Abstract
Background
Data on the prognostic value of left ventricular (LV) morphological and functional parameters including LV rotation in patients with dilated cardiomyopathy (DCM) using cardiovascular magnetic resonance (CMR) are currently scarce. In this study, we assessed the prognostic value of global longitudinal strain (GLS), global circumferential strain (GCS), global radial strain (GRS) and LV torsion using CMR feature tracking (FT).
Methods
CMR was performed in 350 DCM patients and 70 healthy subjects across 5 different European CMR Centers. Myocardial strain parameters were retrospectively assessed from conventional balanced steady-state free precession cine images applying FT. A combined primary endpoint (cardiac death, heart transplantation, aborted sudden cardiac death) was defined for the assessment of clinical outcome.
Results
GLS, GCS, GRS and LV torsion were significantly lower in DCM patients than in healthy subjects (all p < 0.001). The primary endpoint occurred in 59 (18.7%) patients [median follow-up 4.2 (2.0–5.6) years]. In the univariate analyses all strain parameters showed a significant prognostic value (p < 0.05). In the multivariate model, LV strain parameters, particularly GLS provided an incremental prognostic value compared to established CMR parameters like LV ejection fraction and late gadolinium enhancement. A scoring model including six categorical variables of standard CMR and strain parameters differentiated further risk subgroups.
Conclusion
LV strain assessed with CMR FT has a high prognostic value in patients with DCM, surpassing routine and dedicated functional parameters. Thus, CMR strain imaging may contribute to the improvement of risk stratification in DCM.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12968-021-00829-x.
Keywords: Dilated cardiomyopathy, Cardiac magnetic resonance, Strain, LV torsion, Prognosis
Background
As the assessment of myocardial deformation provides incremental prognostic value in heart failure [1, 2], a better understanding of myocardial mechanics in dilated cardiomyopathy (DCM)—including left ventricular (LV) rotational mechanisms—may improve risk stratification and facilitate treatment guidance.
Cardiovascular magnetic resonance (CMR) allows for the assessment of cardiac morphology and function with high spatial resolution and an excellent intrinsic blood-to-tissue contrast [3]. It is regarded as the current reference standard for the evaluation of LV morphology and function [4, 5]. For strain analysis in CMR, feature tracking (FT) has been established as a useful technique as it allows for the retrospective analysis of conventional balanced steady-state free precession (bSSFP) series without the need for additional, dedicated CMR strain sequences [6, 7]. CMR FT strain measurements feature reliable results showing an adequate agreement with echocardiography and CMR tagging [8–11]. Additionally, reference values have been published for adults and children [12–14].
Previous studies showed, that global longitudinal strain (GLS), global circumferential strain (GCS), global radial strain (GRS) and LV torsion are altered in various cardiomyopathies [15, 16]. Although the prognostic value of myocardial strains was shown in prior studies, data on the prognostic value of the different strain parameters—and especially LV torsion—in DCM patients are heterogenous and scarce [15, 17, 18].
Therefore, the aim of this study was to assess GLS, GCS, GRS and LV torsion in a large, multicenter cohort of DCM patients, and to evaluate their prognostic value.
Methods
Study subjects
Diagnosis of DCM was based on the 1995 World Health Organization/International Society and Federation of Cardiology criteria [19]. Inclusion criteria were an impaired systolic function with LV ejection fraction (LVEF) ≤ 45% and dilated LV end-diastolic diameter (LVEDD) obtained from another imaging modality than CMR, the absence of relevant coronary artery disease (defined as ≥ 50% luminal stenosis), myocardial infarction or coronary revascularization, significant valvular disease, hypertensive heart disease and congenital heart defects.
A population of 410 patients with non-ischemic DCM from University Hospital Heidelberg and the EuroCMR Registry was screened. In 45 patients the minimum interval of one year for follow-up data was not reached resulting in exclusion. Another 15 subjects were excluded due to insufficient tracking quality. Finally, a study population of 350 DCM patients was retrospectively included in the study. 306 patients derive from University Hospital Heidelberg, the 44 patients of the EuroCMR Registry from four different imaging centers in Switzerland, Belgium and Lithuania.
In addition, 70 subjects drawn from a study population of proven healthy subjects were analysed at University Hospital Heidelberg. They all underwent a strict selection process including detailed medical history, physical examination, comprehensive blood tests, 12-lead electrocardiogram (ECG) and stress CMR. An oral glucose tolerance test was performed to exclude impaired glucose tolerance. Subjects with a history, signs or symptoms of a cardiac disease were excluded. Additional exclusion criteria were arterial hypertension, cerebrovascular or other relevant diseases, a regular intake of drugs except for thyroid or contraceptive drugs or vitamins.
All participants gave written informed consent and the study was approved by the local ethics committee. A part of the study population was already part of prior CMR trials [15, 20].
Image acquisition and analysis
CMR examinations at the University Hospital Heidelberg were performed on a 1.5T clinical scanner (Achieva®, Philips Healthcare, Best, the Netherlands). Subjects identified from the EuroCMR Registry were examined at various 1.5T and 3T CMR scanners from Philips Healthcare (Intera®, Ingenia®, Achieva®) and Siemens Healthineers (Erlangen, Germany; Aera®, Avanto®, Skyra®, Verio®). Image acquisition was performed in accordance with the Society for Cardiovascular Magnetic Resonance (SCMR) guidelines to allow for data pooling [21].
Analyses of all subjects for ventricular volumes, LVEF and other morphological and functional parameters were derived from short axis and long axis views on commercially available workstations (Viewforum® and IntelliSpace Portal®, Philips Healthcare) and dedicated CMR software (cvi42™, Circle Cardiovascular Imaging, Calgary, Alberta, Canada) at our imaging center from different examiners with many years of expertise in CMR. All ‘standard’ morphological and functional parameters including late gadolinium enhancement (LGE) were analyzed in accordance with actual recommendations [22]. Papillary muscles were part of the LV volume. LGE was acquired in all DCM patients. LGE sequences were routinely interpreted by experienced physicians with level 3 CMR certification of the German Cardiac Society or comparable qualification. LGE extent was not quantified due to the mostly diffuse demarcation of myocardial fibrosis. Every LGE pattern was considered—except for mild, unspecific LGE at the basal right ventricle (RV) insertion.
In EuroCMR Registry patients, baseline parameters as well as anthropometric data were provided by the respective participating centre and retrieved from the EuroCMR Registry database.
Data analysis
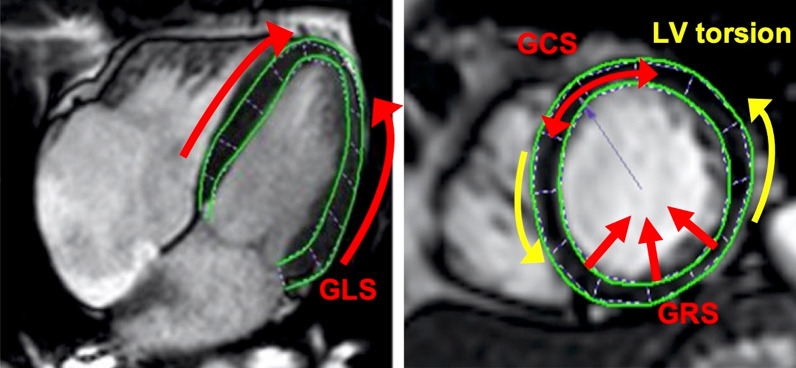
Strain analysis was performed on bSSFP cine images using dedicated 2D CMR FT software (TomTec Imaging Systems GmbH, Unterschleissheim, Germany) at the core lab of the University of Heidelberg. LV endocardial and epicardial borders were manually drawn at end-diastole and the tracking of the myocardium over the whole cardiac cycle was automatically conducted. All measurements were repeated three times [23]. If necessary, the contouring was manually optimized. Patients were excluded if no satisfying myocardial tracking was achievable. Long axis views (2-chamber, 3-chamber, and 4-chamber views) were used for the measurement of GLS, three short axis views at apical, midventricular and basal level were used to evaluate GCS, GRS and LV rotation (Fig. 1). In our study GLS, GCS, GRS and LV rotation of apex and base were calculated using the following approach [15]: First, the three measurements were averaged for every segment resulting in segmental mean curves. Second, the peak of a global mean curve (average of all segmental mean curves over the whole cardiac cycle) was used as the global strain value.
Fig. 1.
Illustration of the assessed strain parameters in long and short axis views. Long axis views (left: 4-chamber view) were used for the measurement of global longitudinal strain (GLS), three short axis views at apical, midventricular and basal level (right: apical short axis view) were used to evaluate global circumferential strain (GCS), global radial strain (GRS) and left ventricular (LV) torsion
Apical and basal slices at standardized 25% and 75% levels were selected for the quantification of rotational parameters, as this approach provides best reproducibility [10]. As viewed from the apex, ‘normal’ LV rotation was defined as a counterclockwise systolic rotation of the apex and a clockwise rotation of the base. LV rotation was expressed as LV twist and LV torsion. The peak difference of LV apical and basal rotation at the same point of the cardiac cycle was defined as LV twist, whereas the LV torsion was calculated as the relation of LV twist/LV length. LV length was defined as the distance between the apical and basal short axis slices used for LV rotation analyses. All rotational parameters were measured at ‘mid-wall’.
Follow-up data and definition of study endpoints
Physicians, blinded to the CMR results, contacted each patient or an immediate family member to obtain follow-up data. Cardiac death, heart transplantation and aborted sudden cardiac death (SCD) by appropriate implanted cardioverter defibrillator (ICD) discharge (including successful antitachycardia pacing = ATP) due to ventricular tachycardia or ventricular fibrillation were defined as the combined primary endpoint. The events of the primary endpoint and hospitalization due to heart failure were defined as the secondary endpoint. In case of simultaneous cardiac events per patient, the worst event was selected (cardiac death > transplantation > aborted SCD due to appropriate ICD discharge > hospitalization due to heart failure). There was lack of follow-up data from 45 patients from the EuroCMR Registry.
Statistical analysis
Continuous parameters were expressed as mean ± standard deviation for parametric and as median with interquartile range (IQR) for nonparametric variables. Normal distribution was assessed using Shapiro–Wilk test. To compare continuous variables between two groups, Student’s t-test and Mann Whitney U test were used as applicable. Categorical variables were expressed as counts and proportions and were compared using the Chi-square test. The Kaplan–Meier method was used for survival curves, which were compared by log-rank tests. Receiver operating characteristics analysis was used to define optimal cut-off values for the prediction of clinical endpoints. Univariate and multivariate Cox proportional hazards regression analyses were performed to calculate hazard ratios (HR) and 95% confidence intervals (CI). Collinearity was examined using a correlation matrix of correlation coefficients (r) for the observed parameters after the method of Pearson et al. for parametric and Spearman et al. for non-parametric parameters. Intra- and interobserver variabilities for GLS, GCS, GRS and LV torsion were assessed by repeated analysis of 20 randomly selected patients of the control group and 20 DCM patients. The intra- and interobserver variability was described using the intra-class correlation coefficient (ICC with 95% CI) with a two-way random model with absolute agreement. A p-value of < 0.05 was regarded as statistically significant. Dedicated statistical software MedCalc (v20.013, MedCalc software, Mariakerke, Belgium) was used for statistical analysis.
Results
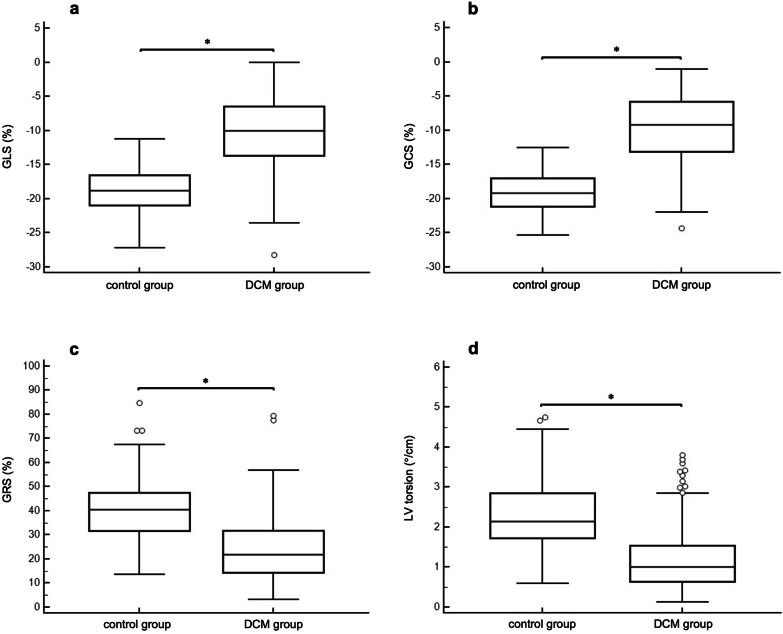
The final study population consisted of 350 subjects (259 male; 52.2 ± 15.2 years). There were no significant differences regarding age and gender between the DCM- and the control group (Table 1). Most patients had dyspnea in New York Heart Association (NYHA) class II and III (n = 260; 74.3%), they presented with various cardiovascular risk factors. Almost every patient was treated with an angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB; 346 patients, 98.9%). LVEF was significantly lower, whereas N-terminal pro-hormone brain natriuretic peptide (NT-pro BNP) was significantly higher in DCM patients compared to the healthy control group (Tables 1 and 2, all p < 0.001). LGE was present in 134 (38.3%) DCM patients. GLS, GCS, GRS and LV torsion were significantly impaired in the DCM cohort (Table 2, Fig. 2). Especially LVEF, GLS and GCS correlated strongly (Additional file 1: Table S1).
Table 1.
Patient characteristics of the healthy—and dilated cardiomyopathy (DCM) group
| Healthy group (n = 70) | DCM group (n = 350) | p-value | |
|---|---|---|---|
| Age [years] | 51.9 ± 15.2 | 52.2 ± 15.2 | 0.87 |
| Male gender [n] | 46 (65.7%) | 259 (73.9%) | 0.16 |
| Weight [kg] | 75.9 ± 10.4 | 79.8 ± 15.7 | < 0.01 |
| Height [cm] | 174.4 ± 8.9 | 175.8 ± 9.3 | 0.26 |
| BMI [kg/m2] | 24.9 ± 3.0 | 25.8 ± 4.3 | < 0.05 |
| NYHA class [n] | |||
| I | 70 (100.0%) | 84 (24.0%) | < 0.001 |
| II | 0 | 152 (43.4%) | < 0.001 |
| III | 0 | 108 (30.9%) | < 0.001 |
| IV | 0 | 6 (1.7%) | < 0.001 |
| Cardiovascular risk factors [n] | |||
| Arterial hypertension | 0 | 154 (44.0%) | < 0.001 |
| Dyslipidemia | 0 | 89 (25.4%) | < 0.001 |
| Diabetes | 0 | 42 (12.0%) | < 0.001 |
| Smoker | 12 (17.1%) | 140 (40.0%) | < 0.001 |
| Family history of CAD | 0 | 77 (22.0%) | < 0.001 |
| Obesity | 4 (5.7%) | 52 (14.9%) | < 0.001 |
| Heart failure therapy | |||
| ACE inhibitor/ARB | 0 | 346 (98.9%) | < 0.001 |
| ß-Blocker | 0 | 300 (85.7%) | < 0.001 |
| Aldosterone receptor antagonist | 0 | 145 (41.4%) | < 0.001 |
| Loop diuretic | 0 | 169 (48.3%) | < 0.001 |
| Heart rate [/min] | 68.5 ± 10.1 | 71.4 ± 15.2 | 0.05 |
| Atrial fibrillation [n] | 0 | 55 (15.7%) | < 0.001 |
| Laboratory findings | |||
| Hemoglobine [g/dl] | 14.5 (13.5–15.4) | 14.3 (13.3–15.4) | 0.14 |
| Creatinine [mg/dl] | 0.9 (0.7–1.0) | 0.9 (0.8–1.1) | 0.10 |
| NT-pro BNP [ng/l] | 39.0 (24.0–61.0) | 373.0 (124.0–1797.0)† | < 0.001 |
ACE angiotensin converting enzyme, ARB angiotensin receptor blocker, BMI body mass index, CAD coronary artery disease, NT-pro BNP N-terminal pro-hormone brain natriuretic peptide, NYHA New York Heart Association
†NT-pro BNP values of 110 patients missing
Table 2.
Comparison of CMR parameters including LV strain and rotational parameters between healthy—and DCM group
| Healthy group (n = 70) | DCM group (n = 350) | p-value | |
|---|---|---|---|
| LVEF [%] | 60.1 ± 5.1 | 36.4 ± 13.7 | < 0.001 |
| LVEDV [ml] | 168.0 ± 38.2 | 248.9 ± 94.4 | < 0.001 |
| LV mass [g] | 77.7 ± 20.9 | 147.3 ± 97.0 | < 0.001 |
| presence of LGE [n] | – | 134 (38.3%) | – |
| GLS [%] | − 17.5 ± 3.8 | − 9.9 ± 4.5 | < 0.001 |
| GCS [%] | − 19.1 ± 3.0 | − 9.4 ± 4.9 | < 0.001 |
| GRS [%] | 41.8 ± 14.6 | 23.5 ± 12.0 | < 0.001 |
| Apical rotation [°] | 6.1 ± 3.0 | 2.0 ± 4.1 | < 0.001 |
| Basal rotation [°] | − 4.8 ± 4.0 | − 2.5 ± 3.6 | < 0.001 |
| LV twist [°] | 10.3 ± 3.7 | 5.5 ± 3.0 | < 0.001 |
| LV torsion [°/cm] | 2.3 ± 0.9 | 1.2 ± 0.7 | < 0.001 |
| Normal directed rotation [n] | 62 (88.6%) | 191 (54.6%) | < 0.001 |
| Reversed apical rotation [n] | 1 (1.4%) | 98 (28.0%) | < 0.001 |
GCS global circumferential strain, GLS global longitudinal strain, GRS global radial strain, LVEDV left ventricular end-diastolic volume, LGE late gadolinium enhancement, LVEF left ventricular ejection fraction
Fig. 2.
Comparison of strain parameters: healthy control group vs. dilated cardiomyopathy (DCM) group. Box-whisker-plots for the comparison of different strain parameters including LV torsion between the healthy control and DCM group. a global longitudinal strain (GLS), b global circumferential strain (GCS), c global radial strain, (GRS) d LV torsion; * p < 0.001
Patients from EuroCMR Registry had a significantly lower LVEF (EuroCMR: 28.7 ± 9.7% vs. Heidelberg: 37.5 ± 13.9%; p < 0.001), while age (EuroCMR: 55.5 ± 15.7 years vs. Heidelberg: 51.7 ± 15.1 years; p = 0.12) and gender (EuroCMR: 63.6% males vs. Heidelberg: 75.4% males; p = 0.10) were similar.
In the DCM subgroup with LGE (n = 134); GLS (− 10.6 ± 4.2% vs. − 8.7 ± 4.6%, p < 0.001), GCS (− 10.4 ± 4.4% vs. − 8.6 ± 5.1%, p < 0.001), GRS (25.5 ± 12.1% vs. 20.3 ± 11.3%, p < 0.001) as well as the LVEF (38.9 ± 13.0% vs. 32.3 ± 13.9%, p < 0.001) but not LV torsion (1.2 ± 0.7°/cm vs. 1.1 ± 0.6°/cm, p = 0.12) were significantly reduced compared to DCM patients without LGE.
In contrast to the healthy control group (p < 0.001), a considerable fraction of DCM patients showed a reversed direction of rotation (45.4%), predominantly affecting the LV apex (n = 98 patients; 28.0%). Instead of a counterclockwise apical rotation, what is regarded to be normal in systole, these patients showed a peak clockwise rotation of the apex resulting in a systolic rotation of apex and base into the same direction. Compared to DCM patients with normally directed rotation, the patients with reversed apical rotation had a significantly lower LVEF (38.5 ± 13.6% vs. 30.8 ± 12.6%; p < 0.001). Regarding LV strain and LV rotation, especially LV torsion was impaired (1.3 ± 0.7°/cm vs. 0.8 ± 0.6°/cm; p < 0.001). In the group of reversed apical rotation, a larger proportion of patients had a left bundle branch block (LBBB) compared to the group of patients with normally directed rotation (42.9% vs. 13.6%, p < 0.001). Consequently, QRS duration was significantly prolonged in patients with reversed apical rotation (125 ± 31 ms vs. 109 ± 23 ms; p < 0.001). Thereby, reversed rotation of the LV apex provided high specificity (specificity = 98.6%, sensitivity = 28.0%) for the diagnosis of DCM.
Some subjects of the control group showed a reversed rotation direction (n = 8, 11.4%), especially of the LV base. One subject had a reversed apical rotation (1.4%). Clinical, CMR or other LV strain parameters showed no significant differences compared to the subjects with normally directed rotation.
Baseline follow-up data
Over a median follow-up period of 4.2 (2.0–5.6) years, the primary endpoint was observed in 59 patients (18.7%), the secondary endpoint in 73 patients (20.9%). In total, 84 events occurred: 25 patients experienced a cardiac death, 7 underwent heart transplantation, 28 had an aborted SCD by an appropriate ICD discharge and 24 patients were hospitalized for heart failure.
The patients, in whom the primary endpoint occurred, had a significantly impaired LVEF, GLS, GCS, GRS and LV torsion (all p < 0.05; Table 3).
Table 3.
Comparison of DCM patients without and with a primary endpoint
| No event (n = 291) | Primary endpoint (n = 59) | p-value | |
|---|---|---|---|
| Age [years] | 51.6 ± 15.2 | 55.2 ± 15.3 | 0.10 |
| Male gender [n] | 214 (73.5%) | 45 (76.3%) | 0.66 |
| Weight [kg] | 80.5 ± 16.0 | 76.4 ± 14.0 | 0.07 |
| Height [cm] | 176.0 ± 8.9 | 174.8 ± 11.1 | 0.44 |
| BMI [kg/m2] | 25.9 ± 4.4 | 24.9 ± 3.4 | < 0.05 |
| Heart rate [1/min] | 70.8 ± 15.2 | 74.4 ± 15.0 | 0.09 |
| Atrial fibrillation [n] | 46 (15.8%) | 11 (18.6%) | 0.58 |
| NYHA class [n] | |||
| I | 72 (24.7%) | 12 (20.3%) | 0.50 |
| II | 133 (45.7%) | 19 (32.2%) | 0.08 |
| III | 80 (27.5%) | 28 (47.5%) | < 0.01 |
| IV | 6 (2.1%) | 0 | – |
| Cardiovascular risk factors [n] | |||
| Arterial hypertension | 127 (43.6%) | 27 (45.8%) | 0.75 |
| Dyslipidemia | 76 (26.1%) | 13 (22.0%) | 0.51 |
| Diabetes | 31 (10.7%) | 11 (18.6%) | 0.14 |
| Smoker | 119 (40.9%) | 21 (35.6%) | 0.50 |
| Family history of CAD | 63 (21.6%) | 14 (23.7%) | 0.66 |
| Obesity | 47 (16.2%) | 5 (8.5%) | 0.08 |
| Heart failure therapy [n] | |||
| ACE inhibitor/ARB | 287 (98.6%) | 59 (100.0%) | 0.57 |
| ß-Blocker | 245 (84.2%) | 54 (91.5%) | 0.11 |
| Aldosterone receptor antagonist | 112 (38.5%) | 34 (57.6%) | < 0.01 |
| Loop diuretic | 128 (44.0%) | 42 (71.2%) | < 0.001 |
| Laboratory findings | |||
| Creatinine [mg/dl] | 0.9 (0.8–1.1) | 1.1 (0.9–1.3) | 0.45 |
| NT-pro BNP [ng/l]† | 275.0 (102.3–1229.8) | 1831 (411.3–4976.3) | < 0.05 |
| CMR parameters | |||
| LVEF [%] | 38.2 ± 13.2 | 27.1 ± 12.7 | < 0.001 |
| LVEDV [ml] | 239 ± 81 | 298 ± 133 | < 0.01 |
| LV mass [g] | 139 ± 50 | 189 ± 205 | 0.07 |
| Presence of LGE [n] | 97 (33.3%) | 37 (62.7%) | < 0.001 |
| GLS [%] | − 10.3 ± 4.5 | − 7.5 ± 3.7 | < 0.001 |
| GCS [%] | − 10.0 ± 4.9 | − 6.6 ± 3.6 | < 0.001 |
| GRS [%] | 24.7 ± 12.2 | 17.7 ± 9.4 | < 0.001 |
| Apical rotation [°] | 1.9 ± 4.2 | 2.3 ± 3.9 | 0.36 |
| Basal rotation [°] | − 2.5 ± 3.7 | − 2.3 ± 3.0 | 0.84 |
| LV twist [°] | 5.6 ± 3.1 | 5.0 ± 2.5 | 0.11 |
| LV torsion [°/cm] | 1.2 ± 0.7 | 1.0 ± 0.6 | < 0.05 |
| Normal directed rotation [n] | 159 (54.6%) | 32 (54.2%) | 0.98 |
| Reversed apical rotation [n] | 89 (27.1%) | 15 (25.4%) | 0.84 |
†NT-pro BNP values of 110 patients missing
In the subgroup of DCM patients with an LVEF ≤ 35% (n = 160), the primary endpoint was observed in 44 patients (27.5%). In these 44 patients a significantly lower GLS (− 5.9 ± 2.7% vs. − 7.4 ± 3.4%, p < 0.01) and GCS (− 5.0 ± 2.0% vs. − 6.2 ± 2.8%, p < 0.01) was observed.
In contrast, only 15 (7.9%) primary endpoint events were observed in the patient group with an LVEF > 35% (n = 190). GLS (− 12.4 ± 3.9% vs. − 11.3 ± 3.6%, p = 0.14), GCS (− 12.9 ± 3.8% vs. − 11.3 ± 2.8%, p = 0.12), GRS (29.5 ± 11.8%, vs. 25.4 ± 9.6%, p = 0.19) and the LV torsion (1.4 ± 0.7°/cm vs. 1.3 ± 0.7°/cm, p = 0.59) were lower in these 15 patients—but only as a trend—compared to the other patients with a LVEF > 35%.
Uni- and multivariate analysis
Several clinical (age, NYHA class), laboratory (log transformed NT-pro BNP), standard CMR (LVEF, LV end-diastolic volume (LVEDV), presence of LGE) and strain parameters (GLS, GCS, GRS, LV torsion) were associated with the primary and the secondary endpoint in univariate analysis (Table 4 and Additional file 1: Table S2).
Table 4.
Univariate survival analysis of the primary endpoint (Cox proportional-hazards regression)
| Primary endpoint | ||||
|---|---|---|---|---|
| HR | 95% CI | p-value | χ2 | |
| Age [years] | 1.02 | 1.00–1.04 | < 0.05 | 4.17 |
| NYHA | 1.49 | 1.04–2.14 | < 0.05 | 4.85 |
| LVEF [%] | 0.94 | 0.93–0.96 | < 0.001 | 35.85 |
| LVEDV [ml] | 1.01 | 1.00–1.01 | < 0.001 | 17.14 |
| lnNT-pro BNP [ng/l] | 1.52 | 1.32–1.75 | < 0.001 | 32.15 |
| LGE [n] | 3.05 | 1.79–5.22 | < 0.001 | 17.58 |
| GLS [%] | 1.22 | 1.14–1.31 | < 0.001 | 36.81 |
| GCS [%] | 1.23 | 1.14–1.32 | < 0.001 | 40.06 |
| GRS [%] | 0.94 | 0.91–0.97 | < 0.001 | 21.41 |
| LV twist [°] | 0.92 | 0.84–1.02 | 0.09 | 2.89 |
| LV torsion [°/cm] | 0.63 | 0.40–0.97 | < 0.05 | 4.90 |
| Reversed apical rotation [n] | 1.08 | 0.61–1.90 | 0.79 | 0.07 |
NT-pro BNP was log transformed (lnNT-pro BNP)
Based on the univariate analysis of LV strains, a multivariate Cox regression model including seven different parameters was assessed (Table 5 and Additional file 1: Table S3). Besides standard CMR parameters used for risk stratification in DCM (LVEF, LVEDV and the presence of LGE), the strain parameters (GLS, GCS, GRS and LV torsion) were included. In this multivariate, backwards regression model only the presence of LGE for the primary endpoint (HR 2.16, 95% CI 1.24–3.77, p < 0.05) and GLS for primary (HR 1.13, 95% CI 1.01–1.26, p < 0.05) and secondary endpoint (HR 1.11, 95% CI 1.01–1.23, p < 0.05) remained as independent predictors.
Table 5.
Multivariate survival analysis of the primary endpoint (Cox proportional-hazards regression)
| Primary endpoint | |||
|---|---|---|---|
| HR | 95% CI | p-value | |
| LVEF [%] | 1.00 | 0.95–1.05 | 0.90 |
| LVEDV [ml] | 1.00 | 1.00–1.01 | 0.50 |
| LGE [n] | 2.16 | 1.24–3.77 | < 0.05 |
| GLS [%] | 1.13 | 1.01–1.26 | < 0.05 |
| GCS [%] | 1.07 | 0.92–1.24 | 0.38 |
| GRS [%] | 0.99 | 0.96–1.03 | 0.71 |
| LV torsion [°/cm] | 1.26 | 0.74–2.13 | 0.40 |
Furthermore, series of Cox proportional-hazards models with stepwise regression were created to evaluate the incremental value of strain imaging using CMR FT (Fig. 3). As demonstrated, the different LV strain parameters, particularly GLS as well as LGE provided incremental information compared to standard risk stratification with LVEF (p < 0.05).
Fig. 3.
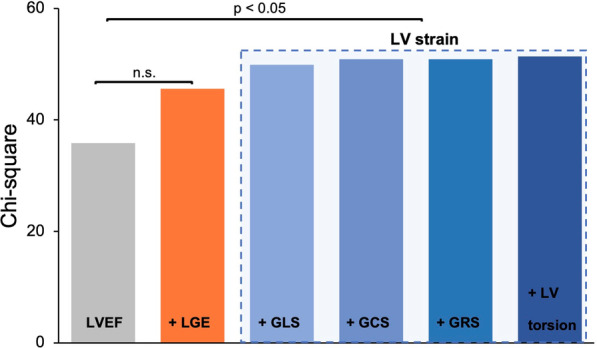
Cox proportional-hazards model of the additional value of LV strain parameters. Strain parameters and late gadolinium enhancement (LGE) improve risk stratification significantly compared to LV ejection fraction (LVEF). p < 0.05
Survival analysis
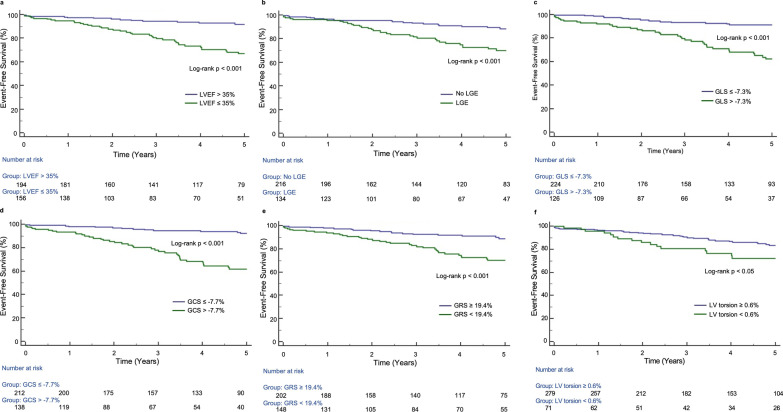
For the Kaplan–Meier survival analyses, LV torsion was used as the only rotational parameter due to its higher prognostic value as described above (Table 4). Cut-off values were determined using ROC curve analyses of the primary endpoint: -7.3% for GLS (AUC = 0.72, p < 0.001), -7.7% for GCS (AUC = 0.73, p < 0.001), 19.4% for GRS (AUC = 0.68, p < 0.001) and 0.6°/cm for the LV torsion (AUC = 0.58, p < 0.05). Consequently, in Kaplan–Meier analysis, patients with a GLS > − 7.3%, a GCS > − 7.7%, a GRS < 19.4% (all log-rank p < 0.001) and a LV torsion < 0.6°/cm (log-rank p < 0.05) had a significantly higher rate of cardiac events (Fig. 4). LVEF ≤ 35% as well as the presence of LGE were also significant predictors of the primary endpoint (all log-rank p < 0.001; Fig. 4).
Fig. 4.
Kaplan–Meier survival analyses for the prediction of primary endpoint. Kaplan–Meier curves for LVEF (a) with cut-off value of ≤ 35%, the presence of LGE (b), GLS (c), GCS (d), GRS (e) and LV torsion (f)
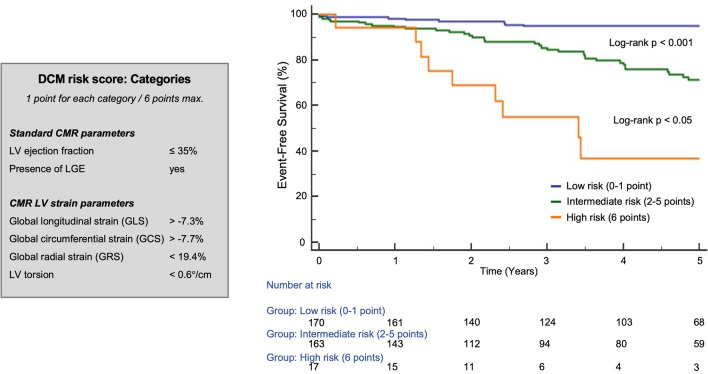
Based on different CMR and strain parameters, a DCM risk score was built, including LVEF ≤ 35% and the presence of LGE as standard CMR parameters. The addition of dichotomized LVEDV led to a worsening of the prognostic power, hence LVEDV was not part of DCM risk score. LV strains and LV torsion completed the six different categories of the scoring model. One point was added for each category worse than the above-mentioned cut-off values. Three main groups were created: a low risk- (0–1 point), an intermediate risk- (2–5 points) and a high-risk group (6 points). In Kaplan–Meier analysis, each group differed significantly from each other (primary and secondary endpoint; p < 0.05; Fig. 5 and Additional file 2: Fig. S1). Even a simplified risk score, only consisting of LVEF ≤ 35%, the presence of LGE and GLS > -7.3% allows for excellent differentiation between patients at low (0–2 points) and high risk (3 points) for a negative clinical outcome (log-rank p < 0.001; Additional file 2: Fig. S2).
Fig. 5.
Kaplan–Meier analysis of a CMR risk score in DCM patients. CMR risk score is based on different CMR (LVEF, LGE) and strain parameters (GLS, GCS, GRS, LV torsion) for the prediction of the primary endpoint (cut-off values as mentioned on the left): low risk (0–1p), intermediate risk (2–5p) and high risk (6p)
Observer variability
Quantification of the different LV strain and LV rotational parameters (GLS, GCS, GRS, LV torsion) using FT in CMR featured a good reproducibility in healthy subjects as well as DCM patients (Additional file 1: Table S4a and S4b). The ICC for the intraobserver variability in healthy subjects ranged between 0.93 (95% CI: 0.72–0.98) for LV torsion and 0.98 (95% CI: 0.93–0.99) for GLS, the ICC for the interobserver variability between 0.85 (95% CI: 0.43–0.96) for LV torsion and 0.96 (95% CI: 0.84–0.99) for GLS. In DCM patients, intra- and interobserver reproducibility was lower but still good: ICC for intraobserver variability ranged between 0.91 (95% CI: 0.65–0.98) for LV torsion and 0.98 (95% CI: 0.80–0.99) for GLS, for interobserver variability between 0.84 (95% CI: 0.40–0.96) for LV torsion and 0.96 (95% CI: 0.77–0.98) for GLS.
Discussion
In this study, including subjects from five different European CMR centers, the alterations of myocardial mechanics in DCM patients were assessed and compared to a control group of proven healthy subjects. To our knowledge, this is the first study assessing GLS, GCS, GRS and LV torsion in a combined approach providing prognostic values for strain as well as LV rotational parameters.
The main findings of our study were: (1) strain and rotational parameters could be assessed reliably using CMR FT with high intra- and interobserver reproducibility. (2) GLS, GCS, GRS as well as LV torsion were significantly impaired in patients with DCM. (3) The analyzed parameters offered a significant prognostic value surpassing standard CMR parameters for risk stratification.
Prognostic value of LV strain parameters
The timely diagnosis of DCM and recognition of myocardial dysfunction is important for the initiation of therapy, risk stratification and to reduce mortality [24]. Currently, global LVEF is used for the quantification of LV systolic function and is commonly used to guide the initiation of medical heart failure therapy. It is also employed as a prognostic marker and as a parameter for ICD and resynchronization therapy eligibility [3].
In accordance with previous studies on myocardial mechanics in DCM, a reduction of all strain parameters including LV torsion was observed [25, 26]. LV strain assessed with CMR FT provided incremental prognostic value and should be considered as clinical routine diagnostics in patients with DCM. Thereby, all strain parameters were significantly associated with the primary and secondary endpoint in univariate analysis. In a multivariate model including standard CMR parameters, GLS remained as the only independent predictor of both clinical endpoints. This finding has been observed in various other cardiac pathologies including acute myocardial infarction, after heart transplantation, myocarditis and in patients with preserved LVEF suggesting that GLS may represent a suitable parameter for global cardiac function assessment and prognostication [27–30]. The assessment of myocardial strains has therefore shown to improve risk stratification surpassing classical clinical, echocardiographic or CMR-derived risk features [1, 15, 31].
In DCM patients, a more precise prediction of clinical outcome using GLS was reported by several investigators—some of these included patients from our study population [15, 17, 20, 32]. This observation was confirmed in our large cohort of DCM patients in which we further examined LV rotation. LV rotational parameters were altered in DCM patients, however, they were no independent risk predictors due to the strong predictive value of LGE and GLS.
A risk score including six categorical variables of standard CMR and strain parameters allowed for further risk stratification in DCM patients.
Remarkably, LGE was an independent predictor of the primary endpoint but not of the secondary endpoint, which additionally includes hospitalization due to heart failure. Of note, GLS was independently associated with both endpoints. Thus, an impaired GLS may represent a better indicator of a worse outcome. Regarding LGE, myocardial fibrosis may not only be a result of an advanced stage of DCM but also increase the risk of adverse events as ventricular arrhythmias.
A comprehensive approach assessing both functional parameters including LV strain and tissue composition, as done in this study, may be beneficial in clinical routine.
In this respect, we were able to prove the additional prognostic value of LV strain in advanced disease stages even in our subgroup analysis: in patients with a LVEF ≤ 35% (n = 160), GLS and GCS were still significant predictors of the primary endpoint. Since the identification of patients eligible for an ICD remains challenging, especially in non-ischemic cardiomyopathies as shown by the DANISH study, the addition of strain parameters may improve the risk stratification [33].
Prognostic value of LV rotational parameters
LV rotation prognostic data are scarce and to our knowledge, we provide the first prognostic data of CMR LV torsion in DCM. The direction of apical rotation may be a hallmark of DCM as many patients showed a reversed apical rotation. The pathogenesis of reversed apical rotation is unclear, though in our study population these patients had significantly more often a LBBB than patients with normal directed rotation. LV rotational mechanics might be influenced by a changed propagation of cardiac excitation: former studies reported of a delayed activation of apical myocardium and an ‘U-shaped’ activation from right to left of at first anterior epicardium followed by septum and then of the lateral wall in patients with LBBB [34, 35]. It might be hypothesized that this changed, delayed excitation results in a reversed apical rotation.
In patients with DCM a reversed apical rotation has been reported, however its prognostic potential was previously unknown [25, 26]. In an echocardiographic study with 50 DCM patients, Popescu et al. observed a lower LVEF in 26 patients with a reversed apical rotation [25]. They proposed, that a reversed apical rotation in DCM patients could be associated with a more severe disease stage. Although LVEF was significantly reduced in patients with reversed apical rotation, we did not observe a significant influence of the direction of rotation on prognosis. Regarding the LV rotational parameters, only LV torsion was a predictor of clinical outcome.
In another study, Rady et al. previously showed an incremental value of LV torsion assessed with speckle-tracking echocardiography compared to right ventricular function and peak VO2 for the prediction of cardiac events in 91 DCM patients and a median follow-up of 272 days [36].
As we were able to prove in this study, LV torsion as a rotational parameter had a significant prognostic value, which, however, is lower than other strain parameters like GLS or GCS. Thereby, GLS and GCS represent the strongest strain parameters with regard to their prognostic value.
Strain imaging for the evaluation of myocardial mechanics
CMR FT provides quick and reliable global strain and rotational values with good intra- and inter-observer variability [6, 8, 37, 38]. Additional information about myocardial mechanics beyond LVEF can be obtained without the need of additional, dedicated CMR sequences. While echocardiography allows for an easy and straightforward assessment of LV morphology and function inclusive of strain measurements using speckle-tracking, CMR offers a more comprehensive approach for risk stratification. In CMR, standard LV parameters as LVEF not only show a better reproducibility but also a higher prognostic value than in echocardiography [39]. Risk stratification can be further enhanced by strain measurements, which are not limited by the patient’s acoustic window. In addition, CMR tissue characterization as LGE adds valuable prognostic information in ischemic and non-ischemic cardiomyopathies [40, 41]. Hence, CMR not only gains importance in clinical practice and current guidelines but is also assessed with regard to therapy optimization in prospective trials [42].
The application of fully automated software approaches for strain analysis could accelerate this development even further [43].
Limitations
CMR FT strain analysis has been previously validated, but there are no data on the agreement of LV rotational measurements between CMR FT and CMR tagging. There are only feasibility studies using CMR FT to assess LV rotation [10, 44]. In general, measurements of cardiac rotation are less robust than longitudinal parameters [10]. Therefore, the tracking was meticulously checked by an experienced reader in this study. Furthermore, we favored global over segmental values for analysis and repeated all strain measurements three times. As FT is dependent on good image quality and an exact endocardial border definition, we had to exclude 15 patients due to an insufficient tracking quality.
As part of the study population was drawn from the EuroCMR Registry, the quality of clinical and CMR-derived data of these patients were dependent on the respective institution. Due to the lack of follow-up data, 45 patients of EuroCMR Registry had to be excluded resulting in a predominance of a single center.
Because NT-pro BNP values were only available in 68.5% of the DCM patients, its prognostic value is possibly underestimated. As we showed a strong correlation between LVEF, GLS and GCS, there is a certain collinearity among the different parameters.
A priori power analysis regarding the prognostic value of LV torsion was not possible due to missing prognostic data. As rotational parameters were significant predictors in the univariate but not the multivariate analyses, one could speculate that they might have become statistically significant in a larger study population. However, the clinical relevance of such small effects would be questionable.
Conclusions
In patients with DCM, global LV strain parameters showed an additive prognostic value. Especially GLS surpassed standard clinical and CMR-derived parameters in the risk stratification of DCM patients. GLS and GCS add further prognostic information in DCM patients even in subjects with a LVEF ≤ 35%. The DCM risk score as a simple scoring system including standard CMR- and strain parameters allows for an exact risk stratification in DCM patients. With its additional prognostic value, an easy-to-use, fast and reproducible assessment of strain and rotational parameters, CMR FT may aid in risk stratification of DCM patients—and may find its way to routine clinical application. Larger, prospective multi-centre trials, using a comparable global strain approach, are needed to confirm our findings.
Supplementary Information
Additional file 1: Table S1. Correlation matrix for correlation coefficients (r) of common CMR and strain parameters including LV torsion. LVEF: left ventricular ejection fraction; GLS: global longitudinal strain; GCS: global circumferential strain; GRS: global radial strain; LV torsion: left ventricular torsion; LVEDV: left ventricular end-diastolic volume. Table S2. Univariate survival analysis of the secondary endpoint (Cox proportional-hazards regression). HR: hazard ratio; CI: confidence interval; LVEF: left ventricular ejection fraction; LVEDV: left ventricular end-diastolic volume; NT-pro BNP: N-terminal pro-hormone brain natriuretic peptide; LGE: late gadolinium enhancement; GLS: global longitudinal strain; GCS: global circumferential strain; GRS: global radial strain. Table S3. Multivariate survival analysis of the secondary endpoint (Cox proportional-hazards regression). HR: hazard ratio; CI: confidence interval; LVEF: left ventricular ejection fraction; LVEDV: left ventricular end-diastolic volume; LGE: late gadolinium enhancement; GLS: global longitudinal strain; GCS: global circumferential strain; GRS: global radial strain. Table S4a. Intra- and Interobserver variability of the strain parameters in healthy individuals. ICC: intra-class correlation coefficient; CI: confidence interval; GLS: global longitudinal strain; GCS: global circumferential strain; GRS: global radial strain; LV: left ventricular. Table S4b. Intra- and Interobserver variability of the strain parameters in DCM patients. ICC: intra-class correlation coefficient; CI: confidence interval; GLS: global longitudinal strain; GCS: global circumferential strain; GRS: global radial strain; LV: left ventricular.
Additional file 2: Figure S1. Kaplan–Meier analysis of a CMR risk score for the prediction of the secondary endpoint. CMR risk score is based on different CMR (LVEF, LGE) and strain parameters for the prediction of the secondary endpoint in DCM patients. Cut-off values as mentioned in Fig. 4: low risk (0-1p), intermediate risk (2-5p) and high risk (6p). CMR: cardiac magnetic resonance imaging; DCM: dilated cardiomyopathy; GLS: global longitudinal strain; GCS: global circumferential strain; GRS: global radial strain; LVEF: left ventricular ejection fraction; LGE: late gadolinium enhancement. Figure S2. Kaplan–Meier analysis of a simplified CMR risk score in DCM patients. The simplified CMR risk score is based on LVEF, LGE and GLS as the single strain parameter for the prediction of the primary endpoint (cut-off values as mentioned in Fig. 4 of the manuscript): low risk (0-2p) and high risk (3p). CMR: cardiac magnetic resonance imaging; DCM: dilated cardiomyopathy; LGE: late gadolinium enhancement; LVEF: left ventricular ejection fraction; GLS: global longitudinal strain.
Acknowledgements
We thank Daniel Asmussen and his team for their excellent acquisitions of CMR images at University Hospital Heidelberg. Furthermore, we would like to thank all participating centers of EuroCMR Registry as well as all associated members.
Abbreviations
- ACE
Angiotensin converting enzyme
- ARB
Angiotensin receptor blocker
- ATP
Anti-tachycardia pacing
- AUC
Area under the curve
- bSSFP
Balanced steady-state free precession
- CI
Confidence interval
- CMR
Cardiovascular magnetic resonance
- DCM
Dilated cardiomyopathy
- ECG
Electrocardiogram
- FT
Feature tracking
- GCS
Global circumferential strain
- GLS
Global longitudinal strain
- GRS
Global radial strain
- HR
Hazard ratio
- ICC
Intraclass correlation coefficient
- ICD
Implanted cardioverter defibrillator
- IQR
Interquartile range
- LBBB
Left bundle branch block
- LGE
Late gadolinium enhancement
- LV
Left ventricle/left ventricular
- LVEDD
Left ventricular end-diastolic diameter
- LVEDV
Left ventricular end-diastolic volume
- LVEF
Left ventricular ejection fraction
- NT-pro BNP
N-terminal pro-hormone brain natriuretic peptide
- NYHA
New York Heart Association
- RV
Right ventricle/right ventricular
- SCD
Sudden cardiac death
- SCMR
Society for Cardiovascular Magnetic Resonance
Authors’ contributions
AO responsible for conception of study design, data acquisition and analysis, statistical assessment, and drafting of the manuscript. JR involved in data acquisition and analysis and revision of the manuscript. MO involved in data analysis, statistical assessment and revision of the manuscript. NA involved in data acquisition and analysis and revision of the manuscript. TF involved in data acquisition and analysis and revision of the manuscript. CG involved in data acquisition and analysis and revision of the manuscript. AS involved in data analysis and revision of the manuscript. OB involved in data acquisition and revision of the manuscript. HM involved in data acquisition and revision of the manuscript. EG involved in data acquisition and revision of the manuscript. NF involved in revision of the manuscript. HAK involved in revision of the manuscript. SJB involved in conception of study design, statistical assessment, and revision of the manuscript. FA involved in data acquisition and analysis, in conception of study design, statistical assessment, and revision of the manuscript. All authors read and approved the final manuscript.
Funding
The study received no funding.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All participants including the patients from EuroCMR Registry—gave written informed consent and the study was approved by the local ethics committee (University of Heidelberg, medical school, approval nr. S154/2015).
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 1.
Competing interests
Christian Galuschky is employee of TomTec Imaging Systems and Thomas Fritz’ was a researcher of Philips Healthcare. All other authors declare that they have no competing interests regarding this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Andreas Ochs, Email: andreas.ochs@med.uni-heidelberg.de.
Johannes Riffel, Email: johannesriffel@web.de.
Marco M. Ochs, marco.ochs@googlemail.com
Nisha Arenja, Email: nisha.arenja@spital.so.ch.
Thomas Fritz, Email: thomas.fritz@crml.be.
Christian Galuschky, Email: cgaluschky@tomtec.de.
Andreas Schuster, Email: andreas.schuster@med.uni-goettingen.de.
Oliver Bruder, Email: o.bruder@contilia.de.
Heiko Mahrholdt, Email: heiko.mahrholdt@rbk.de.
Evangelos Giannitsis, Email: evangelos.giannitsis@med.uni-heidelberg.de.
Norbert Frey, Email: norbert.frey@med.uni-heidelberg.de.
Hugo A. Katus, Email: hugo.katus@med.uni-heidelberg.de
Sebastian J. Buss, Email: sbuss@gmx.net
Florian André, Email: florian.andre@med.uni-heidelberg.de.
References
- 1.Motoki H, Borowski AG, Shrestha K, Troughton RW, Tang WH, Thomas JD, et al. Incremental prognostic value of assessing left ventricular myocardial mechanics in patients with chronic systolic heart failure. J Am Coll Cardiol. 2012;60(20):2074–2081. doi: 10.1016/j.jacc.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 2.Buss SJ, Emami M, Mereles D, Korosoglou G, Kristen AV, Voss A, et al. Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis: incremental value compared with clinical and biochemical markers. J Am Coll Cardiol. 2012;60(12):1067–1076. doi: 10.1016/j.jacc.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Lang C, Atalay MK. Cardiac magnetic resonance imaging and computed tomography: state of the art in clinical practice. R I Med J. 2014;97(2):28–34. [PubMed] [Google Scholar]
- 5.Pesenti-Rossi D, Peyrou J, Baron N, Allouch P, Aubert S, Boueri Z, et al. Cardiac MRI: technology, clinical applications, and future directions. Ann Cardiol Angeiol (Paris) 2013;62(5):326–341. doi: 10.1016/j.ancard.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Moody WE, Taylor RJ, Edwards NC, Chue CD, Umar F, Taylor TJ, et al. Comparison of magnetic resonance feature tracking for systolic and diastolic strain and strain rate calculation with spatial modulation of magnetization imaging analysis. J Magn Reson Imaging. 2015;41(4):1000–1012. doi: 10.1002/jmri.24623. [DOI] [PubMed] [Google Scholar]
- 7.Schuster A, Hor KN, Kowallick JT, Beerbaum P, Kutty S. Cardiovascular magnetic resonance myocardial feature tracking: concepts and clinical applications. Circ Cardiovasc Imaging. 2016;9(4):e004077. doi: 10.1161/CIRCIMAGING.115.004077. [DOI] [PubMed] [Google Scholar]
- 8.Augustine D, Lewandowski AJ, Lazdam M, Rai A, Francis J, Myerson S, et al. Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: comparison with tagging and relevance of gender. J Cardiovasc Magn Reson. 2013;15:8. doi: 10.1186/1532-429X-15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck R, et al. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging. 2010;3(2):144–151. doi: 10.1016/j.jcmg.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Kowallick JT, Lamata P, Hussain ST, Kutty S, Steinmetz M, Sohns JM, et al. Quantification of left ventricular torsion and diastolic recoil using cardiovascular magnetic resonance myocardial feature tracking. PLoS ONE. 2014;9(10):e109164. doi: 10.1371/journal.pone.0109164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams LK, Urbano-Moral JA, Rowin EJ, Jamorski M, Bruchal-Garbicz B, Carasso S, et al. Velocity vector imaging in the measurement of left ventricular myocardial mechanics on cardiac magnetic resonance imaging: correlations with echocardiographically derived strain values. J Am Soc Echocardiogr. 2013;26(10):1153–1162. doi: 10.1016/j.echo.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Taylor RJ, Moody WE, Umar F, Edwards NC, Taylor TJ, Stegemann B, et al. Myocardial strain measurement with feature-tracking cardiovascular magnetic resonance: normal values. Eur Heart J Cardiovasc Imaging. 2015;16(8):871–881. doi: 10.1093/ehjci/jev006. [DOI] [PubMed] [Google Scholar]
- 13.Andre F, Steen H, Matheis P, Westkott M, Breuninger K, Sander Y, et al. Age- and gender-related normal left ventricular deformation assessed by cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson. 2015;17:25. doi: 10.1186/s12968-015-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andre F, Robbers-Visser D, Helling-Bakki A, Foll A, Voss A, Katus HA, et al. Quantification of myocardial deformation in children by cardiovascular magnetic resonance feature tracking: determination of reference values for left ventricular strain and strain rate. J Cardiovasc Magn Reson. 2016;19(1):8. doi: 10.1186/s12968-016-0310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buss SJ, Breuninger K, Lehrke S, Voss A, Galuschky C, Lossnitzer D, et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015;16(3):307–315. doi: 10.1093/ehjci/jeu181. [DOI] [PubMed] [Google Scholar]
- 16.Yoneyama K, Gjesdal O, Choi EY, Wu CO, Hundley WG, Gomes AS, et al. Age, sex, and hypertension-related remodeling influences left ventricular torsion assessed by tagged cardiac magnetic resonance in asymptomatic individuals: the multi-ethnic study of atherosclerosis. Circulation. 2012;126(21):2481–2490. doi: 10.1161/CIRCULATIONAHA.112.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romano S, Judd RM, Kim RJ, Kim HW, Klem I, Heitner JF, et al. Feature-tracking global longitudinal strain predicts death in a multicenter population of patients with ischemic and nonischemic dilated cardiomyopathy incremental to ejection fraction and late gadolinium enhancement. JACC Cardiovasc Imaging. 2018;11(10):1419–1429. doi: 10.1016/j.jcmg.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romano S, Mansour IN, Kansal M, Gheith H, Dowdy Z, Dickens CA, et al. Left ventricular global longitudinal strain predicts heart failure readmission in acute decompensated heart failure. Cardiovasc Ultrasound. 2017;15(1):6. doi: 10.1186/s12947-017-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93(5):841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 20.Riffel JH, Keller MG, Rost F, Arenja N, Andre F, Aus dem Siepen F, et al. Left ventricular long axis strain: a new prognosticator in non-ischemic dilated cardiomyopathy? J Cardiovasc Magn Reson. 2016;18(1):36. doi: 10.1186/s12968-016-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E, Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized P Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson. 2008;10:35. doi: 10.1186/1532-429X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update: society for Cardiovascular Magnetic Resonance (SCMR): board of Trustees Task Force on Standardized Post-Processing. J Cardiovasc Magn Reson. 2020;22(1):19. doi: 10.1186/s12968-020-00610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuster A, Stahnke VC, Unterberg-Buchwald C, Kowallick JT, Lamata P, Steinmetz M, et al. Cardiovascular magnetic resonance feature-tracking assessment of myocardial mechanics: intervendor agreement and considerations regarding reproducibility. Clin Radiol. 2015;70(9):989–998. doi: 10.1016/j.crad.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53(15):e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Popescu BA, Beladan CC, Calin A, Muraru D, Deleanu D, Rosca M, et al. Left ventricular remodelling and torsional dynamics in dilated cardiomyopathy: reversed apical rotation as a marker of disease severity. Eur J Heart Fail. 2009;11(10):945–951. doi: 10.1093/eurjhf/hfp124. [DOI] [PubMed] [Google Scholar]
- 26.Kanzaki H, Nakatani S, Yamada N, Urayama S, Miyatake K, Kitakaze M. Impaired systolic torsion in dilated cardiomyopathy: reversal of apical rotation at mid-systole characterized with magnetic resonance tagging method. Basic Res Cardiol. 2006;101(6):465–470. doi: 10.1007/s00395-006-0603-6. [DOI] [PubMed] [Google Scholar]
- 27.Eitel I, Stiermaier T, Lange T, Rommel KP, Koschalka A, Kowallick JT, et al. Cardiac magnetic resonance myocardial feature tracking for optimized prediction of cardiovascular events following myocardial infarction. JACC Cardiovasc Imaging. 2018;11(10):1433–1444. doi: 10.1016/j.jcmg.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 28.Shenoy C, Romano S, Hughes A, Okasha O, Nijjar PS, Velangi P, et al. Cardiac magnetic resonance feature tracking global longitudinal strain and prognosis after heart transplantation. JACC Cardiovasc Imaging. 2020;13(9):1934–1942. doi: 10.1016/j.jcmg.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer K, Obrist SJ, Erne SA, Stark AW, Marggraf M, Kaneko K, et al. Feature tracking myocardial strain incrementally improves prognostication in myocarditis beyond traditional CMR imaging features. JACC Cardiovasc Imaging. 2020;13(9):1891–1901. doi: 10.1016/j.jcmg.2020.04.025. [DOI] [PubMed] [Google Scholar]
- 30.Romano S, Judd RM, Kim RJ, Heitner JF, Shah DJ, Shenoy C, et al. Feature-tracking global longitudinal strain predicts mortality in patients with preserved ejection fraction: a multicenter study. JACC Cardiovasc Imaging. 2020;13(4):940–947. doi: 10.1016/j.jcmg.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2(5):356–364. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 32.Romano S, Judd RM, Kim RJ, Kim HW, Klem I, Heitner J, et al. Association of feature-tracking cardiac magnetic resonance imaging left ventricular global longitudinal strain with all-cause mortality in patients with reduced left ventricular ejection fraction. Circulation. 2017;135(23):2313–2315. doi: 10.1161/CIRCULATIONAHA.117.027740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375(13):1221–1230. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 34.Auricchio A, Fantoni C, Regoli F, Carbucicchio C, Goette A, Geller C, et al. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation. 2004;109(9):1133–1139. doi: 10.1161/01.CIR.0000118502.91105.F6. [DOI] [PubMed] [Google Scholar]
- 35.Becker RA, Scher AM, Erickson RV. Ventricular excitation in experimental left bundle branch block. Am Heart J. 1958;55(4):547–556. doi: 10.1016/0002-8703(58)90032-2. [DOI] [PubMed] [Google Scholar]
- 36.Rady M, Ulbrich S, Heidrich F, Jellinghaus S, Ibrahim K, Linke A, et al. Left ventricular torsion—a new echocardiographic prognosticator in patients with non-ischemic dilated cardiomyopathy. Circ J. 2019;83(3):595–603. doi: 10.1253/circj.CJ-18-0986. [DOI] [PubMed] [Google Scholar]
- 37.Pedrizzetti G, Claus P, Kilner PJ, Nagel E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson. 2016;18(1):51. doi: 10.1186/s12968-016-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maceira AM, Tuset-Sanchis L, Lopez-Garrido M, San Andres M, Lopez-Lereu MP, Monmeneu JV, et al. Feasibility and reproducibility of feature-tracking-based strain and strain rate measures of the left ventricle in different diseases and genders. J Magn Reson Imaging. 2018;47(5):1415–1425. doi: 10.1002/jmri.25894. [DOI] [PubMed] [Google Scholar]
- 39.Wong TC, Piehler K, Puntil KS, Moguillansky D, Meier CG, Lacomis JM, et al. Effectiveness of late gadolinium enhancement to improve outcomes prediction in patients referred for cardiovascular magnetic resonance after echocardiography. J Cardiovasc Magn Reson. 2013;15:6. doi: 10.1186/1532-429X-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Marco A, Brown PF, Bradley J, Nucifora G, Claver E, de Frutos F, et al. Improved risk stratification for ventricular arrhythmias and sudden death in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2021;77(23):2890–2905. doi: 10.1016/j.jacc.2021.04.030. [DOI] [PubMed] [Google Scholar]
- 41.Li S, Zhou D, Sirajuddin A, He J, Xu J, Zhuang B, et al. T1 Mapping and extracellular volume fraction in dilated cardiomyopathy: a prognosis study. JACC Cardiovasc Imaging. 2021. [DOI] [PubMed]
- 42.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021. [DOI] [PubMed]
- 43.Backhaus SJ, Metschies G, Zieschang V, Erley J, Mahsa Zamani S, Kowallick JT, et al. Head-to-head comparison of cardiovascular MR feature tracking cine versus acquisition-based deformation strain imaging using myocardial tagging and strain encoding. Magn Reson Med. 2021;85(1):357–368. doi: 10.1002/mrm.28437. [DOI] [PubMed] [Google Scholar]
- 44.Kowallick JT, Morton G, Lamata P, Jogiya R, Kutty S, Lotz J, et al. Inter-study reproducibility of left ventricular torsion and torsion rate quantification using MR myocardial feature tracking. J Magn Reson Imaging. 2015. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Correlation matrix for correlation coefficients (r) of common CMR and strain parameters including LV torsion. LVEF: left ventricular ejection fraction; GLS: global longitudinal strain; GCS: global circumferential strain; GRS: global radial strain; LV torsion: left ventricular torsion; LVEDV: left ventricular end-diastolic volume. Table S2. Univariate survival analysis of the secondary endpoint (Cox proportional-hazards regression). HR: hazard ratio; CI: confidence interval; LVEF: left ventricular ejection fraction; LVEDV: left ventricular end-diastolic volume; NT-pro BNP: N-terminal pro-hormone brain natriuretic peptide; LGE: late gadolinium enhancement; GLS: global longitudinal strain; GCS: global circumferential strain; GRS: global radial strain. Table S3. Multivariate survival analysis of the secondary endpoint (Cox proportional-hazards regression). HR: hazard ratio; CI: confidence interval; LVEF: left ventricular ejection fraction; LVEDV: left ventricular end-diastolic volume; LGE: late gadolinium enhancement; GLS: global longitudinal strain; GCS: global circumferential strain; GRS: global radial strain. Table S4a. Intra- and Interobserver variability of the strain parameters in healthy individuals. ICC: intra-class correlation coefficient; CI: confidence interval; GLS: global longitudinal strain; GCS: global circumferential strain; GRS: global radial strain; LV: left ventricular. Table S4b. Intra- and Interobserver variability of the strain parameters in DCM patients. ICC: intra-class correlation coefficient; CI: confidence interval; GLS: global longitudinal strain; GCS: global circumferential strain; GRS: global radial strain; LV: left ventricular.
Additional file 2: Figure S1. Kaplan–Meier analysis of a CMR risk score for the prediction of the secondary endpoint. CMR risk score is based on different CMR (LVEF, LGE) and strain parameters for the prediction of the secondary endpoint in DCM patients. Cut-off values as mentioned in Fig. 4: low risk (0-1p), intermediate risk (2-5p) and high risk (6p). CMR: cardiac magnetic resonance imaging; DCM: dilated cardiomyopathy; GLS: global longitudinal strain; GCS: global circumferential strain; GRS: global radial strain; LVEF: left ventricular ejection fraction; LGE: late gadolinium enhancement. Figure S2. Kaplan–Meier analysis of a simplified CMR risk score in DCM patients. The simplified CMR risk score is based on LVEF, LGE and GLS as the single strain parameter for the prediction of the primary endpoint (cut-off values as mentioned in Fig. 4 of the manuscript): low risk (0-2p) and high risk (3p). CMR: cardiac magnetic resonance imaging; DCM: dilated cardiomyopathy; LGE: late gadolinium enhancement; LVEF: left ventricular ejection fraction; GLS: global longitudinal strain.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.