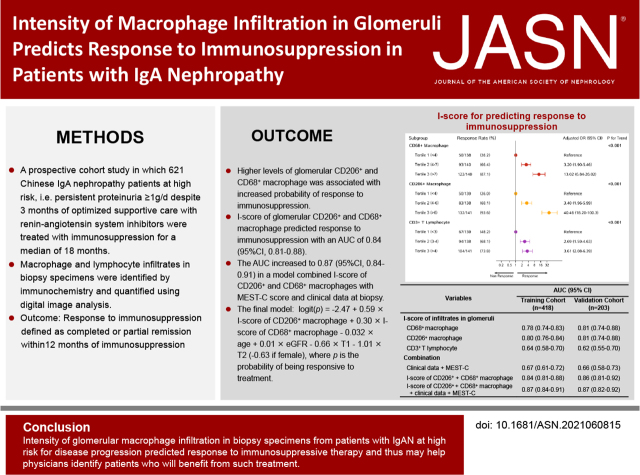
Significance Statement
The lack of a tool for predicting the response to immunosuppressive therapy in IgA nephropathy (IgAN) limits patient-specific risk stratification and early treatment decisions. To derive and validate the models for predicting response to immunosuppressive therapy in IgAN that can be applied at the time of kidney biopsy, the authors conducted a prospective cohort study of 621 Chinese patients with IgAN. Patients had persistent proteinuria of ≥1 g/d, despite 3 months of optimized supportive care with renin-angiotensin system inhibitors, and received immunosuppressive therapy. The authors used immunohistochemistry to identify cellular infiltrates in biopsy specimens and digital image analysis to quantify them. The intensity of glomerular macrophage infiltration, alone or combined with clinical and histologic data, accurately predicted the response to immunosuppression and, thus, may help physicians identify patients with IgAN who will benefit from immunosuppression.
Keywords: IgA nephropathy, prediction, response, immunosuppression, macrophages
Visual Abstract
Abstract
Background
The lack of a tool for predicting the response to immunosuppressive therapy in IgA nephropathy (IgAN) limits patient-specific risk stratification and early treatment decision making. Models for predicting response to immunosuppression in IgAN that can be applied at the time of kidney biopsy are needed.
Methods
This prospective cohort study involved 621 Chinese patients with IgAN who were at high risk for disease progression and had persistent proteinuria ≥1 g/d, despite 3 months of optimized supportive care with renin-angiotensin system inhibitors. Participants received immunosuppressive therapy for a median of 18 months. We used immunochemistry to identify macrophage and lymphocyte infiltrates in biopsy specimens and digital image analysis to quantify them. The outcome was response to immunosuppression, defined as complete or partial remission within 12 months of immunosuppression.
Results
Kidney infiltration of CD68+ and CD206+ macrophages increased in patients with IgAN. Having higher levels of glomerular CD206+ macrophage infiltration was associated with a 40-fold increased probability of response to immunosuppression in adjusted analysis compared with having lower levels. Patients with a higher intensity of glomerular CD68+ infiltrates had a 13-fold increase in probability of responding to immunosuppression. Intensity of glomerular CD206+ and CD68+ macrophage infiltration predicted the response to immunosuppression (area under the curve [AUC], 0.84; 95% CI, 0.81 to 0.88). The AUC increased to 0.87 (95% CI, 0.84 to 0.91) in a model combining the infiltration score of CD206+ and CD68+ infiltrates with the MEST-C score and clinical data at biopsy.
Conclusions
Intensity of glomerular macrophage infiltration predicted response to immunosuppressive therapy in patients with IgAN who were at high risk of progression, and may help physicians identify patients who will benefit from such treatment.
IgA nephropathy (IgAN) is the most common form of primary glomerular disease and remains a leading cause of ESKD in the Asia-Pacific region.1 Up to 30% of patients with IgAN will eventually develop ESKD within 20 years.2
The central role of the immune and autoimmune activation in the pathogenesis of IgAN, including synthesis of galactose-deficient IgA1,3 production of anti-glycan antibodies,4 formation of circulating immune complexes, and glomerular deposition,5,6 indicates a potential benefit of immunosuppression for treating IgAN. However, the recent randomized controlled studies have yielded conflicting results in the use of immunosuppression to treat patients with IgAN.7–12 The Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgAN trial shows that addition of immunosuppressive therapy to the intensive supportive care in patients with IgAN did not improve their clinical outcome.7–9 Whereas the Therapeutic Evaluation of Steroids in IgA Nephropathy Global Study, which mainly recruited Chinese patients with IgAN, found immunosuppression significantly reduced the frequency of the primary composite renal outcome; however, the trial was stopped early due to excess serious adverse events in the steroid group.11 It remains unclear which patients with IgAN would benefit from immunosuppressive therapy.
With the rapid progress in treatments for IgAN, multiple novel immunosuppression strategies that target different pathways in the pathogenesis of IgAN have recently emerged.13–15 Physicians are faced with the difficult task of selecting the most appropriate treatment for each individual patient. An ideal precision-medicine strategy for choosing an immunosuppression therapy would consider three aspects: the risk of disease progression without treatment, the probability of response to immunosuppression, and weighing the benefits of treatment against the adverse events of immunosuppression. Although guidelines recommend risk stratifying patients with IgAN so that immunosuppressive therapy can be reserved for patients at high risk of disease progression,15 there is currently no approach available for accurately predicting the response to immunosuppression therapy at the patient level. The current approaches for predicting the prognosis of IgAN, including pathologic classification (Oxford MEST score)16 and clinical assessment (the International IgAN Prediction Tool),17 may not be appropriate for determining the efficacy of immunosuppression.18–20 Therefore, there is a clear need for a personalized approach to identify those patients who will respond to steroid or other immunosuppression and to improve treatment decisions in IgAN.
A common feature of IgAN is the infiltration of macrophages and T cells in the glomerulus and/or interstitial compartments.21,22 The intensity of the cellular infiltration correlates with the severity of renal histologic lesions and the degree of proteinuria at the time of biopsy.22,23 The marked cellular inflammation in the kidney gives the rationale for pronounced anti-inflammatory therapy using corticosteroids or other immunosuppressive agents. Therefore, we hypothesized that the intensity of inflammatory cell infiltration in the kidney may predict the response to immunosuppression in high-risk patients with IgAN.
To test this hypothesis, we conducted a prospective cohort study in which 621 patients with IgAN who were at high risk of disease progression, i.e., patients with persistent urinary protein excretion ≥1 g/d despite 3 months of optimized supportive care with renin-angiotensin system inhibitors (RASi), were treated with immunosuppression for a median of 18 months. Macrophage and lymphocyte infiltrates were quantified in glomeruli and tubulointerstitium in biopsy specimens from these patients. We found that the intensity of glomerular macrophage infiltrates, expressed as the infiltration score (I-score) of glomerular macrophages at the time of diagnosis, can predict patients’ response to subsequent immunosuppression, and can be used as a promising tool for early identification of patients with IgAN who would benefit from immunosuppression.
Methods
Study Design
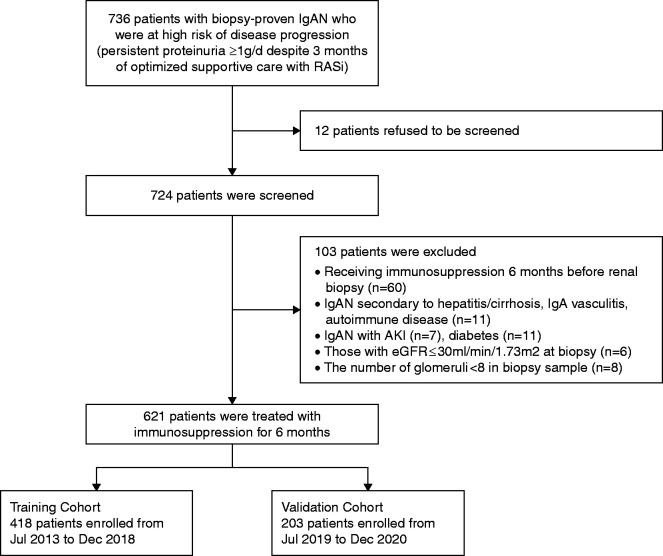
This is a prospective, single-center, two-stage cohort study approved by the Institutional Review Board of the National Clinical Research Center for Kidney Disease (Nanfang Hospital). A total of 724 patients with biopsy sample–proven IgAN who were at high risk of disease progression, i.e., those with persistent proteinuria ≥1 g/d despite 3 months of optimized supportive care, were screened for potential participation at Nanfang Hospital, a tertiary teaching hospital in China. Renal biopsies were performed according to the standard protocol.24 Optimized supportive care included maximization of RAS blockade,25 BP control (target systolic BP <130 mm Hg), dietary advice, and lifestyle modification.15 Among these patients, 103 patients were excluded according to the exclusion criteria. A total of 621 patients were treated with immunosuppression; of these, 418 patients enrolled from July 2013 to December 2018 served as a training cohort, and 203 patients included from January 2019 to December 2020 served as the validation cohort (Figure 1). All participants provided written informed consent.
Figure 1.
Flowchart of enrollment and exclusion in training and validation cohorts. IgAN, immunoglobulin A nephropathy; RASi, renin-angiotensin system inhibitors.
Eligible participants were patients aged ≥18 years with an eGFR of >30 min/ml per 1.73 m2 at the time of kidney biopsy and had proteinuria of ≥1 g/d, despite optimized supportive care with RASi for 3 months.25 We excluded those with clinical or serologic evidence of IgAN secondary to hepatitis/cirrhosis, IgA vasculitis, and autoimmune disease; those complicated with AKI or diabetes; and patients with an eGFR ≤30 min/ml per 1.73 m2.
Eligible participants were then treated with immunosuppression in combination with their RASi therapy for 6 months. The type of immunosuppressive agents was chosen by their physicians who were blinded to the results of the inflammatory cell analysis in biopsy samples. Patients were followed up every 3 months. Urinary protein excretion rate and eGFR were measured every 1–3 months during follow-up.
Response to the Immunosuppressive Therapy
Completed remission was defined as urinary protein that decreased to <0.15 g/d with a stable eGFR (<25% decline in eGFR). Partial remission was defined as urinary protein that decreased by >50% and to a level of <1 g/d and having a stable eGFR. Remission needed to be confirmed by a second measurement of proteinuria and eGFR 30 days later. Patients with a complete or partial remission within 12 months after the initiation of the immunosuppressive therapy were classified as responders. Patients without a complete or partial remission after 12 months of the immunosuppressive therapy were classified as nonresponders.
Quantifying the Intensity of Macrophage and Lymphocyte Infiltration in Renal Tissue
Infiltrating macrophages and lymphocytes in renal tissue were identified using immunohistochemical staining, as described previously.26 Paraffin-embedded tissue sections (4 μm) were incubated with the following primary antibodies: mouse anti-human CD68 mAb (M0876; DAKO, Glostrup, Denmark), mouse anti-human HLA-DR mAb (M0746; DAKO), rabbit anti-human CD206 antibody (ab64693; Abcam, Bristol, United Kingdom), rabbit anti-human CD3 antibody (A0452; DAKO), and mouse anti-human CD20 mAb (IR604; DAKO). The slides were then incubated with the secondary antibody and visualized using a DAKO Envision+ System Kit and counterstained with hematoxylin. The stained sections were scanned with a Digital Pathology Slide Scanner (Leica, Wetzlar, Germany). Positive-stained cells were automatically counted in all nonglobally sclerotic glomeruli at ×400 magnification using Aperio eSlide Manager (Leica).
The intensity of leukocyte infiltration in glomeruli was quantified and expressed as follows. (1) Average cell numbers per glomerulus: The total numbers of positive-stained cells in all nonglobally sclerotic glomeruli divided by the number of nonglobally sclerotic glomeruli. (2) I-score: Because the distribution of glomerular-positive cell counts in a single slide is heavily skewed,27 we proposed an I-score that is conceptually similar to the Hirsh index28 to quantify the intensity of infiltration in glomeruli, which considers both the number of glomeruli affected and the number of positive cells in a glomerulus. The I-score is defined as the count of glomeruli (n) in a slide that has at least n positive cells in each glomerulus, given 20 glomeruli in each slide (see Supplemental Figure 1 for illustration). We bootstrapped the data 100 times by randomly sampling with replacement 20 glomeruli in each slide to calculate I score. The mean I-score was generated using 100 bootstrap samples and used for analysis. We chose 20 glomeruli because this was the average number of glomeruli in a slide in our study. The score ranged from zero to 20, with zero being no infiltration and 20 being the maximal infiltration.
To quantify the infiltrates in the tubulointerstitium compartment, positively stained cells were automatically counted and expressed as the average number per 100 μm2 of tubulointerstitium.
Measurements of Renal Histologic Lesions and Renal Function
All kidney biopsy specimens were reviewed by two independent pathologists who were blinded to patients’ outcomes. Renal histologic lesions were graded according to the Oxford classification MEST-C score.29
Urinary protein excretion was measured using the biuret method using 24-hour urine samples. Serum creatinine were measured using the Roche enzymatic method (Hoffman–La Roche). eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.30
Statistical Analyses
There were no patients with missing data in this study. We compared the level of each glomerular and tubulointerstitial infiltrate (CD68+, CD206+, and CD3+) between the responders and nonresponders using the Mann–Whitney test. We calculated the Spearman correlation between the level of glomerular and tubulointerstitial infiltrates and the MEST-C scores and clinical variables. We performed logistic regression analyses to examine the association between the tertiles of glomerular and tubulointerstitial infiltrates and the treatment response, with and without adjustment for age, sex, eGFR, 24-hour urine protein (log transformed), mean arterial pressure, MEST-C score, class of immunosuppressive therapies, and to estimate the smoothed response curves using natural cubic splines.
We compared the performance of various logistic regression models in predicting the treatment response using the area under the receiver operating characteristic curve (AUC). The prediction models included a univariable model with the level of infiltrates in glomeruli (using the I-score as a continuous variable) and tubulointerstitium; a model with clinical features only; a model with each component of MEST-C or total MEST-C (using all five component of MEST-C as the independent variables); and models using different combinations of I-scores, clinical variables, and MEST-C scores.
We also used stepwise regression with forward selection and backward elimination to obtain the model that minimizes the Alkaike information criterion (AIC). Performance of the regression models in both the training set and the validation set were reported. The predictive accuracy of the model was assessed by discrimination, measured by AUC, and calibration, evaluated by the Hosmer–Lemeshow chi-squared statistic. Reclassification was evaluated using continuous net reclassification improvement and integrated discrimination improvement.
Results are presented according to the Transparent Reporting of a multivariable Prediction model for Individual Prognosis or Diagnosis guidelines.31 Statistical analyses were performed using SAS 9.4 for Windows (SAS Institute, Cary, NC) and STATA 15SE (StataCorp LLC, College Station, TX). A two-sided P value of <0.05 was considered as statistically significant.
Results
Characteristics of the Cohorts
The characteristics at time of kidney biopsy in the training and the validation cohort were stratified by response to immunosuppression and are presented in Table 1 and Supplemental Table 1. The clinical and pathologic variables at the time of initiation of immunosuppressive treatment are shown in Supplemental Table 2. In the training cohort, 94 (22.5%), 120 (28.7%), 184 (44.0%), and 20 (4.8%) patients were treated with corticosteroid, mycophenolate mofetil (MMF), a combination of corticosteroid and MMF, or other immunosuppressive agents, respectively. The characteristics of patients stratified by immunosuppression approaches at the time of renal biopsy are shown in Supplemental Table 3. In the validation cohort, 36 (17.7%), 67 (33.0%), and 100 (49.3%) patients were treated with corticosteroid, MMF, and a combination of corticosteroid and MMF, respectively. In the validation cohort, one patient was enrolled on December 3, 2020 and reached complete remission 2 months after initiation of immunosuppression. The length of treatment in this patient was 4 months before the study was closed. All other patients included in the training and validation cohorts were treated with immunosuppressive agents for at least 6 months. The median (interquartile range [IQR]) time of treatment was 18 (8–23) months in the training cohort and 16 (7–22) months in validation cohort. The median (IQR) time of follow-up in the training cohort was 22 (8–27) months in the responders and 20 (17–27) months in the nonresponders. In the validation cohort, the median (IQR) time of follow-up was 18 (15–22) months in the responders and 16 (14–22) months in the nonresponders. All nonresponders in the validation cohort had at least 12 months of follow-up time (Supplemental Figure 2).
Table 1.
Characteristics at time of biopsy in training cohort stratified by response to immunosuppression
| Variables | Overall (n=418) | Responders (n=265) | Nonresponders (n=153) | P Valuea |
|---|---|---|---|---|
| Age, yr | 34.2±9.7 | 33.5±9.8 | 35.5±9.2 | 0.04 |
| Men, n (%) | 180 (43.1) | 119 (44.9) | 61 (39.9) | 0.32 |
| BMI, kg/m2 | 23.2±3.7 | 22.7±3.7 | 23.3±3.7 | 0.11 |
| Current smoker, n (%) | 20 (4.8) | 12 (4.5) | 8 (5.2) | 0.75 |
| Hypertension, n (%) | 66 (15.8) | 41 (15.5) | 25 (16.3) | 0.81 |
| History of gross hematuria, n (%) | 58 (13.9) | 42 (15.8) | 16 (10.5) | 0.13 |
| MAP, mm Hg | 100.8±13.3 | 99.8±13.5 | 102.6±12.7 | 0.04 |
| eGFR, ml/min per 1.73 m2 | 81.1±29.9 | 85.2±28.9 | 74.0±30.3 | <0.001 |
| eGFR <60 ml/min per 1.73 m2, n (%) | 130 (31.1) | 70 (53.8) | 60 (46.2) | 0.007 |
| 24-hour proteinuria, g/d | 1.4 (0.9–2.5) | 1.4 (0.9–2.6) | 1.3 (0.8–2.4) | 0.04 |
| Serum albumin, g/L | 36.6±6.4 | 36.6±6.7 | 36.7±5.8 | 0.85 |
| Serum triglycerides, mmol/L | 1.7±1.2 | 1.6±1.0 | 1.8±1.4 | 0.17 |
| Serum cholesterol, mmol/L | 5.2±1.6 | 5.2±1.7 | 5.1±1.4 | 0.55 |
| Serum LDL-C, mmol/L | 3.1±1.2 | 3.0±1.0 | 3.2±1.3 | 0.27 |
| Hemoglobin, g/L | 129.1±20.1 | 130.7±20.1 | 126.4±19.9 | 0.03 |
| Oxford MEST-C, n (%) | ||||
| M1 | 394 (94.3) | 248 (93.6) | 146 (95.4) | 0.44 |
| E1 | 45 (10.8) | 31 (11.7) | 14 (9.2) | 0.42 |
| S1 | 359 (85.9) | 227 (85.7) | 132 (86.3) | 0.86 |
| T1 | 158 (37.8) | 103 (38.9) | 55 (35.9) | 0.55 |
| T2 | 124 (29.7) | 63 (23.8) | 61 (39.9) | 0.001 |
| C1+2b | 174 (41.6) | 115 (43.4) | 59 (38.6) | 0.33 |
| Time from urinary abnormality to renal biopsy, mo | 22 (16–20) | 22 (16–33) | 24 (17–38) | 0.68 |
| Time from biopsy to initiation of immunosuppression, mo | 2 (1–4) | 2 (1–3) | 2 (1–4) | 0.56 |
Continuous variables are expressed as mean±SD or median (IQR). Categoric variables are expressed as n (%). BMI, body mass index; MAP, mean arterial pressure; LDL-C, LDL cholesterol; M, mesangial hypercellularity; E, endocapillary hypercellularity; S, segmental glomerulosclerosis; C, crescent formation.
Comparing the covariates between responders and nonresponders.
There were only three patients with ≥25% crescent formation; two were responders, one was a nonresponder.
The response to immunosuppression, within 12 months after initiation of immunosuppression, was observed in 265 of 418 patients (63.4%) in the training cohort, with complete remission in 90 patients (34.0%), and in 139 of 203 patients (68.3%) in the validation cohort, with 44 patients (31.7%) in complete remission. The remission rates, daily dose of immunosuppressive agents, and adverse event rates in patients with IgAN, stratified by immunosuppressive agents, are shown in Supplemental Table 4. Adverse events were observed in 19 patients (4.5%) in the training cohort. Adverse events were infections (n=7, including upper respiratory tract infection [n=4], skin infection [n=2], and herpes zoster [n=1]); gastrointestinal syndromes, such as abdominal distension and increased stool frequency (n=2); newly diagnosed diabetes (n=2); and elevated transaminase (n=8) (Supplemental Table 4).
Pattern of Infiltrates in Renal Tissue from Patients with IgAN
We estimated the level of macrophage and lymphocyte infiltration, including CD68+ macrophages, HLA-DR+ macrophages, CD206+ macrophages, CD3+ T lymphocytes, and CD20+ B lymphocytes, in glomeruli and tubulointerstitium in 418 patients with IgAN in the training cohort and five patients with minimal change disease who served as “normal” controls. As shown in Supplemental Figure 3 and Supplemental Table 5, infiltrated CD68+ macrophages, CD206+ macrophages, and CD3+ T lymphocytes were increased in both glomeruli and tubulointerstitium in patients with IgAN compared with those with minimal change disease. The number of HLA-DR+ macrophages and CD20+ B lymphocytes was not increased. As a result, we choose to focus subsequent studies on infiltration by CD68+, CD206+, and CD3+ cells.
The intensity of glomerular infiltrates correlated with MEST-C scores, urinary protein, and eGFR, with the highest Spearman correlation coefficients (ρ) being 0.21 (Supplemental Table 6). The intensity of tubulointerstitial infiltrates, particularly CD206+ and CD3+ cells, mainly located in the fibrotic area, positively correlated with the scores for interstitial fibrosis/tubular atrophy (T-scores) and proteinuria, and negatively correlated with eGFR, with absolute values of ρ ranging from 0.31 to 0.61 (Supplemental Table 6).
Association between Glomerular Infiltrates and Response to Immunosuppression in Patients with IgAN
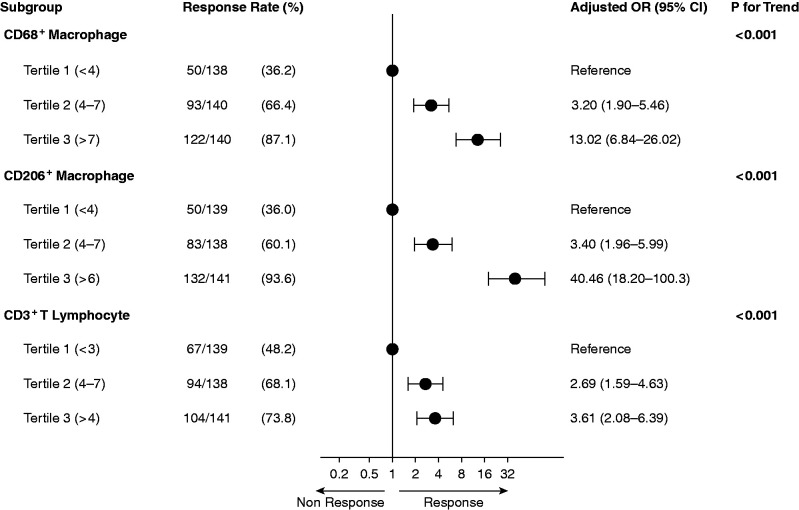
The intensity of glomerular infiltrates, expressed as both cell number per glomerulus and I-score of infiltrates, and cellular infiltration in the tubulointerstitium were significantly greater in responders than that in nonresponders (Table 2). After adjusting for age, sex, eGFR, urinary protein, mean arterial pressure, and MEST-C scores, the intensity of glomerular infiltrates, expressed as either I-scores (Figure 2) or the average number of glomerular infiltrates (Supplemental Figure 4), remained significantly associated with treatment response. I-scores of glomerular macrophage infiltrates were significantly associated with both complete and partial remission in the training cohort (Supplemental Table 7). However, the association between the tubulointerstitial infiltrates and the treatment response disappeared or was significantly attenuated in this group (Supplemental Figure 4). The relationship between the intensity of glomerular infiltrates, particularly CD68+ and CD206+, and logit of response to treatment appeared to be linear (Supplemental Figures 5 and 6).
Table 2.
Renal infiltrates in patients with or without response to immunosuppression in training cohort
| Variables | Overall (n=418) | Responders (n=265) | Nonresponders (n=153) | P Valuea |
|---|---|---|---|---|
| CD68+ macrophages | ||||
| Glomeruli (cells per glomerulus) | 3.3 (1.5–6.4) | 4.7 (2.7–8.2) | 1.5 (0.7–3.2) | <0.001 |
| I-score of CD68+ cell in glomeruli | 6 (4–8) | 7 (5–9) | 4 (3–6) | <0.001 |
| Tubulointerstitium (cells per 100 μm2) | 2.2 (1.2–4.0) | 2.4 (1.4–4.2) | 1.9 (0.8–3.7) | 0.01 |
| CD206+ macrophages | ||||
| Glomeruli (cells per glomerulus) | 2.4 (1.3–3.9) | 3.3 (2.0–4.8) | 1.4 (0.5–2.4) | <0.001 |
| I-score of CD206+ cell in glomeruli | 5 (3–6) | 6 (4–7) | 4 (2–5) | <0.001 |
| Tubulointerstitium (cells per 100 μm2) | 2.3 (1.4–3.9) | 2.4 (1.6–4.1) | 2.1 (1.4–3.6) | <0.001 |
| CD3+ T lymphocytes | ||||
| Glomeruli (cells per glomerulus) | 1.6 (1.2–2.5) | 1.8 (1.3–2.7) | 1.3 (0.9–2.0) | 0.001 |
| I-score of CD3+ cell in glomeruli | 4 (3–5) | 4 (3–5) | 3 (3–4) | <0.001 |
| Tubulointerstitium (cells per 100 μm2) | 2.3 (1.3–3.6) | 2.5 (1.3–3.8) | 2.2 (1.3–3.3) | 0.01 |
Continuous variables were expressed as median (IQR).
Comparing between responders and nonresponders.
Figure 2.
Association between I-score of infiltrates in glomeruli and response to immunosuppression in training cohort. Having higher levels of glomerular infiltrates, expressed as I-scores, significantly associated with an increased probability of response to immunosuppression in adjusted analysis compared with having lower levels. Odds ratio (OR) was adjusted for age, sex, MEST-C score, clinical data (eGFR, proteinuria, mean arterial pressure) at time of renal biopsy, and immunosuppressive strategies.
Predicting the Response to Immunosuppression Using the Intensity of Infiltrated CD68+, CD206+, and CD3+ Cells
Among the univariable models, the glomerular I-scores had the best performance in predicting the response to treatment in the training cohort, with AUCs of 0.80 and 0.78, respectively, for CD206+ and CD68+ cells (Table 3). Tubulointerstitium infiltrates and MEST-C components, and clinical data alone, produced much weaker prediction models, with AUCs ranged from 0.50 to 0.66.
Table 3.
Performance of renal infiltrates, clinical data, or MEST-C score for predicting response to immunosuppression
| Variables | AUC (95% Confidence Interval) | ||
|---|---|---|---|
| Training Cohort (n=418) | Validation Cohort (n=203) | ||
| I-score of infiltrates in glomeruli | |||
| CD68+ macrophages | 0.78 (0.74–0.83) | 0.81 (0.74–0.88) | |
| CD206+ macrophages | 0.80 (0.76–0.84) | 0.81 (0.74–0.88) | |
| CD3+ T lymphocytes | 0.64 (0.58–0.70) | 0.62 (0.55–0.70) | |
| Number of infiltrates in glomeruli | |||
| CD68+ macrophages | 0.76 (0.71–0.80) | 0.77 (0.71–0.84) | |
| CD206+ macrophages | 0.76 (0.72–0.81) | 0.77 (0.70–0.84) | |
| CD3+ T lymphocytes | 0.64 (0.60–0.71) | 0.61 (0.54–0.70) | |
| Number of infiltrates in tubulointerstitium | |||
| CD68+ macrophages | 0.57 (0.51–0.62) | 0.53 (0.45–0.61) | |
| CD206+ macrophages | 0.55 (0.50–0.61) | 0.60 (0.52–0.68) | |
| CD3+ T lymphocytes | 0.58 (0.53–0.64) | 0.57 (0.54–0.63) | |
| MEST-C score alone | |||
| M | 0.51 (0.49–0.53) | 0.50 (0.47–0.54) | |
| E | 0.51 (0.48–0.54) | 0.53 (0.46–0.59) | |
| S | 0.50 (0.47–0.54) | 0.51 (0.48–0.54) | |
| T | 0.60 (0.55–0.65) | 0.60 (0.52–0.67) | |
| C | 0.52 (0.48–0.57) | 0.50 (0.43–0.58) | |
| All MEST-C components (five variables) | 0.62 (0.56–0.67) | 0.62 (0.54–0.70) | |
| Clinical data alonea | 0.66 (0.60–0.71) | 0.65 (0.57–0.72) | |
| Combination | |||
| Clinical data and MEST-Ca,b | 0.67 (0.61–0.72) | 0.66 (0.58–0.73) | |
| I-score of CD206+ and CD68+ macrophages | 0.84 (0.81–0.88) | 0.86 (0.81–0.92) | |
| I-score of CD206+ and CD68+ macrophages, clinical data, and MEST-Ca,b | 0.87 (0.84–0.91) | 0.87 (0.82–0.92) | |
M, mesangial hypercellularity; E, endocapillary hypercellularity; S, segmental glomerulosclerosis; C, crescent formation.
Clinical data included age, sex, eGFR, proteinuria, and mean arterial pressure at the time of renal biopsy.
All five MEST-C components, each as an independent variable, were included in the model.
Multivariable models incorporating clinical variables and MEST-C scores performed poorly with an AUC of 0.67. Using percentage of glomeruli with endocapillary hypercellularity, cellular or fibrocellular crescents, or segmental sclerosis, and mesangial hypercellularity score as the histologic variables did not improve the predictive performance as compared with the MEST-C score (Supplemental Table 8). Addition of glomerular CD68+ and CD206+ scores to clinical data and MEST-C scores improved the AUC to 0.87.
To select a model that is convenient for clinical use, we used stepwise regression to select the variables with a minimized Alkaike information criterion. The final model included six variables, including age, sex, eGFR, T-score, and glomerular I-score of CD68+ and CD206+. The model was logit(P)=−2.47 + 0.59×I-score of CD206+ macrophage+0.30×I-score of CD68+ macrophage−0.032×age+0.01×eGFR−0.66×T1–1.01×T2 (−0.63 if female), where p is the probability of being responsive to treatment. This model produced an AUC of 0.87 (95% CI, 0.84 to 0.90) in the training cohort and 0.87 (95% CI, 0.82 to 0.92) in the validation cohort, respectively, and was consistent in patients with IgAN with or without eGFR decline and among those treated with different immunosuppressive agents (Supplemental Table 9). Consistent results were seen in the validation cohort (Table 3). Compared with the model containing I-scores of CD206+ macrophages only, the final model significantly improved the risk reclassification, with a net reclassification improvement of 0.77 (95% CI, 0.59 to 0.96; P<0.001) and integrated discrimination improvement of 0.10 (95% CI, 0.07 to 0.13; P=0.004). The Hosmer–Lemeshow test for both cohorts showed the close agreement between predicted and observed response to immunosuppression (P=0.13 and 0.11, respectively).
Discussion
In this prospective, large-scale cohort study, we identified glomerular macrophage infiltration as a good marker for predicting the probability of response to immunosuppression in patients with IgAN who were at high risk of disease progression. The glomerular CD68+ and CD206+ cells together could predict the response to immunosuppressive therapy with high accuracy. We also derived a parsimony prediction model that incorporates clinical variables and the glomerular infiltrates, which produced an AUC of 0.87.
Among the inflammatory cells we tested, CD206+, CD68+, and CD3+ infiltrates were increased in glomeruli in IgAN. Glomerular macrophage infiltrates (CD206+, CD68+ cells) exhibited an excellent performance for predicting the probability of response to immunosuppression. Patients with the highest tertile of CD206+ macrophages (I-score greater than six) showed a 40-fold increase in probability of response to immunosuppression, as compared with those with the lowest tertile (I-score less than four). Similarly, patients with the highest tertile of CD68+ cells in glomeruli (I-score greater than 7) had a 13-fold increase in probability of response to immunosuppression compared with those with the lowest tertile (I-score less than four). The predictive performance of glomerular macrophages was comparable among subgroups with various approaches of immunosuppression (Supplemental Table 9). In contrast, the other reported predictors for prognosis of IgAN, including MEST-C score and clinical data, were not able to accurately predict the response of immunosuppression when used alone.
In this study, patients with IgAN who had more glomerular macrophage infiltrates would benefit from immunosuppressive therapy. Inflammatory cell infiltration has been classified as a marker of an active phase in many “proliferative” forms of GN, including IgAN.32,33 Active inflammation in inflammatory disease is commonly depressed by corticosteroids or immunosuppressive agents,34,35 and the therapeutic benefit might be attributable especially to the anti-inflammatory effect of these drugs. Supporting this notion, our results showed that the intensity of glomerular CD68+ (a pan-macrophage marker) and CD206+ (an M2-type macrophage marker) correlated with endocapillary hypercellularity. Glomerular CD206+ infiltrates were also associated with mesangial hypercellularity. Macrophages mediate inflammation, tissue injury, and repair in renal disease.36 Mononuclear phagocytes play an important role in the pathogenesis of the glomerular damage in immunologic glomerular diseases, including IgAN.22,37 However, the effect of CD206+ macrophages in GN remains controversial. A previous report showed that CD206+ M2 macrophages have protective effects in antibody-mediated glomerular injury in mice.38 However, a recent study demonstrated that the accumulation of M2 macrophages expressing CD206+ correlates with renal fibrosis in human kidney disease.39 Why the CD206+ subset of macrophages predict response to immunosuppression more accurately needs further investigation. It has been shown that macrophage depletion in vitro reduces the expression of inflammatory cytokines and adhesion molecules in immune complex–mediated GN.40
In addition to glomerulus infiltration, infiltration of macrophages in the tubulointerstitium is often observed in renal biopsy specimens from patients with IgAN.32,33 In this study, 50% of patients had tubulointerstitial macrophage infiltration in kidney biopsy specimens. However, the intensity of macrophage infiltrates in the interstitium was not able to predict the probability of response to immunosuppression, although there was a weak correlation between tubulointerstitial CD68+ infiltrates and the response to immunosuppression. Consistent with previous studies,27,41, in our results, tubulointerstitial macrophage infiltration significantly associated with T-scores, interstitial fibrosis, and eGFR decline, suggesting tubulointerstitial macrophage infiltration might be a marker for chronic kidney damage, rather than active renal inflammation.27
IgAN is characterized by a wide spectrum of histologic damage, which may be active and potentially reversible by treating with immunosuppression, or chronic and unresponsive to current available treatments,42 making treatment decision at the time of diagnosis (kidney biopsy) a big challenge. Although previously studies43,44 reported that corticosteroid therapy was especially effective for patients with IgAN whose eGFR was <60 ml/min per 1.73 m2, a model for predicting the probability of response to immunosuppression in IgAN remains lacking. Quantification of glomerular macrophage infiltrates in CD206- and CD68-stained sections is a simple and robust assessment that can be used as a tool for improving the treatment decisions in patients with IgAN who are at high risk of disease progression. In addition, the final model combined macrophage infiltration with clinical and histologic data showed improved predictive performance (AUC, 0.87) by using only six variables, which is convenient for clinical practice.
The strengths of our study included its prospective design and appropriate sample size. Enrolling patients who were at high risk on the basis of current guidelines reduced the risk of selection bias. We compared the predictive performance of various types of inflammatory infiltrates and developed a model combining glomerular macrophages, clinical data, and MEST-C scores for prediction. Using an independent validation cohort, we showed consistent results in predicting response to immunosuppression in both training and validation cohorts.
There are several limitations to our study. In our cohort, most patients underwent kidney biopsy 22 months after detecting urinary abnormalities, with a median time of 2 months from biopsy to initiating immunosuppression, suggesting the study cohort may be a “new-onset” IgAN population. Although the improved ability to make treatment decisions early in the disease course may offer an opportunity to intervene before the onset of irreversible tissue damage, the tool may not account for response to immunosuppression over a longer-term horizon. Furthermore, there might be limitations in the immunostaining method, such as the specificity of the antibodies, tissue fixation, and the quality of the tissues. Last, this is a single-center study conducted and validated in a Chinese population. Because the clinical efficacy of MMF in non-Chinese subjects has not been demonstrated, validation studies involving other ethnic populations are warranted.
In conclusion, we have identified intensity of glomerular macrophage infiltration as an independent and strong predictor for probability of response to immunosuppression in patients with IgAN who are at high risk of disease progression. Our model that combined I-scores of glomerular macrophages with MEST-C and clinical data improved our ability to make early treatment decisions in patients with IgAN who were at high risk of disease progression.
Disclosures
F.F. Hou reports having consultancy agreements with, and receiving honoraria from, AbbVie and AstraZeneca; and serving on the editorial boards of Current Opinion in Nephrology and Hypertension, Kidney Diseases, Kidney International, and Kidney Medicine. Y. Liu reports receiving honoraria from New York Medical College and Stony Brook State University. D. Xie reports having serving as a member of the International Society of Nephrology. X. Xu reports having ownership interest in Apple. All remaining authors have nothing to disclose.
Funding
This study was supported by the Guangzhou Regenerative Medicine and Health Guangdong Laboratory Clinical Innovation Research Program grant 2018GZR0201003 (to F.F. Hou), the Guangdong Provincial Key Laboratory of Renal Failure Research grant 2017B030314036 (via the Research Fund Program to F.F. Hou), and the National Innovation Team Program grant 81521003 (to Y. Liu).
Supplementary Material
Acknowledgments
D. Xie and H. Zhao contributed to the data collection and analysis; F.F. Hou designed the study, wrote the manuscript, and conceptualized the study; X. Xu and D. Xie performed the statistical analysis; Y. Liu instructed the research; and N. Jia, Z. Zhou, and C. Su performed the renal pathologic analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “IgA Nephropathy Needs a Diagnostic Marker of Immunologic Activity to Select the Right Patients for Immunotherapies,” on pages 2982–2984.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021060815/-/DCSupplemental.
Supplemental Table 1. Characteristic at renal biopsy in validation cohort stratified by response to immunosuppression.
Supplemental Table 2. Characteristic at time of initiation of immunosuppressive treatment in training cohort stratified by response to immunosuppression.
Supplemental Table 3. Clinical-pathological findings at time of renal biopsy in patients stratified by immunosuppressive agents in training cohort.
Supplemental Table 4. Remission rates, dosage and adverse events of immunosuppression in IgAN patients treated with immunosuppression in training cohort.
Supplemental Table 5. Renal infiltrates in patients with IgAN or minimal change disease.
Supplemental Table 6. The relationship between renal infiltrates and MEST-C score or renal function.
Supplemental Table 7. Association between I-score of infiltrates in glomeruli and complete/partial remission in training cohort.
Supplemental Table 8. Performance of histological changes in glomeruli for predicting response to immunosuppression in the training cohort.
Supplemental Table 9. Performance of the final model for predicting response to immunosuppression in subgroups.
Supplemental Figure 1. Example of developing I-score of glomerular infiltrate in a CD68-stained section.
Supplemental Figure 2. Follow-up time in training cohort (A) and validation cohort (B).
Supplemental Figure 3. Representative photos of infiltrates in glomeruli.
Supplemental Figure 4. Association between renal infiltrates and response to immunosuppression in training cohort.
Supplemental Figure 5. Relationship between the average number of infiltrates in glomeruli (A–C) or tubulointerstitium (D–F) and response to immunosuppression by cubic spline analysis in training cohort.
Supplemental Figure 6. Relationship between the I-score of infiltrates in glomeruli and probability of response to immunosuppression in training (A–C) and validation (D–F) cohorts.
References
- 1.Wyatt RJ, Julian BA: IgA nephropathy. N Engl J Med 368: 2402–2414, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Schena FP: A retrospective analysis of the natural history of primary IgA nephropathy worldwide. Am J Med 89: 209–215, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M, Julian BA, Mestecky J, et al. : Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 71: 1148–1154, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, et al. : Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119: 1668–1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mestecky J, Raska M, Julian BA, Gharavi AG, Renfrow MB, Moldoveanu Z, et al. : IgA nephropathy: Molecular mechanisms of the disease. Annu Rev Pathol 8: 217–240, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Magistroni R, D’Agati VD, Appel GB, Kiryluk K: New developments in the genetics, pathogenesis, and therapy of IgA nephropathy. Kidney Int 88: 974–989, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, et al. ; STOP-IgAN Investigators : Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 373: 2225–2236, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Rauen T, Fitzner C, Eitner F, Sommerer C, Zeier M, Otte B, et al. : Effects of two immunosuppressive treatment protocols for IgA nephropathy. J Am Soc Nephrol 29: 317–325, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rauen T, Wied S, Fitzner C, Eitner F, Sommerer C, Zeier M, et al. ; STOP-IgAN Investigators : After ten years of follow-up, no difference between supportive care plus immunosuppression and supportive care alone in IgA nephropathy. Kidney Int 98: 1044–1052, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Tang S, Leung JCK, Chan LYY, Lui YH, Tang CSO, Kan CHIH, et al. : Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int 68: 802–812, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, et al. ; TESTING Study Group : Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: The TESTING Randomized Clinical Trial. JAMA 318: 432–442, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fellström BC, Barratt J, Cook H, Coppo R, Feehally J, de Fijter JW, et al. ; NEFIGAN Trial Investigators : Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): A double-blind, randomised, placebo-controlled phase 2b trial. Lancet 389: 2117–2127, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Liu LJ, Yang YZ, Shi SF, Bao YF, Yang C, Zhu SN, et al. : Effects of hydroxychloroquine on proteinuria in IgA nephropathy: A randomized controlled trial. Am J Kidney Dis 74: 15–22, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Kidney Disease Improving Global Outcomes (KDIGO) : KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Available at: https://kdigo.org/wp-content/uploads/2017/02/KDIGO-Glomerular-Diseases-Guideline-2021-English.pdf. Accessed May 15, 2021
- 15.Kidney Disease Improving Global Outcomes (KDIGO) : KDIGO 2012 clinical practice guideline for glomerulonephritis. Available at: https://kdigo.org/wp-content/uploads/2017/02/KDIGO-2012-GN-Guideline-English.pdf. Accessed May 15, 2021
- 16.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, et al. ; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Barbour SJ, Coppo R, Zhang H, Liu ZH, Suzuki Y, Matsuzaki K, et al. ; International IgA Nephropathy Network : Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med 179: 942–952, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi SF, Wang SX, Jiang L, Lv JC, Liu LJ, Chen YQ, et al. : Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: Validation of the oxford classification. Clin J Am Soc Nephrol 6: 2175–2184, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natale P, Palmer SC, Ruospo M, Saglimbene VM, Craig JC, Vecchio M, et al. : Immunosuppressive agents for treating IgA nephropathy. Cochrane Database Syst Rev 3: CD003965, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X, Ou J, Zhang H, Xu X, Zhu L, Li Q, et al. : Urinary matrix metalloproteinase 7 and prediction of IgA nephropathy progression. Am J Kidney Dis 75: 384–393, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Alexopoulos E, Seron D, Hartley RB, Nolasco F, Cameron JS: The role of interstitial infiltrates in IgA nephropathy: A study with monoclonal antibodies. Nephrol Dial Transplant 4: 187–195, 1989 [DOI] [PubMed] [Google Scholar]
- 22.Arima S, Nakayama M, Naito M, Sato T, Takahashi K: Significance of mononuclear phagocytes in IgA nephropathy. Kidney Int 39: 684–692, 1991 [DOI] [PubMed] [Google Scholar]
- 23.Topaloglu R, Orhan D, Bilginer Y, Karabulut E, Ozaltin F, Duzova A, et al. : Clinicopathological and immunohistological features in childhood IgA nephropathy: A single-centre experience. Clin Kidney J 6: 169–175, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker PD: The renal biopsy. Arch Pathol Lab Med 133: 181–188, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Hou FF, Xie D, Zhang X, Chen PY, Zhang WR, Liang M, et al. : Renoprotection of Optimal Antiproteinuric Doses (ROAD) Study: A randomized controlled study of benazepril and losartan in chronic renal insufficiency. J Am Soc Nephrol 18: 1889–1898, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Liang M, Xu J, Cao W, Wang GB, Zhou ZM, et al. : Renal expression of advanced oxidative protein products predicts progression of renal fibrosis in patients with IgA nephropathy. Lab Invest 94: 966–977, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Soares MF, Genitsch V, Chakera A, Smith A, MacEwen C, Bellur SS, et al. : Relationship between renal CD68+ infiltrates and the Oxford Classification of IgA nephropathy. Histopathology 74: 629–637, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Hirsch JE: An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A 102: 16569–16572, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, et al. ; IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants : Oxford Classification of IgA nephropathy 2016: An update from the IgA Nephropathy Classification Working Group. Kidney Int 91: 1014–1021, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins GS, Reitsma JB, Altman DG, Moons KG: Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD statement. Ann Intern Med 162: 55–63, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Emancipator SN: IgA nephropathy: Morphologic expression and pathogenesis. Am J Kidney Dis 23: 451–462, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Myllymäki JM, Honkanen TT, Syrjänen JT, Helin HJ, Rantala IS, Pasternack AI, et al. : Severity of tubulointerstitial inflammation and prognosis in immunoglobulin A nephropathy. Kidney Int 71: 343–348, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Locatelli F, Pozzi C, Del Vecchio L, Bolasco PG, Fogazzi GB, Andrulli S, et al. : Role of proteinuria reduction in the progression of IgA nephropathy. Ren Fail 23: 495–505, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Shoji T, Nakanishi I, Suzuki A, Hayashi T, Togawa M, Okada N, et al. : Early treatment with corticosteroids ameliorates proteinuria, proliferative lesions, and mesangial phenotypic modulation in adult diffuse proliferative IgA nephropathy. Am J Kidney Dis 35: 194–201, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Tang PM, Nikolic-Paterson DJ, Lan HY: Macrophages: Versatile players in renal inflammation and fibrosis. Nat Rev Nephrol 15: 144–158, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Schreiner GF, Cotran RS, Pardo V, Unanue ER: A mononuclear cell component in experimental immunological glomerulonephritis. J Exp Med 147: 369–384, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du Q, Tsuboi N, Shi Y, Ito S, Sugiyama Y, Furuhashi K, et al. : Transfusion of CD206+ M2 macrophages ameliorates antibody-mediated glomerulonephritis in mice. Am J Pathol 186: 3176–3188, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Klessens CQF, Zandbergen M, Wolterbeek R, Bruijn JA, Rabelink TJ, Bajema IM, et al. : Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol Dial Transplant 32: 1322–1329, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Chalmers SA, Chitu V, Herlitz LC, Sahu R, Stanley ER, Putterman C: Macrophage depletion ameliorates nephritis induced by pathogenic antibodies. J Autoimmun 57: 42–52, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawasaki Y, Suyama K, Miyazaki K, Kanno S, Ono A, Suzuki Y, et al. : Resistance factors for the treatment of immunoglobulin A nephropathy with diffuse mesangial proliferation. Nephrology (Carlton) 19: 384–391, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Hotta O, Furuta T, Chiba S, Tomioka S, Taguma Y: Regression of IgA nephropathy: A repeat biopsy study. Am J Kidney Dis 39: 493–502, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Moriyama T, Honda K, Nitta K, Yumura W, Nihei H: The effectiveness of steroid therapy for patients with advanced IgA nephropathy and impaired renal function. Clin Exp Nephrol 8: 237–242, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Nagasawa Y, Yamamoto R, Shinzawa M, Shoji T, Hasuike Y, Nagatoya K, et al. : Efficacy of corticosteroid therapy for IgA nephropathy patients stratified by kidney function and proteinuria. Clin Exp Nephrol 24: 927–934, 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.