Abstract
Objective
Determine the utility of aquaporin 4 IgG (AQP4-IgG) testing (live cell-based assay) for Neuromyelitis Optica Spectrum Disorders (NMOSD).
Methods
We included Mayo Clinic patients (1/1/2018-12/31/2019) tested for serum AQP4-IgG by live cell-based flow-cytometric assay. Medical records were reviewed to assess if patients fulfilled 2015 NMOSD criteria.
Results
Of 1371 patients tested, 41 were positive (3%) and all fulfilled NMOSD criteria with AQP4-IgG (specificity = 100%). Only 10/1330 testing negative met NMOSD criteria without AQP4-IgG (sensitivity = 80%) and seven of these 10 were MOG-IgG positive.
Conclusions
AQP4-IgG by live cell-based assay was highly specific and without false positives in a high throughput setting.
Introduction
Neuromyelitis Optica Spectrum Disorder (NMOSD) is an inflammatory CNS demyelinating disease, associated with aquaporin-4 immunoglobulin-G antibodies (AQP4-IgG). We previously showed AQP4-IgG live cell-based assay (M1-isoform) had 83% sensitivity and 100% specificity for NMO diagnosis using older 2006 criteria and similar results (69.7–100% sensitive; 90.6–100% specific) from other centers are reported with the 2006 and 2015 criteria.1–4 In a clinical setting with high testing volumes, the risk of false positivity for diagnostic biomarkers can increase, particularly when ordered in low probability situations. 5 Our aim was to assess the sensitivity, specificity, likelihood ratios (LHR) and frequency of false positives with AQP4-IgG live cell-based assay using updated 2015 NMOSD diagnostic criteria 6 in a high throughput clinical setting at a tertiary referral center.
Methods
Standard protocol approvals, registrations, and patient consents
The study was approved by the Mayo Clinic Institutional Review Board (IRB#: 08-006647). Patients consented to use of their medical records for research purposes.
Data collection
This retrospective observational study involved 1371 consecutive Mayo Clinic patients evaluated for serum AQP4-IgG during routine clinical care (1/1/2018–12/31/2019). Although the test is mostly ordered by neurologists, requests for testing by any physician at any of the three Mayo Clinic sites (Jacksonville[FL], Rochester[MN], Scottsdale[AZ]) were included. Electronic medical records and MRI's were available in all patients and reviewed to determine age at testing, sex, ethnicity, clinical and radiologic phenotypes to determine if they fulfilled 2015 criteria for NMOSD. 6 If AQP4-IgG positive patients did not fulfill 2015 NMOSD criteria either by lacking core clinical characteristics or having an alternative diagnosis, they were designated false positives.
Antibody testing
AQP4-IgG testing was performed with an in-house live cell-based flow-cytometric/fluorescence-activated-cell-sorting (FACS) assay using HEK293 cells transfected with human AQP4 M1-isoform as previously described. 1 Samples were screened at 1:5 dilution. If the IgG-binding-index (IBI: Ratio of median-fluorescence-intensities of AQP4 transfected to non-transfected cells) was ≥2.0, they were retested and titrated from 1:10 dilution, in ten-fold steps, to establish end-point titers (i.e. last dilution with IBI ≥2.0; reference value <1:5). Myelin oligodendrocyte glycoprotein immunoglobulin-G (MOG-IgG) live cell-based assay was tested in 29/41 AQP4-IgG seropositives and all ten NMOSD without AQP4-IgG, using previously described methodology. 5
Data analysis
The sensitivity (true positives/true positives plus false negatives), specificity (true negatives/true negatives plus false positives) and negative likelihood ratio (1-sensitivity/specificity) for NMOSD diagnosis were calculated along with 95% confidence intervals (CI) using ‘R’ version 4.1.
Data availability statement
Anonymized data used for this study are available upon request from authors.
Results
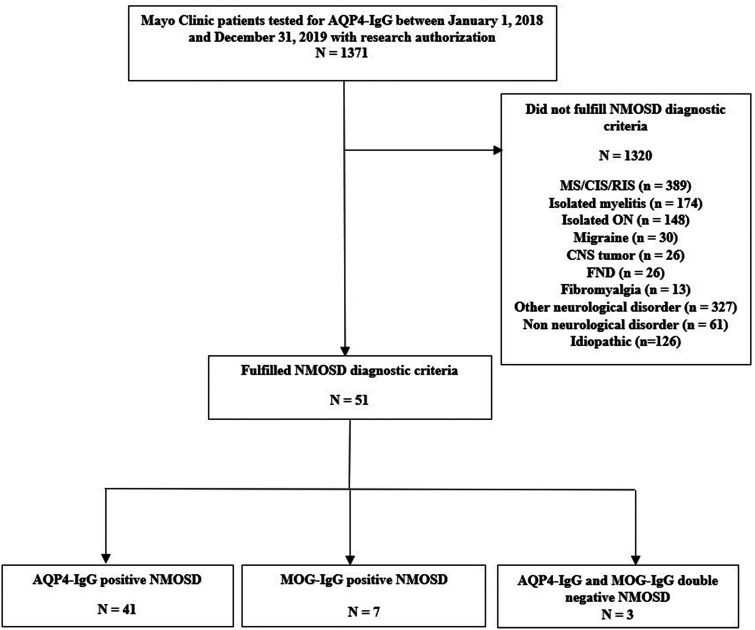
Of the 1371 patients tested for AQP4-IgG, 41 were positive (3%) (median titer, 1000 [range, 5–100,000]) (Figure 1). Demographics are summarized in Table 1.
Table 1.
Demographics and results.
| Characteristic | AQP4-IgG positive NMOSD (True positives) | AQP4-IgG negative without NMOSD (True negatives) | AQP4-IgG negative NMOSD, MOG-IgG positive (MOGAD) † | AQP4-IgG negative NMOSD, MOG IgG negative | Total |
|---|---|---|---|---|---|
| N | 41 * | 1320 | 7 | 3 | 1371 |
| Age at testing (years) | |||||
| Median (range) | 57 (4-90) | 46 (0–88) | 15 (8–28) | 20 (15–61) | 46 (0–90) |
| <18 years | 2 (5%) | 25 (2%) | 4 (57%) | 1 (33%) | 32 (2%) |
| >18 years | 39 (95%) | 1295 (98%) | 3 (43%) | 2 (67%) | 1339 (98%) |
| Sex | |||||
| Female | 34 (83%) | 829 (63%) | 4 (57%) | 1 (33%) | 868 (63%) |
| Male | 7 (17%) | 491 (37%) | 3 (43%) | 2 (66%) | 503 (37%) |
| Race/ethnicity | |||||
| White Caucasian | 19 (46%) | 1081 (82%) | 5 (71%) | 3 (100%) | 1108 (81%) |
| Black | 14 (34%) | 74 (6%) | 1 (14%) | 0 | 89 (7%) |
| Latin American | 2 (5%) | 67 (5%) | 0 | 0 | 69 (5%) |
| Asian | 4 (10%) | 26 (2%) | 0 | 0 | 30 (2%) |
| Other ‡ | 2 (5%) | 31 (2%) | 0 | 0 | 33 (2%) |
| Chose not to disclose | 0 | 41 (3%) | 1 (14%) | 0 | 42 (3%) |
one case was paraneoplastic.
There were no cases of AQP4/MOG-IgG dual positivity.
Includes Native American, Pacific islander, Arab.
Abbreviations: AQP4-IgG, Aquaporin-4-IgG; MOG-IgG, Myelin Oligodendrocyte Glycoprotein-IgG; MOGAD, Myelin Oligodendrocyte Glycoprotein antibody associated disorder; NMOSD, Neuromyelitis optica spectrum disorder.
Figure 1.
Flow chart of patients tested for AQP4-IgG. AQP4-IgG, aquaporin-4-IgG; CIS, clinically isolated syndrome; CNS, central nervous system; FND, functional neurologic disorder; MOG-IgG, myelin oligodendrocyte glycoprotein-IgG; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder; ON, optic neuritis; RIS, radiologically isolated syndrome.
All 41 AQP4-IgG positive patients fulfilled contemporary criteria for NMOSD with AQP4-IgG and their core clinical characteristics are summarized in Table 2. No false positives were identified, and AQP4-IgG specificity was 100% (95% CI, 99.7–100%). Positive LHR not calculable as specificity was 100%. Of these 41 patients, 28 (68%) were receiving immunosuppressants (steroids, azathioprine, mycophenolate or rituximab) at AQP4-IgG testing.
Table 2.
Core clinical characteristics of seropositive and seronegative NMOSD.
| Clinical characteristics | AQP4-IgG positive NMOSD (n = 41) | MOG-IgG positive NMOSD (n = 7) | AQP4 and MOG-IgG negative NMOSD (n = 3) |
|---|---|---|---|
| LETM | 28 (68%) | 6 (86%) | 3 (100%) |
| Optic Neuritis | 14 (34%) | 6 (86%) | 2 (67%) |
| Area Postrema syndrome | 8 (20%) | 0 | 0 |
| Other Brainstem syndrome | 0 | 1 (14%) | 1 (33%) |
| Symptomatic cerebral syndrome | 1 (2%) | 4 (57%) | 0 |
| Symptomatic narcolepsy | 0 | 0 | 0 |
Abbreviations: AQP4-IgG, Aquaporin-4-IgG; LETM, Longitudinally extensive transverse myelitis; MOG-IgG, myelin oligodendrocyte glycoprotein-IgG; NMOSD, Neuromyelitis optica spectrum disorder.
Of the 1330 AQP4-IgG seronegative patients, ten fulfilled clinical diagnostic criteria for NMOSD without AQP4-IgG and are summarized in Table 1. Thus, AQP4-IgG sensitivity for NMOSD diagnosis was 80.4% (95% CI, 66.7–90.2%) and negative likelihood ratio was 0.2 (95% CI, 0.11–0.34). Seven of those ten cases were MOG-IgG positive (median titer, 1:100 [range, 1:20–1:1000]), and three were negative for both AQP4-IgG and MOG-IgG. Three of the ten patients (30%) were receiving immunosuppressants at antibody testing (One MOG-IgG1 positive patient and two dual seronegative patients).
The clinical phenotype of all 7 MOG-IgG positives was consistent with that defined for MOG-IgG associated disease (MOGAD) 5 and no false positives or dual AQP4-IgG and MOG-IgG positives were encountered. Overall, 1072 of 1371 patients were tested for MOG-IgG and 64 were positive including the 10 that fulfilled criteria for NMOSD without AQP4-IgG.
Discussion
Our data show that AQP4-IgG seropositivity has high sensitivity and extremely high specificity for NMOSD diagnosis in a real-world clinical setting, consistent with our prior study using 2006 NMO criteria. 1 The lack of false positive results, despite testing large numbers attests to the value of live cell-based AQP4-IgG assays as a diagnostic biomarker for NMOSD. Although testing of even larger patient populations may be anticipated to yield occasional false positives, these data indicate false positives are rare.
Recently, we showed MOG-IgG was highly specific (97.8%) for MOGAD diagnosis, but the overall positive predictive value of seropositivity was 72%, with particular risk of false positives at low titer when tested in low pre-test probability situations. 5 Thus, more generous ordering of AQP4-IgG by cell-based assay can be considered compared to MOG-IgG which requires more judicious test ordering and result interpretation.
MOG-IgG accounted for 70% of AQP4-IgG seronegative NMOSD, higher than the 41.6% reported previously, 7 but is limited by smaller number of NMOSD patients analyzed. The demographics and clinical features differed for AQP4-IgG and MOG-IgG positive patients justifying separate MOGAD diagnostic criteria, particularly as only 23% of adult MOGAD fulfill criteria for NMOSD without AQP4-IgG. 8
It is worth noting that the percentage of patients seen at the Mayo Clinic during the two year period (January 2018 - December 2019) who met the 2015 NMOSD criteria (3.7%; 51/1371), is similar to that observed during a previous six year study at the Mayo Clinic (October 2005 - November 2011) in which patients met either the 1999 or 2006 criteria for NMOSD (3%; 164/5349). 9 This demonstrates that despite the changes in criteria the percentage of seropositivity has remained quite stable (85% vs 80%, respectively).
Our study had limitations. CSF was not assessed but serum is optimal for AQP4-IgG detection. 10 AQP4-IgG testing was not processed in batch but rather individually as samples arrived and some samples were not tested for MOG-IgG. Some patients were receiving immunotherapy at the time of testing but most AQP4-IgG seronegative NMOSD were MOG-IgG positive making it unlikely this majorly impacted our results. Referral bias is possible and studies in a community setting or other centers are needed, nevertheless, the characteristics of AQP4-IgG positives in our study fit well with typical AQP4-NMOSD. 6
Supplemental Material
Supplemental material, sj-docx-1-mso-10.1177_20552173211052656 for Diagnostic value of aquaporin-4-IgG live cell based assay in neuromyelitis optica spectrum disorders by Vyanka Redenbaugh, Mayra Montalvo, Elia Sechi, Marina Buciuc, James P. Fryer, Andrew McKeon, Vanda A. Lennon, John R. Mills, Brian G. Weinshenker, Dean M. Wingerchuk, John J. Chen, M. Tariq Bhatti, A. Sebastian Lopez Chiriboga, Sean J. Pittock and Eoin P. Flanagan in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Vyanka Redenbaugh – reports no financial disclosures, Mayra Montalvo – reports no financial disclosures, Elia Sechi – reports no financial disclosures, Marina Buciuc – reports no financial disclosures, James P. Fryer – reports no financial disclosures, Andrew McKeon – Dr McKeon received research funding from Alexion, Grifols, and MedImmune and has patents pending for the following IgGs as biomarkers of autoimmune neurological disorders: Septin-5, Septin-7, Kelch-like protein 11, GFAP, PDE10A, and MAP1B. Dr McKeon also reported grants from EUROIMMUN and Grifols outside the submittedVanda A. Lennon – Dr. Lennon receives royalties from RSR/Kronus and other providers of diagnostic testing for AQP4-IgG performed outside of Mayo Clinic and from licensing of AQP4-IgG monoclonal antibodies. John R. Mills – Dr. Mills holds patents on the use of mass spectrometry to measure monoclonal immunoglobulins and has received royalties related to these patents from The Binding Site.Brian G. Weinshenker – Dr Weinshenker reported personal fees from Alexion and Viela Bio for serving on attack adjudication committees for clinical trials in neuromyelitis optica; consulting fees from Chugai, Genentech, and Mitsubishi Tanabe regarding clinical trials for neuromyelitis optica; and consulting and speaking fees from Roche regarding clinical trials for neuromyelitis optica, outside the submitted work. In addition, Dr Weinshenker has a patent for neuromyelitis optica–IgG for a diagnostic test of neuromyelitis optica and associated conditions with royalties paid from RSR Ltd, Oxford University, Hospices Civil de Lyon, and MVZ Labor PD Dr Volkmann und Kollegen GbR. Dr Tobin has received research funding from Mallinckrodt Inc, the Mayo Clinic Center for Multiple Sclerosis and Autoimmune Neurology, and the National Institutes of Health (grant 1R01NS113803-01A1) outside the submitted work.Dean M. Wingerchuck – Dr. Wingerchuk has received research support paid to Mayo Clinic from Alexion and TerumoBCT. He serves on medical advisory boards for MedImmune, Horizon, Novartis, Biogen, Celgene, Genentech, TG Therapeutics, Reistone, and Mitsubishi TanabeJohn J. Chen – consultant to Roche and UCBM. Tariq Bhatti – reports no financial disclosures, A. Sebastian Lopez Chiriboga – reports no financial disclosures, Sean J. Pittock – : Dr Pittock reports receiving grants, personal fees paid to Mayo Clinic, and nonfinancial support from Alexion Pharmaceuticals Inc and MedImmune Inc/Viela Bio; receiving personal fees from Genentech/Roche, UCB, and Astellas, outside the submitted work; holding patent 8,889,102 (application 12-678350) issued and patent 9,891,219B2 (application 12-573942) issued; and serving as a director of the Neuroimmunology Laboratory at Mayo Clinic. He receives no royalties from the sale of myelin oligodendrocyte glycoprotein–IgG1 testing at the Neuroimmunology Laboratory; however, Mayo Clinic Laboratories does receive revenue for conducting such tests. Eoin P. Flanagan - Dr Flanagan has served on advisory boards for Alexion, Genentech and Horizon Therapeutics. He has received speaker honoraria from Pharmacy Times. He received royalties from UpToDate. Dr Flanagan was a site primary investigator in a randomized clinical trial on Inebilizumab in neuromyelitis optica spectrum disorder run by Medimmune/Viela-Bio/Horizon Therapeutics. Dr Flanagan has received funding from the NIH (R01NS113828). Dr Flanagan is a member of the medical advisory board of the MOG project. Dr Flanagan is an editorial board member of the Journal of the Neurological Sciences and Neuroimmunology Reports.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iDs: Vyanka Redenbaugh https://orcid.org/0000-0001-6400-7760
Elia Sechi https://orcid.org/0000-0003-4698-663X
A. Sebastian Lopez Chiriboga https://orcid.org/0000-0001-5653-5600
Eoin P. Flanagan https://orcid.org/0000-0002-6661-2910
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Vyanka Redenbaugh, Departments of Neurology, Mayo Clinic College of Medicine, Rochester, MN, USA.
Mayra Montalvo, Departments of Neurology, Mayo Clinic College of Medicine, Rochester, MN, USA.
Elia Sechi, Departments of Neurology, Mayo Clinic College of Medicine, Rochester, MN, USA; Department of Medical, Surgical and Experimental Sciences, University of Sassari, Sassari, Italy.
Marina Buciuc, Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Rochester, MN, USA.
James P. Fryer, Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Rochester, MN, USA
Andrew McKeon, Departments of Neurology, Mayo Clinic College of Medicine, Rochester, MN, USA; Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Rochester, MN, USA.
Vanda A. Lennon, Departments of Neurology, Mayo Clinic College of Medicine, Rochester, MN, USA Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Rochester, MN, USA; Immunology, Mayo Clinic College of Medicine, Rochester, MN, USA.
John R. Mills, Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Rochester, MN, USA
Brian G. Weinshenker, Departments of Neurology, Mayo Clinic College of Medicine, Rochester, MN, USA
Dean M. Wingerchuk, Department of Neurology, Mayo Clinic College of Medicine, Scottsdale, AZ, USA
John J. Chen, Departments of Neurology, Mayo Clinic College of Medicine, Rochester, MN, USA Ophthalmology, Mayo Clinic College of Medicine, Rochester, MN, USA.
M. Tariq Bhatti, Departments of Neurology, Mayo Clinic College of Medicine, Rochester, MN, USA Ophthalmology, Mayo Clinic College of Medicine, Rochester, MN, USA.
A. Sebastian Lopez Chiriboga, Department of Neurology, Mayo Clinic College of Medicine, Jacksonville, FL, USA.
Sean J. Pittock, Departments of Neurology, Mayo Clinic College of Medicine, Rochester, MN, USA Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Rochester, MN, USA.
Eoin P. Flanagan, Departments of Neurology, Mayo Clinic College of Medicine, Rochester, MN, USA; Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Rochester, MN, USA.
References
- 1.Fryer J, Lennon V, Pittock S, et al. AQP4 Autoantibody assay performance in clinical laboratory service. Neurology-Neuroimmunology Neuroinflammation 2014; 1(1):e11. DOI: 10.1212/NXI.0000000000000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waters P, Reindl M, Saiz A, et al. Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica. J Neurol Neurosurg Psychiatry 2016; 87: 1005–1015. 2016/04/27. DOI: 10.1136/jnnp-2015-312601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prain K, Woodhall M, Vincent A, et al. AQP4 Antibody assay sensitivity comparison in the Era of the 2015 diagnostic criteria for NMOSD. Front Neurol 2019; 10: 1028. 2019/10/23. DOI: 10.3389/fneur.2019.01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarius S, Wildemann B. Aquaporin-4 antibodies (NMO-IgG) as a serological marker of neuromyelitis optica: a critical review of the literature. Brain Pathol 2013; 23: 661–683. 2013/10/15. DOI: 10.1111/bpa.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sechi E, Buciuc M, Pittock SJ, et al. Positive Predictive Value of Myelin Oligodendrocyte Glycoprotein Autoantibody Testing. JAMA Neurol 2021;78(6):741-746. DOI: 10.1001/jamaneurol.2021.0912. [DOI] [PMC free article] [PubMed]
- 6.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–189. DOI: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Zhang C, Jia D, et al. The occurrence of myelin oligodendrocyte glycoprotein antibodies in aquaporin-4-antibody seronegative neuromyelitis Optica Spectrum disorder: a systematic review and meta-analysis. Mult Scler Relat Disord 2021; 53: 103030. 2021/06/13. DOI: 10.1016/j.msard.2021.103030. [DOI] [PubMed] [Google Scholar]
- 8.Kunchok A, Chen JJ, Saadeh RS, et al. Application of 2015 seronegative neuromyelitis Optica Spectrum disorder diagnostic criteria for patients With myelin oligodendrocyte glycoprotein IgG–associated disorders. JAMA Neurol 2020; 77: 1572–1575. DOI: 10.1001/jamaneurol.2020.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiao Y, Fryer JP, Lennon VA, et al. Updated estimate of AQP4-IgG serostatus and disability outcome in neuromyelitis optica. Neurology 2013; 81: 1197–1204. 2013/09/03. DOI: 10.1212/WNL.0b013e3182a6cb5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majed M, Fryer JP, McKeon A, et al. Clinical utility of testing AQP4-IgG in CSF: guidance for physicians. Neurology-Neuroimmunology Neuroinflammation 2016; 33(3):e231. DOI: 10.1212/NXI.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mso-10.1177_20552173211052656 for Diagnostic value of aquaporin-4-IgG live cell based assay in neuromyelitis optica spectrum disorders by Vyanka Redenbaugh, Mayra Montalvo, Elia Sechi, Marina Buciuc, James P. Fryer, Andrew McKeon, Vanda A. Lennon, John R. Mills, Brian G. Weinshenker, Dean M. Wingerchuk, John J. Chen, M. Tariq Bhatti, A. Sebastian Lopez Chiriboga, Sean J. Pittock and Eoin P. Flanagan in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Data Availability Statement
Anonymized data used for this study are available upon request from authors.