Abstract
The CD95 (also called APO-1 or Fas) system plays a major role in the induction of apoptosis in lymphoid and nonlymphoid tissues in response to a variety of extracellular signals, including chemotherapeutic drugs. Here we report that the CD95 ligand (CD95L) is upregulated in hepatoma cells upon treatment with antineoplastic drugs. Upregulation by different chemotherapeutic drugs is functionally relevant for drug-induced apoptosis and is mediated by transcriptional mechanisms. The MEKK1/JNKK pathway and a novel AP-1 element in the CD95L promoter downstream of the TATA box are required for CD95L upregulation. Thus, understanding the mechanisms of CD95-mediated apoptosis through CD95L upregulation upon treatment of hepatocellular carcinomas with chemotherapeutic drugs may contribute to the improvement of anticancer chemotherapy.
Anticancer drugs have previously been shown to induce apoptosis in tumor target cells (17, 43). Several groups have demonstrated that apoptosis was partially mediated via the CD95 (also called APO-1 or Fas)/CD95 ligand (CD95L) pathway (18, 62, 68). Thus, CD95 and CD95L were found to be upregulated by several antineoplastic compounds. Subsequently, cells underwent apoptosis in a suicidal or fratricidal manner, a process similar to activation-induced cell death in activated peripheral T lymphocytes during the downregulation of an immune response (6, 11, 49). Both transcriptional activation and de novo protein synthesis are required for this inducible process. The CD95-CD95L system is not the only system involved in drug-induced cell death (42). However, experiments with CD95 neutralizing agents show that it contributes substantially to this type of apoptosis (18, 62, 68).
A subset of human tumors can be treated successfully with either a single drug or combination chemotherapy. The majority of tumors, however, particularly solid tumors of the gastrointestinal tract, exhibit chemotherapy resistance depending on several as yet unidentified factors. Therefore, chemoresistant tumors remain a major obstacle in chemotherapeutic treatment. Mutations in the intrinsic apoptotic pathway may render tumor cells resistant to anticancer drugs. Hence, the detailed understanding of the signaling pathways involved might lead to the discovery of therapeutic targets to overcome chemotherapy resistance.
The CD95 system is involved in several physiologic and pathophysiologic conditions, such as regulation of the immune response and tumor immune surveillance (32). CD95L, a type II transmembrane protein, induces apoptosis via binding to the CD95 receptor. CD95, a type I transmembrane protein, is a member of the tumor necrosis factor receptor superfamily expressed on various tissues, including T cells, colonic epithelial cells, and hepatocytes (33, 55). In contrast, CD95L expression is restricted to a few cell types, such as T cells, macrophages, and cells of the testis (23). CD95L triggers apoptosis in CD95-bearing cells via formation of a death-inducing signaling complex utilizing the adapter protein FADD (59) and initiation of a signaling cascade of caspases finally leading to apoptotic cell death (32, 33).
Recently, it has been reported that CD95 expression is induced in hepatocellular carcinoma cell lines upon treatment with chemotherapeutic drugs via induction of p53. p53 binds to an intronic enhancer element in the first intron of the CD95 gene (48). The mechanism by which CD95L is upregulated in hepatic tumor cells in response to chemotherapeutic drugs, however, remains to be elucidated.
Targets in the cellular transcription machinery previously demonstrated to be involved in the response to genotoxic stress—as exerted by most chemotherapeutic drugs—include the SAPK/JNK signaling cascade (15, 16) and the transcription factors c-Jun (31), NF-κB (40), p53 (29, 39) and ATF-2 (63). However, the involvement of these transcription factors is still controversial.
Here we show that chemotherapeutic drugs lead to activation of the JNK/SAPK signaling pathway and the transcription factor AP-1. In turn, via a newly identified AP-1 site in the CD95L promoter, recognized by Jun-Fos heterodimers, CD95L expression becomes greatly enhanced starting 20 to 25 h posttreatment. Based on these data, we propose a model for chemotherapy-induced apoptosis in hepatic tumor cells.
Our results help clarify the apoptotic response to anticancer drugs and have implications for the future development of specific compounds for the treatment of tumors not accessible to chemotherapy.
MATERIALS AND METHODS
Cell lines.
The following cell lines were used: (i) HepG2 cells, derived from a human hepatoblastoma expressing low levels of wild-type p53; (ii) Huh7 cells, derived from a human hepatocellular carcinoma, expressing mutated p53 with a point mutation at codon 249 which leads to a shorter half-life of p53; (iii) Hep3B cells, derived from a human hepatocellular carcinoma deficient in p53; and (iv) SKW6.4 cells, a human B lymphoblastoid cell line.
HepG2, Huh7, and Hep3B cells were cultured in Dulbecco's modified Eagle medium (Gibco BRL, Eggenstein, Germany) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Gibco BRL), 10 mM HEPES (Gibco BRL), 5 mM l-glutamine (Gibco BRL), and 100 μg of gentamicin/ml (Gibco BRL). SKW6.4 cells were maintained in RPMI medium (Gibco BRL) containing 10% FCS (Gibco BRL), 10 mM HEPES (Gibco BRL), 2 mM l-glutamine (Gibco BRL), and 100 μg of gentamicin/ml (Gibco BRL).
Isolation and culture of primary human hepatocytes.
Primary human hepatocytes were isolated from healthy liver tissue obtained from patients receiving partial liver resection with a two-step perfusion technique as described and modified from the initial procedure established by Berry and Friend (5). The isolation procedure was approved by the Ethics Committee, Medical Faculty, University of Heidelberg. Briefly, a blood vessel of the resected liver tissue was cannulated and perfused with Ca2+- and Mg2+-free Hanks' balanced salt solution (Gibco BRL) containing 0.5 mM EGTA (Sigma, Deisenhofen, Germany) and 50 mM HEPES (Sigma) for 15 to 20 min. The perfusion was continued using Williams' medium E (WME; Gibco BRL) containing 0.05% collagenase type IV (Sigma) and 5 mM CaCl2 for 15 to 25 min. Cells were mechanically separated from the liver capsule, and the resulting cell suspension was filtered and washed in ice-cold, serum-free WME. To separate hepatocytes from nonparenchymal cells, we performed a centrifugation in Percoll (adjusted to a density of 1.065 g/ml; Biochrom, Berlin, Germany) for 10 min at 50 × g as described previously (60). Cells were washed twice in WME and were seeded in maintenance medium at a density of 1.0 × 105 to 1.5 × 105 viable cells/cm2 on collagen-coated culture plates (collagen type I; Serva Biochemicals, Heidelberg, Germany). Viability was determined by trypan blue dye exclusion. The maintainance medium was changed 2 to 4 h after seeding and every 24 to 48 h thereafter. As maintainance medium we used WME supplemented with 5 mM l-glutamine (Flow Laboratories, Rockville, Md.), 0.6% glucose (Serva), 0.02 M HEPES (Sigma), 50 μg of gentamicin (Sigma)/ml, 100 μg of penicillin (Flow Laboratories)/ml, 100 μg of streptomycin (Flow Laboratories)/ml, 37 μM inosine (Serva), 1.5% dimethyl sulfoxide (Merck, Darmstadt, Germany) and 0.14 U of insuline (Serva)/ml. On days 1 and 2, maintainance medium was supplemented with 10% FCS (Gibco BRL). Cells were incubated at 37°C and 5% CO2.
Plasmids.
Serial deletion constructs of the CD95L promoter were cloned into the pTATA.luc vector (a kind gift from T. Wirth, Institut für medizinische Strahlen- und Zellforschung, Würzburg, Germany) or into the pGL2-basic vector (Promega, Madison, Wis.).
The deletion constructs ranging from −2269/+100 to −36/+100 were established as described previously (41). The −36/+19 vector was constructed as follows: the −36/+100 CD95L promoter fragment was cut with Psp5II, and the smaller fragment was reinserted into the pTATA.luc vector. All constructs were confirmed by automated dideoxy sequencing at Toplab GmbH (Munich, Germany).
The expression constructs for Jun and Fos have been described previously (1, 2). Dominant-negative MEKK-1 (K432M) and dominant-negative JNKK (K611R) were kindly provided by M. Karin and have been described before (67). Dominant-negative MKK3 [MKK3b-(A)] and dominant-negative MKK6 [MKK6b-(A)] were kindly provided by J. Woodgett (53). Dominant-negative c-jun (Δaa 1-192) and the empty control vector pCMV were kindly provided by D. Bohmann (38).
Mutations in the +90 AP-1 site of the −36/+100 construct (APX-4) were introduced using the QuikChange mutagenesis kit (Stratagene, La Jolla, Calif.). The primers for the mutagenesis reaction (MWG Biotech GmbH, Ebersberg, Germany) were APX-4 sense (5′ CCG TTT GCT GGG GCT GGC CTA ATT AAC CAG CTG CCT CTA GAG G 3′) and APX-4 antisense (5′ CCT CTA GAG GCA GCT GGT TAA TTA GGC CAG CCC CAG CAA ACG G 3′). Underlined nucleotides represent the mutated sites compared with the wild-type CD95L promoter sequence. The mutations were confirmed by automated sequencing (Toplab GmbH).
Antibodies.
The neutralizing anti-CD95L antibody NOK-1 and an isotype-matched control antibody were purchased from Pharmingen (Hamburg, Germany). Antibodies for supershift analyses directed against c-Jun, c-Fos, ATF-2, and CEBP were from Santa Cruz Biotechnology Inc. (Heidelberg, Germany). The antibody used for immunofluorescence studies was a c-Jun-specific polyclonal antibody (57). The antibodies recognizing JNK1 and JNK2, and the phosphorylated forms of these kinases, were purchased from Santa Cruz Biotechnology and Promega (Mannheim, Germany), respectively.
Treatment of cells with monoclonal antibody immunoglobulin G3 (IgG3) anti-APO-1 at a concentration of 100 ng/ml has been described previously (4).
Treatment with chemotherapeutic drugs.
Cell cultures were treated with 5-fluorouracil (5-FU; Ribosepharm GmbH, Munich, Germany) or with etoposide (Bristol-Myers Squibb GmbH, Munich, Germany).
Determination of cell death.
Cells were trypsinized with 1% trypsin–EDTA for 5 min, washed twice in phosphate-buffered saline (PBS), and stained by 2.5 μg of propidium iodide (Sigma)/ml. Uptake of the dye was measured in a FACScan flow cytometer (Becton Dickinson GmbH, Heidelberg, Germany) using the CellQuest software. Concomitant changes in forward scatter/side scatter (FSC/SSC) of the cells were evaluated.
For quantification of DNA fragmentation, supernatants were centrifuged at 200 × g, and cells were trypsinized and washed. They were lysed in a hypotonic lysis buffer (0.1% sodium citrate and 0.1% Triton X-100) containing 50 μg of propidium iodide/ml and were incubated at 4°C overnight. The nuclei were then analyzed for DNA content by flow cytometry (52).
51Cr-release assay.
CD95L-mediated cytotoxicity was investigated by the 51Cr-release assay. Hep3B cells (effector cells) were seeded into 96-well plates and were treated with chemotherapeutic drugs for 48 h. After 2 days, the medium of the cultures of the effector cells was changed. SKW6.4 cells (target cells) were incubated for 30 min in Na23CrO4 (100 μCi) (NEN, Neu Isenburg, Germany). Labeled cells were then added to the effector cells at the indicated effector/target ratios. After 12 to 16 h, 100 μl of supernatant was collected from each well and measured in a gamma counter. Specific lysis was calculated according to the formula L = (E−S)/(T−S), in which E is the counts per minute (cpm) of the unknown sample, S is the cpm of the spontaneous lysis of labeled target cells in medium without effector cells, and T is the cpm of the maximal release of the target cells kept in 2 N HCl. The assay was analyzed further only if S/T was ≤30%. Each experiment was done in triplicate.
Detection of CD95L mRNA expression by reverse transcriptase (RT) PCR.
RNA was prepared using the RNeasy kit (Qiagen GmbH, Hilden, Germany) according to the instructions of the manufacturer. For each isolation, 5 × 106 to 1 × 107 cells were used. One microgram of total RNA was reverse transcribed using Moloney murine leukemia virus (MMLV-RT; Gibco BRL) with oligo(dT)15 primers (Roche GmbH, Mannheim, Germany) in a 20-μl reaction mixture containing 10 mM dithiothreitol (DTT) and 500 μM deoxynucleoside triphosphate. Aliquots (5 μl) were amplified in a DNA thermocycler (Stratagene, Heidelberg, Germany) with 0.5 U of Taq DNA polymerase (Roche GmbH) in a 50-μl reaction mixture. Thirty-five reaction cycles were performed. Each cycle consisted of a denaturation step (94°C for 30 s), an annealing step (56°C for 30 s), and an elongation step (72°C for 30 s). The reaction was completed with a 72°C elongation step for 10 min. PCR products were analyzed on 1.5 to 2% agarose gels.
Primers were purchased from MWG Biotech GmbH. Primer sequences were CD95L sense (5′ ATG TTT CAG CTC TTC CAC CTA CAG A 3′) and CD95L antisense (5′ CCA GAG AGA GCT CAG ATA CGT TGA C 3′), yielding a PCR product of 500 bp. The primers span all three introns of CD95L, thereby facilitating the differentiation between cDNA and genomic DNA.
Each reverse transcribed mRNA was internally controlled with a β-actin PCR using the primers sense (5′ TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA 3′) and antisense (5′ CTA GAA TTT GCG GTG GAC GAT GGA GGG 3′), yielding a PCR product of 600 bp.
Transient transfections and luciferase assays.
One day before transfection, cells were plated at a density of 0.6 × 106/9-cm petri dish. Transfection was done using the calcium phosphate precipitation method as described previously (9). Subsequently, cells were divided into six well plates, and treatment with chemotherapeutic drugs was initiated for different periods of time. Cells were lysed after being washed three times with PBS in lysis buffer (Promega). Lysates were measured in a Duolumat (Berthold, Wildbach, Germany) using the dual luciferase assay system (Promega). Renilla luciferase or chloramphenicoltransferase expression vectors, both driven from a basal promoter, were used to normalize transfection efficiencies. In addition, the protein amount was measured using the Bio-Rad protein assay (Bio-Rad GmbH, Munich, Germany) and was used to normalize for the protein content of the transfected cells.
Electrophoretic mobility shift assay and supershift analyses.
Nuclear extracts from HepG2 and Hep3B cells were prepared as described previously (12). Briefly, 4 × 107 cells were lysed in 10 mM Tris-HCl (pH 7.4)–2 mM MgCl2–140 mM NaCl–0.5 mM DTT–0.5 mM phenylmethylsulfonyl fluoride (PMSF)–0.1% Triton X-100. Sucrose density gradient centrifugation was performed, and the nuclear fraction was resolved in 20 mM HEPES (pH 7.9)–25% glycerol–0.42 M NaCl–1.5 mM MgCl2–0.2 mM EDTA–0.5 mM DTT–0.5 mM PMSF. After 30 min of rotation, nuclear membranes were pelleted and the supernatant was stored in liquid nitrogen after determination of the protein content using the Bio-Rad protein assay.
Double-stranded oligonucleotides comprising the AP-1 site at +90 in the CD95L promoter were end labeled with T4 polynucleotide kinase (MBI Fermentas, St. Leon-Roth, Germany) using 5,000 Ci/mmol of [γ-32P]ATP (Amersham GmbH, Braunschweig, Germany). Sequences of the single-stranded oligonucleotides were sense (5′ GGG CTG GCC TGA CTC ACC AGC TGC 3′) and antisense (5′ GCA GCT GGT GAG TCA GGC CAG CCC 3′). Free nucleotides were removed with Microspin G-50 columns (Pharmacia GmbH, Freiburg, Germany).
Binding reactions were carried out at 4°C for 30 min using 5 μg of nuclear protein in a buffer containing 100 ng of bovine serum albumin (Roche GmbH)/μl, 50 ng of poly[d(I-C)] (Roche GmbH)/μl, 2 mM DTT (Gibco BRL), 500 μM Pefabloc (Roche GmbH), 1 μg of aprotinin (Roche GmbH)/μl, 25 mM HEPES (Sigma), 5 mM MgCl2 (Sigma), 35 mM KCl (Sigma), and 3 × 104 cpm of the labeled oligonucleotide. For supershift analyses, 1 μg of antibody was added to the binding reaction. Samples were analyzed on a 6% nondenaturing polyacrylamide gel in 0.5% Tris-borate-EDTA.
In vitro kinase assay and Western blot.
HepG2 cells were treated with 5-FU for different time periods, and in vitro kinase assays or Western blotting for phosphorylated JNK was performed as described previously (67).
Immunofluorescence studies.
Cultured Hep3B cells or freshly isolated human primary hepatocytes were plated on Lab-Tek chamber slides (Renner GmbH, Dannstadt, Germany). After culturing for at least 2 days, the cells were treated with antineoplastic drugs. Subsequently, fixation was performed in methanol and acetone (5 min and 10 s at −20°C, respectively) as described earlier (50). Cells were then incubated with the specific primary antibody against human c-Jun for 1 h at 37°C. After washing three times with PBS, cells were covered for 1 h with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (Dianova, Hamburg, Germany). Nonspecific staining was controlled by incubation with mouse or rabbit immunoglobulins instead of the specific primary antibody or by blocking the primary antibody with the immunogenic peptide. The slides were covered with coverslips and evaluated on a fluorescence microscope (Zeiss GmbH, Ober-kochen, Germany).
RESULTS
Chemotherapeutic drugs induce apoptosis in hepatocellular carcinoma cell lines via the CD95/CD95L system.
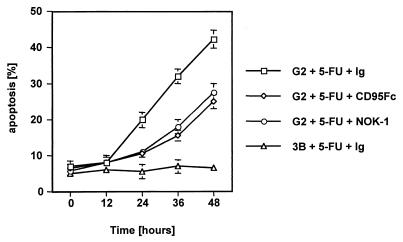
Previous experiments demonstrated that antineoplastic compounds induce apoptosis in tumor cells. The antimetabolite 5-FU is used for adjuvant treatment of hepatocellular cancer. To determine the role of the CD95/CD95L system, we aimed to inhibit drug-induced apoptosis with either a blocking anti-CD95L antibody or a blocking chimeric CD95-Fc construct (11). Cell cultures of HepG2 cells at 70% confluence were treated with the antimetabolite 5-FU in the absence or in the presence of one of the CD95L-blocking reagents, and apoptosis was evaluated by propidium iodide exclusion and FSC/SSC analysis. As shown in Fig. 1, drug treatment leads to a significant increase in apoptosis, starting from 12 to 24 h after administration of 5-FU and reaching over 40% after 48 h. These results were confirmed by staining the cells for subdiploid DNA content according to Nicoletti et al. (reference 52 and data not shown). Although apoptosis was not reduced to background levels, the effect of 5-FU could be blocked substantially by concomitant application of 50 μg of CD95-Fc/ml or 50 μg of NOK-1 antibody/ml, thereby attributing a significant role to the CD95 system in 5-FU-induced apoptosis. Similar results were obtained by treatment with etoposide, an inhibitor of topoisomerases, and 5-FU showed synergy with etoposide in inducing apoptosis in HepG2 cell lines (data not shown). Furthermore, treatment of Hep3B cells with the same concentrations of chemotherapeutic drugs did not lead to a significant induction of apoptosis. Hep3B cells are negative for p53 and do not express the CD95 receptor (48).
FIG. 1.
5-FU causes CD95-mediated apoptosis in HepG2 but not in Hep3B cells. HepG2 or Hep3B cells were grown to near confluence and then treated with 100 μg of 5-FU/ml for the indicated time periods in the presence of either an isotype-matched control antibody (Ig) or the anti-CD95L neutralizing antibody NOK-1 (50 μg/ml). The same experiment was performed with the inclusion of CD95-Fc (50 μg/ml). Apoptosis was determined by propidium iodide exclusion and FSC/SSC measurement. Data represent the mean with standard deviation from triplicate samples. Two experiments with a similar outcome were performed.
CD95L is upregulated upon administration of antineoplastic compounds.
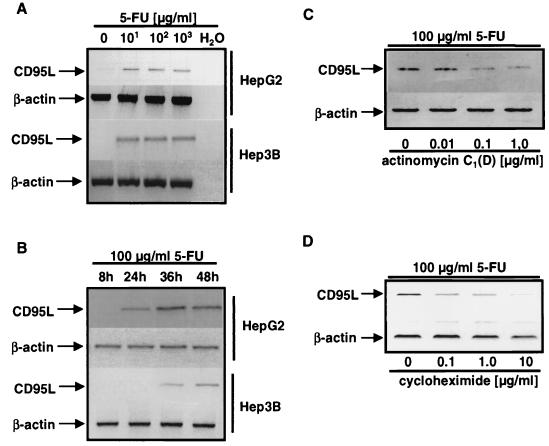
These data show that CD95-CD95L interactions are important for induction of apoptosis following exposure of hepatocellular carcinoma cells to chemotherapeutic drugs. Therefore, we investigated the mechanism of upregulation of CD95L upon drug exposure. As shown in Fig. 2, 5-FU can upregulate CD95L in both HepG2 and Hep3B cells (Fig. 2A and B). Upregulation of CD95L occurs late but is related to the onset of apoptosis in hepatocellular carcinoma cell lines. Upregulation of CD95L occurs earlier in HepG2 than in Hep3B cells (Fig. 2B).
FIG. 2.
CD95L mRNA is induced following stimulation with anticancer drugs, and the induction is regulated on the transcriptional level. PCR analysis of CD95L mRNA in HepG2 and Hep3B cells is shown. Total RNA was extracted and RT-PCR was performed as described in Materials and Methods. (A) HepG2 or Hep3B cells were incubated with the indicated concentrations of 5-FU for 36 h. (B) HepG2 or Hep3B cells were incubated with 100 μg of 5-FU/ml for the indicated time periods. (C and D) 5-FU (100 μg/ml) was given to HepG2 cells for 48 h, and either the transcriptional inhibitor actinomycin C1(D) (C) or the translational inhibitor cycloheximide (D) was added at the indicated concentrations.
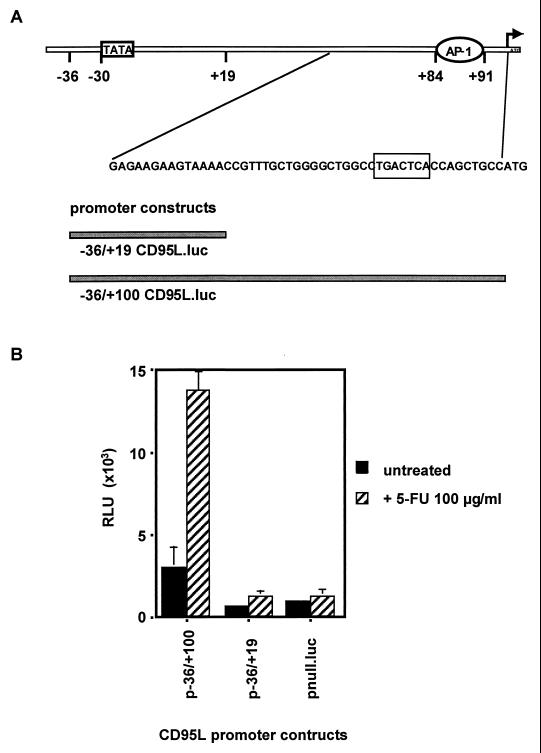
FIG. 5.
A region in the 5′ untranslated region of the CD95L gene comprising nucleotides +20 to +100 is responsible for CD95L induction following treatment with chemotherapeutic drugs. (A) Overview of the 5′ untranslated region of the CD95L gene. Bars represent the −36/+19 and −36/+100 constructs. The circled sequence is the AP-1 site near the first ATG. The arrow indicates the translation start site. (B) Hep3B cells were transfected with the constructs described in A above, and luciferase activity was measured following 48 h of treatment with 5-FU (100 μg/ml). One representative experiment with triplicate samples out of five independent experiments performed is shown. pnull.luc is a promoterless construct used as negative control. Transfection efficiency was controlled by cotransfection of a Renilla luciferase construct. RLU, relative light units.
Upregulation of CD95L mRNA could be observed in HepG2 and Hep3B cells. The upregulation is therefore independent of p53, as the Hep3B cell line is devoid of p53 protein. We were interested in whether CD95L upregulation in hepatocellular carcinoma cells is transcriptionally and translationally regulated. Therefore, we performed blocking experiments with either the transcriptional inhibitor actinomycin C1 (Fig. 2C) or the translational inhibitor cycloheximide (Fig. 2D). Both reagents were used at subtoxic concentrations and had no effect on transcription or translation of the housekeeping gene β-actin. However, upregulation of CD95L upon drug treatment was reduced effectively by actinomycin or cycloheximide, indicating that transcription and de novo protein synthesis are required for the upregulation of CD95L.
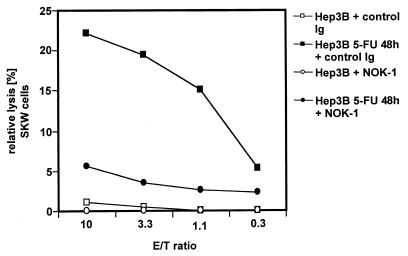
To test whether upregulation on the CD95L mRNA level was coincident with upregulation of CD95L protein on the cell surface, we examined whether treated Hep3B cells could kill CD95-positive target cells. 51Cr-labeled SKW6.4 cells expressing high levels of CD95 were incubated on a 5-FU-treated or -untreated Hep3B cell monolayer overnight. As demonstrated in Fig. 3, treatment of Hep3B cells with 5-FU led to significant killing of CD95-bearing SKW6.4 cells that was almost completely blocked with CD95L neutralizing NOK-1 antibody. These data show that treatment with antineoplastic drugs leads to upregulation of functional cell surface CD95L protein.
FIG. 3.
Treatment of malignant liver cells with chemotherapeutic drugs leads to upregulation of functional CD95L. A 51Cr-release assay with Hep3B cells as effectors and SKW6.4 cells as targets is depicted. Hep3B cells were grown in 96-well plates and treated with 5-FU. After 48 h, the chemotherapeutic drugs were removed from the culture medium and 51Cr-labeled SKW6.4 cells were added either with a control antibody or with the anti-CD95L antibody NOK-1. Following overnight incubation, the supernatants were measured in a gamma counter. The relative lysis was calculated as indicated in Materials and Methods. E/T is the ratio between effector and target cells. Each concentration was done in triplicate. The diagram represents one of three independent experiments with similar outcomes.
Chemotherapeutic drugs exert their effects on the CD95L promoter.
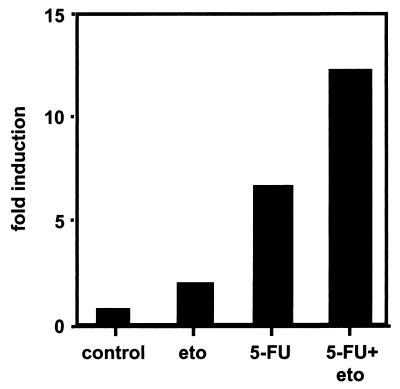
We showed by blocking with actinomycin C1 that chemotherapeutic drugs exert their effects on the CD95L promoter. Thus, we cloned different CD95L promoter deletion mutants in front of a luciferase reporter gene. These constructs were transiently transfected into Hep3B cells, and gene expression was determined in unstimulated and 5-FU/etoposide-stimulated cells. All reporter constructs ranging from 2.27 kb to 136 bp upstream to the first ATG were equally inducible (Table 1). The combination of chemotherapeutic drugs had an additive effect on the induction of these constructs (Fig. 4 and data not shown).
TABLE 1.
Inducibility of different CD95L promoter constructs upon treatment of transiently transfected Hep3B cells with 100 μg of 5-FU/ml for 48 h
| CD95L promoter construct | Fold induction | Standard deviation |
|---|---|---|
| −2269/+100 | 7.0 | 1.2 |
| −1204/+100 | 5.2 | 1.5 |
| −860/+100 | 6.4 | 1.3 |
| −412/+100 | 6.9 | 1.6 |
| −220/+100 | 5.5 | 1.2 |
| −36/+100 | 5.9 | 0.9 |
FIG. 4.
Potentiation of CD95L promoter activity by simultaneously applied anticancer drugs. Hep3B cells were transiently transfected with the −36/+100 CD95L promoter construct. Cells were treated for 48 h with 1.7 μM etoposide (eto) or 100 μg of 5-FU/ml or both agents, as indicated. Cells were lysed, and luciferase activity was measured. Three experiments with similar outcomes were performed. Transfection efficiencies were monitored by cotransfection of a Renilla luciferase construct driven from a basal promoter.
CD95L promoter activity is upregulated in liver cells upon stimulation with antineoplastic drugs via activation of a newly identified AP-1 element.
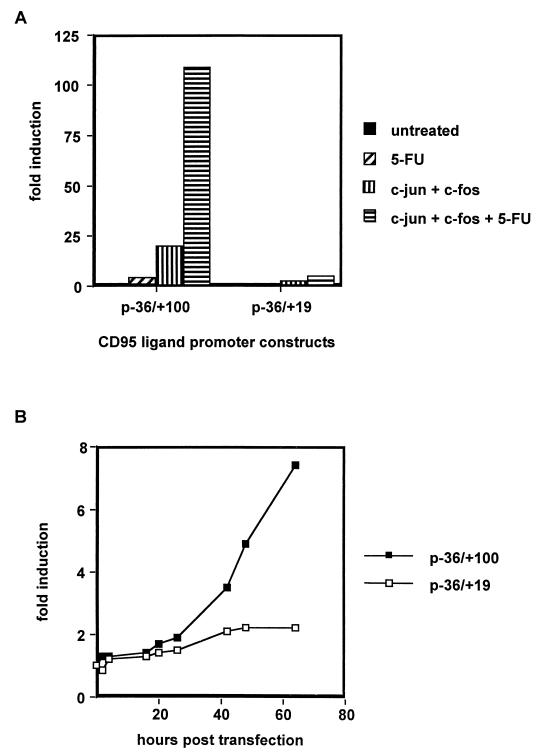
Since all promoter constructs ranging from −2269/+100 to −36/+100 showed equal inducibility upon drug treatment (Table 1), we speculated that a promoter element located between −36 and the translational start site may be responsible for promoter activation. To further determine this site, we generated 3′ deletion constructs of the minimal CD95L (−36/+100) promoter. As shown in Fig. 5B, the construct −36/+19 had a significantly reduced basal activity and was also less inducible than the −36/+100 construct. Therefore, we concluded that the +20/+100 region of the CD95L promoter contains an element responsible for stimulation with chemotherapeutic drugs. Inspection of this region revealed the presence of a consensus binding site for the transcription factor AP-1. The exact localization of this sequence (5′-TGACTCA-3′) is circled in Fig. 5A.
To confirm that this site is indeed a functional AP-1 site, we cotransfected c-jun and c-fos expression constructs with the −36/+100 and −36/+19 luciferase reporters. Figure 6A shows that cotransfection of exogenous c-Jun and c-Fos yielded a pronounced stimulation of the −36/+100 construct but not of the −36/+19 construct. Transfection of either c-jun or c-fos alone also had a stimulating effect (data not shown). Cotransfection with the AP-1 components and treatment with 5-FU did not result in a significant increase of activity of the −36/+19 construct, strongly supporting the localization of the responsible promoter site between +20 and +100 of the CD95L promoter.
FIG. 6.
The −36/+100 and −36/+19 constructs show different kinetics of activation and different inducibility upon cotransfection of c-jun and c-fos. (A) Cotransfection experiments in Hep3B cells with expression vectors for c-jun and c-fos. The cotransfected cells were subsequently treated with 100 μg of 5-FU/ml for 48 h or left untreated. Transfection efficiency was normalized by cotransfection of either an expression vector for chloramphenicoltransferase (CAT) or Renilla luciferase, both under the control of a basal promoter. Luciferase activity was measured with the dual luciferase assay from Promega according to the instructions of the manufacturer. CAT protein content was determined by a commercial CAT enzyme-linked immunosorbent assay (Boehringer GmbH, Mannheim, Germany). Mean values with standard deviation from four independent experiments are shown. Fold induction values in A and B were calculated as follows: relative light units (treated cells)/relative light units (untreated cells). (B) Dual luciferase assay with Hep3B cells cotransfected with the described CD95L promoter constructs and a Renilla luciferase expression vector as a control for transfection efficiency. Cells were treated as described above and were harvested after the indicated time points. In addition, the protein content of the transfected cells was measured. Data are the mean with standard deviation of triplicate samples of one representative experiment. Four independent experiments were performed.
To further investigate the importance of the AP-1 site, kinetics of induction were determined (Fig. 6B). The −36/+100 construct showed a strong activation between 20 and 25 h after initiation of 5-FU treatment. The −36/+19 construct, however, did not react to the same treatment; even after extended incubation with 5-FU, for 64 h, no significant increase could be observed. These results further underline the importance of the newly described AP-1 site in the CD95L promoter for treatment with chemotherapeutic drugs.
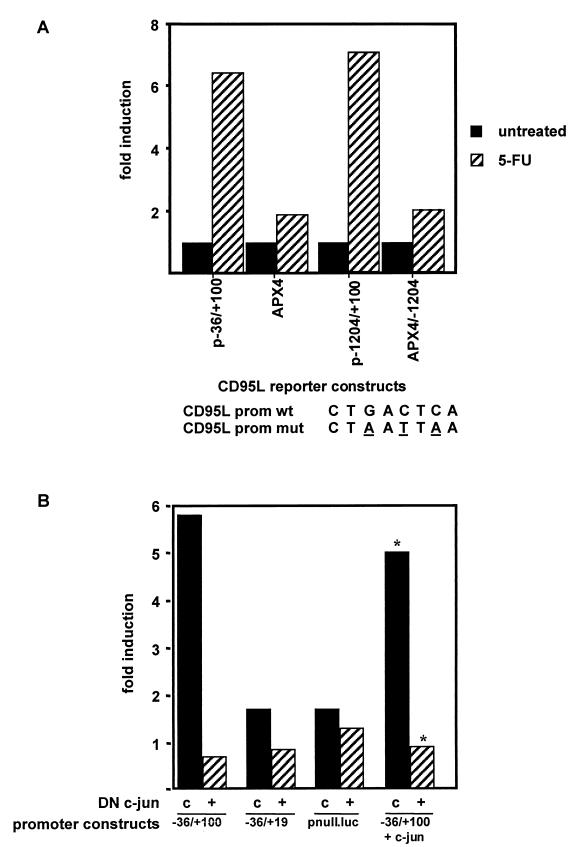
To further elucidate that the site is indeed necessary for the upregulation of CD95L promoter activity seen upon drug treatment, we mutated the AP-1 element by site-directed mutagenesis. The wild-type consensus sequence (CTGACTCA) was mutated at three different positions (CTAATTAA [44]). Introduction of these mutations into our −36/+100 or −1204/+100 reporter constructs almost completely abolished the inducibility of the constructs after 48 h of treatment with 100 μg of 5-FU/ml (Fig. 7A).
FIG. 7.
Mutations in the AP-1 site of the CD95L promoter destroy the inducibility of the promoter constructs, and induction is inhibited by dominant-negative c-Jun. (A) The AP-1 site in the basal promoter was mutated as indicated. To investigate the effect on the basal promoter, Hep3B cells were transfected with the −36/+100 (CD95L wild-type promoter [prom wt]) or the APX4 (mutated −36/+100 CD95L.luc, CD95L mutant promoter [prom mut]) constructs, respectively. To investigate the effect on the full-length promoter, Hep3B cells were transfected with the −1204/+100 (CD95L prom wt) or the APX4/−1204 (mutated −1204/+100 CD95L.luc, CD95L prom mut) constructs, respectively. Transfection efficiency was monitored by cotransfection of Renilla luciferase. Following transfection, cells were treated with 5-FU (100 μg/ml) for 48 h. Luciferase activity was measured and fold induction was calculated. One representative experiment out of five performed is shown. (B) Influence of dominant-negative c-jun (DN c-jun). Hep3B cells were transfected with the −36/+100, −36/+19, or pnull.luc constructs. Cells were either cotransfected with a control plasmid (c) or an expression construct for dominant-negative c-jun (+). As a control, c-jun was also cotransfected in one experiment. After completion of the transfection, cells were split and one-half was treated with 100 μg of 5-FU/ml and the other half was left untreated. Bars show fold induction calculated as follows: relative light units (treated cells)/relative light units (untreated cells). Relative luciferase units were normalized by Renilla luciferase activity using the dual luciferase system. Three independent experiments were performed, and one representative experiment is shown. The asterisks indicate that the absolute luciferase values in this experiment were approximately 75-fold higher than in experiments performed without the cotransfection of c-jun.
Next, we investigated whether transactivation by c-jun is required for the induction of the CD95L promoter. Therefore, we performed cotransfections of a dominant-negative c-jun construct lacking amino acids 1 to 192, a region which represents the transactivation domain, with the different luciferase reporter constructs. The dominant-negative c-jun effectively interferes with the activation of the AP-1 complex because it retains the dimerization domain but can no longer activate AP-1 target genes. The effect of inhibition is shown in Fig. 7B. Cotransfection of Hep3B cells with the −36/+100 reporter, c-jun, and dominant-negative c-jun totally abrogated the activation effect of c-jun on the promoter (Fig. 7B, right two columns). More importantly, dominant-negative c-jun also drastically inhibited promoter activation upon treatment with chemotherapeutic drugs. This underlines the necessity of c-Jun phosphorylation for the observed upregulation (Fig. 7B).
The AP-1 site in the CD95L promoter is preferentially bound by Jun-Fos heterodimers, and AP-1 is activated upon stimulation with chemotherapeutic drugs.
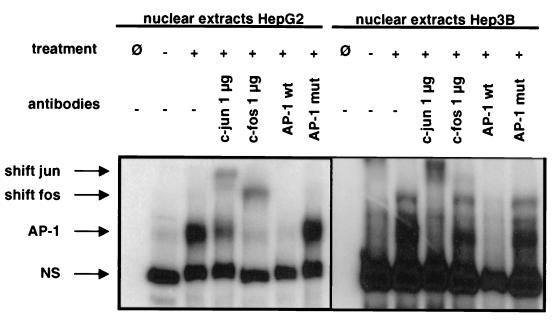
The AP-1 complex is formed by different components affecting the reaction to different extracellular stimuli. Thus, we sought to determine the composition of the dimer binding to the AP-1 site of the CD95L promoter and performed gel shift analyses with oligonucleotides comprising the AP-1 site (CTGACTCA). These oligonucleotides form a complex with nuclear extracts from 5-FU-treated HepG2 cells and from 5-FU-treated Hep3B cells (Fig. 8). The complex formation could be competed with unlabeled wild-type oligonucleotides and with consensus AP-1 oligonucleotides but not with unlabeled oligonucleotides comprising consensus sequences for either NF-κB or SP-1 (data not shown) or an oligonucleotide comprising a mutated AP-1 site (Fig. 8). These data indicate that AP-1 binds specifically to the target promoter site. To further identify the subcomponents of the binding complex, we conducted supershift experiments. As shown in Fig. 8, antibodies to c-Jun and to c-Fos further supershifted the complex. Anti-c-Jun and anti-c-Fos antibodies shifted the complexes in both nuclear extracts from HepG2 and Hep3B cells, respectively, although to different extents. Neither antibodies to C/EBP as an isotype-matched Ig specificity control nor antibodies to ATF2 influenced the mobility of the complexes (data not shown). This shows the specificity of a Jun-Fos heterodimer for this site.
FIG. 8.
Nuclear extracts from liver cell lines treated with chemotherapeutic drugs shift an oligonucleotide comprising the AP-1 sequence in the CD95L promoter. Electrophoretic mobility shift assay and supershift analyses of the +73/+99 region of the human CD95 ligand promoter sequence. Nuclear extracts from HepG2 and Hep3B cells were prepared as described in Materials and Methods. Cells had either been treated with 100 μg of 5-FU/ml for 48 h or been left untreated. Electrophoretic mobility shift analyses were done as described previously. For supershift analyses, antibodies against c-Jun or c-Fos were added. AP-1 wt or AP-1 mut are, respectively, the wild-type or mutant +73/+99 promoter regions used for competition experiments. Ø, negative control without nuclear extracts; −, untreated cells; +, treated cells. Antibodies were added as indicated. NS, nonspecific complexes; AP-1, specific complexes. Shift jun and shift fos indicate the respective shifted complexes.
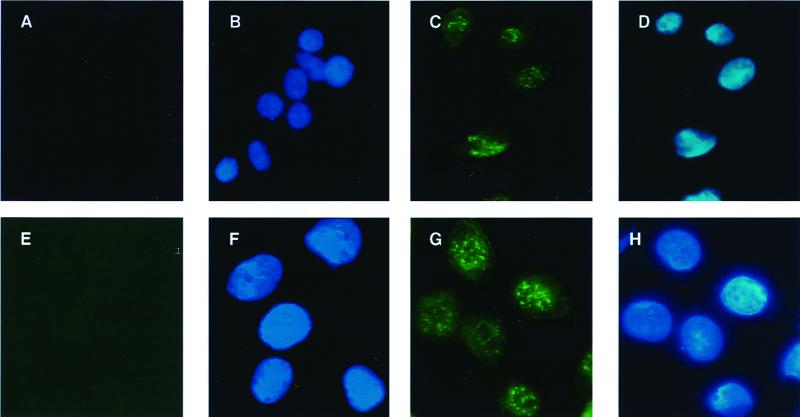
We further examined whether c-Jun expression is also enhanced upon treatment with chemotherapeutic drugs. Therefore, we immunostained Hep3B cells treated for 36 h with 5-FU or left untreated and examined the intensity and spatial distribution of the staining. As shown in Fig. 9, untreated Hep3B cells are negative for c-Jun (Fig. 9A). Upon treatment with 5-FU for 36 h, the cells accumulate AP-1 in the nucleus (Fig. 9C), suggesting a strong activation of AP-1 upon treatment with chemotherapeutic drugs. This induction of c-Jun is also apparent in primary human hepatocytes (Fig. 9E and G). The localization in the nucleus was demonstrated by costaining with DAPI (4′,6′-diamidino-2-phenylindole) (Fig. 9B, D, F, and H).
FIG. 9.
c-Jun protein is upregulated in Hep3B cells and primary human hepatocytes following treatment with chemotherapeutic drugs. (A to D) Hep3B cells were seeded on LabTek culture slides and cultured for 2 days. Subsequently, cells were either left untreated (A and B) or treated with 5-FU (100 μg/ml) for 36 h (C and D) and were fixed and stained with an antibody specific to c-Jun, as described above. DAPI (4′,6′-diamidino-2-phenylindole) staining of the same cells as in panels A and C is shown in panels B and D, respectively. (E to H) Human primary hepatocytes were isolated as described in Materials and Methods and seeded on LabTek culture slides. Cells were left untreated (E and F) or treated with 50 μg of 5-FU/ml for 24 h (G and H) and were fixed and stained with an antibody specific to c-Jun. DAPI staining of the same cells as in panels E and G is shown in panels F and H, respectively.
Involvement of the stress-activated protein kinase (SAPK/JNK) pathway in drug-induced CD95L upregulation.
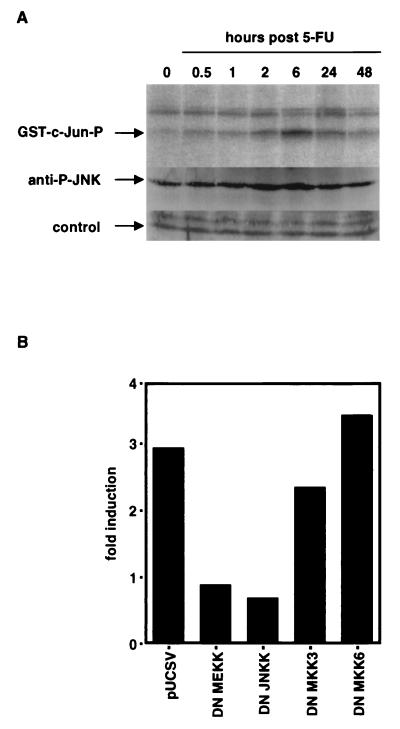
Cell-stressing factors engage the SAPK/JNK cascade in target cells. Chemotherapeutic drugs induce substantial stress in cells. Therefore, we investigated whether sequentially activated kinases are involved in the response to chemotherapeutic drugs. We performed in vitro kinase assays of immunoprecipitated JNKs with glutathione S-transferase (GST)–c-Jun as a substrate. As shown in Fig. 10A, treatment of HepG2 cells with 100 μg of 5-FU/ml increased the ability of the JNKs to phosphorylate the GST–c-Jun fusion protein. The induction was most prominent after 6 h. A similar pattern was observed for the phosphorylation of JNK (Fig. 10A), suggesting a sequential involvement of the JNK pathway. Next, we asked whether the p38 pathway might be involved in this phosphorylation. We cotransfected Hep3B cells with dominant-negative constructs of different SAPK/JNKs. As depicted in Fig. 10B, activation of reporter constructs by 5-FU was inhibited by cotransfection of dominant-negative MEKK-1 and dominant-negative JNKK, suggesting the sequential activation events MEKK-1 → JNKK → JNK → c-Jun. As a control, dominant-negative mutants of the p38 pathway were employed. Both dominant-negative constructs of the p38 pathway (DN-MKK3 and DN-MKK6 [72]) had virtually no effect on upregulation of CD95L promoter activity following drug treatment. In addition, transfections performed in the presence of blocking CD95-Fc constructs did not influence the outcome (data not shown), indicating that the activation of the MEKK-1/JNKK cascade is not a downstream event due to CD95 activation. Based on these results, taken together, we conclude that chemotherapeutic drugs induce CD95L via the JNK pathway.
FIG. 10.
The JNK pathway is activated and dominant-negative (DN) MEKK-1 and JNKK-1 constructs inhibit promoter activation upon treatment with chemotherapeutic drugs. (A) C-Jun and JNK are phosphorylated in HepG2 cells upon treatment with 100 μg of 5-FU/ml. At the indicated time points after treatment with 5-FU, cells were harvested and either activity of JNK was measured by an in vitro kinase immunocomplex assay with GST-c-Jun as a substrate (GST-c-Jun-P) or phosphorylation of JNK was determined by Western blotting with antibodies specific for phosphorylated JNK1/2 (anti-P-JNK). (B) Cotransfection experiments with a control vector (pUCSV) or with dominant-negative mutants for the stress-activated protein kinases JNKK-1, MEKK-1, MKK3, and MKK6. Values of fold induction were calculated as described above. One representative experiment out of three independent experiments is shown.
DISCUSSION
We have shown that chemotherapeutic drugs induce apoptosis in liver cell lines partially via activation of the CD95/CD95L system. This process involves activation of the transcription factor AP-1 stimulating the CD95L promoter. Activation of AP-1 is mediated by the MEKK-1/JNKK pathway. Thus, in malignant liver cells the CD95 system plays an important role in drug-induced apoptosis.
CD95-mediated apoptosis is not dependent on transcriptional events (30). However, expression of both CD95 and CD95L are transcriptionally regulated (8, 15, 35, 41, 46, 54). CD95 is expressed in most tissues, while constitutive CD95L expression is restricted to certain cells, such as cells in the anterior chamber of the eye and testis.
Induced CD95L expression is primarily seen in T cells (11). CD95L mRNA can be upregulated upon several stimuli, e.g., UV irradiation (7, 26), ionizing radiation (20, 61), or withdrawal of survival factors (37). We showed here that the action of chemotherapeutic drugs in liver cells appears to follow a similar mechanism: cell damage leads to activation of SAPK/JNK kinases, activation of AP-1, and upregulation of CD95L, subsequently leading to cell death by CD95-CD95L interaction. This situation closely resembles the activation-induced cell death found in activated T cells (6, 11). However, the CD95 pathway is not the only pathway involved in chemotherapy-induced apoptosis. Possibly, other death systems, such as the TRAIL system, may be involved in drug-induced apoptosis (22, 65). Several groups have published that the CD95 system plays a major role in drug-induced apoptosis. Incomplete blocking of drug-induced apoptosis by CD95-blocking reagents (Fig. 1) suggested that other apoptotic mechanisms are involved. Furthermore, other data contradicted those findings and suggested that death factor-induced apoptosis and anticancer drug-induced apoptosis are independent. This conclusion is derived primarily from investigations of drug-treated cells from caspase 8-, caspase 9-, FADD-, or Apaf-1-null mice (24, 34, 64, 70). In addition, Newton and Strasser published recently that cells from dominant-negative FADD overexpressing mice are not protected from anticancer drug-induced apoptosis (51). However, these conclusions are based on studies of primary cells from spleen or thymus or mouse embryonic fibroblasts. Tumor cells might behave differently as to the apoptotic phenotype, as some might have been selected for an apoptosis-resistant phenotype. Besides, the disruption of the CD95 pathway (as, for example, in lpr or gld mice) and similar resistance to anticancer drug-induced apoptosis do not exclude the possibility that the CD95 system plays a role for certain stress stimuli. Thus, the involvement of the CD95 system in drug-induced apoptosis is controversial and might depend on cell types and drugs used in the experiments. Recently, we have gained support for the involvement of the CD95 system from the following in vivo experiments. Mice injected with 5-FU lost half of their thymocytes approximately 20 h after injection of the drug. This effect could be blocked by neutralizing anti-CD95L antibody (S. T. Eichhorst et al., unpublished data).
Recent work from our laboratory suggests that chemotherapeutic drugs cause expression of CD95 via p53 binding to an intronic enhancer element in the first intron of the CD95 gene (48). Based on these results and on the results presented here, we propose the following mechanism for chemotherapy-induced apoptosis in liver tumors. Chemotherapeutic drugs induce the CD95 gene via a transcriptionally regulated, p53-dependent mechanism. They also engage the SAPK/JNK pathway eventually leading to upregulation of CD95L. Upregulation of CD95 and CD95L then allows the cells to either commit suicide or kill neighboring cells in a fratricidal manner. Cross talk between the two signaling pathways is also possible. Elkeles et al. reported that p53 activates the c-fos gene, thereby generating the partner of c-Jun to form the AP-1 complex required for CD95L activation (14). Moreover, MEKK/JNK signaling stabilizes and activates p53, which in turn could contribute to upregulation of CD95 (19).
Our results suggest that the SAPK/JNK system is of crucial importance in drug-induced CD95L upregulation. This finding is in line with several observations from other laboratories (36). Data by Behrens et al. further argues in this direction. They were able to show that mice with a mutated c-Jun, in which both serines 63 and 73 are replaced by alanines, show reduced kainate-induced neuronal apoptosis (3). This apoptosis defect is due to the impaired phosphorylation of these two residues in c-Jun, supporting a central role for c-Jun phosphorylation in the apoptosis-induction process. Moreover, it has been shown that lack of c-Jun activity increases survival after cisplatin treatment (58). These data may point to a universal, tissue-independent role of c-Jun for apoptosis induction. In contrast, several groups have reported an antiapoptotic function of c-Jun and the JNK cascade (13, 53, 69, 71). In addition to a cell type-specific and stimulus-specific response, this might be explained by the level of involvement of the SAPK/JNK cascade. On the one hand, the SAPK system could play an antiapoptotic role during the execution phase of an apoptotic response. On the other hand, it might nevertheless be involved in transcriptional regulation of proapoptotic molecules during the initiation phase (31). Moreover, the duration of JNK activation may play a role for regulation of the apoptotic program (10). Kasibhatla et al. reported that chemotherapeutic drugs induce CD95L in T-cell lines via activation of AP-1 and NF-κB (28). Our results demonstrate the AP-1 activation but do not the support the role of NF-κB. The difference in the results is most likely due to tissue specificity of transcription factor expression. These data may point towards a differential regulation of CD95L expression, depending on the tissue and transcriptional cofactors present. Thus, Srivastava et al. showed that NFAT is necessary for CD95L upregulation in Jurkat T-cell lines and breast carcinoma (62). Recently, Matsui et al. showed the presence of an AP-1 site in the murine CD95L promoter which works cooperatively with NF-κB to drive T-cell receptor-mediated CD95L expression (45). In line with our data, Preston et al. demonstrated that c-Fos protein is capable of inducing apoptosis in a human colorectal carcinoma cell line (56).
Interestingly, in our experimental system, all tested cytostatic drugs induced CD95L equally well. This is intriguing, because we used drugs with different primary modes of action, antimetabolites or topoisomerase inhibitors. Thus, different types of damage to the cell eventually converged in activation of the JNK/SAPK pathway. It would be highly interesting to determine the links of different types of damage to the activation of the SAPK/JNK pathway and finally to the upregulation of CD95L. Furthermore, in concordance with our observations, it has been demonstrated that MEKK-1 is involved in genotoxin-induced apoptosis (21, 66). The activating kinase activated by chemotherapeutic drugs may be ASK1, as reported by Chen et al. for cisplatin (10).
SAPK/JNK kinases were also shown to be activated downstream of CD95 after interaction with CD95L (25). We can rule out the possibility that the activation of JNK seen in our experiments was due to such a secondary effect, because the Hep3B line, in which most of the transfections were performed, was negative for CD95 (reference 47 and data not shown). Moreover, transfection experiments were also done in the presence of CD95-Fc to block CD95L. The addition of CD95-Fc to these experiments did not affect the outcome of our experiments (data not shown). Therefore, activation of c-jun N-terminal kinases is not due to a downstream CD95 signaling event. Activation of SAPK/JNK alone is also not sufficient for anticancer therapy-induced apoptosis (25).
One of the chemotherapeutic drugs used in our study was 5-FU, often used for treatment of patients with liver tumors and metastases. 5-FU acts by inhibition of thymidilate synthase (TS) in target cells. Houghton et al. studied the behavior of colon cancer cells lacking TS (27). The authors found that these cells undergo apoptosis following “thymineless death” due to the lack of TS (27). Interestingly, as in our experiments, these cells undergo apoptotic death mediated via the CD95/CD95L system. Moreover, it is remarkable that the thymineless death of colon carcinoma cells in the study of Houghton et al. occurred in a time frame similar to that of our experiments.
We propose the CD95-CD95L interaction as a possible mechanism of action of anticancer drugs. Our data could be used to develop specific anticancer therapies.
ACKNOWLEDGMENTS
We thank Sibylle Teurich for excellent technical assistance. Michael Karin kindly provided DN-MEKK and DN-JNKK, Jim Woodgett provided DN-MKK3 and DN-MKK6, Sabine Kirchhoff furnished Renilla luciferase and CAT expression vectors, and Dirk Bohmann provided DN-Jun expression vectors. We thank Thomas Wirth for pTATA.luc and Christina Berndt, Susanne Müerköster, Ingo Schmitz, Elena Ritsou, and Frederick Igney for critical reading of the manuscript.
REFERENCES
- 1.Angel P, Allegretto E A, Okino S T, Hattori K, Boyle W J, Hunter T, Karin M. Oncogene jun encodes a sequence-specific trans-activator similar to AP-1. Nature. 1988;332:166–171. doi: 10.1038/332166a0. [DOI] [PubMed] [Google Scholar]
- 2.Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55:875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- 3.Behrens A, Sibilia M, Wagner E F. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21:326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- 4.Berndt C, Mopps B, Angermuller S, Gierschik P, Krammer P H. CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4(+) T cells. Proc Natl Acad Sci USA. 1998;95:12556–12561. doi: 10.1073/pnas.95.21.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry M N, Friend D S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969;43:506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner T, Mogil R J, LaFace D, Yoo N J, Mahboubi A, Echeverri F, Martin S J, Force W R, Lynch D H, Ware C F, et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 7.Caricchio R, Reap E A, Cohen P L. Fas/Fas ligand interactions are involved in ultraviolet-B-induced human lymphocyte apoptosis. J Immunol. 1998;161:241–251. [PubMed] [Google Scholar]
- 8.Chan H, Bartos D P, Owen-Schaub L B. Activation-dependent transcriptional regulation of the human Fas promoter requires NF-kappaB p50-p65 recruitment. Mol Cell Biol. 1999;19:2098–2108. doi: 10.1128/mcb.19.3.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Seimiya H, Naito M, Mashima T, Kizaki A, Dan S, Imaizumi M, Ichijo H, Miyazono K, Tsuruo T. ASK1 mediates apoptotic cell death induced by genotoxic stress. Oncogene. 1999;18:173–180. doi: 10.1038/sj.onc.1202276. [DOI] [PubMed] [Google Scholar]
- 11.Dhein J, Walczak H, Baumler C, Debatin K M, Krammer P H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 12.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eferl R, Sibilia M, Hilberg F, Fuchsbichler A, Kufferath I, Guertl B, Zenz R, Wagner E F, Zatloukal K. Functions of c-Jun in liver and heart development. J Cell Biol. 1999;145:1049–1061. doi: 10.1083/jcb.145.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkeles A, Juven-Gershon T, Israeli D, Wilder S, Zalcenstein A, Oren M. The c-fos proto-oncogene is a target for transactivation by the p53 tumor suppressor. Mol Cell Biol. 1999;19:2594–2600. doi: 10.1128/mcb.19.4.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faris M, Kokot N, Latinis K, Kasibhatla S, Green D R, Koretzky G A, Nel A. The c-Jun N-terminal kinase cascade plays a role in stress-induced apoptosis in Jurkat cells by up-regulating Fas ligand expression. J Immunol. 1998;160:134–144. [PubMed] [Google Scholar]
- 16.Faris M, Latinis K M, Kempiak S J, Koretzky G A, Nel A. Stress-induced Fas ligand expression in T cells is mediated through a MEK kinase 1-regulated response element in the Fas ligand promoter. Mol Cell Biol. 1998;18:5414–5424. doi: 10.1128/mcb.18.9.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher D E. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 18.Friesen C, Herr I, Krammer P H, Debatin K M. Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nat Med. 1996;2:574–577. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs S Y, Adler V, Pincus M R, Ronai Z. MEKK1/JNK signaling stabilizes and activates p53. Proc Natl Acad Sci USA. 1998;95:10541–10546. doi: 10.1073/pnas.95.18.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fulda S, Scaffidi C, Pietsch T, Krammer P H, Peter M E, Debatin K M. Activation of the CD95 (APO-1/Fas) pathway in drug- and gamma-irradiation-induced apoptosis of brain tumor cells. Cell Death Differ. 1998;5:884–893. doi: 10.1038/sj.cdd.4400419. [DOI] [PubMed] [Google Scholar]
- 21.Gibson S, Widmann C, Johnson G L. Differential involvement of MEK kinase 1 (MEKK1) in the induction of apoptosis in response to microtubule-targeted drugs versus DNA damaging agents. J Biol Chem. 1999;274:10916–10922. doi: 10.1074/jbc.274.16.10916. [DOI] [PubMed] [Google Scholar]
- 22.Gibson S B, Oyer R, Spalding A C, Anderson S M, Johnson G L. Increased expression of death receptors 4 and 5 synergizes the apoptosis response to combined treatment with etoposide and TRAIL. Mol Cell Biol. 2000;20:205–212. doi: 10.1128/mcb.20.1.205-212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffith T S, Brunner T, Fletcher S M, Green D R, Ferguson T A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 24.Hakem R, Hakem A, Duncan G S, Henderson J T, Woo M, Soengas M S, Elia A, De la Pompa J L, Kagi D, Khoo W, Potter J, Yoshida R, Kaufman S A, Lowe S W, Penninger J M, Mak T W. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 25.Herr I, Wilhelm D, Bohler T, Angel P, Debatin K M. JNK/SAPK activity is not sufficient for anticancer therapy-induced apoptosis involving CD95-L, TRAIL and TNF-alpha. Int J Cancer. 1999;80:417–424. doi: 10.1002/(sici)1097-0215(19990129)80:3<417::aid-ijc14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Hill L L, Ouhtit A, Loughlin S M, Kripke M L, Ananthaswamy H N, Owen-Schaub L B. Fas ligand: a sensor for DNA damage critical in skin cancer etiology. Science. 1999;285:898–900. doi: 10.1126/science.285.5429.898. [DOI] [PubMed] [Google Scholar]
- 27.Houghton J A, Harwood F G, Tillman D M. Thymineless death in colon carcinoma cells is mediated via fas signaling. Proc Natl Acad Sci USA. 1997;94:8144–8149. doi: 10.1073/pnas.94.15.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green D R. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell. 1998;1:543–551. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- 29.Kastan M B, Onyekwere O, Sidransky D, Vogelstein B, Craig R W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 30.Klas C, Debatin K M, Jonker R R, Krammer P H. Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol. 1993;5:625–630. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- 31.Kolbus A, Herr I, Schreiber M, Debatin K M, Wagner E F, Angel P. c-Jun-dependent CD95-L expression is a rate-limiting step in the induction of apoptosis by alkylating agents. Mol Cell Biol. 2000;20:575–582. doi: 10.1128/mcb.20.2.575-582.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krammer P H. CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv Immunol. 1999;71:163–210. doi: 10.1016/s0065-2776(08)60402-2. [DOI] [PubMed] [Google Scholar]
- 33.Krammer P H, Galle P R, Moller P, Debatin K M. CD95(APO-1/Fas)-mediated apoptosis in normal and malignant liver, colon, and hematopoietic cells. Adv Cancer Res. 1998;75:251–273. doi: 10.1016/s0065-230x(08)60744-7. [DOI] [PubMed] [Google Scholar]
- 34.Kuida K, Haydar T F, Kuan C Y, Gu Y, Taya C, Karasuyama H, Su M S, Rakic P, Flavell R A. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 35.Latinis K M, Norian L A, Eliason S L, Koretzky G A. Two NFAT transcription factor binding sites participate in the regulation of CD95 (Fas) ligand expression in activated human T cells. J Biol Chem. 1997;272:31427–31434. doi: 10.1074/jbc.272.50.31427. [DOI] [PubMed] [Google Scholar]
- 36.Lee L F, Li G, Templeton D J, Ting J P. Paclitaxel (Taxol)-induced gene expression and cell death are both mediated by the activation of c-Jun NH2-terminal kinase (JNK/SAPK) J Biol Chem. 1998;273:28253–28260. doi: 10.1074/jbc.273.43.28253. [DOI] [PubMed] [Google Scholar]
- 37.Le-Niculescu H, Bonfoco E, Kasuya Y, Claret F X, Green D R, Karin M. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death. Mol Cell Biol. 1999;19:751–763. doi: 10.1128/mcb.19.1.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leppa S, Saffrich R, Ansorge W, Bohmann D. Differential regulation of c-Jun by ERK and JNK during PC12 cell differentiation. EMBO J. 1998;17:4404–4413. doi: 10.1093/emboj/17.15.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 40.Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc Natl Acad Sci USA. 1998;95:13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li-Weber M, Laur O, Hekele A, Coy J, Walczak H, Krammer P H. A regulatory element in the CD95 (APO-1/Fas) ligand promoter is essential for responsiveness to TCR-mediated activation. Eur J Immunol. 1998;28:2373–2383. doi: 10.1002/(SICI)1521-4141(199808)28:08<2373::AID-IMMU2373>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 42.Los M, Wesselborg S, Schulze-Osthoff K. The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Immunity. 1999;10:629–639. doi: 10.1016/s1074-7613(00)80062-x. [DOI] [PubMed] [Google Scholar]
- 43.Lowe S W, Ruley H E, Jacks T, Housman D E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 44.Marks-Konczalik J, Chu S C, Moss J. Cytokine-mediated transcriptional induction of the human inducible nitric oxide synthase gene requires both activator protein 1 and nuclear factor kappaB-binding sites. J Biol Chem. 1998;273:22201–22208. doi: 10.1074/jbc.273.35.22201. [DOI] [PubMed] [Google Scholar]
- 45.Matsui K, Xiao S, Fine A, Ju S T. Role of activator protein-1 in TCR-mediated regulation of the murine fas1 promoter. J Immunol. 2000;164:3002–3008. doi: 10.4049/jimmunol.164.6.3002. [DOI] [PubMed] [Google Scholar]
- 46.McClure R F, Heppelmann C J, Paya C V. Constitutive Fas ligand gene transcription in Sertoli cells is regulated by Sp1. J Biol Chem. 1999;274:7756–7762. doi: 10.1074/jbc.274.12.7756. [DOI] [PubMed] [Google Scholar]
- 47.Muller M, Strand S, Hug H, Heinemann E M, Walczak H, Hofmann W J, Stremmel W, Krammer P H, Galle P R. Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. J Clin Investig. 1997;99:403–413. doi: 10.1172/JCI119174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, Friedman S L, Galle P R, Stremmel W, Oren M, Krammer P H. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med. 1998;188:2033–2045. doi: 10.1084/jem.188.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 50.Neubauer K, Eichhorst S T, Wilfling T, Buchenau M, Xia L, Ramadori G. Sinusoidal intercellular adhesion molecule-1 up-regulation precedes the accumulation of leukocyte function antigen-1-positive cells and tissue necrosis in a model of carbontetrachloride-induced acute rat liver injury. Lab Investig. 1998;78:185–194. [PubMed] [Google Scholar]
- 51.Newton K, Strasser A. Ionizing radiation and chemotherapeutic drugs induce apoptosis in lymphocytes in the absence of Fas of FADD/MORT1 signaling: implications for cancer therapy. J Exp Med. 2000;191:195–200. doi: 10.1084/jem.191.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 53.Nishina H, Vaz C, Billia P, Nghiem M, Sasaki T, De la Pompa J L, Furlonger K, Paige C, Hui C, Fischer K D, Kishimoto H, Iwatsubo T, Katada T, Woodgett J R, Penninger J M. Defective liver formation and liver cell apoptosis in mice lacking the stress signaling kinase SEK1/MKK4. Development. 1999;126:505–516. doi: 10.1242/dev.126.3.505. [DOI] [PubMed] [Google Scholar]
- 54.Norian L A, Latinis K M, Koretzky G A. A newly identified response element in the CD95 ligand promoter contributes to optimal inducibility in activated T lymphocytes. J Immunol. 1998;161:1078–1082. [PubMed] [Google Scholar]
- 55.Peter M E, Scaffidi C, Medema J P, Kischkel F, Krammer P H. The death receptors. Results Probl Cell Differ. 1999;23:25–63. doi: 10.1007/978-3-540-69184-6_3. [DOI] [PubMed] [Google Scholar]
- 56.Preston G A, Lyon T T, Yin Y, Lang J E, Solomon G, Annab L, Srinivasan D G, Alcorta D A, Barrett J C. Induction of apoptosis by c-Fos protein. Mol Cell Biol. 1996;16:211–218. doi: 10.1128/mcb.16.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radler-Pohl A, Sachsenmaier C, Gebel S, Auer H P, Bruder J T, Rapp U, Angel P, Rahmsdorf H J, Herrlich P. UV-induced activation of AP-1 involves obligatory extranuclear steps including Raf-1 kinase. EMBO J. 1993;12:1005–1012. doi: 10.1002/j.1460-2075.1993.tb05741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanchez-Perez I, Perona R. Lack of c-Jun activity increases survival to cisplatin. FEBS Lett. 1999;453:151–158. doi: 10.1016/s0014-5793(99)00690-0. [DOI] [PubMed] [Google Scholar]
- 59.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schroder A J, Blaheta R A, Scholz M, Encke A, Markus B H. Isolation and separation of human adult hepatocytes from resected liver: cellular yield and purity using different methods. ZBL Chir. 1994;119:127–138. [PubMed] [Google Scholar]
- 61.Sheard M A, Vojtesek B, Janakova L, Kovarik J, Zaloudik J. Up-regulation of Fas (CD95) in human p53 wild-type cancer cells treated with ionizing radiation. Int J Cancer. 1997;73:757–762. doi: 10.1002/(sici)1097-0215(19971127)73:5<757::aid-ijc24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 62.Srivastava R K, Sasaki C Y, Hardwick J M, Longo D L. Bcl-2-mediated drug resistance: inhibition of apoptosis by blocking nuclear factor of activated T lymphocytes (NFAT)-induced Fas ligand transcription. J Exp Med. 1999;190:253–265. doi: 10.1084/jem.190.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varfolomeev E E, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann J S, Mett I L, Rebrikov D, Brodianski V M, Kemper O C, Kollet O, Lapidot T, Soffer D, Sobe T, Avraham K B, Goncharov T, Holtmann H, Lonai P, Wallach D. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 65.Walczak H, Miller R E, Ariail K, Gliniak B, Griffith T S, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin R G, Rauch C T, Schuh J C, Lynch D H. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 66.Widmann C, Gerwins P, Johnson N L, Jarpe M B, Johnson G L. MEK kinase 1, a substrate for DEVD-directed caspases, is involved in genotoxin-induced apoptosis. Mol Cell Biol. 1998;18:2416–2429. doi: 10.1128/mcb.18.4.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilhelm D, Bender K, Knebel A, Angel P. The level of intracellular glutathione is a key regulator for the induction of stress-activated signal transduction pathways including Jun N-terminal protein kinases and p38 kinase by alkylating agents. Mol Cell Biol. 1997;17:4792–4800. doi: 10.1128/mcb.17.8.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams B A, Makrigiannis A P, Blay J, Hoskin D W. Treatment of the P815 murine mastocytoma with cisplatin or etoposide up-regulates cell-surface Fas (CD95) expression and increases sensitivity to anti-Fas antibody-mediated cytotoxicity and to lysis by anti-CD3-activated killer-T cells. Int J Cancer. 1997;73:416–423. doi: 10.1002/(sici)1097-0215(19971104)73:3<416::aid-ijc17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 69.Wisdom R, Johnson R S, Moore C. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 1999;18:188–197. doi: 10.1093/emboj/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshida H, Kong Y Y, Yoshida R, Elia A J, Hakem A, Hakem R, Penninger J M, Mak T W. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94:739–750. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]
- 71.Yujiri T, Sather S, Fanger G R, Johnson G L. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science. 1998;282:1911–1914. doi: 10.1126/science.282.5395.1911. [DOI] [PubMed] [Google Scholar]
- 72.Zanke B W, Rubie E A, Winnett E, Chan J, Randall S, Parsons M, Boudreau K, McInnis M, Yan M, Templeton D J, Woodgett J R. Mammalian mitogen-activated protein kinase pathways are regulated through formation of specific kinase-activator complexes. J Biol Chem. 1996;271:29876–29881. doi: 10.1074/jbc.271.47.29876. [DOI] [PubMed] [Google Scholar]