Abstract
A new outbreak of equine Influenza A virus (IAV) was reported in Chile in January 2018, 6 years after its last report in 2012. Equine IAV was detected by rtRT-PCR, followed by virus isolation and full genome sequencing. Genetic characterization of equine IAV classified the virus within clade 1 of the Florida sublineage. Although this is the same sublineage that caused an outbreak in Chile in 2012, the virus has a high similarity to other cocirculating viruses that were recently identified in Europe and Asia. The Chilean 2018 equine influenza (EI) outbreak was caused by an H3N8 strain circulating globally that spread through horse movements.
Keywords: equine influenza virus, IAV, outbreak, phylogeny, South America
1 |. INTRODUCTION
Equine influenza (EI) is an important and highly contagious infectious respiratory disease caused by equine Influenza A virus (IAV) in equids worldwide, with a major impact on the horse industry. The disease is characterized by acute dry cough, fever, nasal discharge, lethargy, and anorexia (Cullinane & Newton, 2013). The infection is caused by the subtypes H7N7 and H3N8 of Influenza A virus. Nowadays, the H7N7 is considered extinct and currently all equine IAV outbreaks worldwide are caused by H3N8 (OIE, 2017a). The H3N8 subtype evolved into two distinct genetic lineages, American and Eurasian. Later, the American lineage diverged into three sublineages South American, Kentucky, and Florida. Currently, the Florida sublineage is predominant and in the early 2000’s it further diverged into two different clades: Florida clade 1 (FC1) and 2 (FC2) (Cullinane & Newton, 2013; Lewis et al., 2011). Additionally, three different antigenic clusters of equine IAV have been described, which are not always related to their phylogenetic clustering (Lewis et al., 2011).
In Chile until 2017, six epizootic events of EI have been reported. The previous outbreaks occurred in 1963, 1977, 1985, 1992, 2006, and 2012. All outbreaks were caused by the H3N8 subtype, except for the 1977 event that was caused by an H7N7 subtype virus (Müller, Pinto, Santibáñez, Celedón, & Valenzuela, 2009; Perglione et al., 2016). On January 10th, 2018 a new epizootic event was reported and officially notified to the Chilean Agricultural and Livestock Services (SAG). The outbreak was first confirmed in Chilean Horses, from Colina, Chacabuco Province, in the northern area of the Metropolitan Region. The aim of this study was to determine the evolutionary origin of the H3N8 epizootic using phylogenetic analyses of whole genome sequences.
2 |. MATERIALS AND METHODS
Data and sample collection: Clinical evaluation and sample collection were performed by equine veterinarians as part of their routine clinical practice. Sampling included Chilean horses, Thoroughbreds, and working horses from vaccinated and nonvaccinated populations. Nasal swabs were obtained using rayon swabs, which were suspended into 2 mL of minimum essential media (MEM) with 0.3% of bovine serum albumin (BSA), trypsin tosyl chlorophenyl ketone (TPCK) and 1% antibiotics (penicillin, streptomycin y amphotericin B) and stored at −80°C until testing.
Official SAG reports of the EI epidemiological situation, data from equine importation in Chile and official World Organization for Animal Health (OIE) reports of EI from neighbouring countries were also compiled to complement the laboratory results.
Diagnostic tests: First, a generic real-time RT-PCR (rtRT-PCR) targeting the M gene of IAV was performed (WHO, 2011). Subsequently, virus isolation from rtRT-PCR-positive samples was attempted, using 10-day-old embryonated hens’ eggs. Briefly, 0.2 ml of rtRT-PCR positive samples was inoculated into the allantoic cavity of embryonated eggs, which were then incubated at 34°C (±1°C) (Quinlivan et al., 2004). After incubation for 72 hr, eggs were euthanized in a CO2 chamber, and chilled at 4°C overnight. The allantoic fluid was harvested and tested by haemagglutination assay using turkey red blood cells and rtRT-PCR to confirm virus isolation. Only one passage was performed, and negative passages were preserved for future studies. The assays were performed at Animal Virology Laboratory, Facultad de Ciencias Veterinarias y Pecuarias, Universidad de Chile, as routine diagnostic tests.
Sequencing: RNA from positive rtRT-PCR samples and isolates were used for whole genome amplification of the IAVs by performing a multisegment RT-PCR for whole genome amplification (Barriga et al., 2016; Zhou et al., 1999). Purified RT-PCR products were sequenced with an Illumina HiSeq 2500 System (Illumina, San Diego, CA, USA). Amplification and DNA purification procedures were performed at Pontificia Universidad Católica de Chile’s Molecular Virology Laboratory and the samples were sequenced by Next Generation Sequencing at the Core Facility at Icahn School of Medicine at Mount Sinai. The same procedure was attempted to obtain full genome sequences collected from earlier equine IAV Chilean outbreaks, including A/equine/Santiago/1/1985 (H3N8), A/equine/Chile/1/1992 (H3N8), A/equine/Lonquen/1/2006 (H3N8), and A/equine/Colina/2/2012 (H3N8). These earlier isolates were provided by Dr. María Orfelia Celedón at Animal Virology Lab, Facultad de Ciencias Veterinarias y Pecuarias, Universidad de Chile.
Genetic Analysis: Phylogenies were reconstructed separately for each segment, and inferred by Bayesian evolutionary analysis. Chi- lean influenza sequences were aligned with relevant reference sequences obtained from NCBI GenBank, and GISAID EpiFlu data-bases using MUSCLE (Edgar, 2004). The reference sequences used for each segment are included in the Supporting Information. Bayesian time divergence estimation using a nucleotide substitution model of HKY+G [4] was used for all segments. For HA and NA segments tree priors: (1) constant coalescent, (2) exponential growth coalescent, and (3) Bayesian skyline coalescent were run and compared using path sampling and stepping-stone sampling marginal likelihood estimation (Baele, Li, Drummond, Suchard, & Lemey, 2013). For the remaining segments, constant coalescent and exponential growth coalescent tree priors were run, and the final prior was selected based on the posterior distribution of the population’s growth parameter (i.e., if the population’s growth parameter included 0 within its 95% highest posterior density (HPD) interval, the tree constructed under coalescent constant population was selected). The analyses were run in BEAST 1.8.4 (Drummond, Suchard, Xie, & Rambaut, 2012). A total of 500,000 iterations were run, sampling every 50,000 trees using the CIPRES platform (Miller, Pfeiffer, & Schwartz, 2012). Traces of the parameters were assessed for convergence with effective sample size (ESS) >200. The maximum clade credibility tree was annotated burning the first 10% of the sampled trees, using TreeAnnotator and were then visualized using Figtree (Rambaut, 2014). Additionally, genetic distances of nucleotide and amino acid sequences were calculated using the Kimura-2 parameters substitution. Alignments were visualized and analysed using MEGA v7.0 software (Kumar, Stecher, & Tamura, 2016).
3 |. RESULTS AND DISCUSSION
One hundred fifty-one horses were sampled from five different locations in Chile within a 500 km distance. Horses showed a range of clinical signs including fever, lethargy, nasal secretion, anorexia, hyperaemic nasal mucosa, and coughing. Twenty-one samples were positive by rtRT-PCR with Ct average of 25.9 (range = 20.6–36.2), from which five isolates were obtained. From them, 13 influenza genomes were sequenced, 10 were obtained directly from clinical samples and three were obtained from viral isolates, which were collected from four different locations. Of these, three genomes were obtained from vaccinated and 10 from nonvaccinated animals. Influenza genomes from 1992 and 2012 outbreaks were also obtained. Sequences were deposited in NCBI Genbank and the accession numbers are included in the Supporting Information.
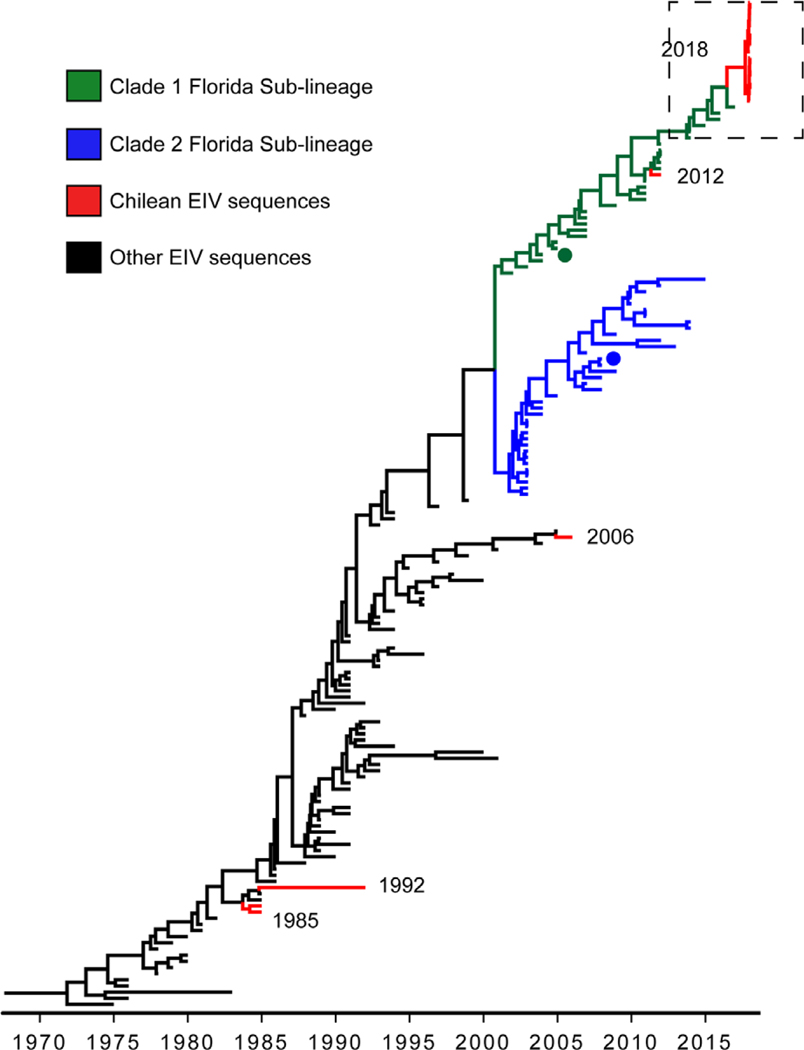
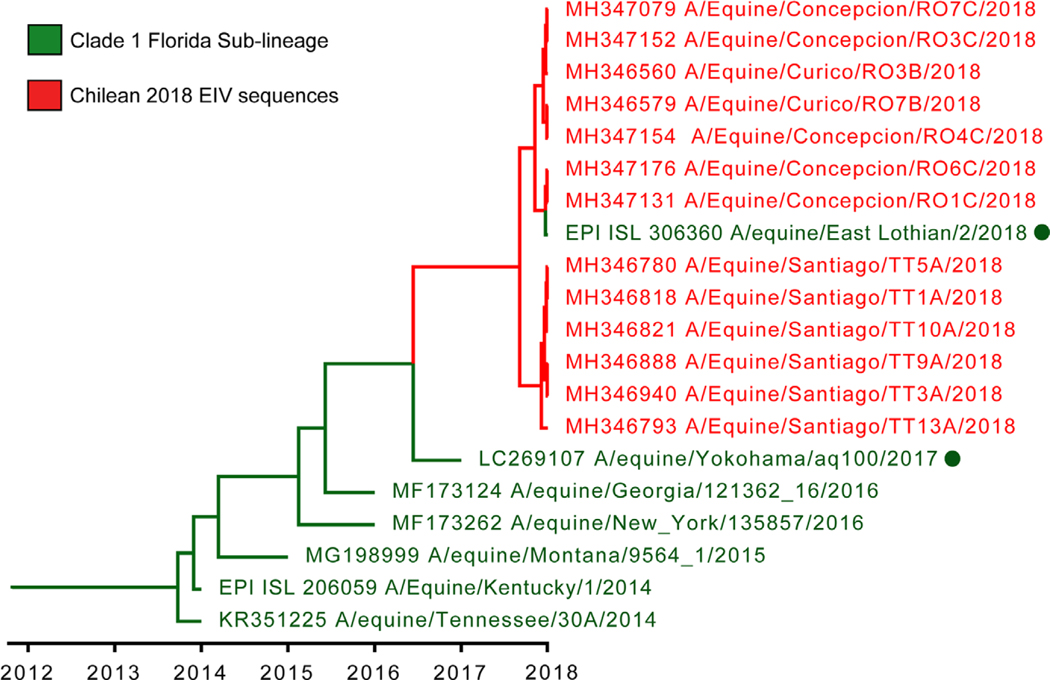
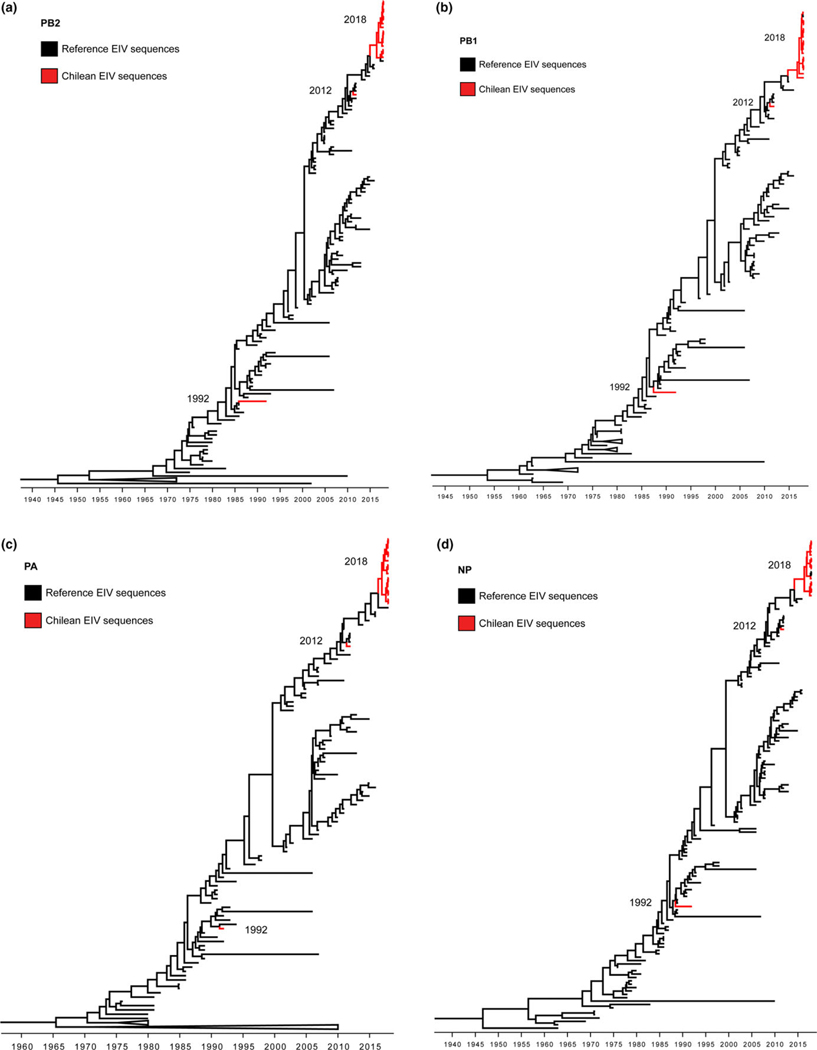
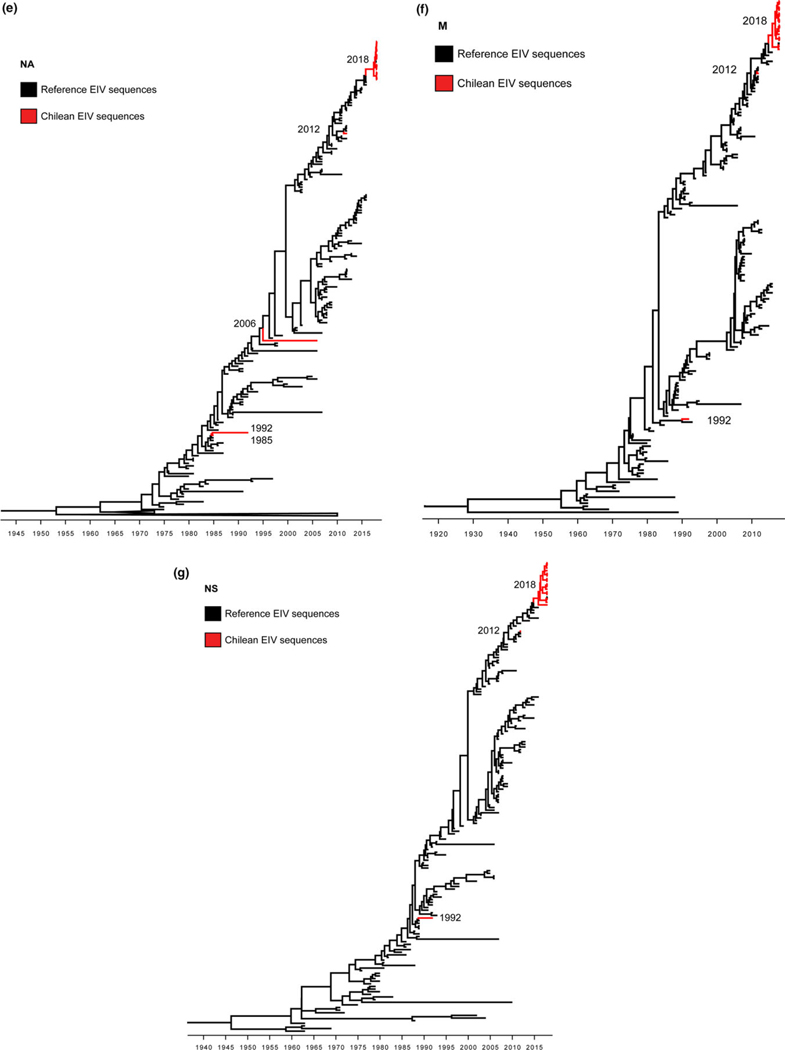
The haemagglutinin (HA) gene phylogeny revealed that Chilean 2018 equine IAVs grouped within FC1 of the American H3N8 linage (Figure 1). These equine IAV strains formed a monophyletic cluster with the strain A/equine/East Lothian/2/2018 (H3N8) (GISAID EpiFlu™ database N° EPI1213182) isolated on February 2018 in East Lothian, UK, from a horse imported recently from the Netherlands. Amino acid and nucleotide sequences of Chilean equine IAVs 2018 and A/equine/East Lothian/2/2018 (H3N8) viruses had ≤100% identity in segments HA and nonstructural (NS), and ≤99.86% in the remaining genes. The closest reference virus to the Chilean equine IAV 2018-East Lothian cluster was A/equine/Yokohama/aq100/2017 (H3N8) (GenBank N° NLC269107) identified in Japan from an imported horse in March 2017. Other close reference sequences identified in the HA phylogeny were collected from the United States between 2014 and 2016 (Figure 2). Inferred time divergence for HA gene, indicated that the time to most recent common ancestor (tMRCA) at 2017 (95% HDP 2017–2018) for the 2018 Chilean equine IAV-East Lothian cluster and 2016 (95% HPD 2015–2017) for the Chilean cluster and the A/equine/Yokohama/aq100/2017(H3N8) virus. The phylogenies of the remaining segments were consistent with the HA phylogeny (Figure 3). Differences between sequences generated from isolates or direct samples, as well as, vaccinated and nonvaccinated animals were not observed.
FIGURE 1.
Maximum clade credibility tree depicting the phylogeny of HA segment of Equine IAV H3 subtype. Florida Sublineage 1 and 2 clades are highlighted in green and blue, respectively and Chilean isolates are highlighted in red. A/equine/Ohio/1/2003-like and A/equine/Richmond/1/2007-like viruses recommended for vaccine preparation are highlighted with dots
FIGURE 2.
Maximum clade credibility tree of HA gene of the Chilean 2018 equine IAV and closest reference sequences. The Chilean equine IAV 2018 belongs to a single monophyletic cluster with A/equine/East Lothian/2/2018 (H3N8). Closely related viruses are highlighted with green dots
FIGURE 3.
Maximum clade credibility trees depicting the phylogeny of PB2 (a), PB1 (b), PA (c), NP (d), NA (e), M (f), and NS (g) segments of Equine Influenza Viruses. Chilean isolates branches are highlighted in red
The HA1 alignment of 2018 Chilean sequences and the reference strain of the FC1 (A/equine/Ohio/01/2003) evidenced nine amino acid substitutions (S6N, G7D, S47P, R62K, N63D, D104N, A138S, N188T, and V223I), one of them in antigenic site B (N188T) and two in antigenic site E (R62K and N63D). Antigenic site B is on the top of HA1. Hence, amino acid changes in this site may modulate the viral antigenicity (Woodward, Rash, Medcalf, Bryant, & Elton, 2015). Additionally, five amino acid substitutions (S6N, S47P, N63D, T78A, and N188T) were identified between 2012 and 2018 Chilean IAV strains (Supporting Information).
The previous EI Chilean outbreak in 2012 was caused by an H3N8 FC1 strain. Although, the equine IAV that caused the Chilean 2018 outbreak is also within the FC1 clade, the inferred phylogenetic relationship with reference sequences indicates that this event corresponds to a new introduction, related to concurrent outbreaks occurring globally in Europe, Asia, and North America. Equine influenza is mostly spread between countries as a consequence of international horse movements (Dominguez, Münstermann, de Guindos, & Timoney, 2016), which explains the circulation of highly similar strains in Chile, the UK, and Japan. In Chile during 2017, 447 horses were imported from 13 different countries, including the United States (40%), Argentina (33%), France (11%), and others (16%). Horses arrived individually or in groups of less than 12 animals, representing 167 entries total and most of them were Thoroughbred horses. Therefore, this virus was likely introduced by imported horses, similar to previous equine IAV introductions in the region (Dominguez et al., 2016; Perglione et al., 2016).
In Chile, the EI outbreak emerged early in 2018, during the Southern hemisphere summer season. The epidemic started in Central Chile, characterized by a Mediterranean climate, with a warm and dry summer (Sarricolea, Herrera-Ossandon, & Meseguer-Ruiz, 2017), climate conditions considered unfavourable for IAV dissemination (Lowen, Mubareka, Steel, & Palese, 2007; Neira et al., 2018). Nonetheless, this equine IAV was rapidly spread across the country. Most likely, transmission was facilitated by the Chilean rodeo qualifiers season, which increases the horses’ movement, and by the lack of vaccination. Although commercial vaccines are available in the country, EI vaccination is mandatory only for horses before exportation. Additionally, the OIE recommends including both clades FC1 and FC2 in vaccine formulations (World Organisation for Animal Health (OIE), 2017b). Three vaccines are registered in Chile (SAG, 2018), and only one vaccine includes FC1 and the remaining strains according to the OIE are considered outdated (OIE, 2017b).
Until March 2018, the virus had been detected in almost all administrative regions of the country. Equine IAV was also detected in Argentina within 3 months of the Chilean index case. In Argentina, EI was first detected at a racetrack in Mendoza, near the border with Chile and it was later detected in other provinces. According to the OIE, the virus involved is an H3N8, FC1 strain (WAHIS, 2018a). Subsequently, in June 2018 the virus also was reported in Uruguay (WAHIS, 2018b). Currently, there are no sequence data available from the 2018 Argentinean and Uruguayan equine IAVs. However, it is plausible that it could have spread from Chile to Argentina as reported in previous outbreaks in the region. In 2012, an equine IAV strain was first introduced into Chile, secondly to Argentina and subsequently to the rest of South America (Perglione et al., 2016).
In conclusion, the 2018 EI epidemic event in Chile was caused by a H3N8 strain of the FC1, contemporaneous to similar viruses circulating globally. The disease was rapidly spread in the country facilitated most likely by horse movement and scarce vaccination. To prevent new equine IAV introductions and spread, vaccination policies should be reviewed at country level, including the implementation of a vaccine update.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the staff of the Instituto de Salud Pública de Chile for biological supplies. We are grateful to Digna Oñate, Rodrigo Tapia, Osmaly Churio, and Felipe Lara for all their support in diagnostic tests and sampling. María Orfelia Celedon provided the historic Equine influenza isolates. We are grateful to the GISAID EpiFlu™ Database, laboratories, and original source of data of equine IAV sequences, especially to the Animal Health Trust, Newmarket, UK, source of the strain A/equine/East Lothian/2/2018. This study was partly funded by the Programa Fondecyt de Iniciación N° 11170877 to V.N; the Programa Beca Doctorado Nacional de CONICYT N° 3344/2016 to J.M; Programa de Investigación Asociativa from the Comisión Nacional de Investigación Científica y Tecnológica, project CONICYT-PIA Anillo ACT 1408 to R.A.M. and V.N. and by the Center for Research in Influenza Pathogenesis (CRIP), a National Institute of Allergy and Infectious Diseases-funded Center of Excellence in Influenza Research and Surveillance (CEIRS), contract number HHSN272201400008C to R.A.M. and H.vB.
Funding information
Beca Doctorado Nacional Fondecyt, Grant/ Award Number: 3344/2016; Programa Fondecyt de Iniciacion, Grant/Award Number: 11170877; Center for Research in Influenza Pathogenesis, Grant/Award Number: HHSN272201400008C; Programa de Investigación Asociativa from the Comisión Nacional de Investigación Científica y Tecnológica, Grant/Award Number: ACT 1408
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- Baele G, Li WLS, Drummond AJ, Suchard MA, & Lemey P. (2013). Accurate model selection of relaxed molecular clocks in bayesian phylogenetics. Molecular Biology and Evolution, 30, 239–243. 10.1093/molbev/mss243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriga GP, Boric-Bargetto D, Cortez-San Martin M, Neira V, van Bakel H, Thompsom M, … Medina RA (2016). Avian influenza virus H5 strain with north American and Eurasian lineage genes in an Antarctic penguin. Emerging Infectious Diseases, 22, 2221–2223. 10.3201/eid2212.161076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinane A, & Newton JR (2013). Equine influenza—A global perspective. Veterinary Microbiology, 167, 205–214. 10.1016/j.vetmic.2013.03.029 [DOI] [PubMed] [Google Scholar]
- Dominguez M, Münstermann S, de Guindos I, & Timoney P. (2016). Equine disease events resulting from international horse movements: Systematic review and lessons learned. Equine Veterinary Journal, 48, 641–653. 10.1111/evj.12523 [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, & Rambaut A. (2012). Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution, 29, 1969–1973. 10.1093/molbev/mss075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC (2004). MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, & Tamura K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, msw054. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis NS, Daly JM, Russell CA, Horton DL, Skepner E, Bryant NA, … Smith DJ (2011). Antigenic and genetic evolution of equine influenza A (H3N8) virus from 1968 to 2007. Journal of Virology, 85, 12742–12749. 10.1128/JVI.05319-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen AC, Mubareka S, Steel J, & Palese P. (2007). Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathogens, 3, 1470–1476. 10.1371/journal.ppat.0030151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, & Schwartz T. (2012). The CIPRES science gateway. p. 1. In: Proc. 1st Conf. Extrem. Sci. Eng. Discov. Environ. Bridg. from Extrem. to campus beyond - XSEDE ‘12. New York, New York, USA: ACM Press. [Google Scholar]
- Müller I, Pinto E, Santibáñez MC, Celedón MO, & Valenzuela PDT (2009). Isolation and characterization of the equine influenza virus causing the 2006 outbreak in Chile. Veterinary Microbiology, 137, 172–177. 10.1016/j.vetmic.2008.12.011 [DOI] [PubMed] [Google Scholar]
- Neira V, Allerson M, Corzo C, Culhane M, Rendahl A, & Torremorell M. (2018). Detection of influenza A virus in aerosols of vaccinated and non-vaccinated pigs in a warm environment. (Huber Victor C, Ed.) PLoS ONE, 13, e0197600. 10.1371/journal.pone.0197600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perglione CO, Gildea S, Rimondi A, Miño S, Vissani A, Carossino M, … Barrandeguy M. (2016). Epidemiological and virological findings during multiple outbreaks of equine influenza in South America in 2012. Influenza and Other Respiratory Viruses, 10, 37–46. 10.1111/irv.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlivan M, Cullinane A, Nelly M, Van Maanen K, Heldens J, & Arkins S. (2004). Comparison of sensitivities of virus isolation, antigen detection, and nucleic acid amplification for detection of equine influenza virus. Journal of Clinical Microbiology, 42, 759–763. 10.1128/JCM.42.2.759-763.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A. (2014). FigTree version 1.4.2 [computer program]. [Google Scholar]
- Sarricolea P, Herrera-Ossandon M, & Meseguer-Ruiz Ó (2017). Climatic regionalisation of continental Chile. Journal of Maps, 13, 66–73. 10.1080/17445647.2016.1259592 [DOI] [Google Scholar]
- Servicio Agricola Ganadero (SAG). (2018). Medicamentos de uso veterinario registrados. Retrieved July 18, 2018 from http://www.sag.gob.cl/content/medicamentos-veterinarios-registrados [Google Scholar]
- WHO. (2011). WHO information for molecular diagnosis of influenza virus in humans - update (pp. 1–38). Geneva, Switzerland: WHO. [Google Scholar]
- Woodward A, Rash AS, Medcalf E, Bryant NA, & Elton DM (2015). Using epidemics to map H3 equine influenza virus determinants of antigenicity. Virology, 481, 187–198. 10.1016/J.VIROL.2015.02.027 [DOI] [PubMed] [Google Scholar]
- World Animal Health Information Database (WAHIS). (2018a). Equine Influenza, Argentina, Final Report. Retrieved July 18, 2018 from http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapEventSummary&reportid=26676 [Google Scholar]
- World Animal Health Information Database (WAHIS). (2018b). Equine Influenza, Uruguay, Follow up report no 3. Retrieved July 18, 2018 from http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapEventSummary&reportid=27112 [Google Scholar]
- World Organisation for Animal Health (OIE). (2017a). WAHIS is modernising: be a partner in the project. In Bulletin 2017–2. Retrieved July 16, 2018 from http://www.oie.int/fileadmin/Home/eng/Publications_%26_Documentation/docs/pdf/bulletin/Bull_2017-2-ENG.pdf [Google Scholar]
- World Organisation for Animal Health (OIE). (2017b). Equine influenza OIE Expert Surveillance Panel on Equine Influenza Vaccine Composition, OIE Headquarters, 22 March 2017 Conclusions and Recommendations. Retrieved July 18, 2018 from http://www.oie.int/scientific-expertise/specific-information-and-recommendations/equine-influenza/ [Google Scholar]
- Zhou NN, Senne DA, Landgraf JS, Swenson SL, Erickson G, Rossow K, … Webster RG (1999). Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. Journal of Virology, 73, 8851–8856. 10.1128/JCM.01549-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.