Abstract
COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection likely ranks among the deadliest diseases in human history. As with other coronaviruses, SARS-CoV-2 infection damages not only the lungs but also the heart and many other organs that express angiotensin-converting enzyme 2 (ACE2), a receptor for SARS-CoV-2. COVID-19 has upended lives worldwide. Dietary behaviors have been altered such that they favor metabolic and cardiovascular complications, while patients have avoided hospital visits because of limited resources and the fear of infection, thereby increasing out-hospital mortality due to delayed diagnosis and treatment. Clinical observations show that sex, age, and race all influence the risk for SARS-CoV-2 infection, as do hypertension, obesity, and pre-existing cardiovascular conditions. Many hospitalized COVID-19 patients suffer cardiac injury, acute coronary syndromes, or cardiac arrhythmia. SARS-CoV-2 infection may lead to cardiomyocyte apoptosis and necrosis, endothelial cell damage and dysfunction, oxidative stress and reactive oxygen species production, vasoconstriction, fibrotic and thrombotic protein expression, vascular permeability and microvascular dysfunction, heart inflammatory cell accumulation and activation, and a cytokine storm. Current data indicate that COVID-19 patients with cardiovascular diseases should not discontinue many existing cardiovascular therapies such as ACE inhibitors, angiotensin receptor blockers, steroids, aspirin, statins, and PCSK9 inhibitors. This review aims to furnish a framework relating to COVID-19 and cardiovascular pathophysiology.
Keywords: COVID-19, Severe acute respiratory syndrome coronavirus 2, Cardiovascular disease, Angiotensin-converting enzyme 2, Hypertension, Risk factor
Introduction
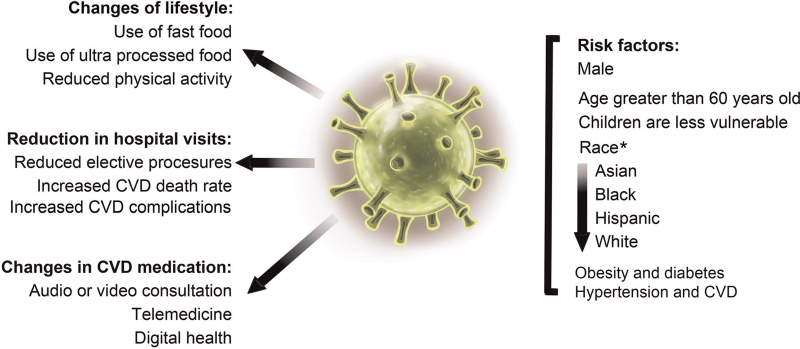
The World Health Organization (WHO) has estimated that, since late 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected approximately 220 million people worldwide, resulting in over 4.5 million deaths. Although COVID-19 was initially defined as an infectious respiratory disease, it is now known that this disease can affect organs other than the lungs, including the kidneys, liver, gastrointestinal tract, central nervous system, and cardiovascular system.[1–3] Early studies showed that patients infected with SARS-CoV or Middle East respiratory syndrome coronavirus (MERS-CoV) developed severe cardiovascular complications. SARS-CoV infection occurred mostly in patients with hyperlipidemia, cardiovascular disorders, and abnormalities of glucose metabolism,[4,5] whereas MERS-CoV infection was found preferentially in patients with underlying cardiovascular disease (CVD), such as congestive heart failure (HF).[6,7] The outbreak of COVID-19 has changed our lifestyle. An online survey from Europe, North America (including the US), and western Asia indicated that reduced activity due to COVID-19-related restrictions led to unhealthier food choices.[8] Social isolation changed dietary behaviors.[9] Adolescents were less physically active and showed increased consumption of ultra-processed foods,[10] leading to other public health issues [Figure 1]. Indeed, obese Italian individuals gained an average of 1.5 kg in weight after 1 month of strict lockdown,[11] thereby increasing their risk of CVD. Here, we offer an overview of our current understanding of the cardiovascular consequences of COVID-19. Patients with CVD may have heightened susceptibility to SARS-CoV-2, and, vice versa, COVID-19 may precipitate cardiovascular complications.
Figure 1.
SARS-CoV-2 infection and the COVID-19 outbreak. Left: The COVID-19 pandemic affected our lifestyle, reduced hospital visits associated with CVD, and changed CVD treatment methods. Right: Some common risk factors for SARS-CoV-2 infection. ∗Race as a risk factor may vary depending on the study and population structure. CVD: Cardiovascular disease; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
Besides advanced age, being male and having underlying medical conditions that are major risk factors for COVID-19-associated mortality, such as cardiovascular complications (myocardial injury, myocarditis, acute coronary syndrome (ACS), cardiac hypertrophy, cardiac arrhythmia, HF, and even venous thromboembolic (VTE) disease) increased the mortality rate by twice as much as other risk factors.[12] Risk factors for CVD such as hypertension, obesity, and diabetes comorbidities are frequently found in COVID-19 patients and are associated with a high mortality rate. CVD and associated risks in COVID-19 patients are tightly linked with hospitalization, mechanical ventilation, admittance to intensive care, and in- and out-hospital deaths. The prevalence of hypertension and CVD in COVID-19 patients reportedly reaches 30% and 14.5%, respectively[13,14]; rates that increase in patients requiring mechanical ventilation or intensive care, and remain 2-fold higher in those who succumb to SARS-CoV-2.[6,13,15] Cardiac injury, as defined by raised troponin levels, occurs more frequently in COVID-19 patients who do not survive (>50%) than in those who survive (1%). Similarly, in a study of 197 hospitalized patients, cases of underlying diabetes (31% vs. 14%), hypertension (48% vs. 23%), and CVD (24% vs. 1%) were found to be higher in non-survivors than in survivors. This suggests that half of these hospitalized COVID-19 patients may have had cardiovascular comorbidities.[13,14] Cardiac injury at admission is associated with high mortality and the need for mechanical ventilation.[16,17] Plasma troponin and natriuretic peptide levels increase with the increasing severity of SARS-CoV-2 infection.[18] COVID-19 patients with severe symptoms experience a greater likelihood of coronary heart disease (CHD), hypertension, and diabetes compared with those with mild symptoms.[15] Patients from intensive care units (ICUs) typically have a high prevalence of CVD events and associated risk factors. For example, a retrospective analysis of 138 COVID-19 patients admitted to ICU showed that 44.4% had arrhythmias and were more likely to have underlying comorbidities, including hypertension (58.3% vs. 21.6%), diabetes (22.2% vs. 5.9%), and CVD (25.0% vs. 10.8%) compared with non-ICU patients.[14] The in-hospital mortality rate for COVID-19 patients was significantly greater among patients with myocardial injury than among those without myocardial injury (60.9% vs. 25.8%).[19]
The numbers of both clinical and basic studies on COVID-19 have grown rapidly since the beginning of the outbreak. We searched approximately 2000 out of about 8000 of the most recent publications in the field from PUBMED and selected approximately 300 of the most recent articles that focused on the link between CVD and SARS-CoV-2. These diseases seldom stand alone but affect each other. One is often a risk factor for the other. This review will focus mostly on clinical observations placed within a pathophysiological context to assist our understanding of one of the deadliest diseases of the century to date.
Impact of the pandemic on hospital visits associated with CVD
Due to the demand for hospital resources for COVID-19 patients, social distancing requirements, and fear of infection during the pandemic, in-person clinical visits were widely switched to virtual (audio or video) consultations, telemedicine, or digital health platforms [Figure 1]. The International Atomic Energy Agency conducted a worldwide survey among 909 inpatient and outpatient centers in 108 countries and reported that cardiovascular procedures decreased by 42% from March 2019 to March 2020, and by 64% from March 2019 to April 2020. The numbers of other procedures also decreased, including transthoracic echocardiography (59%), transesophageal echocardiography (76%), stress tests (78%), and coronary angiography (invasive or computerized tomography (CT); 55%) (P < 0.001 for each procedure).[20] Between January and May 2019 and January and May 2020, there were no differences in acute thoracic and abdominal aortic procedures (incidence rates ratio (IRR) = 0.96, P = 0.39) among 40 departments in Asia, Europe, and the USA, whereas a 35% decline was recorded in the numbers of elective procedures performed (IRR = 0.81, P = 0.001).[21]
In Europe, among 15 centers from 12 countries, only 20,226 consecutive acute admissions to Emergency and Cardiology Departments in 2020 were due to ACS, acute HF, arrhythmia, and pulmonary embolism compared with 30,158 in 2019. Nevertheless, the risk of death was higher in 2020 (odds ratio (OR) = 4.1).[22] A retrospective multicenter registry study conducted in Novara, Italy, included 6609 patients who underwent primary percutaneous coronary intervention (PCI) in 77 European centers in 18 countries. The authors reported a significant reduction in PCIs in 2020 compared with 2019 (IRR = 0.81, P < 0.0001).[23] Data from an Italian National survey of 14 hospitals showed a 39% reduction in hospital beds for surgical patients. Additionally, compared with 2019, surgical activity decreased by 52% in 2020. That fewer procedures were performed did not obviate the risk for nosocomial SARS-CoV-2 infection. Indeed, the report stated that 29 nurses, 12 doctors, and 3 post-operative patients became infected with COVID-19.[24] In 9 locations in western Germany between January 1 and April 30, 2020, there was an overall decline of 20% in cardiovascular-related admissions, including 53% for dizziness/syncope, 38% for HF, 28% for chronic obstructive pulmonary disease (COPD), and 23% for angina; however, there was no change in the numbers of admissions for ST-elevation myocardial infarction (STEMI), cardiopulmonary resuscitation (CPR), and stroke.[25] Before the lockdown of March 23, 2020, ∼700 PCI procedures were performed each week among 126,491 patients from 44 UK hospitals, a number that fell by 49% in subsequent weeks, including a 66% decline in PCI procedures for stable angina, followed by non-STEMI (45%), and STEMI (33%).[26] The COVID-19-associated reduction in elective cardiac-invasive procedures was dependent on the pandemic stage and the availability of healthcare resources (ICU beds, healthcare workers, priority of specific cardiac disorders, budgets). Overall, cardiovascular procedures for patients with acute disease were preserved, whereas those for patients with stable disease were postponed.[27]
Similar observations were reported in South and North America despite the lower number of studies. One study from Duke University showed that there was a 33.1% decrease in cardiovascular-related outpatient visits in the first 15 weeks of the pandemic compared with the same period in 2019. Additionally, 53% of booked visits were canceled in 2020 compared to 35% in 2019.[28] An epidemiological study from Brazil demonstrated that, comparing January to May, 2020, with the same period in 2019, hospital admissions fell by 15% (P = 0.0005) while in-hospital fatality rates due to CVD increased by 9% (P = 0.0318).[29] Despite a decrease in the number of patients presenting to hospitals, total CVD-associated death increased. CVD-related deaths in the UK increased by 8% between March and June 2020 when compared with the previous 6 years. Approximately half of the deaths occurred outside of hospital settings and were mainly due to stroke (35.6%), ACS (24.5%), HF (23.4%), pulmonary embolism (9.3%), and cardiac arrest (4.6%). Most of these deaths were not directly related to COVID-19 infection but were rather the result of delayed medical care or undiagnosed COVID-19.[30] Reduced or late hospital presentation of patients with ACS was associated not only with COVID-19-irrelevant death but also with increases in late complications such as ventricular septal rupture or acute ischemic mitral regurgitation [Figure 1].[31]
Few methods have been used to reduce or prevent CVD-related hospital visits. The Tiantanzhixin application (app), a smartphone-based interactive app launched in August 2019 in Beijing Tiantan Hospital allows real-time, 2-way communication between patients and doctors. STEMI patients who used this app showed shorter systemic delay.[32] Another Chinese app, WeChat, has been used for pre-hospital electrocardiogram (ECG) transfer to help early reperfusion of STEMI patients who were transferred from non-PCI centers.[33] The mAF smartphone app (mAFA) incorporates clinical decision-supported tools for patients with atrial fibrillation,[34] while Apple Heart Study, also app-based, has been used to identify cardiac arrhythmia using a smartwatch.[35]
Common COVID-19 risk factors
Many common risk factors for CVD, including sex, age, race, obesity, diabetes, and hypertension, are also associated with the risk of SARS-CoV-2 infection [Figure 1].
Sex
Multiple studies have shown that men are more likely to develop CHD. A study of 14,786 Finnish men and women aged 25 to 64 showed that men were at a 3-fold greater risk of developing CHD compared with women and their mortality rate was 5 times greater.[36] Similar results were obtained from a US population.[37] Data from the start of the pandemic to May to October 2020 from the US Centers for Disease Control and 5 European countries, including Italy, France, Germany, Spain, and The Netherlands, also showed that the death rate was higher among males than among females (over 2-fold in the US and over 3-fold in European countries).[38] Indirect evidence from 2 independent cohorts (ARISTOTLE, n = 3999 and RE-LY, n = 1088) indicated that being male is a strong independent predictor of high levels of soluble angiotensin-converting enzyme 2 (sACE2).[39] Additionally, the plasma sACE2 level was associated with the plasma levels of CVD biomarkers, including growth differentiation factor 15, N-terminal-proB-type natriuretic peptide (NT-proBNP), high-sensitive cardiac troponin T (hs-cTnT), and D-dimer.[39]
Age
Age is a potent risk factor for CVD.[36,40] A study undertaken from March 1 to April 3, 2020, on a cohort of 887 COVID-19 patients from New York (age (64 ± 17) years) reported that 556 survived without the need for mechanical ventilation, 124 survived with ventilation, and 203 died within 30 days. Multivariate analysis further showed that increased age (hazard ratio (HR) = 1.04 per year), elevated hs-cTnT (HR = 4.57), atrial fibrillation or atrial flutter (AF/AFL, HR = 2.07), history of coronary arterial disease (CAD, HR = 1.56), and active cancer (HR = 1.87) were significant risk factors for COVID-19-associated mortality.[41] Patients over 60 years of age have a markedly greater risk of infection compared with younger patients. In contrast, children are the population least vulnerable to SARS-CoV-2 infection, perhaps due to stronger innate immunity, fewer underlying comorbidities, and differences in the maturation of viral receptors or previous exposure to other coronaviruses.[42] An internet-based survey of 286 COVID-19-positive children (mean age: 8.4 years) from 55 centers in 17 European countries showed that the CVD biomarkers C-reactive protein (CRP), ferritin, procalcitonin, NT-proBNP, interleukin(IL)-6, and D-dimers were all elevated in children admitted to ICUs.[43] Bergamo, Italy, had more than 130,000 cases of COVID-19. Here, there was a 30-fold increase in the number of children diagnosed with the pediatric vascular inflammatory syndrome Kawasaki-like disease. Overall, the affected patients were older than usual ((7.5 ± 3.5) vs. (3.0 ± 2.5) years, P = 0.00035) and exhibited more cardiovascular involvement.[44,45]
Race
According to the US National Center for Health Statistics (NCHS) and National Vital Statistics System, heart disease-associated death rates in the US from 1999 to 2017 were highest among the Black non-Hispanic population, followed by the White non-Hispanic, Hispanic, and Asian or Pacific Islander non-Hispanic populations (https://www.cdc.gov/nchs/hus.htm). Similarly, age-adjusted COVID-19-associated mortality rates for Black individuals was 2.8-fold that of Caucasians.[46] African Americans may have a higher risk for COVID-19 due to social factors, stress, anxiety, environmental factors, and social determinants of health.[47] A retrospective observational study of 7868 consecutive patients from 88 hospitals across the US between January 17 and July 22, 2020, of data from the American Heart Association (AHA) COVID-19 Cardiovascular Registry showed that Black and Hispanic patients accounted for 55.5% of the COVID-19-positive hospitalized population and were much younger than their Caucasian counterparts.[48] In a community-based UK Biobank cohort of 473,555 individuals, 459 deaths were attributed to COVID-19 deaths and 2626 to other causes. Univariate regression models showed that age (OR = 2.76, P = 2.6 × 10−17), male sex (OR = 1.47, P = 1.3 × 10−6), and Black versus White ethnicity (OR = 1.21, P = 3.0 × 10−7) were independently and jointly explanatory (area under the ROC curve (AUC): 0.79) and increased the risk for COVID-19-related mortality.[49] In contrast, Asian COVID-19 patients have a higher risk of developing cardiorespiratory diseases. The mortality rate was reported to be higher for Asian (adjusted odds ratio (adjOR) = 1.31), Black (adjOR = 0.93), and Hispanic patients (adjOR = 0.90) than among the White population.[48] Despite these observations, it is clear that the effect of race on SARS-CoV-2 infection can vary depending on population structure, education level, and socioeconomic factors.
Obesity and diabetes
COVID-19 infections result in increased hospitalization rates and greater severity of illness in patients with metabolic diseases, such as obesity and diabetes. The International Severe Acute Respiratory and Emergency Infection Consortium described rates of 17.4% and 13.4% for diabetes and obesity, respectively, among 95,966 cases.[50] However, the prevalence of diabetes and obesity was reported to be much higher (33.8% and 41.7%, respectively) in a cohort of 5700 COVID-19 patients from 12 New York City hospitals between March 1 and April 4, 2020,[51] likely because the definition of obesity differs between Asians and Americans.[52] Analysis of 7606 patients from the AHA COVID-19 registry through July 22, 2020, showed that obesity classes I to III are risk factors for in-hospital death or mechanical ventilation (OR = 1.28, 1.57, and 1.80, respectively), while class III obesity is associated with the risk of in-hospital death (HR = 1.26). Being overweight and obesity classes I to III are also risk factors for mechanical ventilation (OR = 1.28, 1.54, 1.88, and 2.08, respectively).[53] Similar observations were made in France and China. In a retrospective cohort study from the University Hospital in Lille, France, the authors reported an OR of 7.36 for a requirement for invasive mechanical ventilation among obese COVID-19 patients (body mass index (BMI) of ≥35 kg/m2) versus those with a BMI of <25 kg/m2 after adjusting for age, sex, and comorbidities such as diabetes, hypertension, and dyslipidemia.[54] A BMI greater than 28 kg/m2 is defined as obese in China. The OR for developing severe pneumonia among 383 hospitalized COVID-19 patients was 1.84 for overweight patients (BMI 24.0–27.9 kg/m2) and 3.40 for obese patients compared with those of normal weight (BMI 18.5–23.9 kg/m2).[55] Among age- and sex-matched COVID-19 patients, obesity was associated with a 3-fold increased risk of severe COVID-19 (OR = 3.00) after adjusting for age, sex, smoking status, hypertension, diabetes mellitus, and dyslipidemia.[56] A systematic review and meta-analysis of 41 studies encompassing a total of 219,543 subjects, including 115,635 SARS-CoV-2-positive patients, showed that obesity was a risk factor for COVID-19 (OR = 1.50). Obese COVID-19 patients showed a higher incidence of hospitalization (OR = 1.54), ICU admission (OR = 1.48), invasive mechanical ventilation (OR = 1.47), and in-hospital death (OR = 1.14).[57] Of the total Scottish population of 5,463,300, diabetic patients accounted for 5.8% (319,349), 1082 (0.3%) of whom developed fatal or ICU-treated COVID-19; of these, 972 (89.8%) were more than 60 years old. In contrast, among the 5,143,951 non-diabetic individuals, only 4081 (0.1%) developed fatal or ICU-treated COVID-19. After adjusting for age and sex, diabetes remained a strong risk factor for fatal and ICU-treated COVID-19 (adjOR = 1.395, P < 0.0001).[58] Besides these CVD risk factors, substantial evidence exists worldwide to support the existence of a link between SARS-CoV-2 infection and CVD-associated comorbidities.
Hypertension and CVD
Hypertension is an important component of metabolic syndrome and a significant risk factor for CVD and SARS-CoV-2 infection. In Asia, a study from Beijing, China, showed that 81 (16%) out of 498 consecutive hospitalized COVID-19 patients had pre-existing hypertension. There were more cases of severe COVID-19 among patients with hypertension than among those without hypertension (21% vs. 10%, P = 0.007). Hypertension is associated with an increased risk of illness even after adjusting for age, sex, hospital geographical location, and blood pressure at admission. Hypertensive patients were often older and had higher blood neutrophil counts and CRP, lactate dehydrogenase, and NT-proBNP levels compared with non-hypertensive patients. Like age (OR = 1.062, P < 0.001), hypertension was also a strong risk factor for COVID-19 (OR = 2.310, P = 0.008) in this Chinese population.[59] Other studies from China yielded the same conclusion. In a group of 113 confirmed COVID-19 cases from Shanghai, China, pre-existing hypertension and a high sequential organ failure assessment (SOFA) score were found to be independent risk factors for the development of cardiac injury among COVID-19 patients.[60] Furthermore, among 414 COVID-19 patients from Wuhan, China, patients with hypertension (149) had higher plasma levels of hs-cTnI (P < 0.0001) and NT-proBNP (P < 0.0001) on admission. Moreover, hypertension was found to be a risk factor for in-hospital death (HR = 2.57) after adjusting for age and sex.[61] In a study from Tehran, Iran, the authors reported that 176 (29.4%) out of 598 COVID-19 patients had underlying hypertension. Severe/critical COVID-19 was more frequent among patients with hypertension than in those without hypertension (23.8% vs. 9.7%, P = 0.012).[62] A report by the Chinese Center for Disease Control and Prevention stated that 4.2% of 44,672 patients with COVID-19 had CVD and 12.8% had hypertension.[63] Additionally, although the total fatality rate was 2.3%, that for patients with hypertension, diabetes, and CVD was 6.0%, 7.3%, and 10.5%, respectively.[64] The National Health Commission of China also reported that the mortality rates for COVID-19 patients with a history of CHD and hypertension were 17% and 35%, respectively.[5] Apart from respiratory failure, CVD-related comorbidities were the most frequently observed complications in a multicenter cohort study of 191 COVID-19 patients from Wuhan, China.[13] Cardiac injury (59% vs. 1%), HF (52% vs. 12%), and elevated concentrations of creatinine kinase-myocardial isoform (CK-MB) and hs-cTnI were more common in non-survivors than in survivors.[13] A retrospective study of 150 COVID-19 patients from Wuhan found that CVD was common in patients who died (13 out of 68) but not in those who recovered (0 out of 82).[65] A different study from Wuhan comprising 77 COVID-19 patients who were admitted to the ICU showed that those presenting with myocardial injury were generally older ((68.4 ± 10.1) years vs. (62.1 ± 13.5) years, P = 0.02), had a higher prevalence of underlying CVD (34.1% vs. 11.1%, P = 0.02), and experienced more in-ICU CV complications (41.5% vs. 13.9%, P = 0.008) than non-ICU patients. Myocardial injury at admission increased the risk of 28-day mortality (HR = 2.2, P = 0.004).[66] A study of 138 hospitalized patients with COVID-19 in Wuhan showed that the levels of markers of cardiac damage (CK-MB and hs-cTnI) were greatly increased in ICU patients compared with those not requiring intensive care, while ICU patients were more likely to manifest arrhythmia than non-ICU patients (44.4% vs. 6.9%).[6] Hospitalized COVID-19 patients (n = 416) from Wuhan also demonstrated high levels (19.7%) of cardiac injury, greater age, more comorbidities, more laboratory abnormalities, and more extensive chest X-ray findings.[17] Patients with cardiac injury were more likely to require mechanical ventilation (P < 0.001) and experienced higher mortality rates than those without cardiac injury (51.2% vs. 4.5%, P < 0.001).[17] A meta-analysis of 6 studies that included 1527 COVID-19 patients from China showed that 8% suffered from acute cardiac injury. The incidence of acute cardiac injury was 13-fold higher in ICU/severe COVID-19 patients than non-ICU/severe patients.[6] In Korea, studies of COVID-19 patients from 10 hospitals from February 15 to April 24, 2020, showed that 42% (n = 954) had pre-existing CVD or risk factors for CVD, including hypertension (28.8%) and diabetes (17.0%). Patients with pre-existing CVD or those with risk factors for CVD experienced higher ICU admittance rates (5.3% vs. 1.6%, P < 0.001) or a greater requirement for mechanical ventilation (4.3% vs. 1.7%, P < 0.001) than those without CVD or the risk factors. CVD and associated risk factors (adjOR = 1.79, P = 0.027), diabetes (OR = 2.43, P < 0.001), and congestive HF (OR = 2.43, P = 0.049) independently predicted in-hospital death.[67]
The Premier Healthcare Database from North Carolina, US, contained data for 132,312 patients hospitalized from April 1 to September 30, 2020, 8383 (6.4%) of whom were COVID-19-positive. The death rate was markedly higher in these patients (24.2%) than in those hospitalized for HF (2.5%). As expected, being male (OR = 1.26), age (OR = 1.35), and obesity (OR = 1.25) were associated with in-hospital mortality among patients with HF and COVID-19.[68] Out of a large cohort of 18,472 individuals in the US registry from Ohio and Florida, between March 8 and April 12, 2020, a total of 1735 were found to be COVID-19-positive, 421 were admitted to hospital (24.3%), 161 (9.3%) were admitted to an ICU, and 111 (6.4%) experienced mechanical ventilation. Among the COVID patients, 682 (39.3%) had hypertension; 332 (19.1%) were diabetic; 161 (9.3%) had CAD; and 146 (8.4%) had HF.[69] Among another US cohort of 5894 COVID-19 patients from New York from March 1 to April 15, 2020, 2573 (40%) had a history of hypertension.[70] Of the 1533 acute myocardial infarction (AMI) patients who presented to the MedStar Health system (11 hospitals in Washington DC and Maryland), 86 had COVID-19, were older, tended to be non-White, and had more comorbidities compared with AMI patients who tested negative for COVID-19. In-hospital mortality (P < 0.001) and the levels of inflammatory markers (white blood cells, lactate dehydrogenase, ferritin, and CRP) and NT-proBNP (P = 0.044) were also higher in AMI patients diagnosed with COVID-19 than in those testing negative for the disease.[71] A chest CT-based study of 180 adult COVID-19 patients from New York found that coronary artery calcification was associated with intubation (adjOR = 3.6) and mortality (adjOR = 3.2).[72] A prospective cohort study of 586 COVID-19 patients from Yale University showed a 36.7% incidence of CVD. The highest incidence was reported for hypertension (60.2%), followed by diabetes (39.8%), and hyperlipidemia (38.6%). In this cohort, age (OR = 1.28), previous ventricular arrhythmia (OR = 18.97), use of P2Y12-inhibitors (OR = 7.91), high CRP (OR = 1.81), high hs-cTnT (OR = 1.84), and low albumin (OR = 0.64) were associated with all-cause of mortality.[73] A study of 52 COVID-19 patients from Milwaukee, Wisconsin, who were admitted to ICU reported that 29% had prior cardiac disease. These patients were more likely to have new or worsening left ventricle (LV) dysfunction, while echocardiographic analysis showed that 55.7% had cardiac complications. Prior cardiac disease, right ventricular enlargement, and pulmonary hypertension were all associated with morbidity and mortality in these COVID-19 patients.[74]
An observational and retrospective study from Italy, Europe, reported that hypertension was the most prevalent comorbidity among 351 COVID-19 outpatients (35%).[75] Pre-existing hypertension increased the risk of developing severe disease and death.[76] A study of 692 consecutive patients with COVID-19 from 13 Italian cardiology centers between March 1 and April 9, 2020, showed that 13% (n = 90) had a history of HF. The in-hospital death rate was higher in patients with HF (41.1%) than in those without HF history (22.9%). Multivariate COX regression analysis showed that HF history was a risk factor for COVID-19-associated death (adjusted hazard ratio (adjHR) = 2.25, P = 0.006).[77] Among 6272 patients from the Lombardy region of Italy diagnosed with COVID-19 between February 21 and March 11, 2020, 23.9% of the women and 33.8% of the men had underlying CVD.[78] A study from the same city showed that among 1043 COVID-19 patients admitted to ICU, 49% had hypertension, 21% had CVD, and 21% had diabetes. Of the patients with hypertension, 38% died in the ICU.[79] In France, a study from Paris reported the detection of coronary artery calcification in 50.7% of 209 consecutive hospitalized COVID-19 patients. These patients had more primary outcomes (50.0% vs. 17.5%, P < 0.0001) and episodes of non-invasive ventilation (49.1% vs. 15.5%, P < 0.0001) than those without calcification.[80] In Germany, among 40 COVID-19 patients from 2 medical centers, 19 had hypertension, 11 had diabetes, and 10 had previously diagnosed cardiac disease. Plasma NT-proBNP levels were elevated in 27 patients, and hs-cTnT levels in 25; of these, 18 had no prior cardiac disease. Plasma CK-MB levels were increased in 17 patients, 15 of whom had no history of cardiac disease. Free light chain immunoglobulin (FLC Ig) lambda levels were elevated in 32 patients, FLC Ig kappa levels in 29, and D-dimer levels in 33. Blood hs-cTnT levels were higher in COVID-19 patients admitted to ICU than in non-ICU COVID-19 patients.[81] In Spain, a study of 3080 COVID-19 patients from Madrid reported that patients with a previous history of HF (4.9%) were more prone to developing acute HF (P < 0.001), had higher levels of NT-proBNP, and experienced higher mortality rates (P < 0.001) than those without previous HF. COVID-19 patients with acute HF also had higher mortality rates than those without acute HF (P < 0.001).[82]
Meta-analyses have given a broad overview of the associations between SARS-CoV-2 infection and CVD-related comorbidities. Patients with a history of CVD and hypertension were more likely to have elevated troponin levels.[83] A meta-analysis involving 11 studies reported a combined risk ratio (RR) of 2.49 for severe disease in the presence of hypertension.[84] An analysis of 56 studies and 198 articles that included 159,698 patients infected with COVID-19 showed that acute cardiac injury (OR = 13.29), hypertension (OR = 2.60), HF (OR = 6.72), arrhythmia (OR = 2.75), CAD (OR = 3.78), and CVD (OR = 2.61) were associated with mortality. Arrhythmia (OR = 7.03), acute cardiac injury (OR = 15.58), CHD (OR = 2.61), CVD (OR = 3.11), and hypertension (OR = 1.95) were associated with ICU admission.[85] The results of these meta-analyses indicate that there are strong links between COVID-19 and CVD across patients from Asia, America, and Europe, although the degree of association may vary depending on the study origin and sample size.
Hypertension and ACE2 expression
Hypertension involves endothelial dysfunction that leads to an imbalance between vasodilation and vasoconstriction, elevated levels of reactive oxygen species (ROS) and pro-inflammatory mediators, and reduced nitric oxide (NO) bioavailability.[86,87] SARS-CoV-2 interactions with endothelial cells (ECs) in hypertensive patients drive viral-mediated injury and EC dysfunction; induce chemokine release by ECs, with consequent inflammatory cell adhesion and migration through the endothelial barrier; and lead to a procoagulant state and tissue damage, such as myocardial injury.[88]SARS-CoV-2 infection is mediated by ACE2 located on the host cell surface.[89] ACE2 is a homolog of the metalloproteinase ACE, a key enzyme in the renin-angiotensin system (RAS) that produces angiotensin II (Ang II).[90–92] Ang II promotes EC dysfunction, while SARS-CoV-2 infection-mediated downregulation of ACE2 expression results in the dysregulation of the renin-angiotensin-aldosterone system (RAAS).[93] In the RAAS, ACE converts Ang I into Ang II, which can bind to both Ang II receptor 1 (AGTR1) and AGTR2. In contrast, ACE2 hydrolyzes Ang I into Ang-(1–9) and also generates Ang-(1–7) from Ang II. Ang-(1–7) binds to both AGTR2 and Mas receptor (MasR), an endogenous orphan receptor for Ang-(1–7) [Figure 2].[94] ACE/Ang II/AGTR1 mediate vascular dysfunction, while ACE2/Ang-(1–7)/AGTR2/MasR form the vasoprotective axis that leads to vasodilatory, anti-fibrotic, anti-proliferative, and anti-inflammatory effects.[95–98] Both the overactivity of the vasodeleterious axis and the hypoactivity of the vasoprotective axis are major contributors to hypertension. In addition to producing Ang-(1–7) from Ang II, ACE2 also produces Ang-(1–9) from Ang I, which is then converted into Ang-(1–7) by ACE [Figure 2], indicative of the complexities of ACE and ACE2 functions in the RAAS. A cardiac ACE-mediated increase in Ang II levels drives LV hypertrophy.[99,100] Patients with hypertension are particularly susceptible to an imbalance between the ACE/Ang II/AGTR1 and the ACE2/Ang-(1–7)/AGTR2/MasR axes, which may be further intensified by SARS-CoV-2-mediated downregulation of ACE2 in the myocardium and endothelium.[93,101,102] SARS-CoV-2 infection shifts the balance in the RAAS toward the deleterious axis, resulting in elevated oxidative stress and inflammation in both the endothelium and myocardium. Ang II acts on AGTR1 and promotes the expression of pro-inflammatory cytokines and chemokines via nuclear factor kappa B (NF-κB),[103] favoring vascular permeability and inflammatory cell accumulation, and resulting in tissue injury and hypoxia [Figure 2].[104,105] By binding to AGTR1, Ang II promotes vasoconstriction, pro-fibrotic and pro-thrombosis responses, and cell proliferation. AGTR1 also stimulates nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and ROS production, which impairs endothelial NO synthase function and reduces NO production, thereby facilitating thrombosis and vascular inflammation [Figure 2], which are features of hypertension-associated chronic endothelial dysfunction.[106–108] Thus, AGTR1 activation contributes to the development and progression of hypertension.[99,109,110]
Figure 2.
The RAAS pathway, SARS-CoV-2 infection, and ACEI/ARB function. The ACE/Ang II/AGTR1 axis is shown to the left and the ACE2/Ang-(1–7)/AGTR2/MasR axis to the right. ACE: Angiotensin-converting enzyme; ACEI: ACE inhibitor; AGTR1: Angiotensin II receptor type 1; AGTR2: Angiotensin II receptor type 2; Ang: Angiotensin; ARB: Angiotensin receptor blocker; EC: Endothelial cell; RAAS: Renin-angiotensin aldosterone system; MasR: Mas receptor; NAPDH: Nicotinamide adenine dinucleotide phosphate; NO: Nitric oxide; ROS: Reactive oxygen species; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
ACE2 acts as a counter-regulatory enzyme by converting Ang II into Ang-(1–7), a heptapeptide that binds to both AGTR2 and MasR to reduce blood pressure,[95,97] promote vasodilation, increase kidney Na and water excretion, and elicit anti-inflammatory, anti-proliferative, antioxidant, and anti-apoptotic effects that benefit CVD [Figure 2].[111–114] These activities oppose the ACE/Ang II/AGTR1 axis. ACE2/Ang-(1–7)/AGTR2/MasR signaling stimulates the activity of endothelial NO synthase, increases NO production, decreases Ang II-stimulated NADPH oxidase activity, and modulates the generation of reactive ROS [Figure 2].[115] These activities of Ang-(1–7) can ameliorate hypertrophy and fibrosis in mice.[116] ACE2 overexpression prevents or even reverses HF-related features,[117–120] whereas ACE2 deficiency exacerbates aspects of HF.[121] SARS-CoV-2 infection reduces ACE2 expression and Ang-(1–7) production, leading to reduced Ang-(1–7)-mediated cardioprotective activity and increased Ang II levels.[96] Whereas ACE2 overexpression ameliorates cardiac remodeling,[95] the loss of ACE2 in endothelial and cardiac cells may contribute to acute and, perhaps, chronic exacerbation of CVD in SARS-CoV-2-infected hypertensive patients. Prolonged systemic hypertension results in associated target organ damage, such as LV hypertrophy, which is also an independent risk factor for cardiovascular complications.
The ACE/Ang II/AGTR1 and ACE2/Ang-(1–7)/AT2R/MasR pathways are co-expressed in most tissues and act in both autocrine and paracrine manners. ACE2 is relatively abundantly expressed in the heart, mainly in pericytes and cardiomyocytes, and at much lower levels in ECs and fibroblasts,[93,122–136] although some studies have reported that ACE2 is not expressed in human cardiac ECs.[137] SARS-CoV-2 infection downregulates ACE2, leading to disrupted Ang II metabolism[138] and vascular permeability,[139] increased Ang II availability and Ang-(1–7) deficiency, increased inflammation, endothelial activation, leukocyte recruitment, and even platelet activation [Figure 2].[140] Through a mechanism that likely involves the NF-κB pathway, Ang II enhances inflammatory cytokine production, cellular apoptosis, and fibrosis in response to hypoxia.[97] The reduced presence of ACE2 on the cell surface might be a result of endocytosis and intracellular proteolysis of membrane-bound ACE2.[95] In cardiomyocytes, ACE2-mediated SARS-CoV-2 infection causes myocardial injury through the downregulation of cardioprotective gene expression.[128,141–143] Reduced ACE2 expression in cardiac tissue in COVID-19 patients is associated with an increase in Ang II availability as a result of disrupted Ang II metabolism. Plasma Ang II levels correlate with the viral load.[144] Combined, these observations indicate that SARS-CoV-2 infection worsens outcomes following myocardial injury.
Ace2 gene transfer attenuates atherosclerosis in mice by reducing angiogenesis and regulating monocyte-EC interaction via decreasing the expression of adhesion molecules in ECs through Ang-(1–7) production.[134,135] Blood pressure is also increased in ACE2-deficient mice.[145] Lentiviral-mediated overexpression of ACE2 improved blood pressure and hypertension-associated pathologies.[146] Vascular ACE2 overexpression increased Ang-(1–7) production, restored endothelial function, and reduced blood pressure.[147] Although the underlying mechanisms are unknown, compared with that in their wild-type siblings, blood pressure remains unchanged in 3-month-old ACE2-deficient mice, or is even lower after 6 months.[148] MasR null mice also have normal blood pressure.[149] In wild-type mice, treatment with recombinant human ACE2 did not affect baseline blood pressure or plasma levels of Ang II or Ang-(1–7).[150] These conflicting observations remain to be confirmed and explained.
Cardiac disease and SARS-CoV-2 infection
Cardiac injury and myocarditis
SARS-CoV-2 infection via alveolar epithelial cells causes neutrophil accumulation and enhances sub-endothelial space vascular permeability, which results in alveolar exudate formation,[151,152] pulmonary edema, alveolar gas exchange disorders (such as acute respiratory distress syndrome (ARDS), oxygen depletion, and hypoxia),[153,154] consequently leading to myocardial infarction, sudden cardiac arrest, HF, and abnormal coagulation. Severe pneumonia due to SARS-CoV-2 infection impairs gas exchange and causes hypoxemia, explaining why critically ill COVID-19 patients usually require oxygen therapy and mechanical ventilation for respiratory support. SARS-CoV-2 infection-induced hypoxic injury triggers pulmonary vasoconstriction and pulmonary hypertension, leading to cardiac insufficiency and HF.[155,156] Systemic and local increases in cytokine concentrations can cause myocardial injury,[157] characterized by increased troponin levels that precede myocardial ischemia or non-ischemic processes such as myocarditis.[158] In France, post-mortem histological analysis of hearts from patients with SARS-CoV-2-induced myocarditis showed inflammatory cell accumulation involving mainly macrophages and CD8+ T cells in the ventricles and septum. All nasopharyngeal swabs and distal bronchoalveolar lavages tested negative for SARS-CoV-2 RNA before death. Additionally, myocardial cells in these patients were positive for anti-SARS-CoV2 antibodies.[159] Biopsies of COVID-19 patients from China showed myocardium infiltration of the same mononuclear cells but not CD4+ T cells.[157] Persistent myocarditis led to dilated cardiomyopathy and increased the risk of mortality [Figure 3].[160]
Figure 3.
SARS-CoV-2 infection in patients with common cardiac diseases. Viral infection phenotypes and estimated percentages of COVID-19 patients are shown in each category. hs-cTnT: High-sensitive cardiac troponin T; ICU: Intensive care unit; NT-proBNP: N-terminal-proB-type natriuretic peptide; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
Hs-cTnI, hs-cTnT (cardiac injury), and NT-proBNP (myocardial stress) are biomarkers with prognostic value in COVID-19 patients. Their plasma levels are known indicators of cardiac injury. Initial examination showing increased levels of hs-cTnI and NT-proBNP predicts mortality in COVID-19 patients.[161] Plasma troponin levels are generally higher in patients with severe COVID-19 than in those with mild disease.[18] Across studies, between 7% and 36% of hospitalized COVID-19 patients display elevated cardiac hs-cTnI or hs-cTnT concentrations, and high levels of troponin are associated with the risk for ICU admission and mortality.[14,17,83] In several Chinese cohorts, markers of myocardial injury were found in 7% to 17% of COVID-19 patients[13,14,151] and were present more frequently in those admitted to ICU (22.2% vs. 2.0%, P < 0.001) or who died (59% vs. 1%, P < 0.0001).[13,162] A cohort study from Wuhan, China, showed that myocardial injury was common among COVID-19 patients and was associated with in-hospital mortality.[17] Out of 416 hospitalized COVID-19 patients, 82 (19.7%) had cardiac injury and exhibited higher plasma levels of NT-proBNP, CK-MB, and hs-cTnI compared with patients without myocardial injury. The mortality rate was also 10-fold higher in patients with cardiac injury than in those with normal cardiac function (51.2% vs. 4.5%, P < 0.001). A COX regression model also demonstrated that mortality rates were higher both during the time from symptom initiation and admission to the endpoint.[17] A study of 173 COVID-19 patients (119 over and 54 below 60 years of age) from Tongji Hospital, Wuhan, China, showed that age, but not sex, was positively correlated with cardiac injury (hs-cTnI). Multivariate logistic regression analysis showed that increased blood levels of procalcitonin, IL-2 receptor, IL-6, IL-10, TNF-α, CRP, and D-dimer; higher white blood cell and neutrophil counts; and lower blood lymphocyte and natural killer (NK) cell counts were all associated with cardiac injury.[163] In another 150 COVID-19 patients from the same hospital, including 16 mild and 24 severe cases, blood NT-proBNP, hs-cTnI, hs-CRP, and creatinine levels were all higher in patients with severe COVID-19 than in those with mild disease.[164] Meanwhile, a study of 100 patients with confirmed severe COVID-19 from February 8 to April 10, 2020, from Beijing Hospital found that plasma hs-cTnI levels were higher in individuals aged > 60 years than in those aged ≤60 years (median interquartile range (IQR): 5.2 vs. 1.9, P = 0.018). Hs-cTnI levels were also higher in men than in women (IQR: 4.2 vs. 2.9, P = 0.018), as were plasma NT-proBNP levels (32.1% vs. 9.1%, P = 0.006), although no differences were found between elder and young patients.[91]
An international multicenter cohort study involving 305 patients (205 of whom were male) hospitalized with laboratory-confirmed COVID-19 from 7 hospitals in New York City and Milan identified myocardial injury in 190 (62.3%) of them. In-hospital mortality was higher in patients with myocardial injury with (31.7%) or without (18.6%) transthoracic echocardiographic abnormalities than in those without myocardial injury (5.2%).[165] A study from New York University comprising 2163 consecutive COVID-19-positive adult patients with hs-cTnI ≥ 1 μg/mL showed that patients with myocardial injury were older, more likely to be male, and were associated with higher in-hospital mortality and a greater frequency of critical illness compared with those without myocardial injury.[166] A prospective study of 32 hospitalized COVID-19 patients from Munich, Germany reported that 65.7% of patients had left and/or right ventricular dysfunction. Concomitant biventricular dysfunction was common in patients with increased hs-cTnT levels.[167] In a meta-analysis of 13 studies encompassing a total of 3289 COVID-19 patients, the presence of cardiac injury was found to be associated with mortality (RR = 7.95, P < 0.001), the need for intensive care (RR = 7.94, P = 0.01), and more severe COVID-19 (RR = 13.81, P < 0.001). [168]
The mechanisms by which SARS-CoV-2 infection increases the risk of myocardial injury and myocarditis can be multifactorial, and can involve hypoxemia and oxygen supply and demand mismatch, direct infection of the myocardial tissue by SARS-CoV-2, cardiac damage due to an inflammatory cytokine storm, oxidative stress, microvascular dysfunction, plaque instability, and coagulation abnormalities [Figure 3].[169] SARS-CoV-2 enters cardiomyocytes via ACE2[128] and causes direct damage to the host cells. Endomyocardial biopsy showed cardiomegaly and scattered cardiomyocyte necrosis and mononuclear cell infiltration in the myocardium.[170] Viral particles have been detected in cardiac tissues from COVID-19 patients.[126,127,171,172] SARS-CoV-2 impairs stress granule formation, thereby facilitating viral replication and cellular damage.[173] Following entry, the virus activates inflammatory responses, leading to mononuclear cell and T-cell accumulation. Primed CD8+ T cells cause cardiomyocyte inflammation and cytotoxic cell-mediated toxicity. Pro-inflammatory cytokines are released into the circulation, thereby augmenting lymphocyte activation and cardiomyocyte damage.[158,174] Cellular hypoxia may also worsen cardiomyocyte injury. COVID-19 patients show various degrees of hypoxia, which reduces energy supply for cell metabolism and increases anaerobic metabolism, intracellular acidosis, and oxygen free radical generation.[151] Hypoxia also induces calcium influx, which promotes cardiomyocyte apoptosis.[6]
ACS
ACS includes STEMI, non-STEMI, and unstable angina. Increased thrombotic tendency as reflected by elevated plasma D-dimer levels can promote AMI in COVID-19 patients.[175] An increase in D-dimer levels, which is indicative of fibrin formation, has been used as a biomarker for hypertension and AMI,[175,176] and is also a prognostic marker for mortality in COVID-19.[176] One study of 199 COVID-19 patients reported an association between D-dimer values >1 μg/mL and in-hospital mortality (adjHR = 18.4).[13] In a retrospective cohort of 191 patients with COVID-19 from Wuhan, China, 15 (8%) were found to have CHD.[13] The National Health Commission of China reported that 17% of COVID-19 patients had CHD.[5] Local inflammation and hemodynamic changes may increase the risk of atherosclerotic plaque rupture, resulting in AMI [Figure 3].
During the pandemic surge in 2020, STEMI catheterization procedures fell by 40% in Spain and 38% in the US.[177,178] These drops were associated with multiple factors, including reduced numbers of hospital visits due to patient reluctance, increased medical reperfusion, reduced daily stressors due to social distancing and quarantine, less pollution from traffic and factories, more rest and fewer activities, and, perhaps, less smoking due to warnings from social media.[177] However, case studies showed that delayed STEMI presentation may have increased the risk of mechanical complications associated with AMI.[179] The European Society of Cardiology (ESC) recommends a maximum delay from STEMI presentation to reperfusion of less than 120 minutes for COVID-19 patients. Although non-STEMI ACS is an urgent condition, emergent PCI is usually not necessary. The ESC divided non-STEMI into 4 groups based on the risk level. High-risk non-STEMI should follow the STEMI treatment strategies, whereas intermediate and low-risk non-STEMI may use non-invasive strategies.[178,180]
Cardiomyopathy and HF
HF is one of the most common complications of COVID-19 and is more likely to occur in patients with pre-existing cardiac disease.[181] Patients with HF exhibit high ACE2 expression and an increased risk of heart attack that progresses after infection.[122] Studies have shown that SARS-CoV and MERS-CoV infections cause and aggravate HF.[7,182,183] NT-proBNP is a quantitative cardiac biomarker for hemodynamic stress and HF. High NT-proBNP (>88.64 pg/mL) levels are associated with high mortality rates in COVID-19 patients.[184] Numerous studies have shown HF occurs frequently among COVID-19 patients. Among those who died from SARS-CoV-2 infection, nearly 50% had HF.[16,17,175] For example, in a retrospective case series of 799 moderate to severely ill COVID-19 patients from Tongji Hospital in Wuhan, China, 41 (49%) out of 83 deceased patients had HF.[181] Another retrospective multicenter cohort study from Wuhan showed that HF occurred in 44 (23%) out of 191 COVID-19 patients and 28 (52%) out of 54 non-survivors.[13] A descriptive study of 99 COVID-19 patients from Wuhan again revealed similar levels of HF in patients who died. HF contributed to 40% of deaths in this study. Among the 99 cases, 2 had no history of CVD but nevertheless died of HF and sudden cardiac arrest.[185] Studies from the US and other countries have yielded similar conclusions. A retrospective study of 6439 patients with COVID-19 from Mount Sinai Hospital in New York demonstrated that patients with previous HF experienced longer hospital stays (P < 0.001), had a higher risk of requiring mechanical ventilation (adjOR = 3.64, P < 0.001), and had higher mortality rates (adjOR = 1.88, P = 0.002) compared with those without previous HF.[186] An international study comprising 1272 COVID-19 patients from 69 countries reported that 39% of hospitalized patients had an LV abnormality (dilation, systolic dysfunction, diastolic dysfunction), 33% had a right ventricle abnormality, and 28% experienced biventricular failure.[60]
Many HF patients developed COVID-19 and COVID-19 patients are at increased risk of HF. These observations are suggestive of an interconnection between SARS-CoV-2 and HF pathogenesis. Besides a direct effect of SARS-CoV-2 on cardiomyocytes that leads to cardiomyocyte apoptosis or necrosis,[126–128,141–143,171] a cytokine storm following SARS-CoV-2 infection can induce and aggravate HF, similar to that seen with SARS-CoV and MERS-CoV.[187] Although the direct underlying mechanisms remain unknown, IL-6 released during a cytokine storm can induce diastolic dysfunction by exerting positive inotropic effects on cardiomyocytes via the JAK/STAT3 pathway[188] and increase cardiomyocyte stiffness by reducing the phosphorylation of titin.[189] IL-1β and TNF-α cause contractile abnormalities in cardiomyocytes by altering intracellular Ca2+ homeostasis[190,191] and induce cardiomyocytes apoptosis.[192] IL-1β and TNF-α upregulate AGTR1 expression in cardiac fibroblasts and promote Ang II-induced collagen deposition and fibrosis.[193] TNF-α promotes fibrosis by enhancing the expression of the HF biomarker lysyl oxidase-like 2 via the upregulation of TGF-β.[194] These mechanisms may all contribute to the pathogenic effects of SARS-CoV-2 in human HF [Figure 3].
Cardiac arrhythmia
Arrhythmia can arise secondary to hypoxemia, metabolic disorders, and systemic inflammation following viral infection in patients with or without pre-existing CVD [Figure 3].[175] Arrhythmia includes atrial fibrillations, ventricular tachycardia, and ventricular fibrillation, all symptoms that have been reported in COVID-19 patients.[14,16,195] Patients with severe COVID-19 and with established or undiagnosed CVD are more prone to developing arrhythmia in response to any illness, including SARS-COV-2-induced ischemia or myocardial injury, indirect effects from hypoxia, septic shock, multi-organ failure, and metabolic and electrolyte abnormalities.[196] Arrhythmia occurs in approximately 5% to 17% of COVID-19 patients.[16,195,197]
Two independent studies from Zhongnan Hospital in Wuhan, China, reported that among 138 hospitalized COVID-19 patients, 23 had cardiac arrhythmia. Patients with cardiac arrhythmia were more prevalent in the ICU than in non-ICU settings (44.4% vs. 6.9%, P < 0.001).[14] A retrospective case series of 102 COVID-19 patients reported that COVID-19 patients admitted to ICU were more likely to suffer from arrhythmia (ICU 38.9% vs. non-ICU 13.1%).[198] A study from 8 centers in Heidelberg, Germany, indicated that 20.5% of 166 hospitalized COVID-19 patients had arrhythmia. Atrial fibrillation was the most common observation, while age and CVD were predictors of new onset. Arrhythmia has been associated with increased levels of cardiac biomarkers, hospitalization, admission to ICU, mechanical ventilation, and in-hospital mortality. In multiple regression analyses, the incidence of arrhythmia was associated with hospitalization duration and mechanical ventilation. Blood hs-cTnT (P < 0.001), NT-proBNP (P < 0.001), IL-6 (P = 0.014), lactate dehydrogenase (P = 0.012), hospital duration (P < 0.001), admission to ICU (P = 0.025), and duration in ICU (P = 0.025) were all significantly higher in patients with arrhythmia (n = 34) than in those without arrhythmia (n = 132).[199] In a study of 700 COVID-19 patients from Philadelphia, 53 (8%) were found to have developed arrhythmia-related events during hospitalization, including 9 cardiac arrests, 25 atrial fibrillations, and 9 clinically significant bradyarrhythmias, while 10 patients experienced non-sustained ventricular tachycardias.[195] A retrospective analysis of 4526 hospitalized COVID-19 patients from 4 continents and 12 countries found that 827 had arrhythmia; of these, 69% had hypertension, 42% had diabetes mellitus, 30% had HF, and 24% had CAD. Among those who developed arrhythmia, 81.8% showed atrial arrhythmia, 20.7% had ventricular arrhythmia, and 22.6% had bradyarrhythmia.[200]
Thrombosis and platelet activation
Compared with influenza patients, COVID-19 patients have 9 times as many alveolar capillary microthrombi, leading to a significantly greater number of capillary occlusions.[201] Thrombosis in the lung induces pulmonary hypertension, leading to increased levels of cardiac troponin, CK-MB, and NT-proBNP in patients with severe COVID-19.[14,17,102,202] Therapeutic anti-coagulant treatment can reduce mortality among COVID-19 patients, highlighting the importance of thrombosis in SARS-CoV-2 infection.[203]
ECs express ACE2.[129–135,203] IL-6 and hepcidin enhance ACE2 expression in human pulmonary artery ECs[131] and these 2 molecules are strongly correlated with SARS-CoV-2 infection severity.[204–206] Virus infection causes EC death and consequent inflammatory cell infiltration and microvascular pro-thrombotic events. In a study of 7 COVID-19 patients, the most frequently occurring severe arterial thrombotic events were limb ischemia and floating thrombus of the aorta.[207] The pro-thrombotic state found in COVID-19 patients is related to endothelial damage. SARS-CoV-2 also promotes the transformation of ECs from an anti-thrombotic to a pro-thrombotic state in the microvascular environment, leading to increased levels of von Willebrand factor (VWF) and plasminogen activator inhibitor, a major inhibitor of fibrinolysis [Figure 4].[208] Microvascular thrombosis and endothelial damage in COVID-19 patients contribute to microvascular ischemia, which increases the frequency of myocardial injury and stroke.[209,210] COVID-19 case studies have reported the occurrence of ischemic arterial events such as intra-aortic thrombi, MI, and spontaneous thrombosis of the aortic valve.[211] COVID-19 patients showed left subclavian artery thrombosis and ulcerated plaques with floating thrombus within the aortic arch, and acute ischemia and occlusion of the superficial femoral artery. Computed tomography angiography of the abdominal aorta and lower limbs from one case study revealed the presence of multiple intra-aortic thrombi, occlusion of the distal superior mesenteric artery, splenic and renal ischemic lesions, and occlusion of the right superficial femoral artery and the left supra-articular popliteal artery. Some patients showed acute occlusion of the proximal left circumflex artery with a high thrombus load, or with normal ECG but with high blood troponin and CRP, and acute occlusion of the M2 segment of the middle cerebral artery.[211]
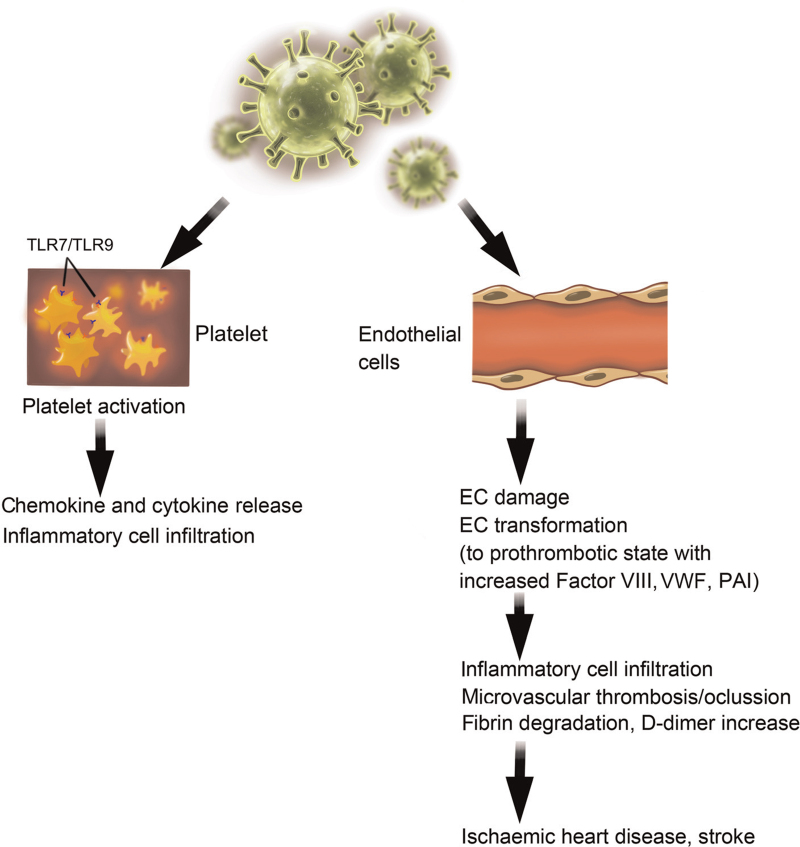
Figure 4.
The effects of SARS-CoV-2 on platelets and endothelial cells (ECs). Left: SARS-CoV-2 may use TLR7 and TLR9 to activate platelets. Right: SARS-CoV-2 infects ECs, resulting in EC damage and transformation. PAI: Plasminogen activator inhibitor; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; TLR: Toll-like receptor; VWF: von Willebrand factor.
Aberrant coagulation makes a substantial contribution to ischemic heart disease and stroke [Figure 4]. COVID-19 patients exhibit abnormal coagulation parameters, such as prothrombin time, fibrin degradation products, activated partial thromboplastin time, and D-dimer values. Increased levels of fibrin degradation products and D-dimer correlated with poor prognosis.[212,213] An observational study of 184 critically ill COVID-19 patients revealed a 31% incidence of thrombotic complications, including 4% arterial and 27% venous.[214] The levels of factor VIII and fibrinogen were elevated in COVID-19 patients, supporting a hypercoagulable state.[215,216] Rises in D-dimer levels in COVID-19 patients indicate ongoing thrombosis.[217] Several studies from Wuhan, China, showed that elevated plasma D-dimer levels are associated with poor prognosis. Blood D-dimer values greater than 2 μg/mL may represent the cut-off value for predicting mortality (sensitivity 92.3%, specificity 83.3%).[176] The burden of underlying coagulopathy reached 50% among deceased COVID-19 patients, and D-dimer levels >1 μg/mL (normal: <0.5 μg/mL) served as an independent predictor of an 18-fold greater risk of in-hospital mortality (OR = 18.4, P = 0.001).[13] Fibrin degradation products and D-dimer levels were also significantly higher in COVID-19 non-survivors than in survivors, and 71.4% of non-survivors had disseminated intravascular coagulation during the course of their disease.[212] Patients with high D-dimers values were more likely to require high-flow oxygen, anticoagulation therapy, antibiotics, and ICU care. These patients also had elevated levels of IL-6, greater numbers of monocytes and lymphocytes, and a greater risk of death.[218] An early report on 1099 COVID-19 patients from 552 hospitals from 30 provinces in China indicated that 46% of these patients had elevated D-dimer levels (>0.5 μg/mL), 60% of whom were severely ill.[15] Among the 150 COVID-19 patients in ICU in a French tertiary hospital, 95% had elevated D-dimer and fibrinogen concentrations.[219]
Platelet activation controls thrombosis.[220] SARS-CoV-2/platelet interactions result in platelet activation and degranulation, thereby potentiating the pro-thrombotic vascular milieu.[221] SARS-CoV-2 RNA has been detected in platelets of COVID-19 patients. These platelets were hyperactivated and aggregated under low-level thrombin stimulation.[222] SARS-CoV-2 RNA likely interacts with platelets via Toll-like receptor 7 (TLR7) and TLR9, thereby activating leukocytes and stimulating inflammatory cytokine expression [Figure 4].[223] Platelet activation and platelet-monocyte aggregate formation were detected in patients with severe, but not mild, COVID-19.[224] Platelets release chemokines (CXCL1, 5, 7 and CCL3, 5, 7),[225] providing additional mechanisms for recruiting mononuclear cells and lymphocytes as part of the cytokine storm [Figure 4]. Platelets from patients with severe COVID-19 were shown to induce tissue factor expression in monocytes from healthy volunteers ex vivo.[224] A study of 36 COVID-19 patients and 31 age- and sex-matched controls from Italy showed that blood D-dimer levels were higher in the former (P < 0.001), but blood neutrophil and platelet counts did not differ. In contrast, the levels of blood platelet activation markers (P-selectin, platelet-derived microparticles, and CD66b+CD41+ platelet/neutrophil complexes) were all higher in COVID-19 patients, as were the levels of blood neutrophil activation markers (neutrophil microparticles, myeloperoxidase (MPO)/DNA complexes, citrullinated histone H3, and matrix metalloproteinase 9). In vitro, plasma from COVID-19 patients induced neutrophil MPO-DNA complex formation, which could be blocked by aspirin.[226] Many studies have also reported that blood platelet counts are reduced in COVID-19 patients. A meta-analysis that included 9 studies comprising 1779 COVID-19 patients showed that blood platelet counts were lower in patients with severe disease, and even lower in those who died.[227] A retrospective study of 1476 consecutively admitted COVID-19 patients during the pandemic in Wuhan, China, showed that platelet counts were reduced in 20.7% of them. In-hospital mortality rates of 92.1%, 61.2%, 17.5%, and 4.7% among these patients correlated with platelet counts of ≤50 × 109/L, >50 to 100 × 109/L, >100 to 150 × 109/L, and >150 × 109/L, respectively.[228] There was also a disparity between survivors and non-survivors, with very low platelet counts being associated with increased mortality.[228–230]
VTE
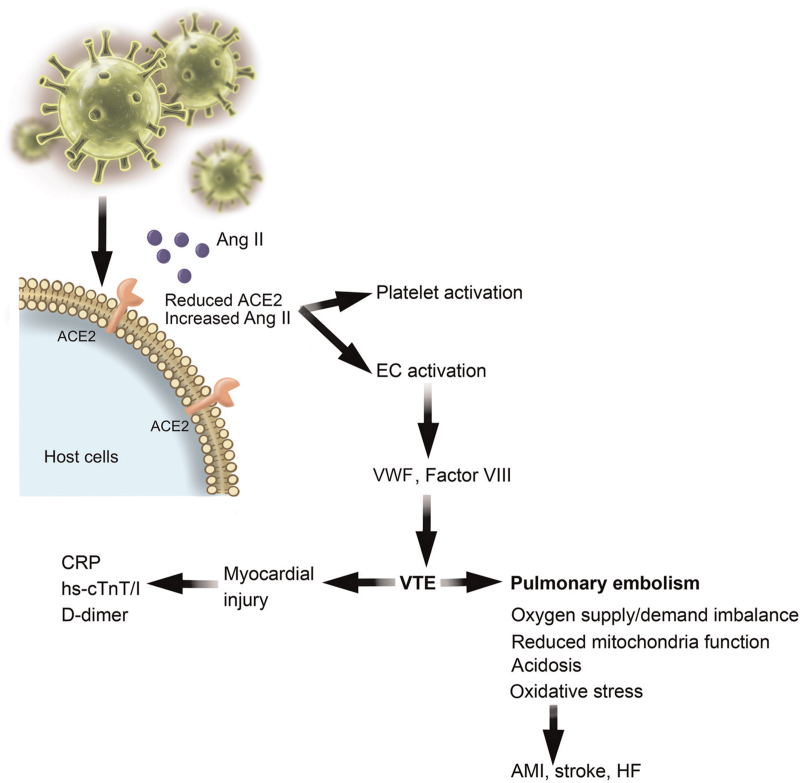
VTE, such as deep vein thrombosis, causes pulmonary embolism[231] and cardiac arrest.[232,233] VTE-associated pulmonary damage and impaired gas exchange lead to an imbalance of myocardial oxygen supply/demand, reduced activity of the mitochondrial electron transport chain, acidosis, and oxidative damage [Figure 5].[234] SARS-CoV-2 may drive thrombotic processes by reducing ACE2 and Ang II clearance. In turn, increased Ang II availability promotes the release of VWF from ECs as well as platelet activation [Figure 5].[235] Patients with co-existing STEMI and COVID-19 showed increased rates of thromboembolic complications that affect multiple vessels, stents, and thrombus grade post-PCI.[236] A case study showed that COVID-19 patients with VTE had greatly elevated CRP (180 mg/L), hs-cTnI (3.24 μg/mL), and D-dimer (21 μg/mL) levels, indicating that SARS-CoV-2 infection and VTE contribute to myocardial injury and that a close association exists between COVID-19 and VTE incidence [Figure 5].[237]
Figure 5.
SARS-CoV-2 infection causes venous thromboembolism (VTE). SARS-CoV-2 infection reduces ACE2 expression concurrently with Ang II accumulation, which activates platelets and endothelial cells (ECs). EC-derived VWF and factor VIII contribute to VTE formation, which induces myocardial injury and pulmonary embolism as a mechanism for AMI, stroke, and HF. ACE: Angiotensin-converting enzyme; AMI: Acute myocardial infarction; Ang: Angiotensin; CRP: C-reactive protein; HF: Heart failure; hs-cTnT: High-sensitive cardiac troponin T; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; VWF: von Willebrand factor.
A study from Wuhan, China, enrolled 81 COVID-19 patients from the ICU of a local hospital, 25% of whom had VTE. These COVID-19 patients with VTE were older and exhibited abnormal coagulation parameters, such as higher D-dimer levels and longer activated partial thromboplastin time than patients without VTE. Blood D-dimer levels greater than 1.5 μg/mL (normal range: <0.5 μg/mL) predicted VTE with a sensitivity of 85.0% and specificity of 88.5%.[238,239] A study from Leiden, Netherlands, showed that patients with severe COVID-19 had a higher incidence of thromboembolic complications. Among 184 COVID-19 patients admitted to the ICU, the rate of thrombotic complications reached 31%, including 27% cases of VTE and 3.7% arterial thrombotic events.[214] After 17 days, the cumulative incidence of thrombotic events reached 49%. Pulmonary embolism was the most frequently reported thrombotic event (87%).[240] A study from Lille, France, showed that the diagnosis of pulmonary thromboembolism in 196 ICU patients with COVID-19 was high (21%), and substantially more common than the 7% for influenza patients or the 6% for all ICU patients.[241] Among a single-center cohort of 198 hospitalized patients from Amsterdam, The Netherlands, 173 patients were confirmed COVID-19-positive, 75 of whom were admitted to the ICU. These ICU patients had higher D-dimer levels, a higher percentage of VTE, deeper venous thrombosis, and increased incidence of symptomatic VTE than patients admitted to the regular ward.[242] When total confirmed cases were considered, 21% of patients had VTE, similar to those from Leiden,[214] Wuhan,[238,239] and Lille.[241] VTE was associated with increased mortality before (HR = 2.7) and after (adjHR = 2.4) adjusting for age, sex, and ICU stay as time-varying variables.[242] A systemic review of 20 studies identified a weighted mean prevalence of 31.3% for VTE among a cohort of 1988 COVID-19 patients.[243] Other types of thrombotic events affecting the arterial system have also been reported, such as MI[15] and stroke.[244] Thrombotic events are consistently associated with a high risk of mortality.[15,240,245]
SARS-CoV-2 infection of cardiomyocytes, ECs, and pericytes
Several mechanisms have been proposed to explain the coronary complications in COVID-19 patients, including coronary plaque rupture, cytokine storm, hypoxic injury, coronary spasm, microthrombi, and endothelial injury. Cardiomyocytes, ECs, and pericytes are among the best-studied cardiac and vascular host cells targeted by SARS-CoV-2 [Figure 6]. SARS-CoV-2 RNA has been found in the blood of COVID-19 patients and was positively correlated with COVID-19 severity.[246] Histological analysis identified the presence of SARS-CoV-2 in myocardium from COVID-19 patients.[171,172] RNA sequencing analysis demonstrated that ventricular cardiomyocytes expressed high levels of cathepsin L and B, which serve for SARS-CoV-2 spike (S) protein priming.[247] Cultured human-induced pluripotent stem cell-derived cardiomyocytes were shown to be susceptible to SARS-CoV-2 infection, which resulted in cytotoxicity.[126–128,143] Direct infection of cardiomyocytes triggers cardiomyocyte inflammation, apoptosis, and necrosis, resulting in myocardial injury and myocarditis [Figure 6]. Although the exact mechanisms by which SARS-CoV-2 damages cardiomyocytes remain incompletely understood, a SARS-CoV-2-induced cytokine storm is thought to be one of the mechanisms underlying COVID-19-induced cardiac injury, coronary spasm, and microthrombi, as discussed above. Another possible mechanism involves a SARS-CoV-2-induced increase in oxygen demand by cardiomyocytes during ARDS as a consequence of hypoxia, which causes oxidative stress.[248]
Figure 6.
SARS-CoV-2 infection in cardiomyocytes, endothelial cells (ECs), and pericytes. Infection of cardiomyocytes and ECs by SARS-CoV-2 involves ACE2 and cathepsins L and B (CatL/CatB) on the host cell surface. Infection of pericytes by SARS-CoV-2 involves ACE2, CD147, and cathepsins L and B. SARS-CoV-2 infection-related consequences for each cell type are shown. ACE: Angiotensin-converting enzyme; ROS: Reactive oxygen species; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; VWF: von Willebrand factor.
The vascular endothelium is among the frontline targets for SARS-COV-2 infection.[249] Indeed, ECs in small or large arteries and veins express ACE2,[129–131,134,135,250] to which the viral S protein binds, although at much lower levels than that seen on pericytes or cardiomyocytes.[122–127] Viral inclusion structures were found in ECs from glomerular capillary loops and airway microvessels from patients with severe COVID-19. In these patients, the lung endothelium was severely injured, with disrupted cell membranes and a high degree of microthrombosis.[251] SARS-CoV-2 infection induces systemic inflammation. The accumulation of inflammatory cells in the endothelium leads to endothelialitis and EC apoptosis, resulting in endothelial dysfunction [Figure 6].[251,252] Circulating cytokines increase the expression of adhesion molecules and chemokines in ECs, thereby augmenting inflammation by promoting leukocyte recruitment [Figure 6]. A case study confirmed SARS-CoV-2 infection of the pulmonary endothelium concomitant with pulmonary endothelialitis and microvascular thrombosis.[201] Endothelial injury and dysfunction in COVID-19 patients may arise either from the direct infection of SARS-CoV-2 and the subsequent induction of intracellular oxidative stress[111,253–255] or indirectly as a consequence of an acute inflammatory response.[131,204–206] Following viral infection, endothelial activation and increased adhesion molecule expression led to enhanced neutrophil activation and ROS production.[256] Oxidative stress due to an imbalance between ROS (or free radicals) and antioxidants causes an increase in pro-thrombotic and cell adhesion molecule-related expression.[257] ROS accumulation promotes oxidative stress and NF-κB signaling, which favors vasoconstriction and vascular permeability [Figure 6].[258,259] SARS-CoV-2 infection of vascular ECs also results in systemic inflammatory endothelial disorder, including the leakage of plasma components from microvessels, intramicrovascular blood clotting, thrombus formation, and excessive release of inflammatory cytokines following vascular endothelial damage.[126,127,129–131,250,254,255] Elevated cytokine levels dampen the anti-thrombotic effects of ECs by activating the coagulation system and causing thrombosis.[260,261] In patients with severe COVID-19, the levels of endothelial and platelet activation markers remain high[262] and the resulting endothelial dysfunction promotes microthrombosis accompanied by thrombocytopenia and elevated blood D-dimer levels.[13]
Pericytes reside outside of the endothelium, share a basement membrane with ECs, thereby helping to maintain basement membrane and vascular barrier integrity, and provide mechanical support to preserve EC stability and function in capillary vessels.[122] A breakdown of the pericyte-EC cross-talk results in a compromised vasculature that is prone to the inflammatory and procoagulant states. This is supported by observations from a pericyte SARS-CoV-2 infection model and COVID-19 patient histology.[136] Pericyte-deficiency (Pdgfbret/ret mice) led to dilated capillaries and elevated production of VWF by ECs, which promoted platelet adhesion and blood coagulation by binding to and stabilizing factor VIII. The loss of pericytes promoted VWF expression in microvascular ECs, platelet aggregation, and fibrin deposition, and disrupted thrombogenic homeostasis [Figure 6].[136] In addition to ACE2,[123,136] cardiac pericytes also express CD147, an extracellular matrix metalloproteinase inducer that also serves as a receptor for SARS-CoV-2.[263] Cardiac pericytes are also primary SARS-CoV-2 targets.[122] SARS-CoV-2-induced pericyte injury may impair endothelial function and induce microvascular dysfunction. One study showed that recombinant SARS-CoV-2 S protein induced cardiac pericyte migration, reduced pericyte activity related to its role in supporting EC network formation, and promoted the secretion of pro-inflammatory molecules as part of the cytokine storm, as well as the production of pro-apoptotic signals targeting ECs [Figure 6].[263]
Inflammation and the cytokine storm in COVID-19 patients
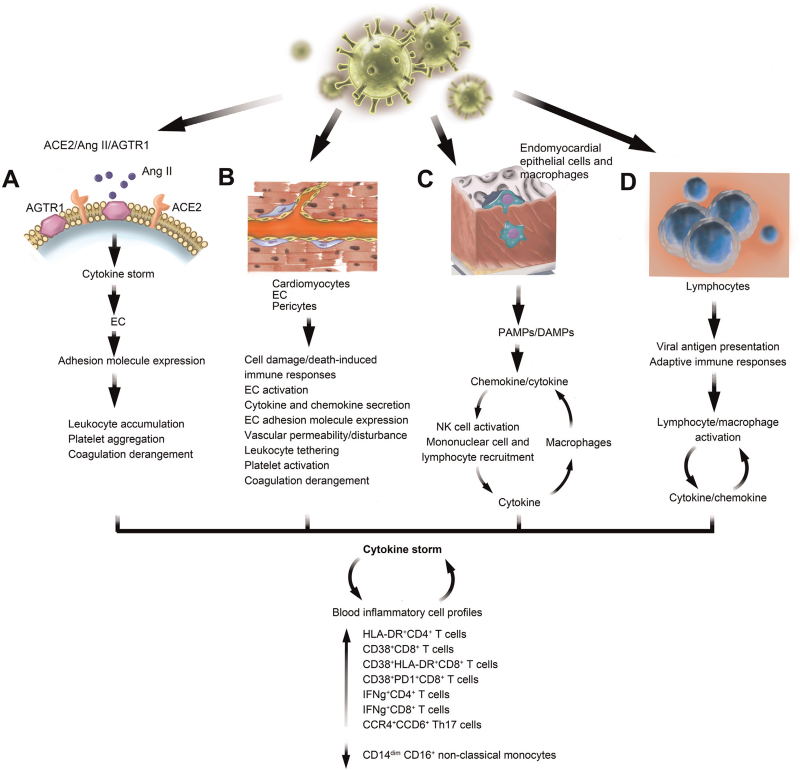
An analysis of the pathology of the first patient who died of COVID-19 showed myocardial inflammation and damage, myocardial cell degeneration and necrosis, and heart interstitial inflammatory infiltrates, including monocytes, lymphocytes, and neutrophils.[157] The cytokine storm observed in COVID-19 patients may originate from multiple sources. Reduced ACE2 expression and increased Ang II accumulation following SARS-CoV-2 infection augment the ACE2/Ang II/AT1R pathway, a mechanism that can promote the cytokine storm, particularly at advanced stages of severe illness, which is characterized by multiple organ failure [Figure 7A].[264] As previously discussed, endocytosis or membrane fusion of SARS-CoV-2 with target cells such as cardiomyocytes, ECs, and cardiac pericytes leads to cell damage or apoptosis, which activates immune responses and promotes the release of inflammatory cytokines [Figure 7B].[111,253–255] ECs produce a wide variety of cytokines and chemokines and have been identified as central regulators of systemic inflammatory responses or the cytokine storm that cause loss of vascular barrier integrity and promote pulmonary edema, endothelialitis, and activation of the coagulation pathway.[265] The marked increase in the levels of inflammatory markers such as IL-2R, IL-6, CRP, and TNF-α links with mortality and promotes inter-endothelial gaps and vascular hyperpermeability,[266,267] a hallmark of the inflammatory response. Cytokines, in turn, activate ECs and increase adhesion molecule expression,[255] leading to vascular disturbances, including leukocyte tethering to the vascular bed, platelet aggregation, and coagulation derangements [Figure 7B]. SARS-CoV-2 infection of alveolar epithelial cells and macrophages leads to the release of pathogen-associated molecular patterns and damage-associated molecular patterns, which also activate chemokine (such as CCLs) and pro-inflammatory cytokine (such as IL-1β, IL-6, TNF-α, IFN-γ) production, contributing to mononuclear cell and lymphocyte recruitment and accumulation[268,269] and further cytokine production by leukocytes [Figure 7C].[270] When epithelial cells are infected, they release cytokines (IL-1β, IFN-α/β) that can stimulate NK cells. Activated NK cells release cytokines to activate macrophages, which, in turn, release their own cytokines (TNF-α and IL-12), thereby activating NK cells and leading to the formation of a positive feedback cycle [Figure 7C].[271] Viral infection is followed by antigen presentation by lymphocytes and adaptive immune system activation. T helper type 1 (Th1) cells release large amounts of IL-12 and IFN-γ for self-stimulation and division, and also activate macrophages that, in turn, activate the innate immune system, which represents an additional feedback mechanism [Figure 7D].[272]
Figure 7.
Cytokine storm resulting from SARS-COV-2 infection in different cell types. (A) The ACE/Ang II/AGTR1 axis-mediated cytokine storm and endothelial cell (EC) activation. (B) SARS-CoV-2 infection of cardiomyocytes, ECs, and pericytes as a source of the cytokine storm. (C) SARS-CoV-2 infection of endomyocardial epithelial cells and macrophages. (D) SARS-CoV-2 infection of lymphocytes and the adaptive immune responses. SARS-CoV-2-induced cytokine storm affects blood inflammatory cell profiles and vice versa. ACE: Angiotensin-converting enzyme; AGTR1: Angiotensin II receptor type 1; Ang: Angiotensin; DAMPs: Damage-associated molecular patterns; PAMPs: Pathogen-associated molecular patterns; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
A cytokine storm can decrease atherosclerotic plaque stability and favor plaque rupture, microthrombosis, and cardiac injury.[273,274] The levels of markers of inflammation such as IL-1, IL-6, TNF-α, CRP, erythrocyte sedimentation rate, and fibrinogen are significantly elevated in COVID-19 patients who develop cardiac complications or those in ICUs.[19,213] In a 3-center study from Paris, France, biomarkers at baseline and 60-day mortality were analyzed in 101 COVID-19 patients admitted to the ICU. Of these, 83 (83%) were under mechanical ventilation. Although the baseline IL-1β level was undetectable, those of IL-6 and CRP were significantly higher in patients with worsening organ failure than in patients in the non-worsening group. Baseline IL-6 and CRP levels were also significantly higher in non-survivors than in survivors. The levels of IL-6 were positively correlated with organ failure severity. After adjusting for the SOFA score and time from symptom onset to blood sampling, IL-6 and CRP levels were also significantly associated with mortality.[275]
Cytokine storm changed blood inflammatory cell profiles in COVID-19 patients and/or blood inflammatory cell profile changes following SARS-CoV-2 infection contributed to the cytokine storm. Histological analysis of the lungs of COVID-19 patients showed the presence of greater numbers of CD4+ T cells but fewer CD8+ T cells compared with lungs from patients with influenza.[201] Flow cytometric analysis of peripheral blood from a patient who died from severe COVID-19 indicated low total numbers of CD4+ and CD8+ T cells; however, these cells were hyperactivated, as evidenced by the high expression of HLA-DR (CD4+) and CD38 (CD8+). This observation, combined with an increase in the concentrations of pro-inflammatory CCR4+CCR6+ Th17 cells,[157] was suggestive of severe immune injury. A study from 4 hospitals in China investigated 258 hypertensive and COVID-19 patients, 207 of whom used anti-viral agents or steroids. Of the 51 patients who did not use anti-viral agents or steroids, 34 had mild disease and 17 had severe disease. Blood levels of CD4+ and CD8+ T cells decreased in the order of normal>mild>severe but cured>severe>died during a 4-week monitoring period. The levels of blood CD38+HLA-DR+ and CD38+PD-1+CD8+ T cells, IFNG+CD8+ T cells, and IFNG+CD4+ T cells were also elevated among patients who survived, as well as those who died during the 4 weeks following symptom onset [Figure 7].[276] As expected, the concentrations of SARS-CoV-2-specific IgG, IgM, and IgA antibodies were higher in survivors than in non-survivors.[276] CD14dimCD16+ non-classical monocytes mediate anti-viral immune responses and play athero-protective, anti-inflammatory, and pro-homoeostatic roles [Figure 7].[277,278] In a cohort of 96 consecutive patients from Tübingen, Germany, including 47 who had CAD and COVID-19, 19 who had CAD only, and 30 who were healthy, the numbers of these non-classical monocytes in the blood were markedly lower in CAD-COVID-19 patients than in those of the other 2 groups (P < 0.0001), and these cells showed decreased expression of adhesion, migration, and T-cell activation markers (CD54, CD62L, CX3CR1, CD80, and HLA-DR). Decreased numbers of these cells were positively associated with ICU admission, respiratory failure, and use of mechanical ventilation.[279]
Impact of SARS-CoV-2 infection on common CVD therapy
ACE2 is required for SARS-CoV-2 entry into host cells. Increased ACE2 expression likely heightens the risk of CVD and increases the likelihood of severe SARS-CoV-2 infection. Nevertheless, myocardium ACE2 expression is decreased in post-mortem autopsy samples from SARS-CoV-2-infected patients, and this reduction is associated with a concomitant increase in myocardial inflammation and fibrosis.[111,253–255] Increased ACE2 expression protects against Ang II-induced vasoconstriction and inflammation but also increases susceptibility to SARS-CoV-2 infection. In particular, the therapeutic RAS blockade agents used in CVD management, such as ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), increase ACE2 expression, thereby increasing the susceptibility to SARS-CoV-2 infection and the pathogenicity of COVID-19. For instance, the ARBs losartan and olmesartan increase ACE2 expression in rat hearts after MI,[280] while enalapril normalizes ACE2 expression in rat LVs following HF.[281] These observations suggest that an increase in ACE2 levels may be protective against SARS-CoV-2 infection due to the increase in Ang II catabolism and Ang-(1–7) production [Figure 2].[282] However, to date, there is a lack of consistent results to support that RAS blockers improve prognosis,[78] or that ACEIs and ARBs affect the susceptibility to SARS-CoV-2 infection.[283] Contradictory results were obtained from both experimental models and patient population studies. Anti-hypertensive treatments with ACEIs or ARBs (lisinopril, losartan, enalapril) were associated with increased ACE2 levels in the LVs of normotensive rats.[14,151] Results from a single-center retrospective study from Wuhan, China, showed that ACEIs and ARBs reduced the blood levels of inflammatory biomarkers (CRP and procalcitonin) and yielded better outcomes in 43 COVID-19 patients compared with those of 83 patients treated with other anti-hypertensive drugs.[284] A retrospective multicenter study of 1128 adult COVID-19 patients with hypertension from 9 hospitals in Wuhan, China, showed that the use of these ACEIs and ARBs was associated with a 58% reduction in the risk for all-cause death (adjHR = 0.42) after adjusting for age, gender, comorbidities, and in-hospital medications.[285] The French National Health Insurance database of almost 2 million hypertensive patients assessed whether the use of anti-hypertensive drugs affected the risk for COVID-19. ACEIs and ARBs were compared with calcium channel blockers. Three exclusive cohorts that included individuals aged 18 to 80 were followed from February 15 to June 7, 2020. Endpoints were time to hospitalization for COVID-19 and time to intubation/death during hospital stay. Within 16 weeks, 2338 individuals were hospitalized, while 526 died or were intubated for COVID-19. ACEIs and ARBs lowered the risk of COVID-19-related hospitalization in both males and females and in all age groups (from 18 to 80). ACEIs and ARBs also lowered the risk of death/intubation in males and females, as well as in patients between the ages of 51 and 80, but not in those aged 18 to 50.[286] Analysis of the Healthcare database from the Health Insurance Review and Assessment Service of Korea, which covers the entire Korean population of 50 million people from January 1, 2015, to April 8, 2020, showed that 69,793 individuals underwent COVID-19 screening. Among 1290 hypertensive and COVID-19-positive patients, 682 had records of ACEI/ARB use while 603 did not. Inverse probability of treatment weighting was used to mitigate selection bias and a Poisson regression model was used to estimate the relative risks to compare the outcomes between ACEI/ARB users and non-users. The use of ACEIs/ARBs was found to be associated with better clinical outcomes (adjRR = 0.60, P = 0.005) and showed a protective effect in overall outcomes in both men (adjRR = 0.84, P = 0.008) and patients with pre-existing respiratory disease (adjRR = 0.74, P = 0 .002); however, no association was found between ACEI/ARB use and all-cause mortality (adjRR = 0.62, P = 0.097) or respiratory events (adjRR = 0.99, P = 0.84).[287] Two clinical trials of losartan as an additional treatment for COVID-19 are currently underway (NCT04311177 and NCT043120-09).
Different population studies have also shown that RAS inhibitors neither increase the risk of SARS-CoV-2 infection in patients with hypertension nor negatively influence disease severity. A case-population study of 1139 COVID-19 patients and 11,390 controls showed that RAS inhibitors do not increase the risk of COVID-19-related hospital admission when compared with other anti-hypertensive drugs (OR = 0.94).[288] A randomized clinical trial of 659 patients hospitalized in Brazil with mild (57.1%) to moderate (42.9%) COVID-19 were grouped 1:1 with or without ACEIs or ARBs for 30 days. The primary outcome was days alive and out of hospital during this 30-day period. No difference was found between patients taking the ACEIs or ARBs and those who did not. Additionally, no differences were identified in the mortality rate, cardiovascular death, or COVID-19 progression.[289] Studies of 3179 patients who had been discharged or had died selected from 5625 COVID-19 inpatients from 61 hospitals across Italy showed that the use of ACEIs, ARBs, or Ca-antagonists was not associated with risk of death. Only a marginal negative association was found between ARB use and risk of death (adjOR = 0.65, P = 0.025), while a marginal positive association was found between diuretic use and risk of death (OR = 1.66, P = 0.020).[290] At the very least, these studies established the safety of ACEIs and ARBs,[70,71,78,291] and support the continued use of ACEIs and ARBs in patients with COVID-19, although the debate continues.[292–295]
Glucocorticoids are not recommended for treating SARS-CoV-2-induced lung injury based on the results of a UK-based study. Although glucocorticoids inhibit lung inflammation, they also increase the risk of CVD events.[296] Studies from SARS-CoV showed that glucocorticoids also inhibit the immune response and virus elimination.[297] In a UK community-based biobank cohort of 473,555 individuals that contained 459 COVID-19 deaths and 2626 non-COVID-19 deaths, besides age, sex, being Black, and comorbidities, the use of oral steroids, but not ACEIs or ARBs, was associated with an increased risk of COVID-19-related death.[49] However, several clinical studies have yielded the opposite conclusions. In Italy, a study of 692 consecutive patients with COVID-19, including 90 (13.0%) who had a history of HF, from 13 Italian cardiology centers between March 1 and April 9, 2020, showed that in-hospital treatment with corticosteroids and heparin had beneficial effects. Adjusted HRs for death were 0.46 (P = 0.001, n = 404) for corticosteroid treatment and 0.41 (P < 0.001, n = 364) for heparin treatment.[77] Patient background, corticosteroid dose, and treatment length may be associated with these discrepancies. In the RECOVERY trial of hospitalized COVID-19 patients from the UK that included 2140 patients who received oral or intravenous low-dose, short-term dexamethasone treatment (6 mg/day for 10 days) and 4321 who received standard care, the 28-day fatality rate was significantly reduced in those receiving dexamethasone (adjRR = 0.83, P < 0.001). The incidence of death was lower in the dexamethasone group than in the standard care group among patients receiving invasive ventilation (adjRR = 0.64) and those receiving oxygen, but not those receiving ventilation (adjRR = 0.82).[298] Compared with standard care alone, higher dose, intravenous dexamethasone treatment (20 mg daily for 5 days or 10 mg daily for 5 days plus standard care) in 151 COVID-19 patients with moderate to severe ARDS from Brazil increased the number of ventilation-free days during the first 28 days (n = 148, P = 0.04).[299] A study of 86 hospitalized COVID-19 patients from Iran showed similar results. Low doses of methylprednisolone (2 mg/day) or dexamethasone (6 mg/day) significantly improved clinical status at day 5 (P = 0.002) and day 10 (P = 0.001) and reduced the overall disease score (3.909 vs. 4.873, P = 0.004) and length of hospital stay (7.43 days vs. 10.52 days, P = 0.015).[300] In a similar randomized controlled trial involving 68 hospitalized COVID-19 patients from Iran, patients receiving high doses of intravenous methylprednisolone (n = 34, 250 mg/day) for 3 days showed a markedly higher percentage of recovery (94.1% vs. 57.1%, P < 0.001), a lower mortality rate (5.9% vs. 42.9%, P < 0.001), and longer survival time (HR = 0.293, log-rank test P < 0.001) compared with those receiving standard care (n = 34).[301] Several meta-analyses indicated that corticosteroids remain effective and safe among severely ill COVID-19 patients, but not among all hospitalized COVID-19 patients.[302–304] Accordingly, the WHO guidelines recommend corticosteroid administration for patients with severe COVID-19 or those critically ill with the disease, but not for patients with non-severe COVID-19.[305]
A recent study from Wuhan, China, assessed the effect of low-dose aspirin on COVID-19 patients who also had CAD. Among 183 patients, 52 took low-dose aspirin and 131 did not. Multivariate analysis indicated that aspirin use did not affect either in-hospital mortality or all-cause mortality.[306] However, some studies have reported different results. Among 28 COVID-19 patients who received aspirin and 204 who did not, those receiving aspirin were significantly older and were more likely to be hypertensive and suffer from cerebrovascular disease and coronary disease (all P < 0.001). Both the 30-day (4.17% vs. 29.2%) and 60-day (8.3% vs. 33.3%) mortality rates were markedly lower in patients of the low-dose aspirin group than in those of the non-aspirin group.[307] In a very recent study of 412 COVID-19 patients from a multicenter (Collaborative Research to Understand the Sequelae of Harm in COVID (CRUSH COVID)) registry from Maryland, USA, 98 patients received aspirin 24 hours or 7 days before admission. Again, aspirin users were significantly older and had a greater probability of suffering from hypertension, diabetes, CAD, renal disease, and beta-blocker use (all P < 0.001). Aspirin use significantly reduced the risk of mechanical ventilation use (HR = 0.56, P = 0.007), ICU admission (HR = 0.57, P = 0.005), and in-hospital mortality (HR = 0.53, P = 0.02).[308] Aspirin has triple effects, namely, it inhibits virus replication and has both anticoagulant and anti-inflammatory properties. Aspirin was suggested to exert protective effects in COVID-19 patients, although the results from a clinical trial (NCT04365309) from Xijing Hospital, Xi’an, China, that was completed in June 2020 have not been released.
Statins and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are both commonly used among CVD patients, but these lipid-lowering drugs have also proved beneficial for COVID-19 patients. Multiple studies have reported that COVID-19 patients have low blood levels of low-density (LDL) and high-density (HDL) lipoproteins. LDL and HDL levels were even lower in patients with severe disease or deceased patients.[309–312] In contrast, blood triglyceride levels are significantly elevated in COVID-19 patients. From 98 COVID-19 patients from Wuhan, China, including 46 who were admitted to the ICU, 32 and 46 who suffered from myocardial injury and HF, respectively, and 36 who died, the blood triglyceride to HDL-C ratio was increased in patients with myocardial injury, HF, severe illness, and fatal outcome, while the baseline triglyceride to HDL-C ratio was correlated with the levels of hs-cTnI (r = 0.251, P = 0.018), NT-proBNP (r = 0.274, P = 0.008), HbA1C (r = 0.239, P = 0.038), and IL-6 (r = 0.218, P = 0.042). Multivariate logistic regression analysis showed that an increased triglyceride to HDL-C ratio was an independent risk factor for myocardial injury (OR = 2.73, P = 0.013), HF (OR = 2.64, P = 0.019), severe illness (OR = 3.01, P = 0.032), and fatal outcome (OR = 2.97, P = 0.014).[313] A retrospective chart review of 139 COVID-19 patients, including 26 deceased and 113 survivors, showed that deceased patients were older (P < 0.001), were more likely to suffer from hypertension (P < 0.006), diabetes mellitus (P = 0.002), CVD (P = 0.001), chronic renal insufficiency (P = 0.003), and atrial fibrillation (P = 0.003). Deceased patients also had lower plasma total cholesterol (P = 0.004), HDL-C (P < 0.001), and LDL-C (P < 0.001) levels but higher triglyceride levels (P < 0.001), compared with those of survivors.[314] Statin use decreased COVID-19 patient fatality and severity by nearly 30%.[315] In a study of 154 elderly nursing home residents with COVID-19, a history of statin use was associated with an absence of symptoms during COVID-19 infection (OR = 2.65, P = 0.028), even after adjusting for age, sex, and comorbidities such as diabetes, hypertension, and functional status.[316] Another study enrolled 13,981 patients with COVID-19, 1219 of whom received statins. The 28-day all-cause of mortality in a propensity score-matching model was 5.2% and 9.4% among statin and non-statin users (adjHR = 0.58, P = 0.001), respectively. Statin therapy was also associated with lower blood CRP and IL-6 concentrations and neutrophil counts.[317] A meta-analysis that included 4 studies and 8990 COVID-19 patients revealed that statin use was associated with a significantly reduced risk of COVID-19-associated death and disease severity (HR = 0.70, P = 0.01) compared with non-use of statins.[315] These results suggest that statin treatment may protect against coronary endothelial dysfunction due to COVID-19.
Statins and PCSK9 inhibitors may mitigate SARS-CoV-2 infection through multiple mechanisms. Statins reduce inflammation and oxidative stress.[318,319] They inhibit the rate-limiting enzyme of the mevalonate pathway, leading to reduced GTPase activity, which is essential for cell migration, activation, signaling, and cytokine expression.[320] Statins stabilize MyD88, suppress TLR function, and attenuate NF-κB activation following a pro-inflammatory trigger (eg, hypoxia).[320,321] Statins also reduce ox-LDL levels and NADPH oxidase activity, which decreases ROS generation by inhibiting NF-κB signaling pathway-associated transcription or by improving endothelial NO synthase coupling.[322] Like ACEIs and ARBs,[323] statin treatment also increases ACE2 expression.[324] Statins may exert similar anti-viral activity to that of ACEIs and ARBs by reducing Ang II availability and increasing Ang-(1–7) production. Statin- or PCSK9 inhibitor-mediated lipid reduction may block SARS-CoV-2 infection by impeding its entry into host cells through the disruption of lipid rafts. Statins, such as pitavastatin, could also reduce SARS-CoV-2 infectivity by inhibiting its main protease, which plays a role in proteolytic maturation and viral replication.[325] Accordingly, statins and PCSK9 inhibitors may act as immunomodulators in COVID-19 because of their antioxidative, anti-arrhythmic, and anti-thrombotic properties. Their use is associated with improved endothelial functions, reduced oxidative stress, less platelet adhesion, and increased atherosclerotic plaque stability.[319,326] Thus, the use of lipid-lowering drugs may not need to be discontinued in COVID-19 patients with CVD.[327]
Study limitations
Due to the rapidly evolving basic and clinical research relating to COVID-19, the contents of this manuscript are subject to modification as we learn more about this devastating disease. Given the broad tissue distribution of ACE2 in many tested organs, it seems likely that SARS-CoV-2 infects not only the lungs and heart but also many other organs that are not discussed in this review. Furthermore, many of the recently published studies are still observational and descriptive, even though this disease was first documented more than a year ago. Our knowledge about SARS-CoV-2 remains incomplete, especially the continuing emergence of different mutants. Despite this, we hope that the contents presented here will aid in our understanding of the disease, particularly the interconnection between COVID-19 and CVD, drug selection, or perhaps most importantly, patient care.
Acknowledgments
The authors thank Ms. Chelsea Swallom for her editorial assistance.
Funding
Dr. Shi receives funding support from the National Heart, Lung, and Blood Institute (R01HL151627) and the National Institute of Neurological Disorders and Stroke (R01AG063839). Dr. Libby receives funding support from the National Heart, Lung, and Blood Institute (1R01HL134892), the American Heart Association (18CSA34080399), and the RRM Charitable Fund, and the Simard Fund.
Author Contributions
Yuanyuan Zhang, Mingjie Wang, Xian Zhang performed literature search and helped with manuscript writing; Tianxiao Liu prepared the illustrations; Peter Libby edited the manuscript; Guo-Ping Shi performed literature search and wrote the manuscript.
Conflicts of Interest
Dr. Libby is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Norvo Nordisk, Novartis, Pfizer, and Sanofi-Regeneron. Dr. Libby is a member of the scientific advisory board for Amgen, Caristo, Cartesian, CSL Behring, DalCor Pharmaceuticals, Dewpoint, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, PlaqueTec, and XBiotech, Inc.
Dr. Libby's laboratory has received research funding in the last 2 years from Novartis. Dr. Libby is on the Board of Directors of XBiotech, Inc. Dr. Libby has a financial interest in Xbiotech, a company developing therapeutic human antibodies. Dr. Libby's interests were reviewed and are managed by Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict-of-interest policies.
Editor note: Peter Libby is an Associate Editor of Cardiology Discovery. Guo-Ping Shi is an Editorial Board Member of Cardiology Discovery. The article was subject to the journal's standard procedures, with peer review handled independently of these editors and their research groups.
References
- [1].Ellison-Hughes GM, Colley L, O’Brien KA, et al. The role of MSC therapy in attenuating the damaging effects of the cytokine storm induced by COVID-19 on the heart and cardiovascular system. Front Cardiovasc Med 2020;7:602183. doi: 10.3389/fcvm.2020.602183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fanelli V, Fiorentino M, Cantaluppi V, et al. Acute kidney injury in SARS-CoV-2 infected patients. Crit Care 2020;24(1):155. doi: 10.1186/s13054-020-02872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu Q, Zhou L, Sun X, et al. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci Rep 2017;7(1):9110. doi: 10.1038/s41598-017-09536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zheng YY, Ma YT, Zhang JY, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alhogbani T. Acute myocarditis associated with novel Middle east respiratory syndrome coronavirus. Ann Saudi Med 2016;36(1):78–80. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ammar A, Brach M, Trabelsi K, et al. Effects of COVID-19 home confinement on eating behaviour and physical activity: results of the ECLB-COVID19 international online survey. Nutrients 2020;12(6):1583. doi: 10.3390/nu12061583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ruiz-Roso MB, de Carvalho Padilha P, Mantilla-Escalante DC, et al. Covid-19 confinement and changes of adolescent's dietary trends in Italy, Spain, Chile, Colombia and Brazil. Nutrients 2020;12(6):1807. doi: 10.3390/nu12061807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ruiz-Roso MB, de Carvalho Padilha P, Matilla-Escalante DC, et al. Changes of physical activity and ultra-processed food consumption in adolescents from different countries during Covid-19 pandemic: an observational study. Nutrients 2020;12(8):2289. doi: 10.3390/nu12082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pellegrini M, Ponzo V, Rosato R, et al. Changes in weight and nutritional habits in adults with obesity during the “lockdown” period caused by the COVID-19 virus emergency. Nutrients 2020;12(7):2016. doi: 10.3390/nu12072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis 2020;63(3):390–391. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].He XW, Lai JS, Cheng J, et al. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. Zhonghua Xin Xue Guan Bing Za Zhi 2020;48(6):456–460. doi: 10.3760/cma.j.cn112148-20200228-00137. [DOI] [PubMed] [Google Scholar]
- [20].Einstein AJ, Shaw LJ, Hirschfeld C, et al. International impact of COVID-19 on the diagnosis of heart disease. J Am Coll Cardiol 2021;77(2):173–185. doi: 10.1016/j.jacc.2020.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Czerny M, Gottardi R, Puiu P, et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on the care of patients with acute and chronic aortic conditions. Eur J Cardiothorac Surg 2021;59(5):1096–1102. doi: 10.1093/ejcts/ezaa452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sokolski M, Gajewski P, Zymlinski R, et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on acute admissions at the emergency and cardiology departments across Europe. Am J Med 2021;134(4):482–489. doi: 10.1016/j.amjmed.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].De Luca G, Verdoia M, Cercek M, et al. Impact of COVID-19 pandemic on mechanical reperfusion for patients with STEMI. J Am Coll Cardiol 2020;76(20):2321–2330. doi: 10.1016/j.jacc.2020.09.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Giamberti A, Varrica A, Agati S, et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on the Italian congenital cardiac surgery system: a national survey. Eur J Cardiothorac Surg 2020;58(6):1254–1260. doi: 10.1093/ejcts/ezaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stöhr E, Aksoy A, Campbell M, et al. Hospital admissions during Covid-19 lock-down in Germany: Differences in discretionary and unavoidable cardiovascular events. PLoS One 2020;15(11):e0242653. doi: 10.1371/journal.pone.0242653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kwok CS, Gale CP, Curzen N, et al. Impact of the COVID-19 pandemic on percutaneous coronary intervention in England: insights from the British Cardiovascular Intervention Society PCI Database Cohort. Circ Cardiovasc Interv 2020;13(11):e009654. doi: 10.1161/CIRCINTERVENTIONS.120.009654. [DOI] [PubMed] [Google Scholar]
- [27].Chieffo A, Tarantini G, Naber CK, et al. Performing elective cardiac invasive procedures during the COVID-19 outbreak: a position statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI). EuroIntervention 2021;16(14):1177–1186. doi: 10.4244/EIJ-D-20-01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wosik J, Clowse M, Overton R, et al. Impact of the COVID-19 pandemic on patterns of outpatient cardiovascular care. Am Heart J 2021;231:1–5. doi: 10.1016/j.ahj.2020.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Normando PG, Araujo-Filho JA, Fonseca GA, et al. Reduction in hospitalization and increase in mortality due to cardiovascular diseases during the COVID-19 pandemic in Brazil. Arq Bras Cardiol 2021;116(3):371–380. doi: 10.36660/abc.20200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wu J, Mamas MA, Mohamed MO, et al. Place and causes of acute cardiovascular mortality during the COVID-19 pandemic. Heart 2021;107(2):113–119. doi: 10.1136/heartjnl-2020-317912. [DOI] [PubMed] [Google Scholar]
- [31].Wilson SJ, Connolly MJ, Elghamry Z, et al. Effect of the COVID-19 pandemic on ST-segment-elevation myocardial infarction presentations and in-hospital outcomes. Circ Cardiovasc Interv 2020;13(7):e009438. doi: 10.1161/CIRCINTERVENTIONS.120.009438. [DOI] [PubMed] [Google Scholar]
- [32].Nan J, Meng S, Hu H, et al. Comparison of clinical outcomes in patients with ST elevation myocardial infarction with percutaneous coronary intervention and the use of a telemedicine app before and after the COVID-19 pandemic at a center in Beijing, China, from August 2019 to March 2020. Med Sci Monit 2020;26:e927061. doi: 10.12659/MSM.927061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu H, Wang W, Chen H, et al. Can WeChat group-based intervention reduce reperfusion time in patients with ST-segment myocardial infarction? A controlled before and after study. J Telemed Telecare 2020;26(10):627–637. doi: 10.1177/1357633X19856473. [DOI] [PubMed] [Google Scholar]
- [34].Xiao J, Zhang Q, Gao YQ, et al. Antifungal and antibacterial metabolites from an endophytic Aspergillus sp. associated with Melia azedarach. Nat Prod Res 2014;28(17):1388–1392. doi: 10.1080/14786419.2014.904308. [DOI] [PubMed] [Google Scholar]
- [35].Turakhia MP, Desai M, Hedlin H, et al. Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: The Apple Heart Study. Am Heart J 2019;207:66–75. doi: 10.1016/j.ahj.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jousilahti P, Vartiainen E, Tuomilehto J, et al. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation 1999;99(9):1165–1172. doi: 10.1161/01.cir.99.9.1165. [DOI] [PubMed] [Google Scholar]
- [37].Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation 2011;124(19):2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nimgaonkar I, Valeri L, Susser E, et al. The age pattern of the male-to-female ratio in mortality from COVID-19 mirrors that of cardiovascular disease in the general population. Aging (Albany NY) 2021;13(3):3190–3201. doi: 10.18632/aging.202639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wallentin L, Lindbäck J, Eriksson N, et al. Angiotensin-converting enzyme 2 (ACE2) levels in relation to risk factors for COVID-19 in two large cohorts of patients with atrial fibrillation. Eur Heart J 2020;41(41):4037–4046. doi: 10.1093/eurheartj/ehaa697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sniderman AD, Furberg CD. Age as a modifiable risk factor for cardiovascular disease. Lancet 2008;371(9623):1547–1549. doi: 10.1016/S0140-6736(08)60313-X. [DOI] [PubMed] [Google Scholar]
- [41].Poterucha TJ, Elias P, Jain SS, et al. Admission cardiac diagnostic testing with electrocardiography and troponin measurement prognosticates increased 30-day mortality in COVID-19. J Am Heart Assoc 2021;10(1):e018476. doi: 10.1161/JAHA.120.018476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lee PI, Hu YL, Chen PY, et al. Are children less susceptible to COVID-19. J Microbiol Immunol Infect 2020;53(3):371–372. doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Valverde I, Singh Y, Sanchez-de-Toledo J, et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation 2021;143(1):21–32. doi: 10.1161/CIRCULATIONAHA.120.050065. [DOI] [PubMed] [Google Scholar]
- [44].Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Müller J, Oberhoffer R, Brudy L, et al. COVID-19 and paediatric patient involvement (cardiovascular aspects). Eur Heart J Suppl 2020;22(Suppl Pt t):19–24. doi: 10.1093/eurheartj/suaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Centers for Disease Control and Prevention (CDC). Risk for COVID-19 Infection, Hospitalization, and Death by Race/Ethnicity; 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html. Accessed May 17, 2021. [Google Scholar]
- [47].Josiah N, Starks S, Wilson PR, et al. The intersection of depression, anxiety, and cardiovascular disease among black populations amid the COVID-19 pandemic. J Clin Nurs 2021;30(9–10):e36–e40. doi: 10.1111/jocn.15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association's COVID-19 Cardiovascular Disease Registry. Circulation 2021;143(24):2332–2342. doi: 10.1161/CIRCULATIONAHA.120.052278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Elliott J, Bodinier B, Whitaker M, et al. COVID-19 mortality in the UK Biobank cohort: revisiting and evaluating risk factors. Eur J Epidemiol 2021;36(3):299–309. doi: 10.1007/s10654-021-00722-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].International Severe Acute Respiratory and Emerging Infections Consortium, Escher M, Hall M, et al. ISARIC Clinical Data Report 10 February 2021. Available from: https://www.medrxiv.org/content/10.1101/2020.07.17.20155218v6. Accessed May 17, 2021. [Google Scholar]
- [51].Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab 2020;31(6):1068–1077.e3. doi: 10. 1016/j. cmet. 2020. 04. 021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hendren NS, de Lemos JA, Ayers C, et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19: results from the American Heart Association COVID-19 Cardiovascular Disease Registry. Circulation 2021;143(2):135–144. doi: 10.1161/CIRCULATIONAHA.120.051936. [DOI] [PubMed] [Google Scholar]
- [54].Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cai Q, Chen F, Wang T, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care 2020;43(7):1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- [56].Gao F, Zheng KI, Wang XB, et al. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care 2020;43(7). e72–72e74. doi: 10.2337/dc20-0682. [DOI] [PubMed] [Google Scholar]
- [57].Yang J, Tian C, Chen Y, et al. Obesity aggravates COVID-19: An updated systematic review and meta-analysis. J Med Virol 2021;93(5):2662–2674. doi: 10.1002/jmv.26677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].McGurnaghan SJ, Weir A, Bishop J, et al. Public Health Scotland C-HPSG and Scottish Diabetes Research Network Epidemiology G. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol 2021;9(2):82–93. doi: 10.1016/S2213-8587(20)30405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wei ZY, Qiao R, Chen J, et al. The influence of pre-existing hypertension on coronavirus disease 2019 patients. Epidemiol Infect 2021;149:e4. doi: 10.1017/S0950268820003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mu S, Wei W, Jin C, et al. Risk factors for COVID-19 patients with cardiac injury: pulmonary ventilation dysfunction and oxygen inhalation insufficiency are not the direct causes. Aging (Albany NY) 2020;12(23):23464–23477. doi: 10.18632/aging.104148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yao Q, Ni J, Hu TT, et al. Clinical characteristics and outcomes in coronavirus disease 2019 (COVID-19) patients with and without hypertension: a retrospective study. Rev Cardiovasc Med 2020;21(4):615–625. doi: 10.31083/j.rcm.2020.04.113. [DOI] [PubMed] [Google Scholar]
- [62].Hosseinzadeh R, Goharrizi M, Bahardoust M, et al. Should all patients with hypertension be worried about developing severe coronavirus disease 2019 (COVID-19). Clin Hypertens 2021;27(1):3. doi: 10.1186/s40885-021-00161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus disease (COVID-19): China, 2020. China CDC Weekly 2020;2(8):113–122. [PMC free article] [PubMed] [Google Scholar]
- [64].Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- [65].Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Qian H, Gao P, Tian R, et al. Myocardial injury on admission as a risk in critically ill COVID-19 patients: a retrospective in-icu study. J Cardiothorac Vasc Anesth 2021;35(3):846–853. doi: 10.1053/j.jvca.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Park BE, Lee JH, Park HK, et al. Impact of cardiovascular risk factors and cardiovascular diseases on outcomes in patients hospitalized with COVID-19 in Daegu Metropolitan City. J Korean Med Sci 2021;36(2):e15. doi: 10.3346/jkms.2021.36.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bhatt AS, Jering KS, Vaduganathan M, et al. Clinical outcomes in patients with heart failure hospitalized with COVID-19. JACC Heart Fail 2021;9(1):65–73. doi: 10.1016/j.jchf.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Mehta N, Kalra A, Nowacki AS, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5(9):1020–1026. doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med 2020;382(25):2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Case BC, Yerasi C, Forrestal BJ, et al. Comparison of characteristics and outcomes of patients with acute myocardial infarction with versus without coronarvirus-19. Am J Cardiol 2021;144:8–12. doi: 10.1016/j.amjcard.2020.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gupta YS, Finkelstein M, Manna S, et al. Coronary artery calcification in COVID-19 patients: an imaging biomarker for adverse clinical outcomes. Clin Imaging 2021;77:1–8. doi: 10.1016/j.clinimag.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Pareek M, Singh A, Vadlamani L, et al. Relation of cardiovascular risk factors to mortality and cardiovascular events in hospitalized patients with coronavirus disease 2019 (from the Yale COVID-19 Cardiovascular Registry). Am J Cardiol 2021;146:99–106. doi: 10.1016/j.amjcard.2021.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jain R, Salinas PD, Kroboth S, et al. Comprehensive echocardiographic findings in critically ill COVID-19 patients with or without prior cardiac disease. J Patient Cent Res Rev 2021;8(1):68–76. doi: 10.17294/2330-0698.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Russo V, Piccinocchi G, Mandaliti V, et al. Cardiovascular comorbidities and pharmacological treatments of COVID-19 patients not requiring hospitalization. Int J Environ Res Public Health 2020;18(1):102. doi: 10.3390/ijerph18010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zuin M, Rigatelli G, Zuliani G, et al. Arterial hypertension and risk of death in patients with COVID-19 infection: Systematic review and meta-analysis. J Infect 2020;81(1):e84–e86. doi: 10.1016/j.jinf.2020.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Tomasoni D, Inciardi RM, Lombardi CM, et al. Impact of heart failure on the clinical course and outcomes of patients hospitalized for COVID-19. Results of the Cardio-COVID-Italy multicentre study. Eur J Heart Fail 2020;22(12):2238–2247. doi: 10.1002/ejhf.2052. [DOI] [PubMed] [Google Scholar]
- [78].Mancia G, Rea F, Ludergnani M, et al. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Dillinger JG, Benmessaoud FA, Pezel T, et al. Coronary artery calcification and complications in patients with COVID-19. JACC Cardiovasc Imaging 2020;13(11):2468–2470. doi: 10.1016/j.jcmg.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Saleh A, Matsumori A, Abdelrazek S, et al. Myocardial involvement in coronavirus disease 19. Herz 2020;45(8):719–725. doi: 10.1007/s00059-020-05001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Rey JR, Caro-Codón J, Rosillo SO, et al. Heart failure in COVID-19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail 2020;22(12):2205–2215. doi: 10.1002/ejhf.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lala A, Johnson KW, Januzzi JL, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lippi G, Wong J, Henry BM. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med 2020;130(4):304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- [85].Hessami A, Shamshirian A, Heydari K, et al. Cardiovascular diseases burden in COVID-19: systematic review and meta-analysis. Am J Emerg Med 2021;46:382–391. doi: 10.1016/j.ajem.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Watson T, Goon PK, Lip GY. Endothelial progenitor cells, endothelial dysfunction, inflammation, and oxidative stress in hypertension. Antioxid Redox Signal 2008;10(6):1079–1088. doi: 10.1089/ars.2007.1998. [DOI] [PubMed] [Google Scholar]
- [87].Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 2007;115(10):1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- [88].Giordo R, Paliogiannis P, Mangoni AA, et al. SARS-CoV-2 and endothelial cell interaction in COVID-19: molecular perspectives. Vasc Biol 2021;3(1):R15–R23. doi: 10.1530/VB-20-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Tipnis SR, Hooper NM, Hyde R, et al. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 2000;275(43):33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- [91].Li J, Zhang Y, Wang F, et al. Cardiac damage in patients with the severe type of coronavirus disease 2019 (COVID-19). BMC Cardiovasc Disord 2020;20(1):479. doi: 10.1186/s12872-020-01758-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 2000;87(5):E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- [93].Oudit GY, Kassiri Z, Jiang C, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 2009;39(7):618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Alenina N, Xu P, Rentzsch B, et al. Genetically altered animal models for Mas and angiotensin-(1-7). Exp Physiol 2008;93(5):528–537. doi: 10.1113/expphysiol.2007.040345. [DOI] [PubMed] [Google Scholar]
- [95].Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sankrityayan H, Kale A, Sharma N, et al. Evidence for use or disuse of renin-angiotensin system modulators in patients having COVID-19 with an underlying cardiorenal disorder. J Cardiovasc Pharmacol Ther 2020;25(4):299–306. doi: 10.1177/1074248420921720. [DOI] [PubMed] [Google Scholar]
- [97].D’Ardes D, Boccatonda A, Rossi I, et al. COVID-19 and RAS: unravelling an unclear relationship. Int J Mol Sci 2020;21(8):3003. doi: 10.3390/ijms21083003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Guo J, Huang Z, Lin L, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc 2020;9(7):e016219. doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Crowley SD, Gurley SB, Herrera MJ, et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A 2006;103(47):17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Ainscough JF, Drinkhill MJ, Sedo A, et al. Angiotensin II type-1 receptor activation in the adult heart causes blood pressure-independent hypertrophy and cardiac dysfunction. Cardiovasc Res 2009;81(3):592–600. doi: 10.1093/cvr/cvn230. [DOI] [PubMed] [Google Scholar]
- [101].Huentelman MJ, Grobe JL, Vazquez J, et al. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol 2005;90(5):783–790. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- [102].Wei JF, Huang FY, Xiong TY, et al. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart 2020;106(15):1154–1159. doi: 10.1136/heartjnl-2020-317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Rojas A, Gonzalez I, Morales MA. SARS-CoV-2-mediated inflammatory response in lungs: should we look at RAGE. Inflamm Res 2020;69(7):641–643. doi: 10.1007/s00011-020-01353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Liu T, Zhang L, Joo D, et al. NF-κB signaling in inflammation. Signal Transduct Target Ther 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med 2010;2(7):247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Farah C, Michel L, Balligand JL. Nitric oxide signalling in cardiovascular health and disease. Nat Rev Cardiol 2018;15(5):292–316. doi: 10.1038/nrcardio.2017.224. [DOI] [PubMed] [Google Scholar]
- [107].Kim DH, Meza CA, Clarke H, et al. Vitamin D and endothelial function. Nutrients 2020;12(2):575. doi: 10.3390/nu12020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Cyr AR, Huckaby LV, Shiva SS, et al. Nitric oxide and endothelial dysfunction. Crit Care Clin 2020;36(2):307–321. doi: 10.1016/j.ccc.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359(9311):995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- [110].Gurley SB, Riquier-Brison A, Schnermann J, et al. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab 2011;13(4):469–475. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Santos RAS, Sampaio WO, Alzamora AC, et al. The ACE2/angiotensin-(1-7)/MAS Axis of the renin-angiotensin system: focus on angiotensin-(1-7). Physiol Rev 2018;98(1):505–553. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Sharma RK, Stevens BR, Obukhov AG, et al. ACE2 (angiotensin-converting enzyme 2) in cardiopulmonary diseases: ramifications for the control of SARS-CoV-2. Hypertension 2020;76(3):651–661. doi: 10.1161/HYPERTENSIONAHA.120.15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zhong J, Basu R, Guo D, et al. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation 2010;122(7):717–728. 18 p following 728. doi: 10.1161/CIRCULATIONAHA.110.955369. [DOI] [PubMed] [Google Scholar]
- [114].Basu R, Poglitsch M, Yogasundaram H, et al. Roles of angiotensin peptides and recombinant human ACE2 in heart failure. J Am Coll Cardiol 2017;69(7):805–819. doi: 10.1016/j.jacc.2016.11.064. [DOI] [PubMed] [Google Scholar]
- [115].Sampaio WO, Henrique de Castro C, Santos RA, et al. Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension 2007;50(6):1093–1098. doi: 10.1161/HYPERTENSIONAHA.106.084848. [DOI] [PubMed] [Google Scholar]
- [116].Sampaio WO, Souza dos Santos RA, Faria-Silva R, et al. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 2007;49(1):185–192. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- [117].Mori J, Patel VB, Abo Alrob O, et al. Angiotensin 1-7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circ Heart Fail 2014;7(2):327–339. doi: 10.1161/CIRCHEARTFAILURE.113.000672. [DOI] [PubMed] [Google Scholar]
- [118].Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 2018;122(4):624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Wong C, Marwick TH. Obesity cardiomyopathy: pathogenesis and pathophysiology. Nat Clin Pract Cardiovasc Med 2007;4(8):436–443. doi: 10.1038/ncpcardio0943. [DOI] [PubMed] [Google Scholar]
- [120].Patel VB, Mori J, McLean BA, et al. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes 2016;65(1):85–95. doi: 10.2337/db15-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Oudit GY, Kassiri Z, Patel MP, et al. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res 2007;75(1):29–39. doi: 10.1016/j.cardiores.2007.04.007. [DOI] [PubMed] [Google Scholar]
- [122].Chen L, Li X, Chen M, et al. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Nicin L, Abplanalp WT, Mellentin H, et al. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J 2020;41(19):1804–1806. doi: 10.1093/eurheartj/ehaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Gallagher PE, Ferrario CM, Tallant EA. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol 2008;295(6):H2373–2379. doi: 10.1152/ajpheart.00426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Tucker NR, Chaffin M, Bedi KC, Jr, et al. Myocyte-specific upregulation of ACE2 in cardiovascular disease: implications for SARS-CoV-2-mediated myocarditis. Circulation 2020;142(7):708–710. doi: 10.1161/CIRCULATIONAHA.120.047911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Perez-Bermejo JA, Kang S, Rockwood SJ, et al. SARS-CoV-2 infection of human iPSC-derived cardiac cells reflects cytopathic features in hearts of patients with COVID-19. Sci Transl Med 2021;13(590):eabf7872. doi: 10.1126/scitranslmed.abf7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Bailey AL, Dmytrenko O, Greenberg L, et al. SARS-CoV-2 infects human engineered heart tissues and models COVID-19 myocarditis. JACC Basic Transl Sci 2021;6(4):331–345. doi: 10.1016/j.jacbts.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Bojkova D, Wagner J, Shumliakivska M, et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc Res 2020;116(14):2207–2215. doi: 10.1093/cvr/cvaa267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Delorey TM, Ziegler CGK, Heimberg G, et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature 2021;595(7865):107–113. doi: 10.1038/s41586-021-03570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Toe QK, Issitt T, Mahomed AS, et al. Human pulmonary artery endothelial cells upregulate ACE2 expression in response to iron-regulatory elements: potential implications for SARS-CoV-2 infection of vascular endothelial cells. Available from: https://www.biorxiv.org/content/10.1101/2021.04.08.437687v1.full.pdf. Accessed May 17, 2021. [Google Scholar]
- [132].McCracken IR, Saginc G, He L, et al. Lack of evidence of angiotensin-converting enzyme 2 expression and replicative infection by SARS-CoV-2 in human endothelial cells. Circulation 2021;143(8):865–868. doi: 10.1161/CIRCULATIONAHA.120.052824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Baker SA, Kwok S, Berry GJ, et al. Angiotensin-converting enzyme 2 (ACE2) expression increases with age in patients requiring mechanical ventilation. PLoS One 2021;16(2):e0247060. doi: 10.1371/journal.pone.0247060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Lovren F, Pan Y, Quan A, et al. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol 2008;295(4):H1377–H1384. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- [135].Zhang YH, Zhang YH, Dong XF, et al. ACE2 and Ang-(1-7) protect endothelial cell function and prevent early atherosclerosis by inhibiting inflammatory response. Inflamm Res 2015;64(3–4):253–260. doi: 10.1007/s00011-015-0805-1. [DOI] [PubMed] [Google Scholar]
- [136].He L, Mäe MA, Muhl L, et al. Pericyte-specific vascular expression of SARS-CoV-2 receptor ACE2-implications for microvascular inflammation and hypercoagulopathy in COVID-19. Available from: https://www.biorxiv.org/content/10.1101/2020.05.11.088500v2. Accessed May 17, 2021. [Google Scholar]
- [137].Nascimento Conde J, Schutt WR, Gorbunova EE, et al. Recombinant ACE2 expression is required for SARS-CoV-2 to infect primary human endothelial cells and induce inflammatory and procoagulative responses. mBio 2020;11(6):e03185–e03220. doi: 10.1128/mBio.03185-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Yan T, Xiao R, Lin G. Angiotensin-converting enzyme 2 in severe acute respiratory syndrome coronavirus and SARS-CoV-2: a double-edged sword. FASEB J 2020;34(5):6017–6026. doi: 10.1096/fj.202000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Leisman DE, Deutschman CS, Legrand M. Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med 2020;46(6):1105–1108. doi: 10.1007/s00134-020-06059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Dolhnikoff M, Ferreira Ferranti J, de Almeida Monteiro RA, et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health 2020;4(10):790–794. doi: 10.1016/S2352-4642(20)30257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Bulfamante GP, Perrucci GL, Falleni M, et al. Evidence of SARS-CoV-2 transcriptional activity in cardiomyocytes of COVID-19 patients without clinical signs of cardiac involvement. Biomedicines 2020;8(12):626. doi: 10.3390/biomedicines8120626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Sharma A, Garcia G, Jr, Wang Y, et al. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. Cell Rep Med 2020;1(4):100052. doi: 10.1016/j.xcrm.2020.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Gurley SB, Allred A, Le TH, et al. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J Clin Invest 2006;116(8):2218–2225. doi: 10.1172/JCI16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Yamazato M, Yamazato Y, Sun C, et al. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension 2007;49(4):926–931. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]
- [147].Rentzsch B, Todiras M, Iliescu R, et al. Transgenic angiotensin-converting enzyme 2 overexpression in vessels of SHRSP rats reduces blood pressure and improves endothelial function. Hypertension 2008;52(5):967–973. doi: 10.1161/HYPERTENSIONAHA.108.114322. [DOI] [PubMed] [Google Scholar]
- [148].Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002;417(6891):822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- [149].Walther T, Wessel N, Kang N, et al. Altered heart rate and blood pressure variability in mice lacking the Mas protooncogene. Braz J Med Biol Res 2000;33(1):1–9. doi: 10.1590/s0100-879x2000000100001. [DOI] [PubMed] [Google Scholar]
- [150].Patel VB, Zhong JC, Grant MB, et al. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res 2016;118(8):1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Wang L, Zhou X, Yin Y, et al. Hyperglycemia induces neutrophil extracellular traps formation through an NADPH oxidase-dependent pathway in diabetic retinopathy. Front Immunol 2018;9:3076. doi: 10.3389/fimmu.2018.03076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Barnes BJ, Adrover JM, Baxter-Stoltzfus A, et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med 2020;217(6):e20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Fadini GP, Morieri ML, Longato E, et al. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest 2020;43(6):867–869. doi: 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Preston IR. Clinical perspective of hypoxia-mediated pulmonary hypertension. Antioxid Redox Signal 2007;9(6):711–721. doi: 10.1089/ars.2007.1587. [DOI] [PubMed] [Google Scholar]
- [156].Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 2005;115(3):500–508. doi: 10.1172/JCI24408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020;17(9):1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Gauchotte G, Venard V, Segondy M, et al. SARS-Cov-2 fulminant myocarditis: an autopsy and histopathological case study. Int J Legal Med 2021;135(2):577–581. doi: 10.1007/s00414-020-02500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Blauwet LA, Cooper LT. Myocarditis. Prog Cardiovasc Dis 2010;52(4):274–288. doi: 10.1016/j.pcad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Stefanini GG, Chiarito M, Ferrante G, et al. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID-19. Heart 2020;106(19):1512–1518. doi: 10.1136/heartjnl-2020-317322. [DOI] [PubMed] [Google Scholar]
- [162].Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Yan X, Wang S, Ma P, et al. Cardiac injury is associated with inflammation in geriatric COVID-19 patients. J Clin Lab Anal 2021;35(1):e23654. doi: 10.1002/jcla.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Chen C, Chen C, Yan JT, et al. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xin Xue Guan Bing Za Zhi 2020;48(7):567–571. doi: 10.3760/cma.j.cn112148-20200225-00123. [DOI] [PubMed] [Google Scholar]
- [165].Giustino G, Croft LB, Stefanini GG, et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol 2020;76(18):2043–2055. doi: 10.1016/j.jacc.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Smilowitz NR, Jethani N, Chen J, et al. Myocardial injury in adults hospitalized with COVID-19. Circulation 2020;142(24):2393–2395. doi: 10.1161/CIRCULATIONAHA.120.050434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Bieber S, Kraechan A, Hellmuth JC, et al. Left and right ventricular dysfunction in patients with COVID-19-associated myocardial injury. Infection 2021;49(3):491–500. doi: 10.1007/s15010-020-01572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Santoso A, Pranata R, Wibowo A, et al. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med 2021;44:352–357. doi: 10.1016/j.ajem.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Kang Y, Chen T, Mui D, et al. Cardiovascular manifestations and treatment considerations in COVID-19. Heart 2020;106(15):1132–1141. doi: 10.1136/heartjnl-2020-317056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Lindner D, Fitzek A, Brauninger H, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol 2020;5(11):1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Bearse M, Hung YP, Krauson AJ, et al. Factors associated with myocardial SARS-CoV-2 infection, myocarditis, and cardiac inflammation in patients with COVID-19. Mod Pathol 2021;34(7):1345–1357. doi: 10.1038/s41379-021-00790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Stephenson E, Savvatis K, Mohiddin SA, et al. T-cell immunity in myocardial inflammation: pathogenic role and therapeutic manipulation. Br J Pharmacol 2017;174(22):3914–3925. doi: 10.1111/bph.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Rojulpote C, Gonuguntla K, Patil S, et al. COVID-19 and the heart. Colomb Med (Cali) 2020;51(2):e4320. doi: 10.25100/cm.v51i2.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost 2020;18(6):1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol 2020;75(22):2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Roffi M, Guagliumi G, Ibanez B. The obstacle course of reperfusion for ST-segment-elevation myocardial infarction in the COVID-19 pandemic. Circulation 2020;141(24):1951–1953. doi: 10.1161/CIRCULATIONAHA.120.047523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Kunkel KJ, Anwaruddin S. Papillary muscle rupture due to delayed STEMI presentation in a patient self-isolating for presumed COVID-19. JACC Case Rep 2020;2(10):1633–1636. doi: 10.1016/j.jaccas.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].European Society for Cardiology. The European Society for Cardiology (2020) ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. Available from: https://www.escardio.org/Education/COVID-19-and-Cardiology. Accessed May 17, 2021. [Google Scholar]
- [181].Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Moni MA, Liò P. Network-based analysis of comorbidities risk during an infection: SARS and HIV case studies. BMC Bioinformatics 2014;15(1):333. doi: 10.1186/1471-2105-15-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Liu CL, Lu YT, Peng MJ, et al. Clinical and laboratory features of severe acute respiratory syndrome vis-a-vis onset of fever. Chest 2004;126(2):509–517. doi: 10.1378/chest.126.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Gao L, Jiang D, Wen XS, et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res 2020;21(1):83. doi: 10.1186/s12931-020-01352-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Alvarez-Garcia J, Lee S, Gupta A, et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol 2020;76(20):2334–2348. doi: 10.1016/j.jacc.2020.09.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Van Linthout S, Tschöpe C. Inflammation – cause or consequence of heart failure or both. Curr Heart Fail Rep 2017;14(4):251–265. doi: 10.1007/s11897-017-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Hilfiker-Kleiner D, Shukla P, Klein G, et al. Continuous glycoprotein-130-mediated signal transducer and activator of transcription-3 activation promotes inflammation, left ventricular rupture, and adverse outcome in subacute myocardial infarction. Circulation 2010;122(2):145–155. doi: 10.1161/CIRCULATIONAHA.109.933127. [DOI] [PubMed] [Google Scholar]
- [189].Savvatis K, Müller I, Fröhlich M, et al. Interleukin-6 receptor inhibition modulates the immune reaction and restores titin phosphorylation in experimental myocarditis. Basic Res Cardiol 2014;109(6):449. doi: 10.1007/s00395-014-0449-2. [DOI] [PubMed] [Google Scholar]
- [190].Thaik CM, Calderone A, Takahashi N, et al. Interleukin-1 beta modulates the growth and phenotype of neonatal rat cardiac myocytes. J Clin Invest 1995;96(2):1093–1099. doi: 10.1172/JCI118095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Yokoyama T, Vaca L, Rossen RD, et al. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J Clin Invest 1993;92(5):2303–2312. doi: 10.1172/JCI116834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Condorelli G, Morisco C, Latronico MV, et al. TNF-alpha signal transduction in rat neonatal cardiac myocytes: definition of pathways generating from the TNF-alpha receptor. FASEB J 2002;16(13):1732–1737. doi: 10.1096/fj.02-0419com. [DOI] [PubMed] [Google Scholar]
- [193].Gurantz D, Cowling RT, Varki N, et al. IL-1beta and TNF-alpha upregulate angiotensin II type 1 (AT1) receptors on cardiac fibroblasts and are associated with increased AT1 density in the post-MI heart. J Mol Cell Cardiol 2005;38(3):505–515. doi: 10.1016/j.yjmcc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- [194].Voloshenyuk TG, Hart AD, Khoutorova E, et al. TNF-α increases cardiac fibroblast lysyl oxidase expression through TGF-β and PI3Kinase signaling pathways. Biochem Biophys Res Commun 2011;413(2):370–375. doi: 10.1016/j.bbrc.2011.08.109. [DOI] [PubMed] [Google Scholar]
- [195].Bhatla A, Mayer MM, Adusumalli S, et al. COVID-19 and cardiac arrhythmias. Heart Rhythm 2020;17(9):1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Colafrancesco S, Alessandri C, Conti F, et al. COVID-19 gone bad: a new character in the spectrum of the hyperferritinemic syndrome. Autoimmun Rev 2020;19(7):102573. doi: 10.1016/j.autrev.2020.102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Cao J, Hu X, Cheng W, et al. Clinical features and short-term outcomes of 18 patients with corona virus disease 2019 in intensive care unit. Intensive Care Med 2020;46(5):851–853. doi: 10.1007/s00134-020-05987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Zylla MM, Merle U, Vey JA, et al. Predictors and prognostic implications of cardiac arrhythmias in patients hospitalized for COVID-19. J Clin Med 2021;10(1):133. doi: 10.3390/jcm10010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Coromilas EJ, Kochav S, Goldenthal I, et al. Worldwide survey of COVID-19-associated arrhythmias. Circ Arrhythm Electrophysiol 2021;14(3):e009458. doi: 10.1161/CIRCEP.120.009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5(7):819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Trinh MA, Chang DR, Govindarajulu US, et al. Therapeutic Anticoagulation Is Associated with Decreased Mortality in Mechanically Ventilated COVID-19 Patients. Available from: https://www.medrxiv.org/content/10.1101/2020.05.30.20117929v1. Accessed May 17, 2021. [Google Scholar]
- [204].Zhou C, Chen Y, Ji Y, et al. Increased serum levels of hepcidin and ferritin are associated with severity of COVID-19. Med Sci Monit 2020;26:e926178. doi: 10.12659/MSM.926178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Nai A, Lorè NI, Pagani A, et al. Hepcidin levels predict Covid-19 severity and mortality in a cohort of hospitalized Italian patients. Am J Hematol 2021;96(1). E32–32E35. doi: 10.1002/ajh.26027. [DOI] [PubMed] [Google Scholar]
- [206].Santa Cruz A, Mendes-Frias A, Oliveira AI, et al. Interleukin-6 is a biomarker for the development of fatal severe acute respiratory syndrome coronavirus 2 pneumonia. Front Immunol 2021;12:613422. doi: 10.3389/fimmu.2021.613422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Kashi M, Jacquin A, Dakhil B, et al. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res 2020;192:75–77. doi: 10.1016/j.thromres.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Schulman S. Coronavirus disease 2019, prothrombotic factors, and venous thromboembolism. Semin Thromb Hemost 2020;46(7):772–776. doi: 10.1055/s-0040-1710337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry 2020;91(8):889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Valderrama EV, Humbert K, Lord A, et al. Severe acute respiratory syndrome coronavirus 2 infection and ischemic stroke. Stroke 2020;51(7):e124–e127. doi: 10.1161/STROKEAHA.120.030153. [DOI] [PubMed] [Google Scholar]
- [211].Guillet H, Gallet R, Pham V, et al. Clinical spectrum of ischaemic arterial diseases associated with COVID-19: a series of four illustrative cases. Eur Heart J Case Rep 2021;5(1):ytaa488. doi: 10.1093/ehjcr/ytaa488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Klok FA, Kruip M, van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 2020;18(7):1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Seheult JN, Seshadri A, Neal MD. Fibrinolysis shutdown and thrombosis in severe COVID-19. J Am Coll Surg 2020;231(2):203–204. doi: 10.1016/j.jamcollsurg.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Li J, Liu Z, Wu G, et al. D-dimer as a prognostic indicator in critically ill patients hospitalized with COVID-19 in Leishenshan Hospital, Wuhan, China. Front Pharmacol 2020;11:600592. doi: 10.3389/fphar.2020.600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Koupenova M, Kehrel BE, Corkrey HA, et al. Thrombosis and platelets: an update. Eur Heart J 2017;38(11):785–791. doi: 10.1093/eurheartj/ehw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Manne BK, Denorme F, Middleton EA, et al. Platelet gene expression and function in patients with COVID-19. Blood 2020;136(11):1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Zaid Y, Puhm F, Allaeys I, et al. Platelets can associate with SARS-Cov-2 RNA and are hyperactivated in COVID-19. Circ Rs 2020;127(11):1404–1418. doi: 10.1161/CIRCRESAHA.120.317703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Bautista-Vargas M, Bonilla-Abadía F, Cañas CA. Potential role for tissue factor in the pathogenesis of hypercoagulability associated with in COVID-19. J Thromb Thrombolysis 2020;50(3):479–483. doi: 10.1007/s11239-020-02172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Hottz ED, Azevedo-Quintanilha IG, Palhinha L, et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood 2020;136(11):1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 2013;13(1):34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- [226].Petito E, Falcinelli E, Paliani U, et al. Association of neutrophil activation, more than platelet activation, with thrombotic complications in coronavirus disease 2019. J Infect Dis 2021;223(6):933–944. doi: 10.1093/infdis/jiaa756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost 2020;18(6):1469–1472. doi: 10.1111/jth.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Thachil J. What do monitoring platelet counts in COVID-19 teach us? J Thromb Haemost 2020;18(8):2071–2072. doi: 10.1111/jth.14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Amgalan A, Othman M. Exploring possible mechanisms for COVID-19 induced thrombocytopenia: unanswered questions. J Thromb Haemost 2020;18(6):1514–1516. doi: 10.1111/jth.14832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Pesavento R, Piovella C, Prandoni P. Heart disease in patients with pulmonary embolism. Curr Opin Pulm Med 2010;16(5):415–418. doi: 10.1097/MCP.0b013e32833b6581. [DOI] [PubMed] [Google Scholar]
- [232].Kürkciyan I, Meron G, Sterz F, et al. Pulmonary embolism as a cause of cardiac arrest: presentation and outcome. Arch Intern Med 2000;160(10):1529–1535. doi: 10.1001/archinte.160.10.1529. [DOI] [PubMed] [Google Scholar]
- [233].Becattini C, Agnelli G, Prandoni P, et al. A prospective study on cardiovascular events after acute pulmonary embolism. Eur Heart J 2005;26(1):77–83. doi: 10.1093/eurheartj/ehi018. [DOI] [PubMed] [Google Scholar]
- [234].Wu L, O’Kane AM, Peng H, et al. SARS-CoV-2 and cardiovascular complications: from molecular mechanisms to pharmaceutical management. Biochem Pharmacol 2020;178:114114. doi: 10.1016/j.bcp.2020.114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Huck V, Niemeyer A, Goerge T, et al. Delay of acute intracellular pH recovery after acidosis decreases endothelial cell activation. J Cell Physiol 2007;211(2):399–409. doi: 10.1002/jcp.20947. [DOI] [PubMed] [Google Scholar]
- [236].Choudry FA, Hamshere SM, Rathod KS, et al. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2020;76(10):1168–1176. doi: 10.1016/j.jacc.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Danzi GB, Loffi M, Galeazzi G, et al. Acute pulmonary embolism and COVID-19 pneumonia: a random association. Eur Heart J 2020;41(19):1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Kollias A, Kyriakoulis KG, Dimakakos E, et al. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol 2020;189(5):846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Cui S, Chen S, Li X, et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Klok FA, Kruip M, van der Meer N, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- [242].Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Di Minno A, Ambrosino P, Calcaterra I, et al. COVID-19 and venous thromboembolism: a meta-analysis of literature studies. Semin Thromb Hemost 2020;46(7):763–771. doi: 10.1055/s-0040-1715456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Shahjouei S, Naderi S, Li J, et al. Risk of stroke in hospitalized SARS-CoV-2 infected patients: a multinational study. EBioMedicine 2020;59:102939. doi: 10.1016/j.ebiom.2020.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a New York City Health System. JAMA 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Chen W, Lan Y, Yuan X, et al. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect 2020;9(1):469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Yang J, Chen T, Zhou Y. Mediators of SARS-CoV-2 entry are preferentially enriched in cardiomyocytes. Hereditas 2021;158(1):4. doi: 10.1186/s41065-020-00168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Tan W, Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int J Cardiol 2020;309:70–77. doi: 10.1016/j.ijcard.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020;181(4):90–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Maccio U, Zinkernagel AS, Shambat SM, et al. SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine 2021;63:103182. doi: 10.1016/j.ebiom.2020.103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Mosleh W, Chen K, Pfau SE, et al. Endotheliitis and endothelial dysfunction in patients with COVID-19: its role in thrombosis and adverse outcomes. J Clin Med 2020;9(6):1862. doi: 10.3390/jcm9061862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Khomich OA, Kochetkov SN, Bartosch B, et al. Redox biology of respiratory viral infections. Viruses 2018;10(8):392. doi: 10.3390/v10080392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Ohishi M, Yamamoto K, Rakugi H. Angiotensin (1-7) and other angiotensin peptides. Curr Pharm Des 2013;19(17):3060–3064. doi: 10.2174/1381612811319170013. [DOI] [PubMed] [Google Scholar]
- [255].Liu PP, Blet A, Smyth D, et al. The science underlying COVID-19: implications for the cardiovascular system. Circulation 2020;142(1):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- [256].Teuwen LA, Geldhof V, Pasut A, et al. COVID-19: the vasculature unleashed. Nat Rev Immunol 2020;20(7):389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Pizzino G, Irrera N, Cucinotta M, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Huertas A, Montani D, Savale L, et al. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19). Eur Respir J 2020;56(1):2001634. doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Masaki T, Sawamura T. Endothelin and endothelial dysfunction. Proc Jpn Acad Ser B Phys Biol Sci 2006;82(1):17–24. doi: 10.2183/pjab.82.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Amraei R, Rahimi N. COVID-19, renin-angiotensin system and endothelial. Dysfunction Cells 2020;9(7):1652. doi: 10.3390/cells9071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Pons S, Fodil S, Azoulay E, et al. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care 2020;24(1):353. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol 2020;7(8). e575–575e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Avolio E, Gamez M, Gupta K, et al. The SARS-CoV-2 spike protein disrupts the cooperative function of human cardiac pericytes-endothelial cells through CD147 receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease. Available from: https://www.biorxiv.org/content/10.1101/2020.12.21.423721v1. Accessed May 17, 2021. [Google Scholar]
- [264].Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr 2020;14(3):247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med 2020;8(6):e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Meduri GU, Kohler G, Headley S, et al. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest 1995;108(5):1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- [268].Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Behrens EM, Koretzky GA. Review: cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheumatol 2017;69(6):1135–1143. doi: 10.1002/art.40071. [DOI] [PubMed] [Google Scholar]
- [271].Wu Y, Tian Z, Wei H. Developmental and functional control of natural killer cells by cytokines. Front Immunol 2017;8:930. doi: 10.3389/fimmu.2017.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res 2020;21(1):163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Xiong TY, Redwood S, Prendergast B, et al. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J 2020;41(19):1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Lavillegrand JR, Garnier M, Spaeth A, et al. Elevated plasma IL-6 and CRP levels are associated with adverse clinical outcomes and death in critically ill SARS-CoV-2 patients: inflammatory response of SARS-CoV-2 patients. Ann Intensive Care 2021;11(1):9. doi: 10.1186/s13613-020-00798-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Zeng Q, Li YZ, Dong SY, et al. Dynamic SARS-CoV-2-specific immunity in critically ill patients with hypertension. Front Immunol 2020;11:596684. doi: 10.3389/fimmu.2020.596684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Narasimhan PB, Marcovecchio P, Hamers A, et al. Nonclassical monocytes in health and disease. Annu Rev Immunol 2019;37:439–456. doi: 10.1146/annurev-immunol-042617-053119. [DOI] [PubMed] [Google Scholar]
- [278].Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010;33(3):375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Mueller K, Langnau C, Günter M, et al. Numbers and phenotype of non-classical CD14dimCD16+ monocytes are predictors of adverse clinical outcome in patients with coronary artery disease and severe SARS-CoV-2 infection. Cardiovasc Res 2021;117(1):224–239. doi: 10.1093/cvr/cvaa328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Ishiyama Y, Gallagher PE, Averill DB, et al. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 2004;43(5):970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- [281].Ocaranza MP, Godoy I, Jalil JE, et al. Enalapril attenuates downregulation of Angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension 2006;48(4):572–578. doi: 10.1161/01.HYP.0000237862.94083.45. [DOI] [PubMed] [Google Scholar]
- [282].Vaduganathan M, Vardeny O, Michel T, et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Mehra MR, Desai SS, Kuy S, et al. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med 2020;382(25):e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [284].Yang G, Tan Z, Zhou L, et al. Effects of angiotensin II receptor blockers and ACE (angiotensin-converting enzyme) inhibitors on virus infection, inflammatory status, and clinical outcomes in patients with COVID-19 and hypertension: a single-center retrospective study. Hypertension 2020;76(1):51–58. doi: 10.1161/HYPERTENSIONAHA.120.15143. [DOI] [PubMed] [Google Scholar]
- [285].Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020;126(12):1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Semenzato L, Botton J, Drouin J, et al. Antihypertensive drugs and COVID-19 risk: a cohort study of 2 million hypertensive patients. Hypertension 2021;77(3):833–842. doi: 10.1161/HYPERTENSIONAHA.120.16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Kim JH, Baek YH, Lee H, et al. Clinical outcomes of COVID-19 following the use of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers among patients with hypertension in Korea: a nationwide study. Epidemiol Health 2021;43:e2021004. doi: 10.4178/epih.e2021004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].de Abajo FJ, Rodríguez-Martín S, Lerma V, et al. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet 2020;395(10238):1705–1714. doi: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Lopes RD, Macedo AVS, de Barros E Silva PGM, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA 2021;325(3):254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Polverino F, Stern DA, Ruocco G, et al. Comorbidities, cardiovascular therapies, and COVID-19 mortality: a nationwide, Italian Observational Study (ItaliCO). Front Cardiovasc Med 2020;7:585866. doi: 10.3389/fcvm.2020.585866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Fosb⊘l EL, Butt JH, Østergaard L, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA 2020;324(2):168–177. doi: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Strauss MH, Hall AS, Lavie CJ. RAAS inhibitors and risk of Covid-19. N Engl J Med 2020;383(20):1992–1993. doi: 10.1056/NEJMc2030446. [DOI] [PubMed] [Google Scholar]
- [293].Islam N, Khunti K, Chowell G. RAAS inhibitors and risk of Covid-19. N Engl J Med 2020;383(20):1992. doi: 10.1056/NEJMc2030446. [DOI] [PubMed] [Google Scholar]
- [294].Mancia G, Rea F, Corrao G. RAAS inhibitors and risk of Covid-19. Reply. N Engl J Med 2020;383(20):1993. doi: 10.1056/NEJMc2030446. [DOI] [PubMed] [Google Scholar]
- [295].Reynolds HR, Adhikari S, Iturrate E. RAAS inhibitors and risk of Covid-19. Reply. N Engl J Med 2020;383(20):1993–1994. doi: 10.1056/NEJMc2030446. [DOI] [PubMed] [Google Scholar]
- [296].Pujades-Rodriguez M, Morgan AW, Cubbon RM, et al. Dose-dependent oral glucocorticoid cardiovascular risks in people with immune-mediated inflammatory diseases: a population-based cohort study. PLoS Med 2020;17(12):e1003432. doi: 10.1371/journal.pmed.1003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Horby P, Lim WS, et al. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Ranjbar K, Moghadami M, Mirahmadizadeh A, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis 2021;21(1):337. doi: 10.1186/s12879-021-06045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Edalatifard M, Akhtari M, Salehi M, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J 2020;56(6):2002808. doi: 10.1183/13993003.02808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Ma S, Xu C, Liu S, et al. Efficacy and safety of systematic corticosteroids among severe COVID-19 patients: a systematic review and meta-analysis of randomized controlled trials. Signal Transduct Target Ther 2021;6(1):83. doi: 10.1038/s41392-021-00521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Cano EJ, Fonseca Fuentes X, Corsini Campioli C, et al. Impact of corticosteroids in coronavirus disease 2019 outcomes: systematic review and meta-analysis. Chest 2021;159(3):1019–1040. doi: 10.1016/j.chest.2020.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Robinson R, Prakash V, Al Tamimi R, et al. Impact of systemic corticosteroids on hospitalized patients with COVID-19: January 2021 Meta-analysis of randomized controlled trials. Available from: https://www.medrxiv.org/content/10.1101/2021.02.03.21251065v1. Accessed May 17, 2021. [Google Scholar]
- [305].Rochwerg B, Agarwal A, Siemieniuk RA, et al. A living WHO guideline on drugs for covid-19. BMJ 2020;370:m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- [306].Yuan S, Chen P, Li H, et al. Mortality and pre-hospitalization use of low-dose aspirin in COVID-19 patients with coronary artery disease. J Cell Mol Med 2021;25(2):1263–1273. doi: 10.1111/jcmm.16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Liu Q, Huang N, Li A, et al. Effect of low-dose aspirin on mortality and viral duration of the hospitalized adults with COVID-19. Medicine (Baltimore) 2021;100(6):e24544. doi: 10.1097/MD.0000000000024544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Chow JH, Khanna AK, Kethireddy S, et al. Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019. Anesth Analg 2021;132(4):930–941. doi: 10.1213/ANE.0000000000005292. [DOI] [PubMed] [Google Scholar]
- [309].Wei X, Zeng W, Su J, et al. Hypolipidemia is associated with the severity of COVID-19. J Clin Lipidol 2020;14(3):297–304. doi: 10.1016/j.jacl.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Wang G, Zhang Q, Zhao X, et al. Low high-density lipoprotein level is correlated with the severity of COVID-19 patients: an observational study. Lipids Health Dis 2020;19(1):204. doi: 10.1186/s12944-020-01382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Hu X, Chen D, Wu L, et al. Declined serum high density lipoprotein cholesterol is associated with the severity of COVID-19 infection. Clin Chim Acta 2020;510:105–110. doi: 10.1016/j.cca.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Tanaka S, De Tymowski C, Assadi M, et al. Lipoprotein concentrations over time in the intensive care unit COVID-19 patients: Results from the ApoCOVID study. PLoS One 2020;15(9):e0239573. doi: 10.1371/journal.pone.0239573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Zhang B, Dong C, Li S, et al. Triglyceride to high-density lipoprotein cholesterol ratio is an important determinant of cardiovascular risk and poor prognosis in coronavirus disease-19: a retrospective case series study. Diabetes Metab Syndr Obes 2020;13:3925–3936. doi: 10.2147/DMSO.S268992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Turgay YÖ, Kaya Ş. The atherogenic index of plasma as a predictor of mortality in patients with COVID-19. Heart Lung 2021;50(2):329–333. doi: 10.1016/j.hrtlng.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Kow CS, Hasan SS. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol 2020;134:153–155. doi: 10.1016/j.amjcard.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].De Spiegeleer A, Bronselaer A, Teo JT, et al. The effects of ARBs, ACEis, and statins on clinical outcomes of COVID-19 infection among nursing home residents. J Am Med Dir Assoc 2020;21(7):909–914 e2. doi: 10.1016/j.jamda.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Zhang XJ, Qin JJ, Cheng X, et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab 2020;32(2):176–187.e4. doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Zeiser R. Immune modulatory effects of statins. Immunology 2018;154(1):69–75. doi: 10.1111/imm.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Norata GD, Tavori H, Pirillo A, et al. Biology of proprotein convertase subtilisin kexin 9: beyond low-density lipoprotein cholesterol lowering. Cardiovasc Res 2016;112(1):429–442. doi: 10.1093/cvr/cvw194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Koushki K, Shahbaz SK, Mashayekhi K, et al. Anti-inflammatory action of statins in cardiovascular disease: the role of inflammasome and toll-like receptor pathways. Clin Rev Allergy Immunol 2021;60(2):175–199. doi: 10.1007/s12016-020-08791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Yuan X, Deng Y, Guo X, et al. Atorvastatin attenuates myocardial remodeling induced by chronic intermittent hypoxia in rats: partly involvement of TLR-4/MYD88 pathway. Biochem Biophys Res Commun 2014;446(1):292–297. doi: 10.1016/j.bbrc.2014.02.091. [DOI] [PubMed] [Google Scholar]
- [322].Nägele MP, Haubner B, Tanner FC, et al. Endothelial dysfunction in COVID-19: current findings and therapeutic implications. Atherosclerosis 2020;314:58–62. doi: 10.1016/j.atherosclerosis.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Kashour T, Bin Abdulhak AA, Tlayjeh H, et al. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers and mortality among COVID-19 patients: a systematic review and meta-analysis. Am J Ther 2020;doi: 10.1097/MJT.0000000000001281. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- [324].Castiglione V, Chiriacò M, Emdin M, et al. Statin therapy in COVID-19 infection. Eur Heart J Cardiovasc Pharmacother 2020;6(4):258–259. doi: 10.1093/ehjcvp/pvaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Reiner Ž, Hatamipour M, Banach M, et al. Statins and the COVID-19 main protease: in silico evidence on direct interaction. Arch Med Sci 2020;16(3):490–496. doi: 10.5114/aoms.2020.94655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Ludman A, Venugopal V, Yellon DM, et al. Statins and cardioprotection – more than just lipid lowering. Pharmacol Ther 2009;122(1):30–43. doi: 10.1016/j.pharmthera.2009.01.002. [DOI] [PubMed] [Google Scholar]
- [327].Barkas F, Milionis H, Anastasiou G, et al. Statins and PCSK9 inhibitors: what is their role in coronavirus disease 2019. Med Hypotheses 2021;146:110452. doi: 10.1016/j.mehy.2020.110452. [DOI] [PMC free article] [PubMed] [Google Scholar]