Abstract
Since the outbreak of the COVID-19 pandemic, there has been a rapid expansion in vaccine research focusing on exploiting the novel discoveries on the pathophysiology, genomics, and molecular biology of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Although the current preventive measures are primarily socially distancing by maintaining a 1 m distance, it is supplemented using facial masks and other personal hygiene measures. However, the induction of vaccines as primary prevention is crucial to eradicating the disease to attempt restoration to normalcy. This literature review aims to describe the physiology of the vaccines and how the spike protein is used as a target to elicit an antibody-dependent immune response in humans. Furthermore, the overview, dosing strategies, efficacy, and side effects will be discussed for the notable vaccines: BioNTech/Pfizer, Moderna, AstraZeneca, Janssen, Gamaleya, and SinoVac. In addition, the development of other prominent COVID-19 vaccines will be highlighted alongside the sustainability of the vaccine-mediated immune response and current contraindications. As the research is rapidly expanding, we have looked at the association between pregnancy and COVID-19 vaccinations, in addition to the current reviews on the mixing of vaccines. Finally, the prominent emerging variants of concern are described, and the efficacy of the notable vaccines toward these variants has been summarized.
Keywords: AstraZeneca, BioNTech/Pfizer, COVID-19 vaccine, Moderna, SARS-CoV-2 variants
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 192 million cases and 4.1 million deaths as of July 22, 2021. 1 This pandemic has brought along a massive burden in morbidity and mortality in the healthcare systems. Despite the implementation of stringent public health measures, there have been devasting effects in other sectors contributing to our economy. This has plunged the global economies toward deep recession and has racked up a debt of approximately 19.5 trillion USD. 2
Immune protection in COVID-19 infection can be conceptualized as a spectrum wherein sterile immunity is at the end of positive spectrum. This is followed by transient infection (<3 days) and asymptomatic infection (~1 week). The negative spectrum of immune protection includes patients who are symptomatic, or hospitalized, or admitted to the intensive care unit for multiorgan support. The extreme end of the negative spectrum of immune protection is encompassed by case fatality. The vaccine will intervene prior to the viral insult and stabilize the population at the positive end of the spectrum of the immune protection. It will also prevent the perpetuating cycle of infection and reinfection via variants of SARS-CoV-2 virus in those who have achieved prior convalescence. One study by Dan et al. showed that in patients infected with COVID-19, immunological memory to SARS-CoV-2 remained intact for up to 6 months. 3 Unfortunately, there is no long-term data on the duration of protected immunity against SARS-CoV-2 in patients after convalescence. Therefore, these patients may also require vaccination but the current priority for vaccination can be stretched relative to the unaffected population.
While the ideal goal of the COVID-19 vaccine roll-out is to instill a global herd immunity; it is important to remember that this goal may never be reached. Furthermore, additional goals of vaccination may be to reduce mortality and stress on healthcare systems by reducing the cases of admitted patients. Various countries have already approved COVID-19 vaccines for human use, and more are expected to be licensed in the upcoming year. It is important that these vaccines are safe, efficacious, and can be deployed on a large scale. It is also prudent to eliminate the concerns of both the scientific and general community regarding its effectiveness, side-effects, and dosing strategies.
Historically, the process of vaccine manufacturing and clinical trials required approximately 10 years, but due to the burden of this disease, various observational studies were expedited so that all crucial information regarding the vaccine pharmacokinetics, pharmacodynamics, dosing, efficacy, and adverse events can be collected within a short period of time. Furthermore, there is a need to provide a compilation of accredited and appraised scientific literature on each of these approved vaccines with an aim to instill public health knowledge and vaccine literacy to members of the scientific and general community. A section dedicated to COVID-19 vaccines and pregnancy is also included in the penultimate section of this review.
Finally, the emergence of the SARS-CoV-2 viral variants of concern (VOC) has attained increased replication, transmission, and infectivity warranting exploration of these genomic mutations as their phenotypes. Hence, the final section of this review will aim to clarify the jargon, highlight the vaccine efficacy (VE) against VOCs, and eliminate any misinformation regarding these variants.
Vaccine physiology
The global burden of the pandemic requires an efficacious vaccine that elicits a lasting protective immune response against SARS-CoV-2. This will be an essential armament for the prevention and mitigation of the downstream morbidity and mortality caused by SARS-CoV-2 infection. As of July 20, 2021, there are approximately 108 vaccines in clinical development and 184 vaccines in pre-clinical development with several vaccines being distributed globally. 4
The technologies employed in the vaccine synthesis and development aim to trigger the adaptive immune system and elicit memory cells that will protect the body from subsequent infections. These technologies may be mRNA-based vaccines such as the Moderna and Pfizer/BioNTech, inactivated virus vector vaccines, DNA vaccines, and numerous other technologies. 5
Due to the urgent implementation of vaccine development, the most obvious target will be the robust proteins expressed on the surface of the virus. Therefore, these technologies target molecular expression of the trimeric SARS-CoV-2 spike (S) glycoprotein. These targets could include its mRNA, DNA, full S1 subunit, or fusion subunits. The S protein is a major component of the virus envelope, it is vital for viral fusion, receptor binding, and virus-entry through recognition of host-cellular receptor. The S protein comprises of two main functional units, the S1 subunit, which contains the receptor-binding domain (RBD) and the S2 subunit which is responsible for virus fusion with the host-cell membrane. 6 The choice to proceed with S protein as the target was reinforced when a study by Dan et al. confirmed that in 169 patients infected with SARS-CoV-2, spike-specific immunoglobulin G (IgG) remained stable for over 6 months. 3 In addition, both spike-specific CD4+ T-cells (CD137+ and OX40+) and spike-specific CD8+ T-cells (CD69+ and CD137+) were present at the 6-month post-convalescence period, but their subpopulations exhibited a steady decline with a half-life of 139 days and 225 days, respectively. 3
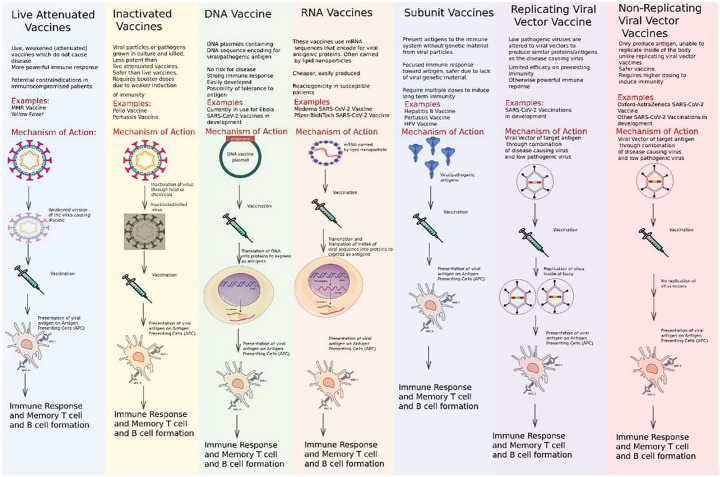
There are subtle differences in the mechanism by which the different vaccine products interact within host cells to induce immunity. Many successful vaccines of the 20 century utilized the target proteins directly such as the tetanus and pertussis vaccine. A summary of the major types of vaccines and their mechanism of action are shown in Figure 1.
Figure 1.
Summary of major vaccine types and their mechanism of action.
DNA, deoxyribonucleic acid; HPV, Human papillomavirus; mRNA, messenger ribonucleic acid; MMR, Measles, Mumps, and Rubella; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
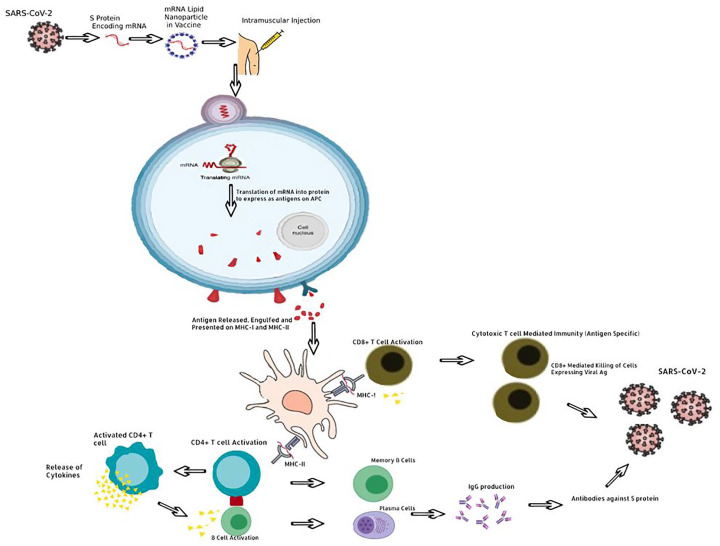
Historically, vaccines usually contained adjuvants which are protein sensitizers that heighten the migratory and sampling response of antigen presenting cells (APCs). Interestingly, the current mRNA vaccines are engineered to code for their own sensitizing protein alongside the S-protein epitopes. Therefore, these new mRNA vaccines usually do not contain any adjuvants. In addition, the mRNA vaccines utilize lipid nanoparticles to deliver the genetic material of a viral S-protein. Contrastingly, vaccines such as the AstraZeneca vaccine may employ a chimpanzee adenovirus vector to carry the DNA genome of the S-protein to the host-cell. 7 Once undergoing the processes of transcription and translation into proteins, these are trafficked and expressed on the host cell surface wherein the adaptive immune system mounts a response via the major histocompatibility complex (MHC) molecules (Figure 2).
Figure 2.
Mechanism of induction of immunity through vaccination.
APC, antigen presenting cells; DNA, deoxyribonucleic acid; MHC, major histocompatibility complex; mRNA, messenger ribonucleic acid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
There are two types of MHC molecules, the first one that will be discussed is the MHC-II, which is found exclusively on APC: these comprise of B-cells, macrophages, and dendritic cells in the lymph nodes. Once the S-protein antigen is presented at the cell surface of the MHC-II molecules, the naïve helper T-cell’s (Th Cells) T-cell receptor (TCR) complex will interact with this antigen leading to activation of CD4+ Th cells. This activation is perpetuated by a secondary activation signal with B7 on the APC recognizing the CD28 on the Th cell which triggers the proliferation of Th cells that can recognize the S-protein antigen. Activated CD4+ Th cells then secrete numerous cytokines, namely interleukin (IL)-2 which activates CD8+ cytotoxic T-cells (Tc cell) and trigger clonal expansion of B-cells in memory B-cells and plasma cells. The cytokines IL-4 and IL-5 facilitate B-cell isotype switching and maturation to plasma cells; promoting secretion of IgG antibodies against S-protein. 8 Formation of antibodies allows the immune system to direct an immune response against cells expressing the S-protein of the virus. The second process involves MHC-I, which activates CD8+ Naïve Tc cells through TCR complex interaction with processed endogenously synthesized S-protein expressed on MHC-I. MHC-I is expressed in all nucleated cells, APCs, and platelets and require a second activation signal provided by IL-2 from activated CD4+ Th cells. This activates CD8+ Tc cells which can mount a cytotoxic response against SARS-CoV-2-infected cells through two mechanisms of apoptosis. The first mechanism is the secretion of perforin which create pores to allow granzyme to enter the targeted cell, thus activating apoptosis. The second mechanism is via the expression of FasL, which binds Fas on target cells and induces apoptosis. 8 A crucial part of this process is the stimulation of memory T-cells and memory B-cells. Importantly, while the SARS-CoV-2 vaccine’s lasting effect is still being researched in the context of the pandemic, theoretically these should provide lasting immunity and allow the immune system to mount a faster and more effective response should a vaccinated individual encounter the virus in the future.
Current prominent COVID-19 vaccines
BioNTech/Pfizer
The BNT162b2 COVID-19 vaccine developed by BioNTech and Pfizer is a lipid nanoparticle-formulated, nucleoside-modified RNA vaccine that encodes a prefusion membrane-anchored SARS-CoV-2 full-length spike protein. 9 It was the first vaccine approved by the US Food and Drug Association (FDA) and now it has been approved in many other countries. 10 The BNT162b2 COVID-19 vaccine may be stored at standard refrigerator temperatures prior to use, but it requires very cold temperatures for long-term storage and shipping (−70°C) to maintain the stability of the lipid nanoparticle. In a phase-1 trial, it was compared to another vaccine candidate BNT162b1, and it was found to have a milder systemic side-effect profile with a similar antibody response. 11 Therefore, it was pushed forward to a blinded phase-2/3 clinical study. 9 In total, 43,548 participants were randomized to receive either two doses of the BNT162b2 vaccine (n = 21,720) or a placebo (n = 21,728) 21 days apart. The participant ages ranged from 16 to 91 years, 35.1% of participants were classified as having obesity and comorbidities within participants included HIV, malignancy, diabetes, and vascular diseases. 9 Based on the results of the study, 7 days after the second BNT162b2 dose, the VE was 95% (95% confidence interval (CI), 90.3–97.6) with only eight observed cases of COVID-19 in the vaccine recipients and 162 cases in the placebo recipients. 9 The efficacy remained consistent across subgroups characterized by age, sex, race, ethnicity, body mass index (BMI), and comorbidities (generally 90–100%). 9 Although there were 10 cases of severe COVID-19 with onset after the first dose, only one occurred in a vaccine recipient and nine in placebo recipients. Like the phase-1 trial results, the safety profile remained favorable with the most common local reaction being mild-to-moderate pain at the injection site while the most common systemic symptoms were fatigue and headache (reported in ⩾50%). 9 In both the vaccine and placebo group, the incidence of severe adverse events did not differ significantly (0.6% and 0.5%, respectively) and no deaths occurred related to the vaccine. As indicated by the manufacturer’s information, contraindications for use include hypersensitivity to the active substance or any of the excipients. 12 These studies show that the mRNA-vaccine BNT162b2 is safe and effective in protecting against COVID-19. However, further investigations are needed to confirm long-term safety and to establish safety and efficacy for populations not included in this study.
Moderna
The mRNA-1273 vaccine, developed by Moderna, relies on mRNA technology to encode prefusion stabilized SARS-CoV-2 spike protein. It is the second COVID-19 vaccine to receive emergency use approval by the US FDA, and it is given as two 100-µg doses intramuscularly into the deltoid muscle, 28 days apart. 13 Storage of the vaccine is done at temperatures between −25°C to −15°C for long-term storage, 2°C to 8°C for 30 days, or 8°C to 25°C for up to 12 hours. Results from the COVE phase-3 trial showed that the mRNA-1273 vaccine was effective at preventing COVID-19 illness in persons 18 years of age or older. A total of 30,420 participants aged 18 years or older were randomized 1:1 to receive either two doses of the vaccine or a placebo, 28 days apart. 14 The mean age of the participants was 51.4 years, and enrollment was adjusted for equal representation of racial and ethnic minorities. In the trial, symptomatic COVID-19 illness occurred in 11 participants within the vaccine group versus 185 participants within the placebo group, showing a 94.1% (95% CI, 89.3–96.8%) efficacy of the vaccine. Efficacy was similar across age, sex, race, and ethnicity as well as in patients with and without risk factors for severe disease (e.g. chronic lung disease, cardiac disease, and severe obesity). Importantly, a secondary endpoint for determining the efficacy of the vaccine in preventing severe COVID-19 was also used. All 30 participants with severe COVID-19 were in the placebo group, indicating a 100% efficacy of no hospital admissions. 14 Regarding the side effects of the vaccine, adverse events at the injection site and systemic adverse events occurred more commonly with the mRNA-1273 group compared to the placebo. The most common local reaction was mild to moderate pain at the injection site (75%). The most common systemic symptoms were fatigue, myalgia, arthralgia, and headache (50%). 14 The overall incidence of serious adverse events did not differ significantly between groups and no deaths occurred in relation to the vaccine. While this vaccine is already being administered, further investigations are still necessary to establish safety and efficacy profiles for populations not included in this study as well as to assess its long-term effects. Current contraindications of the mRNA-1273 vaccine include any persons with known allergy to polyethylene glycol (PEG), another mRNA vaccine component or polysorbate. 15
AstraZeneca
The Oxford and AstraZeneca ChAdOx1 COVID-19 vaccine uses a chimpanzee adenovirus vector to deliver the genetic sequence of a full-length spike protein of SARS-CoV-2 into host cells. 16 The storage for the ChAdOx1 vaccine is favorable, as it may be refrigerated at 2°C–8°C for 6 months. Pooled analysis of four ongoing clinical studies was used to assess efficacy, safety, and immunogenicity of the ChAdOx1 vaccine: COV001 (phase 1/2), COV002 (phase 2/3), COV003 (phase 3), and COV005 (phase 1/2). 17 Across the four studies participants over 18 were randomized to receive either the vaccine or a control (meningococcal group A, C, W, or saline). ChAdOx1 vaccine recipients received two standard doses (SDs) of the vaccine (SD/SD cohort) except for a subset in the COV002 trial who received a half lower dose (LD) followed by an SD (LD/SD cohort). 17 In the four studies, there was a total 23,848 participants, all of whom were used for gathering safety data; only 11,636 participants from the COV002 and COV003 trials were included in the primary efficacy analysis. 17 Of the 11,636 participants in the efficacy analysis, 2741 were in the LD/SD cohort, 88% were between 18 and 55 years old, and comorbidities present included cardiovascular disease, respiratory disease, and diabetes. 17 The results show that in the intended dosing regimen (SD/SD cohort), the VE was 62.1% (95% CI, 41.0–75.7) ⩾14 days after the second injection for symptomatic COVID-19 (27 cases vs 71 cases respectively). 17 In the group that received an LD (LD/SD cohort), the VE was 90.0% (95% CI, 67.4–97.0; 3 cases vs 30 cases, respectively) while across the two dosing regimens the overall efficacy was 70.4% (95.8% CI, 54.8–80.6;30 cases vs 101 cases, respectively). 17 The higher efficacy observed in the LD/SD cohort can be attributed to this group having a longer dosing interval between the two doses in comparison to the SD/SD cohort. Regarding safety, most of the adverse events were mild-moderate with the most frequently reported being injection site pain/tenderness, fatigue, headache, malaise, and myalgia. 18 About 175 serious adverse events were noted, only three of which were possibly linked to intervention: transverse myelitis 14 days after second dose, haemolytic anemia in a control recipient and fever >40°C in a participant still masked to group allocation. One contraindication for use of the vaccine is hypersensitivity to any of its components. In very rare cases, AstraZeneca has been associated internationally with venous thromboembolic events with thrombocytopenia with current estimates being 10–15 cases per million vaccinated patients. 19 This adverse event has been termed thrombosis with thrombocytopenia syndrome (TTS). In summary, these studies demonstrate that the AstraZeneca ChAdOx1 vaccine has a good efficacy and side-effect profile. Limitations include that less than 4% of participants were >70, no one over 55 got the mixed-dose regimen (LD/SD cohort), and those with comorbidities were a minority. Additional investigations are required to analyze long-term effects and assess efficacy and safety in populations not included or underrepresented.
Janssen COVID-19 vaccine
The Janssen (Johnson & Johnson) COVID-19 vaccine, developed by Janssen Pharmaceutical in Netherlands. It is a single-dose intramuscular (IM) vaccine that contains a recombinant, replication incompetent human adenovirus (Ad26) vector encoding the spike protein of SARS-CoV-2 in the stabilized conformation. 20 It can be stored between 2°C and 8°C for up to 6 hours or at room temperature for a duration of 2 hours. The ENSEMBLE Phase-3 trial (n = 43,783) is a randomized, double-blind, placebo-controlled study which included participants ⩾18 years. Efficacy assessment was performed at day 14 and 28. The primary outcome only included moderate and severe (hospitalization and death) infection. Overall, the VE in the moderate to severe cohort was 66.9% (95% CI: 59.0–73.4) at 14 days and 66.1% (95% CI: 55.0–74.8) at 28 days. 20 In the severe cohort, the VE was 76.7% (95% CI: 54.6–89.1) and 85.4% (95% CI: 54.2–96.9) at day 14 and 28 days, respectively. 20 At the time of the study, 96.4% of the strains in the United States, 96.4% were identified as the Wuhan-H1 variant D614G. The VE in the United States for the moderate to severe cohort was 74.4% (95% CI: 65.0–81.6) and 72.0% (95% CI: 58.2–81.7) at 14 days and 28 days, respectively. 20 In the US severe cohort, the VE was 78.0% (95% CI: 33.1–94.6) and 85.9% (95% CI: −9.4 to 99.7) at day 14 and 28 days, respectively. 20 Alternatively, 94.5% of the strains in South Africa were identified as beta variant. The VE in South Africa for the moderate to severe cohort was 52.0% (95% CI: 30.3–67.4) and 64.0% (95% CI: 41.2–78.7) at 14 days and 28 days, respectively. 20 In the South African severe cohort, the VE was 73.1% (95% CI: 40.0–89.4) and 81.7% (95% CI: 46.2–95.4) at day 14 and 28 days, respectively. 20 In Brazil, 69.4% of the strains were identified as P.2 lineage variant and 30.6% were identified as Wuhan-H1 variant D614G. The VE in Brazil for the moderate to severe cohort was 66.2% (95% CI: 51.0–77.1) and 68.1% (95% CI: 48.8–80.7) at 14 days and 28 days, respectively. 20 In the Brazilian severe cohort, the VE was 81.9% (95% CI: 17.0–98.1) and 87.6% (95% CI: 7.8–99.7) at day 14 and 28 days, respectively. 20 The most common localized solitary adverse reaction was the injection site pain (48.6%). Conversely, the most common systemic adverse reactions included headache, fatigue, myalgia, and nausea. 20 In the post authorization phase, adverse reaction included anaphylaxis, thrombosis with thrombocytopenia, Guillain Barré syndrome, and capillary leak syndrome. 20 Overall, the data demonstrate that the Janssen vaccine has a good efficacy and side-effect profile.
Gamaleya
Sputnik V or Gam-COVID-Vac, developed by the Gamaleya Institute, is a recombinant human adenovirus-based vaccine that uses two different vectors (rAd26 and rAd5) to carry the gene encoding for the spike protein of SARS-CoV-2. Only one vector (rAd26) is given at dose 1 and the other (rAd5) at dose 2. This strategy prevents immunity against the vector. It can be stored as either a liquid at −18°C, or it can be freeze-dried and stored at 2°C to 8°C. 21 Regarding the safety and efficacy of the vaccine, both were evaluated in a randomized, double-blind phase-3 trial performed in Moscow, Russia. In the trial, a total of 21,977 participants aged 18 years or older were randomized in a 3:1 ratio to the vaccine or placebo groups. Two doses of the vaccine or placebo were given 21 days apart to the respective groups. 21 The mean age of the participants was 45.3 years, and the majority of participants were Caucasian (98.5%). 21 From 21 days after the first dose of the vaccine, efficacy against symptomatic COVID-19 illness was 91.6% (95% CI, 85.6–95.2%) with 16 confirmed cases of COVID-19 in the vaccine group and 62 confirmed in the placebo group. 21 There were also 20 cases of moderate to severe COVID-19 infection confirmed in the placebo group at least 21 days after the first dose and 0 in the vaccine group, indicating a VE of 100% against moderate to severe infection. 21 The most common adverse effects in both groups were flu-like illness, injection site reactions, headaches, and asthenia, with the majority being grade 1 (94.0%). 21 Serious adverse events were also reported in both the vaccine group and placebo group, but they were deemed not to be associated with the vaccination. Further investigations are still needed to determine the duration of protection of the vaccine and to determine the safety and efficacy of the vaccine in populations not included in the study (e.g. children, adolescents, and pregnant and lactating women).
SinoVac
CoronaVac is an inactivated vaccine developed by SinoVac Biotech containing inactivated SARS-CoV-2. 22 The vaccine can be stored at 2°C to 8°C for up to 3 years making it an attractive option for development. Two phase-1/2 clinical trials assessed the safety, tolerability, and immunogenicity of the CoronaVac vaccine.22,23 The first study (18–59 years old included only) placed 744 participants in either a vaccine or placebo group where they were further divided based on vaccination schedule and dosage (3 and 6 μg). In the second study (⩾60 years old included only), 422 participants were randomized to receive two doses of CoronaVac or placebo 28 days apart and then further divided based on dosage amount only (3 and 6 μg for phase 1; 1.5, 3, and 6 μg for phase 2). Safety results from both trials show a favorable side-effect profile with most symptoms being transient and of mild severity. The most common adverse effect was injection site pain; others included fatigue and fever. In the 18–59 years old study, one serious adverse event of acute hypersensitivity was possibly related to vaccination. 22 No serious adverse events were associated with the vaccine or placebo in the ⩾60-year-old study. Between the dosage amounts in both studies, the tolerability was consistent and the immunogenicity was also similar for the 3 and 6 μg doses (less in 1.5 μg). 23 Multiple phase-3 trials have also taken place to determine the effectiveness of CoronaVac in countries, such as Brazil, Indonesia, and Turkey. In the Brazil trial, 252 cases of COVID-19 were recorded from roughly 9200 health care workers, with 167 in the placebo group and 85 in the vaccine group. 24 The reported efficacy of the vaccine in preventing mild and severe COVID-19 infection was 50.4%. In comparison, the Turkey trial reported that the vaccine was 83.5% effective at preventing symptomatic infection based on 29 COVID-19 cases among 1,322 volunteers while results from the Indonesia trial found that the vaccine was 65.3% effective at preventing symptomatic infection based on 25 COVID-19 cases among 1,600 people. 24 Some reasons for the lower efficacy of CoronaVac in the Brazil trial may include increased likelihood of exposure to the virus since participants were healthcare workers, and insufficient time for participants to reach peak immunity since the doses were administered only 2 weeks apart. 24 The phase-3 SinoVac study in Chile showed the VE 14 days post second dose to prevent symptomatic COVID-19 (67%, 95% CI: 65–69%), hospital admission (85%, 95% CI: 83–87%), intensive care unit (ICU) admission (89%, 95%CI: 84–92%) and death (80%, 95%CI: 73–86%). 25 The Phase-3 SinoVac trial in Brazil showed an overall VE against symptomatic COVID-19 (50.7%, 95% CI: 35.9–62%), moderate cases requiring hospitalization (83.7%, 95% CI: 58–93.7%), and severe cases requiring hospitalization (100%, 95%CI: 56.4–100%). 26 As with any vaccine, a contraindication for CoronaVac is anaphylaxis to it or to one of its constituents.
Other prominent COVID-19 vaccines
Due to the disease burden of SARS-CoV-2, the development and manufacturing of COVID-19 vaccines has been occurring at a remarkable pace which has not been seen before. There are many emerging vaccines with different mechanisms of actions that will be briefly explored. Bharat Biotech, an Indian company, has designed the inactivated COVID-19 vaccine Covaxin (BBV152). Once inside the body, the inactivated viruses can initiate an immune response through the interaction of surface proteins with APCs. Phase-1/2 trials showed no serious side effects and phase-3 trials are currently underway. 27 The state-owned Chinese company Sinopharm has also made an inactivated COVID-19 vaccine called BBIBP-CorV. The Sinopharm phase-3 trial showed that the VE in symptomatic cases for the WIV04 strain-based vaccine (72.8, 95% CI: 58.1–82.4%) and HB02 strain-based vaccine (78.1 95% CI: 64.8–86.3%).28,29 It is approved in Bahrain, U.A.E, and China. NVX-CoV2373 is another promising vaccine produced by Novavax. It is a protein subunit vaccine made by assembling SARS-CoV-2 spike proteins into nanoparticles. A phase-3 trial in the United Kingdom displayed an efficacy rate of 89.3%; however, a phase-2 trial in South Africa had an efficacy just under 50%. 28 This discrepancy is thought to arise because of a new variant in South Africa. Other emerging vaccines include CoVLP produced by Medicago which uses the plant N. benthamiana to create virus-like particles that mimic SARS-CoV-2, CVnCoV produced by CureVac which is an mRNA vaccine, Convidecia produced by CanSino Biologics which is adenovirus based (Ad5), Ad26.COV2.S produced by Johnson & Johnson which is also adenovirus based (Ad26), and ZF2001 created by Anhui Zhifei Longcom which is a protein subunit vaccine. Even though highly effective, COVID-19 vaccines are already in use, it is still important to have a range of vaccines such as those listed above to bring the pandemic under control. Having a diverse profile ensures that vaccines will work for individuals from all ethnic backgrounds and with various underlying health conditions. 30 Getting the virus under control will also require doses for a large proportion of the world. To meet this requirement as soon as possible, having multiple vaccines will help in maximizing the volume of doses that can be produced. In addition, there are many technical issues such as cold storage and transportation, cost, and dosing of certain vaccines that arise when trying to vaccinate remote populations. For example, both the Pfizer-BioNTech and Moderna vaccines are expensive and transported at temperatures of −70°C and −20°C making it difficult to access many locations all at once. Since most vaccines require two doses spaced a few weeks apart, it can be challenging for individuals without regular access to healthcare as well. 30 Such considerations highlight the importance of having a range of single-dose vaccines and vaccines without the need for cold storage. A summary of efficacy, prominent side effects and storage recommendations for all the notable COVID-19 vaccines are shown in Table 1.
Table 1.
Summary of vaccine efficacy, dosing strategy, and side-effects of different COVID-19 vaccines.
| Company | Phase-III efficacy against non-variant COVID-19 strain % (95% CI) | Injection type | Pooled side effects across doses (%frequency, n) | Storage | Reference |
|---|---|---|---|---|---|
| BioNTech/Pfizer (Germany/USA) | Dual dose: 94.1% (89.8–97.6) at ⩾35 days Single dose: 92.6% (69.0–98.3) between days 14–28 |
IM (2 doses) | Phase-II trial results 1. Injection site pain (80.6%, n = 3536) 2. Fatigue (53.1%, n = 2332) 3. Headache (46.6%, n = 2044) 4. Myalgia (28.9%, n = 1270) 5. Arthralgia (16.2%, n = 710) 6. Fever ⩾ 38.0°C (9.5%, n = 416) 7. Vomiting (1.5%, n = 68) * data for 18–55 years old |
−70°C | 31,32 |
| Moderna (USA) | Dual dose: 94.1% (89.3–96.8) at ⩾42 days Single dose: 92.1% (68.8–99.1) between days 14–28 |
IM (2 doses) | Phase-II trial results 1. Pain at the injection site (92.0%, n = 13,970) 2. Fatigue (70.0%, n = 10,630) 3. Headache (64.7%, n = 9825) 4. Myalgia (61.5%, n = 9339) 5. Arthralgia (46.4%, n = 7046) 6. Chills (45.4%, n = 6894) 7. Nausea/vomiting (23.0%, n = 3493) 8. Axillary swelling (19.8%, n = 3007) 9. Fever (15.5%, n = 2354) 10. Injection site swelling (14.7%, n = 2232) 11. Injection site erythema (10.0%, n = 1519) * data for ⩾18 years old |
−25°C and −15°C | 33,34 |
| AstraZeneca (UK) | Dual dose: 66.7% (57·4–74·0) at 104 days Single dose: 76% (59·3–85·9) between days 22–90 |
IM (2 doses) | Phase-II trial results 1. Pain at the injection site (63.7%, n = 7657) 2. Tenderness at the injection site (54.2%, n = 6515) 3. Fatigue (53.1%, n = 6383) 4. Headache (52.6%, n = 6323) 5. Malaise (44.2%, n = 5313) 6. Myalgia (44.0%, n = 5289) 7. Chills (31.9%, n = 3835) 8. Arthralgia (26.4%, n = 3174) 9. Fever ⩾ 38.0°C (7.9%, n = 950) * data for ⩾18 years old with at least one dose |
2°C–8°C | 35,36 |
| Janssen/Johnson & Johnson (Netherlands/US) | Single dose: Symptomatic 66.3% (59.9–71.8) Hospitalization 93% (71–98) |
IM (1 dose) | Phase-I trial results 1. Injection site pain 2. Fatigue 3. Headache 4. Myalgia 5. Nausea 6. Pyrexia * data for 18–55 years old |
2°C–8°C | 20,37 |
| Gamaleya Sputnik V Gam-COVID-Vac (Russia) |
Dual dose: 91.6% (85.6–95.2) Single dose: 73.6% from 15–21 days |
IM (2 doses) | Pooled phase-I and phase-II trial results 1. Hyperthermia (68%, n = 27) 2. Injection site pain (50%, n = 20) 3. Headache (40%, n = 16) 4. Asthenia (38%, n = 15) 5. Myalgia/arthralgia (28%, n = 11) 6. Rhinorrhoea (10%, n = 4) * data for 18–60 years old |
−18°C or 2°C–8°C | 21,38 |
| SinoVac (China) | Dual dose: Symptomatic: 50.7% Moderate hospitalization: 83.7% Severe hospitalization: 100% |
IM (2 doses) | Phase-II trial results 1. Injection site pain (11.2%, n = 27) 2. Diarrhea (2.5%, n = 6) 3. Fever (2.0%, n = 5) 4. Fatigue (1.7%, n = 4) 5. Myalgia (1.3%, n = 3) 6. Headache (0.8%, n = 2) *data for 18–59 years old, 3-μg dose on days 0 and 14 |
2°C–8°C | 22,26 |
| Bharat Biotech COVAXIN BBV152 (India) |
Dual dose: Asymptomatic 63.6% (29·0–82·4) Mild: 77.8% (65·2–86·4) Severe: 93.4% (57·1–99·8) |
IM (2 dose) | Phase-II trial results 1. Fever (3.2%, n = 12) 2. Injection site pain (2.9%, n = 11) 3. Body ache (1.3%, n = 5) 4. Headache (1.1%, n = 4) 5. Weakness (0.8%, n = 3) * data for 12–65 years old, 6 μg + adjuvant |
2°C–8°C | 39,40 |
| Sinopharm BBIBP-CorV (China) |
Dual dose: 78.1% (64.9–86.3) |
IM (2 doses) | Phase-I trial results 1. Injection site pain (12%, n = 10) 2. Injection site swelling (4%, n = 3) 3. Fever (4%, n = 3) 4. Nausea (2%, n = 2) 5. Headache (1%, n = 1) 6. Fatigue (1%, n = 1) * data for 18–59 years-old, 4 μg on days 0 and 21 |
2°C–8°C | 41,42 |
| Novavax (USA) | Dual dose: 89.7% (80.2–94.6) |
IM (2 doses) | Phase-I trial results 1. Local tenderness (71.7%, n = 81) 2. Injection site pain (52.2%, n = 59) 3. Myalgia (42.5%, n = 48) 4. Fatigue (39.8%, n = 45) 5. Headache (38.1%, n = 43) 6. Malaise (25.7%, n = 29) * data for 18–59 years old, 5 μg + adjuvant, 25 μg + adjuvant |
2°C–8°C | 43,44 |
| Medicago (Canada) | – | IM (2 doses) | Phase-I trial results 1. Injection site pain (97.4%, n = 38) 2. Fatigue (48.7%, n = 19) 3. Headache (43.6%, n = 17) 4. Chills (30.8%, n = 12) 5. Injection site swelling (23.1%, n = 9) 6. Myalgia (20.5%, n = 8) 7. Fever (17.9%, n = 7) 8. Injection site redness (17.9%, n = 7) 9. Arthralgia (7.7%, n = 3) * data for 18–55 years old, 3.75 μg dose + adjuvant |
2°C–8°C | 45 |
| CureVac CVnCoV (Germany) |
47% | IM (2 doses) | Phase-I trial results 1. Fatigue (96.3%, n = 52) 2. Injection site pain (88.9%, n = 48) 3. Headache (87.0%, n = 47) 4. Chills (83.3%, n = 45) 5. Myalgia (75.9%, n = 41) 6. Fever (55.6%, n = 30) 7. Arthralgia (50.0%, n = 27) 8. Nausea/vomiting (33.3%, n = 18) 9. Diarrhea (14.8%, n = 8) * data for 18–60 years old, 12-μg dose |
2°C–8°C | 46,47 |
| CanSino (China) | – | IM (1 dose) | Phase-I trial results 1. Injection site pain (56.8%, n = 217) 2. Fatigue (39.2%, n = 150) 3. Headache (28.5%, n = 109) 4. Fever (26.9%, n = 103) 5. Myalgia (16.2%, n = 62) 6. Arthralgia (12.3%, n = 47) * data for 18 years old or older, 1 × 1011 viral particle dose, 5 × 1010 viral particle dose |
2°C–8°C | 48 |
| Anhui Zhifei Longcom (China) | – | IM (2–3 doses) | Phase-I trial results 1. Injection site itch (19%, n = 29) 2. Injection site redness (16%, n = 24) 3. Injection site swelling (14%, n = 21) 4. Injection site pain (12%, n = 18) 5. Fever (8%, n = 12) 6. Headache (2%, n = 3) * data for 18–59 years old, 25-μg, 3-dose regimen |
2°C–8°C | 49 |
CI, confidence interval; COVID-19, coronavirus disease 2019; IM, intramuscular.
Post-vaccination contagion
With the endurance of the COVID-19 vaccine still being heavily researched, a chief concern is the sustainability of the vaccine-mediated immune response. This is important in the consideration of whether vaccinated individuals could still contract, transmit, or be carriers of SARS-CoV-2 virus. Vaccinated individuals currently may not understand the rationale behind why social restriction rules still apply to them. Most COVID-19 mRNA vaccines require at least 3 weeks to mount an immunological response and create the required antibodies and proliferate accessory cells of the adaptive immune system of the appropriate recognition repertoire. 50 This may be particularly relevant in the context of travel, as the World Health Organization (WHO) states that a proof of vaccination should not exempt international travelers from complying with social restrictions and risk-reduction measures. 51
Contraindications for COVID-19 vaccines
All vaccines are contraindicated in cases of documented hypersensitivity to the active substance or any of the excipients. There are a set of general guidelines relative to patients which must be adhered to until further information is provided; predominantly regarding groups such as pregnant or lactating women and immunodeficient patients. The Centers for Disease Control and Prevention (CDC) considers absolute contraindications to patients who have had severe anaphylactic reactions to a previous dose of an mRNA COVID-19 vaccine or PEG, a component of the vaccine. Moreover, immediate allergic reactions of any severity to polysorbate are also a significant contraindication. Importantly, there are many precautions which are not classified as contraindications but must be considered, such as patients who have had allergic reactions to any vaccine or injectable therapy. In the cases of patients with a precaution to the vaccine, they should be counseled on the benefits and risks, but are not contraindicated from vaccination. 15 In the instance of patients with autoimmune diseases, there is currently insubstantial data regarding the efficacy of the vaccine; however, current guidelines suggest that individuals with autoimmune conditions may take the vaccine if they do not have any absolute contraindications. In the case of patients with HIV, limited data from COVID-19 mRNA vaccination trials suggest that they can receive the vaccine barring any contraindications.
COVID-19 vaccines and pregnancy
Prior to discussing the relationship between the current vaccines for COVID-19 and pregnancy, it is crucial to gain an insight of the relationship between pregnancy and COVID-19 itself. Adhikari et al. showed that there was no difference in the frequency of Caesarean section, pre-eclampsia, preterm births, and abnormal fetal cardiotocography in pregnant women with and without SARS-CoV-2 infection. In addition, examination of the placenta revealed were no abnormalities, which were initially suspected due to the cross-matching between the SARS-CoV-2 spike protein and the placental synctyin-1 protein. 52 Similarly, there was no association found between COVID-19 and first-trimester spontaneous abortions. 53 A systematic review and meta-analysis revealed that COVID-19 leads to higher preterm deliveries (odds ratio (OR): 3.01, 95% CI: 1.16–7.85) and an increase in the ICU admission rates (OR: 71.63, 95% CI: 9.81–523.06) in pregnant women. 54
Pregnancy remained an exclusion criterion for all the COVID-19 vaccine trial; therefore, the efficacy of the COVID-19 vaccines in pregnant women is unavailable. However, given the effectiveness of the influenza vaccines elucidated in a meta-analysis conducted by Quach et al., it can be hypothesized that the effects of pregnancy on the vaccine would be minimal, but more data would be needed for confirmation. 55 Pfizer’s animal studies revealed antibodies in the maternal rats, fetus, and offspring, in addition to no effects on fertility pregnancy or fetal development. 56 A similar study was conducted with the Moderna vaccine which led the US FDA to conclude that the vaccine did not have any adverse effects on female reproduction, fetal development, or postnatal development. 34 Furthermore, the Oxford-AstraZeneca vaccine animal studies are still pending. However, as a precaution, the National Immunization Advisory Committee (NIAC) has recommended for the two-dose schedule to not commence before 14 weeks of gestation and to be completed by week 33 of gestation. This precaution reduces any potential associations with miscarriage or pre-term birth. 57
Despite the exclusion of pregnancy in the preliminary stages of the trials, 23 Pfizer, 13 Moderna, and 21 AstraZeneca subjects became pregnant after enrolment into the trial. Among this cohort, there was one miscarriage part of the Pfizer control group, no miscarriages part of the Pfizer vaccine group, one miscarriage part of the Moderna control group, no miscarriages part of the Moderna vaccine group, three miscarriages part of the AstraZeneca control group, and two miscarriages part of the AstraZeneca vaccine group. While these preliminary numbers support the current guidelines regarding the vaccines being safe in pregnancy, it is crucial to be aware of the ongoing studies as new data emerges.
The CDC v-safe COVID-19 Pregnancy Study explored the effect of mRNA vaccine (Pfizer-BioNTech or Moderna) on the pregnancy. The pregnancy loss within those with a completed pregnancy included a spontaneous abortion (<20 weeks) rate of 12.6% (104 out of 827) and stillbirth (⩾20 weeks) incidence of 0.1% (1 out of 725). 58 The neonatal outcomes within the live birth infant cohort showed preterm birth (<37 weeks) incidence at 9.4% (60 out of 636), small for gestational age incidence of 3.2% (23 out of 724), and congenital anomalies were seen in 2.2% (16 out of 724). 58 No neonatal deaths were observed in this study.
Vaccine dosing strategies
Limited vaccine resources have caused some governments to extend the date of the second dose beyond the recommended manufacturer date. On December 30, NHS England had made the decision to prioritize the administration of the first doses, and to extend the second doses of the vaccine to the end of 12 weeks, rather than the recommended 3–4 weeks as shown in the clinical phase-3 trial. Pfizer-BioNTech at the time had no data to support this decision, and thus stated that the safety and efficacy of the vaccine had not been evaluated on different dosing schedules, and importantly, the second dose should not be administered later than 42 days. 59
Newly accrued evidence might warrant changes in the landscape of this vaccination program. Estimation of the effectiveness of the Pfizer-BioNTech after a single dose from the primary data from Israeli population (n = 500,000) showed that from day 0 to day 8 post–vaccination, the likelihood of contracting COVID-19 infection doubled. 60 This result may appear counterintuitive, but it takes 3 weeks for the vaccine to instill efficacy during which this real-world population could have not maintained the stringent public health measures which lead to the increased incidence in COVID-19 in this time-period. Then from day 8 to day 21 the incidence of COVID-19 declined and at day 21 the vaccine effectiveness was documented at 91%. 60 This efficacy was seen to stabilize at 90% for the duration of the study (9 weeks), and the authors of this study extrapolate this stability up to 6 months. 60 This concludes that the single dose of Pfizer-BioNTech is highly protective from day 21 onwards and supports the NHS England’s vaccination policy for extending gaps between the doses. The data from the Early Pandemic Evaluation and Enhanced Surveillance of COVID-19 (EAVE II) trial in the Scottish population revealed that a single dose of Pfizer (n = 650,000) and Oxford-AstraZeneca (n = 490,000) vaccines resulted in a decline in hospitalization at 4 weeks by 84% and 94%, respectively. 61
However, the trials for the Oxford-AstraZeneca vaccine included varied spacing schedules between doses. The findings from these trials displayed that a greater space between the first and second dose provided a superior immune response. This is supported by a combined trial between a UK and Brazil study, which demonstrated a higher VE 14 days after a second dose in patients who had greater than 6 weeks between their first and second dose than patients who had less than 6 weeks by 53.4%.17,62
It was also proposed that to meet the supply shortage that vaccine dose can be halved. Half-dose of Moderna vaccine (50ug) was in a phase-IIa trial. Immune response in the half-dose group compared to those that received a full dose were the same. Therefore, this dosing strategy is supported from an immunogenicity perspective. It is reasonable to infer that the immunogenicity would translate to immune protection, but unfortunately no clinical trial has validated the immune protection for this dosing strategy.
SARS-CoV-2 genome mutations
Mutations are changes in the SARS-CoV-2 viral genome that occur naturally over time. These mutations from the parent SARS-CoV-2 virus create variants. A certain amount of genetic variation is expected as SARS-CoV-2 replicates as such it is important to monitor circulating viral variants to collate key mutations. Fortunately, coronaviruses have a slower rate of mutation of 1 to 2 nucleotides per month. 63 These definitions become complicated when environmental factors apply selective pressures on these variants that enable them to express distinct phenotypes that may facilitate viral fitness. This ability of a variant to express distinct phenotypes is termed as a strain. A compilation of beneficial lineage defining mutations can create a strain that has a higher transmission rate or induce severe disease. This raises the question: will the current vaccines or convalescent immunity from a non-variant SARS-CoV-2 infection provide adequate immunological protection against these new variants?
Coronaviruses mutate spontaneously via antigenic drift. This process typically utilizes the virus-specific transcription regulatory network (TRN) sequence to initiate the change, resulting in a new mRNA sequence virus being formed. Homologous and genetic recombination allows for the virus to gain more ecological features and has been speculated to be the reason why SARS-CoV-2 was zoonotic in origin. 64 A variant of the original SARS-CoV-2 virus with a D614G substitution in the spike protein encoding gene emerged in early February 2020, and by June 2020, D614G became the dominant form of the virus circulating globally. 65 Studies have shown that the D614G mutation resulted in increased infectivity and transmissibility. 66 Since then, there have been many viral lineages to note, most notable VOC include the B.1.1.7/20I/501.Y.V1 variant that was first detected in the United Kingdom in October 2020, the B.1.351/20 H/501Y.V2 variant that was detected in South Africa in December 2020, and the Lineage P.1. (B.1.1.28.1) variant that was detected in Tokyo in January 2021 but is believed to have originated from Brazil.
Currently, there exists two open-source real-time software tools to analyze and assign nomenclature of genetic variations in the SARS-CoV-2 virus: Nextstrain and PANGOLIN.64,67 Both refer to the GISAID (Global Initiative on Sharing All Influenza Data) genomic database but have slight differences with regards to their nomenclature to describe various lineages of the virus. The COVID-19 Genomics UK Consortium has also developed CoV-GLUE, an open-source browser application that allows for easy referral of all sequenced SARS-CoV-2 genetic replacements, insertions, and deletions. 68 Therefore, sequencing every local infection will yield a repository to track the development of new mutations and variants.
Notable mutation drivers in the SARS-CoV-2 genome
Before diving deeper into these variants, it is important to understand the physical alteration in the S-protein at a molecular level and the perceived functional advantages that the SARS-CoV-2 gains. Table 2 highlights some of the notable S-protein mutations as they evolve amid the pandemic.
Table 2.
Summary of the physical and functional alterations of S-protein due to notable amino acid substitutions.
| Mutation | Alterations in S-protein structure | Functional Consequences for VOC | Distribution Earliest/latest (frequency) |
Notes/references |
|---|---|---|---|---|
| D614G | • Substitution of aspartate to glycine at site
614. • Open conformity of S1 spike protein |
• Increased transmissibility • Increased vulnerability to host immune attack (speculated) • Higher nasal viral loads and correlates with the prevalence of anosmia |
1/3/2020 Switzerland (0.4) UK (0.19) France (0.15) Italy (0.11) |
69,70 |
| E484K | • Substitution of glutamate to lysine at site 484 • This change takes place in the receptor-binding motif on RBD |
• Increased ACE2 binding • Potential for escaping recognition from S-protein neutralizing antibodies. • Documented case of reinfection • Moderna and Pfizer have shown small but significant reduction in neutralization |
12/10/2020 Brazil (0.08) South Africa (0.06) 08/02/2021 Brazil (0.58) France (0.22) |
71,72 |
| N501Y | • Substitution from asparagine to tyrosine at position
501 • This change takes place at the RBD of the S-protein |
• Increase ACE2 binding due to increase duration in open
conformation. • Potential for escaping recognition from S-protein neutralizing antibodies. • >Moderna and Pfizer have shown small but significant reduction in neutralization. |
01/06/2020 Netherlands (0.01) 08/02/2021 UK (0.87) France (0.53) Australia (0.43) Brazil (0.42) |
72 –74 |
| HV69-70 del | • Deletion of histidine at site 69 and valine at site 70 in the
S1 domain of S-protein. • Predicted altered structure will be a ‘tucked in’ spike N-terminal domain |
• Potential for escaping recognition from S-protein neutralizing antibodies. The serum virus neutralization (SVN) assay showed reduced neutralization to human SARS-CoV-2 convalescent plasma. | 10/08/2020 Switzerland (0.04) Denmark (0.01) 08/02/2021 UK (0.87) Australia (0.45) France (0.36) Singapore (0.26) |
75,76 |
| P681H | • Substitution of proline to histidine at position 681 is immediately adjacent to the furin cleavage site between S1 and S2 in S-protein | • Enables increased cleavage activity by TMPRSS2. Therefore, increase SARS-CoV-2 entry | 12/10/2020 Nigeria (NA) UK (NA) |
64 |
RBD, receptor-binding domain; VOC, variants of concern.
Notable emerging VOC
Newly emerged variants of SARS-CoV-2 have now become VOC which can be attributed to their new ability of increased transmission and infectivity. Therefore, it is important to collate the data on the mutations they acquired, the extend of spread, and the efficacy of different vaccines to create a repository for further analysis (Table 3).
Table 3.
Summary of data on features, acquired cluster of S-protein mutations, and vaccine efficacy studies for the major COVID-19 variants of concern.
| Names (PANGOLIN, Nexstrain, Media) | Features | Notable mutations in S-Protein | Vaccine efficacy reduction | Countries reported (n) as of August 17, 2021 | References |
|---|---|---|---|---|---|
| B.1.1.7, 20I/501.Y.V1, VOC/20201201, UK strain (Alpha Variant) |
• Increased binding to ACE2 receptor • 30–70% increased transmissibility • Realistic possibility of increased severity Reproduction rate [range 1.5–1.7] • Higher nasal viral load and increased shedding, prolonged viral shedding, and heighten stability in the current environment • Decreased neutralization |
• N501Y • HV69-70 del • P681H • Y144 del, • A570D • E484K • D614G |
• Efficacy data • Novavax 86% • Pfizer/BioNTech Single dose 47.5% (95% CI: 41.6–52.8) • Pfizer/BioNTech Dual dose 93.7% (95% CI: 91.6–95.3) • AstraZeneca Single dose 48.7% (95% CI: 45.2–51.9) • AstraZeneca dual dose 74.5% (95% CI: 68.4–79.4) • Mean loss in neutralization: • At day 43 after dual doses at day 28 • Moderna (n = 12): 1.8-fold • Pfizer/BioNTech (n = 10): 2-fold |
190 | 64,77 –81 |
| B.1.351, 20H/501.Y.V2, South African strain (Beta Variant) |
• Increased severity • Increased transmission • Reinfection is possible as the convalescent immunity cannot mount a response against this new variant |
• E484K • K417N • N501Y • D614G orf1b deletion |
• Efficacy data • Janssen Vaccine (moderate to severe at day 28) 64.0% (95% CI: 41.2–78.7) • Janssen Vaccine (Severe at day 28) 81.7% (95% CI: 46.2–95.4) • Novavax Dual dose 60.1% (95%CI: 19.9–80.1) • AstraZeneca Dual dose 10.4% (95% CI: −76.8 to 54.8) • Mean loss in neutralization compared to wild type: • Moderna (n = 12): 8.6-fold • Pfizer/BioNTech (n = 10): 6.5-fold • BBIBP-CorV: 10-fold |
138 | 20,28,79,81 –84 |
| Lineage P.1, B.1.1.28.1, Brazilian strain (Gamma Variant) |
• Increased severity • Increased transmissibility • Documented case of reinfection |
• N501Y • E484K • D614G • K417N/T • orf1b deletion |
• Pfizer/BioNTech: Significant reduction in
neutralization • Moderna: Significant reduction in neutralization • SinoVac: seroconversion and geometric mean titres in the neutralizing antibody assays |
82 | 26,81,85,86 |
| B.1.617.2 Indian Strain (Delta Variant) |
• Increase transmission • Decrease neutralization |
• L452R • D614R • P681R |
• Efficacy data • Pfizer/BioNTech Single dose 35.6% (95% CI: 22.7–46.4) • Pfizer/BioNTech Dual dose 88.0% (95% CI: 85.3–90.1) • AstraZeneca Single dose 30.0% (95% CI: 24.3–35.3) • AstraZeneca Dual dose 67.0% (95% CI: 61.3–71.8) • BBV152 Dual Dose 65·2% (95% CI: 33·1–83·0) • Mean loss in neutralization compared to wild type: • BBIBP-CorV: 1.38- fold |
148 | 80,81,87 |
CI, confidence interval; COVID-19, coronavirus disease 2019; VOC, variants of concern.
There are more variants emerging as the pandemic progresses, but it is important to note that there is still a myriad of available vaccines in our armamentarium that are adequately efficacious in the performed neutralization assays as well as the real-world data. Furthermore, while vaccines induce the antibody-dependent immunity, they can also stimulate other components of the adaptative immune system such as the Memory B-cells, CD8+ Tc cells, and CD4+ Th cells to mount their own response against the viral variants. This can compensate for the reduction in neutralization rate by the vaccine induced antibodies. Interestingly, the adaptative immune system can proliferate libraries of memory B-cells with mutated antibody repertoires that can predict viral variants. Therefore, it is prudent to commence vaccinations in accordance with the local public health bodies. This combined with the continued implementation of public health measures until target level of herd immunity is acquired can lead toward mitigating the prevalence and incidence of COVID-19 variants.
Conclusion
This review highlighted the current available vaccines and candidates being rolled out amid the ongoing prevention measures and summarized the documented findings with regards to their efficacies, side-effects, and storage requirements. An overview of the physiology of immunogenic responses against the disease provided by the more prominent vaccines were discussed, alongside questions regarding the implementation of vaccines; heterologous prime-boosting, vaccine contraindications, dosing strategies, side effects, and the presence of SARS-CoV-2 mutations and variants.
There are still many unanswered questions that need to be addressed with regards to antibodies produced in individuals including their impact on the clinical course and severity of the disease, how long will they remain in the body to protect from the disease, and if what we have is enough to deal with newly emerging variants. Studies on these topics are rapidly being conducted and published on a global scale, and scientific communities are working on the clock to produce as much information to bring us a better understanding on how to deal with this disease.
For this global pandemic to end, it is imperative that people are vaccinated as quickly as possible until herd immunity can be achieved. One aspect of achieving this, in the face of vaccine hesitancy, is to address the lack of community understanding on how vaccines work, the risks, and the factors that keep this area of research volatile and distribution policies ever-changing. In addition, it is important to remain cautious about the information being released and to trust the accredited sources and experts, rather than the aberrant rumors being spread through social media. Nonetheless, the COVID-19 vaccines have shown to be highly promising and we recommend for everyone that is eligible to take the vaccine at the correct dosing interval when they are given the chance as this would potentiate a positive trend toward pandemic resolution.
Footnotes
Authors’ contributions: CY, AA, Amogh P, Akul P, AP performed acquisition and curation of the data; CY, AA, Amogh P, Akul P, AP, YYL and PK analyzed the data, performed interpretation of the data, and wrote of the original draft; YYL and PK performed the critical revision; All authors have read and approved the final manuscript.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Pramath Kakodkar  https://orcid.org/0000-0002-5288-1247
https://orcid.org/0000-0002-5288-1247
Contributor Information
Charles Yap, School of Medicine, National University of Ireland, Galway, Ireland.
Abulhassan Ali, School of Medicine, National University of Ireland, Galway, Ireland.
Amogh Prabhakar, School of Medicine, National University of Ireland, Galway, Ireland.
Akul Prabhakar, School of Medicine, National University of Ireland, Galway, Ireland.
Aman Pal, School of Medicine, National University of Ireland, Galway, Ireland.
Ying Yi Lim, School of Medicine, National University of Ireland, Galway, Ireland.
Pramath Kakodkar, School of Medicine, National University of Ireland, Galway, University Road, Galway H91 TK33, Ireland.
References
- 1. Johns Hopkins University & Medicine. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Coronavirus Resource Center, Johns Hopkins University & Medicine, 2021, https://coronavirus.jhu.edu/map.html
- 2. McCormick LC, Benhamou M, Pogkas D. The Covid-19 pandemic has added $19.5 trillion to global debt. Bloomberg, 27 January 2021, https://www.bloomberg.com/graphics/2021-coronavirus-global-debt/#:~:text=The%20Covid%2D19%20Pandemic%20Has%20Added%20%2419.5%20Trillion%20to%20Global%20Debt
- 3. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021; 371(6529): eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO. COVID-19 vaccine tracker and landscape, 2021, https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 5. Krammer F. SARS-CoV-2 vaccines in development. Nature 2020; 586(7830): 516–527. [DOI] [PubMed] [Google Scholar]
- 6. Florindo HF, Kleiner R, Vaskovich-Koubi D, et al. Immune-mediated approaches against COVID-19. Nat Nanotechnol 2020; 15(8): 630–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet 2020; 396(10262): 1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clem A. Fundamentals of vaccine immunology. J Glob Infect Dis 2011; 3(1): 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383(27): 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pfizer COVID-19 Vaccine EUA Letter of Authorization. Sect. Section 564(b)(1)(C) (2020). [Google Scholar]
- 11. Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 2020; 383(25): 2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hinton DM. REG 174 INFORMATION FOR UK HEALTHCARE PROFESSIONALS. The UK Department of Health and Social Care and the Medicines & Healthcare products Regulatory Agency, 2021, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/955899/Temporary_Authorisation_HCP_Information_BNT162_6_0_UK_editclean.pdf
- 13. World Health Organization (WHO). Interim recommendations for use of the Moderna mRNA-1273 vaccine against COVID-19: interim guidance. WHO, Geneva, 25 January 2021, https://www.who.int/publications/i/item/interim-recommendations-for-use-of-the-moderna-mrna-1273-vaccine-against-covid-19 [Google Scholar]
- 14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2020; 384(5): 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Interim clinical considerations for use of mRNA COVID-19 vaccines currently authorized in the United States, 2021, https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
- 16. Van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv. Epub ahead of print 13 May 2020. DOI: 10.1101/2020.05.13.093195. [DOI] [Google Scholar]
- 17. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397(10269): 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020; 396(10249): 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. WHO. Statement for healthcare professionals: how COVID-19 vaccines are regulated for safety and effectiveness, 2021, https://www.who.int/news/item/11-06-2021-statement-for-healthcare-professionals-how-covid-19-vaccines-are-regulated-for-safety-and-effectiveness
- 20. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021; 384(23): 2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2020; 397: 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021; 21(2): 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021; 21: 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mallapaty S. China COVID vaccine reports mixed results – what does that mean for the pandemic? Nature. Epub ahead of print 15 January 2021. DOI: 10.1038/d41586-021-00094-z. [DOI] [PubMed] [Google Scholar]
- 25. Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med 2021; 385: 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palacios R, Batista AP, Albuquerque CS, et al. Efficacy and safety of a COVID-19 inactivated vaccine in healthcare professionals in Brazil: the PROFISCOV study, 2021, https://europepmc.org/article/ppr/ppr341815
- 27. Ella R, Vadrevu KM, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis 2021; 21: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Novavax COVID-19 vaccine demonstrates 89.3% efficacy in UK phase 3 trial. Novavax, Inc, 2021, https://ir.novavax.com/news-releases/news-release-details/novavax-covid-19-vaccine-demonstrates-893-efficacy-uk-phase-3
- 29. Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA 2021; 326(1): 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weller C. Four reasons why we need multiple vaccines for Covid-19. Wellcome, 2020, https://wellcome.org/news/four-reasons-why-we-need-multiple-vaccines-covid-19
- 31. Centers for Disease Control and Prevention. Local reactions, systemic reactions, adverse events, and serious adverse events: Pfizer-BioNTech COVID-19 vaccine. Vaccines & Immunizations, 2020, https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
- 32. Vaccines and Related Biological Products Advisory Committee. Briefing document, 2020, https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-october-22-2020-meeting-announcement
- 33. Moderna. Clinical trial results, 2021, https://www.modernatx.com/covid19vaccine-eua/providers/clinical-trial-data
- 34. Vaccines and Related Biological Products Advisory Committee Meeting December 17, 2020. Sect. Section 564 of the Federal Food, Drug, and Cosmetic Act (2020), https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-december-17-2020-meeting-announcement
- 35. COVID-19 Vaccine AstraZeneca, COVID-19 Vaccine (ChAdOx1-S [Recombinant]) (ed European Medicines Agency). European Medicines Agency, 2021, https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca
- 36. Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 2021; 397: 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med 2021; 384: 1824–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet 2020; 396(10255): 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ella R, Reddy S, Jogdand H, et al. Safety and immunogenicity clinical trial of an inactivated SARS-CoV-2 vaccine, BBV152 (a phase 2, double-blind, randomised controlled trial) and the persistence of immune responses from a phase 1 follow-up report. medRxiv. Epub ahead of print 22 December 2020. DOI: 10.1101/2020.12.21.20248643. [DOI] [Google Scholar]
- 40. Ella R, Reddy S, Blackwelder W, et al. Efficacy, safety, and lot to lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): a, double-blind, randomised, controlled phase 3 trial. medRxiv. Epub ahead of print 2 July 2021. DOI: 10.1101/2021.06.30.21259439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis 2021; 21(1): 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. WHO. Evidence assessment: sinopharm/BBIBP COVID-19 vaccine, 2021, https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/2_sage29apr2021_critical-evidence_sinopharm.pdf
- 43. Keech C, Albert G, Cho I, et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med 2020; 383(24): 2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med 2021; 385: 1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ward BJ, Gobeil P, Séguin A, et al. Phase 1 trial of a candidate recombinant virus-like particle vaccine for Covid-19 disease produced in plants. medRxiv. Epub ahead of print 6 November 2020. DOI: 10.1101/2020.11.04.20226282. [DOI] [Google Scholar]
- 46. Kremsner P, Mann P, Bosch J, et al. Phase 1 assessment of the safety and immunogenicity of an mRNA- lipid nanoparticle vaccine candidate against SARS-CoV-2 in human volunteers. medRxiv. Epub ahead of print 9 November 2020. DOI: 10.1101/2020.11.09.20228551. [DOI] [Google Scholar]
- 47. CureVac. CureVac provides update on phase 2b/3 trial of first-generation COVID-19 vaccine candidate, CVnCoV, 2021, https://www.curevac.com/en/2021/06/16/curevac-provides-update-on-phase-2b-3-trial-of-first-generation-covid-19-vaccine-candidate-cvncov/
- 48. Zhu F-C, Guan X-H, Li Y-H, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020; 396(10249): 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang S, Li Y, Dai L, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD protein vaccine against COVID-19 in adults: pooled analysis of two randomized, double-blind, placebo-controlled, phase 1 and 2 trials. medRxiv. Epub ahead of print 22 December 2020. DOI: 10.1101/2020.12.20.20248602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wisnewski AV, Campillo Luna J, Redlich CA. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS One 2021; 16(6): e0249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. World Health Organization (WHO). Statement on the sixth meeting of the International Health Regulations (2005) Emergency Committee regarding the coronavirus disease (COVID-19) pandemic. WHO, 2021, https://www.who.int/news/item/15-01-2021-statement-on-the-sixth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic [Google Scholar]
- 52. Adhikari EH, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open 2020; 3(11): e2029256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cosma S, Carosso AR, Cusato J, et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: a case-control study of 225 pregnant patients. Am J Obstet Gynecol 2021; 224(4): 391.e1–391.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020; 370: m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Quach THT, Mallis NA, Cordero JF. Influenza vaccine efficacy and effectiveness in pregnant women: systematic review and meta-analysis. Matern Child Health J 2020; 24(2): 229–240. [DOI] [PubMed] [Google Scholar]
- 56. Clean COVID-19 Vaccine SmPC-PL (ed European Medicines Agency). European Medicines Agency, 2020, https://ec.europa.eu/health/documents/community-register/2020/20201221150522/anx_150522_en.pdf
- 57. Clinical guidance for COVID-19 vaccination. Health Service Executive Ireland, 2021, https://www.hse.ie/eng/health/immunisation/hcpinfo/covid19vaccineinfo4hps/clinicalguidance.pdf
- 58. Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med 2021; 384(24): 2273–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tauh T, Mozel M, Meyler P, et al. What is the evidence for extending the SARS-CoV-2 (COVID-19) vaccine dosing schedule? BC Med J 2021; 63: 67–70. [Google Scholar]
- 60. Hunter PR, Brainard J. Estimating the effectiveness of the Pfizer COVID-19 BNT162b2 vaccine after a single dose. A reanalysis of a study of ‘real-world’ vaccination outcomes from Israel. medRxiv. Epub ahead of print 3 February 2021. DOI: 10.1101/2021.02.01.21250957. [DOI] [Google Scholar]
- 61. University of Oxford. Coronavirus vaccination linked to substantial reduction in hospitalisation, real-world data suggests, 2021, https://www.ox.ac.uk/news/2021-02-22-coronavirus-vaccination-linked-substantial-reduction-hospitalisation-real-world-data#
- 62. Iacobucci G, Mahase E. Covid-19 vaccination: what’s the evidence for extending the dosing interval? BMJ 2021; 372: n18. [DOI] [PubMed] [Google Scholar]
- 63. Duchene S, Featherstone L, Haritopoulou-Sinanidou M, et al. Temporal signal and the phylodynamic threshold of SARS-CoV-2. Virus Evol 2020; 6(2): veaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rambaut A, Holmes EC, O’Toole Á, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol 2020; 5(11): 1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. World Health Organization (WHO). SARS-CoV-2 variants. WHO, 31 December 2020. [Google Scholar]
- 66. Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell 2020; 182(4): 812–827.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hadfield J, Megill C, Bell SM, et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics 2018; 34(23): 4121–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Singer J, Gifford R, Cotten M, et al. CoV-GLUE: a web application for tracking SARS-CoV-2 genomic variation, 2020, http://cov-glue.cvr.gla.ac.uk/#/home
- 69. Butowt R, Bilinska K, Von Bartheld CS. Chemosensory dysfunction in COVID-19: integration of genetic and epidemiological data points to D614G spike protein variant as a contributing factor. ACS Chem Neurosci 2020; 11(20): 3180–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Korber B, Fischer WM, Gnanakaran S, et al. Spike mutation pipeline reveals the emergence of a more transmissible form of SARS-CoV-2. bioRxiv, 2020, https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/ppbiorxiv-069054
- 71. Nonaka C, Franco M, Gräf T, et al. Genomic evidence of sars-CoV-2 reinfection involving E484K spike mutation, Brazil. Emerg Infect Dis 2021; 27(5): 1522–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 2021; 592: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nelson G, Buzko O, Spilman P, et al. Molecular dynamic simulation reveals E484K mutation enhances spike RBD-ACE2 affinity and the combination of E484K, K417N and N501Y mutations (501Y.V2 variant) induces conformational change greater than N501Y mutant alone, potentially resulting in an escape mutant, bioRxiv, 2021, https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/ppbiorxiv-426558
- 74. Teruel N, Mailhot O, Najmanovich RJ. Modelling conformational state dynamics and its role on infection for SARS-CoV-2 spike protein variants. PLoS Comput Biol 2021; 17(8): e1009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McCarthy KR, Rennick LJ, Nambulli S, et al. Natural deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape. bioRxiv, 2020, https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/ppbiorxiv-389916 [DOI] [PMC free article] [PubMed]
- 76. Kemp SA, Harvey WT, Datir RP, et al. Recurrent emergence and transmission of a SARS-CoV-2 spike deletion ΔH69/V70. bioRxiv. Epub ahead of print 15 December 2020. DOI: 10.1101/2020.12.14.422555. [DOI] [Google Scholar]
- 77. Lai MMC. Coronavirus: organization, replication and expression of genome. Annu Rev Microbiol 1990; 44: 303–333. [DOI] [PubMed] [Google Scholar]
- 78. Horby P, Huntley C, Davies N, et al. NERVTAG, 2021, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/961037/NERVTAG_note_on_B.1.1.7_severity_for_SAGE_77__1_.pdf
- 79. Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021; 593: 130–135. [DOI] [PubMed] [Google Scholar]
- 80. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med 2021; 385(7): 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. WHO. Weekly epidemiological update on COVID-19 – 17 August 2021, https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19–17-august-2021
- 82. Sargent J, Kumar S, Buckley K. Johnson & Johnson announces single-shot Janssen COVID-19 vaccine candidate met primary endpoints in interim analysis of its phase 3 ENSEMBLE trial. Johnson & Johnson, 2021, https://www.jnj.com/johnson-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial
- 83. Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med 2021; 384(20): 1885–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shinde V, Bhikha S, Hoosain Z, et al. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N Engl J Med 2021; 384(20): 1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Naveca F, Nascimento V, Souza V, et al. SARS-CoV-2 reinfection by the new variant of concern (VOC) P.1 in Amazonas, Brazil, Virological.org, 2021, https://virological.org/t/sars-cov-2-reinfection-by-the-new-variant-of-concern-voc-p-1-in-amazonas-brazil/596
- 86. Living evidence – COVID-19 vaccines: agency for clinical innovation, NSW, Australia, 2021, https://aci.health.nsw.gov.au/covid-19/critical-intelligence-unit/covid-19-vaccines
- 87. Jeewandara C, Aberathna IS, Pushpakumara PD, et al. Antibody and T cell responses to Sinopharm/BBIBP-CorV in naïve and previously infected individuals in Sri Lanka. medRxiv. Epub ahead of print 19 July 2021. DOI: 10.1101/2021.07.15.21260621. [DOI] [Google Scholar]