Abstract
Alzheimer’s disease (AD) is the most common reason for progressive dementia in the elderly. It has been shown that disorders of the mammalian/mechanistic target of rapamycin (mTOR) signaling pathways are related to the AD. On the other hand, diabetes mellitus (DM) is a risk factor for the cognitive dysfunction. The pathogenesis of the neuronal impairment caused by diabetic hyperglycemia is intricate, which contains neuro-inflammation and/or neurodegeneration and dementia. Glucagon-like peptide-1 (GLP1) is interesting as a possible link between metabolism and brain impairment. Modulation of GLP1 activity can influence amyloid-beta peptide aggregation via the phosphoinositide-3 kinase/AKT/mTOR signaling pathway in AD. The GLP1 receptor agonists have been shown to have favorable actions on the brain such as the improvement of neurological deficit. They might also exert a beneficial effect with refining learning and memory on the cognitive impairment induced by diabetes. Recent experimental and clinical evidence indicates that dipeptidyl-peptidase-4 (DPP4) inhibitors, being currently used for DM therapy, may also be effective for AD treatment. The DPP-4 inhibitors have demonstrated neuroprotection and cognitive improvements in animal models. Although further studies for mTOR, GLP1, and DPP4 signaling pathways in humans would be intensively required, they seem to be a promising approach for innovative AD-treatments. We would like to review the characteristics of AD pathogenesis, the key roles of mTOR in AD and the preventive and/ or therapeutic suggestions of directing the mTOR signaling pathway.
Keywords: Alzheimer’s disease, Cognitive disorder, Dementia, Glucagon-like peptide-1, Dipeptidyl peptidase-4, Mammalian/mechanistic target of rapamycin
Core Tip: Disorders of mammalian/mechanistic target of rapamycin (mTOR) signaling pathways are related to Alzheimer’s disease (AD). Although further studies for mTOR, glucagon-like peptide-1, and dipeptidyl-peptidase-4 signaling are needed, they seem to be a promising approach for innovative AD-treatments.
INTRODUCTION
Alzheimer’s disease (AD) is a chronic neuro-degenerative disease of the central nervous system (CNS), which is described by a slow and unremitting pathology[1]. The chief clinical appearance of AD is progressive continuing dementia, which is categorized by intellectual symptoms such as diminished cognition, memorial dysfunction, and behavioral complaints[2]. The prevention of AD is a public health concern because of a lack of effective treatments. The onset of AD is associated with an increase in age and to a reduction in mitochondrial ATP synthesis in the hippocampus of the brain[3]. Estrogen has a neuro-protective effect on various nerve cells, however, estrogen also has a carcinogenic effect to non-nerve proliferating cells[4]. Pre-diabetic risk factors, obesity, and metabolic syndrome could promote cognitive dysfunction[5]. Neuro-pathological features of AD are neurofibrillary tangles, molded by hyper-phosphorylated tau protein, which may accumulate into oligomers and/or amyloid plaques[6]. There might be an association between metabolism and brain function. Insulin works as a pro-survival neurotrophic factor with its receptors at cognitive areas in the brain[7]. The commonalities have been found between AD and type 2 diabetes mellitus, which is believed as a high-risk factor for AD[8]. In addition, the animal studies have shown that GLP1 may benefit on the neuro-degeneration[9]. The GLP1 receptor agonists have also been shown as possessing neuro-protective effects in AD, which seem to improve nearly all neuro-pathological features as well as cognitive functions of AD[10]. For example, neurofibrillary tangles, amyloid plaques, and neuro-inflammations in the hippocampus have been reduced in AD model mice[11,12]. In the rat model, it has been shown that a GLP1 receptor agonist also prevents synaptic damage induced by amyloid-beta accumulation, which supports the spatial memory by affecting the phosphoinositide-3 kinase (PI3K)-AKT pathway[11]. Targeting dipeptidyl-peptidase-4 (DPP4) inhibitors that is involved in the GLP1 signaling has been considered as promising therapeutic models to AD[13]. Furthermore, mammalian/mechanistic target of rapamycin (mTOR) has been considered as a center that integrates multiple signaling cascades including the GLP1 receptor signaling, which may also be involved in the progression of AD[14]. We will review the several studies linking potential protective factors to pathogenesis of AD, focusing on the roles of GLP1 and DPP4 inhibitors in the PI3K/AKT/mTOR pathway. In addition, we will summarize the recent researches of the AD-associated biology, by which several diet factors could relate to the pathway. To overview the potential physical activities through the PI3K/AKT/mTOR signaling may contribute to the preventive and/or therapeutic strategy for AD.
PI3K/AKT/MTOR SIGNALING IS INVOLVED IN NEUROPROTECTION OF AD
The mTOR plays a significant role in diverse cellular processes including cell survival, cell proliferation, and cell death[15], which is a particular molecule bound to rapamycin. The rapamycin is an immune-suppressant used for the anti-rejection of tissue-transplantation[16]. Rapamycin also exhibits remarkable potential in the fields of neuro-protection, anti-aging, etc.[17]. It can inhibit the activity of the mTOR[18]. The mTOR is also a nutrient-sensor that mediates the signaling responses to energy status in a cell[19]. Besides, the mTOR activity could be inhibited by nutritional signaling such as caloric restriction[20]. Inhibition of the mTOR could alter cellular responses from cell proliferation to cell quiescence with decreased protein synthesis[21]. Basically, mTOR-inhibition has been shown to increase resistance to stresses resulting in the regulation of age-related diseases, which may contribute to the extension of total life-time[22]. Modulation of the mTOR-function to inhibit cellular apoptosis is deeply involved in the protective effects of pharmacologic agents aiming against diabetes and neurodegenerative diseases[23]. The mTOR activation inhibits autophagy, which is often disrupted in age-related diseases[24]. In the mouse brain neurons, amyloid-beta oligomers have been thought to activate the JNK signaling, leading to insulin resistance[25]. Instead, activation of the PI3K/AKT signaling pathway could bring the inhibition of apoptosis cascade including caspase-signaling[26], leading to the inhibition of the induction of inflammatory cytokines[27]. Following the activation of growth-factor receptors with their ligand, PI3K/AKT gets activated directing to promotion of mitogen-associated protein kinase/extracellular signal-regulated kinases and mTOR[28]. On the other hand, adenosine monophosphate-activated protein kinase (AMPK) is an important signaling mediator of GLP1 receptor, which inhibits mTOR[29]. In fact, the AMPK-loss has resulted in hyper-proliferation and hyperactive mTOR signaling[30].
Therefore, the mTOR signaling could interact with several upstream components including PI3K/AKT and AMPK[31] (Figure 1). Increasing studies have established the involvement of the mTOR signaling in various neuro-degenerative diseases including AD[32]. In particular, activated mTOR signaling is a contributor to the progression of AD[33]. Furthermore, there is a close relationship between mTOR signaling and the presence of amyloid-beta plaques and cognitive impairment[34]. So, the development of mTOR-inhibitors may be useful for the prevention and treatment of AD and/or the other neuro-degenerative diseases. In the CNS, inhibition of the mTOR has been revealed to protect vascular functions in aging[35]. Appropriate dose of rapamycin may diminish neurofibrillary tangles and amyloid-beta plaques improving cognitive functions in AD model mice[36]. Similarly, mTOR inhibition without malnutrition is able to improve the pathology of AD[37]. Moreover, mTOR inhibition protects mitochondrial function, reduces oxidative stress, and maintains glucose homeostasis in aging[20,38]. Conversely, activation of the mTOR may shift metabolisms toward ketone-body consumption[39]. Elevated ketone-body metabolisms and/or the administration of the ketogenic diet have been shown neuro-protective against aging, neurodegeneration, and AD[40].
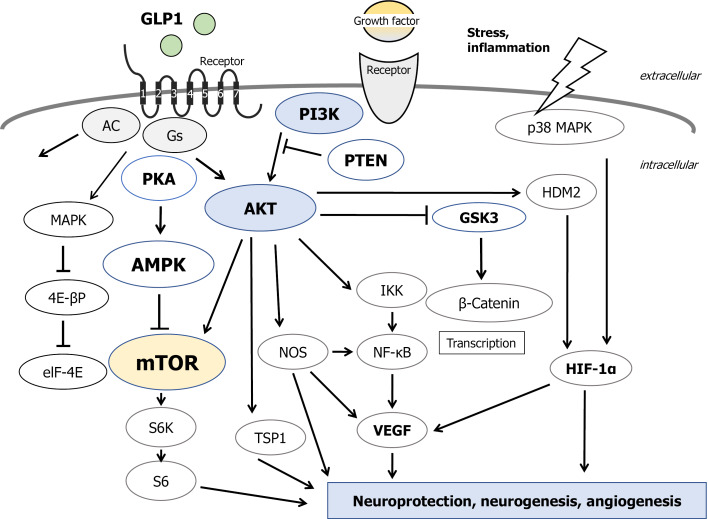
Figure 1.
Several modulator molecules linked to the phosphoinositide-3 kinase/AKT/mammalian/mechanistic target of rapamycin signaling in an extracellular growth-factor response are demonstrated. Example molecules known to act on the glucagon-like peptide-1 (GLP1)-receptor/adenosine monophosphate-activated protein kinase (AMPK)/mammalian/mechanistic target of rapamycin (mTOR) signaling pathway are also shown. Note that some critical events such as immune activation and/or cytokine-induction have been omitted for clarity. Arrowhead means stimulation whereas hammerhead represents inhibition. PI3K: Phosphoinositide-3 kinase; PKA: Protein kinase A; PTEN: Phosphatase and tensin homologue deleted on chromosome 10; DPP4: Dipeptidyl-peptidase-4; GSK3: Glycogen synthase kinase 3; MAPK: Mitogen-activated protein kinase; S6K: S6 kinase; AC: Adenylate cyclase; Gs: Stimulatory G-protein; elF-4E: Eukaryotic translation initiation factor 4E; TSP1: Thrombospondin-1; VEGF: Vascular endothelial growth factor; NOS: Nitric oxide synthase; IKK: I kappa B kinase; NF-kB: Nuclear factor-kappa B; HDM2: Human double minute 2; HIF-1a: Hypoxia inducible factor 1-alpha.
GLP1 AND DIPEPTIDYL PEPTIDASE-IV-INHIBITION EXHIBITS NEUROPROTECTIVE EFFECTS IN AD
GLP1 is an endogenous hormone secreted from intestinal L-cells in response to food-intake[41]. Proteolytic cleavage of the precursor GLP1 (1–37) produce two biological active forms[42]. GLP1 may stimulate insulin-secretion from beta-cells in pancreatic islets under hyperglycemic situations and may decrease glucagon secretion from alfa-cells in pancreatic islets[43]. Signal transduction of GLP1 is mediated by the GLP1 receptor, a G-protein coupled seven-pass-transmembrane domain receptor, heading to cyclic adenosine monophosphate dependent activation of protein kinase A and AMPK. In fact, it has been shown that GLP1 receptor agonists-treatment activates the AMPK signaling within myoblast C2C12 cells[44]. On the contrary, the GLP1 receptor may also operate the downstream signal transduction from the PI3K/AKT pathway so as to work against cellular apoptosis[45]. Accordingly, the GLP1 receptor could dually modulate the activity of mTOR, a key kinase regulating proliferation, survival, and protection in balance. Actually, GLP1 receptor antagonists also stimulate insulin activation by the PI3K/AKT signaling pathway, with the following activation of mTOR and inhibition of GSK3-beta, an essential kinase involved in the phosphorylation of tau protein in AD[46]. GLP1 may also be involved in the regulation of autophagy, the reduction of the oxidative stress, and in the protection of CNS with induction of anti-inflammatory signaling[47]. In addition, GLP1 plays a critical role preventing cardiovascular diseases, in which GLP1 and its analogs may contribute a great deal in the treatment of the diseases[48]. Likewise, it has been shown that GLP1 receptor agonists reduce the infarct size, inflammation, and apoptosis in a rat model of stroke[49].
GLP1 is rapidly degraded by DPP4, a serine aminopeptidase expressed in various organs including brain, pancreas, liver, and gut[50]. Therefore, inhibitors of DPP4 may prolong the bioactive half-life of GLP1 in the circulation, which is additionally effective in amending hyperglycemia[51]. The DPP4 inhibitor, linagliptin, has been shown to protect neurons against amyloid beta-induced cytotoxicity and tau hyper-phosphorylation by restoring insulin downstream signaling in AD[52]. Furthermore, the linagliptin alleviated amyloid-beta-induced mitochondrial dysfunction and intracellular ROS generation by a mechanism involving the activation of AMPK-Sirt1 signaling pathway[53]. Chronic administration of another DPP4 inhibitor, sitagliptin, in AD model mice is associated with increased levels of brain GLP1, reductions in the inflammation-biomarkers, and reduction of amyloid-beta deposition in a dose dependent manner[54,55]. Significant reduction in amyloid-beta-42 Level has been associated with the use of linagliptin implying potential application in AD[56]. Also, linagliptin improved vascular functions by increasing creation of nitric oxide and restraining concentration of apolipoprotein B[56]. DPP4 inhibitors can block the DDP4 to diminish GLP1-degradation, prolong GLP1 active life-time, and sensitize insulin-activity for the aim of lowering hyperglycemia[57], and for neuro-protection (Figure 2).
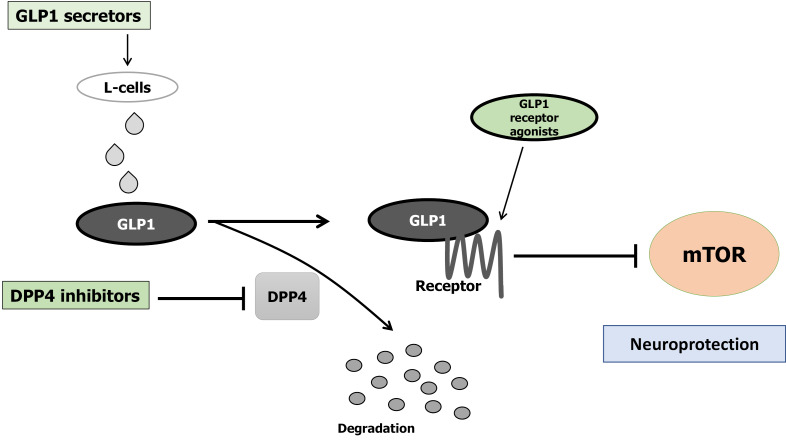
Figure 2.
Implication of decreased dipeptidyl-peptidase-4 activity, increased Glucagon-like peptide-1, increased Glucagon-like peptide-1-receptor agonists, and decreased mammalian/mechanistic target of rapamycin activity for the neuroprotection. Arrowhead means stimulation whereas hammerhead represents inhibition. Note that some critical pathways have been omitted for clarity. GLP1: Glucagon-like peptide-1; mTOR: Mammalian/mechanistic target of rapamycin; DPP4: Dipeptidyl-peptidase-4.
GLP1 and various DPP4 inhibitors (linagliptin, sitagliptin, saxagliptin, etc.) seem to be related to their ability to rescue the insulin cascade. Brain insulin signaling has been reported to dwindle with age[58]. So, restoring insulin signaling might be advantageous to patients with AD. Amazingly, intranasal insulin administration, improves memory in healthy adults without affecting circulating levels of insulin and/or glucose[59-61]. In addition, intranasal insulin improves cognitive performance in patients with early AD[59]. It is possible that therapeutic options for AD arise from this mechanism improving for neural insulin-resistance by the DPP4 inhibitors.
DIET WITH CERTAIN KINDS OF NATURAL PRODUCTS MAY IMPROVE AD
Potential preventive factors against AD including lifestyle factors have been suggested to be neuro-protective by epidemiological research[62]. In particular, diet could play a key role in the neuro-protection of AD[63]. However, the epidemiological analysis of the relations between nutrient and neuroprotection is very intricate. In addition, we think it unlikely that a single component plays a major role in the neuro-protection. The complexity of the human diet and synergistic and/or antagonistic effects among the various nutrients and food ingredients make it more difficult to examine their distinct effects. However, natural products from several plants and animal sources have been used as good preventive factors against AD through different mechanisms and analytical techniques. Here, we partially summarize them in a view point of mTOR inhibition, GLP1 receptor agonists, GLP1 secretion, and DPP4 inhibition. (Figure 3).
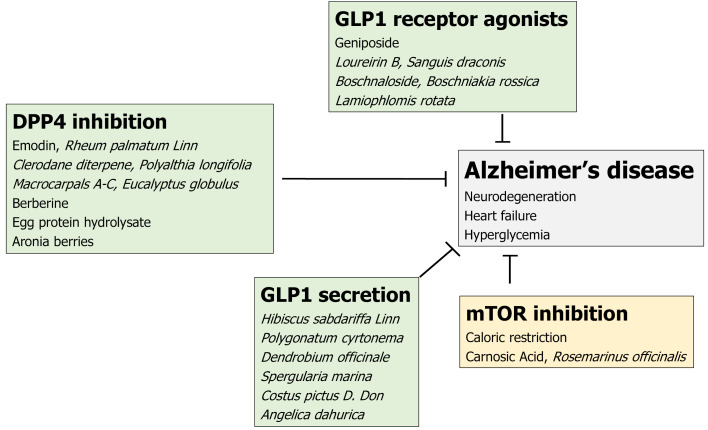
Figure 3.
Simplified diagrams indicating the biochemical properties of several natural products are shown. Several herbs and/or their ingredients may contribute to the neuroprotection against the progression of Alzheimer’s disease. Hammerhead represents inhibition. DPP4: Dipeptidyl-peptidase-4; GLP1: Glucagon-like peptide-1; mTOR: Mammalian/mechanistic target of rapamycin.
First of all, dietary restriction elicits cell protective responses in nearly all cells and tissues including nerve-cells and brain, which could conduct to activation of SIRT1 and inhibition of mTOR and S6K in C57BL/6 mice[64]. Carnosic acid, a polyphenolic diterpene isolated from the herb rosemary (Rosmarinus officinalis) can inhibit the activity of mTOR[65].
Next, GLP1 receptor agonists could protect neurons. Currently, diabetes mellitus treatment based on GLP1 work is being developed. Geniposide, an iridoid glycoside extract from the gardenia fruit, is used in traditional Chinese medicine to alleviate symptoms of liver and inflammatory diseases[23,66]. Geniposide modulates GLP1 receptors signaling[66]. Loureirin B is a natural product derived from Sanguis draconis, which promotes insulin secretion of Ins-1 cells through GLP1 receptor[67]. Lamiophlomis rotata is an orally available Tibetan herb, which specifically reduces pain hypersensitivity states through the activation of GLP1 receptors[68]. Boschnaloside is the major iridoid glycoside in Boschniakia rossica, a well-known traditional Chinese medicine, which can interact with the extracellular domain of the GLP1 receptor[69].
As for compounds stimulating the GLP1 secretion, the ingredient of Hibiscus sabdariffa Linn can increase GLP1 secretion in the ileum[70] Polygonatum cyrtonema polysaccharide stimulates GLP1 secretion from enteroendocrine cells[71]. Polysaccharides from the stems of Dendrobium officinale can decrease fasting blood sugar levels by stimulating GLP1 secretion[72]. Spergularia marina can induce GLP1 secretion, which is a halophyte that grows in mud flats[73]. Costus pictus D. Don, commonly known as insulin plant, is a traditional Indian antidiabetic herbal medicine, which acutely stimulates GLP1 secretion from intestinal L-cells[74]. Angelica dahurica extracts can improve glucose tolerance through the GLP1 secretion[75].
Finally, the intensive search for DPP-4 inhibitors in plant materials has resulted in the identification of macrocarpal A-C from Eucalyptus globulus as a potent inhibitor of DPP4[76]. Furthermore, a variety of other plant derived compounds have been reported to be DPP4 inhibitors. For example, emodin, a natural compound from Rheum palmatum Linn, inhibits DPP4 activity in a dose-dependent manner[77]. Clerodane diterpene can potentiate hypoglycemia via the inhibition of DPP4[78]. Short-term berberine administration can decrease plasma glucose levels through local inhibition of intestinal DPP4[79]. Long-term supplementation with the egg protein hydrolysate exhibits mild in vivo DPP4-inhibitory activities[80]. Furthermore, DPP4 is significantly inhibited by cyanidin 3,5-diglucoside present in aronia berries juice[81].
PERSPECTIVES
It is clear that AD may be a multifactorial and incurable disease. Current treatment strategies against AD are mainly directed at reducing amyloid-beta development and inhibiting amyloid-beta aggregation via the mechanisms including secretase-inhibition and/or impeding tau hyper-phosphorylation[82]. However, medical trials seem to have failed to demonstrate their significant efficacy without any severe side-effects in clinical situations. Since diet with natural products involved in GLP1 signaling, introduced here, are considered safe for long-term use, they could be an encouraging therapeutic approach against AD. In particular, they could exhibit a lower hypoglycemia risk in comparison to other anti-diabetic medications. On the other hand, GLP1 analogues have been found to decrease appetite. It was noticed that pyramidal neurons of the hippocampus and Purkinje cells of the cerebellum have expressed with GLP1 receptor[83]. In addition, several research reports support extra-pancreatic actions of GLP1 and its analogs by crossing the blood brain barrier (BBB), which are independent of its actions on glucose regulation[84]. AD could be considered as a brain disorder that appears to have fused features of insulin deficiency and insulin resistance. Consequently, DPP4 inhibition, GLP1 secretion, GLP1 receptor agonists, and/or mTOR inhibition may all be effective towards the treatment of AD as well as the other neurodegenerative diseases. This approach might accept new targets with simultaneously multiple molecular mechanisms with minimal side effects. Evaluation for intensive experiments should be provided to obtain further insights. Also, long-term studies are mandatory to clarify its efficacy and safety for the treatment of AD as a brain disorder.
CONCLUSION
Current treatment strategies against AD are directed mainly at reducing amyloid-beta development and inhibiting amyloid-beta aggregation via the mechanisms including secretase-inhibition and/or impeding tau hyper-phosphorylation. However, medical trials seem to have failed to demonstrate their significant efficacy without any severe side-effects in clinical situations. Since diet with natural products involved in GLP1 signaling, introduced here, are considered safe for long-term use, they could be an encouraging therapeutic approach against AD. In particular, they could exhibit a lower hypoglycemia risk in comparison to other anti-diabetic medications.
Footnotes
Conflict-of-interest statement: The authors declare that they have no competing financial interests.
Provenance and peer review: Invited manuscript; externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 26, 2021
First decision: May 6, 2021
Article in press: November 28, 2021
Specialty type: Neurosciences
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Byeon H S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL
Contributor Information
Yuka Ikeda, Food Science and Nutrition, Nara Women’s University, Nara 630-8506, Japan.
Nozomi Nagase, Food Science and Nutrition, Nara Women’s University, Nara 630-8506, Japan.
Ai Tsuji, Food Science and Nutrition, Nara Women’s University, Nara 630-8506, Japan.
Yasuko Kitagishi, Food Science and Nutrition, Nara Women’s University, Nara 630-8506, Japan.
Satoru Matsuda, Food Science and Nutrition, Nara Women’s University, Nara 630-8506, Japan. smatsuda@cc.nara-wu.ac.jp.
References
- 1.Melnikova I. Therapies for Alzheimer’s disease. Nat Rev Drug Discov. 2007;6:341–342. doi: 10.1038/nrd2314. [DOI] [PubMed] [Google Scholar]
- 2.Xie Z, Lu H, Yang S, Zeng Y, Li W, Wang L, Luo G, Fang F, Zeng T, Cheng W. Salidroside Attenuates Cognitive Dysfunction in Senescence-Accelerated Mouse Prone 8 (SAMP8) Mice and Modulates Inflammation of the Gut-Brain Axis. Front Pharmacol. 2020;11:568423. doi: 10.3389/fphar.2020.568423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi C, Xu J. Increased vulnerability of brain to estrogen withdrawal-induced mitochondrial dysfunction with aging. J Bioenerg Biomembr. 2008;40:625–630. doi: 10.1007/s10863-008-9195-1. [DOI] [PubMed] [Google Scholar]
- 4.Simpkins JW, Yi KD, Yang SH. Role of protein phosphatases and mitochondria in the neuroprotective effects of estrogens. Front Neuroendocrinol. 2009;30:93–105. doi: 10.1016/j.yfrne.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Chen X, Xu Y, Yang J, Du L, Li K, Zhou Y. Milk consumption and multiple health outcomes: umbrella review of systematic reviews and meta-analyses in humans. Nutr Metab (Lond) 2021;18:7. doi: 10.1186/s12986-020-00527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calsolaro V, Edison P. Novel GLP-1 (Glucagon-Like Peptide-1) Analogues and Insulin in the Treatment for Alzheimer’s Disease and Other Neurodegenerative Diseases. CNS Drugs. 2015;29:1023–1039. doi: 10.1007/s40263-015-0301-8. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Gao L, Zhang Y, Su Y, Kong Z, Wang D, Yan M. Acteoside-improved streptozotocin-induced learning and memory impairment by upregulating hippocampal insulin, glucose transport, and energy metabolism. Phytother Res. 2021;35:392–403. doi: 10.1002/ptr.6811. [DOI] [PubMed] [Google Scholar]
- 8.Hendrix RD, Ou Y, Davis JE, Odle AK, Groves TR, Allen AR, Childs GV, Barger SW. Alzheimer amyloid-β- peptide disrupts membrane localization of glucose transporter 1 in astrocytes: implications for glucose levels in brain and blood. Neurobiol Aging. 2021;97:73–88. doi: 10.1016/j.neurobiolaging.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day SM, Yang W, Wang X, Stern JE, Zhou X, Macauley SL, Ma T. Glucagon-Like Peptide-1 Cleavage Product Improves Cognitive Function in a Mouse Model of Down Syndrome. eNeuro. 2019;6 doi: 10.1523/ENEURO.0031-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bak AM, Egefjord L, Gejl M, Steffensen C, Stecher CW, Smidt K, Brock B, Rungby J. Targeting amyloid-beta by glucagon-like peptide -1 (GLP-1) in Alzheimer’s disease and diabetes. Expert Opin Ther Targets. 2011;15:1153–1162. doi: 10.1517/14728222.2011.600691. [DOI] [PubMed] [Google Scholar]
- 11.Cai HY, Yang JT, Wang ZJ, Zhang J, Yang W, Wu MN, Qi JS. Lixisenatide reduces amyloid plaques, neurofibrillary tangles and neuroinflammation in an APP/PS1/tau mouse model of Alzheimer’s disease. Biochem Biophys Res Commun. 2018;495:1034–1040. doi: 10.1016/j.bbrc.2017.11.114. [DOI] [PubMed] [Google Scholar]
- 12.Solmaz V, Çınar BP, Yiğittürk G, Çavuşoğlu T, Taşkıran D, Erbaş O. Exenatide reduces TNF-α expression and improves hippocampal neuron numbers and memory in streptozotocin treated rats. Eur J Pharmacol. 2015;765:482–487. doi: 10.1016/j.ejphar.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Hussain H, Abbas G, Green IR, Ali I. Dipeptidyl peptidase IV inhibitors as a potential target for diabetes: patent review (2015-2018) Expert Opin Ther Pat. 2019;29:535–553. doi: 10.1080/13543776.2019.1632290. [DOI] [PubMed] [Google Scholar]
- 14.Friedman LG, Qureshi YH, Yu WH. Promoting autophagic clearance: viable therapeutic targets in Alzheimer’s disease. Neurotherapeutics. 2015;12:94–108. doi: 10.1007/s13311-014-0320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 17.Uberti VH, de Freitas BS, Molz P, Bromberg E, Schröder N. Iron Overload Impairs Autophagy: Effects of Rapamycin in Ameliorating Iron-Related Memory Deficits. Mol Neurobiol. 2020;57:1044–1054. doi: 10.1007/s12035-019-01794-4. [DOI] [PubMed] [Google Scholar]
- 18.Tramutola A, Lanzillotta C, Barone E, Arena A, Zuliani I, Mosca L, Blarzino C, Butterfield DA, Perluigi M, Di Domenico F. Intranasal rapamycin ameliorates Alzheimer-like cognitive decline in a mouse model of Down syndrome. Transl Neurodegener. 2018;7:28. doi: 10.1186/s40035-018-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perluigi M, Di Domenico F, Butterfield DA. mTOR signaling in aging and neurodegeneration: At the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol Dis. 2015;84:39–49. doi: 10.1016/j.nbd.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohamed MAE, Abdel-Rahman RF, Mahmoud SS, Khattab MM, Safar MM. Metformin and trimetazidine ameliorate diabetes-induced cognitive impediment in status epileptic rats. Epilepsy Behav. 2020;104:106893. doi: 10.1016/j.yebeh.2019.106893. [DOI] [PubMed] [Google Scholar]
- 24.Peng T, Liu X, Wang J, Liu Y, Fu Z, Ma X, Li J, Sun G, Ji Y, Lu J, Wan W, Lu H. Long noncoding RNA HAGLROS regulates apoptosis and autophagy in Parkinson’s disease via regulating miR-100/ATG10 axis and PI3K/Akt/mTOR pathway activation. Artif Cells Nanomed Biotechnol. 2019;47:2764–2774. doi: 10.1080/21691401.2019.1636805. [DOI] [PubMed] [Google Scholar]
- 25.De Felice FG. Alzheimer’s disease and insulin resistance: translating basic science into clinical applications. J Clin Invest. 2013;123:531–539. doi: 10.1172/JCI64595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Tang B, Feng Y, Tang F, Pui-Man Hoi M, Su Z, Ming-Yuen Lee S. Pinostrobin Exerts Neuroprotective Actions in Neurotoxin-Induced Parkinson’s Disease Models through Nrf2 Induction. J Agric Food Chem. 2018;66:8307–8318. doi: 10.1021/acs.jafc.8b02607. [DOI] [PubMed] [Google Scholar]
- 27.Lin L, Chen H, Zhang Y, Lin W, Liu Y, Li T, Zeng Y, Chen J, Du H, Chen R, Tan Y, Liu N. IL-10 Protects Neurites in Oxygen-Glucose-Deprived Cortical Neurons through the PI3K/Akt Pathway. PLoS One. 2015;10:e0136959. doi: 10.1371/journal.pone.0136959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mejía-García TA, Portugal CC, Encarnação TG, Prado MA, Paes-de-Carvalho R. Nitric oxide regulates AKT phosphorylation and nuclear translocation in cultured retinal cells. Cell Signal. 2013;25:2424–2439. doi: 10.1016/j.cellsig.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Yang S, Lin C, Zhuo X, Wang J, Rao S, Xu W, Cheng Y, Yang L. Glucagon-like peptide-1 alleviates diabetic kidney disease through activation of autophagy by regulating AMP-activated protein kinase-mammalian target of rapamycin pathway. Am J Physiol Endocrinol Metab. 2020;319:E1019–E1030. doi: 10.1152/ajpendo.00195.2019. [DOI] [PubMed] [Google Scholar]
- 30.Crane ED, Wong W, Zhang H, O’Neil G, Crane JD. AMPK Inhibits mTOR-Driven Keratinocyte Proliferation after Skin Damage and Stress. J Invest Dermatol. 2021;141:2170–2177.e3. doi: 10.1016/j.jid.2020.12.036. [DOI] [PubMed] [Google Scholar]
- 31.Gouras GK. mTOR: at the crossroads of aging, chaperones, and Alzheimer’s disease. J Neurochem. 2013;124:747–748. doi: 10.1111/jnc.12098. [DOI] [PubMed] [Google Scholar]
- 32.Yang F, Chu X, Yin M, Liu X, Yuan H, Niu Y, Fu L. mTOR and autophagy in normal brain aging and caloric restriction ameliorating age-related cognition deficits. Behav Brain Res. 2014;264:82–90. doi: 10.1016/j.bbr.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Paccalin M, Pain-Barc S, Pluchon C, Paul C, Besson MN, Carret-Rebillat AS, Rioux-Bilan A, Gil R, Hugon J. Activated mTOR and PKR kinases in lymphocytes correlate with memory and cognitive decline in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22:320–326. doi: 10.1159/000095562. [DOI] [PubMed] [Google Scholar]
- 34.Pozueta J, Lefort R, Shelanski ML. Synaptic changes in Alzheimer’s disease and its models. Neuroscience. 2013;251:51–65. doi: 10.1016/j.neuroscience.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 35.Bao C, Yang Z, Li Q, Cai Q, Li H, Shu B. Aerobic Endurance Exercise Ameliorates Renal Vascular Sclerosis in Aged Mice by Regulating PI3K/AKT/mTOR Signaling Pathway. DNA Cell Biol. 2020;39:310–320. doi: 10.1089/dna.2019.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majumder S, Richardson A, Strong R, Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One. 2011;6:e25416. doi: 10.1371/journal.pone.0025416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thrasivoulou C, Soubeyre V, Ridha H, Giuliani D, Giaroni C, Michael GJ, Saffrey MJ, Cowen T. Reactive oxygen species, dietary restriction and neurotrophic factors in age-related loss of myenteric neurons. Aging Cell. 2006;5:247–257. doi: 10.1111/j.1474-9726.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- 38.Cao R, Li L, Ying Z, Cao Z, Ma Y, Mao X, Li J, Qi X, Zhang Z, Wang X. A small molecule protects mitochondrial integrity by inhibiting mTOR activity. Proc Natl Acad Sci U S A. 2019;116:23332–23338. doi: 10.1073/pnas.1911246116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo J, Bakshi V, Lin AL. Early Shifts of Brain Metabolism by Caloric Restriction Preserve White Matter Integrity and Long-Term Memory in Aging Mice. Front Aging Neurosci. 2015;7:213. doi: 10.3389/fnagi.2015.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Q, Guo M, Wang X, Zhao Y, Zhao Q, Ding H, Dong Q, Cui M. Ischemic preconditioning with a ketogenic diet improves brain ischemic tolerance through increased extracellular adenosine levels and hypoxia-inducible factors. Brain Res. 2017;1667:11–18. doi: 10.1016/j.brainres.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Cremonini E, Daveri E, Mastaloudis A, Oteiza PI. (-)-Epicatechin and Anthocyanins Modulate GLP-1 Metabolism: Evidence from C57BL/6J Mice and GLUTag Cells. J Nutr. 2021;151:1497–1506. doi: 10.1093/jn/nxab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tammen H, Forssmann WG, Richter R. Proteolytic cleavage of glucagon-like peptide-1 by pancreatic beta cells and by fetal calf serum analyzed by mass spectrometry. J Chromatogr A. 1999;852:285–295. doi: 10.1016/s0021-9673(99)00389-1. [DOI] [PubMed] [Google Scholar]
- 43.Meloni AR, DeYoung MB, Lowe C, Parkes DG. GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes Obes Metab. 2013;15:15–27. doi: 10.1111/j.1463-1326.2012.01663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu F, Cao H, Chen Z, Gu H, Guo W, Lin B, Weng J. Short-term GLP-1 receptor agonist exenatide ameliorates intramyocellular lipid deposition without weight loss in ob/ob mice. Int J Obes (Lond) 2020;44:937–947. doi: 10.1038/s41366-019-0513-y. [DOI] [PubMed] [Google Scholar]
- 45.Yao M, Zhang J, Li Z, Bai X, Ma J, Li Y. Liraglutide Protects Nucleus Pulposus Cells Against High-Glucose Induced Apoptosis by Activating PI3K/Akt/ mTOR/Caspase-3 and PI3K/Akt/GSK3β/Caspase-3 Signaling Pathways. Front Med (Lausanne) 2021;8:630962. doi: 10.3389/fmed.2021.630962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2010;31:224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Pozo L, Bello F, Suarez A, Ochoa-Martinez FE, Mendez Y, Chang CH, Surani S. Novel pharmacological therapy in type 2 diabetes mellitus with established cardiovascular disease: Current evidence. World J Diabetes. 2019;10:291–303. doi: 10.4239/wjd.v10.i5.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gardner H, Hamdy O. Oral GLP1 Analog: Where Does the Tide Go? Clin Med Insights Endocrinol Diabetes. 2020;13:1179551420984130. doi: 10.1177/1179551420984130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X, Feng P, Zhang X, Li D, Wang R, Ji C, Li G, Hölscher C. The diabetes drug semaglutide reduces infarct size, inflammation, and apoptosis, and normalizes neurogenesis in a rat model of stroke. Neuropharmacology. 2019;158:107748. doi: 10.1016/j.neuropharm.2019.107748. [DOI] [PubMed] [Google Scholar]
- 50.Smith NK, Hackett TA, Galli A, Flynn CR. GLP-1: Molecular mechanisms and outcomes of a complex signaling system. Neurochem Int. 2019;128:94–105. doi: 10.1016/j.neuint.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilbert MP, Pratley RE. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front Endocrinol (Lausanne) 2020;11:178. doi: 10.3389/fendo.2020.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kosaraju J, Holsinger RMD, Guo L, Tam KY. Linagliptin, a Dipeptidyl Peptidase-4 Inhibitor, Mitigates Cognitive Deficits and Pathology in the 3xTg-AD Mouse Model of Alzheimer’s Disease. Mol Neurobiol. 2017;54:6074–6084. doi: 10.1007/s12035-016-0125-7. [DOI] [PubMed] [Google Scholar]
- 53.Kornelius E, Lin CL, Chang HH, Li HH, Huang WN, Yang YS, Lu YL, Peng CH, Huang CN. DPP-4 Inhibitor Linagliptin Attenuates Aβ-induced Cytotoxicity through Activation of AMPK in Neuronal Cells. CNS Neurosci Ther. 2015;21:549–557. doi: 10.1111/cns.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Amico M, Di Filippo C, Marfella R, Abbatecola AM, Ferraraccio F, Rossi F, Paolisso G. Long-term inhibition of dipeptidyl peptidase-4 in Alzheimer’s prone mice. Exp Gerontol. 2010;45:202–207. doi: 10.1016/j.exger.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Li Y, Tian Q, Li Z, Dang M, Lin Y, Hou X. Activation of Nrf2 signaling by sitagliptin and quercetin combination against β-amyloid induced Alzheimer’s disease in rats. Drug Dev Res. 2019;80:837–845. doi: 10.1002/ddr.21567. [DOI] [PubMed] [Google Scholar]
- 56.Wiciński M, Górski K, Walczak M, Wódkiewicz E, Słupski M, Pawlak-Osińska K, Malinowski B. Neuroprotective Properties of Linagliptin: Focus on Biochemical Mechanisms in Cerebral Ischemia, Vascular Dysfunction and Certain Neurodegenerative Diseases. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20164052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pala L, Pezzatini A, Dicembrini I, Ciani S, Gelmini S, Vannelli BG, Cresci B, Mannucci E, Rotella CM. Different modulation of dipeptidyl peptidase-4 activity between microvascular and macrovascular human endothelial cells. Acta Diabetol. 2012;49 Suppl 1:S59–S63. doi: 10.1007/s00592-010-0195-3. [DOI] [PubMed] [Google Scholar]
- 58.Cole GM, Frautschy SA. The role of insulin and neurotrophic factor signaling in brain aging and Alzheimer’s Disease. Exp Gerontol. 2007;42:10–21. doi: 10.1016/j.exger.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 59.Erichsen JM, Calva CB, Reagan LP, Fadel JR. Intranasal insulin and orexins to treat age-related cognitive decline. Physiol Behav. 2021;234:113370. doi: 10.1016/j.physbeh.2021.113370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hallschmid M. Intranasal Insulin for Alzheimer’s Disease. CNS Drugs. 2021;35:21–37. doi: 10.1007/s40263-020-00781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hallschmid M. Intranasal insulin. J Neuroendocrinol. 2021;33:e12934. doi: 10.1111/jne.12934. [DOI] [PubMed] [Google Scholar]
- 62.Yu JT, Xu W, Tan CC, Andrieu S, Suckling J, Evangelou E, Pan A, Zhang C, Jia J, Feng L, Kua EH, Wang YJ, Wang HF, Tan MS, Li JQ, Hou XH, Wan Y, Tan L, Mok V, Dong Q, Touchon J, Gauthier S, Aisen PS, Vellas B. Evidence-based prevention of Alzheimer’s disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry. 2020;91:1201–1209. doi: 10.1136/jnnp-2019-321913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monacelli F, Acquarone E, Giannotti C, Borghi R, Nencioni A. Vitamin C, Aging and Alzheimer’s Disease. Nutrients. 2017;9 doi: 10.3390/nu9070670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma L, Dong W, Wang R, Li Y, Xu B, Zhang J, Zhao Z, Wang Y. Effect of caloric restriction on the SIRT1/mTOR signaling pathways in senile mice. Brain Res Bull. 2015;116:67–72. doi: 10.1016/j.brainresbull.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 65.Liu J, Su H, Qu QM. Carnosic Acid Prevents Beta-Amyloid-Induced Injury in Human Neuroblastoma SH-SY5Y Cells via the Induction of Autophagy. Neurochem Res. 2016;41:2311–2323. doi: 10.1007/s11064-016-1945-6. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Z, Wang X, Zhang D, Liu Y, Li L. Geniposide-mediated protection against amyloid deposition and behavioral impairment correlates with downregulation of mTOR signaling and enhanced autophagy in a mouse model of Alzheimer’s disease. Aging (Albany NY) 2019;11:536–548. doi: 10.18632/aging.101759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding Y, Xia S, Zhang H, Chen Q, Niu B. Loureirin B activates GLP-1R and promotes insulin secretion in Ins-1 cells. J Cell Mol Med. 2021;25:855–866. doi: 10.1111/jcmm.16138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu B, Gong N, Fan H, Peng CS, Ding XJ, Jiang Y, Wang YX. Lamiophlomis rotata, an orally available Tibetan herbal painkiller, specifically reduces pain hypersensitivity states through the activation of spinal glucagon-like peptide-1 receptors. Anesthesiology. 2014;121:835–851. doi: 10.1097/ALN.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 69.Lin LC, Lee LC, Huang C, Chen CT, Song JS, Shiao YJ, Liu HK. Effects of boschnaloside from Boschniakia rossica on dysglycemia and islet dysfunction in severely diabetic mice through modulating the action of glucagon-like peptide-1. Phytomedicine. 2019;62:152946. doi: 10.1016/j.phymed.2019.152946. [DOI] [PubMed] [Google Scholar]
- 70.Kartinah NT, Fadilah F, Ibrahim EI, Suryati Y. The Potential of Hibiscus sabdariffa Linn in Inducing Glucagon-Like Peptide-1 via SGLT-1 and GLPR in DM Rats. Biomed Res Int. 2019;2019:8724824. doi: 10.1155/2019/8724824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie SZ, Yang G, Jiang XM, Qin DY, Li QM, Zha XQ, Pan LH, Jin CS, Luo JP. Polygonatum cyrtonema Hua Polysaccharide Promotes GLP-1 Secretion from Enteroendocrine L-Cells through Sweet Taste Receptor-Mediated cAMP Signaling. J Agric Food Chem. 2020;68:6864–6872. doi: 10.1021/acs.jafc.0c02058. [DOI] [PubMed] [Google Scholar]
- 72.Kuang MT, Li JY, Yang XB, Yang L, Xu JY, Yan S, Lv YF, Ren FC, Hu JM, Zhou J. Structural characterization and hypoglycemic effect via stimulating glucagon-like peptide-1 secretion of two polysaccharides from Dendrobium officinale. Carbohydr Polym. 2020;241:116326. doi: 10.1016/j.carbpol.2020.116326. [DOI] [PubMed] [Google Scholar]
- 73.Kim K, Lee YM, Rhyu MR, Kim HY. Spergularia marina induces glucagon-like peptide-1 secretion in NCI-H716 cells through bile acid receptor activation. J Med Food. 2014;17:1197–1203. doi: 10.1089/jmf.2013.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patibandla C, Khan ZI, MacGregor L, Campbell MJ, Patterson S. Costus pictus D. Don leaf extract stimulates GLP-1 secretion from GLUTag L-cells and has cytoprotective effects in BRIN-BD11 β-cells. J Ethnopharmacol. 2020;260:112970. doi: 10.1016/j.jep.2020.112970. [DOI] [PubMed] [Google Scholar]
- 75.Park EY, Kim EH, Kim CY, Kim MH, Choung JS, Oh YS, Moon HS, Jun HS. Angelica dahurica Extracts Improve Glucose Tolerance through the Activation of GPR119. PLoS One. 2016;11:e0158796. doi: 10.1371/journal.pone.0158796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kato E, Kawakami K, Kawabata J. Macrocarpal C isolated from Eucalyptus globulus inhibits dipeptidyl peptidase 4 in an aggregated form. J Enzyme Inhib Med Chem. 2018;33:106–109. doi: 10.1080/14756366.2017.1396458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zou J, Luo H, Zeng Q, Dong Z, Wu D, Liu L. Protein kinase CK2α is overexpressed in colorectal cancer and modulates cell proliferation and invasion via regulating EMT-related genes. J Transl Med. 2011;9:97. doi: 10.1186/1479-5876-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang PK, Lin SR, Riyaphan J, Fu YS, Weng CF. Polyalthia Clerodane Diterpene Potentiates Hypoglycemia via Inhibition of Dipeptidyl Peptidase 4. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mi DH, Fang HJ, Zheng GH, Liang XH, Ding YR, Liu X, Liu LP. DPP-4 inhibitors promote proliferation and migration of rat brain microvascular endothelial cells under hypoxic/high-glucose conditions, potentially through the SIRT1/HIF-1/VEGF pathway. CNS Neurosci Ther. 2019;25:323–332. doi: 10.1111/cns.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y, Landheer S, van Gilst WH, van Amerongen A, Hammes HP, Henning RH, Deelman LE, Buikema H. Attenuation of renovascular damage in Zucker diabetic fatty rat by NWT-03, an egg protein hydrolysate with ACE- and DPP4-inhibitory Activity. PLoS One. 2012;7:e46781. doi: 10.1371/journal.pone.0046781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kozuka M, Yamane T, Nakano Y, Nakagaki T, Ohkubo I, Ariga H. Identification and characterization of a dipeptidyl peptidase IV inhibitor from aronia juice. Biochem Biophys Res Commun. 2015;465:433–436. doi: 10.1016/j.bbrc.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 82.Folch J, Petrov D, Ettcheto M, Abad S, Sánchez-López E, García ML, Olloquequi J, Beas-Zarate C, Auladell C, Camins A. Current Research Therapeutic Strategies for Alzheimer’s Disease Treatment. Neural Plast. 2016;2016:8501693. doi: 10.1155/2016/8501693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hamilton A, Hölscher C. Receptors for the incretin glucagon-like peptide-1 are expressed on neurons in the central nervous system. Neuroreport. 2009;20:1161–1166. doi: 10.1097/WNR.0b013e32832fbf14. [DOI] [PubMed] [Google Scholar]
- 84.Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today. 2016;21:802–818. doi: 10.1016/j.drudis.2016.01.013. [DOI] [PubMed] [Google Scholar]