Abstract
Motor abnormalities have been established as a core aspect of psychosis-spectrum disorders, with numerous studies identifying deficits prior to clinical symptom presentation. Additional research is needed to pinpoint standardized motor assessments associated with psychosis-spectrum disorders prior to illness onset to enhance prediction and understanding of etiology. With a long history of findings among people with diagnosable psychosis-spectrum disorders, but little research conducted during the premorbid phase, pegboard tasks are a viable and understudied measure of premorbid for psychosis motor functioning. In the current study, examining data from the Copenhagen Perinatal Cohort, the Simultaneous Pegs Test was performed with children (n = 244, aged 10–13) at genetic high risk for psychosis (n = 94) and controls (n = 150). Findings suggest that children who eventually developed a psychosis-spectrum disorder (n = 33) were less likely to successfully complete the task within time limit relative to controls (χ2 (2, N = 244) = 6.94, p = 0.03, ϕ = 0.17). Additionally, children who eventually developed a psychosis-spectrum disorder took significantly longer to complete the task relative to controls (χ2 (2, N = 244) = 7.06, p = 0.03, ϕ = 0.17). As pegboard performance is thought to tap both diffuse and specific brain networks, findings suggest that pegboard tests may be useful premorbid measures of motor functioning among those on a trajectory towards a psychosis-spectrum disorder.
Keywords: Premorbid, Psychosis, Pegboard, High-risk
1. Introduction
Motor function deficits in individuals with psychosis-spectrum disorders are well-documented (Chan et al., 2010; Peralta and Cuesta, 2011). Common among people with psychotic disorders such as schizophrenia, motor function abnormalities show reliable impairments in performance versus healthy controls (Heinrichs and Zakzanis, 1998; Neelam et al., 2011), and are considered a key feature of illness (Tandon et al., 2009). The relation between motor abnormalities and psychosis, however, is sometimes clouded as factors such as treatment side-effects and other illness related issues can contribute to motor dysfunction in people with psychosis (Crane and Naranjo, 1971; Khot and Wyatt, 1991). Nonetheless, a large body of evidence has documented motor abnormalities prior to antipsychotic medication use or illness onset (Dickson et al., 2012; Erlenmeyer-Kimling et al., 2000; Schiffman et al., 2009; Torrey, 2002; Wolff and O'Driscoll, 1999). Motor deficits are thought to represent aspects of the underlying psychophysiology of psychosis-spectrum disorders, documented prior to clinical symptom manifestation, which may be viable candidates for illness endophenotypes. With increased recognition and precision, it has been argued that measurement of motor function deficits holds the promise of being a low cost addition to the effective screening for risk for psychosis-spectrum disorders (Burton et al., 2016). Additional research documenting the link between motor abnormalities and the premorbid stage of illness prior to the confounds of illness related issues is required to firmly identify specific types of motor abnormalities and standardized assessments associated with future psychosis-spectrum disorders (Morrens et al., 2014).
Pegboard tasks, a measure of perceptual-motor speed, have long been used to study motor function in individuals diagnosed with psychotic disorders such as schizophrenia (Collinson et al., 2004; Flyckt et al., 1999; Fuller and Jahanshahi, 1999; Green and Walker, 1985). Pegboard tasks are thought to measure underlying functioning of the frontal and parietal brain regions in the right hemisphere (Royce et al., 1976), making them particularly relevant for individuals with psychosis, as both structural and functional deficits in the right parietal and frontal regions of the brain have been implicated in psychosis-spectrum disorders (Neuhaus et al., 2011; Pettersson-Yeo et al., 2011; Yao et al., 2013; Yoon et al., 2008; Zetzsche et al., 2008). Research also suggests that abnormal striatal dopamine activity can impact pegboard performance (Bohnen et al., 2007; Mozley et al., 2001), and related deficits play an important role in etiological models of schizophrenia (Howes et al., 2012).
Despite the prominent role of pegboard tasks in psychosis research, as well as a few studies of adolescents with subclinical psychosis (Blanchard et al., 2010; Carrión et al., 2011; Lindgren et al., 2010; Roman-Urrestarazu et al., 2014), little research to date has examined the ability of pegboard tasks to predict conversion to psychosis premorbidly, prior to illness confounds (Cannon et al., 2006; Meier et al., 2014).
The aim of the current investigation was to examine performance on the Simultaneous Pegs Test premorbidly in a subgroup of the Copenhagen Perinatal Cohort known as the “OB Project,” a genetic high-risk longitudinal sample and matched controls of 244 children followed for nearly 50 years. This sample, assessed with a pegboard task in childhood prior to clinical manifestation of illness, provides an opportunity to examine genetic risk and future psychiatric outcome in relation to premorbid pegboard performance. We hypothesized that childhood deficits in performance on the Simultaneous Pegs Test would predict later development of a psychosis-spectrum disorder relative to other non-psychotic mental disorders and to no diagnosis.
2. Method
2.1. Participants
The current study recruited from the Copenhagen Perinatal Cohort, which included 9125 births between September 1, 1959 and December 31, 1961 at Rigshospitalet, Copenhagen Denmark (Schiffman et al., 2009). In 1972, 265 participants (all between the ages of 10 and 13) were recruited from the larger cohort and underwent a more detailed evaluation. These individuals were selected based on parental psychiatric status, described below. Controls matched on race, gender, socioeconomic status, and parent's age were also recruited based on parental psychiatric status. Recruitment, psychiatric evaluations, and social functioning assessments were conducted by researchers at the Institute of Preventive Medicine in Copenhagen. Families provided written informed consent following provision of a description of the research protocol. A complete recruitment, selection, and group categorization flowchart can be found in the supplement to Golembo-Smith et al. (2012). Diagnostic outcome and risk status is presented in Table 1.
Table 1.
Primary diagnosis by age, sex, and genetic risk status of participants.
| Age |
Mother's age |
Sex |
Genetic risk |
Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Male | Female | HR | OR | LR | ||
| Psychosis-spectrum | ||||||||||
| Schizophrenia | 11.5 | 0.77 | 27.5 | 7.7 | 10 | 8 | 15 | 2 | 1 | 18 |
| Any psychosis or delusional disorder | 11.7 | 0.83 | 25.2 | 7.9 | 5 | 3 | 4 | 3 | 1 | 8 |
| Schizotypal PD | 11.6 | 0.57 | 23.4 | 3.6 | 0 | 4 | 1 | 3 | 0 | 4 |
| Paranoid PD | 11.3 | 0.61 | 25.2 | 3.2 | 0 | 2 | 2 | 0 | 0 | 2 |
| Schizoid PD | 10.5 | n/a | 38.6 | n/a | 1 | 0 | 0 | 0 | 1 | 1 |
| Total psychosis-spectrum | 11.5 | 0.74 | 26.6 | 7.3 | 16 | 17 | 22 | 8 | 3 | 33 |
| Other disorders | ||||||||||
| Non-psychotic mood or anxiety disorder | 11.7 | 0.63 | 24.2 | 5.5 | 12 | 15 | 12 | 11 | 4 | 27 |
| Non-psychotic alcohol/drug abuse | 11.9 | 0.63 | 24.2 | 5.7 | 23 | 11 | 9 | 17 | 8 | 34 |
| Non-spectrum personality disorders | 11.7 | 0.80 | 26.9 | 6.4 | 5 | 12 | 7 | 6 | 4 | 17 |
| Total other disorders | 11.8 | 0.68 | 24.8 | 5.9 | 40 | 38 | 28 | 34 | 16 | 78 |
| No mental illness | ||||||||||
| Total no diagnosis | 11.7 | 0.64 | 27.4 | 6.9 | 64 | 69 | 44 | 42 | 47 | 133 |
| All participants | 11.7 | 0.67 | 26.4 | 6.7 | 120 | 124 | 94 | 84 | 66 | 244 |
2.2. Assessment of genetic risk
During initial recruitment (1972), level of genetic risk was determined by parents’ psychiatric status, as assessed by hospital record reviews and clinician interviews. Further validation of parental diagnoses was conducted in 1992 and in 2007. Based on this information, study participants were categorized into one of three genetic risk categories: 1) children with at least one biological parent with a hospital psychiatric diagnosis of schizophrenia (“high-risk,” n = 102), 2) children with at least one biological parent with a hospitalization record for a non-psychotic psychiatric diagnosis (“other-risk,” n = 89), or 3) children with neither biological parent having a record of psychiatric hospitalization (“low-risk,” n = 74).
2.3. Diagnostic outcome
In 1992, when participants were between the ages of 31 and 33, a psychiatrist administered the SCID and also the psychosis section of the Present State Examination (Wing et al., 1974). In addition, Danish psychiatric records were examined. In 2007, an additional diagnostic status update was completed through a scan of the Danish Psychiatric Central Registry for psychiatric admissions between the years 1994 and 2007 (fifteen individuals were assessed using the registry only; others participated in face to face clinical interviews). Using interviews and hospital records, data were available for 244 of the 265 participants (92% of the cohort after 48 years). The final risk groups with diagnostic outcome data comprise the analysis sample and were as follows: high risk, n = 94; other risk, n = 84; low risk, n = 66. Rates of follow-up did not significantly differ by risk group (92%, 94%, and 89% for the high risk, other risk, and low risk groups respectively) or primary demographic characteristics.
As of 2007, 33 participants were diagnosed with a psychosis-spectrum disorder (“spectrum”), 78 were identified as having a non-psychotic disorder (“other disorders”), and 133 were not identified as having a mental health diagnosis (“no mental illness”). Of the psychosis-spectrum disorders, 18 were schizophrenia, 8 were psychosis not otherwise specified or delusional disorder, and 7 were schizotypal, paranoid, or schizoid personality disorders. For the purposes of data analysis, all individuals with a psychosis-spectrum disorder were grouped into one category. (see Table 1).
2.4. Simultaneous Pegs Test (SPT)
In 1972, 244 children were examinedat the Institute of Preventive Medicine (previously Psykologisk Institute) in Copenhagen by an experienced child neurologist who was blind to information about the parents' psychiatric status and of course eventual psychiatric diagnosis. As a part of the research protocol, participants completed the Simultaneous Pegs Test (Stott, 1966). Each child was instructed by the examiner to complete the Simultaneous Pegs Test as rapidly as possible. The task requires participants to simultaneously and bimanually place a number of small plastic pegs of approximately 1 cm in diameter into holes of a squared plastic plate. Criterion for failure was exceeding 16 s on all three trials; however, even if the criterion was not met, total time to complete the task correctly was recorded (Tew, 1979). Both pass/fail criterion and time to completion were analyzed in the current study.
2.5. Intelligence Quotient (IQ)
During the same evaluation as the Pegs Test, participants also were evaluated with the Wechsler Intelligence Scale for Children (WISC) (Sørensen et al., 2010; Wechsler, 1949). The WISC provides a measure of verbal, performance, and full scale intelligence quotients, with a mean of 100 and a standard deviation of 15. Subscales included in this assessment were Similarities, Vocabulary, Block Design, and Maze. Each subscale provides a scaled score based on normative data, with a mean of 10 and a standard deviation of 3. A more detailed description of the 1972 neurological examination has been given elsewhere (Marcus et al., 1985; Golembo-Smith et al., 2012).
2.6. Statistical analysis
Mean seconds to complete the Simultaneous Pegs Test and pass/fail score for each diagnostic outcome group were calculated. Analysis of variance and Pearson correlations were estimated to examine potential associations of Simultaneous Pegs Test scores with sex, genetic risk, handedness, and IQ. Separate multinomial logistic regressions were estimated to examine the predictive validity of the pegboard task. In each model, psychiatric disorder in adulthood (outcome psychosis-spectrum, other disorders, or no mental illness) was regressed on pegboard performance (either pass/fail criterion or seconds to completion) and identified covariates. If significant effects were observed, the coefficients discriminating psychosis spectrum from other disorders and no mental illness were examined to aid interpretation. The moderation of genetic risk on the effects of seconds needed to complete the task was also estimated. Using the outcome from the seconds needed to completion regression model, a receiver operating characteristic (ROC) analysis was performed to determine a statistically derived “optimal” cut score using predictive probability of a psychosis-spectrum outcome. All analyses were conducted using SPSS version 22.0.
3. Results
For mean seconds needed to complete the Simultaneous Pegs Test and pass/fail score by diagnostic outcome, see Table 2. Prior to performing the main analyses, relations of time to completion on the Pegs Test with childhood IQ, gender, and handedness were estimated. As has been observed in the literature (Diaz-Asper et al., 2004), IQ was negatively correlated with seconds needed to complete the pegboard task, r = −0.237, p < 0.001. Neither gender nor laterality were related to completion time on the pegboard task (r = −0.009, p = 0.894 and r = −0.033, p = 0.61, respectively). As expected, genetic risk status was significantly associated with completion time, F(2, 241) = 5.70, p = 0.004, η2 = 0.05. We controlled for the effects of IQ and genetic risk when assessing the relation between adult diagnostic outcome and childhood Simultaneous Pegs Test variables. There was no reason to believe the time to completion variable was non-normally distributed (skew = 0.87, kurtosis = 1.67), thus transformations were not warranted (Curran et al., 1996).
Table 2.
Means, standard deviations, and pass/fail score on Simultaneous Pegs Test (SPT) by psychiatric outcome group.
| Psychosis-spectrum | Other disorders | No mental illness | |
|---|---|---|---|
| Pass/fail SPT | |||
| Pass | 27 (81.82%) | 74 (94.87%) | 124 (93.23%) |
| Fail | 6 (18.18%) | 4 (5.13%) | 9 (6.77%) |
| Seconds needed to complete SPT | |||
| Mean | 13.36 | 12.68 | 12.14 |
| SD | 3.40 | 2.24 | 2.04 |
The model examining the pass/fail criterion on the Pegs Test discriminated among the diagnoses (χ2 [8, N = 244] = 36.01, p < 0.001) and yielded a Nagelkerke pseudo R2 of 0.16. Pass/fail score emerged as a significant predictor, discriminating between psychosis-spectrum and OPD, as well as between psychosis-spectrum and NMI (Table 3).
Table 3.
Coefficients from two multinomial logistic regressions predicting adult diagnostic outcome.
| Model predictors | Wald χ2 |
df | B | Odds Ratio |
95% CI |
|---|---|---|---|---|---|
| Model 1 | |||||
| Other psychopathology group vs. | |||||
| psychosis-spectrum group | |||||
| Intercept | 0.71 | 1 | 1.42 | ||
| Pass/fail score on the SPT | 6.48* | 1 | −1.86 | 0.16 | 0.04–0.65 |
| IQ | 0.29 | 1 | 0.007 | 1.01 | 0.98–1.04 |
| Parent w/ spectrum vs. NMI | 5.30* | 1 | 1.65 | 5.19 | 1.28–21.12 |
| Parent w/ spectrum vs. OPD | 7.93** | 1 | 1.45 | 4.26 | 1.55–11.66 |
| No mental illness group vs. | |||||
| psychosis-spectrum group | |||||
| Intercept | 0.58 | 1 | −1.26 | ||
| Pass/fail score on the SPT | 5.10* | 1 | −1.43 | 0.24 | 0.07–0.83 |
| IQ | 5.43* | 1 | 0.03 | 1.03 | 1.01–1.06 |
| Parent w/ spectrum vs. NMI | 11.35** | 1 | 2.29 | 9.91 | 2.61–37.65 |
| Parent w/ spectrum vs. OPD | 5.89* | 1 | 1.21 | 3.35 | 1.26–8.90 |
| Model 2 | |||||
| Other psychopathology group vs. | |||||
| psychosis-spectrum group | |||||
| Intercept | 0.85 | 1 | 1.87 | ||
| Seconds needed to complete SPT | 3.21† | 1 | −0.16 | 0.85 | 0.72–1.02 |
| IQ | 0.05 | 1 | 0.003 | 1.00 | 0.98–1.03 |
| Parent w/ spectrum vs. NMI | 4.92* | 1 | 1.56 | 4.74 | 1.20–18.76 |
| Parent w/ spectrum vs. OPD | 7.69** | 1 | 1.40 | 4.05 | 1.51–10.91 |
| No mental illness group vs. | |||||
| psychosis-spectrum group | |||||
| Intercept | 0.13 | 1 | 0.74 | ||
| Seconds needed to complete SPT | 6.85** | 1 | −0.23 | 0.80 | 0.67–0.94 |
| IQ | 3.25† | 1 | 0.03 | 1.03 | 1.00–1.06 |
| Parent w/ spectrum vs. NMI | 11.45** | 1 | 2.27 | 9.71 | 2.60–36.23 |
| Parent w/ spectrum vs. OPD | 6.72* | 1 | 1.28 | 3.61 | 1.37–9.53 |
SPT = Simultaneous Pegboard Test, OPD = other psychiatric disorder, NMI = no mental illness.
p < 0.10.
p < 0.05.
p < 0.01.
We also conducted a multinomial logistic regression to assess the ability of seconds needed to complete the Pegs Test to predict adult diagnostic outcome, controlling for genetic risk and IQ. The overall model was significant (χ2 [8, N = 244] = 36.13, p < 0.001), with a Nagelkerke pseudo R2 of 0.16. Seconds needed to complete the test emerged as a significant predictor of diagnostic outcome. Not surprisingly, completion time discriminated between psychosis-spectrum and NMI. Though not a statistically significant effect at traditional (0.05) levels, completion time appeared to discriminate the psychosis-spectrum and OPD diagnoses at trending levels (Table 3). We also assessed a possible interaction between seconds needed to complete the Pegs Test and genetic risk and found no significant difference between the model with and without the interaction terms (p = 0.53).
Probabilistic diagnostic categorizations were determined from the seconds needed model, yielding 21.2% accuracy for those with a spectrum outcome and a total correct classification rate of 60.2% (Table 4). A ROC curve was plotted to predict spectrum versus non-spectrum outcomes (as ROC curves do not allow consideration of three outcomes, other disorders and no mental illness groups were combined). Using predicted probabilities as the ROC curve predictor variable, the AUC was 0.72 [95% C.I. = 0.62–0.82, p < 0.01], in the “fair” range (Hosmer and Lemeshow, 2000) (Fig. 1).
Table 4.
Multinomial regression analysis classification summary.
| Observed | Predicted group membership |
|||
|---|---|---|---|---|
| Psychosis-spectrum | Other disorders | No mental illness | Σ | |
| Psychosis-spectrum | 7 | 3 | 23 | 33 (13.5%) |
| Other disorders | 1 | 17 | 60 | 78 (32.0%) |
| No mental illness | 1 | 9 | 123 | 133 (54.5%) |
| Σ | 9 (3.7%) | 29 (11.9%) | 206 (84.4%) | 244 (100%) |
Fig. 1.
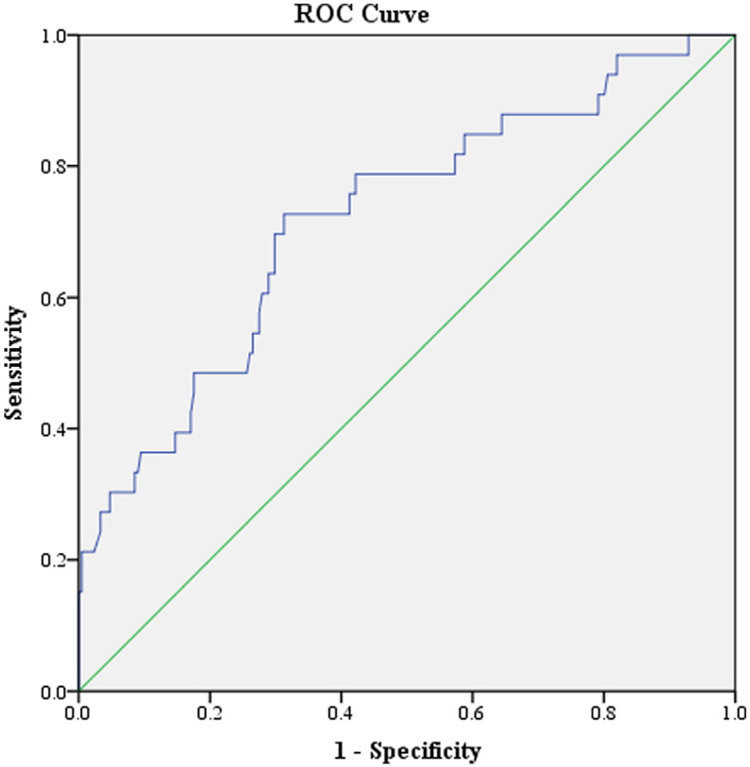
ROC curve predicting psychosis-spectrum vs. other outcomes.
The “optimal” statistically derived cut-off was identified [see Golembo-Smith et al. (2012) for description on cut-off point calculations], yielding a sensitivity of 0.73, specificity of 0.69, positive predictive value of 0.27, and negative predictive value of 0.94. Overall, the inclusive model was able to correctly classify 69% of participants into spectrum vs. not-spectrum outcome groups (Table 5).
Table 5.
ROC analysis classification summary: spectrum vs. all others.
| Observed | Predicted group membership |
||
|---|---|---|---|
| Spectrum | Not spectrum | Σ | |
| Spectrum | 24 | 9 | 33 (13.5%) |
| Not spectrum | 66 | 145 | 211 (86.5%) |
| Σ | 90 (36.9%) | 154 (63.1%) | 244 (100%) |
4. Discussion
Results suggest an association between premorbid pegboard performance and adult onset psychosis-spectrum disorders. Pass/fail and greater time to complete the Simultaneous Pegs Test in childhood was related to development of a psychosis-spectrum disorder relative to children who later developed other psychiatric disorders (trend level significance for the seconds needed analysis) or no mental illnesses. This was true while controlling for IQ and genetic risk, indicating that performance on the pegboard test represents a distal (10+ years) independent predictor of psychosis risk. Simultaneous Pegs Test performance did not differ, however, between children who later developed no mental illness and those who developed non-psychotic mental illnesses, suggestive of specificity of the Simultaneous Pegs Test in measuring deficits in individuals who later developed psychosis-spectrum disorder.
Results suggest direct effects between childhood pegboard performance and adult diagnostic outcome, and between genetic risk and adult diagnostic outcome. Analyses did not support an interaction between genetic risk and pegboard performance. These findings suggest that pegboard performance might predict diagnostic outcome over and above genetic risk; especially when evaluating performance on a pass/fail criterion. Possible neural and environmental explanations as to how pegboard deficits might relay to adult psychosis-spectrum outcomes are described below.
Premorbid studies of individuals at risk for psychosis have consistently found the presence of motor function impairment in individuals who later develop a psychosis-spectrum disorder (Dickson et al., 2012; Erlenmeyer-Kimling et al., 2000; Schiffman et al., 2009; Schiffman et al., 2015; Torrey, 2002; Wolff and O'Driscoll, 1999). Additionally, pegboard task performance deficits are regularly reported in individuals with psychosis (Collinson et al., 2004; Flyckt et al., 1999; Fuller and Jahanshahi, 1999; Green and Walker, 1985), with some research finding differences in individuals with sub-clinical psychosis or at “clinical high-risk” for psychosis (Blanchard et al., 2010; Carrión et al., 2011; Lindgren et al., 2010; Roman-Urrestarazu et al., 2014). The current study adds to the scant literature reporting premorbid pegboard task performance deficits in children who later develop a psychosis-spectrum disorder (Cannon et al., 2006; Meier et al., 2014). Given the ease and low cost of administration as compared to other neuropsychological tests, these findings highlight the potential utility of assessments such as pegboard tasks in measuring motor deficits when assessing for psychosis risk, and might make valuable contributions in clinical high-risk assessment batteries where until now, motor behaviors in this critical population have largely has been assessed with time-intensive coding involving expert raters (e.g., Cannon et al., 2016; Mittal et al., 2011).
Perceptual motor speed deficits as assessed through pegboard tasks may be particularly relevant to our findings (Royce et al., 1976). Previous research using measures other than pegboards has found perceptual motor speed deficits in individuals at clinical high risk for psychosis (Carrión et al., 2011). Additionally, premorbid deficits in perceptual motor speed, as measured by performance in the Coding subtest of the Wechsler Intelligence Scale for Children, have been shown in children who later developed a psychosis-spectrum disorder as compared to controls (Sørensen et al., 2006). Further, a prospective study of children who later developed a psychosis-spectrum disorder and their unaffected siblings found that perceptual-motor speed deficits were significantly greater in children who developed schizophrenia as compared to their unaffected siblings (Niendam et al., 2003). It is possible that pegboard tasks may be measuring underlying deficits in perceptual motor speed that are associated with later conversion to psychosis. Although speculative without in vivo imagining, based on prior literature related to pegboard tasks, as well as imaging work examining the neurology of perceptual motor speed, these deficits could be indicative of structural or functional impairments in the frontal and parietal brain regions in the right hemisphere, as well as of abnormal striatal dopamine activity (Howes et al., 2012; Neuhaus et al., 2011; Pettersson-Yeo et al., 2011; Royce et al., 1976; Yao et al., 2013; Yoon et al., 2008; Zetzsche et al., 2008).
In addition to the possible localized neurological regions, pegboard tasks may also measure presence of neurological soft signs, considered non-localized, general abnormalities where specific brain region ties are either yet unknown or do not exist. In some studies, neurological soft signs have been found to be a biological marker specific to schizophrenia rather than psychopathology generally (Rigucci et al., 2014; Tripathi et al., 2015), and the presence of neurological soft signs in childhood has predicted later conversion to psychosis-spectrum disorders in at least two studies (Schiffman et al., 2009; Schiffman et al., 2015).
There are a few notable limitations to this study. Although cutting edge in 1972, the Simultaneous Pegs Test (Stott, 1966) is no longer a frequently used motor task within the field. Modern versions of a bimanual pegboard task are, however, regularly used in current research (Buddenberg and Davis, 2000). The Simultaneous Pegs Test represented one of the best pegboard tasks at the time of the study. Although the size of this prospective longitudinal sample was relatively large compared to similar longitudinal genetic high risk studies, the total number of individuals in the psychosis-spectrum disorder group (n = 33) was small. This may have limited statistical power to detect possible interactions with genetic risk.
Despite these limitations, detecting premorbid deficits in a prospective sample that included those at risk for psychosis in a motor task highlights the importance of motor function deficits early in the development of psychosis-spectrum disorders, and supports the viability of pegboard performance serving as a possible biomarker of psychosis-spectrum disorders. Future research could consider incorporating pegboard tasks in clinical high-risk research to help facilitate both etiologic understanding, as well as prediction of conversion to a psychotic disorder when used in conjunction with other indicators of risk.
Acknowledgements
This manuscript is dedicated to the memory of Sarnoff A. Mednick, a mentor of the highest quality, and a true visionary in this, and other, fields.
Role of funding source
This work was supported in part by grant R03MH076846 to Jason Schiffman; funding from the Maryland Department of Health and Mental Hygiene, Behavioral Health Administration through the Center for Excellence on Early Intervention for Serious Mental Illness (OPASS# 14-13717G/M00B4400241); and by Sygekassernes Helsefond (Health Insurance Foundation) by grant 9700093 from the Danish Research Council. NIMH, MD HMH, and the Danish Research Council had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest
The authors do not have any actual or potential conflicts of interest to report.
References
- Blanchard MM, Jacobson S, Clarke MC, Connor D, Kelleher I, Garavan H, Harley M, Cannon M, 2010. Language, motor and speed of processing deficits in adolescents with subclinical psychotic symptoms. Schizophr. Res 123 (1), 71–76. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kuwabara H, Constantine GM, Mathis CA, Moore RY, 2007. Grooved pegboard test as a biomarker of nigrostriatal denervation in Parkinson's disease. Neurosci. Lett 424 (3), 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddenberg LA, Davis C, 2000. Test-retest reliability of the Purdue Pegboard Test. Am. J. Occup. Ther 54 (5), 555–558. [DOI] [PubMed] [Google Scholar]
- Burton BK, Hjorthøj C, Jepsen JR, Thorup A, Nordentoft M, Plessen KJ, 2016. Research review: do motor deficits during development represent an endophenotype for schizophrenia? A meta-analysis. J. Child Psychol. Psychiatry 57 (4), 446–456. [DOI] [PubMed] [Google Scholar]
- Cannon M, Moffitt TE, Caspi A, Murray RM, Harrington H, Poulton R, 2006. Neuropsychological performance at the age of 13 years and adult schizophreniform disorder: prospective birth cohort study. Br. J. Psychiatry 189 (5), 463–464. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, … Perkins DO, 2016. An individualized risk calculator for research in prodromal psychosis. Am. J. Psychiatry appi-ajp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión RE, Goldberg TE, McLaughlin D, Auther AM, Correll CU, Cornblatt BA, 2011. Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. Am. J. Psychiatry 168 (8), 806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RCK, Xu T, Heinrichs RW, Yu Y, Wang Y, 2010. Neurological soft signs in schizophrenia: a meta-analysis. Schizophr. Bull 36 (6), 1089–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson SL, Phillips TJ, James ACD, Quested DJ, Crow TJ, 2004. Is lateral bias anomalous in early-onset schizophrenia? Selected comparisons with normal populations. Psychiatry Res. 125 (3), 219–224. [DOI] [PubMed] [Google Scholar]
- Crane GE, Naranjo ER, 1971. Motor disorders induced by neuroleptics: a proposed new classification. Arch. Gen. Psychiatry 24 (2), 179–184. [DOI] [PubMed] [Google Scholar]
- Curran PJ, West SG, Finch JF, 1996. The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. Psychol. Methods 0 (1), 16. [Google Scholar]
- Diaz-Asper CM, Schretlen DJ, Pearlson GD, 2004. How well does IQ predict neuropsychological test performance in normal adults? J. Int. Neuropsychol. Soc 10 (1), 82–90. [DOI] [PubMed] [Google Scholar]
- Dickson H, Laurens KR, Cullen AE, Hodgins S, 2012. Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol. Med 42 (4), 743–755. [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Rock D, Roberts SA, Janal M, Kestenbaum C, Cornblatt B, Adamo UH, Gottesman II., 2000. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: the New York High-Risk Project. Am. J. Psychiatry 157 (9), 1416–1422. [DOI] [PubMed] [Google Scholar]
- Flyckt L, Sydow O, Bjerkenstedt L, Edman G, Erik R, Wiesel FA, 1999. Neurological signs and psychomotor performance in patients with schizophrenia, their relatives, and healthy controls. Psychiatry Res. 86 (2), 113–129. [DOI] [PubMed] [Google Scholar]
- Fuller R, Jahanshahi M, 1999. Concurrent performance of motor tasks and processing capacity in patients with schizophrenia. J. Neurol. Neurosurg. Psychiatry 66 (5), 668–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golembo-Smith S, Schiffman J, Kline E, Sorensen HJ, Mortensen EL, Stapleton L, Hayahsi K, Michaelsen NM, Ekstrom M, Mednick S, 2012. Premorbid multivariate markers of neurodevelopmental instability in the prediction of adult schizophrenia-spectrum disorder: a high-risk prospective investigation. Schizophr. Res 139(1–3), 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M, Walker E, 1985. Neuropsychological performance and positive and negative symptoms in schizophrenia. J. Abnorm. Psychol 94 (4), 460–469. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK, 1998. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 12 (3), 426–445. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S, 2000. Applied Logistic Regression. 2nd ed. John Wiley & Sons, Inc. [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S, 2012. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch. Gen. Psychiatry 69 (8), 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khot V, Wyatt RJ, 1991. Not all that moves is tardive dyskinesia. Am. J. Psychiatry 148 (5), 661–666. [DOI] [PubMed] [Google Scholar]
- Lindgren M, Manninen M, Laajasalo T, Mustonen U, Kalska H, Suvisaari J, Moilanen K, Cannon TD, Huttunen M, Therman S, 2010. The relationship between psychotic-like symptoms and neurocognitive performance in a general adolescent psychiatric sample. Schizophr. Res 123 (1), 77–85. [DOI] [PubMed] [Google Scholar]
- Marcus J, Hans SL, Mednick SA, Schulsinger F, Michelsen N, 1985. Neurological dysfunctioning in offspring of schizophrenics in Israel and Denmark: a replication analysis. Arch. Gen. Psychiatry 42 (8), 753–761. [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Reichenberg A, Keefe RS, Fisher HL, Harrington H, Houts R, Poulton R, Moffitt TE, 2014. Neuropsychological decline in schizophrenia from the premorbid to the postonset period: evidence from a population-representative longitudinal study. Am. J. Psychiatry 171 (1), 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Jalbrzikowski M, Daley M, Roman C, Bearden CE, Cannon TD, 2011. Abnormal movements are associated with poor psychosocial functioning in adolescents at high risk for psychosis. Schizophr. Res. 130 (1), 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrens M, Docx L, Walther S, 2014. Beyond boundaries: in search of an integrative view on motor symptoms in schizophrenia. Front. Psychiatry 5, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozley LH, Gur RC, Mozley PD, Gur RE, 2001. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am. J. Psychiatry 158 (9), 1492–1499. [DOI] [PubMed] [Google Scholar]
- Neelam K, Garg D, Marshall M, 2011. A systematic review and meta-analysis of neurological soft signs in relatives of people with schizophrenia. BMC Psychiatry 11 (139), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus AH, Karl C, Hahn E, Trempler NR, Opgen-Rhein C, Urbanek C, Hahn C, Ta TM, Dettling M, 2011. Dissection of early bottom-up and top-down deficits during visual attention in schizophrenia. Clin. Neurophysiol 122 (1), 90–98. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Rosso IM, Sanchez LE, Hadley T, Nuechterlein KH, Cannon TD, 2003. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. A. J. Psychiatry 160 (11), 2060–2062. [DOI] [PubMed] [Google Scholar]
- Peralta V, Cuesta MJ, 2011. Neuromotor abnormalities in neuroleptic-naive psychotic patients: antecedents, clinical correlates, and prediction of treatment response. Compr. Psychiatry 52 (2), 139–145. [DOI] [PubMed] [Google Scholar]
- Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A, 2011. Dysconnectivity in schizophrenia: where are we now? Neurosci. Biobehav. Rev 35 (5), 1110–1124. [DOI] [PubMed] [Google Scholar]
- Rigucci S, Dimitri-Valente G, Mandarelli G, Manfredi G, Comparelli A, De Filippis S, Gheradelli S, Bersani G, Girardi P, Ferracuti S, 2014. Neurological soft signs discriminate schizophrenia from bipolar disorder. J. Psychiatr. Pract 20 (2), 147–153. [DOI] [PubMed] [Google Scholar]
- Roman-Urrestarazu A, Muray GK, Barnes A, Miettunen J, Jaaskelainen E, Maki P, Nikkinen J, Remes J, Mukkala S, Koivukangas J, Heinimaa M, Moilanen I, Suckling J, Kiviniemi V, Jones PB, Veijola J, 2014. Brain structure in different psychosis risk groups in the Northern Finland 1986 birth cohort. Schizophr. Res 153 (1–3), 143–145. [DOI] [PubMed] [Google Scholar]
- Royce JR, Yeudall LT, Bock C, 1976. Factor analytic studies of human brain damage: I. First and second-order factors and their brain correlates. Multivar. Behav. Res 11 (4), 381–418. [DOI] [PubMed] [Google Scholar]
- Schiffman J, Sorensen HJ, Maeda J, Mortensen EL, Victoroff J, Hayashi K, Michelsen NM, Ekstrom M, Mednick S, 2009. Childhood motor coordination and adult schizophrenia spectrum disorders. Am. J. Psychiatry 166 (9), 1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman J, Mittal V, Kline E, Mortensen EL, Michelsen N, Ekstrom M, Millman ZB, Mednick SA, Sorensen HJ, 2015. Childhood dyspraxia predicts adult-onset nonaffective-psychosis-spectrum disorder. Dev. Psychopathol 27 (4), 1323–1330. [DOI] [PubMed] [Google Scholar]
- Sørensen HJ, Mortensen EL, Parnas J, Mednick SA, 2006. Premorbid neurocognitive functioning in schizophrenia spectrum disorder. Schizophr. Bull 32 (3), 578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen HJ, Mortensen EL, Schiffman J, Ekstrøm M, Denenny D, Mednick SA, 2010. Premorbid IQ and adult schizophrenia spectrum disorder: verbal and performance subtests. Psychiatr. Res 178, 23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott DH, 1966. A general test of motor impairment for children. Dev. Med. Child Neurol 8 (5), 523–531. [DOI] [PubMed] [Google Scholar]
- Tandon R, Nasrallah HA, Keshavan MS, 2009. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr. Res. 110 (1–3), 1–23. [DOI] [PubMed] [Google Scholar]
- Tew B, 1979. Differences between Welsh and Canadian children on parts of the test of motor impairment. Child Care Health Dev. 5 (2), 135–141. [DOI] [PubMed] [Google Scholar]
- Torrey EF, 2002. Studies of individuals with schizophrenia never treated with antipsychotic medications: a review. Schizophr. Res 58 (2–3), 101–115. [DOI] [PubMed] [Google Scholar]
- Tripathi R, Soni A, Tyagi A, Mehta S, Gupta S, 2015. Comparative study of neurological soft signs in patients with schizophrenia or obsessive-compulsive disorder, and healthy controls. East Asian Arch. Psychiatry 25 (2), 64–72. [PubMed] [Google Scholar]
- Wechsler D, 1949. Manual for the Wechsler Intelligence Scale for Children. Psychological Corporation, New York, NY. [Google Scholar]
- Wing JK, Cooper JE, Sartorius N, 1974. The description and classification of psychiatric symptoms. An Instruction Manual for the PSE and CATEGO Systems. Cambridge University Press, Cambridge. [Google Scholar]
- Wolff A-L, O'Driscoll GA, 1999. Motor deficits and schizophrenia: the evidence from neuroleptic-naïve patients and populations at risk. J. Psychiatry Neurosci 24 (4), 304–314. [PMC free article] [PubMed] [Google Scholar]
- Yao L, Lui S, Liao Y, Du MY, Hu N, Thomas JA, Gong QY, 2013. White matter deficits in first episode schizophrenia: an activation likelihood estimation meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 45, 100–106. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Ursu S, Walters R, Wendelken C, Ragland JD, Carter CS, 2008. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am. J. Psychiatry 165 (8), 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetzsche T, Preuss UW, Frodl T, Leinsinger G, Born C, Reiser M, Hegerl U, Moller HJ, Meinsenzahl EM, 2008. White matter alterations in schizophrenic patients with pronounced negative symptomatology and with positive family history for schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci 258 (5), 278–284. [DOI] [PubMed] [Google Scholar]


