Abstract
Objective:
To investigate differences in the amount of tooth movement and root resorption that occurred after tipping and bodily movement of the maxillary first molar in rats.
Materials and Methods:
Ten-week-old female Wistar rats were divided into two groups according to type of tooth movement and subdivided into four subgroups according to the magnitude of applied force. Nickel-titanium closed-coil springs exerting forces of 10, 25, 50, or 100 g were applied to the maxillary left first molars to induce mesial tooth movement. We designed a novel orthodontic appliance for bodily tooth movement. Tooth movement distance and root resorption were measured using microcomputed tomography and scanning electron and scanning laser microscopy.
Results:
The amount of tooth movement in the bodily tooth movement group was less than half that in the tipping tooth movement group. The greatest amount of tooth movement occurred in the 10-g tipping and 50-g bodily tooth movement subgroups, and the amount of tooth movement decreased with the application of an excessive magnitude of force. Conversely, root resorption increased when the heavier orthodontic force was applied in both groups. Root resorption in the tipping tooth movement group was approximately twice that in the bodily tooth movement group.
Conclusions:
Root resorption in the tipping tooth movement group was more pronounced than that in the bodily tooth movement group. Although the amount of tooth movement decreased when extremely heavy forces were applied, root resorption increased in both the tipping and bodily tooth movement groups in rats.
Keywords: Tooth movement, Root resorption, Type of tooth movement
INTRODUCTION
Orthodontic treatment sometimes entails adverse side effects, such as root resorption. More than a century has passed since Sandstedt1 published his detailed drawings of histological slides (the technology to reproduce images of such histological slides had not yet been developed). He observed not only the response of periodontal tissue to orthodontic forces but also consequential root resorption in dogs. The essence of his work was recently reviewed and reintroduced, as the original article was in Swedish.2 The etiology of root resorption has been studied over the past few decades, and the overall progress in this field of research was recently reviewed.3 The type of tooth movement performed (bodily versus tipping) was suggested to be a critical factor for root resorption.4–6 In addition, the amount of tooth movement increased depending on the force magnitude7; however, it decreased when the force magnitude exceeded a certain heavy force level in rats.8 In contrast, in the rat maxillary first molars, root resorption increased depending on the magnitude of force applied, which varied from a light force to an extremely heavy force.8,9 Although many reports and reviews regarding the orthodontic force magnitude, amount of tooth movement, and root resorption have been published,8,10–14 no comprehensive study has yet elucidated the relationships between type of tooth movement, orthodontic force magnitude, and root resorption.
In this study, we investigated the difference between tipping and bodily tooth movement in terms of the velocity of tooth movement and root resorption. We designed a novel orthodontic appliance that induced bodily movement of the maxillary first molar in rats and compared it with a conventional appliance that induced tipping tooth movement. In addition, we compared the effect of various magnitudes of force, which ranged from mild (10 g) to extremely heavy (100 g), on the amount of tooth movement and root resorption.
MATERIALS AND METHODS
This study was conducted with the approval of the Animal Welfare Committee of Nagasaki University (No. 0603170498). Ten-week-old female Wistar rats (SLC, Shizuoka, Japan; body weight: 170–180 g) were used as experimental animals. Rats were housed in plastic cages in a colony room and fed a standard pellet diet and water ad libitum.
Rats were divided into two groups according to the type of tooth movement and then into four subgroups (six animals each) according to the applied magnitude of force (Figure 1). Before the appliance was placed or microcomputed tomography (micro-CT) images were acquired, general anesthesia was induced in the rats with an intramuscular injection of ketamine hydrochloride at a dose of 87 mg/kg (Ketalar 50, Sankyo, Tokyo, Japan) combined with xylazine hydrochloride at a dose of 13 mg/kg (Celactal 2%, Bayer-Japan, Tokyo, Japan).
Figure 1.
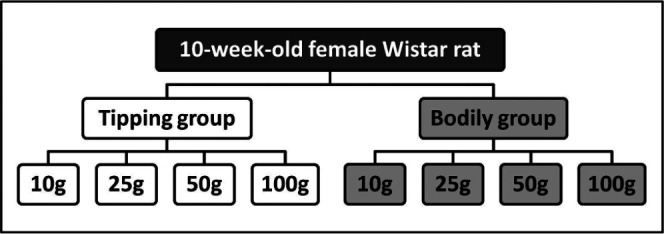
Experimental design. The tipping and bodily tooth movement groups were further subdivided according to the orthodontic force magnitude applied.
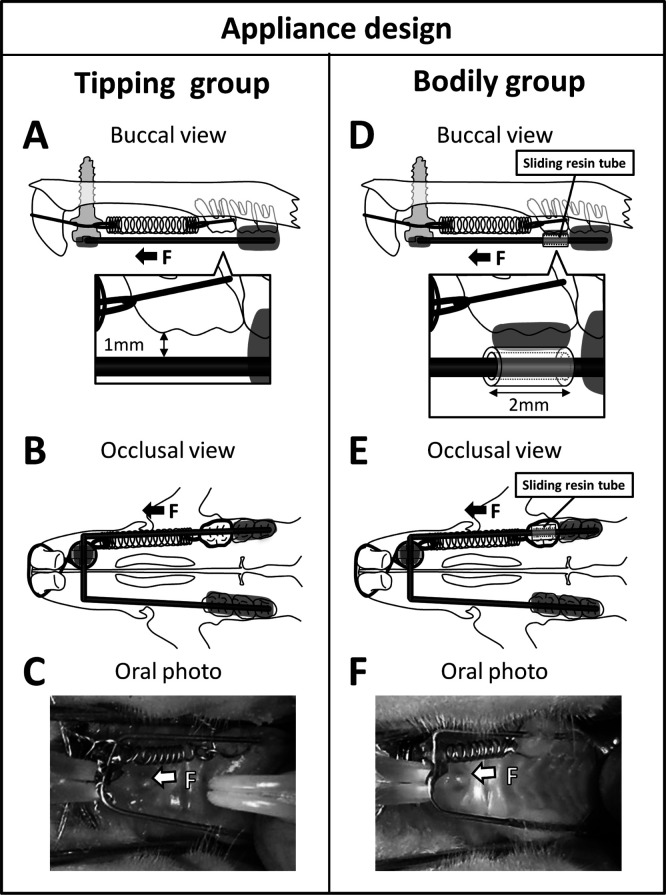
The appliance for bodily tooth movement was designed and placed as follows. A manual screwdriver was used to implant a dual-top miniscrew that was 1.4 mm in diameter and 6 mm in length (Dual-Top, Anchor Screw, Jeil Medical, Seoul, Republic of Korea) into the anterior palatal bone. Subsequently, a 0.016-inch-diameter round cobalt-chromium wire (Elgiloy, Rocky Mountain Morita, Tokyo, Japan) was fixed over the occlusal surfaces of the left second and third molars, all the right molars, and the orthodontic miniscrew using a self-curing resin (Super-Bond, Sun Medical, Shiga, Japan). For bodily tooth movement, we used a sliding tube constructed from the self-curing resin that was 2 mm in length and 0.016 inch in diameter with no visible play. The sliding tube that had been previously threaded onto the archwire was fixed over the occlusal surface of the first molar. Subsequently, coil springs were fixed using 0.008-inch stainless steel ligature wires. Finally, nickel-titanium closed-coil springs exerting 10, 25, 50, or 100 g of force (Sentalloy, Tomy, Fukushima, Japan) were placed between the maxillary left first molar and the miniscrew to induce mesial movement of the first molar. The sliding tube was not used to induce tipping tooth movement. The occlusal surfaces of the molars (except for the left first molar) were raised using the self-curing resin to eliminate any occlusal force on the moved maxillary first molar (Figure 2).
Figure 2.
Appliance design. The schemes represent the orthodontic appliances used to move the maxillary left first molars mesially. (A to C) The tipping tooth movement group. (D to F) The bodily tooth movement group. (A,D) Buccal views of the appliances. (B,E) Occlusal views of the appliances. The sliding tube constructed from the self-curing resin was bonded to the occlusal surface of the maxillary first molar in the appliance for bodily tooth movement (D,E). (C,F) Intraoral images of rats. Arrows indicate the direction of the applied orthodontic force (F).
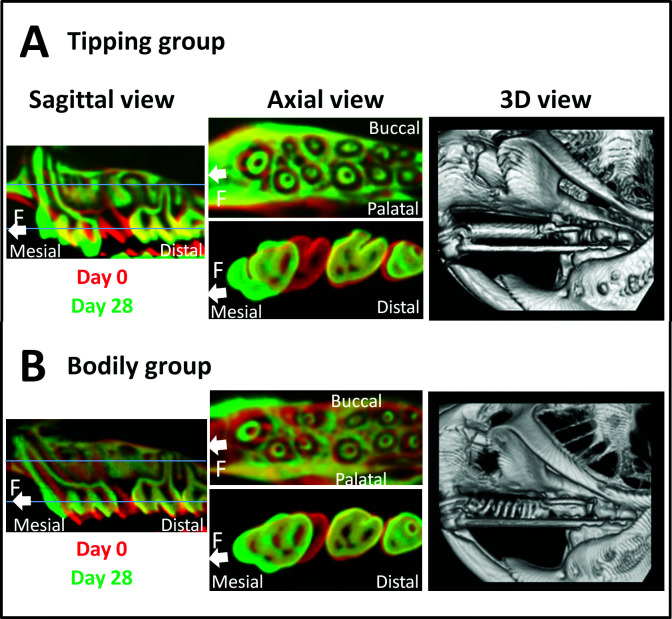
Radiographic micro-CT (RmCT, Rigaku, Tokyo, Japan) images were acquired both with and without the appliance on days 0 and 28, respectively (Figure 3). The resolution (voxel size) of the micro-CT images was 20 µm. To determine three-dimensional (3D) tooth movement, we used 3D analysis software (Figure 3; TRI-BONE, Ratoc System Engineering, Tokyo, Japan). In the images, red and green were used to represent days 0 and 28 of the experimental period, respectively. Subsequently, the superimposed images were used to compare orthodontic tooth movement. The following reference planes were used to acquire the final sliced images: the axial plane, parallel to the tooth axis; the occlusal plane; and the sagittal plane, parallel to the dental arch and tooth axis. Furthermore, superimposed sagittal- and axial-view images were used to determine the amount of orthodontic tooth movement that had occurred (Figure 3). The amount of tooth movement was measured using the distances between the contact points of the first and second molars.
Figure 3.
Micro-CT images. (A) Tipping tooth movement group. (B) Bodily tooth movement group. Superimposition of micro-CT images on days 0 and 28; sagittal views are presented on the left, and two different axial views are presented in the middle. The upper axial image displays a slice of the apical region of the tooth, whereas the lower image displays a slice of the cervical region of the tooth. Reconstructed 3D micro-CT images on day 28 are shown on the right.
At the end of the experiments, rats were sacrificed by an overdose of carbon dioxide. The appliances were checked to confirm that they were still properly fixed, and the molars were then extracted and submerged in 1% sodium hypochlorite to remove any remaining periodontal tissue. The roots of the first molars were divided into three parts (mesial root, middle two roots, and distal two roots) using a diamond disk. Only the mesial and distal roots were used in this study. Root resorption craters on the apical region were not evaluated because of anatomic variations and difficulties in delimiting the craters. The mesial surfaces of the mesial, distobuccal, and distopalatal roots were evaluated using a scanning electron microscope (TM-1000, Hitachi, Tokyo, Japan) and a 3D laser scanning microscope (VK-8500, Keyence, Kyoto, Japan). Because the resorption craters on the distal surface of the roots were scarcely detectable, they were also excluded from this study. All craters scattered on the cervical and middle thirds of the roots (the mesial side) were captured digitally, and the surface area of each was measured using commercial software (Mimics 11.11, Materialise Software, Leuven, Belgium). The depth of each resorption crater was calculated using a laser microscope program.
The volume of root resorption was measured by multiplying the crater area by the average depth. The same investigator (TN) performed all measurements. Every measurement was repeated three times, and the mean value was used as the final measurement.
Analysis of variance followed by the Mann-Whitney test was used to compare the amount of tooth movement and root resorption between the groups. Furthermore, Bonferroni correction was used for multiple comparisons. SPSS software (version 16.0, IBM SPSS, Chicago, Ill, USA) was used to perform the analyses.
RESULTS
The amount of tooth movement differed greatly between the tipping and bodily tooth movement groups. In the tipping tooth movement group, the crown of the first molar was inclined mesially on day 28. Conversely, the apical third of the mesial root had moved distally (Figure 3A). In contrast, in the bodily tooth movement group, the tooth had moved mesially in a parallel manner (Figure 3B).
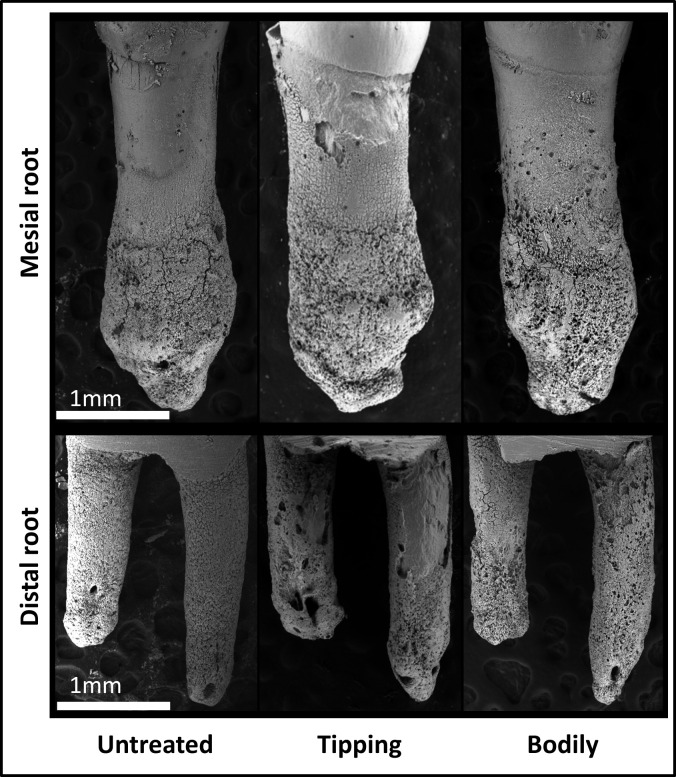
The scanning electron microscope was used to observe root resorption (Figure 4). The mesial surfaces of the untreated negative control roots were covered with undamaged cementum with a characteristic smooth surface. The apical thirds of these roots were covered with thick cementum with a rough and irregular surface that occasionally contained resorption craters. In all experimental groups, isolated lacunae and wide, shallow, and deep resorption craters were found. Small, isolated lacunae were mainly scattered on the mesial roots (on the cervical half of their mesial surfaces). Wide, shallow, and deep resorption craters covered the cervical and middle portions of the distal roots (Figure 4).
Figure 4.
Images of root resorption visualized using scanning electron microscopy. Roots of the untreated control (no force), tipping, and bodily tooth movement groups on day 28 are shown. The mesial roots are shown in the upper panel, and the distal roots are shown in the lower panel. The cervical half of each root was measured for root resorption.
On day 28, the greatest amount of tooth movement was observed in the 10-g tipping tooth movement subgroup (Figure 5A). In the bodily tooth movement subgroups, the amount of tooth movement in the 10-g tipping subgroup was significantly less, but it gradually increased in the 25- and 50-g subgroups (Figure 5B). Furthermore, the 50-g bodily tooth movement subgroup experienced the greatest amount of tooth movement, which was approximately half that of the 10-g tipping tooth movement subgroup. The amount of tooth movement in the 100-g subgroup was significantly less than that seen in the 50-g subgroup (P < .05).
Figure 5.
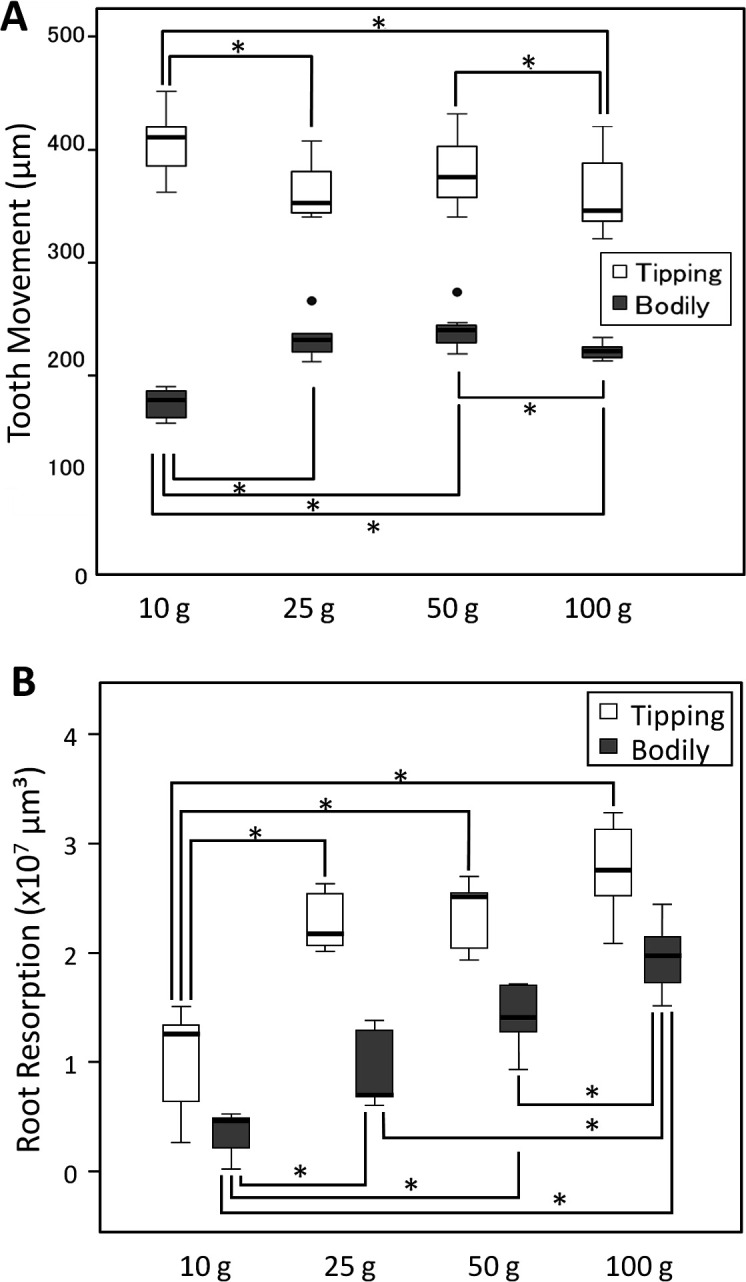
Box plots. (A) The amount of tooth movement with each orthodontic force magnitude applied. White box indicates tipping tooth movement; gray box, bodily tooth movement. (B) Root resorption with each orthodontic force magnitude applied. White box indicates tipping tooth movement; gray box, bodily tooth movement. * P < .05; Mann-Whitney test.
Measurement of root resorption revealed results that contrasted with those of tooth movement (Figure 5B). Root resorption increased with increasing orthodontic force in both the tipping and bodily tooth movement groups, although multiple comparisons after Bonferroni correction were not significant among the 25-, 50-, and 100-g groups. In the tipping tooth movement group, root resorption increased steeply with an increase in the applied force from 10 to 25 g; subsequently, root resorption gradually increased with an increase in the applied force to 100 g. The total root resorption in the tipping tooth movement groups was approximately twice that seen in the 10- and 25-g subgroups and 1.5 times that seen in the 50- and 100-g subgroups compared with that measured in the corresponding bodily tooth movement subgroups.
DISCUSSION
Proffit et al.10 hypothesized that tooth movement increases as pressure increases up to a point, remains at about the same level over a broad range, and then may decrease with extremely heavy pressure.1 Ren et al.15 mathematically tested the magnitude of force versus the volume of bone to be resorbed, and their results partly support this hypothesis. Our results also support the hypothesis that tooth movement decreases when extremely heavy force is applied (Figure 5A). The lightest force caused the greatest amount of tooth movement in the tipping tooth movement, whereas heavier forces caused less tooth movement. With either tipping or bodily tooth movement, the amount of tooth movement decreased when the force magnitude exceeded a certain threshold. Such an excessive orthodontic force may have caused hyaline degeneration and led to a decrease in the amount of tooth movement.16,17 More than 10 g of force may constitute an extremely heavy force for tipping movement of the rat maxillary first molar. Furthermore, an extremely heavy force may decrease blood flow,18 which may suppress phagocytosis of hyaline degenerative tissue by macrophages and subsequent bone resorption by osteoclasts. This traumatic damage to the periodontal tissue may suppress resorption of not only the bone surface but also the underlying bone.
When we applied orthodontic forces between 10 and 100 g to the rat maxillary first molar, tipping tooth movement produced more rapid tooth movement than bodily tooth movement, which may be related to the center of rotation. The center of rotation was theoretically determined in a rat model19; in this study, the crown of the tooth was inclined mesially as a result of tipping tooth movement. The center of resistance was determined in the same study as being located near the center of rotation.19 When a force is applied to the center of resistance, bodily tooth movement will result, as proven by theoretical calculations20 and clinical experiments.21 Displacement of the center of resistance may represent the most accurate measure of the amount of tooth movement. This important question has yet to be resolved, and we are now addressing this issue.
Despite the fact that, in this study, the greatest amount of tooth movement was caused by 10 g of force in the tipping tooth movement group and by 50 g of force in the bodily tooth movement group, root resorption increased in proportion to the increasing force in both the tipping and bodily tooth movement groups. An excessive force may not only decrease the amount of tooth movement, but it may also cause significant damage to the root. Although the sliding mechanics used in this study were monolithic and secure, slight tipping was observed. The tipping tendency increased with the applied force magnitude, which may have been caused by deformation of the wire or an increase in the diameter of the tube. Furthermore, the effect of friction22,23 on the sliding tube used in this study remains unclear. Further study is required to understand the orthodontic force magnitude actually loaded onto the tooth, types of tooth movement, and root resorption.
CONCLUSIONS
The amount of tooth movement decreased with the application of excessive force in both the tipping and bodily tooth movement groups.
Conversely, root resorption increased as the applied force magnitude increased in both tipping and bodily movement of the maxillary first molar in rats.
ACKNOWLEDGMENTS
We appreciate the reviewers very much for their valuable and important comments to modify this paper. This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Sandstedt C. Några Bidrag till Tandregleringens Teori. Stockholm: Kungl Boktryckeriet/PA Nordstedt & Söner; 1901. [Google Scholar]
- 2.Bister D, Meikle MC. Re-examination of ‘Einige Beitrage zur Theorie der Zahnregulierung’ (Some contributions to the theory of the regulation of teeth) published in 1904–1905 by Carl Sandstedt. Eur J Orthod. 2013;35:160–168. doi: 10.1093/ejo/cjs007. [DOI] [PubMed] [Google Scholar]
- 3.Weltman B, Vig KW, Fields HW, Shanker S, Kaizar EE. Root resorption associated with orthodontic tooth movement: a systematic review. Am J Orthod Dentofacial Orthop. 2010;137:462–476; discussion 412A. doi: 10.1016/j.ajodo.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 4.King AD, Turk T, Colak C, et al. Physical properties of root cementum: part 21. Extent of root resorption after the application of 2.5° and 15° tips for 4 weeks: a microcomputed tomography study. Am J Orthod Dentofacial Orthop. 2011;140:e299–e305. doi: 10.1016/j.ajodo.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 5.Wu AT, Turk T, Colak C, et al. Physical properties of root cementum: part 18. The extent of root resorption after the application of light and heavy controlled rotational orthodontic forces for 4 weeks: a microcomputed tomography study. Am J Orthod Dentofacial Orthop. 2011;139:e495–e503. doi: 10.1016/j.ajodo.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Rudolph DJ, Willes PMG, Sameshima GT. A finite element model of apical force distribution from orthodontic tooth movement. Angle Orthod. 2001;71:127–131. doi: 10.1043/0003-3219(2001)071<0127:AFEMOA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Kohno T, Matsumoto Y, Kanno Z, Warita H, Soma K. Experimental tooth movement under light orthodontic forces: rates of tooth movement and changes of the periodontium. J Orthod. 2002;29:129–135. doi: 10.1093/ortho/29.2.129. [DOI] [PubMed] [Google Scholar]
- 8.Gonzales C, Hotokezaka H, Yoshimatsu M, Yozgatian JH, Darendeliler MA, Yoshida N. Force magnitude and duration effects on amount of tooth movement and root resorption in the rat molar. Angle Orthod. 2008;78:502–509. doi: 10.2319/052007-240.1. [DOI] [PubMed] [Google Scholar]
- 9.Noda K, Arai C, Nakamura Y. Root resorption after experimental tooth movement using superelastic forces in the rat. Eur J Orthod. 2010;32:681–687. doi: 10.1093/ejo/cjq016. [DOI] [PubMed] [Google Scholar]
- 10.Proffit WR, Fields HW, Sarver DM. Contemporary Orthodontics 4th ed. St Louis: CV Mosby; 2006. The biologic basis of orthodontic therapy; pp. 343–345. [Google Scholar]
- 11.Frost HM. The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner. 1987;2:73–85. [PubMed] [Google Scholar]
- 12.Owman-Moll P, Kurol J, Lundgren D. Effects of a doubled orthodontic force magnitude on tooth movement and root resorptions. An inter-individual study in adolescents. Eur J Orthod. 1996;18:141–150. doi: 10.1093/ejo/18.2.141. [DOI] [PubMed] [Google Scholar]
- 13.Owman-Moll P, Kurol J, Lundgren D. The effects of a four-fold increased orthodontic force magnitude on tooth movement and root resorptions. An intra-individual study in adolescents. Eur J Orthod. 1996;18:287–294. doi: 10.1093/ejo/18.3.287. [DOI] [PubMed] [Google Scholar]
- 14.Ren Y, Maltha JC, Kuijpers-Jagtman AM. The rat as a model for orthodontic tooth movement—a critical review and a proposed solution. Eur J Orthod. 2004;26:483–490. doi: 10.1093/ejo/26.5.483. [DOI] [PubMed] [Google Scholar]
- 15.Ren Y, Maltha JC, Van't Hof MA, Kuijpers-Jagtman AM. Optimum force magnitude for orthodontic tooth movement: a mathematic model. Am J Orthod Dentofacial Orthop. 2004;125:71–77. doi: 10.1016/j.ajodo.2003.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Brudvik P, Rygh P. The initial phase of orthodontic root resorption incident to local compression of the periodontal ligament. Eur J Orthod. 1993;15:249–263. doi: 10.1093/ejo/15.4.249. [DOI] [PubMed] [Google Scholar]
- 17.Brudvik P, Rygh P. Root resorption beneath the main hyalinized zone. Eur J Orthod. 1994;16:249–263. doi: 10.1093/ejo/16.4.249. [DOI] [PubMed] [Google Scholar]
- 18.Imamura N, Nakata S, Nakasima A. Changes in periodontal pulsation in relation to increasing loads on rat molars and to blood pressure. Arch Oral Biol. 2002;47:599–606. doi: 10.1016/s0003-9969(02)00041-9. [DOI] [PubMed] [Google Scholar]
- 19.Gonzales C, Hotokezaka H, Arai Y, et al. An in vivo 3D micro-CT evaluation of tooth movement after the application of different force magnitudes in rat molar. Angle Orthod. 2009;79:703–714. doi: 10.2319/071308-366.1. [DOI] [PubMed] [Google Scholar]
- 20.Christiansen RL, Burstone CJ. Centers of rotation within the periodontal space. Am J Orthod. 1969;55:353–369. doi: 10.1016/0002-9416(69)90143-2. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida N, Jost-Brinkmann PG, Koga Y, Mimaki N, Kobayashi K. Experimental evaluation of initial tooth displacement, center of resistance, and center of rotation under the influence of an orthodontic force. Am J Orthod Dentofacial Orthop. 2001;120:190–197. doi: 10.1067/mod.2001.115036. [DOI] [PubMed] [Google Scholar]
- 22.Rhee JN, Chun YS, Row J. A comparison between friction and frictionless mechanics with a new typodont simulation system. Am J Orthod Dentofacial Orthop. 2001;119:292–299. doi: 10.1067/mod.2001.112452. [DOI] [PubMed] [Google Scholar]
- 23.Montasser MA, El-Bialy T, Keilig L, Reimann S, Jäger A, Bourauel C. Force loss in archwire-guided tooth movement of conventional and self-ligating brackets. Eur J Orthod. 2014;36:31–38. doi: 10.1093/ejo/cjs110. [DOI] [PubMed] [Google Scholar]