Abstract
Since antiquity, Cannabis has provoked enormous intrigue for its potential medicinal properties as well as for its unique pharmacological effects. The elucidation of its major cannabinoid constituents, Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), led to the synthesis of new cannabinoids (termed synthetic cannabinoids) to understand the mechanisms underlying the pharmacology effects of Cannabis. These pharmacological tools were instrumental in the ultimate discovery of the endogenous cannabinoid system, which consists of CB1 and CB2 cannabinoid receptors and endogenously-produced ligands (endocannabinoids), which bind and activate both cannabinoid receptors. CB1 receptors mediate the cannabimimetic effects of THC and are highly expressed on presynaptic neurons in the nervous system, where they modulate neurotransmitter release. In contrast, CB2 receptors are primarily expressed on immune cells. The endocannabinoids are tightly regulated by biosynthetic and hydrolytic enzymes. Accordingly, the endocannabinoid system plays a modulatory role in many physiological processes, thereby generating many promising therapeutic targets. An unintended consequence of this research was the emergence of synthetic cannabinoids sold for human consumption to circumvent federal laws banning Cannabis use. Here, we describe research that led to the discovery of the endogenous cannabinoid system, and show how knowledge of this system benefitted as well as unintentionally harmed human health.
Keywords: cannabinoid, endocannabinoid, phytocannabinoid, synthetic cannabinoid, Cannabis Use Disorder, CB1/CB2 receptor, allosteric modulation, opioid sparing effects, THC, CBD
1: A Historical Perspective of, and Introduction to, Cannabinoids
Paleobotanical studies indicate that the Cannabis plant was present as long as 11,400 years ago during the Holocene epoch around central Asia (Tarasov et al. 2007; Clarke and Merlin 2013). The earliest evidence of Cannabis use dates back 10,000 years to the end of the ice age in Japan (Okazaki et al. 2011), as well as 4,000 years BCE in China as recorded in the ancient Pharmacopoea text “Shen Nung Pen Ts’ao Ching” (Jiang et al. 2006). Originally grown for its use as a fiber, food, and medicinal plant by shamans, Cannabis spread across the world due to human domestication and its adaptability to a wide range of climates (for an extensive account of the archeobotanical evidence for Cannabis use see Pisanti and Bifulco, 2019). The use of Cannabis as a recreational drug eventually became widespread, with an early description found in an 1857 article by The Hasheesh Eater (Lee 2013). Cannabis now represents the most commonly used psychoactive drug in the world after alcohol and tobacco.
Discussions surrounding the legal, ethical, and societal implications of Cannabis use have been ongoing for at least a century. The last several decades have seen an increasing rise in the frequency of physician-prescribed Cannabis for the treatment of various medical conditions such as chronic pain and psychiatric problems across a growing number of states in the United States (Whiting et al. 2015). The current renaissance of the medical employment of Cannabis as well as the changing legal landscape in 21st Century United States has forced issues associated with the safe use of Cannabis to a now higher prominence. These include; routes of administration, content identification and labelling, drug interactions, dispensing, safety and untoward side effects, contraindications, and use or unintended exposure in specialty populations (the young and the elderly), to name but a few. The inclusion of Cannabis Use Disorder into the third edition of the Diagnostic and Statistical Manual of Mental Disorders (DSMIII) in 1980 makes discussion of its safe use pertinent given the reinforcing value of this ancient plant and as such the potential that exists for dependence. As of 2010, global reports of Cannabis Use Disorder prevalence estimated that 0.2% or 13.1 million people met diagnostic criteria (Degenhardt et al. 2013), compared to the United States general population where a prevalence of 1.5% was reported in a 2015 National Survey of Drug Use and Health (Hasin et al. 2016; SAMHSA 2017). While Cannabis use is a necessary condition to develop Cannabis Use Disorder, not all users develop this disorder; therefore, use alone is not a sufficient predictor. The etiology of Cannabis Use Disorder is thus clearly complex.
Studying the reinforcing and rewarding effects of cannabinoids in preclinical settings remains a challenge. The most widely used preclinical investigative tool, murine species, do not show reliable or robust intravenous or oral self-administration of Δ9-tetrahydrocannabinol (THC), the primary psychoactive constituent of Cannabis (Lefever et al. 2014; Wakeford et al. 2017; Barrus et al. 2018). Rat intracerebroventricular self-administration of THC (Braida et al. 2001; Zangen et al. 2006), as well as intravenous self-administration of the synthetic cannabinoid WIN 55,212–2 (Fattore et al. 2001; Mendizabal et al. 2006) has been reported. Whereas squirrel monkeys readily self-administer THC (Justinova et al. 2003), this phytocannabinoid functioned as a reinforcer in only half of rhesus and cynomolgus monkeys (John et al. 2017). Other behavioral measures have also been employed to assess the rewarding effects of THC, such as conditioned place preference and intracranial self-stimulation, with inconsistent results (Braida et al. 2004; Hempel et al. 2016; Tanda 2016). As such, the limited success of modeling the rewarding effects of Cannabis in research model organisms remains a considerable barrier to preclinical research investigating the neurobiology underlying the abuse liability of cannabinoids as well as assessing drugable targets to treat Cannabis Use Disorder.
The chemicals collectively termed cannabinoids can be organized into three broad classes or categories: phytocannabinoids (plant based; the individual molecular constituents of the Cannabis plant), synthetic cannabinoids (manmade cannabinoids), and endocannabinoids (cannabinoids produced by and within the body).
1a. Phytocannabinoids
The Cannabis plant contains hundreds of phytochemicals, which include phytocannabinoids, terpenes, and phenolic compounds. To date, more than 560 chemicals have been identified in Cannabis, with approximately 120 of these constituents described as terpenophenolic cannabinoids or phytocannabinoids, primarily produced in the glandular trichomes of the plant (ElSohly et al. 2017). Phytocannabinoids are a broad group of closely related chemicals but with diverse structure as well as pharmacological actions. The availability of novel spectrometric methods in the 1960s facilitated the isolation of the primary psychoactive constituent of Cannabis Δ9-THC (Gaoni and Mechoulam 1964) as well cannabidiol (CBD) (Mechoulam and Shvo 1963). For a full review of the chemical elucidation of phytocannabinoids through the 1970s see Mechoulam et al. (1976). The elucidation of these structures sparked an enormous amount of basic research that revealed the effects of these drugs in the brain and body. Moreover, the FDA approved THC (referred to as dronabinol) to treat nausea and emesis associated with cancer chemotherapy, as well as AIDS-related cachexia as an appetite stimulant. The FDA recently approved CBD to treat severe forms of pediatric epilepsy. Although THC and CBD represent the best known phytocannabinoids, other predominant constituents include cannabigerol (CBG), cannabichromene (CBC), and tetrahydrocannabivarin (THCV). The phytocannabinoids exist as acids (e.g., THCA-A, CBDA), which are non-enzymatically decarboxylated to their corresponding neutral forms (e.g., THC, CBD). This decarboxylation begins to occur after the plant is harvested during the drying process over time and/or the application of heat (Flores-Sanchez and Verpoorte 2008). Pharmacokinetic studies of cannabinoids have most often focused on THC. This phytocannabinoid is hydroxylated to the psychoactive metabolite 11-hydroxy-Δ9-THC and then oxidized to the non-psychoactive Δ9-THC-11-oic acid. THC and its metabolites remain sequestered in cell membranes and adipose tissues and are slowly released, which is why Cannabis use can be detected in urine long after use.
1b. Synthetic cannabinoids
Upon elucidation of the primary phytocannabinoids, medicinal chemists modified the structure of THC to understand the mechanisms underlying its pharmacological actions. Structurally diverse compounds that included bicyclic cannabinoids (Compton et al. 1992b) and aminoalkylindoles (Compton et al. 1992a; Wiley et al. 1998; Huffman et al. 2005) served as important tools that contributed to the eventual discoveries of cannabinoid receptors (Devane et al. 1988; Munro et al. 1993; see Section 2) and the endocannabinoids (Devane et al. 1992; Mechoulam et al. 1995; Sugiura et al. 1995; see section 3c). Pharmaceutical companies also developed synthetic cannabinoids as potential medications. For example, the FDA approved nabilone, which is structurally similar to THC, for the treatment of nausea and vomiting associated with cancer chemotherapy.
An unforeseen consequence in studies publishing the synthesis and characterization of the synthetic cannabinoids in scientific journals was their diversion to recreational use and abuse (for full reviews see Ford et al. 2017; Wiley et al. 2017). As these compounds elicit even greater intoxicating effects as THC but would not be detected in common drug screening tests, their use circumvents the law and drug testing of Cannabis. The first generation of synthetic cannabinoids, such as JWH018 (Huffman et al. 2005), were added to plant material and sold over the internet and in convenience stores under various names such as “Spice” and “K-2” as marketing ploys. Administration of JWH018 and other synthetic cannabinoids has been linked to physiological toxicity (Freeman et al. 2013) and psychological complications (Celofiga et al. 2014). The first wave of synthetic cannabinoids were designated Schedule I drugs in 2011, and the United States also made them illegal. Since then, other synthetic cannabinoids emerged in a “cat-and-mouse” game between clandestine laboratories and law enforcement. One such example is the highly potent fubinaca, a Pfizer synthetic cannabinoid made Schedule I in 2014. Illicit synthetic cannabinoids frequently pose a greater public safety threat than Cannabis/THC as they have sparked a large increase in emergency room visits and often life threatening consequences (Gerostamoulos et al. 2015). The adverse effects of recreationally used synthetic cannabinoids are likely a result of their higher efficacy and potency at cannabinoid receptors as well as other non-cannabinoid receptor sites of action (Grim et al. 2016).
1c. Endocannabinoids
The endocannabinoid system refers collectively to cannabinoid receptors (CB1 and CB2) that are acted upon by endogenously produced cannabinoid ligands: the endocannabinoids (as well as by THC, other phytocannabinoids, and synthetic cannabinoids), and their biosynthetic and degradative enzymes (Blankman et al. 2007). The two most extensively studied endogenous ligands of cannabinoid receptors are arachidonylethanolamine (anandamide or AEA) and 2-arachidonoylglycerol (2-AG) (Devane et al. 1992; Mechoulam et al. 1995; Sugiura et al. 1995). The synthesis and degradation of these endocannabinoids are enzymatically regulated (Blankman and Cravatt 2013). The enzymes that regulate AEA and 2-AG are described below in Sections 3a and 3b. Other endogenous cannabinoid ligands have also been described (for a full review see Pertwee 2015). The majority of these ligands are lipids and include 2-arachidonylglyceryl ether (noladin ether) (Hanus et al. 2001), N-arachidonoyl dopamine (NADA) (Bisogno et al. 2000), and virodhamine (Porter et al. 2002).
2: Cannabinoid Receptor Discovery and Function
A major impetus for research geared towards understanding the molecular targets of cannabinoids included the identification of THC as the chief psychoactive constituent of Cannabis. Additionally, extensive efforts in medicinal chemistry provided useful tools to bind and activate specific cannabinoid receptor binding sites in biological tissues. The development of highly selective antagonists for each of these receptors (Rinaldi-Carmona et al. 1994, 1998) has greatly aided the investigation of cannabinoid receptor pharmacology as well as provided insight into the function of the endogenous cannabinoid system. The creation of mutant mice in which the CB1 receptor (Ledent et al. 1999; Zimmer et al. 1999) or CB2 receptor (Buckley et al. 2000) was genetically deleted provided a powerful complementary tool to distinguish receptor targets of cannabinoid agonists and reveal potential functions of the endogenous cannabinoid system. Below we describe research leading to the discovery of the CB1 and CB2 receptors.
2a. CB1 Receptor In Vitro Evidence
i). G proteins and adenylyl cyclase.
The history of cannabinoid receptors, and their phytocannabinoid, endocannabinoid ligands and analogs, has recently been reviewed by authors who have made major discoveries in cannabinoid pharmacology (Mechoulam et al. 2014; Ligresti et al. 2016). A highly comprehensive review of the CB1 and CB2 cannabinoid receptors was submitted by the Cannabinoid Receptor Subcommittee of the International Union of Basic and Clinical Pharmacology (IUPHAR) (Howlett et al. 2002), followed by an evaluation of other targets for the endocannabinoids anandamide and 2-AG (Pertwee et al. 2010). Cellular signaling evoked by the CB1 receptor has been comprehensively reviewed (McAllister and Glass 2002; Turu and Hunyady 2010; Console-Bram et al. 2012; Howlett and Abood 2017).
Cannabinoid receptors were initially identified and pharmacologically characterized based upon the ability of THC and antinociceptive analogs developed by Pfizer Central Research to attenuate cAMP accumulation in neuronal cells and brain (Howlett et al. 1988). The N18TG2 hybrid cell line, derived from rat neonatal dorsal root ganglia and mouse neuroblastoma, played an essential role in the discovery and function of CB1 cannabinoid receptors. Based on the observation that pertussis toxin, which eliminates Gi/o coupling, abrogated the inhibitory effect on cAMP accumulation, the CB1 receptor was determined to be a G protein-coupled receptor (GPCR) (Howlett et al. 1986; Houston and Howlett 1993). Immunoprecipitation studies indicated that CB1 receptors are pre-coupled to Gi/o proteins in membrane preparations without the addition of exogenous agonists (Mukhopadhyay et al. 2000; Mukhopadhyay and Howlett 2001, 2005). These studies demonstrated that agonists promote dissociation of the G protein from the CB1 receptor whereas antagonist/inverse agonists maintain the CB1 receptor-Gi protein interaction in a more stable form. We now know from antibody-capture scintillation proximity assays for [35S]GTPγS binding, that members of the Gi/o family constitute about 75 % of the G protein activation by the high-efficacy receptor agonist CP55,940 (Eldeeb et al. 2016, 2017). Gs, Gq/11, G12 and G13 each contributed 5–10% of the total activation under those assay conditions (Eldeeb et al. 2017). Gz, Gs and Gq/11 were not found to be pre-associated with the CB1 receptor in 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)-solubilized immunoprecipitation protocols (Mukhopadhyay et al. 2000), which are used to isolate and purify the proteins of interest. Specifically, pertussis toxin pre-treatment did not lead to increased Gs activation (Eldeeb et al. 2016). Evidence for agonist-selective regulation of various G proteins has come from G-protein activation studies (Glass and Northup 1999; Prather et al. 2000). Immunoprecipitation studies indicated that full agonists could activate all Gi/o subtypes, whereas “partial agonists” only activate certain Gi subtypes and act as inverse agonists at other subtypes (Mukhopadhyay and Howlett 2005). These studies at the level of G-protein activation suggest that agonists may select differing signal pathways depending upon the G-protein availability in the environment of the CB1 receptor.
Numerous reviews have identified the role of neuronal CB1 receptor signal transduction based upon Gβγ protein release and Gαi-mediated reduction of cAMP in the regulation of neurotransmission (Kano 2014; Lu and Mackie 2016), neurodevelopment (Diaz-Alonso et al. 2012; Gaffuri et al. 2012; Maccarrone et al. 2014) and synaptic plasticity (Garcia et al. 2016; Araque et al. 2017). Following Gi/o activation and dissociation, CB1 receptors can be phosphorylated by G-protein receptor kinases (GRKs), leading to interactions with β-arrestins 1 or 2 (Breivogel et al. 2013; Chen et al. 2014). β-arrestins are scaffolding proteins that internalize the CB1 receptors from the plasma membrane, and/or regulate CB1 receptor-mediated signal transduction that is not related to G proteins (Nogueras-Ortiz and Yudowski 2016). For example, extracellular signal regulated kinase (ERK)1 and 2 phosphorylation and activation can be regulated by both CB1-mediated Gβγ release with diminished cAMP and PKA signaling, followed by an extended phase mediated by β-arrestins (Rubino et al. 2006; Daigle et al. 2008; Dalton and Howlett 2012; Franklin et al. 2013; Mahavadi et al. 2014).
ii). Radioligand binding and cloning of cannabinoid receptors in the CNS.
The biological activity of Δ9-THC and analogs being attributed to cannabinoid receptors was first identified using radioligand binding to a Pfizer analog [3H]CP55,940 (Devane et al. 1988). Using this assay, brain cannabinoid receptors were demonstrated to be among the most abundant GPCRs, being highly expressed in the cortex, hippocampus, basal ganglia, and cerebellum consistent with cannabinoid effects on cognition, memory, hypoactivity, and sedation (Herkenham et al. 1990; Glass et al. 1997; Tsou et al. 1998).
An orphan 7-transmemebrane receptor from rat appeared to exhibit neuroanatomical localization similar to that identified as the brain cannabinoid receptor, and using the [3H]CP55,940 radioligand binding assay, was subsequently identified to be what we now refer to as the CB1 cannabinoid receptor (Matsuda et al. 1990). Based upon this rat clone, the human CB1 receptor was shown to have 97% amino acid sequence identity, but was shorter by one residue (Gerard et al. 1991). The mouse and rat exhibit identical amino acid sequences (Chakrabarti et al. 1995; Ho and Zhao 1996; Abood et al. 1997). Although there appears to be very similar pharmacological properties between human and rodent CB1 receptors, some variation in ligand binding has been noted (McPartland et al. 2007).
The first reported splice variant of the human hCB1 receptor, referred to as hCB1a, is reduced by 167 base pairs in the coding region, thereby reducing the N-terminal extracellular domain by 61 residues, and changing 28 residues in the remaining sequence (Shire et al. 1995). The second reported splice variant, hCB1b, was the result of removal of 99 base pairs from the human mRNA, eliminating 33 residues in the N-terminal domain (Ryberg et al. 2005). It should be noted that these variants are not possible in rodents due to a sequence difference (Bonner 1996). An investigation of the pharmacological profile for the hCB1a variant compared with hCB1 expressed in CHO cells found that agonist ligands and the antagonist SR141716 bound to the receptor with three-fold lower affinity; however, the cellular signaling via cAMP and ERK phosphorylation was not appreciably different (Rinaldi-Carmona et al. 1996; Xiao et al. 2008). CB1a and CB1b variants expressed in HEK293 cells exhibited no significant difference compared with CB1 receptors in receptor binding affinity for Δ9-THC, CP55,940, WIN55,212–2, HU210 or SR141716, and two to three-fold lower affinity for 2-AG, but 200-fold lower affinity for anandamide (Ryberg et al. 2005). Both variants exhibited [35S]GTPγS activation EC50 values and efficacies similar to CB1 receptors; however, 2-AG acted as an inverse agonist in the [35S]GTPγS activation assay (Ryberg et al. 2005). When expressed in CB1−/− mouse hippocampal neurons, hCB1a and hCB1b were less efficacious than CB1 in producing depolarization-induced suppression of excitation (Straiker et al. 2012). These hCB1a and hCB1b mRNAs are expressed in low-abundance, <5% of that of CB1 (Shire et al. 1995; Ryberg et al. 2005; Xiao et al. 2008). However, the protein levels of splice variants were immuno-detectable in human and macaque brains (Bagher et al. 2013). Thus, the relevance of these splice variants to human cannabinoid receptor function is not readily apparent.
CB1 receptors in the CNS function at the pre-synaptic terminals of neurons to curtail release of neurotransmitters, particularly in GABAergic more so than glutamatergic neurons (Katona et al. 1999; Szabo and Schlicker 2005; Puighermanal et al. 2009). However, CB1 receptors are indeed present across all plasma membrane components including lipid rafts (Bari et al. 2005; Barnett-Norris et al. 2005), and intracellularly in endosomes and mitochondria (Benard et al. 2012). In addition to neurons, CB1 receptors are expressed by astrocytes (Han et al. 2012; Oliveira da Cruz et al. 2016), oligodendrocytes and their precursors (Ilyasov et al. 2018), and perhaps other glial subtypes (Stella 2010). It should also be noted that the CB1 receptor can be expressed in tissues outside the nervous systems, including heart, lung, prostate, liver, uterus, ovary, testis, vas deferens, and bone (Galiegue et al. 1995). As such, peripheral CB1 receptors mediate physiological processes such as; gastrointestinal motility and energy balance, reproduction and fertility, pain, and skeletal muscle energy metabolism.
2b. CB1 Receptor In Vivo Evidence
The elucidation of the structures of the many phytocannabinoids present in Cannabis (e.g., THC, CBD, CBG, CBC, THCV) led to a great deal of studies investigating their pharmacological actions. The tremendous breadth of pharmacological actions of these compounds was initially hypothesized to reflect nonspecific or specific interactions (as reviewed in (Martin 1986). The well-described effects of cannabinoids in inhibiting cAMP accumulation through GPCR-dependent mechanisms (see section 2a above) provided strong evidence supporting specific mechanisms. However, given the extremely hydrophobic nature of THC and other cannabinoids, their much higher affinity for cell membranes than for aqueous media is not surprising. Accordingly, early studies hypothesized that cell membrane perturbation mediated the pharmacological effects of THC (Hillard et al. 1985, 1990). This perturbation of neuronal cell membranes was also proposed for the intoxicating effects of ethanol (Lyon et al. 1981) and volatile anesthetics (Seeman 1972). In assays using cholesterol liposomes, THC and other psychoactive cannabinoids elicited perturbation, while CBD elicited stabilizing effects in this artificial membrane system (Lawrence and Gill 1975). However, structure activity relationship (SAR) studies did not bear out a correlation between membrane fluidization and intoxicating effects of cannabinoids (for a full review, see Martin 1986). It should be noted that the high concentration of THC necessary to disrupt membrane fluidity far exceeds typical physiological relevant concentrations. Finally, the n-octanol/water partition coefficients of a series of naturally occurring and synthetic cannabinoids did not correlate with their behavioral activity in measures of spontaneous activity, rectal temperature, tail-flick response, and ring-immobility, suggesting that lipophilicity may represent a component, but not a primary determinant in driving the pharmacological activity of the cannabinoids (Thomas et al. 1990).
SAR studies investigating common in vivo pharmacological effects of cannabinoids demonstrated stereoselectivity in rodents, dogs and nonhuman primates (Martin 1986), which strongly supported a receptor mechanism of action. Early studies reported that dogs displayed particular sensitivity to the ataxic effects of Cannabis extracts (Walton et al. 1938). Accordingly, the dog static ataxia test offered utility to investigate the SAR of synthetic cannabinoids (Adams et al. 1948a, b; Martin et al. 1975, 1984; Pars et al. 1976; Beardsley et al. 1987; Little et al. 1989; Compton and Martin 1990), and was also used to examine in vivo cannabimimetic effects of anandamide (Lichtman et al. 1998) prior to the knowledge of its rapid metabolism by FAAH. Over time, employment of the dog static ataxia assay gave way to rodent high throughput screening and drug discrimination assays.
A high throughput screening, developed by the late Professor Billy Martin for SAR studies and eventually coined the “tetrad test”, evaluates the occurrence of decreased spontaneous activity, hypothermia, catalepsy, and thermal antinociception (Little et al. 1988). Whereas noncannabinoid drugs produce one or a subset of pharmacological actions in this series of tests (Wiley and Martin 2003), THC (Little et al. 1988), potent synthetic THC analogs (Little et al. 1989), synthetic bicyclic cannabinoid analogs (Little et al. 1988; Compton et al. 1992b), synthetic aminoalkylindole analogs (Compton et fal. 1992a), and synthetic anandamide analogs (Thomas et al. 1996) produce the entire constellation of tetrad effects in a stereoselective manor. Indeed, the pharmacological effects of synthetic cannabinoids in the tetrad assay highly correlate with binding affinity to the CB1 receptor (Compton et al. 1993). Additionally, this assay can be used to estimate pA2 and pKB values of cannabinoids (Grim et al. 2017), as well as be modified to determine efficacy, which yields values of efficacy that highly correlate with agonist stimulated [35S]GTPγS binding (Grim et al. 2016).
In contrast to the tetrad assay, the drug discrimination paradigm offers a high degree of specificity in capturing the subjective effects of CB1 receptor agonists (for a full review see Wiley et al. 2018). In this assay, laboratory animals are trained in an operant food motivated task to discriminate between the subjective effects of a psychoactive drug and vehicle (Solinas et al. 2006). A large body of drug discrimination studies examining drugs from a multitude of classes demonstrate its tremendous utility and its exquisite sensitivity and specificity. Specifically, drugs that fully substitute for the training drug act through a similar mechanism of action. In a career spanning over 40 years beginning in the 1970s, Järbe and colleagues pioneered the drug discrimination paradigm to investigate cannabinoids (Järbe and Henriksson 1973, 1974; Henriksson et al. 1975; Jarbe et al. 1977). This work was particularly useful in identifying synthetic cannabinoids with cannabimimetic activity (Järbe and Gifford 2014; Järbe et al. 2016a, b). Studies employing CB1 receptor antagonists confirm that this receptor mediates the discriminative stimulus of THC, synthetic cannabinoids, and MAGL inhibitors (Wiley et al. 1995; Owens et al. 2017). Moreover, other pharmacological agents leading to CB1 receptor activation substitute for these training drugs. Finally, Jarbe and colleagues demonstrated that drug discrimination can determine efficacy of CB1 receptors agonists (Järbe et al. 2014).
2c. CB1 Receptor Allosteric Modulation
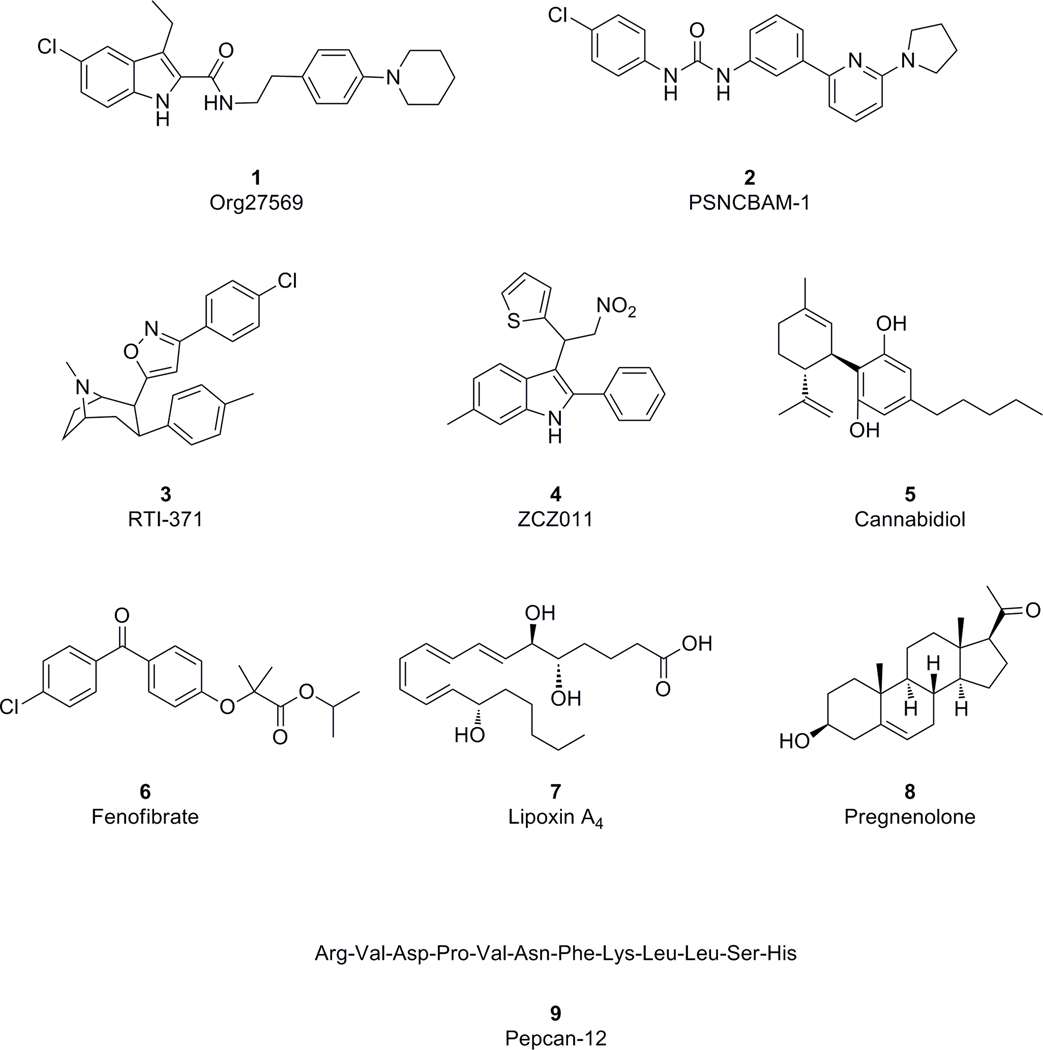
The CB1 cannabinoid receptor has been suggested as a therapeutic target for a number of disorders including chemotherapy-induced nausea, wasting syndrome associated with cancer and AIDS, pain, obesity, neurodegenerative disorders, and substance use disorders (Mackie 2006a; Pacher et al. 2006). Traditionally, therapeutic manipulation of the function of the CB1 cannabinoid receptor is mainly achieved through the application of exogenous compounds that bind to the CB1 receptor orthosteric site where the endogenous cannabinoids such as anandamide and 2-AG bind. Most endogenous compounds bind the orthosteric site, which is the main active site of the receptor. However, agonists or antagonists targeting the orthosteric sites of CB1 receptors have been found with either psychotropic (Grotenhermen and Muller-Vahl 2012) or psychiatric adverse effects (Cridge and Rosengren 2013). These untoward side effects have made orthosteric CB1 ligands challenging to develop into therapeutic agents. To overcome the on-target side effects of CB1 orthosteric ligands, novel ligands interacting with CB1 receptors via a new mechanism of action have been vigorously pursued. To this end, several classes of allosteric modulators, which bind to CB1 receptor sites different from the orthosteric sites, have been discovered (Figure 1). These new CB1 ligands include Org27569 (1) (Price et al. 2005), PSNCBAM-1 (2) (Horswill et al. 2007), RTI-371 (3) (Navarro et al. 2009), ZCZ011 (4) (Ignatowska-Jankowska et al. 2015), and others have been suggested (Laprairie et al. 2015; Priestly et al. 2015) including endogenous (Bauer et al. 2012; Pamplona et al. 2012; Vallee et al. 2014) molecules. Theoretically, a receptor can form a multitude of active and inactive conformations through selective stabilization by various ligands. Allosteric modulators can induce receptor conformations distinct from those stabilized by orthosteric agonists and antagonists but are generally substrates of active or inactive receptor conformations. Thus, novel mechanisms of action from ligand binding to cytosolic signal transduction can be achieved. Preliminary studies of these allosteric modulators have revealed new mechanisms of action in regulating CB1 receptor function. For instance, Org27569 enhances binding of CB1 orthosteric agonists and promotes β-arrestin-1 mediated phosphorylation of ERK1/2, and is a positive allosteric modulator (PAM). This allosteric modulator also inhibits G-protein binding and CB1 agonist-induced G-protein mediated phosphorylation of c-Jun N-terminal kinase (JNK) (Ahn et al. 2013; Baillie et al. 2013). An active form of the receptor may produce signal transduction (e.g. and phosphorylation of some kinases) that differs depending on the nature of the coupling partner (e.g. isoform of G protein and/or β-arrestin). Unlike Org27569, the CB1 allosteric modulator ZCZ011 enhanced the CB1 stimulated G-protein binding and augmented G-protein mediated ERK1/2 phosphorylation induced by CB1 agonists anandamide and CP55,940 (Ignatowska-Jankowska et al. 2015). This evidence suggested functional selectivity in signal transduction and provided for the possibility of separating therapeutic effects from untoward adverse effects when the physiologically important CB1 receptors are manipulated with allosteric modulators.
Figure 1.
Structures of proposed CB1 receptor allosteric agonists.
Translational research using preclinical models of several disorders have shown that the CB1 PAM ZCZ011 and its analogs exhibit exciting promise for potentiating CB1 receptor activity without eliciting adverse effects typically found in the CB1 orthosteric agonists (Ignatowska-Jankowska et al. 2015; Cairns et al. 2017; Slivicki et al. 2018b). On the other hand, the well-characterized CB1 PAM Org27569 failed to show CB1 dependent anorectic activity in a mouse model (Gamage et al. 2014) whereas it attenuated both cue- and drug-induced reinstatement of cocaine- and methamphetamine-seeking behavior in experimental rats (Jing et al. 2014).
To date, growing evidence from in vitro and in vivo characterization indicate that allosteric modulators of the CB1 receptor can regulate the function of the CB1 receptor with novel mechanisms of action. To translate the exciting in vitro pharmacological activities of this class of CB1 ligands into clinical relevant therapies demand further investigations.
2d. CB1 Receptor Functional Selectivity (Biased Agonism)
GPCR signaling is multifaceted and different ligands can induce multiple receptor micro-conformations that generate diverse pharmacological responses (Luttrell 2014). Multiple micro-conformations give rise to a variety of activated receptor sub-states that best couple to different G proteins (e.g. Gi/o subtypes, Gs, Gq, etc) or β-arrestins (1 or 2). Both orthosteric (Lauckner et al. 2005; Mukhopadhyay and Howlett 2005) and allosteric (Khurana et al. 2017) ligands can induce a preference for coupling and downstream signaling. Allosteric modulators that evoke β-arrestin-1 binding to signal include ORG27569 (Ahn et al. 2013) and PSNCBAM-1 (Jagla et al. 2019).
Fay and Farrens (2015) used a fluorescence probe to examine changes in the orientation of helices with ORG27659 binding. They found that ORG27569 binding precludes outward movements of helix 6 that are key to G-protein activation. Further, this and movement of helix 7 (and helix 8 on the carboxy-terminus) may help explain why β-arrestin binds preferentially to G-proteins thereby mediating alternative signal transduction. It is also possible that allosteric modulator binding to the CB1 receptor is in the same region as G-protein or β-arrestin binding, physically precluding some coupling agents from a receptor interaction. Functional selectivity via an allosteric modulator, with or without probe dependence of orthosteric ligand binding, gives one strategy for providing specificity of a therapeutic response.
2e. CB2 Receptors
A major impetus for the development of new synthetic cannabinoids was to create molecules that retained therapeutic actions without the occurrence of cannabimimetic side effects. To this end, the CB2 receptor (Munro et al. 1993) offers great promise. This receptor shares approximately 44% amino acid homology with the CB1 receptor. Similar to the CB1 receptor, it is coupled predominantly to Gi/o proteins, and linked to signaling cascades that involve adenylyl cyclase and cAMP, mitogen-activated protein kinase (MAPK), and the regulation of intracellular calcium. In addition, an extensive characterization of a panel of ligands binding CB2 receptors revealed compelling evidence of biased agonism with respect to GTPγS, cAMP, β-arrestin, pMAPKs and G protein-gated inwardly rectifying potassium channel (GIRKs) (Soethoudt et al. 2017). Several agonists emerged as highly selective for CB2 receptors, including HU910, HU308 and JWH133. Unlike the CB1 receptor, the CB2 receptor is sparsely expressed in the CNS, but it is highly expressed in cells of the immune system. A great deal of effort dedicated to developing selective CB2 receptor agonists as research tools and candidate medications has revealed that these drugs produce antinociceptive actions without the occurrence of cannabimimetic side effects (for reviews see Anand et al. 2009; Donvito et al. 2018). Although a variety of CB2 receptor agonists lacked efficacy in clinical trials, trials are underway with other compounds for a variety of chronic pain conditions (Aghazadeh Tabrizi et al. 2016).
3: The endocannabinoids and their enzymatic regulation
The cannabinoid receptors discussed in Section 2 mediate most of the psychomimetic effects of Cannabis. Yet the evolutionary significance of CB1 and CB2 receptors is greater than their activation by Cannabis in that they are primarily acted upon by endogenous cannabinoid ligands (endocannabinoids), which together form a neuromodulatory network.
Several unique properties of the endocannabinoid system set it apart from the functional profile of a classical neurotransmitter system. These differences include the direction of endocannabinoid cell-to-cell communication, the unique biosynthesis of endocannabinoid lipids in location and temporal regulation, and the manner of achieving endocannabinoid signalling selectivity. The first property, retrograde neurotransmission, distinguishes endocannabinoids from classical neurotransmitters by their release and action sites and as such their direction of cell-to-cell communication. In contrast to classical neurotransmitters that are released from presynaptic terminals to act at postsynaptic neurons, endocannabinoids are released from the postsynaptic neuron and travel retrogradely across the synaptic cleft to act at pre-synaptic CB1 receptors. The activation of presynaptic cannabinoid receptors ultimately dampens presynaptic neurotransmitter release (Mackie 2006b), which is how endocannabinoids modulate synaptic strength. This functional consequence gives the endocannabinoid system its label as a neuromodulatory system.
A second property, biosynthesis of endocannabinoids, is also dramatically different from classical neurotransmitters which are synthesized in the cell body and packaged into secretory vesicles, transported to axon terminals, and stored for release upon propagation of an action potential. Endocannabinoid biosynthesis occurs “on-demand” in response to increased intracellular Ca2+ (Kondo et al. 1998) or activation of the phospholipase C pathway (Prescott and Majerus 1983; Sugiura et al. 1995) at the level of the plasma membrane from phospholipids present within the cell membrane. The manner by which these highly bioactive yet hydrophobic lipids traverse the aqueous environment of the synaptic space as well as following reuptake in the aqueous intracellular environment remains to be fully understood, though carrier proteins such as fatty acid binding proteins (FABPs) are likely candidates (Haj-Dahmane et al. 2018). The location of the endocannabinoid biosynthetic machinery at the cellular membrane and their hydrophobic nature all contribute to their localized sites of action (20 μm area (Wilson and Nicoll 2001)) and short half-life (less than 5 minutes (Willoughby et al. 1997)), all of which makes endocannabinoid signalling directed, short lived, and occurring in response to discrete stimuli.
A third property, signalling specificity, also distinguishes the endocannabinoid system from that of classical neurotransmission. In traditional neurotransmission, differential activation of signalling pathways are achieved through binding of distinct receptor subtypes by one single neurotransmitter (Siegel 1999). However, endogenous cannabinoids produce functional selectivity at CB1 and CB2 receptors. The endocannabinoid ligands and their abundance and action at cannabinoid receptors are a key component of the endocannabinoid system. However, the anatomical and cellular distribution of their biosynthetic and degradative enzymes exerts precise regulatory control of the actions of these endogenous cannabinoid ligands.
3a. N-arachidonylethanolamine (Anandamide)
Anandamide acts as a partial agonist at CB1 and CB2 receptors (Hillard 2000), as well as binding to TRPV1 receptors (Melck et al. 1999; Zygmunt et al. 1999) and GPR55 (Baker et al. 2006). The best-characterized biosynthetic pathway for anandamide is the conversion of N-acylphosphatidylethanolamines (NAPEs) by NAPE phospholipase D-type (NAPE-PLD) (Okamoto et al. 2004). NAPE-PLD is highly expressed in brain as well as kidney, spleen, lung, heart and liver (Degenhardt et al. 2013). However studies using NAPE-PLD knock-out mice show no changes in brain anandamide levels suggesting the existence of alternative biosynthetic pathways (Leung et al. 2006). A unique feature of anandamide biosynthesis is the existence of several further redundant pathways: the conversion of N-acyl-lysophosphatidylethanolamine by a lysophospholipase-D (lyso-PLD) (Sun et al. 2004); the conversion of NAPE or lyso-NAPE by α/β-hydrolase 4 (Simon and Cravatt 2006); and finally the hydrolysis of NAPE by phospholipase C to phosphoanandamide which is then dephosphorylated to anandamide (Liu et al. 2006). The multiple redundant pathways of anandamide biosynthesis perhaps suggest an evolutionarily conserved mechanism for the importance of preserving endocannabinoid tone. The primary deactivation enzyme of anandamide is fatty acid amide hydrolase (FAAH) (Cravatt et al. 1996, 2001), the degradative product of which is arachidonic acid. FAAH is found in soma and dendrites of the postsynaptic neuron and is associated with membranes of cytoplasmic organelles (Gulyas et al. 2004) in areas such as the neocortex, cerebellar cortex, and hippocampus (Egertova et al. 1998). Other enzymes are also responsible for anandamide degradation, specifically through oxidation. Cyclooxygenase-2 (Kozak et al. 2001), lipoxygenases (Hampson et al. 1995), and cytochrome P450 monooxygenases (Snider et al. 2010), all of which convert anandamide to oxygenated derivatives that have biological activity of their own in eicosanoid inflammatory pathways.
3b. 2-Arachidonoylglycerol (2-AG)
2-AG acts as a high efficacy agonist at both CB1 and CB2 receptors (Hillard 2000), as well as binds GABAA receptors (Sigel et al. 2011). The biosynthesis of 2-AG occurs through the conversion of diacylglycerols (DAG) by the diacylglycerol lipases (DAGL) (Bisogno et al. 2003), in which DAGL-α is predominantly expressed on neurons and DAGL-β is expressed on immune cells (Yoshida et al. 2006; Hsu et al. 2012). The distribution of DAGLs markedly differ between development and adulthood. In the developing mouse forebrain projection neuron, DAGLs are located on elongating axons (co-expressed with CB1 receptors) and implicated in growth cone guidance (Bisogno et al. 2003). Post-development, DAGLs accumulate on post-synaptic dendrites and participate in endocannabinoid mediated modulation of synaptic strength (Keimpema et al. 2011). Additional, but less well studied, 2-AG biosynthetic pathways include PLA1 activation of lyso phospholipase C (lyso-PLC) (Higgs and Glomset 1994) and dephosphorylation of arachidonoyl-lyso-phosphatidic acid (Nakane et al. 2002).
2-AG inactivation occurs by a variety of enzymes which either hydrolase 2-AG into its component parts (arachidonic acid and glycerol) or transform it by acylation or phosphorylation. The hydrolysis of 2-AG occurs primarily through monoacylglycerol lipase (MAGL) (Dinh et al. 2002; Blankman et al. 2007), which is highly expressed at presynaptic terminals (Gulyas et al. 2004) in brain areas including the cortex, hippocampus, cerebellum, and thalamus (Dinh et al. 2002) and functions in the bulk clearance of 2-AG. To a lesser extent (< 10%), 2-AG is also hydrolyzed by ABHD6 and ABHD12 (Blankman et al. 2007) as well as FAAH (Di Marzo et al. 1998). Both ABHD6 and ABHD12 are post-synaptic integral membrane proteins but ABHD6 has an intracellular facing active site and the active site of ABHD12 is extracellular. The location of ABHD6/12 and their modest contributions to 2-AG metabolism contribute to the hypothesis that they might act as a form of regulatory break for 2-AG production. Enzymes that participate in the deactivation of 2-AG through transformation include COX-2 (Kozak et al. 2000), cytochrome P450 (Chen et al. 2008), lipoxygenases (Maccarrone et al. 2000), as well as MAG kinases (Kanoh et al. 1986) and MAG acyl transferases (Coleman and Haynes 1986).
2-AG levels are 1,000 times more abundant in brain than those of AEA. This high level of production is particularly pertinent given that the metabolism of 2-AG contributes to the availability of free arachidonic acid, the major precursor for the production of pro-inflammatory eicosanoids. Specifically, MAGL is the rate limiting enzyme for free arachidonic acid production in brain, liver, and lung (Nomura et al. 2011). As such, MAGL not only serves as the major enzyme terminates 2-AG signalling but also plays an important role in the production of free arachidonic acid production in a tissue specific manner. Importantly, MAGL does not mediate the production of arachidonic acid in the gastrointestinal tract. Ultimately 2-AG production and metabolism serves to facilitate both neuromodulation and immunoregulation respectively (an extensive review of 2-AG biosynthesis and degradation can be found in Murataeva et al, 2014).
3c. Other endocannabinoids, hemopressins, and related lipids
Endocannabinoids are not restricted to AEA or 2-AG. They are members of an ever-growing family of bioactive lipids (Di Marzo 2018). Other described endocannabinoids with cannabimimetic properties include; noladin ether (Hanus et al. 2001), NADA (Bisogno et al. 2000), virodhamine (Porter et al. 2002), and LPI (Pineiro and Falasca 2012). Fatty acid amides such as palmitoylethanolamine and oleoylethanolamine while lacking affinity for CB1 or CB2 receptors (O’Sullivan and Kendall 2010), activate GPR55 and GPR119 receptors (Godlewski et al. 2009), as well as enhance AEA and 2-AG activity by competition for FAAH (Ben-Shabat et al. 1998; Jonsson et al. 2001). Hemopressin is a nonapeptide produced from the cleavage of hemoglobin which acts as an inverse agonist at CB1 receptors (Heimann et al. 2007). Hemopressin shows several physiological effects such as antinociception, hypophagy, and hypotension (Heimann et al. 2007; Monti et al. 2016). Indeed, docking studies have shown that hemopressin binds to the same CB1 receptor pocket as SR141716, a CB1 receptor competitive antagonist/inverse agonist used for metabolic syndrome, but withdrawn from the European market in 2009 due to psychiatric side effects (Motaghedi et al. 2011).
4: Drug Interactions
The endocannabinoid system can interact with a wide range of other neurotransmitter systems including opioids, GABA, glutamate, dopamine etc., and modulate the effects of ethanol, NSAIDs, and substrates for various enzymes. Here we will focus on two such interactions: the potential of drugs targeting the endocannabinoid system to increase opioid potency (termed opioid-sparing effects) which minimizes side effects, and cannabinoid competition for cytochrome P450 (CYP) enzymes making them a potential contraindication for diseases with CYP dysregulation or patients taking drugs metabolized through these enzymes.
4a. Opioid-sparing effects
One consequence of the opioid epidemic crisis is the need to identify alternative drug classes of analgesics that can replace opioids or can reduce the dose of opioids necessary to ameliorate pain. Modulating the endocannabinoid system represents a promising strategy to reduce the effective analgesic doses of opioids, while concomitantly decreasing opioid abuse liability as well as unwanted dose-dependent side effects such as constipation and respiratory depression. Substantial pre-clinical evidence suggests that cannabinoid agonists might produce opioid-sparing effects (for reviews see Nielsen et al. 2017; Donvito et al. 2018). THC represents the most widely selected cannabinoid evaluated in combination with opioids in rodent models of pain. The Welch group pioneered this area of research by employing an isobolographic approach revealing that THC synergistically enhances the antinociceptive effects of various opioids in rodent models of acute pain (Welch and Stevens 1992; Smith et al. 1998; Cichewicz et al. 1999, 2001, 2005; Cox et al. 2007). Likewise, the synthetic cannabinoids CP55,940 and WIN55,212–2 augmented the antinociceptive effects of morphine, but did not affect the discriminative stimulus effects of morphine or heroin self-administration in rhesus monkeys (Maguire et al. 2013). The periaqueductal gray (Wilson-Poe et al. 2012, 2013) has been implicated as a potential brain site contributing to the augmented antinociceptive effects resulting from combined administration of opioids and cannabinoids. Inhibitors of endocannabinoid catabolic enzymes also augment the antinociceptive effects of opioids. Combination of the brain penetrant FAAH inhibitor URB597 or peripherally restricted FAAH inhibitor URB937 plus morphine produced synergistic antinociceptive effects in the mouse paclitaxel model of neuropathic pain (Slivicki et al. 2018a). Similarly, combined injections of the MAGL inhibitor MJN110 and morphine produced synergistic antinociceptive effects in the mouse chronic constrictive injury model of neuropathic pain (Wilkerson et al. 2016). Curiously, co-administration of the dual FAAH-MAGL inhibitor and morphine produced an additive antinociceptive effect in this assay (Wilkerson et al. 2017).
In contrast to the well-established findings from pre-clinical studies showing that cannabinoids augment the antinociceptive effects of opioids, translation to clinical settings remains to be established, as discussed in a recent meta-analysis (Nielsen et al. 2017). Based on preclinical studies, clinical case reports, and a highly cited population study (Bachhuber et al. 2014), the idea of opioid-sparing effects of cannabinoid agonists has been touted as rationale for the legalization of “medical” Cannabis. While large controlled clinical studies provide some evidence of analgesic benefits of THC, opioid dose changes have rarely been reported (Seeling et al. 2006; Johnson et al. 2010). However, three recent phase 3 clinical trials failed to achieve statistical significance of the primary endpoint (average pain Numerical Rating Scale) of nabiximols (an oral-mucosal spray consisting of approximately equal parts of THC and CBD) in advanced cancer patients with chronic pain not alleviated by optimized opioid treatment (Fallon et al. 2017; Lichtman et al. 2018). However, nabiximols showed efficacy on secondary endpoints including sleep disruption as well as patient (Subject Global Impression of Change and Patient Satisfaction Questionnaire) and physician (Physician Global Impression of Change) questionnaires. Substantial differences (e.g., endpoints, species differences, type of pain, etc.) exist between preclinical studies of pain and treatment of clinical pain (Negus 2018, 2019). Moreover, preclinical studies typically use opioid-naïve or non-tolerant laboratory animal subjects, whereas patients in clinical trials have generally been on large dose regimens for prolonged periods of time. Thus, it would be advantageous in future clinical investigations to initiate cannabinoid treatment in cancer pain patients prior to establishing an aggressive opioid treatment regimen. In addition, it is possible that cannabinoid drugs produce opioid-sparing effects for only specific types of cancer pain or in certain patients.
4b. Cytochrome P450 enzymes
Cytochrome P450 enzymes are highly expressed in liver and intestine among other tissues and are necessary for the metabolism of steroid hormones, cholesterol, vitamin D, bile acids, and eicosanoids (Hasler et al. 1999). Diseases of CYP dysregulation include hypertension, hepatotoxicity, infection and chronic inflammation among many others (Setchell et al. 1998; Hiratsuka et al. 2006; Capdevila et al. 2007) (for a full review of CYP roles in disease see Pikuleva and Waterman, 2013). CYP activity is also a major factor in the pharmacokinetics of drugs and thus drug responses (Zanger and Schwab 2013). Endocannabinoids, phytocannabinoids (specifically THC and CBD), as well as synthetic cannabinoids are all substrates of various CYP enzymes (for a full review of cannabinoid interactions with CYP enzymes see Zendulka et al, 2016). As such, binding of phytocannabinoids to CYP enzymes could potentially produce treatment failure if taken with clinically co-administered drugs. Competitive inhibition of CYP enzymes raises concerns of drug toxicity from clinical medications. Medications metabolized by CYP enzymes taken in combination with phytocannabinoids or synthetic cannabinoids may interfer with metabolism, thereby increasing drug blood levels and/or extending duration of action. While the perceived risk for clinically significant drug interactions between cannabinoid-based drugs and the metabolism of CYP medications are generally not considered, limited human data quantifying these interactions exist that are necessary to make definitive conclusions. Given the increased use of “medical” and recreational cannabis, a great need exists to understand the metabolic and pharmacodynamic interactions between cannabinoid-based drugs and other pharmaceuticals.
5: Conclusions
Since antiquity, Cannabis has been recognized for its wide range of therapeutic actions as well as for its intoxicating effects. Studies identifying the active constituents of Cannabis provoked an enormous body of research that led to the creation of new research probes revealing mechanisms underlying its pharmacological effects, the discovery of the endocannabinoid system, and cannabinoid-based medications approved by the FDA. However, further research is needed to understand the pharmacological effects and the mechanisms of action underlying the minor phytocannabinoids and terpines, both alone and in combination. Further work geared towards exploiting promising therapeutic targets within the endocannabinoid system (e.g., allosteric sites on the CB1 receptor, CB2 receptors, FAAH, MAGL, FABPs) may yield new medicines. In particular, preclinical research demonstrates that phytocannabinoids, synthetic cannabinoids, and inhibitors of endocannabinoid regulating enzymes produce antinociception and augment the antinociceptive effects of opioids in a great variety of acute and chronic models of pain. An enormous amount of preclinical studies demonstrate potential efficacy of drugs acting upon the endocannabinoid system in various laboratory animal models of disease and injury. Thus, it remains to examine whether this basic knowledge translates into the clinic. Moreover, given the wide availability of Cannabis, Cannabis extracts, and phytocannabinoids in dispensaries throughout the US and legal availability of CBD derived from hemp, a tremendous need exists for evidenced-based practice for therapeutic needs (e.g. mental illness and Cannabis Use Disorder), which includes understanding potential harms and minimizing abuse of synthetic cannabinoids. A great need also exists for further research to understand the long-term consequences of Cannabis on the developing brain, not only in developing fetuses, but also in adolescent and young adults. In sum, great strides have been achieved in the understanding of cannabinoid pharmacology, including the tremendous complexity existing between the endogenous cannabinoid system and the numerous physiological systems it regulates.
Acknowledgements
This work was support by R01 DA039942 (DAK, LD, AHL), R01 DA042157 (LCH), P50 DA006634 (LCH), and VCU School of Pharmacy Start-up funds (AHL).
References
- Abood ME, Ditto KA, Noel MA, et al. (1997) Isolation and expression of mouse CB1 cannabinoid receptor gene: comparison of binding properties with those of native CB1 receptors in mouse brain and N18TG2 neuroblastoma cells. Biochem Pharmacol 53:207–214 [DOI] [PubMed] [Google Scholar]
- Adams R, Aycock BF, Loewe S (1948a) Tetrahydrocannabinol homologs. J Am Chem Soc 70:662–4. doi: 10.1021/ja01182a067 [DOI] [PubMed] [Google Scholar]
- Adams R, Mackenzie S, Loewe S (1948b) Tetrahydrocannabinol homologs with doubly branched alkyl groups in the 3-position. J Am Chem Soc 70:664–8. doi: 10.1021/ja01182a068 [DOI] [PubMed] [Google Scholar]
- Aghazadeh Tabrizi M, Baraldi PG, Borea PA, Varani K (2016) Medicinal chemistry, pharmacology, and potential therapeutic benefits of cannabinoid CB2 receptor agonists. Chem Rev 116:519–60. doi: 10.1021/acs.chemrev.5b00411 [DOI] [PubMed] [Google Scholar]
- Ahn H, Mahmoud MM, Shim JY, Kendall DA (2013) Distinct roles of beta-arrestin 1 and beta-arrestin 2 in ORG27569-induced biased signaling and internalization of the cannabinoid receptor 1 (CB1). J Biol Chem 288:9790–9800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P, Whiteside G, Fowler CJ, Hohmann AG (2009) Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain Res Rev 60:255–66. doi: 10.1016/j.brainresrev.2008.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Castillo PE, Manzoni OJ, Tonini R (2017) Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology 124:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachhuber MA, Saloner B, Cunningham CO, Barry CL (2014) Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern Med 174:1668–73. doi: 10.1001/jamainternmed.2014.4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagher AM, Laprairie RB, Kelly ME, Denovan-Wright EM (2013) Co-expression of the human cannabinoid receptor coding region splice variants (hCB(1)) affects the function of hCB(1) receptor complexes. Eur J Pharmacol 721:341–354 [DOI] [PubMed] [Google Scholar]
- Baillie GL, Horswill JG, Anavi-Goffer S, et al. (2013) CB(1) receptor allosteric modulators display both agonist and signaling pathway specificity. Mol Pharmacol 83:322–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, Pryce G, Davies WL, Hiley CR (2006) In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol. Sci. [DOI] [PubMed] [Google Scholar]
- Bari M, Battista N, Fezza F, et al. (2005) Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells. J Biol Chem 280:12212–12220 [DOI] [PubMed] [Google Scholar]
- Barnett-Norris J, Lynch D, Reggio PH (2005) Lipids, lipid rafts and caveolae: their importance for GPCR signaling and their centrality to the endocannabinoid system. Life Sci 77:1625–1639 [DOI] [PubMed] [Google Scholar]
- Barrus DG, Lefever TW, Wiley JL (2018) Evaluation of reinforcing and aversive effects of voluntary Δ9-tetrahydrocannabinol ingestion in rats. Neuropharmacology 137:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Chicca A, Tamborrini M, et al. (2012) Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. J Biol Chem 287:36944–36967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Scimeca JA, Martin BR (1987) Studies on the agonistic activity of delta 9–11-tetrahydrocannabinol in mice, dogs and rhesus monkeys and its interactions with delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther 241:521–6 [PubMed] [Google Scholar]
- Ben-Shabat S, Fride E, Sheskin T, et al. (1998) An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol 353:23–31 [DOI] [PubMed] [Google Scholar]
- Benard G, Massa F, Puente N, et al. (2012) Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nat Neurosci 15:558–564 [DOI] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, et al. (2003) Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol 163:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Melck D, Gretskaya NM, et al. (2000) N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J 351:817–824 [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Cravatt BF (2013) Chemical probes of endocannabinoid metabolism. Pharmacol Rev 65:849–71. doi: 10.1124/pr.112.006387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF (2007) A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol 14:1347–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner TI (1996) Molecular biology of cannabinoid receptors. J Neuroimmunol 69:15–17 [Google Scholar]
- Braida D, Losue S, Pegorini S, Sala M (2004) Delta9-tetrahydrocannabinol-induced conditioned place preference and introcerebroventricular self-administration in rats. Eur J Pharmacol 506:63–69 [DOI] [PubMed] [Google Scholar]
- Braida D, Pozzi M, Parolaro D, Sala M (2001) Intracerebral self-administration of the cannabinoid receptor agonist CP 55,940 in the rat: interaction with the opiod system. Eur J Pharmacol 413:227–234 [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Puri V, Lambert JM, et al. (2013) The influence of beta-arrestin2 on cannabinoid CB1 receptor coupling to G-proteins and subcellular localization and relative levels of beta-arrestin1 and 2 in mouse brain. J Recept Signal Transduct Res 33:367–379 [DOI] [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey É, et al. (2000) Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. Eur J Pharmacol 396:141–9. doi: 10.1016/S0014-2999(00)00211-9 [DOI] [PubMed] [Google Scholar]
- Cairns EA, Szczesniak AM, Straiker AJ, et al. (2017) The in vivo effects of the CB1-positive allosteric modulator GAT229 on intraocular pressure in ocular normotensive and hypertensive mice. J Ocul Pharmacol Ther 33:582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila JH, Falck JR, Imig JD (2007) Roles of the cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Int 72:683–689 [DOI] [PubMed] [Google Scholar]
- Celofiga A, Koprivsek J, Klavz J (2014) Use of synthetic cannabinoids in patients with psychotic disorders: case series. J Dual Diagn 10:168–173 [DOI] [PubMed] [Google Scholar]
- Chakrabarti A, Onaivi ES, Chaudhuri G (1995) Cloning and sequencing of a cDNA encoding the mouse brain-type cannabinoid receptor protein. DNA Seq 5:385–388 [DOI] [PubMed] [Google Scholar]
- Chen JK, Chen J, Imig JD, et al. (2008) Identification of novel endogenous cytochrome p450 arachidonate metabolites with high affinity for cannabinoid receptors. J Biol Chem 283:24514–24524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zheng C, Qian J, et al. (2014) Involvement of beta-arrestin-2 and clathrin in agonist-mediated internalization of the human cannabinoid CB2 receptor. Curr Mol Pharmacol 7:67–80 [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Haller VL, Welch SP (2001) Changes in opioid and cannabinoid receptor protein following short-term combination treatment with delta(9)-tetrahydrocannabinol and morphine. J Pharmacol Exp Ther 297:121–7 [PubMed] [Google Scholar]
- Cichewicz DL, Martin ZL, Smith FL, Welch SP (1999) Enhancement mu opioid antinociception by oral delta9-tetrahydrocannabinol: dose-response analysis and receptor identification. J Pharmacol Exp Ther 289:859–67 [PubMed] [Google Scholar]
- Cichewicz DL, Welch SP, Smith FL (2005) Enhancement of transdermal fentanyl and buprenorphine antinociception by transdermal Δ9-tetrahydrocannabinol. Eur J Pharmacol 525:74–82. doi: 10.1016/j.ejphar.2005.09.039 [DOI] [PubMed] [Google Scholar]
- Clarke R, Merlin M (2013) Cannabis: evolution and ethnobotany. University of California Press, Berkeley, CA [Google Scholar]
- Coleman RA, Haynes EB (1986) Monoacylglycerol acyltransferase. Evidence that the activities from rat intestine and suckling liver are tissue-specific isoenzymes. J Biol Chem 261:224–228 [PubMed] [Google Scholar]
- Compton DR, Gold LH, Ward SJ, et al. (1992a) Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther 263:1118–26 [PubMed] [Google Scholar]
- Compton DR, Johnson MR, Melvin LS, Martin BR (1992. b) Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J Pharmacol Exp Ther 260:201–9 [PubMed] [Google Scholar]
- Compton DR, Martin BR (1990) Pharmacological evaluation of water soluble cannabinoids and related analogs. Life Sci 46:1575–85. doi: 10.1016/0024-3205(90)90391-4 [DOI] [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, et al. (1993) Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther 265:218–26 [PubMed] [Google Scholar]
- Console-Bram L, Marcu J, Abood ME (2012) Cannabinoid receptors: nomenclature and pharmacological principles. Prog Neuropsychopharmacol Biol Psychiatry 38:4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox ML, Haller VL, Welch SP (2007) Synergy between delta9-tetrahydrocannabinol and morphine in the arthritic rat. Eur J Pharmacol 567:125–130 [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, et al. (2001) Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A 98:9371–9376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, et al. (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87 [DOI] [PubMed] [Google Scholar]
- Cridge BJ, Rosengren RJ (2013) Critical appraisal of the potential use of cannabinoids in cancer management. Cancer Manag Res 5:301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle TL, Kearn CS, Mackie K (2008) Rapid CB1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology 54:36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton GD, Howlett AC (2012) Cannabinoid CB1 receptors transactivate multiple receptor tyrosine kinases and regulate serine/threonine kinases to activate ERK in neuronal cells. Br J Pharmacol 165:2497–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Ferrari AJ, Calabria B, et al. (2013) The global epidemiology and contribution of Cannabis use and dependence to the global burden of disease: results from the GBD 2010 study. PLoS One 8:e76635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Dysarz III FA, Johnson MR, et al. (1988) Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34:605–613 [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, et al. (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science (80- ) 258:1946–1949 [DOI] [PubMed] [Google Scholar]
- Di Marzo V (2018) New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, Sugiura T, et al. (1998) The novel endogenous cannabinoid 2-arachidonoylglycerol is inactivated by neuronal- and basophil-like cells: connections with anandamide. Biochem J 331:15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Alonso J, Guzman M, Galve-Roperh I (2012) Endocannabinoids via CB₁ receptors act as neurogenic niche cues during cortical development. Philos Trans R Soc Lond B Biol Sci 367:32293241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, et al. (2002) Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A 99:10819–10824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donvito G, Nass SR, Wilkerson JL, et al. (2018) The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology 43:52–79. doi: 10.1038/npp.2017.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertova M, Giang DK, Cravatt BF, Elphick MR (1998) A new perspective on cannabinoid signalling: complementary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc Natl Acad Sci U S A 265:2081–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldeeb K, Leone-Kabler S, Howlett AC (2016) CB1 cannabinoid receptor-mediated increases in cyclic AMP accumulation are correlated with reduced Gi/o function. J Basic Clin Physiol Pharmacol 27: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldeeb K, Leone-Kabler S, Howlett AC (2017) Mouse neuroblastoma CB1 cannabinoid receptor-stimulated [(35)S]GTPS binding: total and antibody-targeted Galpha protein-specific scintillation proximity assays. Methods Enzymol 593:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSohly MA, Radwan MM, Gul W, et al. (2017) Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. [DOI] [PubMed] [Google Scholar]
- Fallon MT, Albert Lux E, McQuade R, et al. (2017) Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: two double-blind, randomized, placebo-controlled phase 3 studies. Br J Pain 11:119–33. doi: 10.1177/2049463717710042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta CM, Fratta W (2001) Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212–2 in rats. Psychopharmacology (Berl) 156:410–416 [DOI] [PubMed] [Google Scholar]
- Fay X, Farrens Y (2015) Structural dynamics and energetics underlying allosteric inactivation of the cannabinoid receptor CB1. Proc Natl Acad Sci U S A 112:8469–8474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Sanchez IJ, Verpoorte R (2008) Secondary metabolism in Cannabis. Phytochem Rev 7:615–639 [Google Scholar]
- Ford BM, Tai S, Fantegrossi WE, Prather PL (2017) Synthetic pot: not your grandfather’s marijuana. Trends Pharmacol Sci 38:257–276. doi: 10.1016/j.tips.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JM, Vasiljevik T, Prisinzano TE, Carrasco GA (2013) Cannabinoid agonists increase the interaction between beta-arrestin 2 and ERK1/2 and upregulate beta-arrestin 2 and 5-HT(2A) receptors. Pharmacolgical Res 68:46–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MJ, Rose DZ, Myers MA, et al. (2013) Ischemic stroke after use of the synthetic marijuana “spice.” Neurology 81:2090–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffuri AL, Ladarre D, Lenkei Z (2012) Type-1 cannabinoid receptor signaling in neuronal development. Pharmacology 90:19–39 [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, et al. (1995) Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem 232:54–61 [DOI] [PubMed] [Google Scholar]
- Gamage TF, Ignatowska-jankowska BM, Wiley JL, et al. (2014) In-vivo pharmacological evaluation of the CB1-receptor allosteric modulator Org-27569. Behav Pharmacol 25:182–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R (1964) Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc 86:1646–7. doi: 10.1021/ja01062a046 [DOI] [Google Scholar]
- Garcia AB, Soria-Gomez E, Bellocchio L, Marsicano G (2016) Cannabinoid receptor type-1: breaking the dogmas. F1000 Fac Rev 5:990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard CM, Mollereau C, Vassart G, Parmentier M (1991) Molecular-cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J 279:129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerostamoulos D, Drummer OH, Woodford NW (2015) Deaths linked to synthetic cannabinoids. Forensic Sci Med Pathol 11:478. [DOI] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL (1997) Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77:299–318 [DOI] [PubMed] [Google Scholar]
- Glass M, Northup JK (1999) Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol Pharmacol 56:1362–1369 [DOI] [PubMed] [Google Scholar]
- Godlewski G, Offertaler L, Wagner JA, Kunos G (2009) Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat 89:3–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim TW, Morales AJ, Gonek MM, et al. (2016) Stratification of cannabinoid 1 receptor (CB1R) agonist efficacy: manipulation of CB1R density through use of transgenic mice reveals congruence between in vivo and in vitro assays. J Pharmacol Exp Ther 359:329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim TW, Morales AJ, Thomas BF, et al. (2017) Apparent CB1 receptor rimonabant affinity estimates: combination with THC and synthetic cannabinoids in the mouse in vivo triad model. J Pharmacol Exp Ther 362:210–218. doi: 10.1124/jpet.117.240192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhermen F, Muller-Vahl K (2012) The therapeutic potential of cannabis and cannabinoids. Dtsch Arztebl Int 109:495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, et al. (2004) Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci 20:441–458 [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY, Elemes MW, et al. (2018) Fatty-acid-binding protein 5 controls retrograde endocannabinoid signaling at central glutamate synapses. Proc Natl Acad Sci U S A 115:3482–3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson AJ, Hill WA, Zan-Phillips M, et al. (1995) Anandamide hydroxylation by brain lipoxygenase: metabolite structures and potencies at the cannabinoid receptor. Biochim Biophys Acta 1259:173–179 [DOI] [PubMed] [Google Scholar]
- Han J, Kesner P, Metna-Laurent M, et al. (2012) Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148:1039–1050 [DOI] [PubMed] [Google Scholar]
- Hanus L, Abu-Lafi S, Fride E, et al. (2001) 2-arachidonyl glycerol ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci U S A 98:3662–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Kerridge BT, Saha TD, et al. (2016) Prevalence and correlates of DSM-5 Cannabis use disorder, 2012–2013: findings from the national epidemiologic survey on alcohol and related conditions–III. Am J Psychiatry 173:588–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler JA, Estabrook RMM, Pikuleva IA, et al. (1999) Human cytochromes P450. Mol Aspects Med 20:1–137 [DOI] [PubMed] [Google Scholar]
- Heimann AS, Gomes I, Dale CS, et al. (2007) Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci U S A 104:20588–20593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel BJ, Wakeford AG, Clasen MM, et al. (2016) Delta-9-tetrahydrocannabinol (THC) history fails to affect THC’s ability to induce place preferences in rats. Pharmacol Biochem Behvaior 144:1–6 [DOI] [PubMed] [Google Scholar]
- Henriksson BG, Johansson JO, Järbe TUC (1975) Δ9-tetrahydrocannabinol produced discrimination in pigeons. Pharmacol Biochem Behav 5:771–4. doi: 10.1016/0091-3057(75)90105-7 [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, et al. (1990) Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A 87:1932–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs HN, Glomset JA (1994) Identification of a phosphatidic acid-preferring phospholipase A1 from bovine brain and testis. Proc Natl Acad Sci U S A 91:9574–9578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ (2000) Biochemistry and pharmacology of the endocannabinoids arachidonylethanolamide and 2-arachidonylglycerol. Prostaglandins Other Lipid Mediat 61:3–18 [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Harris RA, Bloom AS (1985) Effects of the cannabinoids on physical properties of brain membranes and phospholipid vesicles: fluorescence studies. J Pharmacol Exp Ther 232:579–588 [PubMed] [Google Scholar]
- Hillard CJ, Pounds JJ, Boyer DR, Bloom AS (1990) Studies of the role of membrane lipid order in the effects of delta 9-tetrahydrocannabinol on adenylate cyclase activation in heart. J Pharmacol Exp Ther 252:1075–1082 [PubMed] [Google Scholar]
- Hiratsuka M, Nozawa H, Katsumoto Y, et al. (2006) Genetic polymorphisms and haplotype structures of the CYP4A22 gene in a Japanese population. Mutat Res 599:98–104 [DOI] [PubMed] [Google Scholar]
- Ho BY, Zhao J (1996) Determination of the cannabinoid receptors in mouse x rat hybridoma NG108–15 cells and rat GH4C1 cells. Neurosci Lett 212:123–126 [DOI] [PubMed] [Google Scholar]
- Horswill JG, Bali U, Shaaban S, et al. (2007) PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB1 receptors with hypophagic effects in rats. Br J Pharmacol 2:805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DB, Howlett AC (1993) Solubilization of the cannabinoid receptor from rat brain and its functional interaction with guanine nucleotide-binding proteins. Mol Pharmacol 43:17–22 [PubMed] [Google Scholar]
- Howlett AC, Abood ME (2017) CB1 and CB2 receptor pharmacology. Adv Pharmacol 80:169–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, et al. (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54:161–202 [DOI] [PubMed] [Google Scholar]
- Howlett AC, Johnson MR, Melvin LS, Milne GM (1988) Nonclassical cannabinoid analgetics inhibit adenylate cyclase: development of a cannabinoid receptor model. Mol Pharmacol 33:297–302 [PubMed] [Google Scholar]
- Howlett AC, Qualy JM, Khachatrian LL (1986) Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol 29:307–313 [PubMed] [Google Scholar]
- Hsu KL, Tsuboi K, Adibekian A, et al. (2012) DAGLβ inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat Chem Biol 8:999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JW, Zengin G, Wu MJ, et al. (2005) Structure–activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB1 and CB2 receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB2 receptor agonists. Bioorg Med Chem 13:89–112 [DOI] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Baillie GL, Kinsey S, et al. (2015) A cannabinoid CB1 receptor-positive allosteric modulator reduces neuropathic pain in the mouse with no psychoactive effects. Neuropsychopharmacology 40:2948–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyasov AA, Milligan CE, Pharr EP, Howlett AC (2018) The endocannabinoid system and oligodendrocytes in health and disease. Front Neurosci 12:733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagla CAD, Scott CE, Tang Y, et al. (2019) Primidinyl bipheylureas act as allosteric modulators to activate cannabinoid receptor 1 and initiate B-arrestin-dependent responses. Mol Pharmacol 95:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU, Henriksson BG, Ohlin GC (1977) Delta9-THC as a discriminative cue in pigeons: effects of delta8-THC, CBD, and CBN. ArchIntPharmacodynTher 228:68–72 [PubMed] [Google Scholar]
- Järbe TUC, Gifford RS (2014) “Herbal incense”: Designer drug blends as cannabimimetics and their assessment by drug discrimination and other in vivo bioassays. Life Sci 97:64–71. doi: 10.1016/j.lfs.2013.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TUC, Gifford RS, Zvonok A, Makriyannis A (2016a) Δ9-Tetrahydrocannabinol discriminative stimulus effects of AM2201 and related aminoalkylindole analogs in rats. Behav Pharmacol 27:211–4. doi: 10.1097/FBP.0000000000000196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- JÄrbe TUC, Henriksson BG (1974) Discriminative response control produced with hashish, tetrahydrocannabinols (δ8-THC and δ9-THC), and other drugs. Psychopharmacologia. doi: 10.1007/BF00429443 [DOI] [PubMed] [Google Scholar]
- JÄrbe TUC, Henriksson BG (1973) Acute effects of two tetrahydrocannabinols (Δ9-THC and Δ8-THC) on water intake in water deprived rats: Implications for behavioral studies on marijuana compounds. Psychopharmacologia 30:315–22. doi: 10.1007/BF00429190 [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Lemay BJ, Halikhedkar A, et al. (2014) Differentiation between low- and high-efficacy CB 1 receptor agonists using a drug discrimination protocol for rats. Psychopharmacology (Berl) 231:489–500. doi: 10.1007/s00213-013-3257-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TUC, Lemay BJ, Thakur GA, Makriyannis A (2016b) A high efficacy cannabinergic ligand (AM4054) used as a discriminative stimulus: Generalization to other adamantyl analogs and Δ 9 -THC in rats. Pharmacol Biochem Behav 148:46–52. doi: 10.1016/j.pbb.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HE, Zhao YX, Ferguson DK, et al. (2006) A new insight into Cannabis sativa (Cannabaceae) utilization from 2500-year-old Yanghai Tombs, Xinjiang, China. J Ethnopharmacol 108:414–422 [DOI] [PubMed] [Google Scholar]
- Jing L, Qiu Y, Zhang Y, Li JX (2014) Effects of the cannabinoid CB1 receptor allosteric modulator ORG 27569 on reinstatement of cocaine-and methamphetamine-seeking behavior in rats. Drug Alcohol Depend 143:251–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John WS, Martin TJ, Nader MA (2017) Behavioral determinants of cannabinoid self-administration in old world monkeys. Neuropsychopharmacology 42:1522–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR, Burnell-Nugent M, Lossignol D, et al. (2010) Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manag 39: [DOI] [PubMed] [Google Scholar]
- Jonsson KO, Vandevoorde SV, Lambert DM, et al. (2001) Effects of homologues and analogues of palmitoylethanolamide upon the inactivation of the endocannabinoid anandamide. Br J Pharmacol 133:1263–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR (2003) Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 169:135–140 [DOI] [PubMed] [Google Scholar]
- Kano M (2014) Control of synaptic function by endocannabinoid-mediated retrograde signaling. Proc Japan Acad Ser B, Phys Biol Sci 90:235–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh H, Iwata T, Ono T, Suzuki T (1986) Immunological characterization of sn-1,2-diacylglycerol and sn-2-monoacylglycerol kinase from pig brain. J Biol Chem 261:5597–5602 [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, et al. (1999) Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19:4544–4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keimpema E, Mackie K, Harkany T (2011) Molecular model of cannabis sensitivity in developing neuronal circuits. Trends Pharmacol Sci 32:551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana L, Mackie K, Piomelli D, Kendall DA (2017) Modulation of CB1 cannabinoid receptor by allosteric ligands: pharmacological and therapeutic opportunities. Neuropharmacology 124:3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Kondo H, Nakane S, et al. (1998) 2-Arachidonoylglycerol, an endogenous cannabinoid receptor agonist: identification as one of the major species of monoacylglycerols in various rat tissues, and evidence for its generation through CA2+-dependent and -independent mechanisms. FEBS Lett 429:152–156 [DOI] [PubMed] [Google Scholar]
- Kozak KR, Crews BC, Ray JL, et al. (2001) Metabolism of prostaglandin glycerol esters and prostaglandin ethanolamides in vitro and in vivo. J Biol Chem 276:36993–36998 [DOI] [PubMed] [Google Scholar]
- Kozak KR, Rowlinson SW, Marnett LJ (2000) Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem 275:33744–33749 [DOI] [PubMed] [Google Scholar]
- Laprairie R, Bagher A, Kelly M, Denovan-Wright EM (2015) Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol 172:4790–4805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner JE, Hille B, Mackie K (2005) The cannabinoid agonist WIN55,212–2 increases intracellular calcium via CB1 receptor coupling to G(q/11) G proteins. Proc Natl Acad Sci U S A 102:19144–19149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DK, Gill EW (1975) The effects of delta1-tetrahydrocannabinol and other cannabinoids on spin-labeled liposomes and their relationship to mechanisms of general anesthesia. Mol Pharmacol 11:595–602 [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, et al. (1999) Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science (80- ) 283:401–4. doi: 10.1126/science.283.5400.401 [DOI] [PubMed] [Google Scholar]
- Lee MA (2013) Smoke signals: a social history of marijuana - medical, recreational and scientific. Scribner, New York, NY [Google Scholar]
- Lefever TW, Marusich JA, Antonazzo KR, Wiley JL (2014) Evaluation of WIN 55,212–2 self-administration in rats as a potential cannabinoid abuse liabity model. Pharmacol Biochem Behvaior 118:30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D, Saghatelian A, Simon GM, Cravatt BF (2006) Inactivation of N-Acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry 45:4720–6. doi: 10.1021/bi060163l [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Lux EA, McQuade R, et al. (2018) Results of a double-blind, randomized, placebo-controlled study of nabiximols oromucosal spray as an adjunctive therapy in advanced cancer patients with chronic uncontrolled pain. J Pain Symptom Manage 55:179–188. doi: 10.1016/j.jpainsymman.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Wiley JL, Lavecchia KL, et al. (1998) Effects of SR 141716A after acute or chronic cannabinoid administration in dogs. Eur J Pharmacol 357:139–48. doi: 10.1016/S0014-2999(98)00558-5 [DOI] [PubMed] [Google Scholar]
- Ligresti A, De PL, Di MV (2016) From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol Rev 96:1593–1659 [DOI] [PubMed] [Google Scholar]
- Little PJ, Compton DR, Johnson MR, et al. (1988) Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther 247:1046–51 [PubMed] [Google Scholar]
- Little PJ, Compton DR, Mechoulam R, Martin BR (1989) Stereochemical effects of 11-OH-Δ8-THC-dimethylheptyl in mice and dogs. Pharmacol Biochem Behav 661–6. doi: 10.1016/0091-3057(89)90014-2 [DOI] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, et al. (2006) A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A 103:13345–13350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HC, Mackie K (2016) An introduction to the endogenous cannabinoid system. Biol Psychiatry 79:516–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM (2014) More than just a hammer: ligand “Bias” and pharmaceutical discovery. Mol Endocrinol 28:281–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon RC, McComb J a, Schreurs J, Goldstein DB (1981) A relationship between alcohol intoxication and the disordering of brain membranes by a series of short-chain alcohols. J Pharmacol Exp Ther 218:669–75 [PubMed] [Google Scholar]
- Maccarrone M, Guzman M, Mackie K, et al. (2014) Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat Rev Neurosci 15:786–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Salvati S, Bari M, Finazzi A (2000) Anandamide and 2-arachidonoylglycerol inhibit fatty acid amide hydrolase by activating the lipoxygenase pathway of the arachidonate cascade. Biochem Biophys Res Commun 278:576–583 [DOI] [PubMed] [Google Scholar]
- Mackie K (2006a) Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol 46:101–122 [DOI] [PubMed] [Google Scholar]
- Mackie K (2006b) Mechanisms of CB1 receptor signaling: endocannabinoid modulation of synaptic strength. Int J Obes 30:S19–S23 [DOI] [PubMed] [Google Scholar]
- Maguire DR, Yang W, France CP (2013) Interactions between mu-Opioid Receptor Agonists and Cannabinoid Receptor Agonists in Rhesus Monkeys: Antinociception, Drug Discrimination, and Drug Self-Administration. J Pharmacol Exp Ther 345:354–62. doi: 10.1124/jpet.113.204099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahavadi S, Sriwai W, Huang J, et al. (2014) Inhibitory signaling by CB1 receptors in smooth muscle mediated by GRK5/beta-arrestin activation of ERK1/2 and Src kinase. Am J Physiol Gastrointest Liver Physiol 306:G535–G545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR (1986) Cellular effects of cannabinoids. Pharmacol Rev 38:45–74 [PubMed] [Google Scholar]
- Martin BR, Dewey WL, Harris LS, et al. (1975) Marihuana like activity of new synthetic tetrahydrocannabinols. Pharmacol Biochem Behav 5:849–53. doi: 10.1016/S0090-3752(76)80023-3 [DOI] [PubMed] [Google Scholar]
- Martin BR, Jeanne Kallman M, Kaempf GF, et al. (1984) Pharmacological potency of R- and S-3′-hydroxy-Δ9-tetrahydrocannabinol: additional structural requirement for cannabinoid activity. Pharmacol Biochem Behav 21:61–5. doi: 10.1016/0091-3057(84)90131-X [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, et al. (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564 [DOI] [PubMed] [Google Scholar]
- McAllister SD, Glass M (2002) CB(1) and CB(2) receptor-mediated signalling: a focus on endocannabinoids. Prostaglandins Leukot Essent Fatty Acids 66:161–171 [DOI] [PubMed] [Google Scholar]
- McPartland JM, Glass M, Pertwee RG (2007) Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br J Pharmacol 152:583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, et al. (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90 [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Hanus LO, Pertwee R, Howlett AC (2014) Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat Rev Neurosci 15:757–764 [DOI] [PubMed] [Google Scholar]
- Mechoulam R, McCallum N, Burstein S (1976) Recent advances in the chemistry and biochemistry of cannabis. Chem Rev 76:75–112 [Google Scholar]
- Mechoulam R, Shvo Y (1963) Hashish I. The structure of cannabidiol. Tetrahedron 19:2073–2078. doi: 10.1016/0040-4020(63)85022-X [DOI] [PubMed] [Google Scholar]
- Melck D, Bisogno T, De Petrocellis L, et al. (1999) Unsaturated long-chain N-acyl-vanillyl-amides (N-AVAMs): vanilloid receptor ligands that inhibit anandamide-facilitated transport and bind to CB1 cannabinoid receptors. Biochem Biophys Res Commun 262:275–284 [DOI] [PubMed] [Google Scholar]
- Mendizabal V, Zimmer A, Maldonado R (2006) Involvement of kappa/dynorphin system in WIN 55, 212–2 self-administration in mice. Neuropsychopharmacology 31:1957–1966 [DOI] [PubMed] [Google Scholar]
- Monti L, Steanucci A, Pieretti S, et al. (2016) Evaluation of the analgesic effect of 4-anilidopiperidine scaffold containing ureas and carbamates. J Enzym Inhib Med Chem 31:1638–1647 [DOI] [PubMed] [Google Scholar]
- Motaghedi R, Lipman EG, Hogg JE, et al. (2011) Psychiatric adverse effects of Rimonobant in adults with Prader-Willi syndrome. Eur J Med 54:14–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Howlett AC (2001) CB1 receptor-G protein association. subtype selectivity is determined by distinct intracellular domains. Eur J Biochem 268:499–505 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Howlett AC (2005) Chemically distinct ligands promote differential CB1 cannabinoid receptor-Gi protein interactions. Mol Pharmacol 67:2016–2024 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, McIntosh HH, Houston DB, Howlett AC (2000) The CB(1) cannabinoid receptor juxtamembrane C-terminal peptide confers activation to specific G proteins in brain. Mol Pharmacol 57:162–170 [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65 [DOI] [PubMed] [Google Scholar]
- Murataeva N, Straiker A, Mackie K (2014) Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br J Pharmacol 171:1379–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane S, Oka S, Arai S, et al. (2002) 2-Arachidonoyl-sn-glycero-3-phosphate, an arachidonic acid-containing lysophosphatidic acid: occurrence and rapid enzymatic conversion to 2-arachidonoyl-sn-glycerol, a cannabinoid receptor ligand, in rat brain. Arch Biochem Biophys 402:51–58 [DOI] [PubMed] [Google Scholar]
- Navarro HA, Howard JL, Pollard GT, Carroll F (2009) Positive allosteric modulation of the human cannabinoid (CB1) receptor by RTI-371, a selective inhibitor of the dopamine transporter. Br J Pharmacol 156:1178–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS (2019) Core outcome measures in preclinical assessment of candidate analgesics. Pharmacol Rev 71:225–266. doi: 10.1124/pr.118.017210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS (2018) Addressing the opioid crisis: the importance of choosing translational endpoints in analgesic drug discovery. Trends Pharmacol Sci 39:327–30. doi: 10.1016/j.tips.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Sabioni P, Trigo JM, et al. (2017) Opioid-sparing effect of cannabinoids: a systematic review and meta-analysis. Neuropsychopharmacology 42:1752–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueras-Ortiz C, Yudowski GA (2016) The multiple waves of cannabinoid 1 receptor signaling. Mol Pharmacol 90:620–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Morrison BE, Blankman JL, et al. (2011) Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science (80- ) 334:809–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan SE, Kendall DA (2010) Cannabinoid activation of peroxisome proliferator-activated receptors: Potential for modulation of inflammatory disease. Immunobiology 215:611–616 [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, et al. (2004) Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem 279:5298–5305 [DOI] [PubMed] [Google Scholar]
- Okazaki H, Kobayashi M, Momohara A, et al. (2011) Early Holocene coastal environment change inferred from deposits at Okinoshima archeological site, Boso Peninsula, Central Japan. Quat Int 230:87–94 [Google Scholar]
- Oliveira da Cruz JF, Robin LM, Drago F, et al. (2016) Astroglial type-1 cannabinoid receptor (CB1): A new player in the tripartite synapse. Neuroscience 323:35–42 [DOI] [PubMed] [Google Scholar]
- Owens RA, Mustafa MA, Ignatowska-Jankowska BM, et al. (2017) Inhibition of the endocannabinoid-regulating enzyme monoacylglycerol lipase elicits a CB1 receptor-mediated discriminative stimulus in mice. Neuropharmacology 125:80–86. doi: 10.1016/j.neuropharm.2017.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58:389–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Ferreira J, Menezes de Lima O, et al. (2012) Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc Natl Acad Sci U S A 109:21134–21139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pars HG, Granchelli FE, Razdan RK, et al. (1976) Drugs derived from cannabinoids. 1. nitrogen analogs, benzopyranopyridines and benzopyranopyrroles. J Med Chem. doi: 10.1021/jm00226a001 [DOI] [PubMed] [Google Scholar]
- Pertwee RG (2015) Endocannabinoids and their pharmacological actions. Handb Exp Pharmacol 231:1–37. doi: 10.1007/978-3-319-20825-1_1 [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, et al. (2010) International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB and CB. Pharmacol Rev 62:588–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikuleva IA, Waterman MR (2013) Cytochromes p450: roles in diseases. J Biol Chem 288:17091–17098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineiro R, Falasca M (2012) Lysophosphatidylinositol signalling: new wine from an old bottle. Biochim Biophys Acta 1821:694–705 [DOI] [PubMed] [Google Scholar]
- Pisanti S, Bifulco M (2019) Medical Cannabis: A plurimillennial history of an evergreen. J Cell Physiol 234:8342–8351 [DOI] [PubMed] [Google Scholar]
- Porter AC, Sauer JM, Knierman MD, et al. (2002) Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther 301:1020–1024 [DOI] [PubMed] [Google Scholar]
- Prather PL, Martin NA, Breivogel CS, Childers SR (2000) Activation of cannabinoid receptors in rat brain by WIN 55212–2 produces coupling to multiple G protein alpha-subunits with different potencies. Mol Pharmacol 57:1000–1010 [PubMed] [Google Scholar]
- Prescott SM, Majerus PW (1983) Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoylmonoacylglycerol intermediate. J Biol Chem 258:764–769 [PubMed] [Google Scholar]
- Price MR, Baillie GL, Thomas A, et al. (2005) Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol 68:1484–1495 [DOI] [PubMed] [Google Scholar]
- Priestly RS, Nickolls SA, Alexander SP, Kendall DA (2015) A potential role for cannabinoid receptors in the therapeutic action of fenofibrate. FASEB 29: [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, et al. (2009) Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci 12:1152–1158 [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, et al. (1994) SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350:240–4. doi: 10.1016/0014-5793(94)00773-X [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, et al. (1998) SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther 284:644–50 [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Calandra B, Shire D, et al. (1996) Characterization of two cloned human CB1 cannabinoid receptor isoforms. J Pharmacol Exp Ther 278:871–878 [PubMed] [Google Scholar]
- Rubino T, Vigano D, Premoli F, et al. (2006) Changes in the expression of G protein-coupled receptor kinases and beta-arrestins in mouse brain during cannabinoid tolerance: a role for RAS-ERK cascade. Mol Neurobiol 33:199–213 [DOI] [PubMed] [Google Scholar]
- Ryberg E, Vu HK, Larsson N, et al. (2005) Identification and characterisation of a novel splice variant of the human CB1 receptor. FEBS Lett 579:259–264 [DOI] [PubMed] [Google Scholar]
- SAMHSA (2017) Results from the 2016 national survey on drug use and health: detailed tables. Prevalence estimates, standard Errors, P Values, and sample sizes. In: Subst. Abus. Ment. Heal. Serv. Adm. Cent. Behav. Heal. Stat. Qual. Rockv. [Google Scholar]
- Seeling W, Kneer L, Buchele B, et al. (2006) DELTA9-tetrahydrocannabinol and the opioid receptor agonist piritramide do not act synergistically in postoperative pain. Anaesthesist 55:391–400 [DOI] [PubMed] [Google Scholar]
- Seeman P (1972) The membrane actions of anesthetics and tranquilizers. Pharmacol Rev [PubMed] [Google Scholar]
- Setchell KD, Schwarz M, O’Connell NC, et al. (1998) Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7α-hydroxylase gene causes severe neonatal liver disease. J Clin Invest 102:1690–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shire D, Carillon C, Kaghad M, et al. (1995) An amino-terminal variant of the central cannabinoid receptor resulting from alternative splicing. J Biol Chem 270:3726–3731 [DOI] [PubMed] [Google Scholar]
- Siegel GJ (1999) Synaptic transmission and cellular signaling: an overview. In: Agranoff MD, Albers BW, Fisher RW, Uhler SK (eds) Basic Neurochemistry. Lippincott-R, Philadelphia, AP [Google Scholar]
- Sigel E, Baur R, Racz I, et al. (2011) Themajor central endocannabinoid directly acts at GABA(A) receptors. Proc Natl Acad Sci U S A 108:18150–18155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF (2006) Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem 281:26465–26472 [DOI] [PubMed] [Google Scholar]
- Slivicki RA, Saberi SA, Iyer V, et al. (2018a) Brain-permeant and -impermeant inhibitors of fatty acid amide hydrolase synergize with the opioid analgesic morphine to suppress chemotherapy-induced neuropathic Nociception Without enhancing effects of morphine on gastrointestinal transit. J Pharmacol Exp Ther 367:551–563. doi: 10.1124/jpet.118.252288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivicki RA, Xu Z, Kulkarni PM, et al. (2018b) Positive allosteric modulation of cannabinoid receptor type 1 suppresses pathological pain without producing tolerance or dependence. Biol Psychiatry 84:722–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FL, Cichewicz D, Martin ZL, Welch SP (1998) The enhancement of morphine antinociception in mice by Δ9- tetrahydrocannabinol. Pharmacol Biochem Behav 60:559. doi: 10.1016/S0091-3057(98)00012-4 [DOI] [PubMed] [Google Scholar]
- Snider NT, Walker VJ, Hollenberg PF (2010) Oxidation of the endogenous cannabinoid arachidonoyl ethanolamide by the cytochrome P450 monooxygenases: physiological and pharmacological implications. Pharmacol Rev 62:136–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soethoudt M, Grether U, Fingerle J, et al. (2017) Cannabinoid CB 2 receptor ligand profiling reveals biased signalling and off-target activity. Nat Commun 8:13958. doi: 10.1038/ncomms13958. doi: 10.1038/ncomms13958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Panlilio L V., Justinova Z, et al. (2006) Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc. doi: 10.1038/nprot.2006.167 [DOI] [PubMed] [Google Scholar]
- Stella N (2010) Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 58:1017–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Wager-Miller J, Hutchens J, Mackie K (2012) Differential signalling in human cannabinoid CB1 receptors and their splice variants in autaptic hippocampal neurones. Br J Pharmacol 165:2660–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, et al. (1995) 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215:89–97 [DOI] [PubMed] [Google Scholar]
- Sun YX, Tsuboi K, Okamoto Y, et al. (2004) Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem J 380:749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Schlicker E (2005) Effects of cannabinoids on neurotransmission. Handb Exp Pharmacol 168:327–365 [DOI] [PubMed] [Google Scholar]
- Tanda G (2016) Preclinical studies on the reinforcing effects of cannabinoids. A tribute to the scientific research of Dr. Steve Goldberg. Psychopharmacology (Berl) 233:1845–66. doi: 10.1007/s00213-016-4244-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasov P, Bezrukova E, Karabanov E, et al. (2007) Vegetation and climate dynamics during the Holocene and Eemian interglacials derived from Lake Baikal pollen records. Palaeogeogr Palaeoclim Palaeoecol 252:440–457 [Google Scholar]
- Thomas BF, Adams IB, Mascarella SW, et al. (1996) Structure-activity analysis of anandamide analogs: Relationship to a cannabinoid pharmacophore. J Med Chem 39:471–9. doi: 10.1021/jm9505167 [DOI] [PubMed] [Google Scholar]
- Thomas BF, Compton DR, Martin BR (1990) Characterization of the lipophilicity of natural and synthetic analogs of delta 9-tetrahydrocannabinol and its relationship to pharmacological potency. J Pharmacol Exp Ther 255:624–30 [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, et al. (1998) Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83:393–411 [DOI] [PubMed] [Google Scholar]
- Turu G, Hunyady L (2010) Signal transduction of the CB1 cannabinoid receptor. J Mol Endocrinol 44:75–85 [DOI] [PubMed] [Google Scholar]
- Vallee M, Vitiello S, Bellocchio L, et al. (2014) Pregnenolone can protect the brain from cannabis intoxication. Science (80- ) 343:94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeford AGP, Wetzell BB, Pomfrey RL, et al. (2017) The effects of cannabidiol (CBD) on Delta(9)-tetradydrocannabinol (THC) self-administration in male and female Long-Evans rats. Exp Clin Psychopharmacol 25:242–248 [DOI] [PubMed] [Google Scholar]
- Walton RP, Martin LF, Keller JH (1938) The relative activity of various purified products obtained from American hashish. J Exp Pharmacol Exp Ther 62:239–251 [Google Scholar]
- Welch SP, Stevens DL (1992) Antinociceptive activity of intrathecally administered cannabinoids alone, and in combination with morphine, in mice. J Pharmacol Exp Ther 262:10–18 [PubMed] [Google Scholar]
- Whiting PF, Wolff RF, Deshpande S, et al. (2015) Cannabinoids for medical use: a systematic review and meta-analysis. J Am Med Assoc 313:2456–2473 [DOI] [PubMed] [Google Scholar]
- Wiley, Jenny L; Lowe JA; Balster RL; Martin B (1995) Antagonism of the discriminative stimulus effects of in rats and rhesus monkeys. J Pharmacol Exp Ther 275:1–6 [PubMed] [Google Scholar]
- Wiley JL, Compton DR, Dai D, et al. (1998) Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther 285:995–1004 [PubMed] [Google Scholar]
- Wiley JL, Martin BR (2003) Cannabinoid pharmacological properties common to other centrally acting drugs. Eur J Pharmacol 471:185–93. doi: 10.1016/S0014-2999(03)01856-9 [DOI] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Thomas BF (2017) Combination chemistry: Structure-activity relationships of novel psychoactive cannabinoids. Curr Top Behav Neurosci 32:231–248. doi: 10.1007/7854_2016_17 [DOI] [PubMed] [Google Scholar]
- Wiley JL, Owens RA, Lichtman AH (2018) Discriminative stimulus properties of phytocannabinoids, endocannabinoids, and synthetic cannabinoids. Curr Top Behav Neurosci 39:153–173. doi: 10.1007/7854_2016_24 [DOI] [PubMed] [Google Scholar]
- Wilkerson JL, Ghosh S, Mustafa M, et al. (2017) The endocannabinoid hydrolysis inhibitor SA-57: Intrinsic antinociceptive effects, augmented morphine-induced antinociception, and attenuated heroin seeking behavior in mice. Neuropharmacology 114:156–167. doi: 10.1016/j.neuropharm.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JL, Niphakis MJ, Grim TW, et al. (2016) The selective monoacylglycerol lipase inhibitor MJN110 produces opioid-sparing effects in a mouse neuropathic pain model. J Pharmacol Exp Ther 357:145–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby KA, Moore SF, Martin BR, Ellis EF (1997) The biodisposition and metabolism of anandamide in mice. J Pharmacol Exp Ther 282:243–247 [PubMed] [Google Scholar]
- Wilson-Poe AR, Morgan MM, Aicher SA, Hegarty DM (2012) Distribution of CB1 cannabinoid receptors and their relationship with mu-opioid receptors in the rat periaqueductal gray. Neuroscience 103:449–9. doi: 10.1016/j.neuroscience.2012.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Poe AR, Pocius E, Herschbach M, Morgan MM (2013) The periaqueductal gray contributes to bidirectional enhancement of antinociception between morphine and cannabinoids. Pharmacol Biochem Behav 103:449–9. doi: 10.1016/j.pbb.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA (2001) Endogenous cannabinoids mediate retrograde signaling at hippocampal synapses. Nature 410:588–592 [DOI] [PubMed] [Google Scholar]
- Xiao JC, Jewell JP, Lin LS, et al. (2008) Similar in vitro pharmacology of human cannabinoid CB1 receptor variants expressed in CHO cells. Brain Res 1238:36–43 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, et al. (2006) Localization of diacyglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci 26:4740–4751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangen A, Solinas M, Ikernoto S, et al. (2006) Two brain sites for cannabinoid reward. J Neurosci 26:4901–4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanger UM, Schwab M (2013) Cytochrome P450 enzymes in drugmetabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138:103–141 [DOI] [PubMed] [Google Scholar]
- Zendulka O, Dovrtelova G, Noskova K, et al. (2016) Cannabinoids and cytochrome P450 interactions. Curr Drug Metab 17:206–226 [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, et al. (1999) Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A 96:5780–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, et al. (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400:452–457 [DOI] [PubMed] [Google Scholar]