Abstract
BACKGROUND
Brain mapping is the most reliable intraoperative tool for identifying surrounding functional cortical and subcortical brain parenchyma. Brain mapping procedures are nuanced and require a multidisciplinary team and a well-trained neurosurgeon. Current training methodology involves real-time observation and operation, without widely available surgical simulation.
OBJECTIVE
To develop a patient-specific, anatomically accurate, and electrically responsive biomimetic 3D-printed model for simulating brain mapping.
METHODS
Imaging data were converted into a 2-piece inverse 3D-rendered polyvinyl acetate shell forming an anatomically accurate brain mold. Functional and diffusion tensor imaging data were used to guide wire placement to approximate the projection fibers from the arm and leg areas in the motor homunculus. Electrical parameters were generated, and data were collected and processed to differentiate between the 2 tracts. For validation, the relationship between the electrical signal and the distance between the probe and the tract was quantified. Neurosurgeons and trainees were interviewed to assess the validity of the model.
RESULTS
Material testing of the brain component showed an elasticity modulus of 55 kPa (compared to 140 kPa of cadaveric brain), closely resembling the tactile feedback a live brain. The simulator's electrical properties approximated that of a live brain with a voltage-to-distance correlation coefficient of r2 = 0.86. Following 32 neurosurgeon interviews, ∼96% considered the model to be useful for training.
CONCLUSION
The realistic neural properties of the simulator greatly improve representation of a live surgical environment. This proof-of-concept model can be further developed to contain more complicated tractography, blood and cerebrospinal fluid circulation, and more in-depth feedback mechanisms.
Keywords: 3D printing, Awake craniotomy, Brain mapping, Neurophysiologic monitoring, Surgical simulation
ABBREVIATIONS
- 3D
3-dimensional
- DAQ
data acquisition
- OR
operating room
- PVA
polyvinyl acetate
- VR
virtual reality
Functional brain mapping was introduced over a century ago by innovators such as Horsley, Sherrington, Cushing, and Penfield,1-4 and is based upon the principle of nerve conduction. Currently, this technique is widely used in neurosurgery with applications including neuro-oncology, epilepsy surgery, and surgery of selective vascular lesions.5-7 Functional brain mapping represents the most reliable tool for identifying and protecting eloquent brain tissue, including speech and motor tracts, during surgery.8-11
Mastering the technique of brain mapping requires rigorous training including live observation and operation, which is complicated by changing expectations of attending physicians and reduced resident exposure and autonomy due to the recent COVID-19 pandemic.12 Surgical education through simulation will reduce the learning curve and error rates, increase resident autonomy, and most importantly increase patient safety and improve patient outcomes.
Simulators have proven validity and demonstrable transfer of skills to the clinical setting.13 There is currently no commercialized surgical simulator of brain mapping; trainees are limited to learn by the classic teaching method of “see one, do one, and teach one,” instead of a more appropriate “see one, simulate one (or many), do one, and teach everyone.”14 The anatomy and nuances of brain mapping surgery can be simulated using current 3-dimensional (3D) printing techniques to replicate functional white matter tracts and cortex. In this article, we present a proof-of-concept of an anatomically accurate and electrically responsive high-definition biomimetic 3D-printed tool for training and simulating brain mapping.
METHODS
Design Ideation
Thirteen neurosurgeons and neurosurgery residents were interviewed in an open-ended format to determine critical design aspects and educational value of a 3D-printed training model (Table 1). This study was approved by our institutional review board (IRB#16-009946), and patient consent was waived as only retrospective anonymized imaging data was used.
TABLE 1.
Example Questions for Interviews With Neurosurgeons
| Category | Question |
|---|---|
| Surgical training | What were the methods used to train surgical skills at your medical school/residency program? |
| Operating procedures | How did you prepare for performing your first procedure in the OR? What challenges did you face in your first awake procedure? |
Model Creation
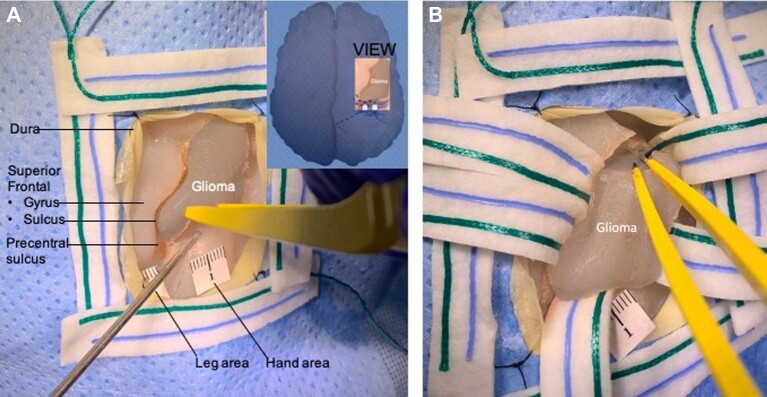
Anonymized brain magnetic resonance imaging (MRI) data were converted into a 2-piece inverse 3D rendered shell, which was printed from polyvinyl acetate (PVA) using MakerBot method 3D printers (MakerBot, New York City, USA, 2019) (Table 2). After printing, the 2 pieces of the shell were attached, forming an anatomically accurate mold of the brain. Then, a preset silicone molded tumor dyed with the FUSEFX BC-03 dye was inserted into the full brain mold in the superolateral surface in the posterior end of the middle frontal gyrus anterior to the motor cortex (Figures 1 and 2). The tumor was further dyed with a layer of blue silicone pigment for a more life-like grayish appearance. The shell was filled with Ecoflex-20 silicone by Smooth-On and dyed with equal parts of the FUSEFX silicone dyes S-301-D and BC-03 to create the opaque pink color. Once the silicone cured for 12 h, the silicone-filled shell was completely submerged in water to dissolve the PVA mold.
TABLE 2.
Printer Settings for MakerBot Method Print of 3D Brain Mold
| Extruder | Setting type | Setting |
|---|---|---|
| Primary filament (extruder in slot 1) | Extruder type | MakerBot Experimental Extruder |
| Extruder temperature | 210°C | |
| Material | PVA | |
| Speed: insets | 50 mm/s | |
| Speed: sparse | 90 mm/s | |
| Retraction density | 1.5 mm | |
| Infill density | 20% | |
| Infill pattern | Diamond (fast) | |
| Travel speed | 150 mm/s | |
| Support filament (extruder in slot 2) | Extruder type | MakerBot Support Extruder |
| Extruder temperature | 210°C | |
| Materials | PVA | |
| Support type | Breakaway | |
| Infill density | 15% | |
| Angle | 40° |
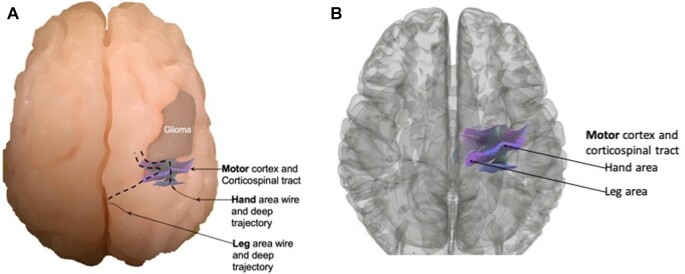
FIGURE 1.
Anatomic placement of glioma tumor near motor tracts. A, Superior view of the model showing the hand/arm and leg area wires exiting the superolateral surface of the brain at the precentral gyrus (motor cortex) and their deep trajectories corresponding with the corticospinal tracts. B, A 3D reconstruction of a magnetic resonance tractography used to guide wire placement posterior to the glioma in an anatomically accurate location.
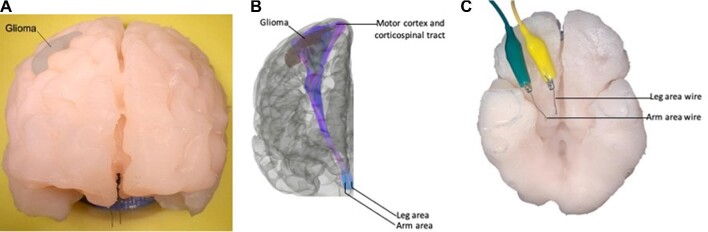
FIGURE 2.
Representative views of the brain and electrical components of the simulator. Anterior A and inferior C views of the simulator showing the leg and arm area wires exiting on the inferior surface in the region of the midbrain's cerebral peduncles. B, Anterior view of a 3D reconstruction of a magnetic resonance tractography used to guide wire placement posterior to the glioma in an anatomically accurate location.
Incorporation of Imaging
Functional MRI and diffusion tensor imaging (DTI) data were analyzed in the open-source software 3D Slicer15 (Slicer 4.10.2, www.slicer.org, 2019) to generate tractography. This was used to guide wire placement in the created mold, to approximate the projection fibers from the arm and leg areas in the motor homunculus that should be mapped and preserved during surgery (Figures 1B and 2B).
Electrical Tract Implementation
The wires generate electric fields that are detected by a handheld probe through electrical coupling. The handheld probe consists of a shielded wire connected to a data acquisition (DAQ) system (myDAQ 19.0.0, National Instruments, Austin, USA, 2019). The electric fields were generated digitally through MATLAB (MATLAB R2020b, The MathWorks, Inc., Natick, USA, 2020) with a 2Vpp square wave running through each tract, at frequencies of 10 and 20 kHz, respectively. Electrical parameters remain constant in both cortical and subcortical regions of the tract. Collected raw data pass from the DAQ device through in-house processing software written in LabVIEW (LabVIEW 2020, National Instruments, Austin, USA, 2020). Processing includes a bandpass filter between 5 and 20 kHz, amplification with a gain of 30, and spectral power analysis to discriminate between the 2 tracts and predict relative location. Following the creation of the model, intellectual property was protected with a provisional patent application.16
Validation
The relationship between the electrical signal acquired and the distance between the probe and the simulated tract was quantified (n = 3 trials) by measuring the response recorded by the simulator between 0 and 3 cm at increments of 0.5 cm using a standard ruler. A baseline reading was taken at a distance greater than 30 cm from the model for calibration purposes.
A literature review was conducted to gather the elasticity, in kilopascals (kPa), of human tissue, as well as animal tissues from Rhesus monkeys, rodents, cows, and pigs. These values were then compared to the elasticity of Ecoflex-20 silicone to demonstrate material accuracy.
A follow-up survey was conducted in Likert format among 32 neurosurgical professionals in order to assess the model's validity and applicability to surgical and educational scenarios.
Statistical Analysis
To determine critical product features during design ideation, 50% of interviews needed to mention a topic for it to be included in the simulator. Electrical data were assessed with a linear regression and estimation of fit via the calculation of r2. A 95% CI based on observed variance was calculated to ensure that no outliers were present. Material results are reported as percent improvement over baseline, as results were compared to values from literature.
RESULTS
Neurosurgeon Interviews and Design Ideation
Via multiple interviews (n = 13) with neurosurgeons who routinely perform awake and asleep craniotomies for brain tumor resection, we identified 3 primary needs to guide the design of the simulator: anatomic accuracy (n = 8/13, 61.5%), tactile feedback (n = 7/13, 54%), and stimulation feedback (n = 7/13, 54%). Emphasis was thus placed on creating an ideal simulator that would replicate not only the brain anatomy and elasticity, but also the brain's electrical conductivity (n = 13/13, 100%).
Physical Model
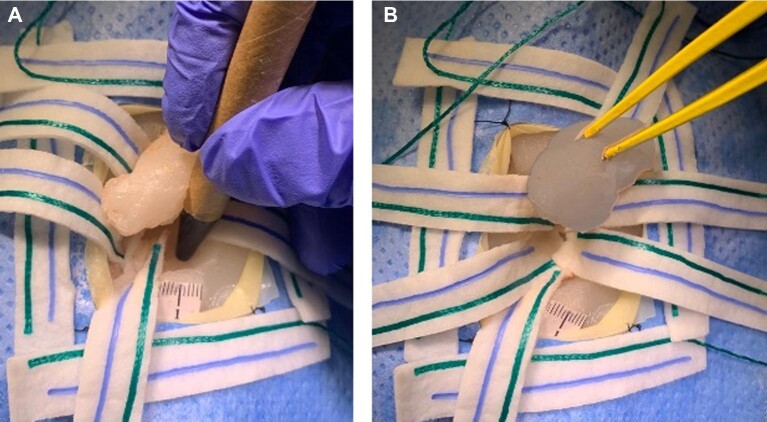
The final brain model used 540 g of PVA, contained 810 g of silicone, and measured 16 × 12 × 10 cm (Figure 3, Table 3). This simulator allows for replication of various surgical scenarios and training performance of microsurgical techniques including brain mapping (Figure 4) and tumor resection (Figure 5). Specifically, the simulator can be used for mapping functional cortex and subcortical regions adjacent to a glioma to perform safe resection (Figure 6).
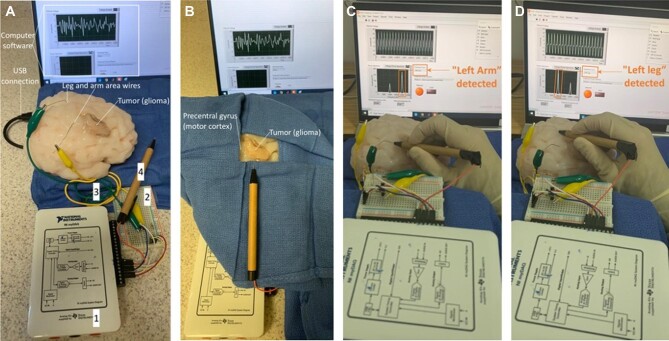
FIGURE 3.
Brain mapping simulator setup for data acquisition and general training and usage. A, Right superolateral view of brain mapping simulator shown with (1) NI myDAQ device, (2) custom circuit interfacing, (3) white matter tract models, and (4) handheld probe. Also depicted are the associated computer software interface and feedback mechanism (on screen connected through USB cable), locations of leg and arm white matter tract models in the simulator body, and location of glioma. B, Draped view of the brain mapping simulator showing a potential surgical training scenario with a glioma near the precentral gyrus (motor cortex) and the handheld electrical probe. C, Arm area stimulation. Surgeon uses handheld probe to stimulate the arm area in motor cortex and receives written and LED feedback on the screen (in orange). D, Leg area stimulation. Surgeon uses handheld probe to stimulate the leg area in motor cortex and receives written and LED feedback on the screen (in orange).
TABLE 3.
Design Features for a Functional Brain Model for Surgical Procedure Training
| Input description | Neurosurgeon needs | Design feature | Metric |
|---|---|---|---|
| Physical embodiment | Anatomically accurate | Brain weight | 810 g |
| Brain width | 12 cm | ||
| Brain length | 16 cm | ||
| Brain height | 10 cm | ||
| Tactile feedback | Elasticity | 55 kPa | |
| Performance | Receives electrical stimulation | Stimulation intensity | 2 Vpp sine wave |
| Linear voltage-to-distance relationship | Slope = –1.96 | ||
| Shelf life | Better shelf life than cadaver | Shelf life | >6 yr |
| Stable at room temperature | 25 °C | ||
| Human factors | Can be used to train for longer than 1 procedure | Duration of single use | Up to 2.8 h |
| Regulatory consideration | FDA regulatory class 1 | Qualifies as a class 1 device for FDA | Class 1 |
These metrics were determined to be the most important to incorporate into the brain mapping simulator.
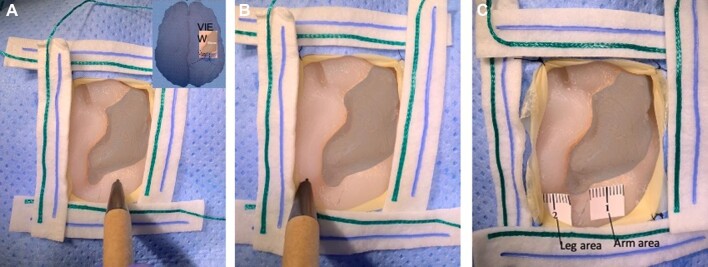
FIGURE 4.
Simulation of brain cortical mapping. Insert showing the intraoperative view. A, Arm area stimulation. B, Leg area stimulation. C, Functional arm (#1) and leg (#2) areas are tagged.
FIGURE 5.
Demonstration of microsurgical tumor resection A and hemostasis B.
FIGURE 6.
Demonstration of subcortical mapping A and final tumor en-bloc resection B.
Validation of Electrical Response
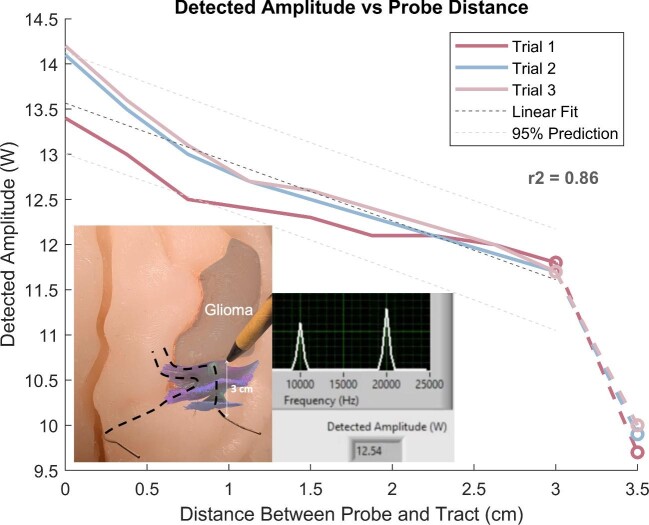
The linear relationship between distance and amplitude was determined to be an acceptable representation of the properties of live brain tissue (Figure 7). A linear regression was performed on the data (n = 3 trials), showing an r2 = 0.86. Additionally, almost all data (n = 25/27, 93%) fell within the 95% CI that was calculated from observed data, indicating that there were few outliers, and that the device produced consistent results.
FIGURE 7.
Detected amplitude vs probe distance. Line graph showing the relationship quantified between the distance of the handheld probe to the nearest tract, and the electric field amplitude detected at that point. Linear regression and estimation of fit were performed to find an r2 value of 0.86. 95% CI shown in dashed lines. Left inset image: close-up of data acquisition process using the simulator and probe. Right inset image: example analyzed output on our custom interface.
Validation of Brain Model Material
Brain tissue hardens postmortem. The elastic modulus of a fixed Rhesus monkey brain measures up to 140 kPa,17 compared to the approximate elasticity of live brain tissue, which is reported between ∼1.9 and 60 kPa.17-21 Our silicone brain model has an elasticity of only 55 kPa,22 which is within the range of reported biological values and over 60% closer to the reported average of 14.8 kPa when compared to cadaveric brain tissue (Figure 8).
FIGURE 8.
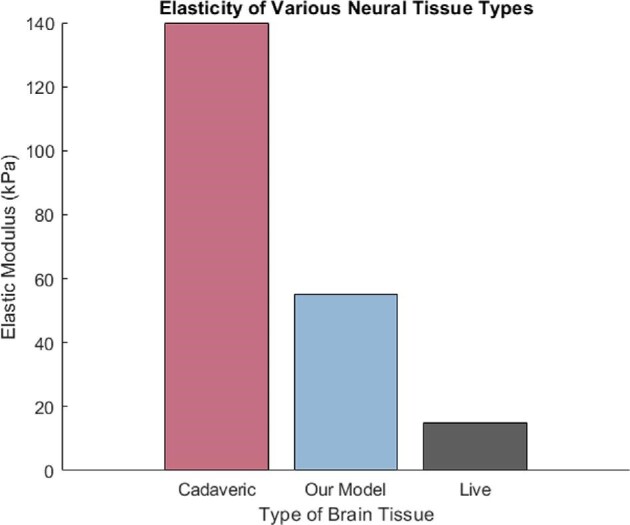
Elasticity of various neural tissue types. Bar graph comparing the elasticity of cadaver brain tissue (the current gold standard of surgical training) (140 kPa), our model's elasticity (55 kPa), and live brain tissue (the ideal target for model's elasticity) (mean = 14.8 kPa).
Validation of Model Translation
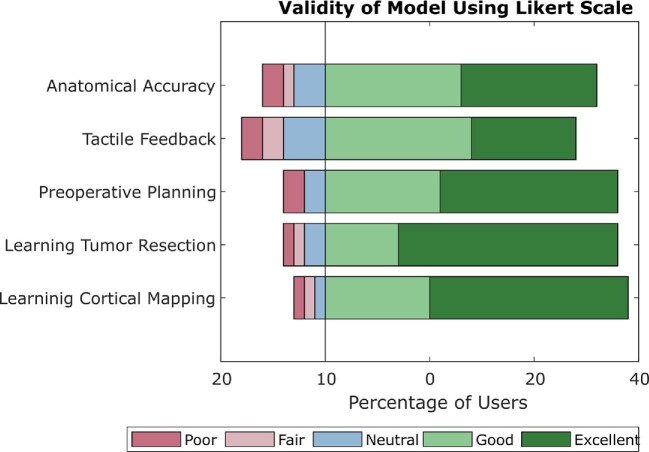
Following many neurosurgeon interviews (n = 32), generally positive results were received about the model's usefulness in educational and surgical practice environments. Over 96% of respondents stated that they wanted to train for neurosurgical procedures on the device, and the majority of respondents saw value and usefulness in many other aspects of the device, such as anatomic accuracy, material accuracy, and mimicry of real surgical scenarios (Table 4, Figure 9).
TABLE 4.
Example Questions for Evaluation of the Validity of the Model
| Category | Question (on a Likert scale unless otherwise indicated) |
|---|---|
| Anatomy | How useful do you perceive the model to be for learning relevant anatomy in a tumor resection surgery? |
| How useful do you perceive the model to be for learning where to properly place the craniotomy, based on the location of the intracranial pathology? | |
| How accurate do you perceive the tactile feedback to be of the neurologic component? | |
| Surgical skills | Would this tool be valuable for training neurosurgeons in the principles of neuro-oncological surgery? |
| Would this tool be valuable for training neurosurgeons in the skills necessary for microsurgical resection of intra-axial brain lesions? | |
| Would this tool be valuable for learning the principles of cortical mapping techniques? | |
| Would this tool be valuable for learning the principles of subcortical mapping techniques? | |
| Would you have wanted this tool as part of your own training in neurosurgery? (Yes/No) | |
| How similar do you perceive the model to be to cortical mapping on a real patient? | |
| Overall | How easy do you perceive the device to be to operate? |
| Would this tool be valuable to inexperienced neurosurgeons for preoperative planning? | |
| Would this tool be valuable to experienced neurosurgeons for preoperative planning? | |
| Would this tool be useful for patient education and preoperative discussions? | |
| How useful do you perceive the tool to be for rehearsing surgical scenarios and enhancing multidisciplinary teamwork (with anesthesiologists, neurologists, neuropsychologists, and neurophysiologists)? | |
| How valuable do you perceive this tool to be for educational purposes and as a supplement to neurosurgical education and training? |
FIGURE 9.
Validity of the model using Likert scale. Stacked bar graph indicating percent positive (green), neutral (blue), and negative (pink) responses to different aspects of the model, including anatomic accuracy, material accuracy or tactile feedback, usefulness in preoperative planning, value in learning the principles of neurological tumor resection, and value in learning the principles of cortical mapping.
DISCUSSION
Value Proposition
Cortical mapping for safe intraoperative monitoring of brain function is the gold standard management for many neurological conditions, including brain tumors, epilepsy, selective vascular lesions (cavernomas, aneurysms, and arteriovenous malformations), and intraparenchymal hematomas.5-7,23-28 Mastering brain mapping requires extensive practice as a neurosurgeon and as a multidisciplinary team including neurosurgeons, neurologists, neurophysiologists, anesthesiologists, etc.29 We present an anatomically accurate, electrically responsive, 3D-printed model to simulate the essential steps of performing a brain mapping operation, therefore enhancing training of the entire surgical team by replicating a multitude of surgical scenarios and improving interdisciplinary communication.
To our knowledge, this is the first model to simulate direct stimulation and electrical conduction, allowing for delineation of functional cortex and subcortical tracts. The consistent linear relationship that was quantified allows for the device to give an accurate prediction of nearby corticospinal tracts. This brings us a step closer to simulating a live neurosurgery, without the use of actual human tissue. The simulator maintains the brain's normal anatomy and reproduces a similar elasticity (Video). This patient-specific 3D-printed physical model can be conveniently created at a low cost for preoperative planning, procedure rehearsal, patient education, and resident training and evaluation, etc.
Based on our experience performing and training brain mapping for over 20 yr, and after conducting interviews with neurosurgeons before and after the design process, we have successfully illustrated that this simulator is an efficacious training tool. We foresee incorporating this model into the resident surgical skills curriculum and using it to evaluate trainee performance with quantifiable milestones to certify their competency prior to a real case. This would translate to more resident autonomy in the operating room (OR), improved surgical precision, and increased patient safety.
Our functional patient-specific 3D-printed model is superior to cadaveric dissection for simulating the resection of intraparenchymal brain tumors. Cadaveric tissue is unable to retain the material and electrical properties of the live brain.30 The ethical, religious, and pricing concerns of cadavers, which can range up to $3000,31 make them an inaccessible resource for many. As has been seen during the COVID-19 pandemic,12 external forces may reduce access to cadavers and limit the ability of trainees to travel for cadaver courses. Our 3D-printed simulator can be transported easily and safely, and used in a variety of environments, while a cadaver's usage is restricted to laboratory space and storage. The lack of biological tissue also eliminates the need for sterilization of tools, personal protective equipment, and cleaning. The cost for the nonreusable components of the model is $250 evaluated from the retail price of materials. The overall manufacturing cost of this simulator can vary widely depending on the availability and cost of software licensing, hardware, and raw materials at a wholesale price.
Alternative Models
3D printing technologies are increasingly used for neurosurgical simulation,32-34 ranging from simulators of superficial extra-axial and intra-axial tumor resection to deep intraventricular neuroendoscopic tumor resection.35,36 To our knowledge, the simulator presented here is the first of its kind as a functional anatomic model that allows stimulation of brain tracts not available in even the best cadaveric specimens. Virtual reality (VR) also plays a role in surgical simulation, especially in laparoscopic and general surgery,37 but current VR technologies cannot accurately replicate the experience of complex microsurgical maneuvers unique to neurosurgery or the neural tissue's haptic feedback or response to surgical manipulation and tension forces.
Model Limitations
The brain is, without any doubt, the most complex arrangement of matter in the universe. Therefore, a limitation of the model is that it does not perfectly replicate the linear relationship between electric propagation and distance observed in live neural tissue. However, at short distances (eg, those used in surgical simulation), it is deemed acceptable. The use of electric fields also presents a large susceptibility to electrical noise in the environment, which could be refined and mitigated in the future. Another limitation of the model is that current microdissection can only be carried out using sharp microdissection and suction. Other technologies such as NICO Myriad and CUSA were not tested for the first prototype of this device, but they will be incorporated into future studies.
Additionally, the process of creating a 3D-printed simulation is time consuming, taking approximately 72 h. Although this is balanced with the longevity of each simulator produced, 3D printing at this time remains relatively complex and requires expertise as well as specific hardware. The current model production process also requires familiarity with various software including printer interfaces, 3D Slicer, LabVIEW, and MATLAB.
Finally, the simulator only includes corticospinal tracts representing a patient's arms and legs. Our goal in this first iteration of the brain mapping simulator was to assess its feasibility, particularly modeling the electrical properties of the brain. As such, a simple prototype was created with straightforward tract representation as a proof-of-concept in order to minimize the number of design variables. The addition of other relevant white matter tracts would increase the validity and applications of the model, presenting a promising area of improvement for the current device.
Future Advancements
Future iterations of the simulator seek to add to its anatomic validity, tactile feedback, and approximation of live brain tissue during surgery. We aspire to incorporate other white matter fiber tracts and connectomic networks that are relevant to mastering the cortical mapping technique.38-40 To accomplish this, patient-specific tractography will be merged with the silicone model at the time of creation and hopefully account for the effect of cerebral edema on nearby tracts.11 A novel, conductive, and malleable material, rather than a single wire, could be placed into the 3D-printed shell before the silicone molding process in order to integrate these biomimetic tracts and improve their material properties.
Along with these white matter tracts, real-time simulated electroencephalography or electrocorticography are being considered to monitor electrical discharges and epileptiform activity while a surgical trainee is stimulating the model.41-43 Additional iterations also seek to introduce blood and cerebrospinal fluid circulation using mechanical pumps, which would replicate intraoperative complications such as vessel injury and seizures.26 This represents a huge leap forward for the field, allowing even the most experienced surgeons to practice a perfect replica of their cases before operating.
Improvements in feedback mechanisms can also help to better simulate the conditions of the OR. Specifically, the use of audio feedback (in addition to visual) during stimulation would more accurately replicate the verbal feedback between neurosurgeon and neuropsychologist intraoperatively. Furthermore, patient-specific models with different lesion types and locations can be manufactured and used for preoperative planning and to rehearse patient positioning and intraoperative team dynamics and OR setup, which is beneficial especially for crowded ORs during awake craniotomies. An additional feedback metric that will be implemented in future versions of the simulator could include amplitude and distance cutoffs for stimulation, in order to avoid seizures and tissue damage. Finally, the simulator could be modified to include fiducial markers to function with existing neuronavigation systems. These advancements would greatly improve the realistic baseline qualities of our device and aid in its ability to replace the current gold standard in neuro-oncolytic tumor resection surgery training.
CONCLUSION
We developed a first-of-its-kind, biomimetic 3D-printed model for simulating craniotomies and brain mapping. Our simulator has the ability to change the current paradigm for resident learning, and future iterations hold tremendous potential for improving neurosurgical training methods. The realistic neural properties of the simulator greatly improve representation of a live surgical environment. This proof-of-concept model can be further developed to contain more complicated tractography, blood and cerebrospinal fluid circulation, and more in-depth feedback mechanisms. Deliberate practice using this model will enhance training of not only the neurosurgical trainee but also the entire surgical team by replicating a multitude of surgical scenarios and improving interdisciplinary communication. We believe these revolutionary devices are the future of neurosurgical education, as well as preoperative planning.
Funding
This study did not receive any funding or financial support.
Disclosures
Dr Quiñones-Hinojosa was supported by the Mayo Clinic Professorship and a Clinician Investigator award, and Florida State Department of Health Research Grant, and the Mayo Clinic Graduate School, as well as the NIH (R43CA221490, R01CA200399, R01CA195503, and R01CA216855). Faith Colaguori, Maité Marin-Mera, Megan McDonnell, and Rebecca Forry were supported by the National Center for Advancing Translational Sciences of the NIH under Award Number UL1TR002378 and The Richard and Lauralee Uihlein Convergence Pilot Program in Neurosurgery and Neuro-Oncology. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Acknowledgments
We would like to thank Dr William (Whit) Smith, Professor James Rains, Dr James Stubbs, and Reagan Newman from the Georgia Institute of Technology for their expertise, guidance, and support throughout the device design process.
Notes
This material was presented at the Capstone Design Expo, Georgia Institute of Technology, Nov 23 2020, virtually, as a poster; Neuroscience-Neurosurgery Grand Rounds, Mayo Clinic, Feb 8 2021, virtually, as a lecture; Southeast Regional Clinical & Translational Science Conference, Georgia CTSA, Mar 4-5, 2021, virtually, as a poster; and Design of Medical Devices Conference, Medtronic, April 11-15, 2021, virtually, as a poster.
Contributor Information
Faith Colaguori, Wallace H. Coulter Department of Biomedical Engineering , Georgia Institute of Technology, Atlanta, Georgia, USA.
Maité Marin-Mera, Wallace H. Coulter Department of Biomedical Engineering , Georgia Institute of Technology, Atlanta, Georgia, USA.
Megan McDonnell, Wallace H. Coulter Department of Biomedical Engineering , Georgia Institute of Technology, Atlanta, Georgia, USA.
Jaime Martínez, Department of Neurologic Surgery, Mayo Clinic, Jacksonville, Florida, USA; Department of Neurologic Surgery, Medical University of South Carolina, Charleston, South Carolina, USA.
Fidel Valero-Moreno, Department of Neurologic Surgery, Mayo Clinic, Jacksonville, Florida, USA.
Aaron Damon, Department of Neurologic Surgery, Mayo Clinic, Jacksonville, Florida, USA.
Ricardo A Domingo, Department of Neurologic Surgery, Mayo Clinic, Jacksonville, Florida, USA.
William Clifton, Department of Neurologic Surgery, Mayo Clinic, Jacksonville, Florida, USA.
W Christopher Fox, Department of Neurologic Surgery, Mayo Clinic, Jacksonville, Florida, USA.
Kaisorn Chaichana, Department of Neurologic Surgery, Mayo Clinic, Jacksonville, Florida, USA.
Erik H Middlebrooks, Department of Radiology, Mayo Clinic, Jacksonville, Florida, USA.
David Sabsevitz, Department of Psychiatry and Psychology, Mayo Clinic, Jacksonville, Florida, USA.
Rebecca Forry, Wallace H. Coulter Department of Biomedical Engineering , Georgia Institute of Technology, Atlanta, Georgia, USA.
Alfredo Quiñones-Hinojosa, Department of Neurologic Surgery, Mayo Clinic, Jacksonville, Florida, USA.
REFERENCES
- 1. Horsley V, Schafer EA. A record of experiments upon the functions of the cerebral cortex. Philos TransR Soc Lond B Biol Sci. 1888;179(31):1-45. [Google Scholar]
- 2. Leyton ASF, Sherrington CS. Observations on the excitable cortex of the chimpanzee, orangutan, and gorilla. Exp Physiol. 1917;11(2):135-222. [Google Scholar]
- 3. Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60(4):389-443. [Google Scholar]
- 4. Pendleton C, Zaidi HA, Chaichana KLet al. Harvey Cushing's contributions to motor mapping: 1902-1912. Cortex. 2012;48(1):7-14. [DOI] [PubMed] [Google Scholar]
- 5. Domingo RA, Vivas-Buitrago T, Sabsevitz DS, Middlebrooks EH, Quinones-Hinojosa A. Awake craniotomy with cortical and subcortical speech mapping for supramarginal cavernoma resection. World Neurosurg. 2020;141(1):260. [DOI] [PubMed] [Google Scholar]
- 6. Quiñones-Hinojosa A, Lyon R, Ames CP, Parsa AT.. Neuromonitoring during surgery for metastatic tumors to the spine: intraoperative interpretation and management strategies. Neurosurg Clin N Am. 2004;15(4):537-547. [DOI] [PubMed] [Google Scholar]
- 7. Quiñones-Hinojosa A, Lyon R, Du R, Lawton MT.. Intraoperative motor mapping of the cerebral peduncle during resection of a midbrain cavernous malformation: technical case report. Neurosurgery. 2005;56(2 Suppl):E439; discussion E439. [DOI] [PubMed] [Google Scholar]
- 8. Ortiz KJ, Hawayek MI, Middlebrooks EHet al. Intraoperative direct stimulation identification and preservation of critical white matter tracts during brain surgery. World Neurosurg. 2021;146:64-74. [DOI] [PubMed] [Google Scholar]
- 9. Walker JA, Quiñones-Hinojosa A, Berger MS. Intraoperative speech mapping in 17 bilingual patients undergoing resection of a mass lesion. Neurosurgery. 2004;54(1):113-118; discussion 118. [DOI] [PubMed] [Google Scholar]
- 10. ReFaey K, Tripathi S, Bhargav AGet al. Potential differences between monolingual and bilingual patients in approach and outcome after awake brain surgery. J Neurooncol. 2020;148(3):587-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quiñones-Hinojosa A, Ojemann SG, Sanai N, Dillon WP, Berger MS.. Preoperative correlation of intraoperative cortical mapping with magnetic resonance imaging landmarks to predict localization of the Broca area. J Neurosurg. 2003;99(2):311-318. [DOI] [PubMed] [Google Scholar]
- 12. Weber AC, Henderson F, Santos JM, Spiotta AM. Letter: for whom the bell tolls: overcoming the challenges of the COVID pandemic as a residency program. Neurosurgery. 2020;87(2):E207. [DOI] [PubMed] [Google Scholar]
- 13. Milburn JA, Khera G, Hornby ST, Malone PS, Fitzgerald JE. Introduction, availability and role of simulation in surgical education and training: review of current evidence and recommendations from the Association of Surgeons in Training. Int J Surg. 2012;10(8):393-398. [DOI] [PubMed] [Google Scholar]
- 14. Kolozsvari NO, Feldman LS, Vassiliou MC, Demyttenaere S, Hoover ML. Sim one, do one, teach one: considerations in designing training curricula for surgical simulation. J Surg Educ. 2011;68(5):421-427. [DOI] [PubMed] [Google Scholar]
- 15. Fedorov A, Beichel R, Kalpathy-Cramer Jet al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colaguori F, Marin-Mera M, McDonnell Met al. Simulator for brain mapping. Mayo Foundation for Medical Education and Research. 2020. Accessed May, 2021. [Google Scholar]
- 17. Metz H, McElhaney J, Ommaya AK. A comparison of the elasticity of live, dead, and fixed brain tissue. J Biomech. 1970;3(4):453-458. [DOI] [PubMed] [Google Scholar]
- 18. Budday S, Nay R, de Rooij Ret al. Mechanical properties of gray and white matter brain tissue by indentation. J Mech Behav Biomed Mater. 2015;46(1):318-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kruse SA, Rose GH, Glaser KJet al. Magnetic resonance elastography of the brain. Neuroimage. 2008;39(1):231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galford JE, McElhaney JH. A viscoelastic study of scalp, brain, and dura. J Biomech. 1970;3(2):211-221. [DOI] [PubMed] [Google Scholar]
- 21. Koeneman JB. Viscoelastic Properties of Brain Tissue. Case Institute of Technology; 1966. [Google Scholar]
- 22. Smooth-On: Ecoflex Series. www.smooth-on.com. 2020. Accessed March, 2021. [Google Scholar]
- 23. De Biase G, Garcia DP, Bohnen A, Quiñones-Hinojosa A. Perioperative management of patients with glioblastoma. Neurosurg Clin N Am. 2021;32(1):1-8. [DOI] [PubMed] [Google Scholar]
- 24. Suarez-Meade P, Marenco-Hillembrand L, Prevatt Cet al. Awake vs. asleep motor mapping for glioma resection: a systematic review and meta-analysis. Acta Neurochir. 2020;162(7):1709-1720. [DOI] [PubMed] [Google Scholar]
- 25. Jackson C, Westphal M, Quiñones-Hinojosa A. Complications of glioma surgery. Handb Clin Neurol. 2016;134(1):201-218. [DOI] [PubMed] [Google Scholar]
- 26. Eseonu CI, Rincon-Torroella J, Lee YM, ReFaey K, Tripathi P, Quinones-Hinojosa A. Intraoperative seizures in awake craniotomy for perirolandic glioma resections that undergo cortical mapping. J Neurol Surg A Cent Eur Neurosurg. 2018;79(3):239-246. [DOI] [PubMed] [Google Scholar]
- 27. Almeida JP, Chaichana KL, Rincon-Torroella J, Quinones-Hinojosa A.. The value of extent of resection of glioblastomas: clinical evidence and current approach. Curr Neurol Neurosci Rep. 2015;15(2):517. [DOI] [PubMed] [Google Scholar]
- 28. Eseonu CI, Rincon-Torroella J, ReFaey Ket al. Awake craniotomy vs craniotomy under general anesthesia for perirolandic gliomas: evaluating perioperative complications and extent of resection. Neurosurgery. 2017;81(3):481-489. [DOI] [PubMed] [Google Scholar]
- 29. Ericsson KA, Harwell KW. Deliberate practice and proposed limits on the effects of practice on the acquisition of expert performance: Why the original definition matters and recommendations for future research. Front Psychol. 2019;10(1):2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Selcuk İ, Tatar I, Huri E.. Cadaveric anatomy and dissection in surgical training. Turk J Obstet Gynecol. 2019;16(1):72-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tabas JA, Rosenson J, Price DD, Rohde D, Baird CH, Dhillon N. A comprehensive, unembalmed cadaver-based course in advanced emergency procedures for medical students. Acad Emerg Med. 2005;12(8):782-785. [DOI] [PubMed] [Google Scholar]
- 32. Clifton W, Damon A.. The three-dimensional printing renaissance of individualized anatomical modeling: Are we repeating history? Clin Anat. 2020;33(3):428-430. [DOI] [PubMed] [Google Scholar]
- 33. Damon A, Clifton W, Valero-Moreno F, Nottmeier E. Orientation planning in the fused deposition modeling 3D printing of anatomical spine models. Cureus. 2020;12(2):e7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clifton WE, Damon AC, Freeman WD. Development of a lumbar drain simulator for instructional technique and skill assessment. Neurocrit Care. 2020;32(3):894-898. [DOI] [PubMed] [Google Scholar]
- 35. Damon A, Clifton W, Valero-Moreno F, Quinones-Hinojosa A. Cost-effective method for 3-dimensional printing dynamic multiobject and patient-specific brain tumor models: technical note. World Neurosurg. 2020;140(1):173-179. [DOI] [PubMed] [Google Scholar]
- 36. Licci M, Thieringer FM, Guzman R, Soleman J. Development and validation of a synthetic 3D-printed simulator for training in neuroendoscopic ventricular lesion removal. Neurosurg Focus. 2020;48(3):E18. [DOI] [PubMed] [Google Scholar]
- 37. Yoon JW, Chen RE, Kim EJet al. Augmented reality for the surgeon: systematic review. Int J Med Robot. 2018;14(4):e1914. [DOI] [PubMed] [Google Scholar]
- 38. Baker CM, Burks JD, Briggs RGet al. A connectomic atlas of the human cerebrum-chapter 1: introduction, methods, and significance. Oper Neurosurg. 2018;15(Suppl_1):S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Briggs RG, Khan AB, Chakraborty ARet al. Anatomy and white matter connections of the superior frontal gyrus. Clin Anat. 2020;33(6):823-832. [DOI] [PubMed] [Google Scholar]
- 40. Burks JD, Conner AK, Bonney PAet al. Anatomy and white matter connections of the orbitofrontal gyrus. J Neurosurg. 2018;128(6):1865-1872. [DOI] [PubMed] [Google Scholar]
- 41. Tatum WO, Feyissa AM, ReFaey Ket al. Periodic focal epileptiform discharges. Clin Neurophysiol. 2019;130(8):1320-1328. [DOI] [PubMed] [Google Scholar]
- 42. ReFaey K, Chaichana KL, Feyissa AMet al. A 360° electronic device for recording high-resolution intraoperative electrocorticography of the brain during awake craniotomy [published online ahead of print: July 5, 2019]. J Neurosurg. doi:10.3171/2019.4.JNS19261. [DOI] [PubMed] [Google Scholar]
- 43. Tatum WO, McKay JH, ReFaey Ket al. Detection of after-discharges during intraoperative functional brain mapping in awake brain tumor surgery using a novel high-density circular grid. Clin Neurophysiol. 2020;131(4):828-835. [DOI] [PubMed] [Google Scholar]