Abstract
Background
Implanted spinal neuromodulation (SNMD) techniques are used in the treatment of refractory chronic pain. They involve the implantation of electrodes around the spinal cord (spinal cord stimulation (SCS)) or dorsal root ganglion (dorsal root ganglion stimulation (DRGS)), and a pulse generator unit under the skin. Electrical stimulation is then used with the aim of reducing pain intensity.
Objectives
To evaluate the efficacy, effectiveness, adverse events, and cost‐effectiveness of implanted spinal neuromodulation interventions for people with chronic pain.
Search methods
We searched CENTRAL, MEDLINE Ovid, Embase Ovid, Web of Science (ISI), Health Technology Assessments, ClinicalTrials.gov and World Health Organization International Clinical Trials Registry from inception to September 2021 without language restrictions, searched the reference lists of included studies and contacted experts in the field.
Selection criteria
We included randomised controlled trials (RCTs) comparing SNMD interventions with placebo (sham) stimulation, no treatment or usual care; or comparing SNMD interventions + another treatment versus that treatment alone. We included participants ≥ 18 years old with non‐cancer and non‐ischaemic pain of longer than three months duration. Primary outcomes were pain intensity and adverse events. Secondary outcomes were disability, analgesic medication use, health‐related quality of life (HRQoL) and health economic outcomes.
Data collection and analysis
Two review authors independently screened database searches to determine inclusion, extracted data and evaluated risk of bias for prespecified results using the Risk of Bias 2.0 tool. Outcomes were evaluated at short‐ (≤ one month), medium‐ four to eight months) and long‐term (≥12 months). Where possible we conducted meta‐analyses. We used the GRADE system to assess the certainty of evidence.
Main results
We included 15 unique published studies that randomised 908 participants, and 20 unique ongoing studies. All studies evaluated SCS. We found no eligible published studies of DRGS and no studies comparing SCS with no treatment or usual care. We rated all results evaluated as being at high risk of bias overall. For all comparisons and outcomes where we found evidence, we graded the certainty of the evidence as low or very low, downgraded due to limitations of studies, imprecision and in some cases, inconsistency.
Active stimulation versus placebo
SCS versus placebo (sham)
Results were only available at short‐term follow‐up for this comparison.
Pain intensity
Six studies (N = 164) demonstrated a small effect in favour of SCS at short‐term follow‐up (0 to 100 scale, higher scores = worse pain, mean difference (MD) ‐8.73, 95% confidence interval (CI) ‐15.67 to ‐1.78, very low certainty). The point estimate falls below our predetermined threshold for a clinically important effect (≥10 points). No studies reported the proportion of participants experiencing 30% or 50% pain relief for this comparison.
Adverse events (AEs)
The quality and inconsistency of adverse event reporting in these studies precluded formal analysis.
Active stimulation + other intervention versus other intervention alone
SCS + other intervention versus other intervention alone (open‐label studies)
Pain intensity
Mean difference
Three studies (N = 303) demonstrated a potentially clinically important mean difference in favour of SCS of ‐37.41 at short term (95% CI ‐46.39 to ‐28.42, very low certainty), and medium‐term follow‐up (5 studies, 635 participants, MD ‐31.22 95% CI ‐47.34 to ‐15.10 low‐certainty), and no clear evidence for an effect of SCS at long‐term follow‐up (1 study, 44 participants, MD ‐7 (95% CI ‐24.76 to 10.76, very low‐certainty).
Proportion of participants reporting ≥50% pain relief
We found an effect in favour of SCS at short‐term (2 studies, N = 249, RR 15.90, 95% CI 6.70 to 37.74, I2 0% ; risk difference (RD) 0.65 (95% CI 0.57 to 0.74, very low certainty), medium term (5 studies, N = 597, RR 7.08, 95 %CI 3.40 to 14.71, I2 = 43%; RD 0.43, 95% CI 0.14 to 0.73, low‐certainty evidence), and long term (1 study, N = 87, RR 15.15, 95% CI 2.11 to 108.91 ; RD 0.35, 95% CI 0.2 to 0.49, very low certainty) follow‐up.
Adverse events (AEs)
Device related
No studies specifically reported device‐related adverse events at short‐term follow‐up. At medium‐term follow‐up, the incidence of lead failure/displacement (3 studies N = 330) ranged from 0.9 to 14% (RD 0.04, 95% CI ‐0.04 to 0.11, I2 64%, very low certainty). The incidence of infection (4 studies, N = 548) ranged from 3 to 7% (RD 0.04, 95%CI 0.01, 0.07, I2 0%, very low certainty). The incidence of reoperation/reimplantation (4 studies, N =5 48) ranged from 2% to 31% (RD 0.11, 95% CI 0.02 to 0.21, I2 86%, very low certainty). One study (N = 44) reported a 55% incidence of lead failure/displacement (RD 0.55, 95% CI 0.35, 0 to 75, very low certainty), and a 94% incidence of reoperation/reimplantation (RD 0.94, 95% CI 0.80 to 1.07, very low certainty) at five‐year follow‐up. No studies provided data on infection rates at long‐term follow‐up.
We found reports of some serious adverse events as a result of the intervention. These included autonomic neuropathy, prolonged hospitalisation, prolonged monoparesis, pulmonary oedema, wound infection, device extrusion and one death resulting from subdural haematoma.
Other
No studies reported the incidence of other adverse events at short‐term follow‐up. We found no clear evidence of a difference in otherAEs at medium‐term (2 studies, N = 278, RD ‐0.05, 95% CI ‐0.16 to 0.06, I2 0%) or long term (1 study, N = 100, RD ‐0.17, 95% CI ‐0.37 to 0.02) follow‐up.
Very limited evidence suggested that SCS increases healthcare costs. It was not clear whether SCS was cost‐effective.
Authors' conclusions
We found very low‐certainty evidence that SCS may not provide clinically important benefits on pain intensity compared to placebo stimulation. We found low‐ to very low‐certainty evidence that SNMD interventions may provide clinically important benefits for pain intensity when added to conventional medical management or physical therapy. SCS is associated with complications including infection, electrode lead failure/migration and a need for reoperation/re‐implantation. The level of certainty regarding the size of those risks is very low. SNMD may lead to serious adverse events, including death. We found no evidence to support or refute the use of DRGS for chronic pain.
Plain language summary
What are the benefits and risks of electrical spinal cord and dorsal root ganglion stimulation for the treatment of chronic pain in adults?
Why this question is important
Persistent (chronic) pain is a common problem that affects people from all walks of life. It can be the result of a wide range of different medical conditions and is sometimes unexplained, but it often causes substantial suffering, distress and disability and can have major impacts on a person's quality of life.
Implanted spinal neuromodulation (SNMD) interventions involve surgically implanting wires (electrodes) into the space around nerves or the spinal cord that are connected to a "pulse generator" device which is usually implanted under the patient's skin. This delivers electrical stimulation to the nerves or spinal cord. It is thought that this stimulation interferes with danger messages being sent to the spinal cord and brain with the goal of reducing the perception of pain. Once implanted with a SNMD device people live with the device implanted, potentially on a permanent basis. We reviewed the evidence to find out whether these interventions were effective at reducing pain, disability and medication use, at improving quality of life and to find out the risk and type of complications they might cause. There are two broad types of SNMD: spinal cord stimulation (SCS), where electrodes are placed near the spinal cord and dorsal root ganglion stimulation (DRGS) where electrodes are placed near the nerve root, where the nerve branches off from the spinal cord.
How we identified and assessed the evidence
First, we searched for all relevant studies in the medical literature. We then compared the results, and summarised the evidence from all the studies. Finally, we assessed the certainty of the evidence. We considered factors such as the way studies were conducted, study sizes, and consistency of findings across studies. Based on our assessments, we rated the evidence as being of very low, low, moderate or high certainty.
What we found
We found 15 published studies that included 908 people with persistent pain due to a variety of causes including nerve disease, chronic low back pain, chronic neck pain and complex regional pain syndrome. All of these studies evaluated SCS; no studies evaluated DRGS.
Eight studies (that included 205 people) compared SCS with a sham (placebo) stimulation, where the electrodes were implanted, but no stimulation was delivered. Six studies that included 684 people compared SCS added with either medical management or physical therapy with medical management or physical therapy on its own. We rated the evidence as being of low, or very low certainty. Limitations in how the studies were conducted and reported, the amount of evidence we found and inconsistency between studies in some instances means that our confidence in the results is limited.
The evidence suggests the following.
Compared to receiving medical management or physical therapy alone, people treated with the addition of SCS may experience less pain and higher quality of life after one month or six months of stimulation. There is limited evidence to draw conclusions in the long term of one year or more. It is unclear whether SCS reduces disability or medication use.
Compared to a sham (placebo) stimulation, SCS may result in small reductions in pain intensity in the short term that may not be clinically important, but this is currently unclear. There is no evidence at medium or long‐term follow‐up points.
SCS can result in complications. These include movement or malfunction of the electrode wires, wound infections and the need for further surgical procedures to fix issues with the implanted devices. We also found instances of serious complications that included one death, nerve damage, lasting muscle weakness, lung injury, serious infection, prolonged hospital stay and the extrusion of a stimulation device through the skin.
Very limited evidence around the costs and economics of SCS suggested that SCS increases the costs of healthcare. It was not clear whether SCS was cost‐effective.
What this means
SCS may reduce pain intensity in people with chronic pain. It is currently not clear how much of this effect is due to the SCS itself and how much is due to so‐called "placebo" effects, which are the result of the experience of undergoing the procedure and the person's expectations that it will help them. Receiving SCS does present a risk of relatively common complications and less common serious complications. We are currently unsure of the precise degree of this risk.
How up‐to‐date is this review?
The evidence in this review is current to September 2021.
Summary of findings
Background
Description of the condition
Chronic pain is a common problem. Global burden of disease data indicate that chronic pain is a leading cause of years lived with disability, with painful conditions comprising 4 of 10 leading causes of disability in both developed and developing countries (Rice 2016; Vos 2015). Chronic pain impacts the physical and mental health and quality of life of those who experience it (Moore 2014; Sylwander 2020), but it also has a substantial economic impact on society, in terms of reduced productivity, participation, and healthcare utilisation (Gaskin 2012; Gustavsson 2012).
Chronic pain is a heterogenous phenomenon with a wide variety of potential causes. These include nociceptive pain conditions, in which there is clear evidence of ongoing peripheral tissue pathology, such as rheumatoid arthritis; neuropathic pain, in which the pain arises as a result of identifiable nerve injury or disease, for example diabetic neuropathy; and many other chronic pain problems, such as fibromyalgia and chronic low back pain, in which the relationship between peripheral tissue pathology and clinical symptoms is less clear. In 2016, the term 'nociplastic pain' was added to the International Association for the Study of Pain (IASP) taxonomy of pain in an attempt to classify this latter group (Kosek 2016). It is likely that different mechanisms underpin these different types of chronic pain, though current understanding of those mechanisms is incomplete (Ossipov 2006; Vardeh 2016). In 2019, chronic pain was formally classified in the International Classification of Diseases (ICD‐11) under the categories of 'chronic primary pain', characterised by disability or emotional distress not attributable to another diagnosis, and 'chronic secondary pain', when the pain is a symptom of an identifiable underlying condition (Detlef‐Treede 2019).
Description of the intervention
Electrical stimulation of neural structures, commonly labelled 'neuromodulation', is becoming an increasingly common intervention for the treatment of chronic pain. In this review, we will focus on neuromodulation procedures that stimulate the spinal cord or the nerve roots that arise directly from the spinal cord, which involve the implantation of electrodes into the epidural space around the spinal cord (spinal cord stimulation (SCS)) or dorsal root ganglion (dorsal root ganglion stimulation (DRGS)). Electrodes can be implanted either surgically or percutaneously. These electrodes remain in situ, and are connected to a portable battery‐powered stimulation unit 'pulse generator' that is either implanted under the skin or worn by the person, and delivers electrical stimulation to the neural structures. The procedure usually consists of a trial phase, with temporary electrode leads attached to an external wearable device, followed by the implantation of permanent electrode leads attached to a pulse generator that is implanted under the skin (Patel 2015).
There are various types of spinal neuromodulation devices (SNMDs), and approaches may differ in terms of the surgical approach taken, the neural structures targeted, the device and equipment, and the parameters of electrical stimulation delivered to the tissues. While novel approaches to stimulation continue to be reported, current stimulation parameters can be classified into the following broad categories (Sdrulla 2018).
Conventional stimulation: involves the delivery of a tonic pulse, at a constant stimulation frequency between 40 Hz and 80 Hz, with a fixed pulse width.
High frequency stimulation: involves the delivery of a tonic pulse, at a frequency between 1 kHz and 10 kHz, with a fixed pulse width.
Burst stimulation: involves the delivery of intermittent trains of stimulation, though the stimulation parameters may vary.
High frequency and burst stimulation approaches differ from conventional stimulation approaches, as they do not induce paraesthesia (tingling) sensations. This potentially improves the tolerability of the intervention, and offers the advantage of allowing the use of sham stimulation under blinded conditions as a comparator in clinical trials, to control for placebo effects (Kjaer 2019). Spinal neuromodulation interventions are typically offered to people whose pain has been refractory to other interventions. They may include pharmacological, surgical, rehabilitation, or a combination of approaches.
How the intervention might work
The fundamental rationale common to all spinal neuromodulation approaches is that the stimulation of neural tissue may impact the processing of nociceptive input from nerve fibres from the painful body area, may alter the excitability (readiness to fire) of nerve cells in the target region, and may have upstream or downstream effects on neural activity in related structures in both the peripheral and central nervous system (Jensen 2019; Sdrulla 2018). It is proposed that neuromodulation techniques can reduce pain by altering the behaviour of the structures that are involved in the generation of the experience of pain. The precise mechanisms by which neuromodulation techniques might reduce pain are not known, and debate continues on which specific nerve structures are activated by SNMD, or which are optimal to achieve the greatest pain relief (Caylor 2019; Jensen 2019; Sdrulla 2018). It is proposed that DRGS and SCS may have distinct analgesic effects due to their respective direct actions on DRG nerve cells, or those in the dorsal column of the spinal cord (Deer 2017; Esposito 2019). While it is possible that different stimulation locations (SCS or DRGS) or parameters (conventional, high frequency, or burst stimulation) might elicit clinical effects through distinct mechanistic pathways, this is yet to be convincingly demonstrated in mechanistic studies (Sdrulla 2018).
Why it is important to do this review
Implanted spinal neuromodulation interventions are becoming a common option for the treatment of chronic pain, particularly for people who have not improved with drug or non‐invasive therapies (Prager 2010). In the Cochrane Library, there are no up‐to‐date reviews specifically focusing on these interventions for non‐cancer and non‐ischaemic pain. A 2013 overview of reviews of interventions for complex regional pain syndrome concluded that there was only very low‐quality evidence that SCS was effective for that condition, and that adverse events appeared to be frequent (O'Connell 2013).
In 2005, a review of health technology appraisals from the Ontario Ministry of Health and Long‐term Care concluded that weak‐to moderate‐quality evidence supported the use of SCS to decrease pain in neuropathic pain conditions (MoH‐LTC 2005). In the UK, the National Institute for Health and Care Excellence (NICE) recommended SCS for people with chronic pain of neuropathic origin who had not responded to conventional medical management, based on a technology appraisal, the searches for which have not been updated since 2014 (NICE 2008). That appraisal considered no placebo‐controlled studies, and only two small trials in populations with failed back surgery syndrome and complex regional pain syndrome. In 2019, a new technology appraisal from NICE focused specifically on a proprietary high‐frequency spinal cord neuromodulation device (SENZA), and recommended the system for chronic neuropathic back or leg pain after failed back surgery, despite conventional medical management, based on limited evidence from two small trials with contradictory findings (NICE 2019). In 2016, the European Academy of Neurology (EAN) guidelines on central neurostimulation therapy in chronic pain conditions, made a weak recommendation to add SCS to medical management in painful diabetic neuropathy, chronic post‐surgical back and leg pain, and complex regional pain syndrome (CRPS) type I, and recommended offering SCS instead of reoperation in chronic low back pain (Cruccu 2016). They highlighted the quality of the evidence as a key issue.
While these guidance documents have made recommendations for the use of these interventions, they have done so from a limited evidence base. Given the relative costs of the treatment, and its invasive nature, there is need for a rigorous, and up‐to‐date review of the efficacy, effectiveness, cost‐effectiveness, and safety of the full range of these interventions, completed to Cochrane standards. This review aims to provide valuable information for people with chronic pain who have been offered, or who are considering these interventions, clinicians who work with people with chronic pain, clinical guideline organisations, and policy makers.
Objectives
To evaluate the efficacy, effectiveness, adverse events, and cost‐effectiveness of implanted spinal neuromodulation interventions for adults with chronic pain.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) comparing implanted spinal neuromodulation devices with placebo (sham) stimulation, no treatment or usual care; or comparing SNMDs + another treatment versus that treatment alone. We included RCTs of parallel, cross‐over, or cluster design, because RCTs are the best design to minimise bias when evaluating the effectiveness of an intervention.
We did not include quasi‐randomised studies or non‐randomised studies, due to the risk of bias inherent in such designs. We excluded non‐randomised studies, studies of experimental pain, case reports, and clinical observations.
Types of participants
We included participants ≥ 18 years old who were identified as having non‐cancer and non‐ischaemic pain of longer than three months duration. We excluded studies of people with cancer, or ischaemic‐related pain, or headache of any origin. We excluded studies in which average baseline (pre‐intervention) pain intensity levels in participants were less than 4/10 or 40/100. For studies in which only some participants met these inclusion criteria, we included these studies if data from participants who met the criteria were presented separately.
Types of interventions
We included studies that used any electrical spinal neuromodulation technique that involves the implanting of electrodes in the epidural space around the spinal cord (spinal cord stimulation (SCS)) or the dorsal root ganglion (dorsal root ganglion stimulation (DRGS)). We did not include interventions of peripheral nerve stimulation (of sites distal to the dorsal root) or transcutaneous stimulation procedures.
Studies must have compared these procedures with either placebo (sham) stimulation, usual care, no treatment, or other treatments, or compared stimulation plus other treatments or usual care versus the same treatment or usual care alone. We did not include studies that compared one form of spinal neuromodulation with another, or compared different stimulation regimens using the same type of spinal neuromodulation. We considered the effectiveness of SCS and DRGS separately, because they differ by procedure, their proposed distinct analgesic effects, and mechanisms.
The key comparisons of interests were:
active stimulation versus placebo stimulation;
active stimulation versus usual care or no treatment;
active stimulation plus another intervention versus that intervention alone.
Types of outcome measures
Primary outcomes
-
Pain intensity, measured using a visual analogue scale (VAS), numerical rating scale (NRS), verbal rating scale, or Likert scale. These must have been reported by the participant to be considered valid, and included. We presented and analysed primary outcomes as change on a continuous scale, or in a dichotomised format as the proportion of participants in each group who attained a predetermined threshold of improvement. For example, we judged cut‐points from which to interpret the likely clinical importance of (pooled) effect sizes according to criteria proposed in the IMMPACT consensus statement (Dworkin 2008). Specifically, we judged reductions in pain intensity compared with baseline as follows:
≥ 30%: moderately important change;
≥ 50%: substantially important change.
Adverse events (AEs; their nature, frequency, and the approach taken to record and classify them). Adverse events included, but were not limited to: electrode lead failure or displacement, infection, need for repeated implantation procedure(s). We treated them as separate outcomes, and included other AEs in an 'other' category.
Secondary outcomes
Disability, measured by validated, self‐report questionnaires or scales, or functional testing protocols
Analgesic medication use
Health‐related quality of life, using any validated tool
Health economic outcomes, including quality‐adjusted life‐years (QALYs), costs, health resource utilisation, and incremental cost‐effectiveness ratios (ICERS)
The planned follow‐up time‐points were:
during use: for trials that report during stimulation outcomes (most likely during the initial trial period of stimulation), we used the outcome reported closest to, but before the end of that period;
short‐term: we used outcomes reported within the first month post permanent implantation. When multiple time points were reported in this timeframe, we took the closest to the implantation date;
medium‐term: we used outcomes reported between four and eight months following permanent implantation. When multiple time points were reported in this timeframe, we took the latest date;
long‐term: we used outcomes reported at one year or longer post‐implantation. When multiple time points were reported in this timeframe, we took the latest date.
The distinction between "during use" and other time‐points was not possible to make and proved artificial in the context of the included studies, as outcomes were evaluated whilst stimulation was active at all time points. As a result, we restricted our analyses to short‐term, medium‐term and long‐term follow‐up. For cross‐over studies, follow‐up was measured from the point of the onset of stimulation.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases from their inception, using a combination of controlled vocabulary, i.e. medical subject headings (MeSH), and free‐text terms to identify published articles:
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library ‐Issue 10 of 12 2020;
MEDLINE (Ovid) ‐1946 to21 Oct 2020;
Embase (Ovid) ‐ 1980 to 2020 week 42;
Web of Science (ISI) SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH ‐1970 to 21‐10‐20;
International HTA Database (https://database.inhata.org) searched on 2/11/20;
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch).
There were no language restrictions. All database searches were based on this strategy, but adapted to individual databases as necessary. We used medical subject headings (MeSH) or equivalent and text word terms. The search strategies can be found in Appendix 1; Appendix 2; Appendix 3.
Searching other resources
In addition, we checked reference lists of reviews and retrieved articles for additional studies. We contacted experts in the field for unpublished and ongoing trials. We contacted study authors for additional information when necessary.
Data collection and analysis
Selection of studies
Two review authors (NOC and WG) independently assessed the titles and abstracts of potential trials identified by the search strategy for their eligibility. We obtained the full text of studies we considered may be eligible, or if the eligibility of a study was unclear from the title and abstract. We excluded studies that did not match the inclusion criteria (see Criteria for considering studies for this review). We resolved disagreements between review authors regarding a study's inclusion by discussion. If we could not reach agreement, we planned that a third review author would assess relevant studies, and a majority decision was made. This was not found to be necessary. We did not anonymise studies prior to assessment. We included a PRISMA study flow diagram to document the screening process (Moher 2009).
Data extraction and management
Two review authors (from NOC, MF, WG) independently extracted data from each included study using a standardised and piloted data extraction form. They resolved discrepancies and disagreements by consensus. In cases where consensus could not be achieved, we planned that a third review author assessed the trial for arbitration, and a majority decision was made. This was not found to be necessary. We extracted the following data from each study included in the review:
country of origin;
study design;
study population (including diagnosis, diagnostic criteria used, symptom duration, age range, gender split, number, details of and reasons for participants excluded during the pre‐randomisation period, if used);
concomitant treatments that may affect outcome: (medication, procedures, etc);
sample size: active and control or comparator groups; loss to follow‐up, including number of people, characteristics, and reasons for withdrawal;
intervention(s) (including type of spinal neuromodulation, device type and manufacturer, details of implantation methods and sites, including details of initial trial period (if any), stimulation parameters, e.g. frequency, intensity, duration, electrode type and position, clinical setting);
type of placebo or comparator intervention;
outcomes (primary and secondary) and time points assessed (only for the comparisons of interest to this review);
declared industry sponsorship, study funding, author conflict of interest statements.
We collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We collected characteristics of the included studies in sufficient detail to populate a table of 'Characteristics of included studies'.
Assessment of risk of bias in included studies
Two review authors (NOC and MF) independently assessed risk of bias for each study using the Cochrane risk of bias 2 (RoB 2) tool, using the criteria outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions, with any disagreements resolved by discussion (Higgins 2020). In cases where consensus could not be achieved, we planned that a third review author would assess the trial for arbitration, and a majority decision made. This was not found to be necessary.
We used the RoB 2 tool (Sterne 2019) to assess the risk of bias around the effect of assignment to the interventions (the intention‐to‐treat effect) for the following results, for DRGS and SCS separately.
Comparisons
Active stimulation versus placebo stimulation
Active stimulation versus usual care or no treatment
Active stimulation plus another intervention vs that intervention alone
Outcomes
Pain intensity (continuous measures)
Pain intensity (dichotomous measures)
Adverse effects
Time points
For pain
Short term
Mid term
Long term
For adverse events
Short term
Medium term
Long term
The RoB 2 tool assesses the risk of bias in individual studies across the following domains:
bias arising from the randomisation process;
bias due to deviations from intended interventions;
bias due to missing outcome data;
bias in the measurement of the outcome; and
bias in the selection of the reported results.
For each domain, we followed the series of signalling questions outlined in the Handbook, and assigned a judgement of low risk of bias, some concerns, or high risk of bias. We planned to use interim guidance from the Cochrane Methods Support Unit to assess risk of bias for cluster‐RCTs but did not find any cluster trials. Since we planned to only take data from the first phase of cross‐over studies (see Unit of analysis issues), we planned to assess them as though they were of parallel design. However, as first phase data were not available for any cross‐over studies we took the decision to analyse them as presented and used the ROB2 tool for cross‐over studies to assess this risk of bias. (https://www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2). This tool adds a supplementary domain "Risk of bias arising from period and carryover effects" with its own set of signalling questions.
When studies used a sham stimulation control as the comparator, as part of the RoB 2 domain 'bias due to deviations from intended interventions', we assessed the credibility of the sham condition, in terms of the likelihood that it was indistinguishable from the active stimulation condition and successfully blinded participants to the treatment condition. We rated the credibility of the sham used in sham‐ or placebo‐controlled studies as follows.
Optimal: high frequency or burst (sub‐perceptual) stimulation is delivered, which would not be expected to induce sensation, and sham stimulation involves implantation of electrodes and the use of a stimulator device that is identical, and appears to be active, but does not induce stimulation. Formal evaluation should indicate that blinding was likely to have been successful.
Suboptimal: stimulation occurs at a frequency that elicits sensation (e.g. paraesthesia), or is compared to a sham condition that is materially distinguishable from the active stimulation condition, or both. Stimulation for which aspects of the intervention other than sensations might compromise blinding, for example, if battery recharging requirements differ substantially between conditions, or sham stimulation for which a formal assessment or explicit report suggests that blinding was likely unsuccessful.
We used these judgements to inform the judgements made on participant blinding. When we made a judgement of suboptimal, we subsequently made a judgement of yes or probably yes for the signalling question 2.1. Were participants aware of their assigned intervention during the trial?
When evaluating the signalling question 2.2. Were carers and people delivering the interventions aware of participants' assigned intervention during the trial?, we considered whether the clinicians implanting the electrodes, involved in the perioperative process, or both, and follow‐up care of the participants were blind to the programmed stimulation parameters.
We reached overall judgements of risk of bias as outlined in Chapter 8 of the Handbook (Higgins 2020):
low risk of bias: the trial is judged to be at low risk of bias for all domains;
some concerns: the trial is judged to raise some concerns for at least one domain, but not to be at high risk of bias for any domain;
high risk of bias: the trial is judged to be at high risk of bias for at least one domain, or the trial is judged to have some concerns for multiple domains, in a way that substantially lowers confidence in the results.
We used the 'RoB Excel' tool and word templates (available at riskofbias.info) to record and manage RoB 2 assessments and processes, and we made available the full data related to this process on Figshare (DOI: 10.17633/rd.brunel.14838678).
Measures of treatment effect
When data were available, we presented outcomes in a dichotomised format. For dichotomised data (responder analyses), we considered analyses based on a 30% or greater reduction in pain intensity to represent a moderately important benefit, and a 50% or greater reduction in pain intensity to represent a substantially important benefit, as suggested by the IMMPACT guidelines (Dworkin 2008). When possible, we calculated risk ratio (RR) and risk difference (RD) with 95% confidence intervals (CIs) for dichotomised outcome measures. For device/procedure‐related adverse events, we prioritised the risk difference as there were zero events in the control arm. We calculated the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) as an absolute measure of treatment effect.
We planned to express the size of the treatment effect for pain intensity, measured with a Visual Analogue Scale (VAS) or = Numerical Rating Scale (NRS), using the mean difference (MD) when all studies utilised the same measurement scale. As all studies measured pain intensity on a 0 to 10 or 0 to 100 VAS or NRS, we normalised all scales to a 0 to 100 scale and expressed the effect size as the mean difference to aid interpretability. We planned to use the standardised mean difference (SMD) when studies used substantially different scales. When we pooled data from different scales for which the direction of interpretation varies, we normalised the direction of the scales to a common direction.
The OMERACT 12 group has developed recommendations for establishing the minimal clinically important difference for pain outcomes (Busse 2015). They recommend 10 mm on a 0 to 100 mm VAS as the threshold for minimal clinical importance for the mean between‐group difference. They advise this should be interpreted with caution, as it remains possible that estimates that fall just below this point may still reflect a treatment that benefits an appreciable number of people. We used this threshold, but interpret it in light of the certainty of the included evidence.
Unit of analysis issues
For studies with more than two eligible comparisons in a single meta‐analysis, we divided the number of participants in each arm by the number of comparisons included from that study to avoid double‐counting. For cluster‐RCTs, we planned to seek direct estimates of the effect from an analysis that accounted for the cluster design. When the analysis in a cluster trial did not account for the cluster design, we planned to use the approximately correct analysis approach, presented in the Handbook (Higgins 2020). For cross‐over studies, we planned to only include data from the first phase of the study, when they were available. This is because it is theorised that spinal cord stimulation may elicit lasting effects beyond the period of stimulation, raising a high risk of carry‐over effects (Duarte 2020). As first‐phase, or phase‐by‐phase data were not available for any of the included cross‐over studies we took the decision to analyse these studies as presented. As we did not have access to individual patient data from any of these studies, we were unable to adjust for the paired nature of the data from these trials as recommended in the Cochrane Handbook (Higgins 2021). As such, while point estimates in this analysis should be accurate representations of the data it is possible that these analyses may be conservative, in terms of overestimating imprecision.
Dealing with missing data
When there were insufficient data presented in the study report to enter into a meta‐analysis, we requested the missing data from the study authors. We preferentially calculated effect sizes derived from intention‐to‐treat analyses. We had planned to exclude studies rated at high risk of bias from the primary meta‐analyses, including those at risk of bias due to missing outcome data. However, all included studies were rated at high risk of bias on one or more domain of the ROB2 tool.
Assessment of heterogeneity
We attempted to deal with clinical heterogeneity by combining studies that examined similar interventions. We did not combine studies that compared spinal neuromodulation techniques to usual care with studies that compared spinal neuromodulation techniques to sham within the same analysis. We assessed heterogeneity using the Chi² test to investigate the statistical significance of such heterogeneity, and the I² statistic to estimate the amount of heterogeneity. When significant heterogeneity (I² ≥ 50%, P < 0.10) was present, we explored subgroup analyses, described in the section Subgroup analysis and investigation of heterogeneity.
Assessment of reporting biases
We considered the possible influence of small‐study biases on review findings. When possible, for studies that reported dichotomised outcomes, we tested for the possible influence of publication bias on each outcome, by estimating the number of participants in studies with no effect required to change the NNTB to an unacceptably high level (defined as an NNTB of 10), as outlined in Moore 2008. When continuous outcomes were reported, we planned to use funnel plots to visually explore small‐study biases when there were at least 10 studies in a meta‐analysis, and the included studies differed substantially in size. However, no analysis contained this number of unique studies.
We identified the number of registered trials that have not been published or had results made available for this review, and report the number of participants that this represents. When results data were available in the trials registers, but there was no study report (published or made available by study authors upon request), we planned to exclude those data in the primary analyses, but conduct sensitivity analyses to evaluate how the inclusion of these data impacted our results and conclusions.
Data synthesis
We pooled the results of included studies using Review Manager 5 (Review Manager 2014). We planned to conduct separate meta‐analyses for SCS and DRGS interventions using a random‐effects model, as there are likely to be a number of sources of clinical heterogeneity between the included studies. However, we identified no studies of DRGS that met our inclusion criteria. We performed analyses at the following follow‐up points:
short term
medium term
long term
In the primary analysis, we pooled data from studies regardless of the specific diagnosis, stimulation site, or parameters. When inadequate data were found to support statistical pooling, we conducted narrative synthesis of the evidence, based upon the same key comparisons.
Subgroup analysis and investigation of heterogeneity
When there was significant heterogeneity (I² ≥ 50%, P < 0.10), we explored subgroup analyses by clinical population, stimulation site, and stimulation parameters.
We planned to explore the effect of clinical condition, using the following distinct subgroups:
neuropathic pain: studies that exclusively include participants with confirmed pain of neuropathic origin;
non‐neuropathic pain: studies that exclusively include participants with pain of non‐neuropathic origin (including conditions that are not clearly neuropathic in origin);
mixed populations: studies that include participants with both neuropathic and non‐neuropathic pain (including conditions that are not clearly neuropathic in origin).
We planned to explore the effect of stimulation parameters, using the following distinct subgroups:
conventional stimulation: the delivery of a tonic pulse, at a constant stimulation frequency between 40 Hz and 80 Hz, with a fixed pulse width;
high‐frequency stimulation: the delivery of a tonic pulse, at a frequency between 1 kHz and 10 kHz, with a fixed pulse width;
burst stimulation: the delivery of intermittent trains of stimulation.
To explore whether there is a difference in mean effects between subgroups, we used the test for subgroup differences (Deeks 2020). We were only able to do this for one comparison (Analysis 1.1) due to a lack of adequate data.
1.1. Analysis.

Comparison 1: SCS vs sham, Outcome 1: Pain Intensity: average post‐intervention. Short‐term follow up. Mean difference
Sensitivity analysis
When sufficient data were available, we planned to conduct the following sensitivity analyses.
Choice of meta‐analysis model: random‐effects models can produce inflated effect sizes in circumstances when many of the included studies are small. We explored this by repeating our analyses using a fixed‐effect model.
Risk of bias: based on the overall ROB 2 judgement for studies, we planned to explore the impact of risk of bias for the primary analyses, by repeating the analyses and excluding studies rated at high risk of bias. As all studies were rated at high risk of bias for one or more domains we were not able to conduct this analysis.
When results data are available in the trials registers, but there was no study report (published or made available by study authors upon request), we planned not include those data in the primary analyses, but to conduct sensitivity analyses to evaluate how the inclusion of these data impacts our results and conclusions. As we did not find any studies with data reported in the trial registry with no published study report we were unable to conduct this analysis.
Incorporating economic evidence
We developed a brief economic commentary based on current methods guidelines, to summarise the availability and principal findings of formal cost‐effectiveness analyses that were conducted as part of the identified RCTs (Shemilt 2019). This included reviewing the methods used, reporting data on costs per quality‐adjusted life‐year (QALY) for each treatment group, and reporting the incremental cost‐effectiveness ratio (ICER) for our comparisons of interest, when reported. We did not develop a new health economic model as part of this review.
Summary of findings and assessment of the certainty of the evidence
Two review authors (NOC, MF) independently used the GRADE system to rate the level of certainty of the evidence (Schünemann 2020).
The GRADE approach uses five considerations (limitations of studies, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for each outcome, and uses the following criteria to describe the confidence in the evidence:
high: we are very confident that the true effect lies close to that of the estimate of the effect;
moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different;
low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect;
very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We decreased the grade rating by one (‐ 1), two (‐ 2), or three (‐ 3) levels, up to a maximum of ‐ 3, (or very low) for any criteria, based on the level of concern it raises.
Summary of findings table
We planned to include Summary of findings tables to present the findings for the following comparisons:
spinal cord stimulation versus placebo stimulation;
spinal cord stimulation versus usual care or no treatment;
dorsal root ganglion stimulation versus placebo stimulation;
dorsal root ganglion stimulation versus usual care or no treatment.
We included key information concerning the sum of available data on the following outcomes at short‐, medium‐and long‐term follow‐up, the magnitude of effect of the interventions examined, and the certainty of the evidence.
Pain intensity (continuous measures)
Pain intensity (dichotomous measures)
Adverse events
For continuous outcomes, we presented the mean difference; for dichotomous outcomes, we presented the risk ratio, risk difference, and the NNTB or NNTH (where the 95% confidence intervals did not include' no effect'), with 95% confidence intervals.
Results
Description of studies
Results of the search
For a full description of our screening process, see the study flow diagram (Figure 1). For a summary of the search results for this review see Appendix 2 and Appendix 3.
1.
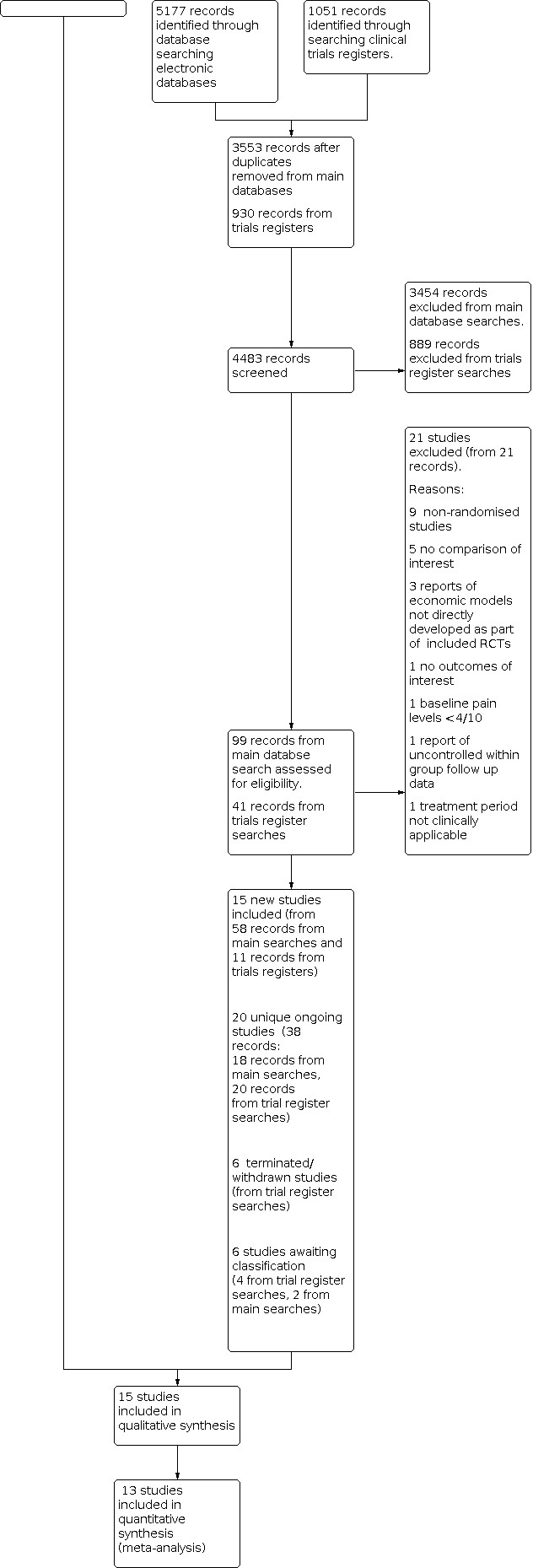
The main database searches were conducted in October 2020 and updated in September 2021 (see Electronic searches) and retrieved 3553 records after de‐duplication. We excluded 3454 of those at the title and abstract screening stage. After full‐text screening of the remaining 99 records we excluded 21 records of 21 studies (see Characteristics of excluded studies and Excluded studies for details). Two studies (Miller 2015; Miller 2016) were awaiting classification as were only available as conference abstracts. This left 58 records describing 15 unique studies and 18 records of ongoing studies.
We searched the trials registries in October 2020 and September 2021. Due to decreased functionality of the WHO registry related to the COVID pandemic we conducted a simplified search strategy of that registry. After deduplication our searches identified 930 records of which 889 were excluded on screening. This left 41 records, 11 of which were of published studies already identified and included, 20 were of ongoing studies, six were of studies identified as being terminated or withdrawn, and four studies were awaiting classification.
The final review includes 15 unique published studies that randomised 908 participants in total, and 20 unique ongoing studies.
Included studies
Country of origin and number of sites
Studies were conducted in the UK (Al‐Kaisy 2018; Eldabe 2021), Belgium (De Ridder 2013), the Netherlands (Kemler 2000; Kriek 2017; Slangen 2014; Tjepkema‐Cloostermans 2016), Germany (Schu 2014), Poland (Sokal 2020), Sweden (Lind 2015), and the USA (SENZA‐PDN). There were four international studies. de Vos 2014 was conducted in centres in the Netherlands, Denmark, Belgium and Germany, Perruchoud 2013 was conducted in Switzerland and the UK, PROCESS in Australia, Belgium, Canada, Italy, Israel, Spain and the UK and PROMISE in Belgium, Canada, Colombia, France, Germany, the Netherlands, Spain, the UK and the USA.
Six studies (Al‐Kaisy 2018; De Ridder 2013; Kemler 2000; Schu 2014; Sokal 2020; Tjepkema‐Cloostermans 2016) were conducted in a single centre. The number of centres in the remaining studies ranged from 2 (Eldabe 2021; Perruchoud 2013; Slangen 2014) to 28 (PROMISE).
Study funding and author declarations of interest
Of the 15 included trials 11 declared some form of industry funding (Al‐Kaisy 2018; de Vos 2014; Eldabe 2021; Kriek 2017; Lind 2015; Perruchoud 2013; PROCESS; PROMISE; Schu 2014; SENZA‐PDN; Slangen 2014). One study was funded by the Dutch Health Insurance council (Kemler 2000), one by the host hospital (Tjepkema‐Cloostermans 2016), one declared no external funding (Sokal 2020), and one provided no information (De Ridder 2013).
Ten studies (Al‐Kaisy 2018; de Vos 2014; Eldabe 2021; Kriek 2017; Perruchoud 2013; PROCESS; PROMISE; Schu 2014; SENZA‐PDN; Slangen 2014) reported one or more authors with relationships with industry involved in SNMD technology. These took the form of non‐financial support, research funding, consultancies, stock options, honoraria, speakers fees and travel grants. De Ridder 2013 declared that the first author had obtained a patent for burst stimulation, which was being evaluated in that study. Lind 2015 reported that authors had no financial interest to declare and Kemler 2000 and Tjepkema‐Cloostermans 2016 reported no information relating to author conflicts of interest.
Study designs
We included nine cross‐over studies (Al‐Kaisy 2018; De Ridder 2013; Eldabe 2021; Kriek 2017; Lind 2015; Perruchoud 2013; Schu 2014; Sokal 2020; Tjepkema‐Cloostermans 2016) with a total of 224 participants randomised. Study size ranged from 10 to 41 participants. Eight of these studies (Al‐Kaisy 2018; De Ridder 2013; Eldabe 2021; Kriek 2017; Perruchoud 2013; Schu 2014; Sokal 2020; Tjepkema‐Cloostermans 2016) compared various types of SCS (high‐frequency, burst, conventional stimulation) to a placebo stimulation condition and one study (Lind 2015) compared conventional stimulation with the stimulator switched off. Seven cross‐over studies (Al‐Kaisy 2018; De Ridder 2013; Lind 2015; Perruchoud 2013; Schu 2014; Sokal 2020; Tjepkema‐Cloostermans 2016) reported no washout period between stimulation conditions, while Kriek 2017 reported a two‐day washout period and Eldabe 2021 reported a nine‐day washout period.
We included six parallel studies (de Vos 2014; Kemler 2000; PROCESS; PROMISE; SENZA‐PDN; Slangen 2014) with a total of 684 participants randomised. Study size ranged from 36 to 218 participants. Of these, five studies compared conventional SCS in addition to other forms of management with the other management alone, and one study (SENZA‐PDN) compared high‐frequency SCS + conventional medical management with conventional medical management alone.
Participants
All studies included both male and female participants. Across all trials 50% of participants were female. Across studies that reported the range, the age of participants ranged from 25 to 74 years. In studies that reported the mean or median age of participants, the age ranged from 37 to 61 years.
Studies included participants with a range of painful conditions. Four studies (Al‐Kaisy 2018; PROCESS; PROMISE; Schu 2014) included participants with failed back surgery syndrome (FBSS), and one study included participants with chronic low back pain with or without leg pain (Eldabe 2021). Of these one study (PROCESS) mandated that leg pain was dominant over back pain in their inclusion criteria and two studies (Al‐Kaisy 2018, PROMISE) required participants' back pain intensity to be greater than their leg pain intensity. Three studies (de Vos 2014; SENZA‐PDN; Slangen 2014) included participants with painful diabetic neuropathy, and two studies (Kemler 2000; Kriek 2017) included participants with complex regional pain syndrome(CRPS) (though Kemler 2000 used the older diagnostic label "reflex sympathetic dystrophy"). Three studies included participants with various diagnoses. Of these De Ridder 2013 included participants with FBSS, failed neck surgery syndrome (FNSS), myelopathy and myelomalacia, Sokal 2020 included participants with CRPS and FBSS and Tjepkema‐Cloostermans 2016 included people with FBSS, peripheral neuropathy, diabetic neuropathic pain, multiple sclerosis (MS), and CRPS. One study (Lind 2015) included participants with irritable bowel syndrome.
Four studies included participants on the basis of a diagnosis of neuropathic pain. These included the three studies in painful diabetic neuropathy (de Vos 2014; SENZA‐PDN; Slangen 2014) and two studies in FBSS (PROCESS; Schu 2014) which specified that participants suffered from neuropathic pain of radicular origin. In the PROCESS study, the neuropathic nature was checked clinically by investigation of the pain distribution, examination of sensory, motor and reflex changes with supporting clinical tests such as electromyography (EMG). One study (Sokal 2020), included a mixed population of participants (CRPS, FBSS with predominant leg pain) with pain of neuropathic and non‐neuropathic origin, although did not describe how that distinction was made. One study in FBSS (PROMISE) reported that pain seemed to be neuropathic in nature in 84.4% of cases as indicated by the Neuropathic Pain questionnaire, Douleur Neuropathique 4 [DN4]. Two studies alluded to the neuropathic nature of the presenting pain in their reports but included conditions that are not necessarily neuropathic in nature such as CRPS, FBSS and FNSS (De Ridder 2013; Kriek 2017). These studies did not report any criteria or process for confirming a neuropathic mechanism. Three studies did not report or discuss the possible neuropathic nature of participants' pain (Al‐Kaisy 2018; Kemler 2000; Perruchoud 2013).
The majority of studies (Al‐Kaisy 2018; De Ridder 2013; de Vos 2014; Kemler 2000; Kriek 2017; Lind 2015; PROMISE; SENZA‐PDN; Slangen 2014; Sokal 2020) stated in their inclusion criteria that participant's pain must be refractory to previous treatments, though the details of those treatments varied. Al‐Kaisy 2018; de Vos 2014; Kemler 2000; PROCESS; PROMISE; SENZA‐PDN; Slangen 2014 all included a minimum level of pain at baseline of at least the equivalent of between 4/10 and 6/10 on a visual analogue scale (VAS) in their inclusion criteria. Four studies included people already implanted with, and receiving SCS at the point of recruitment (Eldabe 2021;nPerruchoud 2013; Schu 2014; Tjepkema‐Cloostermans 2016).
Across studies that reported baseline average pain levels for the pain that was the target of the intervention, these ranged from the equivalent of 4/10 to 8.2/10. Only three studies reported average baseline pain levels below 6/10 (Eldabe 2021; Perruchoud 2013; Schu 2014). In these studies, participants were implanted with a spinal cord stimulator and were receiving conventional stimulation prior to enrolment in the study. Of those studies that reported the average duration of participants' painful symptoms (Al‐Kaisy 2018; de Vos 2014; Kemler 2000; Kriek 2017; PROCESS; PROMISE; SENZA‐PDN; Slangen 2014; Sokal 2020) values ranged from 3 to 8.3 years, indicating longstanding pain.
Interventions and comparisons
All the included trials investigated SCS. We found no eligible completed trials of dorsal root ganglion stimulation(DRGs).
Of the cross‐over studies that compared different simulation parameters with placebo stimulation, five studies (De Ridder 2013; Kriek 2017; Lind 2015; Sokal 2020; Tjepkema‐Cloostermans 2016) compared conventional stimulation with sham, four studies (Al‐Kaisy 2018, Kriek 2017, Perruchoud 2013, Sokal 2020) included a high‐frequency (HF) condition, allowing for a comparison of HF versus sham. Four studies (De Ridder 2013; Eldabe 2021; Kriek 2017; Schu 2014; Tjepkema‐Cloostermans 2016) included a burst stimulation condition, allowing for a comparison of burst stimulation versus sham. Three studies (Eldabe 2021; Kriek 2017; Schu 2014) included a stimulation condition (500 Hz) that did not fit into either our predefined categories for conventional or HF stimulation. Data from that condition were not included in this review. In these studies, the intervention period (first phase) for each stimulation condition ranged from one to six weeks per condition. All of these studies provided outcome data for short‐term follow‐up only.
Of the five parallel studies that compared conventional SCS in addition to other forms of management with the other management alone, four studies compared the addition of SCS with medical management labelled as "conventional medical therapy" (de Vos 2014), "best medical treatment (BMT)" (Slangen 2014), "optimal medical treatment (OMT)" (PROMISE) and "conventional medical management (CMM)" (PROCESS; SENZA‐PDN) with medical management alone. In these studies, the duration of the spinal neuromodulation device (SNMD) intervention mirrored the maximum length of follow‐up, notwithstanding treatment discontinuations; as once implanted these interventions are intended to be used long term. The length of the follow‐up period in these studies ranged from six months to five years.
The details of medical management were reported as follows. In the study by de Vos 2014, medication adjustments and other conventional pain treatments, such as physical therapy, were allowed at any time if required. The PROMISE study reported that OMM was individualised for each patient and optimised at each visit and could include a range of treatments including acupuncture, psychological/behavioural therapies, physiotherapy as well as invasive treatments such as spinal injection, nerve blocks, epidural adhesiolysis and neurotomies. The PROCESS study similarly reported that CMM could include a range of physical, psychological and drug treatments but not spinal surgery or intrathecal drug delivery. Drug therapies included opioids, non‐steroidal anti‐inflammatory drugs (NSAIDs), antidepressants, anticonvulsants/antiepileptics and other analgesic therapies (not defined). SENZA‐PDN reported that CMM may include a variety of non‐invasive or minimally‐invasive treatments that comprise the standard of care for neuropathic limb pain, including, but are not limited to, pharmacological agents, physical therapy, cognitive therapy, chiropractic care, nerve blocks, and other non‐invasive or minimally invasive therapies. Slangen 2014 reported that BMT was based on international guidelines for the treatment of peripheral neuropathic pain but did not offer further detail. In the registry record for that study, the comparator was labelled "treatment as usual".
One study (Kemler 2000) compared SCS in addition to physical therapy versus physical therapy alone. Physical therapy was administered for 30 minutes twice weekly and lasted for six months and consisted of a standardised programme of graded exercises designed to improve strength, mobility and function of the affected hand or foot.
Study blinding
All of the parallel studies (de Vos 2014; Kemler 2000; PROCESS; PROMISE; SENZA‐PDN; Slangen 2014) evaluated open‐label comparisons of SCS + another therapy versus the other therapy alone. As such, neither participants nor clinicians/providers were blinded to the interventions allocated.
Eight of the cross‐over studies (Al‐Kaisy 2018; De Ridder 2013; Eldabe 2021; Kriek 2017; Lind 2015; Perruchoud 2013; Schu 2014; Sokal 2020) employed a sham control during which no stimulation was delivered from the stimulator. Tjepkema‐Cloostermans 2016 employed a low‐amplitude burst paradigm where standard stimulation with 0.1 mA bursts was used as this was expected to be subtherapeutic. This was labelled as a sham in the trial registry record, however, in the final published report was labelled as an active stimulation condition "low amplitude burst stimulation." In their discussion, Tjepkema‐Cloostermans 2016 stated that the difference in amplitude between low (0.1 mA for every participant) and high amplitude (individually adjusted) burst stimulation was less than expected and only minimal in some participants. For this reason, they considered that low amplitude burst was most likely not subtherapeutic in all participants and it is more appropriate to call this form of stimulation low amplitude burst stimulation instead of sham. We have included this study as the sham condition was intended as a sham a priori and an alternative explanation for the lack of contrast in stimulation amplitude may be a lack of efficacy.
For comparisons between conventional stimulation and sham (included by De Ridder 2013; Kriek 2017; Lind 2015; Sokal 2020), we rated blinding as suboptimal since conventional stimulation is associated with paraesthesia sensations and is thus distinguishable from sham. Perruchoud 2013 compared HF stimulation to sham, reported equivalent battery recharging requirements and a formal assessment of blinding was not indicative of problems and so blinding was rated as optimal for this study/comparison. For the comparisons of HF or Burst stimulation to sham, included by Al‐Kaisy 2018; De Ridder 2013; Eldabe 2021; Kriek 2017; Lind 2015; Schu 2014; Sokal 2020; Tjepkema‐Cloostermans 2016, we rated blinding as suboptimal. While these stimulation frequencies are generally sub‐perceptual, no formal evaluation of the success of blinding was reported.
Pre‐implantation trial periods
It is common clinical practice for participants to undergo a trial period of stimulation, in which electrodes are implanted and attached to an external stimulator. If a successful clinical response to trial stimulation is considered to have been established then participants usually proceed to full implantation of the final stimulator device. The timing and details of this trial period with regards to its relationship to the time of recruitment and randomisation varied across studies.
Three cross‐over studies (Al‐Kaisy 2018; Kriek 2017; Sokal 2020) included a trial period of conventional stimulation of between one and two weeks prior to full implantation which was completed after recruitment and prior to randomisation. Those considered to achieve a successful clinical response were subsequently implanted with a permanent stimulator. Four cross‐over studies (Eldabe 2021; Perruchoud 2013; Schu 2014; Tjepkema‐Cloostermans 2016) recruited participants who had been previously implanted were currently being treated with conventional SCS. One cross‐over study (De Ridder 2013) was conducted within the pre‐implantation trial period (that is, participants were randomised to different stimulation parameters during this period before the stimulation device had been permanently implanted), and one study did not report a pre‐implantation trial period (Lind 2015). As a result, it is important to note that in all cross‐over studies except De Ridder 2013 and Lind 2015, participants were pre‐selected on the basis of a positive response to a trial period of conventional stimulation.
All six parallel studies (de Vos 2014; Kemler 2000; PROCESS; PROMISE; SENZA‐PDN; Slangen 2014) included a pre‐implantation trial period in the SCS arm as part of the study intervention. In these studies the pre‐implantation trial period occurred after randomisation had occurred.
Pre‐implantation trial periods ranged from 5 to 28 days in those studies that reported the duration. Not all studies reported criteria for determining the success of these trial periods. Of those that did an average reduction on pain of ≥ 50% was commonly used (Al‐Kaisy 2018; Kemler 2000; Kriek 2017; PROCESS; SENZA‐PDN; Slangen 2014; Sokal 2020). One study (PROCESS) additionally required at least 80% overlap of participant's pain with stimulation‐induced paraesthesia, and one study (Slangen 2014) also considered a score of six or higher on the participant global impression of change (PGIC) scale for pain or sleep as a successful trial. One trial (PROMISE) required "adequate LBP relief with usual activity and appropriate analgesia in the context of post‐operative pain...as assessed by the investigator".
In the parallel design trials (de Vos 2014; Kemler 2000; PROCESS; PROMISE; SENZA‐PDN; Slangen 2014), the proportion of participants randomised to SCS who received trial stimulation ranged from 92% to 100% (median 100%). The proportion of participants who received trial stimulation and were judged to have had a successful trial ranged from 67% to 94% (median 85.5%). The proportion of participants who received a trial period of stimulation and who were subsequently permanently implanted ranged from 67% to 93% (median 83.5%). As a proportion of the total number of participants randomised to SCS between 67% and 93% were received a permanent SCS implant (median 80.7%).
Primary outcomes
None of the cross‐over studies reported data for pain for each phase of the cross‐over study. Therefore, separate first‐phase data were not available in the study reports. For all studies we contacted the authors to request some data that could not be found in the published reports. The authors of three studies (de Vos 2014; Kemler 2000; SENZA‐PDN) provided data upon request.
Pain intensity
All the included studies included pain intensity as an outcome. Pain intensity was measured using 0 to 10 or 0 to100 NRS or VAS in all studies. There was variation across studies in the reported anchors of the scales and in the level of detail provided regarding the precise question asked of participants. Five studies (de Vos 2014; PROCESS; PROMISE; SENZA‐PDN; Slangen 2014) reported the numbers of people who achieved ≥ 50% pain relief and presented responder analyses based on that outcome. Thirteen studies (Al‐Kaisy 2018; De Ridder 2013; de Vos 2014; Eldabe 2021; Kemler 2000; Kriek 2017; Perruchoud 2013; PROCESS; PROMISE; Schu 2014; SENZA‐PDN; Sokal 2020; Tjepkema‐Cloostermans 2016) reported the post‐intervention mean pain score, the average pain score over a period of two to five days at the end of an intervention period, or the average (mean) change from baseline in the pain score. One study (Lind 2015) did not report pain scores in a numeric form in their results.
Adverse events
Adverse events (AEs) were reported in 14 studies (Al‐Kaisy 2018; de Vos 2014; Eldabe 2021; Kemler 2000; Kriek 2017; Lind 2015; Perruchoud 2013; PROCESS; PROMISE; Schu 2014; SENZA‐PDN; Slangen 2014; Sokal 2020; Tjepkema‐Cloostermans 2016). While the level of detail regarding the methods used for monitoring and reporting adverse events varied, for most studies limited information on methods was reported. In five studies (Lind 2015; Perruchoud 2013; Slangen 2014; Sokal 2020; Tjepkema‐Cloostermans 2016), no methods were described for measuring, classifying or reporting AEs. In two studies (Schu 2014; Slangen 2014), only serious adverse events (SAEs)were reported, though the definition used for classifying SAEs was not described. For studies with medium‐ to long‐term follow‐up, AEs were generally reported for the full follow‐up period rather than at each follow‐up time point. In one study (De Ridder 2013), the report did not provide any details for measuring AEs and did not report any data on AEs. In one study (SENZA‐PDN), only SCS‐related adverse events were reported.
Secondary outcomes
Five studies (Kriek 2017; PROCESS; PROMISE; Schu 2014; Sokal 2020) reported measuring disability as an outcome. Of these, four studies used the Oswestry Disability Index (ODI) and one study in CRPS used the Disabilities of Arm Shoulder and Hand Questionnaire (DASH) when the upper extremity was affected, and the Walking Ability Questionnaire when the lower extremity was affected.
Eleven studies (de Vos 2014; Eldabe 2021; Lind 2015; Kemler 2000; Kriek 2017; Perruchoud 2013; PROCESS; PROMISE; SENZA‐PDN; Slangen 2014; Tjepkema‐Cloostermans 2016) reported measuring health‐related quality of life (HRQoL). The EuroQoL 5D (EQ‐5D) was used in eight studies (de Vos 2014; Eldabe 2021; Kemler 2000; Perruchoud 2013; PROCESS; PROMISE; SENZA‐PDN; Slangen 2014), theShort Form 36 (SF‐36) was used in four studies (Kriek 2017; PROCESS; PROMISE; Slangen 2014), and the Nottingham Health profile was also used in one study (Kemler 2000). Lind 2015 and Tjepkema‐Cloostermans 2016 used a 0‐10 VAS to measure HRQoL without reporting the anchors of the scale or the specific question. We did not consider this a valid form of measurement and did not include these data in any analyses. Tjepkema‐Cloostermans 2016 also measured the McGill Pain Questionnaire QoL scale.
Six studies (de Vos 2014; Kriek 2017; Perruchoud 2013; PROCESS; Slangen 2014; Sokal 2020) reported medication use as an outcome. Kriek 2017; PROCESS; Slangen 2014 and Sokal 2020 reported measuring all medication consumption in the trial period. de Vos 2014 used the Medication Quantification Scale III (MQS). Perruchoud 2013 did not report the methods used to measure this outcome. SENZA‐PDN reported measuring medication use in the published protocol but did not report these data.
Two studies (PROCESS; Slangen 2014) reported data on economic aspects of the intervention. The PROCESS study performed an economic evaluation with cost‐effectiveness analysis with long‐term modelling. Slangen 2014 performed an economic evaluation with a 12‐month time horizon. Kriek 2017 reported a cost analysis of SCS in the study protocol but these results were not reported in the published paper.
Ongoing studies
We have identified 20 unique ongoing studies that may be included in future updates of this review. In terms of our comparison "SCS versus placebo" these include eight more cross‐over studies (ACTRN12620000720910; Burst SCS; NCT03546738; NCT03733886; NCT04039633; NCT04894734; PANACEA; PET‐SCS) with a median N of 22 participants(range 10 to 60, total N 246), all of which compare Burst SCS with sham. There are also two parallel design studies, one of which is of high‐frequency SCS (MODULATE‐ LBP, N = 96) and the other is unclear (CITRIP N = 54).
For our comparison SCS + other intervention versus other intervention alone, we have identified six ongoing studies (ChiCTR‐IOR‐17012289; DISTINCT; ISRCTN10663814; NCT04676022; SCS‐PHYSIO; SENZA‐NSRBP) with a median N of 200 participants (range 30 to 300, total N 1100), three of which are of conventional SCS, two of high‐frequency SCS and one of Burst SCS. Three ongoing studies are investigating DRGs, one of which is a cross‐over study (DRKS00022557, N = 50) and two use a parallel design of which one (TSUNAMI DRG, N = 38) is testing high‐frequency DRGs and one (PENTAGONS, N = 56) is unclear. Nine out of 20 of these ongoing studies are reported as industry‐sponsored.
Excluded studies
We excluded 21 studies at the full‐text screening stage. Nine were not RCTs (Alo 2016; Dones 2008; Liem 2013; Marchand 1991; Rigoard 2013; Sagher 2008; Steinbach 2017; Tesfaye 1995; Winfree 2005), five did not include a comparison of interest to this review (Falowski 2019; Kufakwaro 2012; Liu 2020; Gilligan 2020; Liu 2021), three were reports of economic models that were not directly developed as part of the included RCTs (Annemans 2014; Kemler 2010; Taylor 2005), one presented no outcomes of interest (Kemler 2000), one was a report of uncontrolled within‐group follow‐up data (van Beek 2015), and one included participants with average baseline pain levels of < 4/10 on a 0 to 10 Numerical Rating Scale (NRS) (Wolter 2012). In one study (Meier 2015), the clinical stimulation periods were only 12 hours in duration and not considered clinically applicable.
Risk of bias in included studies
A summary of the risks of bias of studies included in each analysis can be found in forest plots of each outcome and in the risk of bias tables (Table 49; Table 50; Table 51; Table 52; Table 53; Table 54; Table 57; Table 55; Table 56; Table 58; Table 59; Table 60; Table 61). Risk of bias assessments for each outcome, including all domain judgements and support for judgements, are located in the risk of bias section (located after the Characteristics of included studies). Additional details on how we applied the Risk of Bias‐2 tool for each trial for each outcome can be found in the supplemental data file available in Figshare (DOI: 10.17633/rd.brunel.14838678).
Risk of bias for analysis 2.1 Pain intensity: average post‐intervention. Short‐term follow‐up. Mean Difference.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| de Vos 2014 | Low risk of bias | Randomisation block stratified by centre. Allocation concealed. No apparent baseline imbalance. | Some concerns | Open‐label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. | Low risk of bias | Low levels of missing data and balanced between groups (7.5% SCS group, 10% CMM group). Authors shared complete dataset which indicates no meaningful bias. | High risk of bias | Open label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Low risk of bias | While no protocol or full SAP is available sharing of the full dataset by the study authors indicates no evidence of selectivity. | High risk of bias | High risk of bias based on bias in the measurement of the outcome. |
| Kemler 2000 | Low risk of bias | Computer generated randomisation. Allocation concealed. Minor imbalance of gender (61%F in SCS group and 83%F in PT group) but compatible with chance. | Some concerns | Open label study without blinding of participants and personnel and no information provided to assess deviations in PT management across groups. | Low risk of bias | Only 1 participant in PT only group lost to follow up at this time‐point | High risk of bias | Open‐label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Some concerns | No trial registry record, SAP or protocol available. | High risk of bias | High risk of bias based on bias in the measurement of the outcome. |
| SENZA‐PDN | Low risk of bias | Computer generated randomisation. Allocation concealed. No apparent baseline imbalance. | Some concerns | Open‐label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. An ITT approach is reported, though there are a number of post‐randomisation exclusions. | High risk of bias | Post randomisation exclusions took place prior to pre‐implantation trial period and were omitted from the analysis. In total at 1 month there are 16% of randomised participants excluded from the SCS group and 9% from the CMM group. It is possible that exclusions may be related to the value of the outcome. | High risk of bias | Open label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Some concerns | While an SAP is available it is unclear whether it was finalised and unchanged ahead of the end of data collection. While authors shared data on request, they remained post‐randomisation exclusions and the N of participants analysed do not clearly match those in the CONSORT flow chart. | High risk of bias | High risk of bias on mutliple domains |
Risk of bias for analysis 2.2 Pain: participants with ≥50% pain relief. Short‐term follow‐up. Risk Ratio.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| de Vos 2014 | Low risk of bias | Randomisation block stratified by centre. Allocation concealed. No apparent baseline imbalance. | Some concerns | Open label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. | Low risk of bias | Low levels of missing data (7.5% in SCS group and 0 in CMM group). Missing data treated as non‐responders. Authors shared complete dataset which indicates no meaningful bias. | High risk of bias | Open‐label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Low risk of bias | While no protocol or full SAP is available sharing of the full dataset by the study authors indicates no evidence of selectivity. | High risk of bias | High risk of bias based on bias in the measurement of the outcome. |
| SENZA‐PDN | Low risk of bias | Computer generated randomisation. Allocation concealed. No apparent baseline imbalance. | Some concerns | Open‐label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. An ITT approach is reported, though there are a number of post‐randomisation exclusions. | High risk of bias | Post randomisation exclusions took place prior to pre‐implantation trial period and were omitted from the analysis. In total at 1 month there are 16% of randomised participants excluded from the SCS group and 9% from the CMM group. While sensitivity analyses are presented they don't account for all exclusions and while statistical significance would likely be unchanged the effect size is likely to be affected.Missingness reasons could reflect intervention tolerability or treatment outcomes. | High risk of bias | Open label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Some concerns | While an SAP is available it is unclear whether it was finalised and unchanged ahead of the end of data collection. While authors shared data on request, they remained post‐randomisation exclusions and the N of participants analysed do not clearly match those in the CONSORT flow chart. | High risk of bias | High risk of bias on multiple domains |
Risk of bias for analysis 2.3 Pain intensity: average post‐intervention. Medium‐term follow‐up. Mean difference.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| de Vos 2014 | Low risk of bias | Randomisation block stratified by centre. Allocation concealed. No apparent baseline imbalance. | Some concerns | Open label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. | Low risk of bias | Low and balanced levels of missing data (10% in each group). Authors shared complete dataset which indicates no meaningful bias. | High risk of bias | Open‐label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Low risk of bias | While no protocol or full SAP is available sharing of the full dataset by the study authors indicates no evidence of selectivity. | High risk of bias | High risk of bias based on bias in the measurement of the outcome. |
| Kemler 2000 | Low risk of bias | Computer generated randomisation. Allocation concealed. Minor imbalance of gender (61%F in SCS group and 83%F in PT group) but compatible with chance. | Some concerns | Open label study without blinding of participants and personnel and no information provided to assess possible deviations in the PT management across groups. | Low risk of bias | Very low levels of missing data (1 participant) at this time‐point. | High risk of bias | Open label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Some concerns | No registry record, protocol or SAP are available | High risk of bias | High risk of bias based on bias in the measurement of the outcome. |
| PROCESS | Low risk of bias | Computer generated randomisation with concealed allocation. Back pain higher in SCS group (SCS 54.5 (24.3: CMM 44.8 (23.2). Higher history of litigation in CMM group (17% VS 10% IN SCS) but likely compatible with chance. | Some concerns | Open label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. | Low risk of bias | Low levels of missing data at this time‐point (SCS 4%, CMM 8%). | High risk of bias | Open label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Low risk of bias | Reporting and analysis consistent with published protocol. | High risk of bias | High risk of bias based on bias in the measurement of the outcome. |
| PROMISE | Low risk of bias | Randomisation block stratified by centre. Allocation concealed. Difference in percentage of participants unable to work: SCS+OMM=58.2%, OMM=43.5% but compatible with chance. | Some concerns | Open label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. | Some concerns | 16% discontinuations in the SCS group and 3% in OMM group. It is not clear how many of these continued to provide data and how many were missing. While BOCF was used to impute missing data, proportion of missing data is imbalanced across groups. ITT analysis appears conservative. | High risk of bias | Open label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Low risk of bias | Reporting and analysis consistent with published protocol. | High risk of bias | High risk of bias based on bias in the measurement of the outcome. |
| SENZA‐PDN | Low risk of bias | Computer generated randomisation. Allocation concealed. No apparent baseline imbalance. | Some concerns | Open‐label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. An ITT approach is reported, though there are a number of post‐randomisation exclusions. | High risk of bias | Post randomisation exclusions took place prior to pre‐implantation trial period and were omitted from the analysis. In total at 6 months there are 16% of randomised participants excluded from the SCS group and 9% from the CMM. While sensitivity analyses are presented they don't account for all exclusions and the effect size is likely to be affected. | High risk of bias | Open‐label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Some concerns | While an SAP is available it is unclear whether it was finalised and unchanged ahead of the end of data collection. While authors shared data on request, they remained post‐randomisation exclusions and the N of participants analysed do not clearly match those in the CONSORT flow chart. | High risk of bias | High risk of bias on multiple domains. |
Risk of bias for analysis 2.4 Pain: participants with ≥50% pain relief. Medium‐term follow‐up. Risk Ratio.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| PROCESS | Low risk of bias | Computer generated randomisation with concealed allocation. Back pain higher in SCS group (SCS 54.5 (24.3: CMM 44.8 (23.2). Higher history of litigation in CMM group (17% VS 10% IN SCS) but likely compatible with chance. | Some concerns | Open label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. | Low risk of bias | Low levels of missing data at this time‐point (SCS 4%, CMM 8%). | High risk of bias | Open‐label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Low risk of bias | Reporting and analysis consistent with published protocol. | High risk of bias | High risk of bias based on bias in the measurement of the outcome. |
| SENZA‐PDN | Low risk of bias | Computer generated randomisation. Allocation concealed. No apparent baseline imbalance. | Some concerns | Open‐label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. An ITT approach is reported, though there are a number of post‐randomisation exclusions. | High risk of bias | Post randomisation exclusions took place prior to pre‐implantation trial period and were omitted from the analysis. In total at 6 months there are 15% of randomised participants excluded from the SCS group and 9% from the CMM group. Reasons for missingness include refusal to be implanted with device and adverse events. | High risk of bias | Open‐label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Some concerns | While an SAP is available it is unclear whether it was finalised and unchanged ahead of the end of data collection. While authors shared data on request, they remained post‐randomisation exclusions and the N of participants analysed do not clearly match those in the CONSORT flow chart. | High risk of bias | High risk of bias based on multiple domains |
| de Vos 2014 | Low risk of bias | Randomisation block stratified by centre. Allocation concealed. No apparent baseline imbalance. | Some concerns | Open label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. | Low risk of bias | Low and balanced levels of missing data (10% in each group). Missing data treated as non‐responders. Authors shared the complete dataset which indicates no meaningful bias. | High risk of bias | Open label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Low risk of bias | While no protocol or full SAP is available sharing of the full dataset by the study authors indicates no evidence of selectivity. | High risk of bias | High risk of bias based on bias in the measurement of the outcome. |
| Slangen 2014 | Low risk of bias | Computer generated randomisation. Allocation concealed. No apparent baseline imbalance. | Some concerns | Open‐label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. | Low risk of bias | Low levels of missing data (14% SCS group, 7% OMM group). Missing data treated as non‐responders. | High risk of bias | Open‐label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Some concerns | No published protocol or SAP available for the study to outline the intentioned for analysis. | High risk of bias | High risk of bias based on bias in the measurement of the outcome. |
| PROMISE | Low risk of bias | Randomisation block stratified by centre. Allocation concealed. Difference in percentage of participants unable to work: SCS+OMM=58.2%, OMM=43.5% but compatible with chance. | Some concerns | Open label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. | Some concerns | 16% discontinuations in the SCS group and 3% in OMM group. It is not clear how many of these continued to provide data and how many were missing. However the classification of participants with missing values as non‐responders reduces the risk of inflating the effect size. | High risk of bias | Open label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Low risk of bias | Reporting and analysis consistent with published protocol. | High risk of bias | High risk of bias based on bias in the measurement of the outcome. |
Risk of bias for analysis 2.5 Pain intensity: average post‐intervention. Long‐term follow‐up. Mean difference.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kemler 2000 | Low risk of bias | Computer generated randomisation. Allocation concealed. Minor imbalance of gender (61%F in SCS group and 83%F in PT group) but compatible with chance. | High risk of bias | Open label study. 4/36 (11%) randomised to PT received a spinal cord stimulator by 5 years and were excluded. 1/18 (5.5%) in SCS group was excluded after 6 months due to failure to implant lead in epidural space despite use of a special lead. | High risk of bias | 14% missing data in SCS group; 28% missing data in PT group. 14% missing data in SCS group; 28% missing data in PT group. LOCF imputation may introduce a bias. | High risk of bias | Open label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Some concerns | No registry record, protocol or SAP available. | High risk of bias | High risk of bias on multiple domains. |
Risk of bias for analysis 2.6 Pain: participants with ≥50% pain relief. Long‐term follow‐up. Risk Ratio.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| PROCESS | Low risk of bias | Computer generated randomisation with concealed allocation. Back pain higher in SCS group (SCS 54.5 (24.3: CMM 44.8 (23.2). Higher history of litigation in CMM group (17% VS 10% IN SCS) but likely compatible with chance. | High risk of bias | Open label study. By 12 months 28 (58%) in the CMM group had crossed to SCS. 5 (9.6%) in SCS group had crossed to CMM. No information about CMM delivered in either group. | High risk of bias | Missing data at this time point (SCS 5/52 (9%), CMM 7/48 14.5%). Analysis based on available data excluding loss to follow up. Analysis based on available data excluding loss to follow up. | High risk of bias | Open label study of a complex, invasive intervention with a subjective self‐reported outcome (Pain VAS). | Low risk of bias | Reported analysis aligns with that in published protocol and reported for this time‐point. | High risk of bias | High risk of biason multiple domains. |
Risk of bias for analysis 2.9 Adverse events: infection. Medium‐term follow‐up.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| de Vos 2014 | Low risk of bias | Randomisation block stratified by centre. Allocation concealed. No apparent baseline imbalance. | Low risk of bias | No information to assess deviations from intended intervention that arose because of experimental context, including no information about medical care received in each group. Any potential deviations in medical management unlikely to impact the incidence of device related adverse events. | Low risk of bias | Balanced levels of missing data (10% per group). Study authors shared data for all participants. | High risk of bias | "The safety and tolerability of SCS therapy over time was evaluated using information on treatment‐emergent adverse events, device complications, and premature withdrawal
from the trial." Limited information on how AEs were classified and the approach taken to surveillance which may impact on the reported values. |
Some concerns | No trial protocol or statistical analysis plan available, though no clear suggestion of selective reporting. | High risk of bias | High risk of bias based on potential bias in the measurement of the outcome. |
| Kemler 2000 | Low risk of bias | Computer generated randomisation. Allocation concealed. Minor imbalance of gender (61%F in SCS group and 83%F in PT group) but compatible with chance. | Low risk of bias | No information to suggest there were or were not deviations from the intended intervention that arose because of the experimental context. No information on the details of the physical therapy delivered. Any potential deviations in PT management unlikely to impact the incidence of device‐related adverse events. | Low risk of bias | Only 1 participant lost to follow up at this point. | High risk of bias | Limited information on how AEs were classified and the approach taken to surveillance, which may impact on the reported values. "Finally, we documented complications of spinal cord stimulation." |
Some concerns | No registry record, protocol or SAP available. | High risk of bias | High risk of bias based on possible bias in the measurement of the outcome. |
| PROMISE | Low risk of bias | Randomisation block stratified by centre. Allocation concealed. Difference in percentage of participants unable to work: SCS+OMM=58.2%, OMM=43.5% but compatible with chance. | Low risk of bias | No information to assess deviations from intended intervention that arose because of experimental context, including no information about medical care received in each group. Any potential deviations in medical management unlikely to impact the incidence of device ‐adverse events. | Low risk of bias | Low levels of missing data in each group (6% in SCS group and 3% in OMM) | High risk of bias | Limited information on how AEs were classified and the approach taken to surveillance which may impact on the reported values. "An independent clinical events committee adjudicated AEs." |
Low risk of bias | Reporting of results for this outcome appears complete and unselective. | High risk of bias | High risk of bias based on possible bias in the measurement of the outcome. |
| SENZA‐PDN | Low risk of bias | Computer generated randomisation. Allocation concealed. No apparent baseline imbalance. | Low risk of bias | Open label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. Any potential deviations in medical management unlikely to impact the incidence of device‐related adverse events. | Low risk of bias | AEs based on full cohort as randomised with low levels of missing data. 4 LTFU and1 withdrew consent in SCS group (4%), 4 LTFU and 2 withdrew consent in CMM group (6%). | High risk of bias | Limited information on how AEs were classified and the approach taken to surveillance which may impact on the reported values. | Low risk of bias | Data reported as per pre‐specification in protocol and SAP | High risk of bias | High risk of bias based on bias in the measurement of the outcome. |
Risk of bias for analysis 2.7 Adverse events: electrode/lead failure or displacement. Medium‐term follow‐up.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| de Vos 2014 | Low risk of bias | Randomisation block stratified by centre. Allocation concealed. No apparent baseline imbalance. | Low risk of bias | Open label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. Any potential deviations in medical management unlikely to impact the incidence of device related adverse events. | Low risk of bias | Balanced levels of missing data (10% per group). Study authors shared data for all participants. | High risk of bias | Limited information on how AEs were classified and the approach taken to surveillance which may impact on the reported values. "The safety and tolerability of SCS therapy over time was evaluated using information on treatment‐emergent adverse events, device complications, and premature withdrawal from the trial." Authors clarified all device‐related AEs on request. |
Some concerns | No trial protocol or statistical analysis plan available, though no clear suggestion of selective reporting. | High risk of bias | High risk of bias based on bias in the measurement of the outcome. |
| Kemler 2000 | Low risk of bias | Computer generated randomisation. Allocation concealed. Minor imbalance of gender (61%F in SCS group and 83%F in PT group) but compatible with chance. | Low risk of bias | No information to suggest there were or were not deviations from the intended intervention that arose because of the experimental context. No information on the details of the physical therapy delivered. Any potential deviations in PT management unlikely to impact the incidence of device‐related adverse events. | Low risk of bias | Only 1 participant reported LTFU in the PT only group. | High risk of bias | Limited information on how AEs were classified and the approach taken to surveillance which may impact on the reported values. | Some concerns | No trial protocol or statistical analysis plan available, though no clear suggestion of selective reporting. | High risk of bias | No trial protocol or statistical analysis plan available, though no clear suggestion of selective reporting. |
| SENZA‐PDN | Low risk of bias | Computer generated randomisation. Allocation concealed. No apparent baseline imbalance. | Low risk of bias | Open label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. ITT analysis used. Any potential deviations in medical management unlikely to impact the incidence of device‐related adverse events. | Low risk of bias | AEs based on full cohort as randomised with low levels of missing data. 4 LTFU and1 withdrew consent in SCS group (4%), 4 LTFU and 2 withdrew consent in CMM group (6%). | High risk of bias | Limited information on how AEs were classified and the approach taken to surveillance which may impact on the reported values. | Low risk of bias | Data reported as per pre‐specification in protocol and SAP | High risk of bias | High risk of bias due to possible bias in the measurement of the outcome |
Risk of bias for analysis 2.8 Adverse events: electrode/lead failure or displacement. Long‐term follow‐up.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kemler 2000 | Low risk of bias | Computer generated randomisation. Allocation concealed. Minor imbalance of gender (61%F in SCS group and 83%F in PT group) but compatible with chance. | Low risk of bias | Open label study without blinding of participants and personnel and no information provided to assess deviations in the PT management across groups. Any potential deviations in PT management unlikely to impact the incidence of device related adverse events. | High risk of bias | At 5 year follow up, 14% LTFU in SCS group and 28% LTFU in PT group. It is possible that LTFU was related to adverse events. | High risk of bias | Limited information on how AEs were classified and the approach taken to surveillance which may impact on the reported values. | Some concerns | No triap protocol or SAP available | High risk of bias | High risk of bias on multiple domains. |
Risk of bias for analysis 2.10 Adverse events: need for repeated implantation procedures. Medium‐term follow‐up.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| de Vos 2014 | Low risk of bias | Randomisation block stratified by centre. Allocation concealed. No apparent baseline imbalance. | Low risk of bias | Open label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. Any potential deviations in medical management unlikely to impact the incidence of device‐related adverse events. | Low risk of bias | Balanced levels of missing data (10% per group). Study authors shared data for all participants. | High risk of bias | Limited information on how AEs were classified and the approach taken to surveillance which may impact on the reported values. "The safety and tolerability of SCS therapy over time was evaluated using information on treatment‐emergent adverse events, device complications, and premature withdrawal from the trial." |
Some concerns | While no protocol or full SAP is available sharing of the full dataset by the study authors indicates no evidence of selectivity. | High risk of bias | High risk of bias based on possible bias in the measurement of the outcome. |
| Kemler 2000 | Low risk of bias | Computer generated randomisation. Allocation concealed. Minor imbalance of gender (61%F in SCS group and 83%F in PT group) but compatible with chance. | Low risk of bias | Open label study without blinding of participants and personnel and no information provided to assess deviations in the PT management across groups. Any potential deviations in PT management unlikely to impact the incidence of device‐related adverse events. | Low risk of bias | Only 1 participant lost to follow up at this timepoint. | High risk of bias | Limited information on how AEs were classified and the approach taken to surveillance which may impact on the reported values. | Some concerns | No registry record, protocol or SAP is available. | High risk of bias | High risk of bias based on possible bias in the measurement of the outcome. |
| PROMISE | Low risk of bias | Randomisation block stratified by centre. Allocation concealed. Difference in percentage of participants unable to work: SCS+OMM=58.2%, OMM=43.5% but compatible with chance. | Low risk of bias | Open label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. Any potential deviations in medical management unlikely to impact the incidence of device‐related adverse events. | Low risk of bias | Low levels of missing data in each group (6% in SCS group and 3% in OMM) | High risk of bias | Limited information on how AEs were classified and the approach taken to surveillance which may impact on the reported values. | Low risk of bias | Protocol consistent with published report | High risk of bias | High risk of bias based on possible bias in the measurement of the outcome. |
| SENZA‐PDN | Low risk of bias | Computer generated randomisation. Allocation concealed. No apparent baseline imbalance. | Low risk of bias | Open‐label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. An ITT approach is reported. Any potential deviations in medical management unlikely to impact the incidence of device‐related adverse events. | Low risk of bias | 4 LTFU and 1 withdrew consent in SCS group (4%), 4 LTFU and 2 withdrew consent in CMM group (6%). Safety cohort is all randomised with low levels of missing data. | High risk of bias | Limited information on how AEs were classified and the approach taken to surveillance which may impact on the reported values. | Low risk of bias | Data reported as per pre‐specification in protocol and SAP and data clarified by authors on request. | High risk of bias | High risk of bias based on bias in the measurement of the outcome. |
Risk of bias for analysis 2.11 Adverse events: need for repeated implantation procedures. Long‐term follow‐up.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kemler 2000 | Low risk of bias | Computer generated randomisation. Allocation concealed. Minor imbalance of gender (61%F in SCS group and 83%F in PT group) but compatible with chance. | Low risk of bias | Open label study without blinding of participants and personnel and no information provided to assess deviations in the PT management across groups. Any potential deviations in PT management unlikely to impact the incidence of device related adverse events. | High risk of bias | 5/36 (14%) LTFU in SCS group and 5/18 (28%) in PT group at 5 year follow up. | High risk of bias | Limited information on how AEs were classified and the approach taken to surveillance which may impact on the reported values. | Some concerns | No trial protocol or SAP available. | Some concerns | High risk of bias based on possible bias in the measurement of the outcome. |
Risk of bias for analysis 2.12 Adverse events: other. Medium‐term follow‐up.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| de Vos 2014 | Low risk of bias | Randomisation block stratified by centre. Allocation concealed. No apparent baseline imbalance. | Some concerns | Some concerns related to bias due to deviations from intended interventions, in the measurement of the outcome and selection of the reported result | Low risk of bias | Balanced levels of missing data (10% per group). Study authors shared data for all participants. | High risk of bias | Limited information on how AEs were classified and the approach taken to surveillance which may impact on the reported values. | Some concerns | While no protocol or full SAP is available sharing of the full dataset by the study authors indicates no evidence of selectivity. | High risk of bias | High risk of bias based on possible bias in the measurement of the outcome. |
| PROMISE | Low risk of bias | Randomisation block stratified by centre. Allocation concealed. Difference in percentage of participants unable to work: SCS+OMM=58.2%, OMM=43.5% but compatible with chance. | Some concerns | Open label study without blinding of participants and personnel and no information provided to assess deviations in the medical management across groups. | Low risk of bias | Low levels of missing data in each group (6% in SCS group and 3% in OMM) | High risk of bias | Limited information on how AEs were classified and the approach taken to surveillance which may impact on the reported values. | Low risk of bias | Reporting and analysis consistent with published protocol. | High risk of bias | High risk of bias based on possible bias in the measurement of the outcome. |
Risk of bias for analysis 2.13 Adverse events: other. Long‐term follow‐up.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| PROCESS | Low risk of bias | Computer generated randomisation with concealed allocation. Back pain higher in SCS group (SCS 54.5 (24.3: CMM 44.8 (23.2). Higher history of litigation in CMM group (17% VS 10% IN SCS) but likely compatible with chance. | High risk of bias | By 12 months 28 (58%) in the CMM group had crossed to SCS. 5 (9.6%) in SCS group had crossed to CMM. No information about CMM delivered in either group. | High risk of bias | Missing data at this time point (SCS 6/52 (11.5%, CMM 7/48 14.5%). Analysis based on available data. Missingness of data may depend on true value. | High risk of bias | Limited information on how AEs were classified and the approach taken to surveillance which may impact on the reported values. | Low risk of bias | Reported analysis aligns with that in published protocol and reported for this time‐point. | High risk of bias | High risk of bias on multiple domains. |
We rated all results that we evaluated as being at high risk of bias overall.
For the comparison of spinal cord stimulation (SCS) versus sham, we judged all results to be at high risk of bias on more than one domain. All results where there were data available used continuous self‐reported outcome measures in the short term and so risk of bias issues were generally consistent across results. Common issues were the lack of washout periods employed to minimise potential carry‐over effects, the likelihood of inadequate blinding compounded by the infrequent use of formal assessment of blinding success and the presence of post‐randomisation exclusions with per‐protocol type analyses. With regard to bias in the measurement of the outcome, we rated comparisons of conventional stimulation versus sham as being at high risk of bias as they involve suprathreshold, perceptible sensations. For high‐frequency and burst stimulations which may be expected to be sub‐perceptual, we rated results as causing"some concerns" unless a formal evaluation of blinding was presented and demonstrated success. As RevMan Web does not currently have the function to present distinct risk of bias (ROB) judgements for different comparisons from the same study, the ROB figures and tables presented in this review give a default "high" risk judgement for all studies unless the stimulation was sub‐perceptual and successful blinding was formally demonstrated. Importantly, this does not impact any overall risk of bias judgments for any studies, or any related GRADE ratings.
For the comparisons SCS + other intervention versus other intervention alone, we rated all results to cause "some concerns" for bias due to deviations from the intended intervention for the outcome pain intensity, on the basis that these were open‐label studies and minimal detail was provided regarding how non‐SCS management was delivered throughout the studies. For device‐related adverse events (AEs), we considered this risk to be low, as any potential deviations in physical therapy or medical management are unlikely to impact the incidence of device‐related adverse events. We judged all of these results to be at high risk of bias in the measurement of the outcome as they are all derived from open‐label studies of the addition of a complex and invasive intervention, with mainly subjective self‐reported outcomes. For adverse events, the included studies presented minimal information on how AEs were classified and the approach taken to surveillance which we considered may have impacted on the reported values.
Effects of interventions
Summary of findings 1. Active stimulation vs placebo (sham).
| Active Stimulation versus placebo for chronic pain in adults | |||||
|
Patient or population: adults with chronic pain Settings: secondary care Intervention/comparison: SCS vs placebo (sham stimulation) | |||||
| Outcomes | Probable outcome with SNMD | Probable outcome with sham | No of participants(studies) | Certainty of the evidence(GRADE) | Comments |
| Short‐term follow‐up (reported within the first month ) | |||||
| Pain intensity continuous outcomes. (VAS 0 ‐ 100) | The mean pain intensity was 8.73 points lower (95%CI ‐15.67, ‐1.78) than in the control group. | Mean post‐intervention pain score in sham group. 55.8 (95%CI 43.3, 64.1) | 164 (6) | ⊕⊝⊝⊝ very low1 |
|
| Pain intensity. Proportion with ≥ 50% pain relief. | No evidence | ||||
| Adverse event: lead failure/ displacement | Not estimable, adverse events not reported by stimulation condition | ||||
| Adverse events: infection | Not estimable, adverse events not reported by stimulation condition. | ||||
| Adverse events: need for reoperation/ reimplantation | Not estimable, adverse events not reported by stimulation condition | ||||
| Adverse events: other | Not estimable, adverse events not reported by stimulation condition | ||||
| Medium term follow‐up (reported between 3 and 6 months) | |||||
| Pain intensity, continuous outcomes(VAS 0 ‐ 100) | No evidence |
||||
| Pain intensity. Proportion with ≥50% pain relief. | No evidence | ||||
| Adverse events: Infection | No evidence | ||||
| Adverse events: Lead failure/ displacement | No evidence | ||||
| Adverse events: Need for reoperation/ reimplantation | No evidence | ||||
| Adverse events: other | No evidence | ||||
| Long term follow‐up (reported at 1 year or longer greater than 1 year) | |||||
| Pain intensity, continuous outcomes (VAS 0 ‐ 100) | No evidence |
||||
| Pain intensity. Proportion with ≥50% pain relief. | No evidence | ||||
| Adverse events: Infection | No evidence | ||||
| Adverse events: Lead failure/ displacement | No evidence |
||||
| Adverse events: Need for reoperation/reimplantation | No evidence |
||||
| Adverse events: other | No evidence | ||||
| CI: confidence interval; SNMD: Spinal neuromodulationVAS: visual analogue scale. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Lowcertainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
1. downgraded twice for serious study limitations, once for imprecision, once for inconsistency and once for the potential for publication bias.
Summary of findings 2. Active stimulation + other intervention vs other intervention alone.
| Active stimulation + other intervention versus other intervention alone for chronic pain in adults (open label studies). | ||||||
|
Patient or population: adults with chronic pain Settings: secondary care Intervention/comparison: SCS + other intervention (medical management or physical therapy) vs other intervention alone | ||||||
| Outcomes | Relative effects (Risk Ratio (RR) 95%CI) | Anticipated absolute effects* | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with SNMD + other interventions | Risk with other intervention alone | |||||
| Short‐term follow‐up (reported within the first month ) | ||||||
| Pain intensity continuous outcomes. (VAS 0‐100, higher scores = worse pain) | ‐ | The mean pain intensity in the intervention groups was 37.41 points lower (95% CI ‐46.39 to ‐28.42) than in the control group. | The mean post‐intervention pain score in control group was 69.3 (95%CI 68‐72) | 303 (3) | ⊕⊝⊝⊝ very low1 |
|
| Pain intensity. Proportion with ≥ 50% pain relief. | RR 15.90 (95%CI 6.70, 37.74) | 69.6% |
4.3% | 249 (2) | ⊕⊝⊝⊝ very low2 |
|
| Difference: 65% more participants with SCS (95%CI 57, 74) NNTB 1.5 (95%CI 1.4, 1.8) | ||||||
| Adverse event: lead failure/ displacement | Not estimable, data not reported. | |||||
| Adverse events: infection | Not estimable, data not reported. | |||||
| Adverse events: need for reoperation/ reimplantation | Not estimable, data not reported. | |||||
| Adverse events: other | Not estimable, data not reported. | |||||
| Medium‐term follow‐up (reported between 4 and 8 months) | ||||||
| Pain intensity, continuous outcomes (VAS 0 ‐ 100, higher scores = worse pain) | The mean pain intensity in the intervention groups was 31.22 points lower (95% CI ‐47.34 to ‐15.10) than in the control group. | The mean post‐intervention pain score in control group was 70.1 (95%CI 67, 73.7) | 635 (5) | ⊕ ⊕⊝⊝ low3 |
||
| Pain intensity. Proportion with ≥50% pain relief. | RR 7.08 (95%CI 3.40, 14.71) | 46.1% | 5.3% | 597 (5) | ⊕ ⊕⊝⊝ low4 |
|
| Difference: 43% more participants with SCS (95%CI 14, 73) NNTB 2.3 (95%CI 1.4, 7.1) | ||||||
| Adverse events:lead failure/ displacement | RR 3.02 (95%CI 0.52, 17.58) | 4% | 0% | 330 (3) | ⊕⊝⊝⊝ very low6 |
|
| Difference: 4% more participants with SCS (95%CI 4% fewer, 11% more) | ||||||
| Adverse events:infection | RR 4.83 (95%CI 1.09, 21.40) | 4.6% | 0% | 548 (4) | ⊕ ⊕⊝⊝ low4 |
|
| Difference: 4% more participants with SCS (95%CI 1, 7) NNTH 25 (95%CI 14, 100) | ||||||
| Adverse events: need for reoperation/ reimplantation | RR 9.79 (95%CI 2.35, 40.76) | 10% | 0% | 548 (4) | ⊕⊝⊝⊝ very low1 |
|
| Difference: 11% more participants with SCS (95%CI 2, 21) NNTH 9 (95%CI 4.8, 50) | ||||||
| Adverse events: other | RR 0.87 (95%CI 0.64, 1.30) | 32.7% | 39.1% | 278 (2) | ⊕ ⊕⊝⊝ low4 |
|
| Difference: 5% fewer participants with SCS (95%CI 16% fewer, 6% more) | ||||||
| Long‐term follow‐up (reported at 1 year or longer greater than 1 year) | ||||||
| Pain intensity, continuous outcomes (VAS 0 ‐ 100, higher scores = worse pain) (5 years follow‐up) | ‐ | The mean pain intensity in the intervention groups was 7 points lower than in the control group (95% CI ‐‐24.76 to 10.76 | Pain intensity reduced from baseline in the control group by ‐10 (95%CI ‐18, 2) points. | 44 (1) | ⊕⊝⊝⊝ very low5 |
|
| Pain intensity. Proportion with ≥ 50% pain relief. (2‐year follow up) | RR 15.15 (95%CI 2.11, 108.91) | 37% | 2% | 87 (1) | ⊕⊝⊝⊝ very low5 |
|
| Difference: 35% more participants with SCS (95%CI 20, 49) NNTB 2.9 (95%CI 2, 5) | ||||||
| Adverse events: lead failure/ displacement | RR 15.31 (95%CI 0.99, 237.16) | 55% | 0% | 44 (1) | ⊕⊝⊝⊝ very low1 |
|
| Difference: 55% more participants with SCS (95%CI 35, 75) NNTH 1.8 (95%CI 1.3, 2.9) | ||||||
| Adverse events: infection | Not estimable, data not reported. | |||||
| Adverse events: need for reoperation/reimplantation | RR 25.81 (95%CI 1.69, 393.31) | 94% | 0% | 44 (1) | ⊕⊝⊝⊝ very low1 |
|
| Difference: 94% more participants with SCS (95%CI 80, 107) NNTH 1.05 (95%CI 0.93, 1.25) | ||||||
| Adverse events: other | RR 0.66 (95%CI 0.42, 1.05) | 34.6% | 52.1% | 100 (1) | ⊕⊝⊝⊝ very low1 |
|
| Difference: 17% fewer participants with SCS (95%CI 37% fewer to 2% more) | ||||||
| * Control group risk estimates come from pooled estimates of control groups. CI: confidence interval; SNMD: Spinal neuromodulationVAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 downgraded once for study limitations, imprecision and inconsistency
2 downgraded for twice for serious study limitations and once for imprecision
3 downgraded once for study limitations and once for inconsistency
4 downgraded once for study limitations and once for imprecision
5 downgraded twice for serious study limitations, once for imprecision and once for inconsistency
6 downgraded once for study limitations and twice for serious imprecision
We found no eligible published studies of DRGS and no studies comparing SCS to no treatment or usual care.
Active Stimulation versus placebo
SCS versus placebo (sham)
See Table 1 for a summary of the primary outcomes for= spinal cord stimulation (SCS) versus sham.
Pain intensity
Short‐term follow‐up
Six studies contributed to this analysis (Analysis 1.1, Al‐Kaisy 2018; Kriek 2017; Perruchoud 2013; Schu 2014; Sokal 2020; Tjepkema‐Cloostermans 2016, N = 164), most of which compared two or more stimulation types with sham and were entered into the analysis more than once. For studies that contributed multiple comparisons, we divided the number of participants in each arm by the number of comparisons included from that study to avoid double counting. We were unable to include data from three eligible studies in this analysis (combined N = 44) due to a lack of reporting of necessary measures of variance (De Ridder 2013; Eldabe 2021) or both point estimates and measures of variance (Lind 2015).
We found evidence of a small effect in favour of SCS (mean difference (MD) ‐8.73, 95% confidence interval (CI) ‐15.67 to 1.78, P = 0.005, I2 = 58%, very low‐certainty evidence, downgraded twice for serious limitations of studies, once for imprecision and once due to the potential for publication bias). The point estimate falls below our threshold of a clinically important effect, though the upper confidence interval exceeds it. Pre‐planned subgroup analysis by stimulation parameters (conventional, high‐frequency and burst) reduced heterogeneity in the high‐frequency group (I2 0%) but not in the conventional (I2 77%) or the burst (I2 76%) group. The test for subgroup differences did not provide evidence for differences (Chi2 1.06, P = 0.59).
Planned sensitivity analysis using a fixed‐effect model resulted in a smaller effect in favour of SCS (MD ‐6.58, 95% CI ‐10.84 to ‐2.32).
No studies reported data for the proportion of participants experiencing 30% or 50% pain relief for this comparison.
Reporting bias
We identified potential studies that were either recorded in the trial registry (ISRCTN33292457; NCT00351208; NCT03462147) or published as a conference abstract (Miller 2015; Miller 2016), but where we did not find a published study report. Combined, these studies may have contributed data from 74 more participants to these comparisons, which in light of the number of participants in this comparison may impact our estimate of effect.
Adverse events (AEs)
Short‐term follow‐up
All studies comparing SCS versus sham stimulation were cross‐over studies. In these trials, all participants were surgically implanted with electrodes for all stimulation conditions. We graded the evidence for all adverse effects as very low certainty, downgraded twice for serious limitations of studies, once for imprecision and once for inconsistency.
There were important differences between the trials that might impact the reported incidence of adverse events. Eldabe 2021, Perruchoud 2013, Schu 2014 and Tjepkema‐Cloostermans 2016 recruited participants who were already implanted successfully with a device and had been receiving SCS for their pain at the point for recruitment, thus reducing the probability of identifying perioperative adverse events. Al‐Kaisy 2018 reported all AEs during the course of the study including the pre‐implantation trial period, Sokal 2020 reported AEs occurring during the pre‐implementation trial period, cross‐over period and up to 17 months of follow‐up after that. Eldabe 2021, Kriek 2017, Schu 2014 and Perruchoud 2013 reported AEs that occurred during the cross‐over period and Eldabe 2021 continued to follow up participants for any serious adverse events (SAEs) for a further six months. Schu 2014 only reported SAEs though did not provide a clear definition for those. De Ridder 2013 reported no data for AEs. Cross‐over periods ranged in duration from 3 to 24 weeks. We have summarised the reported AEs in Table 3.
1. Procedure/device‐related adverse events from studies comparing SCS versus sham.
| Study ID | Total N | Period of measurement | Lead failure/ displacement | Infection | Need for reop | Other* AEs | Details |
| Al‐Kaisy 2018 | 30 | 12 weeks cross‐over period + 17 days pre‐implementation trial period | 2 (during pre‐implentation trial period) | 0 | 1 | 9 | 1 in pre‐implementation trial period, not defined. Pain at the IPG site 3 (1 required re‐operation) Skin heating during recharging 1 Intercostal pain 1 3 additional "minor lead migrations" |
| De Ridder 2013 | 15 | 3‐week cross‐over period | NI | NI | NI | NI | NI |
| Eldabe 2021 | 19 | 6‐week cross‐over period plus 6 months post study surveillance for SAEs | 0 | 0 | 0 | 27 | Increased pain 15 Cramp in foot 2 Pain over device 1 Intermittent jolts of stimulation 2 Loss of adaptive stimulator function 1 Discomfort in neck 1 Paraesthesia 1 Bilateral foot pain 1 Numbness in leg 1 Left hip pain 1 Uncomfortable sensations 1 |
| Kriek 2017 | 33 | 1‐ week cross‐over period | 3 | 1 | 3 | 78 | Device/ stim output issues 68, Itching rash 2, Axial paraesthesia 1, Headache 4, converted to standard stimulation 2, Discontinued stimulation 1 |
| Lind 2015 | 10 | 2‐ week cross‐over period | NI | NI | NI | 7 | Feeling of tiredness during stimulation periods 2, sensations of unsteady gait during stimulation probably related to paresthesia in the legs 2 Uncomfortably high‐intensity stimulation in her legs 1, pain at the implantation site of the stimulator 1, transient headache upon removal of the SCS system 1. |
| Perruchoud 2013 | 33 | 8‐week cross‐over period | NI | NI | NI | 1 | Malaise that was attributed to a vasovagal attack at the programming session of one of the treatment periods. This patient subsequently withdrew consent to the study and was excluded. 1 |
| Schu 2014 | 20 | 3‐week cross‐over period | NI | NI | NI | NI | Only SAEs measured and none reported. |
| Sokal 2020 | 18 | ‐2‐week pre‐trial implementation period, 8‐week cross‐over period, up to 17 months follow‐up | 1 | 0 | 7 | 2 | Device/ electrodes removed due to inadequate pain relief 2 Removal of IPG 3, Allergic reaction at implant site 1, Replacement due to battery issues 1 Electrode replacement due to electrode dysfunction 1 |
| Kriek 2017 | 41 | 6‐week cross‐over period | 1 | NI | NI | 4 | Heavy feeling or pressure in their legs or feet 3 Increased sensation of local stimulation around IPG 1 |
Footnotes:AEs= adverse events;IPG= implanted pulse generator.NI= No Information reported; *as defined and reported in original studies;SAEsserious adverse events
Only Eldabe 2021 (N = 19) reported the incidence ofAEs by stimulation condition to allow comparisons of SCS versus sham at any pre‐specified time point. They reported no cases of infection, lead failure or re‐operation in any condition, though it is important to recognise that participants in that trial were recruited on the basis of experiencing stable pain relief from an existing implanted stimulator. Of otherAEs they reported 14 participants with 15 events in the 500 Hz condition, 11 participants with 11 events in the Burst condition and 12 participants with 12 events in the sham condition. Details of these are summarised in Table 3
Four studies (Al‐Kaisy 2018; Kriek 2017; Schu 2014; Tjepkema‐Cloostermans 2016) reported incidences of lead failure. These ranged from 1 event in 41 participants (2%) (Tjepkema‐Cloostermans 2016) to 4 events in 30 participants (13%) (Al‐Kaisy 2018). Three studies (Al‐Kaisy 2018; Kriek 2017; Sokal 2020) reported the incidence of infection of which Kriek 2017 ranging from no events to 1 event in 33 participants (3%). In these three studies, re‐operation rates ranged from 1 event in 30 participants (3%) (Al‐Kaisy 2018) to 7 events in 18 participants (39%) (Sokal 2020).
In studies where some data were available the rate of other AEs ranged from 1 event in 33 participants to 78 events in 33 participants. Details are reported in Table 3. These results are highly likely to have been substantially affected by variation in the methods and reporting approach taken to AEs, of which there was little detail in the original papers.
Disability
Short‐term follow‐up
Three studies (Kriek 2017; Schu 2014; Sokal 2020) measured disability for this comparison. However, only Schu 2014 reported this outcome in adequate detail to allow analysis. Kriek 2017 reported in their protocol measuring the Disabilities of Arm Shoulder and Hand Questionnaire (DASH) when the upper extremity was affected, and the Walking Ability Questionnaire when the lower extremity was affected, but did not present results in the published study report. Sokal 2020 measured disability using the Oswestry Disability Index (ODI), but did not report point estimates or measures of variance in the published report.
Schu 2014 measured disability using the ODI (0 to 100 scale, higher scores reflect higher levels of disability). Analysis of data from this study (Analysis 1.2), including conventional stimulation and burst stimulation demonstrated a small effect of SCS vs sham (N = 20, MD ‐7.48, 95% CI ‐13.13 to ‐1.82, P = 0.01, I2 0%, very low‐certainty evidence, downgraded twice for serious limitations, once for imprecision and once for inconsistency).
1.2. Analysis.

Comparison 1: SCS vs sham, Outcome 2: Disability: short‐term follow‐up
Health‐related quality of life (HRQoL)
Short‐term follow‐up
Three studies (Eldabe 2021; Perruchoud 2013; Tjepkema‐Cloostermans 2016 pooled N = 73) reported HR‐QoL at short‐term follow‐up. Perruchoud 2013 used EQ‐5D utility index values and Tjepkema‐Cloostermans 2016 used the McGill Pain Questionnaire QoL scale. As a result, we used the standardised mean difference (SMD) as our effect measure. We were unable to include data from Eldabe 2021 in this analysis (N = 19) due to a lack of reporting of necessary measures of variance. We found no evidence for an effect of SCS vs sham on HRQoL (Analysis 1.3, SMD 0.03, 95% CI ‐0.30 to 0.35, P = 0.88, I2 = 0%, very low‐certainty evidence, downgraded twice for serious limitations and once for imprecision).
1.3. Analysis.

Comparison 1: SCS vs sham, Outcome 3: HR‐QoL: short‐term follow‐up
Medication use
Short‐term follow‐up
Three studies (Kriek 2017; Perruchoud 2013; Sokal 2020) reported measuring medication use. None of these studies reported results in adequate detail to allow analysis or pooling of data. Kriek 2017 (N = 33) did not report medication use in the published study report, although it was reported to be measured in the study protocol. Sokal 2020 (N = 23) did not report data in an extractable format but reported that the total number of medications taken did not differ across conditions. Perruchoud 2013 (N = 40) similarly reported that medication use was unchanged in all but one patient who increased their dose of oral morphine. We graded the evidence as very low certainty, downgraded twice for serious limitations, once for imprecision and once for inconsistency.
Active stimulation plus another intervention versus that intervention alone
SCS + other intervention versus other intervention only
See Table 2 for a summary of the primary outcomes for SCS + other intervention versus other intervention only. We included outcomes at medium‐term follow‐up in this table, though this was not prespecified in our protocol. The reason for this was that for all included studies the specified primary endpoint was at medium‐term follow‐up.
Pain intensity
Mean difference (MD)
Three studies (de Vos 2014; Kemler 2000, SENZA‐PDN combined N = 303, Analysis 2.1) provided data after one month of stimulation. Pooling of these studies demonstrated a potentially clinically important mean difference in favour of SCS of ‐37.41 (95% CI ‐46.39 to ‐28.42, P < 0.001, I2 65%) with substantial heterogeneity. The evidence was rated as very low certainty, downgraded once for limitations, once for imprecision and once for inconsistency. Sensitivity analysis using a fixed‐effect model did not substantially impact the estimate of effect. We were unable to include data from one study (PROMISE, N = 218) in this analysis as, although their methods reported that this outcome was evaluated at this time point, the data were not reported.
2.1. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 1: Pain intensity: average post‐intervention. Short‐term follow‐up. Mean Difference
Proportion of participants reporting ≥ 50% pain relief
Two studies (de Vos 2014; SENZA‐PDN combined N= 249; Analysis 2.2) provided data for this outcome. The analysis demonstrated an effect in favour of SCS (RR 15.90 (95% CI 6.70 to, 37.74, P<0.001, I2 0% ; RD 0.65 (95% CI 0.57 to 0.74). This equates to an NNTB of 1.5 (95% CI 1.4 to 1.8). We graded the evidence as very low certainty, downgraded twice for serious limitations and once for imprecision. Sensitivity analysis using a fixed‐effect model did not impact the estimate of effect. We were unable to include data from one study (PROMISE, N=218) in this analysis as, although this outcome was reported to have been evaluated at this time point, the data were not reported.
2.2. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 2: Pain: participants with ≥50% pain relief. Short‐term follow‐up. Risk Ratio
Mean difference (MD)
Five studies (de Vos 2014; Kemler 2000; PROCESS; PROMISE; SENZA‐PDN, combined N = 635, Analysis 2.3) provided data aftersix months of stimulation. Pooling of these studies demonstrated a potentially clinically important mean difference in favour of SCS of ‐31.22 (95% CI ‐47 to 34 to ‐15.10, P < 0.001, I2 = 95%) with substantial heterogeneity. We graded the evidence as low certainty, downgraded once for limitations of studies, and once for inconsistency. Sensitivity analysis using a fixed‐effect model slightly reduced the estimate of effect (MD ‐27.76, 95% CI ‐31.26 to ‐24.26).
2.3. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 3: Pain intensity: average post‐intervention. Medium‐term follow‐up. Mean difference
Proportion of participants reporting ≥ 50% pain relief
Five studies (de Vos 2014; PROCESS; PROMISE; SENZA‐PDN; Slangen 2014, combined n = 597, Analysis 2.4) provided data for this outcome after six months of stimulation. Pooling of these studies demonstrated an effect in favour of SCS (RR 7.08, 95% CI 3.40 to 14.71, P < 0.001, I2 = 43%; RD 0.43, 95% CI 0.14 to 0.73). This equates to an NNTB of 2.3 (1.4 to 7.7). We graded the evidence as as low certainty, downgraded once for limitations of studies and once for imprecision. Sensitivity analysis using a fixed‐effect model resulted in a larger effect size for this comparison (RR 8.24, 95% CI 4.99 to 13.62).
2.4. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 4: Pain: participants with ≥50% pain relief. Medium‐term follow‐up. Risk Ratio
Mean difference (MD)
One study (Kemler 2000, N = 44 for this comparison, Analysis 2.5) provided data for this outcome after 5 years of stimulation. There was no clear evidence for an effect of SCS (MD ‐7, 95% CI ‐24.76 to 10.76, P = 0.44). We graded the evidence as very low certainty, downgraded twice for serious limitations of studies, once for imprecision and once for inconsistency. We were unable to include data from two studies (PROCESS; PROMISE, combined N = 318) in this analysis as, although the methods suggested this outcome was evaluated at this time point, the data were not reported for all participants randomised.
2.5. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 5: Pain intensity: average post‐intervention. Long‐term follow‐up. Mean difference
Proportion of participants reporting ≥50% pain relief
One study (PROCESS, N=87 for this comparison, Analysis 2.6) provided data for this outcome after 24 months of stimulation. The study reported a benefit of SCS (RR 15.15, 95% CI 2.11 to 108.91, P = 0.007; RD 0.35, 95% CI 0.2 to 0.49). This equates to an NNTB of 2.86 (95% CI 2.04 to 5). We graded the evidence as very low certainty, downgraded twice for serious limitations of studies, once for imprecision and once for inconsistency). We were unable to include data from one study (PROMISE, N = 218) in this analysis as, although the methods suggested this outcome was evaluated at this time point, the data were not reported for all participants randomised.
2.6. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 6: Pain: participants with ≥50% pain relief. Long‐term follow‐up. Risk Ratio
Reporting biases
We identified one study (NCT00200122) that was recorded in a trial registry but where we did not identify a full published study report. This study may have contributed data from a further 100 participants to our analyses. We estimated the number of participants in studies with no effect required to change the NNTB to an unacceptably high level (defined as an NNTB of 10), as outlined in Moore 2008 for each time point. This value was 297 (95% CI 210 to 420) at short term, 2033 (95% CI 179 to 3892) at medium‐term and 1490 (95%CI 597 to 2329) at long‐term follow‐up.
Adverse events: device/procedure‐related
Table 4 summarises the detail of other adverse events related to the device/ procedure.
2. Procedure/device‐related adverse events from studies comparing SCS + other interventions vs other interventions alone.
| Study ID | Total N randomised to stimulation | Total N implanted with electrodes in SCS group | Total N who received full device implantation in SCS group | Period of measurement |
Lead failure/ displacement (no. of events) |
Infection (no. of events) |
Need for reop (no. of events) |
Other AEs* (no. of events) |
Other AE details |
| Medium Term reporting | |||||||||
| de Vos 2014 | 40 | 40 | 37 | 6 months | 1 | 1 | 4 | 3 | 1 participant with coagulopathy, which complicated the implantation procedure and prolonged hospitalisation. 2 pain due to IPG |
| Kemler 2000 | 36 | 36 | 24 | 6 months | 5 | 1 | 11 | 13 | 2 dural puncture (1 with headache) Six of the 24 patients treated with spinal cord stimulation (25%) had a total of 11 other complications during the six months after implantation. Four patients had long‐term complications. |
| PROMISE | 110 | 102 | 82 | 6 months | NI | 8 | 12 | 6 | 1 Back pain Device stimulation issues 2 Device deployment issues 1 Device Battery issue 1 Implant site cellulitis 1 Implant site pain 2 Paraesthesia 1 Pelvic pain 1 Pulmonary oedema 1 Urinary tract infection |
| SENZA‐PDN | 113 | 104 | 90 | 6 months | 1 | 3 | 2 | 14 | 2 wound dehiscence 1 impaired healing 1 device extrusion 1 incision site pain 1 IPG site discomfort 1 contact dermatitis 1 urticaria 1 radiculopathy 1 uncomfortable stimulation 1 Gastrooesophageal reflux 1 myalgia 1 arthralgia 1 hyporeflexia |
| Slangen 2014 | 22 | 21 | 17 | 6 months | NI | 1 | NI | 2 | 1 Dural puncture leading to headache. Subsequent large subdural haematoma leading to death. 1 infection leading to device removal Participant did not fully recover and developed autonomic neuropathy |
| Long term reporting | |||||||||
| Kemler | 36 | 36 | 24 | 5 years | 17 | NI | 29 | 67**** | 19, Change of amplitude with bodily movements. 13 Paraesthesia over other body parts 11 Pain/irritation from IPG 7 More pain in other body parts 4 Disturbed urination 3 Movements or cramps resulting from elevated amplitude |
| PROCESS*** | 52 | 52 | 48 (78 implanted after cross‐over from control group) |
12 months | 12 (+1 device migration) | 7 | 20 | 14 | 5 "Technique" issues** 5 Pain at IPG site 4 Fluid collection in neurostimulator pocket |
| PROMISE*** | 110 | 102 | 82 (174 implanted overall after cross‐over from control group) |
2 years | NI | 8 | 12 | 54 | 12 Device stimulation issues 6 implant site pain 5 Back pain 4 Paraesthesia 3 Device dislocation 2 Device deployment issue 2 Procedural pain 2 Product ineffective 2 Therapeutic response decreased 2 Abdominal pain 1 Burning sensation 1 Dermatitis contact 1 Device battery issue 1 Extradural abscess 1 Extradural haematoma 1 Hypoaethesia 1 Implant site cellulitis 1 Implant site swelling 1 Monoparesis 1 Musculoskeletal pain 1 Pelvic pain 1 Postprocedural complication 1 Pulmonary oedema 1 Urinary tract infection |
* Other AEs reported here if there is a high likelihood of being directly related to the device/ procedure.
** "Technique" issues (1) Cap not installed on IPG when only one lead was implanted: (2) suboptimal connection of extension to IPG led to intermittent stimulation; (3) anteriorly implanted electrode caused shocks; (4) lead cut during implant; (5) dural tear during implant
*** Numbers of events for these studies reflects those randomised to SCS and those who crossed over from the control group after 6 months.
**** other device‐related AEs from Kemler 2000 reported a 2 year follow‐up.
All six trials for this comparison (de Vos 2014; Kemler 2000; PROCESS; PROMISE; SENZA‐PDN; Slangen 2014) reported some information regarding AEs though there was variation in the detail reported in terms of the methods used and in the completeness of reporting. In all of these trials the implantation process, including the pre‐implantation trial period occurred post‐randomisation. At long‐term follow‐up, two studies (PROCESS; PROMISE) only reported device/procedure‐related complications for the group randomised to SCS combined and any participants who crossed over to receive SCS from the control group. As a result these data could not be used in our analyses. We describe the number and nature of AEs at this time point for all participants implanted in Table 4. Details of the number and nature of procedure‐ and device‐related adverse events for all studies are presented in Table 4. It is difficult to estimate how many studies measured but failed to report each of these outcomes due to the lack of detail regarding the methods of AE measurement reported in the included studies.
Lead failure or displacement
Incidence of lead failure or displacement was inconsistently reported, and it is likely that for some trials this was reported under the umbrella of device‐related issues. As such it is likely that the data for these comparisons is an incomplete reflection of the true number of events.
Short‐term follow‐up
No studies reported the incidence of lead failure or displacement at short‐term follow‐up.
Medium‐term follow‐up
Three studies (de Vos 2014; Kemler 2000; SENZA‐PDN, pooled N = 330, Analysis 2.7) reported the number of instances of lead failure or displacement after six months of stimulation. The incidence of these events ranged from 1/113 (0.9%) to 5/36 participants (14%). Pooling these studies did not demonstrate clear evidence of an increased risk of lead failure/ displacement with SCS with uncertainty and heterogeneity (RD 0.04, 95% CI ‐0.04 to 0.11, P = 0.31, I2 = 64%). Sensitivity analysis using a fixed‐effect model did not substantially impact the estimate of effect. The evidence was graded to be of very low certainty, downgraded once for limitations of studies, and twice for serious imprecision.
2.7. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 7: Adverse events: electrode/lead failure or displacement. Medium‐term follow‐up
Long‐term follow‐up
One study (Kemler 2000, N = 44, Analysis 2.8) reported the number of cases of lead repositioning or replacement events at five‐year follow‐up. This study reported 17 events in the SCS group (RD 0.55, 95% CI 0.35 to 0.75, P < 0.001) equating to an NNTH of 1.8 (95% CI 1.3 to 2.9). We graded the evidence as very low certainty, downgraded once for limitations of studies, and once for inconsistency and once for imprecision.
2.8. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 8: Adverse events: electrode/lead failure or displacement. Long‐term follow‐up
Infection
Short‐term follow‐up
No studies reported the incidence of infection for the short term follow‐up period.
Medium‐term follow‐up
Four studies (de Vos 2014; Kemler 2000; PROMISE; SENZA‐PDN, pooled N = 548, Analysis 2.9) reported the number of infections after six months of stimulation. Rates ranged from 2.5% to 8% of those implanted with electrodes (including those who did and did not have a successful pre‐implantation trial period). Pooling these studies demonstrates an increased risk of infection (RD 0.04, 95% CI 0.01, 0.07, P =0.003, I2 0%) equating to an NNTH of 25 (95% CI 14.29 to 100). We graded the evidence as low certainty, downgraded once for limitations of studies, and once for imprecision. Sensitivity analysis using a fixed‐effect model did not substantially impact the estimate of effect.
2.9. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 9: Adverse events: infection. Medium‐term follow‐up
Long‐term follow‐up
No studies reported the incidence of infection by group allocation for the long‐term follow‐up period.
Need for reoperation, repeated implantation procedure(s)
All trials that reported on these outcomes reported either lead or device‐related issues some of which required reoperation.
Short‐term follow‐up
No studies reported this outcome for the short term follow‐up period.
Medium‐term follow‐up
Four studies (de Vos 2014; Kemler 2000; PROMISE; SENZA‐PDN, pooled N = 548, Analysis 2.10) reported on the need for repeated implantation/reoperation at this time point. As a percentage of the total number of participants randomised to stimulation, rates of reoperation at medium‐term follow‐up ranged from 2% to 30.5% (4 studies, de Vos 2014; Kemler 2000; PROMISE, SENZA‐PDN 299 participants randomised to stimulation). The pooled analysis indicates an increased risk of repeated procedures with heterogeneity (RD 0.11, 95% CI 0.02 to 0.21, P = 0.02, I2 = 86%). This equates to an NNTH of 9.1 (95% CI 4.8 to 50). We graded the evidence as very low certainty, downgraded once for limitations of studies, once for imprecision and once for inconsistency. Sensitivity analysis using a fixed‐effect model did not meaningfully impact the estimate of effect.
2.10. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 10: Adverse events: need for repeated implantation procedures. Medium‐term follow‐up
Long‐term follow‐up
One study (Kemler 2000, N = 44, Analysis 2.11) provided data for this outcome after five years of stimulation and reported 29 reoperation events, (risk difference (RD) of 0.94, 95% CI 0.80 to 1.07, P < 0.001) equating to an NNTH of 1.05 (95% CI 0.93 to 1.25). We graded the evidence as very low certainty, downgraded once for limitations of studies, once for imprecision and once for inconsistency.
2.11. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 11: Adverse events: need for repeated implantation procedures. Long‐term follow‐up
Serious and other adverse events related to procedure/device
There were some notable cases of SAEs or potential SAEs reported. Slangen 2014 (22 participants randomised to stimulation) reported that one participant experienced a dural puncture headache in the immediate pos‐operative period and a subsequent large subdural haematoma which, despite surgery, led to death. In the same study one participant contracted an infection of the SCS system leading to its removal. Despite antibiotic treatment the study reported that the participant did not fully recover and developed autonomic neuropathy. Dural puncture was also reported by Kemler 2000 (2 cases in 36 participants). de Vos 2014 (40 participants randomised to stimulation) reported one participant who had a coagulopathy leading to procedural complications and prolonged hospitalisation. Both the PROCESS and PROMISE studies (52 and 110 participants randomised to stimulation, respectively) reported one case of pulmonary oedema and urinary tract infection. The PROMISE study also reported a single case of an extradural abscess and an extradural haematoma with that participant experiencing resultant monoparesis at the time of study exit. Kemler 2000 reported one case of disturbed urination. SENZA‐PDN reported two SAEs, on request the authors clarified that one was a wound infection and one was a case of device extrusion.
Adverse events: other
For the analysis of "other adverse events" it was not possible to extract the average number of events per participant in order to treat these as continuous data. We analysed the number of participants who experienced one or more adverse events in each group. Adverse events included non‐SCS‐related AEs as reported in each paper and were reported to broadly consist of adverse drug reactions, new illnesses or injuries.
Short‐term follow‐up
No studies reported this outcome specifically at short‐term follow‐up.
Medium‐term follow‐up
Two studies (de Vos 2014; PROMISE, pooled N = 278; Analysis 2.12) reported on other AEs after six months of stimulation. Pooling these studies found no evidence for a difference in other adverse events (RD ‐0.05, 95% CI ‐0.16 to 0.06, P = 0.82, I2 = 0%). We graded the evidence as low certainty, downgraded once for limitations of studies and once for imprecision. Sensitivity analysis using a fixed‐effect model did not impact the estimate of effect.
2.12. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 12: Adverse events: other. Medium‐term follow‐up
Long‐term follow‐up
One study (PROCESS, N = 100, Analysis 2.13) reported other adverse events after 12 months of stimulation. There was no clear evidence for a difference between groups (RD ‐0.17, 95% CI ‐0.37 to 0.02, P = 0.07). We graded the evidence as very low certainty, downgraded once for limitations of studies, once for imprecision and once for inconsistency. Adverse events were reported as being mainly due to drug adverse events or the development of a new injury, illness or condition.
2.13. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 13: Adverse events: other. Long‐term follow‐up
Disability
Two studies (PROCESS; PROMISE) measured and reported disability as an outcome, both using the ODI.
Short‐term follow‐up
Neither study reported data on disability at short‐term follow‐up.
Medium‐term follow up
Pooling data from two studies (PROCESS; PROMISE, N = 312, Analysis 2.14) found no clear evidence for an effect of SCS on disability (MD ‐15.93, 95% CI ‐35.99 to 4.13, P = 0.12, I2 92%), but there was substantial heterogeneity. We graded the evidence as very low certainty, downgraded once for limitations of studies, once for imprecision and once for inconsistency. Sensitivity analysis using a fixed‐effect model substantially decreased the point estimate of effect and demonstrated an effect in favour of SCS (MD ‐9.74, 95% CI ‐13.97 to ‐5.51).
2.14. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 14: Disability (Oswestry Disabiility Index). Medium‐term follow‐up
Long‐term follow‐up
Neither study reported data on disability at long‐term follow‐up.
Health‐related quality of life
Short‐term follow‐up
One study (de Vos 2014, Analysis 2.15) shared data on request for this outcome in the short term. Using the EQ‐5D self‐reported perception of health scale (0 to100 with higher scores reflecting better health), the data suggest a positive effect on HR‐QoL (N = 55, MD 17, 95% CI 5.74 to 28.26). We graded the evidence as very low certainty, downgraded once for limitations of studies, once for imprecision and once for inconsistency. We were unable to include data from two studies (Kemler 2000, PROMISE, combined N = 272) in this analysis as, although the methods suggested this outcome was evaluated at this time point, the data were not reported.
2.15. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 15: Health‐Related Quality of Life. Short‐term follow‐up
Medium‐term follow‐up
Five studies (de Vos 2014; PROCESS; PROMISE; SENZA‐PDN; Slangen 2014 N=595, Analysis 2.16) contributed to this analysis. We were unable to include data from Kemler 2000 as HRQoL data, measured using the Nottingham Health Profile were only presented as percentage change rather than absolute values. Three studies presented EQ‐5D utility index values, one presented the EQ‐5D 0‐100 VAS scale and one the SF‐36 Physical Component Score. As a result, we pooled results using the SMD. The analysis showed a positive effect of SCS on HR‐QoL with heterogeneity (SMD 0.73, 95% CI 0.46 to 0.99, P < 0.001, I2 = 54%). We graded the evidence as low certainty, downgraded once for limitations of studies and once for inconsistency. It is notable that heterogeneity was largely due to the inclusion of the SENZA‐PDN study. Removal of this study results in an I2 of 0%. That was the only study in this comparison that delivered HF SCS but was also the study rated at high risk of bias on the highest number of domains. Sensitivity analysis using a fixed‐effect model did not meaningfully impact the estimate of effect.
2.16. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 16: Health‐Related Quality of Life. Medium‐term follow‐up
Long‐term follow‐up
Only one study (Kemler 2000, N = 44, Analysis 2.17) reported HRQoL results at long‐term follow‐up. This study reported no evidence for a difference in EQ‐5D visual analogue scale scores at five5‐year follow‐up (N = 44, MD ‐0.09, 95% CI ‐0.74 to 0.56). We graded the evidence as very low certainty, downgraded once for limitations of studies, once for imprecision and once for inconsistency. We were unable to include data from two studies (PROMISE, PROCESS, combined N = 318) in this analysis as, although the methods suggested this outcome was evaluated at this time‐point, the data were not reported for all participants as randomised.
2.17. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 17: Health‐Related Quality of Life. Long‐term follow‐up
Medication use
No studies reported medication use at short‐ or long‐term follow‐up.
Medium‐term follow‐up
Two studies (de Vos 2014; PROCESS, pooled N = 154, Analysis 2.18) reported the number of participants using different classes of analgesics at six months follow‐up. Pooling these studies demonstrated no strong evidence for a difference in the number of participants using of opioids (RR 0.77, 95% CI 0.58 to 1.01, P = 0.06, I2 0%, low‐certainty evidence, downgraded for limitations of studies and imprecision), NSAIDS (RR 0.69, 95% CI 0.43 to 1.09, P = 0.11, I2 0%, low‐certainty evidence, downgraded for limitations of studies and imprecision), antidepressants (RR 0.68, 95% CI 0.46 to 1.00, P = 0.05, I2 0%, lo‐ certainty evidence, downgraded for limitations of studies and imprecision), anticonvulsants (RR 0.80, 95% CI 0.33 to 1.94, P = 0.62, I2 75%, very low‐certainty evidence, downgraded for limitations of studies, imprecision and inconsistency) or paracetamol (acetaminophen) (1 study n = 60; RR 0.58, 95% CI 0.23 to 1.51, P = 0.27, low‐certainty evidence, downgraded for limitations of studies, inconsistency and imprecision). Though it is noted that the point estimates all indicated a possible reduction in the number of participants using these medications in the SCS group. Sensitivity analysis using a fixed‐effect model did not impact these estimates of effect.
2.18. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 18: Medication use: participants using medication. Medium‐term follow‐up
One study (PROCESS, N = 100, Analysis 2.19) measured daily doses of opioids and converted them to a morphine equivalent dose using "routine conversion tables". These were presented as a low and high range equivalent daily mg as for some drugs a range of values was provided. There was no clear evidence of a difference in morphine consumption in either the low range (MD mg, ‐28.60, 95% CI ‐102.65 to 45.45) or high range (MD mg, ‐48.20, 95% CI ‐140.57 to 44.17) values. We graded the evidence as very low certainty, downgraded for limitations of studies, imprecision and inconsistency.
2.19. Analysis.

Comparison 2: SCS + other intervention vs other intervention, Outcome 19: Medication use: morphine oral equivalent daily (mg). Medium‐term follow‐up
The PROCESS study reported that at six months follow‐up 8/52 participants in the SCS group and 1/48 participants in the control group ceased opioids. Slangen 2014 did not report changes in medication use consistently between groups making interpretation difficult.
We were unable to include data from SENZA‐PDN, (N = 226) in this analysis as, although the methods suggested this outcome was evaluated at this time‐point, the data were not reported.
Brief economic commentary
With two of the published RCTs (PROCESS; Slangen 2014), additional publications reported the results of economic evaluations that were planned and conducted as part of the original RCTs. Both compared SCS + medical management with medical management alone. PROCESS included participants with FBSS and Slangen 2014 with painful diabetic neuropathy.
In the PROCESS study, healthcare consumption data related to screening, the use of implantable SCS generators, hospital stays, drug and non‐drug‐related treatments were collected and used to estimate resource consumption and costs for participants using UK and Canadian national figures (from 2005 to 2006). This study was conducted across 12 centres in Europe, Canada, Australia and Israel between 2003 and 2005 and randomised 100 participants. HRQoL was measured using the EQ‐5D. The study reported unadjusted costs at six‐month follow‐up in the SCS group of 12,653 Euros (SD 2756) per participant compared to costs of EUR 2594 (SD 2939) in the CMM group (MD 10059, 95% CI 8742.39 to 11375.61). Improvements in HRQoL at this point were reported as a mean difference, in favour of SCS of 0.23 (95% CI 0.12 to 0.35) on the EQ‐5D index score. After adjustment for participant baseline characteristics, the authors report that SCS increased HRQoL by 0.21 (95% CI 0.00 to 0.33) points at an additional cost of EUR 9997 (95% CI 8435 to 11577). A full cost‐effectiveness analysis, including Quality Adjusted Life Years (QALYs) or incremental cost‐effectiveness ratios (ICERs), was not reported as the authors argued that this would require consideration of costs and quality of life effects beyond the six‐month trial time horizon.
Slangen 2014 conducted an economic evaluation from a societal and healthcare perspective. This study was conducted in two academic hospitals in the Netherlands and randomised 36 participants. EQ‐5D scores were used to calculate QALYs. Cost analysis included all healthcare costs according to Dutch guidelines, inclusive of costs relating to the intervention, costs incurred for other reasons attributable to PDN and its treatment, other healthcare costs and non‐healthcare costs derived by a questionnaire administered to participants. Incremental cost‐effectiveness ratios (ICERs) were derived for full societal costs. From a healthcare perspective costs were calculated based on successfully treated patients, defined as a patient experiencing ≥ 50% relief of pain intensity on a weighted numeric rating scale, for four days during daytime or nighttime, or a score of ≥ 6 on a 7‐point Likert scale (6 = much improved; 7 = very much improved) of the Patient Global Impression of Change (PIC) scale for pain and sleep at 12 months. Cost‐effectiveness was judged using maximum willingness to pay thresholds of EUR 20,000 and 80,000 per QALY gain. The evaluation had a 12‐month time horizon.
Total societal costs amounted to EUR 26,539.20 in the SCS group and EUR 5,313.45 in the medical management group. Intervention costs amounted to EUR 16,579.82 in the SCS group and EUR 341.97 in the medical management group. Implanted/ device materials accounted for 58% of intervention costs, while complications related to SCS resulted in costs of EUR 2388. Non‐healthcare costs were also higher in the SCS group than the medical management group (EUR 7797 and 3140, respectively) and included differences in costs due to informal care, productivity loss and loss of daily activities.
In terms of cost‐effectiveness from a societal perspective, SCS provided the most QALYs with an ICER of EUR 94,159.65 per QALY for SCS versus medical management. Using a range of willingness to pay threshold of EUR 20,000 to 80,000 per QALY, the probability that SCS was cost‐effective ranged between 0 and 46%. From a healthcare perspective, the ICER was EUR 34,518.85 per successfully treated patient.
We did not subject either of the economic evaluations to critical appraisal, and we do not attempt to draw any firm or general conclusions regarding the relative costs or efficiency of SCS + medical management compared with medical management alone. However, very limited evidence demonstrates that adding SCS to medical management to medical management alone is associated with substantial increases in healthcare costs and may be associated with increases in non‐healthcare costs. One small cost‐effectiveness model did not clearly demonstrate cost‐effectiveness. The relatively short‐time horizons of the available studies limit their applicability in assessing cost‐effectiveness. Thus, the included studies are not sufficient to confidently draw conclusions regarding the cost‐effectiveness of SCS.
Discussion
Summary of main results
We included 15 published studies in this review that randomised 908 participants. All the included evidence in this review relates to spinal cord stimulation(SCS). We identified no studies of dorsal root ganglion stimulation (DRGS) and thus have found no evidence to support or refute the use of DRGS for the treatment of chronic pain. We found no studies that compared spinal neuromodulation S(NMD)interventions with no treatment or usual care.
Pain
Active stimulation versus placebo
Spinal cord stimulation (SCS) versus placebo
We included nine studies that randomised 224 participants in total that compared SCS using a variety of stimulation parameters with some form of sham stimulation, in which a potentially therapeutic dose was not delivered to participants. We found very low‐certainty evidence of small short‐term effects of SCS versus sham on pain intensity that may not be clinically important. Heterogeneity of treatment effects was moderate and was not explained by stimulation parameters. These studies did not provide data at medium‐ or long‐term follow‐up.
Active stimulation versus other intervention versus other intervention alone
SCS + other intervention versus other intervention alone
We included six studies that randomised 684 participants in total that evaluated the addition of SCS versus either medical management or physical therapy versus medical management or physical therapy alone. We found evidence that SCS had large effects on pain intensity at both short‐term (very low‐certainty evidence) and medium‐term follow‐up (low‐certainty evidence), both in terms of average pain scores and the proportion of participants experiencing ≥ 50% pain relief. At long‐term follow‐up we found no clear evidence for a benefit of SCS on average pain scores (very low certainty), and evidence of a large effect on the proportion of participants experiencing ≥ 50% pain relief (very low certainty).
Adverse events
SCS is associated with a reasonably common incidence of procedure and device‐related complications including infection, lead failure or displacement, and the need for further surgical procedures. For example, at six months follow‐up our estimates suggest a 4% risk of infection, a 4% risk of lead failure/ displacement and an 11% risk of requiring reoperation/reimplantation. However, the certainty around our estimates of the risk of these events is low to very low. Studies also reported other procedure‐ and device‐related complications.
In the included studies we found reports of some serious adverse events that were highly likely to be associated with the intervention. These included one death resulting from a subdural haematoma following a dural puncture, autonomic neuropathy resulting from a procedure‐related infection, prolonged hospitalisation due to a coagulopathy that resulted in procedural complications, an extradural abscess leading to prolonged monoparesis, a case of pulmonary oedema, wound infection, and an incident of device extrusion.
Secondary outcomes
SCS versus placebo
We found limited data for our secondary outcomes for this comparison. There was insufficient evidence to draw conclusions regarding disability or medication use. We found no evidence for an effect of SCS versus sham on health‐related quality of life (HRQoL) (very low‐certainty evidence).
SCS + other intervention versus other intervention alone
We found no clear evidence for an effect of SCS on disability (very low‐certainty evidence). For HRQoL we found evidence for positive effects of SCS in the short and medium‐term (low certainty evidence) and no evidence for an effect at long‐term follow‐up (very low‐certainty evidence). We found no clear evidence for an effect of SCS on medication use at medium‐term follow‐up (low‐ to very low‐certainty evidence).
We found limited economic evidence. The available evidence indicates that SCS is associated with substantial increases in costs that are dominated by the costs of the device/apparatus and procedure, and the costs of managing complications. The only cost‐effectiveness analysis we included suggested that the cost‐effectiveness of SCS was uncertain at willingness to pay thresholds of both EUR 20,000 to 80,000 per Quality Adjusted Life Years (QALY).
Overall completeness and applicability of evidence
The absence of evidence relating to dorsal root ganglion stimulation (DRGS) prevents us from drawing any conclusions regarding the efficacy, effectiveness or safety of that intervention.
The included studies were conducted across a range of countries and, while limited information was provided on settings, all appeared to be conducted in specialist secondary care. We identified no studies that were conducted in lower‐ to middle‐income countries. Studies included participants with a range of painful conditions, many of which might be classified as neuropathic in nature, or are proposed to be potentially neuropathic e.g. failed back surgery syndrome (FBSS) or complex regional pain syndrome (CRPS). However, few studies in those latter conditions used formal assessments or criteria to determine the presence or absence of neuropathic mechanisms. We did not find adequate data to formally evaluate whether treatment effects differed by clinical condition, but the frequent lack of formal approaches to establishing pain of a likely neuropathic nature (e.g. Finnerup 2016) would have presented a further challenge to that process. The balance across sex of participants was even overall, but almost all studies offered no information on other demographic characteristics of participants such as ethnicity or socioeconomic status. Studies mainly recruited participants whose pain was refractory to prior clinical management and baseline levels of pain were uniformly high across all studies except those where participants were already receiving SCS prior to randomisation.
For the comparison SCS versus sham, three studies randomised participants after they had been considered to demonstrate a positive clinical response to a trial period of stimulation and three studies recruited participants who were already implanted with and were receiving SCS. As a result, these studies might be considered to be of an enriched enrolment design as participants were pre‐selected on the basis of their outcomes following SCS.
Studies comparing SCS versus sham were all small, with short‐term follow‐up and all employed a cross‐over design. We found no studies to inform a comparison of SCS versus placebo in the medium or long term. Some trials failed to report on our outcomes of interest at specific time points despite designating those outcomes in the trial registry record or trial protocol. We needed to request data from the authors of all the included studies, but we only received requested data from the authors of three studies. It is possible that these missing data may not be missing completely at random. We identified a small number of unpublished studies from conference abstracts and trial registry records. However, it is important to consider that this likely underestimates the true number of unpublished studies of SNMD as it cannot include studies that were not pre‐registered or presented at conferences.
While all trials reported adverse events in some form, there was a frequent lack of detail regarding the methods used to measure and classify adverse events (AEs). Reporting of both device‐/procedure‐related and other AEs varied substantially across studies. It is possible that AEs may be underreported in the included trials. In addition, the included trials may not be of adequate size to capture rare adverse events.
There were no adequate data to formally explore the potential differential effectiveness of different stimulation parameters (conventional, high frequency or burst). Preliminary data from our SCS versus sham comparison was not suggestive of marked differences, but this is based on very little data. In our comparisons of SCS + other versus other alone, all but one of the included studies used conventional SCS and one study used high‐frequency spinal cord stimulation(HF‐SCS).
The limited availability of cost‐effectiveness analyses embedded within the included studies substantially limits our ability to draw conclusions. Those data did not clearly demonstrate cost‐effectiveness and should be considered against a historic trend of increasing costs per surgery of SCS (Lad 2010). Similarly, we found little data to inform our analyses for the outcomes of disability and medication use.
Reviewing the ongoing studies that we identified, the majority are similar to included published studies. Most are either small, short‐term cross‐over studies, the majority of which evaluate burst SCS versus sham, or open‐label studies of SCS + medical management versus medical management alone, and vary between evaluating conventional, HF or burst SCS. We found only one parallel sham‐controlled study of SCS (HF) (MODULATE‐ LBP, N = 96), which proposed follow‐up beyond the short term (six months) and only three small studies of DRGS, two where the comparator was sham stimulation (DRKS00022557 N = 50; TSUNAMI DRG, N = 38), and the other medical management (PENTAGONS, N = 56).
Quality of the evidence
SCS versus placebo
All the assessed study results were at high risk of bias overall and were rated to be either at high risk or to present some concerns for multiple domains on the ROB2 tool for all comparisons where we made judgments. For sham‐controlled studies the lack of formal assessment of blinding success, the common use of per‐protocol analyses and the lack of washout periods seriously impact our confidence in those results. That we found only small effects of SCS versus sham despite these biases further reinforces the lack of compelling evidence to support the efficacy of SCS. It is notable that the one study in this comparison that we rated at low risk of bias relating to outcome measurement (blinding) found no evidence for an effect of SCS (Perruchoud 2013).
Studies included in this comparison were all relatively small, delivered short‐term interventions with only short‐term follow‐up, and were essentially exploratory in nature. To reduce the uncertainty there is a need for larger studies comparing SCS with sham over longer, more clinically‐relevant time periods with full reporting of both efficacy outcomes and adverse events. There is evidence that the size of the included studies may lead to an overly positive picture for some interventions (Deschartres 2013; Nuesch 2010). In a review of meta‐analyses, Deschartres 2013 demonstrated that trials with fewer than 50 participants, which reflects all the studies included in this comparison, returned effect estimates that were on average 48% larger than the largest trials and 23% larger than estimates from studies with sample sizes of more than 50. While there were no adequate data to formally explore publication bias, the identification of five potential unpublished studies with a total of 74 participants raises the possibility of such a bias in this evidence base.
SCS + other intervention versus other intervention alone
Open‐label comparisons resulted in much larger effect sizes at short‐ and medium‐term follow‐up. Study results in these comparisons were rated to be at high risk of bias overall and as such the resultant effect sizes may be exaggerated. The finding of large, clinically‐important effects in open‐label studies and a lack of compelling evidence for important effects from sham‐controlled studies raises questions regarding the mechanisms of SCS and how much of the observed effect might be explained by the contextual effects of undergoing this complex and invasive clinical procedure, rather than the specific effects of SCS. It might be argued that contextual (placebo) effects are unlikely to account for such large and sustained effects. However, the use of sophisticated technology, the invasive nature of the procedure, the need for frequent clinical interactions and treatment‐related sensory experiences and, in some cases, the costs of SNMD all have the potential to drive non‐specific effects.
There are parallels from other clinical interventions. Buchbinder 2018 reviewed the evidence for percutaneous vertebroplasty for osteoporotic vertebral compression fractures. Like SNMD, vertebroplasty is an invasive intervention with persistent pain a key outcome and where participants typically report high levels of baseline pain intensity. They found large effects on pain (mean difference ranged from the equivalent of 16 to 33 points on a 0 to 100 pain scale) in usual care comparisons at short‐, medium‐ and long‐term follow‐up, but evidence of no clinically important effects of vertebroplasty versus placebo (moderate to high‐certainty evidence). In contrast, in our review that evidence is of very low certainty. The rating of the results in these comparisons as being at high risk of bias or presenting “some concerns” across ROB2 domains that are not solely related to blinding further increases the risk that the observed effect sizes may be inflated.
There was insufficient evidence to explore small‐study/potential publication biases through the use of funnel plots or associated tests. Using the method outlined by Moore 2008, we estimated the number of participants in studies with no effect required to change the number needed to treat for an additional beneficial outcome (NNTB) to an unacceptably high level (defined as an NNTB of 10) and found that in general, large numbers of participants in negative studies would be needed for that outcome. This is a function of the consistently large effect sizes observed in included studies and subsequent low NNTBs, which, as discussed above, may to some extent reflect a number of study‐level biases.
The evidence for adverse events was of poorer quality than for pain. This was largely due to substantial variation and frequent lack of detail in the detail of reporting of the methods used to record and classify adverse events, and inconsistency in the level of reporting of adverse events.
Eleven of the 15 included studies declared some funding or sponsorship from industry entities with an interest in the manufacture and sale of SCS equipment and the sponsor's role in the study was not always made clear. Ten studies reported one, or commonly, more authors with declared relationships with industry related to SNMD technology. These took the form of non‐financial support, consultancies, stock options, honoraria, speakers fees and travel grants. Another study reported that an author had obtained a patent for the intervention under investigation. Lundh 2017 found moderate‐ to low‐certainty evidence that industry‐sponsored studies of drugs or medical devices more often report more favourable efficacy results and conclusions, though similar harms results compared to non‐industry sponsored studies. While we provide no direct evidence in this review, this potential for bias should be considered when interpreting these results.
Potential biases in the review process
We have conducted searches across a range of databases, included studies regardless of language, searched for unpublished completed studies and contacted study authors to ensure our review is as inclusive of the available evidence as is possible.
Due to the lack of necessary phase‐by‐phase data we deviated from our protocol plan for analysing cross‐over studies, but did not have the required data to make adjustments for the paired nature of these studies. This presents a potential unit of analysis issue. As such, while point estimates in this analysis should be accurate representations of the data it is likely that these analyses may be conservative, specifically in terms of overestimating imprecision. The effect size for the SCS versus sham comparison on pain intensity is slightly more conservative than that seen in a recent review by Duarte 2020 who were able to make adjustments for the paired nature of the data and reported a mean difference equivalent to ‐11.5 (95% CI ‐17.5 to ‐5.5) on a 0 to 100 visual analogue scale (VAS). However, this might better be explained by differences in the studies and data included between the two reviews. We included an additional trial arm from one study (Tjepkema‐Cloostermans 2016) and three comparisons from another study (Sokal 2020), and excluded a study (Wolter 2012) that was included by Duarte 2020. The exclusion of those data from Sokal 2020 and Tjepkema‐Cloostermans 2016 from our analysis results in an effect size very similar to that reported by Duarte 2020 (‐11.1 (95% CI ‐17.4 to ‐4.70), suggesting that our analysis approach may have had minimal impact on our findings.
We planned to analyse on an intention‐to‐treat basis. In many trials, where the pre‐implantation trial period occurred post‐randomisation a proportion of participants (median 19%, range (7% to 33 %) did not receive permanent implantation. In terms of adverse events, our analysis includes all available participants randomised to SCS regardless of whether permanent implantation followed. As a result, these data may underestimate the rate of AEs in those receiving permanent implantation.
We only included economic evaluations that were conducted as part of the included randomised controlled trials (RCTs.) This has limited the amount of health economic evidence from which we can draw conclusions.
Agreements and disagreements with other studies or reviews
Duarte 2020 conducted a systematic review of placebo‐controlled SCS studies. Despite minor differences in inclusion and analytical approach discussed above they found similar results to ours, with modest effects of SCS versus sham, though our analysis is more conservative. Duarte 2020 investigated the potential influence of blinding and found much smaller effects when their analysis was limited to studies they assessed as more likely to be effectively blinded, though that included studies that did not formally evaluate the success of blinding.
In a review of SCS for chronic spinal pain, Grider 2016 concluded that there misquote: "significant level I to II evidence" of the efficacy of SCS in lumbar failed back surgery syndrome (FBSS), moderate evidence for high‐frequency stimulation, and limited evidence for burst stimulation. While the conclusions of that review are nominally more positive than our review, it is important to note that our review is more current, includes more studies and a wider range of conditions, and has arguably taken a more robust approach to assess risk of bias and the certainty of the evidence. It is also noteworthy that Grider 2016 did not evaluate the potential harms of SCS.
Eldabe 2016 conducted a review of the literature relating to the complications of SCS. The authors included a range of study designs including RCTs, systematic reviews and retrospective studies. They reported an overall incidence of complications of 30% to 40%. Mean rates were reported for lead failure (fracture of malfunction) 6% (95% CI 2% to 10%, lead migration 15.5% (95% CI 9% to 22%, infection (4.8% (95% CI 3.4% to 6.4%). These rates are broadly similar to those in this review. Also similar to our findings serious adverse events (SAEs) were generally the result of neurological injury and also included a case of paraplegia as a result of SCS implantation. Eldabe 2016 noted, as we have, that the reporting of complications of SCS is variable in the literature and makes recommendations for more consistent, clearer reporting.
With regards cost‐effectiveness, Niyomsri 2020 reviewed evidence of economic evaluations from controlled studies of SCS or DRGS. Based on 14 studies, the authors concluded that when considering a long‐term time horizon SCS is cost‐effective particularly for the management of FBSS and CRPS. In contrast to our review, there was no requirement for the economic evaluations to have been planned and conducted as part of the original RCTs and the inclusion criteria did not exclude non‐randomised studies.
A recent systematic review (McNicol 2021) scrutinised the design characteristics and quality of reporting of RCTs of SCS for the treatment of pain, in children and adults. Using broader inclusion criteria than our review, McNicol 2021 identified substantial deficiencies in the reporting and methodology of SCS trials, including issues with inadequate study size, the use and adequacy of blinding, clear and appropriate use and reporting of intention‐to=treat analysis, clarity over the sponsor's role and the methods for collecting and the reporting of adverse events. These issues were all present and identified as issues in our review.
Health technology appraisals (HTA) from Canada (MoH‐LTC 2005) and the UK (NICE 2008) have recommended SCS for people with chronic pain of neuropathic origin who had not responded to conventional medical management. These appraisals are not based on the most recent evidence and do not reflect the uncertainty arising from subsequent sham‐controlled studies, regarding the clinical benefit of SNMD over placebo stimulation. A more recent HTA in the UK (NICE 2019), focused on the SENZA high‐frequency spinal cord neuromodulation device, recommended the system for chronic neuropathic back or leg pain after failed back surgery based on limited evidence from two small trials comparing high‐frequency stimulation with conventional stimulation, but did not include any comparison with sham stimulation. The European Academy of Neurology (EAN) guidelines on central neurostimulation therapy in chronic pain conditions (Cruccu 2016) made a "weak recommendation" to add SCS to medical management in painful diabetic neuropathy, chronic post‐surgical back and leg pain, and complex regional pain syndrome (CRPS) type I. Similar to our review, the authors highlighted the quality of the evidence as a key issue in this evidence base.
Authors' conclusions
Implications for practice.
For people with chronic pain
There is low‐ to very low‐certainty evidence that implanted spinal cord stimulation (SCS) devices provide clinically important benefits for pain intensity and benefits on health‐related quality of life (HRQoL) when added to conventional medical management or physical therapy. However, we also found very low‐certainty evidence that SCS may not provide clinically important benefits on pain intensity or HRQoL when compared with placebo (sham) stimulation. These findings raise questions about how much of the observed benefits of SCS may result from the stimulation itself and how much may be the result of the contextual effects of receiving this complex, expensive and invasive intervention. SCS can result in relatively common complications such as infection, electrode lead failure or migration and a need for further surgical procedures. We found instances of serious adverse events (SAEs)resulting from unintended neurological injury including one death, but were not able to accurately estimate the risk of these. We found no clear evidence of benefit from SCS for disability or medication use. The low to very low certainty of our findings means that our confidence in them is limited. We found no evidence at all to support or refute the use of dorsal root ganglion stimulation (DRGS) for chronic pain.
For clinicians
We found very low‐certainty evidence that SCS may not provide clinically important benefits on pain intensity when compared to a sham (placebo) stimulation and low‐ to very low‐certainty evidence that SCS when added to medical care or physical therapy may provide clinically important benefits for pain intensity and health‐related quality of life (HRQoL) for people with persistent pain, but no clear evidence for a beneficial effect for disability or medication use. SCS can result in complications such as infection, electrode lead failure or migration and a need for further surgical procedures. We found instances of SAEs resulting from unintended neurological injury including one death, but were not able to accurately estimate the risk of these. The low to very low certainty of our findings means that our confidence in them is limited. We found no evidence to support or refute the use of DRGS for chronic pain.
For policymakers and funders of the intervention
We found very low‐certainty evidence that SCS may not provide clinically important benefits on pain intensity when compared to a sham (placebo) stimulation and low‐ to very low‐certainty evidence that SCS when added to medical care or physical therapy may provide clinically important benefits for pain intensity and HRQoL for people with persistent pain, but no clear evidence for a beneficial effect for disability or medication use. SCS can result in complications such as infection, electrode lead failure or migration and a need for further surgical procedures. We found instances of SAEs resulting from unintended neurological injury including one death, but were not able to accurately estimate the risk of these. The low to very low certainty of our findings means that our confidence in them is limited. We found limited economic evidence, but the included evidence suggests that SCS is associated with substantial additional healthcare costs, which are dominated by the costs of the device/apparatus and the implantation processes and the costs of managing complications. The only cost‐effectiveness analysis that we included suggested that the cost‐effectiveness of SCS was uncertain at willingness to pay thresholds of both 20,000 to 80,000 Euros perQuality Adjusted Life Years (QALY). We found no evidence at all to support or refute the use of DRGS for chronic pain. While some guidelines make recommendations for spinal neuromodulation (SNMD) interventions for selected participants with chronic pain, they do not specifically consider the uncertainty around clinical benefit compared to placebo stimulation based on current evidence (MoH‐LTC 2005; NICE 2008; NICE 2019).
Implications for research.
General
We have identified that a key area of uncertainty is whether SCS provides clinically important benefits versus placebo. To reduce uncertainty around this question there is a need for larger studies. It can be argued that further small, short‐term cross‐over studies, of the type that dominate the ongoing studies we have identified for this comparison are unlikely to meaningfully improve certainty. Instead, larger parallel trials, that compare SNMD approaches with placebo for a more clinically relevant time period and that fully report both important efficacy outcomes and adverse effects are needed. In our search for ongoing studies we identified one trial with this type of design in people with chronic low back pain (MODULATE‐ LBP, N = 96) but arguably further such trials are needed, and in a broader range of conditions. While further open‐label studies of SNMD might improve the precision of our estimates of effectiveness they will not reduce the uncertainty around the important question of the mechanisms of observed effects.
The field has generated and might continue to develop and promote novel forms of stimulation with claims of superior efficacy. There is currently uncertainty surrounding the efficacy of all existing forms of SNMD. There is a need to establish clear evidence of efficacy for all forms of SCS over placebo. Until there is compelling evidence for the efficacy of existing forms of SNMD any novel stimulation approach should also be validated by comparison with placebo. Future trials should include a formal analysis of healthcare and non‐healthcare costs with long‐term time horizons and conduct formal cost‐effectiveness analysis. Future studies should also be clear as to whether or not participants are recruited on the basis of pain of suspected neuropathic nature and clearly report the approaches used to establish that. If efficacy (versus placebo) is established with certainty then it would be valuable to study how SNMD interventions impact on supported self‐management of persistent pain.
The evidence base is dominated by industry‐sponsored studies and there is a high rate of authors' declarations of industry relationships. Publicly‐funded trials, independent of industry involvement would improve confidence in this evidence base. There is an urgent need to improve the audit trail and transparency for trials in this field with pre‐registration and regular updating of trial status in the registries, routine availability of study protocols and statistical analysis plans, and posting results in the trials registries. Given the apparent trade‐off between clinical benefits and potentiallySAEs there would be value in further formal evaluation of patient preferences. These should be conducted independently of industry involvement.
Design
Placebo‐controlled trials in this, as in all surgical fields, are challenging but can be feasible (Wartolowska 2016). There are specific challenges for SNMD trials that require careful attention, relating to threats to participant blinding associated with the handheld programming units, battery recharging requirements and the presence of paraesthesias (the latter particularly with conventional stimulation). In a recent consensus exercise which aimed to identify important elements for successful sham controls for physical interventions, Braithwaite 2020 found that essential strategies centred around maintaining the credibility of the sham by adhering to expectations and beliefs of participants using clinical interactions, professional behaviours, trial information and environmental setup, whereas exact replication of the intervention itself did not feature as strongly. This suggests that careful consideration needs to be given to the broader clinical interaction in sham‐controlled trials of SCS to maximise the credibility of the sham condition. For all sham‐controlled studies a formal assessment of the success of blinding and/or the credibility of sham controls should be considered essential.
The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT), the Institute of Neuromodulation (ION), and the International Neuromodulation Society (INS) have recently published recommendations (Katz 2021) on the design, conduct, analysis, and interpretation of RCTs of SCS for chronic pain and many of their recommendations are closely aligned with ours. Specifically, they recommend trials disclose all funding sources and potential conflicts; incorporate mechanistic objectives when possible; avoid non‐inferiority designs without internal demonstration of assay sensitivity; achieve and document double‐blinding whenever possible; document investigator and site experience; keep all information provided to patients balanced with respect to the expectation of benefit; disclose all information provided to patients, include verbal scripts; use placebo/sham controls when possible; account for ancillary pharmacologic and nonpharmacologic treatments in a clear manner; provide a complete description of intended and actual programming interactions; make a prospective ascertainment of SCS‐specific safety outcomes; train patients and researchers on appropriate expectations, outcome assessments, and other key aspects of study performance; and provide transparent and complete reporting of results according to applicable reporting guidelines. Future trial reports should fully comply with CONSORT (Schulz 2010).
Outcome assessment
Future trials must include measurement of outcomes known to be important to people with chronic pain. Katz 2021 recommend the following core domains: pain intensity, physical function, emotional functioning, global improvement or satisfaction, concomitant and rescue medications, patient disposition to treatment, sleep and fatigue, health‐related quality of life, costs and cost‐effectiveness. There is a need for improvement in the methodology and reporting of adverse events. Careful, long‐term active surveillance for known complications of SCS is essential and should include all cases of neurological injury and any resultant SAEs. The nature and incidence of all AEs should be reported in full. In addition to clinical trials, there would be value in the establishment of national and international clinical registries to allow more comprehensive surveillance of safety outcomes for these interventions.
What's new
| Date | Event | Description |
|---|---|---|
| 25 April 2022 | Amended | Axes labels for Analysis 2.1 corrected. |
| 7 December 2021 | Amended | Information added to Implications for research section. |
History
Protocol first published: Issue 10, 2020 Review first published: Issue 12, 2021
Risk of bias
Risk of bias for analysis 1.1 Pain Intensity: average post‐intervention. Short‐term follow up. Mean difference.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.1.1 Conventional SCS | ||||||||||||
| Kriek 2017 | High risk of bias | While the study was randomised there was no report of period effects being accounted for. The washout period employed was only for 2 days with no data provided to confirm the absence of carry‐over effects. | High risk of bias | Conventional stimulation is suprathreshold. HF and burst stimulation may be subperceptual however no formal analysis of blinding was conducted. Per protocol analysis was employed. | High risk of bias | 4 participants (12%) discontinued during the stiudy and were excluded from the analysis. Of these 2 experienced lead dislocation and one an increase in pain. | High risk of bias | Conventional stimulation is suprathreshold. HF and burst stimulation may be subperceptual however no formal analysis of blinding was conducted. | Low risk of bias | No SAP Available but outcomes and analysis appear consistent with published protocol. | High risk of bias | High risk of bias on multiple domains |
| Sokal 2020 | High risk of bias | "Randomisation was performed by drawing notes with the name of the modality or treatment arm (i.e., LF tonic, 1kHz, clustered tonic, sham) by an independent examiner. At the next meeting (after two weeks), the selection of notes with names of modalities was modified by the notes that were previously chosen." Comment: Method prone to bias and concealment processes not really clear. Also no clear accounting for period effects and no washout priod employed. |
High risk of bias | Conventional stimulation is suprathreshold and effectively unblinded. High frequency and burst stimulation is likely subthreshold but blinding success was not formally evaluated. No information provided about deviations from intended interventions or on how the analysis was approached. | Low risk of bias | No post‐randomisation exclusions reported. | High risk of bias | Conventional stimulation is suprathreshold. HF and burst stimulation may be subperceptual however no formal analysis of blinding was conducted. | Some concerns | No published protocol or SAP. Registry documents suggest 1 year follow up which does not make sense in the context of this study. | High risk of bias | High risk of bias for mutliple domains |
| Tjepkema‐Cloostermans 2016 | High risk of bias | No detail on methods used to randomise or conceal allocation. Period effects were dismissed based on a lack of statistical significance. No washout period was employed. | High risk of bias | Conventional stimulation is suprathreshold and therefore likely to be unblinded. Burst stimulation may be sub‐perceptual however no formal analysis of blinding was conducted. No information reported about deviations from intended interventions or how they were managed in the analysis. | Low risk of bias | Only 1 participant withdrew post‐randomisation. | High risk of bias | Conventional stimulation is suprathreshold. Burst stimulation may be subperceptual however no formal analysis of blinding success was conducted. | Some concerns | Trial registry reports VAS is primary outcome but the published report demonstrates potential for selection "During all phases of the study, patients were requested to fill in a pain diary, including as VAS scores (on a scale of 0–100) forpain in their back, legs, and feet separately. The average VAS scores for pain during the last three days of each stimulation period (tonic at baseline, low amplitude burst, and high amplitude burst) were used to assess the pain score for that period, thereby the score of the most affected body part, defined as the body part with the highest VAS score at baseline during tonic stimulation, was used." | High risk of bias | High risk of bias on multiple domains |
| Subgroup 1.1.2 High frequency SCS | ||||||||||||
| Al‐Kaisy 2018 | Some concerns | Randomised with allocation concealment. No washout was applied. While carryover effects were reported as not statistically significant there is potential for carryover effects and no reporting of baseline scores by phase. | High risk of bias | While stimulation subperceptual there was no formal assessment of blinding. Battery recharginh issues may have impacted nlinding at 5882 Hz stimulation intensity. There were 20% post randomisation exclusions without specific detail oregarding suring which phase they occurred and a per protocol analysis was employed. | High risk of bias | 20% post randomisation exclusions. Some of these are related to the intervention itself and thus likely related to the true value. Per protocol analysis employed. | Some concerns | Stimulation likely sub‐perceptual but no formal assessment of blinding. Stimulation at 5882 Hz associated with differences in battery recharging requirement which may have impacted blinding negatively. Outcome is subjective and self‐reported. | Some concerns | No statistical analysis plan available. Analysis intentions not reported in sufficient detail to enable assessment in published report. | High risk of bias | High risk of bias on mutliple domains |
| Al‐Kaisy 2018 | Some concerns | Randomised with allocation concealment. No washout was applied. While carryover effects were reported as not statistically significant there is potential for carryover effects and no reporting of baseline scores by phase. | High risk of bias | While stimulation subperceptual there was no formal assessment of blinding. Battery recharginh issues may have impacted nlinding at 5882 Hz stimulation intensity. There were 20% post randomisation exclusions without specific detail oregarding suring which phase they occurred and a per protocol analysis was employed. | High risk of bias | 20% post randomisation exclusions. Some of these are related to the intervention itself and thus likely related to the true value. Per protocol analysis employed. | Some concerns | Stimulation likely sub‐perceptual but no formal assessment of blinding. Stimulation at 5882 Hz associated with differences in battery recharging requirement which may have impacted blinding negatively. Outcome is subjective and self‐reported. | Some concerns | No statistical analysis plan available. Analysis intentions not reported in sufficient detail to enable assessment in published report. | High risk of bias | High risk of bias on mutliple domains |
| Al‐Kaisy 2018 | Some concerns | Randomised with allocation concealment. No washout was applied. While carryover effects were reported as not statistically significant there is potential for carryover effects and no reporting of baseline scores by phase. | High risk of bias | While stimulation subperceptual there was no formal assessment of blinding. Battery recharginh issues may have impacted nlinding at 5882 Hz stimulation intensity. There were 20% post randomisation exclusions without specific detail oregarding suring which phase they occurred and a per protocol analysis was employed. | High risk of bias | 20% post randomisation exclusions. Some of these are related to the intervention itself and thus likely related to the true value. Per protocol analysis employed. | Some concerns | Stimulation likely sub‐perceptual but no formal assessment of blinding. Stimulation at 5882 Hz associated with differences in battery recharging requirement which may have impacted blinding negatively. Outcome is subjective and self‐reported. | Some concerns | No statistical analysis plan available. Analysis intentions not reported in sufficient detail to enable assessment in published report. | High risk of bias | High risk of bias on mutliple domains |
| Kriek 2017 | High risk of bias | While the study was randomised there was no report of period effects being accounted for. The washout period employed was only for 2 days with no data provided to confirm the absence of carry‐over effects. | High risk of bias | Conventional stimulation is suprathreshold. HF and burst stimulation may be subperceptual however no formal analysis of blinding was conducted. Per protocol analysis was employed. | High risk of bias | 4 participants (12%) discontinued during the stiudy and were excluded from the analysis. Of these 2 experienced lead dislocation and one an increase in pain. | High risk of bias | Conventional stimulation is suprathreshold. HF and burst stimulation may be subperceptual however no formal analysis of blinding was conducted. | Low risk of bias | No SAP Available but outcomes and analysis appear consistent with published protocol. | High risk of bias | High risk of bias on multiple domains |
| Perruchoud 2013 | High risk of bias | No information about methods used to randomise or conceal allocation. No washout period employed. | High risk of bias | While steps were taken to blind participants and high‐frequency stimulation is likely sub‐perceptual, clinical personnel were not all blinded. No information provided about deviations from intended interventions. Per protocol analysis employed. | High risk of bias | 5/38 (13%) excluded post randomisation. All appear to have had device issues. Per protocol analysis conducted. | Low risk of bias | Assessor blinded and a formal assessment suggests blinding was successful. | Some concerns | No protocol or statistical analysis plan available. | High risk of bias | High risk of bias on multiple domains. |
| Sokal 2020 | High risk of bias | "Randomisation was performed by drawing notes with the name of the modality or treatment arm (i.e., LF tonic, 1kHz, clustered tonic, sham) by an independent examiner. At the next meeting (after two weeks), the selection of notes with names of modalities was modified by the notes that were previously chosen." Comment: Method prone to bias and concealment processes not really clear. Also no clear accounting for period effects and no washout priod employed. |
High risk of bias | Conventional stimulation is suprathreshold and effectively unblinded. High frequency and burst stimulation is likely subthreshold but blinding success was not formally evaluated. No information provided about deviations from intended interventions or on how the analysis was approached. | Low risk of bias | No post‐randomisation exclusions reported. | High risk of bias | Conventional stimulation is suprathreshold. HF and burst stimulation may be subperceptual however no formal analysis of blinding was conducted. | Some concerns | No published protocol or SAP. Registry documents suggest 1 year follow up which does not make sense in the context of this study. | High risk of bias | High risk of bias for mutliple domains |
| Subgroup 1.1.3 Burst SCS | ||||||||||||
| Kriek 2017 | High risk of bias | While the study was randomised there was no report of period effects being accounted for. The washout period employed was only for 2 days with no data provided to confirm the absence of carry‐over effects. | High risk of bias | Conventional stimulation is suprathreshold. HF and burst stimulation may be subperceptual however no formal analysis of blinding was conducted. Per protocol analysis was employed. | High risk of bias | 4 participants (12%) discontinued during the stiudy and were excluded from the analysis. Of these 2 experienced lead dislocation and one an increase in pain. | High risk of bias | Conventional stimulation is suprathreshold. HF and burst stimulation may be subperceptual however no formal analysis of blinding was conducted. | Low risk of bias | No SAP Available but outcomes and analysis appear consistent with published protocol. | High risk of bias | High risk of bias on multiple domains |
| Schu 2014 | High risk of bias | While the study was randomised there was no report of period effects being accounted for and no washout period was employed. | Some concerns | Conventional stimulation is suprathreshold and the resulting sensations prevent proper blinding compared to sham. Burst and High‐frequency stimulation may be subperceptual but no formal evaluation of blinding employed. No information provided regarding deviations from intended interventions. Intention to treat analysis was conducted. | Low risk of bias | No post randomisation exclusions. | Some concerns | Burst stimulation may be subperceptual but no formal evaluation of blinding employed. | Some concerns | No registry record, protocol or SAP available, | High risk of bias | High risk of bias or some concerns for multiple domains |
| Sokal 2020 | High risk of bias | "Randomisation was performed by drawing notes with the name of the modality or treatment arm (i.e., LF tonic, 1kHz, clustered tonic, sham) by an independent examiner. At the next meeting (after two weeks), the selection of notes with names of modalities was modified by the notes that were previously chosen." Comment: Method prone to bias and concealment processes not really clear. Also no clear accounting for period effects and no washout priod employed. |
High risk of bias | Conventional stimulation is suprathreshold and effectively unblinded. High frequency and burst stimulation is likely subthreshold but blinding success was not formally evaluated. No information provided about deviations from intended interventions or on how the analysis was approached. | Low risk of bias | No post‐randomisation exclusions reported. | High risk of bias | Conventional stimulation is suprathreshold. HF and burst stimulation may be subperceptual however no formal analysis of blinding was conducted. | Some concerns | No published protocol or SAP. Registry documents suggest 1 year follow up which does not make sense in the context of this study. | High risk of bias | High risk of bias for mutliple domains |
| Tjepkema‐Cloostermans 2016 | High risk of bias | No detail on methods used to randomise or conceal allocation. Period effects were dismissed based on a lack of statistical significance. No washout period was employed. | High risk of bias | Conventional stimulation is suprathreshold and therefore likely to be unblinded. Burst stimulation may be sub‐perceptual however no formal analysis of blinding was conducted. No information reported about deviations from intended interventions or how they were managed in the analysis. | Low risk of bias | Only 1 participant withdrew post‐randomisation. | High risk of bias | Conventional stimulation is suprathreshold. Burst stimulation may be subperceptual however no formal analysis of blinding success was conducted. | Some concerns | Trial registry reports VAS is primary outcome but the published report demonstrates potential for selection "During all phases of the study, patients were requested to fill in a pain diary, including as VAS scores (on a scale of 0–100) forpain in their back, legs, and feet separately. The average VAS scores for pain during the last three days of each stimulation period (tonic at baseline, low amplitude burst, and high amplitude burst) were used to assess the pain score for that period, thereby the score of the most affected body part, defined as the body part with the highest VAS score at baseline during tonic stimulation, was used." | High risk of bias | High risk of bias on multiple domains |
Acknowledgements
We would like to thank Joanne Abbott for her support in developing the search strategy, and Anna Erskine and Kerry Harding for their editorial support.
Cochrane Review Group funding acknowledgement: this project was funded by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
We would like to thank Dr Cecile De Vos, Dr Erika Petersen and Dr Marius Kemler, for sharing and clarifying aspects of the data from their study as requested and Dr Rui Duarte for independently reviewing the lists of included and excluded studies.
We would like to thank all of our peer reviewers for their expertise and feedback.
We would like to thank the Cochrane Methods group for their support with implementing Risk of Bias 2.
Editorial and peer‐reviewer contribution
The Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS) supported the authors in the development of this review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Prof. Peter Tugwell, Professor of Medicine, & Epidemiology and Community Medicine, Canada;
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Anna Erskine, Oxford University Hospitals (OUH) NHS Foundation Trust, Oxford, UK;
Assistant Managing Editor (conducted editorial checks and supported editorial team): Kerry Harding, Oxford University Hospitals (OUH) NHS Foundation Trust, Oxford, UK;
Contact Editor (editorial and methods input): Associate Professor Ewan McNicol MCPHS University, Boston, Massachusetts, USA;
Information Specialist (preparing search strategy and running searches): Joanne Abbott, Oxford University Hospitals (OUH) NHS Foundation Trust, Oxford, UK;
Copy‐editing (initial copy‐edit and final proofread): Mrs Heather Maxwell, Copy‐edit group;
Peer‐reviewers (provided comments and recommended an editorial decision): Mia Elena Koponen (clinical/content review); Patrice Forget, University of Aberdeen, NHS Grampian (clinical/content review); Bernhard Frank, Consultant in Pain Medicine, The Walton Centre NHS Foundation Trust, Liverpool, UK (clinical/content review); Alex Green (clinical/content review); Sean Martin (clinical/content review); Thomas Shelton, physiotherapist specialising in Rheumatology at Mid Yorkshire NHS Trust (clinical/content review); Keith Meldrum, Consumer; A Path Forward (consumer review); Danial Sayyad (consumer review); Kerry Dwan, Methods Support Unit, Editorial & Methods Department, The Cochrane Collaboration (methods review); and Iris Gordon, Information Specialist for Cochrane Eyes and Vision Review Group (search review).
Appendices
Appendix 1. Main database search strategies
CENTRAL
#1 MeSH descriptor: [Pain] explode all trees
#2 (((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) Near/4 pain*)):ti,ab,kw (Word variations have been searched)
#3 ((sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* Near/2 neuralg*) or (herp* Near/2 neuralg*) or (diabet* Near/2 neuropath*) or (reflex Near/4 dystroph*) or (sudeck* Near/2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back Near/4 surg*) or (failed back Near/4 syndrome*))):ti,ab,kw (Word variations have been searched)
#4 #1 or #2 or #3
#5 MeSH descriptor: [Spinal Cord Stimulation] this term only
#6 (((spinal cord Near/3 (stimulat* or electrostimulat*)) or (dorsal root Near/3 (stimulat* or electrostimulat*)))):ti,ab,kw (Word variations have been searched)
#7 (((epidural Near/3 (stimulat* or electrostimulat*)) or SENZA or neuromodul*)):ti,ab,kw (Word variations have been searched)
#8 #5 or #6 or #7
#9 #4 and #8
MEDLINE
1 exp Pain/ (399276)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).tw. (153557)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).tw. (45604)
4 1 or 2 or 3 (495370)
5 Spinal Cord Stimulation/ (1055)
6 ((spinal cord adj3 (stimulat* or electrostimulat*)) or (dorsal root adj3 (stimulat* or electrostimulat*))).tw. (5055)
7 ((epidural adj3 (stimulat* or electrostimulat*)) or SENZA or neuromodul*).tw. (17800)
8 5 or 6 or 7 (22179)
9 4 and 8 (3646)
10 randomized controlled trial.pt. (515552)
11 controlled clinical trial.pt. (93892)
12 randomized.ab. (495808)
13 placebo.ab. (211750)
14 drug therapy.fs. (2244366)
15 randomly.ab. (342931)
16 trial.ab. (523841)
17 or/10‐16 (3244736)
18 exp animals/ not humans.sh. (4746729)
19 17 not 18 (2905665)
20 9 and 19 (1062)
21 cancer pain/ or exp headache/ (29372)
22 20 not 21 (1045)
Embase
1 exp Pain/ (1319254)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).tw. (220962)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).tw. (61226)
4 1 or 2 or 3 (1366336)
5 Spinal Cord Stimulation/ (7229)
6 ((spinal cord adj3 (stimulat* or electrostimulat*)) or (dorsal root adj3 (stimulat* or electrostimulat*))).tw. (8201)
7 ((epidural adj3 (stimulat* or electrostimulat*)) or SENZA or neuromodul*).tw. (24602)
8 5 or 6 or 7 (32755)
9 4 and 8 (9323)
10 random$.tw. (1575886)
11 factorial$.tw. (38693)
12 crossover$.tw. (76140)
13 cross over$.tw. (32022)
14 cross‐over$.tw. (32022)
15 placebo$.tw. (309133)
16 (doubl$ adj blind$).tw. (206723)
17 (singl$ adj blind$).tw. (25491)
18 assign$.tw. (401316)
19 allocat$.tw. (157038)
20 volunteer$.tw. (256010)
21 Crossover Procedure/ (64745)
22 double‐blind procedure.tw. (206)
23 Randomized Controlled Trial/ (622842)
24 Single Blind Procedure/ (40570)
25 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 (2356195)
26 (animal/ or nonhuman/) not human/ (5679018)
27 25 not 26 (2085923)
28 9 and 27 (1286)
29 (cancer pain/ or exp headache/) and facial pain/ (1000)
30 28 not 29 (1284)
Web of Science
#9 #8 AND #7
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
# 8 TS=(randomised OR randomized OR randomisation OR randomisation OR placebo* OR (random* AND (allocat* OR assign*) ) OR (blind* AND (single OR double OR treble OR triple) ))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
# 7 #6 AND #3
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
# 6 #5 OR #4
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#5 TOPIC: (((epidural near/3 (stimulat* or electrostimulat*) ) or SENZA or neuromodul*))
#4 TOPIC: (((spinal cord near/3 (stimulat* or electrostimulat*) ) or ("dorsal root" near/3 (stimulat* or electrostimulat*) )))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#3 #2 OR #1
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#2 TOPIC: ((sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* near/2 neuralg*) or (herp* near/2 neuralg*) or (diabet* near/2 neuropath*) or (reflex near/4 dystroph*) or (sudeck* near/2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or ("failed back" near/4 surg*) or ("failed back" near/4 syndrome*) ))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#1 TOPIC: (((chronic* or back or musculoskel* or intractabl* or neuropath* or "phantom limb" or "fantom limb" or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or "post*stroke" or complex or regional or "spinal cord") NEAR/4 pain*))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
International HTA Database (URL:https://database.inahta.org/)
("Pain"[mhe]) OR (pain or sciatica or backache or lumbago or fibromyalgia or neuralgia or neuropathy or whiplash or causalgia or "sudeck atrophy" or "reflex dystrophy") AND (SENZA or neuromodulation or "spinal cord stimulation" or "spinal cord electrostimulation" or "epidural stimulation" or "epidural electrostimulation" or "dorsal root stimulation" or "dorsal root electrostimulation")
Appendix 2. Search results summary
| Database searched | Date of search | Number of results |
| CENTRAL (Cochrane Library) Issue 10 of 12 2020 | 22/10/20 | 876 |
| 8/9/21 | 147 | |
| MEDLINE & Medline in Process (OVID) 1946 to Oct 21 2020 | 22/10/20 | 1045 |
| 8/9/21 | 141 | |
| Embase (OVID) 1980 to 2020 week 42 | 22/10/20 | 1284 |
| 8/9/21 | 109 | |
| Web of Science 1970 to 21‐10‐20 | 22/10/20 | 944 |
| 8/9/21 | 203 | |
| International HTA Database | 2/11/20 | 406 |
| 8/9/21 | 22 | |
| Total | 5177 | |
Appendix 3. Trials register search summary
| Clinical trials.gov. FILTER INTERVENTION STUDIES |
Date searched 22/10/20 | Date Searched 10/9/21 |
| (PAIN OR Chronic OR back OR musculoskeletal OR intractable OR neuropathic) AND (Spinal Cord Stimulation OR electrostimulation OR dorsal root stimulation OR electrostimulation OR epidural stimulation OR SENZA OR neuromodulation OR neuromodulator) | 326 | 39 |
| (phantom limb OR fantom limb OR neck OR myofascial OR temporomandibular) AND (Spinal Cord Stimulation OR electrostimulation OR dorsal root stimulation OR electrostimulation OR epidural stimulation OR SENZA OR neuromodulation OR neuromodulator) | 37 | 9 |
| (central OR poststroke OR post‐stroke OR post stroke OR complex OR regional) AND (Spinal Cord Stimulation OR electrostimulation OR dorsal root stimulation OR electrostimulation OR epidural stimulation OR SENZA OR neuromodulation OR neuromodulator) | 325 | 47 |
| (spinal cord pain OR sciatica OR back‐ache OR backache OR lumbago) AND (Spinal Cord Stimulation OR electrostimulation OR dorsal root stimulation OR electrostimulation OR epidural stimulation OR SENZA OR neuromodulation OR neuromodulator) | 156 | 11 |
| (fibromyalgia OR neuralgia OR diabetic neuropathy OR reflex dystrophy) AND (Spinal Cord Stimulation OR electrostimulation OR dorsal root stimulation OR electrostimulation OR epidural stimulation OR SENZA OR neuromodulation OR neuromodulator) | 59 | 6 |
| (Sudeck’s atrophy OR causalgia OR whip‐lash OR whiplash OR polymyalgia) AND (Spinal Cord Stimulation OR electrostimulation OR dorsal root stimulation OR electrostimulation OR epidural stimulation OR SENZA OR neuromodulation OR neuromodulator) | 0 | 0 |
| (failed back) AND (Spinal Cord Stimulation OR electrostimulation OR dorsal root stimulation OR electrostimulation OR epidural stimulation OR SENZA OR neuromodulation OR neuromodulator) | 33 | 3 |
| TOTAL | 936 | 115 |
| WHO ICTRP* | Date searched 10/11/20 | 10/9/21 |
| SPINAL CORD STIMULATION AND PAIN | 202 | 31 |
| DORSAL ROOT AND PAIN | 50 | 8 |
| SENZA AND PAIN | 16 | 0 |
| EPIDURAL STIMULATION AND PAIN | 1 | 2 |
| NEUROMOD* AND PAIN | 80 | 7 |
| TOTAL | 350 | 48 |
* Simplified search employed due to decreased functionality of database during COVID‐19 pandemic.
Data and analyses
Comparison 1. SCS vs sham.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain Intensity: average post‐intervention. Short‐term follow up. Mean difference | 6 | 328 | Mean Difference (IV, Random, 95% CI) | ‐8.73 [‐15.67, ‐1.78] |
| 1.1.1 Conventional SCS | 3 | 72 | Mean Difference (IV, Random, 95% CI) | ‐7.88 [‐28.14, 12.38] |
| 1.1.2 High frequency SCS | 4 | 146 | Mean Difference (IV, Random, 95% CI) | ‐4.31 [‐10.29, 1.67] |
| 1.1.3 Burst SCS | 4 | 110 | Mean Difference (IV, Random, 95% CI) | ‐13.38 [‐30.09, 3.34] |
| 1.2 Disability: short‐term follow‐up | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐7.48 [‐13.13, ‐1.82] |
| 1.2.1 Conventional | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐4.90 [‐12.72, 2.92] |
| 1.2.2 Burst | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐10.30 [‐18.48, ‐2.12] |
| 1.3 HR‐QoL: short‐term follow‐up | 2 | 146 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.30, 0.35] |
| 1.3.1 Conventional | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.60, 0.64] |
| 1.3.2 Burst | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.66, 0.58] |
| 1.3.3 High frequency | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.41, 0.55] |
Comparison 2. SCS + other intervention vs other intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Pain intensity: average post‐intervention. Short‐term follow‐up. Mean Difference | 3 | 303 | Mean Difference (IV, Random, 95% CI) | ‐37.41 [‐46.39, ‐28.42] |
| 2.2 Pain: participants with ≥50% pain relief. Short‐term follow‐up. Risk Ratio | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 15.90 [6.70, 37.74] |
| 2.3 Pain intensity: average post‐intervention. Medium‐term follow‐up. Mean difference | 5 | 635 | Mean Difference (IV, Random, 95% CI) | ‐31.22 [‐47.34, ‐15.10] |
| 2.4 Pain: participants with ≥50% pain relief. Medium‐term follow‐up. Risk Ratio | 5 | 597 | Risk Ratio (M‐H, Random, 95% CI) | 7.08 [3.40, 14.71] |
| 2.5 Pain intensity: average post‐intervention. Long‐term follow‐up. Mean difference | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐7.00 [‐24.76, 10.76] |
| 2.6 Pain: participants with ≥50% pain relief. Long‐term follow‐up. Risk Ratio | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 15.15 [2.11, 108.91] |
| 2.7 Adverse events: electrode/lead failure or displacement. Medium‐term follow‐up | 3 | 330 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.04, 0.11] |
| 2.8 Adverse events: electrode/lead failure or displacement. Long‐term follow‐up | 1 | 44 | Risk Difference (M‐H, Random, 95% CI) | 0.55 [0.35, 0.75] |
| 2.9 Adverse events: infection. Medium‐term follow‐up | 4 | 548 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.01, 0.07] |
| 2.10 Adverse events: need for repeated implantation procedures. Medium‐term follow‐up | 4 | 548 | Risk Difference (M‐H, Random, 95% CI) | 0.11 [0.02, 0.21] |
| 2.11 Adverse events: need for repeated implantation procedures. Long‐term follow‐up | 1 | 44 | Risk Difference (M‐H, Random, 95% CI) | 0.94 [0.80, 1.07] |
| 2.12 Adverse events: other. Medium‐term follow‐up | 2 | 278 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.16, 0.06] |
| 2.13 Adverse events: other. Long‐term follow‐up | 1 | 100 | Risk Difference (M‐H, Random, 95% CI) | ‐0.17 [‐0.37, 0.02] |
| 2.14 Disability (Oswestry Disabiility Index). Medium‐term follow‐up | 2 | 312 | Mean Difference (IV, Random, 95% CI) | ‐15.93 [‐35.99, 4.13] |
| 2.15 Health‐Related Quality of Life. Short‐term follow‐up | 1 | 55 | Mean Difference (IV, Random, 95% CI) | 17.00 [5.74, 28.26] |
| 2.16 Health‐Related Quality of Life. Medium‐term follow‐up | 5 | 595 | Std. Mean Difference (IV, Random, 95% CI) | 0.73 [0.46, 0.99] |
| 2.17 Health‐Related Quality of Life. Long‐term follow‐up | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.74, 0.56] |
| 2.18 Medication use: participants using medication. Medium‐term follow‐up | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.18.1 Participant using opioids | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.58, 1.01] |
| 2.18.2 Participants using NSAIDS | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.43, 1.09] |
| 2.18.3 Participants using antidepressants | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.46, 1.00] |
| 2.18.4 Participants using anticonvulsants | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.33, 1.94] |
| 2.18.5 Participants using paracetamol (acetominophen) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.23, 1.51] |
| 2.19 Medication use: morphine oral equivalent daily (mg). Medium‐term follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Kaisy 2018.
| Study characteristics | ||
| Methods | Country: UK Design: cross‐over RCT Comparison(s) of interest to this review: SCS (HF) vs sham |
|
| Participants | Diagnosis/ diagnostic criteria: FBSS Duration of pain: mean (range) years: 5.1 (0.5 ‐ 19.5) Age: mean (range) years 47.9 (33‐60) Sex: 8 F 16 M N randomised = 30 |
|
| Interventions | Type of SNMD: high frequency (3 conditions) vs sham Device details: Medtronic Model 09070 Electrode type/ number: dual octapolar leads (Octad, Medtronic, Minneapolis, MN, USA) Stimulation parameters: 1200 Hz @ 180 μsec, 3030 Hz @ 60 μsec, and 5882 Hz @ 30 μsec) Comparator: sham Details of pre‐implantation trial period: pre‐randomisation, up to 17 days trial stimulation. Success criteria: an average reduction in VAS back pain scores 50% of baseline values in a pain diary during the last seven days of the trial period Duration of stimulation: 3 weeks per condition. No washout period employed between conditions. |
|
| Outcomes | Primary:aAverage back pain intensity, 0‐10 NRS average over previous 3 days. NRS anchors not reported. Adverse events. Secondary: none relevant Time points: post each 3‐week stimulation condition period. |
|
| Notes | Study funding: Sponsored by Medtronic, Inc. (MN, USA) Author declarations of interest: quote: “Adnan Al‐Kaisy received travel sponsorship and speaker fees from Medtronic and Nevro Corp, he is the principal investigator in separate studies sponsored by Medtronic, Nevro Corp and Abbot and he has financial interest in Micron Device LLC. Stefano Palmisani received speaker fees and sponsorships to attend professional meetings from Nevro Corp and Medtronic; David Pang received sponsorship to attend professional meetings from Medtronic and Nevro Corp. Ye Tan and Sheryl McCammon are employees of Medtronic. The remaining authors have no conflicts of interest to disclose.” |
|
De Ridder 2013.
| Study characteristics | ||
| Methods | Country: Belgium Design: cross‐over RCT Comparison(s) of interest to this review: SCS (Burst, conventional) vs sham |
|
| Participants | Diagnosis/ diagnostic criteria. chronic limb or back pain including FBSS, FNSS, myelopathy, myelomalacia Duration of pain: no information Age: mean (range) years 54.07 (39‐68) Sex: 11 F 4 M N randomised = 15 |
|
| Interventions | Type of SNMD: Burst, conventional (tonic) or placebo Device details: EON IPG System (St. Jude Medical) Electrode type/ number: Lamitrode: tripole, penta, 44 or 88. 1 per person. Site: Thoracic (n=14) Cervical (n=1) Stimulation parameters: conventional: 40‐50 Hz. Burst: 500 hz bursts at 5 hz stimulation Comparator: placebo stimulation Details of pre‐implantation trial period: the trial data collection/ cross‐over period occurred during this period. 28 days of trial stimulation. Duration of stimulation: 1 week per condition. No washout period reported between conditions. |
|
| Outcomes | Primary: pain intensity, 0‐100 VAS anchors and question not reported. Secondary: none relevant. Time points: post each 1‐week stimulation condition |
|
| Notes | Study funding: No information reported Author conflicts of interest:quote: " Dr. De Ridder has obtained a patent for burst stimulation. The remaining authors have no conflicts of interest." |
|
de Vos 2014.
| Study characteristics | ||
| Methods | Country: Belgium, Denmark, Germany, and the Netherlands Design: parallel RCT Comparison(s) of interest to this review: SCS (conventional) + conventional medical management (CMM) vs CMM alone |
|
| Participants | Diagnosis/ diagnostic criteria: painful diabetic neuropathy (PDN) Duration of pain: mean (SD) years: SCS + CMM group 7 (6) CMM group 7(6) Age: mean(SD) years: SCS+CMM group 58(11), CMM group 61 (12) Sex: 22 F 38 M N randomised = 60 |
|
| Interventions | Type of SNMD: conventional SCS (+conventional medical management) Device details: EonC, Eon, or Eon Mini; St Jude Medical Electrode type/ number: one electrode lead (Octrode or S8 Lamitrode; St Jude Medical, Plano, Tex Stimulation parameters: conventional ‐ no further details reported Comparator: CMM. Medication adjustments and other conventional pain treatments, such as physical therapy, were allowed at any time during the study Details of pre‐implantation trial period: post randomisation. Maximum 7 days. Success criteria: no information. Duration of stimulation: 6 months |
|
| Outcomes | Primary: Pain Intensity 0‐100 VAS anchors quote; "no pain" to "worst pain imaginable" Adverse events quote; "evaluated using information on treatment‐emergent adverse events, device complications, and premature withdrawal from the trial." Secondary: HRQoL: EQ‐5D, MPQ‐QoL Medication use (Medication Quantification Scale III (MQS) Time points: 1, 3, 6 months post randomisation. |
|
| Notes | Study funding: Sponsored by St. Jude Medical Author conflicts of interest: quote;"Dr K. Meier received teaching fees from St Jude Medical and is a paid consultant for Biolab Technology. The other authors report no conflict of interest." |
|
Eldabe 2021.
| Study characteristics | ||
| Methods | Country: United Kingdom Cross‐over RCT |
|
| Participants | Diagnosis/ diagnostic criteria: chronic back pain with or without leg pain. Must have experienced stable pain relief from previously implanted SCS system. Duration of pain: mean (SD) years: NI Age: mean(SD) years: 54 (9) Sex: 12 F 7 M N randomised = 19 |
|
| Interventions | Type of SNMD: SCS Device details: Medtronic’s rechargeable spinal cord stimulator restoreSensor® Electrode type/ number: 1 to 2 epidural leads Stimulation parameters: 500 Hz, Burst Comparator: sham Details of pre‐implantation trial period: N/A Duration of stimulation: 2 weeks per condition. |
|
| Outcomes | Primary: Pain Intensity 0‐100 VAS anchors not reported Adverse events quote; "Safety was assessed by means of a standardized evaluation of adverse events." Secondary: HRQoL: EQ‐5D‐3L, Time points: measured three times per day (morning, midday, and evening) during five consecutive days at baseline (with conventional stimulation) and at the end of each two‐week study period |
|
| Notes | Study funding: quote; "This study was funded by Medtronic Ltd. The funder of the study had no role in study design, data collection, data analysis, interpretation of data, or writing of the paper. The views expressed in this publication are those of the authors and do not necessarily reflect those of Medtronic Ltd." Author conflicts of interest: "Sam Eldabe has received consultancy fees from Medtronic Ltd, Mainstay Medical, Boston Scientific Corp, and Abbott. He has received department research funding from the National Institute of Health Research, Medtronic Ltd and Nevro Corp. Rui V. Duarte has received consultancy fees from Medtronic Ltd and Boston Scientific Corp. Ashish Gulve has received honoraria for consulting as well as advisory board meetings for Nevro Corp, Boston Scientific Corp and Abbott. The other authors declare no competing interests." |
|
Kemler 2000.
| Study characteristics | ||
| Methods | Country: the Netherlands Design: parallel RCT Comparison(s) of interest to this review: SCS (conventional) + Physical Therapy vs Physical Therapy alone |
|
| Participants | Diagnosis/ diagnostic criteria Reflex Sympathetic Dystrophy (now CRPS) Duration of pain, months mean(SD): SCS+PT group 40 (28), PT only group 34 (22) Age, years, mean (SD): SCS+PT group 40 (12), PT only group 35 (8) Sex: SCS+PT group 61% F 39%M, PT only group 83%F 17%M N randomised = 54 |
|
| Interventions | Type of SNMD: conventional SCS Device details: Itrel III, model 7425, Medtronic Electrode type/ number: model 3487A, Medtronic, 1, generally C4 if the hand was affected and T12 if the foot was affected Stimulation parameters: 85Hz, PW 210 μsec Comparator: Physical Therapy: quote; "Physical therapy, which both groups of patients received, consisted of a standardized program of graded exercises designed to improve the strength, mobility, and function of the affected hand or foot. Pain during the exercises was considered acceptable, but if it had not returned to the pre‐session level within 24 hours, the intensity of the exercises was reduced. Physical therapy was administered for 30 minutes twice a week, with a minimum of two days between sessions. The total duration of the physical therapy was six months, starting after the second assessment. To ensure standardization, selected physical therapists were trained to provide the program of exercises. The coordinating physical therapist from our institution visited the other therapists regularly to make sure the treatment was uniform." Details of pre‐implantation trial period: post randomisation. At least 7 days. Success criteria: If the VAS for the intensity of pain during the last four days of the testing period was at least 50 per cent lower than the score before randomisation, or if there was a score of at least 6 (“much improved”) on a seven‐point scale for the global perceived effect of treatment. Duration of stimulation: 6 months in initial study period, up to 5 year follow‐up |
|
| Outcomes | Primary: pain intensity, 0‐10 VAS, anchors 0 = no pain 10 = very severe pain Adverse events Secondary: HRQoL: Notting Health Profile, EQ‐5D Health Economic outcomes: Costs, Costs per QALY Time points: 1, 3 6 months, 2 and 5 years |
|
| Notes | Study funding: Supported by a grant (OG 96‐006) from the Dutch Health Insurance Council. Author conflicts of interest: no information reported |
|
Kriek 2017.
| Study characteristics | ||
| Methods | Country: the Netherlands Design: Cross‐over RCT Comparison(s) of interest to this review: SCS (conventional, HF, Burst) vs sham |
|
| Participants | Diagnosis/ diagnostic criteria: CRPS Duration of pain, years, median (IQR): 3 (1‐5) Age, years, mean (SD): 42.55 (12.83) Sex: 25 F 4 M N randomised = 33 |
|
| Interventions | Type of SNMD: SCS, conventional, high‐frequency, burst Device details: EonTM rechargeable internal pulse generator (IPG) (St. Jude Medical, Plano, TX, USA) Electrode type/ number: cylindrical percutaneous Octrode™ lead (St. Jude Medical, Plano, TX, USA ), 1 Stimulation parameters: conventional 40Hz, High frequency 500 Hz, 1200 Hz, Burst 40 Hz per burst complex. Each burst delivers 5 spikes of 1ms with an ISI of 1ms. Charge balanced during 5 ms pause between bursts. Comparator: placebo stimulation (with the IPG switched off) Details of pre‐implantation trial period: pre‐randomisation. 2 weeks of trial stimulation followed by 3 months of conventional stimulation before randomisation. Success criteria: >50% pain reduction or participant has stated symptoms are much improved Duration of stimulation: 2 weeks per stimulation condition |
|
| Outcomes | Primary: pain intensity, 0‐100 VAS, anchor 0 = no pain 100 = worst pain ever. Average over previous 4 days Adverse events Secondary: Medication consumption. Not reported. Disability: DASH, walking ability. Not reported. Health economic outcomes: costs, direct within and outside healthcare system, productivity costs. Not reported. Time points: timing not clear but appears to be at end of each stimulation condition period |
|
| Notes | Study funding: quote;"This investigator‐initiated study was supported by a grant from St. Jude Medical (Plano, TX, USA). The design, performance, analysis and submission of this trial were independently performed by our research group." Author conflicts of interest: "FH is a paid consultant for Grunenthal GmbH; DdR has a patent on burst stimulation and is a paid consultant for St. Jude Medical. The remaining authors declare no conflict of interest." |
|
Lind 2015.
| Study characteristics | ||
| Methods | Country: Sweden Design: cross‐over RCT Comparison(s) of interest to this review: SCS (conventional) vs sham |
|
| Participants | Diagnosis/ diagnostic criteria: Irritable bowel syndrome Duration of pain, years mean (range): 7.6 (3‐14) Age, years range: 25‐56 Sex: 7 F 3 M N randomised = 10 |
|
| Interventions | Type of SNMD: Conventional SCS Device details: Itrel‐3 Medtronic Electrode type/ number: Quadripolar SCS‐lead, 1, T11‐12 level Stimulation parameters: 50 Hz with other parameters (electrode pole combinations, pulse amplitude 1.3–3.3 V, and pulse width 200–500 s) set to produce adequate paresthesia covering the usual region of pain with comfortable intensity. Comparator: stimulator switched off. Details of pre‐implantation trial period: no pre‐implantation trial period reported. Duration of stimulation: 6 weeks for first phase of cross‐over |
|
| Outcomes | Primary: pain intensity, average pain level for the day, 0‐10 VAS, anchors not reported Adverse events (methods not reported) Secondary: HR‐QoL ‐ 0‐10 VAS, anchors not reported Time points: End of 6‐week first phase. |
|
| Notes | Study funding: quote;"The study was supported by Medtronic providing the entire SCS systems. The study had economic support from Karolinska Institutet, Uppsala University, The Swedish Society of Medicine, and the Bengt Ihre fund...Medtronic Inc., Minneapolis, MN supported the trial with all the implant materials but had no impact on the study design, analysis or interpretation of the results." Author conflicts of interest: "The authors G.L., J.W., B.L. or P.M.H. have no competing financial or other interests to report." |
|
Perruchoud 2013.
| Study characteristics | ||
| Methods | Country: Switzerland and the UK Design: cross‐over RCT Comparison(s) of interest to this review: SCS (HF) vs sham |
|
| Participants | Diagnosis/ diagnostic criteria: patients already receiving SCS for persistent pain Duration of pain: not reported Age, years, mean (SD): 54.2 (10.7) Sex: 17 F 16 M N randomised = 38 |
|
| Interventions | Type of SNMD: high frequency Device details: Medtronic (Minneapolis, MN, USA) impulse generator, either rechargeable (RestoreADVANCED®, RestoreSensor®, or RestoreUltra®) or battery powered (PrimeADVANCED®). Electrode type/ number: no information Stimulation parameters: frequency 5kHz Comparator: sham stimulation Details of pre‐implantation trial period: Not specified. All participants implanted and receiving conventional stimulation at the point of recruitment. Duration of stimulation: 2 weeks per stimulation condition |
|
| Outcomes | Primary: pain intensity, 0‐10 VAS, anchors not reported Adverse events Secondary: HRQoL: EQ‐5D Medication use Time points: end of each stimulation period |
|
| Notes | Study funding: quote; "Medtronic funded the study and the manufacturer provided the technical support for IPG programming. However, no member of Medtronic personnel contributed to the design of the study or the collection or analysis of the data" Author conflicts of interest: "Dr. C. Perruchoud, Dr. S. Eldabe, and Pr. E. Buchser consult for and are members of advisory boards for Medtronic. Dr. C. Perruchoud, Dr. S. Eldabe, and Pr. E. Buchser received consulting fees, honoraria, speaking fees, and travel fees from Medtronic." |
|
PROCESS.
| Study characteristics | ||
| Methods | Country: Australia, Canada, Europe and Israel Design: parallel RCT Comparison(s) of interest to this review: SCS + Conventional Medical Management (CMM) vs CMM alone |
|
| Participants | Diagnosis/ diagnostic criteria: FBSS Duration of pain: (time since back surgery, years, mean(SD) SCS+CMM group 4.7 (5.1), CMM only group 4.6 (4.3) Age, years, mean (SD): SCS+CMM group 48.9 (10), CMM only group 52 (10.7) Sex: 49 F 51 M N randomised = 100 |
|
| Interventions | Type of SNMD: conventional SCS +CMM Device details: Synergy system, Medtronic, Inc., Minneapolis, MN Electrode type/ number: no information Stimulation parameters: no information Comparator: CMM quote;"may receive a combination of physical and psychological therapy/ rehabilitation or drug treatment, but not spinal surgery or intrathecal drug delivery. CMM included oral medication (i.e., opioids, non‐steroidal anti‐inflammatory drugs [NSAIDs], antidepressants, anticonvulsant /antiepileptics, and other analgesic therapies), nerve blocks, epidural corticosteroids, physical and psychological rehabilitative therapy, and/or chiropractic care. In either group, implantable drug delivery systems and re‐operation were not allowed." Details of pre‐implantation trial period: post randomisation. No information for duration. Success criteria: those experiencing at least 80% overlap of their pain with stimulation‐induced paresthesia and at least 50% leg pain relief Duration of stimulation: Up to 24 months |
|
| Outcomes | Primary: pain relief, the proportion of patients achieving at least 50% leg pain relief at 6 months. 0‐100 VAS, anchors not reported. Post‐intervention average pain scores. Adverse events Secondary: Disability, Oswestry Disability Index (ODI) HRQoL: SF‐36, EQ‐5D Cost‐effectiveness analysis Time points: 6, 12, 18 and 24 months |
|
| Notes | Study funding: quote; "All logistical aspects of the study were managed and funded by Medtronic, Inc. The trial was designed and supervised by a Trial Steering Committee that consisted of 4 external advisors and 2 representatives from Medtronic, Inc. Data were collected and analyzed by Medtronic, Inc., under the direction of the committee. The manuscript was written by the independent members who had full, nonrestricted access to the data." Author conflicts of interest:quote; " EHC Hospital of Morges (Eric Buchser’s employer), Rod S. Taylor, the Johns Hopkins University (Richard B. North’s former employer), and the nonprofit Neuromodulation Foundation, Inc. (of which Richard B. North is a director), have received financial reimbursement as consultants for Medtronic, Inc." |
|
PROMISE.
| Study characteristics | ||
| Methods | Country: 28 investigational sites in Belgium, Canada, Colombia, France, Germany, the Netherlands, Spain, the UK, and the USA Design: parallel RCT Comparison(s) of interest to this review: SCS (conventional) + Optimal Medical Management (OMM) vs OMM alone |
|
| Participants | Diagnosis/ diagnostic criteria: FBSS Duration of pain, years, mean(SD): 6.7 (7.2) Age, years, mean (SD): SCS+OMM group 52.8 (12.5), OMM only group 53.9 (11.5) Sex: 132 F 86 M N randomised = 218 |
|
| Interventions | Type of SNMD: conventional SCS Device details: Medtronic, Models 37701, 37702,37712, 37713, 37714, 97702, 97713, and 97714 Electrode type/ number: multicolumn surgical lead (Specify 5‐6‐5; Medtronic), 1 Stimulation parameters: no information Comparator:quote; "Given the lack of international guidance, an OMM guideline was developed by the PROMISE Trial Steering Committee (TSC) to standardize practice in the study. An individual OMM treatment plan was developed for each patient and optimized at each visit. Optimal medical management could include treatments ranging from noninvasive treatments such as acupuncture, psychological/ behavioural therapy, and physiotherapy to invasive treatments such as spinal injections/blocks, epidural adhesiolysis, and neurotomies." Details of pre‐implantation trial period: post‐randomisation, duration "based on standard local practice". Success criteria " defined as a subject having adequate LBP relief with usual activity and appropriate analgesia in the context of postoperative pain (thoracic laminectomy in particular, when applicable), as assessed by the investigator. " Duration of stimulation: Up to 24‐month follow‐up |
|
| Outcomes | Primary: pain intensity. 0‐10 VAS, anchors 0 = no pain, 10 = worst low back pain imaginable Proportion of participants reporting ≥ 50% reduction in LBP. Post‐intervention average pain scores. Adverse events Secondary: HR‐QoL: SF‐36 PCS, EQ‐5D Disability: ODI Time‐points: 1, 3, 6, 12 and 24 months post‐randomisation |
|
| Notes | Study funding: quote;"The study was funded by Medtronic. All logistical aspects of the study were managed and funded by Medtronic. Data were collected by investigational sites and analysed by Medtronic under the direction of the committee and followed a predefined statistical analysis plan. Medtronic personnel made no patient assessments, care decisions, or had any impact whatsoever on the physician or his team’s care decisions. Medtronic funded the study and was involved in the study design, data collection, data analysis, data interpretation, and writing of the report. The corresponding author had access to all the data in the study and had final responsibility for the decision to submit for publication." Author conflicts of interest: " L. Annemans has received grants from Medtronic. S. Basu has received funds to conduct the study and the associated hospital has received Medtronic equipment and hardware from Medtronic. S. Bojanic has received grants from Medtronic. J. Buwembo has received nonfinancial support from Medtronic. M. Desai has received personal fees from Medtronic and Halyard Health, and stock options from dorsaVi, SmartImplantSystems, and MedicalWearables Solutions. M. Eif has received personal fees from Medtronic. N. Mehta has received grants and personal fees from Nevro and Boston Scientific and grants from Medtronic. D. Noriega has received teaching fees from Vexim SAS and SPineart. R. North has received charitable grants from Abbott, Boston Scientific, Medtronic, Nevro, Stimwave, and Algostim/ Nuvectra, personal fees from AlgoStim/Nuvectra, and has patents with royalties paid by Abbott and Nuvectra. J. Pilitsis has received grants and other consultant support from Medtronic, Boston Scientific, Abbott, Nevro, Jazz Pharmaceuticals, GE Global Research, Centauri, Karuna, and the NIH. J.‐M. Remacle has received scientific support from Medtronic and personal fees from Depuy. P. Rigoard has received grants, personal fees, and nonfinancial support from Medtronic, Abbott, and Boston Scientific. R. Taylor has received personal fees and research consultancy fees from Medtronic. F. van Eijs has received personal fees from Medtronic. T. Van Havenbergh has received grants from Medtronic. A. Villareal has received grants and personal fees from Medtronic, grants from Boston Scientific, is a member of the Executive Board of the IASP Special Interest Group in Neuromodulation, and is newsletter liaison of the ASRA Neuromodulation SIG. M.J. Johnson, C. van den Abeele, and Y. Tan are employees of Medtronic. S. Bhatia, C. Burnette, M. Deruytter, B. Edmiston, V. Galan, G.G. March, T. Houden, S. Jaramillo, S.P. Lad, A. Lopez, C. Raftopoulos, T.‐N. Vu, J. Vangeneugden, E. Tallarico, and C. Yepes declare no competing interests." |
|
Schu 2014.
| Study characteristics | ||
| Methods | Country: Germany Design: cross‐over RCT Comparison(s) of interest to this review: SCS (conventional, Burst) vs sham |
|
| Participants | Diagnosis/ diagnostic criteria: FBSS, previously implanted with an SCS system Duration of pain: no information Age, years, mean (SD): 58.6 (10.2) Sex: 13 F 7 M N randomised = 20 |
|
| Interventions | Type of SNMD:cConventional, high frequency and burst SCS Device details: St. Jude Medical SCS system Electrode type/ number: no information, located in mid‐thoracic position (T7‐10) Stimulation parameters: conventional: 40‐50 Hz, HF: 500 HZ, Burst: packets of five pulses (pulse width 1 msec) at 500 Hz, delivered 40 times per second (subsensory amplitude) Comparator: placebo stimulation (device was switched off) Details of pre‐implantation trial period: pre‐randomisation. Duration not specified but all participants treated with conventional stimulation for at least 3 months pre‐randomisation. Success criteria: no information. Duration of stimulation: 1 week per condition. No washout period reported between conditions. |
|
| Outcomes | Primary: Pain intensity 0‐10 NRS, anchors 0 = no pain, 10 = worst imaginable pain Adverse events Secondary: Disability ODI Time points: post each 1 week stimulation period |
|
| Notes | Study funding: quote;"Drs. Slotty and Bara received a fellowship research grant from St. Jude Medical." Author conflicts of interest: "Drs. Schu and Vesper are consultants of St. Jude Medical. Drs. Bara, Schu, Slotty, and Vesper received travel grants from St. Jude Medical. Drs. Slotty and Bara received a fellowship research grant from St. Jude Medical." |
|
SENZA‐PDN.
| Study characteristics | ||
| Methods | Country: 18 investigational sites in the USA Design: parallel RCT Comparison(s) of interest to this review: SCS (HF) + Conventional Medical Management (CMM) vs CMM alonE. |
|
| Participants | Diagnosis/diagnostic criteria: painful diabetic neuropathy (lower limb) Duration of pain, years, mean(SD): SCS+CMM group 7.4 (5.7) , CMM only group 7.1 (5.1) Age, years, mean (SD): SCS+CMM group 60.7 (11.4), OMM only group 60.8 (9.9) Sex: 80 F 136 M N randomised = 216 |
|
| Interventions | Type of SNMD: HF‐SCS Device details: Nevrocorp Senza Electrode type/ number: Nevro Lead 2 Stimulation parameters: 10‐kHz frequency, 30‐μs pulse width delivered via bipole and amplitude range of 0.5 to 3.5 mA. Comparator:quote; "CMM may include a variety of non‐invasive or minimally invasive treatments that comprise the standard of care for neuropathic limb pain. Investigators will follow their standard of care and/or published clinical guidelines (Dworkin, 2010) to administer CMM to both treatment groups. Treatments include, but are not limited to, pharmacological agents, physical therapy, cognitive therapy, chiropractic care, nerve blocks, and other non‐invasive or minimally invasive therapies” Details of pre‐implantation trial period: post‐randomisation, duration 5 to 7 days". Success criteria quote;"Patients reporting 50% or more pain relief using the VAS were eligible for permanent SCS device implant (Nevro Corp)" Duration of stimulation:uUp to 24‐month follow‐up (6 months reported to date) |
|
| Outcomes | Primary: pain intensity. 0‐10 VAS, anchors 0 = no pain, 10 = worst pain imaginable Proportion of participants reporting ≥50% reduction in LBP. Post‐intervention average pain scores. Adverse events Secondary: HR‐QoL: EQ‐5D VAD and index score Time points: 1, 3, 6, 24 months post‐randomisation |
|
| Notes | Study funding: quote;“This study was funded by Nevro Corp. Role of the Funder/Sponsor: The sponsor participated in the design of the study in collaboration with an outside expert advisory committee as well as the conduct of the study by supporting patient optimization in collaboration with the investigators and monitoring data at the sites. The research site investigators and staff were responsible for all data collection and management via entry into a secure database. The sponsor participated in the analysis and interpretation of the data along with the authors and an independent biostatistician. The sponsor also participated in the preparation, review, and approval of the manuscript and decision to submit the manuscript for publication in collaboration with the authors.” Author conflicts of interest: " “Drs Petersen, Scowcroft, White, Sills, Amirdelfan, Guirguis, Xu, Yu, Nairizi, Patterson, Galan, Mehta, Choi, Sayed, Lad, DiBenedetto, Goree,Wu, Argoff, Nasr, Taylor, and Mekhail have received personal fees from Nevro Corp. Dr Petersen has received research support from Medtronic, Neuros Medical, Nevro Corp, and ReNeuron as well as personal fees from Abbott Neuromodulation, Medtronic Neuromodulation, and Neuros Medical. Dr Scowcroft has received research support from Boston Scientific, Nevro Corp, Saluda Medical, and Vertiflex. Drs Brooks and Caraway are employees of Nevro Corp. Dr White has received consulting fees from Eli Lilly and Company and California Institute for Biomedical Research. Dr Amirdelfan has received research support from IPM Medical Group, Biotronik, Vivex Biologics, Saluda Medical, and SPR Therapeutics as well as personal fees from Nalu Medical, Saluda Medical, Biotronik, and Medtronic. Dr Guirguis has received personal fees from Avanos Medical and SPR Therapeutics as well as research support from Abbott Laboratories, Boston Scientific, Neuros Medical, and Avanos Medical. Dr Xu has received research support from the Cleveland Clinic MENTR Program and the National Institutes of Health. Dr Nairizi has received personal fees from Flowonix. Dr Patterson has received personal fees from Abbott Laboratories, AIS Healthcare, Allergan, Amgen, CornerLoc, Nuvectra Medical, and Saluda Medical as well as research support from Abbott Laboratories, Biotronik, Flowonix, Nuvectra Medical, and Vertiflex. Dr Galan has received research support from Medtronic, SPR Therapeutics, St Jude, Biotronik, and PainTEQ. Dr Mehta has received personal fees from Salix Pharmaceuticals, BioDelivery Sciences International, and Sollis Therapeutics as well as research support from Boston Scientific and Medtronic. Dr Sayed has received personal fees from Abbott Laboratories, Medtronic, Boston Scientific, Flowonix, Vertos Medical, and Vertiflex; research support from Abbott Laboratories, Biotronic, Vertos Medical, and Vertiflex; and owns equity in SPR Therapeutics. Dr DiBenedetto has received funding for serving as principal investigator of a study supported by SPR Therapeutics paid to his institution. Dr Goree has received personal fees from Abbott Laboratories and Stratus Medical. Dr Argoff has received research support from Allergan, Amgen, Daiichi Sankyo, Novartis, Teva Pharmaceutical, Eli Lilly and Company, and Vertex Pharmaceuticals as well as personal fees from AbbVie, Teva Pharmaceutical, Eli Lilly and Company, Novartis, Pfizer, Flowonix, Vertex Pharmaceuticals, Elsevier, and SK Life Science. Dr Nasr has received personal fees from Neurogastrx and Exelixis. Dr Taylor has received personal fees from Medtronic and Saluda Medical. Dr Subbaroyan and Mr Gliner were employees of Nevro Corp at the time this work was completed. Dr Subbaroyan has a patent for painful diabetic neuropathy and sensory modulation pending to Nevro Corp and owns stocks in Nevro Corp. Mr Gliner has a patent for HF10 therapy and related issued to Nevro Corp. Dr Mekhail has received personal fees from Boston Scientific, Sollis Therapeutics, Saluda Medical, Abbott Laboratories (formerly Spinal Modulation), Vertos Medical, Nuvectra Medical, and Relievant Medsystems; research support from Avanos Medical (previously Halyard Health), Mallinckrodt Pharmaceuticals, Mesoblast, and Neuros Medical; and was an independent medical monitor for this study. No other disclosures were reported." |
|
Slangen 2014.
| Study characteristics | ||
| Methods | Country: the Netherlands Design: parallel RCT Comparison(s) of interest: SCS (conventional) + Best Medical Treatment (BMT) vs BMT alone |
|
| Participants | Diagnosis/ diagnostic criteria: painful diabetic peripheral neuropathy in lower limbs Duration of pain, years, mean(SD): SCS+ BMT group 6 (5.1), BMT only group 4.9 (3.6) Age, years, mean (SD): SCS+ BMT group 57.1 (12.4), BMT only group 56.5 (8) Sex: 24 M 12F N randomised = 34 |
|
| Interventions | Type of SNMD: conventional SCS +BMT Device details: Synergy Versitrel or Prime Advanced, Medtronic Electrode type/ number: Octapolar lead (Octad lead; Medtronic, Minneapolis, MN), 1, thoracic level Stimulation parameters: no information Comparator: BMT Not clearly defined in the papers.quote; “Treatment as usual in registry” document” Details of pre‐implantation trial period: post‐randomisation. 2 weeks. Success criteria: if the NRS for the intensity of pain during daytime or nighttime for the last 4 days of the trial period was at least 50% lower than the baseline score, or if there was a score of 6 or higher (“much improved” or “very much improved”) on the PGIC scale for pain and sleep. Duration of stimulation: follow up for 6 months |
|
| Outcomes | Primary: pain intensity. Proportion experiencing ≥50% relief of pain intensity on an NRS for 4 days (17) during daytime or nighttime or a score of 6 on a 7‐ point Likert scale (1 = very much worse and 7 = very much improved) of the PGIC scale for pain and sleep. Adverse events, methods not reported Secondary: HR‐QoL EQ‐5D, SF‐36 Medication use Time points: 3 and 6 months |
|
| Notes | Study funding:quote; "This study was supported by Medtronic, which provided a grant for the employment of R.S. for 3 years. No other potential conflicts of interest relevant to this article were reported. Medtronic was not involved in the analysis and interpretation of the data or in writing the manuscript." Author conflicts of interest: "this study was supported by Medtronic, which provided a grant for the employment of R.S. for 3 years." |
|
Sokal 2020.
| Study characteristics | ||
| Methods | Country: Poland Design: cross‐over RCT Comparison(s) of interest to this review: SCS (Conventional, HF, Burst) vs sham |
|
| Participants | Diagnosis/diagnostic criteria: mixed cause chronic pain. FBSS, CRPS "and pain was distributed in the lumbosacral area" Duration of pain, years, mean (range): 8.3 (1‐30) Age, years, range: 35‐74 Sex: 11 F 12 M N randomised = 18 |
|
| Interventions | Type of SNMD: SCS, conventional, high frequency and burst Device details: non‐rechargeable IPG (Precision NoviTM) and in one case (patient 11), a rechargeable IPG (MontageTM) that was produced by Boston Scientific Co., Boston, MA, USA Electrode type/ number: linear 8‐ or16‐contact (Infinion 16TM) electrodes, 1 or 2, T7‐10 Stimulation parameters: conventional: 40‐60Hz, High frequency: 1kHz, Burst: intermittent packets of burst stimuli delivered using the neural targeting algorithm, which consisted of several pulses per packet with PW 250–500 s repeated with frequency = 40 Hz Comparator: Placebo IPG was deactivated except for emergency shutdown of stimulation Details of pre‐implantation trial period: Pre‐randomisation. 2 weeks quote;"for most participants". Success criteria: participants who achieved at least a 50% reduction in pain. Duration of stimulation: 2 weeks per condition, no washout period between conditions |
|
| Outcomes | Primary: pain intensity 0‐10 VAS, specific question and anchors not reported Adverse events, methods not reported Secondary: Disability ODI HRQoL EQ‐5D Medication use Time points: end of each stimulation period |
|
| Notes | Study funding: quote;"This research received no external funding" Author conflicts of interest: " Paweł Sokal reports non‐financial support from Medtronic and Boston Scientific. Agnieszka Malukiewicz and Marcin Ruda´s report non‐financial support from Boston Scientfic. Sara Kiero´ nska, Joanna Murawska, Cezary Guzowski, Marcin Rusinek, Dariusz Paczkowski, and Mateusz Krakowiak report no conflicts of interest." |
|
Tjepkema‐Cloostermans 2016.
| Study characteristics | ||
| Methods | Country: the Netherlands Design: cross‐over RCT Comparison(s) of interest to this review: SCS (conventional, burst) vs sham |
|
| Participants | Diagnosis/ diagnostic criteria:pPreviously implanted with SCS for chronic pain. 32 failed back surgery syndrome (FBSS), 3 peripheral neuropathy (PN), 3 diabetic neuropathic pain (DNP), 1 multiple sclerosis (MS), 1 complex regional pain syndrome (CRPS) Duration of pain pre‐implantation, years, mean (range) 10 (1‐35) Duration since implantation, months, mean (range) 28 (6‐124) Age, years, mean (range): 58 (42‐73) Sex: 16 F 24 M N randomised = 41 |
|
| Interventions | Type of SNMD: SCS, conventional, burst Device details: Eon C pulse generator (St. Jude Medical, Plano) Electrode type/ number: NI Stimulation parameters: cConventional: 0.4 and 19 mA, with pulse widths between 100 and 500 ls, and frequencies between 30 and 120 Hz; Burst: 500 Hz bursts consisting of five pulses of 1 ms with 1‐ms inter pulse interval, delivered 40 times per second. For the high amplitude burst condition, stimulation amplitude just below the individual sensation threshold was used Comparator: sham stimulation: quote; "standard stimulation with 0.1 mA bursts was used. This condition was expected to be subtherapeutic and initially intended as sham stimulation" Details of pre‐implantation trial period: NI Duration of stimulation: 2 weeks per stimulation condition |
|
| Outcomes | Primary: pain intensity, 0‐100 VAS, anchors not specified. Average over previous 3 days Adverse events: no methods reported Secondary: QoL‐ 0‐100 VAS, question and achors not reported MPQ‐QoL Time points: end of stimulation period (average of last 3 days) |
|
| Notes | Study funding: quote; "Financial support for this project was provided entirely by the Medisch Spectrum Twente hospital." Author conflicts of interest: NI |
|
Only outcomes and time points relevant to this review reported
BMT = Best Medical Treatment; CMM = Conventional Medical Management;EQ‐5D = EuroQoL 5D; FBSS = Failed Back Surgery Syndrome; FNSS = Failed Neck Surgery; Syndrome;HRQoL = health‐related quality of life; IQR = interquartile range; LBP = Lower Back Pain; MPQ‐QoL = McGill Pain Questionnaire Quality of Life;NA = not available; NI = No information reported; NRS = Numerical Rating Scale;ODI = Oswestry Disability Index; OMM = Optimal Medical Management;PCS = Physical Component Summary;pgic = Patient Global Impression of Change; QALY = Quality Adjusted Life Year;RCT = randomised controlled trial; RSD = Reflex Sympathetic Dystrophy; SCS = spinal cord stimulation; SD = standard deviation; SF‐36 = Short Form 36;SNMD = spinal neuromodulation device; VAS = Visual Analogue Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alo 2016 | Commentary ‐ not an RCT |
| Annemans 2014 | Economic analysis performed post hoc and independent of RCT |
| Dones 2008 | Commentary ‐ not an RCT |
| Falowski 2019 | Not a comparison of interest |
| Gilligan 2020 | Muscle stimulation: not intervention of interest |
| Kemler 2001 | No outcomes of interest reported |
| Kemler 2010 | Economic analysis performed post hoc and independent of original RCT |
| Kufakwaro 2012 | Not a comparison of interest |
| Liem 2013 | Non‐randomised study |
| Liu 2020 | Not a comparison of interest |
| Liu 2021 | SCS versus nerve block, not a comparison of interest |
| Marchand 1991 | Non‐randomised study |
| Meier 2015 | Treatment period not clinically applicable |
| Rigoard 2013 | Non‐randomised study |
| Sagher 2008 | Commentary ‐ not an RCT |
| Steinbach 2017 | Single case report. Not an RCT |
| Taylor 2005 | Economic analysis developed independent of RCT |
| Tesfaye 1995 | Not an RCT |
| van Beek 2015 | Single arm long term follow‐up data (no control) |
| Winfree 2005 | Commentary ‐ not an RCT |
| Wolter 2012 | Average pain levels at baseline < 4/10 |
RCT = randomised controlled trial; SCS = spinal cord stimulation.
Characteristics of studies awaiting classification [ordered by study ID]
ISRCTN33292457.
| Methods | Cross‐over RCT |
| Participants | Chronic back and leg pain in failed back surgery syndrome (FBSS) patients, n =20 |
| Interventions | HF‐SCS vs sham |
| Outcomes | Primary outcome measure 1. Visual Analogue Scale (VAS) score for back pain: measured at baseline, the mid‐point and end of each period 2. Emergent adverse events Secondary outcome measures 1. VAS score for leg pain: measured at baseline, the mid‐point and end of each period 2. Sleep disturbance: measured at baseline, the mid‐point and end of each period 3. Oswestry Disability Index: measured at baseline and the end of each period 4. Participant diary: measured at baseline, prior to mid‐point and end of each period, following wash‐out 5.Participant's assessment of group assignment: measured at the end of each period 6. Changes in medication usage: measured at baseline, the mid‐point and end of each period |
| Notes | Trial end date 13/12/2012. Registry status "No longer recruiting, results overdue". Last checked 4/1/21 |
Miller 2015.
| Methods | Cross‐over RCT |
| Participants | Chronic intractable pain of trunk and/or limbs with average back pain intensity of ≥ 5, n=20 |
| Interventions | 1.2kHz (HF) SCS vs sham (3 days each condition) |
| Outcomes | Primary: average back pain intensity. Secondary: leg pain, disability, quality of life |
| Notes | No difference reported for all outcomes. Only a conference abstract available. Authors contacted for full study report. |
Miller 2016.
| Methods | Cross‐over RCT |
| Participants | Post laminectomy syndrome. n = 4 |
| Interventions | Conventional SCS (60 Hz), HF SCS (1200 Hz), sham |
| Outcomes | "Pain scores" |
| Notes | Only reported as conference abstract. Author contacted for full study report |
NCT00200122.
| Methods | Parallel RCT |
| Participants | Chronic refractory pain associated with failed back surgery syndrome, epidural fibrosis, peripheral causalgia, complex regional pain syndrome. N =100 |
| Interventions | Device: Spinal Cord Stimulation. Comparator not specified. |
| Outcomes | "The primary endpoint is to assess the pain coverage capability of the RESTORE SCS system. Secondary outcome measures include pain relief, quality of life, function, patient and physician acceptance." |
| Notes | Trial registry record status "Completed" verified in October 2007. No published record found. Correspondence with designated contact (Medtronic) in December 2020: quote: "I have asked our clinical affairs team regarding your request for the publication of the data/ study report. Unfortunately the study was not published; there is no other publicly available information." No reply to follow‐up email (December 2020) requesting reasons for non‐publication. |
NCT00351208.
| Methods | Cross‐over RCT |
| Participants | Chronic pain in the trunk and lLimbs. N= 20 |
| Interventions | Quote: "For a total of 9 weeks, patients will be randomized to 9 stimulation frequency and pulse‐width setting combinations. " No further detail provided. |
| Outcomes | Percent pain relief obtained during the one‐month follow‐up compared to baseline The magnitude of change in other indexes of function, such as MPI, during the one‐month follow‐up period |
| Notes | No published record found. Trial registry recruitment status "unknown". Last checked 4/1/21 |
NCT03462147.
| Methods | Cross‐over RCT |
| Participants | "Back pain", N=10 |
| Interventions | High‐density SCS vs conventional SCS vs sham |
| Outcomes | Quote: "The 3 different study designs will be compared against each other according to a questionnaire including pain, need for medication, sleep quality, quality of life, effectiveness." |
| Notes | Estimated completion date 31/12/2018. Registry recruitment status "Recruiting". Last checked 4/1/21 |
HF = high frequency; RCT = randomised controlled trial; SCS = spinal cord stimulation.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12620000720910.
| Study name | An evaluation of spinal cord stimulation for the treatment of chronic pain, also its effect on mood, sleep, physical activity and analgesic medicine requirements. |
| Methods | Cross‐over RCT |
| Participants | Chronic low back pain, n=10 |
| Interventions | Burst spinal cord stimulation vs placebo stimulation |
| Outcomes | Pain intensity (BPI), Analgesic consumption |
| Starting date | 03/08/20 |
| Contact information | p.drummond@murdoch.edu.au |
| Notes | Industry sponsored: Abbott Medical Australia Pty Ltd |
Burst SCS.
| Study name | Burst Spinal Cord Stimulation (Burst‐SCS) study |
| Methods | Cross‐over RCT |
| Participants | Chronic, intractable pain of the trunk and/or limbs, including unilateral or bilateral pain associated with any of the following: failed back surgery syndrome and intractable low back and leg pain, and for whom Burst‐SCS has been recommended as a treatment option. N = 20 |
| Interventions | Burst SCS vs sham |
| Outcomes | Primary: pain: change in Visual Analogue Scale (VAS) score (Time Frame: pre‐implant visit, up to approximately 2 weeks) Secondary: change in general Pain Disability Index (PDI) score (Time Frame: pre‐implant visit, up to approximately 2 weeks) |
| Starting date | 12/03/2019 |
| Contact information | Vishwanath Sankarasubramanian, PhD(734) 647‐9052, vishwans@med.umich.edu Sana Shaikh, skazi@med.umich.edu |
| Notes |
ChiCTR‐IOR‐17012289.
| Study name | A randomized controlled study of spinal cord electrical stimulation in the treatment of pain in patients with diabetic foot |
| Methods | Parallel RCT |
| Participants | Painful diabetic foot and/or neuropathy. n =30 |
| Interventions | Spinal cord stimulation + clinical routine treatment vs clinical routine treatment |
| Outcomes | Pain VAS (baseline, follow‐up 2 weeks, 1 month, three months, 6 months, 1 year, and every year later) Analgesic dose Complications of entire follow‐up period |
| Starting date | 03/08/2020 |
| Contact information | Wang Yaping, wangyaping6568@126.com |
| Notes |
CITRIP.
| Study name | Efficacy and safety of spinal cord stimulation in patients with chronic intractable pain |
| Methods | Parallel RCT |
| Participants | Chronic trunk or limb pain, n = 54 |
| Interventions | PINS spinal cord stimulation (switched on) vs switched off. |
| Outcomes | Primary: pain VAS at 13 weeks Secondary: changes in VAS (Time Frame: 4, 12, 24 weeks). Change in quality of life as measured by SF‐36 (Time Frame: 4,12, 24 weeks), Number of participants with adverse events (Time Frame: 24 weeks) |
| Starting date | 01/02/2019 |
| Contact information | Fumin Jia, pins_medical@163.con |
| Notes | Industry sponsored: Beijing Pins Medical Co., Ltd |
DISTINCT.
| Study name | Dorsal spinal cord stimulation vs medical management for the treatment of lowbBack pain (DISTINCT) |
| Methods | Parallel RCT |
| Participants | Chronic refractory axial low back pain with a neuropathic component and is not a candidate for spine surgery. N = 270 |
| Interventions | Burst SCS vs comprehensive medical management |
| Outcomes | Primary: the difference in responders between both groups (Time Frame: 6 months) Improvement in function, defined as a ≥ 13% decrease on ODI or score ≤ 20%, OR Improvement in pain, defined as a ≥ 50% decrease on NRS. Secondary: Not provided. |
| Starting date | 12/11/2020 |
| Contact information | Todd Stirman, Todd.stirman@abbott.com Robyn Capobianco, Robyn.capobianco@abbott.com |
| Notes | Industry sponsored: Abbott Medical Devices |
DRKS00022557.
| Study name | Effect of stimulation frequency in dorsal root ganglion stimulation (DRG Stimulation) |
| Methods | Cross‐over trial |
| Participants | Chronic intractable pain, n = 50 |
| Interventions | DRGS Arm 1: Frequency 40 Hz for 4 days, wash‐out for 3 days Arm 2: Frequency 60 Hz for 4 days, wash‐out for 3 days Arm 3: Frequency 80 Hz for 4 days, wash‐out for 3 days Arm 4: Sham stimulation for 4 days, wash‐out for 3 days Arm 5: Ordinary stimulation parameters for 4 days, wash‐out for 3 days |
| Outcomes | Pain intensity (VAS) Medication use |
| Starting date | 01/04/2021 |
| Contact information | Philipp Slotty neuromodulation@med.uni‐duesseldorf.de |
| Notes | No external funding |
ISRCTN10663814.
| Study name | Comparison of spinal cord stimulation in combination with standard pain treatment versus standard pain treatment only in patients with intractable chronic back pain without previous history of spine surgery |
| Methods | Parallel RCT |
| Participants | Chronic low back pain without history of surgery, n = 200 |
| Interventions | Differential target multiplexed spinal cord stimulation vs conventional medical management |
| Outcomes | Primary: responder rate (>50% improvement in pain), primary endpoint 6 months. Secondary: back pain relief (VAS), Disability (ODI), Quality of life (EQ‐5D, SF‐36), Health Economic Outcomes, Adverse events at 3,6,9,12,18,24 months |
| Starting date | 01/01/2020 |
| Contact information | Dr Win Laloo, clinical@sgx-international.com |
| Notes | Industry sponsored: SGX International LLC |
MODULATE‐ LBP.
| Study name | Sham‐controlled RCT on 10 kHz High‐fFrequency spinalcord stimulation for chronic neuropathic low back pain (Modulate‐LBP) (Modulate‐LBP) |
| Methods | Parallel RCT |
| Participants | Chronic low back pain, with neuropathic component: N = 96 |
| Interventions | HF‐SCS (Senza) vs sham SCS |
| Outcomes | Primary: Back pain intensity (VAS) Secondary: Disability (ODI) HR‐QoL (EQ‐5D) Medication usage Adverse events |
| Starting date | August 2018 |
| Contact information | Not shared |
| Notes | StuDy sponsored by NIHR, Guy's and St Thomas' NHS Foundation Trust |
NCT03546738.
| Study name | Spinal cord Burst stimulation for chronic radicular pain following lumbar spine surgery: a randomized double‐blind sham‐controlled crossover trial |
| Methods | Cross‐over RCT |
| Participants | Have undergone ≥1 back surgeries and developed chronic radicular pain that has remained refractory to non‐surgical treatment for ≥6 months. N = 50 |
| Interventions | Burst SCS vs sham SCS |
| Outcomes | Primary: Change in disease‐specific functional outcome from baseline (Time Frame: 12 month) measured with version 2.0 of the Oswestry disability index (ODI) Secondary: Change in generic health‐related quality of life measured with the Euro‐Qol‐5D (5L) (Time Frame: 12 months) Change in back pain (Time Frame: 12 months) Change in leg pain (Time Frame: 12 months) Healthcare Provider's Costs (Time Frame: 12 months) |
| Starting date | 05/09/2018 |
| Contact information | Sasha Gulati, sasha.gulati@ntnu.no Sven M Carlsen, sven.carlsen@ntnu.no |
| Notes |
NCT03733886.
| Study name | A randomised sham‐controlled double‐blinded study of burst spinal cord stimulation for chronic peripheral neuropathic pain |
| Methods | Cross‐over RCT |
| Participants | History, symptoms and clinical findings consistent with peripheral neuropathic pain in an extremity ("probable" or "definite") for at least 3 months. N = 10 |
| Interventions | Burst SCS vs sham SCS |
| Outcomes | Primary: usual pain intensity, numeric rating scale (0‐10); usual pain intensity over the last 24 hours (day 7‐13) Secondary: Numeric rating scale (0‐10); highest pain intensity over the last 24 hours(day 7‐13) with anchor points 0 = No pain and 10 = worst imaginable pain Numeric rating scale (0‐10); highest pain intensity over the last 24 hours (day 7‐13) with anchor points 0 = No pain and 10 = worst imaginable pain Numeric rating scale (0‐10); evening pain intensity (day 7‐13), with anchor points 0 = No pain and 10 = worst imaginable pain Numeric rating scale (0‐10) of pain unpleasantness the last 24 hours, with anchor points 0 = no unpleasantness to 10 = worst imaginable unpleasantness. The Patient‐Specific Functional Scale (Numeric Rating Scale (0‐10)) (day 7‐13). Anchor points 0 = Unable to perform activity to 10 = Able to perform activity. EQ5D index values according to the EQ‐5D UK Time Trade‐off (TTO) value set. EQ5D questionnaire |
| Starting date | 07/11/2018 |
| Contact information | Contact: Bård Lundeland, baalun@ous-hf.no Contact: Audun Stubhaug, astubhau@ous-hf.no |
| Notes | Some changes in outcomes made to registry record. |
NCT04039633.
| Study name | Spinal cord stimulation for refractory pain in erythromelalgia |
| Methods | Cross‐over RCT |
| Participants | Idiopathic erythromelalgia with chronic pain, N = 24 |
| Interventions | Burst SCS vs sham SCS |
| Outcomes | Primary: changes in pain assessed with a 0 ‐to‐10 numerical rating scale (NRS) (Time Frame: 6 months) Secondary:
|
| Starting date | 26/09/2019 |
| Contact information | Sasha Gulati, sasha.gulati@ntnu.no Sven M Carlsen, sven.carlsen@ntnu.no |
| Notes |
NCT04676022.
| Study name | SCS as an option for chronic low back and/or leg pain instead of surgery (SOLIS) |
| Methods | Parallel RCT |
| Participants | CLBP, with or without leg pain with no prior surgeries. N = 140 |
| Interventions | SCS vs conventional medical management |
| Outcomes | Responder rate at 3 months: proportion of participants with 50% or greater reduction in pain |
| Starting date | 26/03/2021 |
| Contact information | Alexander Chernyak alexander.chernyak@bsci.com# Diane Keesey diane.keesey@bsci.com |
| Notes | Industry funded: Boston Scientific Corporation |
NCT04894734.
| Study name | Spinal cord stimulation (SCS) forsSpinal cord injury (SCI) |
| Methods | Cross‐over RCT |
| Participants | Spinal cord injury at T1‐T12 Level, N = 30 |
| Interventions | SCS vs placebo (sham) |
| Outcomes | Change in Multidimensional Pain Inventory (MPI)‐SCI average activity score [Time Frame: Baseline, 9 months] Change in pain as measured by 10‐point Numeric Rating Scales (NRS) [Time Frame: Baseline, 9 months] Change in Quality of Life (QOL) as measured by the Patient‐Reported Outcomes Measurement Information System (PROMIS‐29) [Time Frame: Baseline, 9 months] Change in the number of prescriptions written as measured by Electronic Health Record abstraction. [ Time Frame: Baseline, 9 months] Change in the number of opioid prescriptions filled as measured by Electronic Health Record abstraction [Time Frame: Baseline, 9 months] Change in independence of activities of daily living (ADLs) as measured by the Spinal Cord Independence Measure (SCIM) survey [Time Frame: Baseline, 9 months] |
| Starting date | 31/05/2021 |
| Contact information | Allison Spell, allison.spell@duke.edu Beth Perry, beth.perry@duke.edu |
| Notes | No funding reported |
PANACEA.
| Study name | Prospective, randomised, crossover, controlled, feasibility study to assess the efficacy of BurstDR spinal cord stimulation (SCS) as a treatment for persistent abdominal refractory visceral pain secondary to chronic pancreatitis: PANACEA trial |
| Methods | Cross‐over RCT |
| Participants | Persistent refractory visceral pain secondary to chronic pancreatitis for at least 6 months with or without dermal hyperalgesia or allodynia, N = 30 |
| Interventions | Burst SCS vs conservative management |
| Outcomes | Primary: completion of EQ‐5D5L Health questionnaire (Time Frame: 1 hour), Numeric Rating Scale (NRS) recording pain severity (Time Frame: 1 hour ) |
| Starting date | 09/07/2018 |
| Contact information | Ganesan Baranidharan, PANACEA%20Feasibility%20Study%20to%20Assess%20the%20Efficacy%20of%20BurstDR%20Spinal%20Cord%20Stimulation%20(SCS)" type="EXTERNAL">g.baranidharan@nhs.net |
| Notes |
PARS‐trial.
| Study name | Investigation of efficacy of different spinal cord stimulation paradigms for the treatment of chronic neuropathic pain |
| Methods | Cross‐over RCT |
| Participants | Participants with intractable neuropathic pain (N = 60) |
| Interventions | Burst SCS, 1kHz SCS, 10kHz SCS, placebo SCS |
| Outcomes | Pain "perception" VAS, Quality of life EQ‐5D‐5L |
| Starting date | 16/02/2020 |
| Contact information | Rezvan.Ahmadi@med.uni‐heidelberg.de |
| Notes | Industry sponsored: Stimwave LLC |
PENTAGONS.
| Study name | Diabetic peripheral neuropathy treatment with dorsal root ganglion stimulation – the PENTAGONS trial |
| Methods | Parallel RCT |
| Participants | Painful diabetic neuropathy, n = 56 |
| Interventions | Dorsal root ganglion stimulation vs medical management |
| Outcomes | Primary: pain (VAS) at 30 weeks post randomisation Secondary: pain (VAS) at 8 and 18 weeks post randomisation, Quality of life (EQ‐5D‐5L) at 18 and 30 weeks post randomisation. |
| Starting date | 13/06/2018 |
| Contact information | james.fitzgerald@nds.ox.ac.uk |
| Notes | Industry funder: Abbott Laboratories |
PET‐SCS.
| Study name | PET patterns, biomarkers and outcome in Burst SCS treated FBSS patients (PET‐SCS) |
| Methods | Cross‐over RCT |
| Participants | Chronic pain in the lumbosacral region, as well as unilateral or bilateral leg pain. n = 12 |
| Interventions | Burst SCS vs sham |
| Outcomes | General pain, measured by Brief Pain Inventory (BPI) item 3, 4, 5 and 6 (Time Frame: Measured at day 0 (baseline), day 14 and day 35.) Disability measured by Oswestry Disability Index (ODI). (Time Frame: measured at day 0 (baseline), day 14 and day 35.) |
| Starting date | 11/02/2018 |
| Contact information | Rolf Karlsten, rolf.karlsten@akademiska.se |
| Notes |
SCS‐PHYSIO.
| Study name | Treatment of neuropathic pain with spinal cord stimulation and physiotherapy for more effective pain relief, increased physical activity and improved health related quality of life |
| Methods | Parallel RCT |
| Participants | Chronic neuropathic pain, n = 160 |
| Interventions | SCS vs Physiotherapy |
| Outcomes | Primary: number of patients who report ≥ 50% pain reduction (pain intensity) according to numeric rating scale (score from 0 to 10) (Time Frame: 3 months after implantation). Secondary: Pain intensity according to NRS (6,12,15,21 months after implantation) HRQoL Assessed with RAND Short Form 36 (SF36) and EQ‐5D‐5L (3,6,9,12,15,21 months after implantation) Medicine consumption, number of pills and dosage (9 and 21 months after implantation) Number of hospital and primary care visits (9 and 21 months after implantation) |
| Starting date | 109/05/2018 |
| Contact information | Paulin Andréll, Göteborgs Universitet/ Västra Götalandsregionen |
| Notes | Registered after the official study start date |
SENZA‐NSRBP.
| Study name | Comparison of HF10 therapy combined with Conventional Medical Management (CMM) to CMM alone in the treatment of chronic back pain. (SENZA‐NSRBP) |
| Methods | Parallel RCT |
| Participants | Chronic non‐surgical refractory back pain. N = 300 |
| Interventions | High frequency SCS + conventional medical managemenT vs conventional medical management |
| Outcomes | Primary: responder rates measured using the visual analogue scale (VAS) (as defined by at least a 50% reduction in pain) at 3 months. Secondary: Successful back pain relief is measured using the visual analog scale (VAS) at 1, 3, 6, 9 and 12 months Percentage of patients who experience at least 50% reduction in pain intensity is measured using the VAS at 1, 3, 6, 9 and 12 months Back pain intensity is measured using VAS at baseline, 1, 3, 6, 9 and 12 months Percentage of participants who experience a back pain intensity score of ≤2.5 cm as measured using the VAS scale at 1, 3, 6, 9 and 12 months Quality of life is measured using EQ‐5D questionnaire at 3, 6 and 12 months Health economic outcomes are measured using clinic visits, incidence of adverse events, EQ‐5D at 1, 3, 6, 9 and 12 months |
| Starting date | 05/12/2016 |
| Contact information | Not reported |
| Notes | Industry sponsor: Nevrocorp |
TSUNAMI DRG.
| Study name | A European, prospective, multi‐center, double‐blind, randomized, controlled, clinical trial investigating the effects of high frequency wireless spinal cord stimulation (SCS) over exiting nerve roots in the treatment of chronic back pain |
| Methods | Parallel RCT |
| Participants | Chronic low back pain, N = 38 |
| Interventions | High‐frequency dorsal root ganglion stimulation vs sham |
| Outcomes | Primary: responder rate [ Time Frame: 1 month post‐implant] a > 50% reduction in back pain as measured by VAS with the Freedom SCS system in the HF (test) group as opposed to sham and conventional medical management Secondary: Percentage change from baseline in VAS for back pain (Time Frame: 1, 3, 6, 9 and 12 months) Percentage change from baseline in VAS for leg pain (Time Frame: 1, 3, 6, 9 and 12 months) Change from baseline in functionality using the ODI score ODI (Time Frame: 1, 3, 6, 9 and 12 months) Changes from baseline in quality of life, EQ‐5D‐5L (Time Frame: 1, 3, 6, 9 and 12 months) Incidence of device related adverse events AE's (Time Frame: 1, 3, 6, 9 and 12 months) Prescribed opioid pain medications (Time Frame: 1, 3, 6, 9 and 12 months) Prescribed non‐opioid pain medication (Time Frame: 1, 3, 6, 9 and 12 months) |
| Starting date | 01/03/2018 |
| Contact information | Not available |
| Notes | Industry sponsored: Stimwave Technologies |
Only outcomes relevant to this review are presented here.
BPI = Brief Pain Inventory; EQ‐5D‐5L= 5‐level EQ‐5D version, ODI = Oswestry Disability Index, VAS = Visual Analogue Scale
Differences between protocol and review
The planned distinction between "during use" and other time points was not possible to make and artificial in the context of the included studies, as outcomes were evaluated whilst stimulation was active at all time points. As a result we restricted our analyses to short‐term, mid‐term and long‐term follow‐up. For cross‐over studies, follow‐up was measured from the point of the onset of stimulation. We do not anticipate reinstating "during use" as a time point in future updates.
For cross‐over studies, we planned to only included data from the first phase of the study, when they were available. As first‐phase, or phase‐by‐phase data were not available for any of the included cross‐over studies we took the decision to analyse these studies as presented. As we did not have access to individual data from any of these studies we were unable to adjust for the paired nature of the data from these trials as recommended in the Cochrane Handbook (Higgins 2021). As such, while point estimates in this analysis should be accurate representations of the data it is likely that these analyses may be conservative, in terms of overestimating imprecision. As a result of this decision, we used the ROB2 tool for cross‐over studies to assess the risk of bias for these results. (see Unit of analysis issues).
We planned to express the size of the treatment effect for pain intensity, measured with a VAS or NRS, using the mean difference (MD) when all studies utilised the same measurement scale and to use the standardised mean difference (SMD) when studies used substantially different scales. As all studies measured pain intensity on a 0 to 10 or 0 to 100 VAS or NRS we normalised all scales to a 0 to100 scale and expressed the effect size as the MD to aid interpretability.
We had planned to exclude studies rated at high risk of bias from the primary meta‐analyses, including those at risk of bias due to missing outcome data. However, all included studies were rated at high risk of bias on one or more domain of the ROB2 tool. On that basis, we have included those studies in our analyses but clearly reflect the risk of bias and the certainty of evidence in our intepretation.
In Table 2, we included outcomes at medium‐term follow‐up in this table, though this was not prespecified in our protocol. The reason for this was that for all included studies the specified primary endpoint was at medium‐term follow‐up.
Contributions of authors
| Conceive the review | NOC, WG, AR, LV, CE |
| Draft the protocol | NOC, WG, AR, LV, CE |
| Develop and run the search strategy | NOC PaPaS Information Specialist provided support. |
| Obtain copies of studies | NOC |
| Select which studies to include (2 people) | NOC, WG |
| Extract data from studies (2 people) | NOC, WG, MF |
| Evaluate risk of bias, make GRADE judgements | NOC, MF |
| Enter data into RevMan 5 | NOC |
| Carry out the analysis | NOC |
| Interpret the analysis | NOC, MF, WG, AR, LV, CE |
| Draft the final review | NOC, MF, WG, AR, LV, CE |
| Update the review | NOC, MF, WG, AR, LV, CE |
Sources of support
Internal sources
-
Various, UK
All authors are employed by higher education institutions (Universities, see author affiliations) and receive a salary from their employers
External sources
-
National Institute for Health Research (NIHR), UK
Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS)
Declarations of interest
NOC: none known.
MF: none known
WG: none known.
ASCR: ASCR is an Hon. Consultant in Pain Medicine at Chelsea and Westminster Hospital NHS Foundation Trust and is an employee of Imperial College London. He works in a multi‐disciplinary pain service, providing specialist diagnostic and management service for people living with chronic neuropathic pain. ASCR undertakes consultancy and advisory board work for Imperial College Consultants; in the last 36 months this has included remunerated work for: Pharmanovo, Lateral, Novartis, Pharmaleads, Mundipharma, Orion, Toray, Abide, Confo, Vertex, Shanghai SIMR Biotech, Asahi Kasei & Theranexis (since 2020 fees have been donated to charity). ASCR was the owner of share options in Spinifex Pharmaceuticals, from which personal benefit accrued between 2015 and 2019 upon the acquisition of Spinifex by Novartis. ASCR is named as an inventor on patents (not being pursued):
Rice ASC, Vandevoorde S, Lambert D.M (2005), Methods using N‐(2‐propenyl)hexadecanamide and related amides to relieve pain. WO2005/079771;
Okuse K, et al (2013). Methods of treating pain by inhibition of VGF activity. EP13702262.0/ WO2013 110945.
ASRC is a member of the UK Joint Committee on Vaccination and Immunisation, Varicella sub‐committee, the Neurology, Pain & Psychiatry Expert Advisory Group, Commission on Human Medicines, Medicines & Healthcare Products Regulatory Agency (MHRA) and the Public‐Private Partnership for Analgesic Clinical Trial Translation, Innovations, Opportunities, and Networks (ACTTION) Executive Committee.
LV: none known.
DC: none known.
CE: none known.
Since NOC is an author and PaPaS Co‐ordinating Editor (as was CE at the time of developing the protocol), we acknowledge the input of Peter Tugwell, Senior Editor of the Cochrane Musculoskeletal, Oral, Skin, and Sensory (MOSS) Network, who acted as Sign‐off Editor for this review. NOC and CE had no input into the editorial decisions or processes for this review.
Edited (no change to conclusions)
References
References to studies included in this review
Al‐Kaisy 2018 {published data only (unpublished sought but not used)}
- Al-Kaisy A, Palmisani S, McCammon S, Sanderson KK, Caganillo R, Tan Y, et al.Spinal cord stimulation study evaluating role of higher frequencies (SCS frequency study) (10524). In: Neuromodulation. Vol. 19. 2016:e34.
- Al-Kaisy A, Palmisani S, Pang D, Sanderson K, Wesley S, Tan Y, et al.Prospective, randomized, sham-control, double blind, crossover trial of subthreshold spinal cord stimulation at various kilohertz frequencies in subjects suffering from failed back surgery syndrome (SCS Frequency Study). Neuromodulation 2018;21(5):457‐65. [DOI] [PubMed] [Google Scholar]
De Ridder 2013 {published data only (unpublished sought but not used)}
- De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S.Burst spinal cord stimulation for limb and back pain. World Neurosurgery 2013;80(5):642‐49 e1. [DOI] [PubMed] [Google Scholar]
de Vos 2014 {published and unpublished data}ISRCTN03269533
- Vos C, Dijkstra-Scholten C, Meier K, Zaalberg PB, Vesper J, Lenders MW.Spinal cord stimulation in patients with diabetic neuropathic pain. In: Neuromodulation. Vol. 14. 2011:460.
- Vos C, Meier K, Zaalberg PB, Nijhuis H, Duyvendak W, Vesper J, et l.Spinal cord stimulation in patients with painful diabetic neuropathy. In: Neuromodulation. Vol. 16. 2013:e44. [DOI] [PubMed]
- Vos CC, Meier K, Zaalberg PB, Nijhuis HJ, Duyvendak W, Vesper J, et al.Spinal cord stimulation in patients with painful diabetic neuropathy: a multicentre randomized clinical trial. Pain 2014;155(11):2426-31. [DOI] [PubMed] [Google Scholar]
- Duarte RV, Andronis L, Lenders MW, Vos CC.Quality of life increases in patients with painful diabetic neuropathy following treatment with spinal cord stimulation. Quality of Life Research 2016;25(7):1771‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eldabe 2021 {published data only}
- Eldabe S, Duarte R, Gulve A, Williams H, Garner F, Brookes M, et al.Analgesic efficacy of “Burst” and Tonic (500 Hz) spinalcord stimulation patterns: a randomized placebo-controlled crossover study. Neuromodulation 2021;24:471-8. [DOI] [PubMed] [Google Scholar]
- Tariq A, Eldabe S, Madzinga G, Gulve A, Williams H, Garner F, et al.Analgesic efficacy of “burst” and tonic(500 Hz) spinal cord stimulation patterns: arandomised placebo-controlled study. In: Neuromodulation. Vol. 23. 2020:e1-e325. [DOI] [PubMed]
Kemler 2000 {published and unpublished data}
- Kemler MA, Barendse GA, Kleef M, Vet HC, Rijks CP, Furnée CA, et al.Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. New England Journal of Medicine 2000;343(9):618‐24. [DOI] [PubMed] [Google Scholar]
- Kemler MA, Vet HC, Barendse GA, den Wildenberg FA, Kleef M.Effect of spinal cord stimulation for chronic complex regional pain syndrome Type I: five-year final follow-up of patients in a randomized controlled trial. Journal of Neurosurgery 2008;108(2):292‐8. [DOI] [PubMed] [Google Scholar]
- Kemler MA, De Vet HC, Barendse GA, Van Den Wildenberg FA, Van Kleef M.The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years' follow-up of the randomized controlled trial. Annals of Neurology 2004;55(1):13‐8. [DOI] [PubMed] [Google Scholar]
- Kemler MA, Furnée C A.Economic evaluation of spinal cord stimulation for chronic reflex sympathetic dystrophy. Neurology 2002;59(8):1203‐9. [DOI] [PubMed] [Google Scholar]
Kriek 2017 {published data only (unpublished sought but not used)}ISRCTN36655259
- Kriek N, Groeneweg J, Stronks D, Huygen F.High frequency and burst spinal cord stimulations in patients with complex regional pain syndrome: a randomized placebo controlled trial. In: Pain Practice. Vol. 14. 2014:117.
- Kriek N, Groeneweg JG, Stronks DL, Ridder D, Huygen FJ.Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: a multicentre, double-blind, randomized and placebo-controlled crossover trial. European Journal of Pain 2017;21(3):507‐19. [DOI] [PubMed] [Google Scholar]
- Kriek N, Groeneweg JG, Stronks DL, Huygen FJ.Comparison of tonic spinal cord stimulation, high-frequency and burst stimulation in patients with complex regional pain syndrome: a double-blind, randomised placebo controlled trial. BMC Musculoskeletal Disorders 2015;16:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lind 2015 {published data only (unpublished sought but not used)}
- Hellstrom PM, Lind G, Linderoth B.Spinal cord stimulation in the irritable bowel syndrome-a randomized cross-over trial. In: United European Gastroenterology Journal. Vol. 1. 2013:A240.
- Lind G, Winter J, Linderoth B, Hellström PM.Therapeutic value of spinal cord stimulation in irritable bowel syndrome: a randomized crossover pilot study. American Journal of Physiology 2015;308(10):R887‐94. [DOI] [PubMed] [Google Scholar]
Perruchoud 2013 {published data only (unpublished sought but not used)}
- Perruchoud C, Eldabe S, Batterham AM, Madzinga G, Brookes M, Durrer A, et al.Analgesic efficacy of high-frequency spinal cord stimulation: a randomized double-blind placebo-controlled study. Neuromodulation 2013;16(4):363‐9. [DOI] [PubMed] [Google Scholar]
PROCESS {published data only (unpublished sought but not used)}ISRCTN77527324
- Eldabe S, Buchser E, Kumar K, Taylor R.Function and quality of life in failed back surgery syndrome patients following spinal cord stimulation and conventional medical management. In: European Journal of Pain. Vol. 13. 2009:S45. [DOI] [PubMed]
- Eldabe S, Buchser E, Kumar K, Taylor R.Pain in failed back surgery syndrome patients following spinal cord stimulation and conventional medical management. In: European Journal of Pain. Vol. 13. 2009:S135‐. [DOI] [PubMed]
- Eldabe S, Kumar K, Buchser E, Taylor RS.An analysis of the components of pain, function, and health-related quality of life in patients with failed back surgery syndrome treated with spinal cord stimulation or conventional medical management. Neuromodulation 2010;13(3):201‐9. [DOI] [PubMed] [Google Scholar]
- Kumar K, Eldabe S, Buchser E, Taylor R.Changes in pain, function and quality of life in patients with failed back surgery syndrome treated with spinal cord stimulation or conventional medical management. In: Pain Practice. Vol. 9. 2009:85. [DOI] [PubMed]
- Kumar K, Eldabe S, Buchser E, Taylor R.Function and health-related quality of life in failed back surgery syndrome patients following spinal cord stimulation and conventional medical management. In: Pain Medicine. Vol. 11. 2010:294‐5. [DOI] [PubMed]
- Kumar K, North R, Taylor R, Sculpher M, Van Den Abeele C, Gehring M, et al.Spinal cord stimulation vs. conventional medical management: a prospective, randomized, controlled, multicenter study of patients with failed back surgery syndrome (PROCESS study). Neuromodulation 2005;8(4):213‐8. [DOI] [PubMed] [Google Scholar]
- Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al.Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007;132(1):179‐88. [DOI] [PubMed] [Google Scholar]
- Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al.The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery 2008;63(4):762‐70. [DOI] [PubMed] [Google Scholar]
- Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al.The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery 2008;63(4):762‐70. [DOI] [PubMed] [Google Scholar]
- Manca A, Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, et al.Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). European Journal of Pain 2008;12(8):1047‐58. [DOI] [PubMed] [Google Scholar]
- Meglio M, Cioni S, Sabene S.Spinal cord stimulation versus conventional medical management for failed back surgery syndrome: long-term results from the PROCESS study. In: European Journal of Neurology. Vol. 15. 2008:11, Abstract no: SC109.
- North RB.Spinal cord stimulation vesus conventional medical management: preliminary long-term results from the PROCESS study: a multi-center randomized controlled trial investigating the treatment of patients with failed back surgery syndrome. In: Neurology. Vol. 70. 2008:A162, Abstract no: PO3.147.
PROMISE {published data only (unpublished sought but not used)}
- North R, Desai M, Vangeneugden J, Raftopoulos C, Van Havenbergh T, Deruytter M et al.Postoperative Infections Associated With Prolonged Spinal Cord Stimulation Trial Duration (PROMISE RCT). Neuromodulation 2020;23(5):620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RB, Deruytter MM, Vangeneugden JJM, Raftopoulos C, Van Havenbergh T, Desai MJ, et al.Perioperative infections and prolonged SCS trial duration (PROMISE study). In: Neuromodulation. Vol. 21. 2018:e11.
- Rigoard P, Basu S, Desai M, Taylor R, Annemans L, Tan Y, et alR.Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: a multicenter randomized controlled trial. Pain 2019;160(6):1410‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigoard P, Desai MJ, North RB, Taylor RS, Annemans L, Greening C, Tet al.Spinal cord stimulation for predominant low back pain in failed back surgery syndrome: study protocol for an international multicenter randomized controlled trial (PROMISE study). Trials 2013;14:376. [DOI: 10.1186/1745-6215-14-376] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigoard P, Desai MJ, Taylor RS, Annemans L, Johnson MJ, Tan Y, et al.Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: an international multicenter randomized trial (PROMISE study). In: Pain Physician. Vol. 20. 2017:E614.
- Rigoard P, North R, Taylor RS, Annemans L, Tan Y, Johnson MJ, et al.Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: 12-month results of an international multicenter randomized trial (PROMISE Study). In: Neuromodulation. Vol. 21. 2018:e155.
Schu 2014 {published data only (unpublished sought but not used)}
- Schu S, Bara G, Vesper J.Burst or tonic stimulation' first results of a placebo controlled, doubled blinded, randomized study for the treatment of fbss patients. In: Pain Practice. Vol. 14. 2014:68.
- Schu S, Slotty PJ, Bara G, Knop M, Edgar D, Vesper J.A prospective, randomised, double-blind, placebo-controlled study to examine the effectiveness of burst spinal cord stimulation patterns for the treatment of failed back surgery syndrome. Neuromodulation 2014;17(5):443‐50. [DOI] [PubMed] [Google Scholar]
- Vesper J, Slotty PJ, Schu S.Burst or tonic stimulation? results of a placebo controlled, double blinded, randomized study for the treatment of FBSS patients-3y follow-up. In: Neuromodulation. Vol. 20. 2017:e170.
SENZA‐PDN {published and unpublished data}
- Argoff C, Mekhail N, Nasr C, Taylor R, Caraway D, Gliner B, et al.High frequency spinal cord stimulation (HF-SCS) at 10 kHz for the treatment of neuropathic limb pain from painful diabetic neuropathy. In: Postgraduate Medicine. Vol. 130. 2018:9‐10.
- Argoff C, Mekhail N, Nasr C, Taylor R, Neumann P, Caraway D, et al.A prospective, randomized, controlled trial of high frequency spinal cord stimulation for the treatment of neuropathic limb pain from painful diabetic neuropathy: the Senza-PDN protocol. In: Pain Practice. Vol. 18. 2018:31.
- Mekhail NA, Argoff CE, Taylor RS, Nasr C, Caraway DL, Gliner BE, et al.High-frequency spinal cord stimulation at 10 kHz for the treatment of painful diabetic neuropathy: design of a multicenter, randomized controlled trial (SENZA-PDN). Trials 2020;21(1):87. [DOI: 10.1186/s13063-019-4007-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E, Investigators Senza-PDN.Neuromodulation for treatment of painful diabetic neuropathy: a multicentre randomised controlled trial. In: Diabetologia. Vol. 63. 2020:S94.
- Petersen E, Stauss T, Scowcroft J, Brooks E, White J, Sills S, et al.10 kHz spinal cord stimulation for treatment of painful diabetic neuropathy-a multicenter randomized controlled trial. In: Postgraduate Medicine. Vol. 132. 2020:17-8.
- Petersen E, Stauss T, Scowcroft J, Sills S, White J, Amirdelfan K, et al.10 kHz spinal cord stimulation for treatment of painful diabetic neuropathy-a multicenter randomized controlled trial. In: Neuromodulation. Vol. 23. 2020:3: e31-32.
- Petersen E, Stauss T, Scowcroft J, White J, Sill S, Amirdelfan K, et al.Neuromodulation for treatment of painful diabetic neuropathy - A multicenter randomized controlled trial comparing 10 khz spinal cord stimulation to conventional medical management. In: Endocrine Practice. Vol. 26. 2020:159-60.
- Petersen E, Stauss T, Scowcroft J, White J, Sills S, Amirdelfan K, et al.10 kHz spinal cord stimulation for treatment of painful diabetic neuropathy: a multicenter randomized controlled trial. In: Journal of the Peripheral NervousSsystem. 551 edition. Vol. 25. 2020:4.
- Petersen E, Stauss T, Scowcroft J, White J, Sills S, Amirdelfan K, et al.10 kHz Spinal Cord Stimulation for Treatment of Painful Diabetic Neuropathy-A Multicenter Randomized Controlled Trial. In: Neurology. Vol. 94. 2020:6.
- Petersen E, Stauss T, Scowcroft J, White J, Sills S, Amirdelfan K, et al.10 khz spinal cord stimulation for treatment of painful diabetic neuropathy-a multicenter randomized controlled trial. In: Pain Practice. Vol. 20. 2020:76.
- Petersen E, Stauss T, White JL, Sills SM, Amirdelfan K, Guirguis M, et al.Neuromodulation for treatment of painful diabetic neuropathy: a multicenter randomized controlled trial. In: Diabetes. Vol. 69. 2020.
- Petersen EA, Stauss T, ScowcroftJ, White J, Sills S, Amirdelfa K, et al.10 kHz spinal cord stimulation for treatment of painful diabetic neuropathy - a multicenter randomized controlled trial. In: Neurosurgery. Vol. 67. 2020:177.
- Petersen EA, Stauss TG, Scowcroft JA, Brooks ES, White JL, Sills SM, et al.Effect of high-frequency (10-kHz) spinal cord stimulation in patientswWith painful diabetic neuropathy: a randomized clinical trial. JAMA Neurology 2021;78(6):687-98. [PMID: PMID: 33818600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Slangen 2014 {published data only (unpublished sought but not used)}
- Slangen R, Faber CG, Schaper N C, Joosten EA, Dongen RT, Kessels AG, et al.A trial-based economic evaluation comparing spinal cord stimulation with best medical treatment in painfuldDiabetic peripheralnNeuropathy. Journal of Pain 2017;18(4):405-14. [DOI] [PubMed] [Google Scholar]
- Slangen R, Schaper NC, Faber CG, Joosten EA, Dirksen CD, Dongen RT, et al.Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: a prospective two-center randomized controlled trial. Diabetes Care 2014;37(11):3016-24. [DOI] [PubMed] [Google Scholar]
Sokal 2020 {published data only (unpublished sought but not used)}
- Malukiewicz A, Sokal P.Comparison of tonic, burst and high frequency spinal cord stimulation in chronic pain syndromes: a double-blind, randomised, cross-over, placebo controlled trial. In: Stereotactic Functional Neurosurgery. Vol. 97. 2019:22.
- Sokal P, Malukiewicz A, Kieronska S, Murawska J, Guzowski C, Rudas M, et al.Sub-perception and supra-perception spinal cord stimulation in chronic pain syndrome: a randomized, semi-double-blind, crossover, placebo-controlled trial. Journal of Clinical Medicine 2020;9(9):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tjepkema‐Cloostermans 2016 {published data only (unpublished sought but not used)}
- Tjepkema-Cloostermans MC, Vos CC, Wolters R, Dijkstra-Scholten C, Lenders MW.Effect of burst stimulation evaluated in patients familiar With spinal cord stimulation. Neuromodulation 2016;19(5):492-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alo 2016 {published data only}
- Alo KM.Prospective, multicenter, randomized, double-blinded, partial crossover study to assess the safety and efficacy of the novel neuromodulation system in the treatment of patients with chronic pain of peripheral nerve origin. Neuromodulation 2016;19(1):99-100. [DOI] [PubMed] [Google Scholar]
Annemans 2014 {published data only}
- Annemans L, Van Buyten JP, Smith T, Al-Kaisy A.Cost effectiveness of a novel 10 kHz high-frequency spinal cord stimulation system in patients with failed back surgery syndrome (FBSS). Journal of Long term Effects of Medical Implants 2014;24(2-3):173-83. [DOI] [PubMed] [Google Scholar]
Dones 2008 {published data only}
- Dones I, Broggi G, Loeser JD, Penn RD, Henderson JM, Sagher O.The effects of spinal cord stimulation in neuropathic pain are sustained: A 24 month follow-up of the prospective randomized controlled multi-center trial of the effectiveness of spinal cord stimulation. Comments. Neurosurgery 2008;63(4):768-70. [DOI] [PubMed] [Google Scholar]
Falowski 2019 {published data only}
- Falowski SM, Sharan A, McInerney J, Jacobs D, Venkatesan L, Agnesi F.Nonawake vs awake placement of spinal cord stimulators: a prospective, multicenter study comparing safety and efficacy. Neurosurgery 2019;84(1):198-205. [DOI] [PubMed] [Google Scholar]
Gilligan 2020 {published data only}
- Gilligan C.Restorative neurostimulation for refractory mecjhanical chronic low back pain- results of a randomized active sham-controlled trial. In: Pain Practice. Vol. 20. 2020:71, ID242.
Kemler 2001 {published data only}
- Kemler MA, Reulen JP, Barendse GA, Kleef M, Vet HC, den Wildenberg FA.Impact of spinal cord stimulation on sensory characteristics in complex regional pain syndrome type I: a randomized trial. Anesthesiology 2001;95(1):72‐80. [DOI] [PubMed] [Google Scholar]
Kemler 2010 {published data only}
- Kemler MA, Raphael JH, Bentley A, Taylor RS.The cost-effectiveness of spinal cord stimulation for complex regional pain syndrome. Value in Health 2010;13(6):735-42. [DOI] [PubMed] [Google Scholar]
Kufakwaro 2012 {published data only}
- Kufakwaro N, Kothari S, Reddy R, Petrovic Z, Shetty A.Neuromodulation of dorsal root ganglion: a comparative study to assess efficacy of pulsed-radiofrequency and neurostimulation in treatment of neuropathic pain. Pain Practice 2012;12:174. [Google Scholar]
Liem 2013 {published data only}
- Liem L, Russo M, Huygen FJP, Van Buyten JP, Smet I, Verrills P, et al.A multicenter, prospective trial to assess the safety and performance of the spinal modulation dorsal root ganglion neurostimulator system in the treatment of chronic pain. Neuromodulation 2013;16(5):471-82. [DOI] [PubMed] [Google Scholar]
Liu 2020 {published data only}
- Liu B, Yang Y, Zhang Z, Wang H, Fan B, Sima L.Clinical study of spinal cord stimulation and pulsed radiofrequency for management of herpes zoster-related pain persisting beyond acute phase in elderly patients. Pain Physician 2020;23(3):263‐70. [PubMed] [Google Scholar]
Liu 2021 {published data only}
- Liu J, Zhang A, Ye X, Hu X, He R, Jiang Z.The effect of short-term spinal cord electrical stimulation on patients with postherpetic neuralgiaand its effect on sleep quality. Neuroendocrinology Letters 2021;42(2):81-6. [PubMed] [Google Scholar]
Marchand 1991 {published data only}
- Marchand S, Bushnell M C, Molinanegro P, Martinez S N, Duncan G H.The effects of dorsal column stimulation on measures of clinical and experimental pain in man. Pain 1991;45(3):249257. [DOI] [PubMed] [Google Scholar]
Meier 2015 {published data only}
- Meier K, Nikolajsen L, Sørensen JC, Jensen TS.Effect of spinal cord stimulation on sensory characteristics: a randomized, blinded crossover study. Clinical Journal of Pain 2015;31(5):384-92. [DOI] [PubMed] [Google Scholar]
Rigoard 2013 {published data only}
- Rigoard P, Jacques L, Monlezun O, Delmotte A, Poon K, Munson R.Treatment of the back pain component of failed back surgery syndrome (FBSS) by multi-column spinal cord stimulation: a multicentre prospective study. Neuromodulation 2013;Conference: 11th World Congress of the International Neuromodulation Society Berlin Germany. Conference Start: 20130608 Conference End: 20130613. Conference Publication::e218. [Google Scholar]
Sagher 2008 {published data only}
- Sagher O.The effects of spinal cord stimulation in neuropathic pain are sustained: A 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation: Commentary. Neurosurgery 2008;63(4):770. [DOI] [PubMed] [Google Scholar]
Steinbach 2017 {published data only}
- Steinbach K, Bettstetter H, Link C.High-frequency spinal cord stimulation at 10 kHz for the treatment of chronic neuropathic pain after a II-III degree burn. Pain Medicine 2017;18(9):1826-8. [DOI] [PubMed] [Google Scholar]
Taylor 2005 {published data only}
- Taylor RJ, Taylor RS.Spinal cord stimulation for failed back surgery syndrome: a decision-analytic model and cost-effectiveness analysis. International Journal of Technology Assessment in Health Care 2005;21(3):351-8. [DOI] [PubMed] [Google Scholar]
Tesfaye 1995 {published data only}
- Tesfaye S, Benbow SJ, Watt J, Pang KA, Miles J, Macfarlane IA.Electrical spinal cord stimulator: a new and effective treatment for chronic peripheral neuropathic pain. Diabetologia 1995;38:A7. [Google Scholar]
van Beek 2015 {published data only}
- Beek M, Slangen R, Schaper NC, Faber C G, Joosten E A, Dirksen CD, Dongen RT, et al.Sustained treatment effect of spinal cord stimulation in painful diabetic peripheral neuropathy: 24-month follow-up of a prospective two-center randomized controlled trial. Diabetes Care 2015;38(9):e132‐4. [DOI] [PubMed] [Google Scholar]
Winfree 2005 {published data only}
- Winfree CJ.Spinal cord stimulation for the relief of chronic pain. Current Surgery 2005;62(5):476‐81. [DOI] [PubMed] [Google Scholar]
Wolter 2012 {published data only}
- Wolter T, Kiemen A, Porzelius C, Kaube H.Effects of sub-perception threshold spinal cord stimulation in neuropathic pain: a randomized controlled double-blind crossover study. European Journal of Pain 2012;16(5):648‐55. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ISRCTN33292457 {published data only}ISRCTN33292457
Miller 2015 {published data only}
- Miller N, Sebahar M, Vallejo R, Binyamin R, Lin S, Mekel-Bobrov N.Optimization of stimulation location is essential to sub-perception SCS: results of a double-blind randomized placebo-controlled trial. In: Neuromodulation. Vol. 18. 2015:e99.
Miller 2016 {published data only}
NCT00200122 {published data only}
NCT00351208 {published data only}
NCT03462147 {published data only}
References to ongoing studies
ACTRN12620000720910 {published data only}
- An evaluation of spinal cord stimulation for the treatment of chronic pain, also its effect on mood, sleep, physical activity and analgesic medicine requirements.. Ongoing study. 03/08/20. Contact author for more information.
Burst SCS {published data only}
- Burst Spinal Cord Stimulation (Burst-SCS) study. Ongoing study. 12/03/2019. Contact author for more information.
ChiCTR‐IOR‐17012289 {published data only}
- A randomized controlled study of spinal cord electrical stimulation in the treatment of pain in patients with diabetic foot. Ongoing study. 03/08/2020. Contact author for more information.
CITRIP {published data only}
- Lu Y, Mao P, Wang G, Tao W, Xiong D, Ma K, et al.Spinal cord stimulation for chronic intractable trunk or limb pain: study protocol for a Chinese multicenter randomized withdrawal trial (CITRIP study). Trials 2020;21(1):834. [DOI] [PMC free article] [PubMed] [Google Scholar]
DISTINCT {published data only}
- Moeschler S, Yue J, Schultz D, Deer T, Desai M, Falowski S, et al.DISTINCT: RCT toevaluate BurstDRTM vs medical management for low back pain. In: Neuromodulation. Vol. 24. 2021:e155.
DRKS00022557 {published data only}
- Effect of stimulation frequency in dorsal root ganglion stimulation (DRG Stimulation). Ongoing study. 01/04/2021. Contact author for more information.
ISRCTN10663814 {published data only}
- Comparison of spinal cord stimulation in combination with standard pain treatment versus standard pain treatment only in patients with intractable chronic back pain without previous history of spine surgery. Ongoing study. 01/01/2020. Contact author for more information.
MODULATE‐ LBP {published data only}
- Al-Kaisy A, Royds J, Palmisani S, Pang D, Wesley S, Taylor R, et al.Multicentre, double-blind, randomised, sham-controlled trial of 10 khz high-frequency spinal cord stimulation for chronic neuropathic low back pain (MODULATE-LBP): a trial protocol. Trials 2020;21:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03546738 {published data only}
- Spinal cord Burst stimulation for chronic radicular pain following lumbar spine surgery: a randomized double-blind sham-controlled crossover trial. Ongoing study. 05/09/2018. Contact author for more information.
NCT03733886 {published data only}
- A randomised sham-controlled double-blinded study of burst spinal cord stimulation for chronic peripheral neuropathic pain. Ongoing study. 07/11/2018. Contact author for more information.
NCT04039633 {published data only}
- Spinal cord stimulation for refractory pain in erythromelalgia. Ongoing study. 26/09/2019. Contact author for more information.
NCT04676022 {published data only}
- SCS as an option for chronic low back and/or leg pain instead of surgery (SOLIS). Ongoing study. 26/03/2021. Contact author for more information.
NCT04894734 {published data only}
- Spinal cord stimulation (SCS) forsSpinal cord injury (SCI) . Ongoing study. 31/05/2021. Contact author for more information.
PANACEA {published data only}
- Prospective, randomised, crossover, controlled, feasibility study to assess the efficacy of BurstDR spinal cord stimulation (SCS) as a treatment for persistent abdominal refractory visceral pain secondary to chronic pancreatitis: trial. Ongoing study. 09/07/2018. Contact author for more information.
PARS‐trial {published data only}DRKS00018929
- Ahmadi R, Campos B, Hajiabadi MM, Doerr-Harim C, Tenckhoff S, Rasche D, et al.Efficacy of different spinal cord stimulation paradigms for the treatment of chronic neuropathic pain (PARS-trial): study protocol for a double-blinded, randomized, and placebo-controlled crossover trial.. Trials 2021;22(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
PENTAGONS {published data only}ISRCTN40062191
- Diabetic peripheral neuropathy treatment with dorsal root ganglion stimulation – the trial. Ongoing study. 13/06/2018. Contact author for more information.
PET‐SCS {published data only}
- PET patterns, biomarkers and outcome in treated FBSS patients (). Ongoing study. 11/02/2018. Contact author for more information.
SCS‐PHYSIO {published data only}
- Treatment of neuropathic pain with spinal cord stimulation and physiotherapy for more effective pain relief, increased physical activity and improved health related quality of life. Ongoing study. 109/05/2018. Contact author for more information.
SENZA‐NSRBP {published data only}87648175
- Al-Kaisy A, Eldridge P, Crossman J, Kallewaard JW, Elzinga L, Shiban E, et al.Medical management versus 10 khz spinal cord stimulation and medical management for the treatment of non-surgical back pain. In: Pain physician. Vol. 21. 2018:E229.
- Al-Kaisy A, Eldridge PR, Crossman J, Kallewaard JW, Elzinga LL, Shiban EES, et al.Medical management and 10 kHz spinal cord stimulation for the treatment of non-surgical back pain. In: Neuromodulation. Vol. 21. 2018:3: E138.
- Patel N, Calodney A, Kapural L, Province-Azalde R, Lad SP, Pilitsis J, et al.high-frequency spinal cord stimulation at 10 kHz for the treatment of nonsurgical refractory back pain: design of a pragmatic, multicenter, randomized controlled trial. Pain Practice 2021;21(2):171-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiters P, Al-Kaisy A, Eldridge P, Shiban E, Crossman J, Fritz A K, et al.High Frequency Spinal Cord Stimulation (HFSCS) at 10 kHz plus Conventional Medical Management (CMM) versus conventional medical management alone for the treatment of non-surgical back pain. In: Neuromodulation. Vol. 22. 2019:e8.
TSUNAMI DRG {published data only}
- A European, prospective, multi-center, double-blind, randomized, controlled, clinical trial investigating the effects of high frequency wireless spinal cord stimulation (SCS) over exiting nerve roots in the treatment of chronic back pain. Ongoing study. 01/03/2018. Contact author for more information.
Additional references
Braithwaite 2020
- Braithwaite FA, Walters JL, Moseley GL, Williams MT, McEvoy MP.Towards more credible shams for physical interventions: A Delphi survey. Clinical Trials 2020;17(3):295-305. [DOI] [PubMed] [Google Scholar]
Buchbinder 2018
- Buchbinder R, Johnston RV, Rischin KJ, Homik J, Jones CA, Golmohammadi K, et al.Percutaneous vertebroplasty for osteoporotic vertebral compression fracture. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD006349. [DOI: 10.1002/14651858.CD006349.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Busse 2015
Caylor 2019
- Caylor J, Reddy R, Yin S, Cui C, Huang M, Huang C, et al.Spinal cord stimulation in chronic pain: evidence and theory for mechanisms of action. Bioelectronics in Medicine 2019;5:12. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cruccu 2016
- Cruccu G, Garcia-Larrea L, Hansson P, Keindl M, Lefaucheur JP, Paulus W.EAN guidelines on central neurostimulation therapy in chronic pain conditions. European Journal of Neurology 2016;23:1489-99. [DOI] [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG.Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Deer 2017
- Deer TR, Levy RM, Kramerc J, Poreed L, Amirdelfane K, Grigsby E, et al.Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain 2017;158(4):669-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deschartres 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P.Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ 2013;346:f2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Detlef‐Treede 2019
- Detlef-Treede R, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al.Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International Classification of Diseases (ICD-11). Pain 2019;160(1):19-27. [DOI] [PubMed] [Google Scholar]
Duarte 2020
- Duarte RV, Nevitt S, McNicol E, Taylor RS, Buchser E, North RB, et al.Systematic review and meta-analysis of placebo/sham controlled randomised trials of spinal cord stimulation for neuropathic pain. Pain 2020;161(1):24-35. [DOI] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al.Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105-21. [DOI] [PubMed] [Google Scholar]
Eldabe 2016
- Eldabe S, Buchser E, Duarte RV.Complications of spinal cord stimulation and peripheral nerve stimulation techniques: a review of the literature. Pain Medicine 2016;17(2):325-36. [DOI] [PubMed] [Google Scholar]
Esposito 2019
- Esposito MF, Malayil R, Hanes M, Deer T.Unique characteristics of the dorsal root ganglion as a target for neuromodulation. Pain Medicine 2019;20(Suppl 1):S23-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finnerup 2016
- Finnerup N, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D et al.Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016;157:1599-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaskin 2012
- Gaskin DJ, Richard P.The economic costs of pain in the United States. Journal of Pain 2012;13(8):715-24. [DOI] [PubMed] [Google Scholar]
Grider 2016
- Grider JS, Manchikanti L, Carayannopoulos A, Sharma ML, Balog CC, Harned ME, et al.Effectiveness of spinal cord stimulation in chronic spinal pain: a systematic review. Pain Physician 2016;19(1):E33-54. [PubMed] [Google Scholar]
Gustavsson 2012
- Gustavsson J, Bjorkman J, Ljungcrantz C, Rhodin A, Rivano-Fischer M, Sjolund KF, et al.Socio-economic burden of patients with a diagnosis related to chronic pain - register data of 840,000 Swedish patients. European Journal of Pain 2012;16(2):289-99. [DOI] [PubMed] [Google Scholar]
Higgins 2020
- Higgins JP Savović J, Page MJ, Elbers RG, Sterne JA.Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Higgins 2021
- Higgins JPT Eldridge S, Li T (editors).Chapter 23: Including variants on randomized trials.. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane, 2021. [Google Scholar]
Jensen 2019
- Jensen MP, Brownstone RM.Mechanisms of spinal cord stimulation for the treatment of pain: still in the dark after 50 years. European Journal of Pain 2019;23(4):652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 2021
- Katz N, Dworkin RH, North R, Thomson S, Eldabe S, Hayek SM, et al.Research design considerations for randomized controlled trials of spinal cord stimulation for pain: IMMPACT/ION/INS recommendations. Pain 2021;162(7):1935-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaer 2019
- Kjaer SW, Rice AS, Wartolowska K, Vase L.Neuromodulation: more than a placebo effect? Pain 2019;161(3):491-5. [DOI] [PubMed] [Google Scholar]
Kosek 2016
- Kosek E, Cohen M, Baron R, Gebhart GF, Mico JA, Rice AS, et al.Do we need a third mechanistic descriptor for chronic pain states? Pain 2016;157(7):1382-6. [DOI: 10.1097/j.pain.0000000000000507] [DOI] [PubMed] [Google Scholar]
Lad 2010
- Lad SP, Kalanithi PS, Arrigo RT, Patil CG, Nathan JK, Boakye M, et a[.A socioeconomic survey of spinal cord stimulation (SCS) surgery.. Neuromodulation 2010;13:265-8. [DOI] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L.Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: MR000033. [CENTRAL: MR000033] [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McNicol 2021
- McNicol E, Ferguson M, Bungay K, Rowe EL, Eldabe S, Gewandter JS, Hayek SM, et al.Systematic review of research methods and reporting quality of randomized clinical trials of spinal cord stimulation for pain. Journal of Pain 2021;22(2):127-42. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
MoH‐LTC 2005
- Medical Advisory Secretariat, Ministry of Health and Long-term Care (MoH-LTC).Spinal cord stimulation for neuropathic pain. An evidence based analysis. Ontario Health Technology Assessment Series 2005;5(4):1-78. [PMC free article] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ.Managing potential publication bias. In: Systematic Reviews in Pain Research: Methodology Refined. Vol. 1. Seattle: IASP Press, 2008:1:15-23. [Google Scholar]
Moore 2014
- Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ.The costs and consequences of adequately managed chronic non-cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79-94. [DOI] [PubMed] [Google Scholar]
NICE 2008
- National Institute of Health and Care Excellence (NICE).Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin. Technology appraisal guidance (TA159). www.nice.org.uk/guidance/ta159 2008;(accessed 5 February 2020).
NICE 2019
- National Institute for Health and Care Excellence (NICE).Senza spinal cord stimulation system for delivering HF10 therapy to treat chronic neuropathic pain. Medical technologies guidance (MTG41). www.nice.org.uk/guidance/mtg41 2019;(accessed 5 February 2020).
Niyomsri 2020
- Niyomsri S, Duarte RV, Eldabe S, Fiore G, Kopell BH, McNicol E, et al.A systematic review of economic evaluations reporting the cost-effectiveness of spinal cord stimulation. Value in Health 2020;23(5):656-65. [DOI] [PubMed] [Google Scholar]
Nuesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, TschannenB, Altman DG, et al.Small study effects in meta-analysesof osteoarthritis trials: meta-epidemiological study. BMJ 2010;341(7766):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connell 2013
- O'Connell NE, Wand BM, McAuley J, Marston L, Moseley GL.Interventions for treating pain and disability in adults with complex regional pain syndrome. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No: CD009416. [DOI: 10.1002/14651858.CD009416.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ossipov 2006
- Ossipov MH, Porecca F.Chronic pain: multiple manifestations, multiple mechanisms. Drug discovery today. Disease mechanisms 2006;3(3):301-3. [Google Scholar]
Patel 2015
- Patel VB, Wasserman R, Imani F.Interventional therapies for chronic low back pain: a focused review (efficacy and outcomes). Anaesthesia and Pain Medicine 2015;22(5):e29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prager 2010
- Prager J.Estimates of annual spinal cord stimulator implant rises in the United States. Neuromodulation 2010;13(1):68-9. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5.Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rice 2016
- Rice AS, Smith BH, Blyth FM.Pain and the global burden of disease. Pain 2016;157(4):791-6. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group.CONSORT 2010 Statement: updated guidelinesfor reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al, on behalf of the Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group) and the Cochrane Statistical Methods Group.Chapter 14. 'Summary of findings’ tables and grading the certainty of the evidence. In: JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Sdrulla 2018
- Sdrulla AD, Guan Y, Raja SN.Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Practice 2018;18(8):1048-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shemilt 2019
- Shemilt I, Aluko P, Graybill E, Craig D, Henderson C, Drummond M, et al.Chapter 20: Economic evidence. In: JHiggins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al.RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:I4898. [DOI] [PubMed] [Google Scholar]
Sylwander 2020
- Sylwander C, Larsson I, Andersson M, Bergman S.The impact of chronic widespread pain on health status and long-term health predictors: a general population cohort study. BMC Musculoskeletal Disorders 2020;6(21 (1)):36. [DOI: 10.1186/s12891-020-3039-5.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vardeh 2016
- Vardeh D, Mannion R, Wolff CJ.Toward a mechanism-based approach to pain diagnosis. Journal of Pain 2016;17(9 Suppl 2):T50-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 2015
- Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, et al.Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386(9995):743-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wartolowska 2016
- Wartolowska K, Collins GS, Hopewell S, Judge A, Dean BJ, Rombach I, et al.Feasibility of surgical randomised controlled trials with a placebo arm: a systematic review. BMJ Open 2016;15(5 (3)):e010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
O'Connell 2020
- O'Connell NE, Gibson W, Rice AS, Vase L, Coyle D, Eccleston C.Implanted spinal neuromodulation interventions for chronic pain in adults. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD013756. [DOI: 10.1002/14651858.CD013756] [DOI] [PMC free article] [PubMed] [Google Scholar]


