REPLY
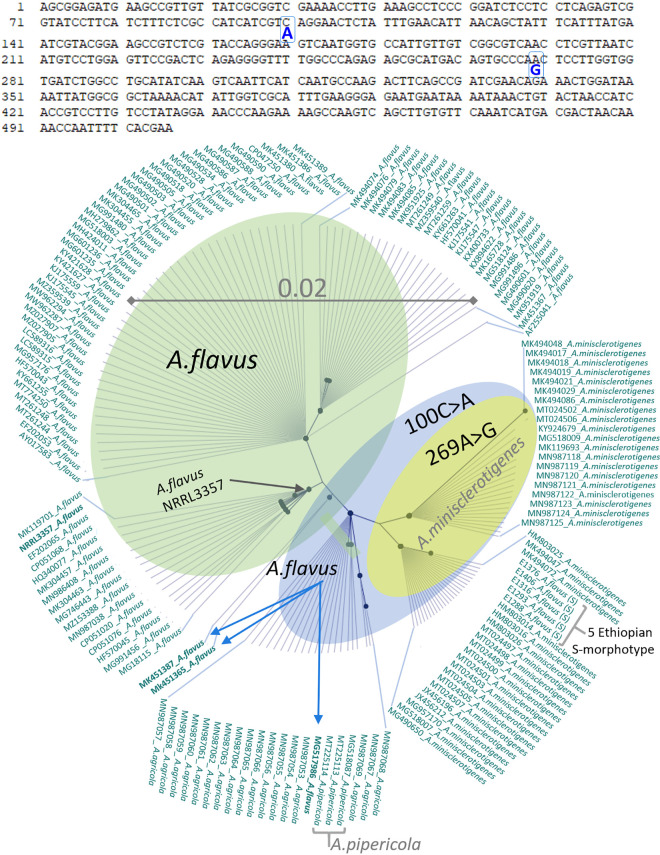
In a recent letter to the editor, Houbraken et al. (23) provide a series of recommendations to the microbiological community to prevent the taxonomic misidentification of genome-sequenced fungal strains. In the era of genomics and bioinformatics, postulating that 1 nucleotide (nt) within a gene can “correctly” identify a species does not seem plausible. However, the authors of the letter call this the “calmodulin barcode,” meaning nucleotide substitutions within a 506-nt region of the calmodulin gene (1). After the evolutionarily conserved rRNA (18S rRNA, internal transcribed spacer [ITS], 28S rRNA) and RNA polymerase II (2–4) showed no differences between Aspergillus flavus S- and L-morphotypes, attention shifted toward the calmodulin gene. Thus, without sequencing 18S rRNA, 28S rRNA, or the largest RNA polymerase II subunit, at least 34 new species of Aspergillus were named by Houbraken, Frisvad, Visagie, and coworkers (1, 5, 6). However, in a phylogenetic tree of 152 Aspergillus section Flavi isolates using the calmodulin 506-nt region, 40 Aspergillus minisclerotigenes isolates had only two nucleotide substitutions in common, namely, 100C>A and 269A>G, both of which are silent mutations (Fig. 1). However, only 269A>G discriminates A. minisclerotigenes from A. flavus, since 100C>A is present in three A. flavus isolates (GenBank accession numbers MK451387, MK451365, and MG517986) identified by the authors of the letter. We all agree that species identification is important; paradoxically, the calmodulin barcode assigns species based on a single-nucleotide polymorphism (SNP), while there are between 133,000 and 179,000 SNPs within A. flavus S- and L-morphotypes, respectively (7).
FIG 1.
Maximum likelihood phylogenetic tree using 506 nt of the calmodulin gene. (Top) Partial sequence of the calmodulin gene of Aspergillus, highlighting the substitutions at positions 100 and 269. (Bottom) Phylogram of 152 isolates of Aspergillus species section Flavi, including 87 A. flavus isolates (14 SNPs), 40 A. minisclerotigenes isolates (10 SNPs), 17 Aspergillus agricola isolates (4 SNPs), 3 Aspergillus pipericola isolates (2 SNPs), and 5 A. flavus S-morphotype isolates (3 SNPs) from Ethiopia; numbers in parentheses correspond to SNPs found within each group. Scale bar, number of substitutions per 100 nt. Blue arrows indicate the 3 A. flavus strains with 100C>A substitutions; the yellow area highlights isolates with 269A>G substitutions. After sequence alignment, the neighbor-joining method was used as the construction method with the Jukes-Cantor substitution model including rate variation, processed in CLC Genomics Workbench v.21.0. (Qiagen).
Another limitation of Aspergillus taxonomy is the chemotypes resulting from 30 genes in the aflatoxin biosynthesis gene cluster (ABC) (8), e.g., A. flavus produces B-aflatoxins and Aspergillus parasiticus produces B and G types (9). Despite that a single nucleotide change in one ABC gene can prevent aflatoxin production (10), the inheritance of the ABC is favored by environmental pressure (11), and Aspergillus spp. are not physically or reproductively isolated; intraspecies and interspecies crosses can result in gain of function, e.g., G-type aflatoxin production (9, 12, 13). Hence, a new species named by one author of the letter was later reversed to its initial name by the same author because of the chemotype, i.e., A. flavus S-morphotype to Aspergillus parvisclerotigenus (14) and back to A. flavus (6). Other groups utilized the calmodulin gene and a single deletion in the ABC to name three new Aspergillus species (15, 16).
Analysis of 25 insertion/deletions (indels) within the ABC revealed almost as many Aspergillus genotypes as the number of isolates (17, 18). That work was performed not to identify new species but to detect predominant genotypes, sequence their genomes (19, 20), and find targets for gene silencing. Isolates in those publications had been identified by Dr. Bruce Horn comparing morphology to type strains in the culture collection at the National Peanut Research Laboratory, as described (17, 21). Furthermore, SNP analysis of five Ethiopian S-morphotype isolates versus the model genome A. flavus NRRL3357 was performed by combining 20,213 nt of evolutionarily conserved genes: rRNA cistron (7,796 nt) and DNA-dependent RNA polymerase largest subunit (RPB1) (5,857 nt) and second largest subunit (RPB2) (6,560 nt). Four S-morphotype isolates had 99.2% identity to A. flavus NRRL3357, with SNP detection processed in CLC Genomics Workbench v.21.0 (Qiagen, Denmark). Genome-wide SNP analysis of 24 Aspergillus strains, including 13 A. flavus isolates (10 L-morphotype and 3 S-morphotype), 10 Aspergillus oryzae isolates, and 1 A. parasiticus, concluded that, despite their morphological differences, L-type and S-type isolates have 99.2% identity; therefore, they belong to the same A. flavus species (7). The fifth S-morphotype isolate from Ethiopia presented a 189-nt insertion in its rRNA cistron. Unfortunately, the full-length rRNA cistron of A. minisclerotigenes is not available for comparison, since it was not reported with its genome (GenBank accession number SWDZ00000000) (22). The current Aspergillus taxonomy is based on PCR amplification of short DNA fragments, does not examine the most evolutionarily conserved genes, adopts single indels within the ABC as a classification tool, and observes chemotypes whose detection can vary by orders of magnitude, depending on the analysis methods. New technologies are available, and we think there is a need for an upgrade to a more robust system of classification.
Data availability.
The data used to generate the figure are openly available in Harvard Dataverse (https://doi.org/10.7910/DVN/LVJ7ZN).
ACKNOWLEDGMENT
We thank the USDA Department of Agriculture for its financial support, Research Project 6044-21000-005.
Footnotes
This is a response to a letter by Houbraken et al. (https://doi.org/10.1128/MRA.01074-20).
Contributor Information
Renee S. Arias, Email: renee.arias@usda.gov.
Antonis Rokas, Vanderbilt University.
REFERENCES
- 1.Samson RA, Visagie CM, Houbraken J, Hong S-B, Hubka V, Klaassen CHW, Perrone G, Seifert KA, Susca A, Tanney JB, Varga J, Kocsubé S, Szigeti G, Yaguchi T, Frisvad JC. 2014. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol 78:141–173. doi: 10.1016/j.simyco.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eickbush TH, Eickbush DG. 2007. Finely orchestrated movements: evolution of the ribosomal RNA genes. Genetics 175:477–485. doi: 10.1534/genetics.107.071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarza P, Yilmaz P, Panzer K, Glöckner FO, Reich M. 2017. A phylogenetic framework for the kingdom Fungi based on 18S rRNA gene sequences. Mar Genomics 36:33–39. doi: 10.1016/j.margen.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Liu YJ, Whelen S, Hall BD. 1999. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol 16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- 5.Pildain MB, Frisvad JC, Vaamonde G, Cabral D, Varga J, Samson RA. 2008. Two novel aflatoxin-producing Aspergillus species from Argentinean peanuts. Int J Syst Evol Microbiol 58:725–735. doi: 10.1099/ijs.0.65123-0. [DOI] [PubMed] [Google Scholar]
- 6.Frisvad JC, Hubka V, Ezekiel CN, Hong S-B, Nováková A, Chen AJ, Arzanlou M, Larsen TO, Sklenář F, Mahakarnchanakul W, Samson RA, Houbraken J. 2019. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud Mycol 93:1–63. doi: 10.1016/j.simyco.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang PK. 2019. Genome-wide nucleotide variation distinguishes Aspergillus flavus from Aspergillus oryzae and helps to reveal origins of atoxigenic A. flavus biocontrol strains. J Appl Microbiol 127:1511–1520. doi: 10.1111/jam.14419. [DOI] [PubMed] [Google Scholar]
- 8.Caceres I, Khoury AA, Khoury RE, Lorber S, Oswald IP, Khoury AE, Atoui A, Puel O, Bailly JD. 2020. Aflatoxin biosynthesis and genetic regulation: a review. Toxins (Basel) 12:150. doi: 10.3390/toxins12030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olarte RA, Worthington CJ, Horn BW, Moore GG, Singh R, Monacell JT, Dorner JW, Stone EA, Xie DY, Carbone I. 2015. Enhanced diversity and aflatoxigenicity in interspecific hybrids of Aspergillus flavus and Aspergillus parasiticus. Mol Ecol 24:1889–1909. doi: 10.1111/mec.13153. [DOI] [PubMed] [Google Scholar]
- 10.Ehrlich KC, Cotty PJ. 2004. An isolate of Aspergillus flavus used to reduce aflatoxin contamination in cottonseed has a defective polyketide synthase gene. Appl Microbiol Biotechnol 65:473–478. doi: 10.1007/s00253-004-1670-y. [DOI] [PubMed] [Google Scholar]
- 11.Carbone I, Jakobek JL, Ramirez-Prado JH, Horn BW. 2007. Recombination, balancing selection and adaptive evolution in the aflatoxin gene cluster of Aspergillus parasiticus. Mol Ecol 16:4401–4417. doi: 10.1111/j.1365-294X.2007.03464.x. [DOI] [PubMed] [Google Scholar]
- 12.Moore GG, Horn BW, Elliott JL, Hell K, Chulze SN, Barros G, Wright G, Naik MK, Carbone I. 2009. Sexual reproduction influences aflatoxin chemotype diversity in worldwide populations of Aspergillus flavus and A. parasiticus. Phytopathology 99:S88. [Google Scholar]
- 13.Olarte RA, Horn BW, Dorner JW, Monacell JT, Singh R, Stone EA, Carbone I. 2012. Effect of sexual recombination on population diversity in aflatoxin production by Aspergillus flavus and evidence for cryptic heterokaryosis. Mol Ecol 21:1453–1476. doi: 10.1111/j.1365-294X.2011.05398.x. [DOI] [PubMed] [Google Scholar]
- 14.Frisvad JC, Skouboe P, Samson RA. 2005. Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin and 3-O-methylsterigmatocystin, Aspergillus rambellii sp. nov. Syst Appl Microbiol 28:442–453. doi: 10.1016/j.syapm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Singh P, Orbach MJ, Cotty PJ. 2018. Aspergillus texensis: a novel aflatoxin producer with S morphology from the United States. Toxins 10:513. doi: 10.3390/toxins10120513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh P, Callicott KA, Orbach MJ, Cotty PJ. 2020. Molecular analysis of S-morphology aflatoxin producers from the United States reveals previously unknown diversity and two new taxa. Front Microbiol 11:1236. doi: 10.3389/fmicb.2020.01236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faustinelli PC, Palencia ER, Sobolev VS, Horn BW, Sheppard HT, Lamb MC, Wang XYM, Scheffler BE, Castillo JM, Arias RS. 2017. Study of the genetic diversity of the aflatoxin biosynthesis cluster in Aspergillus section Flavi using insertion/deletion markers in peanut seeds from Georgia, USA. Mycologia 109:200–209. doi: 10.1080/00275514.2017.1307095. [DOI] [PubMed] [Google Scholar]
- 18.Mohammed A, Faustinelli PC, Chala A, Dejene M, Fininsa C, Ayalew A, Ojiewo CO, Hoisington DA, Sobolev VS, Martínez-Castillo J, Arias RS. 2021. Genetic fingerprinting and aflatoxin production of Aspergillus section Flavi associated with groundnut in eastern Ethiopia. BMC Microbiol 21:239. doi: 10.1186/s12866-021-02290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faustinelli PC, Wang XM, Palencia ER, Arias RS. 2016. Genome sequences of eight Aspergillus flavus spp. and one A. parasiticus sp., isolated from peanut seeds in Georgia. Genome Announc 4:e00278-16. doi: 10.1128/genomeA.00278-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arias RS, Mohammed A, Orner VA, Faustinelli PC, Lamb MC, Sobolev VS. 2020. Sixteen draft genome sequences representing the genetic diversity of Aspergillus flavus and Aspergillus parasiticus colonizing peanut seeds in Ethiopia. Microbiol Resour Announc 9:e00591-20. doi: 10.1128/MRA.00591-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammed A, Chala A, Dejene M, Fininsa C, Hoisington DA, Sobolev VS, Arias RS. 2016. Aspergillus and aflatoxin in groundnut (Arachis hypogaea L.) and groundnut cake in Eastern Ethiopia. Food Addit Contam Part B Surveill 9:290–298. doi: 10.1080/19393210.2016.1216468. [DOI] [PubMed] [Google Scholar]
- 22.Kjærbølling I, Vesth T, Frisvad JC, Nybo JL, Theobald S, Kildgaard S, Petersen TI, Kuo A, Sato A, Lyhne EK, Kogle ME, Wiebenga A, Kun RS, Lubbers RJM, Mäkelä MR, Barry K, Chovatia M, Clum A, Daum C, Haridas S, He G, LaButti K, Lipzen A, Mondo S, Pangilinan J, Riley R, Salamov A, Simmons BA, Magnuson JK, Henrissat B, Mortensen UH, Larsen TO, de Vries RP, Grigoriev IV, Machida M, Baker SE, Andersen MR. 2020. A comparative genomics study of 23 Aspergillus species from section Flavi. Nat Commun 11:1106. doi: 10.1038/s41467-019-14051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houbraken J, Visagie CM, Frisvad JC. 2021. Recommendations to prevent taxonomic misidentification of genome-sequenced fungal strains. Microbiol Resour Announc 10:e01074-20. doi: 10.1128/MRA.01074-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to generate the figure are openly available in Harvard Dataverse (https://doi.org/10.7910/DVN/LVJ7ZN).