Abstract
Background and Aim:
The incidence of hepatocellular carcinoma (HCC) has risen considerably in the U.S. since 1980. The main causes include metabolic disorders (NAFLD, diabetes, obesity, metabolic syndrome), alcohol-related disease (ALD), and hepatitis C and B virus infections (HCV, HBV). Etiology-specific HCC incidence rates by detailed race-ethnicity are needed to improve HCC control and prevention efforts.
Methods:
All HCC cases diagnosed in Florida during 2014–2015 were linked to statewide hospital discharge data to determine etiology. Age-specific and age-adjusted rates were used to assess the intersection between etiology and detailed racial-ethnicities, including White, African American, Afro-Caribbean, Asian, Cuban, Puerto Rican, and Continental Hispanic (Mexican, South and Central American).
Results:
Of 3,666 HCC cases, 2,594 matched with discharge data. HCV was the leading cause of HCC among men and women (50% and 43%, respectively), followed by metabolic disorders (25% and 37%) and ALD (16% and 9%). Puerto Rican and African American men had the highest HCV-HCC rates,7.9 and 6.3 per 100,000, respectively. Age-specific rates for HCV-HCC peaked among baby boomers (those born in 1945–1965). Metabolic-HCC rates were highest among populations above age 70 and among Continental Hispanics. Afro-Caribbean men had high rates of HBV-HCC while Puerto Rican men had high ALD-HCC.
Conclusions:
HCC etiology is associated with specific race/ethnicity. While HCV-related HCC rates are projected to decrease soon, HCC will continue to affect Hispanics disproportionately, based on higher rates of metabolic-HCC (and ALD-HCC) among Continental Hispanics, who demographically represent 80% of all US Hispanics. Multifaceted approaches for HCC control and prevention are needed.
Keywords: hepatocellular carcinoma, etiology, cause, incidence, Florida, Cuban, Puerto Rican, Caribbean, African American, race, ethnicity, subgroup, HCV, HBV, alcohol-liver disease, NAFLD
Introduction
Hepatocellular carcinoma (HCC), representing 78% of all liver cancers, is highly fatal, with only 18% of patients surviving 5 years in the U.S.1,2 Approximately 25,000 new HCC cases are diagnosed annually; incidence rates have increased 48% since 2000.3 A major causal contributor to the current HCC burden is chronic infection with hepatitis C virus (HCV), with a higher prevalence documented among U.S. “baby boomers”, those born between 1945 and 1965.4 However, previous attempts to quantify the attributable risk of HCV as a cause of HCC have produced widely variant results. Population-level studies have attributed approximately 20%−26% of all HCC cases to HCV,5,6 while clinical series found up to 55% of cases to be attributable to HCV.7,8
Other known major HCC etiologies include chronic hepatitis B infection (HBV), alcohol-related liver disease (ALD), and metabolic conditions, including diabetes and non-alcoholic fatty liver disease (NAFLD).9 Clinicians have reported that over 90% of HCC cases have an ascertainable cause, including less common genetic, biliary, or autoimmune conditions.7,8 As with HCV-associated HCC, the respective burden of each distinct HCC etiology in the general population is poorly understood. This lack of knowledge on a population basis may hinder effective cancer prevention and control efforts.
The prevalence of HCV, HBV, ALD, NAFLD and other metabolic precursors of HCC vary substantially by race-ethnicity.10 Accordingly, the rates of HCC incidence 11,12 and mortality (liver cancer) vary by race-ethnicity,7,8 widely documented for broad race-ethnicity categories, hereafter called Level I: White, Black, Hispanic, and Asian/Pacific Islander (API).11,12 A few studies have examined race-ethnicity at a more granular level, looking at rates for specific subgroups within a Level I group, hereafter called Level II. For example, Hispanics, a Level I group, encompass distinct Level II groups such as Puerto Ricans, Cubans, and Mexicans; APIs include Chinese, Vietnamese, Filipinos, etc. while Blacks include African Americans, Afro-Caribbeans and Africans.13,14 Variability in rates across these distinct Level II groups reveal substantial differences, with potential implications for cancer prevention and control. As an example, liver cancer mortality rates among Puerto Ricans are high (17.6 per 100,000 males), especially among baby boomers,13,14 and Puerto Ricans have particularly high prevalence of HCV.15 However, the national viral hepatitis program, using only the Level I racial-ethnic categories, does not target Hispanics as a priority group for viral hepatitis control.16
Moreover, this variation in HCC etiology by race-ethnicity and age group, and patterns of etiology-specific HCC, are in transition given the recent advent of direct-acting antivirals (DAA) that provide sustained virologic response, or “cure”, among HCV-positive patients. Researchers thus predict that the increasing HCC trends will soon reach a turning point and possibly begin to decrease.17 Concurrently, as viral causes of HCC decrease, metabolic-related HCC cases are predicted to increase, given the growing prevalence of metabolic disease.18,19 Thus, detailed knowledge of etiology-specific HCC is important to anticipate the future HCC burden and develop targeted outreach and intervention programs.
In this study we use individual-level data to estimate population-level incidence rates by HCC etiology, HCV-HCC, metabolic-HCC, ALD-HCC, and HBV-HCC, for Level II race-ethnicity, by age group, leveraging the diversity in the populous state of Florida.
Methods
All HCC cases (n=3,666) reported to the statewide cancer registry, the Florida Cancer Data System (FCDS), diagnosed during 2014–2015 were used. Eligible cases included all ICD-O-3 morphologies 8170 – 8180, hepatocellular carcinomas (n=3,273). As American clinical practice guidelines allow for HCC diagnosis without biopsy, based on imaging alone,20 any cancer cases coded C22.0 (liver) with morphologies 8000–8010 were also included (n=393), unless evidence existed of any other primary cancer (e.g. breast, colon, etc.) occurring prior to the HCC diagnosis that could suggest misclassification of liver as the primary site; these 43 cases were excluded.
The FCDS linked registry data with 2014–2015 hospital discharge data, inclusive of diagnosis codes for every medical episode in any hospital setting in Florida, provided by Florida’s Agency for Healthcare Administration (AHCA). Linkage was deterministic, based on first and last name, sex, birth date, social security number, and county of residence. Relevant diagnosis codes for the etiology of HCC were extracted using the list of ICD-9&10-CM codes established by previous research 5,6 (Supplementary Table 1). The FCDS de-identified the data for analysis by the research team.
Some HCC cases had multiple causes,5 (e.g. HCV and ALD), evident after all potential cause codes were incorporated into each HCC case. To assign one predominant-cause category, we used Beste et al.’s hierarchical approach as shown in Table 1,21 largely based on decreasing strength of association (from past research) for each HCC etiology.21 Thus, predominant cause was assigned for each HCC case: 1. HCV; 2. HBV; 3. Hemochromatosis, autoimmune hepatitis, primary sclerosing cholangitis, biliary cholangitis; 4. ALD; 5. Metabolic (including NAFLD, obesity, diabetes, metabolic syndrome); and 6. Other, including remaining codes for genetic, biliary, auto-immune. Lastly, six mutually-exclusive etiology categories were considered for analysis: HCV, HBV, ALD, metabolic, other causes (including causes from steps 3 and 6 above), and cryptogenic. The latter group included HCC cases with matched ACHA data that lacked any recognizable etiology code.
Table 1.
Frequencies of HCC causes and assigned predominant cause for 2,594 Florida Cancer Data System-Agency Health Care Administration (discharge data) matched cases in Florida, 2014–2015.
| n (%) | Predominant Cause† | |
|---|---|---|
| HCV | 379 (14.6%) | HCV (48.0%) |
| HCV+Metabolic‡ | 442 (17.0%) | |
| HCV+ALD | 219 (8.4%) | |
| HCV+ALD+Metabolic‡ | 180 (6.9%) | |
| HCV+HBV+Metabolic‡ | 10 (0.4%) | |
| HCV+HBV+ALD | 6 (0.2%) | |
| HCV+HBV | 5 (0.2%) | |
| HCV+HBV+ALD+Metabolic‡ | 3 (0.1%) | |
| HBV | 45 (1.7%) | HBV (3.9%) |
| HBV+Metabolic‡ | 39 (1.5%) | |
| HBV+ALD | 9 (0.3%) | |
| HBV+ALD+Metabolic‡ | 8 (0.3%) | |
| Genetic, Biliary, Autoimmune | 21 (0.8%) | Other§ (1.0%) |
| Genetic + Metabolic‡ | 5 (0.2%) | |
| ALD | 158 (6.1%) | ALD (14.4%) |
| ALD+Metabolic‡ | 215 (8.3%) | |
| Metabolic‡ | 727 (28.0%) | Metabolic‡ (28.0%) |
| Cryptogenic | 123 (4.7%) | Cryptogenic (4.7%) |
In descending order according to the classification by Beste et al. ref# 21
Metabolic includes NAFLD, obesity, type 2 diabetes, metabolic syndrome
Other includes genetic, biliary, autoimmune
Level I race-ethnicity data was available for all cases. Level II race-ethnicity was analyzed for distinct Hispanic (Cuban, Puerto Rican, and Continental Hispanic [inclusive of Central American, South American and Mexican]), and Black groups; APIs were too few to disaggregate. Birthplace, available for 87% of all cases, was used to create two distinct Level II Black groups: African American (born in the U.S.) and Afro-Caribbeans (born in Caribbean territories and nations, listed elsewhere 22). Level II group identification was discernable for 82.8% of Hispanic and 87.5% of Black cases; the remainder were proportionally allocated into a specific group based on age, sex, county of residence and HCC etiology (when available), using methodology described elsewhere.23
To show the relative weight of each etiology, proportions were computed for the major causes for each racial-ethnic group, sex, and three age groups, categorized as 0–49, 50–69 (approximating baby boomers born between 1945–1965), and 70 and above.
To demonstrate population-level differences in incidence rates, age-adjusted, annualized, sex-stratified etiology-specific HCC rates, were calculated for each Level I and II race-ethnicity group, using the 2000 U.S. population standard and 18 age group bands, as described elsewhere.13 Population denominators corresponding with each age group, race-ethnicity, and sex were obtained from the American Community Survey, pooling years 2014 and 2015.24 Age-specific, sex-stratified, annualized etiology-specific HCC rates were also computed, using 10-year age bands to demonstrate trends by age group.
Data were analyzed using SAS 9.4 and SPSS V22.0. The University of Miami Institutional Review Board provided ethical review for this study.
Results
In 2014–2015, 3,666 new cases of HCC were diagnosed in Florida and linked with statewide discharge data. Of these index cases, 70.8% (n=2,594) matched and constituted our analytical dataset for all etiology-specific analyses. Table 1 represents the distribution of all HCC etiologies, alone or in combination. The most common etiology found alone was metabolic, comprising 28% of all cases (n=727). The most common combination of etiologies, HCV and metabolic, was found in 442 cases, followed by HCV and ALD with 219 cases. Only three patients had all four main causes: HCV, HBV, ALD and metabolic. After a predominant HCC cause was determined for each case, HCV was the leading cause of HCC. (Table 1)
HCV-HCC accounted for 49.6% of male cases and 42.8% of female cases. This HCV-HCC proportion varied substantially by race-ethnicity, from less than 15% among Continental Hispanic women to 64.1% among African American men. Metabolic HCC accounted for a higher proportion among women (37.1%) than men (25.1%). Conversely, HCC associated with alcohol-related disorders was the predominant HCC cause for 16.2% of males and 8.9% of females. HBV-HCC comprised only 4.4% of all male cases when race-ethnicity was combined, but accounted for 48.1% and 26.3% of Afro-Caribbean and Asian male cases, respectively (Table 2).
Table 2.
Distribution of HCC etiology by race/ethnicity and sex for 2,594 FCDS-AHCA matched cases in Florida, 2014–2015.
| Level I Race-Ethnicity |
Level II Race-Ethnicity |
Total N† | HCV | HBV | ALD | Metabolic‡ | Cryptogenic | |
|---|---|---|---|---|---|---|---|---|
| MALE | ||||||||
| White | 1,285 | 49.7% | 2.6% | 16.0% | 26.9% | 4.0% | ||
| Black § | 250 | 60.0% | 12.4% | 12.0% | 12.0% | - | ||
| African American | 220 | 64.1% | 8.2% | 11.8% | 11.8% | - | ||
| Afro-Caribbean | 27 | - | 48.1% | - | - | - | ||
| API | 38 | 34.2% | 26.3% | - | - | - | ||
| Hispanic § | 360 | 44.0% | 3.6% | 18.6% | 28.1% | 4.4% | ||
| Puerto Rican | 123 | 60.2% | - | 18.7% | 14.6% | - | ||
| Cuban | 112 | 43.8% | - | 8.9% | 39.3% | - | ||
| Continental Hispanic | 109 | 29.4% | - | 29.4% | 30.3% | - | ||
| All Race-Ethnicities Combined ¶ | 1,956 | 49.6% | 4.4% | 16.2% | 25.1% | 3.9% | ||
| FEMALE | ||||||||
| White | 374 | 43.0% | - | 11.2% | 36.1% | 7.2% | ||
| Black § | 96 | 60.4% | - | - | 24.0% | - | ||
| African American | 82 | 61.0% | - | - | 23.2% | - | ||
| Afro-Caribbean | 12 | - | - | - | - | - | ||
| API | 28 | 42.9% | - | - | - | - | ||
| Hispanic § | 129 | 27.9% | - | - | 54.3% | 9.3% | ||
| Puerto Rican | 36 | 47.2% | - | - | 41.7% | - | ||
| Cuban | 38 | 26.3% | - | - | 57.9% | - | ||
| Continental Hispanic | 47 | <15% | - | - | 66.0% | - | ||
| All Race-Ethnicities Combined ¶ | 638 | 42.8% | 2.2% | 8.9% | 37.1% | 7.4% | ||
Includes all listed as well as Others (biliary, genetic and auto-immune)
Including NAFLD, obesity, type 2 diabetes, metabolic syndrome
Includes all cases of this race-ethnicity; not just listed groups
All Race-Ethnicities Combined also includes those not listed here (i.e. multiracial, other, and unknown race)
Not reported; observations fewer than 10
API: Asian/Pacific Islander
The median age of HCC diagnosis for all etiologies combined was 64 years; HBV-HCC cases had the youngest median age (59) while metabolic-HCC had the oldest at 72 (Table 3). Almost half of all HCC cases occurred in the baby boomer age category of 50–69. The distribution of etiology varied by age group. Among those over age 70, Metabolic HCC accounted for the largest proportion of cases, 48.0%; for baby boomers, 68.7% of all HCC cases were attributed to HCV (Table 3).
Table 3.
Distribution of HCC etiology by age group and distribution of age group for each HCC etiology for 2,594 FCDS-AHCA matched cases in Florida, 2014–2015.
| All Causes† | HCV | HBV | ALD | Metabolic‡ | Cryptogenic | |
|---|---|---|---|---|---|---|
| Median Age at Diagnosis | 64 | 61 | 59 | 65 | 72 | 65 |
|
| ||||||
| Age Groups | n | n | n | n | n | n |
|
| ||||||
| row % | row % | row % | row % | row % | row % | |
|
|
||||||
| column % | column % | column % | column % | column % | column % | |
|
| ||||||
| Younger than age 50 | 102 | 31 | 20 | 15 | 15 | 20 |
|
|
||||||
| 100% | 30.4% | 19.6% | 14.7% | 14.7% | 19.6% | |
|
|
||||||
| 3.9% | 2.5% | 19.8% | 4.0% | 2.1% | 16.3% | |
|
| ||||||
| Baby boomers (ages 50–69) |
1,293 | 888 | 56 | 166 | 136 | 38 |
|
|
||||||
| 100% | 68.7% | 4.3% | 12.8% | 10.5% | 2.9% | |
|
|
||||||
| 49.8% | 71.4% | 55.4% | 44.5% | 18.7% | 30.9% | |
|
| ||||||
| Older Cohort (ages 70+) | 1,199 | 325 | 25 | 192 | 576 | 65 |
|
|
||||||
| 100% | 27.1% | 2.1% | 16% | 48.0% | 5.4% | |
|
|
||||||
| 46.2% | 26.1% | 24.8% | 51.5% | 79.2% | 52.8% | |
|
| ||||||
| All Ages Combined | 2,594 | 1,244 | 101 | 373 | 727 | 123 |
|
|
||||||
| 100% | 48.0% | 3.9% | 14.4% | 28.0% | 4.7% | |
|
| ||||||
| 100% | 100% | 100% | 100% | 100% | 100% | |
Includes all listed as well as Others (Genetic, biliary, auto-immune)
Including NAFLD, obesity, type 2 diabetes, metabolic syndrome
Inclusive of all etiologies, the age-adjusted HCC incidence rate per 100,000 for all cases in Florida (n=3,666), was 10.3 (95% CI: 9.9–10.7) for men, over three times the rate for women, at 2.9 (95% CI: 2.7–3.1) (Table 4a). Among men, overall HCC rates were highest for Puerto Ricans and African Americans, 18.1 and 15.3, respectively. For women, rates were high for African Americans, APIs, Puerto Ricans, and Continental Hispanics. Lowest overall HCC rates were among Cuban and Afro-Caribbean men and women (Table 4a).
Table 4a.
Age-adjusted† incidence rates per 100,000 by sex and detailed race-ethnicity for all 3,666 cases of HCC. Florida 2014–2015.
| Level I Race-Ethnicity |
Level II Race-Ethnicity |
n | Foreign-born‡ | Annual Population at Risk | Total |
|---|---|---|---|---|---|
| Rate (95% CI) | |||||
| MALE | |||||
| White | 1,824 | 5% | 5,430,462 | 9.7 (9.2–10.1) | |
| Black § | 356 | 13% | 1,576,229 | 12.2 (10.9–13.6) | |
| African American | 311 | 0% | 1,258,435 | 15.3 (13.5–17.2) | |
| Afro-Caribbean | 40 | 100% | 281,596 | 5.5 (3.9–7.9) | |
| API | 52 | 94% | 319,722 | 8.0 (5.9–10.6) | |
| Hispanic § | 493 | 55% | 2,434,105 | 11.5 (10.5–12.6) | |
| Puerto Rican | 160 | 1% | 526,269 | 18.1 (15.4–21.2) | |
| Cuban | 161 | 84% | 725,464 | 8.8 (7.5–10.3) | |
| Continental Hispanic | 169 | 78% | 1,050,196 | 13.2 (10.9 –15.7) | |
| All Race-Ethnicities Combined ¶ | 2,764 | 17% | 9,806,613 | 10.3 (9.9–10.7) | |
| FEMALE | |||||
| White | 542 | 7% | 5,641,656 | 2.6 (2.4–2.9) | |
| Black § | 125 | 15% | 1,716,817 | 3.7 (3.0–4.4) | |
| African American | 106 | 0% | 1,342,891 | 4.5 (3.7–5.5) | |
| Afro-Caribbean | 16 | 100% | 339,212 | 1.8 (1.0 –3.7) | |
| API | 34 | 93% | 370,392 | 4.6 (3.2–6.5) | |
| Hispanic § | 184 | 65% | 2,495,906 | 3.4 (2.9–3.9) | |
| Puerto Rican | 51 | 4% | 541,998 | 4.9 (3.6–6.5) | |
| Cuban | 49 | 93% | 722,244 | 2.2 (1.6–2.9) | |
| Continental Hispanic | 80 | 83% | 1,078,568 | 4.5 (3.5–5.7) | |
| All Race-Ethnicities Combined ¶ | 902 | 26% | 10,275,672 | 2.9 (2.7–3.1) | |
Age-adjusted to the 2000 U.S. Standard Population
Among the 87% with known birthplace
Includes all cases of this race-ethnicity; not just listed groups
All Race-Ethnicities Combined also includes those not listed here (i.e. multiracial, other, and unknown race)
Using only the matched cases (n=2,594), etiology-specific age-adjusted HCC incidence rates (Table 4b) showed the greatest sex differential for ALD-HCC, over five times greater incidence in men than women for all analyzed racial-ethnic groups. Men had approximately four times higher risk for HCV-HCC and twice the risk for metabolic-HCC than their female counterparts.
Table 4b.
Age-adjusted† incidence rates per 100,000 by sex and detailed race-ethnicity for 2,594 matched cases of HCC and selected etiology-HCC categories. Florida 2014–2015.
| Level I Race-Ethnicity |
Level II Race-Ethnicity |
n | Total‡ | HCV-HCC | HBV-HCC | ALD-HCC | Metabolic§-HCC |
|---|---|---|---|---|---|---|---|
| Rate (95% CI) | Rate (95% CI) | Rate (95% CI) | Rate (95% CI) | Rate (95% CI) | |||
| MALE | |||||||
| White | 1,285 | 6.9 (6.5–7.3) | 3.5 (3.2–3.8) | 0.2 (0.1–0.3) | 1.1 (0.9–1.2) | 1.8 (1.6–2.0) | |
| Black ¶ | 250 | 8.4 (7.3–9.5) | 4.7 (3.9–5.5) | 1.0 (0.7–1.5) | 1.1 (0.7–1.6) | 1.3 (0.9–1.9) | |
| African American | 220 | 10.4 (9.1–12.0) | 6.3 (5.3–7.5) | 0.8 (0.5–1.3) | 1.3 (0.8–1.9) | 1.6 (1.0–2.4) | |
| Afro-Caribbean | 27 | 3.8 (2.4–5.9) | - | 1.8 (0.9–3.6) | - | - | |
| API | 38 | 5.8 (4.1–8.1) | 2.0 (1.1–3.5) | 1.4 (0.7–2.6) | - | - | |
| Hispanic ¶ | 360 | 8.3 (7.5–9.3) | 3.4 (2.9–4.0) | 0.3 (0.1–0.5) | 1.6 (1.2–2.0) | 2.6 (2.1–3.2) | |
| Puerto Rican | 123 | 14.0 (11.6–16.8) | 7.9 (6.2–9.9) | - | 2.9 (1.8–4.3) | 2.4 (1.4–3.8) | |
| Cuban | 112 | 6.2 (5.1–7.5) | 2.6 (1.9–3.5) | - | 0.6 (0.3–1.1) | 2.4 (1.8–3.3) | |
| Continental Hispanic | 109 | 8.8 (7.1–10.9) | 1.9 (1.3–2.7) | - | 2.1 (1.4–2.9) | 3.8 (2.5–5.4) | |
| All Race-Ethnicities Combined †† | 1,956 | 7.3 (7.0–7.6) | 3.5 (3.3–3.8) | 0.2 (0.2–0.3) | 1.2 (1.0–1.3) | 1.8 (1.7–2.0) | |
| FEMALE | |||||||
| White | 374 | 1.8 (1.6–2.1) | 0.8 (0.7–1.0) | - | 0.2 (0.2–0.3) | 0.6 (0.5–0.7) | |
| Black ¶ | 96 | 2.8 (2.3–3.5) | 1.7 (1.3–2.2) | - | - | 0.7 (0.4–1.1) | |
| African American | 82 | 3.5 (2.8–4.3) | 2.1 (1.5–2.7) | - | - | 0.8 (0.5–1.3) | |
| Afro-Caribbean | 12 | 1.4 (0.7–3.2) | - | - | - | - | |
| API | 28 | 3.8 (2.5–5.5) | 1.6 (0.8–2.8) | - | - | - | |
| Hispanic ¶ | 129 | 2.4 (2.0–2.9) | 0.7 (0.5–0.9) | - | - | 1.3 (1.0–1.7) | |
| Puerto Rican | 36 | 3.4 (2.4–4.8) | 1.5 (0.9–2.5) | - | - | 1.5 (0.8–2.5) | |
| Cuban | 38 | 1.7 (1.2–2.4) | 0.4 (0.2–0.9) | - | - | 0.9 (0.6–1.5) | |
| Continental Hispanic | 47 | 3.0 (2.2–4.0) | 0.3 (0.1–0.8) | - | - | 2.1 (1.4–2.9) | |
| All Race-Ethnicities Combined †† | 638 | 2.1 (1.9–2.3) | 0.9 (0.8–1.0) | 0.1 (0.0–0.1) | 0.2 (0.1–0.3) | 0.7 (0.6–0.8) | |
Age-adjusted to the 2000 U.S. Standard Population
Includes all listed as well as Cryptogenic and Others (biliary, genetic, auto-immune)
Includes NAFLD, obesity, type 2 diabetes, metabolic syndrome
Includes all cases of this race-ethnicity; not just listed groups
All Race-Ethnicities Combined also includes those not listed here (i.e. multiracial, other, and unknown race)
Rates based on fewer than 10 observations are not reported due to possibility of identification; all suppressed rates per 100,000 for Asians were 0.8 or less, for Afro-Caribbeans, 0.7 or less and for all other populations, 0.3 or less. API: Asian/Pacific Islander
Of all the analyzed HCC etiologies, for both men and women, incidence rates for HCV-HCC were highest, both for race-ethnicity combined and most race-ethnicity groups. Puerto Rican and African American men had the highest HCV-HCC rate, at 7.9 and 6.3 per 100,000 respectively, both significantly higher than in any other population. Metabolic HCC rates were highest in Continental Hispanic men, 3.8 per 100,000 significantly higher than any non-Hispanic population. Other findings suggest high risk for ALD-HCC among Puerto Rican and Continental Hispanic men, 2.9 and 2.1 respectively; and high HBV-HCC among Afro-Caribbean and Asian men, 1.8 and 1.4 per 100,000, respectively (Table 4b).
In Figure 1, age-specific rates of etiology-specific HCC by sex are presented for select racial-ethnic groups. The highest HCV-HCC rates were observed in the age bands from 50 to 69. Conversely, metabolic-HCC rates were highest in age bands above 70. For HCV-HCC, Puerto Rican males in ages 60–69 years had the highest rate, 45.6 per 100,000. The highest age-specific rates for metabolic-HCC was 52.0 per 100,000 among Continental Hispanic men over age 80, and for ALD-HCC was 26.7 among Puerto Rican males ages 70–79. Rates for women were significantly lower than men for all etiologies and racial-ethnic groups. The highest age-specific rate for women was 18.0 per 100,000 for metabolic-HCC among Continental Hispanics in age group 80+.
Figure 1.
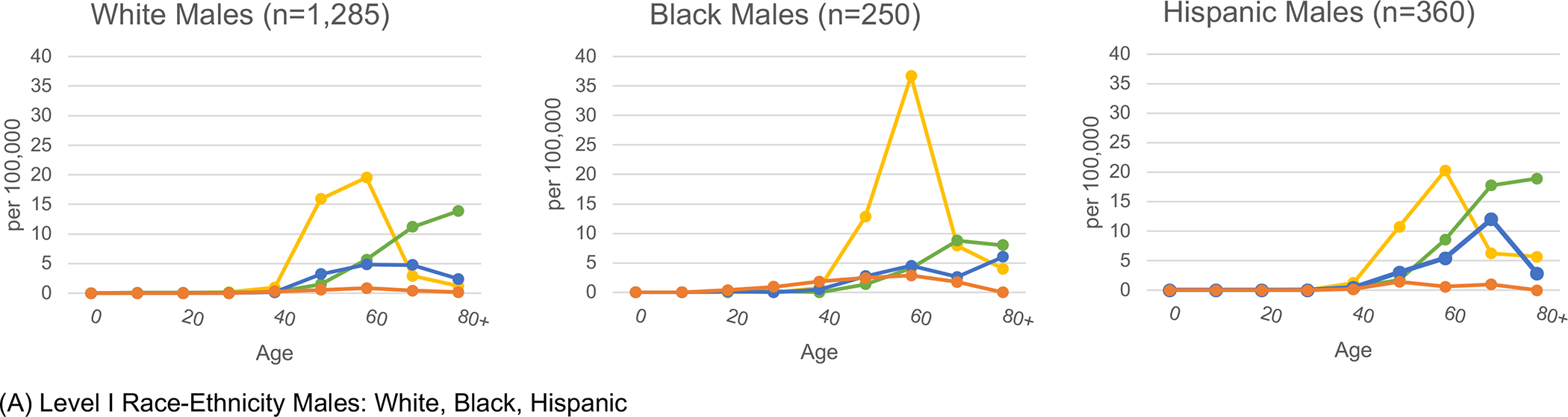
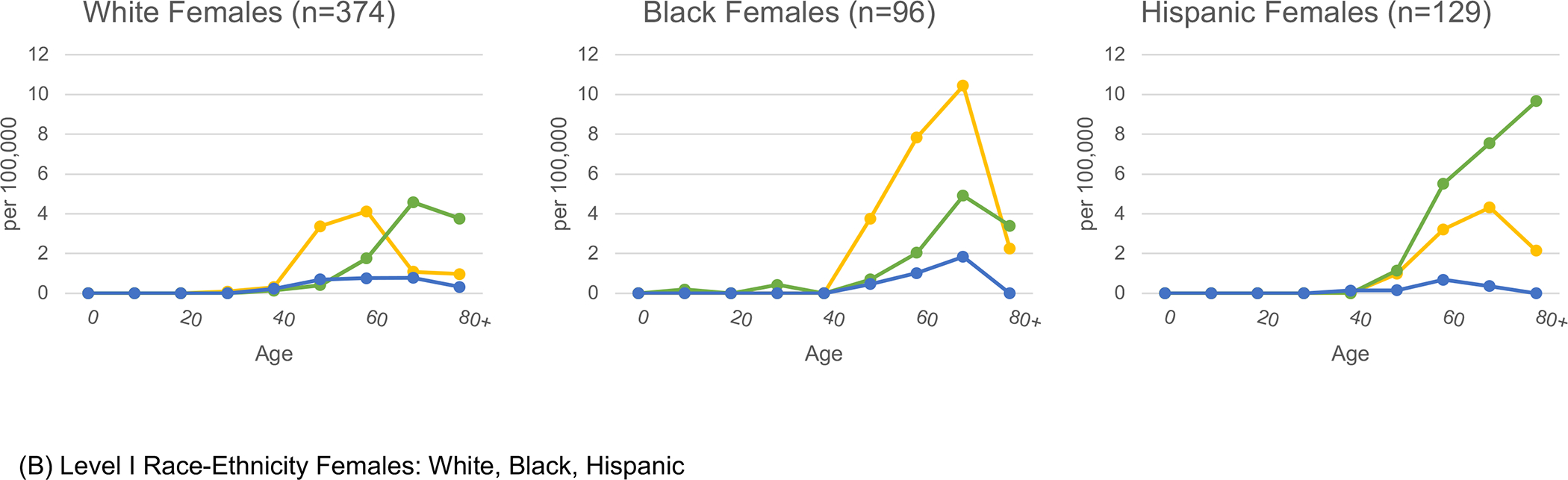
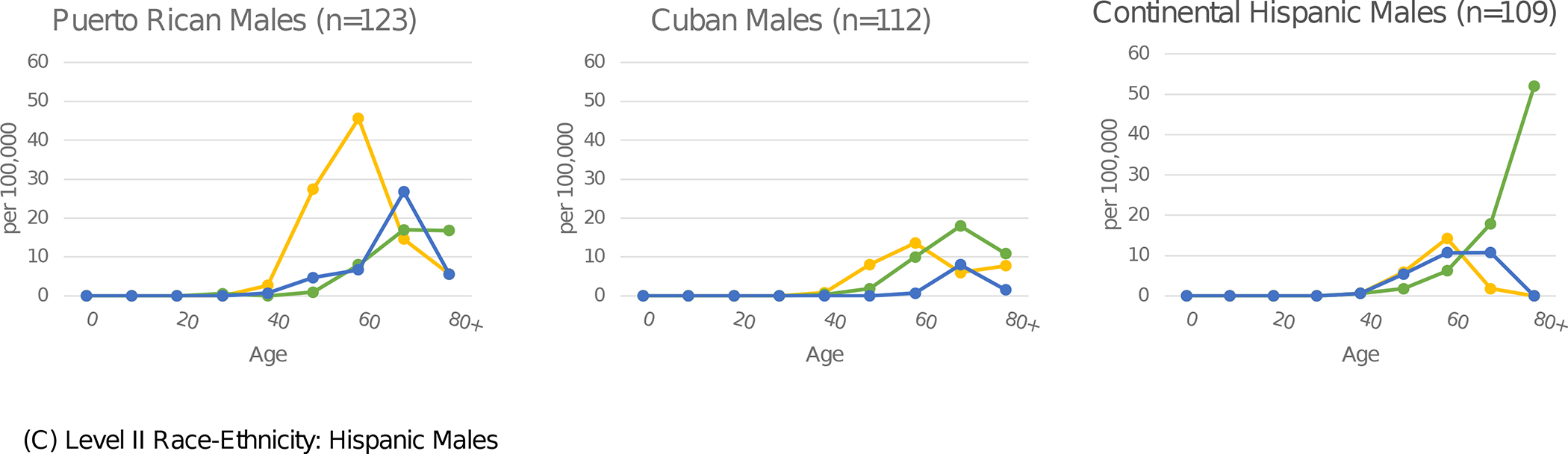
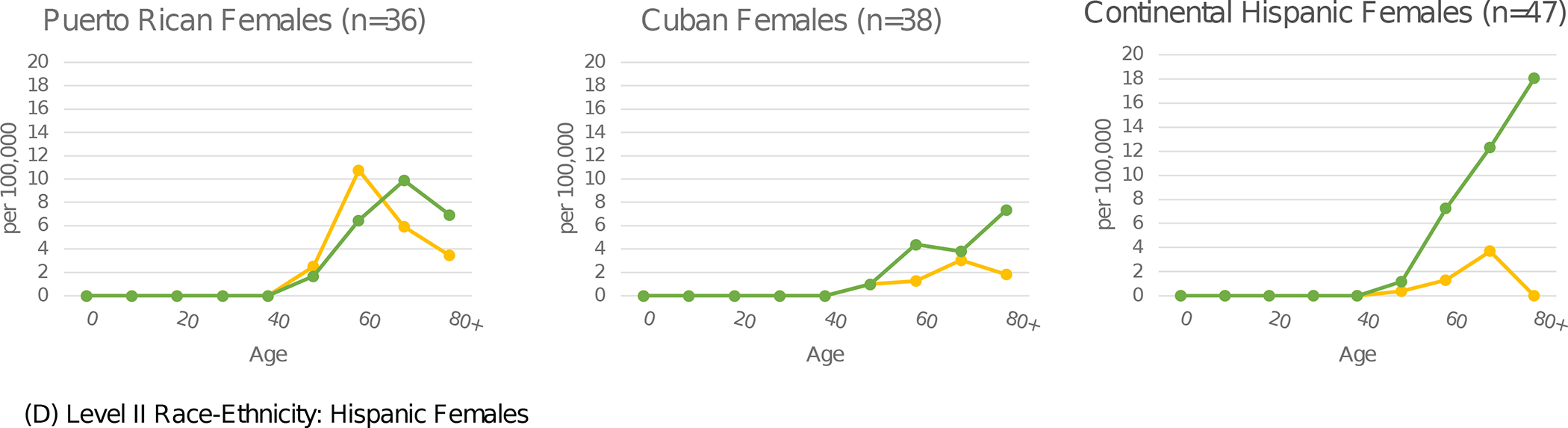
Etiology-specific age-specific rates per 100,000 by sex and detailed race-ethnicity. Florida 2014–2015.
Yellow represents HCV, green represents MTB, blue represents ALD, and orange represents HBV.
Abbreviations: HCV (Hepatitis C Virus); MTB (Metabolic); ALD (Alcoholic Liver Disease); HBV (Hepatitis B Virus)
Discussion
To our knowledge, we present the first analysis of etiology-specific HCC incidence rates by detailed race-ethnicity for all age groups, achieved by leveraging and linking available data from two sources covering the entire population of Florida. Our novel results clarify HCC epidemiology, clearly demonstrating that HCC follows etiology patterns that are associated with sex, birth cohort, and detailed Level II racial-ethnic group.
We document the high impact of HCV-HCC, greater than any other HCC etiology, and concentrated among baby boomers. The weight of HCV in the overall HCC burden observed here aligns more closely with previous clinical hospital series 7,8 than existing population-based studies,5,6 which may have missed numerous cases of HCV-HCC by using mostly Medicare-eligible cases, ages 65 and above, particularly given our median age of 61 for HCV-HCC. Moreover, by disaggregation of the of the Black and Hispanic groups into Level II racial-ethnic groups, we show that rates for HCV-HCC in both sexes are generally higher in U.S.-born groups, whether African American, Puerto Rican, or White, than in groups that are majority immigrant, including Cuban, Continental Hispanic, Asian and Afro-Caribbean. The high risk of HCV-HCC for Puerto Rican and African American men is consistent with recently published liver cancer mortality rates conducted without linkage to medical records,13,14 and correlate with known higher HCV prevalence, particularly among Puerto Ricans,15,25 suggesting a common socio-environmental context that increases HCV risk. While for all U.S.-born baby boomers, higher HCV prevalence is suggested to be related to blood transfusions and/or past IV drug use between 1960–1990,26 for African Americans and Puerto Ricans, higher rates of incarceration,27 where HCV prevalence can be as high as 23%,28 may also play a role.29 Even among women, African Americans had the highest HCV-HCC rates and Puerto Rican women had rates over twice as high as other Hispanic women. To achieve progress in lowering HCV-HCC rates and reducing racial/ethnic disparities, awareness of HCV screening as well as provision of HCV treatment with DAAs, although important for every population at risk, could be prioritized for African American and Puerto Rican populations.
Metabolic disorders were the second leading etiology for HCC, with remarkable variation in patterns by age and Level II race-ethnicity. Rates were highest among those ages 70 and above, surpassing HCC-HCV. Continental Hispanics had the highest metabolic-HCC rates, followed by Puerto Ricans and Cubans; all of the conditions comprising the metabolic HCC category, NAFLD, diabetes, obesity, are high among Hispanics, especially of Mexican origin.30,31 In particular, NAFLD is the most common chronic liver disease in the U.S., affecting up to 100 million Americans 32 and causing approximately 10% of all HCCs 7,8. Hispanics have the highest prevalence of NAFLD,30,31,33 in possible association with a higher prevalence of PNPLA3 polymorphisms.34 However, prevalence of NAFLD is lower among Caribbean Hispanics than other Hispanics, which is consistent with our findings of higher metabolic HCC rates for Continental Hispanics.30 Notably, while the majority of Hispanics in Florida are Caribbean (56%), in the U.S. as a whole, the vast majority are Continental (81%), including the largest group, those of Mexican origin.26 Thus, our rates for Continental Hispanics in Florida likely best approximate rates for the entire U.S. Hispanic population.
Other observed patterns include relatively high ALD-HCC rates among males, particularly Puerto Rican, and seemingly negligible rates among females of all race-ethnicities. For HBV-HCC, our observed higher proportion for APIs was expected, confirming the higher HBV prevalence among APIs documented elsewhere.11,35 However, because the API population in Florida is young and small,24 our findings should be interpreted with caution. High rates of HBV-HCC were also observed among Afro-Caribbean males (females too few to compute), a large group of black immigrants to Florida, originally from Haiti, Jamaica, and other West Indian nations. Our rates correlate with the endemicity of HBV in these countries, ranging from intermediate to high, and highlight the need for an increased focus on the early detection and treatment of HBV among immigrants to reduce future rates of HBV-HCC.36
The major strength of the current study is the production of novel etiology-specific HCC rates on a population basis, leveraging existing surveillance data to characterize HCC profiles specific to each race-ethnicity. While previous studies have used proportions or population-attributable fractions to quantify the etiological burden of different HCC precursors,5,6 population-based incidence rates facilitate more accurate comparisons for each etiology-specific HCC. Taking ALD-HCC among Puerto Rican men as an example, the proportion of ALD-HCC compared to other etiologies is relatively small (18.1%); however, this group has the absolute highest ALD-HCC rate. Similarly, metabolic-HCC is proportionally weighted heavier among females, yet metabolic-HCC rates are uniformly higher in males. Thus, etiology-specific rates provide new insights into the magnitude of each HCC etiology by distinct age group, sex, and Level II detailed race-ethnicity that can be used to guide public health and clinical professionals. Importantly, the incidence rates presented here result not only from the variation in prevalence of each etiology for each population but also from differences in treatment for these underlying liver diseases. For example, differential receipt of antiviral therapy and/or transplantation would impact the development of HCC.
The detailed approach to race-ethnicity by considering Level II groupings whenever feasible, minimizes confounding within the Level I groups, and identifies important distinctions by Level II race-ethnicity that make HCC epidemiology unique in the U.S. Other studies have used mortality data to discern etiology categories for HCC, for level I and even among Hispanic subgroups; however, etiology information, gleaned from death certificates, was largely missing. Thus, their findings, such as only 20% for HCV-HCC, should be interpreted with great caution.37
Our study should be replicated in other states, to expand knowledge of sizable U.S. racial/ethnic populations including Asian groups, who have different cancer risk profiles.23 Likewise, distinguishing between Mexican Americans (US-born) and Mexican immigrants, shown to be distinctly different in mortality studies,38,39 would add additional clarity to the distribution of HCC etiology, as previous research has shown higher HCV prevalence 15 and higher liver cancer mortality among Mexican American baby boomers 13 than their immigrant counterparts. Even within our study, we were compelled to present rates for Mexicans, Central Americans, and South Americans together as Continental Hispanics, while evidence suggests meaningful variation among these groups.13
The current analysis was not without limitations. The availability of AHCA discharge data was restricted to only two years of data which resulted in a small number of cases for some populations, such as Afro-Caribbeans and APIs. Also, with 29.2% of cases unmatched, our etiology-specific rates (based on matched cases) are necessarily underestimated. However, the distribution of matching proportions varied little by race/ethnicity, with 75% match for APIs and Puerto Ricans, and 70–71% for all other race/ethnicities. Not surprisingly, unspecified morphologies (8000–8010) were substantially less likely to match (only 31%) than specified, histologically-confirmed HCC cases (76% matched).
Another limitation is the low yield for NAFLD in discharge data. Despite its high prevalence, NAFLD was described by a relatively nonspecific code in ICD-9-CM: 571.8 “Other Chronic Non-Alcoholic liver disease” until October 2015.40 Thus, population-based studies, including ours, rely upon related conditions 5,6,21 since approximately 50% of patients with diabetes, obesity and metabolic syndrome also have NAFLD.41 We were unable to identify and remove treated HBV-HCC and HCV-HCC cases. Nevertheless, while current available treatment can effectively inhibit HBV replication,42 it does not eradicate it completely.43 Moreover, while the recently introduced direct-acting antivirals (DAAs) can eradicate chronic HCV infection,17,44,45 a risk of HCC remains.45 Aflatoxin exposure, an established risk factor for HCC, especially in Africa, Asia, and parts of Central America,46,47 could not be studied, although existing data suggests low prevalence of aflatoxin in the U.S. and a minimal effect size compared to other etiologies.48 Lastly, the hierarchical classification of predominant cause of HCC may, to a limited degree, affect the precision and accuracy of the estimated rates for etiologies ranked lower in the hierarchy than HCV, such as ALD and metabolic causes. Also, due to limited data, we were unable to analyze specific cause combinations which may be of interest due to known poor prognosis, such as HCV and ALD.
Collectively, the implications of these findings are especially important for anticipating future HCC trends. This timely analysis detects the evident separation of patterns between the two major HCC etiologies: metabolic-HCC, impacting older ages, and HCV-HCC, impacting U.S.-born populations, particularly baby boomers; as this cohort ages, it will be subject to the double burden of HCV- and metabolic-HCC. However, HCV-HCC should be a decreasing problem as the baby boomers age, especially if HCV screening and treatment programs are implemented more effectively. Conversely, metabolic-HCC is likely to persist and potentially increase in the near future given the rising trends for NAFLD, obesity, and diabetes.49,50 Combined, these two trends may exacerbate HCC disparities for Hispanics, especially the majority Mexican-origin group. Lastly, studies such as these, showing disparate HCC incidence in specific groups, provide important data suggesting which groups could benefit from enhanced HCC screening and surveillance of underlying liver disease; additionally, relevant education and awareness can be brought directly to both patients and providers.
As the fastest increasing cancer in the U.S., HCC has a complex epidemiological profile, with etiology inextricably linked to sex, age group, and detailed race-ethnicity. Confronting this challenge will require innovative and multi-faceted approaches, including possibly thinking of HCC itself as different entities according to etiology, to best develop targeted and effective clinical and public health prevention efforts.
Supplementary Material
Key Points.
The etiology of HCC in the US varies substantially by race-ethnicity, sex and birth cohort impacting HCC control and prevention efforts
HCC attributable to HCV currently represents the greatest burden and is highly concentrated amongst baby boomers (born between 1945–1965) and U.S-born groups, specifically Puerto Rican and African American.
Metabolic-HCC is more common in older ages than HCV-HCC and disproportionately affect Hispanics; HBV-HCC is more common among Afro-Caribbeans and Asians; Alcohol-related HCC is high among Puerto Ricans.
The predicted decreasing trend of HCV-HCC and stable or increasing metabolic-HCC will disproportionately affect Hispanics in the US.
Funding Statement:
The author had no financial support for this manuscript.
Disclosure Statement:
The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (FCDS), the statewide cancer registry funded by the Florida Department of Health (DOH) and the Centers for Disease Control and Prevention’s National Program of Cancer Registries (CDC-NPCR). The views expressed herein are solely those of the author(s) and not necessarily reflect those of the DOH or CDC-NPCR.
List of Abbreviations
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- HBV
Hepatitis B virus
- ALD
Alcoholic liver disease
- NAFLD
Non-alcoholic fatty liver disease
- DAA
Direct-acting antivirals
- FCDS
Florida Cancer Data System
- ICD
International Classification of Diseases
- AHCA
Agency for Healthcare Administration
- CI
Confidence interva
Footnotes
Conflict of Interest Statement: The authors declare no potential conflicts of interest.
Contributor Information
Paulo S. Pinheiro, Sylvester Comprehensive Cancer Center, Department of Public Health Sciences, Division of Epidemiology & Population Health Sciences, University of Miami School of Medicine, Clinical Research Building, 1120 N.W. 14th Street, Miami, FL 33136.
Heidy Medina, Department of Public Health Sciences, University of Miami School of Medicine.
Karen E. Callahan, School of Public Health, University of Nevada Las Vegas.
Patricia D. Jones, University of Miami School of Medicine, Department of Medicine, Division of Hepatology.
Clyde Perry Brown, Florida A&M University College of Pharmacy and Pharmaceutical Sciences.
Sean F. Altekruse, Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, Maryland, United States of America.
Katherine A. McGlynn, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Erin N. Kobetz, Sylvester Comprehensive Cancer Center, University of Miami School of Medicine.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2016. Bethesda, MD, 2019. [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov). SEER*Stat Database: Incidence - SEER 21 Regs Limited-Field Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (2000–2016) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. [Google Scholar]
- 3.National Program of Cancer Registries and Surveillance, Epidemiology, and End Results. SEER*Stat Database: NPCR and SEER Incidence - U.S. Cancer Statistics Public Use Database with Puerto Rico, Nov 2018 submission (2005–2016). United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Released June 2019, based on the November 2018 submission. Accessed at www.cdc.gov/cancer/npcr/public-use. [Google Scholar]
- 4.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep 2012;61(RR-4):1–32 [PubMed] [Google Scholar]
- 5.Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016;122(11):1757–65 doi: 10.1002/cncr.29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welzel TM, Graubard BI, Quraishi S, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol 2013;108(8):1314–21 doi: 10.1038/ajg.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones PD, Diaz C, Wang D, Gonzalez-Diaz J, Martin P, Kobetz E. The Impact of Race on Survival After Hepatocellular Carcinoma in a Diverse American Population. Dig Dis Sci 2018;63(2):515–28 doi: 10.1007/s10620-017-4869-3. [DOI] [PubMed] [Google Scholar]
- 8.Rich NE, Hester C, Odewole M, et al. Racial and Ethnic Differences in Presentation and Outcomes of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol 2019;17(3):551–59.e1 doi: 10.1016/j.cgh.2018.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog 2017;16:1 doi: 10.4103/jcar.JCar_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis 2015;19(2):223–38 doi: 10.1016/j.cld.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016;122(9):1312–37 doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cronin KA, Lake AJ, Scott S, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018;124(13):2785–800 doi: 10.1002/cncr.31551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinheiro PS, Callahan K, Jones P, Morris C, Ransdell J, Kwon D, Brown C, et al. Liver Cancer: A Leading Cause of Cancer Death in the United States and the Role of the 1945–1965 Birth Cohort by Ethnicity. JHEP Reports 2019. doi: 10.1016/j.jhepr.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinheiro PS, Callahan KE, Boscoe FP, et al. Cancer Site-Specific Disparities in New York, Including the 1945–1965 Birth Cohort’s Impact on Liver Cancer Patterns. Cancer Epidemiol Biomarkers Prev 2018;27(8):917–27 doi: 10.1158/1055-9965.EPI-18-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuniholm MH, Jung M, Everhart JE, et al. Prevalence of hepatitis C virus infection in US Hispanic/Latino adults: results from the NHANES 2007–2010 and HCHS/SOL studies. J Infect Dis 2014;209(10):1585–90 doi: 10.1093/infdis/jit672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Dept of Health and Human Services. National Viral Hepatitis Action Plan 2017–2020. Jan. 2017. Available from: https://www.hhs.gov/hepatitis/action-plan/national-viral-hepatitis-action-plan-overview/index.html.
- 17.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017;153(4):996–1005.e1 doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol 2014;109(4):542–53 doi: 10.1038/ajg.2014.11|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeves HL, Zaki MY, Day CP. Hepatocellular Carcinoma in Obesity, Type 2 Diabetes, and NAFLD. Dig Dis Sci 2016;61(5):1234–45 doi: 10.1007/s10620-016-4085-6. [DOI] [PubMed] [Google Scholar]
- 20.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68(2):723–50 doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 21.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology 2015;149(6):1471–82.e5; quiz e17–8 doi: 10.1053/j.gastro.2015.07.056. [DOI] [PubMed] [Google Scholar]
- 22.Pinheiro PS, Callahan KE, Ragin C, Hage RW, Hylton T, Kobetz EN. Black Heterogeneity in Cancer Mortality: US-Blacks, Haitians, and Jamaicans. Cancer Control 2016;23(4):347–58 doi: 10.1177/107327481602300406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin H, Pinheiro PS, Xu J, Amei A. Cancer incidence among Asian American populations in the United States, 2009–2011. Int J Cancer 2016;138(9):2136–45 doi: 10.1002/ijc.29958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruggles S, Flood S, Goeken R, et al. IPUMS USA: Version 9.0 [dataset]. In: IPUMS, ed. Minneapolis, MN, 2019. [Google Scholar]
- 25.Kruszon-Moran D, Paulose-Ram R, Denniston M, McQuillan G. Viral Hepatitis Among Non-Hispanic Asian Adults in the United States, 2011–2014. NCHS Data Brief 2015(225):1–8 [PubMed] [Google Scholar]
- 26.Joy JB, McCloskey RM, Nguyen T, et al. The spread of hepatitis C virus genotype 1a in North America: a retrospective phylogenetic study. Lancet Infect Dis 2016;16(6):698–702 doi: 10.1016/S1473-3099(16)00124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Research Council. (2014). The Growth of Incarceration in the United States: Exploring Causes and Consequences. Committee on Causes and Consequences of High Rates of Incarceration, Travis J, Western B, and Redburn S, Editors. Committee on Law and Justice, Division of Behavioral and Social Sciences and Education. Washington, DC: The National Academies Press. [Google Scholar]
- 28.Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology 2015;62(5):1353–63 doi: 10.1002/hep.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larney S, Kopinski H, Beckwith CG, et al. Incidence and prevalence of hepatitis C in prisons and other closed settings: results of a systematic review and meta-analysis. Hepatology 2013;58(4):1215–24 doi: 10.1002/hep.26387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleischman MW, Budoff M, Zeb I, Li D, Foster T. NAFLD prevalence differs among hispanic subgroups: the Multi-Ethnic Study of Atherosclerosis. World J Gastroenterol 2014;20(17):4987–93 doi: 10.3748/wjg.v20.i17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazo M, Bilal U, Perez-Escamilla R. Epidemiology of NAFLD and Type 2 Diabetes: Health Disparities Among Persons of Hispanic Origin. Curr Diab Rep 2015;15(12):116 doi: 10.1007/s11892-015-0674-6. [DOI] [PubMed] [Google Scholar]
- 32.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313(22):2263–73 doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 33.Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2013;178(1):38–45 doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saab S, Manne V, Nieto J, Schwimmer JB, Chalasani NP. Nonalcoholic Fatty Liver Disease in Latinos. Clin Gastroenterol Hepatol 2016;14(1):5–12; quiz e9–10 doi: 10.1016/j.cgh.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Moore MS, Ivanina E, Bornschlegel K, Qiao B, Schymura MJ, Laraque F. Hepatocellular Carcinoma and Viral Hepatitis in New York City. Clin Infect Dis 2016;63(12):1577–83 doi: 10.1093/cid/ciw605. [DOI] [PubMed] [Google Scholar]
- 36.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386(10003):1546–55 doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 37.Pinheiro PS. Letter to Editor: The Need for Complete Population-Based Studies on the Etiology of Liver Disease. Hepatology 2018. doi: 10.1002/hep.30415. [DOI] [PubMed] [Google Scholar]
- 38.Pinheiro PS, Callahan KE, Gomez SL, et al. High cancer mortality for US-born Latinos: evidence from California and Texas. BMC Cancer 2017;17(1):478 doi: 10.1186/s12885-017-3469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinheiro PS, Callahan KE, Stern MC, de Vries E. Migration from Mexico to the United States: A high-speed cancer transition. Int J Cancer 2018;142(3):477–88 doi: 10.1002/ijc.31068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Department of Health and Human Services. International classification of diseases, 9th revision, clinical modifications (ICD-9-CM). In. 6th edition ed. Washington,DC: Health Care Financing Administration; 1997. [Google Scholar]
- 41.Rich NE, Oji S, Mufti AR, et al. Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018;16(2):198–210.e2 doi: 10.1016/j.cgh.2017.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: From discovery to regulatory approval. J Hepatol 2017;67(4):847–61 doi: 10.1016/j.jhep.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017;24(3):1073274817729245 doi: 10.1177/1073274817729245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waziry R, Hajarizadeh B, Grebely J, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol 2017;67(6):1204–12 doi: 10.1016/j.jhep.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 45.Kanwal F, Kramer JR, Asch SM, Cao Y, Li L, El-Serag HB. Long-term risk of hepatocellular carcinoma in HCV patients treated with direct acting antiviral agents. Hepatology 2019. doi: 10.1002/hep.30823. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Chang CC, Marsh GM, Wu F. Population attributable risk of aflatoxin-related liver cancer: systematic review and meta-analysis. Eur J Cancer 2012;48(14):2125–36 doi: 10.1016/j.ejca.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith JW, Kroker-Lobos MF, Lazo M, et al. Aflatoxin and viral hepatitis exposures in Guatemala: Molecular biomarkers reveal a unique profile of risk factors in a region of high liver cancer incidence. PLoS One 2017;12(12):e0189255 doi: 10.1371/journal.pone.0189255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramirez AG, Muñoz E, Parma DL, et al. Lifestyle and Clinical Correlates of Hepatocellular Carcinoma in South Texas: A Matched Case-control Study. Clin Gastroenterol Hepatol 2017;15(8):1311–12 doi: 10.1016/j.cgh.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertuccio P, Turati F, Carioli G, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol 2017;67(2):302–09 doi: 10.1016/j.jhep.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol 2016;34(15):1787–94 doi: 10.1200/JCO.2015.64.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


