t; yield (103 mg, 73%); m.p.: 165–166 °C; [α]D25 = −32.67° (c 0.12, MeOH); 1H NMR (700 MHz, CDCl3): δ (ppm) = 9.13 (s, 1H, NH), 8.33 (s, 1H, NH), 8.09–8.04 (m, 1H, Ar-H), 7.93 (s, 1H, Ar-H), 7.41–7.35 (m, 3H, Ar-H), 7.32–7.30 (m, 1H, Ar-H), 7.29 (d, J = 8.1 Hz, 1H, Ar-H), 7.23 (d, J = 2.4 Hz, 1H, Ar-H), 7.19–7.13 (m, 4H, Ar-H), 6.99 (dd, J = 8.0, 1.9 Hz, 1H, Ar-H), 6.63 (d, J = 1.9 Hz, 1H, Ar-H), 4.89 (d, J = 11.4 Hz, 1H, CHCO), 4.49–4.46 (m, 1H, NCH), 4.19 (dd, J = 11.5, 9.9 Hz, 1H, NCHCH), 2.80–2.75 (m, 1H, NCH2(a)), 2.70–2.66 (m, 1H, NCH2(b)), 2.09 (s, 3H, CH3), 2.06–2.02 (m, 1H, CH2), 1.97–1.91 (m, 1H CH2), 1.90–1.85 (m, 1H CH2), 1.79–1.73 (m, 1H CH2); 13C NMR (176 MHz, CDCl3): δ (ppm) =191.91 (CO), 181.47 (CO), 143.56, 142.53, 141.27, 138.24, 136.68, 134.93, 129.33, 128.97, 128.86, 126.77, 125.58, 124.44, 122.32, 122.10, 121.88, 121.27, 119.90, 119.67, 114.21, 111.44, 110.70, 73.77, 70.74, 65.90, 48.38, 44.74, 31.06, 27.28, 12.00 (CH3); IR (KBr, cm–1) νmax = 3273, 3056, 2962, 2867, 1733, 1661, 1653, 1613, 1536, 1531, 1502, 1486, 1453, 1396, 1324, 1285, 1244, 1222, 1185, 1131, 1072, 936, 922, 813, 794, 763, 741, 694, 526; [anal. calcd. for C33H28ClN5O2: C, 70.52; H, 5.02; N, 12.46; found: C, 70.66; H, 4.91; N, 12.53]; LC/MS (ESI, m/z): found 562.4 [M(35Cl) + H]+, 564.1 [M(37Cl) + H]+; exact mass 561.19 for C33H28ClN5O2.
(1′R,2′S,3R,7a’R)-6-Chloro-2′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-1′-(1-methyl-1H-indol-3-yl)-1′,2′,5′,6′,7′,7a’-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one (8u)
Following the general procedure (GP2), chalcone 5b (85 mg, 0.25 mmol), 6-chloroisatin 6b (46 mg, 0.25 mmol), and l-proline 7c (43 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield light yellow solid compound 8u; yield (124 mg, 87%); m.p.: 150–151 °C; [α]D25 = −23.35° (c 0.11, MeOH); 1H NMR (700 MHz, CDCl3): δ (ppm) = 9.02 (s, 1H, NH), 8.07 (d, J = 7.9 Hz, 1H, Ar-H), 7.89 (s, 1H, Ar-H), 7.41–7.35 (m, 3H, Ar-H), 7.30–7.26 (m, 2H, Ar-H), 7.23–7.20 (m, 1H, Ar-H), 7.17 (dd, J = 16.0, 7.6 Hz, 3H, Ar-H), 7.13 (s, 1H, Ar-H), 6.99 (dd, J = 8.0, 1.9 Hz, 1H, Ar-H), 6.65 (d, J = 1.9 Hz, 1H, Ar-H), 4.85 (d, J = 11.5 Hz, 1H, CHCO), 4.50–4.45 (m, 1H, NCH), 4.16 (dd, J = 11.5, 10.0 Hz, 1H, NCHCH), 3.73 (s, 3H, NCH3), 2.82–2.76 (m, 1H, NCH2(a)), 2.70–2.65 (m, 1H, NCH2(b)), 2.09 (s, 3H, CH3), 2.07–2.01 (m, 1H, CH2), 1.98–1.92 (m, 1H, CH2), 1.90–1.86 (m, 1H, CH2), 1.78–1.73 (m, 1H, CH2); 13C NMR (176 MHz, CDCl3): δ (ppm) = 191.89 (CO), 181.46 (CO), 143.51, 142.51, 141.24, 138.29, 137.38, 134.91, 129.32, 128.93, 128.86, 127.21, 126.92, 125.58, 124.47, 121.86, 121.71, 121.29, 120.07, 119.21, 112.71, 110.64, 109.44, 73.67, 70.80, 66.02, 48.43, 44.66, 32.82 (NCH3), 30.96, 27.19, 12.01 (CH3); IR (KBr, cm–1) νmax = 3216, 3058, 2958, 2872, 1733, 1662, 1614, 1536, 1503, 1484, 1455, 1398, 1326, 1285, 1227, 1184, 1130, 1172, 1012, 935, 922, 815, 795, 765, 740, 694; [anal. calcd. for C34H30ClN5O2: C, 70.89; H, 5.25; N, 12.16; found: C, 71.11; H, 5.16; N, 12.01]; LC/MS (ESI, m/z): found 576.5 [M(35Cl) + H]+, 578.2 [M(37Cl) + H]+; exact mass 575.21 for C34H30ClN5O2.
(1′R,2′S,3R,7a’R)-1′-(5-Bromo-1H-indol-3-yl)-2′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-1′,2′,5′,6′,7′,7a’-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one (8v)
Following the general procedure (GP2), chalcone 5c (102 mg, 0.25 mmol), isatin 6a (37 mg, 0.25 mmol), and l-proline 7c (43 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield light yellow solid compound 8v; yield (136 mg, 90%); m.p.: 178–179 °C; [α]D25 = −37.86° (c 0.10, MeOH); 1H NMR (700 MHz, DMSO-d6) δ (ppm) = 11.16 (s, 1H, NH), 10.35 (s, 1H, NH), 8.10 (s, 1H, Ar-H), 7.85 (d, J = 3.0 Hz, 1H, Ar-H), 7.51–7.46 (m, 3H, Ar-H), 7.44 (d, J = 9.1 Hz, 1H, Ar-H), 7.39 (d, J = 7.9 Hz, 1H, Ar-H), 7.34–7.29 (m, 3H, Ar-H), 7.19 (d, J = 10.5 Hz, 1H, Ar-H), 7.14–7.10 (m, 1H, Ar-H), 6.98–6.94 (m, 1H, Ar-H), 6.65 (d, J = 10.3 Hz, 1H, Ar-H), 4.68 (d, J = 11.7 Hz, 1H, CHCO), 4.09 (t, J = 12.0 Hz, 1H, NCHCH), 4.05–4.01 (m, 1H, NCH), 2.62–2.57 (m, 1H, NCH2(a)), 2.39–2.35 (m, 1H, NCH2(b)), 1.94–1.90 (m, 1H, CH2), 1.89 (s, 3H, CH3), 1.87–1.84 (m, 1H, CH2), 1.80–1.75 (m, 1H, CH2), 1.75–1.69 (m, 1H, CH2); 13C NMR (176 MHz, DMSO-d6) δ (ppm) = 191.33 (CO), 179.87 (CO), 142.37, 141.96, 140.86, 138.03, 135.10, 129.29, 129.08, 128.74, 128.70, 127.85, 125.27, 125.23, 123.96, 123.53, 121.32, 120.99, 120.78, 113.57, 113.33, 111.21, 109.43, 73.20, 71.04, 65.39, 47.42, 43.25, 30.49, 27.08, 11.21 (CH3); IR (KBr, cm–1) νmax = 3270, 2958, 2928, 2869, 1717, 1662, 1653, 1616, 1597, 1538, 1504, 1470, 1457, 1397, 1330, 1268, 1221, 1190, 1104, 936, 884, 793, 754, 694, 678, 659, 610; [anal. calcd. for C33H28BrN5O2: C, 65.35; H, 4.65; N, 11.55; found: C, 65.24; H, 4.77; N, 11.49]; LC/MS (ESI, m/z): found 606.7 [M(79Br) + H]+, 608.4 [M(81Br) + H]+; exact mass 605.14 for C33H28BrN5O2.
(1′R,2′S,3R,7a’R)-1′-(5-Chloro-1H-indol-3-yl)-2′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)-5-nitro-1′,2′,5′,6′,7′,7a’-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one (8w)
Following the general procedure (GP2), chalcone 5d (91 mg, 0.25 mmol), 5-nitroisatin 6c (48 mg, 0.25 mmol), and l-proline 7c (43 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 6 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield yellow solid compound 8w; yield (74 mg, 49%); m.p.: 225–226 °C; [α]D25 = −67.91° (c 0.13, MeOH); 1H NMR (700 MHz, DMSO-d6) δ (ppm) = 11.18 (s, 1H, NH), 11.16 (s, 1H, NH), 8.14 (dd, J = 8.6, 2.3 Hz, 1H, Ar-H), 8.12 (d, J = 2.3 Hz, 1H, Ar-H), 7.93 (d, J = 2.1 Hz, 1H, Ar-H), 7.84 (s, 1H), 7.55 (d, J = 2.5 Hz, 1H, Ar-H), 7.51–7.48 (m, 2H, Ar-H), 7.47–7.44 (m, 1H, Ar-H), 7.36 (d, J = 8.6 Hz, 1H, Ar-H), 7.31 (d, J = 8.6 Hz, 2H, Ar-H), 7.08 (dd, J = 8.6, 2.0 Hz, 1H, Ar-H), 6.88 (d, J = 8.6 Hz, 1H, Ar-H), 4.75 (d, J = 11.4 Hz, 1H, CHCO), 4.15–4.09 (m, 2H, NCH + NCHCH), 2.70–2.65 (m, 1H, NCH2(a)), 2.46–2.42 (m, 1H, NCH2(b)), 1.99–1.94 (m, 1H, CH2), 1.92 (s, 3H, CH3) 1.90–1.88 (m, 1H, CH2), 1.84–1.76 (m, 2H, CH2); 13C NMR (176 MHz, DMSO-d6) δ (ppm) = 191.15 (CO), 180.27 (CO), 148.48, 142.63, 141.67, 140.69, 137.82, 134.92, 129.30, 128.86, 127.70, 126.56, 126.22, 125.19, 124.53, 123.23, 122.78, 121.03, 120.59, 118.14, 113.15, 112.52, 109.86, 72.44, 70.67, 65.39, 47.41, 43.52, 30.02, 27.09, 11.21 (CH3); IR (KBr, cm–1) νmax = 3408, 3378, 3111, 3058, 2973, 2861, 2836, 1748, 1645, 1622, 1598, 1525, 1503, 1479, 1455, 1417, 1392, 1373, 1338, 1293, 1253, 1221, 1188, 1174, 1137, 1100, 1076, 986, 938, 930, 887, 860, 850, 832, 798, 763, 755, 694, 655, 610, 554; [anal. calcd. for C33H27ClN6O4: C, 65.29; H, 4.48; N, 13.84; found: C, 65.39; H, 4.56; N, 13.87]; LC/MS (ESI, m/z): found 607.5 [M (35Cl) + H]+, 609.2 [M (35Cl) + H]+; exact mass 606.18 for C33H27ClN6O4.
(2′R,3′S,4′R)-6-Chloro-4′-(1H-indol-3-yl)-1′-methyl-3′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)spiro[indoline-3,2′-pyrrolidin]-2-one (8x)
Following the general procedure (GP2), chalcone 5a (82 mg, 0.25 mmol), 6-chloroisatin 6b (46 mg, 0.25 mmol), and sacrosine 7d (34 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield light yellow solid compound 8x; yield (48 mg, 35%); m.p.: 140–141 °C; [α]D25 = −19.37° (c 0.10, MeOH); 1H NMR (700 MHz, CDCl3): δ (ppm) = 8.51 (s, 1H, NH), 8.19 (s, 1H, NH), 8.08 (d, J = 9.5 Hz, 1H, Ar-H), 7.73 (s, 1H, Ar-H), 7.45–7.38 (m, 3H, Ar-H), 7.33–7.30 (m, 1H, Ar-H), 7.26 (d, J = 1.2 Hz, 1H, Ar-H), 7.20–7.14 (m, 5H, Ar-H), 6.92 (dd, J = 8.0, 1.9 Hz, 1H, Ar-H), 6.49 (d, J = 1.8 Hz, 1H, Ar-H), 4.80–4.73 (m, 1H, NCH2CH), 4.45 (d, J = 9.6 Hz, 1H, CHCO), 3.86–3.81 (m, 1H, NCH2(a)), 3.49–3.45 (m, 1H, NCH2(b)), 2.29 (s, 3H, NCH3), 2.17 (s, 3H, CH3); 13C NMR (176 MHz, CDCl3): δ (ppm) = 192.45 (CO), 180.34 (CO), 143.46, 142.23, 140.55, 138.20, 136.60, 134.48, 129.44, 129.18, 127.71, 126.87, 126.77, 125.71, 122.53, 122.21, 122.16, 121.44, 120.04, 119.75, 115.62, 111.30, 109.77, 73.69, 64.47, 59.54, 35.99, 35.30 (NCH3), 11.91 (CH3); IR (KBr, cm–1) νmax = 3278, 3072, 2934, 2826, 2797, 1726, 1666, 1654, 1612, 1597, 1534, 1503, 1485, 1552, 1398, 1322, 1284, 1240, 1216, 1179, 1119, 1094, 1069, 936, 910, 882, 814, 798, 766, 718, 693, 592; [anal. calcd. for C31H26ClN5O2: C, 69.46; H, 4.89; N, 13.07; found: C, 69.31; H, 4.97; N, 12.93]; LC/MS (ESI, m/z): found 536.4 [M(35Cl) + H]+, 538.2 [M(37Cl) + H]+; exact mass 535.18 for C31H26ClN5O2.
(2′R,3′S,4′R)-4′-(5-Bromo-1H-indol-3-yl)-6-chloro-1′-methyl-3′-(5-methyl-1-phenyl-1H-pyrazole-4-carbonyl)spiro[indoline-3,2′-pyrrolidin]-2-one (8y)
Following the general procedure (GP2), chalcone 5c (102 mg, 0.25 mmol), 6-chloroisatin 6b (46 mg, 0.25 mmol), and sacrosine 7d (34 mg, 0.37 mmol) in methanol (20 mL) were refluxed for 3 h and purified by column chromatography 100–200 mesh silica gel and MeOH/CH2Cl2 (3:97) to yield light yellow solid compound 8y; yield (73 mg, 47%); m.p.: 158–159 °C; [α]D25 = −35.63° (c 0.12, MeOH); 1H NMR (700 MHz, CDCl3): δ (ppm) = 8.99 (s, 1H, NH), 8.42 (s, 1H, NH), 8.18 (s, 1H, Ar-H), 7.77 (s, 1H, Ar-H), 7.44–7.38 (m, 3H, Ar-H), 7.22 (dd, J = 8.7, 2.0 Hz, 2H, Ar-H), 7.18–7.11 (m, 4H, Ar-H), 6.92 (dd, J = 8.0, 1.8 Hz, 1H, Ar-H), 6.54 (d, J = 1.8 Hz, 1H, Ar-H), 4.70 (q, J = 9.8 Hz, 1H, NCH2CH), 4.36 (d, J = 9.4 Hz, 1H, CHCO), 3.75 (t, J = 9.5 Hz, 1H, NCH2(a)), 3.46 (t, J = 9.5 Hz, 1H, NCH2(b)), 2.27 (s, 3H, NCH3), 2.19 (s, 3H, CH3); 13C NMR (176 MHz, CDCl3): δ (ppm) = 192.35 (CO), 180.67 (CO), 143.63, 142.34, 140.51, 138.14, 135.16, 134.58, 129.47, 129.24, 128.70, 127.64, 126.53, 125.71, 125.00, 123.27, 122.55, 122.51, 121.38, 115.62, 113.00, 112.75, 110.02, 73.75, 64.57, 59.54, 35.58, 35.26 (NCH3), 11.93 (CH3); IR (KBr, cm–1) νmax = 3276, 3071, 2936, 2843, 2795, 1722, 1668, 1652, 1613, 1598, 1537, 1504, 1484, 1554, 1397, 1321, 1280, 1244, 1219, 1181, 1115, 1096, 1068, 938, 916, 883, 813, 793, 765, 714, 694, 593; [anal. calcd. for C31H25BrClN5O2: C, 60.55; H, 4.10; N, 11.39; found: C, 60.41; H, 4.15; N, 11.47]; LC/MS (ESI, m/z): found 614.8 [M(35Cl/79Br) + H]+, 616.1 [M(37Cl/81Br) + H]+, 618.0 [M(37Cl + 81Br) + H]+, exact mass 613.09 for C31H25BrClN5O2.
Acetylcholine Esterase (AChE) Inhibitory Assay (AChEI)
AChEI activity was measured using Ellman’s method as previously described and provided in the Supporting Information.39b,39c
Molecular Docking Study
The protocol for the molecular docking study to investigate the binding mode of the most active compounds are provided in the Supporting Information.43
Acknowledgments
The authors would like to extend their sincere appreciation to Researchers Supporting Project Number (RSP-2021/64), King Saud University, Riyadh, Saudi Arabia.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03978.
AChE assay; molecular docking study protocol; copies of NMR and MS spectrum; and also X-ray single-crystal analysis of compound 8c (PDF)
The authors declare no competing financial interest.
Notes
∇ The current research was carried out in the current affiliation.
Supplementary Material
References
- Hardy J. A.; Higgins G. A. Alzheimer’s disease: the amyloid cascade hypothesis. Science 1992, 256, 184–186. 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Mohandas E.; Rajmohan V.; Raghunath B. Neurobiology of Alzheimer’s disease. Indian J. Psychiatry 2009, 51, 55. 10.4103/0019-5545.44908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow R. H.; Burns J. M.; Khan S. M. The Alzheimer’s disease mitochondrial cascade hypothesis. J. Alzheimer’s Dis. 2010, 20, S265–S279. 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni R. B.; Farías G.; Morales I.; Navarrete L. The revitalized tau hypothesis on Alzheimer’s disease. Arch. Med. Res. 2010, 41, 226–231. 10.1016/j.arcmed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Wood W. G.; Li L.; Müller W. E.; Eckert G. P. Cholesterol as a causative factor in Alzheimer’s disease: a debatable hypothesis. J. Neurochem. 2014, 129, 559–572. 10.1111/jnc.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotova E.; Nicoll J. A.; Kalaria R.; Holmes C.; Boche D. Inflammation in Alzheimer’s disease: relevance to pathogenesis and therapy. Alzheimer’s Res. Ther. 2010, 2, 1–9. 10.1186/alzrt24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery W. R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radical Biol. Med. 1997, 23, 134–147. 10.1016/S0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- Bush A. I.; Tanzi R. E. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics 2008, 5, 421–432. 10.1016/j.nurt.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J. C. The vascular hypothesis of Alzheimer’s disease: bench to bedside and beyond. Neurodegener. Dis. 2010, 7, 116–121. 10.1159/000285520. [DOI] [PubMed] [Google Scholar]
- Neve R. L.; McPhie D. L. The cell cycle as a therapeutic target for Alzheimer’s disease. Pharmacol. Ther. 2006, 111, 99–113. 10.1016/j.pharmthera.2005.09.005. [DOI] [PubMed] [Google Scholar]
- a de Oliveira Viana J.; Monteiro A. F. M.; Filho J. M. B.; Scotti L.; Scotti M. T. The Azoles in Pharmacochemistry: Perspectives on the Synthesis of New Compounds and Chemoinformatic Contributions. Curr. Pharm. Des. 2020, 25, 4702–4716. 10.2174/1381612825666191125090700. [DOI] [PubMed] [Google Scholar]; b Teleb M.; Rizk O. H.; Zhang F.-X.; Fronczek F. R.; Zamponi G. W.; Fahmy H. Design, synthesis and pharmacological evaluation of some substituted dihydropyrimidines with L-/T-type calcium channel blocking activities. Bioorg. Chem. 2019, 83, 354–366. 10.1016/j.bioorg.2018.10.054. [DOI] [PubMed] [Google Scholar]
- Radi S.; El Massaoudi M.; Bacquet M.; Degoutin S.; Adarsh N.; Robeyns K.; Garcia Y. A novel environment-friendly hybrid material based on a modified silica gel with a bispyrazole derivative for the removal of Zn II, Pb II, Cd II and Cu II traces from aqueous solutions. Inorg. Chem. Front. 2017, 4, 1821–1831. 10.1039/C7QI00322F. [DOI] [Google Scholar]
- Ouyang G.; Cai X.-J.; Chen Z.; Song B.-A.; Bhadury P. S.; Yang S.; Jin L.-H.; Xue W.; Hu D.-Y.; Zeng S. Synthesis and antiviral activities of pyrazole derivatives containing an oxime moiety. J. Agric. Food Chem. 2008, 56, 10160–10167. 10.1021/jf802489e. [DOI] [PubMed] [Google Scholar]
- Keter F. K.; Darkwa J. Perspective: the potential of pyrazole-based compounds in medicine. BioMetals 2012, 25, 9–21. 10.1007/s10534-011-9496-4. [DOI] [PubMed] [Google Scholar]
- Ramesh B.; Bhalgat C. M. Novel dihydropyrimidines and its pyrazole derivatives: Synthesis and pharmacological screening. Eur. J. Med. Chem. 2011, 46, 1882–1891. 10.1016/j.ejmech.2011.02.052. [DOI] [PubMed] [Google Scholar]
- Kumar V.; Kaur K.; Gupta G. K.; Sharma A. K. Pyrazole containing natural products: synthetic preview and biological significance. Eur. J. Med. Chem. 2013, 69, 735–753. 10.1016/j.ejmech.2013.08.053. [DOI] [PubMed] [Google Scholar]
- Diculescu V. C.; Chiorcea-Paquim A.-M.; Oliveira-Brett A. M. Applications of a DNA-electrochemical biosensor. TrAC, Trends Anal. Chem. 2016, 79, 23–36. 10.1016/j.trac.2016.01.019. [DOI] [Google Scholar]
- Graillot V.; Tomasetig F.; Cravedi J.-P.; Audebert M. Evidence of the in vitro genotoxicity of methyl-pyrazole pesticides in human cells. Mutat. Res., Genet. Toxicol. Environ. Mutagen. 2012, 748, 8–16. 10.1016/j.mrgentox.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Çetin A.; Bildirici I. A study on synthesis and antimicrobial activity of 4-acyl-pyrazoles. J. Saudi Chem. Soc. 2018, 22, 279–296. 10.1016/j.jscs.2016.05.008. [DOI] [Google Scholar]
- Ramírez–Prada J.; Robledo S. M.; Vélez I. D.; del Pilar Crespo M.; Quiroga J.; Abonia R.; Montoya A.; Svetaz L.; Zacchino S.; Insuasty B. Synthesis of novel quinoline–based 4, 5–dihydro–1H–pyrazoles as potential anticancer, antifungal, antibacterial and antiprotozoal agents. Eur. J. Med. Chem. 2017, 131, 237–254. 10.1016/j.ejmech.2017.03.016. [DOI] [PubMed] [Google Scholar]
- El-Sabbagh O. I.; Baraka M. M.; Ibrahim S. M.; Pannecouque C.; Andrei G.; Snoeck R.; Balzarini J.; Rashad A. A. Synthesis and antiviral activity of new pyrazole and thiazole derivatives. Eur. J. Med. Chem. 2009, 44, 3746–3753. 10.1016/j.ejmech.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Insuasty B.; Tigreros A.; Orozco F.; Quiroga J.; Abonía R.; Nogueras M.; Sanchez A.; Cobo J. Synthesis of novel pyrazolic analogues of chalcones and their 3-aryl-4-(3-aryl-4, 5-dihydro-1H-pyrazol-5-yl)-1-phenyl-1H-pyrazole derivatives as potential antitumor agents. Bioorg. Med. Chem. 2010, 18, 4965–4974. 10.1016/j.bmc.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Bandgar B. P.; Gawande S. S.; Bodade R. G.; Gawande N. M.; Khobragade C. N. Synthesis and biological evaluation of a novel series of pyrazole chalcones as anti-inflammatory, antioxidant and antimicrobial agents. Bioorg. Med. Chem. 2009, 17, 8168–8173. 10.1016/j.bmc.2009.10.035. [DOI] [PubMed] [Google Scholar]
- Pérez-Fernández R.; Goya P.; Elguero J. A review of recent progress (2002-2012) on the biological activities of pyrazoles. Arkivoc 2014, 2014, 233–293. 10.3998/ark.5550190.p008.131. [DOI] [Google Scholar]
- Özdemir A.; Altıntop M. D.; Kaplancıklı Z. A.; Can Ö. D.; Demir Özkay Ü.; Turan-Zitouni G. Synthesis and Evaluation of New 1, 5-Diaryl-3-[4-(methyl-sulfonyl) phenyl]-4, 5-dihydro-1H-pyrazole Derivatives as Potential Antidepressant Agents. Molecules 2015, 20, 2668–2684. 10.3390/molecules20022668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling A.; Jantos K.; Mack H.; Hornberger W.; Drescher K.; Nimmrich V.; Relo A.; Wicke K.; Hutchins C. W.; Lao Y.; et al. Discovery of Novel and Highly Selective Inhibitors of Calpain for the Treatment of Alzheimer’s Disease: 2-(3-Phenyl-1 H-pyrazol-1-yl)-nicotinamides. J. Med. Chem. 2017, 60, 7123–7138. 10.1021/acs.jmedchem.7b00731. [DOI] [PubMed] [Google Scholar]
- a Ahsan N.; Mishra S.; Jain M. K.; Surolia A.; Gupta S. Curcumin Pyrazole and its derivative (N-(3-Nitrophenylpyrazole) Curcumin inhibit aggregation, disrupt fibrils and modulate toxicity of Wild type and Mutant α-Synuclein. Sci. Rep. 2015, 5, 9862 10.1038/srep09862. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Estrada A. A.; Chan B. K.; Baker-Glenn C.; Beresford A.; Burdick D. J.; Chambers M.; Chen H.; Dominguez S. L.; Dotson J.; Drummond J.; et al. Discovery of highly potent, selective, and brain-penetrant aminopyrazole leucine-rich repeat kinase 2 (LRRK2) small molecule inhibitors. J. Med. Chem. 2014, 57, 921–936. 10.1021/jm401654j. [DOI] [PubMed] [Google Scholar]
- Neelarapu R.; Holzle D. L.; Velaparthi S.; Bai H.; Brunsteiner M.; Blond S. Y.; Petukhov P. A. Design, synthesis, docking, and biological evaluation of novel diazide-containing isoxazole-and pyrazole-based histone deacetylase probes. J. Med. Chem. 2011, 54, 4350–4364. 10.1021/jm2001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y. T.; Kim K.; Choi G.-I.; An H.; Son D.; Kim H.; Ha H.-J.; Son J.-H.; Chung S.-J.; Park H.-J.; et al. Pyrazole-5-carboxamides, novel inhibitors of receptor for advanced glycation end products (RAGE). Eur. J. Med. Chem. 2014, 79, 128–142. 10.1016/j.ejmech.2014.03.072. [DOI] [PubMed] [Google Scholar]
- a Turkan F.; Cetin A.; Taslimi P.; Gulçin İ. Some pyrazoles derivatives: Potent carbonic anhydrase, α-glycosidase, and cholinesterase enzymes inhibitors. Arch. Pharm. 2018, 351, 1800200 10.1002/ardp.201800200. [DOI] [PubMed] [Google Scholar]; b Turkan F.; Çetin A.; Taslimi P.; Karaman M.; Gulçin İ. Synthesis, biological evaluation and molecular docking of novel pyrazole derivatives as potent carbonic anhydrase and acetylcholinesterase inhibitors. Bioorg. Chem. 2019, 86, 420–427. 10.1016/j.bioorg.2019.02.013. [DOI] [PubMed] [Google Scholar]
- a Brandão P.; Marques C.; Burke A. J.; Pineiro M. The application of isatin-based multicomponent-reactions in the quest for new bioactive and druglike molecules. Eur. J. Med. Chem. 2021, 211, 113102. 10.1016/j.ejmech.2020.113102. [DOI] [PubMed] [Google Scholar]; b Lotfy G.; Aziz Y. M. A.; Said M. M.; El Sayed H.; El Sayed H.; Abu-Serie M. M.; Teleb M.; Dömling A.; Barakat A. Molecular hybridization design and synthesis of novel spirooxindole-based MDM2 inhibitors endowed with BCL2 signaling attenuation; a step towards the next generation p53 activators. Bioorg. Chem. 2021, 105427 10.1016/j.bioorg.2021.105427. [DOI] [PubMed] [Google Scholar]; c Aziz Y. M. A.; Lotfy G.; Said M. M.; El Ashry E. S. H.; El Tamany E. S. H.; Soliman S.; Abu-Serie M. M.; Teleb M.; Yousuf S.; Dömling A.; Domingo L. R.; Barakat A. Design, Synthesis, Chemical and Biochemical Insights on to Novel Hybrid Spirooxindoles-Based p53-MDM2 Inhibitors with Potential Bcl2 Signaling Attenuation. Front. Chem. 2021, 915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Choi S. S.; Lee S.-R.; Kim S. U.; Lee H. J. Alzheimer’s disease and stem cell therapy. Exp. Neurobiol. 2014, 23, 45. 10.5607/en.2014.23.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Giacobini E. Selective inhibitors of butyrylcholinesterase. Drugs Aging 2001, 18, 891–898. 10.2165/00002512-200118120-00001. [DOI] [PubMed] [Google Scholar]; c Kia Y.; Osman H.; Kumar R. S.; Murugaiyah V.; Basiri A.; Perumal S.; Razak I. A. A facile chemo-, regio-and stereoselective synthesis and cholinesterase inhibitory activity of spirooxindole–pyrrolizine–piperidine hybrids. Bioorg. Med. Chem. Lett. 2013, 23, 2979–2983. 10.1016/j.bmcl.2013.03.027. [DOI] [PubMed] [Google Scholar]; d Kia Y.; Osman H.; Kumar R. S.; Basiri A.; Murugaiyah V. Synthesis and discovery of highly functionalized mono-and bis-spiro-pyrrolidines as potent cholinesterase enzyme inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 1815–1819. 10.1016/j.bmcl.2014.02.019. [DOI] [PubMed] [Google Scholar]; e Kia Y.; Osman H.; Kumar R. S.; Murugaiyah V.; Basiri A.; Perumal S.; Wahab H. A.; Bing C. S. Synthesis and discovery of novel piperidone-grafted mono-and bis-spirooxindole-hexahydropyrrolizines as potent cholinesterase inhibitors. Bioorg. Med. Chem. 2013, 21, 1696–1707. 10.1016/j.bmc.2013.01.066. [DOI] [PubMed] [Google Scholar]; f kia Y.; Osman H.; Kumar R. S.; Basiri A.; Murugaiyah V. Ionic liquid mediated synthesis of mono-and bis-spirooxindole-hexahydropyrrolidines as cholinesterase inhibitors and their molecular docking studies. Bioorg. Med. Chem. 2014, 22, 1318–1328. 10.1016/j.bmc.2014.01.002. [DOI] [PubMed] [Google Scholar]; g Arumugam N.; Almansour A. I.; Kumar R. S.; Kotresha D.; Saiswaroop R.; Venketesh S. Dispiropyrrolidinyl-piperidone embedded indeno [1, 2-b] quinoxaline heterocyclic hybrids: Synthesis, cholinesterase inhibitory activity and their molecular docking simulation. Bioorg. Med. Chem. 2019, 27, 2621–2628. 10.1016/j.bmc.2019.03.058. [DOI] [PubMed] [Google Scholar]; h Barakat A.; Soliman S. M.; Alshahrani S.; Islam M. S.; Ali M.; Al-Majid A. M.; Yousuf S. Synthesis, X-ray Single Crystal, Conformational Analysis and Cholinesterase Inhibitory Activity of a New Spiropyrrolidine Scaffold Tethered Benzo [b] Thiophene Analogue. Crystals 2020, 10, 120. 10.3390/cryst10020120. [DOI] [Google Scholar]; i Goyal D.; Kaur A.; Goyal B. Benzofuran and indole: promising scaffolds for drug development in Alzheimer’s disease. ChemMedChem 2018, 13, 1275–1299. 10.1002/cmdc.201800156. [DOI] [PubMed] [Google Scholar]; j Kumar R. S.; Ali M. A.; Osman H.; Ismail R.; Choon T. S.; Yoon Y. K.; Wei A. C.; Pandian S.; Manogaran E. Synthesis and discovery of novel hexacyclic cage compounds as inhibitors of acetylcholinesterase. Bioorg. Med. Chem. Lett. 2011, 21, 3997–4000. 10.1016/j.bmcl.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Chigurupati S.; Selvaraj M.; Mani V.; Selvarajan K. K.; Mohammad J. I.; Kaveti B.; Bera H.; Palanimuthu V. R.; Teh L. K.; Salleh M. Z. Identification of novel acetylcholinesterase inhibitors: Indolopyrazoline derivatives and molecular docking studies. Bioorg. Chem. 2016, 67, 9–17. 10.1016/j.bioorg.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Barakat A.; Alshahrani S.; Al-Majid A. M.; Ali M.; Altowyan M. S.; Islam M. S.; Alamary A. S.; Ashraf S.; Ul-Haq Z. Synthesis of a New Class of Spirooxindole–Benzo [b] Thiophene-Based Molecules as Acetylcholinesterase Inhibitors. Molecules 2020, 25, 4671. 10.3390/molecules25204671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Mei G.-J.; Shi F. Catalytic asymmetric synthesis of spirooxindoles: recent developments. Chem. Commun. 2018, 54, 6607–6621. 10.1039/C8CC02364F. [DOI] [PubMed] [Google Scholar]; b Saranya P.; Neetha M.; Aneeja T.; Anilkumar G. Transition metal-catalyzed synthesis of spirooxindoles. RSC Adv. 2021, 11, 7146–7179. 10.1039/D1RA00139F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Wang C.-S.; Zhu R.-Y.; Zheng J.; Shi F.; Tu S.-J. Enantioselective construction of spiro [indoline-3, 2′-pyrrole] framework via catalytic asymmetric 1, 3-dipolar cycloadditions using allenes as equivalents of alkynes. J. Org. Chem. 2015, 80, 512–520. 10.1021/jo502516e. [DOI] [PubMed] [Google Scholar]; b Dai W.; Jiang X.-L.; Wu Q.; Shi F.; Tu S.-J. Diastereo-and enantioselective construction of 3, 3′-pyrrolidinyldispirooxindole framework via catalytic asymmetric 1, 3-dipolar cycloadditions. J. Org. Chem. 2015, 80, 5737–5744. 10.1021/acs.joc.5b00708. [DOI] [PubMed] [Google Scholar]; c Wang Y.-M.; Zhang H.-H.; Li C.; Fan T.; Shi F. Catalytic asymmetric chemoselective 1, 3-dipolar cycloadditions of an azomethine ylide with isatin-derived imines: diastereo-and enantioselective construction of a spiro [imidazolidine-2, 3′-oxindole] framework. Chem. Commun. 2016, 52, 1804–1807. 10.1039/C5CC07924A. [DOI] [PubMed] [Google Scholar]
- a Altowyan M. S.; Barakat A.; Al-Majid A. M.; Al-Ghulikah H. Spiroindolone Analogues as Potential Hypoglycemic with Dual Inhibitory Activity on α-Amylase and α-Glucosidase. Molecules 2019, 24, 2342. 10.3390/molecules24122342. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Altowyan M. S.; Barakat A.; Al-Majid A. M.; Al-Ghulikah H. Spiroindolone analogues bearing benzofuran moiety as a selective cyclooxygenase COX-1 with TNF-α and IL-6 inhibitors. Saudi J. Biol. Sci. 2020, 27, 1208–1216. 10.1016/j.sjbs.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Barakat A.; Islam M. S.; Ghawas H. M.; Al-Majid A. M.; El-Senduny F. F.; Badria F. A.; Elshaier Y. A. M.; Ghabbour H. A. Substituted spirooxindole derivatives as potent anticancer agents through inhibition of phosphodiesterase 1. RSC Adv. 2018, 8, 14335–14346. 10.1039/C8RA02358A. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Islam M. S.; Ghawas H. M.; El-Senduny F. F.; Al-Majid A. M.; Elshaier Y. A.; Badria F. A.; Barakat A. Synthesis of new thiazolo-pyrrolidine–(spirooxindole) tethered to 3-acylindole as anticancer agents. Bioorg. Chem. 2019, 82, 423–430. 10.1016/j.bioorg.2018.10.036. [DOI] [PubMed] [Google Scholar]; e Al-Majid A. M.; Soliman S. M.; Haukka M.; Ali M.; Islam M. S.; Shaik M. R.; Barakat A. Design, Construction, and Characterization of a New Regioisomer and Diastereomer Material Based on the Spirooxindole Scaffold Incorporating a Sulphone Function. Symmetry 2020, 12, 1337. 10.3390/sym12081337. [DOI] [Google Scholar]; f Aziz y. a.; Lotfy G.; Said M. M.; El Sayed H.; El Ashry H.; El Tamany H.; Soliman S. M.; Haukka M.; Barakat A. A.; et al. Synthesis and characterization of a new spirooxindole grafted pyrrolidino/piperidine moiety. Record. Pharm. Biomed. Sci. 2021, 5, 1–7. 10.21608/rpbs.2021.50391.1079. [DOI] [Google Scholar]; g Islam M. S.; Haukka M.; Soliman S. M.; Al-Majid A. M.; Rahman A. M.; Bari A.; Barakat A. Regio-and Stereoselective Synthesis of Spiro-heterocycles Bearing the Pyrazole Scaffold via [3+ 2] Cycloaddition Reaction. J. Mol. Struct. 2021, 1250, 131711 10.1016/j.molstruc.2021.131711. [DOI] [Google Scholar]
- a Ríos-Gutiérrez M.; Domingo L. R. Unravelling the mysteries of the [3+ 2] cycloaddition reactions. Eur. J. Org. Chem. 2019, 2019, 267–282. 10.1002/ejoc.201800916. [DOI] [Google Scholar]; b R Domingo L.; Chamorro E.; Perez P. Understanding the high reactivity of the azomethine ylides in [3 + 2] cycloaddition reactions. Lett. Org. Chem. 2010, 7, 432–439. 10.2174/157017810791824900. [DOI] [Google Scholar]; c Domingo L. R.; Kula K.; Ríos-Gutiérrez M. Unveiling the reactivity of cyclic azomethine ylides in [3+ 2] cycloaddition reactions within the molecular electron density theory. Eur. J. Org. Chem. 2020, 2020, 5938–5948. 10.1002/ejoc.202000745. [DOI] [Google Scholar]; d Domingo L. R.; Ríos-Gutiérrez M.; Pérez P. A molecular electron density theory study of the role of the copper metalation of azomethine ylides in [3+ 2] cycloaddition reactions. J. Org. Chem. 2018, 83, 10959–10973. 10.1021/acs.joc.8b01605. [DOI] [PubMed] [Google Scholar]
- a Ellman G. L.; Courtney K. D.; Andres V.; Featherstone R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]; b Mira A.; Yamashita S.; Katakura Y.; Shimizu K. In vitro neuroprotective activities of compounds from Angelica shikokiana Makino. Molecules 2015, 20, 4813–4832. 10.3390/molecules20034813. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Abdel Bar F. M.; Elimam D. M.; Mira A. S.; El-Senduny F. F.; Badria F. A. Derivatization, molecular docking and in vitro acetylcholinesterase inhibitory activity of glycyrrhizin as a selective anti-Alzheimer agent. Nat. Prod. Res. 2019, 33, 2591–2599. 10.1080/14786419.2018.1462177. [DOI] [PubMed] [Google Scholar]
- Elgazar A. A.; Knany H. R.; Ali M. S. Insights on the molecular mechanism of anti-inflammatory effect of formula from Islamic traditional medicine: An in-silico study. J. Tradit. Complementary Med. 2019, 9, 353–363. 10.1016/j.jtcme.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Cheung J.; Rudolph M. J.; Burshteyn F.; Cassidy M. S.; Gary E. N.; Love J.; Franklin M. C.; Height J. J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. 10.1021/jm300871x. [DOI] [PubMed] [Google Scholar]; b Bacalhau P.; San Juan A. A.; Marques C. S.; Peixoto D.; Goth A.; Guarda C.; Silva M.; Arantes S.; Caldeira A. T.; Martins R.; Burke A. J. New cholinesterase inhibitors for Alzheimer’s disease: Structure Activity Studies (SARs) and molecular docking of isoquinolone and azepanone derivatives. Bioorg. Chem. 2016, 67, 1–8. 10.1016/j.bioorg.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Alinezhad H.; Tajbakhsh M.; Zare M. Catalyst-free one-pot synthesis of 1, 4, 5-trisubstituted pyrazoles in 2, 2, 2-trifluoroethanol. J. Fluorine Chem. 2011, 132, 995–1000. 10.1016/j.jfluchem.2011.07.014. [DOI] [Google Scholar]
- a Inc., C. C. G. C. M. O. E. M. S. S. W., Suite #910, Montreal, QC, Canada, H3A 2R7.; b Hsu K.-C.; Chen Y.-F.; Lin S.-R.; Yang J.-M. iGEMDOCK: a graphical environment of enhancing GEMDOCK using pharmacological interactions and post-screening analysis. BMC Bioinf. 2011, 12, 1–11. 10.1186/1471-2105-12-S1-S33. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Studio, D. J. A. I. S. D., CA, USA (2009). “version 2.5”.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.