Abstract
Objective:
To determine whether a progressive multicomponent physical therapy intervention in the home setting can improve functional mobility for deconditioned older adults following acute hospitalization.
Design:
Randomized controlled trial.
Setting:
Patient homes in the Denver, CO, metropolitan area.
Participants:
A total of 22 homebound older adults age 65 and older (mean ± SD; 85.4 ±7.83); 12 were randomized to intervention group and 10 to the control group.
Intervention:
The progressive multicomponent intervention consisted of home-based progressive strength, mobility and activities of daily living training. The control group consisted of usual care rehabilitation.
Measurements:
A 4-meter walking speed, modified Physical Performance Test, Short Physical Performance Battery, 6-minute walk test.
Results:
At the 60-day time point, the progressive multicomponent intervention group had significantly greater improvements in walking speed (mean change: 0.36 m/s vs. 0.14 m/s, p = 0.04), modified physical performance test (mean change: 6.18 vs. 0.98, p = 0.02) and Short Physical Performance Battery scores (mean change: 2.94 vs. 0.38, p = 0.02) compared with the usual care group. The progressive multicomponent intervention group also had a trend towards significant improvement in the 6-minute walk test at 60 days (mean change: 119.65 m vs. 19.28 m; p = 0.07). No adverse events associated with intervention were recorded.
Conclusions:
The progressive multicomponent intervention improved patient functional mobility following acute hospitalization more than usual care. Results from this study support the safety and feasibility of conducting a larger randomized controlled trial of progressive multicomponent intervention in this population. A more definitive study would require 150 patients to verify these conclusions given the effect sizes observed.
Keywords: Home rehabilitation, older adults, functional fitness, rehabilitation interventions
Introduction
Hospitalization is a profound contributor to functional loss and disability in older adults,1,2 but the optimal interventions for restoring this loss of function are not yet known. Considerable evidence exists for hospital-associated deconditioning,3,4 including dramatic and rapid loss of muscle mass and strength.5 Older adults who are hospitalized are 60 times more likely to develop disability than those who are not hospitalized.6 Recent investigations have demonstrated that functional loss occurring as a result of hospital-associated deconditioning is an important modifiable risk factor for disability development7 and re-hospitalizations8 that may effectively be addressed with intensive rehabilitation strategies. However, it is unclear what rehabilitation strategies are both tolerated and maximally effective for optimizing functional gains for posthospitalized older adults; this assertion is supported by a recent systematic review, which concluded that “no randomized controlled trials have been conducted to examine the effectiveness of specific reconditioning interventions in rehabilitation”9 of deconditioned older adults.
Increasingly, older adults utilize home-based rehabilitation services to address hospital-associated deconditioning. Physical therapy services account for 21% of total visits in home health agencies, and nearly a quarter of visit costs.10 Despite the high utilization of physical therapy services, studies have not examined the effectiveness of these services improving function in older adults with multiple comorbidities after hospitalization. Medicare quality measures11 suggest that only 57% of patients receiving home health services improve in their ability to perform basic bed mobility, and only 62% demonstrate improvements in gait ability. The reasons underlying these inadequate outcomes are not fully understood. However, a 2008 survey of home physical therapists12 suggests home health physical therapy may be delivered at an inadequate intensity for producing meaningful strength gains in older adults who are deconditioned from a recent hospitalization.
Post-hospitalization home healthcare typically occurs during the critical time window when patients (1) are at a high risk of health complications with multiple comorbidities and (2) possess a limited capacity to travel to outpatient services. Thus, optimizing the interventions provided in home health physical therapy practices to meet the rehabilitation needs of this vulnerable population is an important step towards addressing low physical function after acute hospitalization. Multicomponent home-based programs have previously been shown to reduce adverse health events in frail community-dwelling older adults more than single-focus programs of mobility or strength training alone.13 However, to our knowledge, these programs have not been studied in the context of home health physical therapy following acute hospitalization.
Therefore, the goals of this small pilot randomized controlled trial were to: (1) determine if delivery of a progressive multicomponent intervention facilitated greater functional gains than usual care interventions; (2) demonstrate whether a high-intensity, progressive multicomponent exercise program is feasible and safe for older adults with hospital-associated deconditioning; and (3) evaluate effect sizes of our intervention to inform sample size estimates for a larger trial. Our primary hypothesis was that a higher intensity exercise program would deliver greater gains in functional performance over the intervention period, and the gains would be maintained 60 days following hospitalization.
Methods
This study was designed as a small randomized controlled trial involving older adults with multiple chronic conditions, who were discharged from acute care with physician referrals for home health physical therapy. The study design was approved by the University of Colorado Multiple Institutional Review Board (IRB# 10-0432). All patient screening, recruitment, and enrollment was performed during acute hospitalization at the University of Colorado Hospital Acute Care for the Elderly unit. Eligibility criteria were constructed to select for medically deconditioned, homebound older adults at risk of functional decline. Participants were eligible if they were ⩾65 years of age, unable to leave the home without physical assistance, referred to home health physical therapy after an acute hospitalization of any duration, had at least three comorbid conditions, and were ambulatory (with or without an assistive device) prior to hospitalization. Exclusion criteria included acute lower extremity fractures with weight-bearing restriction, elective joint replacement surgery, active treatment for cancer diagnosis, current dialysis treatment, acute cardiac surgery, acute stroke, lower extremity amputation, presence of neurologic disorder limiting function (e.g. Parkinson’s disease or Guillain-barré syndrome), a score of less than 20 on the Mini-Mental State Examination or a referral to hospice care.
Patients were randomized to the control or progressive multicomponent intervention group using a computer-generated randomization table. Patients placed in the progressive multicomponent intervention group were referred to our partner home health agency and were seen by a single physical therapist who was trained to provide the progressive intervention. Usual care subjects were seen by physical therapists employed by the same home health agency who were unaware of the investigation. Both groups had two to three physical therapy visits weekly over the 30-day intervention period, as deemed appropriate by the treating therapist. The progressive multicomponent intervention therapist and patients were not blinded to group assignment. Patient outcome assessments were performed in the home by an independent physical therapist blinded to group assignments at baseline (within 72 hours of hospital discharge), 30 days, and 60 days (primary end-point) after hospitalization.
Each progressive multicomponent physical therapy intervention session began with a period of activities of daily living (ADL) training, primarily targeting bathroom transfers, bed mobility, and car transfers. Bathroom transfers consisted of on/off the toilet and tub/shower transfers; these were performed over the first one to two weeks until the patient was deemed fully safe and competent with these activities. Bed mobility and chair transfer training were also practiced over the first two weeks to ensure patient competence. During the later stages of treatment, patients participated in car transfer training, to increase safety when entering and exiting a vehicle.
Along with ADL training, progressive multicomponent physical therapy intervention consisted of an evidence-based mobility training program.14–16 These interventions included indoor walking, gait training on flat ground and stairs, and outdoor walking. Walking activities were progressed from low skill (e.g. walking in a straight line) to more skilled activities, such as walking in a serpentine pattern. Progression was based on increasing speed, accuracy, and by requiring the patient hold and manipulate objects during walking (dual task). Each session began with practicing a well-learned task. Patients were progressed when performance of the task was 80–100% correct. As walking ability improved, the training began to target patient endurance by prescribing daily walking programs of two 10-minute bouts and increased to 20 minutes continuous. Before discharge from home therapy, patients began transition to a more independent physical activity program, without physical therapist supervision, by performing a daily walking program.
The progressive multicomponent strengthening intervention followed the principles of overload,17 utilizing an 8-repetition maximum for each exercise targeting lower and upper extremity muscle groups. The strengthening intervention was performed during each physical therapy visit and also included a scheduled home exercise component consisting of both upper and lower extremity strengthening exercises. Initial intensity of resistance was determined during the first one to two visits. Training volume initially began at two sets of eight repetitions, progressing to three sets during the second week of training. Intensity was reassessed each week and resistance increased as the patient was able to complete three sets of eight repetitions using appropriate form. The strength training was accomplished using body weight resistance and the Shuttle Mini Press® (Contemporary Design Company, Glacier, WA), which is a portable progressive strengthening device that uses resistance bands to provide between 2.72 and 45.36 kilograms of resistance. Lower extremity exercises included a supine leg press, standing hip extension, and body-weight resisted plantar flexion. Upper extremity exercises consisted of seated press and seated row, both using the Shuttle Mini Press®. All progressive multicomponent intervention participants were given a home exercise program to continue basic exercises after discharge.
To best reproduce standard of care in the usual care group, no additional training or education was provided to treating therapists. Therapists treating usual care patients were not made aware of study participation to reduce bias. Usual care therapy, therefore, consisted of the community standard home physical therapy for elderly patients after acute care hospitalization. From chart reviews, we verified that these interventions primarily consisted of low intensity strength training, practice of simple ambulatory skills in the home, and basic in-home functional mobility training, along with a low intensity home exercise program to continue prescribed exercises.
Outcome measures used in this study at All time points have been previously validated for use in medically complex older adults. They consisted of a 4-meter gait speed assessment,18 the modified Physical Performance Test,19 the Short Physical Performance Battery,20 and the 6-minute walk test.21 We also documented the incidence of adverse events (re-hospitalizations and emergency room visits) to ensure safety of the progressive multicomponent intervention.
Analyses were based on the intention-to-treat philosophy using SAS® version 9.2 (SAS Institute, Inc., Cary, NC). To address the main hypotheses, we fit a series of mixed linear models (mixed procedure) with change from baseline in each outcome (gait speed, modified Physical Performance Test, Short Physical Performance Battery, 6-minute walk test) as the dependent variable; intervention arm, follow-up time (30-/60-day) and their interaction was included as primary fixed effects. A linear mixed model, unlike a traditional analysis of variance, allows for the use of both fixed and random effects, and therefore permits controlling for missing data in longitudinal or repeated measure analyses.22 We constructed contrast statements to evaluate group differences at baseline, 60 days (primary end-point), and at 30 days (secondary end-point). The primary comparison at 60 days was used to establish the efficacy of the progressive multicomponent intervention over a home health episode of care, while the additional 30-day secondary comparison helped establish the trajectory of recovery in each treatment group. The number of adverse events (re-hospitalizations and emergency room visits) were totaled and compared, as frequency counts to evaluate preliminary safety outcomes related to the progressive multi-component intervention.
Results
The CONSORT diagram in Figure 1 details the participant pathway throughout the 60-day study period. A total of 61 patients admitted to the Acute Care for the Elderly unit of the University of Colorado Hospital were screened for eligibility. Of those, 22 patients (eight male) were initially enrolled, with two lost to follow-up before the 60-day time point. No significant differences between the two groups existed at baseline for mean age, weight, height, body mass index (Table 1), number of comorbidities (Table 2), or physical function (Table 3).
Figure 1.
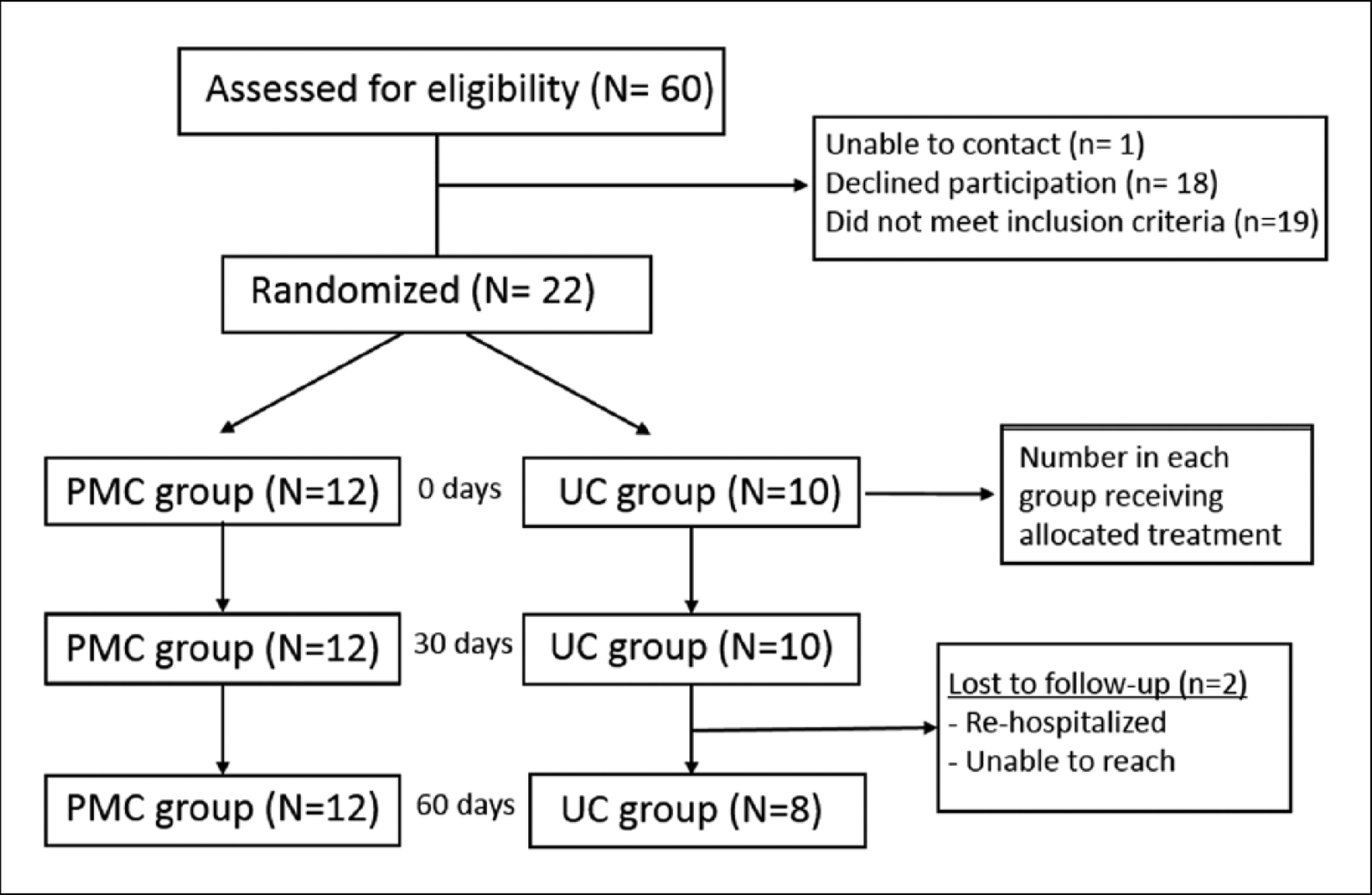
CONSORT diagram showing recruitment, enrollment, and adherence of study participants. Enrollment numbers and withdrawals, or those lost to follow-up, are indicated in the boxes between time points.
PMC: progressive multicomponent intervention group; UC: usual care group.
Table 1.
Baseline demographic information for control and intervention groups. Presented as N (%) or mean ± SD.
| Control group (N = 10) | PMC group (N = 12) | p-value | ||
|---|---|---|---|---|
| Sex | Male | 3 (30) | 5 (41.7) | 0.57a |
| Female | 7 (70) | 7 (58.3) | ||
| Age(y) | 83 ± 6.8 | 87 ± 8.8 | 0.20b | |
| Height (m) | 1.6 ± 0.1 | 1.6 ± 0.1 | 0.49b | |
| Weight (kg) | 62.7 ± 16.1 | 66.8 ± 18.0 | 0.58b | |
| BMI | 24 ± 5.2 | 25 ± 5.7 | 0.80b |
p-values represent chi-square results.
p-values represent independent sample t-test results.
PMC: progressive multicomponent intervention; BMI: body mass index.
Table 2.
Comorbidity information for control and intervention groups. Presented as mean ± SD or N (%). Significance at p < 0.05.
| Control group (N = 10) | PMC group (N = 12) | p-value | |
|---|---|---|---|
| Comorbidities (mean ± SD) | 4.8 ±1.5 | 4.5 ±1.1 | 0.61a |
| OA | 2 (20) | 0 (0) | 0.07b |
| HF | 3 (20) | 4 (33) | 0.87b |
| Chronic lung disease | 3 (30) | 4 (33) | 0.87b |
| DM2 | 4 (40) | 2 (16.7) | 0.22b |
| Non-specific heart disease | 7 (70) | 5 (41.7) | 0.18b |
p-values represent independent sample t-test results.
p-values represent chi-square results.
PMC: progressive multicomponent intervention group; OA: osteoarthritis; HF: heart failure; DM2: type 2 diabetes mellitus.
Table 3.
Mean (95% confidence interval (CI)) values for baseline, 30-, and 60-day outcome scores for control and progressive multicomponent groups.
| Baseline | 30 day | 60 day | ||||
|---|---|---|---|---|---|---|
| Control group | PMC group | Control group | PMC group | Control group | PMC group | |
| Walking speed (m/s) | 0.42 (0.34, 0.50) | 0.39 (0.28, 0.50) | 0.51 (0.38, 0.63) | 0.63 (0.49, 0.76) | 0.55 (0.41, 0.68) | 0.77 (0.59, 0.96) |
| mPPT total score | 9.10 (6.33, 11.87) | 9.50 (6.31, 12.69) | 9.80 (6.16, 13.44) | 15.00 (11.16, 18.84) | 10.20 (6.85, 13.55) | 15.90 (12.18, 19.62) |
| SPPB total score | 3.90 (2.86, 4.94) | 4.50 (2.86, 6.14) | 4.90 (3.13, 6.67) | 6.33 (4.34, 8.33) | 4.40 (2.78, 6.02) | 7.60 (5.69, 9.51) |
| 6MWT (m) | 115.49 (74.11, 156.88) | 84.72 (48.85, 120.59) | 120.81 (76.11, 165.51) | 151.70 (100.42, 202.98) | 129.16 (69.84, 188.48) | 206.59 (134.08, 279.10) |
PMC: progressive multicomponent intervention group; mPPT: modified Physical Performance Test; SPPB: Short Physical Performance Battery; 6MWT: 6-minute walk test.
Within the intervention period, the number of physical therapy treatments were not significantly different between groups, with a mean (SD) of 9.67 visits (2.42) for the progressive multicomponent intervention group compared with 8.00 (3.04) for the control group (p = 0.16). Additionally, the duration of home physical therapy care was 34.80 days (20.25) for the control group and 30.58 (10.24) for the progressive multicomponent intervention group (p = 0.53).
Mean gait speed, 6-minute walk test distance, and scores for modified Physical Performance Test and Short Physical Performance Battery at all time points are presented in Table 3. Change scores for all functional outcome measures are presented in Table 4. At the primary 60-day end-point, the progressive multicomponent intervention group had significantly greater gait speed, modified Physical Performance Test scores, and Short Physical Performance Battery scores compared with the usual care group (Figure 2, available online). Though not statistically significant, the progressive multicomponent intervention group demonstrated a trend towards a greater 6-minute walk test distance at 60 days compared with the usual care group. At the 30-day time point, the progressive multicomponent intervention group had significantly greater modified Physical Performance Test scores (p=0.02), but no other measures were significantly different between groups.
Table 4.
Mean (95% CI) values for change from baseline for both groups, as well as between-group differences at 30 and 60 days (primary end-point) indicate significant differences between groups (p < 0.05).
| Variable | 30-day change from baseline | Between group differences | 60-day change from baseline | Between group differences | ||||
|---|---|---|---|---|---|---|---|---|
| Control group | PMC group | UC-PMC | p-value | Control group | PMC group | UC-PMC | p-value | |
| Walking speed (m/s) | 0.10 (−0.03, 0.22) | 0.23 (0.11, 0.34) | −0.13 (−0.30, 0.04) | 0.12 | 0.14 (−0.02, 0.29) | 0.36 (0.21, 0.51) | −0.22 (−0.44, −0.01) | 0.04 |
| mPPT total score | 0.66 (−2.29, 3.60) | 5.54 (2.85, 8.23) | −4.88 (−8.87, −0.89) | 0.02 | 0.98 (−2.12, 4.09) | 6.18 (3.04, 9.32) | −5.20 (−9.62, −0.77) | 0.02 |
| SPPB total score | 0.96 (−0.52, 2.44) | 1.87 (0.52, 3.22) | −0.91 (−2.93, 1.10) | 0.36 | 0.38 (−1.06, 1.83) | 2.94 (1.46, 4.42) | −2.56 (−4.65, −0.46) | 0.02 |
| 6MWT (m) | 19.88 (−43.57, 83.33) | 62.46 (15.19, 109.73) | −42.58 (−125.11, 39.94) | 0.29 | 19.28 (−59.67, 98.22) | 119.65 (52.55, 186.76) | −100.38 (−208.10, 7.34) | 0.07 |
p-values represent results from mixed model individual contrast statements.
PMC: progressive multicomponent intervention group; UC: usual care group; mPPT: modified Physical Performance Test; SPPB: Short Physical Performance Battery; 6MWT: 6-minute walk test.
Adverse events of emergency room visits and re-hospitalizations were pooled across the 60-day time period. Six combined emergency room visits and re-hospitalizations were recorded in the usual care group (between two patients), while none were recorded in the progressive multicomponent intervention group. None of the adverse events were attributed to the physical therapy intervention.
Discussion
Our findings suggest that a 30-day home-based, high intensity progressive multicomponent intervention exercise program promotes greater gains in physical function for homebound older adults after hospitalization than usual home physical therapy. The trajectory of the gains during and after the intervention period provides useful insight into the dose-dependent effects of exercise on this population. Participants in the higher intensity progressive multicomponent intervention group did not demonstrate the decline in physical function between day 30 and day 60 that was observed in the usual care group, and actually continued to show improvement in Short Physical Performance Battery scores for the month following the end of the intervention—demonstrating a gain nearly three times the minimal clinically important difference (MCID) of the test battery. Comparably, at the 60-day time period, the usual care group had only small improvements in Short Physical Performance Battery scores from baseline—less than what is considered a meaningful change in this measure.
Clinically meaningful changes were also observed in gait speed for both groups at 30 days; however, the progressive multicomponent intervention group continued to make significant gains in walking speed after the conclusion of skilled therapy services. Clinically meaningful gains in gait speed were not observed in the usual care group after the first 30 days. The progressive multicomponent intervention group also had superior improvements in modified Physical Performance Test scores at both the 30-day and 60-day time points. These findings provide preliminary evidence that a higher intensity progressive multicomponent intervention program may more effectively ameliorate the functional decline commonly seen after acute hospitalization. At the same time, restoration of function to levels of healthy, comparably aged older adults was not achieved,20,23,24 suggesting that extending the duration of high intensity treatments in future studies may be required to maximize function in this vulnerable population.
Safety of the study was assessed by evaluating rates of adverse events, hospital re-admissions, and emergency room visits in both treatment arms. There were no adverse events related to treatment in either group. Interestingly, we observed fewer hospital re-admissions and less emergency room utilization in the progressive multicomponent intervention group at 60 days after hospital discharge (six combined re-admission and Emergency Room visits in the usual care group; zero in the progressive multicomponent intervention group). This is an important observation, as re-hospitalizations are an emerging area of interest in healthcare systems and are directly linked to reimbursement for acute care hospitals. The 30-day re-admission rates for the general Medicare population are approximately 16%, but estimates are as high as 24–42%25 for the cohort of older adults who participate in home healthcare services after hospitalization. Emerging research has strongly implicated both ambulatory ability26,27 and physical function8 as independent predictors of re-hospitalization risk; the fact that the progressive multicomponent intervention group in our study had significantly higher gait velocities and Short Physical Performance Battery scores may be an indicator that they were at overall decreased risk of re-hospitalization. However, a future study with larger cohorts is needed to examine the specific relationships between functional improvements and re-hospitalization risk in homebound older adult populations.
The results of this current study expand upon previous research by Tinetti, who examined the effects of an interdisciplinary restorative care intervention compared with a usual care group for older adults hospitalized with multiple diagnoses and comorbid conditions.28 The restorative care group was more likely to remain at home (vs. rehospitalization, death, or nursing home) following an episode of home care and was less likely to require an emergency room visit as compared with the usual care group, but did not demonstrate clinically significant improvements in ADL function. The protocols used in Tinetti’s study were heavily focused on increasing patient participation in, and independence with, home tasks,29 but did not specifically address the deficits in strength, gait ability, or endurance that underlie ADL impairments. This may explain why our results had a much more robust effect on physical function in a similar population of older adults receiving home health services.
The finding of continued physical performance improvement after the withdrawal of supervised exercise is somewhat in contrast with previous findings on exercise effects on older adults. Kalapotharakos30 found that healthy octogenarians who participated in resistance training had significantly improved strength, but did not retain gains over a six-week period of detraining. However, the intensity of training in that study was only 70% of a three-repetition maximum, which is significantly lower than the 80% one-repetition maximum target in our study. We hypothesize that higher intensity interventions facilitate greater gains in functional reserve for this population,3,4 which results in improved participation in ADL and thus better maintenance of physical function gains, even after skilled therapy is discontinued.
There are a number of limitations to our study that should be considered when interpreting the results. First, our sample size was relatively small (n = 22). However, the robust physical performance differences between our groups at the 60-day time-point, combined with the differing trajectories of recovery at that time-point, suggest that there are clear differences in functional performance between the groups. Second, our sample was limited to a single metropolitan geographic area, which may limit the generalizability of our findings to rural settings. Third, the control group in our study was a usual care group that did not have specific exercise protocols to follow, or a specific number of visits. The control group was designed as usual care to more accurately capture typical home health practice patterns. Results may differ in locales with different standards of usual care for post-hospitalization therapy. Another limitation was that baseline testing was performed after randomization, because it was necessary to randomize patients early in the process to allow for scheduling of rehabilitation by the appropriately trained therapist. However, baseline testing was performed by an assessor who was unaware of group assignment. Furthermore, baseline functional status of the two groups was not found to be significantly different. We also did not control for, or measure, activities in either group after the 30-day intervention period. Both groups were given a home exercise program, but it is unknown the level of compliance patients had with this program after physical therapy discharge. Finally, a number of patients declined to participate in the initial study, which may have contributed to study volunteer bias. Many of the refusals stemmed from relative uncertainty about the safety of our interventions; these concerns have been significantly lessened with our demonstrations of safety. In our planned larger study, we feel that our recruitment efforts will be significantly improved because of the strong functional gains demonstrated in combination with minimal adverse events.
From these results, it appears that a 30-day progressive multicomponent intervention delivered at high intensity is feasible and safe for older adults after acute hospitalization and may be more effective than usual care in ameliorating the physical function declines associated with acute hospitalization. A larger trial is needed to confirm and expand these findings to a larger cohort of older adults with hospital-associated deconditioning. Assuming that the standard deviation of the 60-day change in the primary outcome of Short Physical Performance Battery score will be 2.1 (pooled SD used for both groups), a sample size of 150 patients (75/group) would provide 82% power to detect a two-point difference (twice the MCID) between groups (using a two-sided, alpha = 0.05 level, two-group t-test). With an estimated 25% attrition rate during the study, a future randomized controlled trial would require at least 200 participants to replicate the conclusions we observed in this small pilot study.
Supplementary Material
Clinical messages.
Increasing exercise intensity for older adults with hospital-associated deconditioning is a safe and effective strategy for improving physical function in a home health setting.
Older adults who participate in high-intensity, multicomponent training after hospitalization have greater functional gains with greater subsequent maintainence of these gains than older adults receiving usual home health physical therapy.
Acknowledgements
We thank the following individuals for their invaluable contributions to this investigation: Melissa Nolte, SDPT, Catherine Bilyeu, PT, DPT, Visiting Nurses Association, Jennifer Brach, PT, PhD, University of Pittsburgh, and University of Colorado Hospital staff (physical therapists, occupational therapists, and case managers).
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded in part by the National Institutes of Health [T32 AG000279, K23-AG029978], Foundation for Physical Therapy (Florence P. Kendall Post-Professional Doctoral Scholarship and Promotion for Doctoral Studies I and II Awards), Academy of Geriatric Physical Therapy (Fellowship for Geriatric Research), Abington Health Innovators’ Circle Award 2011, and the American Physical Therapy Association (Home Health Section Research Grant).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med 2013; 368: 100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill TM, Gahbauer EA, Han L and Allore HG. The relationship between intervening hospitalizations and transitions between frailty states. J Gerontol A Biol Sci Med Sci 2011; 66: 1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kortebein P Rehabilitation for hospital-associated deconditioning. Am J Phys Med Rehabil 2009; 88: 66–77. [DOI] [PubMed] [Google Scholar]
- 4.Falvey J, Mangione K and Stevens-Lapsley J. Rethinking hospital-associated deconditioning: proposed paradigm shift. Phys Ther. Epub ahead of print 23 April 2015. DOI: 102522/ptj20140511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kortebein P, Ferrando A, Lombeida J, Wolfe R and Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007; 297: 1772–1774. [DOI] [PubMed] [Google Scholar]
- 6.Gill TM, Allore HG, Holford TR and Guo ZC. Hospitalization, restricted activity, and the development of disability among older persons. JAMA 2004; 292: 2115–2124. [DOI] [PubMed] [Google Scholar]
- 7.Baztan JJ, Galvez CP and Socorro A. Recovery of functional impairment after acute illness and mortality: One-year follow-up study. Gerontol 2009; 55: 269–274. [DOI] [PubMed] [Google Scholar]
- 8.Hoyer EH, Needham DM, Atanelov L, Knox B, Friedman M and Brotman DJ. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med 2014; 9: 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timmer AJ, Unsworth CA and Taylor NF. Rehabilitation interventions with deconditioned older adults following an acute hospital admission: A systematic review. Clin Rehabil 2014; 28: 1078–1086. [DOI] [PubMed] [Google Scholar]
- 10.Center for Medicare and Medicaid Services. Home health study report, http://wwwcmsgov/Medicare/Medicare-Fee-for-Service-Payment/HomeHealthPPS/Downloads/HHPPS_LiteratureReviewpdf (accessed 29 September 2014).
- 11.Center for Medicare and Medicaid Services. Home Health Compare, https://www.medicare.gov/homehealthcompare/ (accessed 29 September 2014).
- 12.Mangione KK, Lopopolo RB, Neff NP, Craik RL and Palombaro KM. Interventions used by physical therapists in home care for people after hip fracture. Phys Ther 2008; 88: 199–210. [DOI] [PubMed] [Google Scholar]
- 13.Thomas S, Mackintosh S and Halbert J. Does the ‘Otago exercise programme’ reduce mortality and falls in older adults?: A systematic review and meta-analysis. Age Ageing 2010; 39: 681–687. [DOI] [PubMed] [Google Scholar]
- 14.Brach JS, Van Swearingen JM, Perera S, Wert DM and Studenski S. Motor learning versus standard walking exercise in older adults with subclinical gait dysfunction: A randomized clinical trial. J Am Geriatr Soc 2013; 61: 1879–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brach JS and Vanswearingen JM. Interventions to improve walking in older adults. Curr Transl Geriatr Exp Gerontol Rep 2013; 2: 230–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.VanSwearingen JM, Perera S, Brach JS, Cham R, Rosano C and Studenski SA. A randomized trial of two forms of therapeutic activity to improve walking: Effect on the energy cost of walking. J Gerontol A Biol Sci Med Sci 2009; 64: 1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. Lippincott Williams & Wilkins, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Perera S, Mody SH, Woodman RC and Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriat Soc 2006; 54: 743–749. [DOI] [PubMed] [Google Scholar]
- 19.Host HH, Sinacore DR, Brown M and Holloszy JO. Reliability of a modified Physical Performance Test (PPT) in older adults. Phys Ther 1996; 76: S23. [Google Scholar]
- 20.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49: M85–94. [DOI] [PubMed] [Google Scholar]
- 21.Enright PL, McBurnie MA, Bittner V, et al. The 6-min walk test: A quick measure of functional status in elderly adults. Chest 2003; 123: 387–98. [DOI] [PubMed] [Google Scholar]
- 22.Cnaan A, Laird NM and Slasor P. Tutorial in biostatistics: Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med 1997; 16: 2349–2380. [DOI] [PubMed] [Google Scholar]
- 23.Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79 years: Reference values and determinants. Age Ageing 1997; 26: 15–19. [DOI] [PubMed] [Google Scholar]
- 24.Brown M, Sinacore DR, Binder EF and Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci 2000; 55: M350–M5. [DOI] [PubMed] [Google Scholar]
- 25.Anderson MA, Clarke MM, Helms LB and Foreman MD. Hospital readmission from home health care before and after prospective payment. J Nurs Scholarship 2005; 37: 73–79. [DOI] [PubMed] [Google Scholar]
- 26.Fisher SR, Kuo YF, Sharma G, et al. Mobility after hospital discharge as a marker for 30-day readmission. J Gerontol A Biol Sci Med Sci 2013; 68: 805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen HQ, Chu L, Amy Liu IL, et al. Associations between physical activity and 30-day readmission risk in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2014; 11: 695–705. [DOI] [PubMed] [Google Scholar]
- 28.Tinetti ME, Baker D, Gallo WT, Nanda A, Charpentier P and O’Leary J. Evaluation of restorative care vs usual care for older adults receiving an acute episode of home care. JAMA 2002; 287: 2098–2105. [DOI] [PubMed] [Google Scholar]
- 29.Baker DI, Gottschalk M, Eng C, Weber S and Tinetti ME. The design and implementation of a restorative care model for home care. Gerontol 2001; 41: 257–263. [DOI] [PubMed] [Google Scholar]
- 30.Kalapotharakos VI, Diamantopoulos K and Tokmakidis SP. Effects of resistance training and detraining on muscle strength and functional performance of older adults aged 80 to 88 years. Aging Clin Exp Res 2010; 22: 134–140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


