Significance statement
Protection of solid organ transplant recipients against SARS-CoV-2 by vaccination remains an unmet need, given the low immunogenicity of available vaccines in the presence of immunosuppression. Administration of a third dose to 25 kidney transplant recipients (KTR) resulted in seroconversion in 36% of patients, associated with significant quantitative and functional changes within the spike-antigen–specific B cell and CD4+ T-helper cell compartment. Our data support the need for individual humoral monitoring of immunosuppressed individuals after vaccination and continued efforts to adapt vaccination protocols for this at-risk group.
Keywords: COVID-19, vaccine, B cells, T cells, T-lymphocytes, SARS-CoV-2, kidney transplantation
Abstract
Background
Accumulating evidence sugges ts solid organ transplant recipients, as opposed to the general population, show strongly impaired responsiveness toward standard SARS-CoV-2 mRNA-based vaccination, demanding alternative strategies for protectio n o f this vulnerable group.
Methods
In line with recent recommendations, a third dose of either heterologous ChAdOx1 (AstraZeneca) or homologous BNT162b2 (BioNTech) was administered to 25 kidney transplant recipients (KTR) without humoral response after two doses of BNT162b2, followed by analysis of serological responses and vaccine-specific B- and T-cell immunity.
Results
Nine out of 25 (36%) KTR under standard immunosuppressive treatment seroconverted until day 27 after the third vaccination, whereas one patient developed severe COVID-19 infection immediately after vaccination. Cellular analysis 7 days after the third dose showed significantly elevated frequencies of viral spike-protein receptor-binding domain-specific B cells in humor al responders as compared with nonresponders. Likewise, portions of spike-reactive CD4+ T helper cells were significantly elevated in patients who were seroconverting. Furthermore, overall frequencies of IL-2+, IL-4+, and polyfunctional CD4+ T cells significantly increased after the third dose, whereas memory/effector differentiation remained unaffected.
Conclusions
Our data suggest a fraction of transplant recipients benefit from triple vaccination, where seroconversion is associated with quantitative and qualitative changes of cellular immunity. At the same time, the study highlights that modified vaccination approaches for immunosuppressed patients remain an urgent medical need.
Podcast
This article contains a podcast athttps://www.asn-online.org/media/podcast/JASN/2021_11_23_briggsgriffin112321.mp3
Vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have proven high effectiveness among at-risk populations, such as the elderly,1 patients on maintenance hemodialysis,2 or individuals suffering from chronic inflammatory diseases under selected immunosuppressive regimens.3 In contrast to the latter, kidney transplant recipients (KTR) receiving immunosuppressive medication exhibit strongly reduced humoral and impaired B and T cell responses after mRNA-based vaccination.4–6 These observations are in line with reports of breakthrough infections with severe disease courses in fully vaccinated solid organ transplant recipients, underlining the urge of adapted vaccination protocols for this at-risk population.7,8 To address this clinical challenge, a third vaccination for solid organ transplant recipients has recently become standard of care in France and Israel.9 The first case series reported seroconversion in only 33% of patients who were anti–S1 IgG negative after a third vaccine dose, regardless of whether a vector- or an mRNA-based vaccine was used.8 These findings are in line with recent data from a larger cohort, where seroconversion increased from 40%–67% after three doses of mRNA vaccine.9 In this study, we extend these observations by reporting results in 25 KTR receiving a third vaccination dose of either heterologous ChAdOx1 (Vaxzevria) or homologous BNT162b2 (Comirnaty). In addition to monitoring vaccine-induced humoral responses, we provide comprehensive data on quantitative and qualitative changes within the spike-antigen–specific B and T cell compartments.
Materials and Methods
Study Protocol and Participants
After standard two-dose BNT162B2 (BioNTech/Pfizer) immunization (21 days apart), 25 KTX patients without anti–spike S1 IgG response received a third vaccination as a standard of care clinical measure under strict monitoring by nephrologists with heterologous ChAdOx1 (n=11, 90±7 days after fist vaccine) or homologous BNT162b2 (n=14, 127±1 days after first vaccine), depending on availability. All participants gave written informed consent for sample collection according to the approval of the ethics committees of the Charité-Universitätsmedizin Berlin (EA2/010/21, EA4/188/20), of the county of Saxony-Anhalt (EA7/21) and the University of Greifswald (BB019/21). Patient demographics are summarized in Table 1. Peripheral blood and serum samples were collected 7±2 days after the second and third vaccination for serological, B, and T cell analysis, and 19–27 days after third vaccination for serology only.
Table 1.
Characteristics of enroll ed patients who received a kidney tran splant (n=25)
| Variable | |
|---|---|
| Age, yrs (mean±SD) | 59.7 (13.8) |
| Females (%) | 11 (44.0) |
| White patients (%) | 25 (100) |
| Clinical parameters | |
| Time since Tx, yrs (mean±SD) | 10.4 (8.69) |
| Retransplantation (%) | 5 (20.0) |
| Acute graft rejection (%)a | 0 (0) |
| IS medication | |
| CS+Tac+MMF (%) | 13 (56.0) |
| CS+CyA+MMF (%) | 7 (28.0) |
| mTORi+MMF±CS (%) | 3 (12.0) |
| CyA+mTORi (%) | 1 (4.0) |
| CS+MMF (%) | 1 (4.0) |
| Comorbidities | |
| Hypertension (%) | 23 (92.0) |
| Coronary heart disease (%) | 6 (24.0) |
| History of myocardial infarction (%) | 2 (8.0) |
| Diabetes (%) | 5 (20.0) |
| History of liver disease (%) | 4 16.0) |
| History of malignancy (%) | 6 (24.0) |
Tx, transplant; IS, immunosupression; CS, corticosteroids; Tac, tacrolimus; MMF, mycophenolate mofetil; CyA, cyclosporin A; mTORi, mammalian target of rapamycin inhibitor.
Until 19–27 days after third vaccination.
Serological Assessment
SARS-CoV-2 S1 domain-specific IgG and IgA was determined by ELISA (Euroimmun). Previous or current SARS-CoV-2 infection was excluded for all, but one patient on the basis of medical history in combination with negativity in a SARS-CoV-2 nucleoprotein specific ELISA (Euroimmun). Samples were considered positive with OD ratios of ≥1.1 as per manufacturer’s guidelines. An OD ratio value was determined by calculating the ratio of the OD of the respective test sample over the OD of the internal calibrator provided with the ELISA kit. Virus neutralization capacity of sera was analyzed using a surrogate SARS-CoV-2 neutralization test (GenScript), with >30% being defined as a positive response as described previ ously.10,11
Characterization of Antigen-specific B and T Cells
All experiments have been performed as previous ly described.4,6,12 In brief, peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll-Paque PLUS (GE Healthcare Bio-Sciences, Chicago, IL, USA).
B cells were detected within PBMC by flow cytometry and gated as CD19+CD3-CD14- among single live lymphocytes (gating sho wn in Supplemental Figure 3). Antigen-specific B cells were identified by double staining with recombinant purified RBD (DAGC149, Creative Diagnostics, New York, USA) conjugated to AF647 or AF488, respectively.
For analysis of vaccine-specific T cells, 3–5 × 106 PBMC were stimulated for 16 h with overlapping 15-mers encompassing the complete SARS-CoV-2 spike protein (1 ug/ml per peptide; JPT, Berlin, Germany). Specific CD4+ T helper cells were identified on the basis of CD137 and CD154 coexpression as depicted in Supplemental Figure 4. For detection of surface molecules, antibodies against CD3 (SK7, Biolegend, Carlsbad, CA, USA), CD4 (SK3, Becton Dickinson, Franklin Lakes, NJ, USA), CD8 (SK1, Ebioscience, San Diego, CA, USA), CD45RO (UCHL1, BioLegend), CD62L (DREG-56, BioLegend), and PD1 (EH12.1, Becton Dickinson) were used. Unwanted cells were excluded via a “dump” channel containing CD14+ (M5E2, BioLegend), CD19+ (HIB19, BioLegend), and dead cells (fixable live/dead, BioLegend). After stimulation, cells were fixed in FACS Lysing Solution (Becton Dickinson), permeabilized in FACS Perm II Solution (Becton Dickinson) and intracellularly stained with anti-CD154 (24–31, BioLegend), anti-CD137 (4B4–1, BioLegend), anti–TNF-α (MAb11, BioLegend), anti–IFN-γ (4SB3, Ebioscience), anti–IL-2 (MQ1–17H12, BioLegend), anti-Ki67 (B56, Becton Dickinson), and anti–IL-4 (MP4–25D2, BioLegend). All flow cytometric analyses were performed using a BD FACS Fortessa ×20 (BD Biosciences, Franklin Lakes, NJ, USA).
FACS Data Analysis and Statistics
Flow cytometric data analysis was conducted with FlowJo 10 (Becton Dickinson). The gating strategies for analysis of antigen-specific B and T cells are illustrated in Supplemental Figures 3 and 4. Coexpression of cytokines was analyzed via Boolean gating. Statistical examination and composition of ELISA and FACS data derived graphs were performed using GraphPad Prism 8 (GraphPad, La Jolla, CA, USA). Parameter distribution was assessed using the Kolmogorov–Smirnov test. Depending on normal distribution or not, a t test or Mann–Whitney test was used for two-group comparisons; for multiple comparisons, a two-way ANOVA with Šidák’s post-test or Kruskal–Wallis test with Dunn’s post-test were chosen. For analysis of contingency tables, Fisher's exact test was applied.
Results
Vaccination-induced Humoral and B-cell Immunity
KTR who received a two-dose BioNTech/Pfizer BNT162B2 immunization 3 weeks apart and who did not show a humoral response (anti–S1 IgG) were revaccinated with a heterologous ChAdOx1 (n=11, 90±7 days after first vaccine) or homologous BNT162b2 (n=14, 127±1 days after first vaccine) protocol. In addition to medical history data, humoral, B, and T cell responses were evaluated 7±2 days after the second and third vaccination, and humoral responses were additionally investigated 19–27 days after each vaccination. Anti–S1 specific IgG data were available for all patients, whereas specific IgA and neutralization capacity were only examined in samples from 20 individuals. Patients were on a stable immunosuppressive therapy without changes between or after the vaccination.
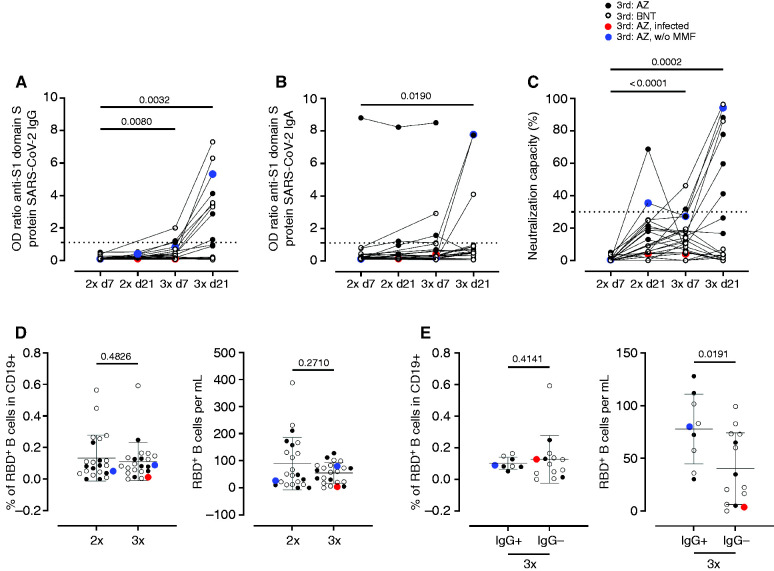
One patient developed severe coronavirus disease 2019 (COVID-19) (World Health Organization ordinal scale 6) 10 days after the third vaccination (indicated in red, throughout the figures). Three out of 25 (12%) KTX developed anti–S1 IgG 7±2 days after the third dose, whereas nine out of 25 (36%) seroconverted until 19–27 days, and were categorized as responders. Of these, three patients had received BNT162b2 and six ChAdOx1 as third vaccine; however, anti–S1 IgG was highly positive (OD ratio >5) only in three responders (two BNT162b2 and one ChAdOx1) (Figure 1A and Supplemental Figure 1A). One of these patients was the only one in our study without mycophenolate mofetil as an immunosuppressant (indicated in blue throughout the figures). Anti–S1 IgA (Figure 1B and Supplemental Figure 1A) only increased 19–27 days after the third vaccination, whereas neutralization capacity was increased 7±2 days and 19–27 days after the third vaccination, as compared with day 7 after the second vaccination, respectively (Figure 1C and Supplemental Figure 1A) in seven out of 20 patients. The patient with the isolated high anti–S1 IgA had high anti–S1 IgA already before third vaccination. The SARS-CoV-2 nucleoprotein–specific ELISA was negative at all samplings.
Figure 1.
Humoral immune responses and specific B cell immunity after third vaccination in KTR. Humoral vaccine-specific immune responses were assessed by ELISA for anti–spike protein S1 IgG (A), spike protein S1 IgA (B), and virus neutralization by a blocking ELISA (C) at the indicated timepoints in KTR after administration of a third dose of either ChAdOx1 (n=11, black filled dots) or BNT162b2 (n=14, back empty dots). Thresholds defining a positive response are indicated by dotted lines. Relative frequencies (left) and absolute counts (right) of RBD-specific CD19+ B cells in all patients (D) and in responders IgG+ and nonresponders IgG- 7±2 days after second or third vaccination with ChAdOx1 or BNT162b2. (A–C) Kruskal–Wallis with Dunn’s post-test. The infected individual is depicted in red. (D, E) Mann–Whitney test.
The relative percentage and absolute number of SARS-CoV-2 spike RBD–specific B cells within the CD19+ population did not change between the second and third vaccination (Figure 1D). The relative percentage of SARS-CoV-2 spike RBD–specific CD19 cells did not differ between responders and nonresponders (Figure 1E), whereas absolute numbers of RBD+ B cells were higher among responders compared with nonresponders 7±2 days after the third vaccination (Figure 1E). There were no differences between antigen-specific B cell responses between patients receiving a homologous versus a heterologous boost (Supplemental Figure 1D).
Quantitative and Qualitative Assessment of Vaccination-specific CD4+ T Cell Responses
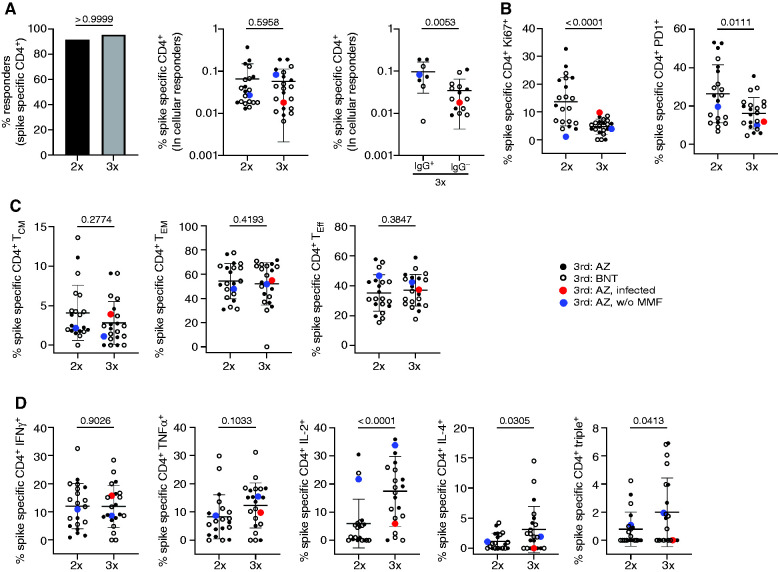
Vaccine-specific CD4+ T helper cells were identified within PBMC after stimulation with an overlapping peptide mix encompassing the complete spike glycoprotein on the basis of coexpression of CD137 and CD154, as demonstrated earlier,6,12 and depicted in Supplemental Figure 4. A T cell response was defined as positive when peptide pool stimulated PBMC contained more than two-fold higher portions of CD137+CD154+ T cells as compared with the unstimulated control with ≥20 events. The overall prevalence of vaccinees displaying spike-specific CD4+ T cell responses was high (>90%) after the second and third inoculation, with no significant changes of antigen-reactive T cell frequencies in cellular responders (Figure 2A). However, individuals who seroconverted after the third vaccination (IgG+) were characterized by significantly higher portions of antigen-reactive T cells than humoral nonresponders (Figure 2A). Analysis of activation-associated markers revealed a significant drop of proliferating Ki67+ and activated PD1+ T cells after the third dose (Figure 2B), whereas no changes in specific memory/effector subset composition were noted between the two timepoints (Figure 2C). Importantly, KTR showed significantly higher frequencies of IL-2 and IL-4 secreting and polyfunctional IFN-γ+TNF-α+IL-2+ (triple+) T cells after the third dose; this observation did not account for IFN-γ or TNF-α alone (Figure 2D). We did not detect significant differences in frequencies of specific T cells, their activation marker expression, memory phenotype, or functional profile in patients who were AstraZeneca versus Biontech/Pfizer boosted (Supplemental Figure 2, A–D). Furthermore, no significant differences in cytokine production by specific T cells were observed in humoral responders versus nonresponders (Supplemental Figure 2E).
Figure 2.
Assessment of T cell reactivity. PBMC of KTR (n=24) were stimulated or not with spike peptide mix. Specific CD4+ T cells were identified and quantified 7±2 days after the second and third vaccination by FACS according to CD137 and CD154 coexpression. Depicted are (A) the percentage of individuals with a cellular response (left, Fisher’s exact test), frequencies of specific CD4+ T cells (middle, paired Wilcoxon test), and frequencies within humoral responders (anti–S1 IgG+) and nonresponders (anti–S1 IgG-) (right, unpaired t test). (B) Frequencies of antigen-specific CD4+ T cells expressing Ki67 (left, paired t test) or PD1 (right, paired t test). (C) Memory/effector subset differentiation of spike-reactive CD4+ T cells (TCM, central memory, left, paired Wilcoxon test; TEM, effector memory, middle, paired t test; Teff, effector right, paired t test, T cells). (D) Expression of IFN-γ (paired t test), TNF-α (paired t test), IL-2 (paired Wilcoxon test), and IL-4 (paired Wilcoxon test in antigen-specific T cells including analysis of IFN-γ+TNF-α+IL-2+ “triple+” polyfunctional (paired Wilcoxon test) cells. Where applicable, graphs show mean±SD.
Discussion
Investigating the humoral, B, and T cell response after a third vaccination with either ChAdOx1 or BNT162b2 in previously nonresponding KTR, we observed an increase in anti–S1 IgG in nine out of 25 (36%) patients, four out of 14 (28%) after homologous, and five out of 11 (45%) after heterologous vaccination. This observation is in accordance with previously published data of third vaccinations in a heterogeneous group of solid organ transplant recipients.8,9 However, these studies did not stratify patient responses according to the type of transplant, or quantify vaccine specific cellular T and B cell immunity. There is increasing evidence that neutralizing capacity is a reliable correlate of protection13–15 and that anti-S1 IgG correlates with neutralizing capacity.10,16 Of the nine responders in our cohort, only three developed high titer anti–S1 IgG (OD IgG >5) whereas one was just above the threshold for positivity. Consistent with lack of protection, one humoral nonresponder developed severe COVID-19 after the third vaccination, underlining the clinical importance of adequate antibody titers in KTR, especially with the emergence of viral variants. It has also been reported that antibody titers decline 3 months after vaccination with an mRNA vaccine, so initial high titers might be necessary for long-term protection.17 The absolute number of antigen-specific B cells significantly increased in responders as compared with nonresponders after the third dose, although it remained largely unchanged compared with the second vaccination. This lack of substantial antigen-specific B and plasmablast induction, as opposed to what has been demonstrated for healthy individuals,4 supports the weak additional mobilizing effect of a third vaccination in the majority of KTR and likely explains its overall limited effectiveness.
The exact contribution of vaccine-induced T cell immunity for protection against SARS-CoV-2 infection is still debated, particularly with respect to direct antiviral effects versus a role in B cell activation. We recently reported that vaccine-specific CD4+ T cells in KTR show broad quantitative and functional limitations.6 In line with data on specific B cells presented herein, spike-reactive T cell frequencies were largely unaffected by a third vaccination. In accordance with the fact that an additional boost does not substantially expand the specific T cell pool in KTR, signs of ex vivo activation, as mirrored by high Ki67 or PD1 expression,6 were significantly reduced after the third dose as compared with the second dose. For reasons that need to be explored further, humoral responders were characterized by a significant increase of spike-specific T cells, a finding supported by recent work of Sahin et al. demonstrating a strong correlation between specific T cell frequencies and antibody titers post vaccination.18 As opposed to their quantities, vaccine-reactive T cells underwent a functional maturation after the third dose with higher portions of IL-2+, IL-4+, and multifunctional cells. Whereas increased IL-4 secretion could lower the threshold for isotype switching to IgG,19 patients might potentially benefit from augmented polyfunctionality being associated with potent SARS-CoV-2 clearance.20
Antigen-specific T and B cells cannot be considered a routine diagnostic measure because it is costly and time consuming. Also neutralization titers are not broadly established in routine diagnostic laboratories, therefore the determination of anti–S1 IgG, which excellently correlates with neutralization capacity10 should be used to guide clinical decision for further booster doses in immunocompromised patients. In patients with a negative or low positive anti–S1 IgG after three doses, a fourth dose should be considered. In any case, the patients should be informed they likely are not protected against severe COVID-19. In our cohort, all but one patient received antimetabolite medication. This is in clear contrast to other previously published cohorts of solid organ recipients, for example, the cohort of Boyarsky et al.21 where only 72% of patients received an antimetabolite and where more patients showed a serological response after two vaccine doses.21 In the study of Kamar et al.9 describing a seroresponse in 68% of solid organ recipients after three doses, 66% of patients were on immunosuppressive treatment with an antimetabolite.9 Also other studies such as the study of Hall et al.22 and of Werbel et al.8 describe a significant and comparable increase in seroresponse after three vaccine doses. The differences in the study populations and their immunosuppressive regimen might explain the different response rates. Our single patient without mycophenolate mofetil showed high IgG titers and high neutralization capacity and a high IL-2 response among antigen-specific T cells, being key for cell proliferation and functional differentiation. This observation supports one potential strategy to improve responder rates by reducing immunosuppression and especially antimetabolite treatment in patients with stable allograft function without previous episodes of rejection or pre-existing HLA immunization before vaccination. Indeed, data from a large multicenter study suggested that withdrawal of mycophenolate mofetil in KTR formerly receiving tacrolimus-based triple immunosuppression does not impair graft or patient survival,23 thereby supporting short-term drug weaning as an option, especially in patients that are on corticosteroids, as in our cohort.
In conclusion, a third vaccination against SARS-CoV-2 leads to a serological response in a fraction of KTR, whereas the majority of patients still lack protective antibody titers. This lack of serological response can be explained by only marginal improvements in cellular responses among antigen-specific B cells and T cells after a third vaccination. Alternative vaccination protocols are urgently needed to protect this at-risk group.
Disclosures
F. Halleck reports receiving honoraria from MSD and Novartis. K. Budde reports having consultancy agreements with AbbVie, Alexion, Astellas, Bristol-Myers Squibb, Chiesi, CSL-Behring, Fresenius, Hansa, Hexal, Novartis, MSD, Otsuka, Pfizer, Quark, Roche, Sandoz, Shire, Veloxis, Vifor, and Vitaeris; reports receiving research funding from AbbVie, Alexion, Astellas, Bristol-Myers Squibb, Chiesi, CSL-Behring, Fresenius, Hansa, Hexal, Novartis, MSD, Otsuka, Pfizer, Quark, Roche, Sandoz, Shire, Veloxis, Vifor, and Vitaeris; reports receiving honoraria from AbbVie, Alexion, Astellas, Bristol-Myers Squibb, Chiesi, Fresenius, Hexal, Novartis, Otsuka, Pfizer, Quark, Roche, Sandoz, Shire, Veloxis Pharma; and reports being a scientific advisor or member of Astellas, Bristol-Myers Squibb, Chiesi, Hansa, Hexal, MSD, Novartis, Pfizer, Roche, and Veloxis. K. Eckardt reports consultancy agreements with Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, Genzyme, Otsuka, Travere, and Vifor; reports receiving research funding from Amgen, AstraZeneca, Bayer, Fresenius, Genzyme, Shire, and Vifor; reports receiving honoraria from Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, Genzyme, Otsuka, Travere, and Vifor; and reports being a scientific advisor or member of the Editorial Boards of British Medical Journal and Kidney International. K. Kotsch repo rts receiving research funding from Chiesi. T. Dörner reports having consultancy agreements with AbbV ie, Bayer Healthcare, Biogen, BMS/Celgene, Boston Pharmaceuticals, Eli Lilly, Janssen, Novartis, and Roche/Genentech; reports receiving research funding from Federal Ministry of Education and Research, Deutsche Forschungsgemeinschaft, Leibnitz Society, Senate of Berlin, and Roche; reports receiving honoraria from AbbVie, Bayer Healthcare, Biogen, BMS/Celgene, Boston Pharmaceuticals, Eli Lilly, Janssen, Novartis, and Roche/Genentech; and reports receiving Speakers Bureau from Eli Lilly, Janssen, Roche, and Samsung/Bioepis. All remaining authors have nothing to disclose.
Funding
This work was supported by the Federal Ministry of Education and Research grant (BCOVIT, 01KI20161) (to E. Schrezenmeier), a German Society of Rheumatology scholarship (to A.-L. Stefanski), the Sonnenfeldstiftung Berlin, Germany (to K. Kotsch), the Deutsche Forschungsgemeinschaft (Do491/7-5, 10-2, 11-1, Transregio 130 TP24) (to T. Dörner), KO-2270/7-1, KO-2270/4-1 (to K. Kotsch), Chiesi GmbH project funding (to K. Kotsch), COLCIENCIAS scholarship 727, 2015 (to H. Rincon-Arevalo), the Ministry for Science, Research and Arts of Baden-Württemberg, Germany, the European Commission (HORIZON2020 Project SUPPORT-E, 101015756) (to H. Schrezenmeier), and the Charité–Universitätsmedizin Berlin and the Berlin Institute of Health funding for the Charité Clinician Scientist Program (to E. Schrezenmeier).
Supplementary Material
Acknowledgments
F. Hallek, A. Sattler, and E. Schrezenmeier designed the study and wrote the manuscript; H. Rincon-Arevelo, A. Sattler, E. Schrezenmeier, and A.-L. Stefanski performed the experiments (B and T cell analysis); F. Bachmann, K. Budde, M. Choi, C. Hammett, A. Potekhin, E. Schrezenmeier, and H. Staub-Hohenbleicher recruited patients; B. Jahrsdörfer and H. Schrezenmeier were responsible for serological studies; K. Budde, T. Dörner, K.-U. Eckardt, F. Hallek, K. Kotsch, and E. Schrezenmeier supervised the work and provided funding; all authors read and approved the manuscript. The authors areg rateful to Dr. Michael Moesenthin, Dr. Peter Bartsch (both Dialysezentrum Burg), Dr. Ralf Kühn, and Dr. Dennis Heutling (both Dialyse Tangermünde) for patient recruitment, and Dr. Petra Glander and Pia Hambach for biobanking of samples.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “COVID-19 Vaccination in Kidney Transplant Recipients: An Ounce Pre-Transplant is Worth a Pound Post-Transplant,” on pages 2977–2978.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021070966/-/DCSupplemental.
Supplemental Figure 1. Humoral immune responses and specific B cell immunity after third vaccination in KTR according to vaccine type.
Supplemental Figure 2. SARS-CoV-2 vaccine specific T helper cell responses in patients who received a KTX stratified according to heterologous/homologous third vaccination or specific IgC serostatus (humoral responder/nonresponder).
Supplemental Figure 3. Detection of SARS-CoV-2 vaccine specific B cells.
Supplemental Figure 4. Detection of SARS-CoV-2 vaccine specific T helper cells.
References
- 1.Schwarz T, Tober-Lau P, Hillus D, Helbig ET, Lippert LJ, Thibeault C, et al. : Delayed antibody and T-cell response to BNT162b2 vaccination in the elderly, Germany. Emerg Infect Dis 27: 2174–2178, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danthu C, Hantz S, Dahlem A, Duval M, Ba B, Guibbert M, et al. : Humoral response after SARS-CoV-2 mRNA vaccination in a cohort of hemodialysis patients and kidney transplant recipients. J Am Soc Nephrol 32: 2153–2158, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geisen UM, Berner DK, Tran F, Sümbül M, Vullriede L, Ciripoi M, et al. : Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 80: 1306–1311, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rincon-Arevalo H, Choi M, Stefanski A-L, Halleck F, Weber U, Szelinski F, et al. : Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol 6: eabj1031, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Boyarsky BJ, Ou MT, Greenberg RS, Teles AT, Werbel WA, Avery RK, et al. : Safety of the first dose of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation 105: e56–e57, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sattler A, Schrezenmeier E, Weber UA, Potekhin A, Bachmann F, Straub-Hohenbleicher H, et al. : Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest 131: 150175, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wadei HM, Gonwa TA, Leoni JC, Shah SZ, Aslam N, Speicher LL: COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant 21: 3496–3499, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werbel WA, Boyarsky BJ, Ou MT, Massie AB, Tobian AAR, Garonzik-Wang JM, et al. : Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: A case series. Ann Intern Med 174: 1330–1332, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A: Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med 385: 661–662, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jahrsdörfer B, Kroschel J, Ludwig C, Corman VM, Schwarz T, Körper S, et al. : Independent side-by-side validation and comparison of 4 serological platforms for SARS-CoV-2 antibody testing. J Infect Dis 223: 796–801, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jahrsdörfer B, Groß R, Seidel A, Wettstein L, Ludwig C, Schwarz T, et al. : Characterization of the SARS-CoV-2 neutralization potential of COVID-19-convalescent donors. J Immunol 206: 2614–2622, 2021 [DOI] [PubMed] [Google Scholar]
- 12.Sattler A, Angermair S, Stockmann H, Heim KM, Khadzhynov D, Treskatsch S, et al. : SARS-CoV-2-specific T cell responses and correlations with COVID-19 patient predisposition. J Clin Invest 130: 6477–6489, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krammer F: A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat Med 27: 1147–1148, 2021 [DOI] [PubMed] [Google Scholar]
- 14.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. : Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 27: 1205–1211, 2021 [DOI] [PubMed] [Google Scholar]
- 15.Earle KA, Ambrosino DM, Fiore-Gartland A, Goldblatt D, Gilbert PB, Siber GR, et al. : Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 39: 4423–4428, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan CW, Chia WN, Qin X, Liu P, Chen MI, Tiu C, et al. : A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol 38: 1073–1078, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Favresse J, Bayart JL, Mullier F, Elsen M, Eucher C, Van Eeckhoudt S, et al. : Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg Microbes Infect 10: 1495–1498, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. : BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 595: 572–577, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Isakson PC, Puré E, Vitetta ES, Krammer PH: T cell-derived B cell differentiation factor(s): Effect on the isotype switch of murine B cells. J Exp Med 155: 734–748, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekine T, Perez-Potti A, Rivera-Ballesteros O, Strålin K, Gorin JB, Olsson A, et al. ; Karolinska COVID-19 Study Group : Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 183: 158–168.e14, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. : Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 325: 2204–2206, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall VG, Ferreira VH, Ku T, Ierullo M, Majchrzak-Kita B, Chaparro C, et al. : Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med 385: 1244–1246, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pascual J, van Hooff JP, Salmela K, Lang P, Rigotti P, Budde K: Three-year observational follow-up of a multicenter, randomized trial on tacrolimus-based therapy with withdrawal of steroids or mycophenolate mofetil after renal transplant. Transplantation 82: 55–61, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.