Abstract
Patients with homonymous hemianopia sometimes show preservation of the central visual fields, ranging up to 10°. This phenomenon, known as macular sparing, has sparked perpetual controversy. Two main theories have been offered to explain it. The first theory proposes a dual representation of the macula in each hemisphere. After loss of one occipital lobe, the back-up representation in the remaining occipital lobe is postulated to sustain ipsilateral central vision in the blind hemifield. This theory is supported by studies showing that some midline retinal ganglion cells project to the wrong hemisphere, presumably driving neurons in striate cortex that have ipsilateral receptive fields. However, more recent electrophysiological recordings and neuroimaging studies have cast doubt on this theory by showing only a minuscule ipsilateral field representation in early visual cortical areas. The second theory holds that macular sparing arises because the occipital pole, where the macula is represented, remains perfused after occlusion of the posterior cerebral artery because it receives collateral flow from the middle cerebral artery. An objection to this theory is that it cannot account for reports of macular sparing in patients after loss of an entire occipital lobe. On close scrutiny, such reports turn out to be erroneous, arising from inadequate control of fixation during visual field testing. Patients seem able to detect test stimuli on their blind side within the macula or along the vertical meridian because they make surveillance saccades. A purported treatment for hemianopia, called vision restoration therapy, is based on this error. The dual perfusion theory is supported by anatomical studies showing that the middle cerebral artery perfuses the occipital pole in many individuals. In patients with hemianopia from stroke, neuroimaging shows preservation of the occipital pole when macular sparing is present. The frontier dividing the infarcted territory of the posterior cerebral artery and the preserved territory of the middle cerebral artery is variable, but always falls within the representation of the macula, because the macula is so highly magnified. For physicians, macular sparing was an important neurological sign in acute hemianopia because it signified a posterior cerebral artery occlusion. Modern neuroimaging has supplanted the importance of that clinical sign but at the same time confirmed its validity. For patients, macular sparing remains important because it mitigates the impact of hemianopia and preserves the ability to read fluently.
Keywords: homonymous hemianopia, cortical magnification factor, fixation stability, primary visual cortex, vision restoration therapy, reading
INTRODUCTION
After invention of the arc perimeter by Förster (1867), mapping of the visual fields became a routine procedure in ophthalmology and neurology. It was soon realized that patients with vision loss from occipital stroke often had sparing of central function in their blind hemifield. Förster (1890) proposed that collateral blood flow preserving cortical tissue representing macular vision might be responsible for this phenomenon. Although he was exactly right, his voice was lost in the din of controversy that followed.
After macular sparing was discovered various theories were offered to explain it, fueling a debate that has simmered for over a century. Polyak (1957, pp. 680–94) provides an excellent summary of various competing schemes and a comprehensive review of the literature up to the mid-twentieth century. He rejects those theories incompatible with contemporary knowledge, even chiding one author for conclusions “liable to bring confusion into a delicate problem requiring, above all, sober facts and clear thinking.” In his discussion, however, Polyak fails to deliver a verdict regarding which of the conflicting mechanisms proposed for macular sparing is correct.
In the modern era, only a single review article about macular sparing has been published (Leff 2004). After a scholarly historical review, it concludes that macular sparing results from incomplete damage to striate cortex and its connections. However, the author is dismissive of collateral flow from the middle cerebral artery, calling the contribution of this blood supply in cases of macular sparing “somewhat overplayed” (p. 275).
Some topics become so mired in confusion that it becomes nearly impossible to extricate them. It seems worthwhile, nonetheless, to attempt to bring clarity and closure to the issue of macular sparing. It is a problem that should be solvable in light of recent advances in neuroimaging and vision science. In this article our goals are to define the phenomenon of macular sparing, to provide a definitive explanation for it, and to explain its significance for clinicians and patients.
INOUYE’S MAP WITH INCLUSION OF THE IPSILATERAL MACULA
The first map of the visual field representation in the brain was published by Tatsuji Inouye (1909). He compiled the map by correlating scotomas with the trajectory of penetrating wounds suffered by veterans of the Boxer Rebellion and the Russo-Japanese War. The inspiring story of this young ophthalmologist’s discovery of the first topographic map in the human cerebral cortex has been chronicled beautifully by Glickstein (1988).
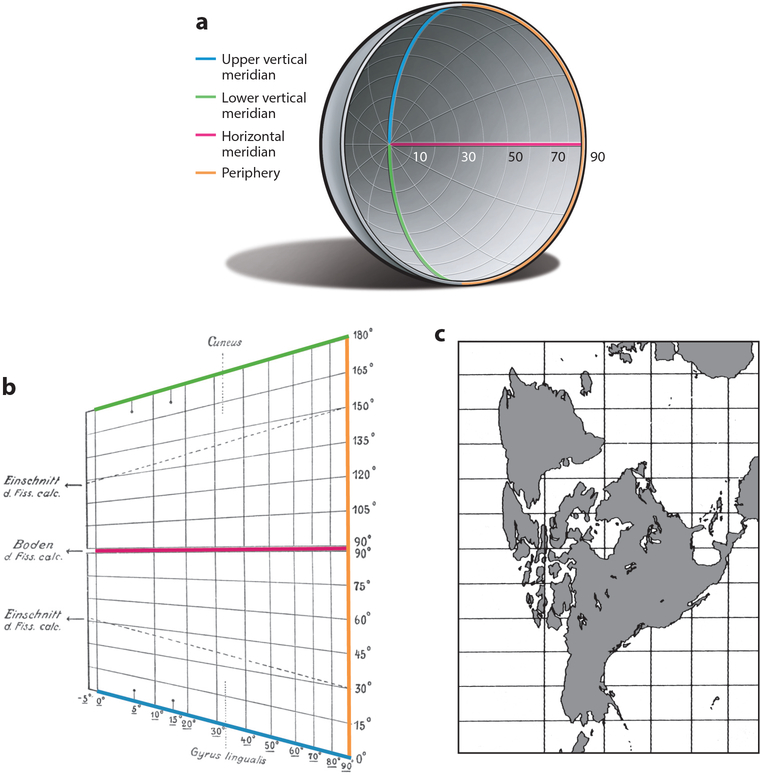
Inouye depicted a cylindrical projection of the quarter sphere formed by each visual hemifield (Figure 1). Such conformal maps, which trace their lineage to Mercator, are a favorite of navigators because a route of constant bearing corresponds to a straight line. Inouye chose a Mercator-style projection because the bullet in each wounded soldier followed a straight path through the skull from entry to exit. A disadvantage is that the center of gaze, a single point in the visual field, becomes transformed into a line. The map also has a rectangular shape. Aware that the visual cortex becomes progressively narrower toward the occipital pole, Inouye shortened the line representing the fovea (North Pole) to give the map a trapezoidal shape. In a true Mercator-style projection it should be equal in length to the line representing the visual field periphery (equator).
Figure 1.
(a) The human visual hemifield corresponds approximately to a quarter sphere. The upper vertical meridian, lower vertical meridian, and horizontal meridian converge at the center of gaze. On a globe, the center of gaze and field periphery are equivalent to the North Pole and equator, respectively. (b) Inouye’s 1909 Mercator-style map of the primary visual cortex (color scheme added), with vertical lines representing parallels and horizontal lines representing meridians. The center of gaze at the occipital pole is transformed into a vertical line labeled 0°. The horizontal meridian runs from the center of gaze along the base of the calcarine fissure to the periphery. A narrow strip (−5° to 0°) is allocated to the ipsilateral central visual field. (c) Mercator-style projection for comparison with Inouye’s map, showing how latitudes become progressively enlarged nearer the North Pole (Snyder 1993, p. 182).
Inouye’s map seems to allocate an escalating amount of tissue to the representation of the central visual field. For example, azimuths from 0° to 10° occupy more territory than azimuths from 10° to 20°, and so on. This progressive inflation of the central visual field is an artifact inherent to all Mercator projections (the Greenland problem). It has been misinterpreted as evidence that Inouye recognized the principle of increasing cortical magnification of more central eccentricities (Glickstein & Whitteridge 1987, Leff 2004). Inouye makes no reference to this concept anywhere in his book.
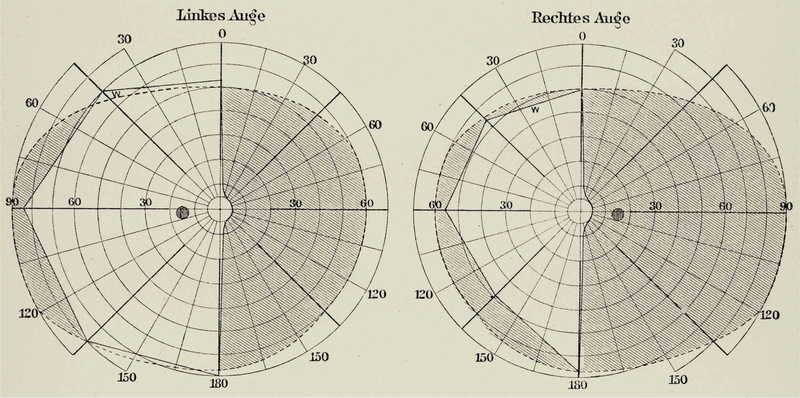
Inouye encountered macular sparing in virtually every soldier, usually subtending 2° to 5°. In a typical case, he shows macular sparing of 5° along the horizontal meridian, with the edge of the hemianopic defect tapering gradually toward the vertical meridian (Figure 2). The borders of the spared macula should actually intersect the vertical meridian smartly at 5°. Inouye failed to capture this feature accurately because precise mapping is so difficult close to the midline. In normal individuals attempting to maintain steady fixation, the position of the center of gaze deviates up to 0.5° from a stationary target (Cherici et al. 2012). When testing is done with one eye covered, which is typical in perimetry, fixation instability is increased (Gonzalez et al. 2012, Otero-Millan et al. 2014).
Figure 2.
Inouye’s Case #4 showing a right homonymous hemianopia (gray shading) in a soldier who died 107 days after a left occipital bullet wound. Macular sparing of 5° is depicted, which tapers toward the vertical meridian from 5° to 40°. This gradual transition is unphysiological, underscoring the treacherous nature of visual field mapping along the vertical meridian in hemianopia.
In patients with hemianopia, the problem of fixation instability is compounded. In 33 hemianopic patients, Trauzettel-Klosinski and her colleagues used a scanning laser ophthalmoscope to record foveal position during attempted continuous fixation. Saccadic intrusions ranging up to 10° were common (Reinhard et al. 2014). The mean amplitude of eye movements, despite subjects’ best effort to maintain fixation, was 1.3° to 2.6° toward the blind hemifield. These data explain Inouye’s erroneous mapping along the vertical meridian. They underscore the challenge of determining if a few degrees of central vision are spared in hemianopia, at least using standard clinical perimetry. Parenthetically, it should be mentioned that visual acuity does not help differentiate between a foveal splitting and a foveal sparing hemianopia because it remains perfectly normal in both.
Macular sparing of less than 2° does occur in hemianopia, but it is obscured by the perimetric noise generated by fixation instability. To avoid getting caught by this messy problem, a conservative approach is to declare the presence of macular sparing only when at least 2° of central vision are preserved. That cutoff assumes that the patient can fixate relatively faithfully and cooperate with testing. In a patient with poor fixation, a higher threshold is warranted before a finding of macular sparing is accepted. With regards to the upper range of macular sparing, it is as vague as the definition of the macula itself. There is no absolute limit, but 10° is a reasonable value because it is seldom exceeded in hemianopic cortical stroke.
REVISIONS TO THE PRIMARY VISUAL CORTICAL MAP
In Inouye’s era, leaders in vision research believed firmly in the concept of bilateral cortical representation of the macula (Henschen 1900, Marchand 1882, Wilbrand & Saenger 1904). They were convinced by the observation of macular sparing after complete destruction of the visual pathway in one hemisphere. Inouye reported at least 2° of macular sparing in nearly every injured soldier, even after loss of the occipital lobe. To explain it, he included 5° of central ipsilateral visual field in his cortical map (Figure 1). Curiously, he allocated it far less tissue than the contralateral central 5°. This discrepancy suggests that Inouye was not entirely comfortable with the idea. An obvious problem is that if the map contains 5° of ipsilateral representation, then every complete hemianopia should be accompanied by exactly 5° of macular sparing. How, then, can one account for wide variation in the magnitude of macular sparing? One must accept the slightly unpalatable concept that the amount of ipsilateral visual field representation differs from person to person.
Gordon Holmes later published a map of the visual field representation in striate cortex, based on data from soldiers wounded in the First World War. He introduced two important modifications to Inouye’s map. He determined that complete destruction of the occipital lobe results in homonymous blindness reaching to the fixation point (Holmes & Lister 1916). He therefore declared that “the macula has not a bilateral representation” and eliminated the strip of tissue that Inouye devoted to the ipsilateral macula (Holmes 1918, p. 382). He also recognized that the macula, being “highly specialized” (Holmes 1918, p. 363), should have an enlarged representation relative to the periphery. His map allocated about 25% of striate cortex to the central 15°.
The Holmes map became the gold standard, completely supplanting the Inouye map. It had the advantage of being much easier to understand, by eliminating Inouye’s confusing Mercator-style projection in favor of an intuitive system of point-to-point matching symbols. Holmes also helped ease Inouye’s work into obscurity. In 1916, in his first paper on the cortical map, he made reference to Inouye’s “excellent monograph” (Holmes & Lister 1916, p. 37). But in 1945, in the Ferrier Lecture containing his final version of the map, he asserted that “there was, prior to 1914, no definite evidence of the local representation of the retina” in striate cortex (Holmes 1945, p. 350). Inouye’s name was purged from the list of accompanying references.
Holmes (1945) was aware of the animal studies of Talbot & Marshall (1941), which provided the first conclusive evidence that central locations in the visual field are more magnified in their cortical representation. The full extent of this enlargement was not realized until the stunning claim by Daniel & Whitteridge (1961) that the central 10° occupies 55% of the surface area of striate cortex in the macaque. This enormous magnification of the macula in the macaque has been confirmed (Van Essen et al. 1984). It has also been shown in the human by correlating visual field defects with lesions of the occipital lobe imaged by magnetic resonance (MR) (Horton & Hoyt 1991).
After the development of functional MR imaging (fMRI) it became possible to probe retinotopic maps in the brain of normal subjects, freeing one from reliance on the cumbersome approach of correlating scotomas with lesions in patients. The first fMRI study reported the surprising result that the emphasis on central vision is even greater than described previously in macaques and humans (Sereno et al. 1995). However, subsequent human fMRI investigations have found a close agreement with data from human clinical studies and macaque electrophysiological mapping (Brewer et al. 2002, DeYoe et al. 1996, Engel et al. 1997, Wandell & Winawer 2011).
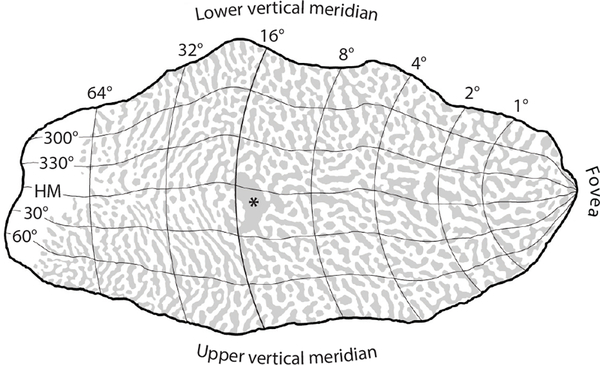
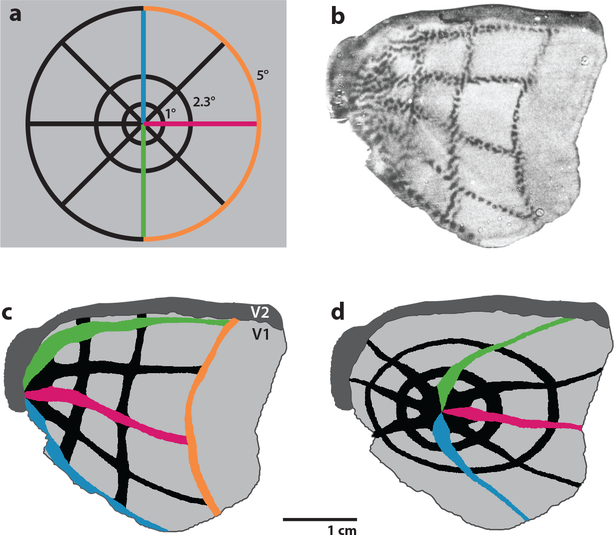
Direct evidence pertaining to this issue comes from reconstruction of the pattern formed by ocular dominance columns in human striate cortex. The columns can be mapped at higher resolution than achievable with fMRI (Cheng et al. 2001) by processing cortical sections for a mitochondrial enzyme, cytochrome oxidase (Wong-Riley 1989). In specimens obtained at autopsy from subjects with a history of monocular blindness, the columns serving the missing eye appear pale because their metabolic demand is reduced. The blind spot representation of the contralateral eye is obvious, providing a reliable landmark centered at 15° eccentricity along the horizontal meridian. Figure 3 shows the retinotopic map derived by Horton & Hoyt (1991) scaled to a complete mosaic of ocular dominance columns (Adams et al. 2007). The blind spot representation is close to the location predicted by the map’s coordinates. This agreement confirms the map’s accuracy. In this subject the central 10° occupied 48% of striate cortex. As explained below, the huge magnification of the macula is a critical factor in the mechanism of macular sparing. Once it is taken into account, the solution to macular sparing falls into place.
Figure 3.
Visual field map of striate cortex with polar coordinates from Horton & Hoyt (1991), scaled to a reconstruction of the pattern formed by ocular dominance columns in a human autopsy specimen (Adams et al. 2007). Rings of iso-eccentricity from the fovea are labeled dorsally, where they intersect the lower vertical meridian. The blind spot representation (*) is at the expected location, confirming the enormous cortical magnification of the macula. About half the cortex is devoted to the central 15°. Abbreviation: HM, horizontal meridian.
DOES ANATOMY SUPPORT AN IPSILATERAL MACULAR REPRESENTATION IN STRIATE CORTEX?
The existence of retinal ganglion cell axons that divide in the optic chiasm to project bilaterally along the visual pathway could potentially support a duplicated representation of the macula in the primary visual cortex. In the cat, Kölliker (1899) observed bifurcating fibers in the chiasm. In the rabbit, Ramon y Cajal (1911) also observed bifurcating fibers. He stated that Henschen believed that they arise from the macula, lending support to Wilbrand’s theory of bilateral macular representation. Ramon y Cajal was skeptical, suggesting instead that the fibers might serve the pupillary light reflex.
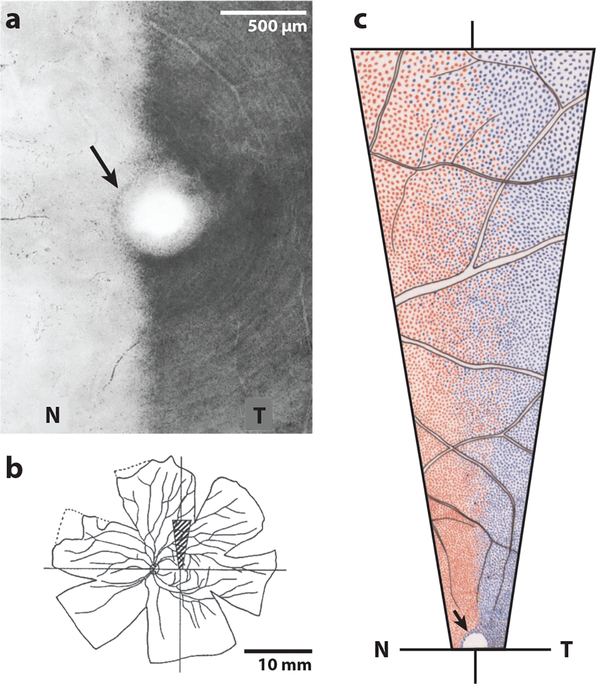
Dividing optic nerve fibers have also been observed in the human (Taban et al. 2005), but they do not contribute to the retinogeniculate pathway. In the macaque, no double-labeled ganglion cells were found in the retina after flooding each lateral geniculate nucleus with different fluorescent retrograde tracers (Fukuda et al. 1989) (Figure 4c). This experiment firmly excludes the existence in primates of retinal ganglion cells that project to the lateral geniculate nucleus in both hemispheres.
Figure 4.
Retinal overlap in ganglion cell populations projecting to each hemisphere in the macaque (Fukuda et al. 1989). (a) Foveal pit (center) with temporal (T) projecting ganglion cells darkly labeled by horseradish peroxidase injection into the optic tract. Some labeled ipsilateral projecting ganglion cells are located along the nasal (N) edge of the fovea (arrow). (b) Flatmount from a different animal showing the left retina; a wedge (hatching) is illustrated at higher power in panel c. (c) Ganglion cells labeled by RITC injection (red) and DAPI injection (blue) into the right and left lateral geniculate bodies, respectively. There is increasing naso-temporal overlap with increasing distance from the fovea. No cells are double-labeled.
However, there exists some untidiness in the chiasmal decussation, raising the possibility of an ipsilateral macular representation driven by retinal ganglion cells that project to the wrong hemisphere. After retrograde tracer injection into the macaque lateral geniculate nucleus, Leventhal and colleagues (1988) observed a ring of labeled ganglion cells around the nasal edge of the fovea in the ipsilateral eye. This means that a small contingent of ganglion cells nasal to the fovea erroneously projects ipsilaterally. No corresponding ring of labeled ganglion cells was present around the temporal edge of the fovea in the contralateral eye. Leventhal and colleagues (1988, p. 67) seized upon this observation to conclude that it might “solve the age-old problem of macular sparing and splitting.” For example, after loss of the right occipital lobe, one would expect macular sparing in the left eye and macular splitting in the right eye.
The observation that some nasal perifoveal ganglion cells fail to decussate is intriguing, but it has nothing to do with the clinical phenomenon of macular sparing. Cortical visual field defects are homonymous, i.e., nearly matching in the two eyes. Patients have either macular splitting or macular sparing in both eyes, not macular splitting in one eye and macular sparing in the other eye. Moreover, the few hundred cells found on the wrong side were located on the sloping foveal rim. Ganglion cells in this position receive signals from photoreceptors located close to the foveal center. Such cells could not possibly contribute to foveal sparing of more than 0.5°, let alone macular sparing (Reinhard & Trauzettel-Klosinski 2003).
The naso-temporal overlap in the projection of retinal ganglion cells increases gradually with increasing distance from the fovea (Figure 4). In the peripheral upper retina, the overlap zone spans a width of ±7.5° from the vertical meridian (Fukuda et al. 1989). As reviewed below, microelectrode studies show that the vertical meridian is represented with knife-like sharpness at the V1–V2 border. This apparent discrepancy between the anatomy and physiology poses an obvious question: how is input from ganglion cells located on the wrong side of the retinal vertical meridian reflected in the properties of cortical cells?
Topographic maps are based on receptive field position, in other words, the location where visual stimulation evokes action potentials. Surrounding each receptive field is a so-called extraclassical receptive field, where stimulation exerts a modulatory influence that can extend for many degrees (Angelucci et al. 2017, Briggs & Usrey 2011, Hallum & Movshon 2014, Henry et al. 2013, Zarella & Ts’o 2017). It is possible that retinal ganglion cells located on the wrong side of the vertical meridian contribute to the extraclassical receptive fields of cortical cells. This hypothesis is supported by the fact that the overlap zone in the retina increases with eccentricity, just as does the size of extraclassical receptive fields in the cortex. Another possibility is that the overlap allows calculation of retinal disparity along the vertical meridian by cells in the same V1, without reliance on callosal projections (Gibaldi et al. 2021). Whatever the case, sloppiness in the hemidecussation of retinal ganglion cells cannot explain macular sparing because the overlap zone is tiny near the fovea and becomes steadily wider in the periphery. Exactly the opposite gradient would be anticipated for an anatomical feature responsible for macular sparing.
A direct projection from the lateral geniculate nucleus via the corpus callosum to the contralateral primary visual cortex also has been proposed to explain macular sparing (Penfield et al. 1935). This theory is vitiated by the finding that occipital lobectomy results in no degeneration of cells in the lateral geniculate nucleus of the opposite hemisphere (Polyak 1933). In addition, no report has ever surfaced of labeled cells in the opposite lateral geniculate nucleus after injection of retrograde tracers into macaque striate cortex.
EVIDENCE FROM PHYSIOLOGICAL STUDIES
In their pioneering recordings from macaque striate cortex, Talbot & Marshall (1941) found that receptive fields strayed less than 0.5° into the ipsilateral hemifield. They noted that there is “no biprojection of the macula” (p.1257).This result was contradicted by Cowey (1964), who reported occasional large foveal receptive fields that crossed the vertical meridian (although never by more than 3°). His recordings were made with silver ball electrodes that rested on the pial surface. Surface potentials lasting approximately 100 ms were evoked by stimulating with small spots in the ipsilateral macula. Cowey (1964,p.373) conceded that these recordings were “anomalous,” but he breathed new life into the theory of bilateral macular representation by concluding that “the amount of crossing of the vertical meridian closely resembles the extent to which central vision is spared in cases of macular sparing after unilateral striate cortex damage in man” (p. 388). Cowey’s result might be explained by the fact that he relied mainly on surface potentials rather than action potentials.
Dow and colleagues (1985) later mapped the vertical meridian more precisely by making hundreds of microelectrode penetrations in a grid pattern centered over macaque foveal cortex. Single-cell recordings showed no center of a receptive field located more than 5 min of arc into the ipsilateral field. Total encroachment by the border of a receptive field never extended more than 25 min across the vertical midline. This result is consistent with Talbot & Marshall’s (1941) original finding that the maximum scatter across the midline is 0.5°.
Recording from single cells, it is sometimes difficult to grasp the prevalence of a particular property or to gauge its robustness. Functional imaging provides a global view of the activity generated simultaneously by innumerable cells. In an elegant demonstration, Tootell and colleagues (1988) used [14C]-2-deoxyglucose to image the representation of the contralateral hemimacula in striate cortex (Figure 5a,b). The fovea was represented precisely at the V1–V2 border, leaving no gap to accommodate the ipsilateral macula. If the macula had an ipsilateral representation in striate cortex, then autoradiography would have shown it intercalated between the fovea and the V1–V2 border (compare Figure 5c,d).
Figure 5.
No ipsilateral macular representation in primary visual cortex (V1). (a) Schematic diagram of flashing visuoscope pattern comprised of rings (1°, 2.3°, 5°) and rays used to activate macaque V1. It was centered on the fovea of one eye and the other eye was occluded. (b) Deoxyglucose autoradiograph of activity in V1 generated by the stimulus in panel a. The light gaps in the dark label correspond to ocular dominance columns of the occluded eye. (c) Drawing of the autoradiograph in panel b, showing the correspondence between the right half of the stimulus in panel a and the resulting pattern of metabolic activity in left V1. The vertical meridian is represented along the V1–V2 border (with a tiny error along the dorsal border, perhaps from ocular cyclorotation). The split fovea is represented at the extreme left edge of V1, where the rays converge. (d) Diagram of the pattern expected if, for example, 4° of ipsilateral macula were represented. The 1° and 2.3° rings would be complete and shifted away from the V1–V2 border. The 5° ring would not be located on the operculum. Data in panel b showing the findings illustrated in panel c, rather than those illustrated in panel d, refute the concept of an ipsilateral macular representation in V1. Panel adapted with permission from Tootell and colleagues (1988).
In higher visual association cortex, receptive fields are much larger. In some areas they extend broadly into the ipsilateral visual field (Gross et al.1972,Tootell et al.1998).Primary visual cortex in the same hemisphere cannot account for this phenomenon given that it harbors no ipsilateral visual field representation. Therefore, the ipsilateral field representation in visual association cortex must be conveyed from the other hemisphere via the corpus callosum. Loss of an occipital lobe (a) destroys the representation of the contralateral hemifield in V1 and (b) destroys the representation of the contralateral hemifield that it provides to visual association cortex in the other occipital lobe. The result is a hemianopic deficit that splits the macula precisely.
FALSE CLAIMS OF MACULAR SPARING AFTER OCCIPITAL LOBE DESTRUCTION
The preponderance of evidence, summarized in the preceding sections, indicates that no representation of the ipsilateral visual field is present in the primary visual cortex. The conclusion seems inescapable, therefore, that loss of one occipital lobe results in a hemianopia that must split the macula because no back-up representation is available in the other occipital lobe to spare the macula. How can one explain that a roster of distinguished investigators has contended that macular sparing can occur even after complete occipital lobe destruction or surgical lobectomy (Dubois-Poulsen et al. 1952, Förster 1929, Halstead et al. 1940, Huber 1962, Inouye 1909, Juba 1934, Penfield et al. 1935, Wilbrand & Saenger 1917)?
They are mistaken—revealing how even skilled examiners can be fooled by imperfect fixation during perimetry. Teuber and colleagues (1960), in their classic monograph on visual deficits in battle-wounded veterans, dismissed the idea of macular sparing after loss of the occipital lobe. They warned about the problem of impaired fixation during perimetry. Nonetheless, such claims have continued to surface, muddling efforts to explain macular sparing by giving rise to false reports that it is present when it is absent. An example is provided by Huber (1962), who examined 11 patients after neurosurgical occipital lobectomy. Using a Goldmann perimeter, he found macular sparing in every patient. Decades later he tested patients again after occipital lobectomy, but this time fixation was controlled with a scanning laser ophthalmoscope (Bischoff et al. 1995). Under these improved testing conditions, Huber found that no patient demonstrated macular sparing and he retracted his earlier report of macular sparing after lobectomy. Other investigators have also shown that macular sparing in patients after occipital lobectomy is a perimetric artifact (Sugishita et al. 1993).
Inadequate control of fixation explains erroneous reports of macular sparing after complete loss of one occipital lobe. However, it is a mistake to extrapolate from these cases to suggest that macular sparing in all patients is a perimetric artifact (Bischoff et al. 1995, Pambakian & Kennard 1997).
FIXATION INSTABILITY CONFLATED WITH VISUAL FIELD RECOVERY
The pitfall of unstable fixation during visual field testing has confounded efforts to explain macular sparing by giving rise to instances where it is mistakenly thought to be present. It has also led to the development of a new pseudotherapy: visual stimulation to restore lost visual field after occipital lobe damage.
Various investigators have reported that patients with geniculostriate damage can shrink their visual field defect through daily practice detecting targets presented just inside their scotoma border (Cavanaugh & Huxlin 2017, Kasten et al. 1998, Zihl & von Cramon 1982). These claims have been challenged by studies demonstrating that fixation during testing was not controlled properly (Balliet et al. 1985, Mansouri et al. 2018, Pollock et al. 2019). In a noteworthy study, Reinhard and colleagues (2005) used fundus perimetry with a scanning laser ophthalmoscope in patients with hemianopia to map the visual fields before and after visual restitution training. With this technique, stimulus presentation relative to the fovea is tightly monitored, essentially eliminating the problem of unreliable fixation. Under these testing conditions, no improvement in visual fields occurred after vision restoration therapy. Nonetheless, vision restoration therapy continues to be advertised on the internet and sold directly to patients.
After developing a hemianopia, patients start to make adaptive eye movements, called surveillance saccades, toward their blind hemifield (Lanyon & Barton 2013; Meienberg et al. 1981; Pambakian et al. 2000, 2005; Papageorgiou et al. 2012; Trauzettel-Klosinski & Reinhard 1998). This adaptive response contributes to fixation instability (Reinhard et al.2014).It also contributes to fallacious improvement from vision restoration therapy. Putative recovery of visual field is usually concentrated along the vertical meridian (Kasten et al.1998).This result is precisely what one would predict from poor fixation control (Plant 2005).
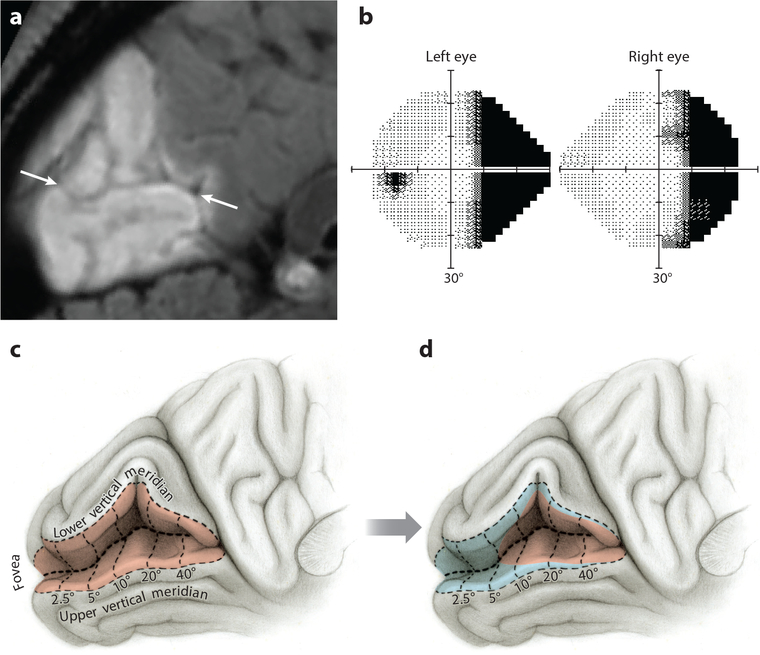
In patients with hemianopia, perimetry often shows sparing of visual field along the vertical meridian, even with no vision therapy. This phenomenon is exemplified by the case of a 52-year old woman after a left posterior cerebral artery stroke (Figure 6a,b). Perimetry showed a zone of intact visual field about 5° wide running along the vertical meridian. If real, this zone would correspond to a swath of viable tissue along the perimeter of V1, increasing exponentially in width toward the foveal representation (Figure 6c,d). Such a pattern of visual field recovery is impossible because (a) occipital cortex is destroyed far beyond V1. Even if metabolically stunned tissue revived within the infarct penumbra, the newly viable tissue would be remote from the V1 border. (b) Putative recovery in V1 would involve centimeters of macular tissue but only millimeters of peripheral tissue. There is no pathological process that could produce such a radical difference in tissue recovery as a function of distance from the foveal representation (Horton et al. 2017, Smirnakis 2016). The simplest explanation for sparing of a zone of constant azimuth along the vertical meridian is inadequate fixation control. This artifact can be confused with macular sparing but of course extends vertically far beyond the macula.
Figure 6.
Hemianopia with recovery along the vertical meridian is unphysiological. (a) A 52-year-old woman with infarction of the left occipital lobe from embolic occlusion of the posterior cerebral artery shown in a sagittal T2 FLAIR magnetic resonance (MR) image. Arrows mark calcarine fissure. (b) Humphrey 24–2 threshold tests performed 10 weeks later showing right homonymous hemianopia, with a strip of apparently intact right visual field approximately 5° in width along the vertical meridian. (c) Opened calcarine fissure showing the visual field map in left primary visual cortex (red shading). The vertical meridian is represented along the V1–V2 border; numbers denote degrees from the fovea. (d) Extent of cortical recovery (blue shading) required to produce the restored field along the vertical meridian mapped in panel b. There is no biological basis for such an eccentricity-dependent gradient in tissue recovery.
Testing multiple points near the vertical meridian simultaneously has been suggested as a way to differentiate between true macular sparing and wandering fixation (Coyle & Milam 1977).This strategy is not available when testing is done by computerized perimetry. In the clinic, automated instruments have replaced manual perimeters because they allow one to obtain accurate measurements of threshold sensitivity in just minutes. The current generation of devices has only a primitive capacity for fixation monitoring. Two approaches are used: eye tracking and blind spot monitoring. Unfortunately, automated perimeters are equipped with relatively crude eye trackers and no auditory feedback is provided to warn patients when their eyes have strayed from the fixation target. Blind spot monitoring misses small eye movements and is especially problematic in hemianopia because the entire temporal field is blind. Most importantly, automated perimeters provide only a global assessment of fixation performance. They provide no information about each point in the visual field and the position of fixation at the instant when that point was tested. Consequently, automated perimetry often fails to determine accurately if macular sparing is truly present and frequently renders unreliable data that feed spurious claims of improvement from restoration therapy (Wall & Schiefer 2018).
An old fashioned bedside technique—confrontation testing—remains among the best means to determine if a hemianopia splits the macula. The patient is asked to fixate a point target, such as a pen tip, about 57 cm away while the other eye is covered securely. Another small target, such as a red dot on a transparent stick, is brought in slowly from the blind hemifield along the tangent plane. The examiner watches assiduously the patient’s fixating eye, to detect and scold any surveillance saccades. If the red dot reaches the point target without detection on even a single trial, the hemianopia is macular splitting. If detected sooner, each centimeter from the target equals 1° of macular sparing.
VARIABILITY IN THE SIZE, LOCATION, AND PERFUSION OF PRIMARY VISUAL CORTEX
Putting aside false reports stemming from flawed testing, genuine macular sparing occurs in many patients after posterior cerebral artery infarction (Holmes & Lister 1916, Leff 2004, Traquair 1925). The prevalence is unclear because testing conditions, patient populations, and criteria for macular sparing differ across studies. Pessin and colleagues (1987) reported macular sparing in 11 out of 13 patients. Zhang and colleagues (2006) found macular sparing in a much lower percentage of patients. Whatever the true prevalence of macular sparing, its inconstancy suggests that the mechanism depends on anatomical properties that are highly variable from one person to another.
In humans the surface area of the primary visual cortex varies by twofold or more (Adams et al. 2007, Andrews et al. 1997, Dougherty et al. 2003, Filimonoff 1932). Moreover, the location of the primary visual cortex within the calcarine cortex also varies widely. Stensaas and colleagues (1974) sliced autopsy brains from 52 subjects to scout the best implantation sites for a neural prosthesis. They found that V1 surface area ranged from 1,284 to 3,702 mm2. In most subjects, striate cortex extended a variable distance onto the lateral convexity (Figure 7a,b). In others, it did not reach the occipital tip but remained nestled entirely within the medial face of the hemisphere, as shown previously (Brodmann 1918) (Figure 7c,d). In an era of racist pseudoscience, a medial location was proposed to represent the final stage of evolution from a simian state to the brain of Modern Man. Less advanced races were imagined to have more cortex exposed on the lateral surface, like monkeys (for discussion, see Polyak 1957, pp. 467–86).
Figure 7.
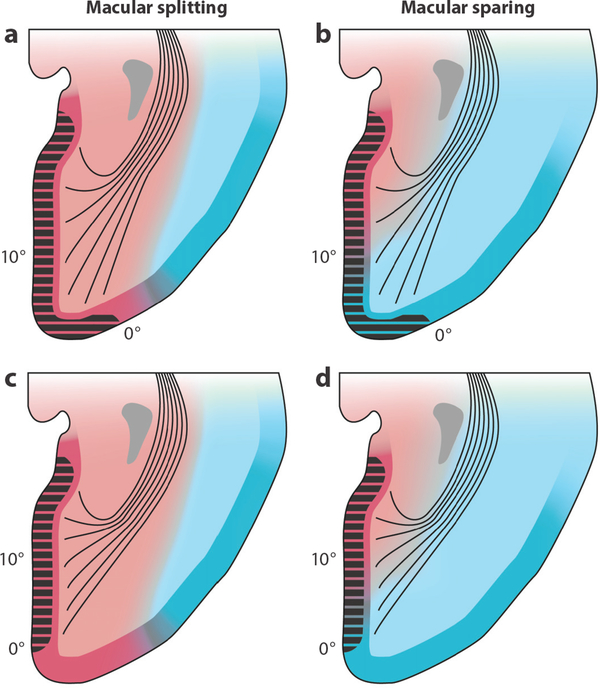
Macular splitting versus macular sparing explained by variation in V1 size and location, coupled with variation in the boundary between posterior and middle cerebral artery perfusion territories. (a,c) Macular splitting occurs if posterior cerebral artery perfusion (red) is extensive, regardless of the size of V1 (black hatching). (b,d) Macular sparing occurs if the middle cerebral artery (blue) supplies posterior V1, where the macula is represented. The precise amount of macular sparing depends on the size of V1 and the location of the frontier between the two circulations but never exceeds 10°.
In a classic study, Beevor (1909) identified the vascular territories of the great cerebral arteries by injecting them with different colored gelatins in 87 autopsy brains. Perfusion of the primary visual cortex was highly variable. The posterior cerebral artery supplied the medial surface of the occipital lobe but failed to reach beyond the pole in 25% of specimens. The middle cerebral artery territory extended posteriorly to the occipital pole, or within a half inch of it, in 40% of cases. To underscore the variability of perfusion territories, Beevor published schematic diagrams showing maximal and minimal distributions for each vessel. He concluded that “in the majority of cases the supply to the calcarine cortex can be easily supplemented by the middle cerebral artery” (Beevor 1909, p. 38).
This conclusion was challenged by Smith & Richardson (1966), who found branches of the middle cerebral artery supplying striate cortex in only 2 out of 32 autopsy specimens. Their study was deficient, however, because arterial territories were not labeled using an injection technique. Instead, vessels were cleaned and examined with a dissector’s loupe to identify terminal twigs. Using this crude approach they failed to appreciate the extent of middle cerebral artery collateral supply to the primary visual cortex derived from fine surface vessels. In an excellent review, Brozici and colleagues (2003) summarized a century of anatomical studies that provide convincing evidence of extensive leptomeningeal anastomoses between major vascular territories formed by pial arterioles. As a result of this rich collateral network, the borders of vascular territories overlap considerably (Adams et al. 2015, Horton & Adams 2018, van der Zwan et al. 1992). Conventional diagrams are misleading because they depict sharp borders dividing the occipital lobe into separate zones supplied exclusively by individual arterial vessels (Margolis et al. 1971, Marinkovic et al. 1987, Smith & Richardson 1966, Zeal & Rhoton 1978). They fail to capture the overlap, variability, and dynamic shifts that occur in cortical perfusion.
The overlap in perfusion at the boundaries of territories served by major trunk vessels has important consequences in stroke. If the posterior cerebral artery is occluded suddenly, then the resulting local blood pressure drop creates a gradient favoring flow from the middle cerebral artery. The middle cerebral artery can expand its perfusion into occipital lobe tissue that normally would be supplied by, or at least shared with, the blocked posterior cerebral artery. This phenomenon means that the boundaries between vascular territories defined by arteriography in healthy subjects can shift to some extent in patients after flow is compromised by occlusion of one vessel (Symon 1961).
NEUROIMAGING OF MACULAR SPARING AND SPLITTING
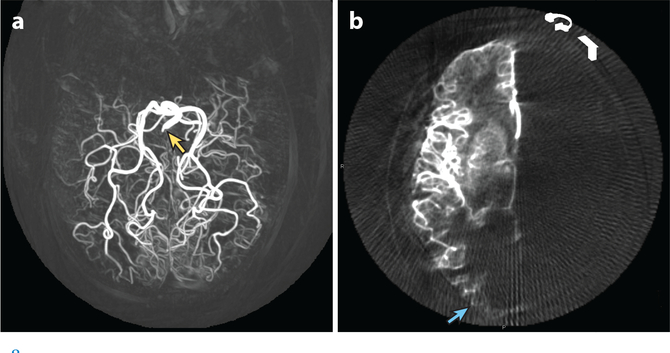
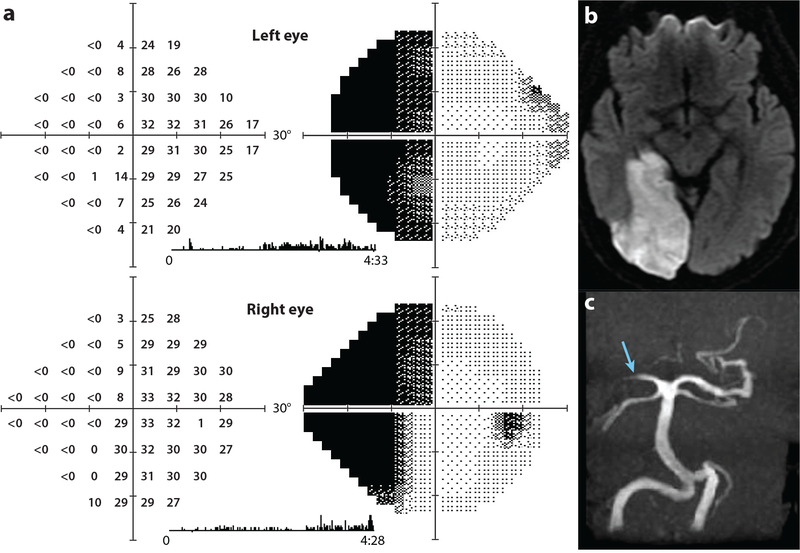
There is variability both in the location of the primary visual cortex and in the middle cerebral artery contribution to perfusion of the occipital lobe. As a result, the amount of primary visual cortex that survives after occlusion of the posterior cerebral artery varies widely. Figure 8a shows an angiogram from a patient whose posterior cerebral artery supplies the lateral convexity of the occipital lobe, as shown in Figure 7a,c. After occlusion of the posterior cerebral artery, one would predict complete infarction of striate cortex, producing a macular splitting hemianopia. Figure 9 shows such a case, a 62-year-old man with atrial fibrillation who developed sudden vision loss. He had an embolic right posterior cerebral artery infarct extending around the occipital pole onto the lateral convexity, causing a left hemianopia. There appeared to be partial sparing of visual field along the vertical meridian. However, by confrontation testing, his hemianopia split the macula, as one would expect from extensive infarction of the occipital pole. Therefore, the sparing of visual field along the vertical meridian must have been due to surveillance saccades.
Figure 8.
3D digital subtraction angiogram reconstructions showing variation in perfusion of the occipital lobe by the posterior cerebral artery versus middle cerebral artery. (a) Maximum intensity projection after contrast agent injection into the basilar artery (yellow arrow). The posterior cerebral artery supplies tissue on the lateral convexity and, thus, all striate cortex. (b) A different patient, after injection into the right internal carotid artery, showing middle cerebral artery supply to the occipital pole (blue arrow), potentially supplying the macular representation. The right posterior cerebral artery territory is not filled, owing to congenital absence of the right posterior communicating artery. Images courtesy of Matthew Amans, M.D.
Figure 9.
Left hemianopia with macular splitting. (a) A 62-year-old man with left homonymous hemianopia tested by automated perimetry 4 months after stroke; thresholds measured in decibels (0 = 105 apostilbs) and corresponding grayscale representations are shown. The patient saw some stimuli along the vertical meridian on the blind side by sneaking saccades to the left. These saccades are captured by the eye monitor trace as vertical tic marks during the 4.5 minutes of testing. By confrontation testing the hemianopia was macular splitting. (b) Axial diffusion–weighted image showing acute right posterior cerebral artery infarction that encompassed the entire V1 by extending onto the lateral convexity of the occipital lobe. (c) Magnetic resonance (MR) angiogram showing occlusion of the right posterior cerebral artery (blue arrow).
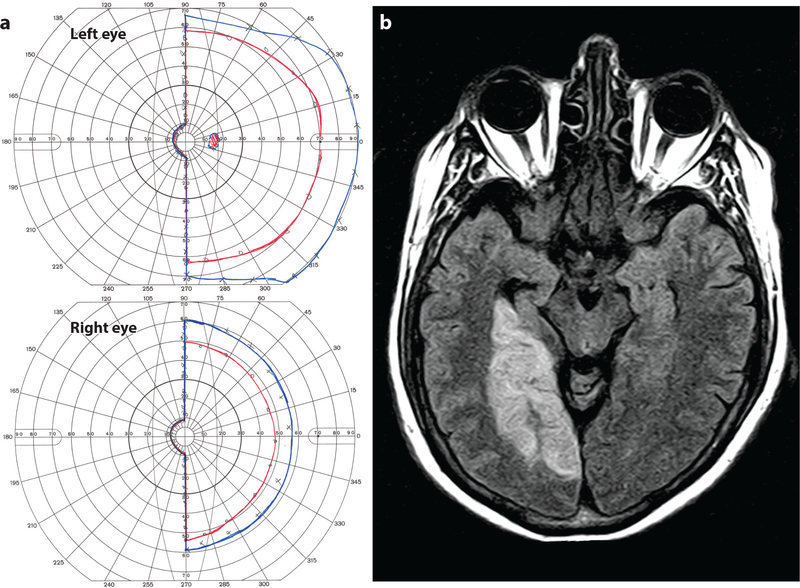
Figure 8b illustrates a subject whose middle cerebral artery supplies the occipital pole via a branch called the temporo-occipital artery. Occlusion of the posterior cerebral artery would not be expected to infarct the entire primary visual cortex. Tissue at the occipital pole, ranging between the extremes diagrammed in Figure 7b,d, would likely escape damage, producing a macular sparing hemianopia. Figure 10a shows an MR image from a 68-year-old man who suffered a right posterior cerebral artery occlusion. The occipital pole was preserved due to perfusion via the terminal branches of the middle cerebral artery. Accordingly, there was a left homonymous hemianopia with macular sparing (Figure 10b).
Figure 10.
Left hemianopia with macular sparing. (a) A 68-year-old man with left homonymous hemianopia and macular sparing; the I4e (red) and V4e (blue) isopters, mapped by Goldmann perimetry, are shown. (b) Axial FLAIR magnetic resonance (MR) image showing right posterior cerebral artery territory infarct with preservation of the occipital pole, presumably due to collateral flow from the middle cerebral artery.
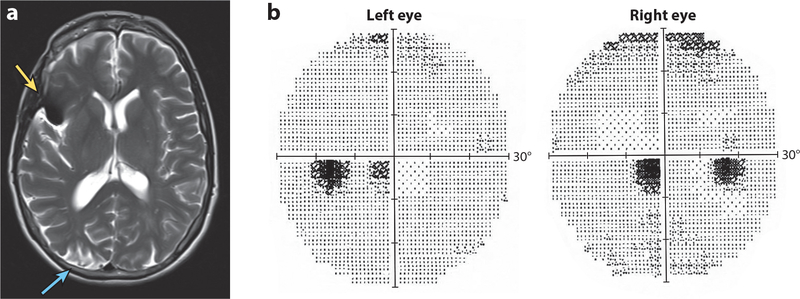
Just as the blood supply from the middle cerebral artery can spare the macula, its loss can blind it. Figure 11a shows an MR image from a 52-year-old woman who underwent clipping of a proximal right middle cerebral artery aneurysm. Her surgery was complicated by infarction of the right occipital pole. After the operation the patient complained of a central visual defect. Visual field testing revealed a left hemimacular scotoma (Figure 11b). This case provides direct evidence that, in some individuals, the macular representation at the occipital pole is supplied by the middle cerebral artery.
Figure 11.
Macular scotoma from impaired middle cerebral artery flow to the macular representation in V1. (a) T2 axial magnetic resonance (MR) image showing artifact (yellow arrow) from a clip placed on a middle cerebral artery aneurysm in a 52-year-old woman. There is a fresh postoperative infarct (blue arrow) from prolonged interruption during clipping of the middle cerebral artery supply to the right occipital pole. (b) Automated visual fields showing a left hemimacular scotoma from the stroke in panel a. This scenario is described in Figure 5c (Horton & Adams 2018).
The findings illustrated in these three cases (Figures 9–11) are consistent with numerous neuroimaging studies showing macular sparing when the occipital pole is preserved or, conversely, macular splitting when the occipital pole is lost (Gray et al. 1997, Kamyar & Trobe 2009, McAuley & Russell 1979, McFadzean et al. 1994, Ogawa et al. 2014, Sugishita et al. 1993, Tanaka et al. 1992, Wong & Sharpe 1999). One might wonder why sparing of central vision is always confined to the macula, given the vast range among patients in the amount of primary visual cortex that remains viable after posterior cerebral artery occlusion. The explanation lies with the macula’s enormous representation in striate cortex (Horton & Hoyt 1991). It fills the entire posterior half of the occipital lobe—the very territory that is potentially salvageable by collateral flow from the middle cerebral artery. Regardless of how much tissue is rescued by the middle cerebral artery, it is always tissue that represents the macula, and never more. The result is a stereotypical phenomenon: macular sparing.
PROOF OF THE MECHANISM OF MACULAR SPARING
The evidence indicates that macular sparing occurs because the representation of the macula in primary visual cortex survives occlusion of the posterior cerebral artery, thanks to collateral flow from the middle cerebral artery. However, the evidence summarized in this review is circumstantial. What would constitute direct proof?
One could perform autopsies in patients with a history of stroke sparing the macula. If striate cortex were preserved at the occipital pole and filled by dye injection into the middle cerebral artery, then the mechanism would be indisputable. Unfortunately, such a study would be difficult to carry out.
In stroke patients, one could map intact striate cortex at the occipital pole using MR imaging. The stria of Gennari can be identified with structural MR imaging, but only with difficulty (Clark et al. 1992, Trampel et al. 2019). fMRI would offer a better approach. After visual field mapping and fMRI, one would then perform cerebral angiography to determine which vessels perfuse the remaining viable striate tissue. This step is critical but problematic. Non-invasive MR and computed tomography (CT) angiography lack sufficient resolution to identify reliably the distal branches of the posterior and middle cerebral arteries. Selective arterial catheterization is necessary to obtain images of sufficient resolution. However, there is seldom a clinical justification to perform invasive angiography in such patients. To treat acute hemianopia, thrombolytic agents are delivered via a peripheral intravenous route (Strbian et al. 2012). It is unusual to perform catheter-based cerebral arteriography in this setting.
These considerations explain in part why direct verification of the mechanism of macular sparing remains elusive. It is clear that macular sparing occurs when striate cortex located in the posterior half of the occipital lobe escapes damage after posterior cerebral artery occlusion. It is less certain in what percentage of such cases the collateral perfusion is derived from the middle cerebral artery. The alternative is that, in some patients, a distal embolus blocking only the calcarine artery (rather than a more proximal embolus in the posterior cerebral artery) produces a hemianopia, with other branches of the posterior cerebral artery continuing to supply the occipital pole (Marinkovic et al. 1987). Given that the organization of the cortical blood supply is highly variable, this mechanism of macular sparing probably does occur in some patients. However, most patients with a macular sparing hemianopia have an occipital infarct involving the territory of the posterior cerebral artery, not just the calcarine artery, so blood flow preserving the occipital pole must come from a different source than the posterior cerebral artery.
SIGNIFICANCE OF MACULAR SPARING
Prior to the invention of CT imaging, macular sparing was highly valued as a localizing sign in clinical neurology because it suggested an occipital stroke (Traquair 1925). Any patient with an isolated, sudden, painless onset of a congruous hemianopia with macular sparing was likely to have a posterior cerebral artery embolus because macular sparing is inherent to the occipital lobe’s dual blood supply.
Of course, macular sparing can occur from many other lesions besides stroke. For example, in almost every soldier, Inouye found macular sparing. Why was macular splitting so rare? Soldiers wounded by bullets that smashed through the occiput, where the macula is represented, usually exsanguinated on the battlefield from rupture of the dural venous sinuses. Those few soldiers who survived extensive occipital lobe damage, including loss of the occipital pole, managed to mislead Inouye through unstable fixation during perimetry.
Any process that injures the cortex and happens to remain confined to the anterior calcarine fissure will produce macular sparing. We have encountered it from brain tumor, arteriovenous malformation, abscess, neurosurgical resection, and so on. In such cases, macular sparing occurs by chance, rather than via a mechanism built into the anatomy of the occipital lobe’s blood supply.
Macular sparing can also occur from lesions of the optic radiations (Pambakian & Kennard 1997, Zhang et al. 2006). However, such cases are frequently misclassified, either because fixation is unstable during perimetry or because the hemianopia partially spares one quadrant. True macular sparing must subtend less than 10° and have absolute, steep, congruous borders that sharply intersect the upper and lower vertical meridian.
For physicians, macular sparing has become moot as a diagnostic finding because it is now standard practice for all patients to undergo CT or MR imaging to define the site and cause of their homonymous hemianopia.
For patients, macular sparing remains highly significant because it dramatically mitigates the functional toll of hemianopia. The retina contains two divisions: (a) a low-resolution periphery that alerts subjects to the location of an object worthy of a saccade and (b) a high-resolution macula used for actually looking at the object (Rosenholtz 2016). If macular vision is spared, then the visual scene remains readily comprehensible because the central area of conscious regard is intact.
During scrutiny of a natural image, most saccades executed by subjects are under 4° in amplitude (Foulsham et al. 2011). Sparing of the macula means that the next target for a saccade usually is visible to the subject, making exploration of the visual scene more efficient and disruption of the scan path less severe (Reinhard et al.2014).Stability of fixation is also better (Trauzettel-Klosinski & Reinhard 1998). Although the ability to localize peripheral targets on one side is impaired, this deficit is tolerable and indeed sometimes goes unnoticed, especially when macular sparing is large. For patients, often the biggest handicap is that driving is no longer safe or permissible. Otherwise, they adapt relatively well.
In contrast, a macular splitting hemianopia is devastating to patients. The difference between macular sparing and splitting is most evident during reading (Figure 12; see Supplemental Videos 1 and 2). If macular function is intact, then reading speed and number of characters per saccade can remain nearly normal (Leff et al.2000).When the macula is split, reading performance declines drastically. Right hemianopia has a more severe impact than left hemianopia because parafoveal vision is needed to plan the next saccade (Trauzettel-Klosinski & Brendler 1998). With left hemianopia, there is greater difficulty finding the beginning of the next line. With macular splitting on either side, half the text surrounding the fixation point is missing, and consequently, the effortless joy of reading becomes transformed into a labored chore.
Figure 12.
Impact of macular sparing versus splitting on reading. (Top) Scan path from a patient with a left hemianopia and 3–4° of macular sparing. He read 366 words per minute, advancing 8.6 characters (including spaces) per saccade. The scan path is shown in Supplemental Video 1. (Bottom) Scan path from the patient illustrated in Figure 9 with a left hemianopia and macular splitting. He read 193 words per minute, advancing 4.7 characters (including spaces) per saccade. He tended to overshoot the left text border. Mean fixation durations were similar (217 ms versus 195 ms). The scan path is shown in Supplemental Video 2.
Supplementary Material
SUMMARY POINTS.
Macular sparing refers to preservation of visual function within a central zone ranging up to 10° in patients with homonymous hemianopia.
Since the discovery of macular sparing, the mechanism behind it has been the subject of debate.
Two major theories have been advanced to explain macular sparing. The first theory proposes a bilateral representation of the macula. After loss of one occipital lobe, a backup copy of the macular representation is available in the remaining occipital lobe to sustain function.
The second theory proposes that macular sparing is due to perfusion of the occipital pole, where the macula is represented, by distal branches of the middle cerebral artery. After occlusion of the posterior cerebral artery, which supplies most of striate cortex, macular cortex remains intact, and thus, central function is preserved.
The second theory was rejected by early investigators because it failed to explain macular sparing observed in subjects after complete loss of one occipital lobe.
More recent studies have shown that macular sparing after complete loss of one occipital lobe is fallacious and due to inadequate control of fixation during visual field testing.
The problem of inadequate control of fixation during testing also accounts for erroneous reports that visual training can induce visual field recovery following cortical stroke or injury.
The perfusion of the occipital lobe is highly variable, as are the size and location of the primary visual cortex. In many individuals, branches of the middle cerebral artery supply primary visual cortex at the occipital pole. Because of its high magnification, the macular representation fills the entire occipital pole, and thus, only the macula is spared after posterior cerebral artery occlusion.
Macular sparing can occur from many different causes, but stroke is the only etiology that occurs frequently and stereotypically by virtue of how the blood supply to the primary visual cortex is organized.
Sudden onset of homonymous hemianopia with macular sparing was an important clinical sign in neurology before the advent of modern neuroimaging because it suggested an acute posterior cerebral artery stroke. Now, clinicians routinely obtain neuroimaging in all patients with hemianopia, so macular sparing is of minor diagnostic importance.
Macular sparing allows patients to read nearly normally, which reduces the functional toll of homonymous hemianopia.
ACKNOWLEDGMENTS
This work was supported by grants EY029703 (to J.C.H.) and EY02162 (to the Beckman Vision Center) from the National Eye Institute and by an unrestricted grant from Research to Prevent Blindness.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Footnotes
Errata
An online log of corrections to Annual Review of Vision Science articles may be found at http://www.annualreviews.org/errata/vision
LITERATURE CITED
- Adams DL, Piserchia V, Economides JR, Horton JC. 2015. Vascular supply of the cerebral cortex is specialized for cell layers but not columns. Cereb. Cortex 25:3673–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DL, Sincich LC, Horton JC. 2007. Complete pattern of ocular dominance columns in human primary visual cortex. J. Neurosci. 27:10391–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews TJ, Halpern SD, Purves D. 1997. Correlated size variations in human visual cortex, lateral geniculate nucleus, and optic tract. J. Neurosci. 17:2859–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci A, Bijanzadeh M, Nurminen L, Federer F, Merlin S, Bressloff PC. 2017. Circuits and mechanisms for surround modulation in visual cortex. Annu. Rev. Neurosci. 40:425–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balliet R, Blood KM, Bach-y-Rita P. 1985. Visual field rehabilitation in the cortically blind? J. Neurol. Neurosurg. Psychiatry 48:1113–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevor CE. 1909. On the distribution of the different arteries supplying the human brain. Philos. Trans. R. Soc. Lond. B 200:1–55 [Google Scholar]
- Bischoff P, Lang J, Huber A. 1995. Macular sparing as a perimetric artifact. Am. J. Ophthalmol. 119:72–80 [DOI] [PubMed] [Google Scholar]
- Brewer AA, Press WA, Logothetis NK, Wandell BA. 2002. Visual areas in macaque cortex measured using functional magnetic resonance imaging. J. Neurosci. 22:10416–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. 2011. Distinct mechanisms for size tuning in primate visual cortex. J. Neurosci. 31:12644–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K 1918. Individuelle Variationen der Sehsphäre und ihr Bedeutung für die Klinik der Hinterhauptschüsse. Allgz. Psychiat. 74:564–68 [Google Scholar]
- Brozici M, van der Zwan A, Hillen B. 2003. Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke 34:2750–62 [DOI] [PubMed] [Google Scholar]
- Cavanaugh MR, Huxlin KR. 2017. Visual discrimination training improves Humphrey perimetry in chronic cortically induced blindness. Neurology 88:1856–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Waggoner RA, Tanaka K. 2001. Human ocular dominance columns as revealed by high-field functional magnetic resonance imaging. Neuron 32:359–74 [DOI] [PubMed] [Google Scholar]
- Cherici C, Kuang X, Poletti M, Rucci M. 2012. Precision of sustained fixation in trained and untrained observers. J. Vis. 12(6):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Courchesne E, Grafe M. 1992. In vivo myeloarchitectonic analysis of human striate and extra striate cortex using magnetic resonance imaging. Cereb. Cortex 2:417–24 [DOI] [PubMed] [Google Scholar]
- Cowey A 1964. Projection of the retina on to striate and prestriate cortex in the squirrel monkey, Saimiri sciureus. J. Neurophysiol. 27:366–93 [DOI] [PubMed] [Google Scholar]
- Coyle JT, Milam DF Jr. 1977. Tangent screen perimetry using twin test objects. Am. J. Ophthalmol. 83:923–24 [DOI] [PubMed] [Google Scholar]
- Daniel PM, Whitteridge D. 1961. The representation of the visual field on the cerebral cortex in monkeys. J. Physiol. 159:203–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, et al. 1996. Mapping striate and extrastriate visual areas in human cerebral cortex. PNAS 93:2382–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, Wandell BA. 2003. Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J. Vis. 3(10):586–98 [DOI] [PubMed] [Google Scholar]
- Dow BM, Vautin RG, Bauer R. 1985. The mapping of visual space onto foveal striate cortex in the macaque monkey. J. Neurosci. 5:890–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Poulsen A, Magis C, de Ajuriaguerra J, Hecaen H. 1952. Les consequences visuelles de la lobectomie occipital chez l’homme. Ann. Ocul. 185:305–47 [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. 1997. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb. Cortex 7:181–92 [DOI] [PubMed] [Google Scholar]
- Filimonoff I 1932. Über die Variabilität der Grosshirnrindenstruktur. Regio occipitalis beim erwachsenen Menschen. J. Psychol. Neurol. 44:1–96 [Google Scholar]
- Förster O 1890. Ueber Rindenblindheit. Arch. Ophthalmol. 36:94–108 [Google Scholar]
- Förster O 1929. Beiträge zur Pathophysiologie der Sehbahn und der Sehsphäre. J. Psychol. Neurol. 39:463–85 [Google Scholar]
- Förster R 1867. Über Gesichtsfeldmessungen. Klin. Monatsbl. Augenh. 5:293 [Google Scholar]
- Foulsham T,Teszka R,Kingstone A.2011.Saccade control in natural images is shaped by the information visible at fixation: evidence from asymmetric gaze-contingent windows. Atten. Percept. Psychophysiol. 73:266–83 [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Sawai H, Watanabe M, Wakakuwa K, Morigiwa K. 1989. Nasotemporal overlap of crossed and uncrossed retinal ganglion cell projections in the Japanese monkey (Macaca fuscata). J. Neurosci. 9:2353–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibaldi A, Benson NC, Banks MS. 2021. Crossed–uncrossed projections from primate retina are adapted to disparities of natural scenes. PNAS 118:e2015651118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein M 1988. The discovery of the visual cortex. Sci. Am. 259:118–27 [DOI] [PubMed] [Google Scholar]
- Glickstein M, Whitteridge D. 1987.Tatsuji Inouye and the mapping of the visual fields on the human cerebral cortex. Trends Neurosci. 10:350–53 [Google Scholar]
- Gonzalez EG, Wong AM, Niechwiej-Szwedo E, Tarita-Nistor L, Steinbach MJ. 2012. Eye position stability in amblyopia and in normal binocular vision. Investig. Ophthalmol. Vis. Sci. 53:5386–94 [DOI] [PubMed] [Google Scholar]
- Gray LG, Galetta SL, Siegal T, Schatz NJ. 1997. The central visual field in homonymous hemianopia. Evidence for unilateral foveal representation. Arch. Neurol. 54:312–17 [DOI] [PubMed] [Google Scholar]
- Gross CG, Rocha-Miranda CE, Bender DB. 1972. Visual properties of neurons in inferotemporal cortex of the macaque. J. Neurophysiol. 35:96–111 [DOI] [PubMed] [Google Scholar]
- Hallum LE, Movshon JA. 2014. Surround suppression supports second-order feature encoding by macaque V1 and V2 neurons. Vis. Res. 104:24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead WC, Walker AE, Bucy PC. 1940. Sparing and nonsparing of “macular” vision associated with occipital lobectomy in man. Arch. Ophthalmol. 24:948–66 [Google Scholar]
- Henry CA, Joshi S, Xing D, Shapley RM, Hawken MJ. 2013. Functional characterization of the extraclassical receptive field in macaque V1: contrast, orientation, and temporal dynamics. J. Neurosci. 33:6230–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschen SE. 1900. Revue Critique de la Doctrine sur le Centre Cortical de la Vision. Paris: G. Steinheil [Google Scholar]
- Holmes G 1945. The Ferrier Lecture: the organization of the visual cortex in man. Proc. R. Soc. Lond. B 132:348–61 [Google Scholar]
- Holmes G, Lister WT. 1916. Disturbances of vision from cerebral lesions, with special reference to the cortical representation of the macula. Brain 39:34. [PMC free article] [PubMed] [Google Scholar]
- Holmes GM. 1918. Disturbances of vision by cerebral lesions. Br. J. Ophthalmol. 2:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC, Adams DL. 2018. Patterns of cortical visual field defects from embolic stroke explained by the anastomotic organization of vascular microlobules. J. Neuroophthalmol. 38:538–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC, Fahle M, Mulder T, Trauzettel-Klosinski S. 2017. Adaptation, perceptual learning, and plasticity of brain functions. Graefes Arch. Clin. Exp. Ophthalmol. 255:435–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC, Hoyt WF. 1991. The representation of the visual field in human striate cortex: a revision of the classic Holmes map. Arch. Ophthalmol. 109:816–24 [DOI] [PubMed] [Google Scholar]
- Huber A 1962. Homonymous hemianopia after occipital lobectomy. Am. J. Ophthalmol. 54:623–29 [PubMed] [Google Scholar]
- Inouye T 1909. Die Sehstörungen bei Schussverletzungen der kortikalen Sehsphäre nach Beobachtungen an Verwundeten der letzten japanischen Kriege. Leipzig, Ger.: W. Engelmann [Google Scholar]
- Juba A 1934. Die korticale Doppelvertretung der Makula und die Projektion der Sehrinde auf den äusseren Kniehöcker des Menschen. Klin. Monatsbl. Augenh. 93:595 [Google Scholar]
- Kamyar R, Trobe JD. 2009. Bilateral mesial occipital lobe infarction after cardiogenic hypotension induced by electrical shock. J. Neuroophthalmol. 29:107–10 [DOI] [PubMed] [Google Scholar]
- Kasten E, Wust S, Behrens-Baumann W, Sabel BA. 1998. Computer-based training for the treatment of partial blindness. Nat. Med. 4:1083–87 [DOI] [PubMed] [Google Scholar]
- Kölliker A 1899. Neue Beobachtungen zur Anatomie des chiasma opticum.In Beiträge zur Onkologie des Auges, ed. von Michel J, pp. 113–28. Würzburg, Ger.: Phys.-Med. Ges. [Google Scholar]
- Lanyon LJ, Barton JJ. 2013. Visual search and line bisection in hemianopia: computational modelling of cortical compensatory mechanisms and comparison with hemineglect. PLOS ONE 8:e54919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff A 2004. A historical review of the representation of the visual field in primary visual cortex with special reference to the neural mechanisms underlying macular sparing. Brain Lang. 88:268–78 [DOI] [PubMed] [Google Scholar]
- Leff AP, Scott SK, Crewes H, Hodgson TL, Cowey A, et al. 2000. Impaired reading in patients with right hemianopia. Ann. Neurol. 47:171–78 [PubMed] [Google Scholar]
- Leventhal AG, Ault SJ, Vitek DJ. 1988. The nasotemporal division in primate retina: the neural bases of macular sparing and splitting. Science 240:66–67 [DOI] [PubMed] [Google Scholar]
- Mansouri B, Roznik M, Rizzo JF III, Prasad S. 2018. Rehabilitation of visual loss: where we are and where we need to be. J. Neuroophthalmol. 38:223–29 [DOI] [PubMed] [Google Scholar]
- Marchand F 1882. Beitrag zur Kenntniss der homonymen bilateralen Hemianopsie und der Faserkreuzung im Chiasma opticum. Arch. Ophthalmol 28:63 [Google Scholar]
- Margolis MT, Newton TH, Hoyt WF. 1971. Cortical branches of the posterior cerebral artery. Anatomic-radiologic correlation. Neuroradiology 2:127–35 [DOI] [PubMed] [Google Scholar]
- Marinkovic SV, Milisavljevic MM, Lolic-Draganic V, Kovacevic MS. 1987. Distribution of the occipital branches of the posterior cerebral artery. Correlation with occipital lobe infarcts. Stroke 18:728–32 [DOI] [PubMed] [Google Scholar]
- McAuley DL, Russell RW. 1979. Correlation of CAT scan and visual field defects in vascular lesions of the posterior visual pathways. J. Neurol. Neurosurg. Psychiatry 42:298–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadzean R, Brosnahan D, Hadley D, Mutlukan E. 1994. Representation of the visual field in the occipital striate cortex. Br. J. Ophthalmol. 78:185–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meienberg O, Zangemeister WH, Rosenberg M, Hoyt WF, Stark L. 1981. Saccadic eye movement strategies in patients with homonymous hemianopia. Ann. Neurol. 9:537–44 [DOI] [PubMed] [Google Scholar]
- Ogawa K, Ishikawa H, Suzuki Y, Oishi M, Kamei S. 2014. Clinical study of the visual field defects caused by occipital lobe lesions. Cerebrovasc. Dis. 37:102–8 [DOI] [PubMed] [Google Scholar]
- Otero-Millan J, Macknik SL, Martinez-Conde S. 2014. Fixational eye movements and binocular vision. Front. Integr. Neurosci. 8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pambakian A, Currie J, Kennard C. 2005. Rehabilitation strategies for patients with homonymous visual field defects. J. Neuroophthalmol. 25:136–42 [PubMed] [Google Scholar]
- Pambakian AL, Kennard C. 1997. Can visual function be restored in patients with homonymous hemianopia? Br. J. Ophthalmol. 81:324–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pambakian AL, Wooding DS, Patel N, Morland AB, Kennard C, Mannan SK.2000.Scanning the visual world: a study of patients with homonymous hemianopia. J. Neurol. Neurosurg. Psychiatry 69:751–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou E, Hardiess G, Mallot HA, Schiefer U.2012.Gaze patterns predicting successful collision avoidance in patients with homonymous visual field defects. Vis. Res. 65:25–37 [DOI] [PubMed] [Google Scholar]
- Penfield W, Evans JP, MacMillan JA. 1935. Visual pathways in man with particular reference to macular representation. Arch. Neurol. Psychiatry 33:816–34 [Google Scholar]
- Pessin MS, Lathi ES, Cohen MB, Kwan ES, Hedges TRIII, Caplan LR. 1987. Clinical features and mechanism of occipital infarction. Ann. Neurol. 21:290–99 [DOI] [PubMed] [Google Scholar]
- Plant GT. 2005. A work out for hemianopia. Br. J. Ophthalmol. 89:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock A, Hazelton C, Rowe FJ, Jonuscheit S, Kernohan A, et al. 2019. Interventions for visual field defects in people with stroke. Cochrane Database Syst. Rev. 5:CD008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak S 1933. A contribution to the cerebral representation of the retina. J. Comp. Neurol. 57:541–67 [Google Scholar]
- Polyak S 1957. The Vertebrate Visual System. Chicago: Univ. Chicago Press [Google Scholar]
- Ramon y Cajal S. 1911. Histologie du Système Nerveux de L’Homme et Des Vertébrés. Paris: A. Maloine [Google Scholar]
- Reinhard J, Schreiber A, Schiefer U, Kasten E, Sabel BA, et al. 2005. Does visual restitution training change absolute homonymous visual field defects? A fundus controlled study. Br. J. Ophthalmol. 89:30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard J, Trauzettel-Klosinski S. 2003. Nasotemporal overlap of retinal ganglion cells in humans: a functional study. Investig. Ophthalmol. Vis. Sci. 44:1568–72 [DOI] [PubMed] [Google Scholar]
- Reinhard JI, Damm I, Ivanov IV, Trauzettel-Klosinski S. 2014. Eye movements during saccadic and fixation tasks in patients with homonymous hemianopia. J. Neuroophthalmol. 34:354–61 [DOI] [PubMed] [Google Scholar]
- Rosenholtz R 2016. Capabilities and limitations of peripheral vision. Annu. Rev. Vis. Sci. 2:437–57 [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, et al. 1995. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268:889–93 [DOI] [PubMed] [Google Scholar]
- Smirnakis SM. 2016. Probing human visual deficits with functional magnetic resonance imaging. Annu. Rev. Vis. Sci. 2:171–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CG, Richardson WF. 1966. The course and distribution of the arteries supplying the visual (striate) cortex. Am. J. Ophthalmol. 61:1391–96 [DOI] [PubMed] [Google Scholar]
- Snyder JP. 1993. Flattening the Earth: Two Thousand Years of Map Projections. Chicago: Univ. Chicago Press [Google Scholar]
- Stensaas SS, Eddington DK, Dobelle WH. 1974. The topography and variability of the primary visual cortex in man. J. Neurosurg. 40:747–55 [DOI] [PubMed] [Google Scholar]
- Strbian D, Ahmed N, Wahlgren N, Kaste M, Tatlisumak T. 2012. Intravenous thrombolysis in ischemic stroke patients with isolated homonymous hemianopia: analysis of Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR). Stroke 43:2695–98 [DOI] [PubMed] [Google Scholar]
- Sugishita M, Hemmi I, Sakuma I, Beppu H, Shiokawa Y.1993.The problem of macular sparing after unilateral occipital lesions. J. Neurol. 241:1–9 [DOI] [PubMed] [Google Scholar]
- Symon L 1961. Studies of leptomeningeal collateral circulation in Macacus rhesus. J. Physiol. 159:68–86.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taban M, Heller KB, Hsu HY, Sadun AA. 2005. Bifurcating axons account for the increase in axonal population in posterior human optic nerve. Neuro-Ophthalmology 29:109–14 [Google Scholar]
- Talbot SA, Marshall WH. 1941. Physiological studies on neural mechanisms of visual localization and discrimination. Am. J. Ophthalmol. 24:1255–64 [Google Scholar]
- Tanaka R, Miyasaka Y, Yada K, Mukuno K. 1992. Bilateral homonymous hemianopsia due to tentorial herniation, with sparing of central vision: case report. Neurosurgery 31:787–90 [DOI] [PubMed] [Google Scholar]
- Teuber HL, Battersby NS, Bender MF. 1960. Visual Field Defects After Penetrating Missile Wounds of the Brain. Cambridge, MA: Harvard Univ. Press [Google Scholar]
- Tootell RB, Mendola JD,Hadjikhani NK, Liu AK,Dale AM.1998.The representation of the ipsilateral visual field in human cerebral cortex. PNAS 95:818–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Switkes E, Silverman MS, Hamilton SL. 1988. Functional anatomy of macaque striate cortex. II. Retinotopic organization. J. Neurosci. 8:1531–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trampel R, Bazin PL, Pine K, Weiskopf N. 2019. In-vivo magnetic resonance imaging (MRI) of laminae in the human cortex. NeuroImage 197:707–15 [DOI] [PubMed] [Google Scholar]
- Traquair HM. 1925. The special vulnerability of the macular fibers and “sparing of the macula.” Br. J. Ophthalmol. 9:53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauzettel-Klosinski S, Brendler K. 1998. Eye movements in reading with hemianopic field defects: the significance of clinical parameters. Graefes Arch. Clin. Exp. Ophthalmol. 236:91–102 [DOI] [PubMed] [Google Scholar]
- Trauzettel-Klosinski S, Reinhard J. 1998. The vertical field border in hemianopia and its significance for fixation and reading. Investig. Ophthalmol. Vis. Sci. 39:2177–86 [PubMed] [Google Scholar]
- van der Zwan A, Hillen B, Tulleken CA, Dujovny M, Dragovic L. 1992. Variability of the territories of the major cerebral arteries. J. Neurosurg. 77:927–40 [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Newsome WT, Maunsell JH. 1984. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vis. Res. 24:429–48 [DOI] [PubMed] [Google Scholar]
- Wall M, Schiefer U. 2018. Reader response: visual discrimination training improves Humphrey perimetry in chronic cortically induced blindness. Neurology 90:436–37 [DOI] [PubMed] [Google Scholar]
- Wandell BA, Winawer J. 2011. Imaging retinotopic maps in the human brain. Vis. Res. 51:718–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbrand H, Saenger A. 1904. Die Neurologie des Auges, Vol. 3 Wiesbaden, Ger.: J. Bergmann [Google Scholar]
- Wilbrand H, Saenger A. 1917. Die homonyme Hemianopsie nebst ihren Beziehungen zu den anderen cerebralen Herderscheinungen, Vol. 7. Wiesbaden, Ger.: J. Bergmann [Google Scholar]
- Wong AM, Sharpe JA. 1999. Representation of the visual field in the human occipital cortex: a magnetic resonance imaging and perimetric correlation. Arch. Ophthalmol. 117:208–17 [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT. 1989. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 12:94–101 [DOI] [PubMed] [Google Scholar]
- Zarella MD, Ts’o DY. 2017. Contextual modulation revealed by optical imaging exhibits figural asymmetry in macaque V1 and V2. Eye Brain 9:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeal AA, Rhoton AL Jr. 1978. Microsurgical anatomy of the posterior cerebral artery. J. Neurosurg. 48:534–59 [DOI] [PubMed] [Google Scholar]
- Zhang X, Kedar S, Lynn MJ, Newman NJ, Biousse V. 2006. Homonymous hemianopias: clinical-anatomic correlations in 904 cases. Neurology 66:906–10 [DOI] [PubMed] [Google Scholar]
- Zihl J, von Cramon D. 1982. Restitution of visual field in patients with damage to the geniculostriate visual pathway. Hum. Neurobiol. 1:5–8 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.