Abstract
Juvenile Idiopathic Arthritis (JIA) is a group of chronic heterogenous disorders that manifests as joint inflammation in patients aged <16 years. Globally, approximately 3 million children and young adults are suffering from JIA with prevalence rates consistently higher in girls. The region of Africa and Middle East constitute a diverse group of ethnicities, socioeconomic conditions, and climates which influence the prevalence of JIA. There are only a few studies published on epidemiology of JIA in the region. There is an evident paucity of adequate and latest data from the region. This review summarizes the available data on the prevalence of JIA and its subtypes in Africa and Middle East and discusses unmet needs for patients in this region. A total of 8 journal publications were identified concerning epidemiology and 42 articles describing JIA subtypes from Africa and Middle East were included. The prevalence of JIA in Africa and Middle East was observed to be towards the lower range of the global estimate. We observed that the most prevalent subtype in the region was oligoarticular arthritis. The incidence of uveitis and anti-nuclear antibody (ANA) positivity were found to be lower as compared to the incidence from other regions. There is a huge unmet medical need in the region for reliable epidemiological data, disease awareness, having regional and local treatment guidelines and timely diagnosis. Paucity of the pediatric rheumatologists and economic disparities also contribute to the challenges regarding the management of JIA.
Background
Juvenile Idiopathic Arthritis (JIA) is the most common chronic heterogenous rheumatological disorder that manifests in patients aged less than 16 years and, in some cases, can cause severe impairment and disability. It constitutes various subtypes with different clinical manifestations, genetic markers, and pathogenesis [1]. According to the most commonly used classification proposed by the International League of Associations for Rheumatology (ILAR), seven different subtypes are recognized to classify patients: oligoarticular, rheumatoid factor (RF) positive polyarticular, RF negative polyarticular, enthesitis related arthritis (ERA), systemic onset, psoriatic arthritis, and undifferentiated arthritis [1, 2].
The precise cause and pathogenesis of JIA are unknown; however, genetic, environmental, and autoimmune factors are hypothesized to play a role in the development of JIA [3, 4]. Socioeconomic status is associated with delayed access to rheumatology care and worsening disease severity in JIA patients, directly affecting their well-being and quality of life [5].
Globally, approximately 3 million children and young adults are estimated to suffer from JIA [6, 7]. The global prevalence of JIA has been estimated to range from 3.8 to 400/100,000 with an incidence of 1.6 to 23/100,000 [8]. Girls were consistently found to be at a higher risk than boys, and oligoarticular subtype was found to be predominant [8].
Africa and Middle East countries constitute a diverse group of ethnicities, socioeconomic backgrounds, and climatic conditions. Few studies have assessed the prevalence of JIA in the region and there is a paucity of adequate and latest data from the region on the epidemiology of JIA. A comprehensive understanding of JIA in the regions is required.
Given the social, economic, and cultural diversity of African and Middle Eastern countries, many studies conducted in this region may underestimate the prevalence of JIA. The aim of this review article was to critically assess and summarize the available published data on epidemiology and demographics of JIA in the Africa and Middle East region and highlight the unmet needs of the region and current efforts being undertaken in the region to generate quality data on JIA and the way forward to address the lacunae. The unmet needs section describes unique challenges from the region by the authors from independent references.
Methods
Our methodology for searching the NCBI PubMed database included the following search strings: “((juvenile idiopathic arthritis) OR JIA) AND (Africa OR (Middle East) OR AfME) AND prevalence.” Search terms also included “Juvenile Chronic Arthritis” and “Juvenile Rheumatoid Arthritis.” Additional searches were conducted to include “(Africa OR (Middle East) OR AfME)” with individual countries in the region.
Publications were included if they evaluated JIA disease prevalence in the individual African or Middle Eastern countries or in African and Middle Eastern regions, using prospective or retrospective study designs or a systematic review or meta-analysis approach between May 1988 to April 2021. We included both population based and hospital-based studies. Prevalence rates were extracted from the articles and were not estimated.
For demographic section, publications were included if they evaluated JIA disease subtype and characteristics in individual African of Middle eastern countries or region between May 1988 to April 2021.
From the articles summarizing epidemiology data from the region, parameters extracted were region/country, prevalence, incidence, sample size, number of cases, classification criteria, age range, study period, and study design (population and setting) were included in (Table 1).
Table 1.
Epidemiology of Juvenile Idiopathic Arthritis in Africa and Middle East
| Sr. No. | Reference | Region/ Country | Prevalence | Incidence | No. of cases | N | Classification Criteria | Age Range | Study Years |
|---|---|---|---|---|---|---|---|---|---|
| Region | |||||||||
| 1 | Usenbo et al., 2015 [10] | Africa | (0.1-3.43)/100,000(NA) | NA | NA | NA | Multiple classification criteria | 0-16 | 1975-2014 |
| Country | |||||||||
| 2 | Khuffash et al., 1988 [13] | Kuwait | 22/100,000(NA) | NA | 41 | 186,363 | ACR (for 3 months) | 0-11 | 1978-1987 |
| 3 | Khuffash et al., 1990 [14] | Kuwait | 18.7/100,000 (15.3-22.6) | 2.8 (2.3-3.4)/100,000 | 108 JCA | 577,540 | ACR (for 3 months) | 0-11 | 1981-1988 |
| 4 | Abdwani et al., 2015 [11] | Oman | Boys 12/100,000 (NA) | 2/100,000 | 107 JIA | 528,480 | ILAR 2004 | 0-13 | 2004-2013 |
| Girls 28/100,000 (NA) | |||||||||
| 20/100,000 (NA) | |||||||||
| 5 | Ozen et al., 1998 [15] | Turkey | 64/100,000 (43-91) | NA | 30 JCA | 46,813 | EULAR (for 6 weeks) | 0-15 | 1997 |
| 6 | El-Soud et al., 2013 [12] | Egypt Sharkia Governate, Egypt | 3.43/100,000 (3.1–4.3) | NA | 132 JIA | 3,844,718 | 2004 ILAR | 0-15 | 2009-2010 |
| boys 2.58/100,000 (2.4–3.6) | |||||||||
| Girls 4.33/100,000 (3.3–5.1) | |||||||||
| 7 | Singwe-Ngandeu et al., 2013 [16] | Cameroon | 1/100,000 (0.7-1.3) | NA | 35 | 34,782 | Not reported | NA | 2004-2012 |
| 8 | Tayel et al., 1999 [17] | Egypt Alexandria | 3.3/100,000 (4-62) | NA | NA | 1500 | EULAR | 10-15years | NA |
ACR, American College of Rheumatology Association; EULAR, The European League Against Rheumatism; ILAR, International League of Associations for Rheumatology; JCA, juvenile chronic arthritis; JIA, juvenile idiopathic arthritis; NA, not applicable.
From the articles summarizing demographic data from the region, parameters such as country, number of cases, female to male ratio, mean age of onset (years), distribution of subtypes, presence, definition and methodology of testing for antinuclear antibody (ANA) positivity, uveitis, Rheumatoid factor (RF) positivity, and human leukocyte antigen HLA-B27 were extracted and included in (Table 2).
Table 2.
Demographic Characteristics
| Sr. No. | Reference | Country | N (no. of cases) | F:M | Mean Age of onset (years) | Subtype | ANA positivity | Uveitis | RF positivity | HLA-B27 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | No. | % | % | Methodology of testing | % | Methodology/ Nature | % | Methodology of testing | % | Methodology of testing | ||||||
| Regional | ||||||||||||||||
| 1 | Consolaro et al., 2019 [22] | Africa and Middle East | 1209 | 1.61 | 6·0 (2·9–9·8)* | Psoriatic arthritis | 37 | 3.1 | NA | NA | 5.9 | NM | NA | NA | NA | NA |
| RF-positive polyarthritis | 61 | 5.0 | ||||||||||||||
| Undifferentiated arthritis | 68 | 5.6 | ||||||||||||||
| ERA | 111 | 9.2 | ||||||||||||||
| Systemic | 204 | 16.9 | ||||||||||||||
| RF-negative polyarthritis | 271 | 22.4 | ||||||||||||||
| Oligoarticular | 457 | 37.8 | ||||||||||||||
| 2 | Al-Mayouf et al., 2021 [28] | Arab (Saudi Arabia, Libya, United Arab Emirates, Jordan, Oman, Egypt, Kuwait) | 702 | 2.04 | 5 (IQR 2.0- 9.0)* | Undifferentiated | 11 | 1.6 | 30.9 | Immunoassay | 8.3 | NM | 9.3 | Immunoassay RF was tested at least twice, with a minimum of 3 months apart. Test results were interpreted according to cutoff values of the local laboratories. | 5.3# | Flow cytometry |
| Psoriatic | 28 | 3.9 | ||||||||||||||
| ERA | 39 | 5.6 | ||||||||||||||
| Oligoarticular Extended | 43 | 6.1 | ||||||||||||||
| Polyarticular RF positive | 48 | 6.8 | ||||||||||||||
| Polyarticular RF negative | 159 | 22.6 | ||||||||||||||
| Systemic | 172 | 24.5 | ||||||||||||||
| Oligoarticular persistent | 202 | 28.8 | ||||||||||||||
| Country | ||||||||||||||||
| 3 | Khuffash et al., 1988 [13] | Kuwait | 41 | 1.28 | NA | Oligoarticular ANA negative | 4 | 9.8 | NA | NM | NA | NA | NA | NA | NA | NA |
| Polyarticular seropositivity | 5 | 12.2 | NA | |||||||||||||
| Oligoarticular ANA positive | 5 | 12.2 | 12.2 | 60.0† | Asymptomatic chronic uveitis detected by slit lamp examination by an ophthalmologist | |||||||||||
| Systemic polyarticular | 6 | 14.6 | NA | NA | NA | |||||||||||
| Systemic oligoarticular | 10 | 24.4 | NA | |||||||||||||
| Polyarticular seronegative | 11 | 26.8 | NA | |||||||||||||
| 4 | Khuffash et al., 1990 [14] | Kuwait | 108 | 1.04 | NA | Oligoarticular seropositive | 3 | 2.8 | NA | NA | NA | NA | NA | NA | NA | NA |
| Oligoarticular ANA positive | 9 | 8.3 | 8 | NM | ||||||||||||
| Polyarticular seropositive | 10 | 9.3 | NA | NA | ||||||||||||
| Systemic polyarticular | 13 | 12.0 | ||||||||||||||
| Systemic oligoarticular | 18 | 16.7 | ||||||||||||||
| Oligoarticular ANA negative | 19 | 17.6 | ||||||||||||||
| Polyarticular seronegative | 36 | 33.3 | ||||||||||||||
| 5 | Abdwani et al., 2015 [11] | Oman | 107 | 2.57 | 6.85 ± 3.86 years | Psoriatic | 1 | 0.9 | 32 | Indirect Immunoflorescence; titer of ≥1:80 obtained on at least 1 clinic visit during the disease course was considered positive | None | Slit lamp examination by ophthalmologist during regular follow up visits at 3, 6 or 12 monthly intervals as per the pediatric screening recommendation | 7.5 | ELISA; RF was considered to be positive when titers were >20 IU/ml (If only one test of RF was performed, which was the case for many patients, then the results of this test were used to assign a JIA subtype rather than apply the subtype category of “other JIA.”) | NA | NA |
| ERA | 3 | 2.8 | ||||||||||||||
| Polyarticular RF positive | 8 | 7.5 | ||||||||||||||
| Systemic JIA | 19 | 17.8 | ||||||||||||||
| Oligoarticular JIA | 34 | 31.8 | ||||||||||||||
| Polyarticular RF negative | 42 | 39.3 | ||||||||||||||
| 6 | Ozen et al., 1998 [15] | Turkey | 30 | 0.67 | NA | Systemic | 1 | 12.5 | NA | NA | NA | NA | NA | NA | NA | NA |
| Polyarticular | 12 | 42.4 | ||||||||||||||
| Oligoarticular | 17 | 45.1 | ||||||||||||||
| 7 | Abou El-Soud et al., 2013 [12] | Egypt | 132 | 1.59 | 12.5 ± 4.56 | Systemic | 18 | 13.6 | 48.5 | Indirect immunofluorescence on Hep-2 cells, with positive titers from 1/40 with at least two determinations 3 months apart during the first 6 months of the disease | 19.7 | Detected by slit lamp examination | 27.20 | Semi-quantitative latex test; titers ≥30 IU/mL were considered positive with at least two determinations 3 months apart during the first 6 months of the disease | 66‡ | Low-resolution PCR analysis |
| ERA | 6 | 4.5 | ||||||||||||||
| Polyarticular RF positive | 11 | 8.3 | ||||||||||||||
| Polyarticular RF negative | 28 | 21.2 | ||||||||||||||
| Oligoarticular | 69 | 52.3 | ||||||||||||||
| 8 | Furia et al., 2020 [47] | Tanzania | 28 | 1.15 | NA | Oligoarticular | 1 | 3.6 | 0.0 | NM | NA | NA | NA | NA | NA | NA |
| Systemic | 6 | 21.4 | ||||||||||||||
| Polyarticular | 21 | 75.0 | ||||||||||||||
| 9 | Aiche et al., 2018 [31] | Algeria | 70 | 1.8 | 7.3* | Psoriatic | 1 | 1.4 | 2.9 | NM | 1.5 | NM | NA | NA | NA | NA |
| Systemic | 7 | 10.0 | ||||||||||||||
| ERA | 8 | 11.4 | ||||||||||||||
| Polyarticular RF positive | 14 | 20.0 | ||||||||||||||
| Polyarticular RF negative | 15 | 21.4 | ||||||||||||||
| Oligoarticular | 25 | 35.7 | ||||||||||||||
| 10 | Al Marri et al., 2017 [32] | Saudi Arabia | 23 | 6.67 | 3.5 | Psoriatic | 1 | 4.3 | 8.7 | NM | NA | NA | 13.0 | NM | NA | NA |
| Polyarticular RF positive | 3 | 13.0 | ||||||||||||||
| Polyarticular RF negative | 5 | 21.7 | ||||||||||||||
| Systemic | 14 | 60.9 | ||||||||||||||
| 11 | Al-Mayouf et al., 2018 [35] | Saudi Arabia | 100 | 1.70 | 4.5* | ERA | 3 | 3.0 | 15.0% | NM | 8.1% | NM | NA | NA | NA | NA |
| Undifferentiated | 3 | 3.0 | ||||||||||||||
| Psoriatic | 6 | 6.0 | ||||||||||||||
| Polyarticular RF positive | 13 | 13.0 | ||||||||||||||
| Oligoarticular | 23 | 23.0 | ||||||||||||||
| Polyarticular RF negative | 25 | 25.0 | ||||||||||||||
| Systemic | 27 | 27.0 | ||||||||||||||
| 12 | Salah et al., 2009 [63] | Egypt | 196 | 1.09 | 6.257±3.41 years | Systemic-onset | 47 | 24.0 | 21.7 | Indirect immunofluorescence; positive at serum dilution between 1:80 to 1:60 | 5.6 | Slit lamp examination; all detected patients had chronic uveitis | NA | NA | NA | NA |
| Polyarthriticular | 68 | 34.7 | ||||||||||||||
| Extended oligoarticular | 18 | 9.2 | ||||||||||||||
| Persistent Oligoarticular | 63 | 32.1 | ||||||||||||||
| Oligoarticular | 81 | |||||||||||||||
| 13 | Al-Abrawi et al., 2018 [33] | Oman | 57 | 2.35 | 5.9* | ERA | 0 | 0.0 | 7.0 | NM | 0 | NM | NA | NA | NA | NA |
| Undifferentiated | 0 | 0.0 | ||||||||||||||
| Psoriatic | 2 | 3.5 | ||||||||||||||
| Polyarticular RF positive | 6 | 10.5 | ||||||||||||||
| Systemic | 13 | 22.8 | ||||||||||||||
| Oligoarticular | 16 | 28.1 | ||||||||||||||
| Polyarticular RF negative | 20 | 35.1 | ||||||||||||||
| 14 | Demirkaya et al., 2018 [45] | Turkey | 466 | 1.49 | 6.3 (2.7–10.8) | Polyarticular RF positive | 11 | 2.4 | 9.9 | NM | 8.1 | NM | NA | NA | NA | NA |
| Undifferentiated | 12 | 2.6 | ||||||||||||||
| Psoriatic | 15 | 3.2 | ||||||||||||||
| Systemic | 64 | 13.7 | ||||||||||||||
| ERA | 70 | 15.0 | ||||||||||||||
| Polyarticular RF negative | 105 | 22.5 | ||||||||||||||
| Oligoarticular | 189 | 40.6 | ||||||||||||||
| 15 | El Miedany et al., 2018 [46] | Egypt | 100 | 0.89 | 9.2 (5.3–11)* | Polyarticular RF positive | 2 | 2.0 | 0.0 | NM | 6.0 | NM | NA | NA | NA | NA |
| Psoriatic | 2 | 2.0 | ||||||||||||||
| ERA | 2 | 2.0 | ||||||||||||||
| Oligoarticular | 10 | 10.0 | ||||||||||||||
| Systemic | 20 | 20.0 | ||||||||||||||
| Polyarticular RF negative | 24 | 24.0 | ||||||||||||||
| Undifferentiated | 40 | 40.0 | ||||||||||||||
| 16 | Hashad et al., 2018 [48] | Libya | 100 | 2.33 | 6.4 (3.1-10.4)* | Psoriatic | 4 | 4.0 | 7.0 | NM | 2.0 | NM | NA | NA | NA | NA |
| Polyarticular RF positive | 5 | 5.0 | ||||||||||||||
| Undifferentiated | 5 | 5.0 | ||||||||||||||
| ERA | 13 | 13.0 | ||||||||||||||
| Systemic | 22 | 22.0 | ||||||||||||||
| Polyarticular RF negative | 25 | 25.0 | ||||||||||||||
| Oligoarticular | 26 | 26.0 | ||||||||||||||
| 17 | Oyoo et al., 2016 [55] | Kenya | 68 | 2.4 | 8.45 | ERA | 4 | 5.9 | 10.9§ | NM | 1.47 | Slit lamp examination by an ophthalmologist | 17.6¶ | One positive or negative RF assay was considered adequate to classify polyarticular patients | NA | NA |
| Systemic JIA | 10 | 14.7 | ||||||||||||||
| Polyarticular RF positive | 12 | 17.6 | ||||||||||||||
| Oligoarticular arthritis | 16 | 23.5 | ||||||||||||||
| Polyarticular RF negative | 26 | 38.2 | ||||||||||||||
| 18 | Scott et al., 2018 [57] | South Africa | 91 | 1.68 | 5.9* | Systemic | 4 | 4.4 | 2.2 | NM | 8.2 | NM | NA | NA | NA | NA |
| Polyarticular RF positive | 6 | 6.6 | ||||||||||||||
| Psoriatic | 6 | 6.6 | ||||||||||||||
| Undifferentiated | 8 | 8.8 | ||||||||||||||
| ERA | 14 | 15.4 | ||||||||||||||
| Polyarticular RF negative | 21 | 23.1 | ||||||||||||||
| Oligoarticular | 32 | 35.2 | ||||||||||||||
| 19 | Sen et al., 2015 [58] | Turkey | 213 | 1.07 | 8.1 (range 8 months-15.4 years) | Psoriatic | 2 | 0.90 | 11.70 | Immunofluorescent antibody method; titers >160 IU/mL were considered positive | 4.20 | Slit lamp examination by an ophthalmologist | 13.10 | Nephelometric method; positivity defined by titers >20 U/mL on at least two occasions during the first six months of disease onset | 2.8^ | PCR |
| Undifferentiated | 0 | 0.00 | ||||||||||||||
| Systemic | 19 | 8.90 | ||||||||||||||
| Polyarticular RF positive | 23 | 10.80 | ||||||||||||||
| ERA | 23 | 10.80 | ||||||||||||||
| Polyarticular RF negative | 67 | 31.50 | ||||||||||||||
| Oligoarticular | 79 | 37.10 | ||||||||||||||
| 20 | Shafaie et al., 2018 [59] | Iran | 102 | 2.19 | 5.2* | ERA | 0 | 0.0 | 2.9 | NM | 1.0 | NM | NA | NA | NA | NA |
| Undifferentiated | 0 | 0.0 | ||||||||||||||
| Polyarticular RF positive | 1 | 1.0 | ||||||||||||||
| Psoriatic | 1 | 1.0 | ||||||||||||||
| Systemic | 15 | 14.7 | ||||||||||||||
| Polyarticular RF negative | 16 | 15.7 | ||||||||||||||
| Oligoarticular | 69 | 67.6 | ||||||||||||||
| 21 | Yener et al., 2020 [61] | Turkey | 116 | 1.58 | NA | Undifferentiated | 0 | 0.0 | 44** | Immunofluorescence; titer of 1/100 was considered positive | 2.6 | Slit lamp examination by an ophthalmologist every 6 months | 22.7## | Two RF values above 10 U/L measured at an interval of 3 months in a 6-month period were considered significant | 21.1†† | Positive or negative for antigen |
| Psoriatic | 4 | 3.4 | ||||||||||||||
| Systemic | 15 | 12.9 | ||||||||||||||
| Polyarticular RF positive | 5 | 4.3 | ||||||||||||||
| Polyarticular RF negative | 17 | 14.7 | ||||||||||||||
| Oligoarticular | 37 | 31.9 | ||||||||||||||
| ERA | 38 | 32.8 | ||||||||||||||
| 22 | Çakan et al., 2017 [41] | Turkey | 265 | 0.95 | NA | Undifferentiated | 5 | 1.9 | 27.20 | Indirect Immunofluorescence; titers ≥1:100 were classified as positive | 4.5 | All cases were of anterior uveitis | 3.8 | Verified by a second analysis at least 3 months later | 26‡‡ | NM |
| Psoriatic JIA | 5 | 1.9 | ||||||||||||||
| Polyarticular RF positive | 10 | 3.8 | ||||||||||||||
| Systemic JIA | 35 | 13.2 | ||||||||||||||
| Persistent oligoarticular | 81 | 30.6 | ||||||||||||||
| Polyarticular RF negative | 36 | 13.5 | ||||||||||||||
| Extended Oligoarticular JIA | 6 | 2.3 | ||||||||||||||
| ERA | 87 | 32.9 | ||||||||||||||
| 23 | Kasapçopur et al., 2004 [51] | Turkey | 198 | 0.87 | 6.62 ± 4.12 | Other | 5 | 2.5 | 18.2 | Hep-2 cell; titers above 1/40 were considered positive | 10.1 | Slit lamp and a detailed ophthalmologic examination by ophthalmologist; single evaluation was considered sufficient for uveitis positivity; repeated every 3 months in uveitis and ANA positive patients | 3.5 | Nephlometric method | NM | Histocompatibility antigen determination |
| Polyarticular RF positive | 7 | 3.5 | ||||||||||||||
| Extended Oligoarticular | 9 | 4.5 | ||||||||||||||
| Psoriatic JIA | 11 | 5.6 | ||||||||||||||
| Polyarticular RF negative | 34 | 17.2 | ||||||||||||||
| Oligoarticular JIA | 37 | 18.7 | ||||||||||||||
| ERA | 43 | 21.7 | ||||||||||||||
| Systemic JIA | 52 | 26.3 | ||||||||||||||
| 24 | Ozdogan et al., 1991 [56] | Turkey | 147 | 0.77 | 8.4±3.9 | Juvenile spondylitis | 3 | 2.0 | 5.6 | Indirect Immunofluorescence using human leukocytes as nuclear substrate and fluorescein anti IgG antisera | 7.5 | Slit lamp examination; Chronic uveitis in 7 patients and acute anterior uveitis in 1 male patient | 10 | Latex slide agglutination test | 45 | Standard microcytotoxicity test |
| Polyarticular sero-positive | 7 | 5.0 | ||||||||||||||
| Polyarticular sero-negative | 19 | 13.0 | ||||||||||||||
| Systemic | 37 | 25.0 | ||||||||||||||
| Pauciarticular | 81 | 55.0 | ||||||||||||||
| 25 | Abdul-Sattar et al., 2014 [30] | Egypt | 52 | 2.06 | NA | Polyarticular RF positive | 5 | 10.0 | NA | NA | NA | NA | NA | NA | NA | NA |
| Oligoarticular persistent | 9 | 17.0 | ||||||||||||||
| Polyarticular RF negative | 11 | 21.0 | ||||||||||||||
| Systemic | 12 | 23.0 | ||||||||||||||
| Oligoarticular extended | 15 | 29.0 | ||||||||||||||
| 26 | Abdul-Sattar et al., 2014 [29] | Egypt | 58 | 2.41 | NA | Polyarticular RF positive | 5 | 8.6 | NA | NA | NA | NA | NA | NA | NA | NA |
| Oligoarticular persistent | 11 | 19.0 | ||||||||||||||
| Polyarticular RF negative | 12 | 20.7 | ||||||||||||||
| Systemic | 13 | 22.4 | ||||||||||||||
| Oligoarticular extended | 17 | 29.3 | ||||||||||||||
| 27 | Albokhari et al., 2019 [36] | Saudi Arabia | 44 | 1.59 | NA | ERA | 0 | 0.0 | NA | NA | NA | NA | NA | NA | NA | NA |
| Psoriatic | 2 | 4.5 | ||||||||||||||
| Oligoarticular | 6 | 13.6 | ||||||||||||||
| Polyarticular | 7 | 15.9 | ||||||||||||||
| Systemic | 12 | 27.3 | ||||||||||||||
| Unknown | 17 | 38.6 | ||||||||||||||
| 28 | Al-Hemairi et al., 2015 [34] | Saudi Arabia | 82 | 1.65 | 7.1 ± 3.6 year | Undifferentiated | 0 | 0.0 | 36.58 | ELISA; titer of 1:80 or more was considered positive. Positivity was confirmed only if two samples were positive at least three months apart | 8.53 | Slit lamp examination by an ophthalmologist | 4.87§§ | RF positivity was confirmed only if two samples were positive, tested three months apart | 100% in ERA | NM |
| ERA | 1 | 1.2 | ||||||||||||||
| Polyarticular RF positive | 4 | 4.9 | ||||||||||||||
| Psoriatic | 4 | 4.9 | ||||||||||||||
| Polyarticular RF negative | 20 | 24.4 | ||||||||||||||
| Oligoarticular | 23 | 28.0 | ||||||||||||||
| Systemic | 30 | 36.6 | ||||||||||||||
| 29 | Amine et al., 2009 [38] | Morocco | 80 | 1.42 | 7.53 | Extended oligoarticular | 4 | 5.0 | NA | NA | NA | NA | NA | NA | NA | NA |
| Systemic | 21 | 26.0 | ||||||||||||||
| Polyarticular | 25 | 31.5 | ||||||||||||||
| Persistent oligoarticular | 30 | 37.5 | ||||||||||||||
| 30 | Bahabari et al., 1997 [39] | Saudi Arabia | 115 | 1.21 | 6(0.75-16) | ERA | 0 | 0.0 | 30.0 | Indirect immunofluorescence; positive at serum dilution between 1:80 to 1:60 | 1.70 | Chronic uveitis | 10.0 | Slide agglutination test (till 1991); ELISA (after 1992) | 6.0 ¶¶ | Standard microcytotoxicity |
| Polyarticular RF positive | 12 | 10.4 | ||||||||||||||
| Polyarticular RF negative | 23 | 20.0 | ||||||||||||||
| Oligoarticular | 30 | 26.1 | ||||||||||||||
| Systemic | 50 | 43.5 | ||||||||||||||
| 31 | Bouaddi et al., 2013 [40] | Morocco | 33 | 0.83 | NA | Polyarticular RF negative | 1 | 3.0 | 76 | NM | NA | NA | 12.10 | NM | NA | NA |
| Oligoarticular | 4 | 12.1 | ||||||||||||||
| ERA | 5 | 15.2 | ||||||||||||||
| Systemic | 8 | 24.2 | ||||||||||||||
| Polyarticular RF positive | 15 | 45.5 | ||||||||||||||
| 32 | Chipeta et al., 2013 [42] | Zambia | 78 | 1.23 | 8.70 years (range: 1–15 years) | Psoriatic | 1 | 1.3 | NA | NA | 11.50 | Chronic uveitis in 3 patients with oligoarticular JIA and in 2 patients with ERA; Acute uveitis in 1 each of ERA and polyarticular JIA | NA | NA | NA | NA |
| ERA | 5 | 6.4 | ||||||||||||||
| Polyarticular RF positive | 9 | 11.5 | ||||||||||||||
| Systemic | 11 | 14.1 | ||||||||||||||
| Oligoarticular | 25 | 32.1 | ||||||||||||||
| Polyarticular RF negative | 27 | 34.6 | ||||||||||||||
| 33 | Hussein et al., 2018 [49] | Egypt | 63 | 0.90 | 6.1 (range 3-14) ±2.8 | ERA | 0 | 0.0 | 20.6 | NM | 6.3 | Slit lamp examination | 69.8 | NM | NA | NA |
| Undifferentiated | 0 | 0.0 | ||||||||||||||
| Psoriatic Arthritis | 0 | 0.0 | ||||||||||||||
| Polyarticular RF negative | 6 | 9.5 | ||||||||||||||
| Systemic | 15 | 23.8 | ||||||||||||||
| Polyarticular RF positive | 16 | 25.4 | ||||||||||||||
| Oligoarticular | 26 | 41.3 | ||||||||||||||
| 34 | Olaosebikan et al., 2017 [54] | Nigeria | 28 | NA | NA | Systemic | 5 | 17.9 | NA | NA | NA | NA | 7.14^^ | Nephlometry | NA | NA |
| Oligoarticular | 9 | 32.1 | ||||||||||||||
| Polyarticular | 14 | 50.0 | ||||||||||||||
| 35 | Weakley et al., 2012 [60] | South Africa | 78 | 1 | 8 (4–10)* | Psoriatic Arthritis | 1 | 1.3 | 3.8*** | Majority ELISA, remaining Hep 2 immunofluorescent | NA | NA | 14.1^^^ | One positive or negative assay for RF was considered sufficient to classify a patient with polyarthritis | 23### | NM |
| Oligoarticular Extended | 4 | 5.1 | ||||||||||||||
| Systemic | 6 | 7.7 | ||||||||||||||
| Polyarticular RF positive | 11 | 14.1 | ||||||||||||||
| Persistent Oligoarticular | 17 | 21.9 | ||||||||||||||
| ERA | 18 | 23.0 | ||||||||||||||
| Polyarticular RF negative | 21 | 26.9 | ||||||||||||||
| 36 | Mostafa et al., 2019 [53] | Egypt | 48 | 2.42 | NA | Psoriatic | 0 | 0.0 | NA | NA | NA | NA | 42.0 | NM | NA | NA |
| ERA | 0 | 0.0 | ||||||||||||||
| Oligoarticular | 8 | 17.0 | ||||||||||||||
| Polyarthritis | 28 | 58.0 | ||||||||||||||
| Systemic | 12 | 25.0 | ||||||||||||||
| 37 | Dagher et al., 2014 [43] | Lebanon | 66 | 1 | 5.2 years (range: 9 months - 14 years). | Polyarticular RF positive | 0 | 0.0 | 23 | NM | 6.1 | NM | NA | NA | NA | NA |
| Oligoarticular extended | 3 | 4.0 | ||||||||||||||
| Undifferentiated | 3 | 5.0 | ||||||||||||||
| ERA | 11 | 17.0 | ||||||||||||||
| Systemic | 15 | 23.0 | ||||||||||||||
| Polyarticular RF negative | 16 | 24.0 | ||||||||||||||
| Oligoarticular persistent | 18 | 27.0 | ||||||||||||||
| 38 | Khawaja et al., 2017 [52] | UAE | 66 | 2.47 | NA | ERA | 1 | 1.5 | NA | NA | 7.6 | NM | NA | NA | NA | NA |
| Psoriatic | 1 | 1.5 | ||||||||||||||
| Oligoarticular extended | 3 | 4.5 | ||||||||||||||
| Polyarticular RF positive | 12 | 18.2 | ||||||||||||||
| Systemic | 13 | 19.7 | ||||||||||||||
| Oligoarticular persistent | 16 | 24.2 | ||||||||||||||
| Polyarticular RF negative | 20 | 30.3 | ||||||||||||||
| 39 | Alzyoud et al., 2020 [37] | Jordan | 210 | 1.23 | 5.08±3.4 (7 months to 14 years) | Polyarticular RF positive | 8 | 3.8 | 33.60 | Indirect immunofluorescence using Hep-2 cells; titers > 1/80 were considered positive | 14.2††† | Slit lamp examination at a dedicated uveitis clinic | 3.80 | Nephelometry; Considered positive when titers were ≥ 15 units/mL. and at least two positive results, 3 months apart, in the first 6 months of observation | NA | NA |
| ERA | 15 | 7.1 | ||||||||||||||
| Polyarticular RF negative | 18 | 8.5 | ||||||||||||||
| Psoriatic arthritis | 18 | 8.5 | ||||||||||||||
| Systemic arthritis | 36 | 17.1 | ||||||||||||||
| Persistent Oligoarticular | 96 | |||||||||||||||
| Extended Oligoarticular | 19 | |||||||||||||||
| Oligoarticular | 115 | 54.7 | ||||||||||||||
| 40 | Demirkaya et al., 2011 [44] | Turkey | 634 | 1.26 | 7.69±4.41 (1-11 years) | Psoriatic | 13 | 2.1 | 30.1 | Titer of 1:80 was chosen as a cut-off point for ANA positivity for at least two positive results at least 3 months apart | 11.6 | Defined in accordance with the criteria of the SUN Working Group^^^^ | 3.1‡‡‡ | NM | 63.3§§§ | NM |
| RF positive polyarthritis | 20 | 3.2 | ||||||||||||||
| Extended Oligoarticular | 26 | 4.1 | ||||||||||||||
| Systemic | 92 | 14.5 | ||||||||||||||
| ERA | 120 | 18.9 | ||||||||||||||
| RF negative polyarthritis | 129 | 20.3 | ||||||||||||||
| Persistent Oligoarticular | 234 | 36.9 | ||||||||||||||
| 41 | Karadag et al., 2020 [50] | Turkey | 281 | NA | NA | RF positive polyarticular | 4 | 1.4 | NA | NA | NA | NA | NA | NA | NA | NA |
| Undifferentiated | 7 | 2.5 | ||||||||||||||
| Systemic | 11 | 3.9 | ||||||||||||||
| Psoriatic | 13 | 4.6 | ||||||||||||||
| RF negative polyarticular | 19 | 6.8 | ||||||||||||||
| ERA | 97 | 34.5 | ||||||||||||||
| Oligoarticular | 130 | 46.3 | ||||||||||||||
| 42 | Yilmaz et al., 2008 [62] | Turkey | 196 | 0.92 | 6.9 ± 3.7 | Psoriatic arthritis | 2 | 1.0 | 14.2 | Indirect Immunofluorescence using Hep-2 cell; titers >1/80 were considered positive | 2 | Slit lamp and detailed ophthalmological examination by ophthalmologist every 4 – 6 months; chronic uveitis occurred in 2 patients with persistent oligoarticular JIA | 8.1 | Nephelometry; Considered positive when titers were 15 units/mL and confirmed with two positive results, 3 months apart, during the first 6 months of observation | 5.6 | Lymphocytotoxicity assay |
| Others | 5 | 2.5 | ||||||||||||||
| RF (+) polyarticular JIA | 13 | 6.6 | ||||||||||||||
| Oligoarticular Extended | 19 | 9.6 | ||||||||||||||
| ERA | 19 | 10.3 | ||||||||||||||
| Systemic JIA | 30 | 15.0 | ||||||||||||||
| Oligoarticular Persistent | 48 | 24.4 | ||||||||||||||
| RF (–) polyarticular JIA | 60 | 30.6 | ||||||||||||||
* Represents values in median
#All patients underwent HLA-B27 testing; number patients tested not available in the article
†3 out of 5 oligoarticular JIA patients tested positive for uveitis, however no full cohort uveitis rate is mentioned
‡ HLA testing was carried out in only ERA (6 cases)
§Not all cases were tested (5/46; 2 oligoarticular, 3 polyarticular RF Positive)
¶Only in RF positive patients
^Note that HLA-B27 test was done in only 47 of the 213 JIA patients
**overall (62.22% in oligo)
##In polyarticular JIA
††in ERA
‡‡HLA-B27 was studied in 169 patients (in all patients with ERA phenotype and male patients over the 6 years of age)
§§Note that only the RF positive polyarticular patients tested positive (n=82)
¶¶HLA-B27 was tested in 32 patients
^^Positive in 2 polyarticular positive RF and this is not specific to JIA patients only
***ANA testing was performed only on oligoathritis patients(n=67)
^^^Performed for polyarticular subtype
###HLA tests were only performed for ERA subtype; all patients tested positive
†††Most of them were Oligoarticular JIA 25/115 (21.7%) and were associated with positive ANA in 16/115 (14%)
‡‡‡Tested in all except systemic; positive in only RF positive cases
§§§Tested only in ERA patients
¶¶¶In patients with uveitis; 24.6% in patients without uveitis; 28.4% combined population
^^^^Both the publications have cited Jabs et al., 2005 for the SUN Working Group Anatomic Classification of Uveitis. SUN working group has classified uveitis based on the primary site of inflammation: anterior uveitis (anterior chamber); intermediate uveitis (vitreous); posterior uveitis (retina or choroid); panuveitis (anterior chamber, vitreous, and retina or choroid).
ANA, anti-nuclear antibody; ARA, American Rheumatology Association; ELISA, Enzyme-Linked Immunoassay; ERA, enthesitis-related arthritis; EULAR, The European League Against Rheumatism; HLA, human leukocyte antigen; ILAR, International League of Associations for Rheumatology; IQR, interquartile range; JIA, juvenile idiopathic arthritis; NA, not available (study did not assess the parameter); NM, not mentioned; PCR, polymerase chain reaction; RF, rheumatoid factor.
Additionally, online databases of the American College of Rheumatology, the Asia-Pacific League of Associations for Rheumatology and the European League Against Rheumatism, Arab League of Associations of Rheumatologists, African league of Associations of Rheumatologists, and South African Rheumatism and Arthritis Association were searched for abstracts presented at annual congresses.
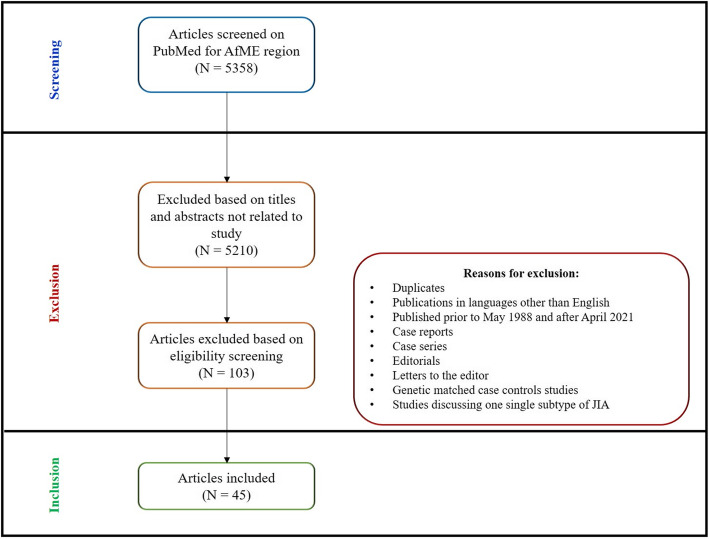
Publications in languages other than English, evaluating JIA incidence alone, or characterizing one subtype of JIA and or that were published prior to 1988 were excluded. Case reports and case series, editorials, letters to the editor and duplicates were also excluded. For the demographics search genetic matched case controls studies and studies discussing one single subtype of JIA were also excluded to limit selection bias. Please refer to Fig. 1.
Fig. 1
Assessment of the risk of bias each study included in our prevalence search was assessed using the Hoy 2012 [9] tool to address of internal and external validity (Table 3). Each parameter was assessed as either low or high risk of bias. Overall assessment of bias was according to number of “high” risk of bias in the parameters per study: low ≤2, moderate [3, 4], and high ≥5.
Table 3.
Risk of Bias Assessment of Included Studies Using the Hoy 2012 Tool
| Sr. No. | Reference | 1.Representation | 2.Sampling | 3.Random Selection | 4.Non-response bias | 5.Data Collection | 6.Case Definition | 7.Reliability Tool | 8.Method of data collection | 9.Prevalence Period | 10.Numerators and Denominators | Summary Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Khuffash et al., 1988 [13] | High | Low | Low | Unclear | Low | High | Low | Low | Low | Low | Moderate |
| 2. | Khuffash et al., 1990 [14] | High | Low | Low | Unclear | Low | High | Low | Low | Low | Low | Moderate |
| 3. | Abdwani et al., 2015 [11] | High | Low | Low | Unclear | Low | Low | Low | Low | Low | Low | Low |
| 4. | Ozen et al., 1998 [15] | Low | Low | Low | Unclear | Low | Low | Unclear | Low | Low | Low | Low |
| 5. | El-Soud et al., 2013 [12] | Low | Low | High | Unclear | Low | Low | Low | Low | Low | Low | Low |
| 6. | Singwe-Ngandeu et al., 2013 [16] | High | Low | Low | Unclear | High | Unclear | Low | Low | Unclear | High | High |
| 7. | Tayel et al., 1999 [17] | Unclear | Low | Low | Low | Low | Low | Low | Low | Unclear | Low | Low |
All articles included in our search were assessed for their quality in terms of methodology, sample size, study design, classification criteria, study period, characteristics and limitations summarized in (Table 4) and (Table 5) to address wide heterogenicity of design of the study types included and limit potential bias with assessment of the results.
Table 4.
Characteristics of Studies - Epidemiology
| Sr. No. | Reference | Country | Study Design | No. of studies included | Sample size | Single or multiple center | Classification Criteria | Time Period | Study features and Limitations |
|---|---|---|---|---|---|---|---|---|---|
| Global/Regional | |||||||||
| 1. | Usenbo et al., 2015 [10] | Africa | Systematic Review | 27 cross-sectional studies | NA | NA | Multiple criteria | 1975-2014 |
•The studies included do not follow a standardized diagnostic criterion •Risk of bias assessed for each study included •Studies on JIA were not pooled in a meta-analysis due to wide statistical heterogeneity |
| Country | |||||||||
| 2. | Khuffash et al., 1988 [13] | Kuwait | Hospital, consultations | NA | 186,363 | Not reported | ACR (for 3 months) | 1978-1987 |
•10-year study period •ACR criteria utilized •Potential referral bias of more severe cases specifically systemic JIA |
| 3. | Khuffash et al., 1990 [14] | Kuwait | Hospital, medical records revised by experts, hospital attendance | NA | 577,540 | Multi-center | ACR (for 3 months) | 1981-1988 |
•Retrospective •Large population cohort •Possible underestimation of undiagnosed cases in the community and nonreferral by primary care practitioners •Children aged between 12 and 16 years were excluded. •Female children possibly underrepresented •No current data is available |
| 4. | Abdwani et al., 2015 [11] | Oman | Hospital based, medical records | NA | 528,480 | Multi-center | ILAR 2004 | 2004-2013 |
•Retrospective •10-year study duration •Potential underestimation, only children <13 years of age were included •Potential referral bias, study might have missed on milder cases |
| 5. | Ozen et al., 1998 [15] | Turkey | Community based survey (parent questionnaire, clinical exam in homes by trained practitioners) | NA | 46,813 | Multi-center | EULAR (for 6 weeks) | 1997 |
•Community-based study from 5 districts in turkey •Possible Exclusion of undiagnosed cases not identifiable from questionnaires may have led to possible underestimation |
| 6. | El-Soud et al., 2013 [12] | Egypt Sharkia Governate, Egypt | Population based prospective study, with retrospective chart review | NA | 3,844,718 | Multi-center | 2004 ILAR | 2009-2010 |
•First population-based study from Sharkia governate •Large population cohort included 19 districts •Possible underestimation of numbers due to undiagnosed cases in the community and nonreferral from primary care practitioners |
| 7. | Singwe-Ngandeu et al., 2013 [16] | Cameroon | Cross sectional medical chart review | NA | 34,782 | Multi-center | Not reported | 2004-2012 |
•Retrospective •Large population cohort •Potential referral bias of more severe cases |
| 8. | Tayel et al., 1999 [17] | Egypt Alexandria | Community based confirmed by clinical examination | NA | 1500 | NA | EULAR | NA |
•Cross sectional •School based •The prevalence period, method of data collection studied is unclear |
Table 5.
Quality Assessment of Articles Selected – Demographics Results
| Sr. No. | Reference | Country | Study Design | N (no. of cases) | Classification Criteria | Time Period | Limitations |
|---|---|---|---|---|---|---|---|
| Regional | |||||||
| 1 | Consolaro et al., 2019 [22] | Africa and Middle East | Retrospective chart review with prospective cross-sectional questionnaire | 1209 | ILAR | 2011-2016 |
•There was disproportionate number of patients included from various geographical areas •Potential underrepresentation of milder forms of JIA and referral bias •Wide variation in tests and evaluation can affect evaluations or tests •Some countries could not be included •Method of grouping some countries in a particular geographical area was arbitrary •Wide variation in healthcare resources across countries |
| 2 | Al-Mayouf et al., 2021 [28] | Arab (Saudi Arabia, Libya, United Arab Emirates, Jordan, Oman, Egypt, Kuwait) | Retrospective chart review with prospective disease activity and disease assessment | 702 | ILAR | 2010-2019 |
•It was a cross-sectional analysis •There is a possibility of patients •selection bias as the participating centers did not enroll the same number of patients •Wide variation in healthcare resources across countries |
| Country | |||||||
| 3 | Khuffash et al., 1988 [13] | Kuwait | Hospital, consultations | 41 | ARA | 1978-1987 |
•10-year study period •ACR criteria utilized •Potential referral bias of more severe cases specifically systemic JIA |
| 4 | Khuffash et al., 1990 [14] | Kuwait | Hospital, medical records revised by experts, hospital attendance | 108 | ARA | 1981-1988 |
•Retrospective •Large population cohort •Possible underestimation of undiagnosed cases in the community and nonreferral by primary care practitioners •Children aged between 12 and 16 years were excluded. •Female children possibly underrepresented •No current data is available |
| 5 | Abdwani et al., 2015 [11] | Oman | Retrospective, Hospital, medical records, multicentre | 107 | ILAR | 2004-2013 |
•Retrospective •10-year study duration •Potential underestimation, only children <13 years of age were included •Potential referral bias, study might have missed on milder cases |
| 6 | Ozen et al., 1998 [15] | Turkey | Community based survey (parent questionnaire, clinical exam in homes) | 30 | EULAR | 1997 |
•Community-based study from 5 districts in turkey •Possible Exclusion of undiagnosed cases not identifiable from questionnaires may have led to possible underestimation |
| 7 | Abou El-Soud et al., 2013 [12] | Egypt | Population based in Sharkia Governate prospective study, with retrospective chart review | 132 | ILAR | 2009-2010 |
•First population-based study from Sharkia governate •Large population cohort included 19 districts •Possible underestimation of numbers due to undiagnosed cases in the community and nonreferral from primary care practitioners |
| 8 | Furia et al., 2020 [47] | Tanzania | Retrospective hospital chart review | 28 | EULAR | 2012-2019 |
•Single centered study •Retrospective study •Possible referral bias and underestimation of milder forms of disease |
| 9 | Aiche et al., 2018 [31] | Algeria | Cross sectional survey parent/PRO | 70 | ILAR | 2012-2013 |
•The objective of the study was to cross-culturally adapt and validate child/adult version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) in JIA patients •Possible selection bias •Only selected centers were invited to participate |
| 10 | Al Marri et al., 2017 [32] | Saudi Arabia | Prospective record review | 23 | ILAR | 1990-2015 |
•Potential referral bias could have caused the overall frequency of familial JIA and recurrence risk •Heterogeneous patients were included and were not compared with controls |
| 11 | Al-Mayouf et al., 2018 [35] | Saudi Arabia | Cross sectional survey parent/PRO | 100 | ILAR | 2012-2016 |
•The objective of the study was to cross-culturally adapt and validate child/adult version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) in JIA patients Possible selection bias •Only selected centers were invited to participate |
| 12 | Salah et al., 2009 [63] | Egypt | Retrospective hospital chart review | 196 | ILAR | 1990-2006 |
•Single center tertiary hospital study •Higher frequency of oligoarticular JRA, polyarticular and systemic onset JRA could be due to referral bias to tertiary care facilities |
| 13 | Al-Abrawi et al., 2018 [33] | Oman | Cross sectional survey parent/PRO | 57 | ILAR | 2012-2013 |
•The objective of the study was to cross-culturally adapt and validate child/adult version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) in JIA patients •Possible selection bias •Only selected centers were invited to participate |
| 14 | Demirkaya et al., 2018 [45] | Turkey | Cross sectional survey parent/PRO | 466 | ILAR | 2012-2014 |
•The objective of the study was to cross-culturally adapt and validate child/adult version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) in JIA patients •Possible selection bias •Only selected centers were invited to participate |
| 15 | El Miedany et al., 2018 [46] | Egypt | Cross sectional survey parent/PRO | 100 | ILAR | 2014-2015 |
•The objective of the study was to cross-culturally adapt and validate child/adult version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) in JIA patients •Possible selection bias •Only selected centers were invited to participate |
| 16 | Hashad et al., 2018 [48] | Libya | Cross sectional survey parent/PRO | 100 | ILAR | 2014-2015 |
•The objective of the study was to cross-culturally adapt and validate child/adult version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) in JIA patients •Possible selection bias •Only selected centers were invited to participate |
| 17 | Oyoo et al., 2016 [55] | Kenya | Retrospective hospital chart review | 68 | ILAR | 2009-2016 |
•Single center tertiary hospital study •Center covers patients from all over Kenya, greater East and Central African region •RF positive polyarthritis patients may be overrepresented which were classified using only one positive assay •Possible underrepresentation of RF negative polyarthritis •Potential referral bias of severe forms of the disease |
| 18 | Scott et al., 2018 [57] | South Africa | Cross sectional survey parent/PRO | 91 | ILAR | 2013-2016 |
•The objective of the study was to cross-culturally adapt and validate child/adult version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) in JIA patients •Possible selection bias •Only selected centers were invited to participate |
| 19 | Sen et al., 2015 [58] | Turkey | Retrospective hospital chart review | 213 | ILAR | 1998-2013 |
•Single center study •The collected data may be incomplete and incorrect due to the retrospective study design •HLA-B27 test was not done for all patients |
| 20 | Shafaie et al., 2018 [59] | Iran | Cross sectional survey parent/PRO | 102 | ILAR | 2012 |
•The objective of the study was to cross-culturally adapt and validate child/adult version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) in JIA patients •Possible selection bias •Only selected centers were invited to participate |
| 21 | Yener et al., 2020 [61] | Turkey | Retrospective hospital chart review | 116 | ILAR | 2012-2018 |
•Single center study •Retrospective cohort study •The study included lower number of patients as compared to other studies conducted in the country |
| 22 | Çakan et al., 2017 [41] | Turkey | Retrospective hospital chart review | 265 | ILAR | 2010-2016 |
•Single center study •The study included lower number of patients •Short follow-up time |
| 23 | Kasapçopur et al., 2004 [51] | Turkey | Retrospective hospital chart review | 198 | ILAR | NA |
•Single center study •Study conducted to determine frequency of ANA positivity and uveitis in newly diagnosed JIA patients |
| 24 | Ozdogan et al., 1991 [56] | Turkey | Retrospective hospital chart review | 147 | EULAR/WHO | 1980-1988 |
•Single center study •Potential referral bias of milder forms of comorbidities such as uveitis |
| 25 | Abdul-Sattar et al., 2014 [30] | Egypt | Cross sectional Medical chart review, school attendance records, HRQOL questionnaire | 52 | ILAR | 2011-2013 |
•Single center study •Included patients aged 7-17 years diagnosed to ILAR criteria •Study aimed to investigate JIA patients school absenteeism and school functioning •Potential selection and referral bias •Cross-sectional study design limits the ability to determine temporal relationships between risk factors and both of school absenteeism and of poor school functioning |
| 26 | Abdul-Sattar et al., 2014 [29] | Egypt | Medical chart review, Health related quality of life (HRQoL) questionnaire | 58 | ILAR | 2010-2012 |
•Single center study •Included patients aged 8-18 years diagnosed to ILAR criteria •Small study sample •Study aimed to identify determinants of impaired HRQOL in children with JIA •Cross-sectional design limits the ability to determine temporal relationships between risk factors and HRQOL |
| 27 | Albokhari et al., 2019 [36] | Saudi Arabia | cross sectional health related quality of life survey | 44 | ILAR | 2017 |
•Single center study •Study aimed to evaluate effect of JIA on HRQOL •Single center study •Potential referral bias and over representation of more severe forms |
| 28 | Al-Hemairi et al., 2015 [34] | Saudi Arabia | Retrospective hospital chart review | 82 | ILAR | 2007-2015 |
•Retrospective record-based study •Single centered •Small sample size •Diagnosis was confirmed by pediatric rheumatologist |
| 29 | Amine et al., 2009 [38] | Morocco | Health related quality of life (HRQoL) survey | 80 | ILAR | 2006-2007 |
•The aim of the study was to assess HRQoL- related impact of JIA •Demographics, subtype, clinical and lab parameters were obtained for patients •Potential selection and referral bias over-representation of severe forms |
| 30 | Bahabari et al., 1997 [39] | Saudi Arabia | Retrospective hospital chart review with prospective follow-up | 115 | ACR | 1978-1993 |
•Multi-center study •18 months follow up •Potential referral bias and under representation of milder forms |
| 31 | Bouaddi et al., 2013 [40] | Morocco | Cross-sectional prospective | 33 | ILAR | 2013 |
•Aim of the study was to assess the impact of JIA on schooling •Single center •Case control •Small sample size |
| 32 | Chipeta et al., 2013 [42] | Zambia | Retrospective hospital chart review | 78 | EULAR/ILAR | 1994-1998 and 2006-2010 |
•Single center •Potential referral bias •Two different classifications were used for each study period •1994-1998 EULAR •2006-2010 ILAR •ANA test was not routinely available |
| 33 | Hussein et al., 2018 [49] | Egypt | Retrospective hospital chart review with prospective follow-up | 63 | ILAR | 2004-2010 |
•Single center •Cross sectional design |
| 34 | Olaosebikan et al., 2017 [54] | Nigeria | Retrospective hospital chart review | 28 | not specified | 2010-2016 |
•Single center •Patients referred to adults rheumatologists due to lack of pediatric rheumatology service •The study included all types of pediatric rheumatology patients, hence unreliable representation of JIA demographics |
| 35 | Weakley et al., 2012 [60] | South Africa | Prospective cross sectional | 78 | ILAR | 2010-2011 |
•Small sample size •Sample bias •Mutli-center |
| 36 | Mostafa et al., 2019 [53] | Egypt | Cross sectional HRQol and functional disability questionnaire | 48 | ILAR | 2018 |
•Aim of the study was to assess functional disability in JIA patients •Single-centered •Potential referral bias and underrepresentation of milder forms |
| 37 | Dagher et al., 2014 [43] | Lebanon | Retrospective chart review | 66 | ILAR | 2010-2014 |
•Single center •Potential referral bias |
| 38 | Khawaja et al., 2017 [52] | UAE | Retrospective hospital chart review ICD codes | 66 | ILAR | 2011-2014 |
•Aim of the study was to assess access to care for JIA patients amongst local and non-local population •Potential referral bias •Selection bias |
| 39 | Alzyoud et al., 2020 [37] | Jordan | Retrospective hospital chart review | 210 | ILAR | 2015-2019 |
•Single center •Potential referral bias •Patients above 14 years of age were not included |
| 40 | Demirkaya et al., 2011 [44] | Turkey | Retrospective cross sectional from registry | 634 | ILAR | 2008-2009 |
•Multi-center •Registry is not representative of all centers from Turkey |
| 41 | Karadag et al., 2020 [50] | Turkey | Retrospective hospital chart review with prospective data collection | 281 | ILAR | 2018-2019 |
•Retrospective chart review •1-year study duration, some patients did not have final diagnosis confirmed •Single center •Potential referral bias |
| 42 | Yilmaz et al., 2008 [62] | Turkey | Retrospective chart review | 196 | ILAR | 1995-2004 |
•Hospital based •Single center •Referral bias may explain low prevalence of oligoarticular JIA and low uveitis |
Search results: epidemiology of JIA in Africa and Middle East
Our PubMed search on epidemiology identified a total of 8 journal publications for all JIA subtypes. The results included 1 systematic review and meta-analysis conducted in Africa between 1975 up to 2014 [10] and seven publications from individual countries [11–17]. One article was excluded from our search as it included only one confirmed JIA case [18].
Discussion: epidemiology
The prevalence of JIA in Africa and Middle east was noted to be towards the lower range of the global estimate, estimated as (3.8 to 400 per 100,000) [8]. We identified the lowest prevalence in Africa with prevalence rate of less than 3.43 per 100,000, [12, 16] and less than 22 per 100,000 in the Gulf, [11, 13, 14] and highest prevalence identified in Turkey i.e., 64 per 100,000 [15].
Our search identified two studies from Kuwait, [13, 14] that used American College of Rheumatology (ACR) criteria of classification [13, 14] in hospital-based surveys and included patients aged <12 years. The ACR 1978 defined Juvenile Rheumatoid Arthritis (JRA) as persistent arthritis in one or more joints for at least 3 months with exclusion of diseases with similar manifestations. The arthritis was considered polyarticular if five or more joints are involved within 6 months of the onset [19]. The 1988 study extended over a 10-year period (1978-1987) and estimated a prevalence rate of 22 per 100,000 [13]. The other study estimated a prevalence of 18.7 per 100,000 (15.3-22.6) and an incidence of 2.8 (2.3-3.4) per 100,000 [95% CI] [14].
One community based epidemiological study from Turkey, screened 46,813 children from 5 different geographical regions, and reported a prevalence of 64 per 100,000 (43-91 [95% CI]) for juvenile chronic arthritis (including spondylarthritis or psoriatic arthritis) [15]. The EULAR criteria was used which defined Juvenile Chronic Arthritis as the chronic arthritis marked by swelling or effusion, or presence of 2 or more of the following: limitation of range of motion, tenderness or pain on motion, and increased heat in one or more joints for at least 6 weeks and included similar onset types such as juvenile Ankylosing Spondylitis and juvenile Psoriatic Arthritis [20].
Abdwani et al, 2015 conducted a multi-center, medical chart review in Oman between 2004 to 2013, using ILAR 2004 criteria in patients aged <13 years. The prevalence was estimated to be 20 per 100,000 and incidence was reported to be 2 per 100,000 [11].
One Egyptian study screened children <15 years of age in a population based epidemiological study in Sharkia Governate (2009-2010), using the 2004 revised ILAR classification. The prevalence was reported to be 3.43 per 100,000 (3.1–4.3) [95% CI] with overall mean age at diagnosis being 10.5 ± 3.6 (range 4–15) years. There was a statistically noticeable difference between urban and rural populations [12]. Another Egyptian community-based study used The European League Against Rheumatism (EULAR) criteria to confirm and classify cases of Juvenile Chronic Arthritis (JCA) in children aged 10-15 years old. A prevalence rate of 3.3 per 100,000 cases [4–62] [95% CI] was reported [10, 17].
Drawing conclusions on the prevalence of JIA in Africa and Middle East should be approached with caution for several reasons. First, due to the limited number of updated prospective epidemiological studies conducted in the region, and second to the wide heterogeneity of different study designs, case ascertainment and variable study qualities that assessed JIA prevalence in the region.
A wide variance of the prevalence rates was also observed. This variance can be explained by the wide diversity of the healthcare systems capabilities across the region, genetic, disease awareness, smaller sample size, and diagnostic challenges that are more prominent in some countries than others. The variance can also be attributed to absence of electronic healthcare system in some countries, difference in methodologies of case ascertainment, and lack of data collection through registries enough to publish findings. The authors provided Table 4 to outline the quality assessment of articles included from the search and Table 3 to assess the risk of bias for each study included from the search
Our search identified studies with different study designs. Community-based surveys were used in Turkey [15] and Egypt [12] while hospital-based chart reviews were utilized in Oman, Kuwait and Cameroon [11, 13, 14, 16]. Community based prevalence studies are known to provide higher prevalence rates compared to hospital-based studies and allow for undiagnosed cases to be included [8, 21]. Five of the seven local country studies were multi-centered [11, 12, 14–16], and two studies didn’t report details [13, 17]. Only one study conducted in Turkey used diagnostic and clinical examinations to confirm cases [15].
Ideally, studies estimating prevalence should use standardized methods and diagnostic criteria [21] for ascertaining the subtypes from the community and include well trained clinicians experienced in the field of rheumatology to confirm diagnosis. Three of the included studies were conducted more than 24 years ago where study methods, JIA disease and study reporting guidelines have drastically changed and developed. Recent studies tend to better describe the methodology and the results clearly due to evolution of reporting guidelines which was not the case with older studies [21].
JIA nomenclature has changed over the years from JRA to JCA to most recently adopting JIA (Juvenile Idiopathic Arthritis). Over the years, different JIA subtype classifications have been proposed and revisions have been implemented. Hence, the data found with use of a certain classification may reflect changes due to time rather than a real difference because of the classification itself [21, 22].
The variation in results may be attributed to the different classifications (ACR, [13, 14] ILAR, [11, 12] and EULAR [15, 17] used and, in some cases not defining the exact classification used [16].
Variability in disease presentation among the subtypes of JIA may make it difficult to compare prevalence estimates for this condition across different study settings. And like other inflammatory arthritis diseases, extended remissions occur, so that prevalence estimates may include individuals who are experiencing symptoms while cases that are in remission may be missed. Less severe subtypes and symptoms like oligoarticular are not further referred for diagnosis by a specialist pediatric rheumatologist. Most of the country specific prevalence studies set the upper age limit of 12 and 15 years for inclusion [11–14, 17] which can lead to underreporting of patients with onset of symptoms during adolescents between 12-16 years of age [21].
A lack of adequate number of rheumatologists and pediatric rheumatologists further adds to the challenge of accurately estimating the incidence and prevalence of rheumatological diseases [23]. This may contribute to the skewness of the results toward higher prevalence in urban areas.
There are too few pediatricians across the Africa and Middle East region to adequately cater to the JIA population in the region, also an appropriate referral hierarchy would be required to address the gap [24]. Paucity of well-trained pediatric rheumatologists, specifically in the rural areas compel many patients to visit other traditional healers [25] or healthcare professionals like general practitioners, family physicians [24] or orthopedics rather than rheumatologists.
Awareness of JIA is increasing and is reflected in the increasing prevalence across the globe and the region [26]. As healthcare systems and economies are developing, more resources are allocated towards improving diagnosis and management of childhood illnesses. Noticeably, most data in the literature describes evidence from the Middle East and North Africa region. There are far fewer data available on prevalence from the sub-Saharan Africa region. The absence of data, however, does not imply absence of the disease.
Robust epidemiological data is needed from the region to assess the impact of JIA on children from Africa and the Middle East through the development of prospective community based epidemiological studies covering regions rather than individual country-based studies needed to accurately determine the prevalence of JIA across the region. In addition, the development of national and regional registries can further facilitate the generation of evidence on JIA prevalence from this region [9].
Other solutions include increased capacity of general health care practitioners and pediatric rheumatologists to address healthcare access for patients underdiagnosed or undertreated. In addition, raise awareness to general and specialized practitioners on MSK examination skills and define uniform case ascertainment or referral criteria [27].
Search Results: Demographics
Our literature search identified 42 articles describing JIA subtypes and demographics from Africa and Middle East. We identified one global study that included 1209 patients from Africa and Middle East, [22] and one multicenter regional study from seven Arab countries, [28] and 40 publications of data from individual countries [11–15, 29–63]. A summary of the demographics is presented in Table 2.
Discussion: Demographics
The findings of this review support that the most prevalent subtype in Africa and Middle East is oligoarticular JIA subtype, followed by polyarticular RF negative, and systemic subtype. Our findings support the global epidemiology, treatment, and outcome of childhood arthritis throughout the world (EPOCA) study findings [22] and the regional Pediatric Rheumatology Arab Group (PRAG) study [28].
Oligoarticular subtype was observed to be the most frequent subtype based on the 15 local studies [12, 15, 29–31, 37, 38, 43, 44, 49, 50, 57, 59, 62, 63]. Followed by polyarticular then systemic JIA.
On a regional scale, the EPOCA study, enrolled 1209 JIA patients using ILAR 2004 criteria, from 15 participating countries from Africa and Middle East region. The study identified oligoarticular JIA (37.8%), RF-negative polyarthritis (22.4%) and systemic JIA (16.9%) as the predominant subtypes in Africa and the Middle East. A predominance of the female gender (61.6%) was observed with mean age of onset of 6.0 (2.9-9.8) and 5.9% of cases had positive signs of uveitis with predominance of uveitis amongst oligoarticular sub-type in 12.4% of the cases from the region [22].
In the PRAG study, 702 JIA patients with a disease duration of more than one year and fulfilled the ILAR criteria were enrolled from 14 pediatric rheumatology centers across seven Arab countries. Oligoarticular JIA (34.9%) was identified as the predominant subtype. Polyarticular JIA (29.5%) and systemic JIA (24.5%) were the second and third most identified subtypes [28].
Oligoarticular subtype has also been the most common across all regions in Europe and North and Latin America except Southeast Asia [8, 22, 64, 65]. A similar finding has also been observed from a JIA epidemiological study conducted in Canada that focused on ethnicity as a risk factor in JIA phenotypes [66]. Arab descent patients had a predominance of oligoarticular subtype [66]. Patients of Arab descent had the highest predominance of systemic disease subtype, almost twice higher than Asian descent patients 23.5% vs. 12%. In contrast, African descent patients had an equal distribution of oligoarticular and RF negative polyarticular disease and had the highest RF positive polyarticular disease prevalence amongst all ethnicities at 16.1% [66].
RF negative polyarticular JIA was the second most identified subtype in Africa and Middle East. The RF negative subtypes were reported to be the predominate subtype in Kuwait, [13, 14] Oman, [11, 33] and Saudi Arabia [35]. One study from Morocco reported predominance of RF-positive polyarthritis [40]. And only one study from Egypt identified undifferentiated subtype (40%) to be predominant [46]. Globally, RF negative polyarticular JIA was recognized to be most prevalent in North America and least in Southeast Asia [22]. Regionally, RF negative polyarticular JIA was identified at 22.6% from the PRAG study, [28] and 22.4% from the EPOCA study [22].
One study from Morocco (45.5%) [40] and one study from Egypt (25.4%) [49] reported a higher prevalence of RF positive polyarthritis as compared to RF negative subtype. The exact cause for a higher frequency of RF positive polyarthritis is unknown but can be attributed to genetics and selection bias. Among the studies that tested and reported rheumatoid factor results, Jordan reported the lowest RF positivity at 3.8% [37]. Regionally, RF positive polyarthritis was identified from the PRAG study at 6.8% [28] and 5% from the EPOCA study [22]. In the Canadian multiethnic cohort study, patients with African descent had the highest prevalence of RF positive polyarthritis and a lower uveitis rate [66]. This observation has been made in multiple studies describing the African population [67, 68]. The subtype frequencies of various geographic regions are presented in Table 6.
Table 6.
Frequency of ILAR Categories by Geographic Area
| Northern Europe (n = 845) |
Western Europe (n = 832) |
Southern Europe (n = 2400) |
Eastern Europe (n = 2044) |
North America (n = 523) |
Latin America (n = 849) |
Africa and Middle East (n = 1209) |
Southeast Asia (n = 379) |
|
|---|---|---|---|---|---|---|---|---|
| Systemic arthritis | 42 (5.0) | 57 (6.9) | 204 (8.5) | 167 (8.2) | 22 (4.2) | 149 (17.6) | 204 (16.9) | 125 (33.0) |
| Oligoarticular | 340 (40.2) | 317 (38.1) | 1360 (56.7) | 848 (41.5) | 185 (35.4) | 261 (30.7) | 457 (37.8) | 41 (10.8) |
| RF-negative polyarthritis | 223 (26.4) | 198 (23.8) | 480 (20.0) | 539 (26.4) | 165 (31.5) | 217 (25.6) | 271 (22.4) | 48 (12.7) |
| RF-positive polyarthritis | 30 (3.6) | 22 (2.6) | 31 (1.3) | 91 (4.5) | 22 (4.2) | 95 (11.2) | 61 (5.0) | 30 (7.9) |
| Psoriatic arthritis | 35 (4.1) | 40 (4.8) | 88 (3.7) | 54 (2.6) | 37 (7.1) | 13 (1.5) | 37 (3.1) | 5 (1.3) |
| Enthesitis related arthritis | 87 (10.3) | 125 (15.0) | 130 (5.4) | 254 (12.4) | 56 (10.7) | 83 (9.8) | 111 (9.2) | 113 (29.8) |
| Undifferentiated arthritis | 88 (10.4) | 73 (8.8) | 107 (4.5) | 91 (4.5) | 36 (6.9) | 31 (3.7) | 68 (5.6) | 17 (4.5) |
Data are number (%)
ILAR = International League of Associations for Rheumatology
Reprinted from Lancet Child Adolesc Health; 2019 3 (4):255-63. Reproduced with permission from copyright holder.
Notably, most of the Saudi Arabia studies reported systemic JIA subtype to be the most frequent [32, 34–36, 39] and in only one study from Turkey (26.3%) [51]. Saudi Arabia was the only country that reported systemic subtype as the most frequent from multiple studies [32, 35, 36, 39]. Higher incidence of systemic JIA was associated with large familial clusters in the country, especially in the southern region [32, 69]. Familial JIA suggest an autosomal recessive mode of inheritance with specific mutations in genetic markers like LACC1 [70, 71]. It has been observed that familial systemic JIA patients were younger at the onset of disease and diagnosed earlier than sporadic JIA cases and had a predominance of refractory disease with progressive disease course [32]. These findings were attributed to a high consanguinity marriage, and potential referral bias (severe cases presentation) [32, 35, 69]. Systemic JIA was identified at 16.9% from Africa and Middle East region in the EPOCA study [22] and identified at higher prevalence of 24.5% was observed in the PRAG study [28]. A lower frequency of systemic JIA subtype was observed in studies from Turkey [50] and South Africa [57] at 3.9% and 4.4%, respectively.
Enthesitis related arthritis (ERA) subtype was most frequent from three retrospective chart studies from Turkey, reported at 34.5% from Istanbul, [50] 32.9% from Denizli region [41] and 32.8% from the Adana region [61]. A third study from Istanbul identified ERA as the second most frequent subtype in 21.7% of the cases analyzed [51]. The lowest frequency of ERA subtype was reported from Saudi Arabia (1.2%), [34] United Arab Emirates (1.5%) [52]. It was observed that several studies from Iran, [59] Oman, [33] Saudi Arabia, [32, 36, 39] and Egypt [49, 53] reported no ERA cases in their cohort. However, two studies from South Africa (23% and 15.4%) [57, 60] reported higher prevalence of ERA subtypes than others. The trend for the high frequency of ERA in South Africa was attributed to the high population of people of Asian and European descent in some regions in South Africa [60].
EPOCA study identified ERA subtype in 9.2% of all cases in Africa and Middle East region, and PRAG study at 5.6% of all JIA cases [22, 28]. This finding of higher predominance of boys in one Turkish study was attributed by high frequency of ERA in Turkey which is more frequent in males than in females [41].
ERA subtype was identified at 9.2% and 5.6% from the EPOCA and PRAG studies, respectively [28]. And globally, ERA has been highest among southeast Asia and lowest in Southern Europe [22, 66]. The possible reason for the lower prevalence of ERA in the Arab and African populations is unknown but can explained by higher incidence of ERA in post-pubertal male, which may be referred to adult rheumatologists and not counted as JIA in pediatric rheumatology literature. Arab ERA patients showed greater articular damage with significant limitation [28]. Intra-country differences were observed in the frequency of JIA subtypes in Turkey [61]. Denizli and Istanbul regions reported ERA as the most common subtype, [41, 61] while oligoarticular was the most prevalent subtype in Adana, [62] Diyarbakir, [58] and from a regional multi-center registry study in Turkey [44]. The heterogenic nature of the Turkish population, cultural, socioeconomic, food habits, and mixed ethnicities have resulted in region wide variations [50, 61].
Psoriatic arthritis and undifferentiated arthritis were the least reported JIA subtype across all the studies from the region, and this observation is aligned with other regions globally [22].
In various studies conducted across the globe, an overall female predominance for JIA was observed [8, 22]. Our literature review also supports that JIA is more likely to occur in girls than in boys in the region [22]. However, notable differences in the ratios exist across the different countries in the region. We observed a higher female to male ratio in most studies conducted in individual countries from Africa and Middle East [11–13, 29–39, 42, 44, 45, 48, 52, 53, 55, 57–59, 61, 63]. Eight studies reported number of male cases to be higher in comparison to female cases. These included five studies from Turkey (female to male ratio - 0.94:1 [41], 0.6:1 [15], 0.87:1 [51], 0.92:1 [62], and 0.77:1 [56],) two from Egypt (female to male ratio - 0.9:1 [49] and 0.88:1 [46]), and one from Morocco (female to male ratio - 0.83:1 [40]). Notably, studies from Lebanon, Kuwait, South Africa, and Tanzania cohorts showed near equal gender distribution [14, 43, 47, 60]. In various studies conducted across the globe, an overall female predominance for JIA was observed [8, 22]. A similar trend was observed in most studies conducted in individual countries from Africa and Middle East [11–13, 29–39, 42, 44, 45, 48, 52, 53, 55, 57–59, 61, 63]. The multinational EPOCA [22] and PRAG [28] studies identified a predominance of girls in the identified JIA cases. The female to male ratio ranged from 1.6:1 [22] to 2:1 [28].
It is noticeable that there is female predominance in many autoimmune diseases, however, the referral bias and study methodologies, case ascertainment and geography can contribute to the variance in gender ratios [72–74]. Male predominance has been reported in some studies that maybe explained by unequal school and medical care provided to male and female children, especially in the rural areas [14, 21]. Globally two studies identified higher prevalence of disease in girls than in boys 19.4 (18.3-20.6) per 100,000 and 11.9 (10.2-11.9) per 100,000 [95% CI], respectively [8]. The higher predominance of JIA in boys has also been linked to high frequency of ERA by one Turkish study [41].
ANA positivity was identified in 30.9% of cases from the PRAG study [28]. From the local studies, the lowest frequency of ANA was reported in a study from Egypt (0%) [46] and highest from Morocco (76%) [40]. Other studies that reported relatively higher ANA positivity rates included 48.5% from Egypt [12], 44% from Turkey [61] and 36.5% from Saudi Arabia [34]. Notably, several local studies reported no ANA-positive patients in all its cohort. Our findings from this review conclude that a wide heterogeneity in ANA positivity among JIA studies can be attributed to genetics, different methods of ANA ascertainment and the unavoidable referral bias.
The human leukocyte antigen (HLA) - B27 was identified regionally in 5.3% cases by the PRAG study [28]. The majority of studies did not test for HLA-B27 in all patients, and some opted to test HLA-B27 in suspected ERA cases only. Among those studies, an Egyptian study reported 66% positivity, a South African study reported 23% positivity, and a Turkish study reported 63.3% positivity in the confirmed ERA cases [12, 44, 60]. One study from Turkey tested HLA-B27 in all ERA phenotype cases and in males over six years of age and reported 26% positivity rate [41]. One study analyzed HLA-B27 in all its patients [39]. One of the studies that analyzed HLA-B27, all JIA subtypes reported 21.1% positivity in overall cohort. However, all HLA-B27 positive patients were of ERA subtype [61].
Our findings from this review observed that uveitis and ANA positivity rates seem to be low for Africa and Middle East region. In individual countries, uveitis’ prevalence ranged from 1% from Iran [59] to 19.7% from Egypt [12]. Uveitis was identified in 8.3% of the PRAG study cases [28] and 5.9% from the EPOCA study [22]. The EPOCA study observed the lowest prevalence of uveitis in Africa and Middle East as compared to other regions [22] (Refer to Table 7). PRAG study reported a higher rate of uveitis i.e., 8.3% [28]. Two studies from Oman reported zero cases of uveitis from their cohorts [11, 33]. We identified one outlier study from Egypt, that reported 19.7% of the cohort with evidence of uveitis predominantly in the oligoarticular subtype. Coincidently, the same study reported high ANA positivity in its cohort in 48.5% cases and a high frequency of both combined ANA positivity and uveitis in oligoarticular subtype 62.3% [12]. Saurenman et al, 2007 also reported a lower relative risk of developing uveitis in Arab and Asian descent patients than European or native North American ethnic groups [66]. Similar findings have been observed in the African population [67, 68].
Table 7.
Demographic Features and Frequency of Uveitis
| Northern Europe (n = 845) |
Western Europe (n = 832) |
Southern Europe (n = 2400) |
Eastern Europe (n = 2044) |
North America (n = 523) |
Latin America (n = 849) |
Africa and Middle East (n = 1209) |
Southeast Asia (n = 379) |
|
|---|---|---|---|---|---|---|---|---|
| Girls | 593 (70.2%) | 538 (64.7%) | 1763 (73.5%) | 1303 (63.7%) | 374 (71.5%) | 550 (64.8%) | 745 (61.6%) | 164 (43.3%) |
| Boys | 252 (29.8%) | 294 (35.3%) | 637 (26.5%) | 741 (36.3%) | 149 (28.5%) | 299 (35.2%) | 463 (38.3%) | 215 (56.7%) |
| Age at onset (years) | 4.7 (2.2 – 9.4) | 6.4 (2.7 – 10.4) | 3.5 (1.9 – 7.3) | 6.7 (3.0 – 10.7) | 7.4 (3.1 – 10.9) | 6.8 (3.6 – 10.5) | 6.0 (2.9 – 9.8) | 7.0 (3.9 – 10.7) |
| Interval onset-referral (years) | 0.3 (0.1 – 0.8) | 0.4 (0.2 – 1.0) | 0.3 (0.1 – 0.9) | 0.3 (0.1 – 1.0) | 0.3 (0.1 – 0.8) | 0.4 (0.2 – 1.0) | 0.4 (0.2 – 1.5) | 0.6 (0.2 – 2.0) |
| Disease duration (years) | 5.0 (2.5 – 8.4) | 3.8 (1.8 – 6.7) | 4.4 (1.9 – 7.7) | 3.4 (1.6 – 6.2) | 4.4 (1.9 – 8.0) | 4.6 (2.1 – 7.3) | 2.8 (1.2 – 5.4) | 3.9 (1.9 – 6.7) |
| Uveitis | 161 (19.1%) | 94 (11.3%) | 450 (18.8%) | 183 (9.0%) | 59 (11.3%) | 54 (6.4%) | 71 (5.9%) | 19 (5.0%) |
Data are n(%) or median (IQR)
Reprinted from Lancet Child Adolesc Health; 2019 3 (4) :255-63. Reproduced with permission from copyright holder.
Across many studies conducted on JIA subtypes worldwide, a wide heterogeneity in the pattern of disease, age of onset, sex, and phenotypes has been observed [22, 66] owing to factors such as immunogenetic, socioeconomic status, environment, and diagnostic criteria [21, 61]. The wide diversity of study design and diagnostic criteria used adds to the challenge of forming a reliable picture of the demographics in the region. Further, there is a lack of uniformity with regards to the type and definition of biomarkers tested (RF, HLA-B27, ANA) and the subtype they are tested in [21, 66]. In some countries, there could be a recruitment bias in studies for patients >10 years of age, as they consult an adult rheumatologist [40]. Factors that may influence the heterogeneity in JIA subtype frequency within the region included: diverse socioeconomic, cultural, nutritional habits and genetics. Migration between the different parts of the region results in mixed ethnicities and different genetic constructs and could significantly contributor to this heterogeneity [66].
The readers should note that the observations should be approached with caution owing to the heterogenicity of the studies pooled. Most of the studies included in this manuscript for reviewing the demographics are single-centered, retrospective study with notable selection biases. Some of the studies included were limited by their sample size.
Region-specific unmet needs
Several factors can contribute to the delays in proper diagnosis and management of JIA which vary region wise. The challenges include access to rheumatology services, access to proper diagnosis and therapies, and lack of awareness of rheumatic musculoskeletal disorders at the policymaker and public level and general pediatricians [23, 24]. Limited access to rheumatologists has been identified as a global challenge, which has also been reported in Africa than in Middle East region. The ratio of practicing rheumatologists ranged 0.3-0.89 rheumatologists per 100,000 in the Gulf and reported lower in Africa 0-0.01 per 100,000 compared to 1.78 per 100,000 in USA [23]. This challenge is further amplified for pediatric patients due to the even greater limitation of pediatric rheumatologists' access and pediatric rheumatology training [24, 75]. The disparities in regulatory approval timelines, health care system settings, economies, and the level of a financial burden on patients may vary considerably across Africa and Middle East.
International guidelines recommend initiating treatment soon after diagnosis and setting remission of disease as the optimal treatment target [76–78]. Those with a longer duration of un-or undertreated disease may only achieve minimal improvement in disease activity. There are limited local and regional guidelines, International guidelines exist but are not always applicable in the region because of the high costs of new therapies and the constraints of regular follow-up. Algeria has developed their national JIA treatment guideline and is published in French [79]. In Egypt, registries have been set up to advance the cause and local guideline is underdevelopment.
A recommendation for management of JIA in less resourced countries has also been developed in a global effort which included experts from South Africa, Kenya and Zambia [80]. At the same time, other countries follow established international guidelines such as ACR, EULAR [35, 76–78]. There are regional collaborations being established throughout the region between countries under PRAG group which is a part of the Arab League of Associations for Rheumatology (ArLAR). The aim of these collaborations is to develop the field of pediatric rheumatology in the region, provide a network of research collaboration to address the unmet needs for patients, develop a consensus on JIA evidence generation and local treatment guidelines. As stated by an ongoing Pediatric Task Force Global Musculoskeletal Health there is a real need to improve research and outcomes for musculoskeletal disorders [81]. There are initiatives like Pediatric Society of the African League Against Rheumatism (PAFLAR) and Global Task Force for Musculoskeletal Health and Pediatric Rheumatology European Society (PReS), who have recognized the need and are working towards reaching out to children with rheumatic diseases who do not have access to proper care [82].
Conclusion
The region of Africa and Middle East is very diverse in terms of socioeconomic conditions, environmental factors, ethnicities, and healthcare infrastructures. There is a paucity of the latest and adequate data on JIA on its epidemiology. In the absence of databases or registries to track disease progression, JIA data for Africa and Middle East are generally derived from hospital-based studies, providing limited accounts of epidemiology. Prospective, population-based studies are preferable in descriptive epidemiology, compared to studies using secondary data that depend upon hospital or public health registry systems. However, such studies are expensive, time-consuming, and consequently rare, especially in lower-income countries. Hence, a comprehensive review was planned to critically analyze the available data from the region. The prevalence rates of the region are relatively lower compared to the global estimates. The reasons for the wide range reported from the region include differences in study designs, methodologies, reach to healthcare facilities, and non-uniform study methodologies. From the demographic data gathered, it was concluded that the oligoarticular subtype was the predominant one over another subtype in Africa and Middle East. It was also noted that the incidence of uveitis and ANA positivity in Africa and Middle East region was lower as compared to the incidence from other parts of the world. The region has an evident unmet need for awareness, delayed diagnosis, lack of an adequate number of rheumatologists, no published local or regional guidelines, and economic disparities. These lacunae need to be addressed to effectively manage JIA in the region.
Acknowledgements
Medical writing support was provided by Vaidehi Wadhwa (Medical Excellence, Emerging Markets, Pfizer Limited).
Abbreviations
- ACR
American College of Rheumatology
- ANA
Anti-nuclear antibody
- ArLAR
Arab League of Associations for Rheumatology
- EPOCA
The multinational epidemiology, treatment, and outcome of childhood arthritis throughout the world
- ERA
Enthesitis related arthritis
- EULAR
The European League Against Rheumatism
- HLA
Human leukocyte antigen
- ILAR
International League of Associations for Rheumatology
- JCA
Juvenile Chronic Arthritis
- JIA
Juvenile Idiopathic Arthritis
- JRA
Juvenile Rheumatoid Arthritis
- PAFLAR
Pediatric Society of the African League Against Rheumatism
- PRAG
Pediatric Rheumatology Arab Group
- PReS
Pediatric Rheumatology European Society
- RF
Rheumatoid Factor
Authors’ contributions
Authors SAM, MM, KB, DH, SH, HL, CS, ES, and NT contributed to conceptualization of the manuscript. All the authors helped with data curation, writing- review and editing. All authors read and approved the final manuscript.
Funding
The development of this manuscript was funded and sponsored by Pfizer. The medical writing support provided by Pfizer.
Availability of data and materials
Not applicable
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
All the authors have read and agreed to the publication of the manuscript.
Competing interests
Sara Habjoka and Nouran Tahoun are employees of Pfizer Ltd. All other authors report no potential conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giancane G, Consolaro A, Lanni S, Davi S, Schiappapietra B, Ravelli A. Juvenile Idiopathic Arthritis: Diagnosis and Treatment. Rheumatol Ther. 2016;3:187–207. doi: 10.1007/s40744-016-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369(9563):767–778. doi: 10.1016/S0140-6736(07)60363-8. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson JL, Pham JT. Juvenile Idiopathic Arthritis: A Focus on Pharmacologic Management. J Pediatr Health Care. 2018;32(5):515–528. doi: 10.1016/j.pedhc.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Saad N, Onel K. Overview of Juvenile Idiopathic Arthritis. The Open Orthopaedics Journal. 2020;14:101–109. [Google Scholar]
- 5.Verstappen SM, Cobb J, Foster HE, Fu B, Baildam vhE, Wedderburn LR, et al. The association between low socioeconomic status with high physical limitations and low illness self-perception in patients with juvenile idiopathic arthritis: results from the Childhood Arthritis Prospective Study. Arthritis Care Res (Hoboken). 2015;67(3):382–9. [DOI] [PMC free article] [PubMed]
- 6.Dave M, Rankin J, Pearce M, Foster HE. Global prevalence estimates of three chronic musculoskeletal conditions: club foot, juvenile idiopathic arthritis and juvenile systemic lupus erythematosus. Pediatr Rheumatol. 2020;18:49. doi: 10.1186/s12969-020-00443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McHugh J. Global prevalence of JIA, JSLE and club foot. Nat Rev Rheumatol. 2020;16(8):408. doi: 10.1038/s41584-020-0465-6. [DOI] [PubMed] [Google Scholar]
- 8.Thierry S, Fautrel B, Lemelle I, Guillemin F. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine. 2014;81(2):112–117. doi: 10.1016/j.jbspin.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Usenbo A, Kramer V, Young T, Musekiwa A. Prevalence of Arthritis in Africa: A Systematic Review and Meta-Analysis. PLoS ONE. 2015;10(8):e0133858. doi: 10.1371/journal.pone.0133858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdwani R, Abdalla E, Al Abrawi S, Al-Zakwani I. Epidemiology of juvenile idiopathic arthritis in Oman. Pediatr Rheumatol Online J. 2015;13:33. doi: 10.1186/s12969-015-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abou El-Soud AM, El-Najjar AR, El-Shahawy EE, Amar HA, Hassan TH, Abd-Allaha SH, et al. Prevalence of juvenile idiopathic arthritis in Sharkia Governorate, Egypt: epidemiological study. Rheumatol Int. 2013;33(9):2315–2322. doi: 10.1007/s00296-013-2707-2. [DOI] [PubMed] [Google Scholar]
- 13.Khuffash FA, Majeed HA. Juvenile rheumatoid arthritis among Arab children. Scand J Rheumatol. 1988;17(5):393–395. doi: 10.3109/03009748809105276. [DOI] [PubMed] [Google Scholar]
- 14.Khuffash FA, Majeed HA, Lubani MM, Najdi KN, Gunawardana SS, Bushnaq R. Epidemiology of juvenile chronic arthritis and other connective tissue diseases among children in Kuwait. Ann Trop Paediatr. 1990;10(3):255–259. doi: 10.1080/02724936.1990.11747439. [DOI] [PubMed] [Google Scholar]
- 15.Ozen S, Karaaslan Y, Ozdemir O, Saatci U, Bakkaloglu A, Koroglu E, et al. Prevalence of juvenile chronic arthritis and familial Mediterranean fever in Turkey: a field study. J Rheumatol. 1998;25(12):2445–2449. [PubMed] [Google Scholar]
- 16.Singwe-Ngandeu M, Mfegue Mengue AL, Ondoa Mekongo M, Ibrahima F, Mbassi Awa HD. Rheumatic diseases in African children: A hospital based study inYaounde, Cameroon. Clinical Rheumatology. 7th Congress of the African League of Associations for Rheumatology, AFLAR and 23rd Congress of the South African Rheumatism and Arthritis Association, SARAA Durban South Africa: Springer; 2013. [Google Scholar]
- 17.Tayel MY, Tayel KY. Prevalence of juvenile chronic arthritis in school children aged 10 to 15 years in Alexandria. J Egypt Public Health Assoc. 1999;74(5-6):529–546. [PubMed] [Google Scholar]
- 18.Singwe-Ngandeu M, Meli J, Ntsiba H, Nouedoui C, Yollo AV, Sida MB, et al. Rheumatic Diseases in Patients Attending a Clinic at a Referral Hospital in Yaounde. Cameroon East Afr Med J. 2007;84(9):404–409. doi: 10.4314/eamj.v84i9.9549. [DOI] [PubMed] [Google Scholar]
- 19.Brewer EJ, Jr, Bass J, Baum J, Cassidy JT, Fink C, Jacobs J, et al. Current proposed revision of JRA Criteria. JRA Criteria Subcommittee of the Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Section of The Arthritis Foundation. Arthritis Rheum. 1977;20(2 Suppl):195–199. [PubMed] [Google Scholar]
- 20.Wood PH. Special meeting on: nomenclature and classification of arthritis in children. In: Munthe E, ed. The care of rheumatic children, EULAR; Basel 1978. p. 47–50.
- 21.Manners PJ, Bower C. Worldwide prevalence of juvenile arthritis why does it vary so much. J Rheumatol. 2002;29(7):1520–1530. [PubMed] [Google Scholar]
- 22.Consolaro A, Giancane G, Alongi A, van Dijkhuizen EHP, Aggarwal A, Al-Mayouf SM, et al. Phenotypic variability and disparities in treatment and outcomes of childhood arthritis throughout the world: an observational cohort study. Lancet Child Adolesc Health. 2019;3(4):255–263. doi: 10.1016/S2352-4642(19)30027-6. [DOI] [PubMed] [Google Scholar]
- 23.Al Maini M, Adelowo F, Al Saleh J, Al Weshahi Y, Burmester GR, Cutolo M, et al. The global challenges and opportunities in the practice of rheumatology: white paper by the World Forum on Rheumatic and Musculoskeletal Diseases. Clin Rheumatol. 2015;34(5):819–829. doi: 10.1007/s10067-014-2841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harper BD, Nganga W, Armstrong R, Forsyth KD, Ham HP, Keenan WJ, et al. Where are the paediatricians? An international survey to understand the global paediatric workforce. BMJ Paediatr Open. 2019;3(1):bmjpo-2018-000397. 10.1136/bmjpo-2018-000397. [DOI] [PMC free article] [PubMed]
- 25.Abdillahi HS, Finnie JF, Van Staden J. Anti-inflammatory, antioxidant, anti-tyrosinase and phenolic contents of four Podocarpus species used in traditional medicine in South Africa. J Ethnopharmacol. 2011;136(3):496–503. doi: 10.1016/j.jep.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Oberle EJ, Harris JG, Verbsky JW. Polyarticular juvenile idiopathic arthritis - epidemiology and management approaches. Clin Epidemiol. 2014;6:379–393. doi: 10.2147/CLEP.S53168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Briggs A, Slater H, Jordan J, Huckel Schneider C, Kopansky-Giles D, Sharma S, et al. Towards a global strategy to improve musculoskeletal health. Australia: Global Alliance for Musculoskeletal Health; 2021. Available at: https://gmusc.com/wp-content/uploads/2021/07/Final-report-with-metadata.pdf.
- 28.Al-Mayouf SM, Hashad S, Khawaja K, Alrasheedi A, Abdwani R, Abushhaiwia A, et al. Cumulative Damage in Juvenile Idiopathic Arthritis: A Multicenter Study From the Pediatric Rheumatology Arab Group. Arthritis Care Res (Hoboken) 2021;73(4):586–592. doi: 10.1002/acr.24436. [DOI] [PubMed] [Google Scholar]
- 29.Abdul-Sattar A, Magd SA, Negm MG. Associates of school impairment in Egyptian patients with juvenile idiopathic arthritis: Sharkia Governorate. Rheumatol Int. 2014;34(1):35–42. doi: 10.1007/s00296-013-2871-4. [DOI] [PubMed] [Google Scholar]
- 30.Abdul-Sattar AB, Elewa EA, El-Shahawy Eel D, Waly EH. Determinants of health-related quality of life impairment in Egyptian children and adolescents with juvenile idiopathic arthritis: Sharkia Governorate. Rheumatol Int. 2014;34(8):1095–1101. doi: 10.1007/s00296-014-2950-1. [DOI] [PubMed] [Google Scholar]
- 31.Aiche MF, Djoudi H, Al-Mayouf S, Consolaro A, Bovis F, Ruperto N, et al. The Algerian Arabic version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) Rheumatol Int. 2018;38(Suppl 1):27–33. doi: 10.1007/s00296-018-3937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al Marri M, Qari A, Al-Mayouf SM. Juvenile idiopathic arthritis in multiplex families: longitudinal follow-up. Int J Rheum Dis. 2017;20(7):898–902. doi: 10.1111/1756-185X.13092. [DOI] [PubMed] [Google Scholar]
- 33.Al-Abrawi S, Al-Mayouf SM, Abdwani R, Abdalla E, Consolaro A, Bovis F, et al. The Omani Arabic version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) Rheumatol Int. 2018;38(Suppl 1):299–306. doi: 10.1007/s00296-018-3965-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Hemairi MH, Albokhari SM, Muzaffer MA. The Pattern of Juvenile Idiopathic Arthritis in a Single Tertiary Center in Saudi Arabia. Int J Inflam. 2016;2016:7802957. doi: 10.1155/2016/7802957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Mayouf SM, AlE'ed A, Muzaffer M, Consolaro A, Bovis F, Ruperto N, et al. The Arabic version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) Rheumatol Int. 2018;38(Suppl 1):43–49. doi: 10.1007/s00296-018-3979-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albokhari SM, Muzaffer MA. Health-Related Quality of Life of Children and Adolescents with Juvenile Idiopathic Arthritis in Western Saudi Arabia. Open J Rheumatol Autoimmune Dis. 2019;9:69–83. [Google Scholar]
- 37.Alzyoud R, Alsuweiti MO, Almaaitah HQ, Aladaileh BN, Alnoubani MK, Alwahadneh AM. Juvenile Idiopathic Arthritis in Jordan: Single-Center Experience. Res Square. 2020:1–17. [DOI] [PMC free article] [PubMed]
- 38.Amine B, Rostom S, Benbouazza K, Abouqal R, Hajjaj-Hassouni N. Health related quality of life survey about children and adolescents with juvenile idiopathic arthritis. Rheumatol Int. 2009;29(3):275–279. doi: 10.1007/s00296-008-0672-y. [DOI] [PubMed] [Google Scholar]
- 39.Bahabri S, Al-Sewairi W, Al-Mazyad A, Karrar A, Al-Ballaa S, El-Ramahai K, et al. Juvenile rheumatoid arthritis: The Saudi Experience. Ann Saudi Med. 1997;17(4):413–418. doi: 10.5144/0256-4947.1997.413. [DOI] [PubMed] [Google Scholar]
- 40.Bouaddi I, Rostom S, El Badri D, Hassani A, Chkirate B, Amine B, et al. Impact of juvenile idiopathic arthritis on schooling. BMC Pediatr. 2013;13:2. doi: 10.1186/1471-2431-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cakan M, Aktay-Ayaz N, Keskindemirci G, Ekinci DY, Karadag SG. Subtype frequencies, demographic features, and remission rates in juvenile idiopathic arthritis - 265 cases from a Turkish center. Turk J Pediatr. 2017;59(5):548–554. doi: 10.24953/turkjped.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Chipeta J, Njobvu P, Wa-Somwe S, Chintu C, McGill PE, Bucala R. Clinical patterns of juvenile idiopathic arthritis in Zambia. Pediatr Rheumatol Online J. 2013;11(1):33. doi: 10.1186/1546-0096-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dagher R, Assi S. Juvenile idiopathic arthritis: a single center Lebanese study. Pediatr Rheumatol. 2014;12:179. [Google Scholar]
- 44.Demirkaya E, Ozen S, Bilginer Y, Ayaz NA, Makay BB, Unsal E, et al. The distribution of juvenile idiopathic arthritis in the eastern Mediterranean: results from the registry of the Turkish Paediatric Rheumatology Association. Clin Exp Rheumatol. 2011;29(1):111–116. [PubMed] [Google Scholar]
- 45.Demirkaya E, Ozen S, Sozeri B, Ayaz NA, Kasapcopur O, Unsal E, et al. The Turkish version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) Rheumatol Int. 2018;38(Suppl 1):395–402. doi: 10.1007/s00296-018-3982-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El Miedany Y, El Mikkawy DME, Youssef SS, El Gaafary M, Nassar N, Consolaro A, et al. The Egyptian Arabic version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) Rheumatol Int. 2018;38(Suppl 1):155–161. doi: 10.1007/s00296-018-3949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furia FF, Godfrey E, Mwamanenge N, Swai P. Spectrum of paediatric rheumatic disorders at a tertiary hospital in Tanzania. Pediatr Rheumatol Online J. 2020;18(1):30. doi: 10.1186/s12969-020-0418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashad S, Zletni MA, Al-Mayouf SM, Etayari H, Ibrahim E, Etfil M, et al. The Libyan Arabic version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) Rheumatol Int. 2018;38(Suppl 1):267–274. doi: 10.1007/s00296-018-3962-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hussein ZM, Wagdy R, Shawki M, Zohni S, Shehawy I. The pattern of juvenile idiopathic arthritis; a retrospective Egyptian study. Egypt J Pediatr Allergy Immunol. 2018;16(1):7–14. [Google Scholar]
- 50.Karadag SG, Sonmez HE, Tanatar A, Cakmak F, Cakan M, Ayaz NA. Profile of new referrals to a single pediatric rheumatology center in Turkey. Rheumatol Int. 2020;40(2):313–321. doi: 10.1007/s00296-019-04421-6. [DOI] [PubMed] [Google Scholar]
- 51.Kasapcopur O, Yologlu N, Ozyazgan Y, Ercan G, Caliskan S, Sever L, et al. Uveitis and anti nuclear antibody positivity in children with juvenile idiopathic arthritis. Indian Pediatr. 2004;41(10):1035–1039. [PubMed] [Google Scholar]
- 52.Khawaja K, Al-Maini M. Access to pediatric rheumatology care for Juvenile Idiopathic Arthritis in the United Arab Emirates. Pediatr Rheumatol Online J. 2017;15(1):41. doi: 10.1186/s12969-017-0170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mostafa WE, Abdul-Sattar AB, Dawa GAE. Prevalence and Factors of Functional Disability in Patients with Juvenile Idiopathic Arthritis. Zagazig Univ Med J. 2019;25(3):456–463. [Google Scholar]
- 54.Olaosebikan BH, Adelowo OO, Animashaun BA, Akintayo RO. Spectrum of paediatric rheumatic diseases in Nigeria. Pediatr Rheumatol Online J. 2017;15(1):7. doi: 10.1186/s12969-017-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oyoo GO, Genga EK, Otieno FO, Omondi EA. Clinical patterns of juvenile idiopathic arthritis: A single tertiary center experience in Kenya. Afr J Rheumatol. 2016;4(2):66–71. [Google Scholar]
- 56.Ozdogan H, Kasapcopur O, Dede H, Arisoy N, Beceren T, Yurdakul S, et al. Juvenile chronic arthritis in a Turkish population. Clin Exp Rheumatol. 1991;9(4):431–435. [PubMed] [Google Scholar]
- 57.Scott C, Okong'o L, Brice N, Murless S, Slamang W, Fadlelmola A, et al. The Afrikaans version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) Rheumatol Int. 2018;38(Suppl 1):19–26. doi: 10.1007/s00296-018-3980-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sen V, Ece A, Uluca U, Gunes A, Yel S, Tan I, et al. Evaluation of children with juvenile idiopathic arthritis in southeastern Turkey: a single center experience. Hippokratia. 2015;19(1):63–68. [PMC free article] [PubMed] [Google Scholar]
- 59.Shafaie N, Ziaee V, Aghighi Y, Raees Karami SR, Moradinejad MH, Consolaro A, et al. The Farsi version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) Rheumatol Int. 2018;38(Suppl 1):171–178. doi: 10.1007/s00296-018-3958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weakley K, Esser M, Scott C. Juvenile idiopathic arthritis in two tertiary centres in the Western Cape. South Africa. Pediatr Rheumatol Online J. 2012;10(1):35. doi: 10.1186/1546-0096-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yener GO, Tekin ZE, Girisgen I, Cetin EN, Akdag B, Yuksel S. Juvenile idiopathic arthritis in a center in the Western Anatolia region in Turkey. Turk Pediatri Ars. 2020;55(2):157–165. doi: 10.14744/TurkPediatriArs.2019.69320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yilmaz M, Kendirli SG, Altintas DU, Karakoc GB, Inal A, Kilic M. Juvenile idiopathic arthritis profile in Turkish children. Pediatr Int. 2008;50(2):154–158. doi: 10.1111/j.1442-200X.2008.02543.x. [DOI] [PubMed] [Google Scholar]
- 63.Salah S, Hamshary A, Lofty H, Adbdel RH. Juvenile Idiopathic Arthritis, the Egyptian Experience. J Med Sci. 2009;9(2):98–102. [Google Scholar]
- 64.Oen K, Tucker L, Huber AM, Miettunen P, Scuccimarri R, Campillo S, et al. Predictors of early inactive disease in a juvenile idiopathic arthritis cohort: results of a Canadian multicenter, prospective inception cohort study. Arthritis Rheum. 2009;61(8):1077–1086. doi: 10.1002/art.24539. [DOI] [PubMed] [Google Scholar]
- 65.Schinzel V, da Silva SGL, Terreri MT, Len CA. Prevalence of juvenile idiopathic arthritis in schoolchildren from the city of Sao Paulo, the largest city in Latin America. Adv Rheumatol. 2019;59(1):32. doi: 10.1186/s42358-019-0078-4. [DOI] [PubMed] [Google Scholar]
- 66.Saurenmann RK, Rose JB, Tyrrell P, Feldman BM, Laxer RM, Schneider R, et al. Epidemiology of juvenile idiopathic arthritis in a multiethnic cohort: ethnicity as a risk factor. Arthritis Rheum. 2007;56(6):1974–1984. doi: 10.1002/art.22709. [DOI] [PubMed] [Google Scholar]
- 67.Haffejee IE, Raga J, Coovadia HM. Juvenile chronic arthritis in black and Indian South African children. S Afr Med J. 1984;65(13):510–514. [PubMed] [Google Scholar]
- 68.Schwartz MM, Simpson P, Kerr KL, Jarvis JN. Juvenile rheumatoid arthritis in African Americans. J Rheumatol. 1997;24(9):1826–1829. [PubMed] [Google Scholar]
- 69.Al-Mayouf SM, Madi SM, AlMane K, Al JS. Comparison of clinical and laboratory variables in familial versus sporadic systemic onset juvenile idiopathic arthritis. J Rheumatol. 2006;33(3):597–600. [PubMed] [Google Scholar]
- 70.Aviel YB, Ofir A, Ben-Izhak O, Vlodavsky E, Karbian N, Brik R, et al. A novel loss-of-function mutation in LACC1 underlies hereditary juvenile arthritis with extended intra-familial phenotypic heterogeneity. Rheumatology (Oxford). 2021. [DOI] [PubMed]
- 71.Wakil SM, Monies DM, Abouelhoda M, Al-Tassan N, Al-Dusery H, Naim EA, et al. Association of a mutation in LACC1 with a monogenic form of systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 2015;67(1):288–295. doi: 10.1002/art.38877. [DOI] [PubMed] [Google Scholar]
- 72.Kronzer VL, Bridges SL, Jr, Davis JM., 3rd Why women have more autoimmune diseases than men: An evolutionary perspective. Evol Appl. 2021;14(3):629–633. doi: 10.1111/eva.13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orstavik KH. Why are autoimmune diseases more prevalent in women. Tidsskr Nor Laegeforen. 2017;137(12-13):866–868. doi: 10.4045/tidsskr.16.0935. [DOI] [PubMed] [Google Scholar]
- 74.Tincani A, Gerardl MC, Fredl M, Andreoll L. Gender differences in rheumatology and the point of view of the Italian Society for Rheumatology (SIR) Ital J Gend Specif Med. 2018;4(4):e79–e88. [Google Scholar]
- 75.Henrickson M. Policy challenges for the pediatric rheumatology workforce: Part III. the international situation. Pediatr Rheumatol Online J. 2011;9:26. doi: 10.1186/1546-0096-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Foster HE, Minden K, Clemente D, Leon L, McDonagh JE, Kamphuis S, et al. EULAR/PReS standards and recommendations for the transitional care of young people with juvenile-onset rheumatic diseases. Ann Rheum Dis. 2017;76(4):639–646. doi: 10.1136/annrheumdis-2016-210112. [DOI] [PubMed] [Google Scholar]
- 77.Ringold S, Angeles-Han ST, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Non-Systemic Polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Care Res (Hoboken) 2019;71(6):717–734. doi: 10.1002/acr.23870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ringold S, Weiss PF, Beukelman T, DeWitt EM, Ilowite NT, Kimura Y, et al. 2013 update of the 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Rheum. 2013;65(10):2499–2512. doi: 10.1002/art.38092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guide De L’arthrite Juvenile Idiopathique: Groupe De Rhumatologie Pediatrique; 2020. Published by: SociétéAlgérienne de Pédiatrie, Available at: https://www.sapediatrie-dz.com/site/congres.php.
- 80.Scott C, Chan M, Slamang W, Okong'o L, Petty R, Laxer RM, et al. Juvenile arthritis management in less resourced countries (JAMLess): consensus recommendations from the Cradle of Humankind. Clin Rheumatol. 2019;38(2):563–575. doi: 10.1007/s10067-018-4304-y. [DOI] [PubMed] [Google Scholar]
- 81.Foster HE, Scott C, Tiderius CJ, Dobbs MB. Members of the Paediatric Global Musculoskeletal Task F. Improving musculoskeletal health for children and young people - A 'call to action'. Best Pract Res Clin Rheumatol. 2020;34(5):101566. doi: 10.1016/j.berh.2020.101566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Migowa AN, Hadef D, Hamdi W, Mwizerwa O, Ngandeu M, Taha Y, et al. Pediatric rheumatology in Africa: thriving amidst challenges. Pediatr Rheumatol Online J. 2021;19(1):69. doi: 10.1186/s12969-021-00557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable