Abstract
The aim of this systematic review was to determine the success rate of nitrous oxide-oxygen procedural sedation (NOIS) in dentistry. A systematic digital search was conducted for publications or reports of randomized controlled trials evaluating the clinical performance of NOIS. Abstracts of research papers were screened for suitability, and full-text articles were obtained for those who met the inclusion and exclusion criteria accordingly. The quality of the studies was assessed using the revised Cochrane risk-of-bias tool (RoB 2). A total of 19 articles (eight randomized clinical trials with parallel intervention groups and 11 crossover trials), published between May 1988 and August 2019, were finally selected for this review. The studies followed 1293 patients reporting NOIS success rates, with a cumulative mean value of 94.9% (95% CI: 88.8–98.9%). Thirteen trials were conducted on pediatric populations (1098 patients), and the remaining six were conducted on adults (195 patients), with cumulative efficacy rates of 91.9% (95% CI: 82.5–98.1%) and 99.9% (95% CI: 97.7–100.0%), respectively. The difference was statistically significant (P = 0.002). Completion of treatment and Section IV of the Houpt scale were the most used efficacy criteria. Within the limitations of this systematic review, the present study provides important information on the efficacy rate of NOIS. However, further well-designed and well-documented clinical trials are required and there is a need to develop guidelines for standardization of criteria and definition of success in procedural sedation. Currently, completion of treatment is the most used parameter in clinical practice, though many others also do exist at the same time. To maximize NOIS efficacy, clinicians should strictly consider appropriate indications for the procedure.
Keywords: Conscious Sedation, Dental Care, Nitrous Oxide, Procedural Sedation
INTRODUCTION
In dentistry, nitrous oxide-oxygen mixture (N2O-O2) is used to induce procedural sedation (previously termed “conscious sedation” [1,2]) in patients unable to receive standard dental treatments [3] or merely to complete therapies comfortably for both the professional and the patient [4]. Procedural sedation is defined as a state of minimal or moderate sedation [5] according to the American Society of Anesthesiologists (ASA) classification [6]. The administration of N2O-O2 can induce procedural sedation due to the potential unspecific depression of the central nervous system caused by this gas mixture. Nitrous oxide delivered at a concentration < 50% is accepted as a minimal sedation drug by the ASA [2] and at a concentration ≤ 50% by the American Academy of Pediatrics (AAP) [7]. In concentrations > 50%, the AAP cautions that “the likelihood for moderate or deep sedation increases” [8]. Therefore, when the proper use of N2O-O2 is effective, the patient shows signs of depressed consciousness (e.g., relaxed, somnolent patient who may appear dissociated and with a feeling of well-being and confidence) [3] but he/she remains in verbal contact throughout treatment and maintains all vital functions [9] (e.g., preservation of airway patency and spontaneous ventilation [1]). However, there is a controversy regarding the efficacy of this technique in scientific literature [10], as it may not always be successful [7,11].
To the best of the authors’ knowledge, the clinical performance of nitrous oxide-oxygen procedural sedation (NOIS) in dentistry has not been sufficiently summarized by an evidence-based method. As a matter of fact, scientific literature lacks reviews that systematically compare and synthesize data from existing studies to determine the efficacy rate of NOIS in dentistry, despite its wide use in this field and the importance for both clinicians and patients to rely on statistically calculated rates for therapy outcomes. Moreover, the efficacy rate of NOIS in dentistry varies widely as reported in published studies. In a recent article [12], it was stated that the efficacy rates of this procedure were placed in a range from 77% to 97%. However, this study had taken into account few reference studies (n = 4) and did not have any systematic analyses included therein. Indeed, other studies showed lower (52% [13]) or higher (100% [14]) rates.
The importance of reporting context-specific evidence of NOIS effectiveness following contemporary research guidelines has been underlined by many authors [13,15,16,17,18,19], and the European Society of Anesthesiology (ESA) [20] stated that there is a need to define the most appropriate procedure-related effective use of nitrous oxide-oxygen. Apparently, there is a lack of information about NOIS efficacy in dental private offices rather than in the hospital settings [15,21], as this sedation technique has been mostly studied in relation to medical procedures other than dental care [22]. In dentistry, it has been described that NOIS is successful in most cases, but not in all, which justifies the importance of investigating its effectiveness in avoiding unwanted consequences for the patient (e.g., aborted, traumatic, or even harmful experience) [11,23].
Therefore, the aim of this study was to systematically review the existing scientific literature and to determine the success rate of NOIS in dentistry.
METHODS
1. Review protocol
The Cochrane Handbook Method Guidelines [24] and Center for Reviews and Dissemination (CRD) guidelines [25] were consulted to prepare the conduct of this study. The protocol was written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and checklist [26]. According to the population, intervention, comparison, outcome, and study design (PICOS) approach [25,27], the guiding question of this review was: “What is the clinical success rate of NOIS in dental patients from randomized clinical trials?”.
The full review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42020155159. The text can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=155159.
2. Search strategy
A digital systematic literature search in PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, EBSCO, LILACS, Summon, and Database of Abstracts of Reviews of Effects (DARE) was conducted on April 2, 2021. The US National Institutes of Health Ongoing Trials Register (clinicaltrials.gov) and World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) were searched on the same date for ongoing studies.
The search strategy was implemented using the following keywords: “nitrous oxide” AND “dental”. No limits were applied to the year of study; however, when possible, search filters were used to find only studies of interest (trials) and articles published in English, Spanish, or Italian. The digital search was implemented by manually searching the reference lists from full-text articles and related reviews.
3. Inclusion criteria
The PICOS criteria related to research question are detailed below:
1. Population: Adult or child who required procedural sedation for dental treatment.
2. Intervention: Inhalation of nitrous oxide-oxygen mixture.
3. Comparison: One or more other drugs, sedation technique, or placebo that was used by dental team professionals. As this category of PICOS approach is optional [28,29], comparison outcomes were not analyzed, these being irrelevant in this review.
-
4. Outcome:
- 4.1. Primary: Clinical success rate of NOIS.
- 4.2. Secondary: Clinical methods (e.g., scales) for defining NOIS success were listed to create a dataset of efficacy criteria prevalence in published trials.
5. Study Design: Randomized controlled clinical trials (RCTs), including crossover trials.
4. Exclusion criteria
Studies presenting at least one of the following characteristics were excluded from this review:
1. Population: Patients with special needs (i.e., patients with physical, medical, developmental, or cognitive conditions who require special consideration when receiving dental treatment).
-
2. Intervention:
- 2.1. Combination of nitrous oxide-oxygen with other drugs (except for local anesthetics), sedation techniques, or placebo.
- 2.2. Administration of nitrous oxide-oxygen through any device other than nasal hood.
3. Comparison: None.
-
4. Outcome:
- 4.1. NOIS success was not defined by trialists or by literature regarding the specific evaluation method used.
- 4.2. Impossibility of extracting data for cumulative efficacy rate calculation (e.g., grouped data presented only as means and/or medians, neglected data, trials that do not evaluate NOIS efficacy).
5. Study Design: Any study design except an RCT.
5. Selection process
Two reviewers (MR and VG) independently screened the above-mentioned databases to select studies that met the inclusion criteria. In order to do this, these authors independently assessed each study found in the review process by grading it as “eligible,” “not eligible” or “might be eligible” [30]. A study was included if both reviewers independently assessed it to be satisfying the inclusion criteria based on the full-text article. Trials’ authors were contacted, if necessary, to request clarifications, raw data, or additional data to those already reported.
In case of disagreement, the full text was analyzed and discussed by all the reviewers to find a consensus.
6. Data extraction
A data extraction form was created using Excel software (version 16.46, Microsoft, Redmond WA, USA) to collect information of interest from articles and facilitate comparison between studies. The two reviewers responsible for screening databases independently used this tool to record all the studies found in the review process. Therefore, data from each study were collected in duplicate and compared at the end of the process.
For included studies, information on trial design, type of interventions, sample size, characteristics of patients, main outcome data, and criteria used to evaluate NOIS efficacy were collected accordingly. Main outcome data were extracted as “number of events / population” proportion (i.e., “number of sedated patients / total number of patients”), in order to obtain an efficacy rate for each trial. Thus, binary data were extracted from these studies.
A “three stage” decision process for inclusion of crossover data in meta-analysis [24,31,32] was followed in the case of crossover trials. To calculate a pooled estimate, reviewers were required to use the same method to analyze data from all included crossover trials [32].
The third reviewer (JMR) checked collected data for consistency and clarity.
7. Quality assessment
The methodological quality of the included studies was assessed using the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2, 2019 version) [33]. The authors independently completed the related form for each included study, grading each trial as being at low, high, or unclear risk of bias. A comparison of the evaluations allowed the reviewers to find a consensus.
8. Measurement of treatment effect
NOIS success was defined according to the criteria (e.g. scales) specified in the individual studies. Sedation scales may be ordinal or dichotomous [34]: as ordinal scales have generally a threshold or cutoff score to establish achievement of procedural sedation state [35], it was possible to collect and group binary data also from included trials that used this type of evaluation (i.e., final dichotomous evaluation: “Yes”=Sedated patient [SP]; “No”=Non-sedated patient [NSP]). Hence, it was feasible to obtain proportions of NOIS efficacy, expressing them as percentages (SP/(SP + NSP) × 100), and to combine trial outcomes, allowing meta-analysis to be carried out.
9. Unit of analysis
The statistical unit was the patient undergoing NOIS for dental treatment.
10. Assessment of heterogeneity
The I2 index was used to describe the degree of heterogeneity among studies [36]. Heterogeneity was considered low if the I2 value was < 50%, moderate between 50% and 75%, and high if ≥ 75%. A random-effects model was used in cases of moderate/high heterogeneity and a fixed-effect model was used in cases of mild heterogeneity.
11. Synthesis of results
Main outcome data were pooled after Freeman-Tukey double arcsine transformation for proportions close to the 0 and 1 values [37] and the NOIS efficacy rate was calculated as previously detailed. Outcomes were combined and calculated using the packages “meta” and “metaprop” [38] for meta-analysis within the statistical R software (version 4.0.1, FOAS, Boston MA, USA). For the objective of this review, the efficacy rates of NOIS were grouped and reported as an event rate with a 95% confidence interval (95% CI).
The included trials were divided into two groups depending on the patients’ type: adult population (≥ 16 years old) and pediatric population (< 16 years old). This differentiation was performed in order to perform a subgroup analysis of NOIS efficacy in those populations, which was completed with the same synthesis methods used for the meta-analysis of the total population. The chi-square test was used to calculate statistical significance when comparing the results. The level of significance was set at P = 0.05 .
The results were visually represented with forest-plot diagrams created with R software, which also allowed for visual expression of heterogeneity among the included studies.
RESULTS
1. Search results
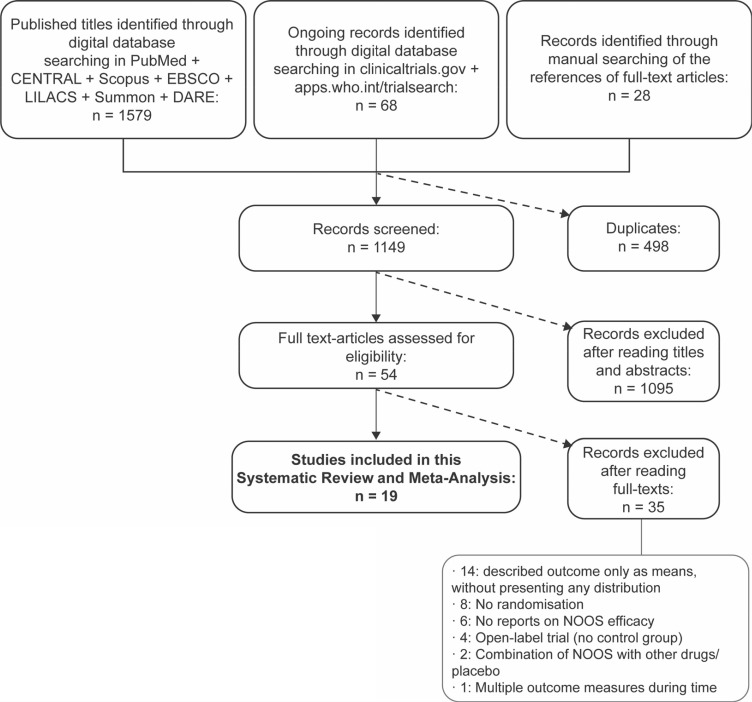
Initial searches using MeSH terms and title/abstract words resulted in 1647 potential studies. After removing duplicates, a total of 1149 references were identified from the digital sources. Screening of titles and abstracts allowed the selection of 54 full-text studies. Hand searches of the bibliographies of full-text articles identified 28 additional articles. After full-text evaluation, 19 RCTs [13,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] published between May 1988 and August 2019 were finally selected for the review. The study screening, selection process, and reasons for exclusion are depicted in Fig. 1, as per the PRISMA guidelines.
Fig. 1. Flow chart (PRISMA, preferred reporting items for systematic reviews and meta-analyses) of the screening and selection of studies process.
2. Included studies
The details of the included studies are listed in Table 1. A total of 1321 patients were enrolled in the trials; the number of drop-outs was 28, and 1293 patients could be completely followed. Of the 19 included studies, 13 were conducted on pediatric populations (1098 patients, 84.9%) and the remaining six were conducted in adult populations (195 patients, 15.1%). Of the 726 patients treated with NOIS, 582 (80.2%) were children and 144 (19.8%) were adults. Eight studies had parallel intervention groups, while the remaining trials had a crossover design.
Table 1. Studies included in this systematic review: characteristics according to PICOS approach.
| Author | Participants | Intervention | Control | Outcome | Study Design |
|---|---|---|---|---|---|
| Gupta P, et al. (2019) [39] |
· Adults (≥ 18 y.o.) · MDAS 19-25 · n = 60 (30/group) |
Slow induction (max: 50% N2O) |
Local anesthesia alone | MDAS (< 19): · NOOS: 100.0% · Control: 96.7% |
N. I. on blinding, Parallel groups |
| Samir PV, et al. (2017) [40] |
· Children (5-12 y.o.) · Frankl 3-4 · n = 60 (30/group) |
Slow induction (max: 40% N2O) (NOOS 1) |
Rapid induction, adjustable proportions (max: 40% N2O) (NOOS 2) | RASS (≤ -2): · NOOS 1: 100.0% · NOOS 2: 100.0% |
N.I. on blinding, Parallel groups |
| Subramaniam P, et al. (2017) [41] |
· Children (5-10 y.o.) · Anxious, potentially cooperatives · n = 60 (30/group |
Slow induction (max: 40% N2O) |
Oral triclofos sodium (70 mg.kg-1) | Houpt, pt. IV (≥ 3): · NOOS: 83.3% · Control: 90.0% |
Non-blinded, Parallel groups |
| Takkar D, et al. (2015) [42] |
· Children (7-10 y.o.) · Frankl 2-3 · n = 40 (20/group |
Slow induction (max: 40% N2O) |
Placebo (O2 100%) | Double-blind, Parallel groups | |
| Allen M, Thompson S. (2014) [43] |
· Adults (18-62 y.o.) · Anxious, potentially cooperatives · n = 40 (NOOS: 19; Control: 21) |
Slow induction (max: 40% N2O) |
Sevoflurane (max: 0.3%) | Single-blind, Parallel groups | |
| Guelmann M, et al. (2012) [44] |
· Children (5-8 y.o) · Anxious, potentially cooperatives · n = 17 |
Rapid induction, adjustable proportions (max: 50% N2O) | Placebo (O2 100%) | OSUBRS (< 3) · NOOS: 100.0% · Control: 94.1% |
Double-blind, Cross-over |
| Zhang G, et al. (2012) [45] |
· Adults (18-42 y.o.) · DAS-R 9-12 · n = 38 |
Rapid induction, fixed proportions (30% N2O) |
Video-eyewear + 30% N2O | Houpt, pt. IV (≥ 3): · NOOS:100.0% · Control: 100.0% |
Non-blinded, Cross-over |
| Özen B, et al. (2012) [46] |
· Children (4-6 y.o.) · Frankl 1-2 · n = 240 (60/group) |
Rapid induction, fixed proportions (50% N2O) |
· 1: Intranasal midazolam (0.20 mg.kg-1) + 50% N2O · 2: Oral midazolam (0.75 mg.kg-1) + 50% N2O · 3: Oral midazolam (0.50 mg.kg-1) + 50% N2O |
Treatment completion: · NOOS: 55.0% · Control 1: 86.6% · Control 2: 78.3% · Control 3: 71.6% |
N.I. on blinding, Parallel groups |
| Abdullah WA, et al. (2011) [47] |
· Adults (18-30 y.o.) · DAS-R 9-14 · n = 20 |
Slow induction (max: 50% N2O) |
Methoxyflurane (max: 0.4%) | RSS (≥ 2): · NOOS: 100.0% · Control: 100.00% |
Non-blinded, Cross-over |
| Soldani F, et al. (2010) [48] |
· Children (6-15 y.o.) · Anxious, potentially cooperatives · n = 29 |
Slow induction (max: 30% N2O) |
Sevoflurane (max: 0.3%) | Treatment completion: · NOOS: 89.7% · Control: 89.3% |
Double-blind, Cross-over |
| Baygin O, et al. (2010) [49] |
· Children (5-8 y.o) · Frankl 1-2 · n = 60 (15/group) |
Slow induction (max: 40% N2O) |
· 1: Oral hydroxyzine hydrochloride (1 mg.kg-1) + 40% N2O · 2: Oral midazolam (0.70 mg.kg-1) + 40% N2O · 3: Oral ketamine (3 mg.kg-1) + oral midazolam (0.25 mg.kg-1) + 40% N2O |
RSS (≥ 2): · NOOS: 66.7% · Control 1: 66.7% · Control 2: 74.0% · Control 3: 66.7% |
Double-blind, Parallel groups |
| Wilson KE, et al. (2007) [50] |
· Children (10-15 y.o.) · Anxious, potentially cooperatives · n = 36 |
Slow induction (max: 30% N2O) |
Transmucosal midazolam (0.2 mg.kg-1) | Houpt, pt. IV (≥ 3): · NOOS: 100.0% · Control: 100.0% |
Non-blinded, Cross-over |
| Wilson KE, et al. (2006) [51] |
· Children (5-10 y.o.) · Anxious, potentially cooperatives · n = 35 |
Slow induction (max: 30% N2O) |
Oral midazolam (0.3 mg.kg-1) | Houpt, pt. IV (≥ 3): · NOOS: 100.0% · Control: 100.0% |
Non-blinded, Cross-over |
| Wilson KE, et al. (2003) [52] |
· Children (12-16 y.o.) · Anxious, potentially cooperatives · n = 40 |
Slow induction (max: 30% N2O) |
Intravenous midazolam (max: 5.0 mg) | Houpt, pt. IV (≥ 3): · NOOS: 97.5% · Control: 95.0% |
Non-blinded, Cross-over |
| Wang CY, et al. (2002) [53] |
· Adults (19-43 y.o.) · Anxious, potentially cooperatives · n = 17 |
Slow induction (max: 50% N2O) |
Sevoflurane (max: 1%) | VAS (≤ 3): NOOS: 100.00% Control: 100.00% |
Single-blind, Cross-over |
| Wilson KE, et al. (2002, a) [54] |
· Children (10-16 y.o.) · Anxious, potentially cooperatives · n = 44 |
Slow induction (max: 30% N2O) |
Oral midazolam (0.5 mg.kg-1) | Houpt, pt. IV (≥ 3): · NOOS: 97.7% · Control: 97.7% |
Non-blinded, Cross-over |
| Wilson KE, et al. (2002, b) [55] |
· Children (10-16 y.o.) · Anxious, potentially cooperatives · n = 26 |
Slow induction (max: 30% N2O) |
Oral midazolam (0.5 mg.kg-1) | Houpt, pt. IV (≥ 3): · NOOS: 96.1% · Control: 96.1% |
Non-blinded, Cross-over |
| Lahoud GY, Averley PA. (2002) [13] |
· Children (3-10 y.o.) · Dedicated clinic for anxiety management · n = 411 (NOOS: 170, Control: 241) |
Rapid induction, fixed proportions (40% N2O) | Sevoflurane (max: 0.3%) | Treatment completion: · NOOS: 52.3% · Control: 89.2% |
Non-blinded, Parallel groups |
| Rodrigo MR, Rosenquist JB. (1988) [56] |
· Adults (18-31 y.o.) · Anxious, potentially cooperatives · n = 20 |
Rapid induction, fixed proportions (33% N2O) | Isoflurane (max: 0.5%) | Custom scale: · NOOS: 100.0% · Control: 100.0% |
Double-blind, Cross-over |
DSTG, Sedation Score from Dental Sedation Teachers Group; Houpt, Houpt Behaviour Rating Scale; MDAS, Modified Dental Anxiety Scale; N2O, Nitrous Oxide; O2, Oxygen; OAA/S, Observer’s Assessment of Alertness/Sedation; OSUBRS, Ohio State University Behavioral Rating Scale; RASS, Richmond Agitation-Sedation Scale; RSS, Ramsay Sedation Score; VAS, Visual Analogue Scale.
In most studies, it was reported that the initial behavior of patients was assessed as uncooperative, with preoperative anxiety, and potentially collaborative under sedation, often using the Frankl scale [57] or the Corah Dental Anxiety Scale (DAS) questionnaire [58].
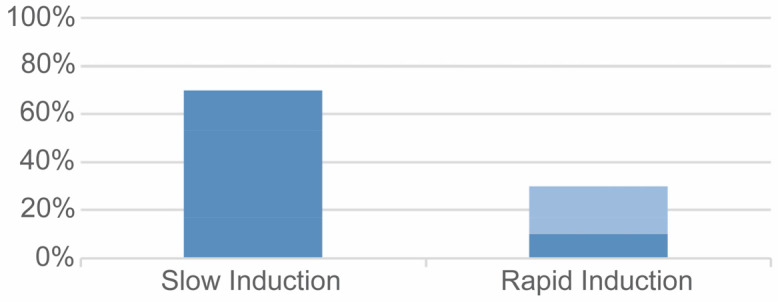
The gas mixture was administered with slow induction and regular increases in nitrous oxide amount in 14 intervention groups (70.0%). The rapid induction technique was used in six intervention groups: in four groups (20.0%), the gas proportions were maintained fixed during the whole process and in two groups (10.0%) the proportions were maintained fixed in the first phase of sedation and then adjusted according to the patient’s condition during treatment (Fig. 2). The proportion of nitrous did not exceed 50% in any trial and in all trials, local anesthesia was used to complete all the therapies.
Fig. 2. Administration techniques in included trials: use of adjustable (dark blue) or fixed (light blue) proportions of gases.
3. Quality analysis
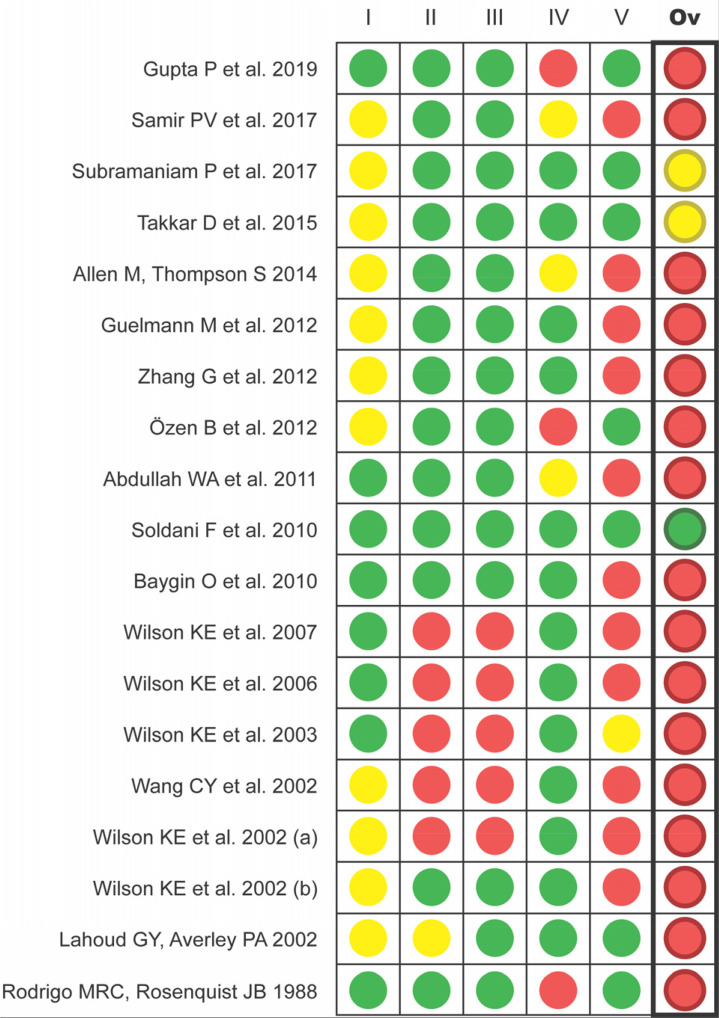
Of the 19 trials included in this review, only one [48] was assessed as having a low overall risk of bias using the Cochrane RoB 2 tool. Two trials (10.5%) [41,42] were assessed to be at unclear risk of bias, and in the remaining 16 studies (84.2%), at least one domain was assessed as being at high risk of bias (Fig. 3).
Fig. 3. Evaluation of the quality of the included studies with Cochrane RoB 2 (I: bias arising from the randomization process; II: bias due to deviations from intended interventions; III: bias due to missing outcome data; IV: bias in measurement of the outcome; V: bias in selection of the reported result; Ov: Overall bias).
4. Measures (success criteria)
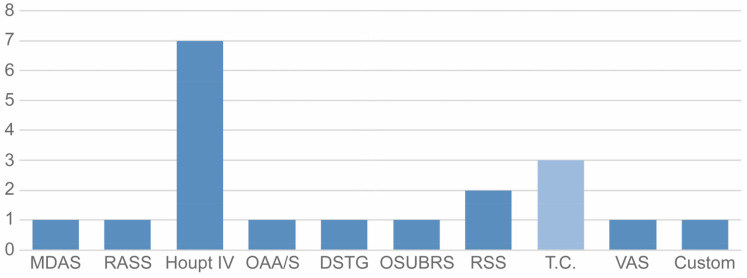
The main outcome variables used in the trials were ordinal or dichotomous. Trialists mostly used the first type of measure (16 studies, 84.2%), employing various ordinal scales: Section IV of Houpt Behavioral Rating Scale [59] was the most common (seven trials, 36.8%). The remaining three studies used the same dichotomous measure: completion of treatment (Yes/No) (Table 1). In total, 10 different measurement scales were used to determine the efficacy of NOIS (Fig. 4).
Fig. 4. Ordinal (dark blue) or dichotomous (light blue) success criteria prevalence in the included trials. DSTG, Sedation Score from Dental Sedation Teachers Group; Houpt, Houpt Behavior Rating Scale; MDAS, Modified Dental Anxiety Scale; OAA/S, Observer’s Assessment of Alertness/Sedation; OSUBRS, Ohio State University Behavioral Rating Scale; RASS, Richmond Agitation-Sedation Scale; RSS, Ramsay Sedation Score; T.C., Treatment Completion; VAS, Visual Analogue Scale.
5. NOIS intervention effect (success rates)
The proportions of successful sedations with the N2O-O2 mixture were variable, ranging from 52.3% to 100% (Table 1). Of all the studies, 10 had a success rate of 100% (five with adults and five with children), while three trials had relatively low efficacy rates (52.3% [13], 55.0% [46], and 66.7% [49]; all with children).
In one study [40], both the intervention and control groups received NOIS but they were administered the mixture using two different techniques. The two groups had a success rate of 100%.
6. Meta-analysis
Treatment arms from included crossover trials were considered as independent, following a “three stage” decision process for inclusion of crossover data in meta-analysis [24,31,32], as using results from paired analysis was impossible and not all of these studies reported data from the first crossover period.
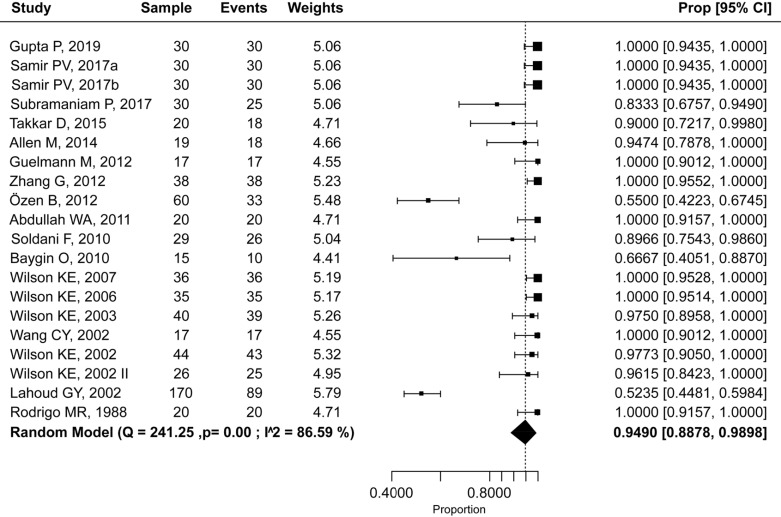
The resulting overall success rate was 94.9% (95% CI: 88.8–98.9%) (Fig. 5). Heterogeneity among all trials was moderate (I2 = 58.6%); thus, a random-effects model was used to perform the calculation.
Fig. 5. Efficacy rate of nitrous oxide-oxygen procedural sedation.
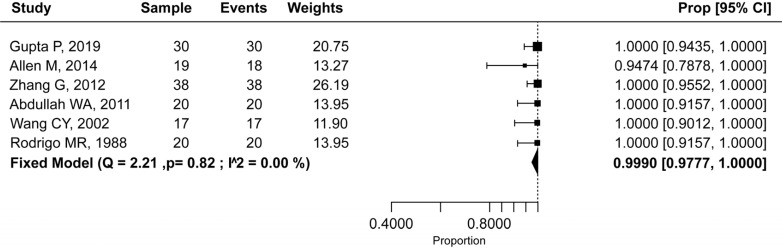
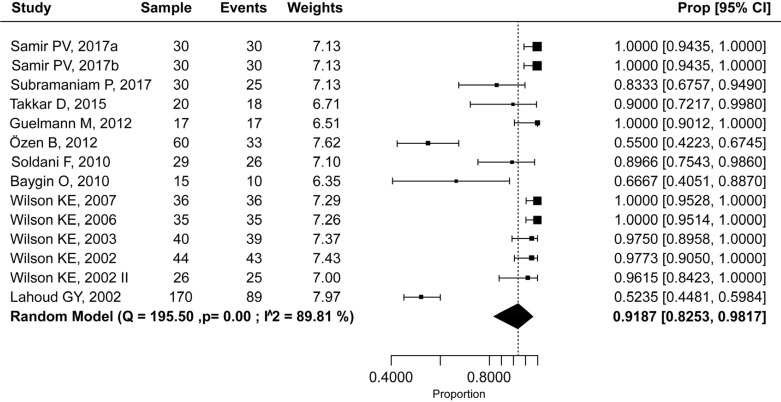
The success rate of NOIS in trials with adults was 99.9% (95% CI: 97.7–100.0%) (Fig. 6), and in studies with pediatric population 91.9% (95% CI: 82.5–98.2%) (Fig. 7), with a statistically significant difference (P = 0.002). Heterogeneity among studies was mild (I2 = 0.0%) and moderate (I2 = 64.6%), respectively. Therefore, a fixed-effects model was used for the adult population and a random-effects model was used for the pediatric population.
Fig. 6. Efficacy rate of nitrous oxide-oxygen procedural sedation in the adult population.
Fig. 7. Efficacy rate of nitrous oxide-oxygen procedural sedation in the pediatric population.
DISCUSSION
The purpose of this systematic review and meta-analysis was to determine the success rate of NOIS in dental patients by analyzing RCTs, as they are one of the most reliable sources of information for clinical practice. After an exhaustive literature search, 19 RCTs were identified following the application of the inclusion and exclusion criteria.
1. Quality
Open-label clinical trials and observational studies were not included in this review, as knowledge of the treatment assignments by patients, clinicians, or evaluators could influence the measurement or communication of the results, thus introducing biases [60]. Indeed, according to the hierarchy of evidence, only systematic reviews of randomized trials provide the highest level of scientific evidence [61].
Careful assessment of the risk of bias using the Cochrane RoB 2 tool gave similar results to those of many other systematic reviews [19,62,63], wherein the general quality of included articles was mostly disappointing. In the Cochrane review by Ashley et al. [19] on sedation techniques, the proportions of trials with high, unclear, and low risk of bias were very similar to those of the present review (81%, 18%, and 1%, respectively). In the present study, the poor information reporting of included articles was an undoubted problem: this implied low scores in the evaluation of design, completion, and outcome communication of the trials (Fig. 3). In general, the overall risk of bias for most studies was at best a mix of low and unclear or likely to have at least one domain with high risk.
2. Main outcomes
According to this systematic review and meta-analysis, the estimated efficacy rates of NOIS in global, adult, and pediatric populations were 94.9% (95% CI: 88.8–98.9%), 99.9% (95% CI: 97.7–100.0%), and 91.9% (95% CI: 82.5–98.2%), respectively. The difference between the adult and pediatric populations was statistically significant (P = 0.002). These results highlight the high efficacy of NOIS in dental patients, with means and data types that were not present in literature.
The findings of this study are consistent with those of other systematic reviews on NOIS. In 2018, Ashley et al. [19] analyzed mean data from published RCTs and observed a behavior improvement in children undergoing dental treatment with NOIS. Nevertheless, the authors stated that the results had limited reliability, since the number of included studies was very low and their quality was excessively poor. In 2017, another systematic review [62] did not identify a statistically significant difference in overall cooperation using NOIS and midazolam, in combination or separately. Thus, the authors concluded that these different techniques could be considered equivalent in terms of efficacy.
3. Characteristics of the included studies
The proportion of nitrous oxide in the gas mixture did not exceed 50% in any trial. Although ASA considers a concentration of < 50% as minimal sedation [2], this maximum was just slightly overpassed by some trialists [39,44,46,47,53] and it was always maintained within the limit established by AAP (≤ 50%) [7]. Furthermore, this proportion was reached only in some patients.
In all trials, local anesthesia was used to complete all the therapies. Therefore, it is safe to assume that all the performed treatments were comparable in terms of pain and comfort perceived by the patient, or that they had negligible differences. Furthermore, the manifestations of dental anxiety are independent of the actual pain experienced by the patient, and conversely, dental anxiety may underlie the pain perceived throughout the entire period of dental treatment [64].
4. Possible reasons for sedation failures
The two studies with the lowest success rates in this review (52.3% [13] and 55.0% [46]) also had the largest samples among all RCTs; thus, the highest w (“weight”) value. This had an impact on the meta-analysis calculation, causing an inclination of the overall results through those rates. The efficacy rate in the pediatric population was the most affected outcome, as both trials were conducted in children. The reason for this evident discrepancy in results (Fig. 5 and 7) is probably due to the NOIS indications considered in these two studies. Indeed, Özen et al. [46] included patients classified as Frankl 1 (“Definitely Negative”, refusal of treatment, or any other overt evidence of extreme negativism), in addition to Frankl 2. The study by Baygin et al. [49] was the other publication that described the inclusion of Frankl 1 patients: this trial obtained the third lowest efficacy rate in this review (66.7%, pediatric population), with an evident discrepancy from all the other studies, too (Fig. 5 and 7). On the other hand, Lahoud and Averley [13] completed their trial in a dedicated clinic for dental anxiety management. Therefore, it is expected that the profile of patients from this trial was similar to that of the two above-mentioned studies. Contrary to these trialists, Abdullah et al. [47] excluded Frankl 1 patients, and they obtained an efficacy rate of 100.00% (Table 1).
As described by Clark and Brunick [65], among NOIS contraindications, there is an inability to understand the procedure, the unwillingness to consent to procedure, the impossibility of establishing communication, and patients who are completely uncooperative (e.g., extreme claustrophobia, psychosomatic behavior problems, inflexible temperament [66] and extremely high anxiety before treatment [67]). The importance of patient selection with NOIS is also underlined by other authors [66,67,68], specifying, for example, that NOIS efficacy is low in cases of severe anxiety or fear [69,70]. These characteristics seem to be compatible with the patients included in the previously described RCTs [13,46,49], that recorded the lowest efficacy rates in this review. Hence, the poor success obtained in those studies is probably attributable to inappropriate selection of the patients to be sedated with NOIS.
Besides this main rationale, scientific literature outlines other possible factors that may result in NOIS failure. Coyle et al. [4] reported that the incidence of adverse effects caused by this drug (e.g., vomiting, inability to communicate during the procedure, or oversedation), as well as incorrect local anesthesia techniques [18,71], could negatively affect procedural sedation. Therefore, the patient’s response to sedative and anesthetic medications should be carefully evaluated in practice [8,72]. In pediatric population, it may be common for the nasal hood to not fit perfectly, thus not creating an airtight seal [73,74]. Moreover, children, as extremely uncollaborative adults, may not accept the nasal mask or they may uncontrollably move during the initial phases of sedation [41]. Some patients (usually children) may not use the mask as instructed, limiting the absorption of the drug: oral breathers, for example, are not considered an appropriate patient profile for this type of sedation [50]. Furthermore, pediatric nasal anatomy may result in unavoidable air entertainment when delivering nitrous oxide–oxygen. In accordance with Subramaniam et al. [41], these issues imply that there will always be a small number of patients for whom treatment with NOIS will be unsuccessful. In many of these situations, if sedation is mandatory, deep sedation or general anesthesia is the procedure of choice [65].
Patients should have the correct set of expectations on procedural sedation, as often they have the impression that they will be ‘‘gently asleep” [75]. Conversely, they must collaborate and participate in every phase of the procedure. Clinicians’ experience with this aspect, both for the ability to use the technique [46] and for affecting patients’ anxiety [76], is hence important.
5. Clinical success criteria
Procedural (or conscious) sedation lacks a clear consensus on the definition of success [19,77] thus, it currently exists more than a singular description [78].
The ESA [1] describes that procedural sedation is effective when “the use of hypnotic and/or analgesic medications enables effective performance of diagnostic or therapeutic procedures”. The ASA in conjunction with other relevant associations [2] listed a series of parameters to define moderate sedation efficacy: induction time, duration of sedation, successful therapy, patient/family satisfaction, and proceduralist satisfaction. Nevertheless, different criteria and methods are used for this scope [15], depending, for example, on the geographical area [79].
Similar to the findings of other systematic reviews [19], Section IV of the Houpt scale [59] was the most used evaluation method by trialists. The various sections of the Houpt scale allow the monitoring and assessment of different patient aspects during sedation (alertness, movement, crying, and completion of treatment). Every section showed high degrees of correlation between expert and/or non-expert evaluators [80] thus the scale has recognized scientific validity. Section IV assesses treatment completion in an ordinal form (grade 1 to 6); nevertheless, treatment completion can also be successfully evaluated in a dichotomous form (Yes/No), and this type of evaluation was the second most used assessment method in this review (Fig. 4).
Completion of dental treatment has been widely used as success criterion for sedation procedures in dentistry [77,81,82,83,84]. The advantage of this parameter is that it is objective and reproducible [42] (unlike, for example, patient cooperation evaluated with scales such as VAS), and it demonstrates a high degree of correlation between expert and non-expert evaluators [80]. On the other hand, this parameter should always be accompanied by an independent assessment of patient's behavior to avoid situations in which treatment completion is considered successful despite, for example, a significant physical restriction [77]. The consequences of interrupted treatment, which could be relevant, as well as patient and clinician satisfaction for completing the therapy, demonstrate the importance of this criterion [85]. For example, the impossibility to conclude planned treatment could imply the need for more powerful sedative drugs or referral of the patient to units equipped for general anesthesia [13].
Scientific literature describes a wide range of other success criteria, some of which were used in the included RCTs, such as the Ramsay Sedation Scale [86] or Richmond Agitation-Sedation Scale [87]. Some methods are based on physiological parameters, such as the bispectral index system [88] or the evaluation of serum cortisol levels [89] and vital signs [90,91].
Although different scales may be used to evaluate procedural sedation, all of them evaluate the same domain: procedural sedation state achievement [35], defined as a state of minimal or moderate sedation [5] that can be induced by the administration of nitrous oxide-oxygen. Therefore, it is reasonable to realize a methodical synthesis of existing RCT results, despite the fact that criteria or scales used for outcome evaluation, may differ among trials. The use of different criteria to evaluate the same outcome is also common in other fields of dentistry and medicine. A typical example is represented by implantology, where this issue is managed in well-known systematic reviews and meta-analyses with methods that have inspired those of this review [92,93,94,95].
6. Crossover trials
Careful quality analysis with validated tools [33,96] revealed a modest level of scientific availability. In this context, eight RCTs with parallel groups were identified, however the other included reports were related to crossover trials.
Crossover trials were included following statistically validated methods [24,31,32]. The most appropriate use of this type of study relates to the investigation of symptomatic treatment of conditions and/or diseases that are chronic or relatively stable (e.g., dental anxiety) and when treatment effects are likely to be reversible and short-lived [31,32] (i.e., cases in which the “carry-over” effect [97] cannot exist). NOIS can be considered among these situations [5,32].
Furthermore, crossover trials are often used with clinical pharmacology [32] and pediatrics [98]. Meta-analyses regarding these topics may consequently require the combination of results from trials with crossover and parallel design [31]. Moreover, the power of a meta-analysis may decrease by combining only trials of the same type [99].
7. Implications of key findings and recommendations
The present review systematically explored the available scientific evidence on the clinical success rate of NOIS. By gathering and summarizing data from RCTs, the findings from this study provide further evidence on the clinical performance of this procedure, providing useful data to dental professionals for clinical activity and communication with patients on expected outcomes. Indeed, statistically calculated rates are often used to guide clinical decision making and to provide a more accurate patient-specific prognosis of the procedure to be performed [95].
Moreover, the findings of this review highlight interesting issues for future research and clinical practice. The variety of clinical criteria used in the trials to define NOIS success was an important finding of this review. As a matter of fact, in scientific literature a wide range of efficacy criteria exists [19,77,78], each with recognized scientific validity or less. Given the evidence found, completion of treatment (ordinally or dichotomously evaluated) was demonstrated to be the most widely used parameter for sedation success, due to its validity and objectivity [42,80]. Nevertheless, there is a need to develop guidelines to standardize efficacy criteria and evaluation methods in procedural sedation, thereby facilitating communication and comparison of data and prospectively defining success.
There is a need for further well-designed and well-documented clinical trials to evaluate NOIS performance. In designing future studies, trialists should consider reference guidelines (e.g., CONSORT) and the evaluation with the RoB 2 tool, and they need to develop reasonable evidence-based assumptions regarding the handling of missing data.
Most of the included trials were of the crossover type and they were conducted in pediatric populations. Considering that evidence provided by interventions on parallel groups is unquestionable, future RCTs should evaluate NOIS effects with such kind of design, and, to a greater degree, in adult populations.
The importance of correct patient selection is underlined by the results of this systematic review. Inappropriate selection was likely the main reason for sedation failure, especially in those studies that recorded the lowest efficacy rates. The found data suggest a potential increase in the risk of NOIS failure in extremely anxious groups, who may have the highest demand for sedation procedures. Clinicians and researchers should strictly follow the most appropriate indications for NOIS that scientific literature has already demonstrated, in order to make procedural sedation the most effective. Indeed, this technique can be recommended as a viable and minimally invasive approach for all patients who comply with the correct indications, as it demonstrates remarkable efficacy rates if the interventions are appropriately selected.
8. Limitations
The relatively low evidence grading was the main limitation of this systematic review. However, the quality assessment was performed using the Cochrane RoB 2 tool, which has recognized validity, but it implies a strict evaluation [96]. Since there is a lack of available RCTs with parallel groups, many of the included studies were crossover trials. Nevertheless, the only RCT with a low overall risk of bias had a crossover design.
Some other limitations can also be considered in this review. For instance, abstracts of studies published in languages other than English, Spanish, or Italian were not examined, which might have led to selection bias. Furthermore, as there is a lack of standardized NOIS processes for clinicians, some variables that are difficult to be comprehensively evaluated, such as administration procedures, clinician experience, and equipments, may have increased the heterogeneity among the included studies.
9. Conclusions
Within the limitations of this systematic review, the present study provides important information on the efficacy rate of NOIS. Further well-designed and well-documented clinical trials are required, and there is a need to develop guidelines for standardization of criteria and definition of success in procedural sedation, since completion of treatment is the most used parameter, but many others also exist. To maximize NOIS efficacy, clinicians should strictly consider the appropriate indications for the procedure.
It is important for clinicians to rely on statistically calculated rates to be fully aware of the expected outcomes and to provide more accurate patient-specific prognosis of the procedure, thus helping in the decision-making process and communication with the patient.
Footnotes
- Marco Rossit: Conceptualization, Data curation, Methodology, Writing — original draft, Writing — review & editing.
- Victor Gil-Manich: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing — original draft, Writing — review & editing.
- José Manuel Ribera-Uribe: Conceptualization, Data curation, Methodology, Supervision, Writing — review & editing.
ACKNOWLEDGMENTS, CONFLICT OF INTEREST, AND SOURCES OF FUNDING STATEMENT: The authors declare that there are no conflicts of interest in this study.
*A first partial version of this review was presented at the CED/IADR (Continental European Division of the International Association for Dental Research) Congress in Madrid on September 21, 2019. The aforementioned version was made by researching studies published only in the past 20 years, using less information sources and less appropriate methods.
This manuscript constitutes the correct, definitive, and comprehensive version of the review, completed in the first half of 2021.
REVIEW PROTOCOL: The Cochrane Handbook Method Guidelines and Center for Reviews and Dissemination (CRD) guidelines were consulted to prepare the conduct of this study. The protocol was written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and checklist. According to the population, intervention, comparison, outcome, and study design (PICOS) approach, the guiding question of this review was: “What is the clinical success rate of NOIS in dental patients from randomized clinical trials?”. The full review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42020155159. The text can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=155159.
References
- 1.Hinkelbein J, Lamperti M, Akeson J, Santos J, Costa J, De Robertis E, et al. European Society of Anaesthesiology and European Board of Anaesthesiology guidelines for procedural sedation and analgesia in adults. Eur J Anaesthesiol. 2018;35:6–24. doi: 10.1097/EJA.0000000000000683. [DOI] [PubMed] [Google Scholar]
- 2.Practice guidelines for moderate procedural sedation and analgesia 2018: a report by the American Society of Anesthesiologists task force on moderate procedural sedation and analgesia, the American Association of Oral and Maxillofacial Surgeons, American College of Radiology, American Dental Association, American Society of Dentist Anesthesiologists, and Society of Interventional Radiology. Anesthesiology. 2018;128:437–479. doi: 10.1097/ALN.0000000000002043. [DOI] [PubMed] [Google Scholar]
- 3.Samir PV, Fere SS. Nitrous oxide-oxygen inhalation sedation: a light on its safety and efficacy in pediatric dentistry. Int J Adv Health Sci. 2015;1:4–10. [Google Scholar]
- 4.Coyle TT, Helfrick JF, Gonzalez ML, Andresen RV, Perrott DH. Office-based ambulatory anesthesia: factors that influence patient satisfaction or dissatisfaction with deep sedation/general anesthesia. J Oral Maxillofac Surg. 2005;63:163–172. doi: 10.1016/j.joms.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Becker DE, Rosenberg M. Nitrous oxide and the inhalation anesthetics. Anesth Prog. 2008;55:124–131. doi: 10.2344/0003-3006-55.4.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurwitz EE, Simon M, Vinta SR, Zehm CF, Shabot SM, Minhajuddin A, et al. Adding examples to the ASA-physical status classification improves correct assignment to patients. Anesthesiology. 2017;126:614–622. doi: 10.1097/ALN.0000000000001541. [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Pediatric Dentistry. Guideline on use of nitrous oxide for pediatric dental patients. Pediatr Dent. 2013;35:174–178. [PubMed] [Google Scholar]
- 8.American Academy of Pediatric Dentistry. Guideline on use of nitrous oxide for pediatric dental patients. Pediatr Dent. 2016;38:211–215. [PubMed] [Google Scholar]
- 9.Clark MS, Campbell SA, Clark AM. Technique for the administration of nitrous oxide/oxygen sedation to ensure psychotropic analgesic nitrous oxide (PAN) effects. Int J Neurosci. 2006;116:871–877. doi: 10.1080/00207450600754012. [DOI] [PubMed] [Google Scholar]
- 10.Mozafar S, Bargrizan M, Golpayegani MV, Shayeghi S, Ahmadi R. Comparison of nitrous oxide/midazolam and nitrous oxide/promethazine for pediatric dental sedation: a randomized, cross-over, clinical trial. Dent Res J (Isfahan) 2018;15:411–419. [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson TM, Griffith TM, Lane KJ, Thikkurissy S, Scott JM. Temperament as a predictor of nitrous oxide inhalation sedation success. Anesth Prog. 2017;64:17–21. doi: 10.2344/anpr-63-03-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramaniam P, Girish Babu KL, Lakhotia D. Evaluation of nitrous oxide-oxygen and triclofos sodium as conscious sedative agents. J Indian Soc Pedod Prev Dent. 2017;35:156–161. doi: 10.4103/JISPPD.JISPPD_82_16. [DOI] [PubMed] [Google Scholar]
- 13.Lahoud GY, Averley PA. Comparison of sevoflurane and nitrous oxide mixture with nitrous oxide alone for inhalation conscious sedation in children having dental treatment: a randomised controlled trial. Anaesthesia. 2002;57:446–450. doi: 10.1046/j.0003-2409.2002.02569.x. [DOI] [PubMed] [Google Scholar]
- 14.Herres J, Chudnofsky CR, Manur R, Damiron K, Deitch K. The use of inhaled nitrous oxide for analgesia in adult ED patients: a pilot study. Am J Emerg Med. 2016;34:269–273. doi: 10.1016/j.ajem.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 15.Hennequin M, Collado V, Faulks D, Koscielny S, Onody P, Nicolas E. A clinical trial of efficacy and safety of inhalation sedation with a 50% nitrous oxide/oxygen premix (KalinoxTM) in general practice. Clin Oral Investig. 2012;16:633–642. doi: 10.1007/s00784-011-0550-y. [DOI] [PubMed] [Google Scholar]
- 16.Bryan R. The success of inhalation sedation for comprehensive dental care within the Community Dental Service. Int J Paediatr Dent. 2002;12:410–414. doi: 10.1046/j.1365-263x.2002.00400.x. [DOI] [PubMed] [Google Scholar]
- 17.Hartling L, Milne A, Foisy M, Lang ES, Sinclair D, Klassen TP, et al. What works and what's safe in pediatric emergency procedural sedation: an overview of reviews. Acad Emerg Med. 2016;23:519–530. doi: 10.1111/acem.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen RS, Bayat A, Steen NP, Jacobsson MLB. Nitrous oxide provides safe and effective analgesia for minor paediatric procedures - a systematic review. Dan Med J. 2013;60:1–8. [PubMed] [Google Scholar]
- 19.Ashley PF, Chaudhary M, Lourenço-Matharu L. Sedation of children undergoing dental treatment. Cochrane Database Syst Rev. 2018;12:CD003877. doi: 10.1002/14651858.CD003877.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The European Society of Anaesthesiology task force on the use of nitrous oxide in clinical anaesthetic practice. The current place of nitrous oxide in clinical practice. Eur J Anaesthesiol. 2015;32:517–520. doi: 10.1097/EJA.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 21.Sado-Filho J, Viana KA, Corrêa-Faria P, Costa LR, Costa PS. Randomized clinical trial on the efficacy of intranasal or oral ketamine-midazolam combinations compared to oral midazolam for outpatient pediatric sedation. PLoS One. 2019;14:e0213074. doi: 10.1371/journal.pone.0213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galeotti A, Garret Bernardin A, D'Antò V, Ferrazzano GF, Gentile T, Viarani V, et al. Inhalation conscious sedation with nitrous oxide and oxygen as alternative to general anesthesia in precooperative, fearful, and disabled pediatric dental patients: a large survey on 688 working sessions. Biomed Res Int. 2016;2016:7289310. doi: 10.1155/2016/7289310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anand KS, Grunau RE, Oberlander TF. Developmental character and long-term consequences of pain in infants and children. Child Adolesc Psychiatr Clin N Am. 1997;6:703–724. [Google Scholar]
- 24.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane; 2021. [updated February 2021]. Available from www.training.cochrane.org/handbook . [Google Scholar]
- 25.Akers J, editor. Centre for Reviews and Dissemination. Systematic Reviews: CRD's guidance for undertaking reviews in health care. 3rd ed. York, UK: University of York; 2013. pp. 1–294. [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JPT, Green S. Chapter 5: Defining the review question and developing criteria for including studies. In: Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0. Cochrane; 2011. [updated March 2011]. Available from www.training.cochrane.org/handbook . [Google Scholar]
- 28.Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579. doi: 10.1186/s12913-014-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Tulder M, Furlan A, Bombardier C, Bouter L Editorial Board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine (Phila Pa 1976) 2003;28:1290–1299. doi: 10.1097/01.BRS.0000065484.95996.AF. [DOI] [PubMed] [Google Scholar]
- 31.Nolan SJ, Hambleton I, Dwan K. The use and reporting of the cross-over study design in clinical trials and systematic reviews: a systematic assessment. PLoS One. 2016;11:e0159014. doi: 10.1371/journal.pone.0159014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31:140–149. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- 33.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 34.Riba H, Al-Zahrani S, Al-Buqmi N, Al-Jundi A. A review of behavior evaluation scales in pediatric dentistry and suggested modification to the Frankl scale. EC Dent Sci. 2017;16:269–275. [Google Scholar]
- 35.Newton T, Pop I, Duvall E. Sedation scales and measures--a literature review. SAAD Dig. 2013;29:88–99. [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin L, Xu C. Arcsine-based transformations for meta-analysis of proportions: pros, cons, and alternatives. Health Sci Rep. 2020;3:e178. doi: 10.1002/hsr2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarzer G. meta : an R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- 39.Gupta PD, Mahajan P, Monga P, Thaman D, Khinda VIS, Gupta A. Evaluation of the efficacy of nitrous oxide inhalation sedation on anxiety and pain levels of patients undergoing endodontic treatment in a vital tooth: a prospective randomized controlled trial. J Conserv Dent. 2019;22:356–361. doi: 10.4103/JCD.JCD_332_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samir PV, Namineni S, Sarada P. Assessment of hypoxia, sedation level, and adverse events occurring during inhalation sedation using preadjusted mix of 30% nitrous oxide + 70% oxygen. J Indian Soc Pedod Prev Dent. 2017;35:338–345. doi: 10.4103/JISPPD.JISPPD_15_17. [DOI] [PubMed] [Google Scholar]
- 41.Subramaniam P, Girish Babu K, Lakhotia D. Evaluation of nitrous oxide-oxygen and triclofos sodium as conscious sedative agents. J Indian Soc Pedod Prev Dent. 2017;35:156–161. doi: 10.4103/JISPPD.JISPPD_82_16. [DOI] [PubMed] [Google Scholar]
- 42.Takkar D, Rao A, Shenoy R, Rao A, Saranya BS. Evaluation of nitrous oxide inhalation sedation during inferior alveolar block administration in children aged 7-10 years: a randomized control trial. J Indian Soc Pedod Prev Dent. 2015;33:239–244. doi: 10.4103/0970-4388.160399. [DOI] [PubMed] [Google Scholar]
- 43.Allen M, Thompson S. An equivalence study comparing nitrous oxide and oxygen with low-dose sevoflurane and oxygen as inhalation sedation agents in dentistry for adults. Br Dent J. 2014;217:E18. doi: 10.1038/sj.bdj.2014.998. [DOI] [PubMed] [Google Scholar]
- 44.Guelmann M, Brackett R, Beavers N, Primosch RE. Effect of continuous versus interrupted administration of nitrous oxide-oxygen inhalation on behavior of anxious pediatric dental patients: a pilot study. J Clin Pediatr Dent. 2012;37:77–82. doi: 10.17796/jcpd.37.1.e2g45201l836689n. [DOI] [PubMed] [Google Scholar]
- 45.Zhang G, Hou R, Zhou H, Kong L, Ding Y, Qin R, et al. Improved sedation for dental extraction by using video eyewear in conjunction with nitrous oxide: a randomized, controlled, crossover clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:188–192. doi: 10.1016/j.tripleo.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Özen B, Malamed SF, Cetiner S, Özalp N, Özer L, Altun C. Outcomes of moderate sedation in paediatric dental patients. Aust Dent J. 2012;57:144–150. doi: 10.1111/j.1834-7819.2012.01673.x. [DOI] [PubMed] [Google Scholar]
- 47.Abdullah WA, Sheta SA, Nooh NS. Inhaled methoxyflurane (Penthrox®) sedation for third molar extraction: a comparison to nitrous oxide sedation. Aust Dent J. 2011;56:296–301. doi: 10.1111/j.1834-7819.2011.01350.x. [DOI] [PubMed] [Google Scholar]
- 48.Soldani F, Manton S, Stirrups DR, Cumming C, Foley J. A comparison of inhalation sedation agents in the management of children receiving dental treatment: a randomized, controlled, cross-over pilot trial. Int J Paediatr Dent. 2010;20:65–75. doi: 10.1111/j.1365-263X.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- 49.Baygin O, Bodur H, Isik B. Effectiveness of premedication agents administered prior to nitrous oxide/oxygen. Eur J Anaesthesiol. 2010;27:341–346. doi: 10.1097/EJA.0b013e3283313cdd. [DOI] [PubMed] [Google Scholar]
- 50.Wilson KE, Welbury RR, Girdler NM. Comparison of transmucosal midazolam with inhalation sedation for dental extractions in children. a randomized, cross-over, clinical trial. Acta Anaesthesiol Scand. 2007;51:1062–1067. doi: 10.1111/j.1399-6576.2007.01391.x. [DOI] [PubMed] [Google Scholar]
- 51.Wilson KE, Girdler NM, Welbury RR. A comparison of oral midazolam and nitrous oxide sedation for dental extractions in children. Anaesthesia. 2006;61:1138–1144. doi: 10.1111/j.1365-2044.2006.04835.x. [DOI] [PubMed] [Google Scholar]
- 52.Wilson KE, Girdler NM, Welbury RR. Randomized, controlled, cross-over clinical trial comparing intravenous midazolam sedation with nitrous oxide sedation in children undergoing dental extractions. Br J Anaesth. 2003;91:850–856. doi: 10.1093/bja/aeg278. [DOI] [PubMed] [Google Scholar]
- 53.Wang CY, Chiu CL, Har KO, Chan C, Rahman ZA. A comparative study of sevoflurane sedation with nitrous oxide sedation for dental surgery. Int J Oral Maxillofac Surg. 2002;31:506–510. doi: 10.1054/ijom.2002.0293. [DOI] [PubMed] [Google Scholar]
- 54.Wilson KE, Welbury RR, Girdler NM. A randomised, controlled, crossover trial of oral midazolam and nitrous oxide for paediatric dental sedation. Anaesthesia. 2002;57:860–867. doi: 10.1046/j.1365-2044.2002.02784.x. [DOI] [PubMed] [Google Scholar]
- 55.Wilson KE, Welbury RR, Girdler NM. A study of the effectiveness of oral midazolam sedation for orthodontic extraction of permanent teeth in children: a prospective, randomised, controlled, crossover trial. Br Dent J. 2002;192:457–462. doi: 10.1038/sj.bdj.4801400. [DOI] [PubMed] [Google Scholar]
- 56.Rodrigo MR, Rosenquist JB. Isoflurane for conscious sedation. Anaesthesia. 1988;43:369–375. doi: 10.1111/j.1365-2044.1988.tb09015.x. [DOI] [PubMed] [Google Scholar]
- 57.Frankl S, Shiere F, Fogels H. Should the parent remain with the child in the dental operatory? J Dent Child. 1962;2:151–163. [Google Scholar]
- 58.Corah NL. Development of a dental anxiety scale. J Dent Res. 1969;48:596. doi: 10.1177/00220345690480041801. [DOI] [PubMed] [Google Scholar]
- 59.Houpt MI, Weiss NJ, Koenigsberg SR, Desjardins PJ. Comparison of chloral hydrate with and without promethazine in the sedation of young children. Pediatr Dent. 1985;7:41–46. [PubMed] [Google Scholar]
- 60.Kahan BC, Cro S, Doré CJ, Bratton DJ, Rehal S, Maskell NA, et al. Reducing bias in open-label trials where blinded outcome assessment is not feasible: strategies from two randomised trials. Trials. 2014;15:456. doi: 10.1186/1745-6215-15-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Charrois TL. Systematic reviews: what do you need to know to get started? Can J Hosp Pharm. 2015;68:144–148. doi: 10.4212/cjhp.v68i2.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sivaramakrishnan G, Sridharan K. Nitrous oxide and midazolam sedation: a systematic review and meta-analysis. Anesth Prog. 2017;64:59–65. doi: 10.2344/anpr-63-03-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar G, Stendall C, Mistry R, Gurusamy K, Walker D. A comparison of total intravenous anaesthesia using propofol with sevoflurane or desflurane in ambulatory surgery: systematic review and meta-analysis. Anaesthesia. 2014;69:1138–1150. doi: 10.1111/anae.12713. [DOI] [PubMed] [Google Scholar]
- 64.Lin CS, Wu SY, Yi CA. Association between anxiety and pain in dental treatment: a systematic review and meta-analysis. J Dent Res. 2017;96:153–162. doi: 10.1177/0022034516678168. [DOI] [PubMed] [Google Scholar]
- 65.Clark MS, Brunick AL Elsevier, editors. Handbook of nitrous oxide and oxygen sedation. 4th ed. Riverport Lane, St. Louis, Missouri: 2015. pp. 1–262. [Google Scholar]
- 66.Isik B, Baygin O, Kapci EG, Bodur H. The effects of temperament and behaviour problems on sedation failure in anxious children after midazolam premedication. Eur J Anaesthesiol. 2010;27:336–340. doi: 10.1097/EJA.0b013e32833111b2. [DOI] [PubMed] [Google Scholar]
- 67.Bozkurt P. Premedication of the pediatric patient: anesthesia for the uncooperative child. Curr Opin Anaesthesiol. 2007;20:211–215. doi: 10.1097/ACO.0b013e328105e0dd. [DOI] [PubMed] [Google Scholar]
- 68.Haas DA. Oral and inhalation conscious sedation. Dent Clin North Am. 1999;43:341–359. [PubMed] [Google Scholar]
- 69.Cantekin K, Yildirim MD, Delikan E, Çetin S. Postoperative discomfort of dental rehabilitation under general anesthesia. Pak J Med Sci. 2014;30:784–788. [PMC free article] [PubMed] [Google Scholar]
- 70.Lourenço-Matharu L, Papineni McIntosh A, Lo JW. Predicting children's behaviour during dental treatment under oral sedation. Eur Arch Paediatr Dent. 2016;17:157–163. doi: 10.1007/s40368-015-0205-9. [DOI] [PubMed] [Google Scholar]
- 71.Donaldson M, Donaldson D, Quarnstrom FC. Nitrous oxide-oxygen administration: when safety features no longer are safe. J Am Dent Assoc. 2012;143:134–143. doi: 10.14219/jada.archive.2012.0123. [DOI] [PubMed] [Google Scholar]
- 72.Mozafar S, Bargrizan M, Golpayegani M, Shayeghi S, Ahmadi R. Comparison of nitrous oxide/midazolam and nitrous oxide/promethazine for pediatric dental sedation: a randomized, cross-over, clinical trial. Dent Res J. 2018;15:411–419. [PMC free article] [PubMed] [Google Scholar]
- 73.Pradeep K, Himanshu A, Preeti D, Aditi S. Making conscious sedation more efficient by custom made nasal hood. Indian J Dent Sci. 2014;6:18–20. [Google Scholar]
- 74.Klein U, Robinson TJ, Allshouse A. End-expired nitrous oxide concentrations compared to flowmeter settings during operative dental treatment in children. Pediatr Dent. 2011;33:56–62. [PubMed] [Google Scholar]
- 75.Jackson DL, Johnson BS. Conscious sedation for dentistry: risk management and patient selection. Dent Clin North Am. 2002;46:767–780. doi: 10.1016/s0011-8532(02)00034-4. [DOI] [PubMed] [Google Scholar]
- 76.Nathan JE. Managing behavior of precooperative children. Dent Clin North Am. 1995;39:789–816. [PubMed] [Google Scholar]
- 77.Hennequin M, Collado V, Faulks D, Koscielny S, Onody P, Nicolas E. A clinical trial of efficacy and safety of inhalation sedation with a 50% nitrous oxide/oxygen premix (Kalinox™) in general practice. Clin Oral Investig. 2012;16:633–642. doi: 10.1007/s00784-011-0550-y. [DOI] [PubMed] [Google Scholar]
- 78.Peyton PJ, Wu CY. Nitrous oxide – related postoperative nausea and vomiting depends on duration of exposure. Anesthesiology. 2014;120:1137–1145. doi: 10.1097/ALN.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 79.Lyratzopoulos G, Blain KM. Inhalation sedation with nitrous oxide as an alternative to dental general anaesthesia for children. J Public Health Med. 2003;25:303–312. doi: 10.1093/pubmed/fdg068. [DOI] [PubMed] [Google Scholar]
- 80.Hosey MT, Blinkhorn AS. An evaluation of four methods of assessing the behaviour of anxious child dental patients. Int J Paediatr Dent. 1995;5:87–95. doi: 10.1111/j.1365-263x.1995.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 81.Lahoud GY, Averley PA, Hanlon MR. Sevoflurane inhalation conscious sedation for children having dental treatment. Anaesthesia. 2001;56:476–480. doi: 10.1046/j.1365-2044.2001.01524-7.x. [DOI] [PubMed] [Google Scholar]
- 82.Hennequin M, Manière MC, Albecker-Grappe S, Faulks D, Berthet A, Tardieu C, et al. A prospective multicentric trial for effectiveness and tolerance of a N2O/O2 premix used as a sedative drug. J Clin Psychopharmacol. 2004;24:552–554. doi: 10.1097/01.jcp.0000138773.48138.c5. [DOI] [PubMed] [Google Scholar]
- 83.Shepherd AR, Hill FJ. Orthodontic extractions: a comparative study of inhalation sedation and general anaesthesia. Br Dent J. 2000;188:329–331. doi: 10.1038/sj.bdj.4800471. [DOI] [PubMed] [Google Scholar]
- 84.Edmunds DH, Rosen M. Inhalation sedation with 25% nitrous oxide. Report of a field trial. Anaesthesia. 1984;39:138–142. doi: 10.1111/j.1365-2044.1984.tb09501.x. [DOI] [PubMed] [Google Scholar]
- 85.Jain S. Sedation: s primer for pediatricians. Pediatr Ann. 2018;47:e254–e258. doi: 10.3928/19382359-20180522-04. [DOI] [PubMed] [Google Scholar]
- 86.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The Richmond agitation–sedation scale. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 88.Medical Advisory Secretariat. Bispectral index monitor: an evidence-based analysis. Ont Health Technol Assess Ser. 2004;4:1–70. [PMC free article] [PubMed] [Google Scholar]
- 89.Miyawaki T, Kohjitani A, Maeda S, Kita F, Higuchi H, Shimada M. Serum cortisol level and depth of propofol-induced sedation. Acta Anaesthesiol Scand. 2004;48:384–385. doi: 10.1111/j.0001-5172.2004.0320a.x. [DOI] [PubMed] [Google Scholar]
- 90.Vasakova J, Duskova J, Lunackova J, Drapalova K, Zuzankova L, Starka L, et al. Midazolam and its effect on vital signs and behavior in children under conscious sedation in dentistry. Physiol Res. 2020;69(Suppl 2):S305–S314. doi: 10.33549/physiolres.934511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sandhu G, Khinda P, Gill A, Singh Khinda V, Baghi K, Chahal G. Comparative evaluation of stress levels before, during, and after periodontal surgical procedures with and without nitrous oxide-oxygen inhalation sedation. J Indian Soc Periodontol. 2017;21:21. doi: 10.4103/jisp.jisp_226_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lindh T, Gunnes J, Tillberg A, Molin M. A meta-analysis of implants in partial edentulism. Clin Oral Implants Res. 1998;9:80–90. doi: 10.1034/j.1600-0501.1998.090203.x. [DOI] [PubMed] [Google Scholar]
- 93.Gomes GH, Misawa MYO, Fernandes C, Pannuti CM, Saraiva L, Huynh-Ba G, et al. A systematic review and meta-analysis of the survival rate of implants placed in previously failed sites. Braz Oral Res. 2018;32:e27. doi: 10.1590/1807-3107bor-2018.vol32.0027. [DOI] [PubMed] [Google Scholar]
- 94.Setzer FC, Kim S. Comparison of long-term survival of implants and endodontically treated teeth. J Dent Res. 2014;93:19–26. doi: 10.1177/0022034513504782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Howe MS, Keys W, Richards D. Long-term (10-year) dental implant survival: a systematic review and sensitivity meta-analysis. J Dent. 2019;84:9–21. doi: 10.1016/j.jdent.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 96.Minozzi S, Cinquini M, Gianola S, Gonzalez-Lorenzo MG, Banzi R. The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J Clin Epidemiol. 2020;126:37–44. doi: 10.1016/j.jclinepi.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 97.Senn S John Wiley and Sons, editors. Cross-over trials in clinical research. 2nd ed. The Atrium, Southern Gate, Chichester, West Sussex: 2002. pp. 1–354. [Google Scholar]
- 98.Campbell H, Surry SAM, Royle EM. A review of randomised controlled trials published in Archives of Disease in childhood from 1982-96. Arch Dis Child. 1998;79:192–197. doi: 10.1136/adc.79.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Curtin F. Meta-analysis combining parallel and cross-over trials with random effects. Res Synth Methods. 2017;8:263–274. doi: 10.1002/jrsm.1236. [DOI] [PubMed] [Google Scholar]