Supplemental Digital Content is available in the text.
Keywords: fibrosis, heart failure, hypertrophy, macrophages, monocytes
Rationale:
The initial hypertrophy response to cardiac pressure overload is considered compensatory, but with sustained stress, it eventually leads to heart failure. Recently, a role for recruited macrophages in determining the transition from compensated to decompensated hypertrophy has been established. However, whether cardiac resident immune cells influence the early phase of hypertrophy development has not been established.
Objective:
To assess the role of cardiac immune cells in the early hypertrophy response to cardiac pressure overload induced by transverse aortic constriction (TAC).
Methods and Results:
We performed cytometry by time-of-flight to determine the identity and abundance of immune cells in the heart at 1 and 4 weeks after TAC. We observed a substantial increase in cardiac macrophages 1 week after TAC. We then conducted Cite-Seq single-cell RNA sequencing of cardiac immune cells isolated from 4 sham and 6 TAC hearts. We identified 12 clusters of monocytes and macrophages, categorized as either resident or recruited macrophages, that showed remarkable changes in their abundance between sham and TAC conditions. To determine the role of cardiac resident macrophages early in the response to a hypertrophic stimulus, we used a blocking antibody against macrophage colony-stimulating factor 1 receptor (CD115). As blocking CD115 initially depletes all macrophages, we allowed the replenishment of recruited macrophages by monocytes before performing TAC. This preferential depletion of resident macrophages resulted in enhanced fibrosis and a blunted angiogenesis response to TAC. Macrophage depletion in CCR2 (C-C chemokine receptor type 2) knockout mice showed that aggravated fibrosis was primarily caused by the recruitment of monocyte-derived macrophages. Finally, 6 weeks after TAC these early events lead to depressed cardiac function and enhanced fibrosis, despite complete restoration of cardiac immune cells.
Conclusions:
Cardiac resident macrophages are a heterogeneous population of immune cells with key roles in stimulating angiogenesis and inhibiting fibrosis in response to cardiac pressure overload.
In This Issue, see p 1083
Meet the First Author, see p 1084
Editorial, see p 1102
The heart responds to stress such as pressure overload or myocardial infarction with an increase in cardiac muscle mass.1,2 This hypertrophic response is initially considered adaptive and involves an increase in microvascular density.3 With sustained stress, the heart eventually transitions into a phase of maladaptive growth, resulting in fibrosis, microvascular rarefaction, and reduced cardiac function.4,5 Most of the research into the mechanisms that drive cardiac hypertrophy and failure focused on cardiomyocyte-specific signaling. More recently, a functional role for monocyte-derived macrophages and T cells has been established in the transition from compensated hypertrophy to heart failure (HF).6,7 In humans and mice, cardiac macrophages can be primarily discriminated according to their expression of CCR2 (C-C chemokine receptor type 2) into resident CCR2- and recruited CCR2+ subsets derived from embryonic and hematopoietic origin, respectively.8,9 However, a subset of CCR2+ resident macrophages that promote monocyte recruitment after injury has been recently identified, highlighting the heterogeneity of cardiac macrophages.10 Cardiac resident CCR2- macrophages mediate cardiac electrical conduction and metabolic stability under homeostatic conditions11,12 and promote cardiac recovery in the early postnatal period through stimulation of cardiomyocyte proliferation and angiogenesis.13 In contrast, recruited CCR2+ macrophages become activated during myocardial injury and promote inflammation.8,10,14 Following transverse aortic constriction (TAC), the number of cardiac macrophages increases due to CCR2+ monocyte recruitment and expansion of resident CCR2- macrophages.6 The notion that cardiac resident macrophages facilitate reparative processes after injury, whereas recruited monocytes and monocyte-derived macrophages generate inflammation and oxidative stress is emerging.13 However, the precise role of resident macrophages in the early adaptation to cardiac pressure overload and the transition to HF is unclear.
Here, we used innovative tools such as cytometry by time-of-flight (CyTOF) and Cite-seq single-cell RNA sequencing (scRNA-seq) to better define the functional heterogeneity of resident and recruited cardiac immune cells. We then depleted cardiac macrophages and identified an important role for resident macrophages in regulating fibrosis and angiogenesis during the early compensatory response to pressure overload. Depletion of resident macrophages before cardiac pressure overload led to an accelerated decline in cardiac function and exaggerated fibrosis development.
Methods
Data Availability
A detailed description of all experimental procedures and statistical tests can be found in the Expanded Materials & Methods section in the Supplemental Material. scRNA-seq datasets are available at Geo Datasets GSE179276.
Results
Pressure Overload–Induced Cardiac Remodeling Profoundly Alters the Cardiac Immune Cell Composition
To better understand the role of cardiac immune cells in the regulation of cardiac hypertrophy, we performed flow cytometry on cardiac noncardiomyocytes isolated from sham or TAC-operated mice 1 week after surgery.15,16 Compared with sham controls, TAC mice showed a substantial increase in the frequency and number of CD45+ (protein tyrosine phosphatase receptor type C) immune cells, 1 week following pressure overload surgery (Figure IA in the Supplemental Material). We observed an accumulation of macrophages, B, and T cells, as well as natural killer cells and dendritic cells in TAC-operated mice compared with sham controls (Figure IB in the Supplemental Material). Changes in immune cells after TAC were specific to the pressure overload and not caused to the surgical intervention in the mediastinum (Figure IC through IE in the Supplemental Material). Given the large increase in macrophage abundance following TAC, we next used CyTOF17 to characterize these cells. Similar to our flow cytometry results, we observed a substantial accumulation of immune cells 1 week after TAC surgery (Figure 1A). However, by week 4 postsurgery, the number of cardiac immune cells receded to levels equivalent to those of sham controls (Figure 1A). Using CyTOF, we identified all major immune cells based on well-established cell surface markers (Figure IF in the Supplemental Material). Visualized stochastic neighbor embedding (ViSNE) analysis of CyTOF data was used to visualize the changes in immune cell clusters in response to pressure overload (Figure 1B). The majority of immune cells that accumulated in the heart after TAC were CD64+F4/80+ (Fc fragment of IgG receptor Ia/adhesion G protein-coupled receptor E1) macrophages, followed by monocytes, dendritic cells, polymorphonuclear leukocytes, and B cells as shown by ViSNE and quantification using manual gating (Figure 1B, Figure IIA in the Supplemental Material).
Figure 1.
Cytometry by time-of-flight of cardiac immune cells shows an expansion of macrophages early after cardiac pressure overload. A, Relative abundance (left) and total number (right) of cardiac CD45+ (protein tyrosine phosphatase receptor type C) immune cells in response to sham or transverse aortic constriction (TAC) surgery at 1 or 4 wk (n=11, 9, 11) determined by cytometry by time of flight (CyTOF). Statistical significance was evaluated by a 1-way ANOVA. All pairwise comparisons were made. Tukey tests were used to correct for multiple comparisons. B, Representative visualized stochastic neighbor embedding (ViSNE) plot showing unsupervised clustering of cardiac immune cells (left) and quantification of their abundance (right) in sham and TAC conditions (n=11, 9, 11). Each dot represents a single cell in the ViSNE plot. Statistical significance by cell type was evaluated by a 1-way ANOVA (normally distributed data) or a Kruskal-Wallis test (non-normally distributed data). All pairwise comparisons were made. Tukey (normally distributed data) or Dunn (non-normally distributed data) tests were used to correct for multiple comparisons. C, Representative ViSNE plots from CyTOF data showing colored expression in arbitrary units (AU) of CD11b (integrin alpha M), CD64 (Fc fragment of IgG receptor Ia), CX3CR1 (C-X3-C motif chemokine receptor 1), CD206 (mannose receptor C type 1), MHC-II (major histocompatibility complex II), CCR2 (C-C chemokine receptor type 2), and Lyve1 (lymphatic vessel endothelial hyaluronan receptor 1) in cardiac immune cells in sham and TAC conditions (n=11, 9, 11). D, Representative CyTOF plots (left) showing cardiac resident macrophages (Res Mϕs) and monocyte-derived macrophages (MoMFs) gated based on CD11b and CD64. The dot color represents the level of CCR2, TIMD4 (T cell immunoglobulin and mucin domain containing 4), and CX3CR1 expression in AU. Quantification (right) of Res Mϕs and MoMFs in sham and TAC conditions (n=8, 5, 6). Statistical significance was evaluated by a Kruskal-Wallis test. All pairwise comparisons were made. Dunn tests were used to correct for multiple comparisons. E, Representative CyTOF plots (left) showing an alternative gating strategy of cardiac macrophages based on CCR2 and MHC-II expression. The dot color represents the level of Ly6C (lymphocyte antigen 6 complex, locus C) and CD206 expression in AU. Quantification (right) of CCR2 and MHC-II macrophage subsets in sham and TAC conditions (n=8, 5, 6). Statistical significance was evaluated by a Kruskal-Wallis test. All pairwise comparisons were made. Dunn tests were used to correct for multiple comparisons. Data are presented as mean±SEM or median±95% CI. DC indicates dendritic cells; Mon, monocytes; NK, natural killer; NKT, natural killer T cells; and PMN, polymorphonuclear leukocytes. Statistical significance is summarized as ns, not significant, *P<0.05, **P<0.01, and ***P<0.001.
We further analyzed the expression and distribution of macrophage markers in cardiac immune cells and found that CD11b+ (Integrin alpha M) CD64+ macrophage express varying levels of CX3CR1 (C-X3-C motif chemokine receptor 1), CD206, MHC-II (major histocompatibility complex II), CCR2, and Lyve1 (lymphatic vessel endothelial hyaluronan receptor 1) (Figure 1C). We then gated resident and monocyte-derived macrophages (MoMF) based on CD11b and CD6418 and verified their expression of TIMD4 (T cell immunoglobulin and mucin domain containing 4) and CX3CR1 or CCR2, respectively (Figure 1D, left). The number of resident macrophages and MoMFs were increased 1 week after TAC. However, by 4 weeks after TAC, these were mostly restored to sham levels (Figure 1D, right). We also used an alternative gating strategy based on the expression of CCR2 and MHC-II and again found an expansion of all macrophages at 1 week after TAC with a larger increase in CCR2- subsets (Figure 1E). Similarly, macrophage activation markers showed enhanced levels at 1 week with return to sham levels by 4 weeks after TAC (Figure IIB in the Supplemental Material). Furthermore, we observed a positive correlation between the number of macrophages present in the heart and the level of cardiac hypertrophy following TAC (Figure IIC in the Supplemental Material). Overall, these findings suggest an important role for macrophages early in the hypertrophic remodeling process.
Single-Cell RNA Sequencing Identifies 12 Different Monocyte/Macrophage Clusters
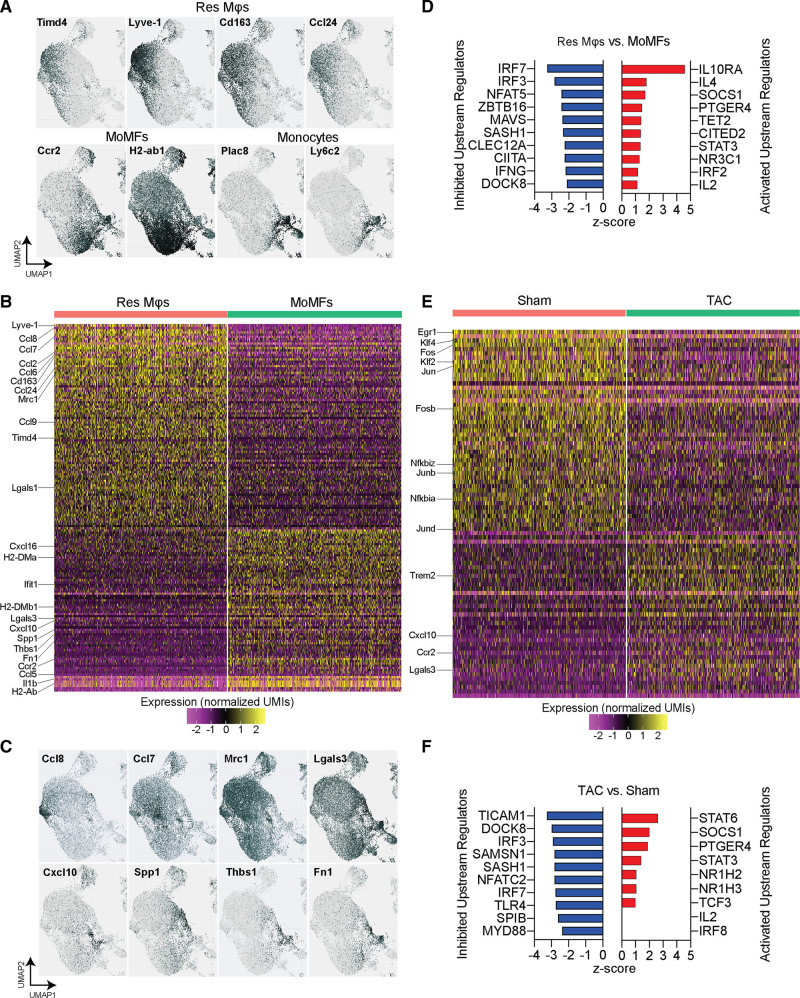
To explore the role of cardiac macrophages during the compensatory hypertrophy phase in response to cardiac pressure overload, we performed multiplexed scRNA-seq of cardiac immune cells (CD45+) from sham and TAC-operated mice 1 week after surgery (Figure 2A). After labeling the immune cells with barcoded antibodies, we combined the cells from 4 sham and 6 TAC hearts and performed droplet-based scRNA-seq. We initially detected 60 981 cells that were captured and sequenced at an average depth of 40 000 reads, based on unique molecular identifier codes. After quality control filtering and deconvolution of barcodes (Figure 2B), we identified 33 566 unique cells that had a single barcode, including 5854 cells from sham samples, and 27 712 cells from TAC samples (Figure 2C). We performed unsupervised graph-based clustering of both sham and TAC cells using uniform manifold approximation and projection, which revealed 25 clusters of CD45+ immune cells present in sham and TAC conditions (Figure 2D). The identity of each cluster was determined based on the differential expression of established immune subset marker genes (Figure 2E, Figure IID and Data Set in the Supplemental Material). In response to cardiac pressure overload, macrophages and monocytes were the most abundant and diverse cell populations in the heart (12 clusters), followed by T cells (5 clusters) and B cells (3 clusters; Figure 2D and 2E). Importantly, based on the relative presence of sequenced cells from each mouse within each cluster, we detected an altered abundance of 8 different macrophage clusters in response to TAC (Figure 2F), indicating a critical role for cardiac macrophages in the response to cardiac pressure overload.
Figure 2.
Single-cell RNA sequencing (scRNA-seq) of cardiac immune cells after pressure overload. A, Design of multiplexed scRNA-seq experiment using hashtag-oligos (HTO) barcoded antibodies to identify mouse of origin of each cell. B, Overview of identified barcodes used to select singlets for further analysis. C, Pie chart indicating the number of single cells sequenced for each condition (upper) and per mouse (lower). D, Uniform manifold approximation and projection (UMAP) projection of single cells clustered in 25 unique clusters with identification of immune cell identity. Sham and transverse aortic constriction (TAC)–derived cells are plotted separately to visualize abundance differences. E, Heatmap of top 5 identified genes that are specifically expressed within clusters using unsupervised clustering. F, Bar graph of relative abundance of each cluster of cells in sham vs TAC conditions (n=4, 6). Statistical significance between the sham and 1-wk TAC groups by cluster was determined by 2-tailed Mann-Whitney U tests. Data are presented as mean±SEM. Single-cell data is shown as scaled, variance-stabilized unique molecular identifiers (UMI) counts. DC indicates dendritic cell; NK, natural killer; and PMN, polymorphonuclear leukocytes. Statistical significance is summarized as ns, not significant and *P<0.05.
Gene Expression Analysis Distinguishes Resident and Monocyte-Derived Macrophages
To probe the spectrum of gene expression profiles among macrophages, we analyzed their gene expression within each cluster and identified both resident and recruited macrophage populations based on established marker gene expression patterns. We focused on the expression of Ccr2 and Timd4 to identify CCR2+ recruited macrophages and TIMD4+CCR2- resident macrophages (Figure 3A). Resident macrophages specifically expressed Lyve1, Cd163, and Ccl24, whereas recruited macrophages were characterized by the expression of antigen processing/presentation genes (MHC-II genes: H2-Aa, H2-Ab1, H2-Eb1, H2-DMa, H2-DMb1, and Cd74), and monocytes expressed Ly6c2 (lymphocyte antigen 6 complex, locus C2) and Plac8 (placenta associated 8) (Figure 3A). To determine gene expression differences between resident and recruited macrophages, we grouped macrophage clusters into resident macrophages and MoMF (Figure IIIA in the Supplemental Material) and analyzed their differentially expressed genes (Figure 3B, Table I in the Supplemental Material). Resident macrophages were characterized by high expression levels of Lyve1, Cd163, Timd4, as well as various C-C chemokine ligands, including Ccl2, 6, 7, 8, 9, and 24, Mrc1 (mannose receptor C-type 1), and Lgals1 (galectin 1). MoMFs were characterized by high expression levels of Ccr2, various MHC-II genes (H2-DMa, H2-DMb1, H2-Ab), Lgals3 (galectin 3), Ccl5, Cxcl10 (C-X-C motif chemokine ligand 10) and 16, Ifit1 (interferon induced protein with tetratricopeptide repeats 1), Il1b (interleukin 1 beta) and fibrosis mediating genes Spp1 (secreted phosphoprotein 1), Thbs1 (thrombospondin 1), and Fn1 (fibronectin 1) (Figure 3B). Visualization of several of these genes shows the distinct expression patterns in macrophage subsets (Figure 3C). Ingenuity Pathway Analysis of differentially regulated genes between resident macrophages and MoMF identified pathways such as cardiac hypertrophy signaling to be activated in resident macrophages, which included genes encoding adrenergic receptors involved in cardiac remodeling19 (Figure IIIB and Table II in the Supplemental Material). Notably, Ingenuity Pathway Analysis predicted the activation of upstream regulators IL10RA (interleukin 10 receptor subunit alpha), IL4 (interleukin4), STAT3 (signal transducer and activator of transcription 3), and IRF2 (interferon regulatory factor 2) pathways, whereas IFNG (interferon gamma), IRF3 (interferon regulatory factor 3), and 7 regulated genes were predicted to be inhibited in resident macrophages, compared with MoMFs (Figure 3D). Ingenuity Pathway Analysis of individual clusters of resident macrophages and MoMFs confirmed their heterogeneity by predicting the top activated upstream regulators IL10RA (clusters 1 and 11), MYD88 (innate immune signal transduction adaptor) (cluster 3), and PTGER4 (prostaglandin E receptor 4) (cluster 4) in resident macrophages, and NFAT5 (nuclear factor of activated T cells 5) (cluster 0), PPARG (peroxisome proliferator activated receptor gamma) (cluster 5), IFNG (cluster 10), and ITGB2 (integrin beta 2) (cluster 14) in MoMFs (Figure IIIC in the Supplemental Material). These analyses indicate distinct cell-intrinsic regulatory mechanisms in resident macrophages and MoMFs that likely reflect their function in tissue homeostasis. Next, we analyzed the differential gene expression between macrophages isolated from sham and TAC-operated mice. In response to TAC, we detected a downregulation of resident macrophage genes, such as Lyve1, Cd163, as well as the transcriptionfactors Klf2 (kruppel like factor 2) and Klf4 (kruppel like factor 4), and the transcription factor AP-1 genes Jun (jun proto-oncogene, AP-1 transcription factor subunit), JunB (junB proto-oncogene, AP-1 transcription factor subunit), JunD (junD proto-oncogene, AP-1 transcription factor subunit), Fos (Fos proto-oncogene, AP-1 transcription factor subunit), and FosB (FosB proto-oncogene, AP-1 transcription factor subunit), and NfκB (nuclear factor kappa B) genes Nfkbiz (NFkB inhibitor zeta) and Nfkbia (NFkB inhibitor alpha) (Figure 3E, Table III in the Supplemental Material). Upregulated genes in response to TAC included Ccr2, Trem2 (triggering receptor expressed on myeloid cells 2), Cxcl10, and Lgals3 (Figure 3E). Ingenuity Pathway Analysis of upstream regulators of differentially expressed genes predicted the activation of the key macrophage regulators NR1H2 (nuclear receptor subfamiliy 1 group H member 2) and NR1H3, as well as STAT3 and STAT6 signaling (Figure 3F). Predicted inhibited regulators included IRF3 and IRF7, as well as NFATC2 (nuclear factor of activated T cells 2) and MYD88 (Figure 3F). These results highlight key changes that occur within resident macrophages and MoMFs as the heart transitions from an adaptive hypertrophic state towards HF.
Figure 3.
Analysis of cardiac macrophage gene expression patterns. A, Identification of cardiac resident macrophages (Res Mϕs) and monocyte-derived macrophages (MoMFs) based on the expression of marker genes Lyve1 (lymphatic vessel endothelial hyaluronan receptor 1), Timd4 (T cell immunoglobulin and mucin domain containing 4), Ccr2, and H2-Ab1. Additional genes that correspond with Res Mϕs (Cd163 and Ccl24 [C-C chemokine ligands]) or monocytes (Ly6c2 [lymphocyte antigen 6 complex, locus C] and Plac8 [placenta associated 8]) are shown. B, Heatmap of differentially expressed genes (DEGs) between Res Mϕs and MoMFs. Selected genes are indicated. C, Gene expression patterns of selected genes that were differentially expressed between Res Mϕs and MoMFs show distinct expression patterns within mϕ clusters. D, List of inhibited and activated upstream regulators identified with Ingenuity Pathway analysis from DEGs between Res Mϕs and MoMFs. E, Heatmap of Differentially Expressed Genes between sham and transverse aortic constriction (TAC)–derived cardiac mϕs. Selected genes are indicated. F, List of inhibited and activated upstream regulators identified with Ingenuity Pathway analysis from DEGs between sham and TAC-derived mϕs. Single-cell data is shown as scaled, variance-stabilized unique molecular identifiers (UMI) counts. CIITA indicates Class II Major Histocompatibility Complex Transactivator; CLEC12A, C-Type Lectin Domain Family 12 Member A; CITED2, Cbp/P300 Interacting Transactivator With Glu/Asp Rich Carboxy-Terminal Domain 2; DOCK8, Dedicator of Cytokinesis 8; IFNG, Interferon Gamma; IL2, Interleukin 2; IL4, Interleukin 4; IL10RA, Interleukin 10 Receptor Subunit Alpha; IRF2, Interferon Regulatory Factor 2; IRF3, Interferon Regulatory Factor 3; IRF7, Interferon Regulatory Factor 7; RF8, Interferon Regulatory Factor 8; MAVS, Mitochondrial Antiviral Signaling Protein; MYD88, MYD88 Innate Immune Signal Transduction Adaptor; NFATC2, Nuclear Factor of Activated T Cells 2; NFAT5, Nuclear Factor of Activated T Cells 5; NR1H2, Nuclear Receptor Subfamily 1 Group H Member 2; NR1H3, Nuclear Receptor Subfamily 1 Group H Member 3; NR3C1, Nuclear Receptor Subfamily 3 Group C Member 1; PTGER4, Prostaglandin E Receptor 4; SAMSN1, SAM Domain, SH3 Domain And Nuclear Localization Signals 1; SASH1, SAM And SH3 Domain Containing 1; SOCS1, Suppressor Of Cytokine Signaling 1; SPIB, Spi-B Transcription Factor; STAT3, Signal Transducer And Activator Of Transcription 3; STAT6, Signal Transducer And Activator Of Transcription 6; TCF3, Transcription Factor 3; TET2, Tet Methylcytosine Dioxygenase 2; TICAM1, Toll Like Receptor Adaptor Molecule 1; TLR4, Toll Like Receptor 4; and ZBTB16, Zinc Finger and BTB Domain Containing 16.
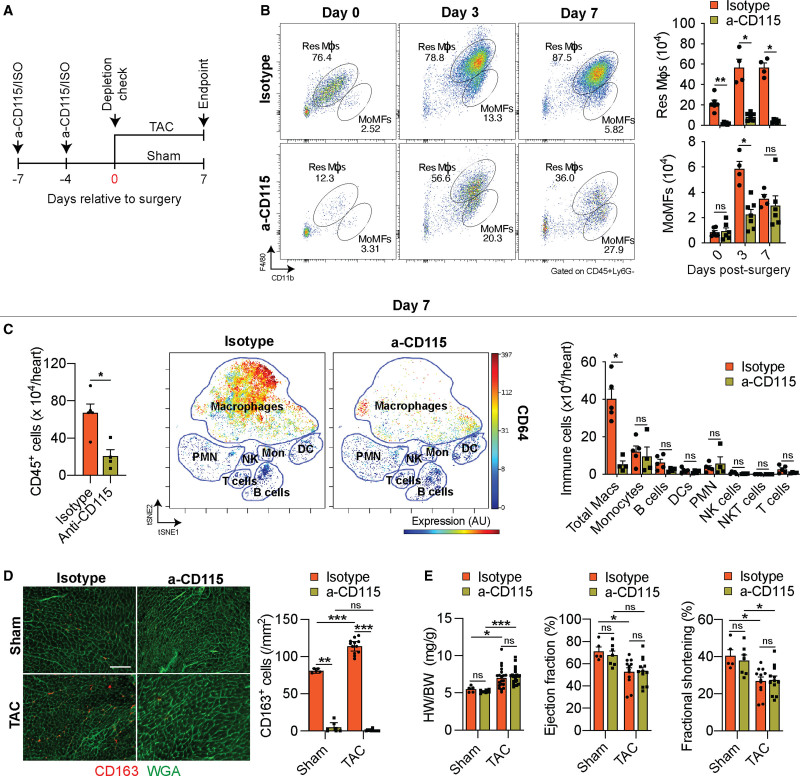
α-CD115–Mediated Macrophage Depletion Preferentially Affects Resident mϕs
To determine if macrophages play a causal role during the initial adaptive hypertrophic response to cardiac pressure overload, we depleted macrophages with a monoclonal α-CD115 antibody that blocks CSF-1 (colony stimulating factor 1) binding to CD115-expressing cells, leading to the depletion of myeloid cells. We first examined the extent to which the α-CD115 antibody depletes cardiac macrophages. We administered 2 doses of either α-CD115 or isotype control antibodies, followed by flow cytometry of cardiac noncardiomyocytes starting at 1 week after the first injection at the time point when we would perform sham or TAC surgery (Figure 4A). Indeed, α-CD115 antibody treatment resulted in a preferential reduction of resident macrophages, although MoMFs were relatively spared (Figure 4B). Although the TAC-induced increase in MoMFs was blunted 3 days after the surgery in α-CD115-treated mice, the number of MoMFs was comparable between α-CD115 and isotype controls 7 days after TAC (Figure 4B). Peripheral blood monocytes fully recovered as early as 4 days following the second injection of α-CD115 antibody (Figure IVA in the Supplemental Material), suggesting that the resilience of MoMFs in the heart is due to a quick replenishment from circulating monocytes. More importantly, the depletion of resident macrophage was sustained throughout the first week after TAC (Figure 4B, Figure IVB in the Supplemental Material). Having validated our cardiac macrophage depletion strategy, we performed TAC surgeries after α-CD115 or isotype control administration, followed by CyTOF analysis one week later. Quantification of the total number of CD45+ immune cells present in TAC hearts showed a 3-fold reduction in α-CD115 treated hearts compared with isotype controls (Figure 4C, left). To determine the potential effects of macrophage depletion on other immune cell types, we quantified all major immune cell types in the heart in response to TAC and found a substantial reduction in cardiac macrophages without an effect on other immune cell types (Figure 4C, middle and right). Although α-CD115 treatment depleted CCR2− resident macrophages, we detected a proportional increase in MHC-II+CCR2+ MoMFs after TAC in α-CD115–treated mice (Figure IVC in the Supplemental Material). Using flow cytometry, we noted a consistent reduction in MHC-II+ total macrophages following α-CD115 treatment (Figure IVD in the Supplemental Material). We next used immunohistochemistry to assess the expression of the resident macrophage marker CD163 (Figure 3A) and found an increase in the abundance of CD163+ macrophages in response to TAC in isotype-treated mice, with a near-complete depletion of CD163+ macrophages in response to α-CD115 antibody treatment (Figure 4D). Despite the successful depletion of resident macrophages, we did not detect changes in cardiac hypertrophy, ejection fraction, and fractional shortening within the first week after cardiac pressure overload (Figure 4E, Figure IVE and IVF in the Supplemental Material). These results show that our antibody-mediated depletion strategy preferentially attenuates resident macrophages while MoMFs have recovered at the time of the surgeries. Furthermore, these results show that depletion of resident macrophages did not affect cardiac hypertrophy or function during the first week after TAC, although depletion might result in cardiac dysfunction at a later stage following pressure overload.
Figure 4.
Cd115 antibody-mediated depletion of cardiac macrophages. A, Schematic showing the timing of isotype or α-CD115 antibody administration relative to sham or transverse aortic constriction (TAC) surgery. B, Representative flow cytometry plots (left) and quantification (right) of cardiac resident macrophages (Res Mϕs) and monocyte-derived macrophages (MoMFs) 0, 3, and 7 d after TAC surgery in isotype or α-CD115 antibody–treated mice (n=4, 6). Statistical significance between the isotype and α-CD115 antibody–treated groups by day was determined by 2-tailed Mann-Whitney U tests. C, Cytometry by time-of-flight–based quantification of cardiac immune cells (left), visualized stochastic neighbor embedding (ViSNE) plot showing the abundance of different immune cells and colored expression of CD64 in arbitrary units (AU, middle), and quantification of immune cell abundance (right) after TAC surgery in isotype or α-CD115 antibody administration (n=5, 4). Statistical significance between the isotype and α-CD115 antibody–treated groups by cell type was determined by 2-tailed Mann-Whitney U tests. D, Representative histological images (left, bar=100 µm) of CD163 staining (red) with WGA (wheat germ agglutinin; green) and quantification (right) of CD163+ cells (n=5, 7, 12, 12). Statistical significance was evaluated by a Kruskal-Wallis test. All pairwise comparisons were made. Dunn tests were used to correct for multiple comparisons. E, Cardiac hypertrophy [left, heart weight (HW) to body weight (BW) ratio], ejection fraction (middle), and fractional Shortening (right, n=5, 7, 12, 12). Statistical significance was evaluated by a 2-way ANOVA. All pairwise comparisons were made. Tukey tests were used to correct for multiple comparisons. Data are presented as mean±SEM or median±95% CI. DC indicates dendritic cell; NK, natural killer; and PMN, polymorphonuclear leukocytes. Statistical significance is summarized as ns, not significant, *P<0.05, **P<0.01, and ***P<0.001.
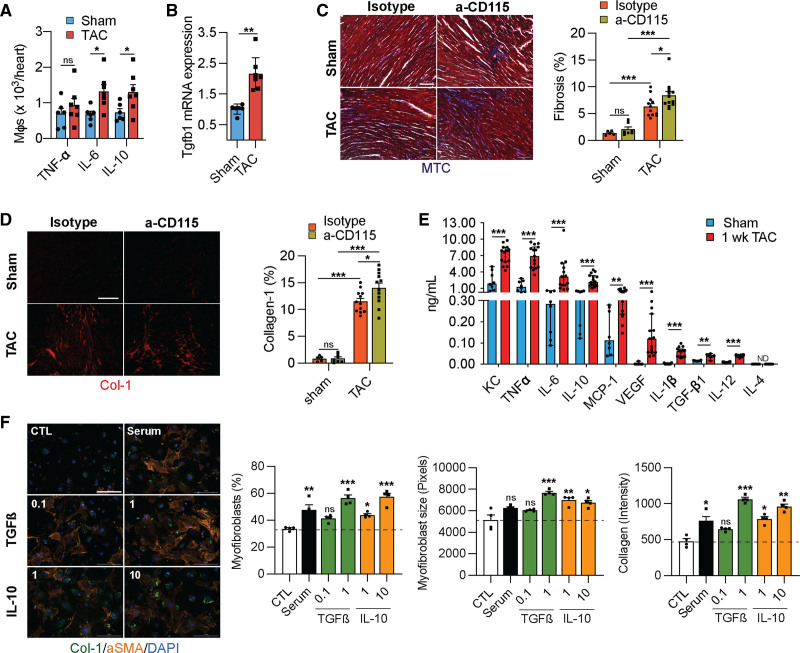
Resident Macrophages Inhibit Fibrosis
Although macrophages appeared to be dispensable for the initial hypertrophy response to pressure overload, it is possible that they regulate initial adaptive processes such as the increase in microvascular density and the development of fibrosis.20–22 To determine if cardiac macrophages regulate angiogenesis and fibrosis during the early response to cardiac pressure overload, we first isolated macrophages from sham or TAC-operated hearts and measured their production of TNF (tumor necrosis factor) α, IL6, and IL10 ex vivo. Using flow cytometry detection of intracellular proteins, we noticed an increase in the production of the proinflammatory cytokine IL6 and the anti-inflammatory cytokine IL10, suggesting an overall immune activation (Figure 5A). To assess the consequences of the activation of macrophages in response to TAC, we isolated mRNA from cardiac macrophages after sham and TAC surgeries and measured their expression of the profibrotic transforming growth factor β1 (Tgfβ1), which increased in response to TAC (Figure 5B). Next, we determined whether depletion of resident macrophages affected the development of fibrosis in response to TAC. Indeed, TAC promoted fibrosis, which was further aggravated in α-CD115 treated mice (Figure 5C and 5D, Figure VA and VB in the Supplemental Material). To identify macrophage-derived factors whose production is stimulated by TAC and could promote fibrosis, we isolated cardiac macrophages after sham or TAC surgery and measured their production of 10 analytes ex vivo using a bead-based immunoassay. Compared with sham, total cardiac macrophages from TAC mice released increased amounts of most cytokines and growth factors including TGFβ1 and IL10, previously implicated in the development of cardiac fibrosis. Indeed, both TGFβ1 and IL10 showed a dose-dependent stimulation of myofibroblast differentiation, myofibroblast growth, and collagen production, at concentrations produced by cardiac macrophages in our ex vivo experiments (Figure 5F). Together, these results show that cardiac macrophages play a critical role in ameliorating fibrosis in response to pressure overload.
Figure 5.
Cardiac macrophages (mϕs) regulate fibrosis. A, Quantification of cytokine-producing mϕs isolated from sham or transverse aortic constriction (TAC)–operated mice. n=6, 7. Statistical significance by cytokine was determined by 2-tailed unpaired Student t test. B, Taqman quantitative polymerase chain reaction for transforming growth factor β1 (Tgfb1) on cDNA from freshly isolated mϕs from sham or TAC-operated mice. n=6, 7. Statistical significance was evaluated by a Kruskal-Wallis test. C, Representative images (left) of Masson Trichrome-stained (MTC) sections derived from sham or TAC-operated mice after isotype or α-CD115 antibody administration. Bar graph (right) shows quantification of fibrosis (blue staining). n=5, 7, 12, 12. Statistical significance was evaluated by a 2-way ANOVA. All pairwise comparisons were made. Tukey tests were used to correct for multiple comparisons. D, Representative images (left) of collagen-1-stained sections (Col-1) derived from sham or TAC-operated mice after isotype or α-CD115 antibody administration. Bar graph (right) shows the percent of collagen-1 staining (n=5, 7, 12, 12). Statistical significance was evaluated by a 2-way ANOVA. All pairwise comparisons were made. Tukey tests were used to correct for multiple comparisons. E, Quantification of cytokines in the supernatants from mϕs isolated from sham or TAC-operated mice (n=8, 15) using a Legendplex assay following 16 h of culture with 10 ng/mL of LPS (lipopolysaccharides). Statistical significance between the sham and TAC groups by cytokine was determined by 2-tailed Mann-Whitney U tests. F, Representative images of fibroblasts stained for collagen-1 (green), αSMA (alpha smooth muscle actin) (orange), and DAPI (4',6-diamidino-2-phenylindole) (blue) following treatment with 0.1 and 10 ng/mL of TGFß or IL (interleukin)-10 (n=4). Bar graphs show quantification of myofibroblast percentage, myofibroblast size, and collagen-1 intensity. Statistical significance was evaluated by a Kruskal-Wallis test. All comparisons were made against the control (CTL). Dunn tests were used to correct for multiple comparisons. Statistical significance is summarized as ns, not significant, *P<0.05, **P<0.01, and ***P<0.001. Scale bar=100 µm for C, D and 200 µm for F. KC indicates platelet-derived growth factor-inducible protein KC; MCP-1, monocyte-chemoattractant protein-1; TNF, tumor necrosis factor; and VEGF, vascular endothelial growth factor.
Resident Macrophages Stimulate Angiogenesis
To assess if cardiac macrophages regulate angiogenesis, we first determined the expression of vascular endothelial growth factor a (Vegfa) in isolated macrophages from sham and TAC-operated hearts and noted an increased expression in the macrophages from TAC hearts (Figure 6A). To determine if cardiac macrophages stimulate angiogenesis, we measured the microvascular density (MVD) in isotype and α-CD115 treated hearts and found an increase in the MVD in response to TAC, which was blunted in α-CD115–treated hearts (Figure 6B). Finally, to establish a direct role for macrophages in regulating MVD, we isolated cardiac macrophages and cocultured them with HUVECs (human umbilical vein endothelial cells). Importantly, macrophages stimulated tube formation with increases in mesh area, and number of nodes, junctions, and meshes compared with the negative control (Figure 6C). As we detected increased VEGF-A production by macrophages from TAC-operated mice (Figure 6A), we treated HUVECs with VEGF-A and confirmed a dose-dependent stimulation of HUVEC tube formation (Figure VC in the Supplemental Material). These results show that cardiac macrophages promote vascular growth in pressure-overloaded hearts.
Figure 6.
Cardiac macrophages (mϕs) regulate angiogenesis. A, Taqman quantitative polymerase chain reaction for Vegfa on cDNA from freshly isolated mϕs from sham or transverse aortic constriction (TAC) operated mice (n=6, 7). Statistical significance by cytokine was determined by 2-tailed unpaired Student t test. B, Representative images (left) of isolectin B4-stained sections (green) derived from sham or TAC-operated mice after isotype or α-CD115 antibody administration. Bar graph (right) shows quantification of microvascular density (vessels/cardiomyocyte; n=5, 7, 12, 12). Statistical significance was evaluated by a 2-way ANOVA. All pairwise comparisons were made. Tukey tests were used to correct for multiple comparisons. C, Representative images of tube formation assay using HUVECs (human umbilical vein endothelial cells) cocultured with negative control (CTL) or with cardiac mϕs compared to positive control. Bar graphs show the quantification of various aspects of tube formation (mesh area, number of nodes, junctions, and meshes; n=3, 6, 3). Statistical significance was evaluated by a Kruskal-Wallis test. All comparisons were made against the negative CTL. Dunn tests were used to correct for multiple comparisons. Data are presented as mean±SEM or median±95% CI. Scale bar=100 µm for C and 200 µm for B. AU indicates arbitrary units; DAPI, 4',6-diamidino-2-phenylindole; and MVD, microvascular density. Statistical significance is summarized as ns, not significant, *P<0.05, **P<0.01, and ***P<0.001.
Recruited Monocyte-Derived Macrophages Stimulate Fibrosis
Because we noticed a preferential depletion of resident macrophages that aggravated fibrosis in α-CD115–treated TAC mice, although MoMFs were relatively spared, we hypothesized that recruited macrophages might be partially responsible for stimulating cardiac fibrosis. To test this hypothesis, we administered α-CD115 or isotype control antibodies to CCR2 knock-out (KO) and C57Bl/6j mice before TAC (Figure 7A). Seven days after TAC, resident macrophages were depleted in C57Bl/6j and CCR2 KO mice, whereas MoMFs were relatively spared in C57Bl/6j mice but barely detectable in CCR2 KO mice by flow cytometry (Figure 7B) and immunohistochemistry (Figure VIA in the Supplemental Material). Similar to our previous results, we did not detect any effect of α-CD115 treatment on cardiac hypertrophy or function at 1-week post-TAC (Figure 7C), although we observed increased cardiomyocyte size on histological sections (Figure VIE in the Supplemental Material). Importantly, depletion of resident macrophages alone aggravated fibrosis in C57Bl/6j in response to TAC, similar to our previous result. Furthermore, isotype-treated CCR2 KO mice showed the lowest fibrosis, consistent with a profibrotic role of MoMFs. However, α-CD115–treated CCR2KO mice that lack both resident macrophages and MoMFs, were protected from the increased fibrosis (Figure 7D and 7E and Figure VIB and VIC in the Supplemental Material). Surprisingly, CCR2KO mice did not show a blunted angiogenesis response to TAC when resident macrophages were depleted (Figure VID in the Supplemental Material). These findings indicate that recruited MoMFs are strong promoters of cardiac fibrosis in a process that ultimately depends on the counteracting role of resident macrophages.
Figure 7.
Recruitment of monocytes promotes fibrosis after pressure overload. A, Experimental design of isotype or α-CD115 antibody administration to either C57Bl/6j or CCR2 (C-C chemokine receptor type 2) KO mice relative to the time of sham or transverse aortic constriction (TAC) surgery. B, Representative flow cytometry plots (left) and quantification (right) of cardiac resident macrophages (Res Mϕs) and monocyte-derived macrophages (MoMFs) in C57Bl/6j or CCR2 knock-out (KO) mice 1 wk after isotype or α-CD115 antibody treatment (n=7, 9, 7, 8). Statistical significance was evaluated by a 2-way ANOVA. All pairwise comparisons were made. Tukey tests were used to correct for multiple comparisons. C, Cardiac hypertrophy; heart weight (HW) to body weight (BW) ratio (left), ejection fraction (middle), and fractional shortening (right) of isotype or α-CD115–treated C57Bl/6j and CCR2 KO mice (n=7, 9, 7, 8). Statistical significance was evaluated by a 2-way ANOVA. D, Representative images (left) and quantification (right) of Masson Trichrome-stained (MTC) sections from isotype or α-CD115–treated C57Bl/6j and CCR2 KO mice (n=7, 9, 7, 8). Statistical significance was evaluated by a 2-way ANOVA. All pairwise comparisons were made. Tukey tests were used to correct for multiple comparisons. E, Representative images (left) of collagen-1 (Col-1)-stained sections derived from sham or TAC-operated mice after isotype or α-CD115 antibody administration. Bar graph (right) shows the percent of collagen-1 staining (n=7, 9, 7, 8). Statistical significance was evaluated by a 2-way ANOVA. All pairwise comparisons were made. Tukey tests were used to correct for multiple comparisons. Data are presented as mean±SEM. Scale bar=100 µm. Statistical significance is summarized as ns, not significant, *P<0.05, **P<0.01, and ***P<0.001.
Depletion of Resident Macrophages Leads to Accelerated HF Development
As depletion of resident macrophages decreased angiogenesis and aggravated fibrosis without detectable changes in cardiac function 1-week post-TAC, we assessed the long-term consequences of α-CD115-treatment. We administered α-CD115 or isotype control antibodies to C57Bl/6j mice before performing sham or TAC surgery and assessed cardiac function 6 weeks after surgeries (Figure VIIA in the Supplemental Material). We noticed that resident macrophages started to partially recover as early as 2 weeks after performing TAC (Figure VIIB through VIID in the Supplemental Material). At 6 weeks post-TAC, there were no differences in the number of total immune cells, macrophages, and other immune cell subsets between α-CD115 and isotype-treated TAC mice (Figure 8B). Similarly, there were no differences in the percent and number of resident macrophages or MoMFs (Figure 8C), suggesting that resident macrophages have been replenished. Although the heart weight to body weight ratio was similar, we detected depressed cardiac function in α-CD115 antibody–treated TAC mice, relative to isotype controls (Figure 8D). At the cardiomyocyte level, we detected enhanced cardiomyocyte cross-sectional areas (Figure 8E). Importantly, the aggravated fibrosis observed in earlier time points was starker 6 weeks after TAC, particularly in the α-CD115 antibody–treated TAC mice (Figure 8F and 8G). These results demonstrate an important role for resident macrophages early in the remodeling process in preventing the deterioration of cardiac function and ameliorating the development of fibrosis following pressure overload.
Figure 8.
Depletion of cardiac macrophages (Mϕ) leads to decreased cardiac function. A, cytometry by time-of-flight (CyTOF)–based quantification of cardiac immune cells (left), visualized stochastic neighbor embedding (ViSNE) plot showing the abundance of different immune cells and colored expression of CD64 in arbitrary units (AU, middle), and quantification of immune cell abundance (right) 6 wk after transverse aortic constriction (TAC) surgery in isotype or α-CD115 antibody administration (n=4). Statistical significance between the isotype and α-CD115 antibody–treated groups by cell type was determined by 2-tailed Mann-Whitney U tests. B, Representative CyTOF plots (left) showing cardiac resident Mϕs (Res Mϕs) and monocyte-derived Mϕs (MoMFs) gated based on CD11b (integrin alpha M) and CD64. The dot color represents the level of CCR2 (C-C chemokine receptor type 2), TIMD4 (T-cell immunoglobulin and mucin domain containing 4), and CX3CR1 (C-X3-C motif chemokine receptor 1) expression. Quantification (right) of Res Mϕs and MoMFs 6 wk after TAC in mice which received isotype or α-CD115 antibody administration (n=4). Statistical significance between the isotype and α-CD115 antibody–treated groups was determined by 2-tailed Mann-Whitney U tests. C, Cardiac hypertrophy (left, heart weight [HW] to body weight [BW] ratio), ejection fraction (middle), and fractional shortening (right) in sham and TAC mice after isotype or α-CD115 antibody administration (n=5, 4, 6, 6). Statistical significance was evaluated by a 2-way ANOVA. All pairwise comparisons were made. Tukey tests were used to correct for multiple comparisons. D, Representative images (left) and area quantification (right) of WGA (wheat germ agglutinin)-stained sections from isotype or α-CD115 antibody administered mice 6 wk after sham and TAC (n=5, 4, 6, 6). Statistical significance was evaluated by a 2-way ANOVA. All pairwise comparisons were made. Tukey tests were used to correct for multiple comparisons. E, Representative images (left) and quantification (right) of Masson Trichrome-stained (MTC) sections from isotype or α-CD115 antibody administered mice 6 wk after sham and TAC (n=5, 4, 6, 6). Statistical significance was evaluated by a 2-way ANOVA. All pairwise comparisons were made. Tukey tests were used to correct for multiple comparisons. F, Representative images (left) and quantification (right) of collagen-1-(Col-1)-stained sections from isotype or α-CD115 antibody administered mice 6 wk after sham and TAC (n=5, 4, 6, 6). Statistical significance was evaluated by a 2-way ANOVA. All pairwise comparisons were made. Tukey tests were used to correct for multiple comparisons. Scale bar=100 µm. DC indicates dendritic cells, Macs, macrophages, Mon, monocytes, NK, natural killer; PMN, polymorphonuclear leukocytes; and tSNE, t-distributed stochastic neighbor embedding. Statistical significance is summarized as ns, not significant, *P<0.05, **P<0.01, and ***P<0.001.
Discussion
Our findings, integrating CyTOF17 and multiplexed scRNA-seq,23 underscore an important role for cardiac immune cells early in the response to cardiac pressure overload. Our results show a substantial increase in the abundance of cardiac macrophages within 1 week after cardiac pressure overload. These findings are consistent with previous research showing that an expansion of CCR2− cardiac macrophages occurs during the first week followed by a detrimental infiltration of CCR2+ monocytes in the later phase of pressure overload hypertrophy.7 Our scRNA-seq experiments identified 12 distinct macrophage and monocyte populations that were classified as resident macrophages or MoMFs, based on previously published marker genes, including Timd4, Lyve1, Ccr2, and MHC-II genes.7–10,18 We identified dynamic changes in 8 of the 12 macrophage clusters suggesting an important function for cardiac macrophages early in the response to cardiac pressure overload. To assess their role in orchestrating a compensatory response to a hypertrophic stimulus, we used an antibody-based strategy to deplete resident macrophages.24 Depletion of resident macrophages had no effect on cardiac hypertrophy or function at 1 week after cardiac pressure overload, suggesting that cardiac macrophages are dispensable for a normal hypertrophy response. However, we noticed reduced MVD and enhanced fibrosis, indicating that resident macrophages are critical for stimulating angiogenesis and inhibiting fibrosis. These early changes appeared to be maladaptive as we detected aggravated fibrosis and worsening of cardiac function at the 6-week time point. Our findings are consistent with previous work showing that resident CCR2− macrophages promote angiogenesis in neonatal injury models.13 In contrast, CCR2+ MoMFs have been shown to promote cardiac inflammation through IL-1ß release8 and neutrophil recruitment14 early after cardiac injury. Recent single-cell genomics studies have identified different populations of immune cells, including the recognition of CCR2+ proinflammatory macrophages as key players in the later phase of pressure overload hypertrophy.25,26
Cardiac macrophages are continually replenished from different pools, and we assessed macrophage populations in the heart to distinguish resident macrophages from MoMFs.8 When we assessed the role of recruited macrophages using CCR2-deficient mice, we found that recruited macrophages stimulate cardiac fibrosis after pressure overload. Recruited macrophages have been implicated in the development of cardiac fibrosis in a CCR2-dependent process.6,10,14 One caveat of using CCR2 expression to distinguish recruited from resident macrophages is that a population of CCR2+ resident cardiac macrophages from hematopoietic origins has been recently identified.10 These CCR2+ resident macrophages promote leukocyte recruitment and myocardial injury, but unlike CCR2+ recruited macrophages, they are recruited to the healthy myocardium early in life.10 Future work should clarify the population dynamics and functional heterogeneity of CCR2+ resident macrophages following cardiac pressure overload. Nevertheless, our results suggest that while recruited macrophages are profibrotic, resident macrophages are reparative as they are needed for tissue homeostasis and beneficial remodeling.7 In a model of myocardial infarction, selective depletion of CX3CR1+ macrophages before myocardial infarction impaired cardiac healing, reduced cardiac function, and increased mortality, suggesting that resident mϕs support a regenerative response after injury.18 Similarly, we found that depletion of resident macrophages reduced the angiogenic response, with a concomitant lower MVD, and an enhanced development of fibrosis. Mechanistically, it is possible that the effects of CD115 blocking on fibrosis are mediated by depletion of IL10–producing macrophages, which we found to increase in response to TAC. In humans and mice, macrophage-derived IL10 activates fibroblasts and promotes collagen deposition leading to diastolic dysfunction.27,28 Here, we show that IL10 and TGFβ are produced by cardiac macrophages in response to pressure overload and that they stimulate myofibroblast differentiation and collagen production.
Recently, several scRNA-seq studies have assessed the response of cardiac cells to stress, including cardiac pressure overload.25,26,29,30 For example, scRNA-seq of >17 000 cardiac immune cells from sham and TAC hearts at 1 and 4 weeks after TAC (one mouse each condition) revealed 1 cluster of reparative/resident, 2 clusters of proinflammatory, and 1 cluster of phagocytic macrophages.25 In another recent study, a comparison between all cardiac cells at 2 and 5 weeks post-TAC revealed that activation of macrophage inflammatory function was the most notable change in this period when the heart loses cardiac function.26 Similarly, we identified multiple types of immune cells residing within and recruited to the heart. The use of cells from 10 mice allowed us to assess the contribution of each mouse within each cluster and test the dynamic changes in clusters of cells in response to TAC. We identified gene expression patterns in specific clusters of macrophages, such as the expression of Thbs1 and Fn1 in cluster 14, as well as Spp1 in cluster 5, suggesting that these cells could mediate cardiac fibrosis. Furthermore, Cxcl10 showed an expression pattern restricted to cluster 10, suggesting that this cluster of macrophages may orchestrate the recruitment of monocytes and Th1 cells, which drive adverse cardiac remodeling.31,32 As infiltration of inflammatory monocytes occurs during the transition to cardiac decompensation following TAC,7 our findings highlight specific subpopulations of cardiac resident macrophage that could mediate this event. Similar to previous studies,18,33 we identified resident macrophages that resemble an alternatively-activated state and express Lyve1, CD163, and MRC1 (CD206).
Here, we used a blocking antibody against CSF1R (colony-stimulating factor 1 receptor or CD115) to block CSF-1 from maintaining a pool of resident macrophages.34 Since macrophages depend on the continuous input of CSF1R for their maintenance, blocking this receptor has been used in prior studies to deplete macrophages in other organs.24,35 Previous work has shown that resident macrophages are exclusively replenished by local proliferation, while CCR2+ MoMFs are maintained through both monocyte recruitment and proliferation.9 Therefore, we took advantage of the rapid replenishment of MoMFs from circulatory monocytes following CD115 antibody treatment to preferentially deplete resident macrophages. It is important to note that this strategy resulted in distinct functional outcomes, compared with liposomal clodronate depletion of macrophages. One important difference between our approach to deplete macrophages and liposomal clodronate is the severe mortality that is observed after TAC in clodronate-treated mice.7 As we did not observe excessive mortality in response to CD115 blocking, it is unclear whether the high mortality in response to clodronate treatment is entirely driven by macrophage-mediated mechanisms or through other off-target effects. A limitation of macrophage depletion strategies, including our own, is the targeting of myeloid populations in other tissues such as the spleen or the lung that could influence a cardiac phenotype.36 Future experiments should employ more precise and tissue-specific strategies to deplete cardiac macrophages to discern specific functions of various populations of macrophages.
In conclusion, we used a combination of CyTOF, scRNA-seq and functional assays to identify the role of cardiac macrophages early in the process of cardiac remodeling in response to pressure overload. We identified different macrophage populations that show dynamic gene expression and abundance differences in the heart after pressure overload. These changes are important for regulating cardiac remodeling, as we identified an important role for resident macrophages to stimulate angiogenesis and limit cardiac fibrosis. Ultimately, a better understanding of the regulatory mechanisms of cardiac macrophage stimulation and recruitment could lead to targeted therapies for HF.
Article Information
Sources of Funding
X.S. Revelo and J.H. van Berlo are supported by grants from the National Institutes of Health (NIH; DK122056, HL130072, and HL155993), the University of Minnesota Informatics Institute, and MnDRIVE. X.S. Revelo is the recipient of a Careers in Immunology Award by the American Association of Immunologists. J.H. van Berlo is supported by an Individual Biomedical Research Award of The Hartwell Foundation. The Mass Cytometry Shared Resource at the University of Minnesota is supported by the Minnesota Partnership for Biotechnology and Medical Genomics.
Disclosures
None.
Supplemental Materials
Expanded Materials and Methods
Supplemental Material Figures I–VII
Supplemental Material Tables I–IV
Supplemental Material Data Set
References 37–39
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CCR2
- C-C chemokine receptor type 2
- CyTOF
- cytometry by time-of-flight
- HF
- heart failure
- Lyve1
- lymphatic vessel endothelial hyaluronan receptor 1
- MoMF
- monocyte-derived macrophage
- Res mϕ
- cardiac resident macrophage
- scRNA-seq
- single-cell RNA sequencing
- TAC
- transverse aortic constriction
- TNF
- tumor necrosis factor
The Supplemental Material is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.121.319737.
For Sources of Funding and Disclosures, see page 1100.
Contributor Information
Preethy Parthiban, Email: parth034@umn.edu.
Chen Chen, Email: chen5706@umn.edu.
Fanta Barrow, Email: fbarrow@umn.edu.
Gavin Fredrickson, Email: fredr300@umn.edu.
Haiguang Wang, Email: wang4226@umn.edu.
Doğacan Yücel, Email: dyucel@umn.edu.
Adam Herman, Email: aherman@umn.edu.
Novelty and Significance
What Is Known?
Cardiac pressure overload initially leads to hypertrophy and angiogenesis, followed by the development of fibrosis and vascular rarefaction as the heart transitions to failure.
Immune cells such as macrophages and T cells reside and accumulate in the heart where they regulate the transition to heart failure.
Cardiac macrophages can be classified broadly as resident or recruited macrophages, with recent fate-mapping and single-cell RNA sequencing experiments showing that cardiac macrophages are composed of multiple subsets of different origins and functions.
What New Information Does This Article Contribute?
Cardiac macrophages, particularly resident cells, accumulate in the heart within 1 week of cardiac pressure overload.
Multiplexed single-cell RNA sequencing of cardiac immune cells revealed 12 transcriptionally distinct clusters of macrophages and monocytes, where cardiac pressure overload altered the abundance of 8 of these macrophage clusters 1 week following injury.
Antibody-mediated depletion of resident macrophages before cardiac pressure overload decreased angiogenesis and aggravated cardiac fibrosis, suggesting that cardiac resident macrophages regulate these processes.
Macrophage-mediated stimulation of angiogenesis and inhibition of fibrosis early after cardiac pressure overload ameliorate the subsequent development of heart failure.
Cardiac pressure overload results in hypertrophy of the myocardium, which is considered adaptive at first. Histopathologic changes like vascular rarefaction and development of fibrosis are important contributors to the development of heart failure. Previous research has shown important roles for recruited macrophages and T cells in mediating the transition to heart failure at later stages after cardiac pressure overload. The precise role of cardiac immune cells in regulating the early response of the heart to cardiac pressure overload is unclear. Our results show a substantial increase in resident macrophage abundance in the first week after cardiac pressure overload. Based on antibody-mediated depletion of resident macrophages we show their importance in stimulating angiogenesis and inhibiting cardiac fibrosis in the first week after cardiac pressure overload. Our results furthermore show that these early adaptations are important to postpone the development of heart failure. These findings suggest that resident macrophages regulate early adaptations, which are critical for the ultimate risk of development of heart failure.
References
- 1.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest. 2013; 123:37–45. doi: 10.1172/JCI62839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018; 15:387–407. doi: 10.1038/s41569-018-0007-y [DOI] [PubMed] [Google Scholar]
- 3.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007; 446:444–448. doi: 10.1038/nature05602 [DOI] [PubMed] [Google Scholar]
- 4.Schiattarella GG, Hill JA. Inhibition of hypertrophy is a good therapeutic strategy in ventricular pressure overload. Circulation. 2015; 131:1435–1447. doi: 10.1161/CIRCULATIONAHA.115.013894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perrino C, Naga Prasad SV, Mao L, Noma T, Yan Z, Kim HS, Smithies O, Rockman HA. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest. 2006; 116:1547–1560. doi: 10.1172/JCI25397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel B, Bansal SS, Ismahil MA, Hamid T, Rokosh G, Mack M, Prabhu SD. CCR2+ monocyte-derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. JACC Basic Transl Sci. 2018; 3:230–244. doi: 10.1016/j.jacbts.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao X, Shen Y, Zhang R, Sugi K, Vasudevan NT, Alaiti MA, Sweet DR, Zhou L, Qing Y, Gerson SL, et al. Distinct roles of resident and nonresident macrophages in nonischemic cardiomyopathy. Proc Natl Acad Sci USA. 2018; 115:E4661–E4669. doi: 10.1073/pnas.1720065115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014; 40:91–104. doi: 10.1016/j.immuni.2013.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, Epelman S, Kreisel D, Liu Y, Itoh A, et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med. 2018; 24:1234–1245. doi: 10.1038/s41591-018-0059-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao HM, Weinheimer C, et al. Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res. 2019; 124:263–278. doi: 10.1161/CIRCRESAHA.118.314028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wülfers EM, Seemann G, Courties G, et al. Macrophages facilitate electrical conduction in the heart. Cell. 2017; 169:510, e20–522. doi: 10.1016/j.cell.2017.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolás-Ávila JA, Lechuga-Vieco AV, Esteban-Martínez L, Sánchez-Díaz M, Díaz-García E, Santiago DJ, Rubio-Ponce A, Li JL, Balachander A, Quintana JA, et al. A network of macrophages supports mitochondrial homeostasis in the heart. Cell. 2020; 183:94, e23–109. doi: 10.1016/j.cell.2020.08.031 [DOI] [PubMed] [Google Scholar]
- 13.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci USA. 2014; 111:16029–16034. doi: 10.1073/pnas.1406508111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Hsiao HM, Higashikubo R, Saunders BT, Bharat A, Goldstein DR, Krupnick AS, Gelman AE, Lavine KJ, Kreisel D. Heart-resident CCR2+ macrophages promote neutrophil extravasation through TLR9/MyD88/CXCL5 signaling. JCI Insight. 2016; 1:87315. doi: 10.1172/jci.insight.87315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Kararigas G, Dworatzek E, Staub E, Martus P, Ruiz Noppinger P, et al. Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol Regul Integr Comp Physiol. 2010; 298:R1597–R1606. doi: 10.1152/ajpregu.00825.2009 [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Zhu W, Bender I, Gong W, Kwak IY, Yellamilli A, Hodges TJ, Nemoto N, Zhang J, Garry DJ, et al. Pathologic stimulus determines lineage commitment of cardiac C-kit+ cells. Circulation. 2017; 136:2359–2372. doi: 10.1161/CIRCULATIONAHA.117.030137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bendall SC, Simonds EF, Qiu P, Amir el-AD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011; 332:687–696. doi: 10.1126/science.1198704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dick SA, Macklin JA, Nejat S, Momen A, Clemente-Casares X, Althagafi MG, Chen J, Kantores C, Hosseinzadeh S, Aronoff L, et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol. 2019; 20:29–39. doi: 10.1038/s41590-018-0272-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grisanti LA, Gumpert AM, Traynham CJ, Gorsky JE, Repas AA, Gao E, Carter RL, Yu D, Calvert JW, García AP, et al. Leukocyte-expressed β2-adrenergic receptors are essential for survival after acute myocardial injury. Circulation. 2016; 134:153–167. doi: 10.1161/CIRCULATIONAHA.116.022304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okyere AD, Tilley DG. Leukocyte-dependent regulation of cardiac fibrosis. Front Physiol. 2020; 11:301. doi: 10.3389/fphys.2020.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deniset JF, Belke D, Lee WY, Jorch SK, Deppermann C, Hassanabad AF, Turnbull JD, Teng G, Rozich I, Hudspeth K, et al. Gata6+ pericardial cavity macrophages relocate to the injured heart and prevent cardiac fibrosis. Immunity. 2019; 51:131, e5–140. doi: 10.1016/j.immuni.2019.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlson S, Helterline D, Asbe L, Dupras S, Minami E, Farris S, Stempien-Otero A. Cardiac macrophages adopt profibrotic/M2 phenotype in infarcted hearts: Role of urokinase plasminogen activator. J Mol Cell Cardiol. 2017; 108:42–49. doi: 10.1016/j.yjmcc.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 23.Stoeckius M, Hafemeister C, Stephenson W, Houck-Loomis B, Chattopadhyay PK, Swerdlow H, Satija R, Smibert P. Simultaneous epitope and transcriptome measurement in single cells. Nat Methods. 2017; 14:865–868. doi: 10.1038/nmeth.4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017; 545:495–499. doi: 10.1038/nature22396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martini E, Kunderfranco P, Peano C, Carullo P, Cremonesi M, Schorn T, Carriero R, Termanini A, Colombo FS, Jachetti E, et al. Single-cell sequencing of mouse heart immune infiltrate in pressure overload-driven heart failure reveals extent of immune activation. Circulation. 2019; 140:2089–2107. doi: 10.1161/CIRCULATIONAHA.119.041694 [DOI] [PubMed] [Google Scholar]
- 26.Ren Z, Yu P, Li D, Li Z, Liao Y, Wang Y, Zhou B, Wang L. Single-cell reconstruction of progression trajectory reveals intervention principles in pathological cardiac hypertrophy. Circulation. 2020; 141:1704–1719. doi: 10.1161/CIRCULATIONAHA.119.043053 [DOI] [PubMed] [Google Scholar]
- 27.Verma SK, Garikipati VNS, Krishnamurthy P, Schumacher SM, Grisanti LA, Cimini M, Cheng Z, Khan M, Yue Y, Benedict C, et al. Interleukin-10 inhibits bone marrow fibroblast progenitor cell-mediated cardiac fibrosis in pressure-overloaded myocardium. Circulation. 2017; 136:940–953. doi: 10.1161/CIRCULATIONAHA.117.027889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hulsmans M, Sager HB, Roh JD, Valero-Muñoz M, Houstis NE, Iwamoto Y, Sun Y, Wilson RM, Wojtkiewicz G, Tricot B, et al. Cardiac macrophages promote diastolic dysfunction. J Exp Med. 2018; 215:423–440. doi: 10.1084/jem.20171274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tucker NR, Chaffin M, Fleming SJ, Hall AW, Parsons VA, Bedi KC, Akkad A-D, Herndon CN, Arduini A, Papangeli I, et al. Transcriptional and cellular diversity of the human heart. Circulation. 2020; 142:466–482. doi: 10.1161/CIRCULATIONAHA.119.045401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gladka MM, Molenaar B, de Ruiter H, van der Elst S, Tsui H, Versteeg D, Lacraz GPA, Huibers MMH, van Oudenaarden A, van Rooij E. Single-cell sequencing of the healthy and diseased heart reveals cytoskeleton-associated protein 4 as a new modulator of fibroblasts activation. Circulation. 2018; 138:166–180. doi: 10.1161/CIRCULATIONAHA.117.030742 [DOI] [PubMed] [Google Scholar]
- 31.Nevers T, Salvador AM, Velazquez F, Ngwenyama N, Carrillo-Salinas FJ, Aronovitz M, Blanton RM, Alcaide P. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J Exp Med. 2017; 214:3311–3329. doi: 10.1084/jem.20161791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngwenyama N, Salvador AM, Velázquez F, Nevers T, Levy A, Aronovitz M, Luster AD, Huggins GS, Alcaide P. CXCR3 regulates CD4+ T cell cardiotropism in pressure overload-induced cardiac dysfunction. JCI Insight. 2019; 4:125527. doi: 10.1172/jci.insight.125527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, Bilbao-Cortes D, Godwin JW, Rosenthal NA. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One. 2012; 7:e36814. doi: 10.1371/journal.pone.0036814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guilliams M, Thierry GR, Bonnardel J, Bajenoff M. Establishment and maintenance of the macrophage Niche. Immunity. 2020; 52:434–451. doi: 10.1016/j.immuni.2020.02.015 [DOI] [PubMed] [Google Scholar]
- 35.MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, Kuns R, Pettit AR, Clouston A, Wainwright B, et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010; 116:3955–3963. doi: 10.1182/blood-2010-02-266296 [DOI] [PubMed] [Google Scholar]
- 36.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009; 325:612–616. doi: 10.1126/science.1175202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yellamilli A, Ren Y, McElmurry RT, Lambert JP, Gross P, Mohsin S, Houser SR, Elrod JW, Tolar J, Garry DJ, et al. Abcg2-expressing side population cells contribute to cardiomyocyte renewal through fusion. FASEB J. 2020; 34:5642–5657. doi: 10.1096/fj.201902105R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hafemeister C, Satija R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019; 20:296. doi: 10.1186/s13059-019-1874-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becht E, McInnes L, Healy J, Dutertre CA, Kwok IWH, Ng LG, Ginhoux F, Newell EW. Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol. 2019; 37:38–44. doi: 10.1038/nbt.4314 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A detailed description of all experimental procedures and statistical tests can be found in the Expanded Materials & Methods section in the Supplemental Material. scRNA-seq datasets are available at Geo Datasets GSE179276.