Significance Statement
Soluble Klotho is produced in the kidney and its deficiency causes a premature aging phenotype that includes hyperphosphatemia, cardiac hypertrophy, accelerated vascular disease, endothelial dysfunction, and sarcopenia. The physiologic mechanisms that regulate soluble Klotho levels are undefined. Using molecular genetic and biochemical approaches, we show that the mouse distal convoluted tubule calcium-sensing receptor (CaSR) activates the protease A Disintegrin and Metalloproteinase 10 (ADAM10) to cleave membrane-bound Klotho, causing its shedding into the circulation in response to CaSR ligands, allosteric activators, and alkaline pH. The renal CaSR interacts with Klotho and responds to physiologic changes in pH in a manner similar to the parathyroid CaSR. The fact that the CaSR and Klotho localize in the plasma membrane and interact with ADAM10 suggests these proteins function in a complex.
Keywords: ADAM10, calcium sensing receptor, distal convoluted tubule, kidney, Klotho
Visual Abstract
Abstract
Background
The kidney is the source of sKlotho and kidney-specific loss of Klotho leads to a phenotype resembling the premature multiorgan failure phenotype in Klotho-hypomorphic mice (kl/kl mice). Klotho and the Ca-sensing receptor (CaSR) are highly expressed in the distal convoluted tubule (DCT). The physiologic mechanisms that regulate sKlotho levels are unknown.
Methods
We measured sKlotho in WT and tubule-specific CaSR−/− (TS-CaSR−/−) mice treated with calcimimetics, alkali, or acid, and Klotho shed from minced mouse kidneys, and from HEK-293 cells expressing the CaSR and Klotho, in response to calcimimetics, calcilytics, alkalotic and acidic pH, and ADAM protease inhibitors. The CaSR, Klotho, and ADAM10 were imaged in mouse kidneys and cell expression systems using confocal microscopy.
Results
The CaSR, Klotho, and ADAM10 colocalize on the basolateral membrane of the DCT. Calcimimetics and HCO3 increase serum sKlotho levels in WT but not in CaSR−/− mice, and acidic pH suppresses sKlotho levels in WT mice. In minced kidneys and cultured cells, CaSR activation with high Ca, calcimimetics, or alkali increase shed Klotho levels via ADAM10, as demonstrated using the ADAM10 inhibitor GI254023X and siRNA. In cultured cells, the CaSR, Klotho, and ADAM10 form cell surface aggregates that disperse after CaSR activation.
Conclusions
We identify a novel physiologic mechanism for regulation of sKlotho levels by the renal CaSR-ADAM10-Klotho pathway. We show that CaSR activators, including alkali, increase renal CaSR-stimulated Klotho shedding and predict that this mechanism is relevant to the effects of acidosis and alkali therapy on CKD progression.
The beneficial effects of αKlotho—i.e., suppression of the premature aging phenotype of Klotho−/− mice, including cardiac hypertrophy, accelerated vascular disease, ectopic calcification, endothelial dysfunction, osteodystrophy, sarcopenia, skin atrophy, and hyperphosphatemia—are attributable to renal production and shedding of its soluble extracellular domain, soluble Klotho (sKlotho) into the circulation.1–6 The importance of the kidney as a source of serum Klotho is demonstrated by the renal artery–to-vein step-up of Klotho levels, bilateral nephrectomy lowering circulating Klotho, and mice with kidney-specific Klotho deletion having patterns of disease that are phenotypically indistinguishable from mice with global Klotho deletion.1,2,4
Klotho is expressed at modest levels in the renal proximal tubule (PT), where membrane-bound Klotho/FGF1 coreceptor complexes bind FGF23 to increase phosphate excretion and reduce vitamin D synthesis, and at considerably higher levels in the distal convoluted tubule (DCT) and connecting tubule (CNT), where the calcium (Ca)-sensing receptor (CaSR) is also expressed at high levels.7,8 In cultured cells, proteases such as A Disintegrin and Metalloproteinase 10 (ADAM10) and ADAM17 cleave the extracellular domain of Klotho. In one report, cleavage was stimulated by insulin.9–13 The DCT and CNT also express ADAM10 and 17. However, the region(s) of the nephron responsible for production of sKlotho are not established, and whether Klotho cleavage is regulated in the kidney via insulin-dependent or other mechanisms and the relative importance of ADAM10 and ADAM17 are unknown.8,14–16
Because Klotho, the CaSR, and ADAM proteases are all expressed in the DCT and CNT, we investigated the possibility that the CaSR could regulate renal Klotho shedding and sKlotho levels. The CaSR is activated by Ca (1–5 mM), other divalent cations, allosteric agents, and pH.17–19 The pH effect on the CaSR has not been studied extensively but has the potential to be important physiologically, as demonstrated by the ability of alkaline pH to suppress parathyroid hormone (PTH) secretion in parathyroid glands.19 We propose that, in the distal nephron, the CaSR, ADAM proteases, and Klotho interact in response to CaSR ligands and systemic pH to provide regulated cleavage and shedding of membrane-targeted Klotho to regulate sKlotho levels.
Methods
Development of Tubule-Specific CaSR−/− Mice
CaSR floxed mice (CaSRflox, Casrtm1Mrpk/J, 129X1/SvJ, stock number 030647) were obtained from The Jackson Laboratories (Bar Harbor, ME).20 These mice were crossed with Six-2Cre mice (University of Texas Southwestern O’Brien Core) and bred to homozygosity to produce tubule-specific CaSR−/− (TS-CaSR−/−) mice. The mice were initially genotyped using tail biopsy specimens and PCR with primers that span exon 3. Once the TS-CaSR−/− line was established, genotyping was performed using real-time PCR with specific probes designed for the CaSR by Transnetyx (Cordova, TN). Selective deletion of the CaSR from the tubules was confirmed by confocal microscopy (Supplemental Figure 1). Reduction in mRNA and protein levels of CaSR, Klotho, and ADAM10 from total kidney extracts were confirmed by quantitative PCR and immunoblots (Supplemental Figure 1).
In Vivo Mouse Studies
On the day of an experiment, mice (wild-type [WT] littermate, C57BL/6, or TS-CaSR−/−, 3–6 months old) were given approximately 400 μl of 0.5 N saline intraperitoneally (IP) 1 hour before administration of drugs or vehicle. Approximately 50 μl of blood was obtained from the submandibular plexus immediately before treatment (T=0). R-568 (0.4 mg/kg), or vehicle (equivalent volume of DMSO as R-568) was administered in 20 μl/g body wt of 0.5 N saline. The biologic activity of R-568 was confirmed by measuring blood PTH levels before and after R-568 administration (Figure 1C) by ELISA (MicroVue Mouse PTH ELISA, catalog number 60-2305). For bicarbonate (HCO3) studies, 20 mEq/kg of sodium bicarbonate (NaHCO3) or 20 μl/g of 1 M sodium chloride was administered IP at T=0. For the ammonium chloride (NH4Cl) studies, 5 mEq/kg of NH4Cl was administered IP at T=0.21 Changes in mouse blood pH were measured before and after treatments using a CCX machine capillary microassay for whole-blood pH and total carbon dioxide (TCO2) values, taken within 1 minute of sampling from the mouse submandibular plexus (Figure 1, D and E). A small volume was reserved for serum Klotho ELISA. After 2 hours for the R-568 studies and 1 hour for the HCO3 and NH4Cl studies, blood was obtained for measurement of sKlotho levels and then animals were euthanized.
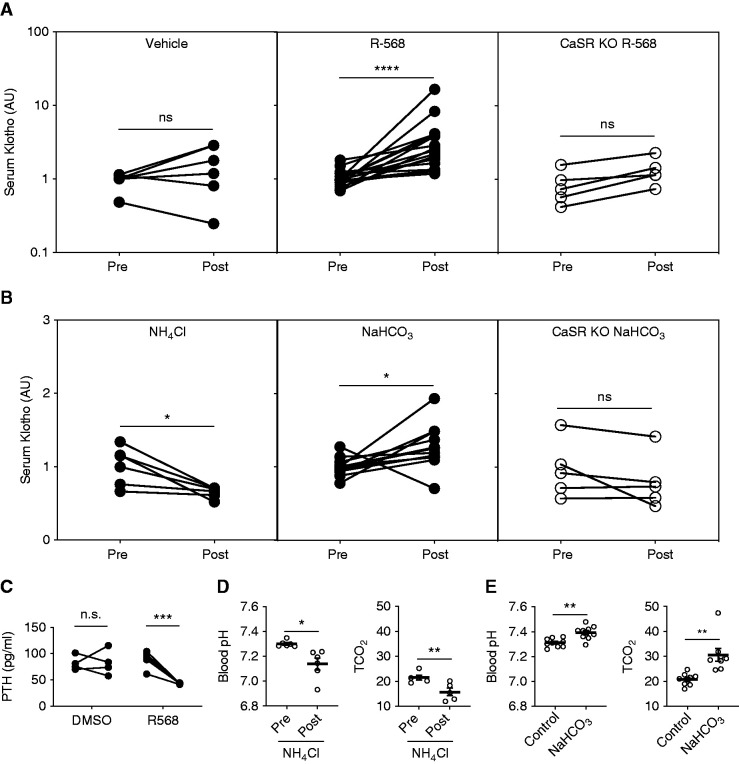
Figure 1.
CaSR activation by a calcimimetic agent or alkaline pH increases soluble αKlotho levels in WT but not TS-CaSR−/− mice. (A) Comparison of serum Klotho before (pre) and 2 hours after (post) treatment with calcimimetic R-568 (0.4 µg/g body wt, IP) or vehicle (equivalent volume of DMSO in saline, IP). R-568 significantly increased serum sKlotho levels (n=12), but fails to do so in response to vehicle (n=6). TS-CaSR−/− (CaSR KO) mice did not significantly respond to R-568 treatment (n=5). The average fold change over pretreatment values are as follows: WT vehicle, 1.6±0.4; WT R-568, 3.6±0.9; CaSR KO R-568, 1.3±0.3. (B) Comparison of serum Klotho before (pre) and 1 hour after (post) administration of 5 μEq/g NH4Cl or 20 μEq/g NaHCO3 (all in water, IP). NH4Cl treatment significantly reduced sKlotho levels (n=6), and HCO3 treatment significantly increased sKotho levels (n=12) in WT mice. The TS-CaSR−/− mice did not respond to HCO3 with an increase in sKlotho (n=5). The average fold change over pretreatment values are as follows: WT NH4Cl, 0.65±0.03; WT NaHCO3, 1.27±0.08; CaSR KO NaHCO3, 0.80±0.16. All serum Klotho values were normalized to the average WT “pre” values on each experimental day. Significance was determined by paired nonparametric, two-tailed t tests, using GraphPad. (C) Plasma PTH is reduced in response to treatment R-568 (n=5), but not DMSO vehicle (n=4). The average PTH levels 30 minutes after treatment were as follows: DMSO, 88.9±7.4 pg/ml; R-568, 43.0±0.55 pg/ml. (D) Blood pH and TCO2 response to NH4Cl (5 mEq/kg body wt, IP). NH4Cl decreased blood pH from 7.30±0.01 to 7.12±0.05, and decreased calculated TCO2 from 21.5±1.0 to 15.7±1.5 mM. (E) Blood pH and TCO2 response to NaHCO3 (20 mEq/kg body wt, IP). NaHCO3 increased blood pH compared with sodium chloride, from 7.32±0.01 to 7.41±0.02, and increased blood TCO2 from 20.8±0.9 to 30.6±2.5 mM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, by paired t test in (A), and by t test in (B–E).
Animal research was performed in accordance with the UT Southwestern Medical Center Animal Institutional Animal Care and Use Committee (IACUC) guidelines. The research study was approved by the Veterans Affairs (VA) North Texas Health Care System Advisory Committee on Research Programs and UT Southwestern Medical Center Animal IACUC (National Institutes of Health Office of Laboratory Animal Welfare assurance number A3472-01). UT Southwestern Medical Center is fully accredited by the AAALAC. Animals were housed and maintained in accordance with the applicable portions of the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals. Veterinary care is under the direction of a full-time veterinarian boarded by the American College of Laboratory Animal Medicine. Mice were euthanized for experiments by first anesthetizing with tribromoethanol and then euthanizing by cervical dislocation.
Tissue Culture and Immunoprecipitation Studies
HEK-293 (ATCC) cells were cultured in DMEM (Sigma) supplemented with 10% FBS (Sigma). For all studies, cells were transfected with Lipofectamine 2000 (Invitrogen), unless stated otherwise, and used for experiments after 48 hours. The expression plasmids for the full-length Klotho were obtained from Dr. Makoto Kuro-o, ADAM10 and ADAM17 from ADDGENE, and human CaSRWT and CaSRR795W were described previously.22,23 Cells were lysed with radioimmunoprecipitation (RIPA) buffer supplemented with Halt Protease Inhibitor Cocktail (Thermo Fisher).
For immunoprecipitation studies, HEK-293 cells stably expressing the hemagglutinin (HA)-tagged CaSRWT or CaSRR795W were transfected with empty vector or full-length Klotho.24 HA-agarose (Thermo Fisher) was used to pull down CaSR and associated proteins in complex from RIPA cell lysate supernatant fractions, following manufacturer’s instructions. Immunoprecipitated proteins were denatured in LDS Sample Buffer with Reducing Solution, warmed to 37°C for 10 minutes, and separated in 8% Bolt mini-gels in an MES buffer system (Thermo Fisher). Separated proteins were wet transferred to nitrocellulose membranes (Whatman) with a pore size of 0.2 μm using a plate electrode Bolt mini-transfer system (Thermo Fisher), and then membranes were immunoblotted with specific antibodies using standard procedures at 4°C.25 The antibodies used were anti-Klotho, generated by Makoto Kuro-o (KM2076, Cosmo Bio); anti-ADAM10 (EMD Millipore); and anti-CaSR, generated by our laboratory (Cell Signaling) as described previously.22,23
For small interfering RNA (siRNA) studies, plasmids and siRNA (20 nM) were cotransfected with DharmaFECT Duo (Dharmacon), following manufacturer’s instructions. Cells were harvested 48 hours after transfection of siRNA (ADAM10 siRNA smart pool, M-004503-02-0005; ADAM17 siRNA smart pool, M-003453-01-0005 [Dharmacon]; or negative control siRNA [ABI]). RNA was extracted using the Direct-Zol RNA Miniprep kit (Zymo Research Corp), and cDNA was reverse transcribed using SuperScript III First-Strand Synthesis Kit (Invitrogen). Gene knockout efficiency was analyzed with the Taqman Gene Expression Assay using the ABI StepOnePlus Real-Time PCR System. The assay identifiers were Hs00153853_m1 (ADAM10), Hs0104191_m1 (ADAM17), and Hs02786624_g1 (glyceraldehyde-3-phosphate dehydrogenase).
Preparation of Minced Kidney Cortex
Each mouse kidney was decapsulated, weighed, and the medulla was removed, minced to a pastelike consistency, washed three times in 1 ml of PBS or HEPES-based buffer (for pH-based studies) in a microcentrifuge tube (300 × g centrifugation speed), aliquoted to six microcentrifuge tubes (approximately 20 mg of wet tissue), and weighed. Buffers and incubation conditions were adapted from a publication by Campion et. al.19 For the measurement of pH-dependent Klotho cleavage, the kidney mince was dispersed in a 20× volume of HEPES-based buffers varying in pH from 7.0 to 7.8, an aliquot of T=0 buffer was collected immediately, and then collected for analysis after 1 hour of rocking at room temperature (RT). For measurements of drug effects on Klotho shedding by minced kidney, washed and weighed wet tissue was transferred to the bottom of a 24-well untreated tissue culture dish, and dispersed in serum-free RPMI containing 0.01% fatty acid–free BSA with the indicated drugs. Tissue was incubated for 1 hour in a humidified incubator at 37°C, 5% CO2. To measure shed Klotho, medium was collected, cleared by centrifugation, and an aliquot from the top was boiled in Laemmli buffer for SDS-PAGE and immunoblotting. BSA in the medium transferred onto nitrocellulose membrane was used as the loading control for immunoblot quantification.
Measurement of Shed Klotho from HEK-293 Cells
For measurement of shed Klotho, 48 hours post-transfection, cells in six-well dishes were washed twice and cultured for 3 hours (for Ca-induced shedding) or 1 hour (for drug treatments) in fresh serum-free DMEM/F12 (1.0 mM Ca) in a humidified cell culture incubator at 37°C, 5% CO2. The time course for shed Klotho response was determined using high Ca, and this was shown to be linear between 1 and 3 hours (Supplemental Figure 2). The medium was collected and precipitated with 10% TCA and processed for immunoblotting, modified from a protocol previously described.13 BSA (10 μg/ml) was added as carrier and coprecipitated with the medium proteins. BSA in resolubilized proteins transferred onto nitrocellulose membrane was stained with Ponceau and used as a loading control for quantification of shed Klotho immunoblotting.
Measurement of Serum Klotho Levels In Vivo
Blood was collected from the submandibular plexus for Klotho measurement before and 1 or 2 hours after treatment with R-568, DMSO vehicle, NaHCO3, or NH4Cl. The optimal collection time was determined by a time-course experiment (Supplemental Figure 3). The amount of Klotho in the serum was measured by immunoprecipitation/immunoblot procedures as previously described,1,26 or by ELISA (product number SEH757Mu; Cloud-Clone Corp.), following manufacturer’s protocols for 20–50× diluted mouse serum. Fold differences in serum Klotho levels between samples were compared using both immunoprecipitation/immunoblot procedures and ELISA to validate use of both methods (Supplemental Figure 4).
Glycerol Density Gradient Centrifugation
Mouse kidneys were homogenized in a 10× volume of tissue homogenization buffer (50 mM MOPS, pH 7.4, 2 mM EDTA, 2 mM EGTA, 150 mM sodium chloride, 1% nonidet P-40, 1× Halt Protease Inhibitor Cocktail, 10 μM E64), with a ground glass homogenizer. Homogenates were centrifuged (20,000 × g, 1 minute at 4°C), and the supernatant fraction was cleared by ultracentrifugation (100,000 × g, 30 minutes at 4°C). Clarified extracts (750 μg total protein determined by Bradford assay) were overlayed on 10%–40% linear glycerol gradients (2 ml total volume) containing tissue homogenization buffer. Samples were centrifuged at 100,000 × g for 3 hours at 4°C in a TLS55 rotor, and 100 μl fractions were collected for immunoblot analysis.
Immunofluorescence and Confocal Microscopy
Cultured HEK-293 cells were transfected with CaSR and Klotho for 24 hours, and then reseeded onto glass coverslips and incubated for 24 hours. For isolated tubules, mouse kidney cortex was cut and minced into a pastelike consistency, and then incubated in a digestion solution containing 5 ml DMEM with 250 units/ml of collagenase type A (LS004154; Worthington) at 37°C for 30 minutes. The digested tissue was centrifuged at 500 × g, washed in 5 ml of DMEM without collagenase, and then filtered through 100-µm and 70-µm cell strainers (FALCON) to remove glomeruli and large underdigested tissue fragments. The digested, dispersed tubules were pipetted onto poly-l-lysine–coated microscope slides for 1 hour for attachment. For staining, tubules and cells that had adhered to slides or glass coverslips were fixed for 10 minutes at RT with 4% paraformaldehyde in PBS, and then blocked with 3% BSA in PBS for 30 minutes. The staining for colocalization of CaSR, ADAM10, and Klotho on cell membranes was accomplished by incubating with primary antibodies without permeabilization overnight at 4°C, and then washing three times in PBS before incubation with secondary antibodies for 1 hour at RT. At the end of the incubations, samples were mounted on glass slides using mounting medium containing 4′,6-diamidino-2-phenylindole.
For tissue staining, frozen OCT–embedded kidney tissues were cut into 5-μm sections and fixed by immersing the slides in acetone/methanol (1:1) for 5 minutes (acetone/methanol prechilled to −20°C). Samples were then washed three times in PBS, dried at RT, and the tissue sections circled with an ImmEdge pen. Blocking solution (100 μl per tissue section) was applied and samples were then incubated, first at RT for 30 minutes, then with specific primary antibodies, and finally with the fluorescence-labeled secondary antibodies, using standard procedures.
Confocal imaging was performed in the Cell Biology and Imaging Core in the O’Brien Kidney Research Core using a Zeiss LSM880 Airyscan laser scanning microscope equipped with plan-apochromat 10×/0.3 NA, 20×/0.8 NA, 25×/0.8 NA, and 63×/1.40 NA oil-immersion objectives (Zeiss, Oberkochen, Germany). Fluorescence images were acquired using ZEN black 2.3 software with a 20×/0.8 NA or 63×/1.40 NA objective (Zeiss Immersion Oil 518F was used for the 63×/1.40 NA objective), and image processing was performed at constant RT. Acquired images were analyzed and regions of interest were further processed using ZEN 2.6 (blue edition) software.
Laser Capture Microscopy
Kidneys were cryosectioned at 30 μm and thaw mounted onto silane-coated polyethylene-naphthalate membrane glass slides (Molecular Devices, Sunnyvale, CA) and stored at −80°C.27 Slides were lightly fixed in 75% ethanol immediately before thionin staining, and then dehydrated in a graded ethanol series followed by 5 minutes in xylene. The Arcturus Veritas Microdissection System (Molecular Devices) was used to isolate nephron regions on the basis of structural characteristics (proximal versus other tubules) and staining for the sodium-chloride cotransporter, NCC (DCT). The anatomic specificity of the laser-capture microdissections (LCMs) was confirmed by quantitative PCR for the expression of known marker genes (sodium-hydrogen exchanger-3 [NHE3] for PT, and sodium-Ca exchanger-1 [NCX1] for DCT). Nephron-region RNAs were isolated using the Ambion Ribopure Kit, and LCM nuclei RNAs were extracted using the PicoPure RNA Isolation Kit (Molecular Devices) with on-column DNase I treatment (Qiagen, Valencia, CA) and stored at −80°C.
Real-Time PCR
The High-Capacity cDNA Kit (Applied Biosystems) and SYBR Green PCR Master Mix (Thermo Fisher) were used to prepare cDNA for measurements of glyceraldehyde-3-phosphate dehydrogenase–corrected gene expression levels (ΔCt) using the ABI StepOnePlus Real Time PCR machine.
We used the following primer sequences.
Klotho: forward, AATTATGTGAATGAGGCTCTGAAAG; reverse, TACGCAAAGTAGCCACAAAGG
Podocin: forward, GGCACAAAGACAGGCCAAA; reverse, GGACTCTGAAGCAGCCTTTTC
NCX1 (Slc8a1): forward, GGAGAGCTCGAATTCCAGAAC; reverse, TCCTCGTCATCGATTACCTTGA
NHE3 (Slc9a3): forward, AATCTCGAGATCGGATCCTG; reverse, CTCTGTTCCAAGGACTGC
CaSR: forward, CGCAGTGGTGGGAGCAACCG; reverse, GGAGGCGTAGCTCACCTGGGG.
Statistical Analyses
For all statistical analyses, GraphPad Prism was used. Comparisons of two groups were by nonparametric, two-tailed t tests; comparisons of more than two groups were by ANOVA, with Dunnett multiple comparisons post-test against control or Tukey multiple comparisons against all groups. P value indications are noted throughout in figure legends. When multiple comparisons were made against all groups, selected comparisons are shown with lines within the graphical representation of mean±SEM. Each scatter dot in all graphs represents a distinct mouse.
Results
CaSR Activation In Vivo Increases Serum Klotho Levels
Mice were treated IP with the calcimimetic R-568 (0.4 mg/kg), NH4Cl (5 mEq/kg), or NaHCO3 (20 mEq/kg). Serum Klotho was measured 2 hours after treatment with R-568 (Figure 1A) or 1 hour after treatment with NH4Cl or NaHCO3 (Figure 1B). R-568 treatment increased serum sKlotho levels to 351% of average paired pretreatment values. This effect of R-568 was eliminated in TS-CaSR−/− mice (Figure 1A). NH4Cl decreased basal serum sKlotho levels to 65% of average pretreatment values (Figure 1B). NaHCO3 increased average serum sKlotho to 130% of paired pretreatment levels (Figure 1B). This response was absent in the TS-CaSR−/− mice. Confocal microscopy and immunoblotting of kidney extracts demonstrated reduction of tubular CaSR in the TS-CaSR−/− mice (Supplemental Figure 1). We confirmed the biologic activity of R-568 in vivo by measuring serum PTH levels (Figure 1C), where R-568 reduced serum PTH from 89 to 43 pg/ml at 30 minutes. NH4Cl (Figure 1D) caused a decrease in blood pH from 7.30 to 7.12, and TCO2 from 21.5 to 15.7 mM. NaHCO3 (Figure 1E) caused an increase in blood pH from 7.32 to 7.41, and a rise in blood TCO2 from 20.8 to 30.6 mM. These results demonstrate that, in vivo, serum sKlotho levels are, in part, regulated by the CaSR and blood pH. Moreover, the lack of serum Klotho response to a specific allosteric CaSR activator or alkaline pH in the TS-CaSR−/− mice supports the hypothesis that the CaSR contributes to regulation of circulating sKlotho levels in vivo.
CaSR Activation by Ca, R-568, or Alkalinity in Minced Renal Cortex or Cultured Cells Increases Klotho Shedding
The in vivo studies showed that activation of the CaSR with R-568 or HCO3 increased circulating sKlotho levels. To refine the mechanism of Klotho shedding, we used minced mouse kidney cortex (Figure 2, A and C) and HEK-293 cells that transiently expressed the CaSR and Klotho (Figure 2, B and D).
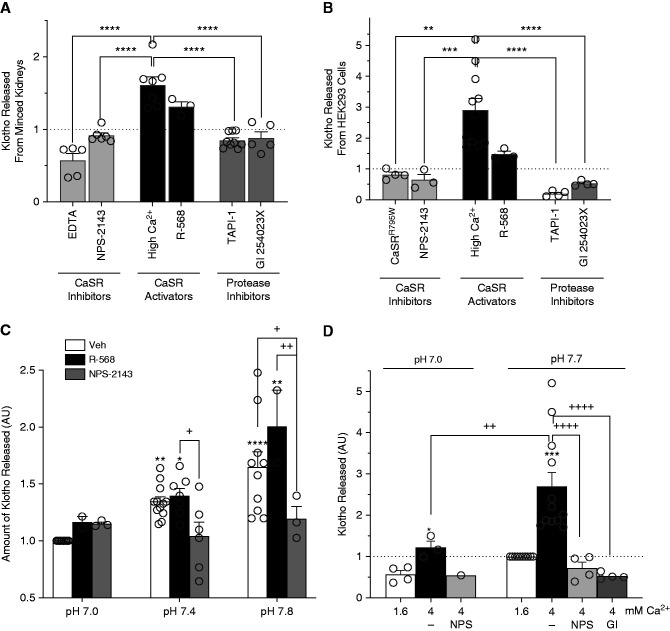
Figure 2.
Klotho shedding responds to CaSR activation in minced kidney cortex and HEK-293 cells transiently transfected with CaSR and Klotho. (A) Klotho shedding by minced kidneys, in response to added CaSR inhibitors (10 mM EDTA or 50 μM calcilytic NPS-2143), CaSR activators (4.0 mM Ca [high Ca2+] or 10 μM calcimimetic R-568), or protease inhibitors (100 μM TAPI-1 or 2 μM GI254023X in the presence of high Ca2+). Shed Klotho responses are expressed as mean±SEM fold increases over average control values (vehicle, dashed line): EDTA, 0.57±0.09; NPS-2143, 0.91±0.04; high Ca2+, 1.61±0.11; R-568, 1.31±0.07; TAPI-1, 0.85±0.03; GI254023X, 0.88±0.08 (n=3–9). R-568 was significantly increased compared with control, NPS-2143, GI254023X, and TAPI-1. (B) Klotho shedding by HEK-293 cells expressing the CaSR and Klotho, in response to CaSR inhibitors (CaSRR795W nonfunctional mutant transfected, or 25 μM calcilytic NPS-2143), CaSR activators (4 mM Ca [high Ca2+] or 1 μM calcimimetic R-568), or protease inhibitors (100 μM TAPI-1 or 2 μM GI254023X in the presence of high Ca2+). Shed Klotho responses are expressed as mean±SEM fold increases over average control values: control, 1.0; CaSRR795W, 0.82±0.08; NPS-2143, 0.65±0.18; high Ca2+, 2.91±0.37; R-568, 1.48±0.10; TAPI-1, 0.20±0.05; GI254023X, 0.53±0.04. R-568 was significantly increased compared with control, CaSRR795W, NPS-2143, GI254023X, and TAPI-1. **P<0.01, ***P<0.001, ****P<0.0001 by ANOVA and multiple comparisons post-test. (C) Klotho shedding by minced kidney in response to HEPES-buffered solution supplemented with 250 μm R-568 or 250 μm NPS-2143 at indicated pH values. Shed Klotho responses are expressed as mean±SEM fold increases over vehicle (Veh) at pH 7.0: for pH 7.0: Veh, 1.0; R-568, 1.16±0.05; NPS-2143, 1.15±0.02; for pH 7.4: Veh, 1.34±0.04; R-568, 1.40±0.07; NPS-2143, 1.04±0.12; for pH 7.8: Veh, 1.65±0.14; R-568, 2.01±0.32; NPS-2143, 1.20±011. (D) Klotho shedding by HEK-293 cells expressing the CaSR and Klotho in response to pH of DMEM (HCO3 buffer) pre-equilibrated in humidified incubators with CO2 content of 20% (pH 7.0) and 5% (pH 7.7). Values are expressed as mean±SEM fold change from standard DMEM which contains 1.6 mM Ca, pH 7.7. Values expressed as mean±SEM are: for pH 7.0: Ca 1.6 mM, 0.57±0.09; Ca 4 mM, 1.22±0.15; Ca 4 mM and NPS-2143, 0.54; for pH 7.7: Ca 1.6 mM, 1; Ca 4 mM, 2.7±0.34; Ca 4 mM and NPS-2143, 0.72±0.15; Ca 4 mM and GI245023X, 0.53±0.04. *P<0.5 compared with pH 7 vehicle treated, ***P<0.001 compared with pH 7.7 vehicle treated, ++P<0.01, ++++P<0.0001 compared with 4 mM high Ca at pH 7.7, as determined ANOVA, with multiple comparisons post-test.
In both minced kidney cortex (Figure 2A) and HEK-293 cells that express the CaSR and Klotho (Figure 2B), high Ca (4.0 mM) and R-568 increased Klotho shedding. In minced kidney cortex, NPS-2143 (calcilytic), which blocks CaSR activation, and EDTA, which chelates Ca, inhibited Klotho shedding in response to 4 mM Cao. HEK-293 cells expressing the nonfunctional CaSR mutant, CaSRR795W, or treated with NPS-2143 failed to increase Klotho shedding in response to 4.0 mM Ca (Figure 2B). In both minced kidney and HEK-293 cells, Ca-stimulated Klotho shedding was inhibited to comparable degrees by two protease inhibitors: a general metalloproteinase inhibitor (TAPI-1, inhibits ADAM10 and ADAM17), and a specific ADAM10 inhibitor (GI254023X). The complete inhibition of Klotho shedding by GI254023X suggests that ADAM10 (and not other metalloproteinases) is primarily responsible for CaSR-stimulated Klotho shedding.
Alkalinity has similar effects on Klotho shedding in these preparations. Raising pH with HEPES-buffered tissue culture medium from 7.0 to 7.8 in minced mouse kidney cortex caused a progressive increase in Klotho shedding (Cao 1.6 mM) and increased responses to R-568. Treatment with NPS-2143 blocked the pH-dependent increases in Klotho shedding (Figure 2C). In HEK-293 cells, pHo values were adjusted to 7 or 7.7 by changing HCO3 and CO2 levels (Figure 2D). The basal and 4.0 mM Ca-stimulated levels of Klotho cleavage were increased at pH 7.7. At pH 7.0, the 4.0 mM Ca-stimulated increase in Klotho shedding was blocked by CaSR inhibition with NPS-2143, which reduces the stimulated level of Klotho shedding to the basal level (Ca 1.6 mM). At pHo 7.7, both NPS-2143 and GI254023X inhibited Klotho shedding below the basal level (1.6 mM Ca). These results demonstrate that, in renal tissue and cultured cells, CaSR activation by CaSR agonists, allosteric CaSR activators, or alkaline pH leads to increased CaSR-dependent Klotho shedding via a metalloproteinase, probably ADAM10.
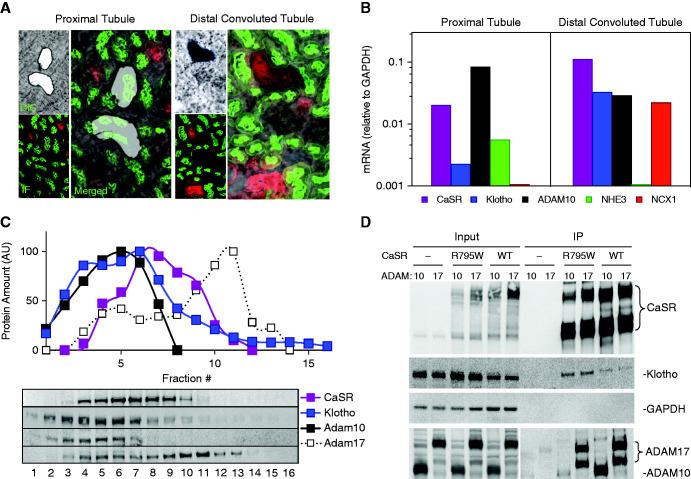
The CaSR, ADAM10, and Klotho Colocalize in the Mouse Kidney
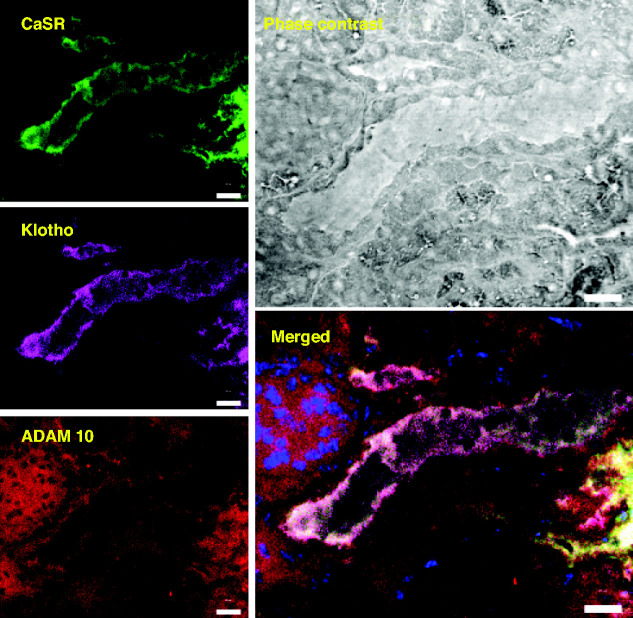
For the CaSR to regulate Klotho release through the ADAM10 protease, these three proteins should be in the same locale in the kidney. Figure 3 shows colocalization of the CaSR, Klotho, and ADAM10 in the DCT of the same mouse kidney section using confocal immunofluorescence. Colocalization of the CaSR on the basolateral and sodium-chloride cotransporter on the apical surfaces of the same tubule demonstrates that the segment is DCT (Supplemental Figures 1 and 5). In these images, the CaSR and Klotho have a basolateral distribution and are seen together only in the DCT segment, whereas ADAM10 has a diffuse membrane and cytoplasmic distribution and is seen in all cell types. Overlap of all three proteins is shown in the merged image. These results indicate that the CaSR, Klotho, and ADAM10 are all present in the DCT with overlap of all three proteins on the basolateral membrane.
Figure 3.
Colocalization of the CaSR, Klotho, and ADAM10 in the kidney. Confocal microscopy of fluorescent triple-labeled mouse kidney cryosections shows selective colocalization of CaSR (green), with ADAM10 (red) and Klotho (magenta). CaSR staining corresponds to DCT-specific sodium-chloride cotransporter staining (Supplemental Figures 1 and 5), consistent with published observations and measured mRNA levels. Scale bar, 20 μm.
To extend colocalization studies of these three proteins, we used laser capture microscopy and microdissection to localize the CaSR, Klotho, and ADAM10 mRNA in mouse PT and DCT (Figure 4, A and B). Transcript levels were measured relative to proteins that are specific to these regions: NHE3 for the PT and the NCX1 for the DCT (Figure 4B). CaSR, Klotho, and ADAM10 transcripts are coexpressed in the PT and DCT. Work by others8,15 and Figure 3 and Supplemental Figure 1 demonstrate that Klotho protein is expressed at relatively low levels in the PT, but at much higher levels in the DCT, so the DCT is the most likely source of sKlotho. Moreover, data from the Humphreys laboratory at Washington University using single-cell RNA sequencing shows that the CaSR, Klotho, and ADAM10 and ADAM17 are all expressed in the DCT and CNT (http://humphreyslab.com/SingleCell/).
Figure 4.
Colocalization of the CaSR, Klotho, ADAM10, and ADAM17 in the kidney. (A) Laser capture microscopy of mouse kidney sections. Enlarged merged image of serial sections visualized by differential interference contrast (DIC) and immunofluorescence (IF) for DCT (specific sodium-chloride cotransporter; NCC in red) and PTs (lotus tetragonolobus agglutinin, LTA in green). (B) Quantitative PCR measurements of mRNA expression for DCT-specific NCX1 and PT-specific NHE3 with the CaSR, Klotho, and ADAM10. (C) Density gradient fractionation of mouse kidney extracts. Representation of the amount of protein as a function of gradient fraction number is shown. ADAM10 cofractionates with the CaSR and Klotho to a greater extent than ADAM17. (D) Coimmunoprecipitation of the CaSR, Klotho, and ADAM10 or ADAM17 from HEK-293 cells. HEK-293 cells or HEK-293 cells that stably express an HA-tagged CaSRWT or nonfunctional mutant CaSRR795W were transiently transfected with full-length Klotho and ADAM10 or ADAM17 (as indicated), extracted in RIPA buffer, and the lysates were incubated with anti-HA agarose. “Input” refers to cell lysates before immunoprecipitation; IP refers to the immunoprecipitated samples corresponding to the lysates in “Input”. The Input and IP samples were separated by SDS-PAGE, transferred onto a nitrocellulose membrane, and proteins were identified with specific antibodies as indicated on the right, except that ADAM10 and ADAM17 were identified with anti-HA antibodies. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
To determine if the CaSR, Klotho, and ADAM10 proteins are in the same cellular compartment in mouse kidneys, we separated total mouse kidney extracts by glycerol density gradient centrifugation. Immunoblots show cofractionation of the CaSR, Klotho, and ADAM10, whereas ADAM17 appears in different fractions, with minor partial overlap with the CaSR in lower fractions (Figure 4C). To address whether the CaSR, Klotho, and ADAM10 or ADAM17 might interact, we performed coimmunoprecipitation experiments using HEK-293 cells stably expressing HA-tagged CaSRWT or CaSRR795W (nonfunctional mutant). These stable cell lines and control HEK-293 cells were transiently transfected with Klotho and Myc-tagged ADAM10 or ADAM17, and the protein aggregates were isolated using anti–HA-agarose (Figure 4D). Both HA-tagged CaSRWT and CaSRR795W coimmunoprecipitate Klotho, ADAM10, and ADAM17. The R795W mutation is localized to the third intracellular loop, a G-protein contact site for the CaSR where changes in affinity to other proteins are not expected. Together with the Klotho shedding experiments (Figure 2), these results support a transient, functional interaction among the CaSR, Klotho, and ADAM10.
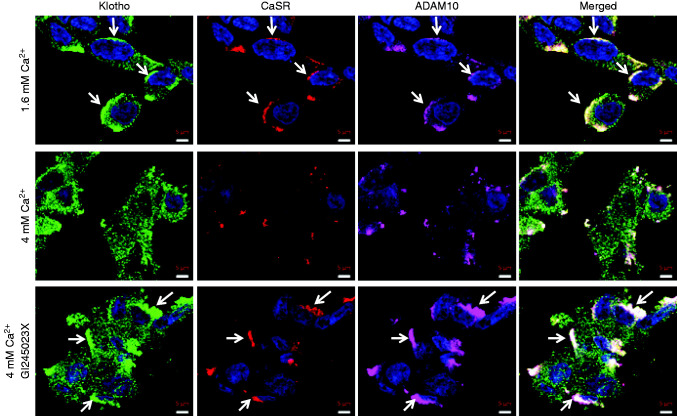
Klotho, CaSR, and ADAM10 Colocalize on the Surface of Cultured Cells
We used confocal microscopy of unpermeabilized HEK-293 cells that transiently coexpressed the CaSR and Klotho to determine if they colocalize on the cell surface with endogenous ADAM10 (Figure 5). In standard medium with 1.6 mM Cao, Klotho (green), the CaSR (red), and ADAM10 (magenta) appear together in aggregates on the cell surfaces (top row), suggesting the CaSR may organize membrane Klotho expression. Klotho is also seen in a punctate distribution independent of the CaSR or ADAM10. In response to 4 mM Cao (middle row), the cells appear more spread, consistent with CaSR activation. The size and number of the aggregates is greatly reduced, but the punctate distribution of isolated Klotho remains. In the presence of 4.0 mM Cao and GI254023X (ADAM10 inhibitor), a condition where Klotho is not cleaved (Figure 2, A, B, and D), the aggregates appear larger than in the unstimulated condition (top row). These results indicate that, under unstimulated conditions, Klotho, the CaSR, and ADAM10 exist in the same region of cell membranes, possibly in complexes, and that the complexes dissociate with CaSR activation and Klotho shedding. Additionally, CaSR activation may be a signal for protein complex formation. Klotho cleavage by ADAM10 appears to provide a signal that leads to loss of the CaSR and ADAM10 from the cell surface. The Klotho in a punctate pattern that is not associated with the other two proteins remains.
Figure 5.
Cell surface expression of Klotho, the CaSR, and ADAM10. Confocal images of HEK-293 cells expressing Klotho, the CaSR, and ADAM10. Cells were fixed using nonpermeabilizing conditions and triple stained with primary antibodies specific for Klotho (green), the CaSR (red), and ADAM10 (magenta). The top row shows cells in basal conditions (1.6 mM Cao), where the three proteins appear together in aggregates (white arrows). The middle row shows cells after 3 hours in 4 mM Cao, where the aggregates are reduced in number, size, and intensity, consistent with the experiments in which significant amounts of Klotho are cleaved from the cell membrane. In the bottom row, the cells are treated with 4 mM Cao and 1 μM GI245023X (ADAM10 inhibitor) for 3 hours, conditions in which ADAM10 will be inactive and the CaSR will be active. Inhibition of ADAM10 activity prevents Klotho cleavage and causes persistence and possibly increased size of the aggregates (white arrows), implicating trafficking or protein complex formation in the CaSR–ADAM10 activation–Klotho cleavage process. Scale bar, 5 μm.
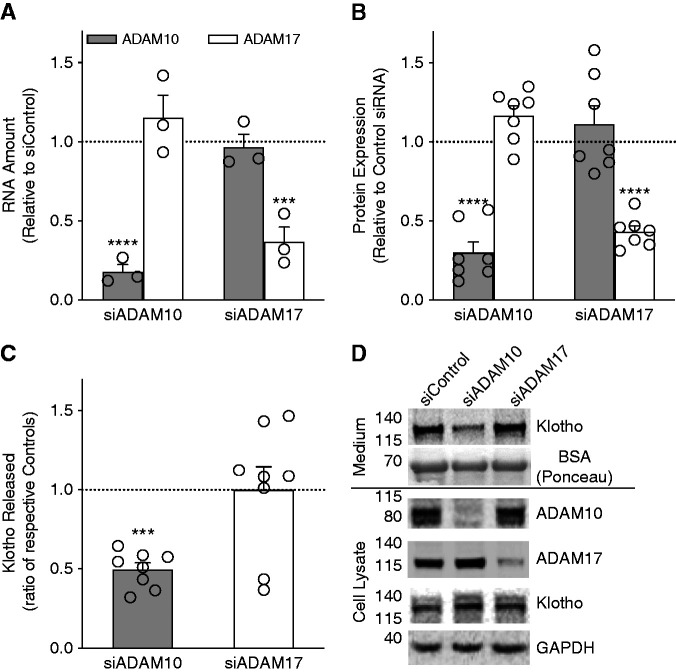
ADAM10 Cleaves Klotho in HEK-293 Cells
Publications by others13,16 showed that, in cultured cells, ADAM10 and ADAM17 can both cause Klotho shedding. Using glycerol gradient centrifugation of mouse kidney extracts, we found that ADAM10 was in the same fractions as the CaSR and Klotho (Figure 4C). To determine whether ADAM10 or ADAM17 cleaves Klotho selectively, we studied siRNA-directed depletion of endogenous ADAM10 or ADAM17 in HEK-293 cells that transiently expressed the CaSR and Klotho. ADAM10 and ADAM17 mRNA and protein were reduced to approximately 25% and 40% of control levels, respectively, in scrambled siRNA transfected cells (Figure 6, A and B). Compared with control, siADAM10 reduced Klotho shedding by 50%, but siADAM17 had no effect (Figure 6C). Representative Western blots show targeted protein reduction in cell lysates and demonstrate selectively reduced Klotho shedding in the medium of cells transfected with siADAM10 (Figure 6D). These results along with those in Figure 2 showing complete inhibition of Klotho shedding by GI254023X (ADAM10 inhibitor) indicate that, in HEK-293 cells and in renal tissue, ADAM10 and not ADAM17 is primarily responsible for CaSR-stimulated Klotho shedding.
Figure 6.
Shedding of Klotho from cultured cells through ADAM10 activation. (A) Expression of ADAM10 (hatched bars) or ADAM17 (open bars) mRNA after incubation of HEK-293 cells with indicated siRNA (siADAM10 or siADAM17) or scrambled control siRNA sequence (dotted line). siADAM10 reduced ADAM10 mRNA to 0.18±0.45 compared with control whereas ADAM17 mRNA was 1.15±0.14 compared with control. siADAM17 reduced ADAM17 mRNA to 0.37±0.0.09 compared with control, whereas ADAM10 mRNA was 0.97±0.0.08 compared with control. Values shown are mean±SEM, n=3 for all conditions. (B) Expression of ADAM10 or ADAM17 protein in HEK-293 cells transiently expressing siRNAs as above. siADAM10 reduced ADAM10 protein to 0.30±0.0.7 compared with control, whereas ADAM17 protein was 1.11±0.11 compared with control. siADAM17 reduced ADAM17 protein to 0.43±0.04 compared with control, whereas ADAM10 protein was 1.16±0.0.06 compared with control. Values shown are mean±SEM, n=7 for all conditions. (C) ADAM10 activity–dependent Klotho shedding from HEK-293 cells transiently transfected with full-length Klotho, CaSR, and ADAM10- or ADAM17-specific siRNAs. Amount of Klotho shed is quantified as the ratio of amount of Klotho released from negative control scrambled siRNA transfected cells, defined as 1.0 (dotted line), and ADAM10 siRNA (hatched bar) or ADAM17 siRNA (open bar). ADAM10 knockdown significantly reduced Klotho levels to 0.50±0.04, consistent with inhibition by TAPI-1 and GI254023X (Figure 2), whereas ADAM17 knockdown had no effect (1.00±0.14). Values are mean±SEM, n=8. Significance was determined by ANOVA with Dunnett multiple comparisons to control scrambled siRNA. ***P<0.001, ****P<0.0001. (D) Representative immunoblots of Klotho shed during 3 hours in serum-free medium, with Ponceau-stained carrier BSA protein shown as loading control (medium, above horizontal bar) and transfected Klotho, endogenous ADAM10, ADAM17, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in HEK-293 cell lysates (below bar, cell lysate).
Discussion
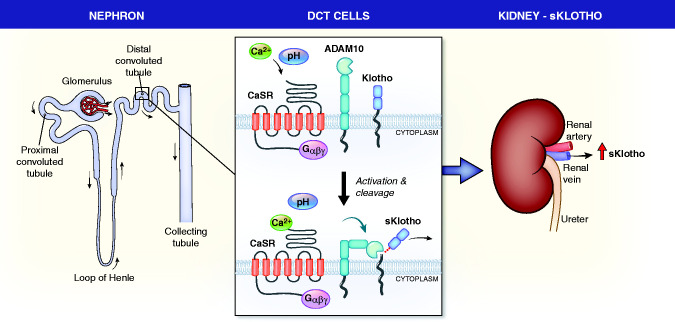
The salient findings from this study are as follows: (1) discovery of a mechanism of regulation of Klotho shedding; (2) description of a molecular model of this regulation via a tripartite complex of the CaSR, ADAM10, and Klotho; and (3) provision of a tangible mechanism for how alkali therapy in humans may retard the progression of CKD. The findings are summarized in Figure 7.
Figure 7.
Proposed model for regulation of sKlotho by the renal CaSR. The left panel shows a diagram of a nephron with the DCT boxed. The middle panel shows two images of the DCT basolateral membrane where the CaSR, ADAM10, and Klotho are all expressed. In the top image, the CaSR is inactive, as is ADAM10, and there is no Klotho cleavage. In the bottom panel, the CaSR is activated by either Ca or increased pH, it activates ADAM10, increasing its protease activity, and ADAM10 cleaves membrane-bound Klotho. In the right panel, sKlotho leaves the kidney by the renal vein.
Regulation of systemic sKlotho levels has not been studied in sufficient detail. We discovered a physiologic mechanism for regulation of serum sKlotho levels through the renal CaSR. CaSR activation by its ligands or alkaline pH stimulates shedding of membrane-bound Klotho from kidney tissue and cultured cells that express both proteins. The probable site for CaSR-stimulated Klotho shedding is DCT epithelial cells where Klotho and the CaSR are expressed at high levels with ADAM10. This finding is consistent with a recent report that the CaSR and Klotho associate in the parathyroid glands to regulate PTH secretion, synthesis, and parathyroid gland growth.28 In mammalian systems, the CaSR senses divalent cations where it contributes to control of PTH secretion and urinary Ca excretion. The parathyroid CaSR may also have a role in sensing pH in the physiologic range that is biologically relevant. Alkaline pH increases CaSR sensitivity to Ca-suppressing PTH secretion, and acid pH has the opposite effect.19 In the kidney, the reason why divalent cations would regulate Klotho shedding is not clear, but a role for pH may provide a broader physiologic rationale and partly explain the beneficial effects of alkali therapy or diets on CKD progression. Common doses of HCO3 can increase venous pH values in patients with CKD from 7.26 to 7.39 and reduce PTH levels.19,29 Finally, we find evidence that the relationship between the CaSR and Klotho is more complex than a simple signaling pathway regulating Klotho shedding. The renal CaSR appears to affect Klotho membrane localization and protein expression. This finding is similar to that described in parathyroid glands.28
In three different experimental systems (mice, mouse kidney tissue, and cultured cells), we show that the CaSR increases sKlotho levels or Klotho shedding. Activation of the CaSR with ligands, calcimimetic agents, or alkaline pH increases Klotho shedding in all of these systems and is blocked by interfering with CaSR signaling pharmacologically, by expressing the nonfunctional CaSRR795W, or genetic deletion. The protease requirements and pharmacology for CaSR-stimulated Klotho shedding are the same for CaSR ligands and alkaline pH, indicating involvement of the same pathway.
Renal Klotho could be cleaved by many proteases, including ADAM10 and ADAM17.30 Using various methodologies, we demonstrated that ADAM10 activity accounts for CaSR-dependent Klotho shedding. Our LCM, fractionation and gradient centrifugation, and immunofluorescence data indicate that the CaSR, ADAM10, and Klotho are expressed in the DCT and function either in a complex or in close proximity to each other.8,15–16,31 The coimmunoprecipitation of both ADAM10 and ADAM17 with the CaSR and Klotho suggests that both proteases may interact with the CaSR, but we find that, in minced kidney and HEK-293 cells, ADAM10 but not ADAM17 is responsible for CaSR-stimulated Klotho shedding. Our kidney extract fractionation data (Figure 4)—showing that ADAM17 is in a different region of the gradient from the CaSR, Klotho, and ADAM10—indicate that differential localization of the proteins may be the reason. Finally, the siRNA data in Figure 6 indicate that, although ADAM17 may interact with the CaSR, it is not responsible for CaSR-stimulated Klotho shedding and is consistent with ADAM17 being in a distinct cellular compartment.
Unlike previous reports, we found no effect of ADAM17 in our system.12,13,16 ADAM17 is expressed at low levels in normal adult kidney, but its expression increases in the settings of fibrosis and inflammation. We used normal kidneys from 4- to 6-month-old mice that may have relatively low levels of ADAM17 expression resulting in minimal activity.32,33 Distinct effects of ADAM10 and ADAM17 can be rationalized by the fact that they associate with different families of accessory proteins: tetraspanins (C8 subfamily) with ADAM10, and rhomboid proteins with ADAM17. The tetraspanin and rhomboid proteins appear to target their respective partners to different regions of the cell, consistent with our density gradient centrifugation and siRNA results (Figures 4 and 6), and can explain the distinct effects of ADAM10 and ADAM17 on Klotho shedding.34,35
The mechanism by which the CaSR stimulates Klotho shedding by ADAM10 may be similar to that used by other G protein–coupled receptors that transactivate the EGF receptor. These receptors activate ADAM10 to cleave pro-EGF to EGF, and the cleaved EGF then activates the EGF receptor.36 Recent work indicates that membrane phosphoserine regulated by the Ca-dependent phospholipid scramblase Anoctamin-6 may play a critical role in ADAM10 activation.37
Physiologic regulation of sKlotho levels and Klotho expression are not well understood. sKlotho expression is reduced in acutely injured kidneys with increased inflammation involving IL-1β and NF-κB and is also reduced by adiponectin.38–40 Inflammatory processes hypermethylate its promoter, leading to repression, whereas transcription is increased by peroxisome proliferator-activated receptor-γ.41,42 In parathyroid glands, like kidneys (Supplemental Figure 1), absence of the CaSR decreased Klotho protein and mRNA by an as-yet-undefined mechanism.28
The level of renal Klotho expression affects circulating sKlotho levels. Genetic deletion of PT Klotho does not affect systemic levels of sKlotho, but hemizygous Klotho deletion results in systemic levels that are 57% of WT, and deletion of nephron Klotho using Six2-Cre results in systemic levels that are 20% of WT.2,8,43 In our TS-CaSR−/− mice, the basal Klotho levels appeared lower than WT, but were not statistically different. The levels of renal protein and mRNA expression were 60% and 50% of WT, respectively. In these mice, the CaSR-stimulated increase in sKlotho was eliminated, rather than reduced, so the reduction in renal Klotho expression does not explain the results from TS-CaSR−/− mice. CaSR-independent mechanisms of increasing sKlotho should remain intact in these mice, although the maximum level would be expected to be reduced.
The kidney continuously produces and sheds sKlotho into the circulation and, in the absence of physiologic or pathophysiologic perturbations, this represents the basal level of sKlotho, the focus of most studies to date. From comparison of the amount of circulating sKlotho and total kidney Klotho, we calculated that circulating sKlotho represents a miniscule proportion of total potentially available renal Klotho protein (Supplemental Figure 6), augmenting the need to understand the mechanisms that regulate sKlotho. The importance of further investigations is underscored by observations that aging and CKD animal models have reduced kidney Klotho but may benefit from increasing circulating levels. Our data in Figure 5 and Supplemental Figure 1 suggest a codependent relationship between CaSR and Klotho membrane protein expression, a prerequisite for Klotho shedding. The relationships among Klotho gene expression, total cell Klotho, membrane expression, and shedding remain to be defined.
This work is the first to demonstrate in vivo physiologically relevant regulation of sKlotho shedding and identify a molecular mechanism for it. In parathyroid glands and renal distal tubules, Klotho and the CaSR interact to regulate important physiologic functions of each tissue. The effect of CaSR signaling on each tissue is different (suppression of PTH secretion in the parathyroid glands and shedding of Klotho in the DCT) but, in both tissues, the level of CaSR expression affects the level of Klotho expression, suggesting at least a common mechanism for CaSR action in both tissues.28 Although more details remain to be defined, these studies provide a basis to investigate approaches to increasing sKlotho levels in early CKD, AKI, or other conditions, such as acute lung injury, where increased levels would be beneficial.2,3,44,45 Opportunities to treat patients with exogenous Klotho are limited at this point for technical reasons, but additional approaches to increase endogenous sKlotho levels may be derived from further research.
Disclosures
L. Liu reports being employed by Reata Pharmaceuticals. R.T. Miller reports serving as a scientific advisor for, or member of, Ackerman Center for Holocaust Studies, JDRF study section, NIH study sections, and UT Dallas; having consultancy agreements with American Society of Nephrology, JDRF, and NIH (National Institute of Diabetes and Digestive and Kidney Diseases); and having patents and inventions for a Ca receptor antibody with University of Florida. O.W. Moe reports having consultancy agreements with, and receiving honoraria from, Allena Pharmaceutical, Alnylam, and Dicernal; and serving as editor of Current Opinion of Nephrology and Hypertension and Seldin and Giebisch’s The Kidney. All remaining authors have nothing to disclose.
Funding
This work was supported by a U.S. Department of VA Merit Review grant BX004691 RTM, the VA North Texas Health Care System Research Service, NIH grants R01 DK081423 (RTM) and R01 DK091392 (OWM), the UT Southwestern O’Brien Kidney Research Center via NIH grant P30 DK-079328, and a Jane and Charles Pak Foundation Center for Mineral Metabolism and Clinical Research at UT Southwestern Medical School Endowed Professor Collaborative Research Support Grant.
Supplementary Material
Acknowledgments
The authors thank Drs. Charles Pak, Dwight Towler, Eleanor Lederer, and Chou-Long Huang for critical review of the manuscript.
Z. Liu, A.N. Chang, O.W. Moe, and R.T. Miller designed the study; Z. Liu, L. Liu, A.N. Chang, J. Yoon, E. Lee, S. Ferre, J. Paster, and J. Zhang carried out experiments; A.N. Chang, Z. Liu, L. Liu, O.W. Moe, and R.T. Miller analyzed the data; Z. Liu and A.N. Chang made the figures; R.T. Miller, A.N. Chang, and O.W. Moe drafted and revised the paper; and all authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021020276/-/DC Supplemental.
Supplemental Figure 1. Expression of the CaSR and Klotho in the DCT and absence of CaSR expression in TS-CaSR−/− mice.
Supplemental Figure 2. R-568 dose response in minced kidney.
Supplemental Figure 3. CaSR-mediated increase in shed Klotho from transiently transfected HEK-293 cells.
Supplemental Figure 4. Expression of the CaSR and Klotho in isolated mouse DCT.
Supplemental Figure 5. Time-course for in vivo response to R-568 and NaHCO3.
Supplemental Figure 6. Comparison of Klotho IP/IB and commercially available ELISA.
References
- 1.Hu MC, Shi M, Zhang J, Addo T, Cho HJ, Barker SL, et al. : Renal production, uptake, and handling of circulating αKlotho. J Am Soc Nephrol 27: 79–90, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindberg K, Amin R, Moe OW, Hu MC, Erben RG, Östman Wernerson A, et al. : The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol 25: 2169–2175, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, et al. : A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One 8: e56695, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akimoto T, Kimura T, Watanabe Y, Ishikawa N, Iwazu Y, Saito O, et al. : The impact of nephrectomy and renal transplantation on serum levels of soluble Klotho protein. Transplant Proc 45: 134–136, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Akimoto T, Yoshizawa H, Watanabe Y, Numata A, Yamazaki T, Takeshima E, et al. : Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol 13: 155, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie J, Cha SK, An SW, Kuro-O M, Birnbaumer L, Huang CL: Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun 3: 1238, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakan H, Nakatani K, Asai O, Imura A, Tanaka T, Yoshimoto S, et al. : Reduced renal α-Klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. PLoS One 9: e86301, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ide N, Olauson H, Sato T, Densmore MJ, Wang H, Hanai JI, et al. : In vivo evidence for a limited role of proximal tubular Klotho in renal phosphate handling. Kidney Int 90: 348–362, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, et al. : Secreted Klotho protein in sera and CSF: Implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565: 143–147, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Hu MC, Kuro-o M, Moe OW: Secreted Klotho and chronic kidney disease. In: Endocrine FGFs and Klothos, edited by Kuro M, New York, Springer, 2012, pp 126–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, et al. : Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett 583: 3221–3224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CD, Tung TY, Liang J, Zeldich E, Tucker Zhou TB, Turk BE, et al. : Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry 53: 5579–5587, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR: Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A 104: 19796–19801, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song JH, Lee MY, Kim YJ, Park SR, Kim J, Ryu SY, et al. : Developmental immunolocalization of the Klotho protein in mouse kidney epithelial cells. Eur J Histochem 58: 2256, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, et al. : Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24: 3438–3450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Loon EPM, Pulskens WP, van der Hagen EAF, Lavrijsen M, Vervloet MG, van Goor H, et al. : Shedding of klotho by ADAMs in the kidney. Am J Physiol Renal Physiol 309: F359–F368, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Nemeth EF, Steffey ME, Hammerland LG, Hung BCP, Van Wagenen BC, DelMar EG, et al. : Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci U S A 95: 4040–4045, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinn SJ, Bai M, Brown EM: pH Sensing by the calcium-sensing receptor. J Biol Chem 279: 37241–37249, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Campion KL, McCormick WD, Warwicker J, Khayat MEB, Atkinson-Dell R, Steward MC, et al. : Pathophysiologic changes in extracellular pH modulate parathyroid calcium-sensing receptor activity and scretion via a histidine-independent mechanism. J Am Soc Nephrol 26: 2163–2171, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toka HR, Al-Romaih K, Koshy JM, DiBartolo S 3rd, Kos CH, Quinn SJ, et al. : Deficiency of the calcium-sensing receptor in the kidney causes parathyroid hormone-independent hypocalciuria. J Am Soc Nephrol 23: 1879–1890, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yagil R, Lerner Z, Etzion Z, Berlyne GM: Acid-base changes in milk and blood of rats in acidosis and alkalosis. Am J Physiol 231: 132–135, 1976 [DOI] [PubMed] [Google Scholar]
- 22.Handlogten, ME, Shiraishi, N, Awata, H, Huang, C, Miller, RT: Extracellular Ca(2+)-sensing receptor is a promiscuous polycation sensor that responds to lead. Am J Physiol Renal Physiol 279: F1083–F1091, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Kato Y, Arakawa E, Kinoshita S, Shirai A, Furuya A, Yamano K, et al. : Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun 267: 597–602, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Huang C, Handlogten ME, Miller RT: Parallel activation of phosphatidylinositol 4-kinase and phospholipase C by the extracellular calcium-sensing receptor. J Biol Chem 277: 20293–20300, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Cha SK, Huang C, Ding Y, Qi X, Huang CL, Miller RT: Calcium-sensing receptor decreases cell surface expression of the inwardly rectifying K+ channel Kir4.1. J Biol Chem 286: 1828–1835, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barker SL, Pastor J, Carranza D, Quiñones H, Griffith C, Goetz R, et al. : The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant 30: 223–233, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, et al. : FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med 19: 1147–1152, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan Y, Liu W, Bi R, Densmore MJ, Sato T, Mannstadt M, et al. : Interrelated role of Klotho and calcium-sensing receptor in parathyroid hormone synthesis and parathyroid hyperplasia. Proc Natl Acad Sci U S A, 115: E3749–E3758, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathur RP, Dash SC, Gupta N, Prakash S, Saxena S, Bhowmik D: Effects of correction of metabolic acidosis on blood urea and bone metabolism in patients with mild to moderate chronic kidney disease: A prospective randomized single blind controlled trial. Ren Fail 28: 1–5, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Schafer AE, Blaxall BC: G protein coupled receptor-mediated transactivation of extracellular proteases. J Cardiovasc Pharmacol 70: 10–15, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riccardi D, Valenti G: Localization and function of the renal calcium-sensing receptor. Nat Rev Nephrol 12: 414–425, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Mulder GM, Melenhorst WB, Celie JW, Kloosterhuis NJ, Hillebrands JL, Ploeg RJ, et al. : ADAM17 up-regulation in renal transplant dysfunction and non-transplant-related renal fibrosis. Nephrol Dial Transplant 27: 2114–2122, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Perna AF, Pizza A, Di Nunzio A, Bellantone R, Raffaelli M, Cicchella T, et al. : ADAM17, a new player in the pathogenesis of chronic kidney disease-mineral and bone disorder. J Ren Nutr 27: 453–457, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Matthews AL, Noy PJ, Reyat JS, Tomlinson MG: Regulation of A disintegrin and metalloproteinase (ADAM) family sheddases ADAM10 and ADAM17: The emerging role of tetraspanins and rhomboids. Platelets 28: 333–341, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthews AL, Szyroka J, Collier R, Noy PJ, Tomlinson MG: Scissor sisters: Regulation of ADAM10 by the TspanC8 tetraspanins. Biochem Soc Trans 45: 719–730, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan Y, Shirakabe K, Werb Z: The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors. J Cell Biol 158: 221–226, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bleibaum F, Sommer A, Veit M, Rabe B, Andrä J, Kunzelmann K, et al. : ADAM10 sheddase activation is controlled by cell membrane asymmetry. J Mol Cell Biol 11: 979–993, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, Meng C, Wang Y, Huang H, Liu W, Zhang JF, et al. : IL-1β inhibits β-Klotho expression and FGF19 signaling in hepatocytes. Am J Physiol Endocrinol Metab 310: E289–E300, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Moreno JA, Izquierdo MC, Sanchez-Niño MD, Suárez-Alvarez B, Lopez-Larrea C, Jakubowski A, et al. : The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol 22: 1315–1325, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rutkowski JM, Pastor J, Sun K, Park SK, Bobulescu IA, Chen CT, et al. : Adiponectin alters renal calcium and phosphate excretion through regulation of klotho expression. Kidney Int 91: 324–337, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin W, Zhang Q, Liu L, Yin S, Liu Z, Cao W: Klotho restoration via acetylation of peroxisome proliferation-activated receptor γ reduces the progression of chronic kidney disease. Kidney Int 92: 669–679, 2017 [DOI] [PubMed] [Google Scholar]
- 42.Yin S, Zhang Q, Yang J, Lin W, Li Y, Chen F, et al. : TGFβ-incurred epigenetic aberrations of miRNA and DNA methyltransferase suppress Klotho and potentiate renal fibrosis. Biochim Biophys Acta Mol Cell Res 1864: 1207–1216, 2017 [DOI] [PubMed] [Google Scholar]
- 43.Xie J, Yoon J, An SW, Kuro-o M, Huang CL: Soluble Klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol 26: 1150–1160, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ravikumar P, Li L, Ye J, Shi M, Taniguchi M, Zhang J, et al. : αKlotho deficiency in acute kidney injury contributes to lung damage. J Appl Physiol (1985) 120: 723–732, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu MC, Shi M, Gillings N, Flores B, Takahashi M, Kuro-O M, Moe OW: Recombinant a-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int 91: 1104–1114, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.