Abstract
Purpose
The impact of intensivist workload on intensive care unit (ICU) outcomes is incompletely described and assessed across healthcare systems and countries. We sought to examine the association of patient-to-intensivist ratio (PIR) with hospital mortality in Australia/New Zealand (ANZ) ICUs.
Methods
We conducted a retrospective study of adult admissions to ANZ ICUs (August 2016–June 2018) using two cohorts: “narrow”, based on previously used criteria including restriction to ICUs with a single daytime intensivist; and “broad”, refined by individual ICU daytime staffing information. The exposure was average daily PIR and the outcome was hospital mortality. We used summary statistics to describe both cohorts and multilevel multivariable logistic regression models to assess the association of PIR with mortality. In each, PIR was modeled using restricted cubic splines to allow for non-linear associations. The broad cohort model included non-PIR physician and non-physician staffing covariables.
Results
The narrow cohort of 27,380 patients across 67 ICUs (predicted mortality: median 1.2% [IQR 0.4–1.4%]; mean 5.9% [sd 13.2%]) had a median PIR of 10.1 (IQR 7–14). The broad cohort of 91,206 patients across 73 ICUs (predicted mortality: 1.9% [0.6–6.5%]; 7.6% [14.9%]) had a median PIR of 7.8 (IQR 5.8–10.2). We found no association of PIR with mortality in either the narrow (PIR 1st spline term odds ratio [95% CI]: 1 [0.94, 1.06], Wald testing of spline terms p = 0.61) or the broad (1.02 [0.97, 1.07], p = 0.4) cohort.
Conclusion
We found no association of PIR with hospital mortality across ANZ ICUs. The low cohort predicted mortality may limit external validity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-021-06575-z.
Keywords: Intensive care unit, Intensivist, Workload, Mortality, Census, Patient-to-intensivist ratio
Take-home message
| We found no association of patient-to-intensivist ratio with hospital mortality across Australian/New Zealand intensive care units. The low cohort predicted mortality may limit external validity. |
Introduction
Studies indicate that care delivered in intensive care units (ICUs) by dedicated intensivists improves short-term outcomes for critically ill patients [1, 2]. However, ICUs vary in size and casemix. If and how these differences should guide safe intensivist staffing strategies is incompletely understood. Such information is increasingly needed in the context of increased ICU demand (e.g., during a pandemic such as coronavirus disease 2019 [COVID-19]).
ICU strain, a complex measure of workload inclusive of resource use and availability, census size and acuity, and patient turnover [3], has been linked to triage and outcomes [4–7]. The value of this concept stems from its integration of many factors which may impact workload; however, its calculation can be complicated. A simpler but potentially more actionable measure of workload is patient-to-intensivist ratio (PIR). Few studies have evaluated the association of PIR with patient outcomes to determine whether certain ratios optimize critical care delivery [8–13]. While they have found associations, findings have been inconsistent. In several studies, higher PIRs were associated with worse outcomes (hospital/shift-specific mortality, ICU length of stay) and, in one, having very low PIRs was also associated with harm. Moreover, prior work has been limited by failure to account for non-intensivist provider and other ICU team member staffing, which may confound any observed impact of PIR.
Here, we assessed the relationship between PIR and outcomes across Australia and New Zealand ICUs, accounting for known confounders such as severity of illness and for the potential impacts of other ICU clinicians. Based on our prior work [8], we hypothesized that there would be a U-shaped association between PIR and hospital mortality.
Methods
This retrospective cohort study included patients admitted to Australia and New Zealand ICUs. Patient-level clinical data were obtained from the Australia and New Zealand Intensive Care Society (ANZICS) Centre for Outcome and Resource Evaluation (CORE) Adult Patient Database (APD) (July 1, 2016–June 30, 2018) which includes > 90% of ICU admissions across the two countries. Staffing data came from the ANZICS Critical Care Resources (CCR) Registry annual survey (administered September 1, 2017–January 31, 2018) including 10 new “workforce” questions (eTable 1).
Cohort
All ICUs were “closed” (admission/discharge decisions at intensivist discretion); most hospitals have one ICU. We excluded ICUs without onsite, weekday daytime intensivists. We included adult (age ≥ 16) ICU admissions from August 1, 2016 to June 30, 2018. Data on admissions prior to July 2016 were not available, so we could not calculate accurate PIRs for dates when they might still be in the ICU. As the 99th percentile of ICU length of stay was 22 days, limiting our analysis to patients admitted on or after August 1, 2016 assured near perfect assessment of PIR. We also excluded ICU admissions that were: (1) not the first of the hospitalization for a given patient, (2) missing ICU or hospital disposition data, or (3) admitted to or transferred from another hospital. All excluded patients were, however, used to calculate our exposure, PIR (see below).
We created two cohorts. A “narrow” cohort was designed to be similar to that used in our prior study in the United Kingdom (UK) (to allow assessment of external validity) [8]. It excluded ICU admissions outside standard daytime hours (8 a.m.–4 p.m.) and ICUs with multiple daytime intensivists. For the “broad” cohort, we only excluded patients who spent no daytime hours in ICU (i.e. those admitted briefly overnight), had limitations on aggressive care at ICU admission, or were admitted to private hospital ICUs, which have very different patient casemixes (e.g., more elective surgery) and care models (e.g., more “open” model intensivist staffing[14] with fewer trainees). Admissions could be included in both cohorts.
Exposure and outcome
The exposure variable for each patient was daytime average PIR, calculated as the number of all patients (including the index patient and any patients excluded due to cohort restrictions) in the ICU during daytime hours divided by the number of daytime intensivists. For the narrow cohort, daytime hours were defined as 8 a.m.–4 p.m. [8]. For the broad cohort, each ICU’s daytime hours were assigned as the interval during which at least one intensivist was continuously present onsite (up to 24 h for ICUs with continuous 24 h onsite coverage). Daily values were averaged over each patient’s ICU stay to determine their exposure. The primary outcome was hospital mortality.
Patient and ICU data
Patient data included age, sex, indigenous ethnicity, chronic medical conditions, location prior to hospital (home/other), predicted probability of death using the Australian and New Zealand Risk of Death [ANZROD] model [15], patient type (medical, elective surgical, emergent surgical), admitting diagnosis, cardiac arrest in the 24 h prior to ICU admission, and mechanical ventilation on ICU day 1. ICU data were type (medical or surgical, defined by > 95% of that patient type, or mixed) and hospital classification (metropolitan, rural/regional, tertiary, private). ICU staffing information included data on intensivists (number of hours intensivists were onsite during weekdays; number of consecutive days worked; if they provided concurrent care outside the ICU), non-intensivist physicians (ratio of senior doctors [specialists, fellows, senior registrars] and, separately, junior doctors [registrars, residents] to intensivists during weekday daytime and weekday overnight hours), and non-physician clinicians (nurse-to-patient ratios for patients on non-invasive positive pressure ventilation; presence of charge, liaison, medical emergency team, clinical support, and/or rostering nurse, respiratory therapist, physical therapist, speech therapist, clinical pharmacist, dietician, social worker, pastoral care, nursing aides, and/or medical students).
Statistical analysis
We used summary statistics to describe cohort characteristics. A two-level, mixed effects logistic regression model was used to evaluate the association of average PIR with hospital mortality using complete cases from the narrow cohort. The model included all patient (level 1) and ICU/hospital (level 2) covariates as fixed effects; individual ICU was included as a random effect. Average PIR and predicted probability of mortality were modeled as restricted cubic splines with four knots [8, 16, 17]. We used post-estimation Wald testing to assess the association of hospital mortality with: (1) average PIR (inclusive of all three spline terms) and (2) the two non-linear spline components of average PIR if an overall association was found.
We then constructed three two-level mixed effects logistic regression models using complete cases from the broad cohort (the individual ICU to which a patient was admitted was modeled as a random effect and all patient/ICU/hospital characteristics were modeled as fixed effects). Model 1 included only the PIR. Model 2 added other physician staffing covariables. Model 3 added non-physician staffing covariables. Other model features and testing to assess the association of PIR with mortality were the same as for the narrow cohort model. To compare the nested Models 1–3, we first used the likelihood ratio test. We then assessed for substantial changes to the regression coefficients for average PIR. We a priori declared the coefficients to be substantially different if any of the three spline terms changed by > 20% [18].
We then assessed for interactions of average PIR with non-PIR staffing covariables in Model 3 by including an interaction of the linear PIR spline term with each of the 23 (7 physician, 16 non-physician) non-PIR staffing covariables. If post-estimation Wald testing demonstrated that all 23 interaction terms together significantly affected the association of average PIR with hospital mortality [16], Model 3 was re-run on cohorts stratified by the following non-PIR staffing covariables: intensivist hours-per-day onsite during weekdays; number of consecutive days worked by the intensivist; whether the intensivist provided care outside the ICU simultaneously; ratio of senior doctors to intensivists during weekday daytime hours; ratio of junior doctors to intensivists during weekday daytime hours; and nurse-to-patient ratio for patients with non-invasive ventilation. We did not consider nurse-to-patient ratio for patients with invasive ventilation as this is nearly uniformly 1-to-1.
Finally, we conducted subgroup analyses to assess whether the association of PIR with mortality differed by ICU size (in tertiles), ICU type, whether patients were mechanically ventilated, whether patients had sepsis, daytime versus overnight admissions, predicted hospital mortality (in quartiles), and ICU length of stay (in quartiles). For each, we first included an interaction term for the subgroup of interest with the linear component of the restricted cubic spline term for average PIR in Model 3 using the full cohort. We then re-ran Model 3 on individual subgroups.
As a prespecified sensitivity analysis, we ran Model 3 including patients admitted to private hospitals (cohort n = 119,710). We also ran several post hoc sensitivity analyses. First, we restricted consideration to patients with an average PIR ≤ 20 to exclude outliers (cohort n = 90,202). Second, we restricted consideration to ICUs with unit-level predicted probabilities of mortality ≥ 10% (cohort n = 13,409), to assess whether the association differed in higher acuity units. Third, we excluded the single ICU with any patients with a very high (≥ 30) PIR (mean[sd] PIR for that ICU = 36.1 [15.7]). Fourth, to minimize restrictions, we assumed missing staffing data indicated the absence of that staff member (rather than dropping those observations as was done in the primary, complete case analyses; cohort n = 113,930) and then, additionally, included patients transferred to/from other hospitals, with limitations to care on ICU admission, and in private hospitals (cohort n = 225,611). Lastly, we considered two alternate definitions of PIR: (i) the PIR only on the first daytime of each patient’s ICU stay; and (ii) a weighted average of daily PIRs (with heavier weighting for daytimes 1–3).
Ethics approval was obtained by the St John of God Health Care Research Ethics Committee, Perth, Western Australia (#1424; September 12, 2018). Statistical analyses were performed using StataMP 16 (StataCorp, College Station, Texas) and Microsoft Excel (Microsoft, Redmond, Washington). Significance was defined as two-sided p < 0.05. As no adjustments were made for multiple comparisons, all secondary and sensitivity analyses must be viewed as hypothesis generating. Our analysis plan was uploaded to Open Science Forum on June 25, 2020 (prior to analysis initiation); in error, it was not made public on the site until April 20, 2021 (after analysis completion) without revision [19].
Results
Narrow cohort
The narrow cohort of patients admitted between 8 a.m. and 4 p.m. to ICUs with a single intensivist consisted of 27,380 complete cases in 67 ICUs (eFigure 1). Most patients had no comorbidities (75.4%), came to the hospital from home (97.9%), were admitted to private hospitals (52.3%), and had a median predicted mortality of 1.2% (interquartile range [IQR]: 0.4–4.1%; mean [standard deviation]: 5.9% [13.2%]; Table 1 and eTable 2). Median average PIR (the median value of PIR averaged over all days) was 10.1 (IQR 7–14, full range of 0–53.5). Hospital mortality was 6.1%. Patients excluded due to missing data were more often medical (54.1% vs 35.7%, p < 0.001) and were more likely to receive mechanical ventilation on ICU day 1 (32.7% vs 21.3%, p < 0.001), yet were less commonly admitted to medical ICUs (1.6% vs 7%, p < 0.001) in private (40.3% vs 52.3%) or rural/regional (8.1% vs 18.5%) hospitals (p < 0.001, eTable 3). There was no association of average PIR with hospital mortality in this cohort using mixed effects logistic regression modeling (PIR 1st spline term odds ratio [95% CI]: 1 [0.94, 1.06], Wald testing of all spline terms p = 0.61; Fig. 1, eTable 4).
Table 1.
Characteristics of primary cohorts
| Narrow cohort | Broad cohort | |||
|---|---|---|---|---|
| Patients, N (%) | ICUs, N (%) | Patients, N (%) | ICUs, N (%) | |
| Patient characteristics | ||||
| Number of patients, N | 27,380 | 67 | 91,206 | 73 |
| Age, mean (sd) | 64 (17.5) | 58.6 (18.2) | ||
| Female | 12,713 (46.4) | 38,024 (41.7) | ||
| Indigenous ethnicity | ||||
| No | 21,366 (78) | 71,575 (78.5) | ||
| Yes | 1194 (4.4) | 6125 (6.7) | ||
| Unknown | 4820 (17.6) | 13,506 (14.8) | ||
| # of comorbidities | ||||
| 0 | 20,640 (75.4) | 69,113 (75.8) | ||
| 1 | 5120 (18.7) | 16,672 (18.3) | ||
| 2 | 1280 (4.7) | 4239 (4.6) | ||
| 3 + | 340 (1.2) | 1182 (1.3) | ||
| At home prior to hospitalization | 26,812 (97.9) | 88,469 (97) | ||
| Predicted probability of death (%) | ||||
| Median (IQR) | 1.2 (0.4, 4.1) | 1.9 (0.6, 6.5) | ||
| Mean (sd) | 5.9 (13.2) | 7.6 (14.9) | ||
| Patient type | ||||
| Medical | 9761 (35.7) | 38,619 (42.3) | ||
| Elective surgical | 10,494 (38.3) | 16,385 (18) | ||
| Emergent surgical | 7125 (26) | 36,202 (39.7) | ||
| Cardiac arrest within 24 h of ICU admission | 449 (1.6) | 3139 (3.4) | ||
| MV on ICU admission day 1 | 5839 (21.3) | 34,930 (38.3) | ||
| Average PIR, median (IQR) | 10.1 (7, 14) | 7.8 (5.8, 10.2) | ||
| ICU characteristics | ||||
| ICU typea | ||||
| Medical ICU | 1914 (7) | 7 (10.4) | 9270 (10.2) | 10 (13.7) |
| Surgical ICU | 2752 (10.1) | 7 (10.4) | 5286 (5.8) | 5 (6.8) |
| Mixed ICU | 22,714 (83) | 53 (79.1) | 76,650 (84) | 58 (79.5) |
| Hospital class | ||||
| Metropolitan | 6230 (22.8) | 19 (28.4) | 23,684 (26) | 26 (35.6) |
| Private | 14,309 (52.3) | 25 (37.3) | ||
| Rural/regional | 5063 (18.5) | 19 (28.4) | 12,463 (13.7) | 19 (26) |
| Tertiary | 1778 (6.5) | 4 (6) | 55,059 (60.4) | 28 (38.4) |
| Outcome | ||||
| ICU | ||||
| Mortality | 1026 (3.7) | 5097 (5.6) | ||
| LOS (days), median (IQR) | 1.5 (0.9, 2.8) | 1.8 (0.9, 3.5) | ||
| Hospital | ||||
| Mortality | 1667 (6.1) | 7712 (8.5) | ||
| LOS (days), median (IQR) | 7.2 (4.1, 12.3) | 8.1 (4.5, 15.2) | ||
| Discharge home for survivors | 22,635 (88) | 75,408 (90.3) | ||
aDetermined by percentage of patients of each type—“medical” with > 95% medical patients; “surgical” with > 95% surgical patients; and, “mixed” if meeting criteria for neither “medical” nor “surgical”
h hours, ICU intensive care unit, IQR interquartile range, LOS length of stay, MV mechanical ventilation, sd standard deviation
Fig. 1.
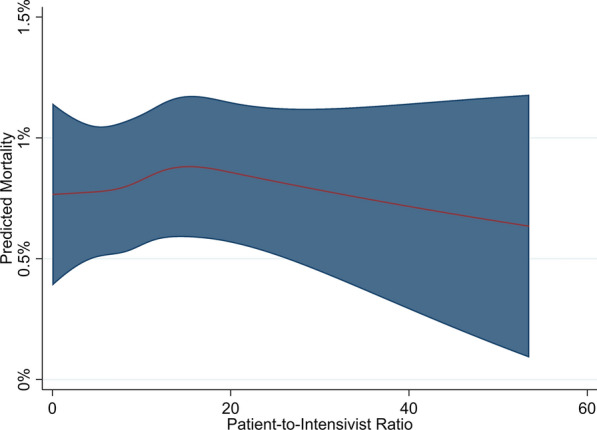
Association of patient-to-intensivist ratio and hospital mortality in narrow cohort. Plot represents the association of patient-to-intensivist ratio with adjusted mortality for the “population average” patient (having the population mean for continuous and mode for categorical characteristics); red line = point estimate, shaded region = 95% confidence interval. P value for association of patient-to-intensivist ratio with mortality = 0.61
Broad cohort
The broad cohort consisted of 91,206 complete cases in 73 ICUs (eFigure 2) and included patients admitted at any time and ICUs with more than one intensivist. Again, most patients were without comorbidities (75.8%), admitted from home (97%), and had low severity of acute illness (predicted mortality: median 1.9% [IQR 0.6–6.5%]; mean [standard deviation]: 7.6% [14.9%]). Median average PIR was 7.8 (IQR 5.8–10.2, full range 0–56; eFigure 3). Hospital mortality was 8.5% (Table 1). Patients excluded due to missing data had a lower median predicted probability of death (1.4% [0.5–4.9%] vs 1.9% [0.6–6.5%], p < 0.001), were less likely to receive mechanical ventilation on ICU day 1 (31.1% vs 38.3%, p < 0.001), and were more likely to be admitted to tertiary care hospitals (66.4% vs 60.4%, p < 0.001, eTable 5).
Nearly one-quarter (24.7%) of ICUs had intensivists with concurrent ICU and non-ICU clinical responsibilities (Table 2). Most ICUs had daytime onsite intensivists for < 12 h (71.2%) and ICUs were fairly evenly split on the number of consecutive days intensivists worked (31.5% 7–8 days, 27.4% 5 days, 20.5% 4 days, and 20.5% ≤ 3 days). Two-fifths (41.1%) of ICUs staffed patients on non-invasive ventilation with one nurse per two patients (the remaining 58.9% had one nurse per patient). Most ICUs always had a charge nurse (89%), while only 4.1% ever had a respiratory therapist.
Table 2.
Staffing characteristics for the broad cohort
| Characteristics | Patients, N (%) | ICUs, N (%) |
|---|---|---|
| # of patients, N | 91,206 | 73 |
| Intensivist has non-ICU responsibilities | 21,163 (23.2) | 18 (24.7) |
| Intensivist hours onsite during weekday daytime (h) | ||
| 24 | 14,267 (15.6) | 10 (13.7) |
| 12 + | 17,999 (19.7) | 11 (15.1) |
| < 12 | 58,940 (64.6) | 52 (71.2) |
| Intensivist consecutive days worked (days) | ||
| 7–8 | 31,052 (34) | 23 (31.5) |
| 5 | 28,355 (31.1) | 20 (27.4) |
| 4 | 16,090 (17.6) | 15 (20.5) |
| 0–3 | 15,709 (17.2) | 15 (20.5) |
| Ratio of weekday daytime senior doctor-to-intensivist ratio | ||
| 0 senior doctors | 23,379 (25.6) | 34 (46.6) |
| > 0–1 senior doctors-to-1 intensivist | 49,742 (54.5) | 31 (42.5) |
| > 1–2 senior doctors-to-1 intensivist | 18,085 (19.8) | 8 (11) |
| Ratio of weekday daytime junior doctor-to-intensivist ratio | ||
| 0 junior doctors | 23,379 (25.6) | 34 (46.6) |
| > 0–1 junior doctors-to-1 intensivist | 49,742 (54.5) | 31 (42.5) |
| > 1–2 junior doctors-to-1 intensivist | 18,085 (19.8) | 8 (11) |
| > 2 junior doctors-to-1 intensivist | 12,467 (13.7) | 8 (11) |
| Ratio of weekday nighttime senior doctor-to-intensivist ratio | ||
| 0 senior doctors | 23,379 (25.6) | 34 (46.6) |
| > 0–1 senior doctors-to-1 intensivist | 49,742 (54.5) | 31 (42.5) |
| Ratio of weekday nighttime junior doctor-to-intensivist ratio | ||
| 0 junior doctors | 23,379 (25.6) | 34 (46.6) |
| > 0–1 junior doctors-to-1 intensivist | 49,742 (54.5) | 31 (42.5) |
| > 1–2 junior doctors-to-1 intensivist | 18,085 (19.8) | 8 (11) |
| > 2 junior doctors-to-1 intensivist | 12,467 (13.7) | 8 (11) |
| Nurse-to-patient ratio for NIPPV 1:2 | 33,217 (36.4) | 30 (41.1) |
| Charge nurse available always | 82,047 (90) | 65 (89) |
| Liaison nurse ever present | 42,221 (46.3) | 29 (39.7) |
| Medical emergency team nurse ever present | 56,823 (62.3) | 36 (49.3) |
| Clinical support nurse ever present | 46,404 (50.9) | 35 (47.9) |
| Rostering nurse ever present | 51,746 (56.7) | 32 (43.8) |
| Respiratory therapist ever present | 3311 (3.6) | 3 (4.1) |
| Physical therapist present overnight | 25,196 (27.6) | 17 (23.3) |
| Speech therapist ever present | 89,635 (98.3) | 71 (97.3) |
| Clinical pharmacist present overnight | 35,170 (38.6) | 28 (38.4) |
| Clinical pharmacist present on weekend days | 14,140 (15.5) | 10 (13.7) |
| Dietician ever present | 90,742 (99.5) | 72 (98.6) |
| Social worker ever present | 91,206 (100) | 73 (100) |
| Pastoral care ever present | 85,765 (94) | 64 (87.7) |
| Nursing aide ever present | 71,582 (78.5) | 58 (79.5) |
| Medical student ever present | 81,037 (88.9) | 63 (86.3) |
NIPPV non-invasive positive pressure ventilation
Model 3 differed significantly from Models 1 and 2 (likelihood ratio Model 1 nested in Model 2, p = 0.28; Model 2 in Model 3, p = 0.001; Model 1 in Model 3, p = 0.004). However, we found no association between average PIR and hospitality mortality in any of the models (Wald testing for association of average PIR with mortality—Model 1, p = 0.91; Model 2, p = 0.58; Model 3, p = 0.4; Fig. 2; eTable 6). Given the null association, we did not assess whether PIR coefficients changed significantly from Models 1 to 3. There was a significant interaction between the 23 staffing covariables and average PIR (p < 0.001); however, no association was found between average PIR and hospital mortality in any staffing subgroup where models converged (eTable 7). Similarly, no association was found between average PIR and mortality across patient and ICU subgroups (Table 3). Finally, no association was found in any of the sensitivity analyses (eFigures 4–11).
Fig. 2.

Adjusted association of patient-to-intensivist ratio and hospital mortality in the broad cohorta. a Model 1 (including patient characteristics)b. b Model 2 (including patient and intensivist staffing characteristics)c. c Model 3 (including patient, intensivist staffing, and non-intensivist staffing characteristics)d. a—Plots represent the association of patient-to-intensivist ratio with adjusted mortality for the “population average” patient (having the population mean for continuous and mode for categorical characteristics); red line = point estimate, shaded region = 95% confidence interval. b—P value for association of patient-to-intensivist ratio with mortality = 0.91. c—p value for association of patient-to-intensivist ratio with mortality = 0.58. d—p value for association of patient-to-intensivist ratio with mortality = 0.40
Table 3.
Adjusted odds ratios of patient-to-intensivist ratio with hospital mortality across patient subgroups
| p value for interaction term in full broad cohort model | 1st PIR spline term, OR (95% CI) |
2nd PIR spline term, OR (95% CI) |
3rd PIR spline term, OR (95% CI) |
p value for Wald testing for PIR spline terms within subgroups | |
|---|---|---|---|---|---|
| ICU size in tertiles (beds) | 0.037 | ||||
| ≤ 13 | 0.99 (0.92, 1.07) | 1.1 (0.84, 1.44) | 0.67 (0.28, 1.65) | 0.66 | |
| 13–22 | 1.02 (0.93, 1.12) | 0.98 (0.75, 1.28) | 1.02 (0.45, 2.29) | 0.82 | |
| ≥ 23 | Model did not converge | ||||
| Patient type | 0.039 | ||||
| Medical | 1.04 (0.97, 1.12) | 0.87 (0.69, 1.1) | 1.46 (0.7, 3.04) | 0.37 | |
| Non-cardiac surgical | 0.98 (0.92, 1.04) | 1.13 (0.93, 1.37) | 0.67 (0.37, 1.2) | 0.45 | |
| Cardiac surgical | 0.94 (0.73, 1.22) | 1.71 (0.73, 4.04) | 0.12 (0.01, 2.04) | 0.23 | |
| Mechanical ventilation | 0.1 | ||||
| No | 1.08 (1.01, 1.16) | 0.85 (0.68, 1.06) | 1.48 (0.75, 2.93) | 0.07 | |
| Yes | 0.98 (0.93, 1.04) | 1.11 (0.91, 1.34) | 0.72 (0.4, 1.31) | 0.47 | |
| Sepsis | 0.17 | ||||
| No | 1.02 (0.97, 1.07) | 0.98 (0.84, 1.15) | 1.02 (0.62, 1.68) | 0.63 | |
| Yes | Model did not converge | ||||
| Time of admission | 0.58 | ||||
| Daytime (7a–6:59p) | 1.02 (0.97, 1.08) | 1.02 (0.85, 1.22) | 0.88 (0.5, 1.55) | 0.15 | |
| Overnight (7p–6:59a) | 1.04 (0.97, 1.11) | 0.93 (0.75, 1.16) | 1.2 (0.61, 2.35) | 0.52 | |
| ANZROD probability of death quartiles | < 0.001 | ||||
| Q1 (≤ 0.6%) | 1.38 (0.83, 2.29) | 0.33 (0.07, 1.5) | 28.57 (0.31, 2655.34) | 0.51 | |
| Q2 (0.6–1.8%) | 1.08 (0.87, 1.34) | 0.64 (0.32, 1.28) | 4.18 (0.49, 35.35) | 0.22 | |
| Q3 (1.9–6.4%) | 1.04 (0.93, 1.15) | 0.93 (0.66, 1.32) | 1.17 (0.4, 3.41) | 0.85 | |
| Q4 (≥ 6.5%) | 1.01 (0.96, 1.06) | 1.05 (0.9, 1.24) | 0.8 (0.48, 1.32) | 0.11 | |
| ICU LOS quartiles | < 0.001 | ||||
| Q1 (≤ 0.9 days) | 1.15 (1, 1.32) | 0.69 (0.44, 1.09) | 2.76 (0.68, 11.2) | 0.29 | |
| Q2 (1.0–1.8 days) | 1.02 (0.9, 1.16) | 0.98 (0.65, 1.47) | 1.02 (0.29, 3.57) | 0.97 | |
| Q3 (1.9–3.4 days) | 1.1 (1, 1.21) | 0.77 (0.57, 1.04) | 2.08 (0.82, 5.27) | 0.24 | |
| Q4 (≥ 3.5 days) | 0.99 (0.94, 1.05) | 1.11 (0.91, 1.35) | 0.69 (0.38, 1.27) | 0.13 | |
All models are Model 3 (including patient, intensivist staffing, and non-intensivist staffing characteristics)
CI confidence interval, ICU intensive care unit, LOS length of stay, OR odds ratio, PIR patient-to-intensivist ratio, Q quartile
Discussion
Contrary to our hypothesis, we found no association between average PIR and hospital mortality among patients admitted to Australia and New Zealand ICUs. These findings contrast with those from our similar work in the UK which demonstrated a U-shaped association [8].
Our null results add to a growing evidence base addressing patient outcomes in relation to intensivist workload. Two prior studies identified a clear association between patient mortality and physician patient load. The first, by Neuraz et al., demonstrated a two-fold increase in shift-specific mortality among French ICU patients cared for by doctors with > 14 vs < 8 patients [adjusted odds ratio: 2.0 (1.3–3.2)] [13]. Similarly, our prior work found a U-shaped association between PIR and hospital mortality among UK ICU patients, with patients whose PIR was both less than and greater than ~ 7.5 more likely to die [8]. In contrast, a large study of US ICUs found no association of ICU census on the day of a patient’s admission and hospital mortality [10]. Finally, a study from a single US quaternary care center found that ICU length of stay, but neither ICU nor hospital mortality, increased as intensivist bed-load increased [9]. Bed-to-intensivist and patient-to-intensivist ratios are not synonymous; yet, they often correlate with one another.
While at first these works appear contradictory, on closer inspection a pattern emerges. Access to ICU beds varies substantially across the developed world, leading to variability in patient acuity [20, 21]. The two studies with significant associations between mortality and physician workload were in French and UK ICUs. In the first, the mean predicted hospital mortality across all evaluated shifts was 47.2% (standard deviation [sd] 0.5%) [13, 22]; in the second, mean predicted mortality at ICU admission was 24.1% (sd 26.8%) [8, 23]. In contrast, in the two studies in which no association was found between intensivist patient load and mortality, the overall acuity of patients was much lower (mean predicted mortality for the multicenter US study, 13.8% [10, 24], and this study in Australia/New Zealand, 7.6% [sd 14.9%][15]). The study by Dara et al. in which ICU length of stay, but not mortality, was associated with intensivist workload had a mid-range predicted mortality of 18.2–20% [9, 24]. We hypothesize that measures of intensivist workload (e.g., PIR) are strongly associated with patient mortality when patient acuity is high but not when acuity is low.
This framework has face validity. When patients are either too sick (e.g., predicted mortality > 90%) or too well (e.g., < 10%), it is less likely that specific individual organizational factors will be impactful enough to noticeably affect population-level mortality rates. Small changes may occur, but prohibitively large sample sizes would be needed to appreciate these differences. Conversely, it is the patients who are sick enough to need but not too sick to benefit from high-quality, thoughtful care for whom the true impact on mortality of intensivist workload is most likely to be observed.
It is important that an incorrect message not be drawn from our study, namely that intensivists in Australia/New Zealand and other regions with lower overall ICU acuity can assume responsibility for a limitless number of patients. First, our cohort ICUs had, for the most part, a narrow range of beds, suggesting some attention to ensuring an appropriate workload is part of ICU design across these countries, which is reinforced by national guidelines [25]. Second, our study only evaluated the association of PIR with hospital mortality. It is quite possible that other outcomes of great interest to stakeholders (e.g., morbidity, longer-term mortality, time intensivists spend with patients/families, quality of communication with non-physician staff, and/or intensivist burnout) may be associated with PIR. Finally, ICUs with high PIRs may have strategies (e.g., redistribution of clinician responsibilities, telehealth) for which we could not account that mitigate negative associations of PIR with outcome; simply adding more patients to an intensivist without these systems in place (as may happen in times of increased demand, such as COVID-19), therefore, may be problematic.
The strengths of this study stem from its reliance on a comprehensive database inclusive of nearly all ICU admissions in Australia and New Zealand as well as detailed clinical and staffing information. Our study, however, has limitations. First, planned exclusions substantially reduced cohort size which may impact the generalizability of our findings; however, our results were robust across numerous sensitivity analyses including those aimed at liberalizing inclusion criteria. Second, patients with missing data were different from those with complete information which may have introduced selection bias. Similarly, exclusion of patients in ICUs with no weekday daytime intensivists (or missing data on intensivist number) limits generalizability to these sites. Third, for ICUs with greater than one daytime intensivist, we assumed patients were evenly divided across intensivists; if and to what degree this assumption may have biased our results is unknown. Similarly, for non-PIR staffing variables, we did not have patient-specific information; only ICU-level staffing averages were available. Fourth, we considered only average PIR, not the variability of workload across a patient’s ICU course nor the impact of simultaneous patient load (e.g., 12 patients present all day versus 6 patients at any one time, but rapid turnover resulting in 12 patients across the day). How large swings in workload versus a more constant demand may impact patient outcomes is unknown and worthy of future study. Fifth, despite adjustment for available patient- and ICU/hospital-level covariables, residual confounding (e.g., care quality provided by intensivists, nursing workload as measured by the Nursing Activities Score [26]) may remain and contribute to our null findings. Sixth, our staffing survey was conducted from September 1, 2017 to January 31, 2018; staffing changes over the time period of study (August 1, 2016–June 30, 2018) were not captured. Seventh, as discussed above, our cohort’s overall severity of illness was quite low which may have impacted our ability to identify small magnitude mortality differences. And, finally, our use of PIR as a marker of intensivist workload is less nuanced than other measures of strain [3]. The simplicity of PIR is powerful, allowing easy calculation and, thus, usability; however, it may be insufficient to fully quantify workload.
Conclusions
As the COVID-19 pandemic has made abundantly clear, optimally delivered ICU care is not a limitless resource. More ICU beds can be built rapidly, but expanding the critical care workforce takes years of planning and training of key staff. As a result, existing ICU clinicians (e.g., physicians) are often simply asked to take on more patients to meet increasing demand. In the height of an emergency, we may have no choice. But, we must understand the consequences of such decisions. As our findings reveal, in lower acuity ICUs, asking intensivists to assume responsibility for a larger number of patients may be safe from the standpoint of patient survival. Yet, future work is needed to understand whether patient morbidity suffers and if non-patient stakeholders (e.g., families, non-physician clinicians, intensivists) are negatively impacted.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
For contributing their data for analysis, we greatly appreciate the following sites: Albury Base Hospital ICU, Alfred Hospital ICU, Alice Springs Hospital ICU, Armadale Health Service ICU, Auckland City Hospital CV ICU, Auckland City Hospital DCCM, Austin Hospital ICU, Ballarat Health Services ICU, Bankstown-Lidcombe Hospital ICU, Bathurst Base Hospital ICU, Bendigo Health Care Group ICU, Box Hill Hospital ICU, Brisbane Private Hospital ICU, Bunbury Regional Hospital ICU, Bundaberg Base Hospital ICU, Caboolture Hospital ICU, Cabrini Hospital ICU, Cairns Hospital ICU, Calvary Hospital (Canberra) ICU, Calvary John James Hospital ICU, Calvary Mater Newcastle ICU, Campbelltown Hospital ICU, Canberra Hospital ICU, Central Gippsland Health Service ICU, Christchurch Hospital ICU, Coffs Harbour Health Campus ICU, Concord Hospital (Sydney) ICU, Dandenong Hospital ICU, Dunedin Hospital ICU, Epworth Eastern Private Hospital ICU, Epworth Freemasons Hospital ICU, Epworth Hospital (Richmond) ICU, Fairfield Hospital ICU, Fiona Stanley Hospital ICU, Flinders Medical Centre ICU, Flinders Private Hospital ICU, Footscray Hospital ICU, Frankston Hospital ICU, Gold Coast Private Hospital ICU, Gold Coast University Hospital ICU, Gosford Hospital ICU, Goulburn Base Hospital ICU, Grafton Base Hospital ICU, Greenslopes Private Hospital ICU, Griffith Base Hospital ICU, Hawkes Bay Hospital ICU, Holy Spirit Northside Hospital ICU, Hornsby Ku-ring-gai Hospital ICU, Hutt Hospital ICU, Ipswich Hospital ICU, John Fawkner Hospital ICU, John Flynn Private Hospital ICU, John Hunter Hospital ICU, Joondalup Health Campus ICU, Kareena Private Hospital ICU, Knox Private Hospital ICU, Latrobe Regional Hospital ICU, Launceston General Hospital ICU, Lismore Base Hospital ICU, Liverpool Hospital ICU, Logan Hospital ICU, Lyell McEwin Hospital ICU, Macquarie University Private Hospital ICU, Manly Hospital & Community Health ICU, Manning Rural Referral Hospital ICU, Maroondah Hospital ICU, Mater Adults Hospital (Brisbane) ICU, Mater Private Hospital (Brisbane) ICU, Melbourne Private Hospital ICU, Middlemore Hospital ICU, Mildura Base Hospital ICU, Monash Medical Centre-Clayton Campus ICU, Mount Hospital ICU, Mount Isa Hospital ICU, National Capital Private Hospital ICU, Nelson Hospital ICU, Nepean Hospital ICU, Newcastle Private Hospital ICU, Noosa Hospital ICU, North Shore Hospital ICU, North Shore Private Hospital ICU, North West Regional Hospital (Burnie) ICU, Norwest Private Hospital ICU, Orange Base Hospital ICU, Peninsula Private Hospital ICU, Port Macquarie Base Hospital ICU, Prince of Wales Hospital (Sydney) ICU, Prince of Wales Private Hospital (Sydney) ICU, Princess Alexandra Hospital ICU, Queen Elizabeth II Jubilee Hospital ICU, Redcliffe Hospital ICU, Robina Hospital ICU, Rockhampton Hospital ICU, Rockingham General Hospital ICU, Rotorua Hospital ICU, Royal Adelaide Hospital ICU, Royal Brisbane and Women's Hospital ICU, Royal Darwin Hospital ICU, Royal Melbourne Hospital ICU, Royal North Shore Hospital ICU, Royal Perth Hospital ICU, Royal Prince Alfred Hospital ICU, Shoalhaven Hospital ICU, Sir Charles Gairdner Hospital ICU, South West Healthcare (Warrnambool) ICU, Southern Cross Hospital (Wellington) ICU, St Andrew's Hospital (Adelaide) ICU, St George Hospital (Sydney) ICU, St John Of God Health Care (Subiaco) ICU, St John Of God Hospital (Murdoch) ICU, St Vincent's Hospital (Melbourne) ICU, St Vincent's Hospital (Sydney) ICU, St Vincent's Private Hospital (Sydney) ICU, St Vincent’s Private Hospital Fitzroy ICU, Sunnybank Hospital ICU, Sunshine Hospital ICU, Sutherland Hospital and Community Health Services ICU, Sydney Adventist Hospital ICU, Sydney Southwest Private Hospital ICU, Tamworth Base Hospital ICU, Taranaki Health ICU, Tauranga Hospital ICU, The Memorial Hospital (Adelaide) ICU, The Northern Hospital ICU, The Prince Charles Hospital ICU, The Townsville Hospital ICU, The Wesley Hospital ICU, Timaru Hospital ICU, Toowoomba Hospital ICU, University Hospital Geelong ICU, Wagga Wagga Base Hospital and District Health ICU, Waikato Hospital ICU, Warringal Private Hospital ICU, Wellington Hospital ICU, Western District Health Service (Hamilton) ICU, Westmead Hospital ICU, Westmead Private Hospital ICU, Whangarei Area Hospital, Northland Health Ltd ICU, Wimmera Health Care Group (Horsham) ICU, Wollongong Hospital ICU, Wyong Hospital ICU.
Author contributions
All authors conceived of and contributed to the design of the project, the interpretation of results, and the critical revision of the paper. DVP and EL assembled the datasets. HBG performed data analyses and was responsible for primary drafting of the manuscript.
Funding
HBG received funding from the University of Miami Hospital and Clinics through UHealth-DART. HW is supported by a Canada Research Chair [Tier 2] from the Canadian Institutes of Health Research and research merit award from the Department of Anesthesiology and Pain Medicine at the University of Toronto. EL is supported by a National Health and Medical Research Council Early Career Fellowship Grant.
Availability of data and material
ANZICS CORE is the custodian of data submitted by contributing hospitals. Data may be made available for research purposes on request.
Code availability
Code for this project is available upon request from Dr. Gershengorn.
Declarations
Conflicts of interest
All authors report no conflicts of interest.
Ethics approval
Ethics approval was obtained by the St John of God Health Care Research Ethics Committee, Perth, Western Australia (#1424).
Consent to participate
A waiver of consent was obtained.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilcox ME, Chong CA, Niven DJ, Rubenfeld GD, Rowan KM, Wunsch H, Fan E. Do intensivist staffing patterns influence hospital mortality following ICU admission? A systematic review and meta-analyses. Crit Care Med. 2013;41:2253–2274. doi: 10.1097/CCM.0b013e318292313a. [DOI] [PubMed] [Google Scholar]
- 2.Kerlin MP, Adhikari NK, Rose L, Wilcox ME, Bellamy CJ, Costa DK, Gershengorn HB, Halpern SD, Kahn JM, Lane-Fall MB, Wallace DJ, Weiss CH, Wunsch H, Cooke CR, Organization AAHCoI An official american thoracic society systematic review: the effect of night time intensivist staffing on mortality and length of stay among intensive care unit patients. Am J Respir Crit Care Med. 2017;195:383–393. doi: 10.1164/rccm.201611-2250ST. [DOI] [PubMed] [Google Scholar]
- 3.Halpern SD. ICU capacity strain and the quality and allocation of critical care. Curr Opin Crit Care. 2011;17:648–657. doi: 10.1097/MCC.0b013e32834c7a53. [DOI] [PubMed] [Google Scholar]
- 4.Anesi GL, Liu VX, Gabler NB, Delgado MK, Kohn R, Weissman GE, Bayes B, Escobar GJ, Halpern SD. Associations of intensive care unit capacity strain with disposition and outcomes of patients with sepsis presenting to the emergency department. Ann Am Thorac Soc. 2018;15:1328–1335. doi: 10.1513/AnnalsATS.201804-241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anesi GL, Gabler NB, Allorto NL, Cairns C, Weissman GE, Kohn R, Halpern SD, Wise RD. Intensive care unit capacity strain and outcomes of critical illness in a resource-limited setting: a 2-hospital study in South Africa. J Intensive Care Med. 2020;35:1104–1111. doi: 10.1177/0885066618815804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabler NB, Ratcliffe SJ, Wagner J, Asch DA, Rubenfeld GD, Angus DC, Halpern SD. Mortality among patients admitted to strained intensive care units. Am J Respir Crit Care Med. 2013;188:800–806. doi: 10.1164/rccm.201304-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159:447–455. doi: 10.7326/0003-4819-159-7-201310010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gershengorn HB, Harrison DA, Garland A, Wilcox ME, Rowan KM, Wunsch H. Association of intensive care unit patient-to-intensivist ratios with hospital mortality. JAMA Intern Med. 2017;177:388–396. doi: 10.1001/jamainternmed.2016.8457. [DOI] [PubMed] [Google Scholar]
- 9.Dara SI, Afessa B. Intensivist-to-bed ratio: association with outcomes in the medical ICU. Chest. 2005;128:567–572. doi: 10.1378/chest.128.2.567. [DOI] [PubMed] [Google Scholar]
- 10.Iwashyna TJ, Kramer AA, Kahn JM. Intensive care unit occupancy and patient outcomes. Crit Care Med. 2009;37:1545–1557. doi: 10.1097/CCM.0b013e31819fe8f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward NS, Read R, Afessa B, Kahn JM. Perceived effects of attending physician workload in academic medical intensive care units: a national survey of training program directors. Crit Care Med. 2012;40:400–405. doi: 10.1097/CCM.0b013e318232d997. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox ME, Harrison DA, Short A, Jonas M, Rowan KM. Comparing mortality among adult, general intensive care units in England with varying intensivist cover patterns: a retrospective cohort study. Crit Care. 2014;18:491. doi: 10.1186/s13054-014-0491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neuraz A, Guérin C, Payet C, Polazzi S, Aubrun F, Dailler F, Lehot JJ, Piriou V, Neidecker J, Rimmelé T, Schott AM, Duclos A. Patient mortality is associated with staff resources and workload in the ICU: a multicenter observational study. Crit Care Med. 2015;43:1587–1594. doi: 10.1097/CCM.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 14.Carson SS, Stocking C, Podsadecki T, Christenson J, Pohlman A, MacRae S, Jordan J, Humphrey H, Siegler M, Hall J. Effects of organizational change in the medical intensive care unit of a teaching hospital: a comparison of 'open' and 'closed' formats. JAMA. 1996;276:322–328. doi: 10.1001/jama.1996.03540040066035. [DOI] [PubMed] [Google Scholar]
- 15.Paul E, Bailey M, Pilcher D. Risk prediction of hospital mortality for adult patients admitted to Australian and New Zealand intensive care units: development and validation of the Australian and New Zealand Risk of Death model. J Crit Care. 2013;28:935–941. doi: 10.1016/j.jcrc.2013.07.058. [DOI] [PubMed] [Google Scholar]
- 16.Harrell FE., Jr . Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis (Springer series in statistics) Switzerland: Springer International Publishing; 2015. [Google Scholar]
- 17.Gauthier J, Wu QV, Gooley TA. Cubic splines to model relationships between continuous variables and outcomes: a guide for clinicians. Bone Marrow Transplant. 2020;55:675–680. doi: 10.1038/s41409-019-0679-x. [DOI] [PubMed] [Google Scholar]
- 18.Kleinbaum D, Kupper L, Muller K, Nizam A. Confounding and interaction in regression applied regression analysis and other multivariable methods. 3. Boston: Duxbury Press; 1998. pp. 186–211. [Google Scholar]
- 19.Gershengorn HB, Pilcher DV, Litton E, Anstey M, Garland A, Wunsch H (2020) Association of intensivist staffing strategies with patient outcomes in Australia and New Zealand intensive care units: study design and data analysis plan. https://osf.io/tkdu3/
- 20.Wunsch H, Angus DC, Harrison DA, Collange O, Fowler R, Hoste EA, de Keizer NF, Kersten A, Linde-Zwirble WT, Sandiumenge A, Rowan KM. Variation in critical care services across North America and Western Europe. Crit Care Med. 2008;36(2787–2793):e2781–2789. doi: 10.1097/CCM.0b013e318186aec8. [DOI] [PubMed] [Google Scholar]
- 21.Vincent JL, Marshall JC, Namendys-Silva SA, François B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H, Jimenez E, Sakr Y, investigators I Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2:380–386. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- 22.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 23.Harrison DA, Parry GJ, Carpenter JR, Short A, Rowan K. A new risk prediction model for critical care: the Intensive Care National Audit and Research Centre (ICNARC) model. Crit Care Med. 2007;35:1091–1098. doi: 10.1097/01.CCM.0000259468.24532.44. [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman JE, Wagner DP, Draper EA, Wright L, Alzola C, Knaus WA. Evaluation of acute physiology and chronic health evaluation III predictions of hospital mortality in an independent database. Crit Care Med. 1998;26:1317–1326. doi: 10.1097/00003246-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 25.(2011) Minimum standards for intensive care units. https://www.cicm.org.au/CICM_Media/CICMSite/CICM-Website/Resources/Professional%20Documents/IC-1-Minimum-Standards-for-Intensive-Care-Units.pdf
- 26.Miranda DR, Nap R, de Rijk A, Schaufeli W, Iapichino G, System TWGTIS Nursing activities score. Crit Care Med. 2003;31:374–382. doi: 10.1097/01.CCM.0000045567.78801.CC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
ANZICS CORE is the custodian of data submitted by contributing hospitals. Data may be made available for research purposes on request.
Code for this project is available upon request from Dr. Gershengorn.


