Abstract
Cannabis sativa L. produces over 200 known secondary metabolites that contribute to its distinctive aroma. Studies on compounds traditionally associated with the scent of this plant have focused on those within the terpenoid class. These isoprene-derived compounds are ubiquitous in nature and are the major source of many plant odors. Nonetheless, there is little evidence that they provide the characteristic “skunk-like” aroma of cannabis. To uncover the chemical origins of this scent, we measured the aromatic properties of cannabis flowers and concentrated extracts using comprehensive two-dimensional gas chromatography equipped with time-of-flight mass spectrometry, flame ionization detection, and sulfur chemiluminescence. We discovered a new family of volatile sulfur compounds (VSCs) containing the prenyl (3-methylbut-2-en-1-yl) functional group that is responsible for this scent. In particular, the compound 3-methyl-2-butene-1-thiol was identified as the primary odorant. We then conducted an indoor greenhouse experiment to monitor the evolution of these compounds during the plant’s lifecycle and throughout the curing process. We found that the concentrations of these compounds increase substantially during the last weeks of the flowering stage, reach a maximum during curing, and then drop after just one week of storage. These results shed light on the chemical origins of the characteristic aroma of cannabis and how volatile sulfur compound production evolves during plant growth. Furthermore, the chemical similarity between this new family of VSCs and those found in garlic (allium sativum) suggests an opportunity to also investigate their potential health benefits.
Introduction
Cannabis sativa L. is one of the most popular recreational drugs that has recently seen significant changes of legality within the United States and other countries.1−5 This plant produces a wide variety of secondary metabolites, including tetrahydrocannabinol (THC), cannabidiol (CBD), and a plethora of volatile organic compounds (VOCs) such as terpenes and terpenoids that create the plant’s unique aroma.6−13 Although typically consumed recreationally for the psychoactive effects brought upon by THC, many consumers use cannabis for its medicinal properties.14−16Cannabis has been shown to contain compounds potentially effective in relieving chronic pain,17,18 rare/extreme forms of epilepsy,19 and as a promising treatment of pancreatic cancer.20 These possible benefits, along with the changing opinion within the general public, have led to a significant increase in legal cannabis production across many states.21 As such, there is a need to comprehensively understand the chemical origins of this plant’s unique and pungent aroma.
Over 200 aroma compounds have previously been reported in cannabis, highlighting the complexity of its odor.9−11,22 The compounds with the highest concentrations typically include the terpenoids β-myrcene, α- and β-pinene, d-limonene, β-caryophyllene, terpinolene, and humulene, which can individually contribute upward of 50% of the aroma concentration.9−11,13,23 The types and relative concentrations of these compounds contribute significantly to the scent of cannabis, which is becoming increasingly diverse as cultivars are crossbred. For instance, OG Kush, a cannabis indica cultivar, possesses a strong, pungent, fuel-like aroma that arises from high concentrations of β-myrcene and β-caryophyllene. On the other hand, Jack Herer, a cannabis sativa cultivar, has high amounts of terpinolene and d-limonene, creating a woody and citrus aroma. In the middle ground is Sherbert, which is a hybrid crossed between a cannabis sativa and cannabis indica cultivar and has high d-limonene and linalool concentrations, leading to a citrus and floral aroma.24 Although these compounds contribute strongly to the aroma of cannabis and give each cultivar its unique scent, questions remain regarding the chemical origins of the “skunk-like” scent, which is in part due to the difficulty of analyzing samples with such complexity.
To ameliorate this issue, we employed a custom-built comprehensive 2-dimensional gas chromatography (GC × GC) system with three detectors operating simultaneously: A time-of-flight mass spectrometer (TOF-MS), flame ionization detector (FID), and sulfur chemiluminescence detector (SCD). GC × GC provides much better separation efficiency compared with traditional 1-dimensional techniques—i.e., traditional gas chromatography—and thus is an ideal method for detecting low concentration compounds in exceedingly complex volatile analysis.25 Furthermore, the combination of sulfur chemiluminescence, mass spectrometry, and flame ionization equips us with the means to detect, identify, and quantify low concentration analytes that would otherwise be extremely difficult to elucidate. In particular, sulfur chemiluminescence allows us to detect volatile sulfur compounds (VSCs) very easily, which can then be identified using mass spectrometry.
We specifically focused on identifying VSCs for two reasons: First, the aroma of cannabis is often described as “skunk-like,” and as skunks are well known to possess several potent VSCs in their defensive aerosol spray, we suspected there could be similar compounds in cannabis.26 Secondly, VSCs are also important in the chemistry of other plants known for their oftentimes pungent aromas and flavors, including hops (Humulus lupulus),27−30 garlic (Allium sativum),31−33 and durian (Durio zibethinus).34,35 Hops, which are used to add flavor to beer, can contain polyfunctional thiols such as 4-mercapto-4-methyl-2-pentanone and 3-mercaptohexan-1-ol.36,37 These compounds impart strong aromas and flavors at very low (ppb) concentrations. Garlic is used ubiquitously in culinary preparation due to its pleasant fragrance and flavor as well as potential health benefits arising from VSCs such as diallyl disulfide and triallyl disulfide.31,32,38 Lastly, durian has an extremely divisive and potent aroma due to numerous VSCs, including ethyl (2S)-2-methylbutanoate, 1-(ethylsulfanyl)ethane-1-thiol, and ethanethiol.34,35 As VSCs play an important part in the strong odors of these three plants, we focused on measuring this class of compounds in cannabis to determine if they likewise contribute to its pungent aroma.
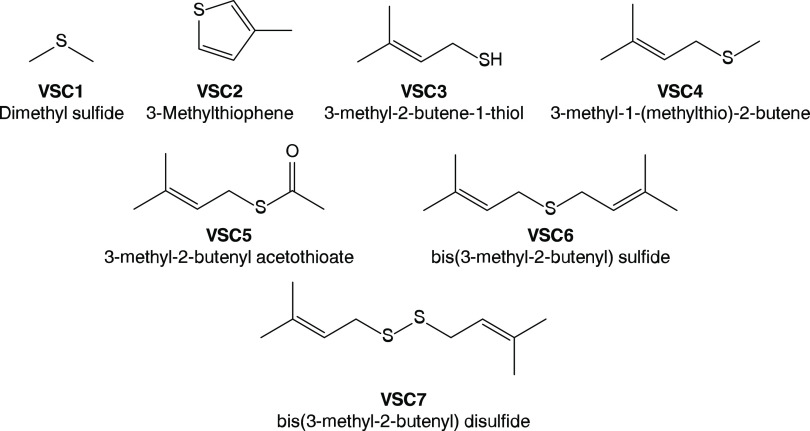
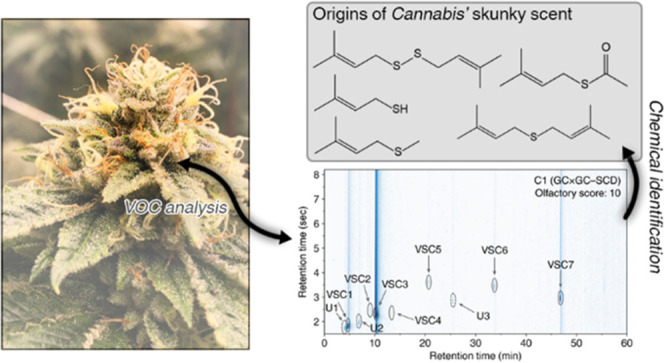
Our results uncovered numerous VSCs, as shown in Figure 1, some of which have not been identified in nature. The compounds VSC3–VSC7 each contain the prenyl functional group—the dimethyl analogue of the allyl group—the latter of which is found ubiquitously in garlic VSCs with reported health benefits.32,33,39,40 After identifying these compounds, we formulated the flower aroma profile of the cultivar Bacio Gelato using botanically derived isolates in a laboratory setting that confirmed 3-methyl-2-butene-1-thiol (VSC3) to be the major component of this aroma. We then measured VSCs in three cannabis extract products, an increasingly popular form of consumption, to determine if these compounds are retained throughout the hydrocarbon extraction process. We lastly conducted an indoor greenhouse trial to determine when these compounds are produced by the cannabis plant by monitoring the concentrations of VSCs as a function of growth. Our results establish two-dimensional gas chromatography coupled with mass spectrometry, flame ionization detection, and sulfur chemiluminescence as a powerful tool for elucidating the chemistry of the complex odor of cannabis.
Figure 1.
Chemical structures of VSCs identified in cannabis. VSC3–VSC7 each contains the prenyl functional group and contributes to the characteristic skunk-like aroma of cannabis.
Experimental Section
Samples of cannabis flowers were curated from different sources. Cultivars Cali Berry, Apple Fritter, Gouda Berry, and Jetlag OG were purchased from Cookies dispensary (San Bernardino, CA). Black Jack and Area 41 were purchased from Hyperwolf Riverside dispensary (Riverside, CA). Gushers, Gelato, and Bacio Gelato were purchased from Catalyst (Long Beach, CA), The Circle (Long Beach, CA), and Sherbinskis (Los Angeles, CA) dispensaries, respectively. WiFi Cake and Chem 91 were procured from Jungle Boys dispensary (Santa Ana, CA). The procured samples were stored at ≈55% relative humidity and ≈21 °C in mason jars. GC × GC data collection and analysis was conducted within a day of procurement for all samples. For the indoor greenhouse trial, four OG varietal clones (Clone Guy Industries) were purchased from Empire Connect dispensary (San Bernardino, CA).
Two separate GC × GC–TOF–MS/FID/SCD experiments were conducted for each flower sample: The first was a high-sample mass experiment (VSC analysis) aimed at identifying and quantifying low concentration compounds. The second was a low-sample mass experiment (VOC analysis) that was used to identify and quantify the major components of each sample.
VSC Analysis Sample Preparation
As VSCs are highly volatile, it was necessary to prepare samples for GC × GC analysis as quickly as possible. For flower samples, 200 mg of the flower for each cultivar was put into a 20 mL headspace vial followed by immediately mechanically breaking the flower for 20 s with a disposable plastic spatula. The sample vial was then immediately capped and crimped using an electric crimper. Triplicates of the data were collected for each sample and averaged (GC × GC–SCD chromatograms for each experiment are found in Figures S19–S31). For cannabis extract samples, approximately 80 mg of the extract was added directly to the 20 mL headspace vial. In the case of the indoor greenhouse trial, up until the curing stage, a single flower was cut from each of the four plants to minimize damage to the plant during growth. These four data points were then averaged each week.
VOC Analysis Sample Preparation
The major volatile aroma compounds of the flowers were extracted using methanol as a solvent. Approximately 200 mg of flower was placed into a scintillation vial, followed by mechanical grinding with a plastic disposable spatula. Briefly, 2 mL of methanol was added to the flower and agitated for 15 min, followed by transferring 4 μL of the solution using a filtered syringe to a 20 mL headspace vial and crimped using an electric crimper.
Comprehensive Two-Dimensional Gas Chromatography
GC × GC analysis was performed using the INSIGHT reverse fill flush flow modulator (SepSolve Analytical). This was coupled for data generation to an Agilent 7890B GC equipped with a BPX5 (20 m × 0.18 mm ID × 0.18 μm film thickness) 1st dimension column and Mega Wax (4.8 m × 0.32 mm ID × 0.15 μm film thickness) 2nd dimension column and BenchTOF-Select time-of-flight mass spectrometer (Markes International). Time-of-flight mass spectrometry (TOF-MS) was used to identify compounds. Quantification of compounds was done using a flame ionization detector (FID). Sample introduction was done using a Centri Sample Concentration Platform (Markes International).
VSC analysis samples were incubated and agitated at a temperature of 45 °C for 10 min, followed by six 1 mL headspace injections from the headspace vial to a cryogen-free cold trap held at 25 °C. The low incubation temperature prevents the possible reactivity of VSCs of interest. After the six injections were complete, the cold trap was rapidly heated to 300 °C to desorb the sample in a narrow band onto the analytical columns. The GC × GC column configuration was an apolar to polar setup. The GC oven ramp rates used were as follows: The oven was initially set to 45 °C and held for 3 min. The oven was then ramped at a rate of 5 °C per minute to 90 °C, followed by a 2.0 °C ramp rate to 130 °C, followed lastly by a 5 °C ramp rate to 240 °C. The modulation period set for the flow modulator was 7.2 s.
VOC analysis samples were incubated and agitated at a temperature of 70 °C for 10 min, followed by a single 5 mL headspace injection from the headspace vial to a cryogen-free cold trap held at 25 °C. The cold trap was rapidly heated to 300 °C to desorb the sample in a narrow band onto the analytical columns. The GC × GC column configuration was an apolar to polar setup. The GC oven ramp rates used were as follows: The oven was initially set to 45 °C and held for 3 min. The oven was then ramped at a rate of 5 °C per minute to 90 °C, followed by a 2.0 °C ramp rate to 130 °C, followed last by a 5 °C ramp rate to 240 °C. The modulation period set for the flow modulator was 7.2 s.
Data was collected, integrated, and analyzed using the ChromSpace software platform (Sepsolve Analytical). VSC4, VSC6, and VSC7 were synthesized by Synerzine (75%, 54%, and 80%, respectively), while VSC3 and VSC5 were purchased from Excellentia (95%, 1% in Triacetin) and Santa Cruz Biotechnology (97%), respectively, to confirm similar elution times, mass spectra, and generate calibration curves to approximate concentrations. Then, 5 or 6-point calibration curves of each were generated to quantify VSCs (see SI) using GC × GC–FID. Non-sulfur-containing compounds were quantified from GC × GC–FID data with calibration curves using a 40-compound terpene standard (LGC Standards) (Table S2). Figures showing GC × GC–SCD or GC × GC–MS chromatograms have been realigned to account for void time (1.5 s) in the second dimension.
Formulation of Reverse Engineered Aroma of Flower and Olfactory Testing
A four-member olfactory testing panel was used to rate the pungency of cannabis flowers and extracts using a blind olfactory test. The panel set references as a group to help standardize the grading system of the samples. Standards used included compounds commonly found in high concentration in cannabis, including β-myrcene, α- and β-pinene, d-limonene, terpinolene, linalool, humulene, and β-caryophyllene. Additionally, VSC3–VSC7 were also used as references (0.01% dilution in triacetin) and were used to obtain aroma descriptors shown in Table 2. The trained panel was used to determine and distinguish the difference or similarities between flower, extract, and reverse engineered formulations. All members received the same training and understood the use of descriptors to describe samples. Samples were stored in scintillation vials and capped prior to testing. The panel then ranked each sample on a scale of 0–10, with 10 being the most pungent aroma. The results were averaged and compared with the concentrations of VSCs.
Table 2. Volatile Sulfur Compounds (VSCs) Detected in Cannabis, CAS Numbers, Aroma Descriptions, and Retention Times.
| compound ID | compound name | CAS number | aroma descriptora | 1tR, 1tR (min, s) |
|---|---|---|---|---|
| VSC1 | dimethyl sulfide | 75-18-3 | sulfurous, vegetable, cabbage | 4.684, 1.851 |
| VSC2 | 3-methylthiophene | 616-44-4 | fatty, winey | 9.009, 8.967 |
| VSC3 | 3-methyl-2-butene-1-thiol | 5287-45-6 | intense, sulfurous, skunk-like | 10.082, 2.318 |
| VSC4 | 3-methyl-1-(methylthio)-2-butene | 5897-45-0 | intense, sulfurous, savory | 13.226, 2.285 |
| VSC5 | 3-methyl-2-butenyl acetothioate | 33049-93-3 | intense, sulfurous, skunk-like | 20.328, 3.4479 |
| VSC6 | bis(3-methyl-2-butenyl) sulfide | N/A | mild, alliaceous | 25.465, 2.848 |
| VSC7 | bis(3-methyl-2-butenyl) disulfide | 24963-39-1 | mild, alliaceous | 46.706, 2.965 |
Aroma descriptors reported by olfactory testing panel.
Data obtained from the VOC analysis experiments for the cultivar Bacio Gelato (C1) was used to reverse-engineer the chemical makeup of the aroma. All reagents were purchased from Sigma-Aldrich except 3-methyl-2-butene-1-thiol (VSC3) (1% in Triacetin), which was purchased from Excellentia. The top 10 compounds were added together in their respective concentrations to emulate the aroma of the flower sample. The solution was split into two scintillation vials to act as a control and experimental group. An approximate 0.01% addition of VSC3 was then added and stirred into the experimental group. The testing panel was again used to compare the Bacio Gelato aroma formulation with and without VSC3 to determine how this compound affects the aroma.
Indoor Greenhouse Trial
Four cannabis clones of the OG varietal (Clone Guy Industries) were purchased from the Empire Connect dispensary in San Bernadino, CA. The clones were allowed to grow in the vegetative stage for four weeks before being transplanted into a cocoa-based medium (Canna Brand). They were then installed into a custom-designed and -built hydroponic feeding system (Canna Precision Inc). Reverse osmosis purified water was used as the medium that was flushed and filled once a day. Nutrients used was Mr. Nice Guys brand and followed their suggested feeding schedule. The temperature and relative humidity were maintained at ≈22 °C and ≈55%, respectively. During the vegetative stage, continuous lighting (24 h/day) was maintained until the plants reached an appropriate size. The light cycle was then changed to alternate between 12 hours of light and darkness to induce the flowering stage of growth. Once inflorescence developed to an appropriate size (week 2 of the flowering stage), each plant had a sample cut to be measured on our GC × GC–TOF-MS/FID/SCD. After the flowers were deemed fully mature, the plants were cut down and hung to cure and dry for 11 days at a temperature and relative humidity of ≈22 °C and ≈45%, respectively, to emulate the process used during commercial cannabis preparation. The water activity of the flowers was monitored during the curing process using a Pawkit water activity meter (Meter Food Group). Data was collected on the final day of the curing process. The flowers were then cut from the stems, dried leaves trimmed, and stored in glass mason jars. A final dataset was collected ten days after the curing process ended.
Results
Detecting and Identifying VSCs in Cannabis Using Sulfur Chemiluminescence
Numerous VSCs were identified in our initial screening of cultivars using 2-dimensional gas chromatography coupled with sulfur chemiluminescence. We then compared VSC concentrations in 13 different cannabis cultivars to identify trends between their aroma characteristics and individual compounds (Table 1). The aromas of the cultivars were ranked on a score of 0–10 by a four-member olfactory testing panel (individual results can be found in Table S4), with 10 representing the most pungent cannabis aroma and 0, the least pungent. The two cultivars with the greatest difference in their olfactory scores were Bacio Gelato (C1) and Black Jack (C13), with scores of 10 and 0, respectively. As such, we investigated differences in their corresponding GC × GC–SCD chromatograms (Figure 2). Intense peaks seen in C1 are completely absent in C13, indicating that the former contains many more VSCs than the latter. In particular, the intense peak located at 1tR = 10.082 min and 2tR = 2.313 s (identified as 3-methyl-2-butene-1-thiol, VSC3) dominates the SCD chromatogram. The only similar peak between these two datasets occurs at 1tR = 4.67 min and 2tR = 1.88 s that was identified as dimethyl sulfide (VSC1). As these two cultivars have very different aroma characteristics, i.e., C1 has an extremely pungent, skunk-like aroma, whereas C13 has a mild, woody aroma, VSC1 was eliminated as the primary source of the intense aroma detected in C1. Comparing the raw peak intensities of the SCD chromatograms of the other cultivars to their olfactory scores revealed lower peak intensities trending with lower scores, as shown in Figure S13.
Table 1. Cultivars, Sample Ages, Concentrations of VSCs, and Olfactory Scores.
| sample ID | cultivar | [VSC3] (μg/mg) | [VSC4] (μg/mg) | [VSC5] (μg/mg) | [VSC6] (μg/mg) | [VSC7] (μg/mg) | Olfactory scorea |
|---|---|---|---|---|---|---|---|
| C1 | Bacio Gelato | 1.35(33) × 10–2 | 1.79(3) × 10–4 | 5.99(2.46) × 10–4 | 3.23(76) × 10–3 | 5.60(1.40) × 10–3 | 10.0(0) |
| C2 | Clone Guy OG | 4.62(1.50) × 10–3 | ND | 6.95(2.46) × 10–4 | 1.70(48) × 10–3 | 1.71(53) × 10–3 | 8.6(4) |
| C3 | Gelatob | 3.48(1.25) × 10–3 | ND | 1.87(49) × 10–3 | 1.06(15) × 10–3 | 1.10(26) × 10–3 | 8.9(5) |
| C4 | Area 41 | 2.94(25) × 10–3 | ND | 5.27(52) × 10–4 | 1.31(15) × 10–3 | 1.40(52) × 10–3 | 8.1(3) |
| C5 | Jetlag OG | 2.05(27) × 10–3 | ND | 2.85(90) × 10–4 | 1.14(13) × 10–3 | 1.00(13) × 10–3 | 7.5(4) |
| C6 | Gushers | 2.04(82) × 10–3 | ND | 1.46(10) × 10–4 | 7.70(10) × 10–4 | 8.99(2.50) × 10–4 | 7.0(7) |
| C7 | WiFi Cake | 1.30(46) × 10–3 | ND | 4.74(1.04) × 10–4 | 7.94(17) × 10–4 | 7.16(2.93) × 10–4 | 6.5(4) |
| C8 | Apple Fritter | 1.34(17) × 10–3 | ND | 2.18(42) × 10–4 | 7.73(8) × 10–4 | 5.37(40) × 10–4 | 5.3(6) |
| C9 | Chem 91 | 6.50(87) × 10–4 | ND | 1.88(6) × 10–4 | 4.27(6) × 10–4 | 2.77(41) × 10–4 | 1.6(5) |
| C10 | Gelatob | 6.59(88) × 10–4 | ND | 5.30(94) × 10–4 | 6.48(3) × 10–4 | 4.49(1.48) × 10–4 | 2.6(9) |
| C11 | Cali Berry | 6.50(42) × 10–4 | ND | 2.00(10) × 10–4 | 4.87(3) × 10–4 | 1.74(46) × 10–4 | 1.1(3) |
| C12 | Gouda Berry | ND | ND | ND | ND | ND | 0.0(0) |
| C13 | Black Jack | ND | ND | ND | ND | ND | 0.0(0) |
Olfactory score represents pungency of characteristic aroma of cannabis from 0 to 10, where 10 represents the highest pungency.
C3 and C10 were the same product lot measured at different sample ages.
Figure 2.
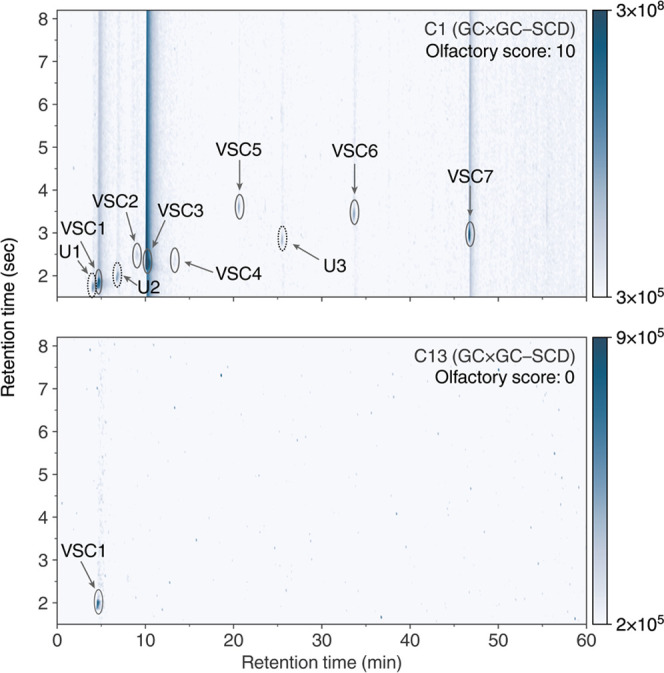
2D chromatograms of GC × GC–SCD data for cultivars with the largest difference in olfactory scores, Bacio Gelato (C1, top), and Black Jack (C13, bottom). Color bars indicate detector response intensity. Significant peaks are circled and annotated, as seen in Table 2 in black. Unknown VSCs are indicated by the black, dashed circles.
Time-of-flight mass spectrometry was used to determine the chemical structures of the detected VSCs. Standards were used to ensure similar elution times and mass spectra. A complete list of VSCs identified is shown in Table 2 with structures, names, CAS numbers (if applicable), first- and second-dimension retention times, and respective aroma properties. Of all cultivars, Bacio Gelato (C1) possesses the highest concentration of VSCs, as shown in Table 1, and thus we focus on this cultivar to describe the identification and quantification of VSCs. We note that unknown VSC eluents exist due to unresolved mass spectra and are listed in Table S3.
Figure 3 shows C1 GC × GC–SCD and GC × GC–TOF–MS data. VSC3 (3-methyl-2-butene-1-thiol) elutes at 1tR = 10.082 min and 2tR = 2.313 s and has a molar mass of 102.20 g/mol; the mass spectral peak at m/z ≈ 102 thus corresponds to the molecular ion. The major peak located at m/z ≈ 69 corresponds to the prenyl ion, C5H10+. This is further substantiated by the fact that the other compounds containing a prenyl moiety have an intense peak at m/z ≈ 69. The final major peak is located at m/z ≈ 41, which most likely corresponds to the C3H6+ fragment. This spectrum was then compared with entries in the aforementioned spectral libraries, revealing excellent agreement with the spectra of VSC3 (Figure S8). Lastly, analysis of a VSC3 standard (Excellentia, 1% in triacetin) confirmed the elution retention time in the 2-dimensional data (Figure S13). This compound has an intense, sulfuric, skunky aroma even in extremely dilute concentrations. We note that this compound has been detected in beer previously and is the primary compound leading to the flavor and aroma of “skunked beer”.41
Figure 3.
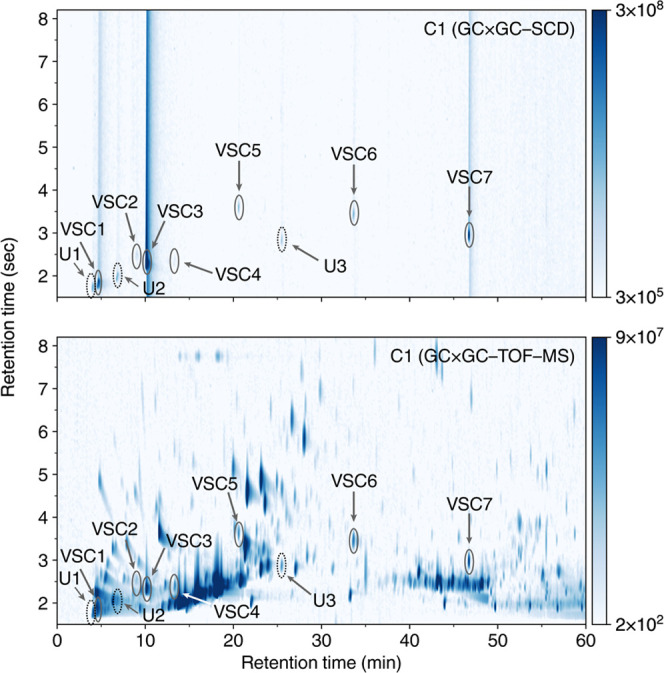
Cultivar C1 GC × GC–SCD (top) and GC × GC–TOF–MS (bottom) chromatograms showing the location of VSCs. GC × GC–SCD provides a convenient method for detecting and identifying these eluents in the more complex GC × GC–TOF–MS chromatogram.
VSC1 and VSC2 were quickly identified as dimethyl sulfide and 3-methylthiophene, respectively, by comparing mass spectral data with entries in the NIST Spectral Library v17 (2017) and Wiley Registry of Mass Spectra (11th Edition). As these compounds do not contribute strongly to the scent of cannabis, we focus our discussion on compounds VSC3–VSC7.
Eluents VSC4–VSC7 were not found in the mass spectral databases and therefore were determined by analysis of mass spectral data, first- and second-dimension retention times, and standards. Each of these eluents contains similar ions in their data, suggesting similar chemical structures. Indeed, like VSC3, we found each of these compounds contains the prenyl functional group.
VSC4 was identified as 3-methyl-1-(methylthio)-2-butene. Figure S9 shows the mass spectral data closely resembling that of VSC3, with major peaks located at m/z ≈ 41 and 69, indicating that the prenyl moiety is found in this structure but with a molecular ion of m/z ≈ 116. This mass corresponds to the formula C6H12S, which corresponds to the methylated analogue of VSC3, 3-Methyl-1-(methylthio)-2-butene. This was confirmed by a standard yielding a similar elution time as that found in C1 (Figure S14). This compound has rarely been described previously, although it is reported to be an important component of the foul-smelling defensive secretion of the beetle Ceroglossus buqueti.42 The aroma of this compound is potent, resembling that of VSC3 but with a more savory, umami scent.
VSC5 was found to be 3-Methyl-2-butenyl acetothioate. Like VSC3, the ions m/z ≈ 41, 69, and 102 are present but with a largest m/z is ≈ 144, which corresponds to the formula C7H12OS (Figure S10). Additionally, this eluent is found at a higher second-dimension retention time than VSC3, suggesting greater polarity and most likely includes an electronegative atom such as oxygen. These data suggest VSC5 to be the compound 3-Methyl-2-butenyl acetothioate (CAS # 33049-93-3), which was confirmed by a standard of 3-Methyl-2-butenyl acetothioate (Figure S15). The aroma of this compound closely resembles that of VSC3, however, with less potency.
VSC6 was confirmed to be bis(3-methyl-2-butenyl) sulfide. The mass spectrum presents similar peaks as the previous compounds (m/z ≈ 41, 69, 101) but with a molecular ion m/z ≈ 170. This ion corresponds to the formula C10H18S (Figure S11). As the main ions are the same as VSC3, the prenyl moiety is most likely present. Additionally, ion m/z ≈ 69 has a significantly higher relative intensity compared with that in VSC3, suggesting multiple prenyl moieties in the structure. Bis(3-methyl-2-butenyl) sulfide contains two symmetrical prenyl moieties bridged by the sulfur atom, which would yield similar fragments upon ionization and thus generate a higher m/z ≈ 69 ion relative intensity peak like that seen in the data. A standard of bis(3-methyl-2-butenyl) sulfide revealed the same mass spectra and elution times confirming the assignment (Figure S16). This compound has a much lower odor intensity thanVSC3–VSC5 and has a more alliaceous aroma.
Lastly, VSC7 was identified as the disulfide analogue of VSC3, bis(3-Methyl-2-butenyl) disulfide. The mass spectrum shows a molecular ion of m/z ≈ 202, corresponding to the formula C10H18S2. The major ions m/z ≈ 41, 69 again indicate similar functionality as VSC3–VSC6. Indeed, a standard of VSC7 confirmed the correct assignment (Figures S12 and S17). This compound has a similar aroma to VSC6, with a mild, alliaceous scent.
Discussion
Structural Commonalities between VSCs in Cannabis and Garlic
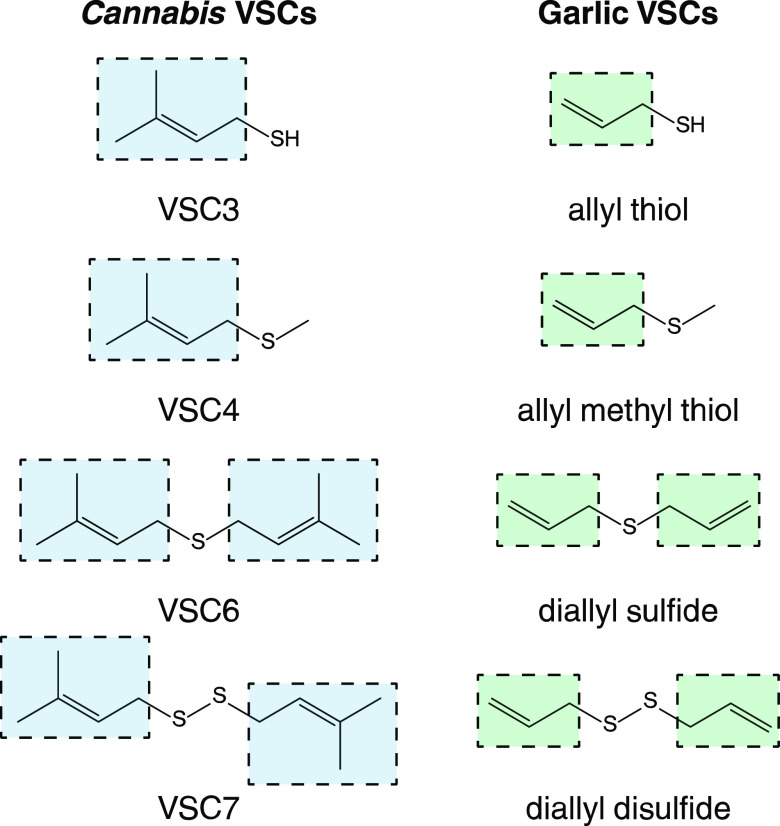
The VSCs in cannabis reported here are structurally similar to those found in garlic (Figure 4). Many garlic VSCs contain the allyl functional group, which is related to the prenyl group by replacing the two methyl groups on the terminal C3 carbon with hydrogens. These compounds contribute to garlic’s aroma, flavor, and possible health benefits.32,33,39,40 For instance, the allylic analogue of VSC3, allyl thiol, is a histone deacetylase (HDAC) inhibitor, which may engender it with anticarcinogenic properties.43−45 Diallyl disulfide, which is structurally analogous to VSC7, may help protect against colorectal cancer and contribute beneficially toward cardiovascular health.43,46 In the latter case, diallyl disulfide is converted in the body into hydrogen sulfide (H2S), which is a cardioprotective vascular cell signaling molecule that has beneficial vasoactivity.47 A key step in this conversion involves nucleophilic substitution at the α-carbon of the allyl group. VSC7 likewise contains an α-carbon on its prenyl groups neighboring a sulfur atom and therefore may undergo similar conversion in the human body. The structural similarities between VSCs in cannabis and garlic thus warrant further investigation to determine if the former possess similar health benefits to those of the latter.
Figure 4.
VSCs containing the prenyl moiety in cannabis (left) and VSCs containing the allyl moiety in garlic (right) show structural similarities that may suggest similar biological properties.
Correlating VSCs to the Aroma of Cannabis
Identifying VSCs in cannabis allowed us to then correlate their concentrations in the cultivars to their aromatic properties. Specifically, we observed a strong correlation between VSC3 concentration and the pungency of the characteristic “skunk-like” aroma of cannabis from the olfactory tests. We note that VSC4–VSC7 generally trend similarly as VSC3 across the samples but at lower concentrations. Nonetheless, certain compounds were only detected in specific cultivars. VSC4 was only detected in C1, while two unknowns (U4 and U5 in Table S3) were only observed in C7. This suggests that certain cultivars may produce unique VSC metabolites that others do not, although we caution that several factors must be considered.
First, the product age significantly affects the concentrations of VSCs, as evident from the data collected on the cultivar Gelato at two different times (C3 and C10), as well as the general trend that samples with older product ages tend to have lower VSC concentrations (product ages shown in Table S4). C3 was measured four days after the packaging date, while C10 was measured 46 days after. C3 had a concentration nearly 3 times greater than C10, showing that after about a month and a half, the majority of VSC3 volatilizes. This data suggests that cultivars with older product ages may have produced VSCs in greater quantities at an earlier time but are now at lower concentrations, some of which may be below our limits of detection.
Second, the packaging containers that the flowers are stored in likely play a role in the retention of VSCs as well. Gushers has a moderately high VSC3 concentration relative to the other cultivars measured, yet it was the oldest sample measured. This sample was packaged in a plastic jar with a heat-sealed airtight aluminum film to protect the product before use, which may contribute to its higher concentration of VSCs. On the other hand, most other samples were packaged in mylar-like zipper storage bags, which may not be as conducive to containing VSCs as the sealed plastic jars.
Third is the possibility that specific plant growth conditions and genetic differences are conducive to production of certain VSCs. It is well known that different types of abiotic stress, such as temperature, humidity, or light intensity, can modify how plants produce secondary metabolites.48 As these cultivars were produced by different cultivators (Table S4), it is possible that the growth conditions used may have facilitated different production of secondary metabolites. Cultivation experiments monitoring different stress or nutrient regiments may help provide insight into how they affect VSC production during growth.
Confirming the Aroma of Cannabis via Reverse Engineering
To definitively confirm the contribution of VSCs to the aroma of cannabis, we reverse-engineered the C1 cultivar aroma by creating a formulation of the top ten components with and without VSC3. VSC3 was chosen as it has the largest concentration range among cultivars and scales most closely with the olfactory scores. The top ten compounds were added together into a scintillation vial to emulate the major aroma of the cultivar. We found that although the scent was mildly reminiscent of the flower, it did not possess the pungent, skunk-like aroma. Addition of VSC3 at approximately 1% dilution (1% VSC3 in triacetin) resulted in an immediate olfactory change that strongly emulated the scent of the flower. Interestingly, although VSC3 evaluated independently had a sulfuric, skunk-like aroma, the combination of the formulation and VSC3 was described most closely to the characteristic scent of cannabis, indicating that the combination of VSCs and other major components combine synergistically to yield this scent.
We note that when the scintillation vial was left uncapped for even short periods of time (∼15 to 20 min), the scent associated with VSC3 was almost undetectable, indicating that this compound volatilizes quickly. These olfactory tests confirm that VSC3 is the primary source of the characteristic scent of cannabis, while the remaining compounds VSC4– VSC7 may further intensify or modulate this aroma.
Detection of VSCs in Concentrated Cannabis Extract Products
The discovery of VSCs in cannabis flowers opens the question as to whether concentrated extracts also contain these compounds. Cannabis concentrates are an increasingly popular form of consumption, such as those found in vapes or “dabbing.”49 These concentrates are often produced using a hydrocarbon solvent, such as butane, to extract the desired cannabinoids and terpenoids from freshly cut cannabis plants at low temperatures. We measured the VSCs present in three butane hash oil (BHO) concentrates (Sherbinskis brand) (Figure 5).
Figure 5.
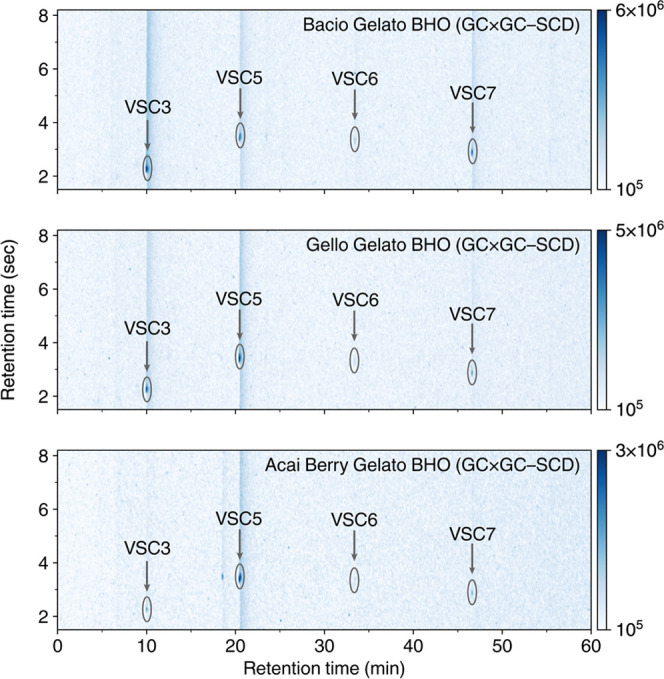
GC × GC–SCD 2D chromatograms for BHO extracts measured. Peak intensity indicates a higher detector response. Significant peaks are circled and annotated. The occurrence of these compounds indicates they can be found in cannabis extract products.
We observe not only significant concentrations of VSC3, the major VSC in the flower samples, but also VSC5, as shown in Table 3. In fact, both Gello Gelato and Acai Berry Gelato have higher VSC5 concentrations than VSC3, a result not seen in the flower samples above. Both samples have pungent aromas, indicating that VSC5 contributes strongly to the characteristic “skunk-like” scent of cannabis like VSC3. This was further substantiated by the olfactory testing panel results (Table 3), showing very high scores for each extract, confirming that the high concentrations for VSC3 and VSC5 correlate with the pungency of the samples. These results show that concentrated extracts can retain these volatile compounds throughout the extraction process and thus can have very strong aromas like cannabis flowers.
Table 3. BHO Samples, Their Respective VSC Concentrations, and Average Olfactory Scores.
| sample | [VSC3] (μg/mg) | [VSC5] (μg/mg) | [VSC6] (μg/mg) | [VSC7] (μg/mg) | Olfactory score |
|---|---|---|---|---|---|
| Bacio Gelato BHO | 5.91 × 10–3 | 5.08 × 10–3 | 1.68 × 10–3 | 5.87 × 10–3 | 9.8(2) |
| Gello Gelato BHO | 3.45 × 10–3 | 7.71 × 10–3 | 9.51 × 10–4 | 2.17 × 10–3 | 9.7(2) |
| Acai Berry Gelato BHO | 1.95 × 10–3 | 7.09 × 10–3 | 7.95 × 10–4 | 8.13 × 10–4 | 9.6(2) |
Evolution of VSC Concentrations as a Function of Plant Growth and Storage
To understand how VSC concentrations change during the lifespan of a cannabis plant, we conducted an indoor greenhouse trial monitoring them as a function of time. Four cannabis clones were grown in a controlled hydroponically fed system (see methods) over the course of 14 weeks. After maintaining the plants in the vegetative state for four weeks, the lighting was changed to alternate between 12 h of light and 12 h of darkness to induce the flowering stage. We monitored the evolution of VSC3 concentration in the four plants starting in week two of the flowering stage (the first week where flowers were large enough to cut and measure) until complete (week 10), through the curing process, and ten days after (Figure 6).
Figure 6.
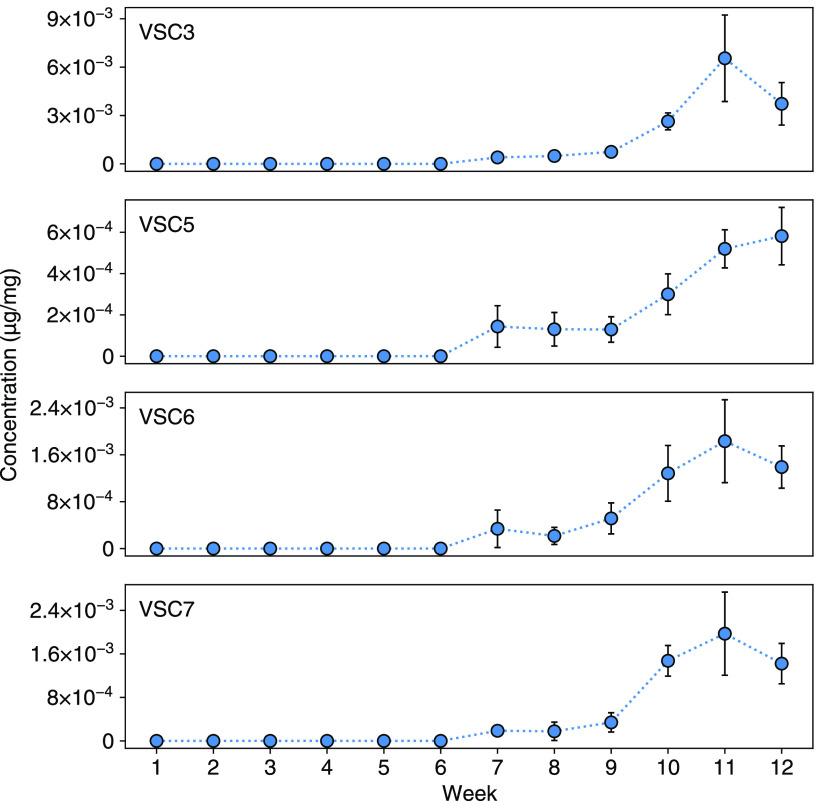
Concentration of detected VSCs as a function of week during indoor greenhouse trial. Week 1 represents the first week of the flowering stage of plant growth. Week 11 data was taken on the dried and cured flower. Week 12 data was taken 10 days after the end of curing. The rapid increase in VSC concentrations toward the end of the flowering stage was concomitant with increased odor intensity.
We did not detect any of the VSCs contributing to the skunky aroma of cannabis until the seventh week of the flowering stage, at which point VSC3, VSC5, VSC6, and VSC7 were detected at low concentrations. After this initial detection, the concentration of each increased rapidly through week 10—the final week of the flowering stage, with the others also increasing. At this point, the plants were cut and allowed to cure and dry for 11 days where the concentration of VSC3, VSC6, and VSC7 reached a maximum at the end of this process. Once the flowers were considered sufficiently dry (water activity of approximately 0.58), they were cut from the stems and stored in mason jars at room temperature. A final data point was measured 10 days after, which showed a substantial decrease in all VSC concentrations except VSC5, which was comparable to the previous data point. We hypothesize that VSC5 may not volatilize as readily as the others due to hydrogen bonding between the thioacetate group and other oxygenated functional groups within the plant. However, further experiments are needed to confirm this.
The rapid increase of VSCs was concomitant with an intense rise in the pungency of the skunk-like aroma of the flowers. The final datapoint taken 10 days after curing and drying had a substantially less potent odor, again correlating with the drop in concentrations for these compounds. In particular, the drop in VSC3 concentration suggests rapid volatilization, which most likely contributes to the extremely diffuse and detectable aroma associated with cannabis.
Conclusions
We investigated the origins of the characteristic skunk-like aroma of cannabis using 2-dimensional gas chromatography coupled with time-of-flight mass spectrometry, flame ionization detection, and sulfur chemiluminescence. Our results found that the primary compounds that contribute to this scent are a new family of volatile sulfur compounds (VSCs), with 3-methyl-2-butene-1-thiol correlating most strongly with the aroma of 13 cannabis cultivars. We then analyzed three concentrated cannabis extract products to determine if VSCs are retained during the hydrocarbon extraction process. Indeed, we observe high concentrations of both 3-methyl-2-butene-1-thiol and 3-methyl-2-butenyl acetothioate, indicating that cannabis extract products can likewise have a pungent “skunky” aroma. Lastly, an indoor greenhouse trial was conducted, revealing that the concentrations of the discovered VSCs increase significantly toward the end of the flowering stage of growth, reach a maximum during curing, and then drop substantially after only 10 days of storage. Our results highlight how two-dimensional gas chromatography coupled with mass spectrometry, sulfur chemiluminescence, and flame ionization detection can be used to analyze the complex mixture of volatile compounds in cannabis. Furthermore, identification of the reported VSCs definitively confirms the chemical origins of the odor of cannabis and provides a new family of secondary metabolites that can be investigated regarding their biosynthetic pathways and medicinal benefits.
Acknowledgments
The authors thank C. Tafoya and A. Kahn for help preparing samples and S. Galli for help developing GC × GC instrumentation. No external funding was used for this study.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c04196.
Calibration curves and data for quantification of VSCs and VOCs, mass spectral data of VSCs identified GC × GC–SCD chromatograms comparing VSCs in cannabis to analytical standards, tables containing the highest concentration aroma compounds for all compounds (PDF)
Author Contributions
I.W.H.O. conceived and conducted the GC × GC experiments, analyzed the results, and wrote the manuscript. M.A.O. conducted GC × GC experiments and data analysis and edited the manuscript. R.J.P. designed and managed the indoor greenhouse trial. J.D.R. and M.A.G. provided guidance on the indoor greenhouse trial and edited the manuscript. K.A.K. and T.J.M. edited the manuscript and oversaw the project.
The authors declare the following competing financial interest(s): A patent related to the findings reported has been filed by I. W. H. O., K. A. K., and T. J. M.
Supplementary Material
References
- Adinoff B.; Cooper Z. D. Cannabis legalization: progress in harm reduction approaches for substance use and misuse. Am. J. Drug Alcohol Abuse 2019, 45, 707–712. 10.1080/00952990.2019.1680683. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. T.; Yarnell S.; Radhakrishnan R.; Ball S. A.; D’Souza D. C. Marijuana Legalization: Impact on Physicians and Public Health. Annu. Rev. Med. 2016, 67, 453–466. 10.1146/annurev-med-050214-013454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart R.; Pacula R. L. Early evidence of the impact of cannabis legalization on cannabis use, cannabis use disorder, and the use of other substances: Findings from state policy evaluations. Am. J. Drug Alcohol Abuse 2019, 45, 644–663. 10.1080/00952990.2019.1669626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D.; Goodman S.; Wadsworth E.; Rynard V.; Boudreau C.; Hall W. Evaluating the impacts of cannabis legalization: The International Cannabis Policy Study. Int. J. Drug Policy 2020, 77, 102698 10.1016/j.drugpo.2020.102698. [DOI] [PubMed] [Google Scholar]
- Caulkins J. P.; Kilborn M. L. Cannabis legalization, regulation, & control: a review of key challenges for local, state, and provincial officials. Am. J. Drug Alcohol Abuse 2019, 45, 689–697. 10.1080/00952990.2019.1611840. [DOI] [PubMed] [Google Scholar]
- ElSohly M. A.; Slade D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Flores-Sanchez I. J.; Verpoorte R. Secondary metabolism in cannabis. Phytochem. Rev. 2008, 7, 615–639. 10.1007/s11101-008-9094-4. [DOI] [Google Scholar]
- Fischedick J. T.; Hazekamp A.; Erkelens T.; Choi Y. H.; Verpoorte R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry 2010, 71, 2058–2073. 10.1016/j.phytochem.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Marchini M.; Charvoz C.; Dujourdy L.; Baldovini N.; Filippi J.-J. Multidimensional analysis of cannabis volatile constituents: Identification of 5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane as a volatile marker of hashish, the resin of Cannabis sativa L. J. Chromatogr. A 2014, 1370, 200–215. 10.1016/j.chroma.2014.10.045. [DOI] [PubMed] [Google Scholar]
- Andre C. M.; Hausman J.-F.; Guerriero G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira A.; Berman P.; Futoran K.; Guberman O.; Meiri D. Tandem Mass Spectrometric Quantification of 93 Terpenoids in Cannabis Using Static Headspace Injections. Anal. Chem. 2019, 91, 11425–11432. 10.1021/acs.analchem.9b02844. [DOI] [PubMed] [Google Scholar]
- Booth J. K.; Page J. E.; Bohlmann J. Terpene synthases from Cannabis sativa. PLoS One 2017, 12, e0173911 10.1371/journal.pone.0173911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommano S. R.; Chittasupho C.; Ruksiriwanich W.; Jantrawut P. The Cannabis Terpenes. Molecules 2020, 25, 5792 10.3390/molecules25245792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K. M.; Bisson J.; Singh G.; Graham J. G.; Chen S.-N.; Friesen J. B.; Dahlin J. L.; Niemitz M.; Walters M. A.; Pauli G. F. The Essential Medicinal Chemistry of Cannabidiol (CBD). J. Med. Chem. 2020, 63, 12137–12155. 10.1021/acs.jmedchem.0c00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchi P. K.; Dasmahapatra A. K. Destabilization of the Alzheimer’s amyloid-β protofibrils by THC: A molecular dynamics simulation study. J. Mol. Graph. Model. 2021, 105, 107889 10.1016/j.jmgm.2021.107889. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A.; Albayram O.; Draffehn A.; Michel K.; Piyanova A.; Oppenheimer H.; Dvir-Ginzberg M.; Rácz I.; Ulas T.; Imbeault S.; Bab I.; Schultze J. L.; Zimmer A. A chronic low dose of Δ9-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat. Med. 2017, 23, 782–787. 10.1038/nm.4311. [DOI] [PubMed] [Google Scholar]
- Nugent S. M.; Morasco B. J.; O’Neil M. E.; Freeman M.; Low A.; Kondo K.; Elven C.; Zakher B.; Motu’apuaka M.; Paynter R.; Kansagara D. The Effects of Cannabis Among Adults With Chronic Pain and an Overview of General Harms. Ann. Intern. Med. 2017, 167, 319–331. 10.7326/M17-0155. [DOI] [PubMed] [Google Scholar]
- Ligresti A.; De Petrocellis L.; Di Marzo V. From Phytocannabinoids to Cannabinoid Receptors and Endocannabinoids: Pleiotropic Physiological and Pathological Roles Through Complex Pharmacology. Physiol. Rev. 2016, 96, 1593–1659. 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]
- Stockings E.; Zagic D.; Campbell G.; Weier M.; Hall W. D.; Nielsen S.; Herkes G. K.; Farrell M.; Degenhardt L. Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence. J. Neurol. Neurosurg. Psychiatry 2018, 89, 741. 10.1136/jnnp-2017-317168. [DOI] [PubMed] [Google Scholar]
- Moreau M.; Ibeh U.; Decosmo K.; Bih N.; Yasmin-Karim S.; Toyang N.; Lowe H.; Ngwa W. Flavonoid Derivative of Cannabis Demonstrates Therapeutic Potential in Preclinical Models of Metastatic Pancreatic Cancer. Front. Oncol. 2019, 9, 660 10.3389/fonc.2019.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers H. M.; Sproul E.; Quinn J. C. The greenhouse gas emissions of indoor cannabis production in the United States. Nat. Sustainable 2021, 4, 644–650. 10.1038/s41893-021-00691-w. [DOI] [Google Scholar]
- Rice S.; Koziel J. A. Characterizing the Smell of Marijuana by Odor Impact of Volatile Compounds: An Application of Simultaneous Chemical and Sensory Analysis. PLoS One 2015, 10, e0144160 10.1371/journal.pone.0144160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micalizzi G.; Vento F.; Alibrando F.; Donnarumma D.; Dugo P.; Mondello L. Cannabis Sativa L.: a comprehensive review on the analytical methodologies for cannabinoids and terpenes characterization. J. Chromatogr. A 2021, 1637, 461864 10.1016/j.chroma.2020.461864. [DOI] [PubMed] [Google Scholar]
- Fischedick J. T. Identification of Terpenoid Chemotypes Among High (-)-trans-Δ(9)-Tetrahydrocannabinol-Producing Cannabis sativa L. Cultivars. Cannabis Cannabinoid Res. 2017, 2, 34–47. 10.1089/can.2016.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber B.; Weggler B. A.; Jaramillo R.; Murrell K. A.; Piotrowski P. K.; Dorman F. L. Comprehensive two-dimensional gas chromatography in forensic science: A critical review of recent trends. TrAC Trends Anal. Chem. 2018, 105, 292–301. 10.1016/j.trac.2018.05.017. [DOI] [Google Scholar]
- Andersen K. K.; Bernstein D. T. Some chemical constituents of the scent of the striped skunk (Mephitis mephitis). J. Chem. Ecol. 1976, 1, 493–499. 10.1007/BF00988589. [DOI] [Google Scholar]
- Lermusieau G.; Collin S. Volatile Sulfur Compounds in Hops and Residual Concentrations in Beer—A Review. J. Am. Soc. Brew. Chem. 2003, 61, 109–113. 10.1094/ASBCJ-61-0109. [DOI] [Google Scholar]
- Collin S.; Vermeulen C. Combinatorial Synthesis and Screening of Novel Odorants Such as Polyfunctional Thiols. Comb. Chem. High Throughput Screen. 2006, 9, 583–590. 10.2174/138620706778249712. [DOI] [PubMed] [Google Scholar]
- Takazumi K.; Takoi K.; Koie K.; Tuchiya Y. Quantitation Method for Polyfunctional Thiols in Hops (Humulus lupulus L.) and Beer Using Specific Extraction of Thiols and Gas Chromatography–Tandem Mass Spectrometry. Anal. Chem. 2017, 89, 11598–11604. 10.1021/acs.analchem.7b02996. [DOI] [PubMed] [Google Scholar]
- Takoi K.; Degueil M.; Shinkaruk S.; Thibon C.; Maeda K.; Ito K.; Bennetau B.; Dubourdieu D.; Tominaga T. Identification and Characteristics of New Volatile Thiols Derived from the Hop (Humulus luplus L.) Cultivar Nelson Sauvin. J. Agric. Food Chem. 2009, 57, 2493–2502. 10.1021/jf8034622. [DOI] [PubMed] [Google Scholar]
- Block E. The Organosulfur Chemistry of the Genus Allium—Implications for the Organic Chemistry of Sulfur. Angew. Chem., Int. Ed. Engl. 1992, 31, 1135–1178. 10.1002/anie.199211351. [DOI] [Google Scholar]
- Vaidya V.; Ingold K. U.; Pratt D. A. Garlic: Source of the Ultimate Antioxidants—Sulfenic Acids. Angew. Chem. Int. Ed. 2009, 48, 157–160. 10.1002/anie.200804560. [DOI] [PubMed] [Google Scholar]
- Borlinghaus J.; Foerster Née Reiter J.; Kappler U.; Antelmann H.; Noll U.; Gruhlke M. C. H.; Slusarenko A. J. Allicin, the Odor of Freshly Crushed Garlic: A Review of Recent Progress in Understanding Allicin’s Effects on Cells. Molecules 2021, 26, 1505. 10.3390/molecules26061505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-X.; Schieberle P.; Steinhaus M. Characterization of the Major Odor-Active Compounds in Thai Durian (Durio zibethinus L. ‘Monthong’) by Aroma Extract Dilution Analysis and Headspace Gas Chromatography–Olfactometry. J. Agric. Food Chem. 2012, 60, 11253–11262. 10.1021/jf303881k. [DOI] [PubMed] [Google Scholar]
- Li J.-X.; Schieberle P.; Steinhaus M. Insights into the Key Compounds of Durian (Durio zibethinus L. ‘Monthong’) Pulp Odor by Odorant Quantitation and Aroma Simulation Experiments. J. Agric. Food Chem. 2017, 65, 639–647. 10.1021/acs.jafc.6b05299. [DOI] [PubMed] [Google Scholar]
- Kishimoto T.; Kobayashi M.; Yako N.; Iida A.; Wanikawa A. Comparison of 4-Mercapto-4-methylpentan-2-one Contents in Hop Cultivars from Different Growing Regions. J. Agric. Food Chem. 2008, 56, 1051–1057. 10.1021/jf072173e. [DOI] [PubMed] [Google Scholar]
- Roland A.; Viel C.; Reillon F.; Delpech S.; Boivin P.; Schneider R.; Dagan L. First identification and quantification of glutathionylated and cysteinylated precursors of 3-mercaptohexan-1-ol and 4-methyl-4-mercaptopentan-2-one in hops (Humulus lupulus). Flavour Fragr. J. 2016, 31, 455–463. 10.1002/ffj.3337. [DOI] [Google Scholar]
- Warren J. M.; Parkinson D.-R.; Pawliszyn J. Assessment of Thiol Compounds from Garlic by Automated Headspace Derivatized In-Needle-NTD-GC-MS and Derivatized In-Fiber-SPME-GC-MS. J. Agric. Food Chem. 2013, 61, 492–500. 10.1021/jf303508m. [DOI] [PubMed] [Google Scholar]
- Iciek M.; Kwiecień I.; Chwatko G.; Sokołowska-Jeżewicz M.; Kowalczyk-Pachel D.; Rokita H. The effects of garlic-derived sulfur compounds on cell proliferation, caspase 3 activity, thiol levels and anaerobic sulfur metabolism in human hepatoblastoma HepG2 cells. Cell Biochem. Funct. 2012, 30, 198–204. 10.1002/cbf.1835. [DOI] [PubMed] [Google Scholar]
- Bayan L.; Koulivand P. H.; Gorji A. Garlic: a review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [PMC free article] [PubMed] [Google Scholar]
- Burns C. S.; Heyerick A.; De Keukeleire D.; Forbes M. D. E. Mechanism for Formation of the Lightstruck Flavor in Beer Revealed by Time-Resolved Electron Paramagnetic Resonance. Chem. Eur. J. 2001, 7, 4553–4561. . [DOI] [PubMed] [Google Scholar]
- Xu S.; Errabeli R.; Will K.; Arias E.; Attygalle A. B. 3-Methyl-1-(methylthio)-2-butene: a component in the foul-smelling defensive secretion of two Ceroglossus species (Coleoptera: Carabidae). Chemoecology 2019, 29, 171–178. 10.1007/s00049-019-00286-0. [DOI] [Google Scholar]
- Bradley J. M.; Organ C. L.; Lefer D. J. Garlic-Derived Organic Polysulfides and Myocardial Protection. J. Nutr. 2016, 146, 403S–409S. 10.3945/jn.114.208066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nian H.; Delage B.; Pinto J. T.; Dashwood R. H. Allyl mercaptan, a garlic-derived organosulfur compound, inhibits histone deacetylase and enhances Sp3 binding on the P21WAF1 promoter. Carcinogenesis 2008, 29, 1816–1824. 10.1093/carcin/bgn165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler L. M.; Zhou X.; Xu W.-S.; Scher H. I.; Rifkind R. A.; Marks P. A.; Richon V. M. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 11700. 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J. A. Preclinical Perspectives on Garlic and Cancer. J. Nutr. 2006, 136, 827S–831S. 10.1093/jn/136.3.827S. [DOI] [PubMed] [Google Scholar]
- Benavides G. A.; Squadrito G. L.; Mills R. W.; Patel H. D.; Isbell T. S.; Patel R. P.; Darley-Usmar V. M.; Doeller J. E.; Kraus D. W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 17977. 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akula R.; Ravishankar G. A. Influence of abiotic stress signals on secondary metabolites in plants. Plant. Signal. Behav. 2011, 6, 1720–1731. 10.4161/psb.6.11.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan-Atrash J.; Luo W.; Strongin R. M. Toxicant Formation in Dabbing: The Terpene Story. ACS Omega 2017, 2, 6112–6117. 10.1021/acsomega.7b01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.