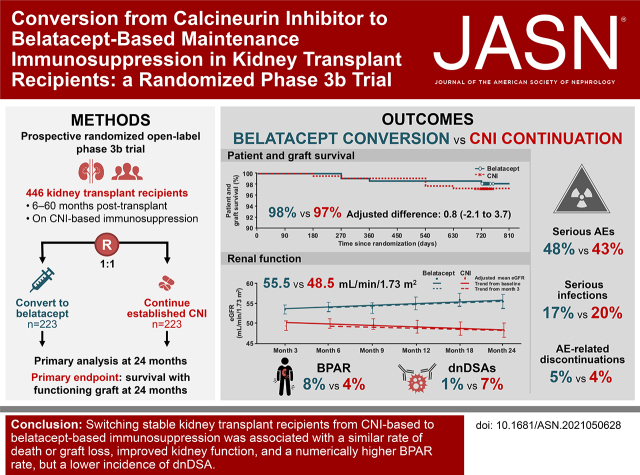
Significance Statement
This randomized trial demonstrates the safety and efficacy of conversion from calcineurin inhibitor (CNI)– to belatacept-based maintenance immunosuppression in renal transplant recipients 6–60 months post-transplant. Patients converted to belatacept showed sustained improvement in renal function associated with an acceptable safety profile consistent with prior experience and a smaller treatment difference in acute rejection postconversion compared with that observed in earlier studies in de novo renal allograft recipients. These results favor the use of belatacept as an alternative to continued long-term CNI-based maintenance immunosuppression, which is particularly relevant for CNI-intolerant patients, including those who experience nephrotoxicity. These data help inform clinical practice guidelines regarding the conversion of such patients to an alternative immunosuppressive drug regimen.
Keywords: renal transplantation, randomized controlled trials, acute rejection, renal function, immunosuppression, abatacept, calcineurin inhibitors, kidney transplantation, transplant recipients
Visual Abstract
Abstract
Background
Calcineurin inhibitors (CNIs) are standard of care after kidney transplantation, but they are associated with nephrotoxicity and reduced long-term graft survival. Belatacept, a selective T cell costimulation blocker, is approved for the prophylaxis of kidney transplant rejection. This phase 3 trial evaluated the efficacy and safety of conversion from CNI-based to belatacept-based maintenance immunosuppression in kidney transplant recipients.
Methods
Stable adult kidney transplant recipients 6–60 months post-transplantation under CNI-based immunosuppression were randomized (1:1) to switch to belatacept or continue treatment with their established CNI. The primary end point was the percentage of patients surviving with a functioning graft at 24 months.
Results
Overall, 446 renal transplant recipients were randomized to belatacept conversion (n=223) or CNI continuation (n=223). The 24-month rates of survival with graft function were 98% and 97% in the belatacept and CNI groups, respectively (adjusted difference, 0.8; 95.1% CI, −2.1 to 3.7). In the belatacept conversion versus CNI continuation groups, 8% versus 4% of patients experienced biopsy-proven acute rejection (BPAR), respectively, and 1% versus 7% developed de novo donor-specific antibodies (dnDSAs), respectively. The 24-month eGFR was higher with belatacept (55.5 versus 48.5 ml/min per 1.73 m2 with CNI). Both groups had similar rates of serious adverse events, infections, and discontinuations, with no unexpected adverse events. One patient in the belatacept group had post-transplant lymphoproliferative disorder.
Conclusions
Switching stable renal transplant recipients from CNI-based to belatacept-based immunosuppression was associated with a similar rate of death or graft loss, improved renal function, and a numerically higher BPAR rate but a lower incidence of dnDSA.
Clinical Trial registry name and registration number: A Study in Maintenance Kidney Transplant Recipients Following Conversion to Nulojix® (Belatacept)-Based, NCT01820572
Calcineurin inhibitor (CNI)–based immunosuppression has largely eliminated early graft loss from rejection in renal transplant recipients.1 However, CNIs are potentially nephrotoxic, resulting in impaired renal function and increased cardiovascular risk and leading to diminished long-term patient and graft survival.2 The development of anti-HLA de novo donor-specific antibodies (dnDSAs) with current standard immunosuppression therapies, including CNIs, is another major threat to long-term graft survival, as chronic antibody-mediated rejection is believed to be a major cause for graft loss.3 In a study evaluating long-term dnDSA incidence in kidney transplant recipients, nearly all on CNI-based immunosuppression, approximately 25% of patients developed dnDSAs 10 years after transplant.4 Immunosuppression strategies are needed for minimizing CNI exposure to reduce late transplant failure rates.5 One such strategy is the conversion from CNI to alternative maintenance immunosuppression.5 Conversion to mammalian target of rapamycin inhibitors has shown variable degrees of improvement in renal function in several studies; however, risks of rejection, development of dnDSAs, and in some cases, graft failure were shown to be higher than those with CNIs.5–8 In addition, discontinuations due to dose-limiting mammalian target of rapamycin inhibitor–associated toxicities were common.6
Belatacept, a fusion protein composed of the human IgG1 Fc fragment linked to the modified extracellular domain of cytotoxic T lymphocyte–associated antigen 4, selectively inhibits T cell activation by blocking the CD28-CD80/86 costimulatory pathway.9 Compared with kidney transplant recipients receiving cyclosporin, those receiving belatacept showed an improved cardiovascular and metabolic profile, reduced incidence of chronic allograft nephropathy, reduced incidence of dnDSAs, and improved renal function and in living or standard criteria deceased donor kidney recipients, better long-term (7-year) patient and graft survival.10–15 However, a higher incidence of biopsy-proven acute rejection (BPAR) was noted with belatacept than with cyclosporin. Although the overall safety profile was similar between belatacept and cyclosporin, belatacept was associated with an increased risk of post-transplant lymphoproliferative disorder (PTLD), particularly in Epstein–Barr virus (EBV)–seronegative individuals.10,11,13–15
Several studies exploring conversion from CNI- to belatacept-based immunosuppression in kidney transplant recipients have shown improved renal function postbelatacept conversion, although in association with acute rejection rates of 4%–13%.11,16–21 In an earlier phase 2 study conducted to evaluate conversion from CNI- to belatacept-based regimens in clinically stable renal allograft recipients 6–36 months post-transplantation,22 conversion from CNI- to belatacept-based therapy was well tolerated without graft loss at 12 months postrandomization, and improved renal function was noted, albeit with a higher BPAR rate (7% versus 0% in the CNI group). The majority of BPAR episodes occurred during the first 6 months.22,23 On this basis, a prospective, randomized, open-label phase 3b study was undertaken to evaluate the efficacy and safety of conversion of renal allograft recipients from CNI- to belatacept-based maintenance immunosuppression.
Methods
Trial Design and Participants
This active comparator, prospective, parallel-group, randomized, open-label phase 3b study was conducted at 85 centers in ten countries. Adult patients who had received living or deceased donor kidney transplants 6–60 months prior to enrollment were eligible. All patients were EBV seropositive and had received CNI-based immunosuppression, along with a mycophenolate and daily corticosteroids, for a month or longer. A key inclusion criterion was stable renal function, defined as the absence of new-onset proteinuria during the 12-week period prior to enrollment or proteinuria ≤500 mg/d in diabetic patients or ≤1000 mg/d in nondiabetic patients prior to the 12-week period and an eGFR between ≥30 and ≤75 ml/min per 1.73 m2 (assessed at screening and at one additional time point 2–12 weeks prior to screening; detailed inclusion criteria are available in the study protocol). Patients were excluded if they were EBV seronegative or if EBV serostatus was unknown; had a history of BPAR within 3 months prior to enrollment; had experienced antibody-mediated acute rejection, recurrent acute rejection, or greater than or equal to Banff 97 grade IIA acute rejection in the current allograft; had previous graft loss due to BPAR; or had a positive T cell lymphocytotoxicity crossmatch prior to the current transplant.
Written informed consent was obtained at the time of enrollment. This study was conducted in accordance with Good Clinical Practice per the International Conference on Harmonization and applicable regulatory requirements. The study protocol and any amendments were reviewed and approved by the institutional review board/independent ethics committee for each site prior to study initiation.
Randomization and Masking
Eligible kidney transplant recipients were randomized in a central system in a 1:1 ratio to either switch to belatacept or continue with their established CNI treatment. Patients were stratified by screening eGFR in a 1:2 ratio (≥30 to <45 or ≥45 to <75 ml/min per 1.73 m2) to maintain similar distributions across treatment groups.
Study Assessments
The belatacept dosing regimen in this study was identical to that in the phase 2 conversion study.22 Patients in the conversion group received belatacept 5 mg/kg as a 30-minute intravenous infusion every 2 weeks (days 1, 15, 29, 43, and 57) for the first 8 weeks and every 28 days thereafter as a maintenance regimen. The CNI dose was tapered to 40%–60% by day 15 and 20%–30% by day 22, and it was discontinued by 29±3 days postrandomization. In the CNI continuation group, dosing was continued to maintain trough serum concentrations in the range of 50–250 ng/ml for cyclosporin or 4–11 ng/ml for tacrolimus. All patients continued to receive a mycophenolate (mofetil or sodium salt) and daily corticosteroids. After randomization, patients were followed for 24 months.
Renal biopsies were performed to assess all clinically suspected episodes of acute rejection per protocol-specified criteria; surveillance biopsies were not permitted. Acute rejection was diagnosed on the basis of the local pathologist’s interpretation of the biopsy findings and treated per local standard of care. Samples for donor-specific antibody testing were collected prior to the first dose of belatacept and at 12 and 24 months.
Safety was assessed from baseline throughout the study period and at follow-up 8 weeks after the last dose. Safety monitoring of serious viral infections (cytomegalovirus [CMV] or BK virus infections) was performed at the discretion of the individual investigators and was not specified in the protocol. After completion of the study, patients in the belatacept conversion arm who continued commercially available belatacept were followed up at 8 weeks postcompletion. Patients who discontinued belatacept and switched to nonbelatacept-based regimens also underwent serum testing for the presence of antibelatacept antibodies at weeks 8, 12, and 24 after discontinuation.
Study End Points
The primary end point of this study was the percentage of patients surviving with a functioning graft at 24 months postrandomization. Secondary end points included individual patient and graft survival outcomes at 12 and 24 months; incidence and severity of BPAR at 12 and 24 months; renal function, assessed by mean eGFR and mean change from baseline eGFR at 12 and 24 months; mean changes from baseline systolic and diastolic BP at 12 and 24 months; the proportion of patients with preexisting donor-specific antibodies at baseline and dnDSAs at 12 and 24 months; and safety monitoring for adverse events (AEs). Post hoc subgroup (sensitivity) analyses were performed to assess survival with graft function, BPAR, and mean percentage change from baseline eGFR at 24 months on the basis of demographic, therapeutic, and renal function variables.
Statistical Analyses
Because of the absence of previously published and adequately sized randomized trial data for this patient population, an acceptable noninferiority margin for the treatment difference in the primary end point could not be established for regulatory purposes. Therefore, this study was not powered to demonstrate any statistically significant treatment differences in survival with graft function that might exist at month 24. These factors determined the selection of the descriptive primary end point and the key secondary end points.
However, it was estimated that a sample size of approximately 220 patients per treatment group would provide sufficient power to rule out a clinically unacceptable difference in patient and graft survival. With a confidence level (one sided) of 0.975 and assuming a 93% patient and graft survival rate at month 24 in both treatment groups, a sample size of 220 patients per arm was estimated to provide a 90% probability to rule out a treatment difference of 8.3% in the primary end point.
Efficacy analyses were based on the intention-to-treat population. Safety was assessed in all patients who received at least one dose of study treatment. Efficacy outcomes were summarized within each treatment group using point estimates and the corresponding 95% confidence intervals (95% CIs). The difference between treatment arms was presented as proportions of patients who survived with a functioning graft along with 95.1% CIs calculated on the basis of the O’Brien and Fleming α-spending function adjustment. Graft loss was defined as a sustained level of GFR<15 ml/min per 1.73 m2 for ≥4 weeks, resumption of regularly scheduled dialysis for a period of ≥56 days, or retransplantation. The incidence and severity of clinically suspected BPAR up to 24 months postrandomization were summarized using point estimates and 95% CIs. eGFR was calculated using the four-variable Modification of Diet in Renal Disease equation.24 Adjusted estimates for differences in proportions were adjusted for the screening calculated GFR using the minimum risk weights methodology.25 Adjusted renal function estimates were based on a repeated measures model with treatment, month (categorical variable), baseline eGFR (continuous variable), and interaction of treatment by month as covariates. For patient and graft survival and eGFR, analyses were performed both with imputation to zero for data missing due to death or graft loss and without imputation. Descriptive summaries were provided for systolic and diastolic BP.
Results
Patient Disposition and Baseline Characteristics
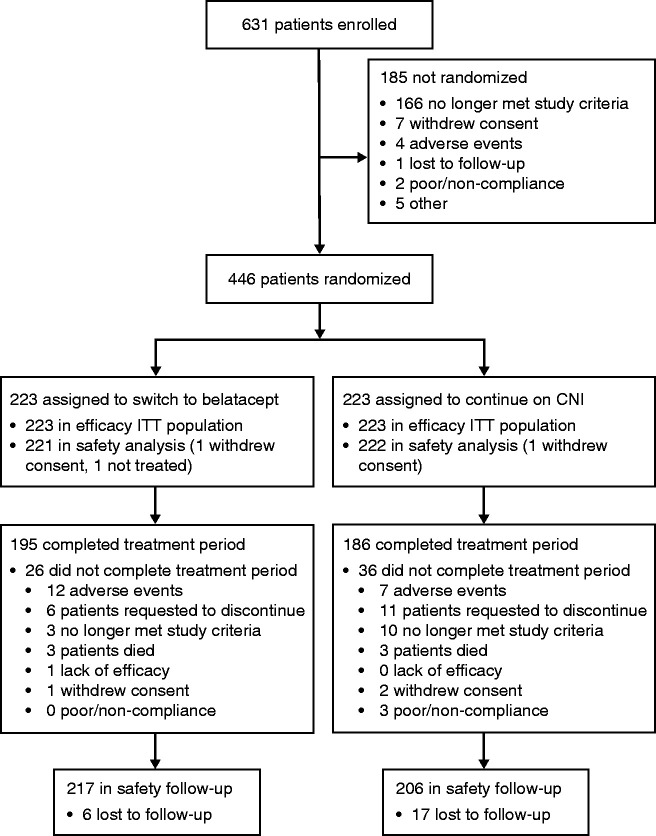
Between April 17, 2013 and May 22, 2017, 631 renal transplant recipients were enrolled, and 446 were randomly assigned to the belatacept conversion (n=223) or the CNI continuation group (n=223) (Figure 1). Of 185 patients not randomized, 166 did not meet study criteria, and seven withdrew consent. Overall, 221 patients in the belatacept group and 222 in the CNI group were included in the safety analysis; three patients (n=2, belatacept; n=1, CNI) did not receive treatment.
Figure 1.
The trial profile of patient disposition shows 446 patients randomized to belatacept conversion (n=223) or CNI continuation (n=223); these patients constituted the ITT population. The 24-month treatment period was completed by 87% of patients in the belatacept group and 83% in the CNI group. ITT, intention to treat.
Baseline characteristics were well balanced between the two treatment groups (Table 1). In the overall population (n=446), 82% of patients had no prior transplants, 66% had received allografts from deceased donors, 62% had a baseline eGFR ≥45 ml/min per 1.73 m2, and 94% had hypertension. Mean serum creatinine concentrations were similar at baseline in both groups but varied inversely with the corresponding mean baseline eGFRs, and they were numerically higher among Black patients (Supplemental Table 1). All patients were EBV seropositive, 62% were CMV seropositive, and 18% were CMV-seronegative recipients of a CMV-positive donor kidney. Overall, 73% had ≤5% panel reactive antibodies prior to the current transplant, and 52% had zero to three donor-recipient HLA mismatches. Most patients (89%) were receiving tacrolimus at study entry.
Table 1.
Baseline characteristics
| Characteristics | Belatacept Conversion, n=223 | CNI Continuation, n=223 |
|---|---|---|
| Age, yr | 53.4 (11.3) | 52.6 (11.7) |
| <65 | 184 (83%) | 186 (83%) |
| ≥65 | 39 (17%) | 37 (17%) |
| Sex | ||
| Men | 150 (67%) | 151 (68%) |
| Women | 73 (33%) | 72 (32%) |
| Race | ||
| White | 191 (86%) | 187 (84%) |
| Black | 24 (11%) | 24 (11%) |
| Asian | 1 (<1%) | 3 (1%) |
| Other | 7 (3%) | 9 (4%) |
| Geographic region | ||
| Europe | 97 (43%) | 92 (41%) |
| North America | 91 (41%) | 94 (42%) |
| South America | 35 (16%) | 37 (17%) |
| Prior transplants | ||
| 0 | 183 (82%) | 182 (82%) |
| 1 | 37 (17%) | 39 (17%) |
| 2 | 3 (1%) | 2 (1%) |
| Baseline eGFR based on laboratory values, ml/min per 1.73 m2 | ||
| <30 | 1 (<1%) | 0 |
| 30 to <45 | 87 (39%) | 83 (37%) |
| 45 to <75 | 131 (59%) | 136 (61%) |
| ≥75 | 4 (2%) | 4 (2%) |
| Mean | 49.6 (12.1) | 50.7 (11.6) |
| Mean (SD) baseline creatinine, mg/dl | 1.47 (0.35) | 1.44 (0.33) |
| Time from transplant to randomization, mo | 21.5 (14.0) | 21.6 (13.5) |
| Panel reactive antibodies, n/N (%) | ||
| ≤5% | 164/211 (78) | 160/212 (75) |
| >5% | 47/211 (22) | 52/212 (25) |
| Missing | 12/223 (5) | 11/223 (5) |
| Type of transplant donor | ||
| Living, related | 37 (17%) | 41 (18%) |
| Living, unrelated | 38 (17%) | 36 (16%) |
| Deceased donor | 148 (66%) | 146 (65%) |
| Primary cause of ESKD | ||
| Glomerular disease | 45 (20%) | 54 (24%) |
| Polycystic kidney disease | 43 (19%) | 45 (20%) |
| Diabetes mellitus | 38 (17%) | 30 (13%) |
| Hypertensive nephrosclerosis | 24 (11%) | 27 (12%) |
| Tubular and intestinal diseases | 9 (4%) | 9 (4%) |
| Renovascular and other vascular diseases | 8 (4%) | 6 (3%) |
| Congenital, rare familial, and metabolic disorders | 5 (2%) | 3 (1%) |
| Neoplasms | 0 | 1 (<1%) |
| Other | 50 (22%) | 48 (22%) |
| Not reported | 1 (<1%) | 0 |
| Specific disease history | ||
| Hypertension | 208 (93%) | 210 (94%) |
| Hypercholesterolemia | 100 (45%) | 104 (47%) |
| Type 2 diabetes mellitus | 60 (27%) | 63 (28%) |
| Type 1 diabetes mellitus | 14 (6%) | 4 (2%) |
| Donor-recipient HLA mismatches | ||
| 0–3 | 117 (52%) | 116 (52%) |
| 4–6 | 106 (48%) | 107 (48%) |
| Donor-recipient CMV serostatus | ||
| Positive-positive | 94 (42%) | 99 (44%) |
| Negative-negative | 47 (21%) | 39 (17%) |
| Negative-positive | 39 (17%) | 43 (19%) |
| Positive-negative | 39 (17%) | 42 (19%) |
| Unknown | 4 (2%) | 0 |
| Baseline immunosuppressants a | ||
| Tacrolimus + MMF | 118 (53%) | 125 (56%) |
| Tacrolimus + MPS | 82 (37%) | 73 (33%) |
| Cyclosporin + MMF | 13 (6%) | 15 (7%) |
| Cyclosporin + MPS | 10 (4%) | 10 (4%) |
Data are n (%) or mean (SD) unless otherwise stated. MMF, mycophenolate mofetil; MPS, mycophenolate sodium.
Baseline tacrolimus and cyclosporin concentrations at study entry are provided in Supplemental Table 2.
Clinical database lock occurred on September 10, 2019, after trial completion; 381 patients (195 [87%] in the belatacept group; 186 [83%] in the CNI group) completed the 24-month treatment period. The most common reason for discontinuation was AEs in the belatacept group (5%) and patient request to discontinue in the CNI group (5%). The median durations of study drug exposure were 24.8 and 24.2 months for the belatacept and CNI groups, respectively. In the CNI continuation arm, mean serum trough concentrations of tacrolimus and cyclosporin during the study period approximated concentrations observed at study entry (Supplemental Table 2).
Primary Efficacy Outcome: Patient/Graft Survival
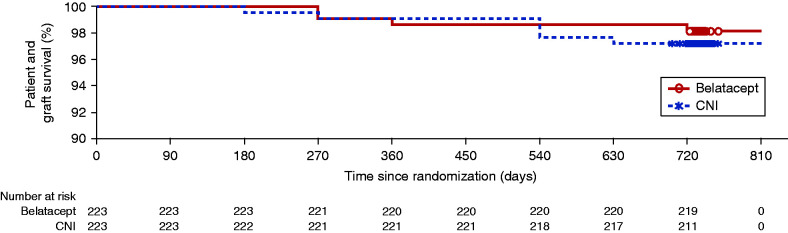
At 24 months, 219 (98%) patients in the belatacept conversion group and 217 (97%) patients in the CNI continuation group were alive with a functioning graft (Figure 2, Table 2), corresponding to an adjusted treatment difference of 0.8 (95.1% CI O’Brien and Fleming α-spending function, −2.1 to 3.7). Kaplan–Meier analyses indicated a similar time to the first occurrence of death or graft loss in both groups. In the intention-to-treat population, eight patients died (four in each group), all with functioning grafts. In the belatacept group, three patients died due to unwitnessed, presumed acute cardiac events, and one patient (randomized to belatacept conversion but was never treated because he withdrew consent) died of infection during month 24. The four deaths in the CNI continuation group were attributed to gram-negative bacterial sepsis, disseminated histoplasmosis, acute myocardial infarction, and strangulated small bowel obstruction (n=1 each). One additional death was reported in each group poststudy. One patient, who developed noncentral nervous system (non-CNS) PTLD postbelatacept conversion, discontinued treatment, received chemotherapy, and completed the study alive with graft function but died 15 months poststudy of an unrelated cause (motor vehicle accident); one patient in the CNI group was diagnosed with lung cancer at week 104 and died 3 months poststudy.
Figure 2.
Kaplan-Meier analysis of the time to first occurrence of death or graft loss of the ITT population showed a similar time to death or graft loss in the belatacept conversion group and in the CNI continuation group. No patient was imputed as having graft loss or death due to loss of follow-up.
Table 2.
Treatment effect on patient and graft survival (primary end point), BPAR, and renal function at 24 months
| End Points | Belatacept Conversion, n=223 | CNI Continuation, n=223 |
|---|---|---|
| Patient and graft survival | ||
| Patients surviving with a functioning graft | 219 (98%) | 217 (97%) |
| Adjusted difference from CNI (95.1% CI) | 0.8 (−2.1 to 3.7) | |
| Graft loss or death | 4 (2%) | 6 (3%) |
| Graft loss | 0 | 2 (1%) |
| Death | 4 (2%) | 4 (2%) |
| Death with a functioning graft | 4 (2%) | 4 (2%) |
| BPAR | ||
| Patients with cellular (Banff IA or higher) or antibody-mediated BPAR | 18 (8%) | 9 (4%) |
| Adjusted difference from CNI (95.1% CI) | 4.1 (−0.4 to 8.5) | |
| All Banff grade (1A or higher) acute cellular rejection events | 20 (9%) | 6 (3%) |
| Mild acute (IA) | 2 (1%) | 4 (2%) |
| Mild acute (IB) | 1 (<1%) | 0 |
| Moderate acute (IIA) | 7 (3%) | 1 (<1%) |
| Moderate acute (IIB) | 6 (3%) | 0 |
| Severe acute (III) | 4 (2%) | 1 (<1%) |
| All humoral rejection events | 5 (2%) | 5 (2%) |
| Humoral only | 0 | 3 (1%) |
| Humoral and cellular | 5 (2%) | 2 (1%) |
| Renal function | ||
| Mean adjusted eGFR, ml/min per 1.73 m2 (95% CI) | ||
| Month 12 | 55.0 (53.5 to 56.6) | 49.3 (47.7 to 50.8) |
| Month 18 | 55.9 (54.3 to 57.6) | 48.9 (47.2 to 50.5) |
| Month 24 | 55.5 (53.8 to 57.3) | 48.5 (46.7 to 50.3) |
| Mean adjusted change from baseline at month 24 in eGFR, ml/min per 1.73 m2 | +5.2 | −1.9 |
| Adjusted difference from CNI (95.1% CI) | 7.0 (4.5 to 9.6) | |
| P value | <0.001 | |
Data are n (%) unless otherwise stated.
By month 24, two patients in the CNI group had experienced functional graft loss. One of these events occurred in a patient who developed CNI-associated thrombotic microangiopathy that necessitated reinitiation of maintenance dialysis for >3 months, thereby meeting the protocol definition of functional graft loss; however, following multiple doses of eculizumab, this patient recovered sufficient renal function to complete the study off dialysis. Two additional patients in the CNI group experienced functional graft loss poststudy (on days 759 and 837) (not included in Table 2). Patient and graft survival rates were consistent between the belatacept and CNI groups across all subgroups analyzed (Supplemental Table 3).
Secondary Efficacy Outcomes: BPAR, Renal Function, and Donor-Specific Antibodies
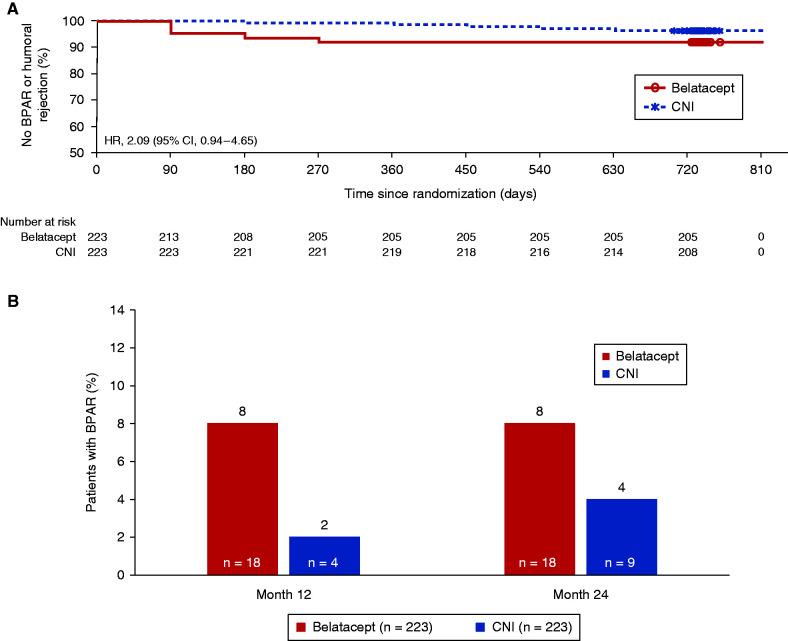
At 24 months, 18 (8%) patients in the belatacept group had experienced one or more BPAR episodes compared with nine (4%) patients in the CNI group; the Cox model hazard ratio estimate was 2.09 (95% CI, 0.94 to 4.65) (Figure 3A, Table 2). In the belatacept group, isolated episodes of acute cellular rejection (Banff grade IA or higher) occurred in 16 patients, and the remaining two patients each experienced a second event, leading to 20 total rejection events. All events were successfully treated with increased corticosteroid dosing and in six cases, with concomitant lymphocyte-depleting therapy. In the CNI continuation group, nine patients experienced isolated BPAR episodes by 24 months; of these, three developed isolated antibody-mediated (humoral) rejection, one of whom experienced subsequent functional graft loss. All other events responded to increased corticosteroid dosing; two patients received concomitant lymphocyte-depleting therapy. Two additional patients in the CNI group were diagnosed with Banff IA acute cellular rejection by the local pathologist, but the central pathologist determined that there was either no cellular rejection (n=1) or the cellular rejection was borderline/suspicious (n=1) (not included in Table 2). In the belatacept group, all BPAR episodes occurred during the first 7 months after randomization (Figure 3B). In the CNI continuation group, four of nine events were reported during the first year (2% at month 12), and another five were reported during the second year (4% at month 24). In sensitivity analyses, BPAR rates across most patient subgroups were consistent with those reported in the overall population (Supplemental Table 4).
Figure 3.
Treatment effect on time and incidence of BPAR. (A) The Kaplan-Meier analysis of time to first BPAR event showed an HR of 2.09 (95% CI, 0.94 to 4.65) for belatacept conversion relative to CNI continuation. (B) The incidence of BPAR at 24 months was 8% in the belatacept conversion group and 4% in the CNI continuation group. In the belatacept group, all episodes occurred during the first 7 months after randomization, whereas in the CNI group, the rate was 2% at month 12 and 4% at month 24. HR, hazard ratio.
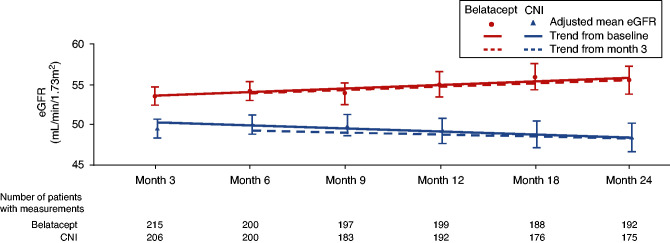
A sustained improvement in renal function was noted in the belatacept group over the 24-month period, and the mean eGFR in this group was higher than that in the CNI group at all time points, beginning at month 3 (Figure 4, Table 2). At 24 months, the adjusted mean change from baseline eGFR was 5.2 ml/min per 1.73 m2 in the belatacept group versus −1.9 ml/min per 1.73 m2 in the CNI group, resulting in a treatment difference of 7.0 ml/min per 1.73 m2 (P<0.001). Analysis of the trend in baseline-adjusted mean eGFR over 24 months showed an estimated positive slope of 0.68 ml/min per 1.73 m2 following belatacept conversion. Over the same period, the trend in baseline-adjusted mean eGFR was negative in the CNI continuation group, corresponding to a slope of −0.11 ml/min per 1.73 m2 (Figure 4). Results from the analysis of mean eGFR changes from month 3 were similar to those of mean eGFR changes from baseline. At 24 months, 48% and 22% of patients in the belatacept and CNI groups, respectively, experienced 10% improvement in eGFR (Supplemental Table 5). The adjusted mean percentage change from baseline eGFR was greater in the belatacept group versus the CNI group for all subgroups analyzed. When patients were stratified into baseline eGFR subgroups of <45, 45 to <60, or ≥60 ml/min per 1.73 m2, at month 24, the adjusted mean percentage changes from baseline eGFR were 22.6%, 10.8%, and 6.2%, respectively, with belatacept conversion. In the CNI continuation group, the corresponding adjusted mean percentage changes from baseline eGFR were 0.2%, 0.7%, and −7.3%, respectively (Supplemental Figure 1).
Figure 4.
Plots of adjusted mean eGFR over time from randomization (solid lines) and month 3 (dotted lines) indicated a sustained improvement in renal function in the belatacept conversion group during the 24-month period. The trend in baseline-adjusted mean eGFR over 24 months showed an estimated positive slope of 0.68 ml/min per 1.73 m2 in the belatacept group and a negative slope of −0.11 ml/min per 1.73 m2 in the CNI group. For patients who died or had graft loss, missing eGFR values were imputed to zero. Adjusted mean eGFR values (error bars representing 95% CIs) over time are shown along with trend lines on the basis of a linear mixed model using treatment group, baseline eGFR, month (continuous), and interaction term treatment by month as fixed effects. These models also included a random intercept and slope for month. A separate model was fitted for the trend analysis from baseline (shown as solid lines) and month 3 (shown as dashed lines).
Mean eGFR was numerically lower in patients with BPAR than in those without BPAR in both arms (Supplemental Figure 2); however, small numbers of patients with events (n=18, belatacept; n=9, CNI) precluded additional analysis of BPAR on eGFR.
Among patients with available urine protein-creatinine ratio (UPCR) data, more patients had a UPCR≥0.5 mg/mg at month 24 than at baseline in both groups (Supplemental Table 6); 24-month mean (SD) UPCR values were 0.255 (0.314) and 0.217 (0.323) mg/mg in the belatacept and CNI groups, respectively.
Mean changes from baseline systolic and diastolic BP were small and not clinically meaningful in either group. At 24 months, mean changes in systolic and diastolic BP were −1.3 and −1.7 mm Hg, respectively, in the belatacept group versus +1.2 and +0.5 mm Hg, respectively, in the CNI group; patients in each group received an average of 2.3 antihypertensive medications. Mean changes from baseline fasting serum lipid concentrations were generally small and not clinically meaningful in most patients. New-onset diabetes after transplant occurred in 12 (5%) and ten (4%) patients in the belatacept and CNI groups, respectively.
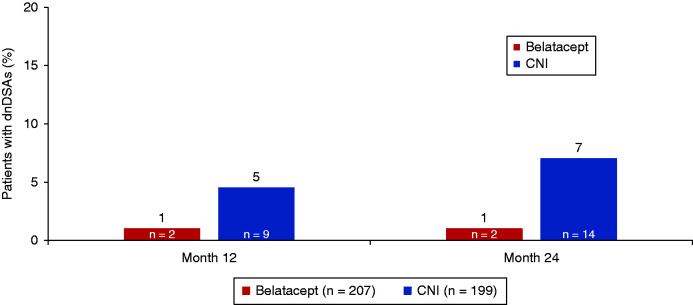
Preexisting donor-specific antibodies were identified at baseline in ten (5%) of 207 patients in the belatacept group and 26 (13%) of 199 patients in the CNI group. By 24 months, two patients (1%) in the belatacept group and 14 patients (7%) in the CNI group had developed dnDSAs that were not present at baseline (Figure 5). Only class 1 dnDSAs were detected in the belatacept group (Supplemental Table 7). Among the 14 patients in the CNI group who developed dnDSAs, class 1 antibodies were identified in six patients, class 2 antibodies were identified in 12 patients, and both classes 1 and 2 antibodies were identified in four patients.
Figure 5.
Analysis of the prevalence of dnDSAs indicated that 1% of patients in the belatacept group and 7% of patients in the CNI group developed dnDSAs at 24 months postrandomization.
Safety
At least one AE was reported in 211 (95%) of 221 patients in the belatacept conversion group and 204 (92%) of 222 patients in the CNI continuation group (Table 3). The most common AEs in the belatacept versus CNI groups were diarrhea (48 [22%] versus 63 [28%]) and nasopharyngitis (44 [20%] versus 50 [23%]). Serious AEs were reported in 107 (48%) patients in the belatacept group and 95 (43%) patients in the CNI group. AEs led to treatment discontinuation in 12 (5%) patients in the belatacept group and eight (4%) patients in the CNI group. None of the seven deaths reported in the safety population (n=3, belatacept; n=4, CNI) were attributed to study treatment.
Table 3.
Most common AEs (>10%), serious AEs (>3%), and malignancies at 24 months
| Variable | Belatacept Conversion, n=221 | CNI Continuation, n=222 |
|---|---|---|
| AEs | 211 (95%) | 204 (92%) |
| Diarrhea | 48 (22%) | 63 (28%) |
| Nasopharyngitis | 44 (20%) | 50 (23%) |
| Urinary tract infection | 42 (19%) | 34 (15%) |
| Cough | 31 (14%) | 20 (9%) |
| Hypertension | 29 (13%) | 21 (9%) |
| Headache | 27 (12%) | 23 (10%) |
| Bronchitis | 23 (10%) | 18 (8%) |
| Peripheral edema | 22 (10%) | 35 (16%) |
| Arthralgia | 21 (10%) | 23 (10%) |
| Serious AEs | 107 (48%) | 95 (43%) |
| Kidney transplant rejectiona | 19 (9%) | 7 (3%) |
| Basal cell carcinoma | 11 (5%) | 5 (2%) |
| Urinary tract infection | 7 (3%) | 7 (3%) |
| Pneumonia | 5 (2%) | 7 (3%) |
| Major categories of serious AEs | ||
| Any serious infection | 37 (17%) | 44 (20%) |
| Any serious viral infection | 5 (2%) | 9 (4%) |
| Influenza | 2 (1%) | 2 (1%) |
| BK virus infection | 1 (<1%) | 2 (1%) |
| CMV infection | 0 | 2 (1%) |
| Gastroenteritis norovirus | 1 (<1%) | 1 (<1%) |
| Herpes zoster | 2 (1%) | 0 |
| Gastroenteritis rotavirus | 0 | 1 (<1%) |
| Gastroenteritis viral | 0 | 1 (<1%) |
| Pneumonia respiratory syncytial viral | 0 | 1 (<1%) |
| Any serious fungal infection | 0 | 1 (<1%)b |
| Any malignancy, excluding PTLD | 17 (8%) | 12 (5%) |
| All malignancies, n (%) | ||
| Basal cell carcinoma | 11 (5%) | 5 (2%) |
| Squamous cell carcinoma of skin | 4 (2%) | 1 (<1%) |
| Squamous cell carcinoma | 3 (1%) | 5 (2%) |
| Bowen disease | 2 (1%) | 1 (<1%) |
| Prostate cancer, including recurrence | 1 (<1%) | 1 (<1%) |
| PTLDc | 1 (<1%) | 0 |
| Papillary thyroid carcinoma | 1 (<1%) | 0 |
| Lung adenocarcinoma | 0 | 1 (<1%) |
Data are n (%).
Not based on AE records; not all biopsies were reported as serious AEs.
Patient experienced disseminated histoplasmosis, which resulted in death.
Patient developed non-CNS PTLD, received chemotherapy, and completed the study with a functioning graft.
Malignancies other than PTLD were reported in 17 (8%) and 12 (5%) patients in the belatacept and CNI groups, respectively; these were mostly attributed to nonmelanoma skin cancers. Basal cell carcinoma occurred more frequently in the belatacept group (11 patients [5%] versus five patients in the CNI group [2%]) and accounted for most of the difference in overall malignancy rate between groups.
One case of non-CNS PTLD was reported in the belatacept group (<1%) versus none in the CNI group. In the belatacept and CNI groups, serious infections were reported in 37 (17%) and 44 (20%) patients, respectively, with serious viral infections occurring in five (2%) and nine (4%) patients, respectively; CMV viremia or infection occurring in two and zero patients, respectively; and BK virus infections occurring in two and one patient, respectively. No cases of tuberculosis, CNS infections, or progressive multifocal leukoencephalopathy were reported. Infusion-related reactions occurred in 13 (6%) patients with belatacept conversion within 24 hours of belatacept infusion; all events were mild to moderate in intensity, and none required hospitalization or discontinuation from assigned therapy.
Discussion
This study is the largest prospective, randomized, comparative trial of conversion from a CNI-based regimen to belatacept in maintenance renal transplant recipients. The rate of survival with graft function at 2 years postconversion was similar to that with CNI continuation in clinically stable allograft recipients whose study participation began 6–60 months post-transplant. Sustained improvements in renal function were observed over 24 months in the belatacept conversion group relative to the CNI continuation group. Although a higher BPAR rate was observed following belatacept conversion than with CNI continuation, all rejection events in the belatacept group occurred within the first 6–7 months postconversion and responded to treatment. Despite a higher rejection rate with belatacept conversion, these patients had a lower incidence of dnDSAs than patients who continued CNI treatment, and none developed class 2 dnDSAs, which are the drivers of chronic antibody-mediated rejections.26 Importantly, conversion to belatacept was associated with a safety profile consistent with prior studies of belatacept use from the time of renal transplantation.10,13–15 No new or unexpected AEs were reported overall. The 2-year incidence of serious infections following belatacept conversion was comparable with that with CNI continuation, and premature treatment discontinuation rates were similar across groups.
These data are consistent with earlier phase 2 conversion trial results, in which similar death or graft loss rates and similar degrees of improvement in renal function were observed with belatacept conversion versus CNI continuation, despite a higher BPAR rate during the first year.22 In the phase 2 trial, the observed postconversion improvement in renal function persisted through 3 years of follow-up.23 Similar renal function improvements have been reported in previous belatacept conversion studies.16–21 However, early conversion to belatacept, attempted within 3–6 months post-transplant, was associated with higher acute rejection rates,11,16,27 as well as higher rates of infection-related hospitalizations and opportunistic infections, particularly CMV infection in high-risk (donor-positive/recipient-negative) recipients.16 Reported BPAR rates following later conversion (6 months or longer post-transplant) have been substantially lower (1-year rates: 4%–13%),16,17,27 consistent with rejection rates observed in this phase 3 study (8.1% at 2 years) and the phase 2 study (8.4% at 3 years),23 both of which enrolled patients ≥6 months post-transplantation. Both studies reported lower serious and opportunistic infection rates than those reported in the literature following early conversion. Overall, these findings suggest a favorable benefit-risk ratio for conversion to belatacept after 6 months post-transplantation.
Although a higher BPAR incidence was observed in this study at 2 years, all postconversion events occurred relatively early (i.e., ≤7 months, consistent with previous studies).18,22,27 In contrast, BPAR events in the CNI continuation group occurred at a similar frequency during the first and second years of follow-up, although no modifications to the maintenance regimen were required per protocol. All postconversion rejection events were successfully treated in the belatacept group; none were associated with subsequent graft loss, unlike in the CNI continuation group, in which one patient with isolated humoral rejection experienced functional graft loss during the study. These outcomes are consistent with previous studies of belatacept use from the time of transplantation in which the majority of BPAR episodes occurred within 6 months post-transplant and responded to treatment.10,13–15
Sustained improvement in renal function was observed after belatacept conversion, beginning at 3 months, persisting and even increasing throughout the 2-year study period. In contrast, eGFR in the CNI continuation group remained the same or decreased during the study period. These results were consistent regardless of whether the eGFR changes were assessed from baseline or from month 3, suggesting that the observed improvement in eGFR following conversion from CNI to belatacept may not, by itself, be fully explained by early improvement in renal cortical blood flow following CNI discontinuation.1 Similar GFR improvements were reported in previous belatacept conversion studies22,27,28 and those of belatacept use from the time of transplantation.10,13–15 These results add further evidence that conversion from CNI-based to belatacept-based treatment may better preserve long-term allograft function.
The rates of AEs or serious AEs were similar across treatment groups, with slightly higher cumulative rates at 2 years with belatacept conversion than with CNI continuation (95% versus 92% and 48% versus 42%, respectively). The frequency and types of AEs were consistent with those in the phase 2 conversion study22 and in the BENEFIT and BENEFIT-EXT studies.10,13 No new or unexpected events were reported in either group in this study. This was notable because the belatacept conversion group underwent a major change in immunosuppressive regimen. The most frequent AEs were infections involving the respiratory and urinary tracts, which occurred at similar rates in both arms. PTLD, a previously recognized concern with belatacept,14,15 was reported in one patient in the belatacept group. This PTLD event did not involve the CNS and was successfully treated.
Recent reports have cited an increased risk of CMV infection associated with belatacept-based immunosuppression and a prolonged course of viral replication in CMV high-risk patients (donor/recipient serostatus positive/negative).29 In this study, 18% and 19% of patients in the belatacept and CNI groups, respectively, were CMV-seronegative recipients of CMV-positive donor kidneys and considered at high risk for CMV infection. However, no cases of CMV infection were reported following belatacept conversion, whereas two cases were reported in the CNI continuation group. This could partly be attributed to the later conversion of these patients (>6 months post-transplant), consistent with recently reported data.30
No clinically meaningful changes in systolic or diastolic BP, new-onset diabetes after transplant rates, or fasting lipid profiles were observed in either treatment group during the 24-month study period.
The presence of donor-specific antibodies is associated with an increased risk of antibody-mediated rejection, considered a major cause of long-term graft failure in kidney transplant recipients.3,4 In this study, conversion to belatacept was associated with a lower incidence of dnDSA formation compared with CNI-based treatment (1% versus 7% at 24 months); these results are consistent with prior observations in newly transplanted patients.12 Despite more rejections, no increase in the frequency of dnDSA formation was detected from 12 to 24 months postconversion, although increases in donor-specific antibodies (5%–7%) were observed in the CNI group over the same time period.
These results indicate that conversion from CNI- to belatacept-based maintenance immunosuppression in stable renal allograft recipients 6–60 months after transplantation was well tolerated and associated with similar patient and graft survival rates. Renal function was improved with belatacept conversion and increased over time compared with CNI continuation. Costimulation blockade with belatacept resulted in a numerically higher BPAR rate; yet, lower dnDSAs were reported with belatacept conversion compared with CNI continuation. The overall benefit-risk profile for belatacept in this study was similar to that previously described in newly transplanted patients.10,13 Conversion from CNI to belatacept may offer the alternative clinical management strategy for renal allograft recipients for whom continued therapy with tacrolimus or cyclosporin is no longer considered the optimal approach to long-term immunosuppression.
Disclosures
L. Allamassey is currently employed by Bristol-Myers Squibb. S.P. Berger has received grant/research support from Chiesi and Novartis and has received research support from Bristol-Myers Squibb. S. P. Berger reports consultancy agreements with Novartis; research funding from Chiesi and Novartis; honoraria from Novartis; and scientific advisor or membership via an advisory board for Novartis and the supervisory board for the Dutch Transplant Foundation. K. Budde has received research grants, travel support, and honoraria from Abbvie, Alexion, Astellas, Bristol-Myers Squibb, Chiesi, CSL Behring, Fresenius, Hansa, Hexal, Hookipa Biotech, Merck Sharp & Dohme, Novartis, Otsuka, Pfizer, Quark, Roche, Sandoz, Shire, Siemens, Veloxis, Vifor, and Vitaeris; has consultancy agreements with Abbvie, Alexion, Astellas, Bristol-Myers Squibb, Chiesi, CSL Behring, Fresenius, Hansa, Hexal, MSD, Novartis, Otsuka, Pfizer, Quark, Roche, Sandoz, Shire, Veloxis, Vifor, and Vitaeris; and scientific advisor or membership with Astellas, Bristol-Myers Squibb, Chiesi, Hansa, Hexal, MSD, Novartis, Pfizer, Roche, and Veloxis. A. Djamali served as a consultant and has received research grants from CareDx and CSL Behring. A. Djamali reports consultancy agreements with CareDx and CSL; research funding from CareDx and Takeda; honoraria from CareDx and CSL; and scientific advisor or membership with CareDx and CSL. S. Gao is currently employed by Janssen Pharmaceuticals and has ownership interest in Bristol-Myers Squibb and Janssen Pharmaceuticals. S. Gao is a former employee of Bristol-Myers Squibb. N. Kamar has served on advisory boards and received speaker fees from AbbVie, Amgen, Astellas, Biotest, Chiesi, CSL Behring, Fresenius Medical Care, Gilead, Merck Sharpe & Dohme, Neovii, Novartis, Sandoz, Sanofi, Shire, and Takeda; has consultancy agreements with Astellas, Neovii, and Novartis; research funding from Astellas, Neovii, and Novartis; honoraria from Abbvie, Astellas, Biotest, Chiesi, CSL Behring, Merck Sharp and Dohme, Neovii, Novartis Pharma, Sandoz, Sanofi, Shire, and Takeda; and patents and inventions with UpToDate. N. Leca has received grants from Bristol-Myers Squibb; research funding from Angion, Behring, CareDx, CSL, Natera, Novartis, and Transplant Genomics; and honoraria form CareDx. M. Polinsky is a full-time employee of and owns stock in Bristol-Myers Squibb. M.C. Rial reports research funding from Bristol-Myers Squibb, Pfizer, and Otsuka. L. Rostaing reports consultancy agreements with Veloxis; honoraria from Novartis, Fresenius Medical Care, Hansa, BMS, and Biotest; and scientific advisor or membership with Novartis and BMS. F. Vincenti reports research funding from Astellas, Novartis, Pfizer, Merck, Angion, Viela Bio, and CSL Behring, and honoraria from Veloxis. H. Haller reports consultancy agreements with Bayer Pharma. MedWiss, Phenos, Alexion, Boehringer, AstraZeneca, and Vifor-Fresenius; honoraria from Alexion, AstraZeneca, Novartis, Bayer Pharma, MedWiss, Phenos, Boehringer, and Vifor-Fresenius; scientific advisor or membership with Der Internist, Der Nephrologe, Bayer Pharma, and Alexion; and speakers bureau with Amgen, Novartis, Bayer Pharma. MedWiss, Phenos, Alexion, Boehringer, AstraZeneca, and Vifor-Fresenius. All remaining authors have nothing to disclose.
Funding
This study was supported by Bristol-Myers Squibb.
Supplementary Material
Acknowledgments
We thank the patients and investigators who participated in this trial. We acknowledge Ms. Deborah Pocetti, RPh, for her contribution as the clinical trial manager for this study. Professional medical writing and editorial assistance were provided by Dr. Kakoli Parai, PhD, Dr. Vasupradha Vethantham, PhD, and Ms. Kelsey Hogan, MS, of Ashfield MedComms, an Ashfield Health company, funded by Bristol-Myers Squibb.
This study was designed by the funder, Bristol-Myers Squibb. Data were collected by the investigators and analyzed by the funder in collaboration with all authors. Data were interpreted jointly by the funder and the authors. Writing and editorial support were funded by the study sponsor. All authors had access to the data, and the corresponding author had final responsibility for the decision to submit for publication.
L. Allamassey, K. Budde, M. Polinsky, M.C. Rial, and F. Vincenti contributed to study conception and design; A. Agarwal, S.P. Berger, K. Budde, J.W. de Fijter, A. Djamali, H. Haller, N. Kamar, N. Leca, R. Prashar, M.C. Rial, L. Rostaing, and F. Vincenti assisted with data acquisition; L. Allamassey, K. Budde, S. Gao, and M. Polinsky assisted with data analysis; and all authors contributed to data interpretation and draft development of the manuscript and approved the final submitted version.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “It Is Time for Patient-Reported Outcome Measures to Be Included in the Approval Process for Solid Organ Transplant Medications,” on pages 2985–2986.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with Bristol-Myers Squibb’s policy on data sharing described at www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021050628/-/DCSupplemental.
Supplemental Figure 1. Subgroup analyses of change in eGFR from baseline to month 24.
Supplemental Figure 2. Effect of BPAR on eGFR over time with imputation.
Supplemental Material. IM103-116 investigators and recruitment numbers
Supplemental Table 1. Baseline serum creatinine concentrations (milligrams per deciliter) by baseline eGFR and race.
Supplemental Table 2. Serum trough concentrations of tacrolimus and cyclosporin at study entry (all patients) and during the trial period (CNI continuation arm only).
Supplemental Table 3. Patient and graft survival at 24 months in patient subgroups.
Supplemental Table 4. BPAR at 24 months in patient subgroups.
Supplemental Table 5. Improvement from baseline in eGFR.
Supplemental Table 6. Urine protein-creatinine ratio at baseline and at month 24.
Supplemental Table 7. Impact on donor-specific antibodies.
References
- 1.Azzi JR, Sayegh MH, Mallat SG: Calcineurin inhibitors: 40 years later, can’t live without …. J Immunol 191: 5785–5791, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Malvezzi P, Rostaing L: The safety of calcineurin inhibitors for kidney-transplant patients. Expert Opin Drug Saf 14: 1531–1546, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Schinstock CA, Mannon RB, Budde K, Chong AS, Haas M, Knechtle S, et al. : Recommended treatment for antibody-mediated rejection after kidney transplantation: The 2019 Expert Consensus from the Transplantation Society Working Group. Transplantation 104: 911–922, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everly MJ, Rebellato LM, Haisch CE, Ozawa M, Parker K, Briley KP, et al. : Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation 95: 410–417, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Sawinski D, Trofe-Clark J, Leas B, Uhl S, Tuteja S, Kaczmarek JL, et al. : Calcineurin inhibitor minimization, conversion, withdrawal, and avoidance strategies in renal transplantation: A systematic review and meta-analysis. Am J Transplant 16: 2117–2138, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Lim WH, Eris J, Kanellis J, Pussell B, Wiid Z, Witcombe D, et al. : A systematic review of conversion from calcineurin inhibitor to mammalian target of rapamycin inhibitors for maintenance immunosuppression in kidney transplant recipients. Am J Transplant 14: 2106–2119, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Schena FP, Pascoe MD, Alberu J, del Carmen Rial M, Oberbauer R, Brennan DC, et al. ; Sirolimus CONVERT Trial Study Group : Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation 87: 233–242, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Liefeldt L, Brakemeier S, Glander P, Waiser J, Lachmann N, Schönemann C, et al. : Donor-specific HLA antibodies in a cohort comparing everolimus with cyclosporine after kidney transplantation. Am J Transplant 12: 1192–1198, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, et al. : Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant 5: 443–453, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. : A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 10: 535–546, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Noble J, Jouve T, Janbon B, Rostaing L, Malvezzi P: Belatacept in kidney transplantation and its limitations. Expert Rev Clin Immunol 15: 359–367, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Bray RA, Gebel HM, Townsend R, Roberts ME, Polinsky M, Yang L, et al. : De novo donor-specific antibodies in belatacept-treated vs cyclosporine-treated kidney-transplant recipients: Post hoc analyses of the randomized phase III BENEFIT and BENEFIT-EXT studies. Am J Transplant 18: 1783–1789, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durrbach A, Pestana JM, Pearson T, Vincenti F, Garcia VD, Campistol J, et al. : A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant 10: 547–557, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, et al. : Belatacept and long-term outcomes in kidney transplantation. N Engl J Med 374: 333–343, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Durrbach A, Pestana JM, Florman S, Del Carmen Rial M, Rostaing L, Kuypers D, et al. : Long-term outcomes in belatacept- versus cyclosporine-treated recipients of extended criteria donor kidneys: Final results from BENEFIT-EXT, a phase III randomized study. Am J Transplant 16: 3192–3201, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertrand D, Terrec F, Etienne I, Chavarot N, Sberro R, Gatault P, et al. : Opportunistic infections and efficacy following conversion to belatacept-based therapy after kidney transplantation: A French multicenter cohort. J Clin Med 9: 3479, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta G, Raynaud M, Kumar D, Sanghi P, Chang J, Kimball P, et al. : Impact of belatacept conversion on kidney transplant function, histology, and gene expression - a single-center study. Transpl Int 33: 1458–1471, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Choi M, Bachmann F, Wu K, Lachmann N, Schmidt D, Brakemeier S, et al. : Microvascular inflammation is a risk factor in kidney transplant recipients with very late conversion from calcineurin inhibitor-based regimens to belatacept. BMC Nephrol 21: 354, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar D, Raynaud M, Chang J, Reeve J, Yakubu I, Kamal L, et al. : Impact of belatacept conversion on renal function, histology, and gene expression in kidney transplant patients with chronic active antibody-mediated rejection. Transplantation 105: 660–667, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Santeusanio AD, Bhansali A, Weinberg A, Shapiro R, Delaney V, Florman S, et al. : Conversion to belatacept within 1-year of renal transplantation in a diverse cohort including patients with donor-specific antibodies. Clin Transplant 34: e13823, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Sethi S, Najjar R, Peng A, Choi J, Lim K, Vo A, et al. : Outcomes of conversion from calcineurin inhibitor to belatacept-based immunosuppression in HLA-sensitized kidney transplant recipients. Transplantation 104: 1500–1507, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Rostaing L, Massari P, Garcia VD, Mancilla-Urrea E, Nainan G, del Carmen Rial M, et al. : Switching from calcineurin inhibitor-based regimens to a belatacept-based regimen in renal transplant recipients: A randomized phase II study. Clin J Am Soc Nephrol 6: 430–439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grinyó JM, Del Carmen Rial M, Alberu J, Steinberg SM, Manfro RC, Nainan G, et al. : Safety and efficacy outcomes 3 years after switching to belatacept from a calcineurin inhibitor in kidney transplant recipients: Results from a phase 2 randomized trial. Am J Kidney Dis 69: 587–594, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Mehrotra DV, Railkar R: Minimum risk weights for comparing treatments in stratified binomial trials. Stat Med 19: 811–825, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Haas M, Mirocha J, Reinsmoen NL, Vo AA, Choi J, Kahwaji JM, et al. : Differences in pathologic features and graft outcomes in antibody-mediated rejection of renal allografts due to persistent/recurrent versus de novo donor-specific antibodies. Kidney Int 91: 729–737, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Darres A, Ulloa C, Brakemeier S, Garrouste C, Bestard O, Del Bello A, et al. : Conversion to belatacept in maintenance kidney transplant patients: A retrospective multicenter European study. Transplantation 102: 1545–1552, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Brakemeier S, Kannenkeril D, Dürr M, Braun T, Bachmann F, Schmidt D, et al. : Experience with belatacept rescue therapy in kidney transplant recipients. Transpl Int 29: 1184–1195, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Karadkhele G, Hogan J, Magua W, Zhang W, Badell IR, Mehta A, et al. : CMV high-risk status and posttransplant outcomes in kidney transplant recipients treated with belatacept. Am J Transplant 21: 208–221, 2021 [DOI] [PubMed] [Google Scholar]
- 30.Bertrand D, Chavarot N, Gatault P, Garrouste C, Bouvier N, Grall-Jezequel A, et al. : Opportunistic infections after conversion to belatacept in kidney transplantation. Nephrol Dial Transplant 35: 336–345, 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.