Abstract
Objectives
To describe the epidemiology of granulomatosis with polyangiitis (GPA) in Denmark. To investigate if cardiovascular (CV) related comorbidity and death were increased among Danish AAV patients registered with a diagnosis of granulomatosis with polyangiitis (GPA) in Denmark. To investigate if there was a temporal relation between diagnosis of GPA and CV disease and death.
Methods
A population-based cohort study was performed using the Danish Civil Registration System, the Danish National Patient Registry and the Danish Cause of Death Register in the period January 1, 1995, to December 31, 2015. Patients registered twice or more with a diagnosis of GPA were included. Annual incidence rate (IR), point prevalence (PP) and standardized mortality rate (SMR) were calculated. The entire adult population in Denmark served as control population. CV morbidity and death caused by CV disease was registered.
Results
We identified 1829 individuals with GPA. The median annual IR was 20.5/1,000,000 and PP increased from 64 to 277/1,000,000 in 2015. Overall SMR was 2.14. Among patients with GPA 171 had a hospital diagnosis of acute myocardial infarction (AMI). Compared to the control population, the hazard ratio (HR) of AMI was 2.47 (95% CI 1.24–4.94) during the first 3 months after the GPA diagnosis. From 3 months to one year declining to 1.41 (95%CI 0.80–2.49) and after 10 years the HR was still slightly increased to 1.64 (95%CI 1.20–2.23). The risk of a diagnosis of heart failure (HF) was markedly increased with a HR at 7.22 (95% CI 4.55–11.46) during the first 3 months after a GPA diagnosis, after three months up to one year 2.94 (95%CI 1.87–4.69), and 2.07 (95% CI 1.54–2.78) after 10 years. The total number of CV deaths in the GPA cohort was 307. During the first three months after a GPA diagnosis, the HR was increased to 9.51 (95%CI 7.12–12.70) declining to 2.51 (95% CI 1.77–3.58) after one year, but still increased to 1.56 (95% CI 1.23–1.98) after 10 years. Powered by Editorial Manager® and ProduXion Manager® from Aries Systems Corporation.
Conclusion
In a population-based study on GPA, we found stable incidence, increasing prevalence and an overall increased SMR. The risk of CV comorbidity and of CV death among patients with a register diagnosis of GPA was increased.
Keywords: ANCA associated Vasculitis, Epidemiology, Cardiovascular disease, GPA, Incidence, Prevalence
Highlights
-
•
This is a nationwide register based study on granolumatosis with polyangiitis in the period 1995–2015.
-
•
We found stable incidence, increasing prevalence and increased mortality rate.
-
•
Furthermore, we found increased cardiovascular mortality and morbidity especially early in the disease cause.
-
•
This is relevant for the initial diagnostic set up regarding these rare patients.
1. Introduction
Antineutrophil cytoplasmic autoantibody (ANCA) associated vasculitis (AAV) are systemic vasculitides comprising three major clinical syndromes: Granulomatosis with polyangiitis (GPA), (formerly known as Wegener's granulomatosis), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA) (formerly known as Churg-Strauss syndrome). It is a complex group of diseases characterized by necrotising inflammation, few or no immune deposits in predominantly small vessels, circulating autoantibodies directed against neutrophil cytoplasmic constituents (i.e. ANCAs) and a wide range of clinical presentations. Histologically, GPA is characterized by small vessel vasculitis and extravascular necrotizing granulomatous inflammation, MPA by small vessel vasculitis without granulomatous inflammation and EGPA by small vessel vasculitis, extravascular granulomas and eosinophilic tissue inflammation. Common organ specific manifestations include pauci-immune glomerulonephritis, sino-nasal lesions and pulmonary nodules. The clinical spectrum of AAV ranges from limited disease to life-threatening fulminant disease.
Despite the clinical and microscopic differences described, it may be difficult in some cases clearly to distinguish between the AAV diagnoses as indicated by the Chapel Hill Consensus Conference nomenclature system [1]. However, local clinical practice and laboratory practice in particular have long been a stable foundation for a relatively uniform diagnostic approach in relation to GPA in Denmark [2].
The highest incidences for AAV in general are found in Northern Europe and the overall annual incidence measures vary from 10 to 20/1,000,000 [[3], [4], [5], [6], [7]]. Annual incidence rates (IR) for GPA vary from 2.1 to 15.6/1,000,000 [[3], [4], [5], [6], [7], [8]]. The GPA/MPA ratio differs significantly among different populations with a higher proportion of GPA in Scandinavia and Northern Europe compared to Southern Europe [9].
The prevalence of all AAV diagnoses have increased significantly according to several studies during the recent years and this tendency is partly under the influence of increased attention to the AAV diagnosis but important contributors are also efficient treatment modalities and better survival [[10], [11], [12]].
In the present study we focus on GPA, and among untreated GPA patients the overall mortality is above 80% during the first year of disease but even if treated the mortality is still increased [13,14]. In a newly published meta-analysis, the meta-standardized mortality rate (SMR) for GPA was significantly increased to 2.71 (95% CI 2.26–3.24) [15]. Increased mortality may be related to disease activity, organ damage and severe infections, but increased mortality due to atherosclerosis among patients with GPA has also been reported in some studies [5,16].
Cardiovascular (CV) diseases are more prevalent among patients with GPA than in the background population [[17], [18], [19]]. The same picture has been reported in other autoimmune diseases as rheumatoid arthritis and systemic lupus erythematosus [20,21], but it is unclear if there is a common mechanism e.g. inflammation, or if the risk is due to the specific disease manifestations or the pharmacological treatment. Furthermore, presenting features of CV disease may be different in patients with systemic vasculitis as compared to the general population, potentially resulting in delayed diagnosis [22].
The aim of the present study was to determine the IR, point prevalence (PP) and SMR of GPA in Denmark in a 21-year period and to investigate mortality and morbidity caused by cardiovascular diseases by the use of registry data.
2. Patients and methods
A population-based cohort study was performed using the Danish Civil Registration System, the Danish National Patient Registry (DNPR) and the Danish Cause of Death Register (DCDR) in the period January 1, 1995, to December 31, 2015 [23,24]. Patients were identified using the unique individual identification number assigned to all Danish inhabitants. The study population consisted of patients registered twice or more in the DNPR with a diagnosis of Wegener's granulomatosis according to ICD8 code: 446.29 before 1995 and after 1995 with ICD10: M313. The entire adult population in Denmark served as control population. Outcomes included CV morbidity defined as heart failure (HF) ((ICD-10 code I42, I50, J81 and I110) and acute myocardial infarction (AMI) (ICD-10 code I21-25). Additionally, deaths registered in the DCDR caused by CV disease (ICD-10 code I1-999) were included.
2.1. Statistical analysis
IR were calculated as new cases per 1,000,000 person-years and PP as prevalent cases per 1.000.000. 95% confidence intervals (95% CI) were calculated assuming that the number of cases followed the Poisson distribution. Normally distributed data were analyzed using parametric statistics and reported with 95% CIs. Hazard ratios (HR) and the corresponding 95% CIs were calculated for the specified time intervals after GPA diagnosis using Cox regression models. All the statistical analyses were performed using STATA and SAS software.
2.2. Ethics
This study was approved by the Danish Data Protection Agency (2007-58-0015, local reference No. GEH-2014–017, I-Suite No. 02736). Register studies do not require further approval in Denmark.
3. Results
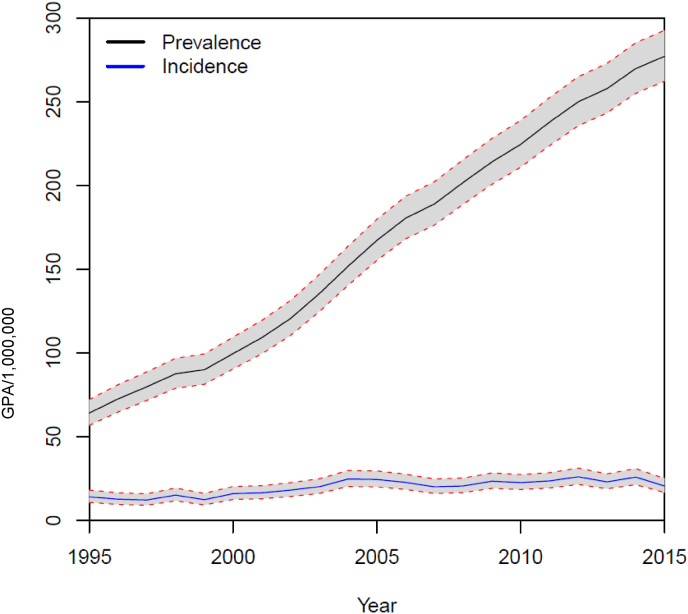
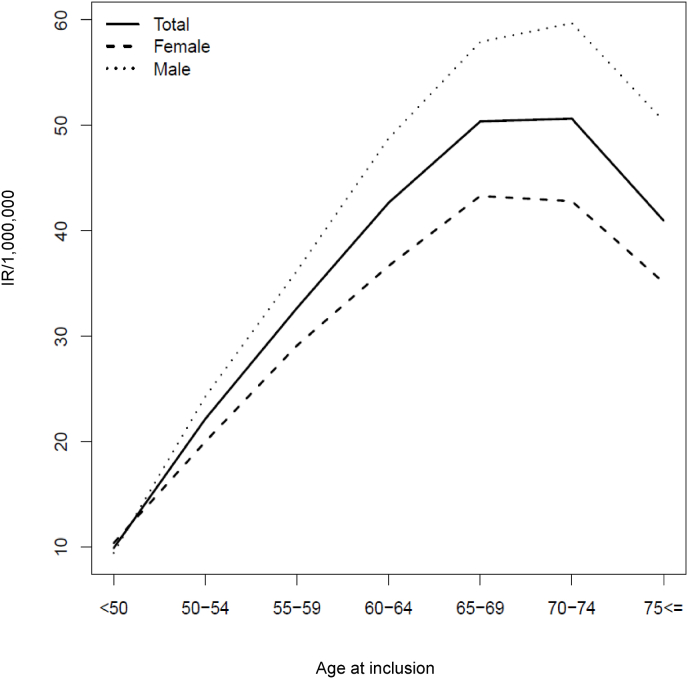
During the study period, 1829 unique individuals with a diagnosis of GPA were identified. The median annual IR was 20.5/1000.000 without an obvious increment during the study period (Fig. 1). There was a slight male preponderance with 960 male patients and 869 females and a corresponding male: female ratio at 11:10. There was a peak incidence for both genders between 65 and 75 years (Fig. 2).
Fig. 1.
Stable incidence and increasing prevalence of GPA during the study period. 95% confidence intervals are shown.
Fig. 2.
Age and gender specific incidence rates of GPA during the study period.
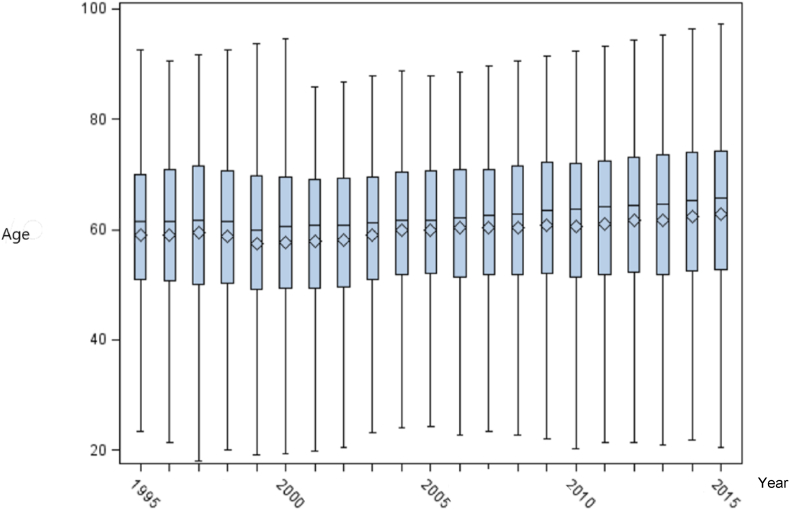
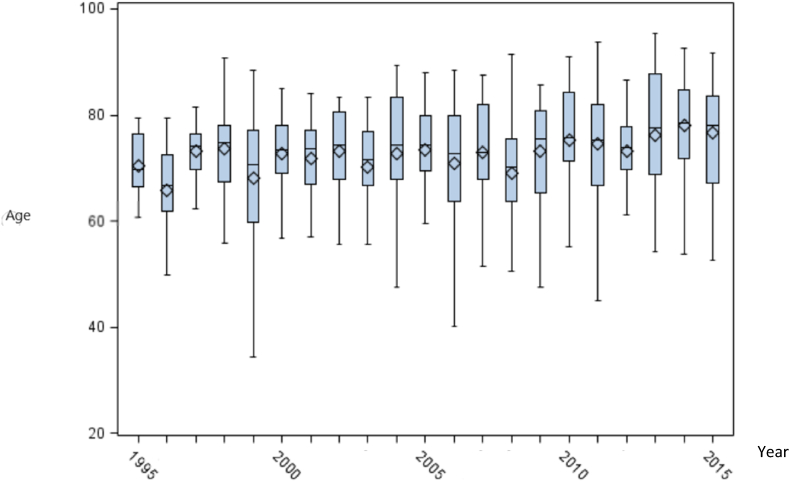
The prevalence figures increased from 64 to 277/1,000,000 during the 21 years study period (Fig. 1). In absolute numbers, the cohort increased from 274 individuals in 1995 to 1315 individuals in 2015. The mean age of patients with GPA was stable during the study period (Fig. 3).
Fig. 3.
Age of patients newly diagnosed with GPA was stable in the study period. The boxes shows the upper and lower quartiles and the whiskers show the range.
3.1. Cardiovascular co-morbidity
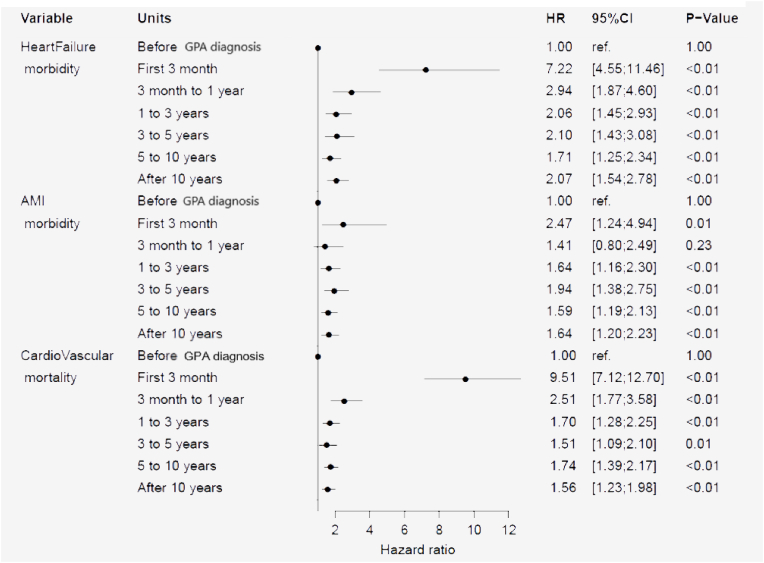
Among the 1829 patients with GPA, 171 patients subsequently were diagnosed at a hospital with AMI. Compared to the background population, the HR of AMI was 2.47 (95% CI 1.24–4.94), (p = 0.01) during the first 3 months after the GPA diagnosis (Fig. 4). From 3 months to one year the HR declined to 1.41 (95% CI 0.80–2.49) (p = 0.23), but still after 10 years the HR was slightly increased to 1.64 (95% CI 1.20–2.23), (p < 0.01). The risk of a diagnosis of HF was markedly increased with a HR at 7.22 (95% CI 4.55–11.46), (p < 0.01) during the first 3 months after a GPA diagnosis. After three months up to one year declining HR to 2.94 (95% CI 1.87–4.69), (p < 0.01) but still increased to 2.07 (95% CI 1.54–2.78), (p < 0.01) after 10 years.
Fig. 4.
Hazard ratio (HR) for development of heart failure, acute myocardial infarction (AMI) and mortality due to cardiovascular disease after a diagnosis of GPA.
3.2. Mortality
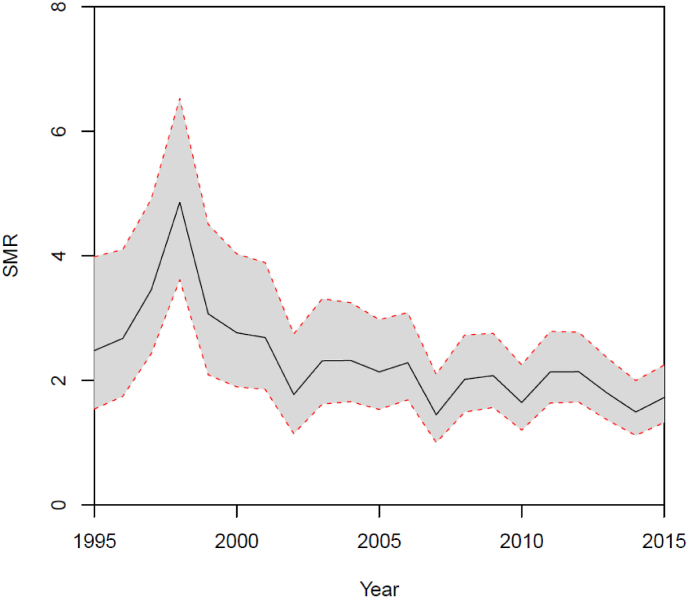
A total of 775 patients with a diagnosis of GPA died during the study period (340 females and 435 males). The SMR is shown in Fig. 5 with a median value of 2.14 (95% CI 2.20–2.29) for both genders. There was a peak in mortality during 1998 with SMR at 4.85 (95% CI 3.61–6.52) for both genders including a total of 44 deceased GPA patients in that year. Except for this peak in mortality, the SMR was stable during the 21 years of observation. Age of death was stable during the study period (Fig. 6). In absolute numbers, 17 deaths among 214 GPA were registered in 1995. In 1998 44 deaths among 377 GPA patients were registered and in 2015 there were 55 deaths among 1315 GPA patients.
Fig. 5.
Standardized mortality ratio (SMR) in patients with GPA was stable after year 2000. 95% confidence intervals are shown.
Fig. 6.
Age at death for patients diagnosed with GPA was stable during the study period. The boxes shows the upper and lower quartiles and the whiskers show the range.
The total number of CV deaths in the GPA cohort was 307 as opposed to 549,539 deaths in the background population. During the first three months after a GPA diagnosis the HR of death was increased to 9.51 (95% CI 7.12–12.70), (p < 0.01) declining to 2.51 (95% CI 1.77–3.58), (p < 0.01) after one year, but still increased to 1.56 (95% CI 1.23–1.98), (p < 0.01) after 10 years (Fig. 4). The HR of CV death increased with age in patients with GPA (data not shown).
4. Discussion
In this registry-based study on GPA during 21 years we found a stable IR, a markedly increasing PP and a significantly increased risk of CV disease, especially early in the disease course. We found an increased mortality among GPA patients with a median SMR of 2.14 and a pronounced CVD related mortality especially in the first months after a GPA diagnosis.
Danish patients with GPA are treated in public hospitals covered by public health insurance, and since 1995 all in- and outpatient contacts have been registered utilizing the ICD diagnosis codes mentioned above. In the present study we only included patients with 2 registrations of a GPA diagnosis in order to exclude simple coding errors and ensure the data quality as validation on the GPA diagnoses in the DNPR have not been performed. This method has been utilized by Faurschou et al. with a positive predictive value of 0.91 [25]. This method may imply that short, fatal disease courses with only one diagnosis registration in DNPR may not be ascertained in the present study, probably a rare scenario. GPA diagnoses in the DNPR on 104 individuals have been validated in a recent Danish study reporting positive predictive values of necrotizing vasculitides including GPA and MPA of 87% and for the whole group of AAV 98% [26]. Hence, the registry-data included in the present study presumably have relatively high validity.
Misclassification due to trouble distinguishing GPA from the other AAV diseases and perhaps from other connective tissue diseases may nevertheless have occurred in some cases, but there is no expectation that this would apply to many individuals or that it would point in a particular direction [27].
In the present register based study, we found a relatively stable and high median IR of GPA at 20.5/1.000,000 in the study period. Our results resemble those from another Danish register study where patients with a diagnosis of GPA or MPA from 2000 to 2015 were included. In contrast to our study, they found an increasing incidence during their study period [26]. Other studies has found corresponding values for incidence measures, e.g. a Norwegian study from Northern part of the country covering the years 1999–2013 reported an IR of 15.6/1000,000 [8]. Pearce et al. reported IR at 14/1,000,000 in United Kingdom [28]. The peak age of incident cases was above the age of 50 in accordance with recent studies demonstrating the importance of paying special attention to elderly with GPA as this subgroup apparently has increased susceptibility to disease and treatment related morbidity [29].
Our prevalence figures were, although higher than many of the previous reports, still in accordance with the GPA PP at 218/1,000,000 in Minnesota reported by Berti et al. [30]. In our population based register study, we found a remarkable increase in PP during the 21-year study period, and the GPA patient cohort was apparently not in steady state at the end of study period. Possible causes for the observed high PP include the increased life expectancy for GPA patients as well as for the entire Danish population during the study period [31]. Improvement of medical diagnostics including availability and routine use of ANCA testing enabling a GPA diagnosis earlier in the disease course [32], and potentially, the diagnosis of less severe cases with less morbidity and mortality [33]. Also improved medical treatment options have presumably contributed to increasing prevalence of GPA [16,34].
We found a significantly high HR of CV morbidity especially early in the GPA disease course. This might be explained by presence of traditional risk factors such as, diabetes, tobacco use or hypertension as well as influence of medical treatment possibly including steroids. We were unfortunately unable to investigate this with our registry-data. Inflammation might also be a contributing factor possibly by involvement of microvasculature, myocarditis and even vasculitis of coronary vessels [35]. Another important factor might be an increased awareness of CV disease in patients with GPA as international recommendations state [36]. It is noteworthy that the risk of CV disease was still increased after 10 years of disease duration. An increased surveillance of GPA patients may result in more and earlier diagnosis of complicating conditions.
The result indicates a strong association between systemic vascular inflammatory disease and CV involvement. This is in accordance with a meta-analysis of relevant observational studies on cardiovascular risk in AAV patients by Houben et al. who found an increased overall risk of cardiovascular disease, relative risk at 1.65 (95% CI 1.23–2.22 [37]. At the time of diagnosis of GPA systemic inflammatory activity usually is present and the results point towards an association of inflammation with CV risk-factors probably including premature and pronounced atherosclerosis [38]. The persistence of increased HR of CV involvement after 10 years indicates a permanent influence on the risk factors and treatment strategy should take this into account as recommended by EULAR [36].
The peak in SMR in 1998 (Fig. 4) is puzzling, especially because there is a stable SMR in the following years. We tend to explain this phenomenon by left censorship, reflecting more advanced disease and hence increased mortality among a relatively low number of patients as indicated by 44 deaths among 377 GPA patients in 1998 as compared by 55 deaths among 1315 GPA patients in 2015.
In our study, there was a persistent increased mortality among GPA patients with a median SMR of 2.14. This result is in accordance with results from Tan et al. reporting an increased meta-SMR of 2.63 (95% CI 2.02–3.43) for GPA. The mortality in the included studies of GPA showed the same picture of increased mortality [15]. Another study from Wallace et al. reported an overall SMR for AAV at 2.3 (95% CI: 1.9–2.8) [39]. Lugmani et al. reported a bimodal pattern of mortality in patients with GPA, with a 9-fold increased risk of death in the first year of disease and a new peak in mortality after 8 years of disease [40].
Limitations of this study include the use of not validated registry diagnoses of GPA, but we aim to compensate only analyzing data on individuals with at least two registrations of the GPA. Unfortunately, the register did not give us access to data concerning GPA disease activity or information about comorbidities apart from CV disease. Finally, information about medical treatment would have been of interest.
The strengths of the present study include analysis of unselected national data covering 21 years with consistent findings in the study period. The entire Danish population serves as control group.
The present study provides a foundation for prospective studies where we at a cohort level will describe the epidemiological developments taking into account measures of disease activity and severity, other comorbidity and medical treatment.
Sample credit author statement
Laustrup H: Conceptualization, methodology and writing- original draft preparation Lund P: Methodology, software and data curation, Voss A: Conceptualization, writing-reviewing, editing and supervision.
Funding
The study was supported by the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Helle Laustrup reports article publishing charges was provided by Odense University Hospital.
References
- 1.Jennette J.C., Falk R.J., Bacon P.A., Basu N., Cid M.C., Ferrario F., et al. Revised international Chapel Hill Consensus conference nomenclature of vasculitides. Arthritis Rheum. 2012;65(1):1–11. doi: 10.1002/art.37715. 2013. [DOI] [PubMed] [Google Scholar]
- 2.Baslund B. Anti-neutrophil cytoplasmic autoantibodies (ANCA) and vasculitis. Clin. Rev. Allergy. 1994;12(3):297–304. doi: 10.1007/BF02802324. [DOI] [PubMed] [Google Scholar]
- 3.Watts R.A., Scott D.G. Epidemiology of the vasculitides. Semin. Respir. Crit. Care Med. 2004;25(5):455–464. doi: 10.1055/s-2004-836139. [DOI] [PubMed] [Google Scholar]
- 4.Watts R.A., Lane S.E., Scott D.G., Koldingsnes W., Nossent H., Gonzalez-Gay M.A., et al. Epidemiology of vasculitis in Europe. Ann. Rheum. Dis. 2001;60(12):1156–1157. doi: 10.1136/ard.60.12.1156a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammad A.J., Jacobsson L.T., Westman K.W., Sturfelt G., Segelmark M. Incidence and survival rates in Wegener's granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome and polyarteritis nodosa. Rheumatology. 2009;48(12):1560–1565. doi: 10.1093/rheumatology/kep304. [DOI] [PubMed] [Google Scholar]
- 6.Lane S.E., Watts R., Scott D.G. Epidemiology of systemic vasculitis. Curr. Rheumatol. Rep. 2005;7(4):270–275. doi: 10.1007/s11926-005-0036-5. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi S., Fujimoto S. Epidemiology of vasculitides: differences between Japan, Europe and North America. Clin. Exp. Nephrol. 2013;17(5):611–614. doi: 10.1007/s10157-013-0813-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nilsen A.T., Karlsen C., Bakland G., Watts R., Luqmani R., Koldingsnes W. Increasing incidence and prevalence of ANCA-associated vasculitis in Northern Norway. Rheumatology. 2020;59(9):2316–2324. doi: 10.1093/rheumatology/kez597. [DOI] [PubMed] [Google Scholar]
- 9.Corral-Gudino L., Borao-Cengotita-Bengoa M., Lerma-Marquez J.L., Del Pino-Montes J. Differences in the incidence of microscopic polyangiitis and granulomatosis with polyangiitis (Wegener's). Is there a latitudinal gradient? J. Rheumatol. 2011;38(11):2494–2496. doi: 10.3899/jrheum.110650. [DOI] [PubMed] [Google Scholar]
- 10.Mohammad A.J., Jacobsson L.T., Mahr A.D., Sturfelt G., Segelmark M. Prevalence of Wegener's granulomatosis, microscopic polyangiitis, polyarteritis nodosa and Churg-Strauss syndrome within a defined population in southern Sweden. Rheumatology. 2007;46(8):1329–1337. doi: 10.1093/rheumatology/kem107. [DOI] [PubMed] [Google Scholar]
- 11.Herlyn K., Buckert F., Gross W.L. Reinhold-Keller E. Doubled prevalence rates of ANCA-associated vasculitides and giant cell arteritis between 1994 and 2006 in northern Germany. Rheumatology. 2014;53(5):882–889. doi: 10.1093/rheumatology/ket440. [DOI] [PubMed] [Google Scholar]
- 12.Watts R.A., Lane S., Scott D.G. What is known about the epidemiology of the vasculitides? Best Pract. Res. Clin. Rheumatol. 2005;19(2):191–207. doi: 10.1016/j.berh.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Fauci A.S., Haynes B.F., Katz P., Wolff S.M. Wegener's granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann. Intern. Med. 1983;98(1):76–85. doi: 10.7326/0003-4819-98-1-76. [DOI] [PubMed] [Google Scholar]
- 14.Walton E.W. Giant-cell granuloma of the respiratory tract (Wegener's granulomatosis) Br. Med. J. 1958;2(5091):265–270. doi: 10.1136/bmj.2.5091.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan J.A., Dehghan N., Chen W., Xie H., Esdaile J.M., Avina-Zubieta J.A. Mortality in ANCA-associated vasculitis: ameta-analysis of observational studies. Ann. Rheum. Dis. 2017;76(9):1566–1574. doi: 10.1136/annrheumdis-2016-210942. [DOI] [PubMed] [Google Scholar]
- 16.Flossmann O., Berden A., de G.K., Hagen C., Harper L., Heijl C., et al. Long-term patient survival in ANCA-associated vasculitis. Ann. Rheum. Dis. 2011;70(3):488–494. doi: 10.1136/ard.2010.137778. [DOI] [PubMed] [Google Scholar]
- 17.Tervaert J.W. Translational mini-review series on immunology of vascular disease: accelerated atherosclerosis in vasculitis. Clin. Exp. Immunol. 2009;156(3):377–385. doi: 10.1111/j.1365-2249.2009.03885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faurschou M., Mellemkjaer L., Sorensen I.J., Svalgaard T.B., Dreyer L., Baslund B. Increased morbidity from ischemic heart disease in patients with Wegener's granulomatosis. Arthritis Rheum. 2009;60(4):1187–1192. doi: 10.1002/art.24386. [DOI] [PubMed] [Google Scholar]
- 19.Morgan M.D., Turnbull J., Selamet U., Kaur-Hayer M., Nightingale P., Ferro C.J., et al. Increased incidence of cardiovascular events in patients with antineutrophil cytoplasmic antibody-associated vasculitides: a matched-pair cohort study. Arthritis Rheum. 2009;60(11):3493–3500. doi: 10.1002/art.24957. [DOI] [PubMed] [Google Scholar]
- 20.Skeoch S., Bruce I.N. Atherosclerosis in rheumatoid arthritis: is it all about inflammation? Nat. Rev. Rheumatol. 2015;11(7):390–400. doi: 10.1038/nrrheum.2015.40. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Kaplan M.J. Cardiovascular disease in systemic lupus erythematosus: an update. Curr. Opin. Rheumatol. 2018;30(5):441–448. doi: 10.1097/BOR.0000000000000528. [DOI] [PubMed] [Google Scholar]
- 22.Cohen Tervaert J.W. Cardiovascular disease due to accelerated atherosclerosis in systemic vasculitides. Best Pract. Res. Clin. Rheumatol. 2013;27(1):33–44. doi: 10.1016/j.berh.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen C.B. The Danish Civil registration system. Scand. J. Publ. Health. 2011;39(7 Suppl):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt M., Schmidt S.A., Sandegaard J.L., Ehrenstein V., Pedersen L., Sørensen H.T. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin. Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faurschou M., Ahlstrom M.G., Lindhardsen J., Baslund B., Obel N. Impact of pre-existing co-morbidities on mortality in granulomatosis with polyangiitis: a cohort study. Rheumatology. 2016;55(4):649–653. doi: 10.1093/rheumatology/kev390. [DOI] [PubMed] [Google Scholar]
- 26.Nelveg-Kristensen K.E., Szpirt W., Carlson N., McClure M., Jayne D., Dieperink H., et al. Increasing incidence and improved survival in ANCA-associated vasculitis-a Danish nationwide study. Nephrol. Dial. Transplant. 2020 doi: 10.1093/ndt/gfaa303. PMID: 33313875. [DOI] [PubMed] [Google Scholar]
- 27.Ntatsaki E., Watts R.A., Scott D.G. Epidemiology of ANCA-associated vasculitis. Rheum. Dis. Clin. N. Am. 2010;36(3):447–461. doi: 10.1016/j.rdc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Pearce F.A., Grainge M.J., Lanyon P.C., Watts R.A., Hubbard R.B. The incidence, prevalence and mortality of granulomatosis with polyangiitis in the UK Clinical Practice Research Datalink. Rheumatology. 2017;56(4):589–596. doi: 10.1093/rheumatology/kew413. [DOI] [PubMed] [Google Scholar]
- 29.Berti A., Felicetti M., Monti S., Ortolan A., Padoan R., Brunori G., et al. Disease and treatment-related morbidity in young and elderly patients with granulomatosis with polyangiitis and microscopic polyangiitis. Semin. Arthritis Rheum. 2020;50(6):1441–1448. doi: 10.1016/j.semarthrit.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Berti A., Cornec D., Crowson C.S., Specks U., Matteson E.L. The epidemiology of antineutrophil cytoplasmic autoantibody-associated vasculitis in olmsted county, Minnesota: a twenty-year US population-based study. Arthritis Rheum. 2017;69(12):2338–2350. doi: 10.1002/art.40313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denmark in Figures. Statistics Denmark; Copenhagen: 2019 may. [Google Scholar]
- 32.Rasmussen N., Wiik A., Jayne D.R. A historical essay on detection of anti-neutrophil cytoplasmic antibodies. Nephrol. Dial. Transplant. 2015;30(Suppl 1):i8–13. doi: 10.1093/ndt/gfv070. [DOI] [PubMed] [Google Scholar]
- 33.Watts R.A., Robson J. Introduction, epidemiology and classification of vasculitis. Best Pract. Res. Clin. Rheumatol. 2018;32(1):3–20. doi: 10.1016/j.berh.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Watts R.A., Gonzalez-Gay M.A., Lane S.E., Garcia-Porrua C., Bentham G., Scott D.G. Geoepidemiology of systemic vasculitis: comparison of the incidence in two regions of Europe. Ann. Rheum. Dis. 2001;60(2):170–172. doi: 10.1136/ard.60.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soulaidopoulos S., Madenidou A.V., Daoussis D., Melissaropoulos K., Mavrogeni S., Kitas G., et al. 2020. Cardiovascular Disease in the Systemic Vasculitides. Current Vascular Pharmacology. [DOI] [PubMed] [Google Scholar]
- 36.Yates M., Watts R.A., Bajema I.M., Cid M.C., Crestani B., Hauser T., et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann. Rheum. Dis. 2016;75(9):1583–1594. doi: 10.1136/annrheumdis-2016-209133. [DOI] [PubMed] [Google Scholar]
- 37.Houben E., Penne E.L., Voskuyl A.E., van der Heijden J.W., Otten R.H.J., Boers M., et al. Cardiovascular events in anti-neutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis of observational studies. Rheumatology. 2018;57(3):555–562. doi: 10.1093/rheumatology/kex338. [DOI] [PubMed] [Google Scholar]
- 38.Moriya J. Critical roles of inflammation in atherosclerosis. J. Cardiol. 2019;73(1):22–27. doi: 10.1016/j.jjcc.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Wallace Z.S., Fu X., Harkness T., Stone J.H., Zhang Y., Choi H. All-cause and cause-specific mortality in ANCA-associated vasculitis: overall and according to ANCA type. Rheumatology. 2020;59(9):2308–2315. doi: 10.1093/rheumatology/kez589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luqmani R., Suppiah R., Edwards C.J., Phillip R., Maskell J., Culliford D., et al. Mortality in Wegener's granulomatosis: a bimodal pattern. Rheumatology. 2011;50(4):697–702. doi: 10.1093/rheumatology/keq351. [DOI] [PubMed] [Google Scholar]