Abstract
In this study, the therapeutic efficacy of quercetin in combination with remdesivir and favipiravir, were evaluated in severe hospitalized COVID-19 patients. Our main objective was to assess the ability of quercetin for preventing the progression of the disease into critical phase, and reducing the levels of inflammatory markers related to SARS-Cov-2 pathogenesis. Through an open-label clinical trial, 60 severe cases were randomly divided into control and intervention groups. During a 7-day period, patients in the control group received antivirals, i.e., remdesivir or favipiravir, while the intervention group was treated with 1000 mg of quercetin daily in addition to the antiviral drugs. According to the results, taking quercetin was significantly associated with partial earlier discharge and reduced serum levels of ALP, q-CRP, and LDH in the intervention group. Furthermore, although the values were in normal range, the statistical outputs showed significant increase in hemoglobin level and respiratory rate in patients who were taking quercetin. Based on our observations, quercetin is safe and effective in lowering the serum levels of ALP, q-CRP, and LDH as critical markers involved in COVID-19 severity. However, according to the non-significant borderline results in comparing the mortality, the ICU-admission rate, and the duration of ICU-admission, further studies can be helpful to compensate the limitations of our study and clarify the therapeutic potential of quercetin in COVID-19 treatments.
Keywords: COVID-19, Quercetin, Remdesivir, Favipiravir
Abbreviations:
- ARDS
Acute respiratory distress syndrome
- ALP
Alkaline phosphatase
- BUN
Blood urea nitrogen
- Cr
Creatinine
- CK-mb
Creatine kinase-mb
- ESR
Erythrocyte sedimentation rate
- FIASMA
Functional inhibitors of acid sphingomyelinase
- LFTs
Liver function tests
- LDH
Lactate dehydrogenase
- q-CRP
Quantitative C-reactive protein
- SIRS
Systemic inflammatory response syndrome
- SGOT
Serum glutamic oxaloacetic transaminase
- SGPT
Serum glutamic pyruvic transaminase
1. Introduction
Coronavirus disease 2019 is a respiratory viral infection caused by severe acute respiratory syndrome coronavirus-2. The virus was first detected in December 2019 in Wuhan, China. Most COVID-19 patients demonstrate mild symptoms and usually recover without hospitalization. However, almost 15%–20% of the cases develop severe lung infiltration that may lead to multiple organ failure and acute respiratory distress syndrome, which is called critical COVID-19 (Huang et al., 2020). Thus far, approximately more than 240 million infected cases and almost 5 million deaths due to SARS-Cov-2 have been reported globally. Available therapeutic approaches are symptomatic management and O2 therapy. Given the importance of controlling the mortality rate, uncovering the cellular and molecular mechanisms involved in the progression of disease into the storm phase is necessary. Immune dysregulation is a significant challenge, which drives the disease into cytokine storm and mortality phases. Macrophages are critical players in severe lung injury caused by the activation of NLRP3 inflammasome following the virus entry. Massive secretion of inflammatory cytokines, such as IL-1β, IL-6, IL-18, TNFα, and CXCL-10, by macrophages leads to systemic inflammatory response syndrome, thrombotic complications, and multiorgan failure in severe COVID-19 cases (Mangalmurti and Hunter, 2020). While there is no specific drug targeting SARS-Cov-2, several antivirals, including nucleotide analogues remdesivir or favipiravir, and immune-based therapies, including tocilizumab, are prescribed with various controversial efficacies reported (Frediansyah et al., 2020). Quercetin, a plant flavonoid, is a well-known natural supplement found in green tea, apples, red wine, berries, onions, and several green leaf plants. It has been confirmed that quercetin may affect the viral entry and regulate the virus-mediated immune response (Colunga Biancatelli et al., 2020). Inhibition of the virus entry into the host cells and viral replication as well as inhibitory effects on NLRP3 inflammasome activation are all considered anti-inflammatory effects of quercetin in viral infections (Colunga Biancatelli et al., 2020). On the other hand, quercetin may be effective in inhibiting the clinical deterioration of SARS-Cov-2 infected patients through regulating the acid sphingomyelinase/ceramide system, which is remarkably involved in viral internalization into the respiratory epithelial cells during SARS-Cov-2 infection (Marín-Corral et al., 2021). Potential usefulness of acid/sphingomyelinase ceramide system inhibitors in reducing the intubation and mortality risk of COVID-19 were reported by recently published clinical trials. Taking an antidepressant, such as fluoxetine or paroxetine (Hoertel et al., 2021a), or more broadly a FIASMA medication upon hospital admission (Hoertel et al., 2021b), may be significantly and substantially associated with reduced risk of intubation or death in COVID-19 inpatients. Two clinical trials, including a randomized placebo-controlled trial (Lenze et al., 2020) and an open-label pragmatic trial (Seftel and Boulware, 2021), report a significant reduced risk of clinical deterioration associated with a 2-week prescription of the FIASMA antidepressant fluvoxamine among COVID-19 outpatients. Furthermore, pharmacological inhibition of acid sphingomyelinase by amitriptyline or secretolytic therapy of bronchopulmonary complications related to excessive mucus secretion and impaired mucus transport by ambroxol have been shown to be effectively related to the inhibition of SARS-Cov-2 entry into the lung epithelial cells by preventing the ACE2/sphingomyelin/ceramide interactions (Carpinteiro et al., 2020, 2021). The inhibitory potential of quercetin on sphingomyelinase ceramide system has been investigated in experimental models of traumatic hemorrhagic shock and reperfusion. It was observed that quercetin could prevent the sphingomyelinase activity and inhibit the progression of lung edema (Chamorro et al., 2016). Consequently, through a similar mechanism with antidepressants, quercetin may be considered as an inhibitor of acid sphingomyelinase ceramide system, leading to prevention of SARS-Cov-2 entry into the host epithelial cells. In this trial, our main objective was to evaluate the therapeutic efficacy of quercetin in combination with antiviral drugs remdesivir and favipiravir in severe non-ICU admitted patients.
2. Materials and methods
2.1. Study design, inclusion and exclusion criteria
The project was designed to evaluate the inhibitory effectiveness of quercetin on SARS-Cov-2 infection. A combination therapy of nucleotide analogues, i.e., remdesivir or favipiravir, with quercetin was administered for patients with severe SARS-Cov-2 infection. A total number of 60 patients with definite COVID-19 diagnosis who were admitted in Ahvaz Razi Teaching Hospital were enrolled into an open-label clinical trial from December 2020 to January 2021. The study plan was explained, and written informed consents were obtained from the patients who agreed to participate in the study. Ethics Committee of Ahvaz Jundishapur University of Medical Sciences approved the study (IR.AJUMS.REC.1399.087). Moreover, the project was registered as a clinical trial in Iranian Registry of Clinical Trials (IRCT20200419047128N2 (. Non-ICU admission, uninterrupted O2 therapy supported by reservoir bags, arterial blood O2 saturation under 93% (based on IDSA guidelines defining severe COVID-19), nasopharyngeal detected SARS-Cov-2 reported by RT-PCR, lung involvement with crazy paving pattern, ground glass opacities, unilateral or bilateral consolidation, and an age ˃18 years were considered as the inclusion criteria. Furthermore, patients who did not agree to participate in the study, patients under 18 years old, patients with negative PCR report despite respiratory involvement, pregnant patients, and patients with a history of allergic reaction to quercetin-containing sources were excluded. Based on the national therapeutic protocols of COVID-19 at the time of the study, patients with low PO tolerability and O2 saturation lower than 90% were received remdesivir, while patients with O2 saturation higher than 90% and optimal PO tolerability were received favipiravir. Reducing the length of hospitalization, decreasing the mortality rate, and the number of ICU-admissions, were defined as the primary endpoints. Also, reducing the lung involvement with normal O2 saturation and respiratory rate were considered as the secondary endpoints of this study.
2.2. Study procedure
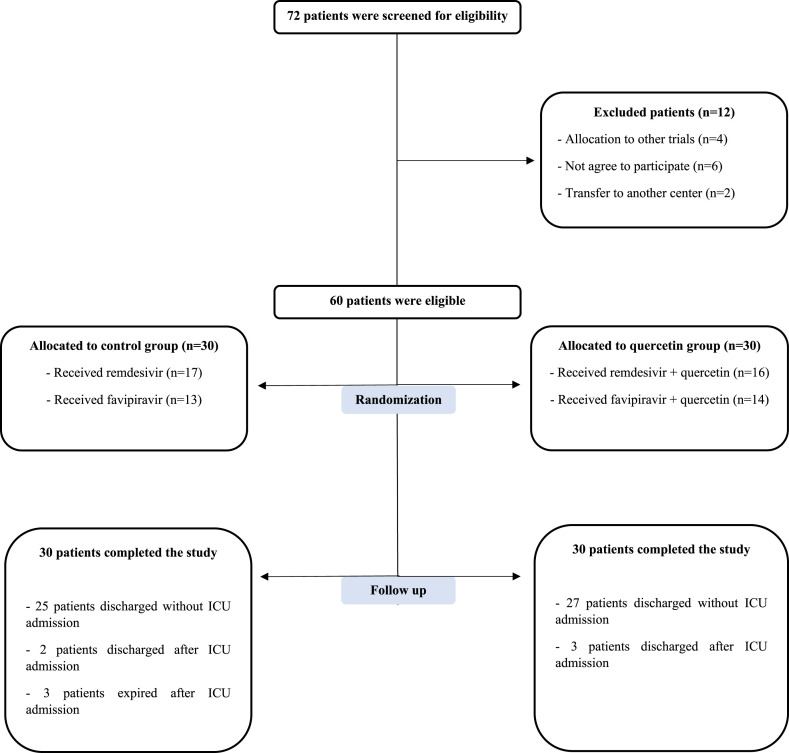
A nasopharyngeal swab sampling was performed for SARS-Cov-2 detection by RT-PCR. RNJia Virus Kit (ROJE Technologies, Iran) and COVITECH COVID-19 multiplex qPCR kit (ACECR, Iran) were used for RNA extraction and qPCR quantification, respectively. After passing the eligibility criteria, patients were admitted to the Infectious Diseases Ward to start the treatment process, and they were randomly (1:1) divided into control and quercetin groups (Fig. 1 ). Demographic and clinical information, including age, gender, history of underlying diseases, vital signs, and clinical symptoms at admission, were recorded (Table 1 ).
Fig. 1.
Consort flowchart of the trial. A total number of 72 cases were screened for eligibility. 60 patients were passed the enrolling criteria and finally allocated to control and intervention groups (30 cases per group).
Table 1.
Demographic information, clinical symptoms and the duration of symptoms before randomization.
| Variable | Definition | Control (n = 30) | Quercetin (n = 30) | P |
|---|---|---|---|---|
| Age (years) | Min | 32 | 35 | |
| Max | 79 | 75 | 0.56 | |
| Std. deviation | 13189 | 1032 | ||
| Mean | 527 | 509 | ||
| Gende | Male | 17(56.7%) | 17(56.7%) | 1.00 |
| Female | 13(43.3%) | 13(43.3%) | ||
| Underlying diseases | Diabetes | 8(26.7%) | 7(23.3%) | 0.76 |
| Hypertension | 4 (13.3%) | 8 (26.7%) | 0.33 | |
| Cardiovascular diseases | 4 (13.3%) | 5 (16.7%) | 1.00 | |
| Asthma | 1 (3.3%) | 3 (10%) | 0.61 | |
| BMI >25:* | 10(33.3%) | 9(30%) | 0.78 | |
| atty liver history | 4 (13.3%) | 4 (13.3%) | 1.00 | |
| Clinical symptoms | Cough | 27(90%) | 24(80%) | 0.47 |
| before randomization | Dyspnea | 28(93.3%) | 29(96.7%) | 1.00 |
| Chest pain | 18(60%) | 20(66.7%) | 0.59 | |
| Headache | 15(50%) | 15(50%) | 1.00 | |
| Weakness and lethargy | 27(90%) | 23(76.7%) | 0.29 | |
| Fever | 15(50%) | 24(80%) | 0.015* | |
| Runny nose | 5(16.7%) | 9(30%) | 0.22 | |
| Diarrhea | 9(30%) | 12(40%) | 0.41 | |
| Nausea | 9(30%) | 8(26.7% | 0.77 | |
| Vomiting | 11(36.7%) | 9(30%) | 0.58 | |
| Duration of symptoms | Min | 3 | 3 | |
| before randomization | Max | 20 | 14 | 0.043* |
| Std. deviation | 353 | 263 | ||
| Mean | 943 | 777 |
Based on the statistical outputs of Fisher's exact and Chi-square tests, there was not any significant differences between control and intervention groups in terms of age, gender, history of underlying diseases, and clinical symptoms at the time of admission. However, the number of patients with body temperature >38 °C was significantly higher in quercetin group rather than control at the time of admission (P: 0.015). Furthermore, the duration of symptoms onset before randomization was partially higher in control group (P: 0.043). The number of overweight and obese participants were measured using Body Mass Index based on the CDC guidelines. Overweight: BMI between 25 and <30. Obesity: BMI between 30 and <35. Control group: 6 overweight and 4 obese patients. Quercetin group: 7 overweight and 2 obese patients.
2.3. Statistical analysis
Descriptive and inferential statistics including the Fisher's exact test, Chi-square and independent T-tests, were performed to analyze the demographic and clinical data. The laboratorial and clinical data were compared parametrically using repeated measures analysis and mixed-design analysis of variance (ANOVA). SPSS 23.0 was used for data analysis and designing the graphs. P-values lower than 0.05 were considered statistically significant.
2.4. Patients’ follow-up and clinical outcomes of the study
Due to the impacts of corticosteroid consumption in the patients, all the cases (diabetic and non-diabetic) were controlled by blood sugar check within the first 48 h of admission, and based on the BS range, insulin was prescribed to control the blood sugar level. Moreover, the probability of diabetic ketoacidosis was controlled in diabetic patients by uric acid test and arterial blood gas measurement. Patients with history of hypertension and cardiovascular diseases were regularly visited by cardiologist to assess the probability of any interference between their anti-hypertensive drugs and the treatments used in the study. According to the cardiology consultation, the type of anti-hypertensive drug was changed if any interference with the antiviral drugs was seen. Fortunately, no drug interference was seen. The patients with asthma and salbutamol inhalation were monitored by pulmonologist during the study. Moreover, the patients with history of fatty liver diseases were in grade I of the disease and were not taking medications. The intervention period was 7 days, however, the participants who completed the study were followed for 14 more days. The patients in the control group received remdesivir or favipiravir, while the patients in the intervention group received quercetin in addition to remdesivir or favipiravir (Table 2 ). Peripheral blood samples were collected before and after the intervention. Clinical and laboratorial analyses were performed before, during, and after the study, and the serum levels of IL-1β, TNFα, and IL-6 cytokines were measured using ELISA kits from Karmania Pars Gene Company, Kerman, Iran (Cat numbers: KPG-hIL-1β, KPG-hTNF-α, and KPG-hIL-6). No interference was observed between discharge time and the study process. Actually, none of the participants were discharged earlier than 7 days as the project was being conducted. All the patients in the control and intervention groups were monitored by the clinical consulter of the project to control the possible side effects related to the use of remdesivir. Based on the clinical therapeutic guidelines, daily LFTs and BUN/Cr check were done for all the participants to evaluate the risk of liver hepatotoxicity (5–10 time increase in serum levels of SGPT and SGOT) and kidney damage (Glomerular filtration rate <30 mL/min/1.73 m2) in the patients. The risk of coagulation disorders that might appear with the sign of hemoptysis, was followed in the patients by performing daily PT/PTT and INR check. Moreover, daily electrocardiogram and cardiology examination were done for the patients to control the risk of bradycardia and reduction in pulse rate (<40 BPM). For the patients who were taking favipiravir, famotidine was prescribed to reduce the risk of dyspepsia. Also, the electrolyte imbalances such as hypomagnesemia that might increase the risk of QT prolongation, was treated by MgSO4 based on the serum magnesium level of the patients (Table 2). Uric acid level was assessed to control the risk of favipiravir-associated hyperuricemia. Fortunately, no serious side effect leading to drug discharge was observed in the patients. The tolerability of the antiviral drugs and quercetin used in this trial, was followed and evaluated by the clinical advisor of the project. Although the values were close to statistical significance, there were not any significant differences between control and intervention groups in the number of ICU-admitted patients, the duration of ICU admission, the number of deaths and discharged patients. However, the duration of hospitalization after the intervention to discharge time was partially lower in quercetin group (Table 3 ). Also, the number of patients received favipiravir or remdesivir, were not significantly different (Table 3).
Table 2.
Dosage and time period of antiviral drugs, quercetin, and other therapies used in the study.
| Group | Dosage and duration of administration |
|---|---|
| Control and intervention | Remdesivir (Ronak Pharmaceutical Co. Tehran, Iran) |
| First day: 200 mg (IV injection) | |
| Second to fifth day: 100 mg (IV injection daily) | |
| Favipiravir (Cytovex, Abidi Pharmaceutical Co. Tehran, Iran) | |
| First day: 3200 mg (1600 mg PO BD) | |
| Second to fifth day: 1200 mg (600 mg PO BD) | |
| Intervention | Quercetina (Jarrow FORMULAS, USA) |
| 1000 mg daily for 7 days (500 mg PO BD) | |
| Control and intervention | Vitamin. D: 1000 IU PO daily |
| MgSO4: 250 mg PO BD (based on serum Mg level) | |
| Famotidine: 40 mg PO daily | |
| Zinc sulfate: 30 mg PO daily | |
| Vitamin C: 500–1000 mg PO daily | |
| Dexamethasone: 8 mg daily IV injection |
PO: Oral consumption BD: Bidaily.
Several formulation strategies such as enzymatic modifications and the use of vitamins have been tested to enhance the bioavailability and dissolution of quercetin in the human body. In this study, 500 mg veggie caps dietary supplement of quercetin (manufactured by Jarrow Formulas Company, USA) was used. According to the manufacturer, this dietary supplement is suitable for vegetarians or vegans, and no wheat, gluten, soybeans, dairy, egg, fish/shellfish, or peanuts/tree nuts is found in this product. Moreover, the product is also non-GMO and contains only organic ingredients. Based on the recent reports, vitamin C was prescribed for all the patients enrolled in the study to improve the efficacy and bioavailability of quercetin (Ahmed et al., 2020; Colunga Biancatelli et al., 2020).
Table 3.
Clinical outcomes of the study and the number of patients based on the type of antiviral drug received. The analyses were applied by proportion test.
| Control (n = 30) | Quercetin (n = 30) | P | |
|---|---|---|---|
| Number of ICU-admitted patients | 5 (16.6%) | 3 (10%) | 0.44 |
| Duration of ICU-admission | 8–15 days | 6–10 days | 0.053 |
| Number of deaths | 3 (10%) | 0 (0%) | 0.076 |
| Number of discharged patients | 27 (90%) | 30 (100%) | 0.076 |
| Duration from 7th day of intervention to discharge (days) | mean: 4.63 | mean: 3.13 | |
| min: 1 | min: 1 | 0.039* | |
| max: 15 | max: 12 | ||
| Number of patients received favipiravir | 13 (43.3%) | 16 (53.3%) | 0.43 |
| Number of patients received remdesivir | 17 (56.6%) | 14 (46.6%) | |
3. Results
Although the intervention period was 7 days, the participants were followed for 14 days after completing the trial to follow their clinical symptoms (Table 4 ). Complete blood count and biochemical and coagulation assessment, including BUN, Cr, SGOT, SGPT, Total/Direct Bilirubin, ALP, LDH, quantitative CRP, ESR, D-dimer, CK-mb, and quantitative Troponin, were measured before and after the intervention in both groups. Moreover, vital signs, including O2 saturation, pulse rate, respiratory rate, body temperature, and blood pressure, were monitored and recorded daily. Based on the confirmed inhibitory effects of quercetin on the expression of cytokines involved in SARS-Cov-2 immunopathogenesis, serum levels of IL-1β, TNFα, and IL-6 were measured before and after the intervention. Comparisons within period, comparisons between groups and group/period interaction analysis were applied to evaluate the therapeutic efficacy of our intervention in different times, between the groups and mixed time/group effect (Table 5 ).
Table 4.
Comparisons of the clinical symptoms in the control and quercetin groups in 7th day and 14th day after the intervention. The statistical analysis was applied by proportion test.
| 7 days after intervention |
14 days after intervention |
|||||
|---|---|---|---|---|---|---|
| Clinical symptoms | Control | Quercetin | P | Control | Quercetin | P |
| Cough | 13 (43.3%) | 12 (40%) | 0.79 | 7 (23.3%) | 4 (13.3%) | 0.31 |
| Dyspnea | 14 (46.6%) | 8 (26.6%) | 0.1 | 6 (20%) | 2 (6.6%) | 0.12 |
| Chest pain | 3 (10%) | 4 (13.3%) | 0.68 | 1 (3.3% | 2 (6.6%) | 0.55 |
| Headache | 8 (26.6%) | 5 (16.6%) | 0.34 | 6 (20%) | 2 (6.6%) | 0.27 |
| Weakness and lethargy | 22 (73.3%) | 13 (43.3%) | 0.018* | 12 (40%) | 7 (23.3%) | 0.16 |
| Fever | 8 (26.6%) | 3 (10%) | 0.09 | 2 (6.6%) | 1 (3.3%) | 0.55 |
| Runny nose | 2 (6.6%) | 3 (10%) | 0.64 | 0 (0%) | 0 (0%) | – |
| Diarrhea | 6 (20%) | 4 (13.3%) | 0.31 | 0 (0%) | 0 (0%) | – |
| Nausea | 11 (36.6%) | 6 (20%) | 0.15 | 4 (13.3% | 1 (3.3%) | 0.16 |
| Vomiting | 4 (13.3%) | 2 (6.6%) | 0.38 | 0 (0%) | 0 (0%) | – |
Table 5.
Comparisons of laboratorial and clinical parameters in control and quercetin groups, before and after the intervention. Repeated measures analysis using mixed-design ANOVA test was applied to compare the data. The mean values with standard deviations in 4 different times are provided. Statistical outcomes including P-values of ANOVA test and F statistics of Fisher test are also presented.
| A |
B |
C |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test |
Control (Day 1) Mean (SD) |
Control (Day 7) Mean (SD) |
Quercetin (Day 1) Mean (SD) |
Quercetin (Day 7) Mean (SD) |
P | F | P | F | P | F |
| WBC | 9363 (3876) | 9213 (4088) | 8260 (2839) | 8390 (2804) | 0.98 | 0.001 | 0.22 | 1.48 | 0.73 | 0.11 |
| RBC | 4.58 (0.64) | 4.56 (0.74) | 5.11 (0.62) | 5.10 (0.66) | 0.78 | 0.08 | 0.001 | 11.41 | 0.95 | 0.003 |
| HCT | 38.55 (4.77) | 37.78 (5.43) | 39.19 (3.35) | 39.94 (4.35) | 0.98 | 0.00 | 0.19 | 1.69 | 0.11 | 2.59 |
| HGB | 12.8 (1.6) | 12.3 (1.8) | 13.4 (1.4) | 13.5 (1.7) | 0.38 | 0.77 | 0.027 | 5.11 | 0.04 | 4.24 |
| PLT | 218300 (64625) | 224533 (64627) | 239667 (68561) | 280833 (76612) | 0.01 | 5.87 | 0.01 | 6.86 | 0.07 | 3.18 |
| PT | 13.3 (1.2) | 13.6 (1.7) | 12.7 (0.9) | 12.5 (0.5) | 0.85 | 0.03 | 0.001 | 11.54 | 0.28 | 1.14 |
| PTT | 36.5 (12.7) | 38.4 (16.1) | 37.3 (3.3) | 37.6 (3.1) | 0.56 | 0.03 | 0.93 | 0.00 | 0.66 | 0.19 |
| BUN | 20.4 (9.9) | 18 (5.7) | 14.3 (5.1) | 15 (5.6) | 0.32 | 1.05 | 0.005 | 8.42 | 0.059 | 3.7 |
| Cr | 1.1 (1.2) | 1.2 (1.7) | 0.8 (0.1) | 0.7 (0.1) | 0.73 | 0.11 | 0.17 | 1.91 | 0.25 | 1.31 |
| SGOT | 63.8 (32.5) | 56.9 (44.9) | 50.3 (21.4) | 40.5 (17) | 0.06 | 3.47 | 0.027 | 5.12 | 0.75 | 0.1 |
| SGPT | 58.9 (48.5) | 60.5 (51) | 51.2 (21.3) | 41.8 (11) | 0.28 | 1.16 | 0.14 | 2.19 | 0.13 | 2.28 |
| ALP | 192 (84) | 202 (80) | 200 (37) | 179 (35) | 0.27 | 1.19 | 0.63 | 0.22 | 0.002 | 10.02 |
| Total Bilirubin | 1.06 (0.38) | 1.2 (0.33) | 0.98 (0.36) | 0.92 (0.33) | 0.4 | 0.71 | 0.019 | 5.78 | 0.052 | 3.92 |
| Direct Bilirubin | 0.27 (0.13) | 0.37 (0.34) | 0.2 (0.1) | 0.2 (0.2) | 0.058 | 3.75 | 0.059 | 3.71 | 0.42 | 0.65 |
| q-Troponin | 0.02 (0.006) | 0.03 (0.007) | 0.02 (0.006) | 0.02 (0.006) | 0.72 | 0.058 | 0.53 | 0.48 | 0.56 | 0.33 |
| D-dimer | 834 (867) | 516 (314) | 872 (452) | 513 (207) | 0.00 | 15.01 | 0.86 | 0.029 | 0.8 | 0.05 |
| q-CRP | 62.9 (30.3) | 34.1 (17.3) | 66.9 (23.9) | 21.4 (13.8) | 0.00 | 182.8 | 0.39 | 0.73 | 0.004 | 9.2 |
| ESR | 50 (17) | 32 (14) | 41 (12) | 23 (7) | 0.00 | 141.9 | 0.004 | 9 | 0.89 | 0.017 |
| CK-mb | 18 (3) | 15 (4) | 14 (4) | 11 (3) | 0.00 | 20.24 | 0.00 | 25.24 | 0.96 | 0.002 |
| LDH | 754 (290) | 537 (154) | 792 (234) | 439 (128) | 0.00 | 84.45 | 0.5 | 0.44 | 0.032 | 4.85 |
| SpO2 | 88 (3) | 93 (3) | 90 (2) | 95 (3) | 0.00 | 76.24 | 0.051 | 3.97 | 0.14 | 1.16 |
| Body Temperature | 37.5 (0.6) | 37.4 (0.3) | 37.6 (0.4) | 37.3 (0.3) | 0.005 | 8.69 | 0.77 | 0.08 | 0.24 | 1.39 |
| Respiratory rate | 27 (6) | 25 (5) | 27 (5) | 29 (6) | 0.63 | 0.23 | 0.11 | 2.51 | 0.011 | 6.91 |
| Pulse rate | 82 (12) | 82 (13) | 79 (13) | 75 (14) | 0.35 | 0.87 | 0.061 | 3.53 | 0.29 | 1.12 |
| Systolic/Diastolic blood pressure | 12/7 (1/0.7) | 12/7 (1/0 .6) | 12/7 (1/0.5) | 12/7 (0.6/0.5) | 0.63 | 0.23 | 0.53 | 0.38 | 0.77 | 0.08 |
| IL-1β | 64 (27) | 52 (22) | 61 (27) | 39 (24) | 0.00 | 26.55 | 0.2 | 1.63 | 0.13 | 2.26 |
| IL-6 | 33 (10) | 27 (7) | 27 (8) | 18 (6) | 0.00 | 39.96 | 0.00 | 14.55 | 0.22 | 1.48 |
| TNF-α | 21 (11) | 16 (7) | 24 (9) | 15 (7) | 0.00 | 30.59 | 0.53 | 0.39 | 0.1 | 2.76 |
A: Comparisons within periods B: Comparisons between groups C: Group × Period interaction. WBC: cells/mm3. RBC: 106/UL. HCT: %. HGB: g/dl. PLT: cells/mm3. PT/PTT: second (s). BUN/Cr: mg/dl. SGOT/SGPT/ALP: U/L. Total/Direct Bilirubin: mg/dl. q-Troponin: ng/ml. D-dimer: ng/ml.
q-CRP: mg/L ESR: mm/h CK-mb/LDH: U/L SpO2: % Body Temperature: °C Respiratory/Pulse rate: BPM Systolic/Diastolic BP: mm/Hg.
IL-1β/IL-6/TNF-α: pg/ml.
3.1. Earlier discharge time was observed in the patients who were taking quercetin
The statistical assessments indicated that daily taking of 1000 mg quercetin for 1 week, was associated with partially shorter hospitalization time. The average of days between the 7th day of intervention to discharge time was significantly lower in the patients who were taking quercetin in comparison with the patients in the control group (Table 3).
3.2. Statistical comparisons of the clinical and laboratorial data “within the periods” and “between the groups”
According to the statistical outputs, it was observed that the levels of PLT, D-dimer, q-CRP, ESR, CK-mb, LDH, body temperature and O2 saturation rate, were remarkably changed during the time of intervention. Also, serum concentrations of IL-1β, IL-6, and TNF-α cytokines were significantly changed in 4 different times that was measured in control and intervention groups, before and after the intervention. Moreover, comparisons between the groups revealed that the levels of HGB, PLT, PT, BUN, SGOT, total bilirubin, ESR, CK-mb, and IL-6 were considerably different between the control and the intervention groups. The related statistical analyses are given in Table 5.
3.3. Taking quercetin was associated with a more effective decrease in the levels of q-CRP, LDH, and ALP in the intervention group
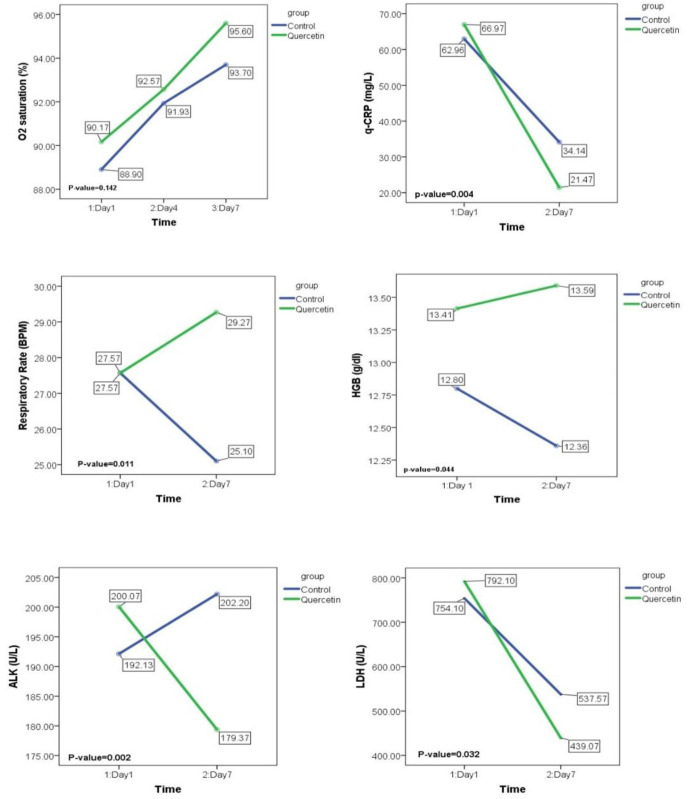
To evaluate the therapeutic efficacy of quercetin, mixed-design analysis of variance test was applied to show the period × group interactions. As presented in Table 5, it was observed that taking 500 mg quercetin bidaily for 1 week, could significantly decrease the serum levels of q-CRP and LDH in the intervention group rather that the control group (p: 0.004 & 0.032, respectively for q-CRP and LDH). Moreover, serum concentration of ALP demonstrated a remarkable decrease in the patients who were taking quercetin in addition to the antiviral drugs (p: 0.002). Although the measured values were in normal range, the statistical analysis showed that the levels of hemoglobin concentration and also the respiratory rate were partially increased in the intervention group in comparison to the control group (p: 0.04 & 0.011, respectively for HGB and respiratory rate). The statistical graphs displaying the comparisons of HGB, q-CRP, LDH, ALP, and respiratory rate between the control and quercetin groups before and after the intervention, are displayed in Fig. 2 .
Fig. 2.
Statistical graphs of the mixed-design ANOVA comparisons of the variables that were significantly changed during the intervention. The reported p-values are the outputs of period × group interactions which were presented in the results section. Due to its clinical importance, changes in O2 saturation level during the study are also displayed.
4. Discussion
Due to the complicated pathophysiology of COVID-19, there are various therapeutic choices to be prescribed for the patients which are dependent on the stage of the disease. Patients with severe COVID-19 show heterogenous immune responses to the inflammatory cascade caused by the virus-mediated immune dysregulation. After the first exposure, viral antigens are recognized by innate immunity receptors, such as TLRs and NLRs, to induce an effective antiviral interferon response; however, this is not the only outcome. Concurrent with the release of interferons, the expression of co-stimulatory molecules and antigen presentation signals will be induced to provide adaptive immune responses, leading to antibody secretion and antiviral T cells activation. Exactly at this point, the immune system faces the dilemma of cytokine storm or recovery by walking on the sword of NLRP3 inflammasome. Uncontrolled NLRP3 induction leads to the over secretion of IL-1β and IL-18, and the respiratory infiltration of mononuclear phagocytes, neutrophils, and T cells, resulting in serious lung injury and inflammatory storm (Mortaz et al., 2020; Nile et al., 2020; Ros et al., 2020). NLRP3 inflammasome is, in effect, an uncontrolled inflammatory weapon which is considered as an important SARS-Cov-2 associated therapeutic target (Zhao et al., 2021). According to the recent reports on the inhibitory effects of quercetin on NLRP3 inflammasome, a combination therapy of quercetin with antiviral drugs, i.e., remdesivir and favipiravir has been carried out in this trial (Saeedi-Boroujeni and Mahmoudian-Sani, 2021; van den Berg and Te Velde, 2020). From the beginning of the COVID-19 pandemic, there are controversial reports regarding to the therapeutic effectiveness of antiviral drugs in promoting clinical improvement in SARS-Cov-2 patients. A study by J. Grein et al. that was conducted with a total number of 61 patients who were received at least one dose of remdesivir, showed that compassionate use of remdesivir was associated with clinical improvement in 68% of the patients hospitalized for severe COVID-19 (Grein et al., 2020). Moreover, the results of a double-blind, randomized, placebo-controlled trial of intravenous remdesivir in 1062 hospitalized adults with COVID-19, demonstrated that remdesivir was superior in shortening the time to clinical recovery in the patients with lower respiratory tract infection (Beigel et al., 2020). Based on the results of systematic reviews and meta-analysis studies, remdesivir has shown no benefit in reducing the mortality rate, however, in terms of reducing the proportion receiving ventilation and serious harms, remdesivir may be effective in hospitalized adults with severe form of COVID-19 (Kaka et al., 2021; Singh et al., 2021). On the other hand, there are several controversial reports on the efficacy of favipiravir in the therapeutic protocols related to COVID-19 management. The effectiveness of favipiravir has been confirmed in moderate COVID-19 patients with SpO2 ≥ 94%, obviously with shortening the time to clinical improvement by approximately 3 days in these patients (Shinkai et al., 2021). Another study conducted by H. M. Dabbous et al. showed that favipiravir was superior to chloroquine in decreasing the hospital stay and the need for mechanical ventilation in mild to moderate COVID-19 cases who were hospitalized (Dabbous et al., 2021a). Moreover, it was reported that, the combination treatment with favipiravir and methylprednisolone was significantly associated with good clinical outcomes by reducing the need for oxygen supplementation and ventilation in severe COVID-19 patients (Murohashi et al., 2020). Taken together, it can be concluded from the findings in the literature, that remdesivir may be effective in shortening the time to clinical improvement in severe COVID-19 cases with no benefits in reducing the mortality. Also, favipiravir should be prescribed for the moderate cases as quickly as possible after the onset of the symptoms to be effective in improving the clinical condition of the patients, and decreasing the need for mechanical ventilation (Dabbous et al., 2021b; Ucan et al., 2021). Quercetin, chemically known as 3,3′,4′,5,7-pentahydroxyflavone, is a plant flavonol, which is a member of the flavonoid family (Derosa et al., 2021). Gene set enrichment analyses have revealed that quercetin is able to regulate the expression of 85% of SARS-Cov-2 structural proteins (Glinsky, 2020). Moreover, molecular docking screening has shown that quercetin effectively reduces the lytic release replication and frustrates the SARS-Cov-2 replication cycle through binding to 3CL and PL proteases of the virus (Jo et al., 2020). Quercetin suppresses TNF/TNFR and NLRP3 downstream signals, such as Nf-κB and IL-1β (Li et al., 2016). Significant reductions in viral load, replication, and virus-mediated cholinergic hyperresponsiveness are confirmed as the effects of quercetin in clinical trial reports on hepatitis C, herpes simplex, and rhinoviruses infections (Agrawal et al., 2020).
Consequently, we decided to conduct a therapeutic method based on the combination of antiviral drugs and quercetin in severe COVID-19 patients. The prophylactic and therapeutic use of quercetin in combination with vitamin D3 and ascorbic acid is recommended as a complementary therapy to increase the efficiency of antiviral drugs and the plasma exchange method in severe COVID-19 cases. Improved clinical conditions and good prognosis were reported in SARS-Cov-2 infected patients after taking 800 mg of quercetin daily in combination with 50 mg of zinc acetate and 165 mg of bromelain for 5 days before antiviral prescription (Ahmed et al., 2020). Based on our observations, in addition to the antiviral drugs, taking 1000 mg of quercetin daily for 1 week was associated with lower hospital stay in the intervention group (Table 3). Moreover, taking quercetin reduced the serum levels of q-CRP, LDH, and ALP in the intervention group (Fig. 2/Table 5). Inversely, although the values were in normal range, statistical evaluations demonstrated that the respiratory rate and hemoglobin level were partially increased during the intervention in the patients who were taking quercetin (Fig. 2/Table 5). In line with our results, Di Pierro et al. showed that taking 1000 mg quercetin for 30 days in 152 patients, was remarkably associated with the reduction in length of hospitalization, in need of non-invasive oxygenation, in progression into intensive care units, and in the number of deaths (Di Pierro et al., 2021a). Although the number of enrolled patients and the time of quercetin administration were lower in our study, the results were close to statistical significance when we compared the number of ICU-admitted patients, the number of deaths, and the duration of ICU-admission in the control and quercetin groups (Table 3). The ability of quercetin in virus clearance and ameliorating the clinical symptoms was further evaluated by Di Pierro et al. It was observed that taking quercetin for 2 weeks in addition to standard care, statistically shortened the timing of molecular test conversion from positive to negative. Also, taking quercetin remarkably reduced the levels of LDH, Ferritin, CRP, and D-dimer (Di Pierro et al., 2021b). To compare the improvement of clinical symptoms between the groups, we followed the patients for 14 more days after the intervention period to evaluate the presence of the symptoms. Although the statistical comparisons did not demonstrate significant differences, the patients in quercetin group showed better clinical improvements in terms of the symptoms (Table 3). Approximately 15%–58% of COVID-19 patients show elevated serum LFTs as a secondary outcome (Moon and Barritt, 2020). Therefore, to avoid any liver and even cardiac damage, we checked LFTs daily and measured CK-mb/troponin before and after the study. Fortunately, no serious rise in LFTs was observed in the patients that require drug discharge. Providing a definitive conclusion requires further clinical observations; however, other clinical trial reports have also confirmed the hepatic safety of quercetin in COVID-19 patients (Moon and Barritt, 2020; Onal et al., 2021).
5. Conclusion
This is the first study reporting the therapeutic efficacy of quercetin in combination with antiviral drugs, i.e., remdesivir and favipiravir in severe form of COVID-19 disease. The results showed that quercetin was able to reduce the hospitalization period. Also, the serum levels of q-CRP, LDH, and ALP were decreased more effectively after taking quercetin. Based on the observations, there were no significant differences in mortality, duration of ICU-admission, and the number of ICU-admitted cases, although our study may be underpowered to detect an effect on these outcomes if it exists. Due to the complicated pathophysiology of SARS-Cov-2, more research is needed to provide a definite conclusion. Nonetheless, based on these results, it can be stated that quercetin may be therapeutically effective in reducing the time to clinical improvement upon combination with antiviral drugs.
Funding
This work was supported by the Deputy of Research and Technology of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, IRAN. Grant number: U-99020.
CRediT authorship contribution statement
Mojtaba Shohan: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Roohangiz Nashibi: Visualization, Supervision. Mohammad-Reza Mahmoudian-Sani: Methodology, Investigation. Farhad Abolnezhadian: Supervision. Mehri Ghafourian: Writing – review & editing. Seyed Mohammad Alavi: Supervision. Asaad Sharhani: Formal analysis, Visualization. Ali Khodadadi: Supervision, Conceptualization, Funding acquisition.
Declaration of competing interest
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors specially thank the clinical staff of Infectious Diseases Ward of Ahvaz Razi Teaching hospital and Danial pathobiology laboratory for their valuable collaboration. This article is taken from Mr. Mojtaba shohan's dissertation that was supported by the Deputy of Research and Technology of Ahvaz Jundishapur University of Medical Sciences (No. U-99020).
References
- Agrawal P.K., Agrawal C., Blunden G. Quercetin: antiviral significance and possible COVID-19 integrative considerations. Natural Product Communications. 2020;15 [Google Scholar]
- Ahmed A., Abdelseed H., Albalawi Y., Almutairi Y., Alsalameen E., Alkattan A. medRxiv; 2020. Evaluation of the Effect of Zinc, Quercetin, Bromelain and Vitamin C on COVID-19 Patients. [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S. Remdesivir for the treatment of covid-19. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpinteiro A., Edwards M.J., Hoffmann M., Kochs G., Gripp B., Weigang S., Adams C., Carpinteiro E., Gulbins A., Keitsch S. Pharmacological inhibition of acid sphingomyelinase prevents uptake of SARS-CoV-2 by epithelial cells. Cell Reports Medicine. 2020;1:100142. doi: 10.1016/j.xcrm.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpinteiro A., Gripp B., Hoffmann M., Pöhlmann S., Hoertel N., Edwards M.J., Kamler M., Kornhuber J., Becker K.A., Gulbins E. Inhibition of acid sphingomyelinase by ambroxol prevents SARS-CoV-2 entry into epithelial cells. J. Biol. Chem. 2021;296 doi: 10.1016/j.jbc.2021.100701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro V., Pandolfi R., Moreno L., Barreira B., Martínez-Ramas A., Morales-Cano D., Ruiz-Cabello J., Lorente J.A., Duarte J., Cogolludo Á. Effects of quercetin in a rat model of hemorrhagic traumatic shock and reperfusion. Molecules. 2016;21:1739. doi: 10.3390/molecules21121739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colunga Biancatelli R.M.L., Berrill M., Catravas J.D., Marik P.E. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19) Front. Immunol. 2020;11:1451. doi: 10.3389/fimmu.2020.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbous H.M., Abd-Elsalam S., El-Sayed M.H., Sherief A.F., Ebeid F.F., Abd El Ghafar M.S., Soliman S., Elbahnasawy M., Badawi R., Tageldin M.A. Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study. Arch. Virol. 2021;166:949–954. doi: 10.1007/s00705-021-04956-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dabbous H.M., El-Sayed M.H., El Assal G., Elghazaly H., Ebeid F.F., Sherief A.F., Elgaafary M., Fawzy E., Hassany S.M., Riad A.R. Safety and efficacy of favipiravir versus hydroxychloroquine in management of COVID-19: a randomised controlled trial. Sci. Rep. 2021;11:1–7. doi: 10.1038/s41598-021-85227-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Derosa G., Maffioli P., D'Angelo A., Di Pierro F. A role for quercetin in coronavirus disease 2019 (COVID‐19) Phytother Res. 2021;35:1230–1236. doi: 10.1002/ptr.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pierro F., Derosa G., Maffioli P., Bertuccioli A., Togni S., Riva A., Allegrini P., Khan A., Khan S., Khan B.A. Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: a prospective, randomized, controlled, and open-label study. Int. J. Gen. Med. 2021;14:2359. doi: 10.2147/IJGM.S318720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pierro F., Iqtadar S., Khan A., Mumtaz S.U., Chaudhry M.M., Bertuccioli A., Derosa G., Maffioli P., Togni S., Riva A. Potential clinical benefits of quercetin in the early stage of COVID-19: results of a second, pilot, randomized, controlled and open-label clinical trial. Int. J. Gen. Med. 2021;14:2807. doi: 10.2147/IJGM.S318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frediansyah A., Tiwari R., Sharun K., Dhama K., Harapan H. Antivirals for COVID-19: A critical review. Clinical Epidemiology and global health. 2020 doi: 10.1016/j.cegh.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinsky G.V. Tripartite combination of candidate pandemic mitigation agents: vitamin D, quercetin, and estradiol manifest properties of medicinal agents for targeted mitigation of the COVID-19 pandemic defined by genomics-guided tracing of SARS-CoV-2 targets in human cells. Biomedicines. 2020;8:129. doi: 10.3390/biomedicines8050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N., Sánchez-Rico M., Vernet R., Beeker N., Jannot A.-S., Neuraz A., Salamanca E., Paris N., Daniel C., Gramfort A. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Mol. Psychiatr. 2021:1–14. doi: 10.1038/s41380-021-01021-4. [DOI] [PubMed] [Google Scholar]
- Hoertel N., Sánchez‐Rico M., Gulbins E., Kornhuber J., Carpinteiro A., Lenze E.J., Reiersen A.M., Abellán M., De La Muela P., Vernet R. Association between FIASMAs and reduced risk of intubation or death in individuals hospitalized for severe COVID‐19: an observational multicenter study. Clin. Pharmacol. Ther. 2021 doi: 10.1002/cpt.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S., Kim S., Shin D.H., Kim M.-S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzym. Inhib. Med. Chem. 2020;35:145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaka A.S., MacDonald R., Greer N., Vela K., Duan-Porter W., Obley A., Wilt T.J. Major update: remdesivir for adults with COVID-19: a living systematic review and meta-analysis for the American College of Physicians Practice Points. Ann. Intern. Med. 2021;174:663–672. doi: 10.7326/M20-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze E.J., Mattar C., Zorumski C.F., Stevens A., Schweiger J., Nicol G.E., Miller J.P., Yang L., Yingling M., Avidan M.S. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. Jama. 2020;324:2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Yao J., Han C., Yang J., Chaudhry M.T., Wang S., Liu H., Yin Y. Quercetin, inflammation and immunity. Nutrients. 2016;8:167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalmurti N., Hunter C.A. Immunity; 2020. Cytokine Storms: Understanding COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Corral J., Rodríguez-Morató J., Gomez-Gomez A., Pascual-Guardia S., Muñoz-Bermúdez R., Salazar-Degracia A., Pérez-Terán P., Restrepo M.I., Khymenets O., Haro N. Metabolic signatures associated with severity in hospitalized COVID-19 patients. Int. J. Mol. Sci. 2021;22:4794. doi: 10.3390/ijms22094794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon A.M., Barritt A.S. Springer; 2020. Elevated Liver Enzymes in Patients with COVID-19: Look, but Not Too Hard. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaz E., Tabarsi P., Varahram M., Folkerts G., Adcock I.M. The immune response and immunopathology of COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murohashi K., Hagiwara E., Kitayama T., Yamaya T., Higa K., Sato Y., Otoshi R., Shintani R., Okabayashi H., Ikeda S. Outcome of early-stage combination treatment with favipiravir and methylprednisolone for severe COVID-19 pneumonia: a report of 11 cases. Respiratory investigation. 2020;58:430–434. doi: 10.1016/j.resinv.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onal H., Arslan B., Ergun N.U., Topuz S., Semerci S.Y., Kurnaz M., Bolu Y., Bozkurt M., Suner N., Kocatas A. Treatment of COVID-19 patients with quercetin: a prospective, single-centre, randomized, controlled trial. Authorea Preprints. 2021 doi: 10.3906/biy-2104-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros U., Pedrera L., Garcia-Saez A.J. Partners in crime: the interplay of proteins and membranes in regulated necrosis. Int. J. Mol. Sci. 2020;21:2412. doi: 10.3390/ijms21072412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi-Boroujeni A., Mahmoudian-Sani M.-R. Anti-inflammatory potential of Quercetin in COVID-19 treatment. J. Inflamm. 2021;18:1–9. doi: 10.1186/s12950-021-00268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seftel D., Boulware D.R. Oxford University Press US; 2021. Prospective Cohort of Fluvoxamine for Early Treatment of Coronavirus Disease 19, Open Forum Infectious Diseases. ofab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai M., Tsushima K., Tanaka S., Hagiwara E., Tarumoto N., Kawada I., Hirai Y., Fujiwara S., Komase Y., Saraya T. Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial. Infect. Dis. Ther. 2021:1–21. doi: 10.1007/s40121-021-00517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Khera D., Chugh A., Khera P.S., Chugh V.K. Efficacy and safety of remdesivir in COVID-19 caused by SARS-CoV-2: a systematic review and meta-analysis. BMJ open. 2021;11 doi: 10.1136/bmjopen-2020-048416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucan A., Cerci P., Efe S., Akgun H., Ozmen A., Yagmuroglu A., Bilgin M., Avci D. Benefits of treatment with favipiravir in hospitalized patients for COVID-19: a retrospective observational case–control study. Virol. J. 2021;18:1–9. doi: 10.1186/s12985-021-01577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg D.F., Te Velde A.A. Severe COVID-19: NLRP3 inflammasome dysregulated. Front. Immunol. 2020;11:1580. doi: 10.3389/fimmu.2020.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N., Di B., Xu L.-l. Cytokine & Growth Factor Reviews; 2021. The NLRP3 Inflammasome and COVID-19: Activation, Pathogenesis and Therapeutic Strategies. [DOI] [PMC free article] [PubMed] [Google Scholar]