Abstract
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of multiple malignancies, especially in non-small cell lung cancer (NSCLC). With the extensive application of ICIs in clinical practice, clinicians have to manage their toxicities, which are often termed immune-related adverse events (irAEs). Several ICIs, such as nivolumab, pembrolizumab, atezolizumab, and durvalumab, have been approved by the US Food and Drug Administration (FDA) to treat advanced NSCLC, accompanied by a broad spectrum of toxicity reactions. However, ICIs-associated neurological toxicities, regarding polyneuropathy, Bell palsy, encephalopathy, and myasthenia gravis, as uncommon emerging toxicities have not been well recognized, present a challenge for clinicians to improve awareness of supervision, recognition, and management before death from them. Herein, we have summarized the incidence, diagnosis, clinical manifestations, potential mechanisms, treatments, and outcomes of ICIs-related neurotoxicity and optimized the management approach for NSCLC patients. Prompt recognition and proper management are indispensable to reduce the morbidity of these patients with immune-related neurological toxicities.
Keywords: Immune checkpoint inhibitors, neurotoxicity, polyneuropathy, myasthenia gravis, encephalopathy, Guillain-Barre syndrome, non-small cell lung cancer
Introduction
Lung cancer has been regarded as 1 of the most common cancers with both high incidence and mortality worldwide. 1 Among them, non-small cell lung cancer (NSCLC) comprises a high incidence of more than 85%. 2 However, nearly all patients treated with traditional chemotherapy and radiotherapy, and the molecular targeting agents will display disease progression due to acquired resistance, which is inevitable. 3 Cancer immunotherapy, arising vigorously after 2000, is a remedial modality that exploits the immune system to recognize, target, and eliminate cancer cells. 4 Immune checkpoint inhibitors (ICIs), mainly composed of programmed cell death protein 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1), and cytotoxic T-lymphocyte antigen-4 (CTL-4) monoclonal antibodies (mAbs), have been proved effective toward numerous malignancies over a hundred of clinical trials. 5 Four ICIs have been approved by the US Food and Drug Administration (FDA) for patients with NSCLC, composing nivolumab, pembrolizumab, durvalumab, and ipilimumab. 6 Although clinical trials of ICIs in patients with NSCLC have indicated superior overall survival (OS), median progression-free survival, and objective response rate (ORR), immune-related adverse events (irAEs) are observed, which may even be life-threatening. 7
Immune-related adverse events caused by ICIs are related to multiple organs and numerous systems. The most common irAEs documented from clinical trials include rash, pruritis, thyroiditis, autoimmune hypophysitis, pneumonitis, colitis, and hepatitis. 8 Moreover, some less common irAEs have also been reported, including cardiotoxicity, ocular toxicity, and neurotoxicity. 8 Among all these adverse effects, ICI-related neurological toxicities are rare, which may lead to the permanent interruption of therapeutic regimen, treatment of corticosteroids, exacerbation of cancers, or even death. To date, as the case reports have documented various ICI-related neurotoxicity in patients with NSCLC, a safety profile of ICIs needs to be consummated, and the incidence of neurotoxicity needs to be adequately assessed. 9 Our review collected 20 case reports of ICI-related neurological adverse events (NAEs) in patients with NSCLC and recorded their NAE types, symptoms, treatments, and outcomes (Table 1). Multiple accessory examinations are essential in the early identification of these toxicities and are summarized (Table 2).
Table 1.
Published case reports of ICI–related neurological adverse events in NSCLC.
| Reference | Case reports/series | Age/sex | Cancer histological classification/stage | ICI drugs/dosage | Time of onset/time to event | Symptoms | Neurological adverse events | Withdraw the drug | Treatment/drugs/dosage | The time of recovery | CTCAE grade | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | Abe et al | 58/M | Lung adenocarcinoma/recurrent stage | Nivolumab | Within 1 week | Irritated, fidgety, communicative disorders, compulsive sequential movements | Akathisia | YES | Oral prednisolone, one pulse methylprednisolone, oral and IV sedative drugs | NR | 2 | Failed to relieve his symptoms |
| 11 | Richard et al | 74/M | Squamous NSCLC/stage IV | Nivolumab | Within 1 week | Gradual decrease in mental status, repeated fall, mumbling, inability to follow commands and stand on his own intuition, agitated, confused, visual hallucinations | Encephalitis | NO | IV decadron, IV Solu-Medrol, oral steroid | NR | 2 | Improved after 6-week steroid taper |
| 12 | Nakatani et al | 73/F | Squamous cell lung cancer/stage IV | Nivolumab | 20 weeks later | Ptosis, lower limb weakness, photophobia | Lambert-Eaton myasthenic syndrome | YES | Cholinesterase inhibitors, oral prednisolone, pyridostigmine, anti-acetylcholinesterase inhibitors | 115 weeks later | 2 | Stable |
| 13 | Schneider et al | 78/M | Squamous cell lung cancer/stage IV | Nivolumab | 28 weeks later | Apathy, aphasia, withdrawal from pain and verbal response, paresis of the left facial nerve | Autoimmune encephalitis | YES | Antiepileptic treatment, intravenous methylprednisolone | 60 weeks later | 3-4 | Improved |
| 14 | Leitinger et al (seronegative autoimmune encephalitis and cerebral vasculitis were suspected) | 67/F | Squamous cell lung cancer/stage IV | Nivolumab | 2 weeks later | Seizures, dyspnea, fear, confusion, fluent aphasia, perseveration, disorientation, speech arrest, cannot execute complex request | Necrotizing encephalopathy | YES | IV methylprednisolone, immunoglobulins, antiviral treatment, antiepileptic treatment | NR | 5 | Died (8 weeks later) |
| 15 | Jacob et al | 68/F | Squamous cell lung cancer/stage IV | Nivolumab | 13 weeks later | Fatigue, loss of motor and sensory function in arms and legs, respiratory muscle paralysis | Guillain-Barré syndrome | YES | Ventilator support, IVIg, plasma exchange | NR | 5 | Died (15 weeks later) |
| 16 | Läubli et al | 53/M | Lung adenocarcinoma/stage IV | Nivolumab | NR | Progressive gait disturbance, speech difficulties | Cerebral vasculitis/necrotizing encephalitis | YES | Steroids, excision of the intracranial lesion | NR | 2 | Improved after excision of the lesion |
| 17 | Fukumoto et al | 66/M | NSCLC/stage IV | Nivolumab | 3 weeks later | Muscle weakness of the lower limbs, became bed-bound | Acute demyelinating polyneuropathy | YES | Steroids, IVIg | 17 weeks later | 3-4 | Improved |
| 18 | Tan et al | 45/M | NSCLC/advanced stage | Nivolumab | 2 weeks later | Exertional dyspnea, ptosis, and ophthalmoplegia | Myasthenic crisis and myositis | YES | Pyridostigmine, methylprednisolone, immune globulin, intubation | 24 weeks later | 3-4 | Improved |
| 19 | Chen et al | 57/M | Squamous cell lung cancer/stage IV | Ipilimumab and nivolumab | 4 weeks later | Ptosis, dyspnea, and muscle weakness | Myasthenia gravis, myositis, polyneuropathy | NO | IV steroids, oral pyridostigmine | NR | 2 | Improved but died because of hospital-acquired pneumonia |
| 20 | Chen et al | 65/M | Squamous cell lung cancer/stage IIIB, cT4N2M0 | Nivolumab | 5 weeks later | Weakness of 4 extremities, ptosis, limited eye movement, dropped head, drooling with dysphagia, respiratory failure | Myasthenia gravis | YES | Oral steroids and pyridostigmine | NR | 5 | Died (9 weeks later) |
| 21 | Ong et al | 68/M | Lung adenocarcinoma/stage IV | Pembrolizumab | 4 weeks later | Paresthesia, limb and facial weakness | Guillain-Barré-like syndrome | YES | Intravenous steroids, IVIg | 12 weeks later | 2 | Improved |
| 22 | Polat et al | 65/M | NSCLC/stage IV | Nivolumab | 4 weeks later | Blurry vision, ptosis, and intermittent diplopia | Myasthenia gravis | YES | Pyridostigmine | 20 weeks later | 2 | Improved |
| 23 | Sciacca et al | 81/M | Lung adenocarcinoma/stage IV | Nivolumab | 4 weeks later | Bilateral ptosis, nasal speech, and proximal limb weakness. | Myasthenia gravis | YES | Oral steroids | 8 weeks later | 2 | Improved |
| 24 | Hussein et al | 47/F | Poorly differentiated lung cancer with neuroendocrine features/stage IV | Nivolumab | 5 weeks later | Nausea, vomiting, disorientation, generalized tonic clonic seizure | PRES | YES | Treated with supportive therapy | NR | 2 | Improved |
| 25 | Hibino et al | 83/M | Squamous cell lung cancer/stage IV, cT1cN3M1c | Pembrolizumab | 5 weeks later | Easy fatigability of the eyelids and eye movement, bilateral ptosis, diplopia, posterior neck myalgia, neck extensor weakness | Myasthenia gravis with myositis | YES | Oral steroids, pyridostigmine | 14 weeks later | 2 | Improved |
| 26 | Mori et al | 64/M | NSCLC/stage IV | Atezolizumab | 48 weeks later | Sudden visual loss | Optic neuritis | YES | IV methylprednisolone 1 g for 3 days, then 30 mg PO prednisolone administration | NR | 2 | Improved |
| 27 | Narumi et al | 75/M | Lung squamous cell carcinoma/stage IIIA, T3N1M0 | Nivolumab | 8 weeks later | Acute paralysis in bilateral lower limbs, sensory loss below Th10 level, urinary retention | NMOSD | YES | Steroid pulse therapy, plasmapheresis | 34 weeks later | 3-4 | Improved |
| 28 | Horio et al | 63/M | Lung adenocarcinoma/stage IVB, cT1cN3M1c | Pembrolizumab | Within 1 week | Homonymous hemianopia | Trousseau syndrome | NO | Unfractionated heparin | NR | 5 | Improved but died because of hemorrhagic infarctions |
| 29 | Tan et al | 66/M | Metastatic NSCLC | Atezolizumab | 12 weeks later | Ataxic wide-based gait | Cerebellar ataxia | YES | Prednisolone 1 mg/kg | NR | 2 | Improved |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; ICI, immune checkpoint inhibitor; IV, intravenous; IVIg, intravenous immunoglobulin; NMOSD, neuromyelitis optica spectrum disorder; NR, not reference; NSCLC, non-small cell lung cancer; PO, oral; PRES, posterior reversible encephalopathy syndrome.
Table 2.
Laboratorial, electrophysiological, and imaging examinations of ICI–related neurological adverse events in NSCLC.
| Reference | Age/sex | Neurological adverse events | EEG/EMG/SFEMG | NCS | RNS | Image examination | Antibodies of paraneoplastic neurological syndrome/serum laboratory data | CSF test | Co-occurring non-neurological irAEs |
|---|---|---|---|---|---|---|---|---|---|
| Abe et al 10 | 58/M | Akathisia | NR | NR | NR | Brian MRI: negative | Negative for all | Negative | NO |
| Richard et al 11 | 74/M | Encephalitis | EEG: mild slowing, no evidence of seizure activity | NR | NR | Chest X ray: negative MRI: not conducted |
Not conducted | Negative | Euthyroid sick syndrome |
| Nakatani et al 12 | 73/F | Lambert-Eaton myasthenic syndrome | NR | Low-amplitude CMAP | Waning phenomenon in 3 Hz RNS, waxing phenomenon in 10 and 20 Hz RNS | Chest CT: right hilar lymphadenopathy, primary tumor in the right lower lobe | AChRAbs: negative, anti-P/Q-type VGCC antibody: positive, anti-thyroglobulin antibody: positive | Negative | Hypothyroidism |
| Schneider et al 13 | 78/M | Autoimmune encephalitis | EEG: moderate background slowing, focal delta slowing over the left temporal region | NR | NR | CT: negative Brain MRI: negative |
Negative for all | Reduced level of glucose, elevated lactate: 4.1 mmol/L, protein level: 1027 mg/L, pleocytosis: 16 lymphocytes/µL | NO |
| Leitinger et al 14 | 67/F | Necrotizing encephalopathy | EEG: rule out complex-partial status epilepticus, moderate diffuse slowing | NR | NR | MRI: edematous disseminated lesions | Negative for all | Inflammatory CSF findings and increased IgG level | NO |
| Jacob et al 15 | 68/F | GBS | NR | NR | NR | Brain CT: partial response Spine MRI: negative |
NR | CSF: no nucleated cells, normal glucose, elevated protein level, albuminocytological dissociation (consistent with a diagnosis of GBS) | NO |
| Läubli et al 16 | 53/M | Cerebral vasculitis/necrotizing encephalitis | NR | NR | NR | Brain MRI: new parietotemporal lesion in proximity of the formerly irradiated masses | Antibodies against neuronal antigens: negative, anti-SSA/Ro and anti-SSB/La antibodies: positive | NR | NO |
| Fukumoto et al 17 | 66/M | Acute demyelinating polyneuropathy | NR | Decreased distal sensory nerve action potentials on median and ulnar nerves, prolonged distal latency | NR | NR | IgM antibodies to GM2 and GalNAc-GD1a: positive, CK: 36 IU/L | CSF: 4 leucocytes/μL, protein level: 339 mg/dL | NO |
| Tan et al 18 | 45/M | Myasthenic crisis and myositis | NR | NR | No decremental response | Brain MRI: no evidence of stroke or metastasis | AChRAbs: 2.00 nmol/L, transaminitis, elevated muscle enzymes | NR | NO |
| Chen 19 | 57/M | Myasthenia gravis, myositis, and polyneuropathy | EMG: active denervation and myopathic changes in sample muscles, SFEMG over right orbicularis oculi: mean consecutive difference of 74 µs | Sensorimotor polyneuropathy of axonal degeneration | No decremental response | NR | AChRAbs: 0.70 nmol/L, CK: 2682 U/L | Slightly lower protein level at 13 mg/dL | NO |
| Chen et al 20 | 65/M | Myasthenia gravis | EMG: negative | Polyneuropathy of median, ulnar, peroneal, tibial, and sural nerves | Negative | Brain MRI: negative | AChRAbs: not detected, CK: 2216 U/L, AST: 153 U/L, ALT: 110 U/L, LDH: 484 U/L, troponin-I: 2.62 ng/mL | NR | NO |
| Ong et al 21 | 68/M | Guillain-Barré-like syndrome | NR | Prolonged tibial motor distal latency, partial conduction block in peroneal motor nerves, sparing of the sural sensory response | NR | Spine MRI: degenerative changes | Paraneoplastic autoantibodies: negative | NR | NO |
| Polat et al 22 | 65/M | Myasthenia gravis | NR | Normal after the treatment of pyridostigmine | Normal after the treatment of pyridostigmine | MRI: negative Chest X ray: negative |
AChRAbs and anti-MUSK: negative after myasthenia gravis disappear | NR | NO |
| Sciacca et al 23 | 81/M | Myasthenia gravis | SFEMG over orbicularis oculi: abnormal (mean jitter, 36 µs; 15% pairs with abnormal jitter) | NR | Negative | NR | AChRAbs: 0.40 nmol/L, ALT: 296 U/L, AST: 325 U/L | NR | NO |
| Hussein et al 24 | 47/F | PRES | NR | NR | NR | MRI: PRES | NR | NR | NO |
| Hibino et al 25 | 83/M | Myasthenia gravis with myositis | NR | NR | Negative | Brain MRI and abdominal CT: negative | Autoimmune antibodies: negative, CK:4361 IU/L, aldolase: 134.8 IU/L, myoglobin: 4572.0 ng/mL, LDH: 580 IU/L, AST: 269 IU/L, ALT: 222 IU/L | NR | Myositis and hepatitis. |
| Mori et al 26 | 64/M | Optic neuritis | NR | NR | NR | MRI: high-intensity lesion in left optic nerve | NR | Protein level: 69 mg/dL | Hypopituitarism |
| Narumi et al 27 | 75/M | NMOSD | NR | NR | NR | Spinal MRI: hyperintense lesions between C5-6 and Th12-L1 | Paraneoplastic autoantibodies: negative, AQP4 antibody: positive | WBC: 1195/µL, protein level: 380.9 mg/dL, glucose concentration: 40 mg/dL | NO |
| Horio et al 28 | 63/M | Trousseau syndrome | NR | NR | NR | Brain MRI: intratumor hemorrhage, small infarct near tumor, multiple cerebral infarcts | NR | NR | Brain hemorrhagic infarction |
| Tan et al 29 | 66/M | Cerebellar ataxia | NR | NR | NR | Brain CT: negative Brain MRI: small vessel disease |
Negative | Negative | NO |
Abbreviations: AChRAbs, anti-acetylcholine receptor antibodies; ALT, alanine aminotransferase; AQP4, aquaporin 4; AST, aspartate aminotransferase; CK, creatine kinase; CMAP, compound muscle action potential; CSF, cerebrospinal fluid; CT, computational tomography; EEG, electroencephalograph; EMG, electromyograph; GBS, Guillain-Barré syndrome; LDH, lactate dehydrogenase; MRI, magnetic resonance image; MUSK, muscle-specific tyrosine kinase; NCS, nerve conduction study; NMOSD, neuromyelitis optica spectrum disorder; NR, not reference; NSCLC, non-small cell lung cancer; PRES, posterior reversible encephalopathy syndrome; RNS, repetitive nerve stimulation; SFEMG, single-fiber electromyography; VGCC, voltage-gated calcium channel; WBC, white blood cell.
We select the 4 most common NAEs based on case reports assembled and summarize their incidence, potential mechanisms, diagnosis, treatment, and several notable questions of various types of ICI-related neurological toxicities, giving a comprehensive direction in both clinical and basal experiments.
Incidence of NAEs
To date, due to the lower incidence of ICI-related NAEs, data available on it in clinical trials of NSCLC are limited, and most of these adverse effects are documented in case reports. Although ICI-related NAEs are relatively rare, approximately accounting for 1% to 3% of all irAEs in monotherapies, they may strongly affect the prognosis and even lead to patients’ death with cancers. 30 Different groups of statistics confine the incidence of immune-related neurotoxicity in ipilimumab-treated patients to 1% to 1.6%, and anti-PD-1 agents (nivolumab + pembrolizumab) to 3% to 3.2%.30-33 This rate can be much higher with combined ICIs, surpassing 10%. It has been estimated that within 9208 patients distributed in 59 trials, the incidence of ICI-related NAEs in combined therapy (nivolumab + ipilimumab) is up to 12%. 34 Researches based on NSCLC patients rarely document ICI-related neurological effects. Based on the data available in a multicenter analysis, we discover the incidence of nivolumab-related neurological toxicities in 1.3% (3/230) and pembrolizumab in 0% (0/41).35-37 In another retrospective research of 134 NSCLC patients, ICI-related neurological toxicities have been associated with nivolumab. 38 Neurological toxicities only manifest as myasthenia gravis (MG), which happens to only 1 patient, indicating an incidence of 0.7%. 38 Besides, a retrospective study with a large sample size of patients with NSCLC only indicates a gross incidence of anti-PD-1-related neuromuscular diseases, rheumatisms, fever, anorexia, pancreatitis, and asthenia. 39
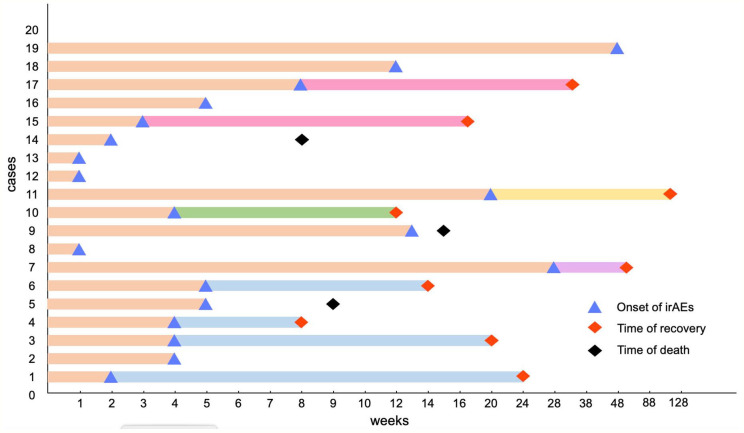
The occurrence of neurological toxicities can happen in any progress of applications of ICIs, which ranges widely from soon after the first dose to a prolonged period after the cessation of drugs. The length of time to occurrence relies on the medical history, categories of drug application, and applied dosages of drugs. Based on a retrospective study in a tertiary-care center, a vast majority of patients (89%) first run into ICI-related neurological toxicities within 12 weeks. 40 According to the case reports we summarize, most ICI-related neurological toxicities occur 1 to 5 weeks after the first dose of administration (Figure 1).10,14,16,17 However, some cases indicate a relatively rare latter occurrence of neurological toxicities, surpassing 20 weeks after the initiation of ICIs (Figure 1).12,13 Late-emerging irAEs have been noticed in long-term responders to PD-1/PD-L1 checkpoint inhibitors. 41 A systematic review also reported that early and late ICI-related encephalitis were possible. 42 In addition, not all irAEs will disappear when antitumor treatment is stopped; some late-irAEs could occur a year after first application. Interestingly, a multicenter study identified that neurological late-irAEs are the only type of irAEs which tends to occur late (>12 months) rather than early (⩽12 months) in melanoma and NSCLC patients. In this condition, any suspicious symptoms occurring during medication should be noticed even after a long time of drug withdraw.41,43 Wide ranges of time in neurological toxicity initiation possibly augment clinicians’ difficulties in detecting and diagnosing ICI-related NAEs.
Figure 1.
Time to onset of immune checkpoint inhibitor–associated NAEs. This figure is built based on type of NAEs. The time when immune checkpoint inhibitor–related neurological toxicity occurred is recorded as a dot: yellow dots represent myasthenia gravis; green dots represent encephalitis; blue dots represent Guillain-Barré syndrome; purple dot represents Lamber-Eaton syndrome; red dots represent other neurological adverse events. In 20 patients, the occurrence time of 1 patient has not been mentioned. IrAEs indicates immune-related adverse events.
Potential Mechanisms of irAEs
Although the development of immunotherapy thrives, the mechanisms of irAEs remain unclear. According to the existing knowledge, the fundamental principles are an imbalance between autoimmunity and immune tolerance, together with an uncontrolled infiltration of inflammatory T cells, production of autoimmune antibodies, and the assembly of cytokines. 44
Immune checkpoints are molecules expressed on the surface of cells, acting as immune regulators to maintain immune tolerance and limit autoimmunity. PD-1 and cytotoxic T lymphocyte-associated antigen (CTLA-4) are 2 of the most significant receptors that existed on the surface of cytotoxic T cells, binding their ligands of PD-L1/L2 and CD80/86, reducing Akt activation, thus inhibiting the cytokine and protein synthesis, glucose uptake, prohibiting T-cell activation and proliferation.44,45 It has been proved that blockade of PD-1 and CTLA-4 pathways influences the induction of tolerance of peripheral CD4+ T cell, disrupting the homeostasis of peripheral tolerance.46-49
The specific mechanisms of ICI-related NAEs remain unknown. However, the existence of autoantibodies detected in the serum of patients with ICI-related NAEs provides a potential assumption. Most antigens to autoantibodies detected in the serum of patients with autoimmune neurological toxicities could be identified in nearly all tumor cells called cross-presentation of onconeural antigens. 50 Researchers have identified various common antigens expressed in tumor cells and neurons in patients with paraneoplastic neurological syndromes.51-55 The ligand of anti-Hu (ANNA-1) is expressed on the nucleus of all neurons and tumor cells of patients with neurological syndromes in prostate cancer, NSCLC, and neuroblastoma.55-57 Similarly, genes coding for P/Q type voltage-gated calcium channel (VGCC) has been identified, which express both on the presynaptic membrane and cells of lung carcinomas after the treatment of ICIs. 53 With the application of ICIs, hyperactivation of the immune system leads to the attack of autoantibodies to self-antigen expressed inside or on the surface of neurons, disrupting linkage between neuromuscular transmission, influencing normal neuronal functions.30,58
In addition to the autoimmune attack to cross-presentation onconeural antigens, it is noteworthy that PD-1 and its ligands are not only expressed on hematopoietic cells but also on a vast majority of cell lines, including pancreatic islet cells, epithelial cells, reticular cells, vascular endothelial cells, and neurons.59,60 It is considering about nervous system, targets of ICI distributed in distinct densities in different anatomical regions. Researches point out that PD-1 is transcribed across the central nervous system, especially the basal ganglia and cortex.61,62 However, CTLA-4 is mostly expressed in the spinal cord and brainstem. 62 In this condition, the “off-target” effects of PD-1/PD-L1 and CTLA-4 mAbs could be understood.
Despite the cross-presentations of onconeural antigens, another theory called “epitope spreading” (ES) appears on the scene. 63 In the process of tissue dissociation when applying ICIs, tumor cells, together with disrupted nontumor antigens, will release secondary antigens, evoking an expanded immune response. Unlike the primary response, secondary antigens are delivered by antigen-presenting cells near the dissolved tissue, prime T and B cells, and mediating autoimmune response. This potential theory has been mentioned in the applications of CTLA-4 and PD-1/PD-L1 blockade. However, the specificity of it to the nervous system needs to be further explored.63-65
Categories of ICIs-Related Neurological Toxicities
Immune checkpoint inhibitor–related neurological toxicities have various manifestations, which affect the central nervous system or peripheral nervous system. It is estimated that the peripheral nervous system is often affected twice as the central nervous system. 66 The manifestations of ICI-related NAEs in the central nervous system include cerebellitis, 67 meningitis, encephalitis, 68 posterior reversible encephalopathy syndrome (PRES), 69 transverse myelitis, 70 cerebral vasculitis, 16 and multiple sclerosis. 71 In the peripheral nervous system, chronic immune demyelinating polyneuropathy (acute inflammatory demyelinating polyneuropathy [AIDP]), 72 facial nerve palsies, 73 Guillain-Barré syndrome (GBS), Tolosa-Hunt syndrome, 32 MG, 30 and Lambert-Eaton myasthenic syndrome (LEMS) 30 could also be observed.
Encephalitis
Encephalitis is a neurological inflammatory disorder induced by various possible reasons with complex diagnoses. 68 Immune checkpoint inhibitor–related encephalitis can occur during any drug administration cycle, and it is estimated to account for 0.1% to 1% of all irAEs in patients with PD-1/PD-L1 monotherapy. 13 Patients with ICI-related encephalitis often have a fever, headache, fatigue, working memory loss, altered mental status (consciousness or personality), psychiatric symptoms, and stiff neck. 45 It has been documented in 2 cases that encephalitis occurred within 1 week after the first dose of ICI in patients with metastatic NSCLC, and melanoma. 74 In NSCLC, initial symptoms appear in a 74-year-old man within 1 week, while in another 78-year-old man, 12 days after the 14th application of nivolumab, he suffers tonic convulsion of his right hand and becomes apathy and aphasia.13,35 In this situation, diagnosis should be made as soon as possible. Doctors should first evaluate the Glasgow Coma Scale score and perform a physical examination to assess the patient’s state of consciousness as well as the existence of pathological reflexes. 13 Magnetic resonance imaging (MRI) of the brain could suggest encephalitis features, which appear as the hyperintense focus on T2 fluid-attenuated inversion recovery confined to unilateral or bilateral medial temporal lobes or scattered to gray or white matter. Indications of electrocardiograph (ECG) are nonspecific, which adjusts to an electroencephalograph (EEG) as well. The EEG rarely shows specific manifestations. Most are unspecific moderate background slowing and focal delta slowing. However, its sensitivity is relatively high in the case reports we collected (Figure 2). Research claims that only anti-N-methyl D-aspartate (NMDA) receptor encephalitis has a specific extreme delta brush, which helps diagnose. 75 Adjuvant investigations should be done to exclude diseases that mimic the symptoms, including detailed medical history, laboratory analysis of blood and cerebrospinal fluid (CSF), complete physical and neurological examination, MRI including diffusion-weighted imaging (DWI), and serological autoantibodies. 14 The most common differential diagnosis of autoimmune encephalitis is herpes simplex virus (HSV) encephalitis. What is difficult for differentiating is the polymerase chain reaction (PCR) result of HSV in CSF may be harmful when it is done too early. A repetitive test should be done until the disease is ascertained. 76 Considering treatments, if the patient is suspected epileptic or in the postictal state, antiepileptic drugs (levetiracetam, midazolam, lamotrigine) should be administrated. 13 Steroidal treatment indicates its effectiveness in ICI-related encephalitis. Corticosteroids, for instance, methylprednisolone, can be administered intravenously or orally according to the severity of symptoms. Anti-infectious treatment should be preventively used.
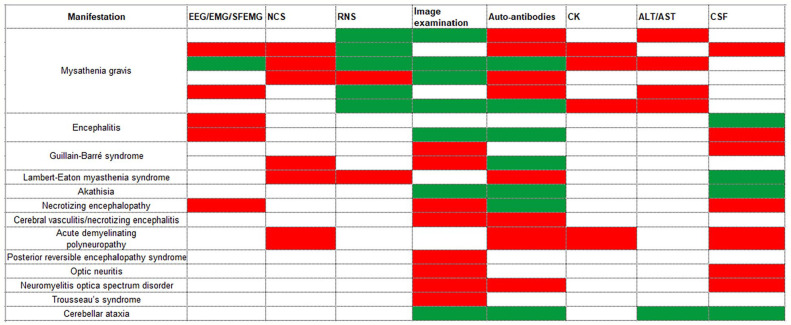
Figure 2.
Summarized a variety of diagnostic results in published case reports. Diagnostic approaches include EEG/EMG/SFEMG, NCS, RNS, image examinations, serological autoantibodies, CK, ALT/AST, and CSF. White blocks mean this examination has not been mentioned in case reports; green blocks represent negative results; red blocks represent positive results. ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; CSF, cerebrospinal fluid; EEG, electroencephalograph; EMG, electromyograph; ICI, immune checkpoint inhibitor; NAEs, neurological adverse events; NCS, nerve conduction study; RNS, repetitive nerve stimulation; SFEMG, single-fiber electromyography.
Myasthenia Gravis
Immune checkpoint inhibitor–related MG is a postsynaptic disorder in neuromuscular junctions, which shares its idiopathic modality. 77 It is reported to be the most common neuromuscular disorder in PD-1 inhibitor-related NAEs. 78 Based on the data collected from case reports and retrospective clinical databases, nivolumab-induced MG occupies 21%, whereas pembrolizumab accounts for 33% in patients with all kinds of neuromuscular disorders. 79 The MG onset occurs early after the administration of ICIs in patients with NSCLC, mostly following the first to third cycles. 80 Based on the 6 case reports we summarized in NSCLC, all the patients develop their symptoms within 8 weeks (4 cycles). Among which, 66.7% (4/6) patients suffer symptoms within 4 weeks.18-20,22,23,25 The ICI-related MG could affect muscles throughout the whole body. Ocular, bulbar, facial, respiratory muscles, and working muscles of limb and neck are most frequently affected.80,81 Clinical manifestations always cover fatigue, blurry visions, bilateral ptosis, diplopia, nasal speech, dysphagia, dysarthria, mild/severe dyspnea, and weakness of limbs. 82 Thorough workups are necessary for the diagnosis of MG. Neurological examination is the fundamental workup that should be done first, including Jolly, Icepack, and Tensilon. Doctors could verify the weakness of specific muscle groups and can further classify the strength them. 19 Low-frequency repetitive nerve stimulation (RNS) is the most common electrophysiological examination conducted in neuromuscular-transmitted disease. Aberrant RNS displays a decrement or decrease in compound muscle action potential (CMAP) amplitude in generalized MG, the detection rate of which is 75%. 83 However, in 6 ICI-related MG patients with NSCLC, 5 are negative, indicating a relatively low sensitivity18-20,23,25 (Figure 2). Nerve conduction study (NCS) could also assist in diagnosing, indicating the denervation or degeneration in ICI-related patients with NSCLC, but it is unremarkable on most occasions.19,20 Single-fiber electromyography (SFEMG) over proper muscles may indicate abnormal jitters in 95% to 99% of patients with MG, similar to ICI-induced MG.19,23 However, the specificity of abnormal jitters is relatively low, as it also appears in other muscular or neural diseases.84-86 The sensitivity of serological studies in AchR antibodies is higher than RNS, approximately up to 85% in generalized MG. 87 Under this circumstance, the concentration of AchR antibody needs to be measured. The mean frequency of positive AchR antibody in collected patients with NSCLC is 66.7%. Therefore, for suspicion patients, AchR antibodies is worthy of being quantified18-20,22,23,25, (Figure 2). However, in the early stage of MG (within 24 hours), it is difficult to detect AchR antibodies. Meanwhile, 15% of patients are negative with that at any time. Among these patients, 40% of them are positive in anti-muscle-specific tyrosine kinase (MUSK) antibodies. 88 Another serum biochemistry test, creatine kinase (CK) chemistry examination, always indicates an abnormal CK, overwhelming several times of upper limit in ICI-induced patients with NSCLC.20,25
The prior treatment to ICI-induced MG is withdrawing or lowering the dose of ICI, which should be individualized according to its severity. After the cessation of ICI, patients with MG will acquire great improvement. 23 Under this circumstance, the cholinesterase inhibitors (pyridostigmine bromide), a first-line therapy for MG, is recommended in 30 to 90 mg 3 times a day.22,77 It is noteworthy that overdose of pyridostigmine bromide prolongs depolarization and exacerbates muscular weakness, resulting in a cholinergic crisis. In addition, corticosteroids are mostly adhibited and recommended in ICI-related MG, which are applied with or after cholinesterase inhibitors.18,19,25,77 When the symptoms cannot be approximately controlled by monotherapy of pyridostigmine bromide, IV prednisone (0.75-1.00 mg/kg/d) could be tapered gradually to oral steroids in patients with improved symptoms.25,89 Patients with NSCLC who have severe ICI-related MG, plasmapheresis and IV immunoglobulin (IVIg) will relieve their muscular weakness within a few days. 86 Plasmapheresis could rapidly decrease serological autoantibodies concentration while IVIg could competitively bind to the Fc receptors. Most patients with NSCLC could acquire partial relief or complete remission after multiple treatments to ICI-related MG.19,20,22,23,25
Guillain-Barré Syndrome
Guillain-Barré syndrome is an autoimmune-peripheral neuropathy that manifests as extensive demyelinating of peripheral nerves and nerve roots. It is reported as the third most common PD-1-related neuromuscular disorder. 78 According to the Japanese Adverse Drug Event Report database (JADER), patients with GBS/MFS (Miller-Fisher syndrome) account for 0.1% of all ICI-related NAEs. 90 Another World Health Organization pharmacovigilance database VigiBase reported an incidence of 0.45% in combined ICI-related GBS during 2008 to 2018. 91 However, the incidence of ICI-related GBS in patients with NSCLC has not been reported alone. Compared with MG, the onset time of ICI-related GBS is more variable, ranging from 4 weeks of ICI application to several months after completing the treatment cycle. 92 The most remarkable symptoms of GBS are rapidly progressive limb weakness and loss/decrease of tendon reflexes, which peak within 28 days.93-96 Patients with ICI-related GBS in NSCLC usually suffer mild sensory problems, containing paresthesia and numbness.95,96 The muscle weakness shows in symmetrical, arising from the lower limbs to upper arms, as well as bulbar muscles. 97 Concomitant symptoms of muscular weakness are extensive loss or decrease of deep tendon reflexes, especially the most common tendon, biceps, and knee reflex, which are documented in 2 case reports with NSCLC.94-96 Cranial nerve deficits, particularly facial paralysis, ophthalmoplegia, and bulbar paralysis, can be observed. 98 Patients with ICI-related GBS may also strike from the autonomic dysfunction, which influences multiple systems. 98 To diagnose ICI-related GBS, auxiliary examinations are required. Brain and spinal MRI in 2 patients we collected showed pathological features, indicating a high sensitivity (Figure 2). Elevated protein levels combined with normal cell counts are a hallmark of GBS. It is reported that 64% of patients with GBS have an elevated protein level, and the cell count of 85% is normal. 99 However, patients with new-onset symptoms have a relatively low incidence of elevated protein detection, increasing from 50% to approximately 90% after 2 weeks. 99 Clinically, the best-known subtypes of GBS are AIDP and acute motor axonal neuropathy (AMAN), which distinguish from each other by the existence of sensory signs. 100 The NCS is another necessary workup assisting the diagnosis of GBS. The NCS of patients of AIDP subtypes may reveal distal motor latency (DML), prolonged motor conduction velocity (MCV), conduction blocks, and abnormal temporal dispersion. 100 The results of NCS of AMAN patients always show no demyelinating features with the decreased amplitude of distal CMAP. 94 According to the treatment, IVIg and plasmapheresis are proved efficient, which applies to ICI-related GBS in patients with NSCLC according to the 2 case reports we collected.95,96 The IVIg is usually given for 5 days (0.4 g/kg/d). 93 The normal regimen for plasma exchange is 5 sessions over 2 weeks, which is recommended to be applied within 4 weeks since the onset of GBS. 93 Studies also indicate that the combination of plasmapheresis and IVIg does not have a significant difference compared with plasmapheresis alone. 101 The efficacy of oral corticosteroids remains unclear, but the synergistic effects of combined use of IVIg and IV methylprednisolone cannot be excluded in patients. 102 In a case report of pembrolizumab-related GBS with NSCLC, a combination of IV methylprednisolone and IVIg is proved to be effective. 95 Prognosis of ICI-related GBS in patients with NSCLC is difficult to assess due to the individual variation. Most patients could get partial release within 2 to 4 weeks after the cease of disease progression. However, in some patients, diseases progress in a short period, resulting in death.94-96
Lambert-Eaton Myasthenic Syndrome
Lambert-Eaton myasthenic syndrome is a presynaptic disorder of neuromuscular junctions. It is reported that 50% to 60% of LEMS occurs with malignancies, which appears as a paraneoplastic syndrome. 103 Lambert-Eaton myasthenic syndrome is induced by autoantibody against presynaptic P/Q-type VGCCs, which hampers over 95% of functional receptors responsible for signal transmission of neuromuscular junction. 104 Due to the expression of mimic ectopic antigens in small cell lung cancer (SCLC), LEMS is commonly observed in patients suffering from it.105,106 However, ICI-related LEMS in patients with NSCLC is only documented as a case report, and its incidence has not been reported yet. 12 Symptoms of ICI-related LEMS mainly appear as limb weakness, areflexia/hyporeflexia of deep tendons, and autonomic dysfunction. 30 Imperceptible fatigue could be the incipient symptom, followed by weakness of proximal legs, which is regarded as the first symptoms by 80% of LEMS patients. 107 Meanwhile, the weakness of arms may appear soon after the weakness of proximal legs, which suggests the principle of weakness spreading: from proximal, cranial parts to distal, caudal parts. 107 Compared with MG, bulbar, axial, and ocular muscle weakness is mild or even absent. 108 However, the incipient symptoms of nivolumab-related LEMS in a patient with NSCLC manifest as ptosis and photophobia. 12 As for autonomic dysfunction, xerostomia is the most common one, which is always accompanied by a metallic taste. 108 Constipation, dry eyes, and orthostatic hypotension may exist as well. 30 Loss or decrease of deep tendon reflexes strengthens the suspicion of LEMS. 102 In addition to clinical symptoms, to diagnose ICI-related LEMS in patients with NSCLC, antibody testing and electrodiagnostic studies need to be done for certain. P/Q type VGCC antibodies can be observed in nearly all patients with lung cancer accompanied by LEMS.109,110 However, in the serum of patients with LEMS, P/Q type VGCC could be negative. 111 Similar symptoms could be induced by other blockers of a presynaptic receptor, suggesting that the seropositive P/Q type VGCC is not the only diagnostic criteria. 112 The most prominent EMG characteristics of LEMS patients with NSCLC are called “post-exercise facilitation.” 113 Initially, EMG shows a low amplitude of resting CMAP, which could be even lower after low-frequency (2-5 Hz) RNS. A rapidly progressive increase of CMAP after a high-frequency RNS (>10 Hz) or a maximal muscle contraction is then observed.12,103,114 Results of auxiliary examinations of the only 1 LEMS patient in NSCLC can be observed in the heat map (Figure 2). Symptomatic treatment and immunomodulatory therapy need to be applied to ICI-related LEMS patients with NSCLC. The strategy of symptomatic treatment is to increase the release of neurotransmitters. The most common agent is the 3,4-diaminopyridine (3,4-DAP), an inhibitor of presynaptic K+ channels. 115 Blockade of presynaptic K+ channels prolongs the opening of VGCC, allowing the entry for more Ca2+, which therefore increases the release of intracellular Ca2+ dependent-Ach. Acetylcholinesterase inhibitor pyridostigmine is less commonly used in combination with 3,4-DAP. 116 If the application of 3,4-DAP could not achieve a preconceived effect, immunomodulatory therapy needs to be added. It is reported that combined application of azathioprine and prednisolone is more effective than prednisolone alone. 117 Application of azathioprine needs to start from 15 mg/d to 30 mg/d, to maximal 80 mg/d to 100 mg/d, divided into 3 to 4 times a day. 30 In the case report of nivolumab-related LEMS in a patient with NSCLC, pyridostigmine’s initial application only slightly relieves her wadding gait after the failure of oral prednisolone and another acetylcholinesterase inhibitor ambenonium, 3,4-DAP finally improves her manifestations. 12 If the patient fails to respond to all these management, IVIg or plasma exchange is necessary, which shows relatively long-lasting improvements by reducing the concentration of circulating autoantibodies. 118
Other NAEs
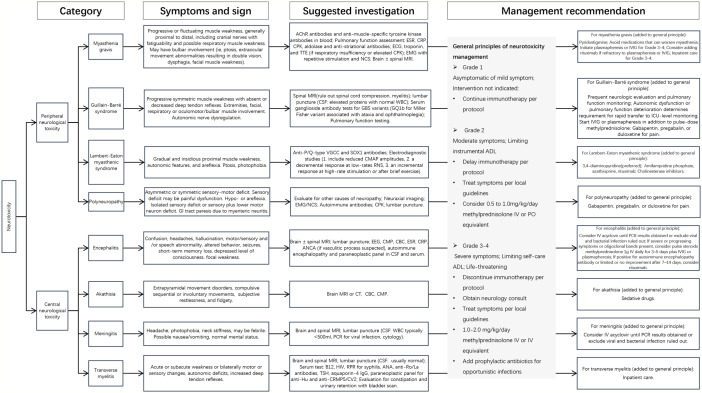
In addition to the 4 relatively common ICI-related NAEs we mentioned, several rare ICI-related NAEs have not been mentioned in retrospective or prospective studies, and all of them are documented as case reports. Akathisia, necrotizing encephalitis, cerebral vasculitis, PRES, and neuromyelitis optica spectrum disorders (NMOSDs) induced by nivolumab manifested motor disturbance of cerebral dysfunction. Compulsive movements, nausea, vomiting, dyspnea, and urinary retention were also observed. In most instances, the application of steroids slightly or apparently improved the symptoms. Sedative drugs, antiepileptic treatments, IVIg, and plasmapheresis were applied based on the manifestations.10,14,16,24,27 One patient died with no apparent response to IVIg, antiepileptic treatments, and corticosteroids. 14 Trousseau syndrome induced by pembrolizumab indicated right homonymous hemianopia. The MRI showed multiple cerebral infarcts because of brain metastasis. The patient died from hemorrhagic infarction soon after the second dose of pembrolizumab. 28 Atezolizumab-induced cerebella ataxia appeared with a progressive ataxic gait improved by long-term prednisolone application. 29 Another patient treated with atezolizumab developed fatigue, diarrhea, anorexia, and pain in bilateral upper limbs leading the cessation of the application. She suddenly had visual loss a year later, which was diagnosed with optic neuritis. She recovered after the steroid pulse therapy and oral administration of prednisolone. 26 Results of all these patients’ accessory examinations are recorded in the heat map, reflecting the positive and negative rate of each workup in different NAEs (Figure 2). Consensus guidelines have been published by the National Comprehensive Cancer Network (NCCN) Panel; therefore, we summarize a comprehensive diagnosis and treatment algorithm (Figure 3).
Figure 3.
Algorithm for the diagnosis and management of neurotoxicity. AChR indicates acetylcholine receptor; ADL, activities of daily life; ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibody; CBC, complete blood count; CMAP, compound muscle action potential; CMP, comprehensive metabolic panel; CPK, creatinine phosphokinase; CRP, C-reactive protein; CSF, cerebrospinal fluid; CT, computed tomography; ECG, electrocardiogram; EEG, electroencephalogram; EMG, electromyography; ESR, erythrocyte sedimentation rate; GBS, Guillain-Barré syndrome; GI, gastrointestinal; HIV, human immunodeficiency virus; ICU, intensive care unit; IV, intravenous; IVIG, intravenous immunoglobulin; MG, myasthenia gravis; MRI, magnetic resonance imaging; NCS, nerve conduction study; PCR, polymerase chain reaction; PO, oral; RPR, rapid plasma reagin; TSH, thyrotropin; TTE, transthoracic echocardiogram; VGCC, voltage-gated calcium channels; WBC, white blood cell.
Notable Questions of Interest in Clinicians
Is the incidence of ICI-related NAEs higher in cancer patients with brain metastasis?
Immune checkpoint inhibitors have been proved effective in patients with various advanced malignancies. In previous clinical trials, cancer patients received ICI with untreated brain metastasis are mostly excluded from the penetration across blood-brain barrier (BBB), historically poor prognosis, and potential side effects to the nervous system. 119 The incidence of ICI-related NAEs in patients with brain metastasis has not been documented as the lack of multicenter studies with large samples. However, according to a small sample study with 23 patients (18 with prior local central nervous system [CNS] therapy) who suffer from melanoma with untreated brain metastasis (⩽20 mm), the incidence of pembrolizumab-related ataxia and headache is 22% and 17%, respectively, which is higher in patients without brain metastasis we mentioned above. 120 Meanwhile, in the same study, the incidence of headache in NSCLC patients with brain metastasis is 22%, and that of dizziness is 11%. 121 Nevertheless, the increasing incidence of ICI-related NAEs in patients with brain metastasis compared with patients without intracranial malignancies could owe to the perilesional edema after the local surgery or small sample size. It is noteworthy that all the pembrolizumab-related NAEs in patients with brain metastasis of NSCLC are of grade 1 to 2, suggesting a relatively safe profile of ICI application in patients with brain metastasis. 121 In this situation, we could only summarize the application of ICI in a patient with primary lesion and brain metastasis is relatively safe according to the rare occurrence of severe NAEs that are uncontrolled and lead to death.
Does application of ICIs exacerbate preexisting autoimmune symptoms?
In consideration of the treatment of patients with preexisting autoimmune disease, the application of ICI needs to be assessed meticulously. Both MG and LEMS could appear after the application of ICI, which may result from unmasking of dormant diseases or appearance of de novo diseases. It is reported that the application of PD-1/PD-L1 inhibitors exacerbates the symptoms of preexisting paraneoplastic syndrome in 50% (8/16) of patients suffered from cancer. 106 A study based on SEER database identified patients with cutaneous melanoma combined with preexisting autoimmune disease received significant higher risk of irAEs in most organ systems. 122 A meta-analysis of 6 reports discovered that preexisting autoimmune disease is a risk factor of irAE. 123 All these data indicate that preexisting autoimmune disease augments the effect of ICI-related NAEs. Other autoimmune diseases, including myositis and myocarditis, are much higher in nivolumab-induced MG than idiopathic MG.124,125 Meanwhile, facial muscle weakness, bulbar symptoms, and panting also appear more frequently in nivolumab-related MG than idiopathic MG. 125 Therefore, ICI also increases the possibility of acquiring other types of autoimmune diseases in cancer patients with preexisting autoimmune disease. According to the case reports we collected in NSCLC, there are no documentations of patients with preexisting autoimmune diseases who receive the ICI treatment. Studies focusing on the safety profile of ICI application to patients with preexisting autoimmune diseases need to be conducted in this condition.
How to deal with patients with a relapse of NAEs?
In patients treated with ICI, a notable phenomenon of relapse of ICI-related NAEs is observed. Studies indicate a higher incidence of it with the application of CTLA-4 inhibitors or a combined therapy than PD-1/PD-L1 monotherapy. 40 This rate is also higher in patients who have ICI-related NAEs affected both the central and peripheral nervous system. 40 It is noteworthy that patients with relapse of NAE are not treated with immunosuppressive therapy or only receive oral corticosteroids for a short period, which suggests an application of immunosuppressants to all patients with severe ICI-induced NAEs. 40 We observe a relapse of PRES in a patient with NSCLC after a few weeks while the primary and relapsing treatments are not mentioned. 24
How is the prognosis of different descriptions of NAEs?
The prognosis of different types of ICI-related NAEs remains unclear. Based on the case reports of ICI-related NAEs in patients with NSCLC, ICI-induced MG prognosis is relatively favorable, symptoms of 5 of 6 patients are improved by applying steroids pyridostigmine.18-20,22,23,25, The patient who died from MG receives similar treatment with others while achieving bad results due to the refusal of a mechanical ventilator, leading to hypercapnic respiratory failure. 19 In a case report of ICI-related GBS, symptoms of the patient progress rapidly and show no obvious improvement to IVIg and plasma exchange, suggesting that an early diagnosis and treatment is necessary. 15 In addition to the unresponsiveness to most therapeutic modalities, the patient died from necrotizing encephalopathy owes to the second application of ICI, which aggravates her previous symptoms. After the first application of nivolumab, symptoms have already appeared, which are considered partially attributed to hyponatremia and application of sertraline. 14 In this condition, nivolumab is applied continuously, leading to the death of the patient. 14 It has been reported that ICI-associated NAEs progress rapidly after the second application of ICI, although the symptoms after the first cycle are mild or can be relieved by excluding other risk factors. 126 Clinicians need to carefully assess the severity of ICI-related NAEs and determine whether the stoppage or continuous ICI application is better for prognosis.
Conclusions
Immune checkpoint inhibitor–related NAEs are the disorder of central and peripheral nervous system, rare but may be fatal. A combination of PD-1/PD-L1 and CTLA-4 inhibitors increases the risk of ICI-induced NAEs compared with monotherapy. Time of occurrence ranges widely, but most concentrate within 1 to 5 weeks after the first dose initiation. Encephalitis, MG, GBS, and Lambert-Eaton are relatively common NAEs, which have been discussed in detail on clinical symptoms, diagnostic examinations, and treatment modalities. Steroids are main treatment modality to ICI-related NAEs. Intravenous immunoglobulin and plasmapheresis are necessary when manifestations deteriorate. To better understand the diagnosis and treatment of ICI-related NAEs in NSCLC, further studies are needed.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Sichuan Science and Technology Department Key Research and Development Project (2019YFS0539), 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC18022).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: KC and YW collected data, reviewed the literature, analyzed all data, and wrote the manuscript. YZ collected data and wrote and revised the manuscript. RX collected data, rechecked the manuscript. and assisted in drawing. LT designed and revised the manuscript. All authors read and approved the final manuscript.
ORCID iD: Ke Cheng  https://orcid.org/0000-0002-6034-8872
https://orcid.org/0000-0002-6034-8872
References
- 1. Califano R, Gomes F, Ackermann CJ, Rafee S, Tsakonas G, Ekman S. Immune checkpoint blockade for non-small cell lung cancer: what is the role in the special populations? Eur J Cancer. 2020;125:1-11. [DOI] [PubMed] [Google Scholar]
- 2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008; 83: 584-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suda K, Mizuuchi H, Maehara Y, Mitsudomi T. Acquired resistance mechanisms to tyrosine kinase inhibitors in lung cancer with activating epidermal growth factor receptor mutation—diversity, ductility, and destiny. Cancer Metastasis Rev. 2012;31:807-814. [DOI] [PubMed] [Google Scholar]
- 4. Papaioannou NE, Beniata OV, Vitsos P, Tsitsilonis O, Samara P. Harnessing the immune system to improve cancer therapy. Ann Transl Med. 2016;4:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125:3384-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khan S, Gerber DE. Autoimmunity, checkpoint inhibitor therapy and immune-related adverse events: a review. Semin Cancer Biol. 2020;64:93-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sacher AG, Gandhi L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non-small-cell lung cancer: a review. JAMA Oncol. 2016;2:1217-1222. [DOI] [PubMed] [Google Scholar]
- 8. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697. [DOI] [PubMed] [Google Scholar]
- 9. Horinouchi H, Yamamoto N, Fujiwara Y, et al. Phase I study of ipilimumab in phased combination with paclitaxel and carboplatin in Japanese patients with non-small-cell lung cancer. Invest New Drugs. 2015;33:881-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abe J, Sato T, Tanaka R, Okazaki T, Takahashi S. Nivolumab-induced severe akathisia in an advanced lung cancer patient. Am J Case Rep. 2016;17:880-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Richard K, Weslow J, Porcella SL, Nanjappa S. A case report of steroid Responsive Nivolumab-Induced Encephalitis. Cancer control: J Moffitt Cancer Cent. 2017;24:1073274817729069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakatani Y, Tanaka N, Enami T, Minami S, Okazaki T, Komuta K. Lambert-Eaton myasthenic syndrome caused by nivolumab in a patient with squamous cell lung cancer. Case Rep Neurol. 2018;10:346-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schneider S, Potthast S, Komminoth P, Schwegler G, Böhm S. PD-1 checkpoint inhibitor associated autoimmune encephalitis. Case Rep Oncol. 2017;10:473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leitinger M, Varosanec MV, Pikija S, et al. Fatal necrotizing encephalopathy after treatment with nivolumab for squamous non-small cell lung cancer: case report and review of the literature. Front Immunol. 2018;9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacob A, Unnikrishnan DC, Mathew A, Thyagarajan B, Patel S. A case of fatal Guillain-Barre syndrome from anti-PD1 monoclonal antibody use. J Cancer Res Clin Oncol. 2016;142:1869-1870. [DOI] [PubMed] [Google Scholar]
- 16. Läubli H, Hench J, Stanczak M, et al. Cerebral vasculitis mimicking intracranial metastatic progression of lung cancer during PD-1 blockade. J Immunother Cancer. 2017;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fukumoto Y, Kuwahara M, Kawai S, Nakahama K, Kusunoki S. Acute demyelinating polyneuropathy induced by nivolumab. J Neurol Neurosurg Psychiatry. 2018;89:435-437. [DOI] [PubMed] [Google Scholar]
- 18. Tan RYC, Toh CK, Takano A. Continued Response to One Dose of Nivolumab Complicated by Myasthenic Crisis and Myositis. J Thorac Oncol: official publication of the International Association for the Study of Lung Cancer. 2017;12:e90-e91. [DOI] [PubMed] [Google Scholar]
- 19. Chen JH, Lee KY, Hu CJ, Chung CC. Coexisting myasthenia gravis, myositis, and polyneuropathy induced by ipilimumab and nivolumab in a patient with non-small-cell lung cancer: A case report and literature review. Medicine. 2017;96:e9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen YH, Liu FC, Hsu CH, Chian CF. Nivolumab-induced myasthenia gravis in a patient with squamous cell lung carcinoma: Case report. Medicine. 2017;96(27):e7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ong S, Chapman J, Young G, Mansy T. Guillain-Barré-like syndrome during pembrolizumab treatment. Muscle Nerve. 2018;58:e8-e10. [DOI] [PubMed] [Google Scholar]
- 22. Polat P, Donofrio PD. Myasthenia gravis induced by nivolumab therapy in a patient with non-small-cell lung cancer. Muscle Nerve. 2016;54:507. [DOI] [PubMed] [Google Scholar]
- 23. Sciacca G, Nicoletti A, Rampello L, Noto L, Parra HJ, Zappia M. Benign form of myasthenia gravis after nivolumab treatment. Muscle Nerve. 2016;54:507-509. [DOI] [PubMed] [Google Scholar]
- 24. Hussein HM, Dornfeld B, Schneider DJ. Nivolumab-induced posterior reversible encephalopathy syndrome. Neurol Clin Pract. 2017;7:455-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hibino M, Maeda K, Horiuchi S, Fukuda M, Kondo T. Pembrolizumab-induced myasthenia gravis with myositis in a patient with lung cancer. Respirol Case Rep. 2018;6:e00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mori S, Kurimoto T, Ueda K, et al. Optic Neuritis Possibly Induced by Anti-PD-L1 Antibody Treatment in a Patient with Non-Small Cell Lung Carcinoma. Case Rep Ophthalmol. 2018;9:348-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Narumi Y, Yoshida R, Minami Y, et al. Neuromyelitis optica spectrum disorder secondary to treatment with anti-PD-1 antibody nivolumab: the first report. BMC Cancer. 2018;18:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horio Y, Takamatsu K, Tamanoi D, et al. Trousseau’s syndrome triggered by an immune checkpoint blockade in a non-small cell lung cancer patient. Eur J Immunol. 2018;48:1764-1767. [DOI] [PubMed] [Google Scholar]
- 29. Tan YY, Rannikmae K, Steele N. Case report: immune-mediated cerebellar ataxia secondary to anti-PD-L1 treatment for lung cancer. Int J Neurosci. 2019;129:1223-1225. [DOI] [PubMed] [Google Scholar]
- 30. Guidon AC. Lambert-Eaton myasthenic syndrome, botulism, and immune checkpoint inhibitor-related myasthenia gravis. Continuum (Minneap Minn). 2019;25:1785-1806. [DOI] [PubMed] [Google Scholar]
- 31. Spain L, Walls G, Julve M, et al. Neurotoxicity from immune-checkpoint inhibition in the treatment of melanoma: a single centre experience and review of the literature. Ann Oncol. 2017;28:377-385. [DOI] [PubMed] [Google Scholar]
- 32. Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS ONE. 2013;8:e53745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer (Oxford, England). 2016;60:210-225. [DOI] [PubMed] [Google Scholar]
- 34. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:1270-1271. [DOI] [PubMed] [Google Scholar]
- 35. Richard K, Weslow J, Porcella SL, Nanjappa S. A case report of steroid responsive nivolumab-induced encephalitis. Cancer Control. 2017;24:1073274817729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fife BT, Pauken KE, Eagar TN, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ksienski D, Wai ES, Croteau N, et al. Efficacy of nivolumab and pembrolizumab in patients with advanced non-small-cell lung cancer needing treatment interruption because of adverse events: a retrospective multicenter analysis. Clin Lung Cancer. 2019;20:e97-e106. [DOI] [PubMed] [Google Scholar]
- 38. Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncology. 2018;4:374-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cortellini A, Chiari R, Ricciuti B, et al. Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer. 2019;20:237-247.e231. [DOI] [PubMed] [Google Scholar]
- 40. Dubey D, David WS, Reynolds KL, et al. Severe neurological toxicity of immune checkpoint inhibitors: growing spectrum. Ann Neurol. 2020;87:659-669. [DOI] [PubMed] [Google Scholar]
- 41. Nigro O, Pinotti G, De Galitiis F, et al. Late immune-related adverse events in long-term responders to PD-1/PD-L1 checkpoint inhibitors: a multicentre study. Eur J Cancer. 2020;134:19-28. [DOI] [PubMed] [Google Scholar]
- 42. Nersesjan V, McWilliam O, Krarup LH, Kondziella D. Autoimmune encephalitis related to cancer treatment with immune checkpoint inhibitors: a systematic review. Neurology. 2021;97:e191-e202. [DOI] [PubMed] [Google Scholar]
- 43. Boland P, Pavlick AC, Weber J, Sandigursky S. Immunotherapy to treat malignancy in patients with pre-existing autoimmunity. J Immunother Cancer. 2020;8:e000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gianchecchi E, Delfino DV, Fierabracci A. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmun Rev. 2013;12:1091-1100. [DOI] [PubMed] [Google Scholar]
- 45. Hottinger AF. Neurologic complications of immune checkpoint inhibitors. Curr Opin Neurol. 2016;29:806-812. [DOI] [PubMed] [Google Scholar]
- 46. Fife BT, Guleria I, Gubbels Bupp M, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science (New York, NY). 2003;299:1057-1061. [DOI] [PubMed] [Google Scholar]
- 48. Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142-1151. [DOI] [PubMed] [Google Scholar]
- 49. Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519-6523. [DOI] [PubMed] [Google Scholar]
- 50. Graus F, Dalmau J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2019;16:535-548. [DOI] [PubMed] [Google Scholar]
- 51. Raheja D, Specht C, Simmons Z. Paraproteinemic neuropathies. Muscle Nerve. 2015;51:1-13. [DOI] [PubMed] [Google Scholar]
- 52. Saiz A, Dalmau J, Butler MH, et al. Anti-amphiphysin I antibodies in patients with paraneoplastic neurological disorders associated with small cell lung carcinoma. J Neurol Neurosurg Psychiatry. 1999;66:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kesner VG, Oh SJ, Dimachkie MM, Barohn RJ. Lambert-Eaton myasthenic syndrome. Neurol Clin. 2018;36:379-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peterson K, Rosenblum MK, Kotanides H, Posner JB. Paraneoplastic cerebellar degeneration. I. A clinical analysis of 55 anti-Yo antibody-positive patients. Neurology. 1992;42:1931-1937. [DOI] [PubMed] [Google Scholar]
- 55. Graus F, Keime-Guibert F, Reñe R, et al. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. 2001;124:1138-1148. [DOI] [PubMed] [Google Scholar]
- 56. Levine TD, Gao F, King PH, Andrews LG, Keene JD. Hel-N1: an autoimmune RNA-binding protein with specificity for 3′ uridylate-rich untranslated regions of growth factor mRNAs. Mol Cell Biol. 1993;13:3494-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sakai K, Gofuku M, Kitagawa Y, et al. A hippocampal protein associated with paraneoplastic neurologic syndrome and small cell lung carcinoma. Biochem Biophys Res Commun. 1994;199:1200-1208. [DOI] [PubMed] [Google Scholar]
- 58. Kao JC, Liao B, Markovic SN, et al. Neurological complications associated with anti-programmed death 1 (PD-1) antibodies. JAMA Neurol. 2017;74:1216-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pan PC, Haggiagi A. Neurologic immune-related adverse events associated with immune checkpoint inhibition. Curr Oncol Rep. 2019;21:108. [DOI] [PubMed] [Google Scholar]
- 61. Psimaras D, Velasco R, Birzu C, et al. Immune checkpoint inhibitors-induced neuromuscular toxicity: from pathogenesis to treatment. J Peripher Nerv Syst. 2019;24:S74-S85. [DOI] [PubMed] [Google Scholar]
- 62. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med. 2017;23:540-547. [DOI] [PubMed] [Google Scholar]
- 64. Kwek SS, Dao V, Roy R, et al. Diversity of antigen-specific responses induced in vivo with CTLA-4 blockade in prostate cancer patients. J Immunol. 2012;189:3759-3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Memarnejadian A, Meilleur CE, Shaler CR, et al. PD-1 blockade promotes epitope spreading in anticancer CD8+ T cell responses by preventing fratricidal death of subdominant clones to relieve immunodomination. J Immunol. 2017;199:3348-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Naito T, Osaki M, Ubano M, Kanzaki M, Uesaka Y. Acute cerebellitis after administration of nivolumab and ipilimumab for small cell lung cancer. Neurol Sci. 2018;39:1791-1793. [DOI] [PubMed] [Google Scholar]
- 68. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Posterior reversible encephalopathy syndrome during ipilimumab therapy for malignant melanoma. J Clin Oncol. 2012;30:76-78. [DOI] [PubMed] [Google Scholar]
- 70. Liao B, Shroff S, Kamiya-Matsuoka C, Tummala S. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol. 2014;16:589-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gerdes LA, Held K, Beltrán E, et al. CTLA4 as immunological checkpoint in the development of multiple sclerosis. Ann Neurol. 2016;80:294-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fellner A, Makranz C, Lotem M, et al. Neurologic complications of immune checkpoint inhibitors. J Neurooncol. 2018;137:601-609. [DOI] [PubMed] [Google Scholar]
- 73. Johnson DB, Friedman DL, Berry E, et al. Survivorship in immune therapy: assessing chronic immune toxicities, health outcomes, and functional status among long-term ipilimumab survivors at a single referral center. Cancer Immunol Res. 2015;3:464-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Williams TJ, Benavides DR, Patrice KA, et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol. 2016;73:928-933. [DOI] [PubMed] [Google Scholar]
- 75. Schmitt SE, Pargeon K, Frechette ES, Hirsch LJ, Dalmau J, Friedman D. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology. 2012;79:1094-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Weil AA, Glaser CA, Amad Z, Forghani B. Patients with suspected herpes simplex encephalitis: rethinking an initial negative polymerase chain reaction result. Clin Infect Dis. 2002;34:1154-1157. [DOI] [PubMed] [Google Scholar]
- 77. Gilhus NE. Myasthenia Gravis. The New England journal of medicine. 2016;375:2570-2581. [DOI] [PubMed] [Google Scholar]
- 78. Kao JC, Brickshawana A, Liewluck T. Neuromuscular Complications of Programmed Cell Death-1 (PD-1) Inhibitors. Curr Neurol Neurosci Rep. 2018;18:63. [DOI] [PubMed] [Google Scholar]
- 79. Johansen A, Christensen SJ, Scheie D, Hojgaard JLS, Kondziella D. Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies: Systematic review. Neurol. 2019;92:663-674. [DOI] [PubMed] [Google Scholar]
- 80. Grob D. Myasthenia gravis. A review of pathogenesis and treatment. Arch Intern Med. 1961;108:615-638. [DOI] [PubMed] [Google Scholar]
- 81. Grob D. Course and management of myasthenia gravis. J Am Med Assoc. 1953;153:529-532. [DOI] [PubMed] [Google Scholar]
- 82. Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37:141-149. [DOI] [PubMed] [Google Scholar]
- 83. Chiou-Tan FY, Gilchrist JM. Repetitive nerve stimulation and single-fiber electromyography in the evaluation of patients with suspected myasthenia gravis or Lambert-Eaton myasthenic syndrome: review of recent literature. Muscle Nerve. 2015;52:455-462. [DOI] [PubMed] [Google Scholar]
- 84. Oh SJ, Kim DE, Kuruoglu R, Bradley RJ, Dwyer D. Diagnostic sensitivity of the laboratory tests in myasthenia gravis. Muscle Nerve. 1992;15:720-724. [DOI] [PubMed] [Google Scholar]
- 85. Juel VC. Single fiber electromyography. Handb Clin Neurol. 2019;160:303-310. [DOI] [PubMed] [Google Scholar]
- 86. Armstrong SM, Schumann L. Myasthenia gravis: diagnosis and treatment. J Am Acad Nurse Pract. 2003;15:72-78. [DOI] [PubMed] [Google Scholar]
- 87. Lindstrom JM, Seybold ME, Lennon VA, Whittingham S, Duane DD. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976;26:1054-1059. [DOI] [PubMed] [Google Scholar]
- 88. McConville J, Farrugia ME, Beeson D, et al. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann Neurol. 2004;55:580-584. [DOI] [PubMed] [Google Scholar]
- 89. Bosch EP, Subbiah B, Ross MA. Cholinergic crisis after conventional doses of anticholinesterase medications in chronic renal failure. Muscle Nerve. 1991;14:1036-1037. [PubMed] [Google Scholar]
- 90. Kenichiro Sato TM, lwata Atsushi, Toda Tatsushi. Neurological and related adverse events in immune checkpoint inhibitors: a pharmacovigilance study from the Japanese Adverse Drug Event Report database. J Neurooncol. 2019;145:1-9. [DOI] [PubMed] [Google Scholar]
- 91. Johnson DB, Manouchehri A, Haugh AM, et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J immunotherapy of cancer. 2019;7:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yost MD, Chou CZ, Botha H, Block MS, Liewluck T. Facial diplegia after pembrolizumab treatment. Muscle Nerve. 2017;56:E20-E21. [DOI] [PubMed] [Google Scholar]
- 93. Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388:717-727. [DOI] [PubMed] [Google Scholar]
- 94. Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27:S21-S24. [DOI] [PubMed] [Google Scholar]
- 95. Ong S, Chapman J, Young G, Mansy T. Guillain-Barré-like syndrome during pembrolizumab treatment [published online ahead of print February 14, 2018]. Muscle Nerve. doi: 10.1002/mus.26101. [DOI] [PubMed] [Google Scholar]
- 96. Jacob A, Unnikrishnan DC, Mathew A, Thyagarajan B, Patel S. A case of fatal Guillain-Barre syndrome from anti-PD1 monoclonal antibody use. J Cancer Res Clin Oncol. 2016;142:1869-1870. [DOI] [PubMed] [Google Scholar]
- 97. Sejvar JJ, Kohl KS, Gidudu J, et al. Guillain-Barre syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29:599-612. [DOI] [PubMed] [Google Scholar]
- 98. Samadi M, Kazemi B, Golzari Oskoui S, Barzegar M. Assessment of autonomic dysfunction in childhood Guillain-Barré syndrome. J Cardiovasc Thorac Res. 2013;5:81-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barre syndrome and validation of Brighton criteria. Brain. 2014;137:33-43. [DOI] [PubMed] [Google Scholar]
- 100. Van der Meché FG, Van Doorn PA, Meulstee J, Jennekens FG, GBS-Consensus Group of the Dutch Neuromuscular Research Support Centre. Diagnostic and classification criteria for the Guillain-Barré syndrome. Eur Neurol. 2001;45:133-139. [DOI] [PubMed] [Google Scholar]
- 101. Hughes RA, Swan AV, Raphael JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barre syndrome: a systematic review. Brain. 2007;130:2245-2257. [DOI] [PubMed] [Google Scholar]
- 102. van Koningsveld R, Schmitz PI, Meché FG, Visser LH, Meulstee J, van Doorn PA. Effect of methylprednisolone when added to standard treatment with intravenous immunoglobulin for Guillain-Barré syndrome: randomised trial. Lancet. 2004;363:192-196. [DOI] [PubMed] [Google Scholar]
- 103. Hulsbrink R, Hashemolhosseini S. Lambert-Eaton myasthenic syndrome—diagnosis, pathogenesis and therapy. Clin Neurophysiol. 2014;125:2328-2336. [DOI] [PubMed] [Google Scholar]
- 104. Payne M, Bradbury P, Lang B, et al. Prospective study into the incidence of Lambert Eaton myasthenic syndrome in small cell lung cancer. J Thorac Oncol. 2010;5:34-38. [DOI] [PubMed] [Google Scholar]
- 105. O’Neill JH, Murray NM, Newsom-Davis J. The Lambert-Eaton myasthenic syndrome. A review of 50 cases. Brain. 1988;111:577-596. [DOI] [PubMed] [Google Scholar]
- 106. Manson G, Maria ATJ, Poizeau F, et al. Worsening and newly diagnosed paraneoplastic syndromes following anti-PD-1 or anti-PD-L1 immunotherapies, a descriptive study. J Immunother Cancer. 2019;7:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Titulaer MJ, Wirtz PW, Kuks JB, et al. The Lambert-Eaton myasthenic syndrome 1988-2008: a clinical picture in 97 patients. J Neuroimmunol. 2008;201-202:153-158. [DOI] [PubMed] [Google Scholar]
- 108. Sanders DB. Lambert-Eaton myasthenic syndrome: diagnosis and treatment. Ann N Y Acad Sci. 2003;998:500-508. [DOI] [PubMed] [Google Scholar]
- 109. Lennon VA, Kryzer TJ, Griesmann GE, et al. Calcium-channel antibodies in the Lambert-Eaton syndrome and other paraneoplastic syndromes. N Engl J Med. 1995;332:1467-1474. [DOI] [PubMed] [Google Scholar]
- 110. Titulaer MJ, Klooster R, Potman M, et al. SOX antibodies in small-cell lung cancer and Lambert-Eaton myasthenic syndrome: frequency and relation with survival. J Clin Oncol. 2009;27:4260-4267. [DOI] [PubMed] [Google Scholar]
- 111. Tarr TB, Wipf P, Meriney SD. Synaptic pathophysiology and treatment of Lambert-Eaton myasthenic syndrome. Mol Neurobiol. 2015;52:456-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Oh SJ, Hatanaka Y, Claussen GC, Sher E. Electrophysiological differences in seropositive and seronegative Lambert-Eaton myasthenic syndrome. Muscle Nerve. 2007;35:178-183. [DOI] [PubMed] [Google Scholar]
- 113. Odabasi Z, Demirci M, Kim DS, et al. Postexercise facilitation of reflexes is not common in Lambert-Eaton myasthenic syndrome. Neurology. 2002;59:1085-1087. [DOI] [PubMed] [Google Scholar]
- 114. Oh SJ, Kurokawa K, Claussen GC, Ryan HF., Jr. Electrophysiological diagnostic criteria of Lambert-Eaton myasthenic syndrome. Muscle Nerve. 2005;32:515-520. [DOI] [PubMed] [Google Scholar]
- 115. Keogh M, Sedehizadeh S, Maddison P. Treatment for Lambert-Eaton myasthenic syndrome. Cochrane Database Syst Rev. 2011;2011:Cd003279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Maddison P, Lang B, Mills K, Newsom-Davis J. Long term outcome in Lambert-Eaton myasthenic syndrome without lung cancer. J Neurol Neurosurg Psychiatry. 2001;70:212-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lang B, Newsom-Davis J, Wray D, Vincent A, Murray N. Autoimmune aetiology for myasthenic (Eaton-Lambert) syndrome. Lancet. 1981;2:224-226. [DOI] [PubMed] [Google Scholar]
- 118. Tim RW, Massey JM, Sanders DB. Lambert-Eaton myasthenic syndrome: electrodiagnostic findings and response to treatment. Neurol. 2000;54:2176-2178. [DOI] [PubMed] [Google Scholar]
- 119. Tran TT, Jilaveanu LB, Omuro A, Chiang VL, Huttner A, Kluger HM. Complications associated with immunotherapy for brain metastases. Curr Opin Neurol. 2019;32:907-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kluger HM, Chiang V, Mahajan A, et al. Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. J Clin Oncol. 2019;37:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tully KH, Cone EB, Cole AP, et al. Risk of immune-related adverse events in melanoma patients with preexisting autoimmune disease treated with immune checkpoint inhibitors: a population-based study using SEER-Medicare data. Am J Clin Oncol. 2021;44:413-418. [DOI] [PubMed] [Google Scholar]
- 123. Yamaguchi A, Saito Y, Okamoto K, et al. Preexisting autoimmune disease is a risk factor for immune-related adverse events: a meta-analysis [published online ahead of print June 23, 2021]. Support Care Cancer. doi: 10.1007/s00520-021-06359-7. [DOI] [PubMed] [Google Scholar]
- 124. Suzuki S, Utsugisawa K, Yoshikawa H, et al. Autoimmune targets of heart and skeletal muscles in myasthenia gravis. Arch Neurol. 2009;66:1334-1338. [DOI] [PubMed] [Google Scholar]
- 125. Suzuki S, Ishikawa N, Konoeda F, et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology. 2017;89:1127-1134. [DOI] [PubMed] [Google Scholar]
- 126. Garcia CA, El-Ali A, Rath TJ, et al. Neurologic immune-related adverse events associated with adjuvant ipilimumab: report of two cases. J Immunother Cancer. 2018;6:83. [DOI] [PMC free article] [PubMed] [Google Scholar]