Abstract
In mammalian taste buds, Type I cells comprise half of all cells. These are termed “glial-like” based on morphologic and molecular features, but there are limited studies describing their function. We tested whether Type I cells sense chemosensory activation of adjacent chemosensory (i.e., Types II and III) taste bud cells, similar to synaptic glia. Using Gad2;;GCaMP3 mice of both sexes, we confirmed by immunostaining that, within taste buds, GCaMP expression is predominantly in Type I cells (with no Type II and ≈28% Type III cells expressing weakly). In dissociated taste buds, GCaMP+ Type I cells responded to bath-applied ATP (10-100 μm) but not to 5-HT (transmitters released by Type II or III cells, respectively). Type I cells also did not respond to taste stimuli (5 μm cycloheximide, 1 mm denatonium). In lingual slice preparations also, Type I cells responded to bath-applied ATP (10-100 μm). However, when taste buds in the slice were stimulated with bitter tastants (cycloheximide, denatonium, quinine), Type I cells responded robustly. Taste-evoked responses of Type I cells in the slice preparation were significantly reduced by desensitizing purinoceptors or by purinoceptor antagonists (suramin, PPADS), and were essentially eliminated by blocking synaptic ATP release (carbenoxolone) or degrading extracellular ATP (apyrase). Thus, taste-evoked release of afferent ATP from type II chemosensory cells, in addition to exciting gustatory afferent fibers, also activates glial-like Type I taste cells. We speculate that Type I cells sense chemosensory activation and that they participate in synaptic signaling, similarly to glial cells at CNS tripartite synapses.
SIGNIFICANCE STATEMENT Most studies of taste buds view the chemosensitive excitable cells that express taste receptors as the sole mediators of taste detection and transmission to the CNS. Type I “glial-like” cells, with their ensheathing morphology, are mostly viewed as responsible for clearing neurotransmitters and as the “glue” holding the taste bud together. In the present study, we demonstrate that, when intact taste buds respond to their natural stimuli, Type I cells sense the activation of the chemosensory cells by detecting the afferent transmitter. Because Type I cells synthesize GABA, a known gliotransmitter, and cognate receptors are present on both presynaptic and postsynaptic elements, Type I cells may participate in GABAergic synaptic transmission in the manner of astrocytes at tripartite synapses.
Keywords: glia, paracrine, purinoceptor, sensory, synaptic transmission
Introduction
Mammalian taste buds are formed from tightly packed, elongate epithelial cells. About half the cells are chemosensory, whereas the other half, termed Type I, are glial-like supportive cells. The chemosensory cells consist of Type II cells, responsible for detecting sweet, bitter, and umami tastants, and Type III cells that detect sour and possibly bitter taste stimuli (Liman et al., 2014; Roper and Chaudhari, 2017; Taruno et al., 2021). Salt detection is believed to be attributed to “Type II-like” and Type III taste bud cells (Chandrashekar et al., 2010; Lewandowski et al., 2016; Roebber et al., 2019; Nomura et al., 2020). Accordingly, Type II taste bud cells express GPCRs selective for sweet, bitter, and umami tastants, which activate a common set of downstream effectors (Chandrashekar et al., 2006). Taste-evoked transduction ultimately results in the secretion of the taste transmitter, ATP, in a unique, nonvesicular manner involving large-pore CAHLM1/3 ion channels (Finger et al., 2005; Y. J. Huang et al., 2007; Ma et al., 2018; Romanov et al., 2018). Type III taste bud cells express proton-selective ion channels to detect sour stimuli, exhibit several neuronal features, including voltage-dependent calcium channels, and secrete the neurotransmitter serotonin (5-HT) (Medler et al., 2003; Y. J. Huang et al., 2007; Tu et al., 2018). Both ATP and 5-HT also exert autocrine and/or paracrine effects in the taste bud (Y. J. Huang et al., 2007; Y. A. Huang et al., 2009).
Few details are known concerning the function of Type I taste bud cells, despite their being the most numerous cells. Type I cells have been termed “glial-like” and possess extensive cytoplasmic lamellae that ensheath Type II and III cells (Pumplin et al., 1997; Yang et al., 2020). These cells express the ectonucleotidase, NTPase2, that breaks down ATP released by Type II cells during taste excitation (Bartel et al., 2006; Vandenbeuch et al., 2013). Type I taste bud cells have also been proposed to help regulate the ionic environment in taste buds, in particular the concentrations of K+ and Cl– (Dvoryanchikov et al., 2009; Cherkashin et al., 2016; Guarascio et al., 2021).
There is growing appreciation that glia in the brain are intimately associated, anatomically and functionally, with synapses. There is bidirectional communication between neurons and surrounding astrocytes at synaptic junctions, including the observation that astrocytes respond to neurotransmitter release by mobilizing intracellular Ca2+. Importantly, glial Ca2+ signals are coordinated with neural signals in time and intensity, including in sensory pathways (Wang et al., 2006; Zhao et al., 2012; Andersen et al., 2016; Leonard et al., 2018). In response to synaptic activity, astrocytes release signaling molecules, gliotransmitters, that may modulate transmission at the synapse (Parpura et al., 2004; Orellana et al., 2011; Araque et al., 2014). The notion of the “tripartite synapse” incorporates astrocytes as a third key player in synaptic function (Halassa et al., 2007; Stevens, 2008).
Inspired by the neuronal-glial interactions at tripartite synapses in the CNS, we asked whether glial-like Type I taste bud cells might cooperate structurally and functionally with surrounding chemosensory cells and afferent fibers, forming a type of “tripartite synapse” in the taste bud. We used Gad2-Cre to express the genetically encoded calcium indicator, GCaMP3, selectively in Type I taste bud cells. Using Ca2+ imaging of isolated taste bud cells and of taste buds embedded in lingual tissue slices, we examined whether Type I cells sense the synaptic signals of neighboring Type II chemosensory cells. Our data show that Type I cells respond secondarily to tastant-evoked synaptic ATP secretion by Type II cells. We speculate that the resulting Ca2+ signals in Type I cells result in secretion of one or more gliotransmitters. These findings are consistent with the hypothesis that Type I cells participate in shaping the sensory output of taste buds.
Materials and Methods
Mice
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Miami and conducted according to the National Institutes of Health's Guide for the care and use of laboratory animals. For functional assays, mice were killed by CO2 asphyxiation followed by cervical dislocation, and lingual slices prepared as described below. For histologic analyses, mice were deeply anesthetized, and then transcardially perfused with PFA.
Gad2-Cre and RCL-GCaMP3 mice were purchased from Jax (strains 010802 and 014538, respectively). We crossed these strains to produce Gad2;;GCaMP3 mice in which GCaMP3 is expressed in Type I taste bud cells after Cre-mediated recombination, as validated below. Mice of both sexes, 2-6 months of age, were used. We observed no significant differences between male and female mice; data were combined from both sexes. Plcb2-GFP mice were as described earlier, with selective expression of GFP only in Type II cells of taste buds (J. W. Kim et al., 2006).
Buffers and reagents
Lingual slices were cut and imaging was conducted in high-Ca-Tyrode's buffer (in mm as follows: 130 NaCl, 5 KCl, 8 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, 10 Na pyruvate, 5 NaHCO3, pH 7.3, 310-330 mOsmol/L). We have found that elevated [Ca2+] increases the stability and reliability of recordings. High-K Tyrode's buffer substituted 50 mm KCl for equimolar NaCl. Regular Tyrode's buffer included 2 mm CaCl2 and 135 mm NaCl. Ca-free Tyrode buffer was as follows (in mm): 135 NaCl, 5 KCl, 10 HEPES, 10 glucose, 10 Na pyruvate, 5 NaHCO3, 2 BAPTA, 2 EGTA, pH 7.3, 310-330 mOsmol/L. All reagents, including taste stimuli and carbenoxolone (C4790), were from Sigma, except PPADS tetrasodium salt (Tocris Bioscience, #0625) and Suramin sodium (Santa Cruz Biotechnology, #200833). Drug solutions were prepared immediately before use. PBS (in mm as follows: 154 NaCl, 1 KH2PO4, 3 Na2HP04) was used for immunostaining.
Immunostaining
Circumvallate papillae were dissected from mice after perfusion-fixation with 4% PFA. Cryosections were permeabilized in 1% Triton for 2 h, blocked for 2-3 h in 10% donkey serum and incubated with primary antibodies overnight at 4°C. After washing in PBS with 0.01% Tween, sections were incubated with secondary antibodies for 1-2 h, as previously described (Dvoryanchikov et al., 2009).
Antibodies were chicken anti-GFP (1:3000; Aves, 1020), rabbit anti-NTPDase2 (1:1000, J. Sévigny, Université Laval, #mN2-36I6TG), rabbit anti-PLCβ2 (1:1000, Santa Cruz Biotechnology, SC206), and goat anti-Car4 (1:500, R&D Systems, AF2414). Secondary antibodies were donkey anti-chicken Alexa488, donkey anti-rabbit Cy3 (Jackson ImmunoResearch Laboratories, 703-545-155 and 711-165-152, respectively), donkey anti-rabbit Alexa594, donkey anti-goat Alexa647 and donkey anti-rabbit Alexa647 (Thermo Fisher Scientific, A-21 207, A-21 447, A-31 573 respectively), all diluted 1:1000.
To visualize afferent fibers in taste buds, we injected AAV.PHP.S::CAG-mScarlet-I into Plcb2-GFP mice as separately detailed (Asencor et al., 2021). At 21 d after injection, mice were perfusion-fixed and cryosections of circumvallate papillae were immunostained for NTPDase2. GFP and mScarlet-I were imaged by intrinsic fluorescence.
Images were captured on a Fluoview Fv1000 laser scanning confocal microscope with a 60× water-immersion objective and digital zoom. Micrographs were minimally corrected to decrease background. We also captured higher-resolution images on a Leica Microsystems STELLARIS/5 confocal microscope fitted with a 63× oil-immersion objective. These latter images were processed using a scaled-gradient-projection method to reassign out-of-focus light to the computed points of origin (Zanella et al., 2013).
Taste bud dissociation
Lingual epithelium from anterior tongue and circumvallate papilla was peeled after ∼20 min of enzymatic treatment (Collagenase A 1 mg/ml, Dispase II 2.5 mg/ml, Elastase 0.25 mg/ml, DNase I 0.5 mg/ml) in Tyrode's buffer. Delaminated epithelium was redigested in the same enzyme cocktail for 2 min followed by incubation in Ca2+/Mg2+ free Tyrode's for 5 min. Taste buds were drawn into a 50- to 55-µm-diameter fire-polished micropipette, transferred to a coverslip in a drop of polyvinylpyrrolidone (0.2%) in Tyrode's buffer, and cleaned of contaminating nonsensory epithelial cells by 5 sequential transfers to fresh coverslips with Tyrode's buffer (Dvoryanchikov et al., 2009). Isolated taste buds were then treated with 0.25% trypsin for ∼20 min at 37°C with slow shaking, transferred to fresh Tyrode's buffer containing trypsin inhibitor (1 mg/ml), and dissociated by trituration with a 35- to 40-µm-diameter glass pipette. Taste cells were transferred onto a coverslip, pre-coated with Cell-Tak (Corning #354240) in a recording chamber.
The above procedure was empirically developed to obtain Type I cells with high viability, confirmed by propidium iodide exclusion (data not shown). Incubation times for enzymes and Ca-free buffers were minimized to emulate protocols for isolating viable Schwann cells (Kaewkhaw et al., 2012; Andersen et al., 2016). Schwann and Type I cells both ensheath with cytoplasmic extensions. Longer digestions led to greater yield of cells but lower viability of Type I taste cells.
Lingual slice preparations
We adapted a slice preparation previously used (Dando et al., 2015; Roebber et al., 2019). Circumvallate papillae were rapidly dissected and embedded in 6% low-melting agarose (Sigma, #A9918) at ∼37°C. Slices (100 μm thick) were cut on a vibratome (Leica Microsystems, VT100s), collected in high-Ca Tyrode's buffer, adhered to coverslips precoated with Cell-Tak, placed in a recording chamber, and continuously perfused with high-Ca Tyrode's buffer at 2 ml/min by gravity perfusion.
Ca2+ imaging
Imaging was conducted using a Fluoview FV1000 laser scanning confocal microscope with a 20× water immersion objective, as previously detailed (Roebber et al., 2019). Images were captured at 1 frame/s, digitized, and stabilized using ImageJ with TurboReg plugin. Fluorescence changes over baseline (ΔF/F0) were identified and analyzed as previously detailed (Roebber et al., 2019). Taste stimuli for slices or dissociated taste bud cells were 5-30 μm cycloheximide, 1-3 mm denatonium, and 0.3 mm quinine. All taste stimuli and ATP (10-100 μm) were dissolved in high-Ca Tyrode's buffer. The higher concentrations of cycloheximide and denatonium were used only for the slice preparation as bath-applied stimuli do not access the apical tips of taste cells as easily as in the dissociated preparation.
Criteria for including Ca2+ responses in our analyses were as follows: (1) peak ΔF/F is > 3× SD of prestimulus baseline fluctuation and persisting for ≥ 6 frames; (2) responses could be replicated ≥ 2 times by the same stimulus for the same cell; and (3) peak ΔF/F occurred within 30 s of stimulus onset. The final criterion was introduced because ATP and analogs need to permeate into interstitial spaces in the slice to activate cells. In contrast, taste stimuli readily access the exposed taste pore during bath application.
Experimental design and statistical analysis
Biological replicates are reported as number of cells from number of taste buds and mice (each mouse representing an independent experiment). Different experimental conditions (e.g., drug effects) were tested sequentially and analyzed as paired comparisons for the same cell. Statistical analyses, including ratio t test (two-tailed, paired), one-way ANOVA on paired measurement with Bonferroni correction for multiple comparisons, and Iterative Grubbs' method for identifying outliers, were conducted in GraphPad Prism 9. Results were considered significant at p < 0.05.
Results
GCaMP3 expression in Type I (glial-like) cells
The GABA-synthesizing enzyme, Gad2, is strongly expressed in Type I cells of mouse taste buds (Dvoryanchikov et al., 2011). Thus, to explore the function of Type I taste bud cells, we used Gad2-Cre mice to drive expression of the calcium reporter, GCaMP3 in Type I cells. In Gad2;;GCaMP3 mice, GCaMP3 expression targeted to Type I cells was validated by immunostaining for NTPDase2, a marker for Type I cells (Bartel et al., 2006), and anti-GFP to visualize GCaMP in circumvallate taste buds. The lamellar morphology of Type I cells precludes counting them, but we observed that the overwhelming majority of GCaMP signal overlapped the NTPDase2 immunofluorescence in circumvallate (Fig. 1Aa–Ac), fungiform, and palatal taste buds (data not shown). Because GCaMP is a cytosolic protein while NTPDase2 is on the plasma membrane, there are instances, particularly on the periphery of the taste bud, where newly differentiated Type I cells with substantial cytoplasm appear to display GCaMP with relatively little NTPDase2 (Fig. 1Ac, 1Ca). In the taste bud interior, Type I cells typically have acquired an ensheathing morphology with little cytoplasm.
Figure 1.
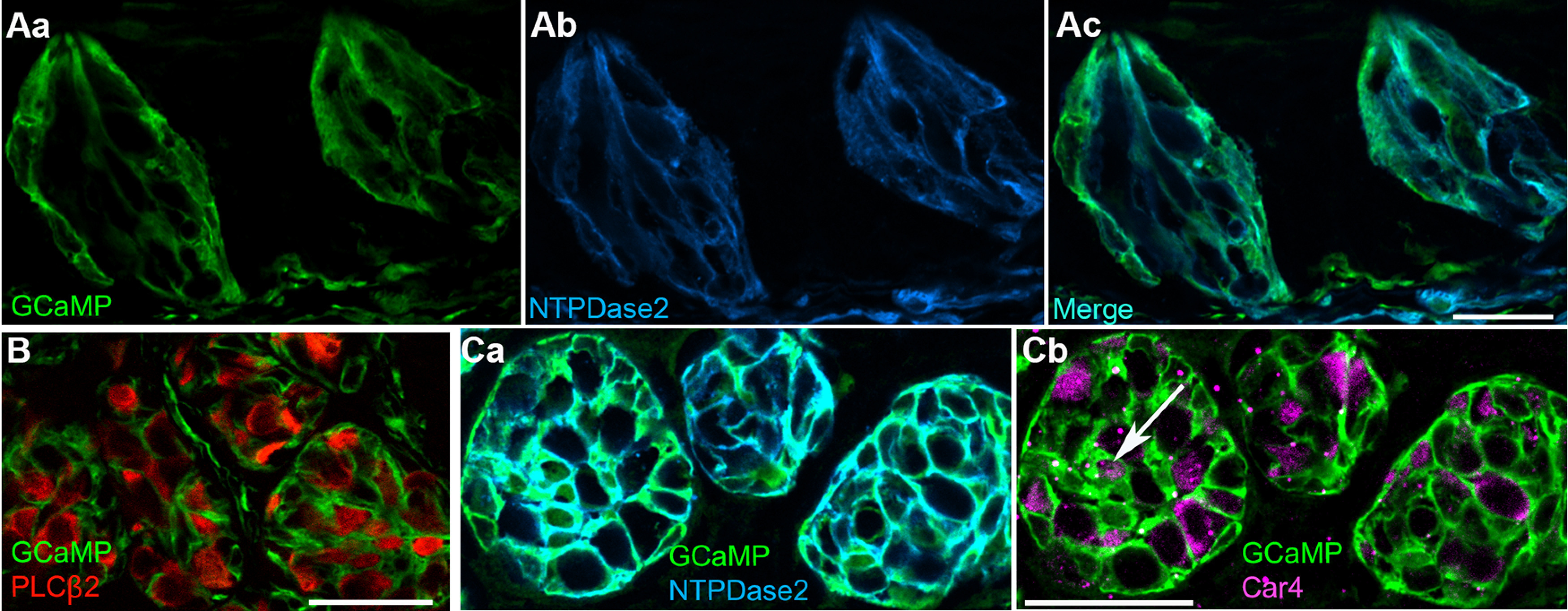
GCaMP3 is expressed principally in Type I (glial-like) cells. Cryosections of circumvallate papillae of Gad2;;GCaMP3 mice were immunostained for GFP and taste cell markers, as noted. Aa, Ab, Vertical section through two taste buds, immunostained for GFP (i.e., GCaMP3, green) and a Type I cell marker, NTPDase2 (blue). Ac, The merged image shows nearly complete coexpression of GCaMP and NTPDase2 within the taste bud. B, Cross section through several taste buds, immunostained for GFP (GCaMP, green) and a Type II cell marker, PLCβ2 (red), shows GCaMP+ Type I cells ensheathing PLCβ2+ Type II cells. No coexpression was detected across multiple images. Ca, Cb, Cross section through taste buds, triple immunostained for GFP (GCaMP, green), NTPDase2 (blue), and a Type III cell marker, Car4 (magenta). GCaMP+ Type I cells ensheath Type III cells. Occasional Type III cells (arrow) express low levels of GCaMP. All images are single confocal plane. Scale bar, 20 µm.
To examine whether GCaMP3 is also expressed in taste cells other than Type I cells, we immunostained for PLCβ2 or Car4, markers for Type II and Type III cells, respectively. Importantly, no Type II cells (0 of 262 PLCβ2+ cells; 42 taste buds; 3 mice) showed GFP immunostaining above background (Fig. 1B). A minority of Type III taste cells (22 of 79 Car4+ cells; 13 taste buds; 2 mice) appeared to weakly express GCaMP. We believe this is the maximum extent of GCaMP expression within taste buds outside of Type I cells. It is difficult to resolve the thin lamellae of Type I cells from the plasma membrane of other taste bud cells. There are also GCaMP3-immunopositive cells in the stroma below taste buds (see Fig. 1A). However, they were not included in our analyses. In summary, GCaMP3 is predominantly expressed in Type I taste bud cells in Gad2;;GCaMP3 mice in fungiform, palatal, and circumvallate taste buds.
Isolated Type I cells respond to ATP
ATP and 5-HT are transmitters secreted from Type II and III taste bud cells, respectively (Y. J. Huang et al., 2007). We tested whether Type I cells respond to either of these transmitters. For this, we dissociated taste buds from Gad2;;GCaMP3 mice and used Ca imaging to investigate responses evoked by bath-applied ATP and 5-HT. Because of their ensheathing and lamellar shape, Type I cells are much more fragile than Types II and III cells. Hence, we optimized the enzymatic and mechanical dissociation procedure to maximize the viability of Type I cells (see Materials and Methods).
We examined responses in GCaMP+ cells that were well separated from any surrounding cells by at least one cell diameter to ensure we were recording direct activation by the applied transmitters. Cells dissociated from either circumvallate taste buds or combined fungiform and palatal taste buds were exposed to bath-applied ATP (10-50 μm) or 5-HT (200 nm) (Fig. 2A–D). Of 156 GCaMP+ circumvallate taste cells (3 mice), 115 (∼74%) responded to ATP, and none responded to 5-HT (Fig. 2C). Of 42 GCaMP+ cells from fungiform and palatal taste buds (2 experiments), 20 (48%) responded to ATP, while 0 responded to 5-HT. Overall, the pattern of responses was similar for Type I cells from circumvallate versus fungiform and palatal taste buds.
Figure 2.
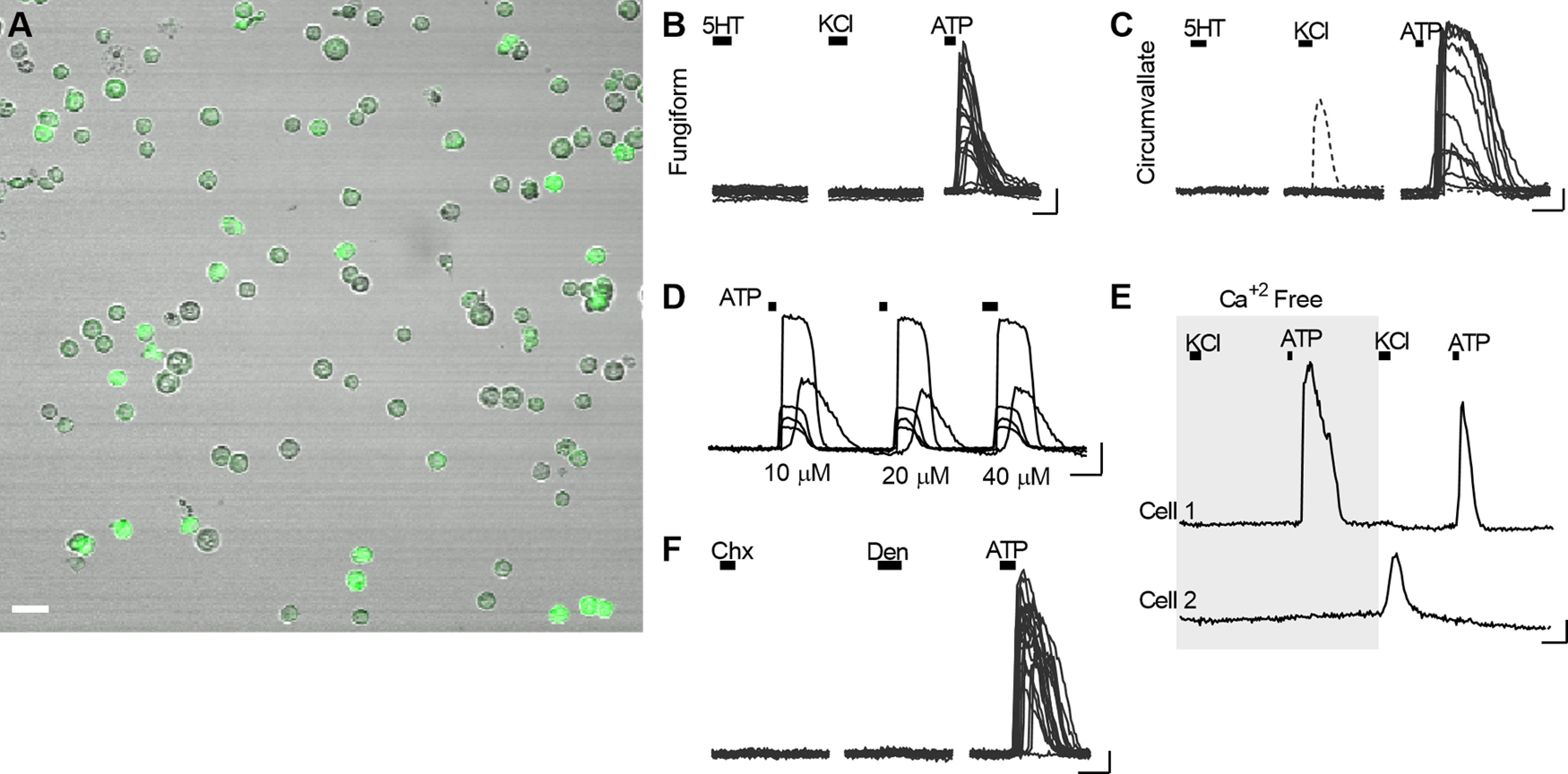
Isolated Type I cells respond to ATP. A, Combined fluorescence and Nomarski optics micrograph of taste cells dissociated from circumvallate taste buds of a Gad2;;GCaMP3 mouse, showing numerous GCaMP+ cells. Dissociated preparations from palatal and fungiform taste buds were also obtained (not shown). B, Ca2+ responses of dissociated fungiform and palatal taste bud cells, stimulated sequentially with 200 nm 5-HT, 50 mm KCl, and 50 μm ATP (bars above traces). Traces from 18 ATP-responsive cells are overlaid. C, Responses of 13 circumvallate taste bud cells, stimulated sequentially with 200 nm 5-HT, 50 mm KCl, and 10 μm ATP. Traces from 12 ATP-responsive cells and one of the infrequent KCl-responsive cells are overlaid. D, Type I cells, stimulated with ATP (bars, 10, 20, 40 μm) respond repeatedly. Traces of 5 ATP-responsive cells are superimposed. E, Bathing cells in Ca2+-free buffer did not alter ATP responses (cell 1) but did eliminate responses to KCl depolarization (cell 2). F, GCaMP+ (Type I) taste bud cells, stimulated sequentially with 10 μm cycloheximide (Chx) and 1 mm denatonium (Den), did not respond to these taste stimuli. All 21 GCaMP+ cells in the field responded to 10 μm ATP. Scale bar, 10 µm. Calibration: 20 s, 0.1 ΔF/F.
Importantly, responses to ATP were unchanged in the absence of extracellular Ca2+ (Fig. 2E), indicating that they depend on Ca2+ mobilization from stores and arise from P2Y, not P2X receptors. Indeed, P2Y receptors have previously been reported in Type I cells (Cherkashin et al., 2016).
We also tested whether isolated taste bud cells responded to KCl depolarization (50 mm KCl), which might indicate the presence of voltage-gated Ca2+ channels. A small fraction of circumvallate GCaMP3+ taste cells responded to KCl (11 of 156 cells; 3 mice) (Fig. 2C), and these responses were dependent on extracellular Ca2+ (Fig. 2E). This suggests that a minority of isolated GCaMP+ cells were Type III taste cells insofar as only Type III taste cells have voltage-gated Ca2+ channels (Medler et al., 2003; Chaudhari and Roper, 2010). This minority of isolated GCaMP+/KCl-responsive (presumptive Type III cells) is consistent with the incidence of GCaMP+ Type III cells detected by immunohistochemistry (Fig. 1Cb). In contrast to circumvallate taste buds, those from fungiform papillae appeared to contain no GCaMP+ cells that responded to KCl (Fig. 2B).
Isolated Type I cells do not respond to taste stimuli
To further test the identity of GCaMP+ cells, we bath-applied taste stimuli to isolated taste cells and recorded Ca responses. Taste GPCRs for sweet, bitter, or umami tastants are expressed in Type II, not Type I cells (Chaudhari and Roper, 2010). Bitter tastants were selected for this assay because they are effective at low concentration and introduce minimal osmotic perturbation. Bath-applied cycloheximide (5-10 μm) or denatonium (1 mm) elicited responses only in 1 of 141 isolated GCaMP+ cells (3 experiments) (Fig. 2F), consistent with the absence of GCaMP3 expression in Type II taste bud cells from Gad2;;GCaMP3 mice.
Type I cells in a semi-intact preparation respond to applied ATP
Next, we confirmed that Type I cells in their native environment in taste buds respond to ATP. For this, ∼100 µm slices of circumvallate or fungiform papillae from Gad2;;GCaMP3 mice were prepared for Ca imaging as previously described (Dando et al., 2015; Roebber et al., 2019). GCaMP+ cells displayed robust responses to bath-applied ATP (10-100 μm; Fig. 3A). Because Type I cells have convoluted shapes, we took care to select ROIs from distant areas within a taste bud in an attempt to quantify Ca2+ responses from separate cells (Fig. 3B). Although responses to ATP were similar in circumvallate and fungiform taste buds in slices, the latter preparation has smaller and far fewer taste buds. Hence, we proceeded for all subsequent experiments only with cells from circumvallate taste buds, which are more numerous and yield larger numbers of cells.
Figure 3.
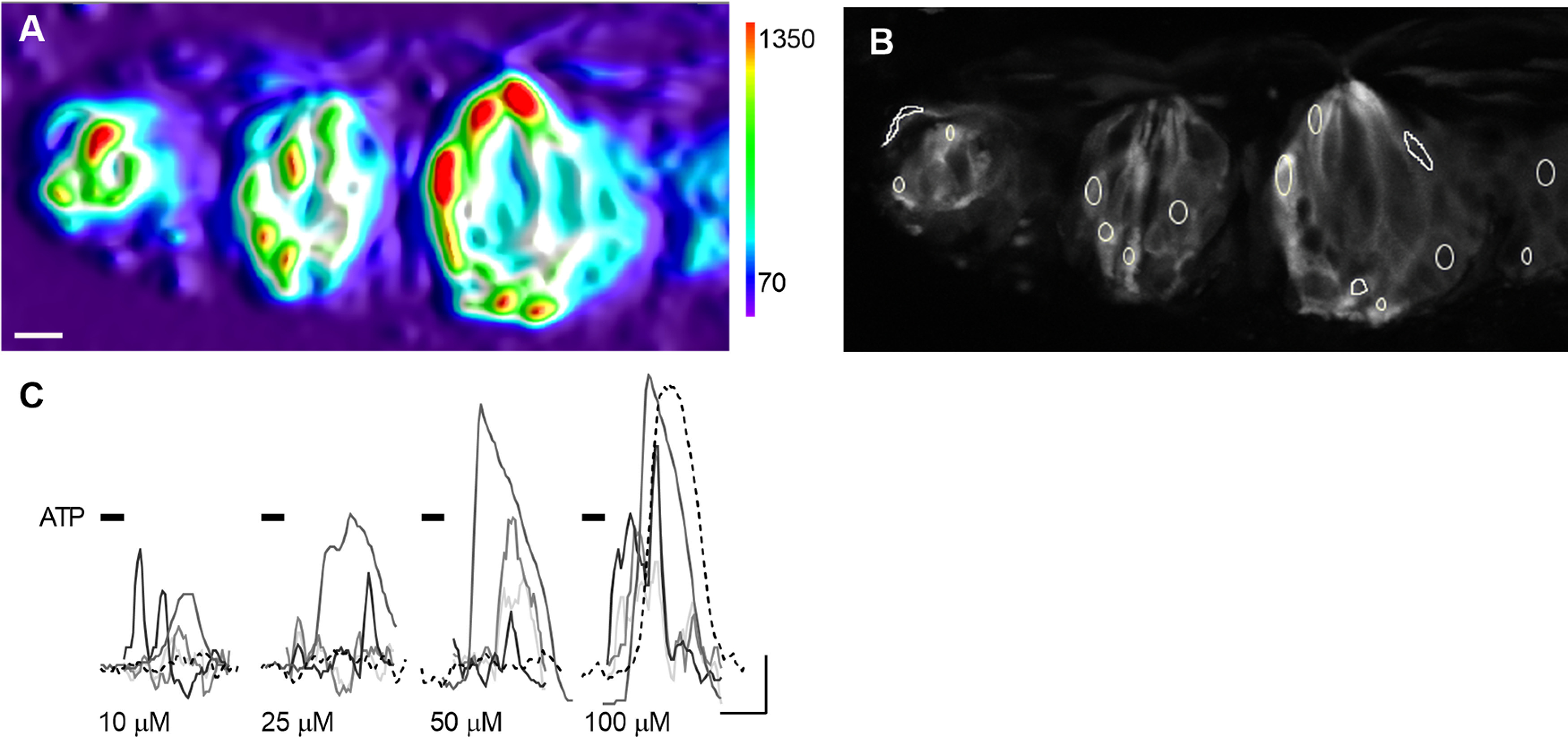
ATP evokes Ca2+ responses in Type I cells within circumvallate slice. A, 3D Surface Plot shows maximum fluorescence at each pixel, when slice was stimulated with ATP. Scale at right is in arbitrary fluorescence units. Three taste buds are seen in this view, each with several responding Type I cells. B, Grayscale image of the same view as in A, to display GCaMP fluorescence with ROIs designating separate cells. C, Traces from 5 cells, stimulated sequentially with 10, 25, 50, and 100 μm ATP. Some cells (dashed line) are not activated until ATP reaches 100 μm. Scale bar, 10 µm. Calibration: 20 s, 0.2 ΔF/F.
Although some GCaMP+ circumvallate taste cells responded to the lowest concentration of ATP (Fig. 3C), others even in the same bud only responded at 100 μm ATP. We believe this may reflect a restricted access of ATP to the interior of the taste bud, attributed to cellular and enzymatic barriers that prevent or reduce permeation of drugs and compounds into taste buds (Dando et al., 2015). Consequently, to ensure that ATP-sensitive cells would respond to bath-applied ATP, we selected 100 μm ATP for subsequent assays in the lingual slice preparation.
Parenthetically, we noticed that GCaMP+ cells occasionally were present in the nonsensory epithelium surrounding taste buds in the lingual slice preparation. Such cells often responded robustly to ATP. However, because these cells clearly are not taste cells, we have not included them in our analyses of Type I taste cell function.
Type I cells respond to endogenously secreted ATP
Taste stimuli activate TasR GPCRs on Type II cells and elicit secretion of the taste transmitter, ATP (Y. J. Huang et al., 2007; Romanov et al., 2018; Taruno et al., 2021). We tested whether taste-evoked release of ATP from Type II taste cells might indirectly activate Type I taste cells in intact taste buds in the lingual slice preparation. As shown above, taste stimuli did not directly stimulate isolated Type I taste cells. However, in marked contrast, taste stimulation of taste buds in the lingual slice elicited strong responses in GCaMP+ Type I taste cells, described next.
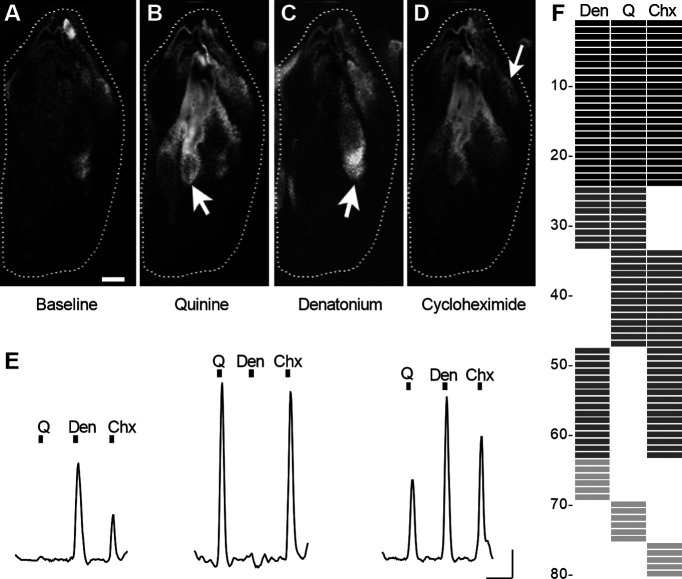
To test taste stimulation in the lingual slice preparation, we used the bitter tastants, cycloheximide, denatonium, and quinine. As explained above, these compounds are active at low concentrations that minimize osmotic changes. Moreover, bitter taste stimuli are particularly effective for circumvallate taste buds, and cycloheximide, denatonium, and quinine activate multiple Tas2Rs and cells (Meyerhof et al., 2010; Lossow et al., 2016). Cycloheximide (5-30 μm), den (3 mm), or quinine (0.3 mm) reliably elicited responses in Type I taste cells in the lingual slice preparation (Fig. 4A–D). Type I cells responded to 1, 2, or all 3 bitter tastants (Fig. 4E). Of 80 bitter-responsive Type I cells (22 taste buds; 5 mice), 30% responded to all three, 49% to two, and 21% to just one of the bitter compounds (Fig. 4F). Intriguingly, this is similar to the distribution of bitter-sensing Type II taste bud cells (Caicedo and Roper, 2001). We also observed tastant-evoked responses in Type I cells in slices of fungiform papillae (2 experiments; not shown), although detailed analyses were not possible because of the limited numbers and sizes of taste buds. Because cycloheximide produced responses in the largest proportion of Type I circumvallate taste cells, we continued subsequent experiments with this tastant alone.
Figure 4.
GCaMP+ (Type I) cells in lingual slices respond to bath-applied bitter tastants. A, Circumvallate taste bud showing baseline-subtracted fluorescence, using a grayscale to display intensity of resting GCaMP fluorescence. B-D, GCaMP fluorescence at peak of response to bath-applied quinine (Q) 0.3 mm (B), Den 3 mm (C), and Chx 30 μm (D). Arrows indicate three cells in this taste bud that responded to one or more stimuli. Scale bar, 5 μm. E, Traces from another taste bud, similarly exposed to three bitter stimuli, displaying the varying pattern of responses to two or three stimuli. Black bar represents stimulus application. F, Summary of the response profiles of 80 bitter-sensitive taste cells as shown in B-E. Each row represents one cell. Each column is the response to a different bitter compound. Individual cells responding to 1, 2, or 3 stimuli are shown in progressively darker shades. Calibration: 50 s, 0.1 ΔF/F.
Type I cells do not express taste GPCRs to detect tastants directly. Hence, we interpret taste-evoked Ca responses in Type I cells as reflecting indirect activation, secondary to ATP released from Type II cells. As shown above (Fig. 1), few, if any, Type II taste cells express GCaMP3 in Gad2;;GCaMP3 mice. However, immunostaining (Fig. 1Cb) and Ca imaging on isolated cells (Fig. 2C) had suggested that a small percentage of Type III cells may also express GCaMP. To discern whether taste-elicited responses in the lingual slice might originate in Type III cells, we sequentially stimulated lingual slices with tastants and KCl (50 mm). Type III cells are the only taste bud cells that show depolarization (KCl)-induced Ca2+ responses (Medler et al., 2003; DeFazio et al., 2006). To maximize the incidence of taste-evoked responses in this assay, we used a mix of sweet and bitter tastants that are effective at low concentration, avoiding osmotic effects (3 μm cycloheximide, 1 mm denatonium, 100 μm SC45647, 1 mm saccharin). Of the 58 cells (12 taste buds, 2 mice) that responded to bath application of this stimulus protocol, 76% responded exclusively to taste mix, 17% to KCl-mediated depolarization, and 7% to both (Fig. 5B).
Figure 5.
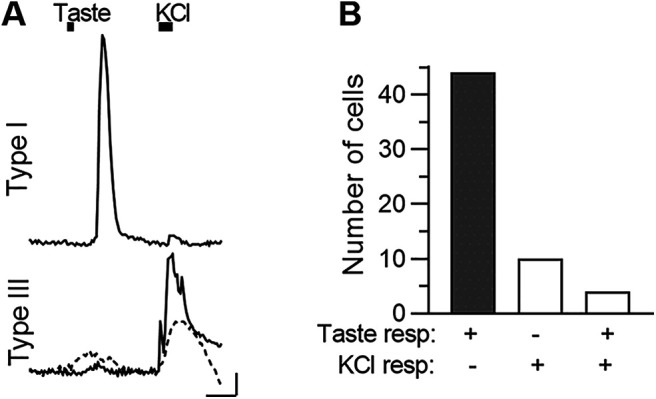
In the intact taste bud, most taste-responsive GCaMP+ cells are Type I. A, Top, Representative trace from a circumvallate taste bud cell, that responded robustly to a taste mix (Chx 3 μm, 1 mm Den, 100 μm SC45647, 1 mm saccharin), but displayed a negligible response to KCl (50 mm) depolarization. Bottom, Traces from two cells, both of which responded strongly to KCl, but with small or no response to taste mix. Top, Typical of Type I cells. Bottom two are Type III cells. Black bars above traces represent stimulus application. B, Summary of two experiments, displaying numbers of cells which responded only to taste mix (shaded bar), only to KCl, or to both stimuli. Calibration: 20 s, 0.1 ΔF/F.
In brief, at most, 24% of the 58 GCaMP+ cells in this analysis plausibly were Type III cells; the majority were Type I cells. Importantly, when putative Type III GCMP+ cells were stimulated with tastants, Ca2+ responses were prolonged and of low amplitude (Fig. 5A). Most taste-evoked responses in such putative Type III cells did not meet our stringent criteria for inclusion (see Materials and Methods). Type I cell responses were distinctly of greater amplitude (compare Figs. 4E, 5A, top, vs Fig. 5A, bottom).
In summary, although GCaMP+ Type I cells do not respond to bitter stimuli when directly stimulated (Fig. 2), yet when presented with the same stimuli in the slice preparation, Type I cells respond robustly to these same stimuli. We infer that taste-evoked Type I cell responses in the lingual slice represent initial activation of Type II cells, followed by taste-evoked ATP secretion, which then activates adjacent, ensheathing Type I cells. This is similar to how synaptic glia are activated by secreted neurotransmitter from an active presynaptic neuron (Halassa et al., 2007; Stevens, 2008; Farhy-Tselnicker and Allen, 2018).
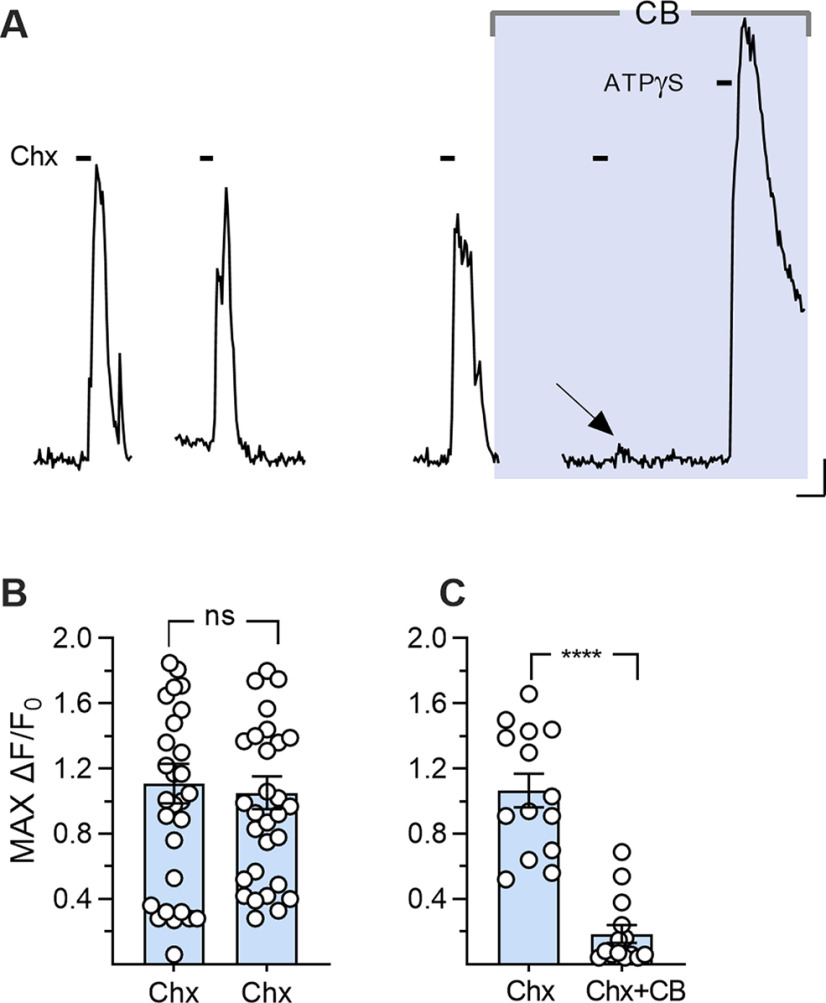
Degrading extracellular ATP damps Type I cell responses to tastants
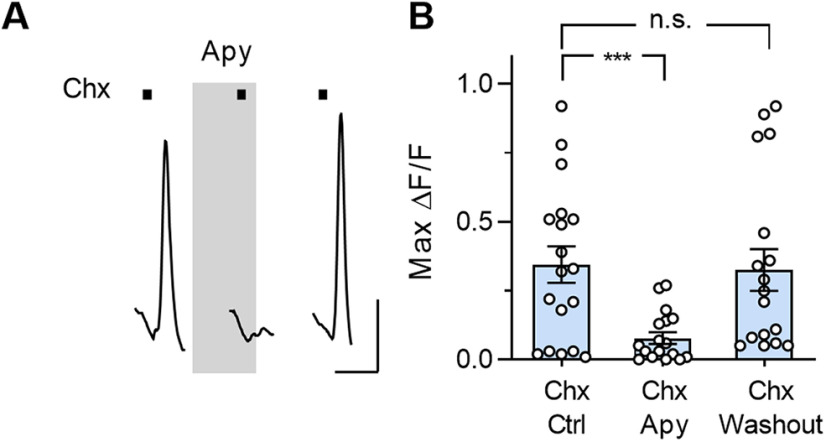
To test the above interpretation of the origin of taste-evoked responses in Type I cells, we incubated lingual slices in a potent ecto-ATPase, apyrase (30 units/ml) (Fig. 6A) to reduce or eliminate ATP released by Type II cells. Apyrase treatment consistently and reversibly decreased or abolished taste-evoked responses in Type I taste cells; the mean response amplitude decreased by 74 ± 2% (18 cells, 3 mice; Fig. 6B). We interpret this result as indicating that tastant-evoked responses in Type I cells are ATP-dependent and secondary to ATP secretion from bitter-sensing Type II cells.
Figure 6.
Degrading endogenously secreted ATP reduces bitter-evoked responses in Type I cells. A, Ca2+ responses in a Type I cell, evoked by bath-applied Chx (5 μm, bars), before, during, and after incubating the slice in apyrase (Apy). B, Summary data; apyrase significantly reduced Chx-evoked responses in Type I cells, and this reduction was reversible when apyrase was washed out (t(17) = 4.4, ***p = 0.0008, one-way ANOVA, paired) n = 18 cells. Calibration: 50 s, 0.1 ΔF/F.
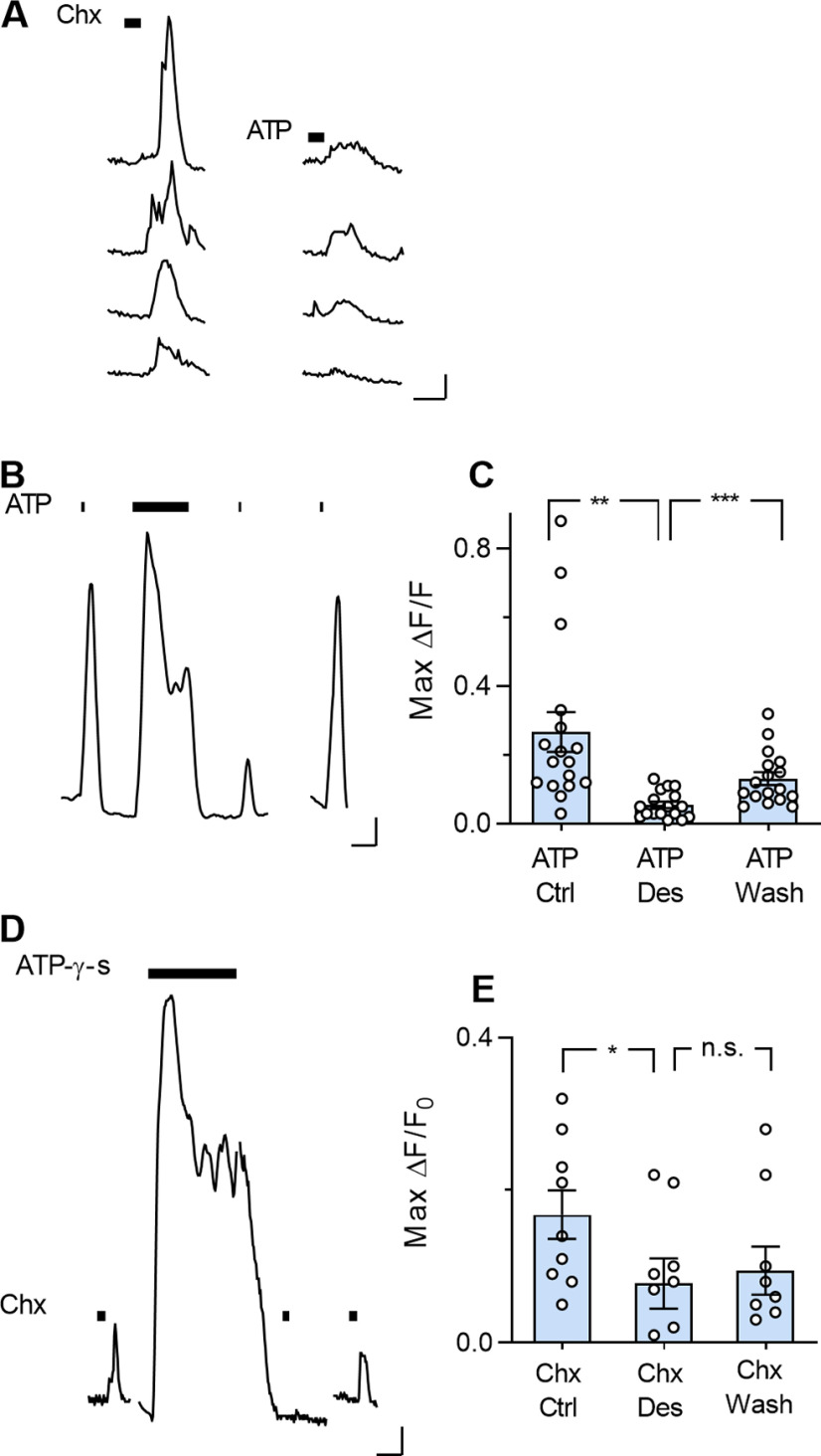
Indeed, most bitter-responsive Type I cells were also sensitive to bath-applied ATP. Surprisingly, their responses to ATP were highly variable (Fig. 7A), possibly because physical and enzymatic barriers within the taste bud and the ensheathing nature of the cells results in cell membranes that are relatively inaccessible to the bath. In contrast, Type I cells that are more superficially located in the taste bud may be easily stimulated by ATP.
Figure 7.
Desensitizing purinoreceptors reduces taste-evoked responses in Type I cells. A, Ca2+ responses in four Type I cells in the lingual slice when the preparation was stimulated (bars) with Chx (5 μm) and ATP (100 μm). B, ATP (100 μm)-evoked Ca2+ responses in another Type I cell before, during, and after prolonged (60 s) exposure to the same concentration of ATP (which desensitized ATP receptors and blocked subsequent responses to ATP). A 5 min wash restored ATP sensitivity (final ATP stimulation). C, Summary data; desensitizing purinoceptors in the slice preparation significantly reduced subsequent ATP responses (one-way ANOVA with Bonferroni correction (t(16) = 3.85, **p = 0.004; t(16) = 4.72, ***p = 0.0007). n = 17 cells. D, Ca2+ responses in a Type I cell when the preparation was stimulated with Chx (5 μm, bars) before, during, and after a 100 s bath application of ATP-γ-S (10 μm), a nonhydrolyzable analog of ATP, to desensitize purinoreceptors. E, Summary data; prolonged exposure to ATP-γ-S significantly reduced Chx-evoked responses in Type I taste cells (t(8) = 3.5, *p = 0.02, one-way ANOVA with Bonferroni correction) n = 9 cells. Outliers were identified by Iterative Grubbs' method and removed. Calibration: 20 s; 0.1 ΔF/F.
Desensitizing ATP receptors dampens Type I cell responses
ATP responses in circumvallate taste bud cells desensitize (Baryshnikov et al., 2003). Thus, we further tested the ATP dependence of taste-evoked Type I cell responses by desensitizing purinoceptors. Prolonged bath application of the transmitter (100 μm ATP, 60-100 s) clearly reduced subsequent ATP-evoked responses in Type I cells for 10's of seconds (Fig. 7B). Subsequent (≥5 min) rinsing with Tyrode's buffer allowed the recovery of ATP-evoked responses (Fig. 7B,C). Similarly, prolonged exposure to the nonhydrolyzable ATP analog, ATP-γ-S (10 μm), desensitized Type I cell purinoceptors, and reduced ATP-evoked responses (data not shown).
Next, we examined the effect of purinoceptor desensitization on taste-evoked responses. Bathing the lingual slice in 10 μm ATP-γ-S for 60-100s significantly reduced cycloheximide-evoked responses in Type I taste cells (Fig. 7D,E).
In sum, the ability of the ecto-ATPase, apyrase, and of purinoceptor desensitization to significantly reduce taste-evoked Type I cell responses strongly implicate the Type II cell transmitter, ATP, as a mediator for these responses.
Purinergic inhibitors damp Type I cell responses
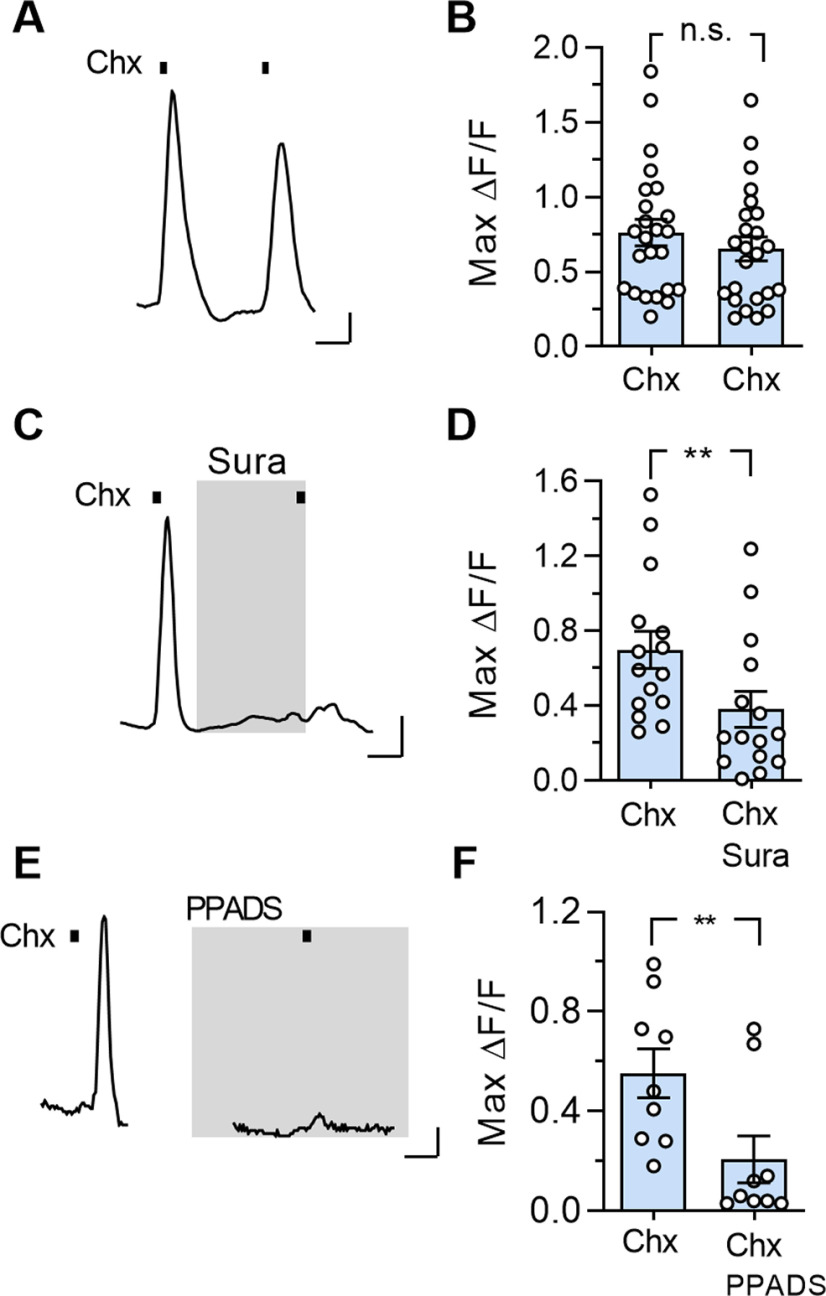
As a further test of the hypothesis that taste-evoked responses in Type I cells were generated secondarily to stimulus-evoked ATP release from Type II cells, we used two broadly selective purinoceptor inhibitors, suramin and PPADS, in the lingual slice preparation. First, we confirmed that repeated stimulation with cycloheximide (5 μm) elicited repeatable responses in Type I cells (Fig. 8A,B). Next, we imaged responses of Type I cells to cycloheximide (5 μm) before and in the presence of suramin (50-150 μm) or PPADS (10 μm). Cycloheximide-evoked responses of Type I cells to cycloheximide were decreased by 46% by suramin (15 cells, 8 taste buds, 3 mice), and by 63% by PPADS (n = 9 cells, 7 taste buds, 2 mice) (Fig. 8C–F).
Figure 8.
Purinoceptor antagonists reduce taste-evoked responses in Type I cells. A, Ca2+ responses in a Type I cell in the lingual slice preparation, evoked by repeated bath application of Chx (5 μm). B, Summary data showing no significant difference between two sequential Chx responses (t(23) = 1.42, p = 0.17, two-tailed, ratio paired t test). C, Chx (5 μm)-evoked Ca2+ responses in a Type I cell before and during application of suramin (150 μm, sura), a purinoceptor inhibitor. Suramin is not reversible in the time frame of the experiment. D, Summary data; suramin (50-150 μm) significantly reduced Chx-evoked responses in Type I cells (t(14) = 2.99, **p = 0.01, two-tailed, ratio paired t test). n = 15 cells. E, Chx (5 μm)-evoked Ca2+ responses in another Type I cell before and during application of PPADS (10 μm), another nonselective purinoceptor inhibitor. F, Summary data; PPADS (10 μm) significantly reduced Chx-evoked responses in Type I cells (t(8) = 3.52, **p = 0.008, two-tailed, ratio paired t test) n = 9 cells. Calibration: A, E, 20 s, 0.1 ΔF/F; C, 20 s, 0.2 ΔF/F.
Blocking ATP release from Type II cells inhibits Type I cell response
The preceding experiments demonstrate that taste-evoked responses in Type I cells are mediated through purinoceptors and dependent on ATP secreted by Type II cells. ATP is released from Type II cells through large-pore CALHM1/3 channels (Romanov et al., 2018). Taste-evoked ATP release from Type II cells can be abolished by carbenoxolone, a CALHM1/3 channel blocker (Y. J. Huang et al., 2007; Taruno et al., 2013; Ma et al., 2018). Thus, we used carbenoxolone to assess whether taste-evoked Type I responses depend on synaptic release of ATP from Type II cells via CALHM1/3. In slices of circumvallate papillae, we identified Type I cells that, over a span of 10 min, displayed repeatable responses to cycloheximide (5 μm) (Fig. 9A,B). We then bathed the slice in 10 μm carbenoxolone for 10 min and retested responses to cycloheximide in the continued presence of the drug (Fig. 9A,C). Cycloheximide-evoked responses in Type I cells were significantly decreased by carbenoxolone (83% decrease, 14 cells, 6 taste buds, 5 mice). We did not attempt to wash out the drug as it has been shown to have a long-lasting effect on taste cells (Ma et al., 2018). Positive control stimuli, ATP or ATP-γ-S, continued to elicit responses in Type I cells in these slices even when taste-evoked responses were blocked (Fig. 9A).
Figure 9.
Inhibiting ATP release reduces taste-evoked responses in Type I cells. A, Ca2+ responses in a Type I cell to Chx (5 μm), 3 times before and once during incubation of the slice in carbenoxolone (CB, 10 μm; shaded area). Shaded gap represents ≈10 min incubation in carbenoxolone before taste stimulation. Arrow indicates a residual response to Chx in the presence of CB. Far right, Response to ATPγ-S as a positive control. B, Summary data showing no significant difference between the first two sequential Chx responses (t(30) = 0.58, p = 0.56, two-tailed, ratio paired t test). n = 31 cells. C, Summary data comparing responses to Chx immediately before and during carbenoxolone (10 μm). The drug significantly reduced Chx-evoked responses in Type I cells (t(13) = 7.57, ****p = 0.000004, two-tailed, ratio paired t test). n = 14 cells. Calibration: 20 s, 0.2 ΔF/F.
Discussion
Type I cells of taste buds are termed supportive or “glial-like.” They ensheath chemosensory taste bud cells with lamellar processes, and they participate in transmitter clearance (Bartel et al., 2006; Vandenbeuch et al., 2013) and in K+ and Cl– regulation (Dvoryanchikov et al., 2009; Cherkashin et al., 2016; Guarascio et al., 2021). Here, we demonstrate another glial-like role, sensing the activation of chemosensory (Type II) cells, similarly to synaptic glia. Using Gad2;;GCaMP3 mice in which Type I taste bud cells express the Ca reporter, GCaMP3, and a semi-intact slice preparation, we show that Type I cells in their native environment respond to ATP, the neurotransmitter of Type II taste cells. Moreover, when chemosensory Type II cells are stimulated by tastants, we show that synaptically released ATP also activates Type I taste cells via P2Y receptors. Our results strongly support a model of Type II to Type I interactions by showing (1) Type I cells which respond to cycloheximide also respond to ATP; (2) degrading endogenously secreted ATP (with apyrase) within the lingual slice inhibits Type I cell responses to tastants; and (3) desensitizing purinoceptors or using purinergic antagonists (suramin, PPADS) abolishes tastant-evoked responses in Type I cells. Importantly, blocking taste-evoked ATP release with carbenoxolone strongly inhibits tastant-evoked signals in Type I cells. The relationship we postulate is like that in tripartite synapses in the CNS: presynaptic transmitter release (from Type II cells) stimulates postsynaptic (afferent) neuronal targets as well as ensheathing astrocytes (Type I cells).
Taste bud cells express several ionotropic and metabotropic purinoceptors, as shown by RT-PCR, immunohistochemistry, patch-clamp recordings, and Ca2+ imaging (Y. V. Kim et al., 2000; Baryshnikov et al., 2003; Hayato et al., 2007). Among P2Y receptors, distinct nucleotide and drug sensitivities allowed the functional identification of P2Y1 on Type II and P2Y4 on Type III cells (Y. A. Huang et al., 2009), whereas P2Y2 and P2Y4 are the predominant forms on Type I cells (Bystrova et al., 2006; Cherkashin et al., 2016). However, we were not able to distinguish which specific purinoceptors on Type I cells mediated the “tripartite interaction” (also see below).
In addition to its role as afferent transmitter, ATP functions in cell-cell communication within the taste bud. In a series of studies on isolated cells and in a slice preparation, ATP was shown to act as a paracrine factor from Type II and III cells (Y. A. Huang et al., 2009, 2011; Dando et al., 2012); and adenosine, a breakdown product of ATP, serves as an autocrine factor for Type II cells, enhancing sweet taste signals. Perhaps most significantly, ATP mediates autocrine positive feedback onto Type II cells. That is, released ATP activates P2X2/3 receptors, enhancing the depolarization of the Type II cell, and facilitating further ATP release (Y. A. Huang and Roper, 2010; Y. A. Huang et al., 2011; Romanov et al., 2018).
Our current study now documents that taste-evoked ATP signaling within the taste bud also involves glial-like Type I cells. To examine this cell-cell communication, we used two broad spectrum purinergic antagonists, suramin and PPADS (Fig. 8C–F). However, we note that rodent P2Y2 and P2Y4, the dominant receptors on Type I cells, are unaffected by PPADS (Raqeeb et al., 2011; von Kugelgen and Hoffmann, 2016). Thus, the site of PPADS action may be PPADS-sensitive P2X2/X3 (autocrine) receptors (Brown et al., 2001) on Type II cells. Because ATP release from Type II cells is subject to positive feedback (see above), blocking P2X receptors with PPADS would decrease ATP release, and, consequently, reduce the signal in Type I cells. Suramin, on the other hand, should interfere with both Type II and Type I cells in the current experiments. Both inhibitors help to substantiate the purinergic cross-talk between receptor and glial cells.
ATP secretion from Type II cells is unusual and nonvesicular. Instead, at sites of contact with the afferent nerve fiber, an “atypical” mitochondrion, with large tubular cristae, is found directly under the plasma membrane in the Type II cell cytoplasm (Yang et al., 2020). These mitochondria are the site of synthesis and nonvesicular storage of ATP for release through CALHM1/3 channels, which also are localized in this same membrane microdomain (Romanov et al., 2018). Importantly, the complex of atypical mitochondria and CALHM channels is only located along the track of afferent fibers contacting the Type II cell, and these structures together form the synapse of Type II cells (Yang et al., 2020; Taruno et al., 2021). ATP release from Type II cells is inhibited by carbenoxolone (Y. J. Huang et al., 2007; Dando and Roper, 2009; Murata et al., 2010; Dvoryanchikov et al., 2011), reflecting a block of CALHM1/3 channels (Ma et al., 2018; Romanov et al., 2018). Our demonstration that taste-evoked responses in Type I cells also are carbenoxolone-sensitive suggests that they originate from the same source of ATP (atypical mitochondria and CALHM channels) as the afferent channel synapse rather than a more global cytosolic pool of ATP.
We used Gad2-Cre mice to drive expression of GCaMP3 in Type I cells (Gad2;;GCaMP3 mice), based on our earlier findings by single-cell RT-PCR, that Gad2 is primarily in Type I cells and only occasionally in Type III cells (Dvoryanchikov et al., 2011). Here, we similarly found Gad2-Cre driven expression of GCaMP in nearly all Type I cells, no Type II cells, and ≈ 28% of Type III cells (at low levels). Because Type I and III cells represent 63% and 11%, respectively, of all circumvallate taste bud cells (Ogata and Ohtubo, 2020), we estimate that ∼4% of the GCaMP+ taste bud cells in our slices were Type III, while ≈96% were Type I cells. Functionally, Type III cells were easily distinguished by their responses to KCl (Fig. 5); they were not included in our reported analyses. Consistent with earlier reports (Bystrova et al., 2010; Cherkashin et al., 2016), we found that Type I cells exhibited Ca2+ responses to ATP but not to 50 mm KCl. Parenthetically, a recent report suggested that Gad2-Cre drives tdTomato reporter expression in all three taste bud cell types (Larson et al., 2021). We believe their choice of tdTomato reporter, with its very bright fluorescence, and some Cre-independent expression (Papathanou et al., 2019; our unpublished observations; www.Jax.org for strain 007909) may underlie the difference between their histology findings and ours. Further, Type I cells are much more susceptible to damage during cell isolation than Types II and III cells (see Materials and Methods). This may have resulted in a greater representation of tdTomato-labeled Type III cells during taste bud dissociation in the Larson et al. (2021) study. The majority of our functional data are from intact taste buds in lingual slice preparations, avoiding cell isolation.
Type I cells are considered the glia of taste buds, tightly ensheathing chemosensory cells, clearing neurotransmitters, and serving additional potential supporting roles. Similarly to Type I cells, CNS astrocytes are sensitive to extracellular neurotransmitters, including ATP, mobilize intracellular calcium in response (Parpura et al., 1994; Pasti et al., 1997), and release their own transmitters. The earliest identified so-called gliotransmitters were glutamate, ATP, and GABA, and these were shown to modulate astrocytic and/or neuronal activity (Araque et al., 1998; Nedergaard et al., 2003; Halassa et al., 2007; Martin et al., 2015; Papouin et al., 2017). Astrocytic gliotransmitters can modulate neuronal activity at the circuit level, including for sensory processing (Zhao et al., 2012; Lines et al., 2020). The bidirectional communication pathways between neurons and astrocytes at central synapses have led to the concept of the tripartite synapse, comprising the presynaptic and postsynaptic neurons and the synaptic astrocyte (Araque et al., 1998; Eroglu and Barres, 2010).
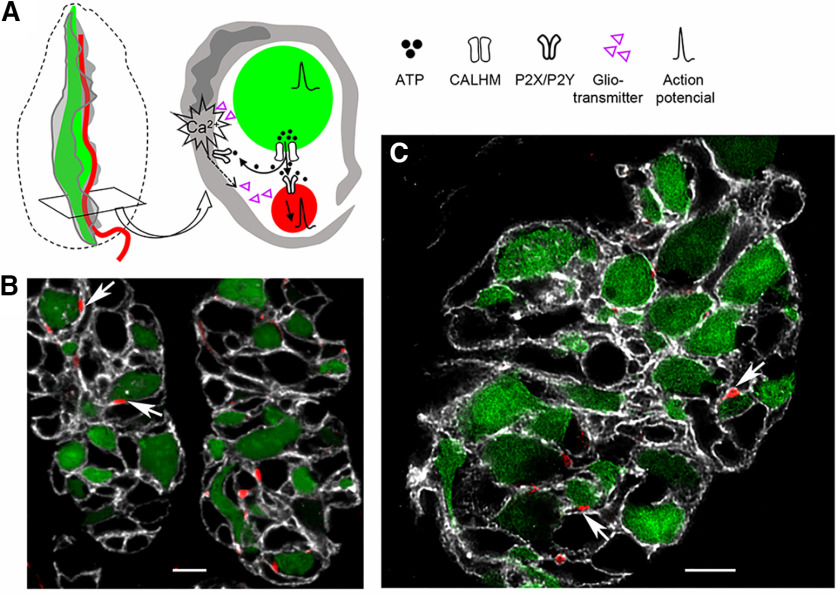
We hypothesize that Type I taste bud cells, akin to CNS astrocytes, sense nearby activity of chemosensory taste bud cells and influence presynaptic and/or postsynaptic targets (Fig. 10A). When Type II cells are activated by sweet, bitter, umami, or salty stimuli, the secreted ATP would activate both the afferent nerve and an adjacent Type I cell that ensheaths both. ATP secretion occurs at sites where Type II cells and nerve terminals are apposed (Romanov et al., 2018); Type I cells are intimately juxtaposed at these sites (Fig. 10B,C). Thus, ATP could readily reach the Type I cell associated with presynaptic and postsynaptic elements (i.e., Type II cell and nerve, respectively). Ultrastructural studies document the paucity of extracellular space within the taste bud and the tight ensheathing of chemosensory taste bud cells by Type I cells (Pumplin et al., 1997; Yang et al., 2020). Although in theory, this arrangement could interfere with cell-cell communication within the bud, our current functional data confirm that ATP can indeed diffuse in the extracellular spaces of the intact taste bud. We also note that gaps are apparent in NTPDase2-labeled ensheathing of Type II cells (Fig. 10B,C).
Figure 10.
A model for interactions of Type I (glial-like) cells at the synapse between chemosensory cells and afferent fibers. A, Schematic representation of the postulated interactions between Type I cells (gray), Type II cells (green), and gustatory sensory afferent fibers (red). A Type I cell ensheathes a Type II cell and its afferent fiber. Taste stimulation excites action potentials in the Type II cell, resulting in ATP secretion through CALHM1/3 ion channels. Secreted ATP targets P2X2/P2X3 receptors on the afferent fiber and P2Y receptors on the Type I cell. The latter initiate intracellular Ca2+ mobilization, in response to which, Type I cells are postulated to release one or more gliotransmitters (purple triangles), such as GABA. This transmitter, in turn, may influence the activity of Type II cells and/or the afferent fiber, thereby modulating neuronal activity. B, A single plane confocal image of two cross-sectioned circumvallate taste buds from a Plcb2-GFP mouse, injected with AAV-PHP.S;;mScarlet to label afferent fibers. Both fluorescent proteins were imaged for native fluorescence. Type II cells (green) and afferent nerve fibers (red) are seen enwrapped by Type I cells (NTPDase2+, white). C, A deconvolved confocal image of another circumvallate taste bud, at higher resolution. B, C, Arrows indicate examples of the three elements that potentially form a “tripartite synapse.” Scale bar, 5 µm.
What is the consequence of Ca2+ elevation in Type I cells? We suggest that GABA may be released as a Type I cell gliotransmitter. First, GABA is synthesized and accumulates plentifully in Type I cells (Dvoryanchikov et al., 2011). Second, GABA exerts a negative feedback loop, reducing ATP secretion by acting on GABAA and GABAB receptors on Type II cells (Dvoryanchikov et al., 2011). Third, GABAA receptors are plentiful on gustatory afferent neurons that carry taste signals from taste buds to central circuits in the brainstem (Dvoryanchikov et al., 2013, 2017; Chaudhari, 2021). Several gaps remain in this scenario. It is not yet known whether the Ca2+ signal in Type I cells causes secretion of GABA or any other transmitter. It is also unclear whether GABA accumulates in vesicles or whether nonvesicular mechanisms, such as GABA-permeating channels or transporters, may be involved (Bazargani and Attwell, 2016; Woo et al., 2018).
Our present data begin to illustrate the functional participation of glial-like Type I cells in taste neurotransmission. This participation may take the form of modulating presynaptic transmitter release, or shaping the postsynaptic excitatory signal through tonic or acute release of GABA or other transmitters (Chaudhari, 2021). Indeed, the participation of astrocytes in shaping sensory signals is an area of much recent investigation (Kozlov et al., 2006; Cirillo et al., 2012; Leonard et al., 2018; Cho and Huh, 2020; Ung et al., 2020).
In addition to our proposed synaptic model, ATP-evoked Ca2+ mobilization in Type I cells activates Ca-dependent Cl– channels in the apical tips of Type I cells and thus may participate in spatial buffering of ions to permit repetitive firing of chemosensory taste cells (Dvoryanchikov et al., 2009; Cherkashin et al., 2016; Guarascio et al., 2021).
Footnotes
This work was supported by National Institutes of Health, National Institute on Deafness and Other Communication Disorders Grants R01 DC006308 and R01 DC017303 to N.C. We thank Andoni Asencor for technical assistance, labeling nerves with AAV.
The authors declare no competing financial interests.
References
- Andersen ND, Srinivas S, Pinero G, Monje PV (2016) A rapid and versatile method for the isolation, purification and cryogenic storage of Schwann cells from adult rodent nerves. Sci Rep 6:31781. 10.1038/srep31781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG (1998) Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci 10:2129–2142. 10.1046/j.1460-9568.1998.00221.x [DOI] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A (2014) Gliotransmitters travel in time and space. Neuron 81:728–739. 10.1016/j.neuron.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asencor AI, Dvoryanchikov G, Pantelis T, Chaudhari N (2021) AAV-PHP.S-mediated delivery of reporters to cranial ganglion sensory neurons. bioRxiv. doi: 10.1101/2021.09.14.460327 [DOI] [Google Scholar]
- Bartel DL, Sullivan SL, Lavoie EG, Sevigny J, Finger TE (2006) Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of Type I cells in taste buds. J Comp Neurol 497:1–12. 10.1002/cne.20954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baryshnikov SG, Rogachevskaja OA, Kolesnikov SS (2003) Calcium signaling mediated by P2Y receptors in mouse taste cells. J Neurophysiol 90:3283–3294. 10.1152/jn.00312.2003 [DOI] [PubMed] [Google Scholar]
- Bazargani N, Attwell D (2016) Astrocyte calcium signaling: the third wave. Nat Neurosci 19:182–189. 10.1038/nn.4201 [DOI] [PubMed] [Google Scholar]
- Brown SG, Kim YC, Kim SA, Jacobson KA, Burnstock G, King BF (2001) Actions of a series of PPADS analogs at P2X1 and P2X3 receptors. Drug Dev Res 53:281–291. 10.1002/ddr.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrova MF, Yatzenko YE, Fedorov IV, Rogachevskaja OA, Kolesnikov SS (2006) P2Y isoforms operative in mouse taste cells. Cell Tissue Res 323:377–382. 10.1007/s00441-005-0098-8 [DOI] [PubMed] [Google Scholar]
- Bystrova MF, Romanov RA, Rogachevskaja OA, Churbanov GD, Kolesnikov SS (2010) Functional expression of the extracellular-Ca2+-sensing receptor in mouse taste cells. J Cell Sci 123:972–982. 10.1242/jcs.061879 [DOI] [PubMed] [Google Scholar]
- Caicedo A, Roper SD (2001) Taste receptor cells that discriminate between bitter stimuli. Science 291:1557–1560. 10.1126/science.1056670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS (2006) The receptors and cells for mammalian taste. Nature 444:288–294. 10.1038/nature05401 [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS (2010) The cells and peripheral representation of sodium taste in mice. Nature 464:297–301. 10.1038/nature08783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N (2021) Is there a role for GABA in peripheral taste processing. Curr Opin Physiol 20:105–111. 10.1016/j.cophys.2021.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD (2010) The cell biology of taste. J Cell Biol 190:285–296. 10.1083/jcb.201003144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkashin AP, Kolesnikova AS, Tarasov MV, Romanov RA, Rogachevskaja OA, Bystrova MF, Kolesnikov SS (2016) Expression of calcium-activated chloride channels Ano1 and Ano2 in mouse taste cells. Pflugers Arch 468:305–319. 10.1007/s00424-015-1751-z [DOI] [PubMed] [Google Scholar]
- Cho J, Huh Y (2020) Astrocytic calcium dynamics along the pain pathway. Front Cell Neurosci 14:594216. 10.3389/fncel.2020.594216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo G, De Luca D, Papa M (2012) Calcium imaging of living astrocytes in the mouse spinal cord following sensory stimulation. Neural Plast 2012:425818. 10.1155/2012/425818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando R, Roper SD (2009) Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol 587:5899–5906. 10.1113/jphysiol.2009.180083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando R, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD (2012) Adenosine enhances sweet taste through A2B receptors in the taste bud. J Neurosci 32:322–330. 10.1523/JNEUROSCI.4070-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando R, Pereira E, Kurian M, Barro-Soria R, Chaudhari N, Roper SD (2015) A permeability barrier surrounds taste buds in lingual epithelia. Am J Physiol Cell Physiol 308:C21–C32. 10.1152/ajpcell.00157.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N (2006) Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci 26:3971–3980. 10.1523/JNEUROSCI.0515-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoryanchikov G, Sinclair MS, Perea-Martinez I, Wang T, Chaudhari N (2009) Inward rectifier channel, ROMK, is localized to the apical tips of glial-like cells in mouse taste buds. J Comp Neurol 517:1–14. 10.1002/cne.22152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoryanchikov G, Huang YA, Barro-Soria R, Chaudhari N, Roper SD (2011) GABA, its receptors, and GABAergic inhibition in mouse taste buds. J Neurosci 31:5782–5791. 10.1523/JNEUROSCI.5559-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoryanchikov G, Pereira E, Williams C, Roper SD, Chaudhari N (2013) GABA as afferent taste neurotransmitter. Chem Senses 38:e81. [Google Scholar]
- Dvoryanchikov G, Hernandez D, Roebber JK, Hill DL, Roper SD, Chaudhari N (2017) Transcriptomes and neurotransmitter profiles of classes of gustatory and somatosensory neurons in the geniculate ganglion. Nat Commun 8:760. 10.1038/s41467-017-01095-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Barres BA (2010) Regulation of synaptic connectivity by glia. Nature 468:223–231. 10.1038/nature09612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy-Tselnicker I, Allen NJ (2018) Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural Dev 13:7. 10.1186/s13064-018-0104-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC (2005) ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310:1495–1499. 10.1126/science.1118435 [DOI] [PubMed] [Google Scholar]
- Guarascio DM, Gonzalez-Velandia KY, Hernandez-Clavijo A, Menini A, Pifferi S (2021) Functional expression of TMEM16A in taste bud cells. J Physiol 599:3697–3714. 10.1113/JP281645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG (2007) The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13:54–63. 10.1016/j.molmed.2006.12.005 [DOI] [PubMed] [Google Scholar]
- Hayato R, Ohtubo Y, Yoshii K (2007) Functional expression of ionotropic purinergic receptors on mouse taste bud cells. J Physiol 584:473–488. 10.1113/jphysiol.2007.138370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Roper SD (2010) Intracellular Ca(2+) and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol 588:2343–2350. 10.1113/jphysiol.2010.191106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Dando R, Roper SD (2009) Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci 29:13909–13918. 10.1523/JNEUROSCI.2351-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Stone LM, Pereira E, Yang R, Kinnamon JC, Dvoryanchikov G, Chaudhari N, Finger TE, Kinnamon SC, Roper SD (2011) Knocking out P2X receptors reduces transmitter secretion in taste buds. J Neurosci 31:13654–13661. 10.1523/JNEUROSCI.3356-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD (2007) The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA 104:6436–6441. 10.1073/pnas.0611280104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaewkhaw R, Scutt AM, Haycock JW (2012) Integrated culture and purification of rat Schwann cells from freshly isolated adult tissue. Nat Protoc 7:1996–2004. 10.1038/nprot.2012.118 [DOI] [PubMed] [Google Scholar]
- Kim JW, Roberts C, Maruyama Y, Berg S, Roper S, Chaudhari N (2006) Faithful expression of GFP from the PLCbeta2 promoter in a functional class of taste receptor cells. Chem Senses 31:213–219. 10.1093/chemse/bjj021 [DOI] [PubMed] [Google Scholar]
- Kim YV, Bobkov YV, Kolesnikov SS (2000) Adenosine triphosphate mobilizes cytosolic calcium and modulates ionic currents in mouse taste receptor cells. Neurosci Lett 290:165–168. 10.1016/s0304-3940(00)01342-2 [DOI] [PubMed] [Google Scholar]
- Kozlov AS, Angulo MC, Audinat E, Charpak S (2006) Target cell-specific modulation of neuronal activity by astrocytes. Proc Natl Acad Sci USA 103:10058–10063. 10.1073/pnas.0603741103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson ED, Vandenbeuch A, Anderson CB, Kinnamon SC (2021) GAD65Cre drives reporter expression in multiple taste cell types. Chem Senses 46:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard EM, Salman S, Nurse CA (2018) Sensory processing and integration at the carotid body tripartite synapse: neurotransmitter functions and effects of chronic hypoxia. Front Physiol 9:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski BC, Sukumaran SK, Margolskee RF, Bachmanov AA (2016) Amiloride-insensitive salt taste is mediated by two populations of type III taste cells with distinct transduction mechanisms. J Neurosci 36:1942–1953. 10.1523/JNEUROSCI.2947-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Zhang YV, Montell C (2014) Peripheral coding of taste. Neuron 81:984–1000. 10.1016/j.neuron.2014.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lines J, Martin ED, Kofuji P, Aguilar J, Araque A (2020) Astrocytes modulate sensory-evoked neuronal network activity. Nat Commun 11:3689. 10.1038/s41467-020-17536-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossow K, Hubner S, Roudnitzky N, Slack JP, Pollastro F, Behrens M, Meyerhof W (2016) Comprehensive analysis of mouse bitter taste receptors reveals different molecular receptive ranges for orthologous receptors in mice and humans. J Biol Chem 291:15358–15377. 10.1074/jbc.M116.718544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Taruno A, Ohmoto M, Jyotaki M, Lim JC, Miyazaki H, Niisato N, Marunaka Y, Lee RJ, Hoff H, Payne R, Demuro A, Parker I, Mitchell CH, Henao-Mejia J, Tanis JE, Matsumoto I, Tordoff MG, Foskett JK (2018) CALHM3 is essential for rapid ion channel-mediated purinergic neurotransmission of GPCR-mediated tastes. Neuron 98:547–561.e10. 10.1016/j.neuron.2018.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Bajo-Graneras R, Moratalla R, Perea G, Araque A (2015) Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349:730–734. 10.1126/science.aaa7945 [DOI] [PubMed] [Google Scholar]
- Medler KF, Margolskee RF, Kinnamon SC (2003) Electrophysiological characterization of voltage-gated currents in defined taste cell types of mice. J Neurosci 23:2608–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M (2010) The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses 35:157–170. 10.1093/chemse/bjp092 [DOI] [PubMed] [Google Scholar]
- Murata Y, Yasuo T, Yoshida R, Obata K, Yanagawa Y, Margolskee RF, Ninomiya Y (2010) Action potential-enhanced ATP release from taste cells through hemichannels. J Neurophysiol 104:896–901. 10.1152/jn.00414.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA (2003) New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26:523–530. 10.1016/j.tins.2003.08.008 [DOI] [PubMed] [Google Scholar]
- Nomura K, Nakanishi M, Ishidate F, Iwata K, Taruno A (2020) All-electrical Ca(2+)-independent signal transduction mediates attractive sodium taste in taste buds. Neuron 106:816–829.e816. 10.1016/j.neuron.2020.03.006 [DOI] [PubMed] [Google Scholar]
- Ogata T, Ohtubo Y (2020) Quantitative analysis of taste bud cell numbers in the circumvallate and foliate taste buds of mice. Chem Senses 45:261–273. 10.1093/chemse/bjaa017 [DOI] [PubMed] [Google Scholar]
- Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C, Saez JC (2011) ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem 118:826–840. 10.1111/j.1471-4159.2011.07210.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papathanou M, Dumas S, Pettersson H, Olson L, Wallen-Mackenzie A (2019) Off-target effects in transgenic mice: characterization of dopamine transporter (DAT)-Cre transgenic mouse lines exposes multiple non-dopaminergic neuronal clusters available for selective targeting within limbic neurocircuitry. eNeuro 6:ENEURO.0198-19.2019. 10.1523/ENEURO.0198-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouin T, Dunphy J, Tolman M, Foley JC, Haydon PG (2017) Astrocytic control of synaptic function. Philos Trans R Soc Lond B Biol Sci 372:20160154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG (1994) Glutamate-mediated astrocyte-neuron signalling. Nature 369:744–747. 10.1038/369744a0 [DOI] [PubMed] [Google Scholar]
- Parpura V, Scemes E, Spray DC (2004) Mechanisms of glutamate release from astrocytes: gap junction 'hemichannels,' purinergic receptors and exocytotic release. Neurochem Int 45:259–264. 10.1016/j.neuint.2003.12.011 [DOI] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G (1997) Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci 17:7817–7830. 10.1523/JNEUROSCI.17-20-07817.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin DW, Yu C, Smith DV (1997) Light and dark cells of rat vallate taste buds are morphologically distinct cell types. J Comp Neurol 378:389–410. [DOI] [PubMed] [Google Scholar]
- Raqeeb A, Sheng J, Ao N, Braun AP (2011) Purinergic P2Y2 receptors mediate rapid Ca(2+) mobilization, membrane hyperpolarization and nitric oxide production in human vascular endothelial cells. Cell Calcium 49:240–248. 10.1016/j.ceca.2011.02.008 [DOI] [PubMed] [Google Scholar]
- Roebber JK, Roper SD, Chaudhari N (2019) The role of the anion in salt (NaCl) detection by mouse taste buds. J Neurosci 39:6224–6232. 10.1523/JNEUROSCI.2367-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Lasher RS, High B, Savidge LE, Lawson A, Rogachevskaja OA, Zhao H, Rogachevsky VV, Bystrova MF, Churbanov GD, Adameyko I, Harkany T, Yang R, Kidd GJ, Marambaud P, Kinnamon JC, Kolesnikov SS, Finger TE (2018) Chemical synapses without synaptic vesicles: purinergic neurotransmission through a CALHM1 channel-mitochondrial signaling complex. Sci Signal 11:eaao1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD, Chaudhari N (2017) Taste buds: cells, signals and synapses. Nat Rev Neurosci 18:485–497. 10.1038/nrn.2017.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B (2008) Neuron-astrocyte signaling in the development and plasticity of neural circuits. Neurosignals 16:278–288. 10.1159/000123038 [DOI] [PubMed] [Google Scholar]
- Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK (2013) CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495:223–226. 10.1038/nature11906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taruno A, Nomura K, Kusakizako T, Ma Z, Nureki O, Foskett JK (2021) Taste transduction and channel synapses in taste buds. Pflugers Arch 473:3–13. 10.1007/s00424-020-02464-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu YH, Cooper AJ, Teng B, Chang RB, Artiga DJ, Turner HN, Mulhall EM, Ye W, Smith AD, Liman ER (2018) An evolutionarily conserved gene family encodes proton-selective ion channels. Science 359:1047–1050. 10.1126/science.aao3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung K, Tepe B, Pekarek B, Arenkiel BR, Deneen B (2020) Parallel astrocyte calcium signaling modulates olfactory bulb responses. J Neurosci Res 98:1605–1618. 10.1002/jnr.24634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Anderson CB, Parnes J, Enjyoji K, Robson SC, Finger TE, Kinnamon SC (2013) Role of the ectonucleotidase NTPDase2 in taste bud function. Proc Natl Acad Sci USA 110:14789–14794. 10.1073/pnas.1309468110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kugelgen I, Hoffmann K (2016) Pharmacology and structure of P2Y receptors. Neuropharmacology 104:50–61. 10.1016/j.neuropharm.2015.10.030 [DOI] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M (2006) Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci 9:816–823. 10.1038/nn1703 [DOI] [PubMed] [Google Scholar]
- Woo J, Min JO, Kang DS, Kim YS, Jung GH, Park HJ, Kim S, An H, Kwon J, Kim J, Shim I, Kim HG, Lee CJ, Yoon BE (2018) Control of motor coordination by astrocytic tonic GABA release through modulation of excitation/inhibition balance in cerebellum. Proc Natl Acad Sci USA 115:5004–5009. 10.1073/pnas.1721187115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Dzowo YK, Wilson CE, Russell RL, Kidd GJ, Salcedo E, Lasher RS, Kinnamon JC, Finger TE (2020) Three-dimensional reconstructions of mouse circumvallate taste buds using serial blockface scanning electron microscopy: I. Cell types and the apical region of the taste bud. J Comp Neurol 528:756–771. 10.1002/cne.24779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella R, Zanghirati G, Cavicchioli R, Zanni L, Boccacci P, Bertero M, Vicidomini G (2013) Towards real-time image deconvolution: application to confocal and STED microscopy. Sci Rep 3:2523. 10.1038/srep02523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Wang D, Wang JH (2012) Barrel cortical neurons and astrocytes coordinately respond to an increased whisker stimulus frequency. Mol Brain 5:12. 10.1186/1756-6606-5-12 [DOI] [PMC free article] [PubMed] [Google Scholar]