Abstract
Background
Patients with autoimmune disease and on immunotherapy were largely excluded from seminal anti-SARS-CoV-2 vaccine trials. This has led to significant vaccine hesitancy in patients with neuroinflammatory diseases (NID); including, but not limited to: multiple sclerosis (MS), neuromyelitis optica spectrum disorders (NMOSD), neurosarcoidosis and myelin oligodendrocyte antibody-mediated disease (MOG-AD). Data is urgently needed to help guide clinical care in the NID population.
Methods
This was a cross-sectional observational study evaluating adults with a neurologist-confirmed diagnosis of a neuroinflammatory disease (NID) and a neurologically asymptomatic control population. Participants were recruited from multiple academic centers participating in the MS Resilience to COVID-19 Collaborative study. We analyzed participant responses from a vaccine-specific questionnaire collected between February and May 2021.
Results
1164 participants with NID and 595 controls completed the vaccine survey. Hesitancy rates were similar between NID and control groups (n = 134, 32.7% NID vs. n = 56, 30.6% control; p = 0.82). The most common reasons for hesitancy in NID participants were lack of testing in the autoimmune population and fear of demyelinating/neurologic events. Unvaccinated patients who had discussed vaccination with their doctor were less likely to be hesitant (n=184, 73.6% vs. n=83, 59.7%; p = 0.007). 634 NID patients and 332 controls had received at least one dose of a vaccine against SARS-CoV-2 at the time of survey completion. After adjusting for age, BMI, and comorbidities, there was no difference in self-reported side effects (SE) between groups with the first dose (n = 256, 42.2% NID vs. 141, 45.3% control; p = 0.20) or second dose (n = 246, 67.0% NID vs. n = 114, 64.8% control, p = 0.85) of the mRNA vaccines nor with the viral-vector vaccines (n = 6, 46% NID vs. n = 8, 66% control; p = 0.39). All reported SEs fell into the expected SE profile. There was no difference in report of new/recurrent neurologic symptoms (n = 110, 16.2% vaccinated vs. 71, 18.2% unvaccinated; p = 0.44) nor radiologic disease activity (n = 40, 5.9% vaccinated vs. n = 30, 7.6% unvaccinated) between vaccinated and unvaccinated NID participants.
Conclusions
We found no difference in patient-reported vaccine side effects and no evidence of NID worsening after vaccination. Large-scale real-world evidence is needed for further validation.
1. Introduction
Achieving herd immunity against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is a vital component to overcoming the Coronavirus Disease of 2019 (COVID-19) pandemic. Vaccine hesitancy is a major barrier to accomplishing this goal and is considered a top threat to global health by the World Health Organization (WHO) (World Health Organization 2019). Hesitancy is especially apparent in patients with autoimmune diseases, such as multiple sclerosis (MS), who were largely excluded from vaccine seminal trials and are often on immunotherapy that may reduce vaccine response (Marsh et al., 2021). Preliminary data suggest that hesitancy in the autoimmune population is primarily due to fear of side effects and worsening of their underlying disease (Boekel et al., 2021; Doornekamp et al., 2020).
While data relating to COVID-19 vaccines remain limited in the neuroinflammatory disease population, one study to date in MS reported no safety concerns nor increased risk of MS relapse after the Pfizer/BioNTech BNT162b2 vaccine (Achiron et al., 2021). For many other well-established vaccines, it has been shown that the benefits of vaccination in patients with MS and related disorders strongly outweigh the risks of contracting the associated infection (Farez et al., 2019; Hapfelmeier et al., 2019). Accordingly, the WHO and Centers for Disease Control (CDC) have encouraged vaccination against COVID-19 for patients with autoimmune disease, as has the National MS Society (NMSS) for those with MS (Center for Disease Control and Prevention 2021; National Multiple Sclerosis Society 2021; World Health Organization 2021).
In this study, we assessed vaccine hesitancy and evaluated early vaccine safety/tolerability in patients with MS and other neuroinflammatory diseases (NID) by leveraging an existing network of MS Centers that are part of the MS Resilience to COVID-19 (MSReCOV) Collaborative.
2. Methods
This was a cross-sectional observational study evaluating participants recruited from multiple MS centers participating in the MSReCOV Collaborative (Levin et al., 2021), including Columbia University Irving Medical Center, University of Pittsburgh Medical Center, University of Pennsylvania School of Medicine, Yale University School of Medicine, State University of New York at Buffalo, University of Rochester Medical Center, University of Toronto and The Multiple Sclerosis Center of Northeastern New York. Participants were 18 years or older with a diagnosis of a neuroinflammatory disease or were part of the neurologically asymptomatic control group. Neuroinflammatory disease was defined as a neurologist-confirmed diagnosis of an autoimmune and/or demyelinating condition affecting the central nervous system; this included participants with multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD), myelin oligodendrocyte antibody-mediated disease (MOG-AD), neurosarcoidosis, transverse myelitis, optic neuritis, or an autoimmune encephalitis. A subset of the control group included subjects from the Genes & Environment in MS (GEMS) study which recruits asymptomatic first-degree relatives of MS patients (Xia et al., 2016). Participants completed online surveys using the Research Electronic Data Capture (REDCap) platform, including a vaccine-specific questionnaire (Supplemental Material). Ethics boards of each institution approved the study protocol. All participants provided electronic consent.
We sent participants an initial vaccine questionnaire between February and May of 2021 with a plan for repeat deployment every three months. Data from the initial vaccine survey distribution are included in this analysis. Baseline demographic and clinical factors were obtained from surveys that participants completed upon enrollment in the umbrella MSReCOV collaborative study, with enrollment dates ranging from April 2020 to May of 2021. The Charlson Comorbidity Index (CCI) was used to classify the number and severity of comorbid conditions (Charlson et al., 1987). Functional status for NID participants was obtained using Patient Determined Disease Steps (PDDS), which correlates with Expanded Disability Status Scale (EDSS) and is a validated patient-reported outcome of MS disability (Hohol et al., 1995; Learmonth et al., 2013). The PDDS scoring scale ranges from 0 to 8, based on patient's response to prompts about severity of their disease-related physical symptoms. Scores include: 0, normal; 1, mild disability; 2, moderate disability; 3, gait disability; 4, early cane use; 5, late cane use; 6, bilateral support; 7, wheelchair/scooter use; and 8, bedridden. Patients were considered to be hesitant if they responded either “No” or “Not Sure” to the survey question: “Do you plan to be vaccinated once a COVID-19 vaccine is made available to you?”. When determining the effect of disease modifying therapy (DMT) on the rate of vaccine side effects, patients were grouped as either being on high-efficacy therapy (ocrelizumab, rituximab, ofatumumab, alemtuzumab, cladribine, fingolimod, ozanimod, siponimod, natalizumab), standard-efficacy therapy (glatiramer acetate, interferons, teriflunomide, dimethyl fumarate, diroximel fumarate) or no therapy at the time of vaccination. Groupings were based on mechanism of action and available evidence regarding the effect of each medication on vaccine responses (2; Doornekamp et al., 2020; Achiron et al., 2021; Arnold et al., 2021).
Descriptive analyses were performed using R 3.6.3 (https://www.r-project/). Differences in measured variables by two groups were assessed using an independent T-test for continuous variables with a normal distribution and non-parametric equivalent Wilcoxon Ranked-Sum Test for continuous variables without a normal distribution. When comparing categorical variables between two groups, a Pearson's Chi-squared Test or Fisher's Exact Test was used based on sample size. Logistic regression was used to control for covariates that may confound vaccine side effects, such as age, comorbidities, disability level, DMT and vaccine type. A p-value of <0.05 was considered statistically significant.
3.0. Results
3.1. Participant Characteristics
1164 patients with NID and 595 neurologically asymptomatic control patients completed the initial vaccine survey. Sociodemographic information, neurologic history and functional status are provided in Table 1 . Notably, patients in the NID group were older, had a higher mean CCI, and lower mean BMI than control patients. These factors were controlled for in subsequent analyses. NID patients had a mean disease duration of 18.8 (SD ±13.3) years and mean PDDS of 2.21 (SD ± 2.5).
Table 1.
Participant Characteristics
| Sociodemographic Factora |
MS/NID N (%) |
Control N (%) |
p |
|---|---|---|---|
| Age: mean (SD)b | 52 (±13.5) | 47 (±14.7) | < 0.001 |
|
Sex: Female Male |
848 (79.9) 213 (20.1) |
413 (72.7) 152 (26.8) |
0.002 |
|
Race: African American, African American Indian, Alaskan, Hawaiian Asianb Caucasian Multi-racial Other/Unknown |
38 (3.6) 3 (0.3) 10 (1.0) 987 (92.8) 11 (1.0) 14 (1.3) |
13 (2.3) 5 (0.9) 1 (0.2) 532 (94.2) 9 (1.6) 5 (0.8) |
0.123 |
|
Ethnicity: Hispanic/Latino Non-Hispanic Unknown |
26 (2.4) 1018 (95.7) 20 (1.9) |
13 (2.3) 542 (95.9) 10 (1.8) |
0.971 |
| BMI: mean (SD) | 28.0 (±11.0) | 29.2 (±7.9) | 0.002 |
|
Comorbidities:c Cardiovascular Diabetes Pulmonary GI/Hepatobiliary Renal Malignancy Connective Tissue Disease |
199 (20.2) 48 (4.5) 18 (1.7) 36 (3.4) 6 (0.57) 25 (2.4) 30 (2.8) |
83 (14.8) 35 (6.1) 15 (2.6) 18 (3.2) 6 (1.1) 31 (5.4) 32 (5.6) |
0.755 0.217 0.260 1.0 0.361 0.311 0.010 |
| CCI: mean (SD) | 1.2 (±1.28) | 0.9 (±1.55) | < 0.001 |
|
Smoking History: Never Smoker Prior Smoker Current Smoker |
710 (66.8) 287 (27.0) 66 (6.2) |
431 (76.3) 114 (20.1) 20 (3.5) |
< 0.001 0.320 0.400 |
|
Location: New York Pennsylvania Connecticut Washington New Jersey Massachusetts Illinois Virginia Other (US) Canada Other (non-US) |
327 (30.7) 436 (41.0) 94 (8.8) 5 (0.5) 69 (6.5) 6 (0.6) 7 (0.7) 6 (0.6) 95 (8.9) 12 (1.1) 3 (0.3) |
185 (32.7) 20 (3.5) 27 (4.8) 14 (2.5) 7 (1.2) 13 (2.3) 11 (1.9) 10 (1.8) 267 (47.3) 1 (0.2) 1 (0.2) |
0.005 |
|
Vaccination: Yes No Not Sure/Clinical Trial |
742 (64.1) 413 (35.7) 3 (0.3) |
408 (68.9) 184 (31.1) 0 (0.0) |
0.084 |
| Clinical Factor | |||
|---|---|---|---|
|
Disease: Multiple Sclerosis Neuromyelitis Optica Other Neuroinflammatoryd |
1123 (96.4) 17 (1.5) 24 (2.1) |
||
| Disease Duration: mean (SD) | 18.8 y (±13.3) | ||
|
Disability Levele PDDS |
2.21 (2.5) |
||
|
DMTf No therapy B-cell therapy Interferons Glatiramer Acetate Natalizumab Fumarates S1P receptor modulators Teriflunomide Cladribine Alemtuzumab Other |
121 (17.6) 208 (30.2) 82 (11.9) 75 (10.9) 52 (7.5) 50 (7.3) 44 (6.4) 28 (4.1) 7 (1.0) 2 (0.3) 20 (2.9) |
||
Abbreviations: MS= multiple sclerosis, NID= neuroinflammatory disease; SD= standard deviation; BMI= Body Mass Index; CCI= Charlson Comorbidity Index; PDDS: patient determined disease steps; DMT = disease modifying therapy. S1P= sphingosine-1-phosphate.
Demographic information was not available for a subset of participants (n=130, 3.8%) who completed the vaccine survey due to deployment issues. All percentages are based on patients with available information.
Asian: Asian Indian, Chinese, Filipino, Japanese, Korean, Vietnamese or other.
Disease Categories included: Cardiovascular (Hypertension*, Myocardial Infarction, Congestive Heart Failure, Peripheral Vascular Disease), GI/Hepatobiliary (Ulcer, Liver disease, moderate or severe liver disease), Diabetes (Diabetes without complications, Diabetes with end organ damage), Malignancy (Solid tumor, Leukemia, Lymphoma, multiple myeloma, metastatic Tumor). *Data not available for hypertension for 122 participants all at one site, including 99 in the NID group and 24 in the control group. Percentages are based on those who were asked about the diagnosis of hypertension only.
Other NID included: MOG-AD, Neurosarcoidosis, Optic Neuritis (not part of another disease), Transverse Myelitis (not part of another disease), Autoimmune encephalitis, or other autoimmune disease of the central/peripheral nervous system.
Disability level was determined at the time of vaccine survey completion for all participants using the patient determined disease steps (PDDS) scoring scale, ranging from 0 (no disability) to 8 (bedridden) as outlined further in the methods section of the main text.
Disease modifying therapy at the time of vaccination is shown. B-cell therapies included: ocrelizumab (n=168, 24%), rituximab (n = 38, 5.5%), ofatumumab (n=2, 0.3%). Interferons included: interferon beta-1a IM (n=47, 6.28) and SC (n=17, 2.5%), and pegylated interferon beta-1a (n=12, 1.7%), interferon beta-1b (n=6, 0.9%). Fumarates included dimethyl fumarate (n=45, 6.5%) and diroximel fumarate (n=5, 0.7%). S1P receptor modulators included: fingolimod (n=40, 5.1%), siponomid (n=3, 0.4%), and ozanimod (n=1, 0.2%).
3.2. Vaccine hesitancy
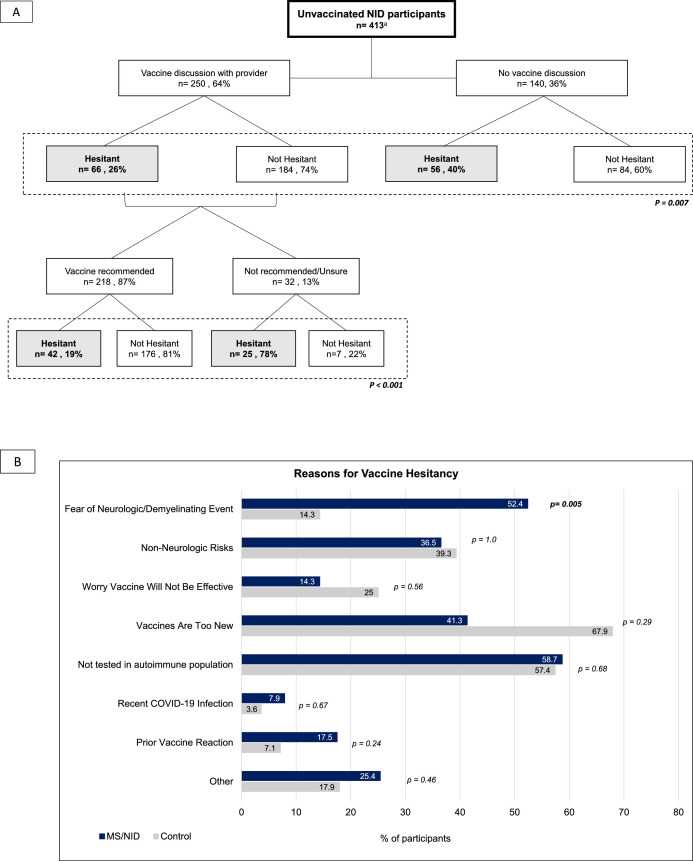
597 patients (34%) were unvaccinated at the time of survey completion, including 413 (35.6%) NID patients and 184 (31.1%) controls. Hesitancy rates among unvaccinated participants were similar in the NID and control group (n=134, 32.7% NID vs. n=56, 30.6% control; p=0.82). Among patients who planned to be vaccinated, a larger portion of NID patients than controls were planning to delay vaccination once eligible (n=47, 17.1% NID vs. n=6, 5.5% control, p=0.0026); the majority by 1-6 months (n=42, 89.4%), with 4 (8.3%) by 6-12 months, and 1 (2.1%) by > 1 year. Among NID patients who had not yet been vaccinated, 250 (64%) had discussed vaccination with their neurologists. Hesitancy rates were lower among the NID participants who had discussed vaccination with their neurologists (n=66, 26.4%) than the NID participants who had not discussed vaccination with their neurologists (n=56, 40.2% hesitant); p=0.007. Among those who had the discussion, hesitancy rates were lower among participants who reported that vaccination was recommended by their neurologists during their discussion than among those who reported that their neurologist did not recommend vaccination or was unsure about what what recommendation to make (n=42, 19.3% hesitant when recommended vs. n=25, 78.0% hesitant when not recommended/unsure; p < 0.0001). [Fig. 1 ; A].
Fig. 1.
Vaccine Hesitancy. (A): Vaccine Discussion and Hesitancy Rate. NID participants who had discussed vaccination with their neurological healthcare professional were less hesitant than the participants who had not discussed vaccination with their neurologists p=0.007. Among those who had the discussion, the hesitancy rate was lower when the neurologists recommended vaccination during their discussion (p < 0.0001). P-values in the figure refer to comparison of hesitancy rates between indicated groups. *23 patients were not sure if they had discussed the vaccine with their neurologists and were excluded from this figure. (B) Vaccine Hesitancy Reasons. Patients who responded “No” to the question, “Do you plan to be vaccinated against COVID-19?” were asked to select the reason(s) why they did not want to be vaccinated. Participants were able to select more than one reason. Responses from both the MS/NID group (navy, n= 134) and control group (n=56) are displayed here. MS/NID patients were more likely to report fear of a neurologic and/or demyelinating event as reasons they would not get vaccinated. *Answers provided as free text for “Other” in both groups most commonly included: perceiving oneself as low risk for contracting COVID-19, vaccines only having emergency use authorization, and fear of unknown long term side effects. Abbreviations: MS (multiple sclerosis), NID (neuroinflammatory disease).
The most common reasons for hesitancy in NID patients were lack of vaccine testing in the autoimmune population (n=37, 58.7%), fear of a new demyelinating event and/or neurologic symptom (n=33, 52.4%), and worry that the vaccines are too new (n=26, 41.3%) [Fig. 1; B]. Approximately half of NID patients (n=32, 50.8%) who did not plan to be vaccinated did not previously receive the influenza and/or other recommended vaccines, most commonly due to personal preference (n=14, 45.2%) or fear of an autoimmune reaction (n=11, 35.5%).
3.3. Vaccination rate
742 (64.08%) NID patients and 408 (n=68.92) control participants had received at least one dose of a vaccine against SARS-CoV-2 at the time of survey completion. The majority of participants in both groups had received a 2-dose mRNA-mediated vaccine, with either the Pfizer/BioNTech (BNT162b2) or the Moderna (mRNA-1273) vaccine; however, the proportion of the mRNA vaccination was slightly higher in the NID group (n=624, 98.4%) than in the control group (n=317, 94.9%), p = 0.007. Due to survey completion errors, vaccine type was not available for a subset of participants in the NID group (n=119, 16% vaccinated) and control group (n=74, 18% vaccinated); these patients were excluded from the safety/tolerability analysis.
Most NID patients were on DMT at the time of vaccination (n=568, 82.4%). The most commonly prescribed DMT mechanistic categories were B-cell therapies (n=208, 30.2%), interferons (n=82, 11.9%), and glatiramer acetate (n=75, 10.9%) [Table 2 ]. 111 (16.6%) NID patients adjusted the timing of their DMT administration, and 6 (0.9%) switched therapies entirely to be vaccinated. Ocrelizumab was the most common DMT among participants who adjusted their DMT dosing administration (n=46, 41.4%) ;with the median time from infusion to first vaccine dose of 6.5 (IQR 5.4, 7.5) months.
Table 2.
Vaccine Safety/Tolerability
| Factor |
MS/NID N(%) |
Control N (%) |
p |
|
Vaccine Typea Pfizer/BioNTech Moderna AstraZeneca Johnson & Johnson Not Sure/Clinical Trial |
357 (56.3) 267 (42.1) 0 (0.0) 9 (1.4) 1 (0.2) |
169 (50.6) 148 (44.3) 2 (0.6) 13 (3.9) 2 (0.6) |
0.009 |
|
Side effects with the 1stdose of a 2-dose vaccine?: Yes No |
256 (42.2) 350 (57.8) |
141 (45.3) 170 (54.7) |
0.410b |
|
Side effect type: Arm soreness Fevers, chills, aches, flu-like Allergic Reaction Neurologic complications Fatigue Headache Otherc |
225 (37.1) 72 (11.8) 6 (0.9) 1 (0.2) d 18 (2.9) 35 (5.7) 36 (5.9) |
127 (40.8) 38 (12.2) 6 (1.9) 0 (0) 15 (9.3) 6 (1.9) 11 (3.5) |
0.345 0.948 0.237 0.555 0.214 0.022 0.170 |
|
Side effect timing: Within 1 week 1-4 weeks 2-6 months >6 months |
244 (95.3) 7 (2.7) 0 (0) 0 (0) |
136 (96.5) 4 (2.8) 0 (0) 0 (0) |
1.0 |
|
Side effects with 2nddose of a 2-dose vaccine? Yes No |
246 (67.0) 121 (33.0) |
114 (64.8) 62 (35.2) |
0.672b |
|
Side effect type: Arm soreness Fevers, chills, aches, flu-like Allergic Reaction Neurologic complications Fatigue Headache Otherc |
191 (52.0) 150 (40.9) 2 (0.5) 6 (1.6)d 34 (9.2) 30 (8.1) 27 (7.4) |
97 (55.1) 85 (48.3) 1 (0.6) 1 (0.6) 13 (7.4) 3 (1.7) 8 (4.5) |
1.0 0.454 1.0 0.280 0.455 0.006 0.229 |
|
Side effect timing: Within 1 week 1-4 weeks 2-6 months >6 months |
243 (98.8) 2 (0.8) 1 (0.4) 0 (0) |
110 (96.5) 3 (2.6) 0 (0) 0 (0) |
0.330 |
|
Side effects with a 1-dose vaccine? Yes No |
6 (46.2) 7 (53.8) |
8 (66.7) 4 (33.3) |
0.310b |
|
Side effect type: Arm soreness Fevers, chills, aches, flu-like Allergic Reaction Neurologic complications Fatigue Headache Other c |
4 (30.8) 3 (23.1) 0 (0) 0 (0) 1 (16.7) 1 (16.7) 1 (16.7) |
5 (38.5) 4 (30.8) 0 (0) 0 (0) 2 (25) 3 (37.5) 2 (25) |
0.176 0.236 1.0 1.0 0.593 0.322 0.593 |
|
Side effect timing: Within 1 week 1-4 weeks 2-6 months >6 months |
6 (100) 0 (0) 0 (0) 0 (0) |
8 (100) 0 (0) 0 (0) 0 (0) |
0.593 |
Comparison of patient-reported side effects following all doses of vaccination against SARS-CoV-2 are reported here. p-values represent comparison between the NID and control group. There were no significant differences in reported side effect rates between the groups. However, when adjusting for age, comorbidities, and vaccine type, there was a higher rate of side effects reported after the second dose of the mRNA vaccines. The only difference in side effect type between groups was an increase in headaches reported by the NID group after the mRNA vaccines.
Vaccine types included: Pfizer/BioNTech sponsored (BNT162b2), Moderna sponsored (mRNA-1273), Johnson & Johnson/Janssen sponsored (Ad26.COV2.S), and AstraZeneca sponsored (ChAdOx1 nCoV-19).
When adjusting for age, comorbidities, BMI, and vaccine type there was no difference in side effect rate between NID and controls with the first dose (p=0.205) or second dose (p=0.846) of the 2-dose mRNA vaccines. When adjusting for age, comorbidities, BMI and vaccine type there was no difference in side effect rate with the 1-dose viral-vector vaccines (p=0.393). .
“Other” free text responses for all vaccine doses fell into the categories of: lymphadenopathy, joint pain, myalgias, chest pain, light-headedness/dizziness and rash. There were no differences between sub-categories of “other” responses between NID and control.
Neurologic complications listed by the MS/NID group in free text form who indicated a neurologic complication as side effect of a 2-dose vaccine series included “worsening of numbness and coordination”, “worsening of MS symptoms - weakness of muscles and loss of balance”, and “mental pain related to MS.” The remaining participants (n=1 first dose, and n=4 second dose) did not specify.
3.4. Vaccine Safety/Tolerability
There was no difference in self-reported side effects between the NID and control group with the first (n=256, 42.2% NID vs 141, 45.3% control; p=0.41) and second doses of the mRNA vaccines (n= 246, 67% NID vs n=114, 64.8% control; p=0.67) nor the only dose of the viral-vector vaccines (n=6, 46% NID vs. n=8, 66% control; p=0.31). After adjusting for age, BMI, and comorbidities to control for differences between groups, there was still no difference in reported side effect rates between the NID and control group with any dose. Headache was more common in the NID participants after the mRNA vaccine , although no other difference was seen in the side effect profile between groups [Table 2] . No patients reported an episode of transverse myelitis, Guillain-Barre syndrome, or Bell's Palsy in either group. In both the NID and control groups, younger age correlated with an increased rate of reported side effects with the first (p=<0.0001) and second (p=0.03) dose of the mRNA vaccines.
Within the NID group, there was no correlation seen between disability level and reported side effects with any dose of the vaccine after adjusting for age, BMI, and comorbidities. With the second dose of the mRNA vaccines, NID patients on high-efficacy therapy had a lower rate of reported side effects (n=39, 23.6%, RR 0.51, p=0.002) than NID patients not on medication at the time of vaccination (n=27, 46.6%). There was no difference in rate of reported side effects for those on standard-efficacy therapy (n=31, 37.6%, RR 0.81, p=0.34) compared to those not on medication for the second dose. However, when adjusting for age, BMI, and comorbidities, treatment with standard-efficacy therapy was associated with a lower risk of reported side effects (p=0.03) than NID patients not on medication for the mRNA vaccines, and there was no longer an association between high-efficacy therapy and reported side effects with any dose.
3.5. Vaccinated vs Unvaccinated NID
All NID participants evaluated here were enrolled in the previously established MSReCOV study, which regularly deploys surveys at predetermined time intervals. At the time of the vaccine survey completion, all NID participants were asked about interval neurologic symptoms, MRI results, and COVID diagnoses since completion of their most recent study survey. The inter-survey time interval in the vaccinated NID group (mean 11.4 weeks, SD 2.5) was slightly longer than the inter-survey time interval in the unvaccinated NID group (mean 10.6 weeks, SD 2.5; p=<0.001).
During this inter-survey time interval, there was no difference in the rates of reported new or recurrent neurologic symptoms between the vaccinated and unvaccinated NID groups (n=110, 16.2% vaccinated vs. 71, 18.2% unvaccinated; p=0.44). There was also no difference in the type of new neurologic symptoms reported between groups. Among vaccinated patients reporting an interval new neurologic symptom, onset occurred after vaccination in 10 (1.5%) participants, with median time of onset of 33 [IQR 16, 53] days after vaccination. Symptoms reported by these 10 individuals included: sensory changes (n=4; 1 described “MS hug”, the others did not specify), weakness (n=1); sensory changes and weakness (n=2), difficulty with walking (n=1), weakness and vision changes (n=1), "other" (n=1; described in free text as difficulty with speech). 3 of the 10 patients had adjusted the timing of their DMT for vaccination (1 rituximab, 1 natalizumab, 1 interferon-beta IM) and 1 required acute treatment with steroids/PLEX for their symptoms (weakness/vision changes, onset 42 days after vaccination, no DMT adjustment for vaccine). 6 of these patients underwent MRI due to the new neurological symptom, including the patient who required acute treatment. None of the MRI showed a new or enhancing lesion.
Similarly, there was no difference in reported radiologic disease activity among the vaccinated and unvaccinated NID participants during the inter-survey time interval: 40 (5.9%) vaccinated and 30 (7.6%) unvaccinated NID participants reported new or enhancing lesions on an interval MRI (p=0.32). The majority of MRIs with evidence of disease activity in the vaccinated NID participants were obtained prior to vaccination, though 10 (1.5%) vaccinated NID participants had an MRI with new or enhancing lesion at some point after vaccination, with a median time from vaccine to MRI of 21 days [IQR 12.8, 45]. None of these 10 patients reported new neurologic symptoms during the same time interval.
Lastly, fewer vaccinated NID participants reported an interval COVID-19 diagnosis (n=12, 1.6% vaccinated vs n=25, 6.0% unvaccinated; p = 0.001). All COVID-19 diagnoses in the vaccinated NID group occurred before their first vaccine dose, except for one NID participant who contracted COVID-19 within days of the first dose of a 2-dose mRNA vaccine. The control group exhibited similar patterns of COVID-19 diagnosis (n=8, 2.0% vaccinated vs. n=13, 7.1% unvaccinated; p=0.003). All infections in the vaccinated control group occurred prior to the participants’ first or only vaccine dose.
4.0. Discussion
The lack of available data evaluating the effect of vaccines against SARS-CoV-2 in patients with neuroinflammatory disease is a major cause of vaccine hesitancy and a potential obstacle to optimizing patient safety and public health. In our study, we found similar rates of hesitancy between a large group of patients with NID and a neurologically asymptomatic control group. Our results confirmed that the most common reasons for vaccine hesitancy in the NID group were related to lack of data in the autoimmune population and fear of worsening of underlying neurologic disease. Importantly, we found a lower vaccine hesitancy rate among participants who had discussed vaccination with their neurologic healthcare provider than those who did not have such discussion. The difference in vaccine hesitancy rate was also more pronounced when the clinicians had recommended vaccination. Taken together, our findings suggest that clinicians can decrease vaccine hesitancy in the NID population through patient education and counseling. Our results highlight the need for further SARS-CoV-2 vaccine studies in the NID population to improve patient reassurance.
In our early analysis, we found no overall difference in reported side effect rates between the NID and control group for all vaccine types and doses. This remained true after adjusting for population differences, including age, BMI, and comorbidities. When examining the side effect profiles, headache was the only side effect reported at higher rates in NID patients than the controls. This finding could be due to worsening of underlying headache disorders, which are more prevalent in patients with multiple sclerosis (i.e. majority of the NID group) (Mirmosayyeb et al., 2020). Notably, there was no new side effect signal for either the NID or control group when compared to published SARS-CoV-2 vaccine trials (Baden et al., 2020; Folegatti et al., 2020; Polack et al., 2020; Voysey et al., 2021).
With the second dose of the mRNA vaccines, we found that being on high-efficacy therapy (prior to adjustment for population differences) or standard-efficacy therapy at the time of vaccination correlated with a lower rate of side effects. While difficult to know based on our current study, we speculate that this finding could be related to an attenuated immune response. Thus far, decreased humoral response to the COVID-19 mRNA vaccine has been most convincingly shown for certain high-efficacy therapies, such as B-cell depleting therapies, S1P-receptor modulating therapies, and within the first four months of cladribine dosing (Achiron et al., 2021). Further studies will be needed to determine the impact of immunotherapy on the rate and severity of side effects experienced.
There was no difference in the rate of recurrent/new neurologic symptoms or radiologic disease activity between vaccinated and unvaccinated patients with NID during the inter-survey time interval within the MSReCOV study. Among those who experienced new symptoms, the types of symptoms reported were also the same between vaccinated and unvaccinated participants. However, we note that for this initial evaluation, a large proportion of the inter-survey time interval occurred prior to vaccination and therefore may not accurately represent the effect of vaccination on underlying disease activity. Continued monitoring is necessary prior to drawing any conclusions. When honing in on the small subset of patients (1.5%) with onset of a neurologic symptom after vaccination, we could not conclude whether these symptoms were vaccine-related. It is reassuring that the majority of these events occurred at least one month after vaccination (60%) and had no known MRI correlation. Further, 9 out of 10 individuals did not require any acute symptomatic treatment.
In our study, no patients with NID were diagnosed with COVID-19 after completing their vaccination. It is worth noting that many patients were on DMTs that could reduce vaccine efficacy (2; Achiron et al., 2021; Arnold et al., 2021; Kelly et al., 2021; Ruddy et al., 2021). We note the relatively short follow-up duration and therefore strongly caution against false reassurance from our results, as our goal of this initial report is for timely dissemination of early findings to guide clinical care.
Several strengths of this study are worth noting. For one, we leveraged a previously established network of MS centers (e.g. the MSReCOV Collaborative) for wide and rapid survey deployment. This enabled us to generate prompt real-world data focused on the understudied topic of COVID-19 vaccination in patients with NID. Second, our cohort was large and had detailed information available regarding demographics, clinical history, disability level and several vaccine-related topics. Finally, we utilized a sizable population of neurologically asymptomatic participants as a control group, allowing us to assess if any vaccine-related side effects were unique to the NID population.
Our study also had several limitations. First, patients with NID differed from controls in age and comorbidities, both of which may affect side effect rates. We controlled for these differences when evaluating side effect profiles to reduce confounding. Second, our findings may have limited generalizability, as study participants predominantly identified as being of non-Hispanic European descent and a subset of the control participants were neurologically asymptomatic first-degree relatives of individuals with autoimmune disease. Third, the findings described here relied on self-report; though we note that survey response is a reasonable research method that enables rapid data collection for a timely investigation. Fourth, there may be recall bias as a large proportion of patients had been vaccinated prior to survey distribution, again highlighting the choice for survey deployment in existing research participants. Lastly, in attempt for prompt data acquisition, the overall follow up time after vaccination is limited. As the pandemic persists and the need for booster rises, we are continuing with longitudinal follow-up to evaluate the safety/tolerability and efficacy of SARS-CoV-2 vaccines in this patient population.
5. Conclusions
Overall, our findings suggest that SARS-CoV-2 vaccines in patients with neuroinflammatory disease are safe and unlikely to worsen underlying disease activity. This is consistent with evidence from other vaccines in MS (Farez et al. 2019; Hapfelmeier et.al. 2019, 2; Achiron et al., 2021). In contrast, we note that COVID-19 is associated with a variety of inflammatory and non-inflammatory neurologic complications, which should be considered in the risk/benefit discussion regarding vaccination (Ellul et al., 2020; Guilmot et al., 2021; Keyhanian et al., 2020). Our study highlights the utility of patient education by clinicians and the need for inclusion of this population in future vaccine trials to ensure that the vast majority of this vulnerable population is well protected from novel infections.
Author disclosures
Samantha Epstein, Annie Lee, Fatoumata Diallou, Adelle Ricci, Megan Dahl, Katelyn Kavak and Elle Levit report no relevant disclosures.
Zongqi Xia serves on the advisory board for Roche/Genentech; he has sponsored research agreements with Octave Biosciences.
Erin E Longbrake has served on an advisory board/consulted for: Genentech/Roche, Genzyme, Alexion, Biogen. She has received research funding from Genentech.
Christopher Perrone has served on an advisory board/consulted for: EMD Serono and Novartis.
Bianca Weinstock-Guttman has participated in speaker's bureaus and/or served as a consultant for Biogen, EMD Serono, Novartis, Genentech, Celgene/Bristol Meyers Squibb, Sanofi Genzyme, Bayer, Janssen and Horizon. Dr. Weinstock-Guttman also has received grant/research support from the agencies listed in the previous sentence.
Claire S. Riley has served on an advisory board/consulted for: Genentech/Roche; Biogen; Novartis; Bristol Myers Squibb; Janssen Global; TRV Cell Therapy.
Philip L. De Jager serves on advisory board for: Biogen; Merck Serono; and Roche; he has sponsored research agreements with Roche, Biogen, and Puretech.
Rebecca Farber has served on advisory board / consulted for: Roche/ Genentech; EMD Serono; Alexion; Viela Bio.
Sarah F. Wesley has sponsored agreements with the NIH and Novartis.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- World Health Organization . 2019. Ten threats to global health in.https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 [online]. Available at: [Google Scholar]
- Marsh EB, Kornberg M, Kessler K, et al. COVID-19 and Vaccination in the Setting of Neurologic Disease: An Emerging Issue in Neurology. Neurology. 2021 doi: 10.1212/WNL.0000000000012578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekel L, Hooijberg F, van Kempen ZLE, et al. Perspective of patients with autoimmune diseases on COVID-19 vaccination. Lancet Rheumatol. 2021;3:e241–e243. doi: 10.1016/S2665-9913(21)00037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doornekamp L, van Leeuwen L, van Gorp E, Voeten H, Goeijenbier M. Determinants of Vaccination Uptake in Risk Populations: A Comprehensive Literature Review. Vaccines (Basel) 2020;8 doi: 10.3390/vaccines8030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Mult Scler. 2021;27:864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: Vaccine-preventable infections and immunization in multiple sclerosis. Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. 2019;93:584–594. doi: 10.1212/WNL.0000000000008157. [DOI] [PubMed] [Google Scholar]
- Hapfelmeier A, Gasperi C, Donnachie E, Hemmer B. A large case-control study on vaccination as risk factor for multiple sclerosis. Neurology. 2019;93:e908–e916. doi: 10.1212/WNL.0000000000008012. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention . 2021. COVID-19 Vaccines for People with Underlying Medical Conditions [online]https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/underlying-conditions.html Available at: [Google Scholar]
- National Multiple Sclerosis Society . 2021. COVID-19 Vaccine Guidance for People Living With MS. [online]. Available at: https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance#section-0. [Google Scholar]
- World Health Organization . 2021. COVID-19 advice for the public: Getting vaccinated. [online]. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice. [Google Scholar]
- Levin SN, Venkatesh S, Nelson KE, et al. Manifestations and impact of the COVID-19 pandemic in neuroinflammatory diseases. Ann Clin Transl Neurol. 2021;8:918–928. doi: 10.1002/acn3.51314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, White CC, Owen EK, et al. Genes and Environment in Multiple Sclerosis project: A platform to investigate multiple sclerosis risk. Annals of Neurology. 2016;79:178–189. doi: 10.1002/ana.24560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology. 1995;45:251–255. doi: 10.1212/wnl.45.2.251. [DOI] [PubMed] [Google Scholar]
- Learmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurology. 2013;13:37. doi: 10.1186/1471-2377-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord. 2021;14 doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J, Winthrop K, Emery P. COVID-19 vaccination and antirheumatic therapy. Rheumatology. 2021 doi: 10.1093/rheumatology/keab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmosayyeb O, Barzegar M, Nehzat N, Shaygannejad V, Sahraian MA, Ghajarzadeh M. The prevalence of migraine in multiple sclerosis (MS): A systematic review and meta-analysis. Journal of Clinical Neuroscience. 2020;79:33–38. doi: 10.1016/j.jocn.2020.06.021. [DOI] [PubMed] [Google Scholar]
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine. 2020 doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly H, Sokola B, Abboud H. Safety and efficacy of COVID-19 vaccines in multiple sclerosis patients. J Neuroimmunol. 2021;356 doi: 10.1016/j.jneuroim.2021.577599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy JA, Connolly CM, Boyarsky BJ, et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Annals of the Rheumatic Diseases. 2021 doi: 10.1136/annrheumdis-2021-220656. annrheumdis-2021-220656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilmot A, Maldonado Slootjes S, Sellimi A, et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. Journal of Neurology. 2021;268:751–757. doi: 10.1007/s00415-020-10108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyhanian K, Umeton RP, Mohit B, Davoudi V, Hajighasemi F, Ghasemi M. SARS-CoV-2 and nervous system: From pathogenesis to clinical manifestation. J Neuroimmunol. 2020;350 doi: 10.1016/j.jneuroim.2020.577436. 577436–577436. [DOI] [PMC free article] [PubMed] [Google Scholar]