Significance Statement
A Task Force from the National Kidney Foundation and American Society of Nephrology developed recommendations for reassessing inclusion of race in the estimation of GFR in the United States. The Task Force recommends immediate implementation of the Chronic Kidney Disease Epidemiology Collaboration creatinine equation refit without the race variable in all laboratories because the calculation does not include race, it included diversity in its development, its potential adverse consequences do not disproportionately affect any one group, and it is immediately available to all laboratories. A second recommendation calls for national efforts to facilitate increased, routine, and timely use of cystatin C, especially to confirm eGFR in adults for clinical decision making. A third recommendation encourages research on GFR estimation with new endogenous filtration markers and interventions to eliminate racial and ethnic disparities.
Keywords: eGFR, health disparities, creatinine, ethnicity, chronic kidney disease, clinical nephrology, glomerular filtration rate, drug excretion, epidemiology and outcomes
Visual Abstract
Abstract
Background
In response to a national call for re-evaluation of the use of race in clinical algorithms, the National Kidney Foundation (NKF) and the American Society of Nephrology (ASN) established a Task Force to reassess inclusion of race in the estimation of GFR in the United States and its implications for diagnosis and management of patients with, or at risk for, kidney diseases.
Process & Deliberations
The Task Force organized its activities over 10 months in phases to (1) clarify the problem and evidence regarding eGFR equations in the United States (described previously in an interim report), and, in this final report, (2) evaluate approaches to address use of race in GFR estimation, and (3) provide recommendations. We identified 26 approaches for the estimation of GFR that did or did not consider race and narrowed our focus, by consensus, to five of those approaches. We holistically evaluated each approach considering six attributes: assay availability and standardization; implementation; population diversity in equation development; performance compared with measured GFR; consequences to clinical care, population tracking, and research; and patient centeredness. To arrive at a unifying approach to estimate GFR, we integrated information and evidence from many sources in assessing strengths and weaknesses in attributes for each approach, recognizing the number of Black and non-Black adults affected.
Recommendations
(1) For US adults (>85% of whom have normal kidney function), we recommend immediate implementation of the CKD-EPI creatinine equation refit without the race variable in all laboratories in the United States because it does not include race in the calculation and reporting, included diversity in its development, is immediately available to all laboratories in the United States, and has acceptable performance characteristics and potential consequences that do not disproportionately affect any one group of individuals. (2) We recommend national efforts to facilitate increased, routine, and timely use of cystatin C, especially to confirm eGFR in adults who are at risk for or have CKD, because combining filtration markers (creatinine and cystatin C) is more accurate and would support better clinical decisions than either marker alone. If ongoing evidence supports acceptable performance, the CKD-EPI eGFR–cystatin C (eGFRcys) and eGFR creatinine–cystatin C (eGFRcr-cys_R) refit without the race variables should be adopted to provide another first-line test, in addition to confirmatory testing. (3) Research on GFR estimation with new endogenous filtration markers and on interventions to eliminate race and ethnic disparities should be encouraged and funded. An investment in science is needed for newer approaches that generate accurate, unbiased, and precise GFR measurement and estimation without the inclusion of race, and that promote health equity and do not generate disparate care.
Implementation
This unified approach, without specification of race, should be adopted across the United States. High-priority and multistakeholder efforts should implement this solution.
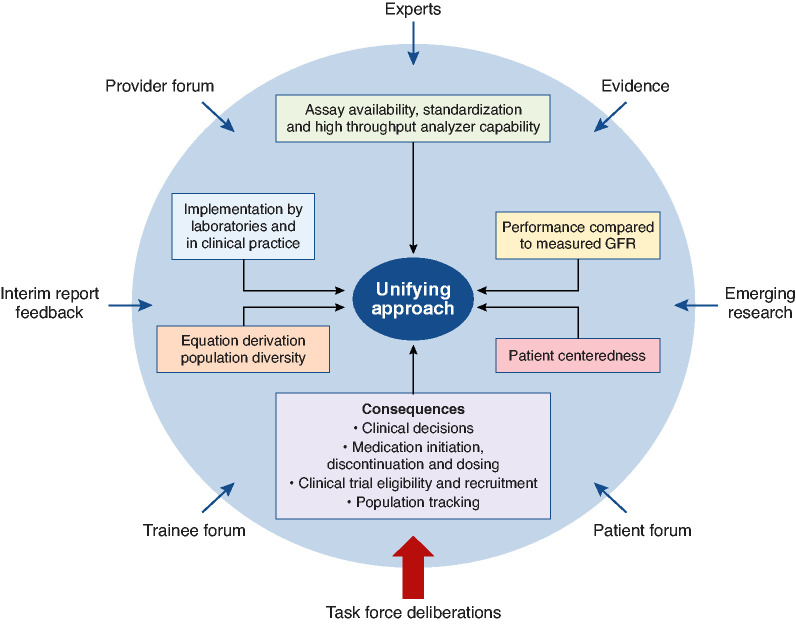
On July 2, 2020 the National Kidney Foundation (NKF) and the American Society of Nephrology (ASN) announced they would establish a Task Force to reassess the inclusion of race in the estimation of GFR in the United States and its implications for diagnosis and management of patients with, or at risk for, kidney diseases. The rationale for the Task Force includes the following: race is a social and not a biologic construct, the problematic nature of applying race to clinical algorithms, and the need to advance health equity and social justice. These factors have been described.1 The Task Force organized its activities in three phases: (1) clarify the problem and evidence regarding eGFR equations in the United States, (2) evaluate approaches to address the use of race in GFR estimation, and (3) provide recommendations.1 The Task Force published an interim report that detailed phase 1, described the nearly 50-year evolution of estimating equations, and included scientific evidence and values held by members.1 In this final report, we outline a path forward in GFR estimation that integrates filtration marker/assay availability and standardization, implementation challenges, equation derivation population diversity, equation performance, avoidance of foreseeable adverse clinical consequences, and patient centeredness. In alignment with the NKF and ASN, the Task Force unanimously agreed that race should be removed from estimating equation calculation and reporting. Thus, the Task Force used a comprehensive process of evidence ascertainment to identify the most patient-centered solution that did not include race (Figure 1).
Figure 1.
Process and input to create a unifying approach to GFR estimation was comprehensive. Sources (blue arrows) used to identify and evaluate attributes (boxes) of 26 approaches. Each source provided information about multiple topics: equity and disparities; race and racism; GFR measurement, estimation, and equation performance; laboratory standardization; consequences; patient perspectives; and new science. Information was integrated by the Task Force in its considerations of the attributes during deliberations to arrive at a unifying approach.
Information and Evidence Sources (Phase 1 and 2)
The Task Force sought to have an inclusive, comprehensive, and in-depth process that, after establishment of the Task Force, took 10 months (September 2020 to June 2021). This final report describes the multiple sources (expert testimony; new social and scientific evidence; oral testimonies from patients, providers, and trainees; and community feedback on the interim report) used to gather information, describes the information assembled on 26 approaches and evaluates their attributes, and explains how we integrated this information into a unifying solution.
Scientific Evidence
The Task Force conducted >40 sessions to assemble and review the data and evidence, including extensive expert testimony. In the 16 sessions that heard testimony, covering a broad range of related topics, 97 experts presented a diversity of views, representing 21 US states and seven other countries.1
The Task Force constructed statements of evidence and value, established criteria to evaluate approaches (in the interim report), and identified 26 potential approaches for GFR estimation that were available or in development, with an option to combine approaches according to clinical indication (Table 1, Supplemental Table 2). The Task Force gathered additional community input in recognition of differing views, and information regarding nascent science.
Table 1.
Inventory of possible approaches to estimating and reporting GFR, attributes, and challenges
| Approach | Acronym | Race Includeda | Challenges by Attributeb,c | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| Creatinine used as biomarker | ||||||||
| 1. CKD-EPI eGFRcr (age, sex, race)6 | CKD-EPIcr | ✓ ©® | * | * | * | * | ** | ** |
| 2. MDRD Study (age, sex, race)7 | MDRDcr | ✓ ©® | * | * | ** | * | ** | ** |
| 3. eGFRcr (CKD-EPI) (age, sex, race) with “Black” estimate reported as “high muscle mass” and non-Black estimate reported as “low muscle mass” | CKD-EPIcr_MM | ✓ © | * | ** | * | *** | *** | *** |
| 4. eGFRcr (CKD-EPI) (age, sex, race) with “Black” estimate reported as “high value” and non-Black reported as “low value” | CKD-EPIcr_H/L | ✓ © | * | ** | * | ** | *** | ** |
| 5. eGFRcr (CKD-EPI) (age, sex, race) with the Black coefficient removed and eGFR value for non-Black is reported for all | CKD-EPIcr_NB | ✓ © | * | * | * | ** | **/*** | ** |
| 6. eGFRcr (CKD-EPI) (age, sex, race), with the Black coefficient used and eGFR value for Black individuals is reported for all | CKD-EPIcr_B | ✓ © | * | * | * | ** | **/*** | ** |
| 7. Blended eGFRcr (CKD-EPI) (age, sex, race) using single coefficient weighted for percentage Black individuals in specific population reported for all | CKD-EPIcr_blend | ✓ © | * | *** | * | *** | *** | *** |
| 8. CG estimated creatinine clearance (age, sex, weight)8 | CG_Clcr | * | *** | *** | *** | *** | *** | |
| 9. eGFRcr (FAS) (age, sex, population-specific Scr/Q)43 | FAScr | * | *** | *** | *** | *** | *** | |
| 10. eGFRcr (EKFC) (age, sex, population-specific Scr/Q)27 | EKFCcr | * | *** | *** | *** | *** | *** | |
| 11. eGFR (LM) (age, sex)44 | LMcr | * | ** | *** | *** | *** | *** | |
| 12. eGFRcr (CKD-EPI) refit without race variable38 | CKD-EPIcr_R | * | * | * | * | * | * | |
| 13. eGFRcr (CKD-EPI) refit with height and weight without race variable45 | CKD-EPI_R_HW | * | *** | * | *** | *** | *** | |
| Creatinine in combination with cystatin C or other markers | ||||||||
| 14. eGFRcr-cys (CKD-EPI) with race coefficient (age, sex, race)46 | CKD-EPIcr-cys | ✓ ©® | ** | * | * | * | * | * |
| 15. eGFRcr-cys (CKD-EPI) (age, sex, race) with “Black” estimate reported as “high muscle mass” and non-Black estimate reported as “low muscle mass” | CKD-EPIcr-cys_MM | ✓ © | ** | ** | * | *** | *** | *** |
| 16. eGFRcr-cys (CKD-EPI) (age, sex, race) with “Black” estimate reported as “high value” and non-Black estimate reported as “low value” | CKD-EPIcr-cys_H/L | ✓ © | ** | ** | * | *** | * | * |
| 17. eGFRcr-cys (CKD-EPI) (age, sex, race) with the Black coefficient removed and value for non-Black estimate reported for all | CKD-EPIcr-cys_NB | ✓ © | ** | * | * | * | ** | ** |
| 18. eGFRcr-cys (CKD-EPI) (age, sex, race), with Black coefficient used and value for Black reported for all | CKD-EPIcr-cys_B | ✓ © | ** | * | * | ** | ** | ** |
| 19. Blended eGFRcr-cys (CKD-EPI) (age, sex, race), using a single coefficient weighted for percentage of Black patients in the specific population, is reported for all | CKD-EPIcr-cys_blend | ✓ © | ** | *** | * | *** | *** | *** |
| 20. eGFRcr-cys (CKD-EPI) refit without race variable38 | CKD-EPIcr-cys_R | ** | * | * | * | * | * | |
| 21. eGFRcr-cys (FAS) (age, sex, population-specific Q)47 | FAScr-cys | ** | *** | *** | *** | *** | *** | |
| 22. eGFRcr-cys-β2m-βtp (age, sex)48 | CKD-EPI_4M | *** | *** | * | ** | ** | ** | |
| Cystatin C or other filtration Markers | ||||||||
| 23. eGFRcys (CKD-EPI) (age, sex)49 | CKD-EPIcys | ** | ** | * | ** | * | * | |
| 24. eGFRcys (FAS) (age, sex, population-specific Q)47 | FAScys | ** | *** | *** | *** | *** | *** | |
| 25. eGFRcys (CAPA) (age, sex)50 | CAPAcys | ** | ** | *** | *** | *** | *** | |
| 26. eGFRcys-β2m-βtp (age, sex)48 | CKD-EPI_3M | *** | *** | * | ** | ** | ** | |
eGFRcr, eGFR computed using creatinine; CG, Cockcroft and Gault; FAS, full age spectrum; Scr, serum creatinine; Q, Q factor coefficient; EKFC, European Kidney Function Consortium; LM, Lund–Malmö; eGFRcr-cys, eGFR computed using creatinine and cystatin C; β2m, β2-microglobulin assay; βtp, β-trace protein; eGFRcys, eGFR computed with cystatin C; CAPA, Caucasian, Asian, pediatric, and adult.
✓ Race included in calculation © or reporting ® of equation.
For details of origin of attributes, see Delgado et al. 1 For details on basis for categorization, please see Supplemental Appendices 1–3.
Attributes are labeled as follows: 1, filtration marker assay; 2, implementation challenges; 3, equation population diversity; 4, equation performance; 5, consequences; 6, patient centeredness. For the difficulties assessment, * represents minimal challenges, ** represents some challenges, and *** represents many challenges; **/*** represents no consensus among Task Force members between some and many challenges.
Community Input
The Task Force sought input from the community at large regarding the effect of particular approaches on clinical outcomes and health equity, through written and oral testimony, in separate web-based forums for (1) students and trainees; (2) clinicians, scientists, and other allied health professionals; and (3) patients, family members, and other public stakeholders (450 individuals from 18 states and three countries). The testimony addressed the limitations of GFR-estimating equations and the importance of considering the potential role of including race in eGFR equations in perpetuating or preventing health care disparities. Trainees, patients, and health care professionals were unified in their desire to promote equity in health and health care delivery.
New Science Input
The Task Force called for research from the scientific community on innovative solutions in estimating equations and in assessing kidney function. Sixteen investigators, nationally and globally, provided new information on kidney function measurement, accuracy of estimating equations, and biomarkers without a race variable (Supplemental Table 1). National Institutes of Health (NIH) program leaders also provided commentary.
Process for Evaluation of Approaches (Phase 2)
A solution based upon the possible identified approaches would ideally promote racial equity and have the previously noted desirable attributes (Figure 1). Task Force members divided into attribute-defined subgroups, on the basis of their expertise, to examine in detail how each of the 26 eGFR approaches aligned with each desired attribute (Table 1). Each attribute subgroup presented its findings over several weeks to the entire Task Force for review and discussion.
After review and discussion of each attribute independently, it was clear to the Task Force that not all approaches were viable in the short or intermediate time frame, and that the consequences for decision making and racial disparities in general medical care, medication use, nephrology care, and clinical trial eligibility and recruitment for some approaches were complex. The Task Force recognized that attributes can be inter-related and influence each other. As such, the Task Force eliminated, by consensus, some of the 26 approaches from further consideration. The decisions were not made on the basis of any single identified limitation but considered multiple independently identified difficulties, including the use of race in equation calculations or in reporting (Table 1). For example, equations that include novel filtration markers would be less likely to have standardized assays across laboratories and less likely to be available on multiplex analyzers in all laboratories, leading to challenges in implementation by most clinical laboratories. This, in turn, would diminish uptake of such an approach, including for population tracking. The Task Force selected five approaches after extensive discussion of inter-relationships and paying attention to projected time frames to overcome any perceived limitation. However, the Task Force did review information on all 26 approaches for completeness. Leaders in the NKF and ASN with expertise in health equity, health care justice, quality of patient care, research, policy, and advocacy reviewed the final recommendations of the Task Force.
Attribute Considerations
Attribute information on the five selected approaches are shown in Tables 1–6 and Figures 2–3, and for all approaches in Supplemental Tables 3–7.
Table 6.
Estimated consequences of approaches (attribute 5): Population surveillance
| Population Tracking/Monitoring | What Potential Barriers Would Delay the Process for Operationalizing the Approach? | Estimated Time to Systematically Address the Change across Data Sources? (months or years) |
|---|---|---|
| Creatinine-based GFR equations | ||
| USRDS | • Minimal effect because serum creatinine is reported in CMS Form 2728 | NA |
| IHS | • Minimal effect because serum creatinine and eGFR without race correction are reported in the IHS Audit | NA |
| ICD/CPT codes data (e.g., from claims [Medicare, Optum], or EHRs [CURE-CKD, Kaiser]) | • Lag in systematic revision and implementation of laboratory protocols/standards of care using the recommended eGFR approach • Lag in incorporating to the EHR/patient record • Potential inconsistency across the country in staging patients and reporting as the recommended eGFR equation is operationalized |
Months to years |
| Survey data (NHANES) | • Minimal effect because serum creatinine is reported | NA |
| Creatinine/cystatin-based GFR equations | ||
| USRDS | • Lag in revising CMS 2728 Form • Lag in systematic implementation across multiple sources reporting to Medicare (revising laboratory protocols/standards of care using the recommended eGFR equation, procuring reagents, incorporating to the EHR/patient record) • Potential inconsistency in reporting from multiple data sources as the recommended GFR equation is operationalized |
Months to years (depending on production and costs to estimate cystatin C) |
| IHS | • Lag in revising the IHS Audit form • Lag in systematic implementation across IHS |
Months to years (depending on cystatin C production and costs) |
| ICD/CPT codes data (e.g., from claims [Medicare, Optum], or EHRs [CURE-CKD, Kaiser]) | • Lag in systematic implementation across multiple data sources (revising laboratory protocols/standards of care using the recommended GFR approach, procuring reagents, incorporating to the EHR/patient record) • Potential inconsistency in reporting from multiple data sources as the recommended GFR approach is operationalized |
Months to years (depending on cystatin C production and costs) |
| Survey data (NHANES) | • Lag in systematic implementation (revising laboratory [e.g., NHANES Nephrology Component] protocols, procuring reagents) • Lag in systematic programming for reporting cystatin C and in preparing survey analytic files |
Years (depending on production and costs to estimate cystatin C) |
USRDS, United States Renal Data System; CMS, Centers for Medicare and Medicaid Services; NA, not applicable; IHS, Indian Health Service; HER, electronic health record; CURE-CKD, Center for Kidney Disease Research, Education and Hope; NHANES, National Health and Nutrition Examination Survey.
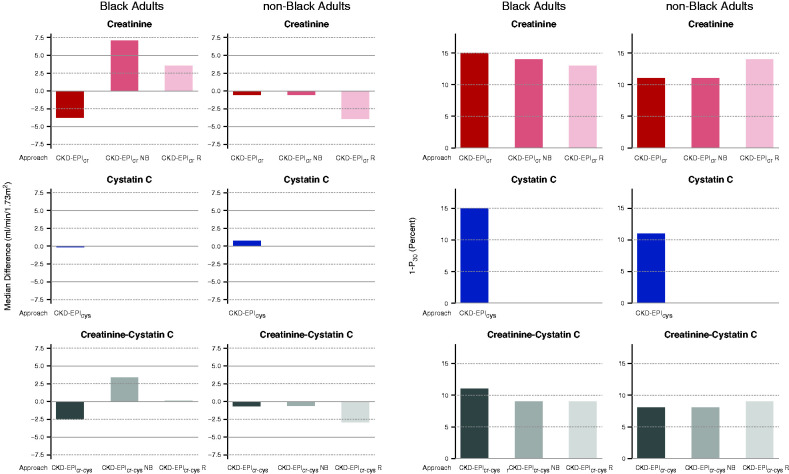
Figure 2.
Performance of approaches CKD-EPIcr, CKD-EPIcr_NB, CKD-EPIcr_R, CKD-EPIcr-cys, CKD-EPIcr-cys_NB, CKD-EPIcr-cys_R, and CKD-EPIcys compared was examined with mGFR for Black and non-Black adults. (Left six panels) Bias as shown as median difference between mGFR and eGFR. Units are milliliters per minute per 1.73 m2. A positive number indicates underestimate of mGFR and a negative number indicates overestimate of mGFR. Solid gray line is the line of identity. Dashed gray lines are drawn at the median difference of 5 and −5 ml/min per 1.73 m2, which is defined as a small bias (shown in Table 2). (Right six panels) Accuracy as shown as percentage of estimates >30% of mGFR (1−P30). Dashed gray lines are drawn at 1−P30 of 10%, which is the definition of small inaccuracy (greatest accuracy), as shown in Table 2. For all panels, the left column shows results for Black adults and the right column shows results as modified from Inker et al.38
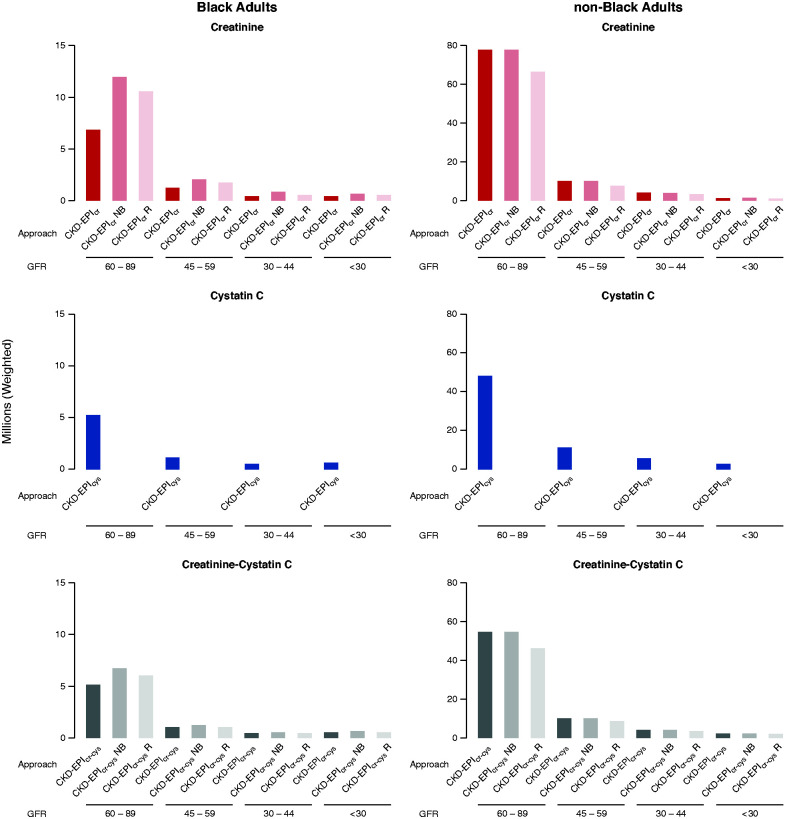
Figure 3.
Estimated number of US Black and non-Black adults is larger at higher eGFR categories according to approaches CKD-EPIcr, CKD-EPIcr_NB, CKD-EPIcr_R, CKD-EPIcr-cys, CKD-EPIcr-cys_NB, CKD-EPIcr-cys_R, and CKD-EPIcys. Using serum creatinine or cystatin C, GFR was estimated from 4563 participants (≥20 years) from the 1999–2000 and 2001–2002 cycles of National Health and Nutrition Examination Surveys.29 Prevalence estimates for eGFR categories as shown were applied to the 2019 US estimate of 246.6 million adults aged ≥20 years. Units of GFR are in milliliters per minute per 1.73 m2. Data are shown in Inker et al.38 Results are consistent with Duggal et al.,22 Diao et al.,25 and Walther et al.26 for approach CKD-EPIcr_NB.38 For approach names, see Table 1.
1. Filtration Marker Assay Availability, Standardization, and High-Throughput Analyzer Capability
All approaches were evaluated for characteristics of the filtration marker assays according to availability in laboratories, of reference standards, and of high-throughput analyzers (Table 2, Supplemental Table 3). Creatinine is standardized, widely available on high-throughput analyzers, and one of the most common laboratory tests performed in the United States. Thus, all approaches based solely on creatinine would not encounter barriers regarding the assay for measurement. Cystatin C is available only in some laboratories, reagents are available for some high-throughput analyzers, and standardization is available. Therefore, cystatin C is an unfeasible assay to replace current eGFR reporting methods for immediate implementation in all laboratories. Furthermore, adding cystatin C to the commonly ordered basic and comprehensive metabolic panels would require changes in Current Procedural Terminology (CPT) diagnostic codes used for reimbursement because of its higher cost. β-Trace protein assays are available only in research laboratories and standardized methods are not currently available for either β-trace protein nor β2-microglobulin assays.
Table 2.
Attribute 1–4 summary: Filtration marker assay, implementation challenges, equation marker, performance compared with mGFR assay
| Approaches | Attribute | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Assay | 2. Implementation | 3. Equation Derivation Population Diversity | 4. Performance in External Validation for Black Adultsa | ||||||
| Availability | Standardized | High-Vol Throughput | Laboratories | Clinical practice | Bias | Differential Bias | Accuracy | ||
| 1. CKD-EPIcr | W | Y | Y | In current use | RACDG | Reference | |||
| 5. CKD-EPI_NB | W | Y | Y | CR | NP | RACDG | ↓↓ | ↓↓↓ | ND |
| 12. CKD-EPIcr_R | W | Y | Y | CE | NP | RACDG | ND* | ↓↓↓ | ND |
| 17. CKD-EPIcr-cys_NB | S | Y | N | NF | NP | RACDG | ND* | ↓ | ↑ |
| 20. CKD-EPIcr-cys_R | S | Y | N | CE, NF | NP | RACDG | ↑ | ↓ | ↑ |
| 23. CKD-EPIcys | S | Y | N | NC, NF | NP | RACDG | ↑ | ND | ND |
See Supplemental Tables 3 and 4 for all approaches. Vol, volume; W, widespread; Y, yes; R, race groups; A, age; C, CKD status; D, diabetes; G, gender; CR, change to reporting only; NP, no problems anticipated; ND, no difference (see footnote below); CE, change to equation; S, specialized laboratories; N, no; NF, new filtration marker.
ND indicates there was no difference in performance compared with approach 1 (CKD-EPIcr), as indicated by nonoverlapping confidence intervals for performance. We evaluated the absolute magnitude of the bias. If the direction of the bias changed but the absolute magnitude was the same, this is indicated with an asterisk (*). If a difference was observed, then the magnitude of the bias or inaccuracy is indicated by the number of arrows. Down arrow indicates worse performance and up arrow indicates better performance compared with approach CKD-EPIcr. For details, see Supplemental Appendix 1 and Supplemental Tables 3B and 4. For bias, small, medium, and large are defined as the median difference between mGFR and eGFR of 0 to ±5, ±5 to ±10, and more than ±10 ml/min per 1.73 m2. For differential bias, small, medium, and large are defined as difference in bias between Black and non-Black individuals of <2.5, 2.6–5, and >5 ml/min per 1.73 m2. For accuracy, small, medium, and large is indicated as percentage of estimates >30% of mGFR (1−P30) of 10%, 10%–20%, and >20%. See Supplemental Tables 3A for other approaches.
2. Implementation by Laboratories and in Clinical Practice
Several of the alternative approaches would pose challenges to implementation by laboratories because they include information not readily or currently accessible to laboratories, such as height and weight (Table 1, approaches CG_Clcr and CKD-EPI_R_HW), or proportion of Black individuals living in any one community (approaches CKD-EPIcr_blend and CKD-EPIcr-cys_blend). Additionally, coefficients such as the Q factor, which corresponds to healthy population age and is sex matched, and specific average for a serum concentration (i.e., serum creatinine or serum cystatin C) in any given region—as described in approaches FAScr, EKFCcr, and FAScr-cys—would pose additional challenges.
Other approaches would challenge practical implementation because information is limited on the eGFR value reported (e.g., approach MDRDcr does not allow reporting eGFR values that are >60 ml/min per 1.73 m2). The Cockcroft and Gault equation (approach CG_Clcr) cannot be expressed for standardized creatinine values. Approaches that report two values would require health care providers to make individual decisions on suitability of an eGFR approach for their patients. Deciding on which value to use, or falsely presuming that a true value always is somewhere in between estimates (approaches CKD-EPIcr_MM, CKD-EPIcr_H/L, CKD-EPIcr-cys_MM, CKD-EPIcr-cys_H/L), can introduce subjectivity and biases in either direction that may unnecessarily complicate clinical practice and may lead to the wrong conclusion that the true GFR value falls between the two values, confusing some clinicians and leading to variation in eGFR values across clinical settings.2,3 Of the 12 approaches that included race, nine included race in calculation of GFR, but adjusted reporting of the results to exclude race. There is no evidentiary basis for keeping a race coefficient in the eGFR calculation and renaming the two eGFR values (approach CKD-EPIcr_MM, CKD-EPIcr_H/L, CKD-EPIcr-cys_MM, and CKD-EPIcr-cys_H/L), nor for any approaches with changes in reporting, because these are semantic label changes that may not mitigate the bias that using race in computation introduces.
3. Diversity in Characteristics of Equation Derivation Populations
To better understand the factors that drive kidney function estimation, there needs to be diversity in the populations used to develop the relevant equations. Individual studies used in development of GFR-estimating equations varied in representation by age, sex, race, ethnicity, geography, and in presence of, and risk factors for, kidney disease. Furthermore, variables, such as social determinants of health (e.g., income, education), hypothesized to contribute to the relationship between race and filtration markers were not consistently reported.4,5
The Task Force focused on identifying a solution that addresses the diversity of the US population. The majority of approaches that were developed with US cohorts were inclusive of Black individuals (except approach CG_Clcr), although the proportion of Black and non-Black individuals within these cohorts varied greatly and did not necessarily mirror US population census statistics of 13% Black individuals. For example, among the 1628 Modification of Diet in Renal Disease (MDRD) equation (MDRDcr) study participants, 12% were Black, whereas 31% of participants across the ten studies within the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) study developmental and internal validation cohorts were identified as Black.6,7 The Cockcroft and Gault creatinine clearance equation was originally developed in only White men, and the majority of GFR-estimating equations derived in European cohorts did not specify whether Black individuals were included, or specifically excluded Black participants, in equation development or internal validation (approaches CG_Clcr, FAScr, EKFCcr, LMcr, FAScr-cys, FAScys, and CAPAcys).8 Other racial and ethnic minority groups (e.g., Asian, Hispanic/Latinx, Native American, and Pacific Islander groups) are also under-represented in eGFR research. Similar considerations may be needed to identify suitable approaches for non-US populations.
All studies showed both age (adults between the ages of 18–65 years) and sex diversity among the populations used to develop the equations. A number of approaches did not report sufficient data to suggest comparable population diversity with regard to body composition, the presence of kidney disease, and/or the presence of risk factors for kidney disease development, including diabetes and hypertension (approaches MDRDcr, CG_Clcr, FAScr, EKFCcr, LMcr, FAScr-cys, CKD-EPIcys, and FAScys).
A total of 12 out of 26 approaches included race as a variable in equation calculation or reporting. Of the 12 approaches that did not include race as a variable, seven were not developed in a population that included Black individuals (approaches CG_Clcr, FAScr, EKFCcr, LMcr, FAScr-cys, FAScys, and CAPAcys). Six approaches were developed in a diverse population where race was not explicit (with two where race was in the calculation but not in reporting: CKD-EPIcr_NB and CKD-EPIcr-cys_NB) and, of those, the Task Force selected five approaches for further in-depth review (approach CKD-EPI_3M was not selected).
4. Performance in External Validation (Bias, Precision, and Accuracy)
To uniformly compare each approach’s performance in external validation with regard to statistical bias, precision, and accuracy, we used a single dataset that represented Black and non-Black individuals and that was not used to develop any of the approaches being considered (Supplemental Table 4). Bias is defined as the median difference between measured GFR (mGFR) and eGFR. Precision is defined as the interquartile range of the difference of mGFR minus eGFR. Accuracy is defined as the percentage of estimates >30% of mGFR (1−P30), with 1−P30 reflecting clinically relevant, large errors. We evaluated equations by the size of the difference compared either with mGFR or to the reference equation, CKD-EPI equation in which eGFR is computed using creatinine (approach 1), but only considered equations to have substantially different performance if confidence intervals around the mean estimate did not overlap. (For additional details, see Supplemental Appendixes 1–3.)
Supplemental Table 4 shows the bias and accuracy for all of the approaches; Table 2 and Figure 2 shows these factors in the selected approaches. We found that, for the creatinine-only approaches, relative to CKD-EPIcr, three had poor performance in bias and/or accuracy (approaches CKD-EPIcr_B, CG_Clcr, and LMcr), and five approaches had intermediate performance in either bias or accuracy (approaches CKD-EPIcr, CKD-EPIcr_NB, CKD-EPIcr_B, FAScr, EKFCcr, CKD-EPIcr_R). For equations that included two or more filtration markers, all approaches had good (approaches CKD-EPIcr-cys_NB, CKD-EPI_4M) or intermediate performance (approaches CKD-EPIcr-cys_B, FAScr-cys), and none had poor performance. Approaches CKD-EPIcr-cys, CKD-EPIcr-cys_NB, and FAScr-cys all showed improved accuracy relative to approach CKD-EPIcr. Cystatin-alone approaches (CKD-EPIcys, CAPAcys, and FAScys) demonstrated less differential bias between race groups, but accuracy is not improved and inaccuracy may be larger.
5. Potential Consequences
An accurate, nonracially biased, feasible, and cost-effective assessment of GFR is fundamental for individual clinical decision making in general medical care and in nephrology care. Such assessment is also essential in defining criteria for participation in clinical research and for tracking the population burden of kidney disease and associated health care delivery. Refining an ideal estimating equation will require research on long-term consequences, as has been done in previous disparities work, and was not available for this assessment.
Compared with White patients, Black patients have worse outcomes with respect to BP control, timely nephrology referral, fistula placement before hemodialysis initiation, waitlisting for kidney transplantation, and receiving a transplant. These disparities were documented before the first use of race in eGFR equations and persist.9–13 Currently, Black patients also are less likely to receive cardiac catherization, medications such as metformin and sodium-glucose cotransporter–2 inhibitors (SGLT-2is), adequate doses of pain and cancer medication, and be included in clinical trials.14–21 eGFR reporting influences such clinical decisions. Keeping in mind currently available markers and existing research, the Task Force considered whether approaches would benefit or harm patients and effectually reduce, create, or worsen disparities such as these.
Clinical Decision Making.
The potential consequences for clinical decision making were organized into general medical care (including medication initiation, discontinuation, and dosing) and nephrology care (Tables 3–5). Most research studies to date that have evaluated these consequences are national or single institutional simulations of alternative approaches, compared with approach CKD-EPIcr, that estimate the number of Black adults potentially affected by shifting eGFR across thresholds commonly used for clinical decision making. These simulations, although not measuring actual clinical practice with an eGFR approach change (for which clinician, patient, and health system behavior play a role), provide valuable data for evaluating potential consequences. We considered these simulations when evaluating the potential effect on patient populations from changing the current recommended eGFR approach 1, which includes the race variable, to other approaches (Figure 3, Table 3).22–28 Most of the comparisons are between approaches CKD-EPIcr and CKD-EPIcr_NB; with a few with approach CKD-EPIcys; and one study compared CKD-EPIcr_NB, CKD-EPIcr_R, CKD-EPIcr-cys_NB, CKD-EPIcr-cys_R, and CKD-EPIcys. Tables 3–5 show these data. (Supplemental Tables 5–7 show data for all approaches.) For some approaches, it was not possible to evaluate and compare consequences due to unavailability of data to the Task Force.
Table 3.
Possible consequences of approaches for clinical decision making (attribute 5): General medical care evaluation and management
| Approach | Implications | General Medical Care | Risk | ||||
|---|---|---|---|---|---|---|---|
| CKD Screening or Detection (<60 ml/min per 1.73 m2)a | Nephrology Referral (<30 ml/min per 1.73 m2) | Radiographic Diagnostic Assessment (<30 ml/min per 1.73 m2) | Mortality (all) | ESKD (all) | Incident CKD (>60 ml/min per 1.73 m2) | ||
| 1. CKD-EPIcr | N Black adults below/above thresholdb | 1–2.1Mb | 0.1–0.6M | 0.0–0.6M | 31Mc | 31Mc | 27Md |
| 5. CKD-EPIcr_NB | N Black adults changed | 1–2M | Approximately 0.12M | Approximately >0.12M | NQ | NQ | >2M |
| Possible benefits to Black adults | Large increase in no. diagnosed |
Small increase in no. referred | Small decreased harm of assessment | Overpredict risk | |||
| Possible harms to Black adults | Large increase in no. false diagnoses of CKD | Small increase in no. referred | Small decrease in no. assessed | Excess risk hidden | |||
| Possible non- Black adult changes | None | None | None | None | None | None | |
| Comment | Decreased eGFRcr in Black individuals may influence systematic differences in care, which may contribute to increasing or decreasing disparities and mask disparities in predicting risk when comparing individuals with the same eGFR across racial groups. | ||||||
| 12. CKD-EPIcr_R | No. Black adults changed % | Approximately 0.64M (31%) | Approximately 0.04M (9%) | Approximately 0.04M (9%) | NQ | NQ | NQ |
| Possible benefits to Black adults | Moderate increase in no. diagnosed |
Minimal increase in no. referred |
Minimal decrease in harm of assessment | Overpredict risk | |||
| Possible harms to Black adults | Moderate increase in no. of false diagnoses of CKD | Minimal increase in no. referred |
Minimal decrease in no. assessed | Excess risk hidden | |||
| Possible non- Black adult changes | Large decrease in no. diagnosed with CKD; 3.14M (23%) |
Small decrease in no. referred 0.29M (26%) |
Small decrease in no. assessed/harm of assessment; 0.29M (26%) |
||||
| Comment | Less change in eGFR than approach 5 and reduced bias in Black individuals, with subsequent decreased influence on masking disparities. Similar differential bias between Black and non-Black individuals because approach 5 leads to similar differences in care between groups. | ||||||
| 17. CKD-EPIcr-cys_NB | No. Black adults changed % | +0.22M (11%) | +0.12M (27%) | Approximately 0.12M (27%) | NQ | NQ | NQ |
| Possible benefits to Black adults | Small increase in no. diagnosed | Small increase in no. referred | Small decrease in harm of assessment | Similar risk predictions and associations seen | |||
| Possible harms to Black adults | Small increase in no. false diagnoses of CKD | Small decrease in no. assessed | |||||
| Possible non- Black adult changes | 0.74M (5%) | Moderate increase; 0.75M (68%) |
Moderate increase; 0.75M (68%) |
||||
| Comment | Less differential bias between Black and non-Black individuals leading to less differential care. Increased accuracy in all individuals, potentially leading to better decision making. | ||||||
| 20. CKD-EPIcr-cys_R | No. Black adults changed % | 0.09M (4%) | 0.1M (22%) | 0.1M (22%) | NQ | NQ | NQ |
| Possible benefits to Black adults | Minimal increase in no. diagnosed | Minimal increase in no. referred | Minimal decrease in harm of assessment | Similar risk predictions and associations seen | |||
| Possible harms to Black adults | Minimal increase in no. false diagnoses of CKD | Minimal decrease in no. referred | Minimal decrease in no. assessed | ||||
| Possible non-Black adult changes | Large decrease of approximately 1.36M (9%) |
Moderate increase of approximately 0. 61M (55%) | Moderate increase of approximately 0.61M (55%) | ||||
| Comment | Less differential bias between Black and non-Black individuals leading to less differential care. Increased accuracy in all individuals potentially leading to better decision making. | ||||||
| 23. CKD-EPIcys | No. Black adults changed % | 0.09M (4%) | 0.12M (26%) | 0.12M (26%) | NQ | NQ | |
| Possible benefits to Black adults | Minimal increase in no. diagnosed | Small increase in no. referred | Greater risk observed | Greater risk observed | Greater risk observed | ||
| Possible harms to Black adults | Minimal increase in no. false diagnoses of CKD | Small increase in no. referred | |||||
| Possible non-Black adult changes | Large increase; 4.29M (29%) |
Large increase; 1.46M (133%) |
Large increase; 1.46M (133%) |
||||
| Comment | No differential bias between Black and non-Black individuals. Less accurate than eGFRcr-cys and large changes in care for non-Black individuals compared with current | ||||||
See Supplemental Table 5 for all approaches. “Large” indicates estimated changes in the number of people to be below a threshold of >1 million; “moderate” represents 500,000–999,000; “small” represents 100,000–499,000; “minimal” represents <100,000. M, million; K, thousand; eGFRcr, eGFR computed using creatinine; NQ, not quantified; NHANES, National Health and Nutrition Examination Survey.
CKD is defined as GFR <60 ml/min per 1.73 m2 or presence of kidney damage that is present for at least 3 months. For this table, we are using only a one-time measurement of GFR <60 ml/min per 1.73 m2 to isolate the effect of new GFR equations on CKD prevalence.
All values are approximate and are based on combination of reports using simulations of NHANESs or clinical datasets.22–26,38 This might not indicate what occurs in practice and cannot incorporate health care professionals or patient behavior.
Estimated number of Black adults living in the United States from 2019 US Census.
Estimated number of Black adults with GFR >60 ml/min per 1.73 m2 living in the United States estimated from the 2019 census and multiplied by portion without CKD, as defined by GFR <60 ml/min per 1.73 m2 and albuminuria from NHANES data.38
Table 5.
Possible consequences of approaches for clinical decision making (attribute 5): Nephrology evaluation and management
Approximately <120K (.26M)
| Approach | Implications | Nephrology Care | Candidate Kidney Donor Assessment | |||||
|---|---|---|---|---|---|---|---|---|
| Medical Nutrition Benefit (13–50) | Kidney Disease Education Coverage (<30) | Vascular Access Referral (<18) | Initiation of Dialysis (<15) | Transplant Referral (<20) | N Eliminated or Accepted via an Initial Single Screening (<60) | Risk Prediction for ESKD in Donors (>60) | ||
| 1. CKD-EPIcr | No. US Black adults below/(above) threshold | Approximately 1M | Approximately 0.1–0.6M | Approximately 0.1M | Approximately 0.1M | Approximately 0.1M | 1–2.1M | Approximately 27M |
| 5. CKD-EPIcr_NB | No. Black adults changed (%) | Approximately 0.4M (+49) | 0.26M (0–29) | 0.26M (+0–29) | 0.29M (+0–29) | 1–2M (+16–102) | >2M (−9) | NQ |
| Possible benefits to Black adults | Small increase in no. eligible for benefit | Small increase in no. eligible for benefit | Minimal increase in no. with AVF placed at time of HD initiation |
Unclear; no absolute eGFR for initiation | Minimal increase in no. with earlier referral |
|||
| Possible harms to Black adults | — | — | Minimal increase in no. with AVF put in early or un-needed |
Unclear; no absolute eGFR for initiation | Large increase in no. eliminated if single screening | Large increase in no. eliminated due to overprediction of risk |
||
| Possible non- Black adult changes | None | None | None | None | None | None | None | |
| Comment | Decreased eGFRcr in Black individuals may influence systematic differences in care, which may contribute to increasing or decreasing disparities and mask disparities in predicting risk when comparing individuals with the same eGFR across racial groups. Magnitude of decreased GFR is less at lower levels of GFR than higher levels. | |||||||
| 12. CKD-EPIcr_R | No. Black adults changed (%) | NQ | +0.04M (9) | NQ | NQ | NQ | +0.64M (31) | NQ |
| Possible benefits to Black adults | Small increase in no. eligible for benefit | Minimal increase in no. eligible for benefit | Minimal increase in no. with AVF placed at time of HD initiation | Unclear; no absolute eGFR for initiation | Slightly increase in no. with earlier referral | |||
| Possible harms to Black adults | — | — | Minimal increase in no. with AVF put in early or un-needed | Unclear; no absolute eGFR for initiation | Moderate increase in no. eliminated if single screening used |
Moderate increase in no. eliminated due to overprediction of risk | ||
| Possible non- Black adult changes | Small decrease in no. eligible for benefit | Small decrease in no. eligible for benefit 0.29M (26%) |
Minimal decrease in no. with AVF placed at time of HD initiation | Unclear; no absolute eGFR for initiation | Large no. potentially now accepted; approximately 3.41M (23%) |
Large no. potentially accepted due to perceived lower risk | ||
| Comment | Less change in eGFR than approach 5 and reduced bias in Black individuals, with subsequent decreased effect on masking disparities. Similar differential bias between Black and non-Black individuals as approach 5, leading to similar differences in care between group. | |||||||
| 17. CKD-EPIcr-cys_NB | No. Black adults changed (%) | NQ | +0.12M (27) | NQ | NQ | NQ | +0.22M (11) | NQ |
| Possible benefits to Black adults | Small increase in no. eligible for benefit | Small increase in no. eligible for benefit | Minimal increase in no. with AVF placed at time of HD initiation | Unclear; no absolute eGFR for initiation | Earlier referral | |||
| Possible harms to Black adults | — | — | Minimal increase in no. with AVF put in early or un-needed | Unclear; no absolute eGFR for initiation | Small increase in no. eliminated if single screening used |
Small no. eliminated due to overprediction of risk | ||
| Possible non- Black adult changes | Moderate increase in no. eligible for benefit | Moderate increase 0.75M (68%) |
Moderate increase in no, with AVF put in earlier | Unclear; no absolute eGFR for initiation | Moderate increase in earlier referral | Moderate increase in no. eliminated if single screening used; 0.74M (5%) |
Moderate increase in no. eliminated due to overprediction of risk | |
| Comment | Less differential bias between Black and non-Black individuals leading to less differential care. Increased accuracy in all individuals potentially leading to better decision making. | |||||||
| 20. CKD-EPIcr-cys_R | No. Black adults changed (%) | NQ | +0.1M (22) | NQ | NQ | NQ | −0.09M (4) | NQ |
| Possible benefits to Black adults | Small increase in no. eligible for benefit | Small increase in no. eligible for benefit | Small increase in no. with AVF placed at time of HD initiation | Unclear; no absolute eGFR for initiation | Small increase in earlier referral | — | ||
| Possible harms to Black adults | — | — | Slightly increase in no. with AVF put in early or un-needed | Unclear; no absolute eGFR for initiation | — | Minimal no. now potentially now accepted | Minimal mo. accepted with underprediction of risk | |
| Possible non- Black adult changes | Moderate decrease in no. eligible for benefit |
Moderate decrease in no. eligible for benefit 0.61M (55%) |
Minimal decrease in no. with AVF placed at time of HD initiation | Unclear; no absolute eGFR for initiation | Moderate decrease referred | Large no. now potentially now accepted; approximately 1.36M (9%) |
Large no. potentially accepted due to perceived lower risk | |
| Comment | Less differential bias between Black and non-Black individuals leading to less differential care. Increased accuracy in all individuals potentially leading to better decision making. | |||||||
| 23. CKD-EPIcys | No. Black adults changed (%) | NQ | +0.12M (26) | NQ | NQ | NQ | +0.09M (4) | NQ |
| Possible benefits to Black adults | Small increase in no. eligible for benefit | Small increase in no. eligible for benefit | Small increase in no. with AVF placed at time of HD initiation | Unclear; no absolute eGFR for initiation | Earlier referral | |||
| Possible harms to Black adults | — | Slight increase in no. with AVF put in early or un-needed | Unclear; no absolute eGFR for initiation | Minimal increase in no. eliminated if single screening used | Minimal no. potentially eliminated due to overprediction of risk | |||
| Possible non- Black adult changes | Large increase in no. eligible for benefit | Large increase in no. eligible for benefit +1.46M (133%) | Large increase in no. with AFV placed earlier | Unclear; no absolute eGFR for initiation | Large increase in no. eliminated if single screening; 4.29M (29%) |
Large increase in no. now potentially eliminated due to overprediction of risk | ||
| Comment | No differential bias between Black and non-Black individuals. However, less accurate than eGFRcr-cys and large changes in care for non- Black individuals. | |||||||
See Supplemental Table 7 for all approaches. All values are approximate and are based on combination of reports using simulations of NHANESs or clinical datasets.22–26,38 This might not indicate what occurs in practice and cannot incorporate health care professionals or patient behavior. “Large” indicates estimated changes in the number of people to be below a threshold of >1 million; “moderate” represents 500,000–999,000; “small” represents 100,000–499,000; “minimal” represents <100,000. M, million; K, thousand; AVF, arteriovenous fistula; HD, hemodialysis; eGFRcr, eGFR computed using creatinine; NQ, not quantified but relative magnitude estimated based on data in Table 3; eGFRcr-cys, eGFR computed using creatinine and cystatin C.
For each approach, we also examined how medication use and dosing might change from approach CKD-EPIcr on the basis of bias and accuracy compared with mGFR. We also considered differential bias compared with reference approach CKD-EPIcr in examining the potential implications for both Black and non-Black adults, an important undertaking to examine potential systematic differences that could lead to disparities in health and health care. Finally, we considered the drawbacks of using race to guide clinical decision making, including the potential perpetuation of implicit and explicit bias.
Overall, the effect on clinical decisions differs at high versus lower eGFR; more Black adults will be affected for clinical decisions that occur at higher eGFR thresholds (e.g., kidney donor candidate evaluation, eGFR threshold ≥90 ml/min per 1.73 m2) compared with lower eGFR thresholds (e.g., transplant referral, eGFR threshold <20 ml/min per 1.73 m2) (Figure 3). Thus, the number of Black adults that would be affected by using alternative approaches for meeting a clinical decision threshold is greatest for kidney donor candidate evaluation decisions, CKD screening or detection, and risk prediction; intermediate for medication considerations, kidney disease education, nephrology referral, and radiographic diagnostic assessment (e.g., cardiac catheterization); and smallest for vascular access referral, initiation of dialysis, and transplant referral (Tables 3–5).22–25
General Medical and Nephrology Care.
For CKD screening, approach CKD-EPIcr_NB could increase the number of Black adults meeting a threshold by 16% to >100% (local versus national studies) and the number of Black adults meeting eGFR <30 ml/min per 1.73 m2 by up to 52%, with potential benefit or harm resulting from an increased diagnosis or overdiagnosis, respectively. Approach CKD-EPIcr_NB could change the number of Black adults meeting a threshold by 102% or 9%, with attendant harms of eliminating potential kidney donors in screening or by risk prediction, respectively (Table 5). Approach CKD-EPIcr_NB could increase by 29%–52% the number of Black adults being eligible for Medicare’s medical nutrition or kidney disease education benefit, vascular access referral, or transplant referral. The larger differential bias between race groups using approach CKD-EPIcr_NB compared with approach CKD-EPIcr would lead to systematic difference between groups in these decisions. This approach overpredicted risk of kidney failure in Black individuals using existing kidney failure risk models and potentially obscured previously documented disparities.
Approach CKD-EPIcr_R without the race variable would have less change on the reported eGFR value from approach CKD-EPIcr, as compared with the change with the use of approach CKD-EPIcr_NB. This may lead to attenuated effects on clinical decisions and risks for Black adults, as might be seen with approach CKD-EPIcr_NB, but may also have consequence for non-Black adults. In addition, the differential bias is the same as for approach CKD-EPIcr_NB, thus also leading to concerns about systematic differences in care. Approaches CKD-EPIcr-cys_NB and CKD-EPIcys have greater accuracy than approaches CKD-EPIcr_NB or CKD-EPIcr_R, would yield less change in eGFR values with less differential bias, and would thus have attenuated effects on clinical decisions and risk, and systematic differences in care. Approach CKD-EPIcys exhibits no differential bias between groups, but decreased accuracy compared with all of the eGFRcr-cys approaches. For Black individuals, eGFR values are similar, leading to minimal differences in decision making, with approach CKD-EPIcys compared with approach CKD-EPIcr. However, differences for non-Black individuals would be greater, leading to potential effects on care.
Medication Initiation, Discontinuation, and Dosing.
Table 4 shows the effect on medication usage and dosing using the performance data of mGFR and CKD-EPIcr compared with eGFR approaches (Figure 2 and Supplemental Table 7). In general, consistent with the expected change in eGFR, there are more potential harms to non-Black adults for inappropriate drug continuation or overdosing, and more harms to Black adults for inappropriate drug discontinuation and underdosing. Approaches CKD-EPIcr-cys_NB and CKD-EPIcr-cys_R are more accurate, with small differential bias between Black and non-Black adults. Thus, changes from the current approach would be an improvement.
Table 4.
Possible consequences of approaches for clinical decision making (attribute 5): Medication-related decision making
| Approach | Group | Drug Initiation To Decrease CKD Progressiona | Inappropriate Drug Continuation and Overdosing (adverse effects/toxicity)b,c | Inappropriate Drug Discontinuation and Underdosing (less effective)d,e | Bias from mGFR | Absolute Difference from Reference (approach 1) (ml/min per 1.73 m2) | Accuracy Compared with Reference (approach 1) |
|---|---|---|---|---|---|---|---|
| 1. CKD-EPIcr | Black | Reference | Overestimate: 3.7 | Reference | |||
| Non-Black | Overestimate: 0.5 | ||||||
| 5. CKD-EPIcr_NB | Black | ↑↑ | – | ↑↑ | Underestimate: 7.1 | 10.8 | NC |
| Non-Black | NC | NC | NC | Overestimate: 0.5 | 0 | NC | |
| 12. CKD-EPIcr_R | Black | ↑↑ or NC | – | ↑↑ or NC | Underestimate: 3.6 | 7.3 | NC |
| Non-Black | ↓ | ↑ | – | Overestimate: 3.9 | 3.4 | ↓ | |
| 17. CKD-EPIcr-cys_NB | Black | ↑ or NC | – | ↑↑or NC | Underestimate: 3.4 | 7.1 | ↑ |
| Non-Black | NC | NC | NC | Overestimate: 0.6 | 0.1 | ↑ | |
| 20. CKD-EPIcr-cys_R | Black | – | – | – | Underestimate: 0.1 | 3.8 | ↑ |
| Non-Black | ↓ | ↑ | – | Overestimate: 2.9 | 2.4 | ↑ | |
| 23. CKD-EPIcys | Black | – | – | – | Overestimate: 0.1 | 3.6 | NC |
| Non-Black | NC | NC | NC | Underestimate: 0.6 | 1.1 | NC | |
See Supplemental Table 6 for all approaches. Approaches are compared with approach CKD-EPIcr. Performance data compared with mGFR are shown in Figure 2 and Supplemental Table 7. Small bias as compared with mGFR and low differential bias between Black and non-Black individuals. Arrows, extent of over- or underestimate of mGFR (and thus potential for benefits or harm for drug decision making); –, there was a change from approach CKD-EPIcr but alternative approach does not increase potential for issue; NC, no change from approach CKD-EPIcr; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; GLP-1, glucagon-like peptide-1.
Drug initiation: Medications (e.g., ACE inhibitor, ARB, SGLT-2i, GLP-1 receptor agonist) more or less likely to be initiated for decreasing CKD progression and risk of cardiovascular disease.
Medications more likely to be continued when it is not appropriate (e.g., glyburide, metformin and bisphosphonates, and dulaglutide and dabigatran are contraindicated when eGFR is <60, <30, and <15 ml/min per 1.73 m2, respectively).
Increased potential for overdosing (adverse effects/toxicity): Potential for overdosing leading to potential severe adverse effects or toxicities. For example, chemotherapies (e.g., carboplatin, cisplatin, cytarabine, melphalan), anticoagulants (dabigatran, rivaroxaban), immunosuppressives/immunotherapies (e.g., methotrexate, lenalidomide).
Medications more likely to be discontinued when it is not appropriate (e.g., metformin at eGFR <30 ml/min per 1.73 m2, SGLT-2 inhibitors at various eGFR thresholds based on product label or practice guidelines, dabigatran at eGFR <15 ml/min per 1.73 m2).
Increased potential for underdosing (less effective): Potential for underdosing and decreased effectiveness for several chemotherapies, antibiotics, anticoagulants, and many other medications.
This overall summary does not reflect the effect on specific populations. For example, national data suggest that approach CKD-EPIcr_NB would increase CKD diagnosis in an estimated 235,000 (one in 20) Black individuals with type 2 diabetes, and could promote initiation of medications to reduce CKD progression.26 Most of these patients meet eligibility for the same medications under other clinical indications (one in four for cardiovascular or nine in ten for weight-loss indications) and, therefore, this change is far less likely than estimated.26 Conversely, these data also suggest approximately 40,000 Black adults with type 2 diabetes might not qualify for initiation of SGLT-2is, continued metformin use, or glucagon-like peptide-1 receptor agonist therapy.26 Approaches CKD-EPIcr_R and CKD-EPIcr-cys_R, however, may lead to less CKD diagnosis and thus less consideration for angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, SGLT-2i, or glucagon-like peptide-1 receptor agonist therapy in non-Black individuals. Veterans Administration data also show that a large percentage of Black individuals with CKD using several common medications would be reclassified to a lower eGFR, perhaps leading to dose reduction or medication discontinuation.23
For patients with cancer, approach CKD-EPIcr_NB would also increase the number of Black individuals ineligible for chemotherapy or those recommended to receive less than a full dose or a potentially less accurate dose adjustment (by 61%–163%) for several chemotherapies, which may lead to decreased survival.29 Consequently, Black individuals may experience increased disparities because potentially lifesaving cancer therapies may not be initiated or they may be undertreated.29
Although approach CG_Clcr had many challenges for widespread use for kidney function reporting, it is commonly used for medication-related decision making (Supplemental Tables 4 and 6).30 Use of total body weight with approach CG_Clcr results in moderate overestimation of mGFR in Black and non-Black individuals. Pharmacists use approach CG_Clcr for many medication dosing decisions by applying different body weights (e.g., ideal, adjusted, total) to improve equation performance across the spectrum of patient weights.31
Individual patient kidney clearance of medications correlates best with nonindexed GFR.31,32 Application of this individualized measure is especially important for overweight or underweight patients where indexed eGFR (ml/min per 1.73 m2) can differ from non-indexed mGFR.33 This situation may lead to increased risk of medication underdosing in overweight or overdosing in underweight patients. Compiled data from nine studies with a diversity of patients, with a limited spectrum of BSA, showed that those in the highest BSA group had an indexed eGFR that was, on average, 19.7, 20.9, and 23 ml/min per 1.73 m2 lower than nonindexed mGFR for approaches CKD-EPIcr, CKD-EPIcr-cys, and CKD-EPIcys, respectively.33 They found a significant trend for larger underestimation of mGFR with both indexed and nonindexed eGFR at higher BSAs, such that the average indexed value was consistently lower (further away from nonindexed mGFR) than the average nonindexed value, supporting the practice of deindexing GFR for medication dosing. The average nonindexed mGFR was 90.6 ml/min, but these data were not stratified by race or GFR category. Any eGFR approach that underestimates mGFR has the potential to amplify the underestimation observed in patients who are overweight or obese and—because obesity is rising among all persons in the United States, with the highest prevalence among Black adults, particularly Black women—applying indexed eGFR approaches that underestimates mGFR in Black adults who are overweight and obese can potentially exacerbate disparities in medication-related dosing decisions.34,35
Clinical Trials and Research.
To assess the potential effect of the eGFR equations under consideration on clinical trial eligibility, enrollment, adverse event reporting, and safety monitoring, we evaluated two landmark trials including “Black and non-Black” participants across a range of eGFR values: the Systolic Blood Pressure Intervention Trial (SPRINT) and the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial. SPRINT, a trial that demonstrated significant cardiovascular benefit with intensive BP control, included both a general population and CKD group in which CKD was defined as an eGFR of 20–59 ml/min per 1.73 m2 on the basis of the MDRD equation.36 CREDENCE, a trial that showed cardiovascular and renoprotection with canagliflozin in patients with type 2 diabetes and CKD, included patients with an eGFR range of 30–90 ml/min per 1.73 m2 on the basis of the CKD-EPI equation.37
Using the creatinine-based equations that would remove race and report high muscle mass and low muscle mass or high value and low value (approaches CKD-EPIcr_MM and CKD-EPIcr_H/L), trial inclusion and outcomes would likely be similar, if not identical, to the original equations used if higher values are exclusively assigned to Black patients, and introduce additional subjectivity. Alternatively, if eGFR assignment is based on muscle mass, which was not assessed in these studies and, in general, is poorly defined, the effect and generalizability are unclear. Similar issues would emerge with the replacement of race with reporting these high/low parameters with the eGFRcr-cys equation (approaches CKD-EPIcr-cys_MM and CKD-EPIcr-cys_H/L).
In equations that use solely the Black coefficient or the non-Black coefficient for all study populations (approaches CKD-EPIcr_NB, CKD-EPIcr_B, CKD-EPIcr-cys_NB, and CKD-EPIcr-cys_B), there would be proportional shifts in eGFR that would mainly affect inclusion of populations at the defined trial cutoff margins. For example, if the Black coefficient is excluded, more Black participants would be below the current lower end of the eGFR cutoff and, therefore, would have been excluded from the trials. However, some candidates excluded due to low eGFR might be replaced by candidates who were excluded due to eGFR above the inclusion range because they would now fall into the eligibility range of the trials. Notably, in both studies, the subgroup analyses suggest that these shifts in study population would not have significantly affected overall study outcomes. However, in CREDENCE, there were more events in those with lower eGFR and, for Black participants, many of these trial outcomes would have been lost with the exclusion of those with newly calculated lower eGFR, according to study investigators. A similar pattern would be expected with the use of eGFRcr-cys equations, although these data comparisons are not available to fully assess.
Although a blended eGFR equation based on local or regional population race/ethnicities is a novel idea, for multisite clinical trials, this strategy would create challenges in implementation and generalizability. Additionally, this approach has the potential to lead to confusion about eGFR among patients and health care providers when eGFR used for investigation may be different from regional clinically based eGFR. The blended equation using cystatin C is likely to have similar implementation issues and would need further investigation to understand its effect.
The effect of utilization of the Caucasian, Asian, pediatric, and adult; full age spectrum; European Kidney Function Consortium; or Lund–Malmö equations (approaches FAScr, EKFCcr, LMcr, FAScr-cys, FAScys, and CAPAcys) on these two clinical trials is unclear given the lack of inclusion of Black, and specifically US Black, populations in the development of these equations.
The CKD-EPI equations refit without race lead to minor shifts in eGFR for Black and non-Black populations, which would likely have a very small effect on study involvement and a negligible effect on study results (approach CKD-EPIcr_R). The CKD-EPI equation refit without race, but with height and weight included, may be problematic given lack of consistency in the ascertainment of these anthropometric measures (approach CKD-EPI_R_HW).
With the equations including cystatin C (approaches CKD-EPIcr-cys, CKD-EPIcr-cys_blend, and CKD-EPIcys), the lack of direct comparisons in populations with current eGFR data limit our ability to assess the effect on clinical trials. However, eGFRcr-cys refit along with newer equations using novel markers (approaches CKD-EPIcr-cys_R, CKD-EPI_4M, and CKD-EPI_3M) have the potential to characterize GFR without race modifiers in study populations, but require further investigation to assess their effect on clinical trials.
Population Surveillance.
To examine the potential effect any one approach may have on population surveillance of CKD and estimates of CKD burden, we focused on ramifications of reporting from the United States Renal Data System, Indian Health Service, the International Statistical Classification of Diseases and Related Health Problems (ICD)/CPT claims, survey data using National Health and Nutrition Examination Survey, and electronic health record data (e.g., Medicare; Optum; Center for Kidney Disease Research, Education and Hope; and Kaiser Permanente) (Table 6). We examined whether approach changes would create barriers or delay the implementation process and estimated the time to systematically address an approach change across data sources.
Population surveillance relies primarily on existing testing modalities to identify people with CKD and, generally, creatinine-based approaches would pose minimal effects. Few potential barriers would delay the process for operationalizing a creatinine-based approach, and a minimal time delay for consistency across the country in staging patients and reporting would only affect data from ICD/CPT claims and electronic health records. A number of barriers delaying the implementation of approaches that included cystatin C were identified across all four metrics of population surveillance (Table 6).
6. Patient Centeredness
Clinical algorithms, although seemingly appropriate at the population level, may require further consideration when assigning kidney disease severity using any single value of eGFR for an individual, particularly during CKD screening. Testimonies from patients highlighted a desire for earlier CKD detection, transparent communication of this detection with patients, tracking of eGFR trajectory over time, rapid referral to nephrology, and prompt transplant referral for individuals with advanced CKD. Approaches CKD-EPIcr_R, CKD-EPIcr-cys_R, and CKD-EPIcys would potentially support these processes. Communication should include education on the inherent imperfection of a GFR estimate and the imprecision of even the reference standard mGFR.2 Careful consideration for individual evaluations should not rely on a single result for clinical decision making but should be informed by trends in eGFR values (which may require more frequent testing) and other clinical information, including confirmatory testing to determine a more reliable baseline function using different filtration assays or clearance methods.2 Additionally, education on the precision and limitations of GFR estimation, including variability among individuals, has the potential to enhance information transfer, health literacy, shared decision making between patients and health care providers, and the provider-patient relationship.
Recommendations for a Unifying Approach to Estimation of GFR (Phase 3)
The Task Force reached a consensus that estimating equations for US adults that do not incorporate race are desirable and needed. Because all available estimating equations have limitations, the selected approach should not disproportionately affect any one group of individuals, but rather bias and inaccuracy should be minimal and of equal effect across all patient groups, and not concentrated within one group. Therefore, the phase 2 evaluation highly informed the following phase 3 recommendations and other important considerations for clinical decision making.
Recommendation 1
The Task Force recommends for US adults (>85% of whom have normal kidney function) that the CKD-EPIcr_R equation that was developed without the use of the race variable be implemented immediately, including in all laboratories. In addition to not including race in the calculation and reporting, it included diversity in its development, is immediately available to all laboratories in the United States, and has acceptable performance characteristics and potential consequences that do not disproportionately affect any one group of individuals.38
Recommendation 2
The Task Force recommends national efforts to facilitate increased, routine, and timely use of cystatin C, especially to confirm eGFR in adults who are at risk for or have CKD. Combining filtration markers (creatinine and cystatin C) is more accurate and would support better clinical decisions than either marker alone. Thus, if ongoing evidence supports acceptable performance, the CKD-EPIcys and CKD-EPIcr-cys_R without the race variable should be adopted to provide more accurate first-line or confirmatory testing, as appropriate for the clinical setting.
Recommendation 3
The Task Force recommends that research on GFR estimation with new endogenous filtration markers and on interventions to eliminate race and ethnic disparities in kidney disease be encouraged and funded. We implore US society at large and funding agencies to invest in developing the science needed for new approaches for accurate, unbiased, and precise GFR estimation, and for improving estimation of physiologic function in other areas of medicine, with the ultimate goal of promoting health equity.
Other Considerations
The Task Force supports the use of clearance measurements using creatinine, exogenous filtration markers, or estimated GFR using alternative endogenous filtration markers as confirmatory tests for important clinical decisions based on GFR.28 Wider-spread use of confirmatory tests will increase recognition of the limits of precision in GFR assessment.2,3 We encourage clinical laboratories to discontinue use of serum creatinine assays that use the Jaffe reaction in favor of enzymatic reaction assays to further limit serum creatinine variability and increase eGFR accuracy to improve standardization. We also encourage eGFR reporting indexed to standard body surface area (ml/min per 1.73 m2), with serum creatinine values extending to two decimal places and the notation that “use of nonindexed eGFR values (ml/min) should be considered for drug dosing decisions”. Although the Task Force efforts were focused on the examination of the use of race in GFR-estimating equations, we also support that kidney disease evaluations should include an assessment for albuminuria, as recommended in the Kidney Disease Improving Global Outcomes guidelines.28
Gaps in Knowledge and Future Science
The Task Force identified gaps in current knowledge and areas for future investigation to fully realize an ideal solution (Table 7). First is a critical need to identify new filtration markers and methods to assess GFR that are more accurate than current methods; that are not sensitive to social and demographic factors, such as race and sex; and are applicable for all ethnic and geographic groups. Understanding the effect of new methods can only be achieved if they are evaluated in representative populations that include multiethnic and racial groups across a spectrum of health, socioeconomic status, and geography. Second, for current (e.g., creatinine and cystatin C) and future endogenous filtration markers, we suggest thorough investigations into understanding the non-GFR determinants of their serum concentration if they are to remain as mainstays in GFR estimation. Third, investigation of the effect that different approaches might have on every race and ethnic group in the United States is needed.39,40 Fourth, for cystatin C, exploring performance in more heterogenous (e.g., hospitalized) cohorts is necessary because some reports suggest it is an inflammatory marker or acute phase reactant.41 Fifth, additional research is required to specifically define best approaches for GFR assessment for real-time clinical decision making (e.g., point of care). Sixth, it is necessary to define the criteria for, and evaluate the consequences of, adding new filtration markers to the basic metabolic panel used in clinical practice. Seventh, reappraisal of the relation of kidney drug clearance with nonindexed mGFR, indexed and nonindexed eGFR across GFR categories, and a diversity of patients is needed to help guide drug-related decision making using eGFR approaches in an era of rising obesity. Finally, and most important to help eliminate the entire spectrum of disparities in kidney health and delivery of health care, is the need to address GFR threshold versus risk-based criteria for obtaining medical benefits and to enhance research emphasis and funding aimed at reducing racial and ethnic disparities.1
Table 7.
Key questions for future research
| Key Questions |
|---|
| 1. What new endogenous filtration markers are not sensitive to social and demographic factors (e.g., race, ethnicity, sex)? How do they perform with regard to accuracy, bias, and precision in estimating GFR in representative populations that include multiethnic and racial groups across a spectrum of health, socioeconomic status, and geography? |
| 2. For current (e.g., creatinine and cystatin C) and future endogenous filtration markers, what are the non-GFR determinants of their serum concentration? |
| 3. What are the performance characteristics (accuracy, bias, and precision) of cystatin C in more heterogeneous (e.g., hospitalized) populations? |
| 4. What is the effect of recommended approaches for estimation of GFR on all race and ethnic groups? |
| 5. Are there sound new GFR approaches for real-time decision making (e.g., point of care)? |
| 6. What are the criteria for, and consequences of, adding new filtration markers to the basic metabolic panel used in everyday inpatient and outpatient clinical practice? |
| 7. What is the relation of kidney drug clearance with nonindexed mGFR and indexed and nonindexed eGFR across GFR categories in diverse populations, including the full spectrum of body size and composition? |
| 8. What is the effect of using risk-based versus GFR threshold criteria for obtaining access to medical benefits, such as transplantation, nephrology referral, and nutrition services? |
| 9. What interventions focus on the most important drivers and are effective in prevention and elimination of race and ethnic disparities? |
Implementation Considerations
A concerted effort, including multiple and committed stakeholders, must occur with the implementation of these recommendations. National and local laboratories, health care systems, vendors, health care providers, health education institutions, public and private payers, and organizations that generate clinical practice guidelines must be engaged. Support from payers; policymakers; and federal agencies of the US Department of Health and Human Services, including Centers for Disease Control and Prevention, Centers for Medicare and Medicaid Services, Food and Drug Administration, and Health Resources and Services Administration will be crucial to ensure broad national adoption. Currently, GFR reporting is not uniform, and previous guideline recommendations have taken years, if not decades, to implement.28,42 The recommended equations should be adopted promptly to provide standardized reporting of eGFR and thus to enable uniform and consistent clinical practice. This timeline—from evidence, to guidelines, to uptake in clinical practice—must be shortened. We also foresee the need for assistance to many health providers and health systems in initiating the process of change to adopt the new equations, to provide cystatin C testing, address reimbursement considerations, and understand the relationship between the new eGFR values and values from earlier equations used in a health care setting. The commonly ordered basic and comprehensive metabolic panels include creatinine and substituting/adding cystatin C would require changes in coding and reimbursement. The Task Force supports increased use of cystatin C and supports NKF and ASN’s role in advocating for improved methods and resources such that it can be available in all laboratories.
Challenges in implementation are vast, time consuming, and compete against other priorities. Professional societies in all specialties of medicine, academic institutions, health care systems, and relevant industry partners must be committed and unified in driving these recommended changes as the best currently available clinical approach for assessment of GFR.
Disclosures
M. Baweja reports having other interests in/relationships with Physicians for Human Rights and Young Center for Immigrant Children’s Rights. D. C. Crews reports having other interests in/relationships with American Board of Internal Medicine (via the nephrology board), American College of Physicians (via the Council of Subspecialist Societies), and NKF of Maryland/Delaware (via the board of directors); serving as cochair of Bayer HealthCare Pharmaceuticals Inc., on the patient and physician advisory board steering committee for the Disparities in Chronic Kidney Disease Project, in the advisory group for Health Equity Collaborative, as associate editor of Kidney360, and on advisory boards for Optum Labs and Partner Research for Equitable System Transformation after COVID-19 (PRESTAC); serving on the editorial boards for CJASN, JASN, and Journal of Renal Nutrition; receiving research funding from Somatus Inc.; and having consultancy agreements with Yale New Haven Health Services Corporation Center for Outcomes Research and Evaluation. C. Delgado reports that her contribution is the result of work supported with the resources and the use of facilities at the San Francisco Veterans Affairs Medical Center. N. D. Eneanya reports having consultancy agreements with Davita and Somatus, and serving as a scientific advisor for, or member of, Healthcare: The Journal of Delivery Science and Innovation and Kidney Medicine. C. A. Gadegbeku reports serving as a scientific advisor for, or member of, the ASN Council. L. A. Inker reports serving as a scientific advisor for, or member of, Alport Foundation, Diametrix, and Goldfinch; having other interests in/relationships with ASN (as a member), National Kidney Disease Education Program (as a member), and NKF (as a member); having consultancy agreements with Diamtrix and Tricidia; and receiving research funding from NIH, NKF, Omeros, Reata, and Retrophin. M. L. Mendu reports having ownership interest in, and serving as a scientific advisor for, or member of, RubiconMD. G. Miller reports having consultancy agreements with Baebies; and receiving honoraria from, and serving as a scientific advisor for/member of, Clinical Chemistry. M. M. Moxey-Mims reports serving as an associate editor for JASN, on the scientific advisory boards for NephCure International and NKF, and on the editorial board for Pediatric Nephrology; and receiving research funding from Rockwell Medical and Travere. N. R. Powe reports serving as an associate editor for JASN; and receiving honoraria from, and serving as a scientific advisor for/member of, Patient-Centered Outcomes Research Institute, Robert Wood Johnson Foundation, University of Washington, Vanderbilt University, and Yale University. G. V. Roberts reports receiving research funding from Ambulatory Kidney to Improve Vitality Human Factors Project funded by the Veterans Administration, Center for Disease Innovation, and Kidney Precision Medicine Project funded by National Institute of Diabetes and Digestive and Kidney Diseases; serving on a speakers bureau for American Association of Kidney Patients; having other interests in/relationships with American Association of Kidney Patients, APOLLO Recruitment and Dissemination Committees, ASN COVID-19 Response Team and Transplant Subcommittee, ASN Nephrologists Transforming Dialysis Safety Quality, Assessment, Improvement and Education Work Group, Can-SOLVE CKD International Research Advisory Committee, International Nephrology Society Patient Group, KHI Patient Family Partnership Council, NKF Ambassador, and NKF Kidney Advocacy Committee Diversity Workgroup; receiving honoraria from serving on the APOL1 Community Advisory Board, APOLLO Community Advisory Committee, C-Path Patient AKI, Kidney Precision Medicine Project Patient Advisor for Community Advisory Committee, and Northwest Renal Dietitians Annual Conference; serving as a scientific advisor for, or member of, APOLLO Community Advisory Board, ASN COVID-19 Response Team, ASN COVID-19 Transplant Subcommittee, Can-SOLVE CKD International Research Advisory Committee, Center for Dialysis Innovation Patient Advisory Board, C-Path Patient Engagement Committee, Home Dialyzors United Advisory Committee, Kidney Health Initiative (KHI) Patient and Family Partnership Council, and Kidney Research Institutes Patient Advisory Committee; having consultancy agreements with Critical Path Institute (C-Path) AKI Project and Options Unlimited International LLC (information technology management consulting firm); and having an ownership interest in Microsoft (via stock) and Options Unlimited International. W. L. St. Peter reports receiving honoraria from American Nephrology Nursing Association, Integritas Group, Letters and Sciences, and OptumLabs; serving on the Centers for Medicare and Medicaid Services Technical Expert Panel on Development of a Quality Measure Assessing Delay in Progression of CKD, and Technical Expert Panel for Quality Insights Kidney Care Pilot Project; serving as a scientific advisory board member for the NKF; and having consultancy agreements with Total Renal Care Inc. C. Warfield reports having other interests in/relationships with Home Dialyzors United (via the board of directors)and NKF Indiana (via the board of directors). Because M. M. Moxey-Mims and N. R. Powe are associate editors of JASN, they were not involved in the peer-review process for this manuscript. Another editor oversaw the peer-review and decision-making process for this manuscript.
Funding
No funding was provided to the Task Force members. The ASN and NKF provided administrative assistance for scheduling and convening of Task Force members.
Supplementary Material
Acknowledgments
We thank Ms. Nilka Ríos Burrows (MT) from the Division of Diabetes Translation, Centers for Disease Control and Prevention (Atlanta, GA) for information and discussion on the potential implications of eGFR approaches for population surveillance and tracking of kidney disease. The authors thank all who testified and provided expertise and evidence at the Task Force sessions and forums, and Mr. Killian Gause and Ms. Riley Hoffman for assistance during the sessions. The authors also thank Dr. Flor Alvarado and Dr. Abinet Aklilu for assistance in compiling information on diversity in equation development, and Sara Couture (MS) and Shiyuan Miao (MS) for their assistance with the construction of figures and tables. The authors thank the leadership of the NKF and ASN for their support of the Task Force and the entire health care community for their patience in identifying an evidence-based, unifying path forward for estimation of GFR.
This article is being published concurrently in JASN and American Journal of Kidney Diseases. The articles are identical except for stylistic changes in keeping with each journal’s style. Either of these versions may be used in citing this article.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related perspective, “Removing Race from Kidney Disease Diagnosis,” on pages 2987–2989.
Disclaimer: This report and its recommendations concerning the inclusion of race in the estimation of GFR in the United States are advisory only. They are not clinical guidelines, do not define a standard of care, and do not constitute the practice of medicine. Variations in practice will inevitably and appropriately occur when clinicians take into account the needs of individual patients, available resources, and limitations unique to an institution or type of practice. Every health care professional reviewing the recommendations in the report is responsible for evaluating the appropriateness of applying them to any particular clinical situation.
This article is being published concurrently in the Journal of the American Society of Nephrology and American Journal of Kidney Diseases. The articles are identical except for stylistic changes in keeping with each journal's style. Either of these versions may be used in citing this article.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021070988/-/DCSupplemental.
Supplemental Appendix 1. Methods for performance in external validation (bias, precision and accuracy).
Supplemental Appendix 2. Methods for potential consequences on clinical decision making.
Supplemental Appendix 3. Methods for potential consequences on medication dosing.
Supplemental Table 1. Topics and panelists/discussants during phase two.
Supplemental Table 2. Equation specification in each approach.
Supplemental Table 3. Summary of attributes 1 to 4: Filtration marker assay, implementation challenges, equation marker, performance compared with measured GFR*assay.
Supplemental Table 3A. Attribute 1-4 summary: Filtration marker assay, implementation challenges, equation marker, performance compared with measured GFR assay.
Supplemental Table 3B. Metric adjudication key for attributes 1 to 4.
Supplemental Table 4. Performance compared with measured GFR in CKD-EPI 2021 validation.
Supplemental Table 5. Possible consequences of approaches for clinical decision making (attribute 5): General medical care evaluation and management.
Supplemental Table 6. Possible consequences of approaches for clinical decision making (attribute 5). Medication-related decision making.
Supplemental Table 7. Possible consequences of approaches for clinical decision making (attribute 5): Nephrology care and management.
References
- 1.Delgado C, Baweja M, Burrows NR, Crews DC, Eneanya ND, Gadegbeku CA, et al. : Reassessing the inclusion of race in diagnosing kidney diseases: An interim report from the NKF-ASN task force. J Am Soc Nephrol 32: 1305–1317, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwong Y-TD, Stevens LA, Selvin E, Zhang YL, Greene T, Van Lente F, et al. : Imprecision of urinary iothalamate clearance as a gold-standard measure of GFR decreases the diagnostic accuracy of kidney function estimating equations. Am J Kidney Dis 56: 39–49, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sehgal AR: Race and the false precision of glomerular filtration rate estimates. Ann Intern Med 173: 1008–1009, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, et al. ; CRIC Study Investigators : Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 60: 250–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eneanya ND, Kostelanetz S, Mendu ML: Race-free biomarkers to quantify kidney function: Health equity lessons learned from population-based research. Am J Kidney Dis 77: 667–669, 2021 [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention : A closer look at African American men and high blood pressure control: A review of psychosocial factors and systems-level interventions, 2010. Available at: https://www.cdc.gov/bloodpressure/docs/african_american_sourcebook.pdf. Accessed September 17, 2021
- 10.Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, et al. : The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 137: 479–486, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Rocco MV, Bleyer AJ, Burkart JM: Utilization of inpatient and outpatient resources for the management of hemodialysis access complications. Am J Kidney Dis 28: 250–256, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Allon M, Ornt DB, Schwab SJ, Rasmussen C, Delmez JA, Greene T, et al. ; Hemodialysis (HEMO) Study Group : Factors associated with the prevalence of arteriovenous fistulas in hemodialysis patients in the HEMO study. Kidney Int 58: 2178–2185, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Eggers PW: Racial differences in access to kidney transplantation. Health Care Financ Rev 17: 89–103, 1995 [PMC free article] [PubMed] [Google Scholar]
- 14.Daumit GL, Hermann JA, Coresh J, Powe NR: Use of cardiovascular procedures among black persons and white persons: A 7-year nationwide study in patients with renal disease. Ann Intern Med 130: 173–182, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Hoffman KM, Trawalter S, Axt JR, Oliver MN: Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A 113: 4296–4301, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meghani SH, Byun E, Gallagher RM: Time to take stock: A meta-analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Med 13: 150–174, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Bandera EV, Lee VS, Rodriguez-Rodriguez L, Powell CB, Kushi LH: Racial/ethnic disparities in ovarian cancer treatment and survival. Clin Cancer Res 22: 5909–5914, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang M, Wang D, Coresh J, Selvin E: Trends in diabetes treatment and control in U.S. adults, 1999–2018. N Engl J Med 384: 2219–2228, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le P, Chaitoff A, Misra-Hebert AD, Ye W, Herman WH, Rothberg MB: Use of antihyperglycemic medications in US adults: An analysis of the National Health and Nutrition Examination Survey. Diabetes Care 43: 1227–1233, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Shin J-I, Sang Y, Chang AR, Dunning SC, Coresh J, Inker LA, et al. : The FDA metformin label change and racial and sex disparities in metformin prescription among patients with CKD. J Am Soc Nephrol 31: 1847–1858, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eberly LA, Yang L, Eneanya ND, Essien U, Julien H, Nathan AS, et al. : Association of race/ethnicity, gender, and socioeconomic status with sodium-glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open 4: e216139, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duggal V, Thomas IC, Montez-Rath ME, Chertow GM, Kurella Tamura M: National estimates of CKD prevalence and potential impact of estimating glomerular filtration rate without race. J Am Soc Nephrol 32: 1454–1463, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed S, Nutt CT, Eneanya ND, Reese PP, Sivashanker K, Morse M, et al. : Examining the potential impact of race multiplier utilization in estimated glomerular filtration rate calculation on African-American care outcomes. J Gen Intern Med 36: 464–471, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diao JA, Wu GJ, Taylor HA, Tucker JK, Powe NR, Kohane IS, et al. : Clinical implications of removing race from estimates of kidney function. JAMA 325: 184–186, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diao JA, Powe NR, Manrai AK: Removing race from kidney function estimates-reply. JAMA 325: 2018–2019, 2021 [DOI] [PubMed] [Google Scholar]
- 26.Walther CP, Winkelmayer WC, Navaneethan SD: Black race coefficient in GFR estimation and diabetes medications in CKD: National estimates. J Am Soc Nephrol 32: 1319–1321, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pottel H, Bjork J, Courbebaisse M, Couzi L, Ebert N, Eriksen BO, et al. : Development and validation of a modified full age spectrum creatinine-based equation to estimate glomerular filtration rate: A cross-sectional analysis of pooled data. Ann Intern Med 174: 183–191, 2021 [DOI] [PubMed] [Google Scholar]
- 28.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 29.Casal MA, Ivy SP, Beumer JH, Nolin TD: Effect of removing race from glomerular filtration rate-estimating equations on anticancer drug dosing and eligibility: A retrospective analysis of National Cancer Institute phase 1 clinical trial participants. Lancet Oncol 22: 1333–1340, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) : Guidance for industry pharmacokinetics in patients with impaired renal function-study design, data analysis, and impact on dosing draft guidance. Clinical Pharmacology Revision 2, 2020. Available at: https://www.fda.gov/media/78573/download. Accessed July 24, 2020
- 31.Park EJ, Pai MP, Dong T, Zhang J, Ko CW, Lawrence J, et al. : The influence of body size descriptors on the estimation of kidney function in normal weight, overweight, obese, and morbidly obese adults. Ann Pharmacother 46: 317–328, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Nix DE, Mayersohn M, Erstad BL: Should estimates of glomerular filtration rate and creatinine clearance be indexed to body surface area for drug dosing? Am J Health Syst Pharm 74: 1814–1819, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Titan S, Miao S, Tighiouart H, Chen N, Shi H, Zhang L, et al. : Performance of indexed and nonindexed estimated GFR. Am J Kidney Dis 76: 446–449, 2020 [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention : Adult obesity facts. Available at: https://www.cdc.gov/obesity/data/adult.html. Accessed July 24, 2021
- 35.U.S. Department of Health and Human Services Office of Minority Health : Obesity and African Americans. Available at: https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=25. Accessed July 24, 2021
- 36.Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. ; SPRINT Research Group : A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 373: 2103–2116, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. ; CREDENCE Trial Investigators : Canagliflozin and Renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 [DOI] [PubMed] [Google Scholar]
- 38.Inker LA, Eneanya ND, Coresh J, et al. : New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitch WE, Collier VU, Walser M: Creatinine metabolism in chronic renal failure. Clin Sci (Lond) 58: 327–335, 1980 [DOI] [PubMed] [Google Scholar]
- 40.Hsu J, Johansen KL, Hsu CY, Kaysen GA, Chertow GM: Higher serum creatinine concentrations in black patients with chronic kidney disease: beyond nutritional status and body composition. Clin J Am Soc Nephrol 3: 992–997, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang M, Li Y, Yang X, Shan H, Zhang Q, Ming Z, et al. : Serum cystatin C as an inflammatory marker in exacerbated and convalescent COPD patients. Inflammation 39: 625–631, 2016 [DOI] [PubMed] [Google Scholar]
- 42.College of American Pathologists; Clinical Chemistry Committee : Kidney biomarkers: The kidney profile order, urine albumin-creatinine ratio (uACR), and estimated glomerular filtration rate (eGFR), 2020. Available at: https://documents.cap.org/documents/2020-a-kidney-biomarkers.pdf. Accessed January 3, 2021
- 43.Pottel H, Hoste L, Dubourg L, Ebert N, Schaeffner E, Eriksen BO, et al. : An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant 31: 798–806, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Björk J, Grubb A, Sterner G, Nyman U: Revised equations for estimating glomerular filtration rate based on the Lund-Malmö Study cohort. Scand J Clin Lab Invest 71: 232–239, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Levey AS, Tighiouart H, Titan SM, Inker LA: Estimation of glomerular filtration rate with vs without including patient race. JAMA Intern Med 180: 793–795, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. ; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pottel H, Delanaye P, Schaeffner E, Dubourg L, Eriksen BO, Melsom T, et al. : Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant 32: 497–507, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inker LA, Couture SJ, Tighiouart H, Abraham AG, Beck GJ, Feldman HI, et al. ; CKD-EPI GFR Collaborators : A new panel-estimated GFR, including β2-microglobulin and β-trace protein and not including race, developed in a diverse population. Am J Kidney Dis 77: 673–683.e1, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inker LA, Eckfeldt J, Levey AS, Leiendecker-Foster C, Rynders G, Manzi J, et al. : Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis 58: 682–684, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grubb A, Horio M, Hansson L-O, Björk J, Nyman U, Flodin M, et al. : Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin Chem 60: 974–986, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.