Abstract
Clinical Trial registry name and registration number: Zeus study, NCT02403115
Keywords: systemic lupus erythematosus, lupus nephritis, glomerulonephritis, rheumatology, clinical trial
The characterization of antibodies that cause autoimmune GN is essential for clarifying disease pathogenesis, defining biomarkers, and developing novel therapies. The detection of either pathogenic autoantibodies or new renal antigens in membranous nephropathy (MN) illustrates how better comprehension of the disease process can rapidly change the clinical approach.1
In a recent clinical study,2 we suggested that a “second wave” of antibodies subsequent to the initial autoimmune response may substantially modify the clinical course of disease. Such antibodies recognize proteins of the inflammatory milieu or cells implicated in the recovery of inflammation. Among others, antibodies targeting superoxide dismutase (SOD2), a detoxifying enzyme that protects against the oxidative process that results from deposition of glomerular autoantibodies, have been recently reported in this journal.2 Experimental findings support the existence of second wave antibodies; SOD2 expression increases in a mouse model of MN induced by the injection of anti-thrombospondin type 1 domain containing 7A (THSD7A) IgG4,3 and it is released by podocytes stimulated in vitro by IgG4 purified from patients with MN.4 In both experimental models, upregulation of SOD has been explained as a secondary response to the oxidative process. We hypothesize that anti-SOD2 antibodies may interfere with the antioxidant protective effect of SOD2, resulting in worse outcome in terms of both evolution to fibrosis and renal failure.2
In this study, we investigated serum levels of anti-SOD2 IgG2 in lupus nephritis (LN), which is an important example of secondary autoimmune GN. Histologic findings in LN generally present proliferative glomerular lesions (classes 2–4). However, class 5 of LN is characterized by subepithelial deposits of autoantibodies, similar to MN. The only difference between MN and LN is the isotype of immune deposits: IgG4 in MN and IgG2 in LN.
We studied 1052 patients with systemic lupus erythematosus (SLE), 459 with and 573 without LN, enrolled at different times from the onset of symptoms: 0–1, 2–12, 13–24, 25–48, 48–96, and >96 months. These cohorts are part of a nationwide study (the Zeus study), which aims to characterize the antibody multicomponents in SLE, LN, rheumatoid arthritis, Sjogren syndrome, and antiphospholipid syndrome.5 The main characteristics were median age of 40 (interquartile range, 28–54) years, predominance of women (88%), disease activity (SLEDAI) of four (interquartile range, two to eight), and different types of therapy administered (Supplemental Table 1). Patients with LN (n=91) recruited at T0 had follow-up at 36 months that included measurements of major markers of SLE autoimmunity (serum complement C3 and C4, ANA, ENA, anti-dsDNA, and proteinuria) as well as follow-up every 6 months in the first year and then yearly. At the same time points, a custom-designed ELISA determined serum anti-SOD2 IgG2.2
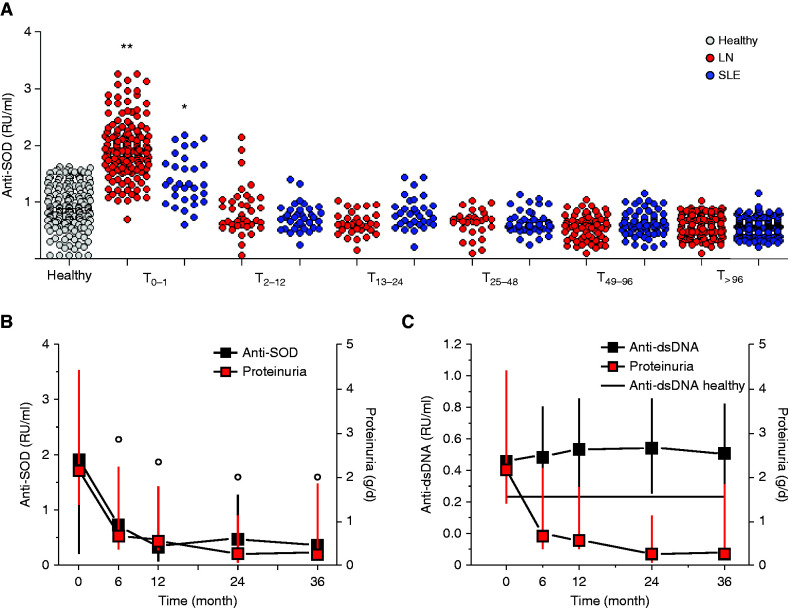
In the cross-sectional analysis, serum levels of anti-SOD2 IgG2 at T0 were significantly higher in LN compared with SLE (Figure 1A). Moreover, among patients with LN, serum levels of anti-SOD2 IgG2 were significantly higher at T0 than in other patients recruited months after the onset of the disease. No correlation with SLEDAI and C3 activity was observed.
Figure 1.
Circulating levels of anti-SOD2 IgG2. (A) Cross-sectional study. Circulating levels of anti-SOD2 IgG2 were determined in two cohorts of patients with SLE with (459) and without (573) LN recruited at different times from the beginning of symptoms. One hundred eighty-two normal controls were studied as well. Clinical characteristics of all patients are reported in Supplemental Table 1. (B and C) Prospective study. Ninety-one LN patients recruited at the time of the diagnosis were prospectively followed for 36 months. Anti-SOD2 IgG2 (B) and anti-dsDNA IgG2 (C) levels were determined every 6 months in the first year and then every 12 months. In both cases, levels were calculated as relative intensity value (RU/ml) given the absence of WHO international standards. The line in (C) indicates normal limits for anti-dsDNA IgG2. **P<0.001 versus healthy, SLE and LN any other time point; *P<0.001 versus healthy, SLE any other time point; °P<0,001 versus T0. dsDNA, double-strain DNA.
We report a similar finding in the prospective analysis of patients with LN with an entire follow-up of 36 months (Figure 1B). Also in this case, serum level of anti-SOD2 IgG2 dropped to almost normal levels at T6, with a persistence of such values until the end of the follow-up (T36). The kinetics of reduction of anti-SOD2 IgG2 were in accordance with the reduction of proteinuria reported after administration of immunosuppressive treatments in new-onset LN. Of interest, anti-dsDNA IgG2 serum levels were persistently maintained at high values during the same period (Figure 1C).
Taken together, these findings suggest that circulating anti-SOD2 IgG2 antibodies are elevated in new-onset LN. It might be hypothesized that the increase is related to the activation of SOD2, in response to the oxidative stress that active disease induces. In that context, presence of anti-SOD2 antibodies might potentially hamper the detoxifying effect of SOD2 and indirectly contribute to sustaining glomerular and tubular injury.
In our findings, anti-SOD2 IgG2 levels decrease in accordance with proteinuria in LN, suggesting a few hypotheses on their significance. One possibility is that anti-SOD2 antibodies decrease in response to immunosuppression, affecting levels of biomarkers that indicate both disease activity and therapeutic efficacy. On the other hand, the results cannot exclude a pathologic cause-effect relationship between anti-SOD2 and proteinuria. We suggest that anti-SOD2 be added to the multicomposition of antibodies involved in SLE and LN, including anti-dsDNA, antinucleosome, and antienolase IgG2 antibodies.5 In fact, except for anti-dsDNA IgG2, other antibody levels were similarly reduced after therapies, leading us to repose the fundamental question. Which antibody of the multicomposite panel that characterizes LN and SLE plays the main pathogenic role?
Overall, these data support the hypothesis that anti-SOD2 antibodies may be considered as second wave antibodies that could actively contribute to the pathogenesis of autoimmune glomerulonephritides.
Disclosures
F. Franceschini reports honoraria from AbbVie, Amgen, Janssen, and Lilly. G. Garibotto reports consultancy agreements with Fresenius Kabi and scientific advisor or membership via editorial boards of BMC Nephrology, Journal of Clinical Medicine, Journal of Nephrology, and Journal of Renal Nutrition; a council member of the International Society of Renal Nutrition and Metabolism; and secretary for the International Society of Renal Nutrition and Metabolism. P. Migliorini reports research funding from Diametra. C. Montecucco reports speakers bureau for AbbVie, BMS, Janssen, Lilly, Novartis, Roche, and Sanofi. G. Moroni reports consultancy agreements with Glaxo. M. Mosca reports consultancy agreements with AbbVie, AstraZeneca GSK, Lilly, Pfizer, and UCB; honoraria from AstraZeneca and GSK; scientific advisor or membership with the editorial boards of Clinical and Experimental Medicine and Lupus Science and Medicine; and speakers bureau for AstraZeneca and GSK. F. Pratesi reports scientific advisor or membership with Vaccines. M. Prunotto reports Galapagos Ltd. as current employer. A. Ravelli reports consultancy agreements with AbbVie, Angelini, BMS, Hoffman LaRoche, Novartis, Pfizer, and Reckitt Benckiser (<$10,000 USD each); research funding from the Italian Public Entity Agenzia Italiana del Farmaco (Italian Agency for Drugs; https://aifa.gov.it); honoraria from AbbVie, Angelini, BMS, Hoffman LaRoche, Novartis, Pfizer, and Reckitt Benckiser (<$10,000 USD each); speakers bureau for AbbVie, Angelini, BMS, Pfizer, Hoffman LaRoche, Novartis, Pfizer, and Reckitt Benckiser (<$10,000 USD each); and other interests/relationships as the president of the Pediatric Rheumatology European Society (https://www.pres.eu/). A. Ravelli has received payment for expert testimony from the pharmaceutical companies indicated in the past 3 years. D. Santoro reports honoraria from Alexion, Astellas, Glaxo, and Travere. R. Sinico reports consultancy agreements with GSK Italy and Otsuka. A. Tincani reports consultancy agreements with Janssen (previously Alexion), Novartis, and UCB; honoraria from Alexion, Novartis, and UCB; scientific advisor or membership with Autoimmunity Reviews, Clinical and Experimental Rheumatology, and Reumatismo; and other interests/relationships with American College of Rheumatology as a member and the Italian Society of Rheumatology as a member. S. Volpi reports other interests/relationships with the European Society for Immunodeficiency. All remaining authors have nothing to disclose.
Funding
The Giannina Gaslini Institute (trial sponsor) provided logistic and financial support to the study through grants from the Ministry of Health (“Ricerca corrente” and “Cinque per mille of Imposta sul Reddito delle Persone Fisiche (IRPEF)-Finanziamento della ricerca sanitaria”). People working at the Zeus project belong to the “Fondazione Malattie Renali del Bambino,” from which we acknowledge financial support.
Supplementary Material
Acknowledgments
We thank all the Zeus study participants (doctors, nurses, and laboratory personnel) and all patients who enrolled.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021050659/-/DCSupplemental.
Supplemental Table 1. Clinical data relative to 479 and 573 patients with lupus nephritis and SLE, respectively.
Supplemental Figure 1. The same data of Figure 1B relative to the patients with LN enrolled in the prospective study are here reported a as single line for each patient: anti-SOD2 levels and proteinuria.
References
- 1.Sethi S: New ‘antigens’ in membranous nephropathy. J Am Soc Nephrol 32: 268–278, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghiggeri GM, Seitz-Polski B, Justino J, Zaghrini C, Payré C, Brglez V, et al. ; Italian Study Group for Membranous Nephropathy : Multi-autoantibody signature and clinical outcome in membranous nephropathy. Clin J Am Soc Nephrol 15: 1762–1776, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomas NM, Hoxha E, Reinicke AT, Fester L, Helmchen U, Gerth J, et al. : Autoantibodies against thrombospondin type 1 domain-containing 7A induce membranous nephropathy. J Clin Invest 126: 2519–2532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buelli S, Perico L, Galbusera M, Abbate M, Morigi M, Novelli R, et al. : Mitochondrial-dependent autoimmunity in membranous nephropathy of IgG4-related disease. EBioMedicine 2: 456–466, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruschi M, Moroni G, Sinico RA, Franceschini F, Fredi M, Vaglio A, et al. : Serum IgG2 antibody multicomposition in systemic lupus erythematosus and lupus nephritis (part 1): Cross-sectional analysis. Rheumatology (Oxford) 60: 3176–3188, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.