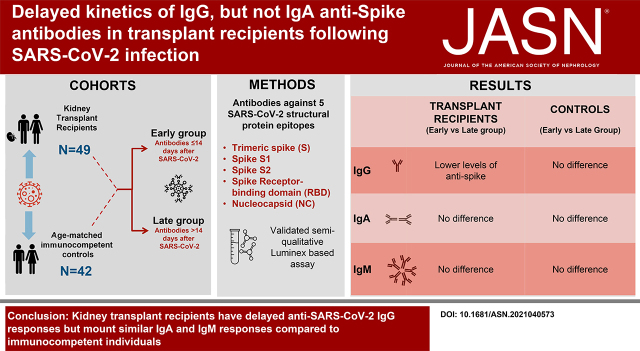
Significance Statement
Analyses of the incidence, relative kinetics, and spectrum of anti–SARS-CoV-2 antibodies in kidney transplant recipients are not as detailed as they are for immunocompetent controls. In this multicenter, cross-sectional study of 49 kidney transplant recipients with PCR-confirmed SARS-CoV-2 infection, we found that anti–SARS-CoV-2 IgG production is delayed but that IgM and IgA responses are similar compared with those observed in immunocompetent controls. Therefore, antiviral humoral immunity is delayed but preserved in kidney transplant recipients. This finding is important in understanding the immune response against SARS-CoV-2 in patients on chronic immunosuppression and may provide insights into devising strategies to monitor antibody responses to infection and vaccination.
Keywords: end stage kidney disease, immunology, kidney transplantation, antibodies, SARS-Co-V-2, COVID-19
Visual Abstract
Abstract
Background
Kidney transplant recipients are at increased risk of severe outcomes during COVID-19. Antibodies against the virus are thought to offer protection, but a thorough characterization of anti–SARS-CoV-2 immune globulin isotypes in kidney transplant recipients following SARS-CoV-2 infection has not been reported.
Methods
We performed a cross-sectional study of 49 kidney transplant recipients and 42 immunocompetent controls at early (≤14 days) or late (>14 days) time points after documented SARS-CoV-2 infection. Using a validated semiquantitative Luminex-based multiplex assay, we determined the abundances of IgM, IgG, IgG1–4, and IgA antibodies against five distinct viral epitopes.
Results
Kidney transplant recipients showed lower levels of total IgG antitrimeric spike (S), S1, S2, and receptor binding domain (RBD) but not nucleocapsid (NC) at early versus late time points after SARS-CoV-2 infection. Early levels of IgG antispike protein epitopes were also lower than in immunocompetent controls. Anti–SARS-CoV-2 antibodies were predominantly IgG1 and IgG3, with modest class switching to IgG2 or IgG4 in either cohort. Later levels of IgG antispike, S1, S2, RBD, and NC did not significantly differ between cohorts. There was no significant difference in the kinetics of either IgM or IgA antispike, S1, RBD, or S2 on the basis of timing after diagnosis or transplant status.
Conclusions
Kidney transplant recipients mount early anti–SARS-CoV-2 IgA and IgM responses, whereas IgG responses are delayed compared with immunocompetent individuals. These findings might explain the poor outcomes in transplant recipients with COVID-19.
Podcast
This article contains a podcast athttps://www.asn-online.org/media/podcast/JASN/2021_11_23_briggsgriffin112321.mp3
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection of kidney transplant recipients is associated with increased mortality compared with immunocompetent individuals.1–3 Although poor outcomes in patients with transplants suggest an impaired immune response to SARS-CoV-2 infection, studies of anti–SARS-CoV-2 antibody responses have provided conflicting results. Some reports indicate that transplant recipients generate normal levels of total IgG upon SARS-CoV-2 infection,4–7 but the antibody decline might be more rapid than in immunocompetent subjects.8,9 Recent studies documented a lower antibody response (6.2%–17%) after the first dose of mRNA vaccination in kidney transplant recipients,10–12 contrasting with the robust early immunogenicity observed in the general population.13–15 Patients with ESKD receiving dialysis demonstrated a mostly intact early response to vaccination, with 87% seroconversion.11 Importantly, little is known about the dynamics of various immune globulin classes and isotypes after natural infection in transplant recipients. Studies published to date have utilized various antibody detection assays, which further complicates data interpretation and comparison.
In this study, we adopted a recently developed and validated high-throughput multiplex antibody detection assay16 to interrogate the spectrum of antibody responses to SARS-CoV-2 in a cohort of kidney transplant recipients and in nontransplanted, immunocompetent individuals.
Methods
Study Population
Our study included all consecutive consenting adult kidney transplant recipients followed up at Mount Sinai or Montefiore Medical Center (both in New York, NY) with an ongoing or prior SARS-CoV-2 infection diagnosed through RT‐PCR of nasopharyngeal swab samples. Serum samples were collected from April 2020 to February 2021 during hospitalization or at follow-up clinic visits. Serial samples were collected from nine patients.
Immunocompetent subjects with a coronavirus disease 2019 (COVID-19) PCR-positive nasopharyngeal swab were enrolled from the Emory Hospitals and outpatients between March 2020 and January 2021. From these control subjects, we identified individuals who were matched for age and time after PCR-based diagnosis with the kidney transplant recipients. Two control subjects were later excluded on the basis of a history of autoimmune disease. We recorded epidemiologic, clinical, and laboratory data in an ad hoc database. We graded disease severity per previously published reports.17
For analysis, subjects were divided into early (first 14 days) and late (15 days and later) cohorts on the basis of time between PCR diagnosis and sample collection for antibody testing.
The study received appropriate approval of the ethics and scientific committees of the participating centers (institutional review board [IRB] titles/numbers: STUDY-20–01922, Mount Sinai Medical Center; IRB-2020–11662, Montefiore Medical Center; IRB-58271, Emory University; and IRB-56413, Stanford University). All patients and controls provided informed consent.
Blood Collection, Serum Isolation, and Storage
Blood was collected in sterile tubes, allowed to clot, and then centrifuged to separate the serum. Samples were aliquoted and stored at −80°C until analyses.
Anti–SARS-CoV-2 Antibody Measurement
Detection of SARS-CoV-2–specific IgG antibodies directed against the full trimeric spike protein; the individual spike 1 (S1), spike 2 (S2), and receptor binding domains (RBDs) of the spike protein; and the nucleocapsid (NC) protein was performed with the One Lambda single-antigen bead assay, as previously described (LABScreen COVID Plus; One Lambda, Canoga Park, CA),16 and then analyzed on a Luminex FLEXMAP 3D instrument (Luminex Corp., Austin, TX). IgA, IgM, and IgG1/G2/G3/G4 antibody detection was performed with R-Phycoerythrin AffiniPure goat anti-human serum IgA α-chain specific (Jackson ImmunoResearch, West Grove, PA; catalog no. 109–115–011), PE-conjugated anti-human IgM (One Lambda, West Grove, PA; catalog no. IGM-PEC1), mouse anti-human IgG1 (Invitrogen; catalog no. MH1013), mouse anti-human IgG2 (Invitrogen; catalog no. 05–3500), mouse anti-human IgG3 (Invitrogen; catalog no. 05–3600), and mouse anti-human IgG4 (Invitrogen; catalog no. A-10651), respectively.18
Statistical Analyses
Graphs and statistics were completed in GraphPad Prism (GraphPad Software, La Jolla, CA). We expressed results as means and SDs or SEM sunless stated otherwise. Two-way comparison of two or more matched groups was computed using two-way ANOVA using the Kruskal–Wallis test as appropriate. Distributions were compared using the Kolmogorov–Smirnov two-tailed unpaired t test, and categorical variables were compared by the two‐sided chi‐squared or two‐sided Fisher exact test, where applicable. P values were computed to assess significance of individual comparisons, with a value of P=0.05 considered as statistically significant.
Results
To better understand the antibody response to SARS-CoV-2 infection in transplant recipients, we analyzed 58 serum samples collected from 49 recipients of kidney transplants with PCR-confirmed diagnosis of COVID-19. The majority of patients were men (57%), with a median age of 57 (interquartile range, 42–65) years. Immunosuppression at time of sample draw mainly consisted of calcineurin inhibitors and steroids with or without antiproliferative agents. Subjects were divided into early (first 14 days) and late (15 days and later) cohorts on the basis of time after PCR diagnosis. The early cohort had significantly higher dialysis requirements and lower lymphocyte counts compared with the late cohort at the time of sample acquisition. Both the early and late cohorts of transplant recipients had similar antimetabolite exposure pre– and post–COVID-19 diagnosis; the mean daily mycophenolate dose pre–COVID-19 was 1250 mg (SD: 583 mg) compared with a post–COVID-19 diagnosis dose of 421 mg (SD: 526 mg) (Table 1). Characteristics of the early and late control groups are also presented in Table 1.
Table 1.
Demographics
| Characteristic | Transplant Early, n=16 | Transplant Late, n=33 | Immunocompetent Controls Early, n=19 | Immunocompetent Controls Late, n=23 | P Valuea |
|---|---|---|---|---|---|
| Age, median (IQR) | 60 (41–67) | 54 (42–65) | 59 (31–66) | 56 (36–64) | 0.90 |
| Sex, no. (% men) | 11/16 (69%) | 17/33 (52%) | 11/19 (58%) | 6/23 (26%) | 0.05b |
| Race and ethnicity | 0.04 | ||||
| Non-Hispanic White participants | 0/16 | 4/33 (12%) | 5/19 (26%) | 9/23 (39%) | |
| Non-Hispanic Black participants | 6/16 (38%) | 10/33 (30%) | 4/19 (21%) | 7/23 (30%) | |
| Hispanic participants | 10/16 (63%) | 15/33 (45%) | 10/19 (53%) | 5/23 (21%) | |
| Other | 0/16 | 4/33 (12%) | 0/19 | 2/23 (9%) | |
| Years since transplantation, median (IQR) | 2.2 (0.1–6.1) | 4.3 (2.0–8.3) | N/A | N/A | 0.06 |
| Calcineurin inhibitor at time of sample draw | 15/16 (94%) | 30/33 (91%) | N/A | N/A | 0.70 |
| Mycophenolate mofetil at time of sample draw | 5/16 (31%) | 12/33 (36%) | N/A | N/A | 0.70 |
| Steroids at time of sample draw | 14/16 (88%) | 31/33 (94%) | N/A | N/A | 0.40 |
| Mycophenolate mofetil dose decreased at time of sample drawc | 12/13 (92%) | 21/25 (84%) | N/A | N/A | 0.50 |
| Daily preinfectious mycophenolate mofetil dose, mg, mean (SD)c | 1115 (582) | 1320 (593) | N/A | N/A | 0.30 |
| Daily mycophenolate mofetil dose (mg) at time of sample draw, mean (SD)c,d | 346 (473) | 460 (557) | N/A | N/A | 0.60 |
| Required dialysis after SARS-CoV-2 diagnosise | 11/16 (69%) | 12/33 (36%) | 1/10 (10%) | 3/3 (100%) | 0.004b,f,g,h |
| Peak creatinine after SARS-CoV-2 diagnosis if dialysis not required, median (IQR)e | 1.6 (1.5–2.7) | 2 (1.1–3) | 0.8 (0.7–0.9) | N/A | 0.004g |
| Lymphocyte count (1000/μl) at time of sample draw, median (IQR)i | 0.4 (0.2–1) | 1.1 (0.7–1.6) | 1.2 (0.8–1.3) | 1.1 (0.3–1.1) | 0.03f,g |
| Peak clinical severity score, median (IQR) | 5 (4–7) | 4 (3–6) | 6 (1.5–6) | 1.5 (1.5–5) | 0.01h |
| Days postdiagnosis, median (IQR) | 4 (2–7) | 44 (21–60) | 5 (2–7) | 36 (22.5–49) | <0.001b,f |
Transplant early indicates that samples are drawn from kidney transplant recipients 14 or fewer days after SARS-CoV-2 diagnosis. Transplant late indicates that samples are drawn from kidney transplant recipients >14 days after SARS-CoV-2 diagnosis. Immunocompetent controls early indicates that samples are drawn from immunocompetent controls 14 or fewer days after SARS-CoV-2 diagnosis. Immunocompetent controls late indicates that samples are drawn from immunocompetent controls >14 days after SARS-CoV-2 diagnosis. Peak clinical severity score: one, not hospitalized with resumption of normal activities; two, not hospitalized but unable to resume normal activities; three, hospitalized, not requiring supplemental oxygen; four, hospitalized, requiring supplemental oxygen; five, hospitalized, requiring nasal high-flow oxygen therapy, noninvasive mechanical ventilation, or both; six, hospitalized, requiring extracorporeal membrane oxygenation, invasive mechanical ventilation, or both; and seven, death. IQR, interquartile range; N/A, not applicable.
P value for difference between groups. Superscripts are present if the P value for difference between transplant early and transplant late, transplant early and immunocompetent controls early, transplant late and immunocompetent controls late, or immunocompetent controls early and immunocompetent controls late is 0.05.
P=0.05 for comparison between immunocompetent controls early and immunocompetent controls late.
No prediagnosis mycophenolate mofetil for three of 16 transplant early subjects and eight of 33 transplant late subjects.
Mycophenolate dose decreased to 0 mg/d in eight of 13 transplant early subjects and 13 of 25 transplant late subjects.
Data unavailable for nine of 19 immunocompetent controls early and 20 of 23 immunocompetent controls late.
P=0.05 for comparison between transplant early and transplant late.
P=0.05 for comparison between transplant early and immunocompetent controls early.
P=0.05 for comparison between transplant late and immunocompetent controls late.
Data unavailable for 13 of 19 immunocompetent controls early and 20 of 23 immunocompetent controls late.
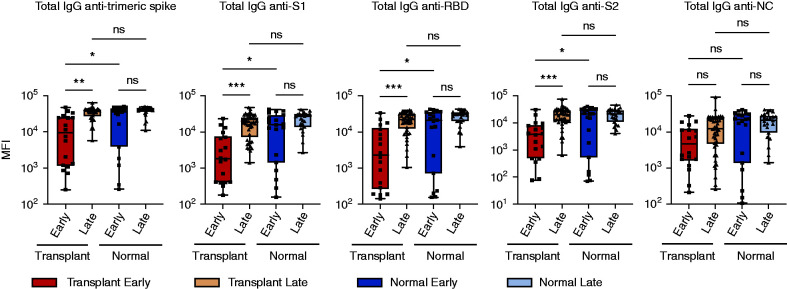
We assessed antibodies directed against the SARS-CoV-2 trimeric spike protein (spike), S1, spike RBD, S2, and NC epitopes using a bead-based multiplex assay.16 As shown in Figure 1, there were lower levels of total IgG antispike, S1, S2, and RBD but not NC in samples from SARS-CoV-2–positive transplant recipients obtained earlier compared with later. IgG antibodies directed against all spike epitopes were also lower when compared with samples from matched immunocompetent subjects obtained within the first 14 days after diagnosis. These antibodies were predominantly IgG1 and IgG3 compared with class switching to IgG2 or IgG4 in either cohort (Supplemental Figure 1). Patterns of IgG1 and IgG3 differences between early versus late time points and patients with transplants versus immunocompetent subjects largely mirrored those seen for total IgG. Interestingly, late levels of total IgG antitrimeric spike, S1, S2, RBD, and NC from transplant recipients were not significantly different from those obtained >14 days after confirmed infection from matched immunocompetent controls. There was a nonstatistically significant trend toward increasing IgG levels directed toward spike epitopes between early and late immunocompetent controls. Taken together, these data indicate a delayed IgG response specific to the SARS-CoV-2 spike protein in kidney transplant recipients that reaches normal levels in the convalescent phase.
Figure 1.
Generation of IgG antispike but not anti-NC antibodies is dependent on time after diagnosis. Levels of total IgG specific for trimeric spike, S1, RBD, S2, or NC epitopes. Serum is from (dark red) transplant recipients ≤14 days after diagnosis, (brown) transplant recipients >14 days after diagnosis, (dark blue) immunocompetent individuals ≤14 days after diagnosis, and (light blue) immunocompetent individuals >14 days after diagnosis. MFI, mean fluorescence intensity; ns, not significant. *P=0.05; **P=0.01; ***P<0.001.
As a means to determine the capacity to generate protective IgG immune responses to coronaviruses in transplant recipients versus immunocompetent controls, we also assessed convalescent levels of IgG directed against four common cold coronaviruses in all four cohorts. As shown in Supplemental Figure 2, levels of these antibodies were comparable between all four cohorts; the sole exception was anti-OC43 spike S1 in the transplant and control late groups. These data are consistent with those for antibody response to SARS-CoV-2 that show that kidney transplant recipients eventually reach normal antiviral antibody levels, although their kinetics may be delayed.
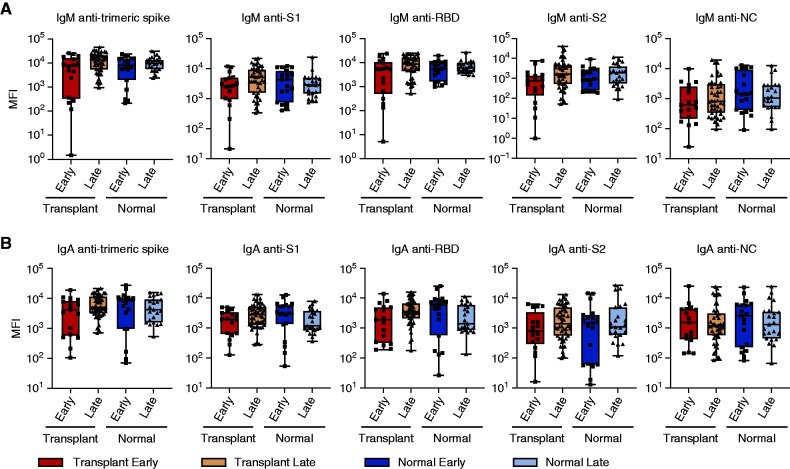
We also assessed IgM and monomeric IgA from the serum of the same subjects (Figure 2). There was a nonstatistical trend toward lower levels of IgM antispike epitopes early versus later after infection in transplant recipients but not in immunocompetent subjects. In contrast to IgG levels, there was no significant difference in IgA antispike, S1, RBD, or S2 IgA in early versus late disease. Antibody levels from transplant recipients were similar to those found in SARS-CoV-2 immunocompetent controls at both time points. Taken together, these data suggest that, unlike IgG responses, class switching to IgA occurs with a normal time course following infection with SARS-CoV-2.
Figure 2.
Early generation of IgM and IgA to multiple SARS-CoV-2 epitopes. Levels of total (A) IgM or (B) IgA specific for trimeric spike, S1, RBD, S2, or NC epitopes. Serum is from (dark red) transplant recipients ≤14 days after diagnosis, (brown) transplant recipients >14 days after diagnosis, (dark blue) immunocompetent individuals ≤14 days after diagnosis, and (light blue) immunocompetent individuals >14 days after diagnosis. Comparisons with a statistically significant difference are indicated. MFI, mean fluorescence intensity.
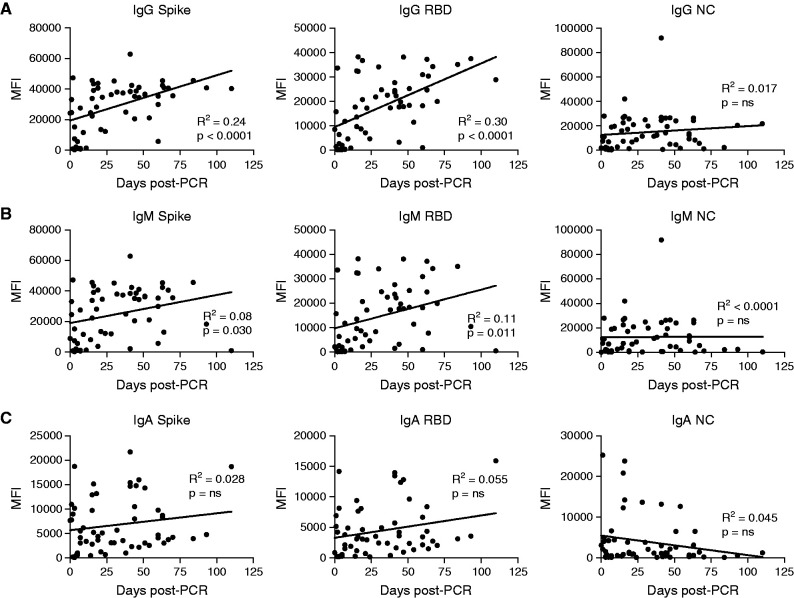
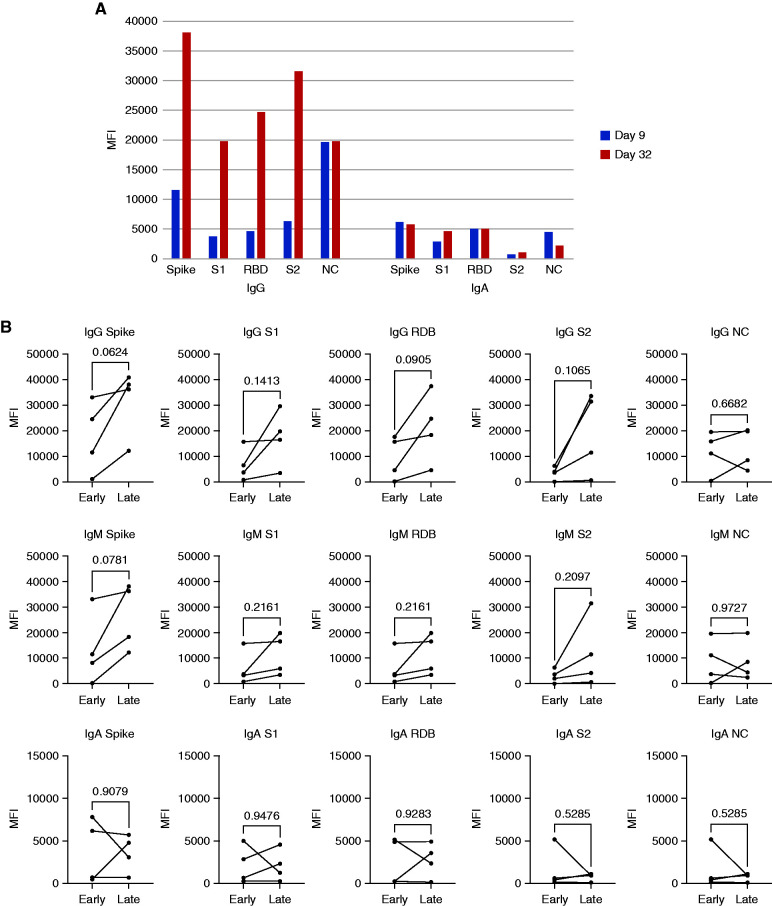
We next assessed changes in total IgG, IgM, and IgA over time. Scatterplots demonstrate that IgG antispike (all epitopes tested) but not anti-NC antibodies are higher at later time points after infection (Figure 3A) (data not shown). There is also a time-dependent rise in IgM antifull spike, anti-S1, and anti-RBD but not anti-S2 or NC (Figure 3B). In contrast to the time-dependent rise in IgG, analysis of IgA levels versus time indicated that IgA antispike (all epitopes tested) does not significantly rise with time and that IgA anti-NC decreases with time after initial infection (Figure 3C). Longitudinal samples, including early (≤14 days) and late (>14 days), were available in four kidney transplant recipients. In one subject with 23 days between the samples collected, the kinetics of IgA were more rapid than those of IgG for antispike but not anti-NC antibodies (Figure 4A). We next analyzed all four subjects with both an early and late sample available. Consistent with the full cohort data, there was a trend toward increasing IgG antispike but not anti-NC, although this did not reach statistical significance. There was a similar trend toward increased IgM antispike in these four individuals. In contrast, IgA antispike levels did not have a consistent change. Together, these data suggest that class switching to IgA occurs very early in the disease course and may decay at a more rapid rate than IgG isotypes.
Figure 3.
Kinetic analysis of IgG, IgM, and IgA. (A) IgG, (B) IgM, and (C) IgA levels specific for trimeric spike, RBD, or NC on the basis of MFI plotted against the number of days after PCR-based diagnosis of SARS-CoV-2 infection for samples obtained up to 100 days after diagnosis. The line is derived from simple linear regression. P values indicate the significance of the line slope. ns, not significant. MFI, mean fluorescence intensity.
Figure 4.
Longitudinal analysis of antibody isotype kinetics in individual subjects. (A) Time course of IgG and IgA antibodies in a single patient. Sera in blue columns are from day 9 after diagnosis, and sera in orange columns are from day 32 after diagnosis. (B) Comparison of early versus late time points from four subjects. Lines indicate samples from the same subject. P values are calculated using a two-tailed paired t test. MFI, mean fluorescence intensity.
Discussion
Increased mortality in kidney transplant recipients with COVID-19 and impaired early response to the SARS-CoV-2 vaccine have been matters of concern.1–3,10 Interestingly, the data in this study indicate that although antiviral IgG production in kidney transplant recipients is delayed, the later levels of total and of various subclasses of IgG are similar to those observed in immunocompetent individuals.
IgM production was not delayed in response to SARS-CoV-2 infection, suggesting that extrafollicular and T cell–independent humoral responses are not significantly impaired by immunosuppressive maintenance therapy. Conversely, although IgG production can occur in the context of an extrafollicular response,19–21 it is primarily a germinal center product with class switching of B cells that requires help from T follicular helper cells.22 As immunosuppressive drugs used to prevent allograft rejection largely target both T cells and B cells, antiviral IgG responses in organ transplant recipients are commonly impaired.23 Conversely, IgG production against SARS-CoV-2 was delayed but not significantly impaired in our cohort of patients. This atypical response might have been facilitated by the tapering of immunosuppression during infection in individuals with COVID-19. Prior studies have shown an impaired antibody response against the SARS-CoV-2 vaccine in organ transplant recipients on antimetabolites.10,24–26 In our cohort, over 80% underwent significant reduction or withdrawal of mycophenolate mofetil after infection, which likely explains the normal IgG levels at later time points. Although not mutually exclusive, an alternative hypothesis is that in individuals with COVID-19, IgG production is predominantly extrafollicular and potentially T cell independent.21 Nonetheless, the fact that IgG production was impaired during the acute phase of the disease may explain, at least in part, the previously reported increased morbidity and mortality associated with COVID-19 in this population.
The presence of IgG anti–SARS-CoV-2 spike protein antibodies in a majority of our cohort of infected individuals at over 1 month after infection is in stark contrast to the antibody response after vaccination in this population, where less than half of these individuals reach a positive response after two standard doses of mRNA vaccine.24–27 There are several potential explanations for this discrepancy. First, natural infection occurs via a respiratory route, leading to the orchestration of an innate immune response that involves activation of local dendritic cells and epithelial cells of the respiratory tract. This context is potentially more prone to an effective immune response than the muscle in which the vaccine is delivered. Second, the vaccine has only spike protein epitopes, whereas natural infection has four structural and 23 nonstructural proteins that are coordinately expressed.28 Third, natural infection results in a significant amount of systemic inflammation with Toll-like receptor activation not seen with vaccination. In many subjects of our cohort, this was prolonged due to the severity of illness, potentially leading to a more robust response. In this proinflammatory context, reduction of immunosuppression might have been particularly important in boosting an antibody response. Understanding which of these mechanisms is responsible may provide insight into improved vaccination strategies in this at risk population.
Secretory IgA plays a crucial role in protecting mucosal surfaces against pathogens.29 Importantly, serum IgA has more potent neutralizing activity against SARS-CoV-2 than IgG.30 However, little is known about anti–SARS-CoV-2 IgA response in kidney transplant recipients. Our study demonstrates that transplant recipients effectively class switch to IgA early in the disease course. IgA class switching may occur through T cell–independent pathways31 and could explain why this Ig class is less affected by immunosuppressive therapy. In particular, lack of correlation between plasmablasts and T follicular helper cell expansion observed in SARS-CoV-2–infected individuals is consistent with germinal center–independent induction of IgA occurring during COVID-19, but more studies are needed to confirm this intriguing hypothesis.21,30,32
There are several limitations to this study, including the relatively small sample size and its statistical power, which might reduce the generalizability of our findings. Yet, the inclusion of immunocompetent controls for each time period after infection strengthens our conclusions. Matching for peak clinical severity of disease was not perfect in the late immunocompetent group, which has the potential of leading to altered antibody strength in this group. Lack of serial samples for most of the included individuals is another limitation. However, the trends observed in individuals with serial serum collection substantiate the conclusions obtained by the remainder of the cross-sectional data. Kidney transplant recipients were the only transplant type evaluated. Given differences in maintenance immunosuppression, these findings may not be generalizable to recipients of other organs.
Collectively, our data indicate that kidney transplant recipients mount early IgM and IgA responses against SARS-CoV-2, whereas IgG responses are delayed. This may at least in part explain the poor outcomes of kidney transplant recipients with SARS-CoV-2 infection. Our data are likely to extend to other individuals on chronic immunosuppression.
Disclosures
E. Akalin reports consultancy agreements with CareDx and Immucor; research funding from Angion, Astellas, CareDx, and the National Institutes of Health; honoraria from CareDx and Immucor; and scientific advisor or membership with CareDx and Immucor. H.M. Gebel reports consultancy agreements with Immucor and One Lambda, a division of Thermo Fisher, and scientific advisor or membership with the Scientific Registry of Transplant Recipients. A. Girnita reports consultancy agreements with Hookipa Biotech GmbH (Vienna, Austria), INTEGRIS Baptist Medical Center (Oklahoma City, OK), and Kezar Life Science (San Francisco, CA). F.E.-H. Lee is the founder of Micro-plex, Inc.; receives grants from BMGF and Genentech; is a member of the scientific advisory board of Be Bio Pharma; has received research funding via grants from BMGF and Genentech; has received honoraria from Be Bio Pharma; and receives license royalties from BLI, Inc. J.S. Maltzman has received honoraria from FOCIS and One Lambda, Inc./Thermo Fisher; has received research funding from One Lambda/Thermo Fisher; is a member of the American Society of Nephrology (ASN) Kidney Week Education Committee (ended November 2020); was on the board of directors of the American Society of Transplantation (AST; ended June 2021); was part of the AST Research Network (ended June 2021); is Secretary/Treasurer of the Federation of Clinical Immunology Societies; is on the Transplantation Science Committee of the Transplantation Society; is a member of the ASN Qihan Biotech scientific advisory board; and has a family member who is employed by and has an equity interest in Genentech/Roche. M. C. Menon reports ownership interest in Renalytix AI and scientific advisor or membership with the JASN editorial board as a former editorial fellow, the Journal of Clinical Medicine editorial board, and Clinical Transplantation as an associate editor. I. Sanz reports consultancy agreements with BMS, Celgene, GSK, Janssen, Kyverna, and Visterra; ownership interest in Kyverna; research funding from Exagen and GSK; honoraria from BMS/Celgene, GSK, Janssen, and Visterra; and scientific advisor or membership with Kyverna. E.S. Woodle reports consultancy agreements with Novartis and Sanofi; research funding from Amgen, Bristol Myers Squibb, Novartis, and Veloxis; honoraria from Novartis and Sanofi; and speakers bureau with Sanofi. All remaining authors have nothing to disclose.
Funding
This work was funded by National Institutes of Health, National Institute of Allergy and Infectious Diseases grants 3U01AI063594‐17S1 (to P. Cravedi and M.C. Menon) and P01AI125180-05S1 (to F.E.-H. Lee and I. Sanz) and Thermo Fisher Scientific/One Lambda, Inc. (to J.S. Maltzman).
Supplementary Material
Acknowledgments
The authors sincerely thank Dr. Robert Bray (Emory University), Dr. Jar-How Lee (Terasaki Institute), and Dr. Sander S. Florman (Mount Sinai) for their input as well as staff and patients for their efforts.
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
P. Cravedi, A. Girnita, and J.S. Maltzman conceived, designed, and oversaw the project; P. Ahearn, P. Cravedi, J.S. Maltzman, L. Wang, and T. Yalamarti performed the analyses and interpreted the data; Y. Azzi, M. Billah, S. Hartzell, A. Jain, and M.C. Menon recruited the patients with transplants and processed the samples; N.S. Haddad, F.E.-H. Lee, A. Morrison-Porter, and I. Sanz recruited control subjects and contributed samples; E. Akalin, M. Fernandez-Vina, H.M. Gebel, F.E.-H. Lee, I. Sanz, and E.S. Woodle helped in critically interpreting the data; P. Ahearn, P. Cravedi, A. Girnita, and J.S. Maltzman wrote the manuscript; and all authors reviewed and edited the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021040573/-/DCSupplemental.
Supplemental Figure 1. Measurement of IgG subtype antibodies specific for SARS-CoV-2.
Supplemental Figure 2. Measurement of IgG to common cold coronaviruses.
References
- 1.Cravedi P, Mothi SS, Azzi Y, Haverly M, Farouk SS, Pérez-Sáez MJ, et al. : COVID-19 and kidney transplantation: Results from the TANGO International Transplant Consortium. Am J Transplant 20: 3140–3148, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, et al. : Covid-19 and kidney transplantation. N Engl J Med 382: 2475–2477, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azzi Y, Bartash R, Scalea J, Loarte-Campos P, Akalin E: COVID-19 and solid organ transplantation: A review article. Transplantation 105: 37–55, 2021 [DOI] [PubMed] [Google Scholar]
- 4.Hartzell S, Bin S, Benedetti C, Haverly M, Gallon L, Zaza G, et al. : Evidence of potent humoral immune activity in COVID-19-infected kidney transplant recipients. Am J Transplant 20: 3149–3161, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Favà A, Donadeu L, Sabé N, Pernin V, González-Costello J, Lladó L, et al. : SARS-CoV-2-specific serological and functional T cell immune responses during acute and early COVID-19 convalescence in solid organ transplant patients. Am J Transplant 21: 2749–2761, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prendecki M, Clarke C, Gleeson S, Greathead L, Santos E, McLean A, et al. : Detection of SARS-CoV-2 antibodies in kidney transplant recipients. J Am Soc Nephrol 31: 2753–2756, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azzi Y, Parides M, Alani O, Loarte-Campos P, Bartash R, Forest S, et al. : COVID-19 infection in kidney transplant recipients at the epicenter of pandemics. Kidney Int 98: 1559–1567, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavarot N, Leruez-Ville M, Scemla A, Burger C, Amrouche L, Rouzaud C, et al. : Decline and loss of anti-SARS-CoV-2 antibodies in kidney transplant recipients in the 6 months following SARS-CoV-2 infection. Kidney Int 99: 486–488, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burack D, Pereira MR, Tsapepas DS, Harren P, Farr MA, Arcasoy S, et al. : Prevalence and predictors of SARS-CoV-2 antibodies among solid organ transplant recipients with confirmed infection. Am J Transplant 21: 2254–2261, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. : Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 325: 1784–1786, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yi SG, Knight RJ, Graviss EA, Moore LW, Nguyen DT, Ghobrial RM, et al. : Kidney transplant recipients rarely show an early antibody response following the first COVID-19 vaccine administration. Transplantation 105: e72–e73, 2021 [DOI] [PubMed] [Google Scholar]
- 12.Benotmane I, Gautier-Vargas G, Cognard N, Olagne J, Heibel F, Braun-Parvez L, et al. : Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int 99: 1487–1489, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. ; COVE Study Group : Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384: 403–416, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. : BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 384: 1412–1423, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. ; C4591001 Clinical Trial Group : Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383: 2603–2615, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bray RA, Lee JH, Brescia P, Kumar D, Nong T, Shih R, et al. : Development and validation of a multiplex, bead-based assay to detect antibodies directed against SARS-CoV-2 proteins. Transplantation 105: 79–89, 2021 [DOI] [PubMed] [Google Scholar]
- 17.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. : A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 382: 1787–1799, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamdani G, Goebel JW, Brailey P, Portwood EA, Hooper DK, Girnita AL: IGG3 anti-HLA donor-specific antibodies and graft function in pediatric kidney transplant recipients. Pediatr Transplant 22: e13219, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Raval FM, Mishra R, Garcea RL, Welsh RM, Szomolanyi-Tsuda E: Long-lasting T cell-independent IgG responses require MyD88-mediated pathways and are maintained by high levels of virus persistence. mBio 4: e00812–e00813, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanzavecchia A, Sallusto F: Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr Opin Immunol 19: 268–274, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Woodruff MC, Ramonell RP, Nguyen DC, Cashman KS, Saini AS, Haddad NS, et al. : Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol 21: 1506–1516, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crotty S: T follicular helper cell differentiation, function, and roles in disease. Immunity 41: 529–542, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gangappa S, Wrammert J, Wang D, Li ZN, Liepkalns JS, Cao W, et al. : Kinetics of antibody response to influenza vaccination in renal transplant recipients. Transpl Immunol 53: 51–60, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grupper A, Rabinowich L, Schwartz D, Schwartz IF, Ben-Yehoyada M, Shashar M, et al. : Reduced humoral response to mRNA SARS-Cov-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant 21: 2719–2726, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. : Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 325: 2204–2206, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marinaki S, Adamopoulos S, Degiannis D, Roussos S, Pavlopoulou ID, Hatzakis A, et al. : Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant 21: 2913–2915, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A: Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med 385: 661–662, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mariano G, Farthing RJ, Lale-Farjat SLM, Bergeron JRC: Structural characterization of SARS-CoV-2: Where we are, and where we need to be. Front Mol Biosci 7: 605236, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Planque S, Salas M, Mitsuda Y, Sienczyk M, Escobar MA, Mooney JP, et al. : Neutralization of genetically diverse HIV-1 strains by IgA antibodies to the gp120-CD4-binding site from long-term survivors of HIV infection. AIDS 24: 875–884, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claër L, et al. : IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med 13: eabd2223, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, et al. : Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26: 812–826, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Berkowska MA, Driessen GJ, Bikos V, Grosserichter-Wagener C, Stamatopoulos K, Cerutti A, et al. : Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood 118: 2150–2158, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.