Abstract
Background
Inflammatory bowel diseases (IBD), including Crohn disease (CD) and ulcerative colitis (UC), are complex disorders with multiple comorbidities. We conducted international patient and physician surveys to evaluate current experiences and perceptions of patients with CD or UC and physicians who treat IBD.
Methods
The IBD Global Assessment of Patient and Physician Unmet Need Surveys comprised a patient survey and a physician survey, fielded in North America and Europe between August 16, 2019, and November 10, 2019. Adults with CD or UC (targeted 1:1 ratio) were recruited from physicians, patient advocacy groups, and recruitment panels; physicians were recruited by recruitment agencies and panels.
Results
In total, 2398 patients with IBD (1368 CD, 1030 UC) and 654 physicians completed surveys. Anxiety and depression were the most common comorbidities among patients with IBD. Patients and physicians were generally aligned on treatment goals and patient-physician communication. Patients with IBD reported high quality-of-life impact by rectal urgency and need to use the toilet, which were rated as lower-impact by physicians. Patients defined remission based on symptoms; physicians defined remission based primarily on clinical tests. Patients expected current treatments to control their disease for a longer duration than did physicians. Patients expressed more concern about corticosteroid use compared with physicians; many physicians reported prescribing corticosteroids for more than 4 months per year in some patients.
Conclusions
Patients could benefit from education about disease remission and expectations for current therapies. High corticosteroid use is concerning to patients, and physicians should minimize the use of corticosteroids for extended periods of time.
Keywords: inflammatory bowel disease, Crohn disease, ulcerative colitis
INTRODUCTION
Crohn's disease (CD) and ulcerative colitis (UC) are chronic, immune-mediated inflammatory bowel diseases (IBDs) that result in considerable morbidity and a significantly diminished quality of life (QOL).1-3 In general, IBD causes a substantial economic burden on health care systems, in the form of both direct and indirect costs.4 The primary goals of treatment for CD and UC are to adequately control the immune dysfunction and thereby eliminate the inflammatory burden to achieve symptom control, establish corticosteroid-free remission, and improve QOL.1, 2 Patients with IBD may be treated with a variety of agents, including 5-aminosalicylates (5-ASAs), antibiotics, corticosteroids, immunomodulators, biologics, and new small-molecule agents.1, 2 Despite the effectiveness of biologics such as tumor necrosis factor (TNF) inhibitors, anti-α4β7 integrin agents, and anti-interleukin-12/23 (IL-12/23) agents,5-11 patients may experience loss of response for many reasons, such as antidrug antibody formation, and increased risk for other complications such as serious infections or malignancies.5-9,12-15 Corticosteroids are effective as induction therapy and for treating exacerbations of IBD but are neither effective nor safe in maintenance of remission. Common adverse effects include (but are not limited to) infections, osteoporosis, hyperglycemia, hypertension, psychiatric disturbances, and difficulty with wound healing.1, 2, 16, 17 Despite these risks, excessive or prolonged corticosteroid use has been documented in 15% to 17% of patients with IBD, suggesting a need for improved disease management.16, 18
As a chronic disease, IBD requires long-term management and extended interactions and communication between patients and physicians. Good patient-physician communication is particularly important for optimizing patient involvement in IBD management. Indeed, most patients report that their physician is the primary source of information about their disease.19-21 Surveys designed to understand IBD from the perspective of patients and physicians have revealed differences and misalignments on the fundamental concepts of IBD management.19, 20 For example, physician estimates of IBD severity have been shown to be lower than patient reports.19 Similarly, physicians may tend to underestimate the impact or burden of IBD on patients.19, 20 To better understand the global experience and perceptions of patients with CD or UC and physicians who treat IBD, we conducted 2 online surveys. Herein, we report the results of the IBD Global Assessment of Patient and Physician Unmet Need Surveys (IBD GAPPS).
MATERIALS AND METHODS
The IBD GAPPS comprised 2 online surveys: 1 for patients and 1 for physicians. Each took approximately 30 minutes to complete. Surveys were fielded in Canada, France, Germany, Italy, Spain, the United Kingdom, and the United States. Surveys were programmed online in English, and translations into other languages were electronically applied to the survey links. Surveys in non-English languages were proofread and approved by local translators.
All results were summarized using descriptive statistics. When patients and physicians were asked to rate items, all scales ranged from 1 to 7, with 1 representing none, minimal, least, and so on, and 7 representing the most, highest, or greatest, and so on. Except for the risk/benefit ratings (explained in detail below in “Treatment Choices and Concerns”), results presented as percentages of patients or physicians reporting a high degree of agreement or severity included only those reporting scores of 6 or 7.
Participant Selection and Characterization
Recruitment of patients and physicians was implemented using mixed methodology. Patients were recruited by physicians, patient advocacy groups, and recruitment panels. A 1:1 ratio of patients with CD to patients with UC was targeted. Physicians were recruited by third-party recruitment agencies. Eligible physicians were gastroenterologists who saw ≥12 patients with CD and ≥12 patients with UC in the previous month. At least 30% of the physicians’ caseload included moderate to severe disease (as determined by the physicians). Eligible patients were adults (aged ≥18 years) with a diagnosis of CD or UC who had received treatment for their IBD. Patients self-classified their disease severity as mild, moderate, severe, or “do not know.” The severity of IBD was also assessed based on treatment history; moderate to severe disease was defined a priori as prior hospitalization because of IBD; surgery for CD; receipt of a TNF inhibitor, anti-integrin agent, Janus kinase inhibitor, anti-interleukin-12/23, and/or immunomodulator for IBD; or receipt of corticosteroids for >2 months of the last 12 months. The proportion of patients with mild disease was capped at 20%, so most patients had moderate to severe IBD.
Patients were asked about prior and current medications and physicians were asked to estimate the percentage of their patients currently receiving medications from a list (Supplementary Table S1). Comorbidities were self-reported by patients (Supplementary Table S2).
Disease Symptoms and Burden
Patients were asked if they had experienced symptoms from a list (Supplementary Table S3), to rate these symptoms for severity (scale of 1-7), and to select which 5 of these symptoms interfered most with their QOL/day-to-day activities. Physicians noted 5 symptoms that they believed interfered the most with QOL (Supplementary Table S4). Both patients and physicians rated the impact of these symptoms on emotional well-being.
Remission and Duration of Response
Patients and physicians indicated their definition of remission from a list (Supplementary Tables S5, S6). Patients were asked if they had heard of the term “mucosal healing” (defined as a reduction in damage, inflammation, ulcers, and blood observed in the gastrointestinal tract); those who responded yes were asked to rate the importance of mucosal healing in achieving remission. Patients rated the strength of their belief that remission was a feasible treatment goal.
Physicians estimated the percentage of patients who achieved remission with IBD treatments and rated their satisfaction with current treatment options. Physicians were asked about the frequency of loss of response to biologic therapies and the average duration of these treatments. For patients who lost response to a TNF inhibitor, physicians were asked about actions taken (Supplementary Table S7) and to rank subsequent treatment options (Supplementary Table S8). The impact of therapeutic drug monitoring was not addressed.
Expectations of Current Medications
Patients were asked if they had experienced loss of response to a medication. Patients who answered yes were asked about subsequent actions taken after the loss of response (Supplementary Table S9). Patients were also asked about their expectations for the current treatment duration and to rate the extent to which they believed their current treatment was a long-lasting solution for their IBD. Patients indicated the number of months in the past year that they required corticosteroids for their IBD.
Physicians rated their satisfaction with the durability of current IBD options and their belief that current therapy options can be classified as “durable” therapies. Physicians were asked about the minimum duration of a durable therapy, how long patients with moderate/severe IBD maintained corticosteroid-free remission with each treatment option, and how many months a patient with moderate/severe IBD required corticosteroids to maintain disease control.
Treatment Goals and Satisfaction
Patients and physicians were asked to provide their top 3 disease-related (Supplementary Tables S10, S11) and QOL-related treatment goals (Supplementary Table S12). Patients were asked about their satisfaction with their current treatment based on treatment goals, and physicians estimated the percentage of patients that fell into categories from a selection of statements (Supplementary Table S13). Both patients and physicians were asked to provide the sources of information used to influence treatment goals (Supplementary Table S14).
Treatment Choices and Concerns
Both patients and physicians ranked drivers of treatment choice by allocating 100 points between the following factors: achieving a durable response, speed of onset, tolerability, patient preference for mode of administration, and long-term safety profile. Financial and health insurance preference were not addressed. Physicians rated the overall risk/benefit profile for each drug class by rating risk and benefit independently on a scale of 1 (no risk/no benefit) to 5 (high risk/high benefit). Net benefit was calculated as the difference between the estimated risk and the estimated benefit, expressed as a percentage.
Patients rated their level of concern about adverse effects with their current treatment (Supplementary Table S15) and whether they had ever opted against IBD-specific treatment because of concerns about adverse effects, and if so, which medication they had declined. Physicians rated their level of concern about treatments for their patients from a list (Supplementary Table S16). Patients and physicians were asked to select the time beyond which they become most concerned about corticosteroid use. Patients were asked if they had ever chosen not to take their prescribed medication and if so, to provide the reason from a list (Supplementary Table S17). Physicians estimated the proportion of patients who took their medication as instructed, who occasionally missed a dose, and who frequently missed doses of their medications and to rate each on a scale of 1 (none), 2 (some patients), 3 (approximately 50% of patients), 4 (most patients), or 5 (all patients). Physicians then ranked the top 3 reasons that patients changed their dosing frequency without a doctor’s permission (Supplementary Table S18).
Patient-Physician Communication
Patients rated their satisfaction with their main health care provider (gastroenterologist/IBD specialist or other provider). Patients and physicians also rated their level of agreement with a list of communication issues (Supplementary Tables S19, S20).
ETHICAL CONSIDERATIONS
This study was approved by the Western Institutional Review Board. All participants provided consent to participate in the study.
RESULTS
Patient Characteristics
Patient surveys were completed between August 22, 2019, and November 10, 2019 by a total of 2398 patients with IBD (1368 CD, 1030 UC). Most patients resided in the United States (39%), followed by Spain (12%), France (10%), Germany (10%), the United Kingdom (10%), Italy (9%), and Canada (9%). Characteristics of patients with CD and with UC are reported in Table 1. Patients with CD most commonly reported current use of TNF inhibitors, whereas patients with UC were most commonly receiving 5-ASAs. In addition, patients with CD reported greater use of current biologic therapy than patients with UC. More than one-quarter of patients in each group were currently taking corticosteroids. The percentage of patients with moderate/severe disease (per clinical criteria) or hospitalization (ever) was higher for patients with CD vs patients with UC. Patient-reported IBD severity (moderate/severe disease: 85% CD, 76% UC) was aligned with severity assessed according to clinical criteria. Anxiety and depression were the most commonly reported comorbidities.
TABLE 1.
Patient Characteristics
| Patients With CD (n = 1368) | Patients With UC (n = 1030) | |
|---|---|---|
| Current age, mean (SD), y | 42.4 (14.2) | 44.2 (14.1) |
| Age at diagnosis, mean (SD), y | 32.4 (14.3) | 36.8 (14.2) |
| Female, % | 60 | 55 |
| IBD severity | ||
| Moderate/severe, %* | 93 | 78 |
| Ever required hospitalization, % | 71 | 47 |
| Treatment, % current/% ever | ||
| Corticosteroid | 27/76 | 29/74 |
| 5-ASA | 24/57 | 45/68 |
| Immunomodulator | 26/52 | 19/37 |
| TNF inhibitor | 32/52 | 22/34 |
| Nonpharmacologic treatment | 16/28 | 16/24 |
| Anti-integrin | 9/15 | 7/11 |
| Anti-interleukin-12/23 | 9/14 | 2/4 |
| JAK inhibitor | 2/5 | 4/5 |
| Other | 3/7 | 2/5 |
| Comorbid conditions, % | ||
| Anxiety | 33 | 31 |
| Depression | 31 | 25 |
| Upper gastrointestinal problems | 17 | 10 |
| Rheumatoid arthritis | 9 | 7 |
| Celiac disease | 6 | 4 |
| Type 1 diabetes | 6 | 4 |
*Patient-reported. For inclusion, the proportion of patients with mild disease was capped at 20%.
JAK indicates Janus kinase.
Physician Characteristics
Physician surveys were completed between August 16, 2019, and November 10, 2019 by 654 physicians. Physician respondents were from the United States (33%), the United Kingdom (15%), Germany (12%), Italy (12%), Spain (10%), France (9%), and Canada (8%). Physician characteristics are reported in Table 2. Physicians had a mean (SD) total caseload of 268 (162) patients in the previous month, including a mean (SD) of 43 (33) patients with CD and 43 (34) patients with UC. Physicians reported that 67% and 62% of their patients with CD or UC, respectively, had moderate/severe disease. Most physicians were practicing in a university/teaching hospital, private practice, or regional/community hospital setting. Most had been in clinical practice for less than 20 years. Physician-reported current therapy for their patients with CD was closely aligned with patient-reported current therapy. Physician reports for their patients with UC were also generally aligned with patient reports, except that physicians reported that 60% of their patients with UC currently received 5-ASAs, whereas only 45% of patient respondents with UC reported currently using 5-ASAs.
TABLE 2.
Physician Characteristics
| All Physicians (N = 654) | |
|---|---|
| Caseload in last month, mean | |
| CD caseload | 42.9 |
| UC caseload | 43.3 |
| Caseload severity, % (CD/UC) | |
| Mild | 33/38 |
| Moderate | 43/41 |
| Severe | 25/22 |
| Primary care setting, % | |
| University/teaching hospital | 41 |
| Private practice | 31 |
| Regional/community hospital | 20 |
| Private hospital | 4 |
| Regional center | 2 |
| Health center | 2 |
| Other | <1 |
| Year of qualification for primary specialty, % | |
| 1980-1990 | 14 |
| 1991-2000 | 26 |
| 2001-2010 | 39 |
| 2011-2016 | 20 |
Perceptions of Disease Symptoms and Burden
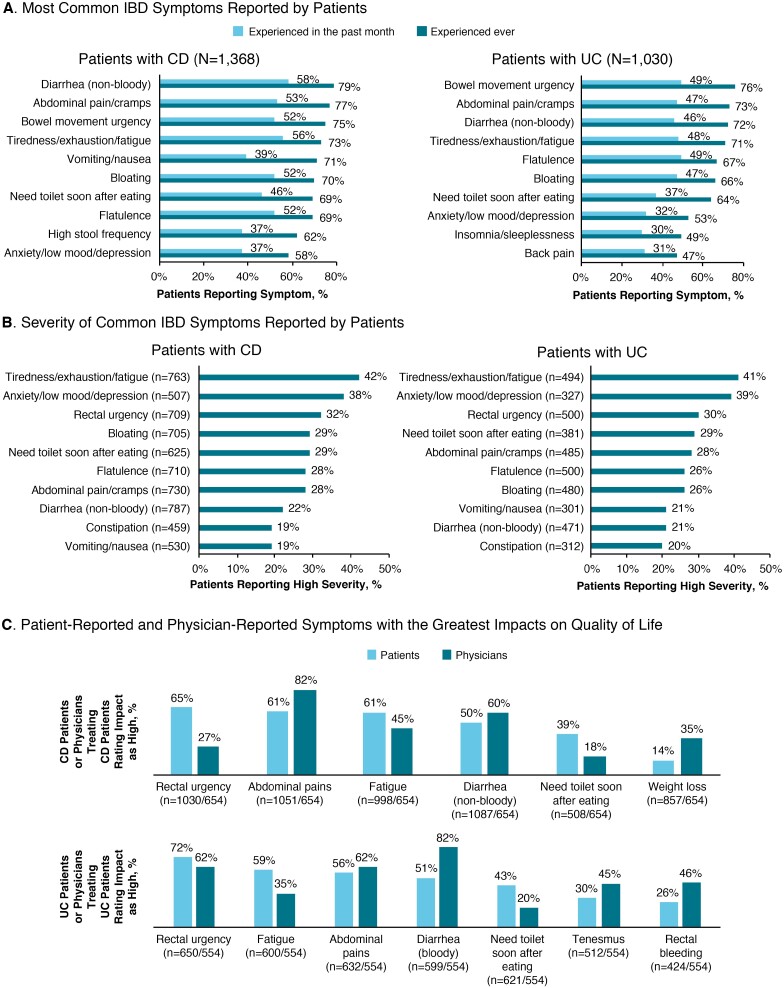
One-third of patients felt that their IBD was well controlled, most (61%) felt that their IBD was only partially controlled, and 6% reported that their disease was not controlled. Patients with CD most commonly reported experiencing nonbloody diarrhea and fatigue in the previous month, whereas patients with UC most commonly reported bowel movement urgency, flatulence, and fatigue (Fig. 1A). The symptoms most commonly identified as having a high severity (score of 6 or 7) were similar between patients with CD and patients with UC, most often fatigue and anxiety/low mood/depression (Fig. 1B). Patient and physician reports of the top 5 symptoms interfering with QOL were not well aligned. Whereas most patients with CD reported rectal urgency as a top symptom impacting QOL, less than one-third of physicians recognized the burden of this symptom. In addition, a considerably higher percentage of patients with UC vs physicians reported fatigue and needing the toilet after eating as the top 5 symptoms impacting QOL (Fig. 1C).
FIGURE 1.
Perceptions of burden of disease. A, IBD symptoms reported by patients with the greatest frequency in the past month or ever. B, IBD symptoms reported by patients as having high severity (left panels = CD; right panels = UC). C, Symptoms reported by patients and physicians to have the greatest impact on QOL (upper panel = CD; lower panel = UC).
Forty-three percent and 36% of patients with CD or UC, respectively, reported a high impact of their disease on their emotional mood. Impacts of IBD on patients’ lives were also recognized by physicians; 72% and 64% of physicians reported a high disease impact on the QOL of their patients with CD and with UC, respectively. The IBD-related emotions causing the greatest impact (score of 6 or 7) were similar between patients with CD and with UC, including worry that their IBD would get worse (45% CD, 44% UC); frustration with having to put up with symptoms (42% CD, 39% UC); feeling stressed as a result of their IBD (34% CD, 30% UC); often feeling anxious/nervous (34% CD, 30% UC); worrying about family/friends who had to take care of them (32% CD, 25% UC); feeling embarrassed (27% CD, 28% UC), depressed (28% CD, 23% UC), or helpless (28% CD, 23% UC); worrying about feeling worse than the doctor thought (26% CD, 23% UC); and feeling alone or isolated (23% CD, 19% UC).
Perceptions of Remission and Duration/Loss of Response
Nearly one-quarter (22%) of patients with IBD reported never having discussed remission with their primary physician; only 7% of physicians reported that they did not typically discuss remission with their patients with IBD. Among the patients who reported having discussed remission with their main physician, they noted that it was most commonly discussed at treatment initiation (24%) or treatment success (22%), followed by routinely (19%), at diagnosis (19%), and at treatment failure (14%).
Patients defined remission primarily by resolution of IBD symptoms (45% of all patients), followed by an ability to de-escalate treatment (25%), test results (19%), or no longer needing treatment (10%). In contrast, physicians most commonly reported defining remission using test results (64% for CD, 70% for UC), followed by resolution of IBD symptoms (29% for CD, 23% for UC). Tests most commonly used by physicians to define remission included colonoscopy or sigmoidoscopy (56% for CD, 58% for UC) and biopsies (36% and 37%, respectively), followed by biochemical tests (8% and 6%).
Few patients (38% CD, 36% UC) had previously heard of the term “mucosal healing.” Among the patients who were familiar with mucosal healing, approximately two-thirds (63% CD, 69% UC) believed that mucosal healing was important (score of 6 or 7) to achieving remission. Most patients (75% CD, 78% UC) somewhat or strongly agreed that remission was a feasible treatment goal.
Physicians estimated that only 37% to 55% of their patients with moderate/severe CD or UC achieved remission with current biologic therapy, with the highest remission rates reported with TNF inhibitors (Supplementary Fig. 1A). When asked about their satisfaction with the rate of remission with current treatments, 25% of physicians reported high satisfaction (score of 6 or 7) with current treatments for CD and 36% reported high satisfaction with remission rates for UC.
Loss of response to medications was common, reported by 69% of patients with CD and 58% of patients with UC. Among the patients who had ever lost response to a medication, most (65% CD, 61% UC) reported being switched to a new drug; fewer patients stayed on the current drug but added another drug (30% CD, 29% UC), escalated the dose or frequency of the current drug (28% CD, 24% UC), stopped the medication without adding another drug (12% CD, 10% UC), or had no change in medication (1% CD, 3% UC). Similarly, 66% and 55% of physicians estimated that their patients with moderate/severe CD or UC, respectively, lost response to biologic therapies fairly or very frequently. Physicians reported that 21% to 31% and 22% to 29% of their patients with moderate/severe CD or UC, respectively, lost response to current treatments (Supplementary Fig. 1B).
In general, physicians most commonly estimated that current therapies had a durability of response of at least 1 or at least 2 years (Supplementary Fig. 1C), but few physicians (18% for CD, 24% for UC) were very satisfied (score of 6 or 7) with the durability of current medications. For their patients with IBD who lost response to a TNF inhibitor, physicians most frequently reported escalating the dose (36%) or switching to a medication with a different mechanism of action (32%; Supplementary Fig. 1D). However, when considering agents that they would most likely use after loss of response to a TNF inhibitor, physicians most commonly reported that they would contemplate switching their patients to a different TNF inhibitor as a first option (Supplementary Fig. 1E).
Expectations of Current Treatments
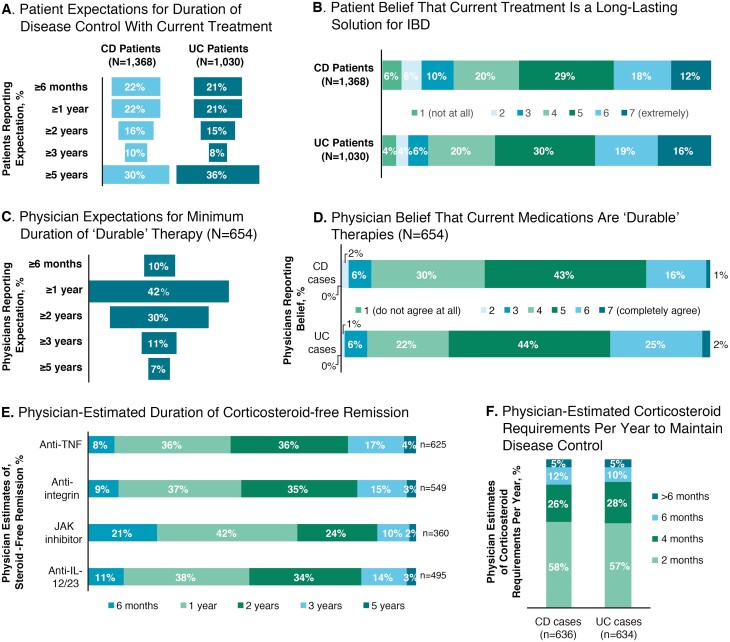
Approximately one-third of patients with IBD expected their current IBD treatment to control their disease for at least 5 years (Fig. 2A). Many patients expected their current treatment to provide a long-lasting solution to their IBD, with 30% of patients with CD and 35% of patients with UC expressing a strong belief (score of 6 or 7) in this expectation (Fig. 2B). Physicians had lower expectations for the durability of current treatments than did patients. Most physicians (72%) reported that an IBD treatment considered durable should provide remission for at least 1 or at least 2 years (Fig. 2C), and relatively few (17% for CD, 27% for UC) strongly agreed (score of 6 or 7) that current treatment options for moderate/severe IBD could be classified as durable therapies (Fig. 2D). Most physicians estimated that current biologic therapies provided at least 1 or at least 2 years of corticosteroid-free remission (Fig. 2E).
FIGURE 2.
Patient and physician expectations with current medications. A, Percentages of patients with CD or UC reporting the expected duration of disease control with their current treatment. B, Percentage of patients with CD (upper panel) or UC (lower panel) expressing a belief that their current treatment was long-lasting. C, Physician-reported expectations for treatment durability (overall). D, Physician-reported beliefs regarding the durability of current treatments for their patients with CD (upper panel) and patients with UC (lower panel). E, Physician-estimated duration of corticosteroid-free remission (overall). F, Physician-estimated months of corticosteroid use required to maintain disease control per year with current treatments in patients with CD (left) and patients with UC (right). IL indicates interleukin; JAK, Janus kinase.
When asked about the duration of corticosteroid use required to maintain disease control in a typical year, 43% and 17% of physicians estimated at least 4 months and at least 6 months, respectively, for patients with CD, and 43% and 15% for patients with UC (Fig. 2F). Similarly, corticosteroid use of at least 4 months or at least 6 months, respectively, was reported by 35% and 18% of patients with CD and 39% and 19% of patients with UC to help manage their disease within the last year.
Drivers of Treatment Choice
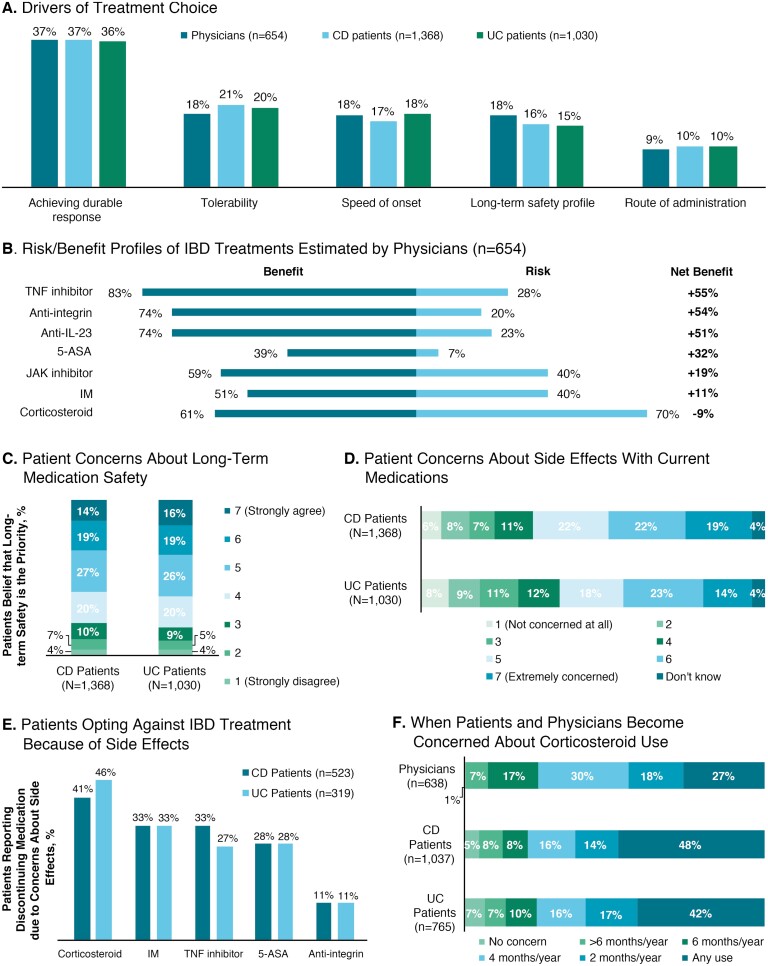
Patients’ and physicians’ rankings of the drivers of treatment choice (based on allocation of points from a total of 100 points across each of 5 drivers) were generally in agreement, with achievement of a durable response as the most common driver (mean percentage of points allocated: 36%-37%), followed by tolerability (18%-21%), speed of onset (17%-18%), long-term safety profile (15%-18%), and route of administration (9%-10%; Fig. 3A). When physicians estimated the risk/benefit profiles, most physicians (83%) rated TNF inhibitors as having a high benefit and the greatest net benefit (+55%) compared with other agents (Fig. 3B). Corticosteroids were rated by most physicians (70%) as having a high risk and the poorest net benefit (–9%).
FIGURE 3.
Selection of treatment for IBD. A, Most common reasons provided by physicians and patients with CD or UC for choice of IBD treatment. B, Risk/benefit profiles and net benefit (%) for current treatments. C, Degree of concern expressed by patients with CD or UC regarding long-term safety of medications. D, Degree of concern expressed by patients with CD or UC regarding adverse effects with current medications. E, Percentages of patients with CD or UC who opted against specific treatments because of adverse effects. F, Percentage of physicians, patients with CD, and patients with UC reporting level of concern with duration of corticosteroid use. IL indicates interleukin; IM, immunomodulator; JAK, Janus kinase.
When asked about the long-term safety of their IBD medication, 33% of patients with CD and 35% of patients with UC strongly agreed (score of 6 or 7) that the long-term safety of the medication was a priority, even if the medication was not as effective as it could be (Fig. 3C). Fifty-nine percent of physicians strongly agreed that they considered their patients’ concerns about long-term adverse effects when making treatment decisions. Patients were also concerned about the adverse effects of their current IBD therapy, with 41% of patients with CD and 37% of patients with UC expressing great concern (score of 6 or 7) about adverse effects (Fig. 3D). Approximately one-third of patients (38% CD, 31% UC) reported that they had ever opted against taking an IBD-specific medication that had been recommended because of concerns about adverse effects. A higher percentage of patients had declined corticosteroids because of adverse effects than any other medication (Fig. 3E). Patients expressed greater concern about corticosteroid use than physicians, with 48% of patients with CD and 42% of patients with UC concerned about any use of corticosteroids compared with 27% of physicians (Fig. 3F). Many patients (27% CD, 20% UC) noted that they had chosen to not take their prescribed medication at some point; among these patients, nonadherence was most common because of adverse effects (33% CD, 31% UC).
Patients and physicians were aligned on how well they thought patients adhered to therapy; 80% of physicians reported that most or all of their patients took their medication as prescribed, whereas 68% of patients with CD and 75% of patients with UC reported taking their medications as prescribed. Thirty-two percent of physicians reported that most or all of their patients occasionally missed doses, and only 5% reported that most or all patients frequently missed doses of their medications. The most common reasons provided by physicians for reduced adherence included that the patient felt that the treatment was working and they could reduce their dose (43% of physicians), the patient felt that the treatment was not working (23%), and the patient could not tolerate the adverse effects associated with their treatment (14%).
Treatment Goals
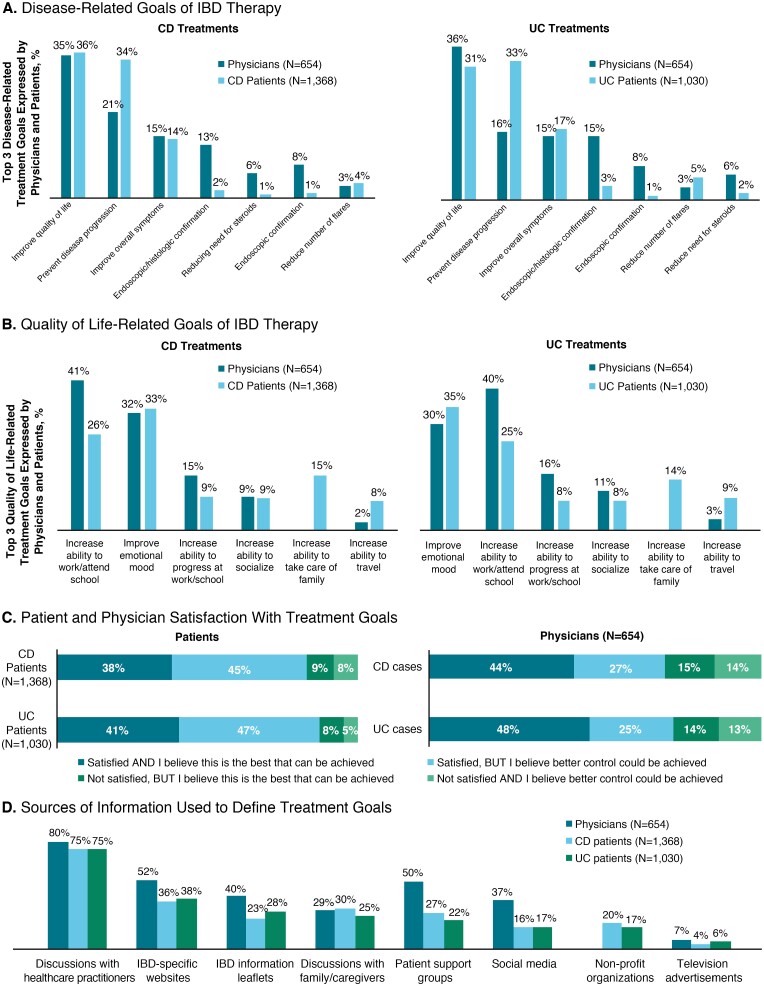
Patients and physicians were asked to provide their top 3 disease-related and QOL-related treatment goals. Patients and physicians agreed that improving QOL and preventing disease progression were important goals. However, many physicians considered clinical test results (ie, endoscopic/histologic results or confirmation) to be among the top 3 treatment goals, which was reported rarely by patients (Fig. 4A). Physicians most commonly identified increasing a patient’s ability to work or attend school as one of the top 3 primary QOL-related goals of IBD treatment, whereas most often patients prioritized improving emotional mood (Fig. 4B). Approximately one-half of patients believed that better control could be achieved for their disease, whereas only approximately one-third of physicians shared this belief (Fig. 4C). Both patients and physicians used a variety of sources of information to define treatment goals; however, both most commonly reported health care providers as the primary source of information (Fig. 4D).
FIGURE 4.
Treatment goals. A, Disease-related goals of IBD therapy reported by physicians and patients (left panels = CD; right panels = UC). B, QOL-related goals of IBD therapy reported by physicians and patients (left panels = CD; right panels = UC). C, Satisfaction with treatment goals as reported by patients (left panel) and physicians (right panel) (upper bars = CD; lower bars = UC). D, Sources of information used by physicians and patients with CD or UC. HCP, healthcare practitioners.
Patient-Physician Communication
Overall, patients were satisfied with their primary physician, with 60% of patients with CD and 58% of patients with UC reporting high satisfaction (score of 6 or 7). Similar percentages of patients and physicians included agreement (net difference ≤5% in rates of high agreement) that physicians were aware of patient concerns about treatment (55% vs 56%, respectively), that patients were satisfied with their involvement in disease management (56% for both), that patients were involved in setting treatment goals (51% vs 54%), that patients felt comfortable discussing symptoms with their physician (61% vs 62%), and that patients were well informed about new treatment options (46% vs 51%). Responses differed between patients and physicians on the following: patients were asked about symptoms at every appointment (67% vs 74%, respectively), physicians understood how much IBD affected patients’ lives (51% vs 60%), and patients/physicians had enough time during routine appointments (53% vs 31%). Although the majority of physicians (62%) believed that they made treatment decisions together with the patient, some patients (11% CD, 11% UC) felt that their physicians did not consult them.
DISCUSSION
IBD GAPPS provides an international snapshot of current beliefs and expectations around IBD and its management from both the patient and physician perspective. Patients and physicians agreed on some aspects of IBD management, particularly with respect to patient-physician communication. Notably, most patients were satisfied with communication with their primary physician. This is important because CD and UC are chronic diseases that often require long-term management and therefore can lead to decades-long patient-physician relationships.22 One effect of strong patient-physician interactions is a potential reduction in health care utilization for gastrointestinal symptoms.22
We noted 4 key areas of misalignment between patients and physicians with implications for clinical management: ratings of symptom severity, definitions of remission, expectations about treatment durability, and corticosteroid use. The most severe symptoms most commonly reported by patients were anxiety/depression and fatigue. Anxiety and depression have long been recognized as comorbidities in IBD; in fact, appropriate treatment of anxiety and depression has been suggested to be 1 of the 2 most significant interventions for IBD.23 Our survey revealed that the greatest discrepancy between patients and physicians was that physicians did not seem to appreciate the severity of rectal urgency in patients with CD or the need to use the toilet shortly after eating in both patients with CD and patients with UC. Issues of needing and finding toilets have been noted as having a high impact on QOL by patients with IBD in another study, suggesting that these issues may contribute an additional psychosocial burden to these patients.24 This survey underlines a need for enhanced physician education on the impact of IBD on mental health and for the development of tools to better assess mental health status and to assist in referrals to specialists for treatment of psychosocial comorbidities. Psychosocial interventions could begin at the time of diagnosis with consideration for referrals to specialty providers.
Patients and physicians were also not aligned on the definition of remission. Patients defined remission based on the resolution of symptoms, and a need for patient education on mucosal healing was revealed. Indeed, proper patient education has been shown to be the other most significant intervention for IBD,23 and more detailed discussions of how remission is measured at clinic visits is warranted. These findings suggest a need for improved vocabulary for physicians explaining mucosal healing and an understanding of alignment with patients’ goals to assist with patient education. The results also indicated that a high percentage of physicians used biopsies to assess remission, raising the question of whether physicians responded to the survey question based on theory or on real practice.
Discrepancies between patients and physicians about the expectations of and satisfaction with the durability of current treatments were observed. We found that physicians had lower expectations of treatment durability compared with patients. Notably, most physicians were not satisfied with current medications to control IBD, highlighting the urgent need for new and durable treatments for IBD. Physicians may be more realistic about expectations for treatment durability, whereas patients are more optimistic. This observation suggests an opportunity for physicians to educate their patients on both current and novel treatment options for IBD.
Overuse of corticosteroids was observed in patients with CD and UC; for example, 42% of physicians believed that their patients with CD would require the use of corticosteroids for at least 4 months per year. This observation is of particular concern because a recent study showed that patients reported more oral corticosteroid use than their physicians did.25 In another study, the disagreement over rates of corticosteroid use was shown to be more common in community practices than in IBD centers.25 Notably, many patients who participated in our survey, similar to another study,24 were concerned about their corticosteroid use, in contrast to most of the physicians who participated in our survey. Corticosteroid use in patients with IBD has been so prevalent and widespread that the first recommendation of both the Canadian and the U.S./European Union “Choosing Wisely” programs for IBD treatment states that physicians should not use corticosteroids (eg, prednisone) for maintenance therapy in IBD.26, 27 The long-term use of corticosteroids suggests that despite the availability of modern IBD therapies, symptoms remain uncontrolled in a large proportion of patients.
Overuse of 5-ASAs was commonly reported by patients with CD, despite the lack of evidence for their utility as induction or maintenance therapy.28, 29 A recent survey of physicians showed that personal beliefs of possible efficacy in patients with mild disease, low cost and good safety profile, and patient preferences to avoid aggressive immunosuppression are drivers of 5-ASA treatment decisions for patients with CD.30 Despite these perceived benefits, the use of 5-ASA in patients with CD may delay the use of effective therapies during the window of opportunity when the course of the disease can be affected.30 In addition, high 5-ASA usage is associated with high cumulative costs of treatment.30 These observations further emphasize the need for durable treatment in this large patient population.
The limitations of the study design included the deliberate weighting of the survey sample to include a high proportion of patients with moderate/severe disease; the sample therefore does not represent the full patient population with IBD. In addition, generalizability may be limited by the fact that patients who take an active role in managing their disease by interacting with patient advocacy groups or recruitment panels may differ from the overall IBD population. A cross-sectional study design (rather than a longitudinal design) collects a snapshot of data; therefore, trends over time cannot be assessed. There was no relationship between the patients and physicians who participated in the surveys, so no correlations or associations can be made between statements made by the patients surveyed and the physicians surveyed. Comorbidities were self-reported by patients and were not necessarily confirmed by a physician diagnosis. Costs were not addressed in the surveys. Finally, the surveys have not been assessed for content validity, comprehensibility, or translatability.
CONCLUSIONS
The results of this international study reveal that IBD remains uncontrolled for many patients. Although patients and physicians were generally aligned on treatment goals, 4 key areas of misalignment were observed. First, they differed on which symptoms they considered to be most severe and bothersome, with results from the patient survey highlighting anxiety and depression as important areas that may require greater attention, including early referral to specialists from treating physicians. Second, a significant discrepancy regarding the definition of remission between patients and physicians was observed, which may affect expectations and clinical outcomes; this information could be discussed during clinic visits. Third, expectations of the durability of current treatments also differed between patients and physicians, with physician expectations based on their knowledge of treatments but patient expectations being more optimistic. Patients could benefit from better education about current treatments during clinic visits. Physicians indicated a preference for TNF inhibitors, even after a loss of response with a prior TNF inhibitor; the choice of medication was driven by many factors. Fourth, patients expressed more concern about the use of corticosteroids than physicians, and many physicians prescribed corticosteroids for more than 4 months per year. Physicians should avoid prescribing corticosteroids for extended periods of time, as supported by current treatment guidelines. The results of this study reinforce the urgent need for new and durable treatments for IBD.
Supplementary Material
ACKNOWLEDGMENTS
This survey study was conducted by Adelphi Real World and was supported by Bristol Myers Squibb Company. The authors thank the Crohn’s & Colitis Foundation for its input on the patient survey and its contribution to U.S. respondent recruitment. All authors contributed to and approved the article; writing and editorial assistance was provided by Cindy Gobbel, PhD, and Julie Gage, PhD, of Peloton Advantage, LLC, an OPEN Health company (Parsippany, NJ), and was funded by Bristol Myers Squibb Company.
Glossary
Abbreviations
- 5-ASAs
5-aminosalicylates
- CD
Crohn disease
- IBD
inflammatory bowel disease
- IBD GAPPS
IBD Global Assessment of Patient and Physician Unmet Need Surveys
- QOL
quality of life
- TNF inhibitor
tumor necrosis factor inhibitor
- UC
ulcerative colitis
Supported by: This work was supported by Bristol Myers Squibb Company..
Conflicts of interest: DTR has received research funding from Takeda and has served as a consultant to AbbVie, Abgenomics, Allergan, Arena Pharmaceuticals, Biomica, Bristol Myers Squibb Company, Dizal Pharmaceuticals, Ferring Pharmaceuticals, Genentech/Roche, Janssen Pharmaceuticals, Lilly, Mahana Therapeutics, Medtronic, Merck, Napo Pharmaceuticals, Pfizer, Prometheus Laboratories, Shire, Takeda, and Target PharmaSolutions. CS has served on speaker’s bureaus for Janssen, AbbVie, UCB, Takeda, and Pfizer; has served as an investigator on clinical research studies for Janssen, AbbVie, and Takeda; and has served as a consultant to Celgene. BS has served as a consultant for AbbVie, Boehringer, Celgene, Falk, Janssen, Lilly, Pfizer, Prometheus, and Takeda; has received speaker’s fees from AbbVie, CED Service, Falk, Ferring, Janssen, Novartis, and Takeda; and has served as a representative of Charité–Universitätsmedizin Berlin. MS has received honoraria for advisory activities/lecture fees from Pfizer, Takeda, Amgen, Celgene, Chiesi, Gebro, Tillots, Kern, Ferring, and Faes. AH has served as a consultant, advisory board member, or speaker for AbbVie, Atlantic, Bristol Myers Squibb Company, Celltrion, Falk, Ferring, Janssen, MSD, Napp Pharmaceuticals, Pfizer, Pharmacosmos, Shire, and Takeda and has served on a global steering committee for Genentech. BB has served as an advisor and speaker for Ferring, Janssen, AbbVie, Takeda, Pfizer, Novartis, and Merck; has served as an advisor for Robarts Clinical Trials, Celgene, Microbiome Insights, Merck, Amgen, Pendopharm, Genentech, Bristol Myers Squibb Company, Allergan, and Protagonist; has received research support from Janssen, AbbVie, GSK, Bristol Myers Squibb Company, Amgen, Genentech, Merck, BI, Qu Biologic, Celgene, and Alvine; and owns stock options from Qu Biologic. YB has received honoraria from AbbVie, Biogaran, Biogen, Boehringer Ingelheim, Celgene, Ferring, Gilead, Hospira, Janssen, Mayoly Spindler, MSD, Norgine, Pfizer, Roche, Samsung Bioepis, Sandoz, Sanofi, Shire, Takeda, and UCB and has received grants from Pfizer and Takeda. AAr has served as a consultant for AbbVie, Allergan, Amgen, Biogen, Bristol Myers Squibb Company, Celgene, Celltrion, Ferring, Gilead, Janssen, Lilly, MSD, Mylan, Pfizer, Roche, Samsung Bioepis, Sandoz, Sofar, and Takeda; has received lecture fees from AbbVie, Amgen, Biogen, Bristol Myers Squibb Company, Chiesi, Ferring, Janssen, MSD, Mitsubishi Tanabe, Nikkiso, Pfizer, Samsung Bioepis, Sandoz, Takeda, and TiGenix; and has received research grants from MSD, Pfizer, and Takeda. AAf has served as a consultant for AbbVie, Takeda, Janssen, Celgene, and UCB and has received speaker’s fees from AbbVie, Takeda, Janssen, UCB, and Pfizer.
REFERENCES
- 1. Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 2. Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 3. Dahlhamer JM, Zammitti EP, Ward BW, et al. Prevalence of inflammatory bowel disease among adults aged ≥18 years—United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1166–1169. [DOI] [PubMed] [Google Scholar]
- 4. Mehta F. Report: economic implications of inflammatory bowel disease and its management. Am J Manag Care. 2016;22:s51–s60. [PubMed] [Google Scholar]
- 5. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. [DOI] [PubMed] [Google Scholar]
- 6. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–265.e1. [DOI] [PubMed] [Google Scholar]
- 7. Loftus EV Jr, Colombel JF, Feagan BG, et al. Long-term efficacy of vedolizumab for ulcerative colitis. J Crohns Colitis. 2017;11:400–411. [DOI] [PubMed] [Google Scholar]
- 8. Sands BE, Sandborn WJ, Panaccione R, et al. ; UNIFI Study Group . Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381:1201–1214. [DOI] [PubMed] [Google Scholar]
- 9. D’Amico F, Parigi TL, Fiorino G, et al. Tofacitinib in the treatment of ulcerative colitis: efficacy and safety from clinical trials to real-world experience. Therap Adv Gastroenterol. 2019;12:1756284819848631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rutgeerts P, Gasink C, Chan D, et al. Efficacy of ustekinumab for inducing endoscopic healing in patients with Crohn’s disease. Gastroenterology. 2018;155:1045–1058. [DOI] [PubMed] [Google Scholar]
- 11. Löwenberg M, Vermeire S, Mostafavi N, et al. Vedolizumab induces endoscopic and histologic remission in patients with Crohn’s disease. Gastroenterology. 2019;157:997–1006.e6. [DOI] [PubMed] [Google Scholar]
- 12. Danese S, Allez M, van Bodegraven AA, et al. Unmet medical needs in ulcerative colitis: an expert group consensus. Dig Dis. 2019;37:266–283. [DOI] [PubMed] [Google Scholar]
- 13. Moss AC, Brinks V, Carpenter JF. Review article: immunogenicity of anti-TNF biologics in IBD—the role of patient, product and prescriber factors. Aliment Pharmacol Ther. 2013;38:1188–1197. [DOI] [PubMed] [Google Scholar]
- 14. Thomas SS, Borazan N, Barroso N, et al. Comparative immunogenicity of TNF inhibitors: impact on clinical efficacy and tolerability in the management of autoimmune diseases. a systematic review and meta-analysis. Biodrugs. 2015;29:241–258. [DOI] [PubMed] [Google Scholar]
- 15. Hindryckx P, Novak G, Vande Casteele N, et al. Incidence, prevention and management of anti-drug antibodies against therapeutic antibodies in inflammatory bowel disease: a practical overview. Drugs. 2017;77:363–377. [DOI] [PubMed] [Google Scholar]
- 16. Waljee AK, Wiitala WL, Govani S, et al. Corticosteroid use and complications in a US inflammatory bowel disease cohort. Plos One. 2016;11:e0158017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15:457–465. [DOI] [PubMed] [Google Scholar]
- 18. Selinger CP, Parkes GC, Bassi A, et al. A multi-centre audit of excess steroid use in 1176 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:964–973. [DOI] [PubMed] [Google Scholar]
- 19. Rubin DT, Siegel CA, Kane SV, et al. Impact of ulcerative colitis from patients’ and physicians’ perspectives: results from the UC: NORMAL survey. Inflamm Bowel Dis. 2009;15:581–588. [DOI] [PubMed] [Google Scholar]
- 20. Schreiber S, Panés J, Louis E, et al. Perception gaps between patients with ulcerative colitis and healthcare professionals: an online survey. BMC Gastroenterol. 2012;12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peyrin-Biroulet L, Van Assche G, Sturm A, et al. Treatment satisfaction, preferences and perception gaps between patients and physicians in the ulcerative colitis CARES study: a real world-based study. Dig Liver Dis. 2016;48:601–607. [DOI] [PubMed] [Google Scholar]
- 22. Owens DM, Nelson DK, Talley NJ. The irritable bowel syndrome: long-term prognosis and the physician-patient interaction. Ann Intern Med. 1995;122:107–112. [DOI] [PubMed] [Google Scholar]
- 23. Husain A, Triadafilopoulos G. Communicating with patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:444–450. [DOI] [PubMed] [Google Scholar]
- 24. Lönnfors S, Vermeire S, Greco M, et al. IBD and health-related quality of life—discovering the true impact. J Crohns Colitis. 2014;8:1281–1286. [DOI] [PubMed] [Google Scholar]
- 25. Ghosh S, Bressler B, Petkau J, et al. Healthcare providers underestimate patients’ glucocorticoid use in Crohn’s disease. Dig Dis Sci. 2019;64:1142–1149. [DOI] [PubMed] [Google Scholar]
- 26. Nguyen GC, Boland K, Afif W, et al. Modified Delphi process for the development of choosing wisely for inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:858–865. [DOI] [PubMed] [Google Scholar]
- 27. Lenti MV, Armuzzi A, Castiglione F, et al. ; IG-IBD . Are we choosing wisely for inflammatory bowel disease care? The IG-IBD Choosing Wisely campaign. Dig Liver Dis. 2020;52:44–50. [DOI] [PubMed] [Google Scholar]
- 28. Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD). Pharmacol Rep. 2011;63:629–642. [DOI] [PubMed] [Google Scholar]
- 29. Jeong DY, Kim S, Son MJ, et al. Induction and maintenance treatment of inflammatory bowel disease: a comprehensive review. Autoimmun Rev. 2019;18:439–454. [DOI] [PubMed] [Google Scholar]
- 30. Ma C, Ascoytia C, McCarrier KP, et al. Physicians’ perspectives on cost, safety, and perceived efficacy determine aminosalicylate use in Crohn’s disease. Dig Dis Sci. 2018;63:2555–2563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.