Abstract
Background
To date, there are no data available on the safety of COVID-19 vaccines in Latin American patients with Multiple Sclerosis (MS).
Objective
Characterize safety of COVID-19 vaccines in Latin American (LATAM) patients with Multiple Sclerosis (pwMS).
Methods
A cross-sectional study between February 1, 2021, and April 30, 2021. Individuals with MS from LATAM countries were invited to participate in a self-administered web-based survey, through MS patient organizations from the region.
Results
393 vaccinated pwMS from 10 different Latin American countries were included. The vaccines administered were: inactivated virus vaccines (IVV) in 38.2% of patients, adenovirus vector vaccines (AdV) in 48.8% and mRNA vaccines 13%. All patients received at least one dose of any of the COVID-19 vaccines and 123 (31.3%) declared receiving a second dose. Mean (SD) age 41.5 (11.8) years, 82.4% female, MS disease duration: 8.4 (8.2) years. No serious adverse events were reported with any of the COVID-19 vaccines after either the first or second dose. A lower frequency of adverse events was found with IVV (22%) in comparison with AdV (46.4%) and mRNA (35.3%) (p < 0.01). Five participants reported having an MS relapse after IVV first dose.
Conclusion
COVID-19 vaccines applied in LATAM proved safe for MS patients.
Keywords: Multiple sclerosis, COVID-19, safety, vaccines
Introduction
On March 11, 2020, the World Health Organization (WHO) declared the novel coronavirus (COVID-19) outbreak a global pandemic. 1 One year later, over 21 million cases (out of 115 million in the world, 18.8%) have been reported in the Latin American and Caribbean region, led by Brazil (10.6 million). Managing multiple sclerosis (MS) during the COVID-19 pandemic poses an exceptional challenge on healthcare providers, patients and caregivers longing for specific counseling. 2 The COVID-19 pandemic has certainly interfered with the routine care of patients with MS (pwMS) from Latin America Affecting access to treatments, rehabilitation services and laboratory and resonance monitoring. 3 In addition, the RELACOEM study, the largest registry from the region, recently reported that the overall mortality of their cohort was higher than that of the general population. 4
Ending 2020, multiple Latin American countries started their vaccination plans using different COVID-19 vaccines. In South America, more than 14.2 million doses have been applied, (over 9 million in Brazil). 2 Given that both autoimmune diseases and immunotherapies are potentially problematic regarding vaccination, many pwMS and their physicians face an ongoing dilemma on whether to vaccinate or not. 5 However, epidemiological studies have repeatedly demonstrated the safety of the vast majority of vaccines. 6 Since COVID-19 vaccines have become available, many questions have emerged. Although it is highly recommended that pwMS be vaccinated, it is uncertain if vaccination can produce specific adverse effects to this population or whether immune mechanisms may be altered, leading to the activation of the disease. Currently, the available information comes from the Israeli cohort with mRNA vaccine (Pfizer/Biontech), but in Latin America, there are also other types of vaccines being used with scarce information within the pwMS community. 6 Therefore, our objective was to evaluate the safety of COVID-19 vaccines and the occurrence of relapses after vaccination in Latin American pwMS.
Methods
Patients
We performed a cross-sectional study between February 1, 2021, and April 30, 2021. Individuals with MS from Latin American countries were invited to participate in an anonymous, voluntary, self-administered web-based survey. The organizer research center Esclerosis Multiple Argentina (EMA, an MS patient organization from Argentina) invited other MS patient organizations from the region to participate. Each organization subsequently contacted their affiliates and forwarded them the link to the survey. In order to participate, pwMS had to be ≥18 years old, be registered in an MS patient organization and be vaccinated with at least one dose of any of the available vaccines against COVID-19 in their country. During the study period, 393 pwMS completed the survey. The institutional review board of each participating organization approved the study protocols. All patients provided electronic informed consent.
Questionnaire
The questionnaire was developed to focus on: (a) demographic characteristics of vaccinated MS patients; (b) history of previous vaccinations; (c) type of COVID-19 vaccine administered; and (d) COVID-19 adverse events. This survey, collected in the Spanish language, was designed by three MS specialists (RA, FL and ACh) and reviewed by a panel composed of two neurologists (BS and AC), a neuropsychologist (BE) and a representative of a patient organization (JB). A pilot study administered to 17 MS patients was carried out prior to the final survey. Following review, no questions were canceled or added at this stage; only minor changes were made to improve Spanish grammatical expressions. To provide a valid and comprehensive assessment of MS-related disability we used the Patient Determined Disease Steps (PDDS). This is a simple, efficient and valid patient-reported scale that ranges nine ordinal levels from 0 (normal) to 8 (bedridden) and can be converted into EDSS scores. 7 To record the type of vaccine administered, the questionnaire contained a drop-down list with all available vaccines in Latin America. The brand, along with the vaccine's country of origin, was indicated. In addition, a free text option was provided for patients to fill in if they were unable to identify the vaccine. Safety information was recorded after each vaccination dose. The pre-specified list of adverse events comprised the following: (1) pain at the injection site; (2) fever; (3) muscle and/or joint pain; (4) flu-like symptoms; (5) fatigue and/or general weakness; (6) headache; (7) gastrointestinal symptoms (nausea, vomiting or diarrhea); (8) post-vaccination COVID-19 infection; and (9) acute MS relapse. In addition, patients had a free text option to report any other possible adverse event. The final questionnaire is available as supplementary material.
Statistical analysis
Descriptive analyses were carried out. Results are presented as frequencies, percentages, mean and standard deviation (SD) values. Comparisons between patients with and without adverse events after vaccination were performed using Chi-square or Fisher's exact tests for categorical variables and t-test or Mann–Whitney U test for continuous variables, when appropriate. For all analyses, p-values < 0.05 were considered statistically significant. Data analysis was conducted using SPSS Statistics® v22.
Results
Patient population
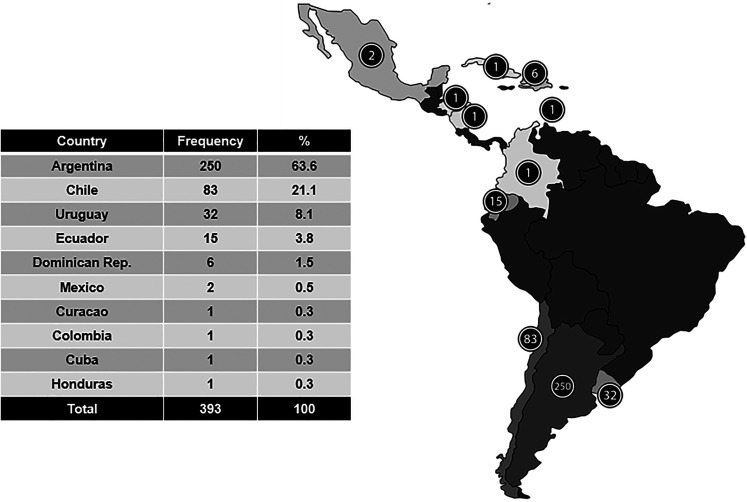
Between February 1, 2021, and April 30, 2021, a total of 393 vaccinated pwMS from 10 different Latin American countries responded to the survey (Figure 1). The mean (SD) age of the participants was 41.5 (11.8) years, 82.4% were female and the MS disease duration was 8.4 (8.2) years. Three hundred and forty-six patients (88%) reported use of disease-modifying therapy (DMT), and 27 (6.9%) claimed to have temporarily stopped the treatment to get COVID-19 vaccine. Additionally, 28 (7.1%) pwMS declared postponing the initiation of a DMT to get vaccinated. The most frequently postponed DMT was ocrelizumab in 10 out of 28 participants. Complete baseline characteristics of the included patients are shown in Table 1. Regarding previous vaccination history, 43% reported not receiving an annual flu vaccine and 61.3% declared to have never received immunization for pneumonia. The main reason reported for not getting the flu or pneumonia vaccine was the lack of recommendation by the patients’ neurologist in 45% and 53.5%, respectively.
Figure 1.
Participation in the survey by country of origin.
Table 1.
Demographic and clinical characteristics of the survey participants (n = 393).
| Variable | n (%) |
|---|---|
| Age, mean (SD), years | 41.9 (11.8) |
| Female | 324 (82.4) |
| Health insurance | |
| Public | 98 (24.9) |
| Private | 295 (75.1) |
| Employment status | |
| Employed | 271 (68.6) |
| Unemployed or not in the workforce | 122 (30.9) |
| (Retired, pensioner, student, other) | |
| MS duration, mean (SD), years | 8.5 (8.2) |
| Disease course | |
| RRMS | 310 (78.9) |
| SPMS | 12 (3.1) |
| PPMS | 28 (7.1) |
| Unknown to participant | 43 (10.9) |
| PDDS, median (IQR) | 2 (4) |
| Currently on a DMT | 346 (88.0) |
| Interferons | 65 (16.5) |
| Glatiramer acetate | 25 (6.4) |
| Teriflunomide | 32 (8.1) |
| Dimethyl fumarate | 32 (8.1) |
| Natalizumab | 18 (4.6) |
| Fingolimod | 95 (24.2) |
| Ocrelizumab | 29 (7.4) |
| Rituximab | 7 (1.8) |
| Cladribine | 20 (5.1) |
| Alemtuzumab | 7 (1.8) |
| Other | 16 (4.1) |
| None | 47 (12.0) |
| Confirmed previous COVID-19 infection | 33 (8.4) |
| Vaccine received | |
| mRNA | 51 (12.9) |
| Pfizer/BioNTech | 49 (12.5) |
| Moderna | 2 (0.5) |
| Inactivated virus | 150 (38.0) |
| CoronaVac/Sinovac | 80 (20.4) |
| Sinopharm | 70 (17.8) |
| Adenovirus | 192 (48.6) |
| Sputnik V | 116 (29.5) |
| Covishield | 33 (8.4) |
| Oxford AztraZeneca | 42 (10.6) |
| Janssen - Johnson & Johnson | 1 (0.3) |
DMT: disease-modifying therapy; Other: corticosteroids; PDDS: patient determined disease steps; PPMS: primary progressive multiple sclerosis; RRMS: relapsing-remitting multiple sclerosis; SPMS: secondary progressive multiple sclerosis.
COVID-19 vaccine administered
All included patients received at least one dose of any of the COVID-19 vaccines and 123 (31.3%) declared receiving a second dose. The different vaccines administered were: inactivated virus vaccines [(IVV); CoronaVac; BBIBP-CorV)] in 150 (38.2%) patients, adenovirus vector vaccines [(AdV); Gam-COVID-Vac; AZD1222; ChAdOx1nCoV-19; Ad26.COV2.S] in 192 (48.8%) and mRNA vaccines (BNT162b2; ARNm-1273) in 51 (13%). The mean time from vaccination to survey completion was 22.2 ± 23.5 days after the first dose and 16.5 ± 19.2 for those receiving a second dose. The reported reasons behind receiving only one vaccine dose were: insufficient time had elapsed to receive the second dose in 57.3% of cases; lack of appointments for second doses (recommended times were exceeded) in 8.1%; and second dose discouraged in 1 (0.3%) participant due to first-dose reported side effects.
Safety
No serious adverse events were reported with any of the COVID-19 vaccines after either the first or second dose. The safety profile of all COVID-19 vaccines in our MS patient population was characterized by short-term, transient, mild-to-moderate adverse events that did not require hospitalization. Presentation of any adverse event was 35.6% and 23.6% after the first and second doses, respectively. These included pain at the injection site, headache, fever, flu-like symptoms, fatigue and muscle or joint pain. A lower frequency of adverse events was found with IVV (22%) in comparison with AdV (46.4%) and mRNA (35.3%) (p < 0.01). Side effect frequency for each COVID-19 vaccine is shown in Table 2.
Table 2.
Adverse events reported for each COVID-19 vaccine.
| Variable | IVV | AdV | mRNA | p |
|---|---|---|---|---|
| Study population, n | 150 | 192 | 51 | – |
| Any adverse events, n (%) | 33 (22) | 89 (46.4) | 18 (35.3) | <0.01 |
| Pain at the injection site, n (%) | 15 (10) | 58 (30.2) | 13 (25.5) | <0.01 |
| Headache, n (%) | 24 (16) | 47 (24.5) | 12 (23.5) | 0.15 |
| Fever/chills, flu-like symptoms, n (%) | 2 (1.3) | 30 (15.6) | 6 (11.8) | <0.01 |
| Nausea and vomiting, n (%) | 1 (0.7) | 13 (6.7) | 1 (2) | 0.01 |
| Fatigue and/or general weakness, n (%) | 3 (2) | 17 (8.8) | 3 (6.1) | 0.03 |
| Diarrhea, n (%) | 2 (1.3) | 5 (2.69) | 2 (4.1) | 0.5 |
| Myalgia, n (%) | 17 (11.3) | 55 (28.6) | 10 (19.6) | <0.01 |
Ten pwMS (2.5%) were infected by SARS-CoV-2 between the first and second vaccine dose, all pwMS had mild or no symptomatology. All referred to testing by nasopharyngeal swab. No SARS-CoV-2 infections were reported after the second vaccine dose.
Five (1.3%) participants reported having an MS relapse, all occurring after first dose of IVV. Mean time to relapse: 14.8 ± 4.2 days and were associated with the flu-like symptomatology in all cases. None of these patients had stopped or postponed their MS treatment before vaccination (Table 3).
Table 3.
Demographic and clinical characteristics of the patients with relapse (n = 5).
| Gender/age | MS phenotype | MS duration (years) | PDDS | Current DMT | Vaccine received | Type of relapse | Time to relapse |
|---|---|---|---|---|---|---|---|
| F 35 | RRMS | 0.5 | 2 | Dimethyl fumarate | Sputnik V | Optic neuritis | 25 |
| F 46 | RRMS | 1.2 | 1 | Glatiramer acetate | Pfizer/BioNTech | Weakness | 13 |
| F 28 | RRMS | 5 | 2 | Ocrelizumab | Pfizer/BioNTech | Weakness | 12 |
| F 34 | RRMS | 3 | 0 | Interferon | CoronaVac/Sinovac | Diplopia | 15 |
| F 29 | RRMS | 2.5 | 1 | No treatment | Covishield | Vertigo and numbness | 9 |
DMT: disease-modifying therapy; F: female; PDDS: patient determined disease steps; RRMS: relapsing-remitting multiple sclerosis.
Discussion
In general, most vaccines are considered to be safe for MS patients. In this study, we evaluated through a survey the safety of the vaccination against COVID-19 in people with MS from Latin America. This work included patients who received vaccines from different platforms: inactivated virus, adenovirus vectors and mRNA vaccines. Our findings showed that pwMS presented similar rates of adverse events to those reported for the general and MS population, regardless of the vaccine type. 6 None of the COVID-19 vaccines analyzed in this study were associated with serious adverse events after either the first or second dose. Furthermore, we did not find an increased incidence of post-vaccination demyelinating events in our population. We believe that our study augments the scarce available literature on the safety of COVID-19 vaccines. For instance, at the moment, there is a scarce report of COVID-19 vaccines in pwMS involved exclusively mRNA vaccines.6,8
In our study, the largest number of people received adenovirus-based vaccines: Gam-COVID-Vac, ChAdOx1 and Ad26.COV2.S. These adenovirus-based vaccines showed the highest frequency of adverse events compared to IVV and mRNA vaccines. However, all adverse events reported were considered mild. The efficacy and safety of Gam-COVID-Vac was addressed in two open, non-randomized phase 1/2 studies at two hospitals in Russia. 9 In this trial, the most common adverse event was pain at the injection site (58%). Accordingly, in our study, we found a similar rate of adverse events, also with pain at the injection site as the most frequent (30.2%). Other adverse events, reported less frequently in our study, included headache, fever/chills, nausea, vomiting, fatigue, diarrhea and myalgia. These results are in line with the aforementioned phase 1/2 study. 9 Likewise, the efficacy and safety of ChAdOx1 and Ad26.COV2.S were addressed in blinded, randomized, controlled trials.10,11 In these trials, injection-site pain was the most common local reaction, and headache and fatigue were the most common systemic event.10,11 Similarly, a recent study reporting the safety of the ChAdOx1nCoV-19 vaccine in 33 MS patients found that 94% of them presented adverse events after vaccination, including sore arm and flu-like symptoms as the most frequent. In more than two-thirds of the patients the symptoms lasted for up to 48 h, resolving within seven days. No severe adverse events occurred. 12
The second most frequently administered types of vaccines in our cohort were the IVVs: CoronaVac and Sinopharm. These vaccines are inactivated candidates against COVID-19 created from African green monkey kidney cells (Vero cells) that have been inoculated with SARS-CoV-2 (CN02 strain for CoronaVac and HB02 strain for Sinopharm). The safety, tolerability and immunogenicity of CoronaVac and Sinopharm were demonstrated in randomized, double-blind, placebo-controlled, phase 1/2 clinical trials performed in China.13,14 No serious adverse events were registered. The most common symptom reported after vaccination was injection-site pain and fever.13,14 In contrast, in our study, headache was the most frequently reported adverse event with IVVs. In accordance with previously reported data, compared to other COVID-19 vaccine platforms such as mRNA and viral-vectored vaccines, in our study, the occurrence of fever after vaccination with IVVs was lower. On the other hand, five patients from our study who received a first dose of an IVV reported post-vaccination MS relapse, with a time to relapse of 18 ± 13 days. To date, this is the first report from the real world showing the presence of relapses after IVV COVID-19 vaccination. Previous reports of MS relapses were described by Etemadifar and cols in a patient receiving the Sputnik V vaccine 15 and by Achiron and cols in 13 MS patients receiving an mRNA vaccine. 6
The least frequently reported vaccines in this study were the mRNA BNT162b2 and mRNA-1273. These RNA-based vaccines contain mRNA that encodes the SARS-CoV-2 full-length spike, modified by two proline mutations to lock it in the prefusion conformation. The safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine and the mRNA-1273 SARS-CoV-2 were addressed in two phases three randomized, observer-blinded, placebo-controlled trials.16,17 In both clinical trials, as in our real-world study, the most frequent adverse event was pain at the injection site followed by headache. In our population of MS patients, no severe adverse events were recorded with these vaccines. Finally, regarding the risk of MS disease activity following vaccination with mRNA vaccines, although we know that most vaccines have a very low risk of causing relapses, yellow fever and H1N1 swine flu vaccines are regarded as vaccines that could potentially generate disease activity.18–20 In relation to COVID-19 vaccines, as mentioned, MS relapses were reported with BNT162b2 in 2% of patients after the first vaccination dose and in 4.8% of patients after the second vaccination dose. 6 In our study, lower relapse rates were reported after receiving COVID-19 vaccines. However, it should be noted that this was a cross-sectional study with only short-term post-vaccination data collected.
We identified an important pitfall in patient care associated with the low rates of vaccination with annual flu and pneumonia vaccines. This self-reported low prevalence is thought to be secondary to an inadequate physician's recommendation, although other factors may also be involved. Their identification and the implementation of specific strategies to address vaccine hesitancy may increase influenza and pneumococcal vaccination coverage among pwMS. This issue can also be of great interest among other professionals who also deal with chronic autoimmune diseases, such as rheumatologists.21,22 Throughout the short follow-up period, 10 pwMS were infected by SARS-CoV-2 between the first and second vaccine dose, all of them with a mild or asymptomatic disease. Recent publications showed that anti-CD20 treatment and fingolimod led to a reduced humoral response to mRNA-based SARS-CoV-2 vaccines in pwMS.23–25 In contrast, Apostolidis et al. reported that all pwMS treated with anti-CD20 treatment generated antigen-specific T cell responses after vaccination. 26 Future studies are needed to further evaluate the humoral and T-cell memory responses in pwMS in other COVID-19 vaccines beyond mRNA-based ones.
The main limitations of our study include the relatively short follow-up period and the inclusion of a small number of patients, which can be associated with a sampling bias. In addition, some patients who responded to the survey received only one of the two doses of the vaccine. This could also result in a lower rate of adverse effects. Finally, in order to avoid including pseudorelapses, we only considered relapses diagnosed by a neurologist. This may have underestimated the actual number of relapses since many patients did not leave their home during the pandemic.
Conclusion
Our study suggests that the vaccines used in Latin America against COVID-19 appear to be safe in a short-term period. This is the first report of COVID-19 vaccination safety in pwMS patients from our region. Taken together, our findings support the recommendation to promote vaccination for pwMS during the SARS-CoV-2 pandemic. Further studies are certainly needed to analyze the long-term safety of these vaccines in MS patients.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ricardo Alonso has received personal compensation for consulting, serving on the scientific advisory board, lecturing as well as professional/travel accommodation stipends among other activities from Biogen Idec, Genzyme, Merck-Serono, Novartis, Teva and Roche. Anibal Chertcoff has received speaking honoraria and travel stipends from Merck-Serono and Roche. María Bárbara Eizaguirre has received personal compensation for consulting, lecturing as well as stipends for national and international conferences among other activities from Biogen Idec, Genzyme, Novartis and Roche. Berenice Anabel Silva has received economic retribution for the development of educational, scientific activities and travel grants to Congresses from Biogen, Novartis, Merck, Genzyme, Teva, Bayer, Tuteur and Roche. Felisa Leguizamón has received economic retribution for the development of educational activities as well as travel stipends from Bayer, Biogen Idec, Biosidus, Genzyme, Novartis, Merck, Raffo, Tuteur, and Teva. Rest of the authors report no conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iDs: Ricardo Alonso https://orcid.org/0000-0001-9955-8343
Aníbal Chertcoff https://orcid.org/0000-0002-8645-6134
Maria B Eizaguirre https://orcid.org/0000-0002-7817-2772
Adriana Carrá https://orcid.org/0000-0003-3874-4884
Berenice A Silva https://orcid.org/0000-0001-5866-2419
Contributor Information
Ricardo Alonso, Centro Universitario de Esclerosis Múltiple - CUEM, Hospital Ramos Mejía, Buenos Aires, Argentina; Sanatorio Güemes, Buenos Aires, Argentina.
Aníbal Chertcoff, Esclerosis Múltiple Argentina - EMA, Buenos Aires, Argentina.
Felisa del V Leguizamón, Hospital Británico, Buenos Aires, Argentina.
Lorna Galleguillos Goiry, Hospital Álvarez, Buenos Aires, Argentina.
Maria B Eizaguirre, Centro Universitario de Esclerosis Múltiple - CUEM, Hospital Ramos Mejía, Buenos Aires, Argentina.
Roberto Rodríguez, Clínica Alemana, Santiago de Chile, Chile.
Marta Sosa, Renacer, Fundación Dominicana de EM, Santo Domingo, Dominican Republic.
Susana Carballido, Asociación de Lucha contra la Esclerosis Múltiple de Colombia - ALEM, Medellín, Colombia.
Verónica Cruchet, Esclerosis Múltiple Uruguay - EMUR, Montevideo, Uruguay.
Agnes de Jong-Martis, Corporación Esclerosis Múltiple Chile, Santiago de Chile, Chile.
Adriana Carrá, Esclerosis Múltiple Argentina - EMA, Buenos Aires, Argentina; Fundación Favaloro, Buenos Aires, Argentina.
Berenice A Silva, Centro Universitario de Esclerosis Múltiple - CUEM, Hospital Ramos Mejía, Buenos Aires, Argentina.
References
- 1.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed 2020; 91: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cimerman S, Chebabo A, Cunha CAD, et al. One year after the arrival of COVID-19 in Latin America: what have we learned in Brazil and other countries? Braz J Infect Dis 2021; 25: 101571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chertcoff A, Bauer J, Silva BA, et al. Changes on the health care of people with multiple sclerosis from Latin America during the COVID-19 pandemic. Mult Scler Relat Disord 2021; 54: 103120. [DOI] [PubMed] [Google Scholar]
- 4.Alonso R, Silva B, Garcea O, et al. COVID-19 in multiple sclerosis and neuromyelitis optica spectrum disorder patients in Latin America: COVID-19 in MS and NMOSD patients in LATAM. Mult Scler Relat Disord 2021; 51: 102886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsur SW, Zaher EA, Tsur M, et al. Current immunological and clinical perspective on vaccinations in multiple sclerosis patients: are they safe after all? Int J Mol Sci 2021; 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult Scler 2021; 27: 864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Learmonth YC, Motl RW, Sandroff BM, et al. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol 2013; 13: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotan I, Wilf-Yarkoni A, Friedman Y, et al. Safety of the BNT162b2 COVID-19 vaccine in multiple sclerosis (MS): early experience from a tertiary MS center in Israel. Eur J Neurol 2021; 28: 3742–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet 2020; 396: 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020; 396: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against covid-19. N Engl J Med 2021; 384: 2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen-Philbey K, Stennett A, Begum T, et al. Experience with the COVID-19 AstraZeneca vaccination in people with multiple sclerosis. Mult Scler Relat Disord 2021; 52: 103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA 2020; 324: 951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021; 21: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etemadifar M, Sigari AA, Sedaghat N, et al. Acute relapse and poor immunization following COVID-19 vaccination in a rituximab-treated multiple sclerosis patient. Hum Vaccin Immunother 2021: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huttner A, Eperon G, Lascano AM, et al. Risk of MS relapse after yellow fever vaccination: a self-controlled case series. Neurol Neuroimmunol Neuroinflamm 2020; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farez MF, Correale J. Immunizations and risk of multiple sclerosis: systematic review and meta-analysis. J Neurol 2011; 258: 1197–1206. [DOI] [PubMed] [Google Scholar]
- 20.Farez MF, Correale J. Yellow fever vaccination and increased relapse rate in travelers with multiple sclerosis. Arch Neurol 2011; 68: 1267–1271. [DOI] [PubMed] [Google Scholar]
- 21.Boucher VG, Pelaez S, Gemme C, et al. Understanding factors associated with vaccine uptake and vaccine hesitancy in patients with rheumatoid arthritis: a scoping literature review. Clin Rheumatol 2021; 40: 477–489. [DOI] [PubMed] [Google Scholar]
- 22.Colmegna I, Valerio V, Boucher VG, et al. Barriers and facilitators to influenza and pneumococcal vaccine hesitancy in rheumatoid arthritis: a qualitative study. Rheumatology (Oxford) 2021. [DOI] [PubMed] [Google Scholar]
- 23.Sormani MP, Inglese M, Schiavetti I, et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine 2021: 103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.G S, L S, Z Cet al. et al. Serological response to SARS-CoV-2 vaccination in multiple sclerosis patients treated with fingolimod or ocrelizumab: an initial real-life experience. J Neurol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord 2021; 14: 17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]