Abstract
Objective
To evaluate the effectiveness and safety of intrathecal baclofen treatment of spasticity, administered via a cervical catheter tip.
Design
A review of PubMed and the Cochrane Library up to September 2020. No restriction in study design. Two reviewers independently evaluated eligibility, extracted data and evaluated risk of bias. Studies were included in which patients were treated with intrathecal baclofen for spasticity, with the catheter tip at or above the first thoracic level, independent of diagnosis and age.
Results
Thirteen studies were eligible, with a moderate to critical risk of bias. Improvement in spasticity was seen only in the upper extremity in 6% of subjects, only in the lower extremity in 2%, in both upper and lower extremities in 50% and without specification of location in 41%. Upper extremity function improved in 88% of cases. Neither drug-related (1%) nor technical (21%) complications occurred more often than in lower placement of the tip. Effects on respiratory function and sleep apnoea were not investigated.
Conclusion
Cervically administered intrathecal baclofen seems to improve upper extremity spasticity and function, without causing more complications than thoracolumbar intrathecal baclofen. However, the mainly drug-related complications have not been thoroughly investigated and the available literature is of poor methodological quality. Further research is needed to confirm the efficacy and safety of this procedure.
LAY ABSTRACT
Spasticity is a common complication in central neurological disorders. It can lead to discomfort and functional limitations. To reduce spasticity, administration of baclofen via a catheter into the spinal canal has been used successfully for several years. However, this treatment often has limited effects on the upper limbs. The catheter tip is often situated in the thoracolumbar region. This review suggests that baclofen treatment via a cervically located catheter tip reduces spasticity of both arms and legs. Also, arm function improved in patients with a cervical catheter tip. Neither drug-related nor technical complications occurred more often than in lower placement of the cervical catheter tip. Few studies were found on this subject, and the available literature is of poor quality. Therefore, more research is needed to confirm the positive effect of this procedure on spasticity of the arms and to monitor for complications.
Key words: intrathecal baclofen, tip placement, cervical, upper extremity, spasticity
Spasticity is a common complication after central nervous system injury with involvement of the upper motor neuron. The prevalence varies from 45% in stroke, 65% in spinal cord injury to 80% in multiple sclerosis (1–3).
Spasticity is most commonly defined as “velocity-dependent increase in the tonic stretch reflex”, but has been redefined in 2005 by the European working group EUSPASM as “disordered sensori-motor control, resulting from an upper motor neuron lesion, presenting as intermittent or sustained involuntary activation of muscles” (4, 5). Depending on the severity, spasticity can negatively influence voluntary movement and lead to discomfort and functional limitations (5, 6).
In functionally limiting spasticity, non-invasive treatment options, such as elimination of spasm provoking stimuli and physical therapy, should be considered first. In case of insufficient effect, oral medication can be started for general spasticity, or botulinum toxin, phenol or surgery for focal spasticity (7). Baclofen is the most commonly used oral antispasmodic (8). It is a centrally-acting gamma aminobutyric acid (GABA)-B agonist that works as muscle relaxant by diminishing reflex transfer at the spinal cord level. As orally provided baclofen poorly crosses the blood–brain barrier, high doses may be needed to achieve a functional effect, which may induce side-effects, such as fatigue, respiratory depression and confusion. Baclofen can also be administered locally via an intrathecal catheter, resulting in fewer side-effects than systemic baclofen treatment (6). The lower occurrence of side-effects can be explained, on the one hand, by a lower required baclofen dose (as the blood–brain barrier no longer has to be crossed), resulting in fewer general side-effects, and, on the other hand, by a lower cerebral baclofen concentration, resulting in fewer central nervous side-effects.
The effect of intrathecal baclofen (ITB) on spasticity of the lower extremity (LE) has been well described in numerous studies, whereas the effect on spasticity of the upper extremity (UE) is less certain (9, 10). This difference in effectiveness might be related to the position of the intrathecal catheter tip and the baclofen gradient in the cerebrospinal fluid. The catheter tip is often positioned at the thoracic or lumbosacral level, assuming that the cerebrospinal fluid flow distributes baclofen in the intrathecal space to the required site of action. However, 2 studies that have been conducted on ITB distribution in humans show a steep concentration gradient of baclofen (11, 12). Kroin et al. found a lumbar-to-cisternal decrease in concentration of approximately 75% (11), and Heetla et al. reported a decrease in concentration from T10 to 30% at 5 cm tip distance, 12% at 10 cm and 0.7% at cerebral level (12). This baclofen concentration gradient suggests that, for optimal treatment of UE spasticity, the catheter tip should be located at the high thoracic or cervical level.
Balsara et al. and Albright et al. suggest that catheter tip position should be dependent on treatment goal: T10–12 for diplegia and C5–T2 for tetraplegia (8, 13). However, in clinical studies there is no consensus on the relationship between catheter tip position and the effect on spasticity (14, 15).
The demand for adequate treatment of UE spasticity has increased, as there is a growing number of patients with cervical spinal cord injury (16–18). A cervically positioned catheter tip could ameliorate spasmolytic effects on the UE due to higher cervical concentration of baclofen. However, it might lead to an insufficient effect on LE spasticity. Cervical ITB could also influence respiratory function and sleep-related disorders, either positively or negatively (19–21). Furthermore, a high cervical concentration of baclofen could increase cerebral side-effects and thereby influence cognitive and emotional functions. In this systematic review, the effectiveness and safety of baclofen treatment via a cervical catheter tip is analysed.
METHODS
Search strategy
A literature search in PubMed and The Cochrane Library was conducted to find relevant English or Dutch articles, without a restriction on publication period or study design. Key words used were “intrathecal baclofen”, “upper extremity”, “arm”, “upper limb”, “cervical” and “tip” (Table I). Reference lists were checked for eligible studies. Trial registers (Prospero, ClinicalTrials.gov, www.trialregister.nl) were searched for ongoing trials.
Table I.
Search strategy
| Database | Date of search | Query | Results |
|---|---|---|---|
| PubMed | 25 Sept 2020 | (((((intrathecal baclofen) AND ((upper extremity) OR (upper extremit*))) OR ((intrathecal baclofen) AND (arm OR arms))) OR ((intrathecal baclofen) AND ((upper limb) OR (upper limb*)))) OR ((intrathecal baclofen) AND (cervical))) OR ((intrathecal baclofen) AND (tip)) | 180 |
| PubMed | 25 Sept 2020 | (intrathecal baclofen) AND (tip) | 41 |
| PubMed | 25 Sept 2020 | (intrathecal baclofen) AND (cervical) | 56 |
| PubMed | 25 Sept 2020 | (intrathecal baclofen) AND ((upper limb) OR (upper limb*)) | 68 |
| PubMed | 25 Sept 2020 | (intrathecal baclofen) AND (arm OR arms) | 19 |
| PubMed | 25 Sept 2020 | (intrathecal baclofen) AND ((upper extremity) OR (upper extremit*)) | 61 |
| The Cochrane Library | 6 Jan 2020 | intrathecal baclofen | 13 |
Study selection
Studies were included in which patients with spasticity were treated with ITB, with the catheter tip at a cervical vertebral level or at the first thoracic vertebral level. Inclusion was independent of diagnosis and age, because of the limited studies available on cervical ITB. Reviews were excluded because none of them specifically described the effect of cervical ITB. Prior to exclusion, the reviews were checked for relevant references, which were added to the screening list for eligibility in the current review. Also, relevant references of the other included studies were added to this screening list. Two reviewers (NJ, EM) independently examined study eligibility. In case of unknown catheter tip height (n = 21), authors were asked for information about catheter position by e-mail, with a reminder after 2 weeks. Three reactions were received: 2 studies concerned low tip placement, in one study tip location was unclear. The low response is probably due to dated studies: 18 of 21 studies with unknown tip height were published before 2015. The author of a study in which only one participant received cervical ITB was requested to send specific information regarding this case; however, no reaction was obtained and the study was excluded (22). Also, specific outcomes regarding the cervical ITB patients (n = 9, total n = 166) in a study was requested, without response (14). Therefore, this study was also excluded. Three studies were added to the review via references. Of these studies, one was not available in the databases searched (23), and the other 2 did not focus on upper limb function (24, 25), which explained why they were not selected using the key words used in the current study.
Data collection and analysis
Two reviewers (NJ, EM) independently assessed risk of bias of the selected studies and extracted data regarding study design, intervention details, number of participants, intervention details and outcomes (spasticity, UE function, complications, additional findings). In case of disagreement, a third reviewer was involved in the discussion (MB). Risk of bias was evaluated as low, moderate, serious or critical, using the ROBINS tool for the cohort study and the tool developed by Murad et al. for case reports and case series (26, 27).
Measurement scales
The following scales were used in the selected studies:
Ashworth Scale (AS): a 5-point scale [1–5], in which a score of 1 indicates no increase in muscle tone and 5 indicates that the affected part(s) is rigid in flexion or extension (28).
Modified Ashworth Scale (MAS): this scale conforms with AS, but is scored from 0 (no increase in tone) to 4 (rigid). A “1+” (catch, followed by minimal resistance) category is added between scores 1 and 2 to make the scale more discrete (29).
Spasm Frequency Score (SFS): a 5-point scale that assesses the frequency at which spasms occur, ranging from 0 (no spasms) to 4 (spontaneous spasms occurring more than 10 times per hour) (30).
Reflex Score (RS): a 6-point scale [0–5] that assesses the severity of reflexes; 0 indicates areflexia and 5 hyperreflexia with frank clonus (31).
Melbourne assessment of Unilateral Upper Limb scale (MUUL): range 0–122, assesses reach, grasp, release and manipulation; a higher score indicates better function (32).
Barry Albright scale for the evaluation of Dystonia (BAD): a 5-point scale for 8 body regions (total score 0–32), in which 0 means no dystonia and 4 severe dystonia that prevents functional use of the body part (33).
Burke-Fahn-Marsden rating scale (BFM): consists of a movement subscale [0–120], which evaluates involuntary movements of 9 body regions, and a disability subscale [1–30], which evaluates disability in 7 daily activities. In both subscales, higher values indicate more severe dystonia (34).
Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS): rates cervical dystonia, consisting of severity [0–35], disability [0–32] and pain [0–20] subscores (35).
RESULTS
Description of studies
Thirteen eligible studies were found (Fig. 1): 1 cohort study (15), 11 case series (23–25, 31, 36–42), and 1 case report (43). There were no randomized controlled trials on cervical ITB. No ongoing trials were found in trial registers. In total, 121 patients with a tip at or above vertebral level T1 were included. Risk of bias was moderate in 5 (15, 23, 40–42), serious in 6 (15, 25, 31, 36–38, 43) and critical in 2 (24, 39) studies (Appendix 1 and Appendix 2). Risk of bias of the study of McCall & MacDonald was evaluated for 2 different outcomes: it was moderate for Ashworth and serious for complications (15). Study characteristics and outcomes are shown in Table II.
Fig. 1.
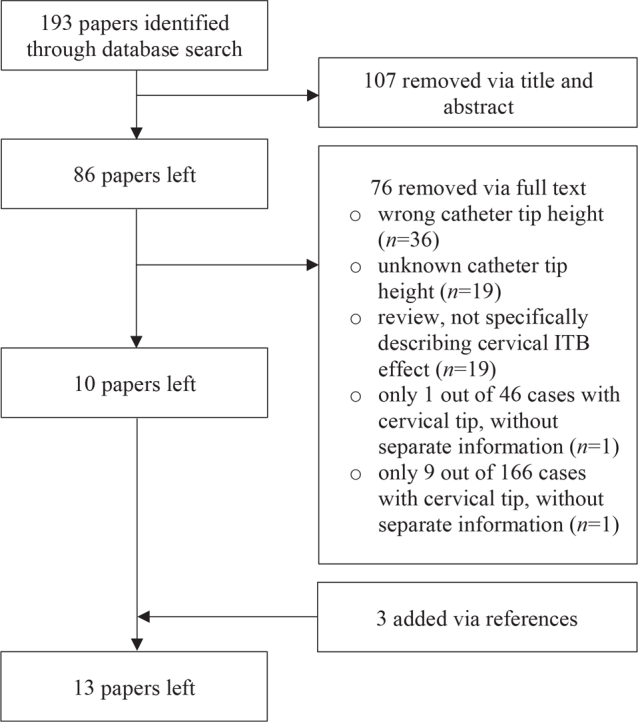
Study selection.
Table II.
Study characteristics and outcomes
| Author, year (ref) Study design Risk of bias N. of participants [with catheter tip at a cervical vertebral level or at the first thoracic vertebral level] |
Tip height, dose [number of casesa] | Spasticity [number of casesa] | UE function [number of casesa] | Complications [number of casesa] | Other outcomes [number of casesa] |
|---|---|---|---|---|---|
| Chang and Ehsan 2018 (36) case series serious n = 2 [2] |
C5–C6, bolus 100 μg + continuous 133 μg/day [1], C5, continuous 650 μg/day [1] | MAS: pre-ITB 3 (UE), post-ITB 1–2 (overall); spasms: reduced [1] Spasticity control: improved (UE), unchanged (LE) [1] | Improved [1] | No information | Motor strength proximal UE: pre-ITB 2–3/5, post-ITB 4/5 [1] Pain: decreased [1] ROM: improved [1] Mood, comfort and ease of care: improved [1] |
| Aljuboori et al. 2018 (37) case series serious n = 5 [4] |
C2–C7, unknown [4] | Tone: stable [1], improved in UE and LE [1], improved in UE and trunk, stable in LE [1], improved in LE, stable in UE and trunk [1] | Improved [1] | Drug-related: 0 Non-drug-related: 0 |
Gait: improved [1] |
| Roscher et al. 2016 (43) case report serious n = 1 [1] |
C5–C6, unknown [1] | MAS: pre-ITB 2 (UE), post-ITB 0 (UE) [1] | NA | Drug-related: 0 Non-drug-related: 0 |
NA |
| Ughratdar et al. 2012 (38) case series serious n = 20 [5] |
C5-T1, unknown [5] | Patient and caregivers: improved [5] | NA | Drug-related: 0 Non-drug-related: infection [1] |
Facilitating nursing care: improved [5] |
| Muquit et al. 2012 (39) case series critical n = 20 [20] |
Catheter passed for up to 10 cm from lowest 2 cervical levels, bolus 50 μg [20] | NA | NA | Drug-related: 0 Non-drug-related: 0 |
NA |
| Hamed et al. 2011 (40) case series moderate n = 3 [3] |
T1, bolus 50 μg [3], continuous 200–700 μg/day [3] | MAS: pre-ITB 3–4 (UE and LE), post-ITB 0–3 (UE), 1–2 (LE) [3] | NA | No information | No weakness or function loss: contralateral [3], in LE [1] Speech: improved [1] Self-care: improved [1] Gait: improved [2] |
| Motta et al. 2009 (41) case series moderate n = 11 [11] |
C1, continuous mean 311 μg/day (SD 133) [11] | NA | MUUL: pre-ITB 46.42 (dominant limb, SD 19.6), 32.19 (non-dominant limb, SD 18.9), post-ITB 55.44 (dominant limb, SD 17.4), 40.61 (non-dominant limb, SD 15) [11] Caregivers: improved [11] | Drug-related: 0 Non-drug-related: catheter rupture [1] |
BAD-related to UE: pre-ITB 6.7 (SD 1.2); post-ITB 5 (SD 1.3) [11] Caregivers: improved patient management [11] |
| Motta et al. 2008 (23) case series moderate n = 19 [19] |
C1, continuous [18] and boli [1], dose unknown | NA | Patients, caregivers: much improved [1], improved [5], slightly improved [9], unchanged [3], worsened [1] | Drug-related: 0 Non-drug-related: CSF leakage [4], infection [1], catheter breakage [1] |
BAD: pre-ITB 23.84 (SD 4.11), post-ITB 17.79 (SD 3.3) [19] BFM: pre-ITB 98, 57 (SD 13.07), post-ITB 77.60 (SD 20.56); no change in everyday activities [19] Patients, caregivers [improvement]: dystonia [18], hygiene [13], dressing [18], control head [11], control trunk [13], sitting [17], autonomy [7], mood [9], sleeping [10], pain [10] |
| McCall and MacDonald 2006 (15) retrospective cohort study moderate (for Ashworth) serious (for complications) n = 48 ([23] |
C5–C7, mean 306 μg/day [23] | Ashworth: pre-ITB 4.0 (UE, SD 0.8), 4.0 (LE, SD 0.9), post-ITB 3.0 (UE, SD 0.9), 3.1 (LE, SD 1.0) [23] | NA | Drug-related: somnolence leading to aspiration pneumonia [1] Non-drug-related: broken or retracted catheter [2], malfunction of baclofen pump and catheter system of unidentified origin [3], pump flipping over [1], pump infection [2], pseudomeningocele [1] |
NA |
| Dykstra et al. 2005 (42) case series moderate n = 2 [2] |
C1–C3, continuous 186.1 μg/day [1], boli 50 μg every 4 h [1] | NA | Handwriting: improved [1] | Drug-related: 0 Non-drug-related: CSF leakage [1], catheter breakage [1] |
TWSTRS: pre-ITB 49–72; post-ITB 18–32 [2] Cervical ROM: improved [2] Neck pain: almost gone [1] |
| Chappuis et al. 2001 (24) case series serious n = 15 [15] |
C5–T5, continuous, dose unknown [15] | Tone: better controlled in UE, head and neck [unknown] | Significant improved [unknown] | Drug-related: somnolence, urine retention [unknown] Non-drug-related: unknown | Speech and swallowing: improved (unknown) |
| Conçalves et al. 1994 (31) case series serious n = 11 [11] |
C4, bolus 12.5–75 μg [11], continuous mean 145 μg/day [4]; multistep base infusion 72 μg/day plus 3–4 boli (mean dose 25 μg) [7] | Ashworth: pre-ITB 3-4, post-ITB 1–3 [11] Spasm: pre-ITB 0–4, post-ITB 0–1 [11] Reflexes: pre-ITB 3–5, post-ITB 2–3 [11] | NA | Drug-related: 0 Non-drug-related: infection [1], catheter migration [1], transient pump malfunction [1] |
Bladder control: improved [3] Level of consciousness: improved [3] Speech: improved [3] Transfer: improved [2] Gait: improved [1] |
| Broseta et al. 1990 (25) case series critical n = 19 [5] |
C4, bolus 12.5–100 μg [5], continuous 25–210 μg/day [4], multistep 260 μg/day [1] | Ashworth: pre-ITB 3–5, post-ITB 1–3 [5] Spasm: pre-ITB 0–4, post-ITB 0 [5] Reflexes: pre-ITB 3–4, post-ITB 2–3 [5] | NA | Drug-related: 0 Non-drug-related: seroma [1], skin erosion on pump attachment [1] |
Level of consciousness: improved [2] Pain: improved [3] Gait: improved [2] |
Only participants with catheter tip at a cervical vertebral level or at the first thoracic vertebral level.
BAD: Barry Albright scale for the evaluation of dystonia; BFM: Burke-Fahn-Marsden rating scale; ITB: intrathecal baclofen; LE: lower extremity; MAS: Modified Ashworth scale; MUUL: Melbourne assessment of unilateral upper limb scale; N: number; NA: not available; ROM: range of motion; TWSTRS: Toronto Western Spasmodic Torticollis Rating Scale; UE: upper extremity.
Description of results
Spasticity
Spasticity was evaluated in 9 studies (15, 24, 25, 31, 36–38, 40, 43). In total, the effect of cervical ITB on spasticity has been described in 54 patients.
In all but one patient, improvement was reported. This concerned improvement only in UE spasticity in 3 cases (6%), mixed improvement in UE and LE spasticity in 27 cases (50%), improvement in only LE spasticity in 1 case (2%), and spasticity improvement without specification of location in 22 cases (41%). In the only patient without improvement, a stable tone was reported with cervical ITB treatment.
Spasticity was most often evaluated using the (modified) Ashworth scale. All 6 studies that used AS/MAS reported a lower score with ITB treatment: MAS score improved from 2–4 without ITB to 0–3 with ITB (36, 40, 41); AS improved from 3–5 to 1–3.1 (15, 25, 31). SPS improved in both studies in which it was evaluated: range from 0–4 before treatment to 0–1 after treatment (25, 31). Also, RF improved: from 3–5 before start of ITB to 2–3 with ITB (25, 31). Four studies described a subjective improvement in tone or spasticity control (24, 36–38). Spasticity did not become worse in any study.
Six studies specifically reported spasticity effects on UE (15, 24, 36, 37, 40, 43). Aljuboori et al. described UE tone improvement in 2 cases and a stable tone in the other 2 cases, of which 1 showed LE tone improvement (37). The other studies mentioned only positive effects: MAS improved from 2–4 without ITB to 0–3 with ITB (15, 36, 40, 43) and subjective tone improvement was noted (36, 25).
Four studies compared changes in spasticity effects on UE and LE (Table II) (15, 36, 37, 40). In 2 patients improvement in UE tone occurred, without change in LE (36, 37). One patient showed improvement only in LE tone (37). In most patients however, spasticity of both UE and LE improved with ITB treatment (15, 37, 40). In these studies Aljuboori et al. did not quantify the degree of improvement in tone (37). Hamed et al. described MAS improvement from 3–4 in UE and LE before ITB to 0–3 in UE and 1–2 in LE after ITB (40). McCall and MacDonald found a significant improvement on the AS for both UE (4.0 (SD 0.8) to 3.0 (SD 0.9)) and LE (4.0 (SD 0.9) to 3.1 (SD 1.0)) with cervical ITB treatment, whereas their thoracic tip control group showed only a significant difference on LE (3.5 (SD 0.7) to 2.3 (SD 1.2)) (15). Improvement in UE AS (2.6 (SD 1.5) to 2.1 (SD 1.2)) was not significant in the thoracic group.
Upper extremity function
Six studies reported an effect of the ITB treatment on UE function (Table II) (23, 24, 36, 37, 41, 42), describing a total of 33 patients. UE function improved in 29 patients (88%), remained stable in 3 (9%), and worsened in 1 (3%). Only one study used a scale (MUUL) to demonstrate the effect, which was positive: dominant limb score improved from 46.42 (SD 19.6) to 55.44 (SD 17.4) (41). Other studies noted subjective positive effects on handwriting, dressing, bringing hand to mouth and driving a powered wheelchair (36, 37, 42). One study reported improvement in 15 patients, but unchanged function in 3 patients and worsened function in 1; specification of these effects was not reported (23).
Complications
Complications of the cervical ITB treatment were divided into 2 groups: drug-related (i.e. side-effects of baclofen) and non-drug-related (other adverse events, such as pump and/or catheter-related problems or infection).
In all but 2 studies included in this review (36, 40), complications were reported. This concerned a total of 116 cases. Only 1 case with drug-related complications was reported (1% of the 116 included cases) (15). However, Chappuis et al. found that somnolence and urine retention were the most seen complications, although without reporting the number of cases (24). The reported drug-related case concerned somnolence leading to aspiration pneumonia (15). The somnolence was related to the use of both cervical ITB and oral baclofen and improved after discontinuation of oral baclofen without change in ITB. Two studies explicitly denied respiratory depression; however, they did not mention testing this complication (31, 41).
Non-drug-related complications occurred more often: they were reported in 8 studies, involving a total of 24 cases (21% of the 116 patients with reported complications). Most common were catheter fracture (n = 5, 4%), infection (n = 5, 4%) and cerebrospinal fluid leakage (n = 5, 4%).
Other outcomes
Several other effects of ITB treatment were observed (Table II). Subjective functional improvements were seen in gait (n = 6), ease of (self-)care (n = 31) and speech and/or swallowing (n = 4) (23–25, 31, 36–38, 40, 41). Also, mood (n = 10) and level of consciousness (n = 5) ameliorated (23, 25, 31, 36).
One study stated a preserved contralateral and/or LE function in unaffected extremities (40). Chang & Ehsan reported improvement in motor strength in UE in one case from 2–3/5 to 4/5 (36).
Dystonia was assessed in 3 studies, using the BAD, BFM, TWSTRS, and a patient and caregiver questionnaire (23, 41, 42). Improvement was seen in all dystonia evaluations.
DISCUSSION
ITB via a thoracolumbar catheter is an effective and safe treatment for LE spasticity (9). However, it often has limited effects on the upper limbs (44–46). This review suggests that baclofen treatment via a cervically located catheter tip reduces spasticity and improves function of the UE without causing more complications, but confirmatory research is needed because of the moderate to poor methodological quality of the current studies.
Studies
This literature search found few studies on cervically administered ITB. Most studies have a low level of evidence: 11 case series (23–26, 31, 36–42), 1 case report (43). One retrospective cohort study was included (15). All studies had moderate to critical risk of bias. Common and important risks of bias were inadequate ascertainment of outcome, insufficient evaluation of co-interventions, and insufficient reporting of research procedure. No randomized controlled trials have been published on this subject. Moreover, the available stud ies do not answer all our questions. Complications were often described in a limited way, without specific evaluation of respiratory function, sleep-related disorders, effects on cognitive or emotional function or gait. Regarding efficacy, only 9 studies described effects on spasticity (15, 24, 25, 31, 36–38, 40, 41). Three studies concerned cervical ITB in patients with dystonia (23, 41, 42) and one study focused on surgical aspects of cervical catheter placement, rather than the effect on spasticity (39). Six studies evaluated effects on UE function (23, 24, 36, 37, 41, 42). To describe efficacy, different measurement tools were used, which complicated comparison of the studies. With the currently available data, it is impossible to perform a meta-analysis.
Pharmacodynamics in intrathecal baclofen
The effect of ITB on spasticity of the LE has been described in numerous studies, whereas the effect on the UE seems to be less certain (9). This difference in effectiveness might be related to the steep baclofen gradient in the cerebrospinal fluid. The ITB gradient has been demonstrated in 2 studies, in which baclofen was administered at L3 and T10, with concentrations dropping to 24% at cisternal level and 0.7% at cerebral level, respectively (11, 12). The catheter tip is often positioned in the thoracic and lumbosacral level and consequently, baclofen concentration at the cervical region could be too low to cause spasmolytic effects on the UE (9, 47).
Factors that contribute to ITB drug distribution are infusion rate and location of infusion. Concerning infusion rate, a lumbar quick bolus raises cervical concentration more than lumbar slow continuous infusion (47, 48). To achieve maximal distribution, a low concentration of baclofen could be administered at a fast infusion rate. The disadvantage of this is that the pump empties quickly. As regards infusion location, it is important to know that cerebrospinal fluid flows rapidly at the cervical region, whereas there is hardly any flow at the lumbar level (49). Therefore, lumbar-administered baclofen is less likely to distribute towards the cervical spine, whereas cervical administration will probably lead to wider distribution.
Spasticity
Based on the steep concentration gradient of ITB, as demonstrated by Kroin et al. and Heetla et al. (11, 12), it was expected that cervically administrated baclofen would lead to better control of UE spasticity. However, with cervical administration, effect on LE spasticity might be insufficient. This was not confirmed in this review: in most patients both UE and LE spasticity improved (15, 36, 37, 40). The effect on LE despite administration at a high intrathecal level might be explained by the faster cerebrospinal fluid flow at cervical region compared with the flow at lumbar level (49), which causes high baclofen concentrations at greater tip distance.
Only one study evaluated effect differences between cervical and thoracic tip placement: improvement in UE and LE spasticity was seen with a cervical tip, whereas only LE spasticity improved with a thoracic tip (15). This finding fits the assumption that thoracically administered baclofen causes too low cervical concentrations and therefore does not act on the UE. However, this is a retrospective study with a moderate risk of bias in spasticity measurement.
Upper extremity function
An important reason to treat UE spasticity is functional limitations. It was expected that better control of UE spasticity would lead to a functional amelioration. All included studies that assessed UE function stated functional improvement with cervical ITB treatment (23, 24, 36, 37, 41, 42). One study reported deteriorated function in one patient, without further specification (23). Only one study used a scale to measure functional improvement, which demonstrated improvement on the MUUL from 46.42 (SD 19.6) to 55.44 (SD 17.4) (41). However, an improvement of at least 14 points on the MUUL scale is needed to reflect a true change in function (minimal clinically important difference) rather than an error in measurement (32). The subjective improvement reported in multiple studies suggests a positive effect of cervical ITB on UE function, although objective research is needed to confirm this effect and to specify the degree of improvement.
Complications
According to the literature, ITB causes drug-related side-effects in 4.4–54% of patients, most commonly drowsiness, dizziness, constipation and hypotonia (50, 51). Also, brainstem effects have been reported, e.g. respiratory depression, hypotension, bradycardia and coma (51). Other central nervous side-effects include confusion and psychological symptoms (6, 9). The catheter tip is usually placed at the lumbothoracic level, because it is thought that higher tip placement raises the concentration of baclofen at the brainstem and cerebral level and thereby reduces central nervous side-effects (6, 9).
In this review, drug-related complications were reported in 1% of all cases. This is less than reported in other literature (50), possibly due to more careful titration due to fear of side-effects with a cervical tip, or due to insufficient registration of side-effects. Moreover, Chappuis et al. reported urine retention and somnolence in an unknown number of cases (24). Because of the unknown number of cases, this could not be included in the calculated percentage of complications.
The reported somnolence could be explained by co-administered oral baclofen in the titration phase of the ITB; after discontinuation of oral baclofen mental status improved (15). In this study, no drug-related events were reported in the thoracic ITB group. Regard ing respiratory function and sleep-related disorders, cervical ITB might influence either positively, due to reduced spasticity of thoracic muscles, or negatively, due to central side-effects or peripheral muscle weakness. Previous research demonstrated positive effects of continuous thoracic ITB on respiratory function (21); however, also a worsening of sleep apnoea syndrome on boluses (20). The current review found no reported effects on respiratory function and sleep apnoea syndrome.
Another theoretically possible complication of ITB is deterioration in gait function due to reduced LE strength. This particularly involves patients with spasticity of the UE and preserved LE function, such as in central cord syndrome. The unwanted effect of ITB on the LE could occur despite cervical tip placement, as LE spasticity can be treated using cervical ITB, as mentioned above. In this review, gait improvement was reported; however, this concerned cases with LE spasticity (25, 31, 37, 40). The ITB effect on gait function in patients with only UE spasticity remains unknown. Since no weakness or function loss was observed on the healthy contralateral side (40), a limited negative effect of cervical ITB would be expected, although confirmatory studies are needed.
Non-drug-related complications happened more often than drug-related problems, which is also the case in lower positioned ITB (9). Technical complications might happen more often in cervical ITB, due to the long intrathecal part of the catheter, which could increase risk of migration and fracture (6). However, McCall & MacDonald found no relation between tip height and technical complications (15). In this review, 21% of patients had a non-drug-related complication; according to the literature this occurs in 20–36% of patients with ITB treatment (50). To prevent a long intrathecal catheter, a C1–2 entry site could be an alternative for the traditional lumbar entry site (37). This might be particularly useful in patients with (future) scoliosis correction, since the entrance at vertebral level C1–2 ensures that the ITB catheter does not cross the surgical region of the thoracolumbar vertebrae and the thoracolumbar spinal canal.
CONCLUSION
This review shows that ITB administered via a cervically located catheter tip results in improvement in UE spasticity and function (15, 23, 24, 36, 37, 40–43). Cervical tip placement has shown to be more effective to treat UE spasticity than a thoracic tip placement (15). Also LE spasticity improved with cervical ITB (15, 36, 37, 40), possibly due to the faster cerebrospinal fluid flow in the cervical region compared with the lumbar level, which causes higher concentrations of baclofen at greater distance from the tip. The effects on gait remain unknown.
The higher cervical concentration does not seem to cause more drug-related complications; however, this has not been thoroughly investigated. Effects on respiratory function and sleep apnoea mainly remain unknown. Technical complications do not occur more often in patients with a cervical tip placement.
The available literature is of a low level of evidence and has a high risk of bias. Therefore, more research is needed to confirm the efficacy and safety of cervically administered ITB.
Appendix 1. Risk of bias of included case series and case reports, evaluated according to Murad et al. (27)
| Domains | Leading explanatory questions | Chang (36) | Aljuboori (37) | Roscher (43) | Ughratdar (38) | Muquit (39) | Hamed (40) | Motta (41) | Motta (23) | Dykstra (42) | Chappuis (24) | Conçalves (31) | Broseta (25) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | 1. Does the patient(s) represent(s) the whole experience of the investigator (centre) or is the selection method unclear to the extent that other patients with similar presentation may not have been reported? | – | +/– | – | + | – | + | + | +/– | +/– | +/– | – | +/– |
| Ascertainment | 2. Was the exposure adequately ascertained? | + | +/– | +/– | – | – | + | +/– | +/– | + | +/– | +/– | +/– |
| 3. Was the outcome adequately ascertained? | – | – | + | – | – | + | + | + | +/– | unknown | – | +/– | |
| Causality | 4. Were other alternative causes that may explain the observation ruled out? | – | – | – | – | – | +/– | +/– | +/– | +/– | +/– | – | – |
| 5. Was there a challenge/rechallenge phenomenon? | – | – | – | – | – | + | – | – | +/– | – | – | – | |
| 6. Was there a dose-response effect? | + | unknown | + | +/– | +/– | + | +/– | +/– | + | +/– | +/– | +/– | |
| 7. Was follow-up long enough for outcomes to occur? | +/– | + | +/– | + | +/– | +/– | + | + | + | unknown | + | +/– | |
| Reporting | 8. Is the case(s) described with sufficient details to allow other investigators to replicate the research or to allow practitioners make inferences related to their own practice? | – | – | – | – | – | + | + | + | +/– | – | – | +/– |
| Overall Risk of Bias judgement |
Low/Moderate/Serious/Critical/No Information | serious | serious | serious | serious | critical | moderate | moderate | moderate | moderate | critical | serious | serious |
Appendix 2. Risk of bias of the included cohort study, evaluated according to ROBINS-I (26)
| Evaluated outcome | Signalling questions | McCall (15) – efficacy |
McCall (15) – safety |
||
|---|---|---|---|---|---|
| Ashworth scale |
Complications |
||||
| Answer | Risk of bias | Answer | Risk of bias | ||
| Bias due to confounding | Is there potential for confounding of the effect of intervention in this study? | Y | high | Y | high |
| Was the analysis based on splitting participants' follow-up time according to intervention received? | N | low | N | low | |
| Did the authors use an appropriate analysis method that controlled for all the important confounding domains? | N | high | NI | unknown | |
| Did the authors control for any post-intervention variables that could have been affected by the intervention? | N | low | NA | NA | |
| Did the authors use an appropriate analysis method that controlled for all the important confounding domains and for time-varying confounding? | PY | moderate | NI | unknown | |
| Where confounding domains that were controlled for measured validly and reliably by the variables available in this study? | PY | moderate | NA | NA | |
| Risk of bias judgement | moderate | serious | |||
| Bias in selection of participants into the study | Was selection of participants into the study (or into the analysis) based on participant characteristics observed after the start of intervention? | N | low | N | low |
| Do start of follow-up and start of intervention coincide for most participants? | Y | low | Y | low | |
| Risk of bias judgement | low | low | |||
| Bias in classification of interventions | Were intervention groups clearly defined? | Y | low | Y | low |
| Was the information used to define intervention groups recorded at the start of the intervention? | N | high | N | high | |
| Could classification of intervention status have been affected by knowledge of the outcome or risk of the outcome? | N | low | N | low | |
| Risk of bias judgement | moderate | moderate | |||
| Bias due to deviations from intended interventions | Were important co-interventions balanced across intervention groups? | NI | unknown | NI | unknown |
| Was the intervention implemented successfully for most participants? | Y | low | PY | moderate | |
| Did study participants adhere to the assigned intervention regimen? | NI | unknown | NI | unknown | |
| Risk of bias judgement | moderate | moderate | |||
| Bias due to missing data | Were outcome data available for all, or nearly all, participants? | Y | low | PN | moderate |
| Were participants excluded due to missing data on intervention status? | N | low | N | low | |
| Were participants excluded due to missing data on other variables needed for the analysis? | N | low | N | low | |
| Risk of bias judgement | low | moderate | |||
| Bias in measurement of outcomes | Could the outcome measure have been influenced by knowledge of the intervention received? | PN | moderate | PN | moderate |
| Were outcome assessors aware of the intervention received by study participants? | Y | high | Y | high | |
| Were the methods of outcome assessment comparable across intervention groups? | Y | low | Y | low | |
| Were any systematic errors in measurement of the outcome related to intervention received? | PY | moderate | PY | moderate | |
| Risk of bias judgement | moderate | moderate | |||
| Bias in selection of the reported result | Is the reported effect estimate likely to be selected, on the basis of the results, from multiple outcome measurements within the outcome domain? | NI | unknown | PN | moderate |
| Is the reported effect estimate likely to be selected, on the basis of the results, from multiple analyses of the intervention-outcome relationship? | N | low | N | low | |
| Is the reported effect estimate likely to be selected, on the basis of the results, from different subgroups? | N | low | N | low | |
| Risk of bias judgement | moderate | low | |||
| Overall risk of bias judgement | Low/Moderate/Serious/Critical/No Information | moderate | serious | ||
Y: yes; PY: probably yes; N: no; PN: probably no; NI: no information; NA: not applicable
REFERENCES
- 1.Schinwelski MJ, Sitek EJ, Waz P, Slawek JW. Prevalence and predictors of post-stroke spasticity and its impact on daily living and quality of life. Neurol Neurochir Pol 2019; 53: 449–457. [DOI] [PubMed] [Google Scholar]
- 2.Holtz KA, Lipson R, Noonan VK, Kwon BK, Mills PB. Prevalence and effect of problematic spasticity after traumatic spinal cord injury. Arch Phys Med Rehabil 2017; 98: 1132–1138. [DOI] [PubMed] [Google Scholar]
- 3.Patejdl R, Zettl UK. Spasticity in multiple sclerosis: contribution of inflammation, autoimmune mediated neuronal damage and therapeutic interventions. Autoimmun Rev 2017; 16: 925–936. [DOI] [PubMed] [Google Scholar]
- 4.Lance JW. Symposium synopsis. In: Feldman RG, Young RR, Koella WP, editors. Spasticity: disordered motor control. Chicago: Year Book Medical Publishers; 1980, p. 485–494. [Google Scholar]
- 5.Pandyan AD, Gregoric M, Barnes MP, Wood D, Van Wijck F, Burridge J, et al. Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil 2005; 27: 2–6. [DOI] [PubMed] [Google Scholar]
- 6.Brennan PM, Whittle IR. Intrathecal baclofen therapy for neurological disorders: a sound knowledge base but many challenges remain. Br J Neurosurg 2008; 22: 508–519. [DOI] [PubMed] [Google Scholar]
- 7.Federatie Medisch Specialisten . Cerebrale en/of spinale spasticiteit. 2016. [cited 2020 Sep 24] Available from: https://richtlijnendatabase.nl/richtlijn/cerebrale_en_of_spinale_spasticiteit/cerebrale_en_of_spinale_spasticiteit_-_startpagina.html.
- 8.Balsara K, Jea A, Raskin JS. Neurosurgical management of spastic conditions of the upper extremity. Hand Clin 2018; 34: 547–554. [DOI] [PubMed] [Google Scholar]
- 9.Dykstra D, Stuckey M, DesLauriers L, Chappuis D, Krach L. Intrathecal baclofen in the treatment of spasticity. Acta Neurochir Suppl 2007; 97: 163–171. [DOI] [PubMed] [Google Scholar]
- 10.Rekand T, Grønning M. Treatment of spasticity related to multiple sclerosis with intrathecal baclofen: a long-term follow-up. J Rehabil Med 2011; 43: 511–514. [DOI] [PubMed] [Google Scholar]
- 11.Kroin JS, Ali A, York M, Penn RD. The distribution of medication along the spinal canal after chronic intrathecal administration. Neurosurgery 1993; 33: 226–230. [PubMed] [Google Scholar]
- 12.Heetla HW, Proost JH, Mol mans BH, Staal MJ, van Laar T. A pharmacokinetic-pharmacodynamic model for intrathecal baclofen in patients with severe spasticity. Br J Clin Pharmacol 2016; 81: 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albright AL, Turner M, Pattisapu JV. Best-practice surgical techniques for intrathecal baclofen therapy. J Neurosurg 2006; 104: 233–239. [DOI] [PubMed] [Google Scholar]
- 14.Sivakumar G, Yap Y, Tsegaye M, Vloeberghs M. Intrathecal baclofen therapy for spasticity of cerebral origin – does the position of the intrathecal catheter matter? Childs Nerv Syst 2010; 26: 1097–1102. [DOI] [PubMed] [Google Scholar]
- 15.McCall TD, MacDonald JD. Cervical catheter tip placement for intrathecal baclofen administration. Neurosurgery 2006; 59: 634–640. [DOI] [PubMed] [Google Scholar]
- 16.Barbara-Bataller E, Mendez-Suarez JL, Aleman-Sanchez C, Sanchez-Enriquez J, Sosa-Henriquez M. Change in the profile of traumatic spinal cord injury over 15 years in Spain. Scand J Trauma Resusc Emerg Med 2018; 26: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKinley W, Santos K, Meade M, Brooke K. Incidence and outcomes of spinal cord injury clinical syndromes. J Spinal Cord Med 2007; 30: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hentz VR, Leclercq C. The management of the upper limb in incomplete lesions of the cervical spinal cord. Hand Clin. 2008; 24: 175–184. [DOI] [PubMed] [Google Scholar]
- 19.Bensmail D, Quera-Salva MA, Roche N, Benyahia S, Bohic M, Denys P, et al. Effect of intrathecal baclofen on sleep and respiratory function in patients with spasticity. Neurology 2006; 67: 1432–1436. [DOI] [PubMed] [Google Scholar]
- 20.Bensmail D, Marquer A, Roche N, Godard AL, Lofaso F, Quera-Salva MA. Pilot study assessing the impact of intrathecal baclofen administration mode on sleep-related respiratory parameters. Arch Phys Med Rehabil 2012; 93: 96–99. [DOI] [PubMed] [Google Scholar]
- 21.Kishima H, Yanagisawa T, Goto Y, Oshino S, Maruo T, Tani N, et al. Respiratory function under intrathecal baclofen therapy in patients with spastic tetraplegia. Neuromodulation 2016; 19: 650–654. [DOI] [PubMed] [Google Scholar]
- 22.Overgard TM, Kjaersgaard-Hansen L, Soe M, Illum NO. Positive experience with intrathecal baclofen treatment in children with severe cerebral palsy. Dan Med J 2015; 62: 4999. [PubMed] [Google Scholar]
- 23.Motta F, Stignani C, Antonello CE. Effect of intrathecal baclofen on dystonia in children with cerebral palsy and the use of functional scales. J Pediatr Ortho 2008; 28: 213–217. [DOI] [PubMed] [Google Scholar]
- 24.Chappuis D, Boortz-Marx R, Stuckey M, Baxter T, DesLauriers L. Safety and efficacy of intrathecal baclofen infused in the cervical and high thoracic area: a preliminary report [abstract]. Am J Phys Med Rehabil 2001; 80: 314. [Google Scholar]
- 25.Broseta J, Garcia-March G, Sanchez-Ledesma MJ, Anaya J, Silva I. Chronic intrathecal baclofen administration in severe spasticity. Stereotact Funct Neurosurg 1990; 54–55: 147–153. [DOI] [PubMed] [Google Scholar]
- 26.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: 4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018; 23: 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandyan AD, Johnson GR, Price CI, Curless RH, Barnes MP, Rodgers H. A review of the properties and limitations of the Ashworth and modified Ashworth scales as measures of spasticity. Clin Rehabil 1999; 13: 373–383. [DOI] [PubMed] [Google Scholar]
- 29.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987; 67: 206–207. [DOI] [PubMed] [Google Scholar]
- 30.Penn RD, Savoy SM, Corcos D, Latash M, Gottlieb G, Parke B, et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med 1989; 320: 1517–1521. [DOI] [PubMed] [Google Scholar]
- 31.Concalves J, Garcia-March G, Sanchez-Ledesma MJ, Onzain I, Broseta J. Management of intractable spasticity of supraspinal origin by chronic cervical intrathecal infusion of baclofen. Stereotact Funct Neurosurg 1994; 62: 108–112. [DOI] [PubMed] [Google Scholar]
- 32.Randall M, Carlin JB, Chondros P, Reddihough D. Reliability of the Melbourne assessment of unilateral upper limb function. Dev Med Child Neurol 2001; 43: 761–767. [DOI] [PubMed] [Google Scholar]
- 33.Barry MJ, VanSwearingen JM, Albright AL. Reliability and responsiveness of the Barry-Albright dystonia scale. Dev Med Child Neurol 1999; 41: 404–411. [DOI] [PubMed] [Google Scholar]
- 34.Krystkowiak P, du Montcel ST, Vercueil L, Houeto JL, Lagrange C, Cornu P, et al. Reliability of the Burke-Fahn-Marsden scale in a multicenter trial for dystonia. Mov Disord 2007; 22: 685–689. [DOI] [PubMed] [Google Scholar]
- 35.Boyce MJ, Canning CG, Mahant N, Morris J, Latimer J, Fung VS. The Toronto western spasmodic torticollis rating scale: reliability in neurologists and physiotherapists. Parkinsonism Relat Disord 2012; 18: 635–637. [DOI] [PubMed] [Google Scholar]
- 36.Chang EV, Ehsan A. Placement of baclofen pump catheter tip for upper extremity spasticity management. Neuromodulation 2018; 21: 714–716. [DOI] [PubMed] [Google Scholar]
- 37.Aljuboori Z, Archer J, Huff W, Moreno A, Jea A. Placement of baclofen pump catheter through a C1-2 puncture: technical note. J Neurosurg Pediatr 2018; 21: 389–394. [DOI] [PubMed] [Google Scholar]
- 38.Ughratdar I, Muquit S, Ingale H, Moussa A, Ammar A, Vloeberghs M. Cervical implantation of intrathecal baclofen pump catheter in children with severe scoliosis. J Neurosurg Pediatr 2012; 10: 34–38. [DOI] [PubMed] [Google Scholar]
- 39.Muquit S, Ughratdar I, Ingale H, Vloeberghs M. Cervical catheter placement for intrathecal baclofen test dose: is it safe? Childs Nerv Syst 2012; 28: 919–922. [DOI] [PubMed] [Google Scholar]
- 40.Harned ME, Salles SS, Grider JS. An introduction to trialing intrathecal baclofen in patients with hemiparetic spasticity: a description of 3 cases. Pain Physician 2011; 14: 483–489. [PubMed] [Google Scholar]
- 41.Motta F, Antonello CE, Stignani C. Upper limbs function after intrathecal baclofen therapy in children with secondary dystonia. J Pediatr Orthop 2009; 29: 817–821. [DOI] [PubMed] [Google Scholar]
- 42.Dykstra DD, Mendez A, Chappuis D, Baxter T, DesLauriers L, Stuckey M. Treatment of cervical dystonia and focal hand dystonia by high cervical continuously infused intrathecal baclofen: a report of 2 cases. Arch Phys Med Rehabil 2005; 86: 830–833. [DOI] [PubMed] [Google Scholar]
- 43.Roscher M, Munin MC. Poster 291: Improved upper extremity spasticity during continuous intrathecal baclofen trial with high cervical catheter placement: a case report. PMR 2016; 8: 255. [DOI] [PubMed] [Google Scholar]
- 44.Korenkov Al, Niendorf WR, Darwish N, Glaeser E, Gaab MR. Continuous intrathecal infusion of baclofen in patients with spasticity caused by spinal cord injuries. Neurosurg Rev 2002; 25: 228–230. [DOI] [PubMed] [Google Scholar]
- 45.Meythaler JM, Guin-Renfroe S, Brunner RC, Hadley MN. Intrathecal baclofen for spastic hypertonia from stroke. Stroke 2001; 32: 2099–2109. [DOI] [PubMed] [Google Scholar]
- 46.Meythaler JM, DeVivo MJ, Hadley M. Prospective study on the use of bolus intrathecal baclofen for spastic hypertonia due to acquired brain injury. Arch Phys Med Rehabil 1996; 77: 461–466. [DOI] [PubMed] [Google Scholar]
- 47.Heetla HW, Staal MJ, Proost JH, van Laar T. Clinical relevance of pharmacological and physiological data in intrathecal baclofen therapy. Arch Phys Med Rehabil 2014; 95: 2199–2206. [DOI] [PubMed] [Google Scholar]
- 48.Skalsky AJ, Fournier CM. Intrathecal baclofen bolus dosing and catheter tip placement in pediatric tone management. Phys Med Rehabil Clin N Am 2015; 26: 89–93. [DOI] [PubMed] [Google Scholar]
- 49.Enzmann DR, Pelc NJ. Normal flow patterns of intracranial and spinal cerebrospinal fluid defined with phase–contrast cine MR imaging. Radiology 1991; 178: 467–474. [DOI] [PubMed] [Google Scholar]
- 50.Ertzgaard P, Campo C, Calabrese A. Efficacy and safety of oral baclofen in the management of spasticity: a rationale for intrathecal baclofen. J Rehabil Med 2017; 49: 193–203. [DOI] [PubMed] [Google Scholar]
- 51.Hsieh JC, Penn RD. Intrathecal baclofen in the treatment of adult spasticity. Neurosurg Focus 2006; 21: 5. [DOI] [PubMed] [Google Scholar]


