Supplemental Digital Content is available in the text.
Keywords: atherosclerosis, chaperone-mediated autophagy, inflammasome, lysosomes, macrophages
Rationale:
The NLRP3 (NLR [NOD-like receptor] family, pyrin domain containing 3) inflammasome is an important driver of atherosclerosis. Our previous study shows that chaperone-mediated autophagy (CMA), one of the main lysosomal degradative process, has a regulatory role in lipid metabolism of macrophages. However, whether the NLRP3 inflammasome is regulated by CMA, and the role of CMA in atherosclerosis remains unclear.
Objective:
To determine the role of CMA in the regulation of NLRP3 inflammasome and atherosclerosis.
Methods and Results:
The expression of CMA marker, LAMP-2A (lysosome-associated membrane protein type 2A), was first analyzed in ApoE−/− mouse aortas and human coronary atherosclerotic plaques, and a significant downregulation of LAMP-2A in advanced atherosclerosis in both mice and humans was observed. To selectively block CMA, we generated macrophage-specific conditional LAMP-2A knockout mouse strains in C57BL/6 mice and ApoE−/− mice. Deletion of macrophage LAMP-2A accelerated atherosclerotic lesion formation in the aortic root and the whole aorta in ApoE−/− mice. Mechanistically, LAMP-2A deficiency promoted NLRP3 inflammasome activation and subsequent release of mature IL (interleukin)-1β in macrophages and atherosclerotic plaques. Furthermore, gain-of-function studies verified that restoration of LAMP-2A levels in LAMP-2A–deficient macrophages greatly attenuated NLRP3 inflammasome activation. Importantly, we identified the NLRP3 protein as a CMA substrate and demonstrated that LAMP-2A deficiency did not affect the NLRP3 mRNA levels but hindered degradation of the NLRP3 protein through CMA pathway.
Conclusions:
CMA function becomes impaired during the progression of atherosclerosis, which increases NLRP3 inflammasome activation and secretion of IL-1β, promoting vascular inflammation and atherosclerosis progression. Our study unveils a new mechanism by which NLRP3 inflammasome is regulated in macrophages and atherosclerosis, thus providing a new insight into the role of autophagy-lysosomal pathway in atherosclerosis. Pharmacological activation of CMA may provide a novel therapeutic strategy for atherosclerosis and other NLRP3 inflammasome/IL-1β–driven diseases.
Meet the First Author, see p 1084
Atherosclerosis is recognized as a chronic inflammatory disease.1,2 The NLRP3 (NLR [NOD-like receptor] family, pyrin domain containing 3) inflammasome and its products IL (interleukin)-1β and IL-18 greatly contribute to the vascular inflammatory response that drives atherosclerosis initiation and progression.3,4 The NLRP3 inflammasome is a multiprotein platform consisting of NLRP3, ASC (apoptosis-associated speck-like protein containing a CARD [C-terminal caspase-recruitment domain]), and the cysteine protease caspase-1.5, 6 Assembly of the NLRP3 inflammasome activates caspase-1, which cleaves pro–IL-1β and pro–IL-18 into mature IL-1β and IL-18, respectively.7,8 IL-1β is an important proatherogenic factor and targeting IL-1β using an IL-1β–neutralizing antibody has proven beneficial for cardiovascular diseases in the CANTOS trial (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study).4,9 Because the NLRP3 inflammasome serves as a central producer of IL-1β,4 understanding the regulation of NLRP3 inflammasome activation has important therapeutic significance. Although the upstream machinery of NLRP3 inflammasome activation has been described,4,8 the exact downstream mechanism by which NLRP3 is regulated is rather complex and not yet completely understood.
Autophagy is a catabolic process in which cellular components are targeted and delivered to lysosomes for degradation.10,11 Three forms of autophagy, macroautophagy (commonly known as autophagy), microautophagy, and chaperone-mediated autophagy (CMA) coexist in mammalian cells.12 Defective degradation of the NLRP3 inflammasome is an important downstream mechanism of its aberrant activation in many inflammatory diseases. Under the condition of ubiquitination promoted by drug stimulation or gene manipulation, the NLRP3 inflammasome has been reported to be degraded by the macroautophagy pathway13,14 and ubiquitin-proteasome (UPS) pathway.15 However, whether CMA, another specialized autophagic pathway for protein degradation, is also involved in regulation of the NLRP3 inflammasome remains unknown.
As a special form of autophagy, CMA is quite different from macroautophagy in terms of its cargo selectivity and delivery.16 The function of CMA is more specific because CMA degrades only specific client proteins bearing specific CMA motifs (pentapeptide motifs collectively called the KFERQ motif),16–18 whereas the substrates of macroautophagy are relatively extensive and nonspecific.10,11 In CMA, the cargo is not delivered in a double-membraned vesicle as in macroautophagy. Instead, CMA substrates are recognized and targeted by a cytosolic chaperone protein—HSC70 (heat shock cognate 71 kDa protein)—forming the HSC70-substrate complex, which binds lysosomal LAMP-2A (lysosome-associated membrane protein type 2A) for subsequent lysosomal uptake and degradation.16,17,19 LAMP-2A is the rate-limiting component of CMA, and its level directly determines positively the extent of CMA activity.16,20,21 Compared with the well-studied process macroautophagy, which has been proven to play a protective role against atherosclerosis by inhibiting inflammasome-dependent inflammation22 and promoting cholesterol efflux from macrophage foam cells,23 the role of CMA in atherosclerosis has not been reported.
Considering the function of CMA in protein degradation, we hypothesized that CMA is involved in regulation of NLRP3 inflammasome activation by affecting its degradation, which in turn influences the progression of atherosclerosis. In our present study, we assessed the presence of CMA and changes in the level of CMA in atherosclerotic plaques, the cells that contribute to this process, whether CMA affects the development of atherosclerosis, and whether the NLRP3 inflammasome is implicated in this process.
Methods
See Supplemental Material for Details.
Data Availability
This article adheres to the Transparency and Openness Promotion Guidelines. The data that support the findings of this study are available from the corresponding authors upon reasonable request. Please see the Major Resources Table in the Supplemental Material.
Results
CMA Was Most Related to Macrophages Among the Cell Types in Atherosclerotic Plaques
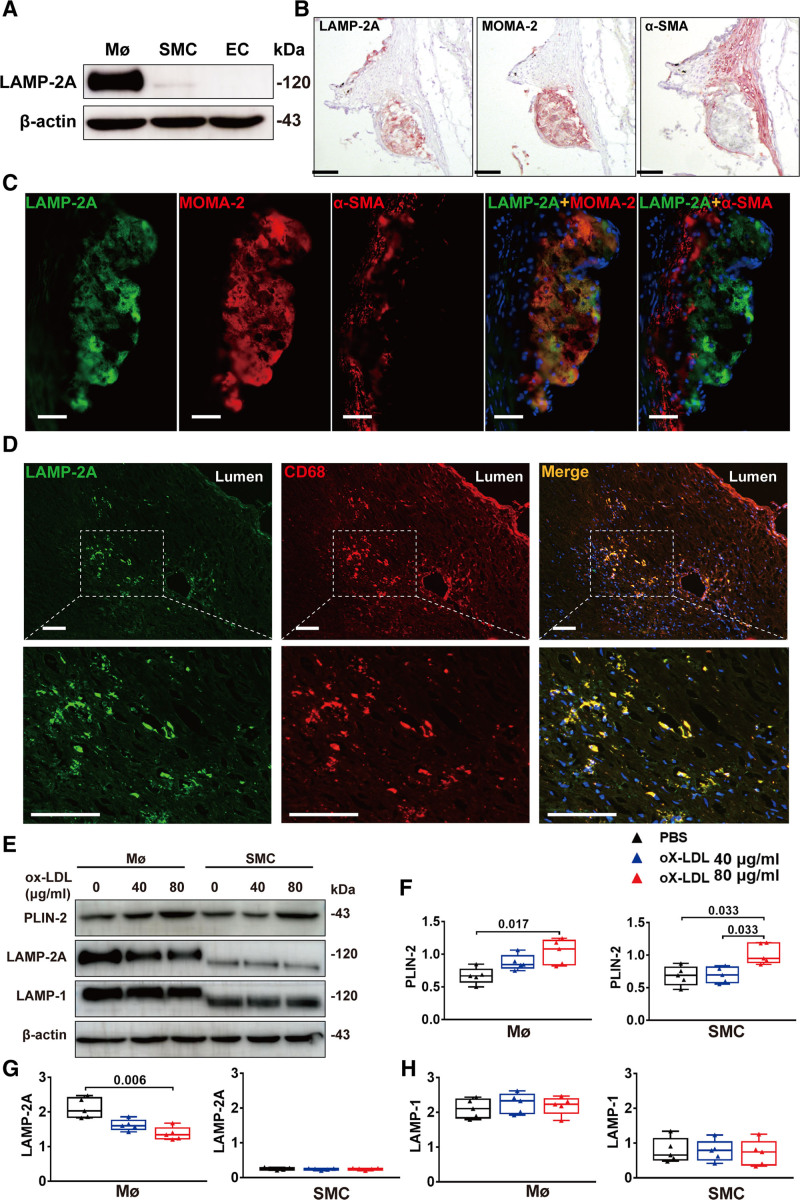
As atherosclerosis is a pathological process involving multiple cell types, among which macrophages, vascular smooth muscle cells (VSMCs), and endothelial cells are the major cell populations within atherosclerotic plaques,24 we first determined which cell types are most related to CMA with the marker LAMP-2A. Immunoblot analysis of mouse primary peritoneal macrophages, primary aortic VSMCs, and primary aortic endothelial cells for LAMP-2A showed that the protein level of LAMP-2A was high in macrophages and very low in endothelial cells and SMCs (Figure 1A). Immunohistochemical staining of consecutive sections from the aortic roots of ApoE−/− mice for LAMP-2A, α-SMA (specific for monocyte and macrophage), and MOMA-2 (specific for monocyte macrophages) also showed that LAMP-2A mainly colocalized with macrophages, the most abundant cell type within atherosclerotic lesions (Figure 1B). VSMCs constitute a very small portion of early atherosclerotic lesions and did not appear to colocalize with LAMP-2A (Figure 1B). The colocalization of LAMP-2A and macrophages was further verified by double immunofluorescence analysis for LAMP-2A and MOMA-2 or F4/80 (another macrophage marker, Figure S1A and S1B in the Supplemental Material) in both mouse atherosclerotic lesions (Figure 1C) and coronary atherosclerotic plaques obtained from human autopsy specimens (Figure 1D). Besides, in the colon tissues in a mouse model of colitis, we also observed colocalization of LAMP-2A and macrophages, but not LAMP-2A and SMCs (Figure S1C in the Supplemental Material). As recent studies suggest that cholesterol-loaded VSMCs may be transdifferentiated to macrophage-like cells and express macrophage foam cell markers, such as MOMA-2, F4/80, or CD68,25 we further examined whether macrophage-like VSMCs express higher levels of LAMP-2A than cholesterol-unloaded VSMCs. We treated mouse primary peritoneal macrophages and primary aortic VSMCs with ox-LDL (oxidized low-density lipoprotein) and found that although both VSMCs macrophage took up ox-LDL (Figure 1E and 1F), cholesterol-loaded VSMCs expressed only a very small amount of LAMP-2A (Figure 1E and 1G), probably due to the fact that there were far fewer lysosomes in VSMCs than in macrophages (Figure 1E and 1H). Collectively, these data suggested that macrophages were the predominant cell type in atherosclerotic plaques that expresses a CMA marker.
Figure 1.
Detection of chaperone-mediated autophagy marker LAMP-2A (lysosome-associated membrane protein type 2A) in vivo and in vitro. A, Representative Western blot analysis of LAMP-2A in mouse primary peritoneal macrophages (MØ), primary aortic smooth muscle cells (SMCs), and primary aortic endothelial cells (ECs). B, Representative immunohistochemical images for detecting LAMP-2A, MOMA-2 (specific for monocytes and macrophages), and α-SMA (smooth muscle actin; specific for SMCs) in three consecutive frozen sections from the aortic root of ApoE−/− mice (male) fed a high-fat diet (HFD) for 8 wk. n=5 per group. The positive reactions of tissue sections were displayed as red color. Scale bar=100 μm. C, Representative immunofluorescence analysis for detecting LAMP-2A (green particles), MOMA-2 (red particles), and α-SMA (red particles) in frozen sections from the aortic root of ApoE−/− mice (male) fed a HFD for 8 wk. n=5 per group. Scale bar=100 μm. D, Representative immunofluorescence analysis for detecting the colocalization (yellow particles) of LAMP-2A (green particles) and CD68 (specific for monocyte macrophages, red particles) in frozen sections from human coronary atherosclerotic plaques. Scale bar=100 μm. E, Representative Western blot images and (F–H) quantitative analysis of protein expression of PLIN-2 (perilipin-2), LAMP-2A, and LAMP-1 of mouse peritoneal macrophages from wild-type (WT) and L2A-mØKO mice (male) treated with or without ox-LDL (oxidized low-density lipoprotein; 40 and 80 μg/mL) for 24 h. Five independent experiments were performed. Data were presented as medians and quartiles. ox-LDL 80 μg/mL group and ox-LDL 40 μg/mL group were compared with PBS group respectively. Statistical analysis was conducted using Kruskal-Wallis 1-way ANOVA with Nemenyi post hoc test.
CMA Was Impaired During Progression of Atherosclerosis
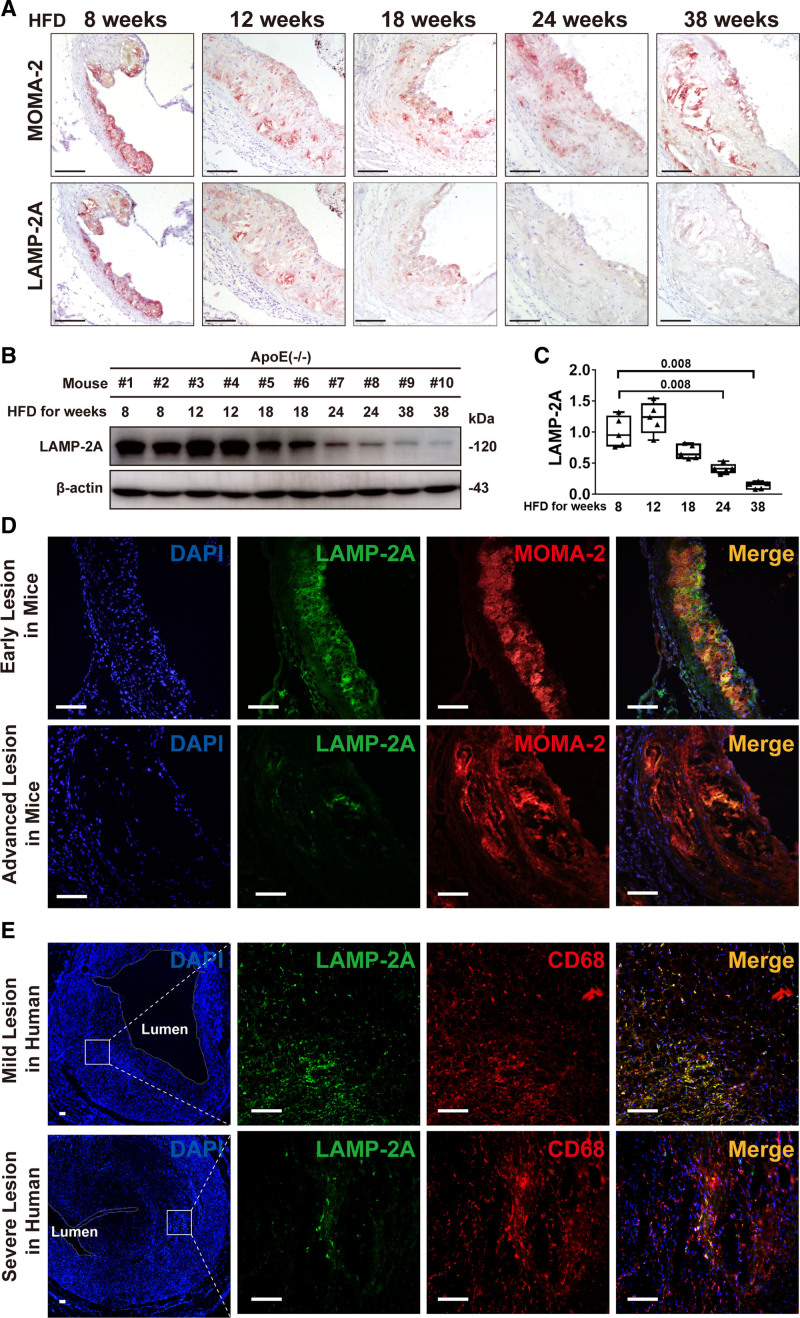
To explore the involvement of CMA in atherosclerosis, we built a model of progressive atherosclerosis in ApoE−/− mice by high-fat diet (HFD) feeding for different durations. As LAMP-2A was predominantly expressed in macrophages (Figure 1), we analyzed LAMP-2A protein levels by immunohistochemical staining of consecutive sections of aortic roots taken at each stage of atherogenesis for MOMA-2 and LAMP-2A. HFD resulted in massive macrophage infiltration in atherosclerotic lesions at all stages, whereas LAMP-2A levels gradually decreased in mice fed an HFD for 18 weeks and longer or was even undetectable in mice fed a HFD for 32 weeks (Figure 2A), which was further verified by immunoblotting of whole-aorta lysates for LAMP-2A (Figure 2B and 2C). Immunofluorescence analysis also confirmed the marked decline in LAMP-2A in advanced lesions in both mice (Figure 2D) and humans (Figure 2E). This result is consistent with previous studies showing that dietary lipids26 and aging27 compromise CMA. In addition, the decrease in LAMP-2A was not the consequence of a reduction in the number of lysosomes (Figure S2 in the Supplemental Material). Taken together, our findings demonstrated that progressive atherosclerosis was characterized by dysfunctional CMA.
Figure 2.
Alterations in chaperone-mediated autophagy marker LAMP-2A (lysosome-associated membrane protein type 2A) in atherosclerotic lesions of mice and humans. A, Representative immunohistochemical images for detecting MOMA-2 (upper, specific for monocytes and macrophages) and LAMP-2A (lower) in 2 consecutive (upper and lower) frozen sections from the aortic root of ApoE−/− mice (male) fed a high-fat diet (HFD) for 8, 12, 18, 24, and 38 wk, respectively (n=5 in each group). The positive reactions of tissue sections were displayed as red color. Scale bar=100 μm. B, Representative Western blot analysis of LAMP-2A in whole-aorta lysates (including the aortic root) from ApoE−/− mice (male) fed an HFD for 8, 12, 18, 24, and 38 wk, respectively (n=5 in each group). C, Quantitative analysis of western blot analysis in 5 groups of mice. Five independent experiments were performed. Data were presented as medians and quartiles. Mice fed with HFD for 12, 18, 24, and 38 wk were compared with those fed with HFD for 8 wk, respectively. Statistical analysis was conducted using Kruskal-Wallis 1-way ANOVA with Nemenyi post hoc test. D, Double immunofluorescence analysis for detecting LAMP-2A (green particles) and MOMA-2 (red particles) in early lesions in mice (male) fed an HFD for 8 wk and in advanced lesions in mice (male) fed an HFD for 24 wk. n=5 in each group. Scale bar=100 μm. E Double immunofluorescence analysis for detecting LAMP-2A (green particles) and CD68 (red particles) in mild lesions (area stenosis ≤30%) and severe lesions (area stenosis ≥ 90%) in human coronary atherosclerotic plaques. n=5 in each group. Scale bar=100 μm.
Deficient CMA of Macrophages Promoted Development of Atherosclerosis In Vivo
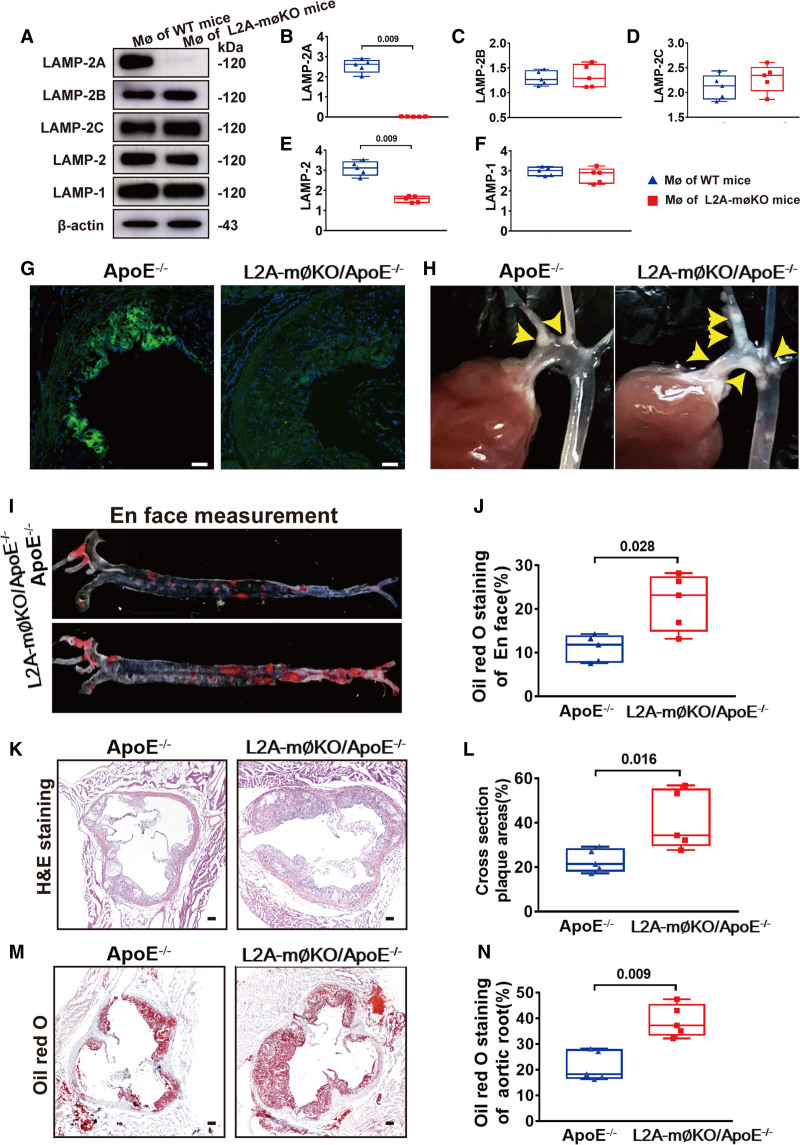
To investigate the consequences of defective CMA in atherosclerosis, we generated a macrophage-specific conditional LAMP-2A knockout mouse. The Cre-loxP system was used to conditionally disrupt the 50 bp region in exon 8 of the LAMP-2 gene that encodes the LAMP-2A protein (referred to as L2A).28 L2Afl/fl mice were crossbred with LysM-Cre mice to generate L2Afl/fl/LysM-Cre mice (referred to as macrophage [MØ]-specific LAMP-2A [lysosomeassociated membrane protein type 2A; L2A]-knockout mice) or further crossed with ApoE−/− mice (C57BL/6 background) to generate L2Afl/fl/LysM-Cre/ApoE−/− mice (referred to as L2A-mØKO/ApoE−/− mice). The LAMP-2A protein was undetectable in both primary peritoneal macrophages from L2A-mØKO mice (Figure 3A and 3B) and aortic root plaques from L2A-mØKO/ApoE−/− mice (Figure 3C and 3D), whereas the levels of other LAMPs (LAMP-1, LAMP-2B, and LAMP-2C) were comparable in the L2A-mØKO mice and wild-type (WT) mice (Figure 3A and 3B). These results confirmed a selective depletion of LAMP-2A in macrophages.
Figure 3.
Quantification of aortic atherosclerotic lesions in control and L2A-mØKO mice (macrophage (MØ)-specific LAMP-2A (lysosome associated membrane protein type 2A; L2A)-knockout mice). A, Representative western blot analysis to determine the efficiency of LAMP-2A knockout in primary peritoneal MØs from wild-type (WT, C57 background, male) and MØ-specific L2A-knockout (L2A-mØKO, C57 background, male) mice. n=5 in each group. B–F, Quantification of protein expression of LAMP-2A, LAMP-2B, LAMP-2C LAMP-2, and LAMP-1 in control WT mice (male) and L2A-mØKO mice (male). Five independent experiments were performed. Data were presented as medians and quartiles. Statistical analysis of B–F was conducted using Mann-Whitney test. G, Representative immunofluorescence analysis to detect LAMP-2A (green particles) in frozen aortic root sections from control ApoE−/− mice and L2A-mØKO/ApoE−/− mice (male). Bar=100 μm. H, Representative photographic images of atherosclerotic plaques (yellow arrows) in the aortic arch and their branches from ApoE−/− mice and L2A-mØKO/ApoE−/− mice (male). I–J, Representative Oil-red-O staining and en face analysis of atherosclerotic lesions in the whole aorta (n=5 in each group). Data were presented as medians and quartiles. Statistical analysis was performed using Mann-Whitney test. K and L, Hematoxylin and eosin (HE) staining and cross-sectional analysis of atherosclerotic lesions in the aortic root (n=5 per group). Data were presented as medians and quartiles. Statistical analysis was conducted using Mann-Whitney test. Scale bar=100 μm. M and N, Oil-red-O staining and cross-sectional analysis of atherosclerotic lesions in the aortic root (n=5 per group). Data were presented as medians and quartiles. Statistical analysis was performed using Mann-Whitney test. Scale bar=100 μm.
As previous studies suggested a possible crosstalk between macroautophagy and CMA,16 we further analyzed the effect of CMA defect on macroautophagy. Western blot analysis showed that LAMP-2A deficiency had no effect on the expression levels of most proteins involved in macroautophagy, but resulted in a considerable decline of SQSTM1/p62 levels (Figure S3A and S3G in the Supplemental Material), suggestive of increased macroautophagy. To further investigate macroautophagy activity in WT and LAMP-2A–deficient macrophages, we analyzed macroautophagy flux by addition of bafilomycin A1 (Figure S3H and S3I in the Supplemental Material) or using mRFP-GFP-LC3 adenovirus transfection (Figure S3J through S3L in the Supplemental Material). The result showed that basal macroautophagy activity was slightly higher in CMA-deficient macrophages than in WT macrophages, and macroautophagy activity was further increased upon starvation (Figure S3H through S3L in the Supplemental Material), probably due to a compensatory effect of these two forms of autophagy for each other.29 The functioning macroautophagy also indicated the existence of a normal functional lysosomal compartment. Furthermore, the content of lysosomal structural proteins (LAMP-1, Figure S3A and S4C in the Supplemental Material), mature hydrolases (cathepsin B and cathepsin D, Figure S4A, S4D, S4E, and S4H in the Supplemental Material), lysosome acidification (Figure S4F in the Supplemental Material), and enzymatic activities (total macrophage β-hexosaminidase activities, Figure S4G in the Supplemental Material) were comparable between LAMP-2A–deficient macrophages and WT macrophages, suggesting lysosome function was not impaired in LAMP-2A–deficient macrophages. All these findings enabled us to investigate the direct effects of CMA dysfunction on atherosclerosis in this animal model.
There was no statistically significant difference in body weight or blood glucose, serum triglyceride, or cholesterol levels between L2A-mØKO/ApoE−/− mice and WT mice (ApoE−/− mice; Table S2 in the Supplemental Material). However, L2A-mØKO/ApoE−/− mice exhibited markedly more whole-aorta atherosclerosis than WT mice (Figure 3H through 3J). Hematoxylin and eosin and oil-red-O staining of the aortic root also showed a significant increase (52%) in the lesion area in L2A-mØKO/ApoE−/− mice compared to WT mice (Figure 3K through 3N). Taken together, these data demonstrated that LAMP-2A deficiency in macrophages accelerated atherosclerotic plaque formation.
Deficient CMA Promoted Secretion of IL-1β and IL-18 In Vitro and In Vivo
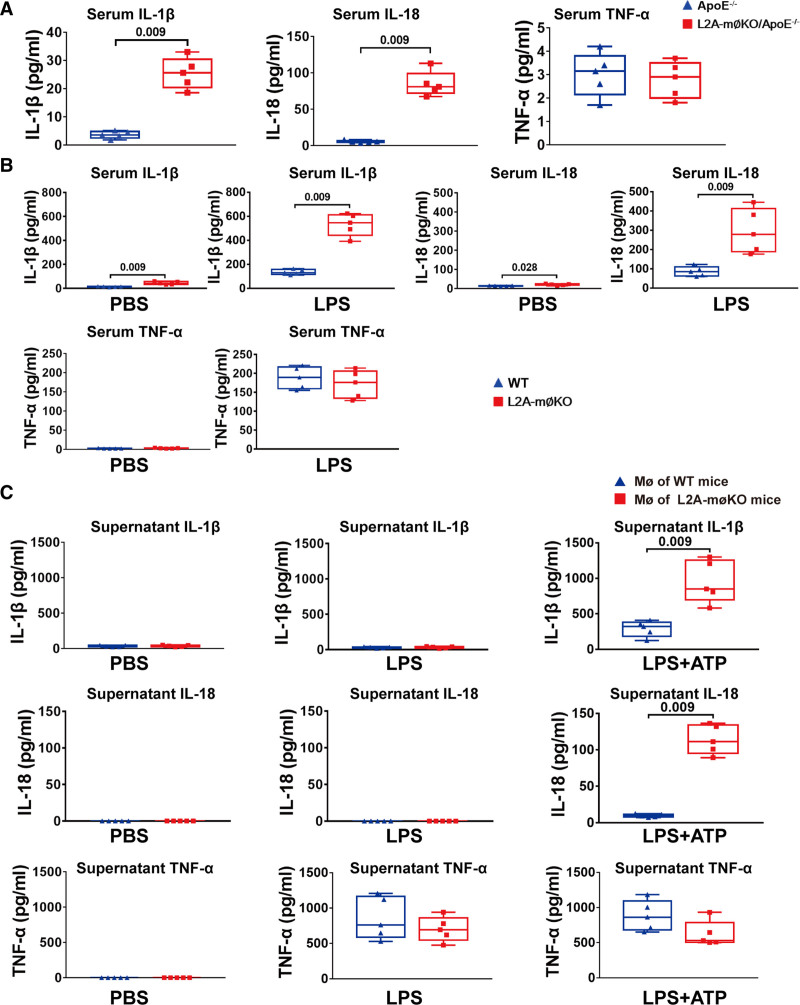
We next investigated the mechanism by which CMA affects atherosclerosis. As atherosclerosis is widely accepted to be a chronic inflammatory disease,1–3 we first determined the effect of LAMP-2A deficiency on systemic inflammatory factors in vivo. Interestingly, we found that serum IL-1β and IL-18 levels, but not TNF (tumor necrosis factor)-α levels, were increased in L2A-mØKO/ApoE−/− mice fed an HFD for 16 weeks compared with similarly treated ApoE−/− mice (Figure 4A). After lipopolysaccharide injection, both groups of mice exhibited a remarkable increase in serum levels of IL-1β, IL-18, and TNF-α relative to their baseline levels, but the increase in serum levels of IL-1β and IL-18 was significantly higher in L2A-mØKO/ApoE−/− than ApoE−/− mice (Figure 4B). Because both IL-1β and IL-18 are NLRP3 inflammasome-dependent cytokines,4–6 whereas TNF-α is not, these results indicated that macrophage LAMP-2A deficiency might activate the NLRP3 inflammasome, an inflammatory process that has been proven to trigger vascular wall inflammatory responses and atherosclerosis progression.3,4
Figure 4.
Comparison of proinflammatory factors in wild-type (WT) and L2A-mØKO mice (macrophage (MØ)-specific LAMP-2A (lysosome associated membrane protein type 2A; L2A)-knockout mice) determined by ELISA. A, Serum levels of IL (interleukin)-1β, IL-18, and TNF (tumor necrosis factor)-α in ApoE−/− mice and L2A-mØKO/ApoE−/− mice (male) fed a high-fat diet for 16 wk. n=5 per group. Data were presented as medians and quartiles. Statistical analysis was conducted using Mann-Whitney test. B, WT mice (n=5) and L2A-mØKO mice (n=5, male) were intraperitoneally injected with PBS or lipopolysaccharide (LPS; 1.0 mg/kg), and 2 h later, the serum levels of IL-1β, IL-18, and TNF-α were measured by ELISA. Data were presented as medians and quartiles. Comparison was made only between WT and L2A-møKO groups. Statistical analysis was carried out using Mann-Whitney test. C, ELISA was used to detect IL-1β, IL-18, and TNF-α levels in the supernatants of mouse peritoneal macrophages from WT and L2A-mØKO mice (male). Macrophages were primed with PBS or LPS (100 ng/mL) for 8 h, followed by stimulation with ATP (5 mmol/L) for 30 min in the latter group. Five independent experiments were performed. Data were presented as medians and quartiles. Comparison was made only between WT and L2A-møKO groups. Statistical analysis was carried out using Mann-Whitney test.
To confirm this hypothesis, primary peritoneal macrophages were isolated from both L2A-mØKO and WT mice and then treated with lipopolysaccharide alone or in combination with the NLRP3 inflammasome activator ATP. IL-1β and IL-18 secretion was significantly increased in LAMP-2A–deficient macrophages in response to NLRP3 inflammasome stimulation (Figure 4C). In contrast, TNF-α secretion was not influenced by LAMP-2A deletion (Figure 4C). Furthermore, the selective hypersecretion of IL-1β and IL-18 in LAMP-2A–deficient macrophages was not due to transcriptional effects because the mRNA levels of IL-1β, IL-18, and TNF-α in LAMP-2A–deficient and control macrophages responded similarly to lipopolysaccharide (or in the presence of ATP; Figure S5A through S5C in the Supplemental Material). This was further validated by exploring the influence of LAMP-2A on alterations in the mitogen-activated protein kinase (MAPK) and IκBa (nuclear factor κB inhibitor alpha)/NF-κB (nuclear factor κB) pathways. LAMP-2A depletion had no effect on the lipopolysaccharide-induced phosphorylation of JNK (C-Jun N-terminal kinase), ERK (extracellular signal-regulated kinase), and p38 (Figure S5E through S5H in the Supplemental Material), nor did it affect NF-κB p65 nuclear translocation within 40 min (Figure S6L in the Supplemental Material). Together, this specific proinflammatory phenotype suggested that deficient CMA activated NLRP3 inflammasome-dependent processing of pro–IL-18 and pro–IL-18 rather than directly influencing their production.
Deficient CMA Promoted NLRP3 Inflammasome Activation
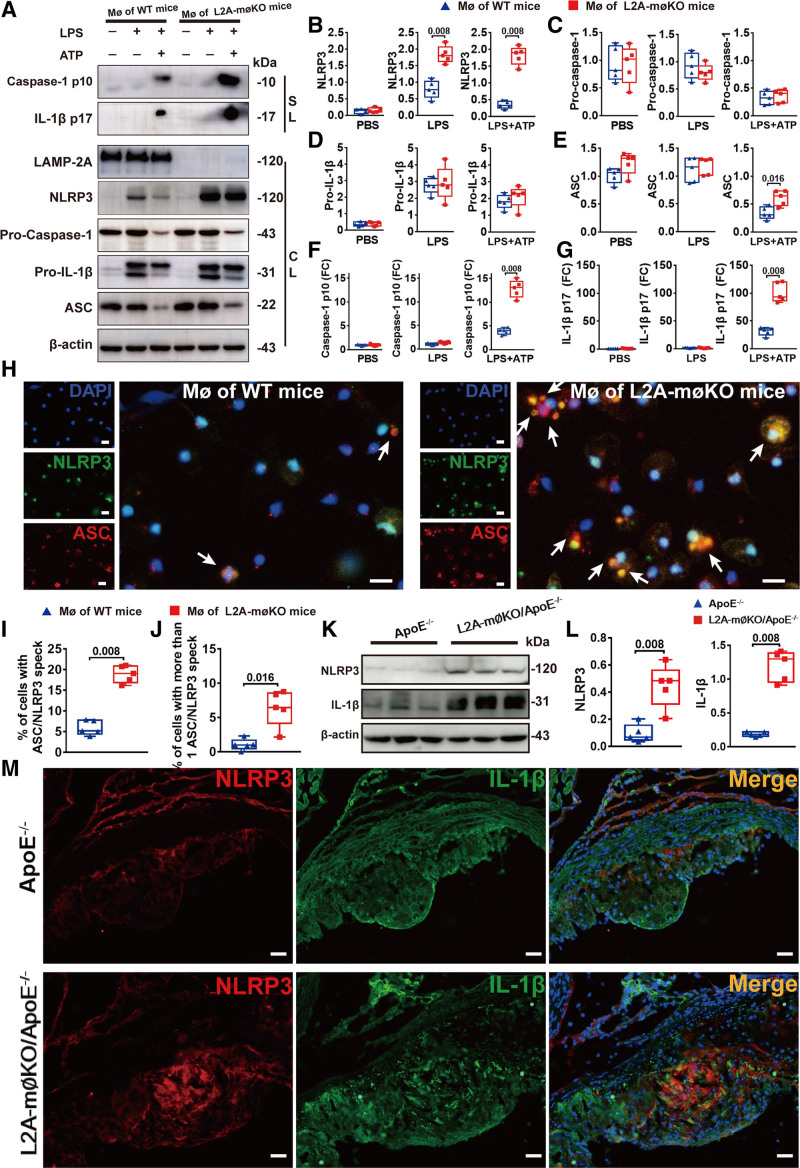
To validate the role of CMA in NLRP3 inflammasome activation, we further analyzed the protein levels of the NLRP3 inflammasome components of NLRP3, ASC, and caspase-1 in LAMP-2A–deficient macrophages and WT macrophages treated with lipopolysaccharide with or without ATP. Immunoblot analysis showed that the level of NLRP3, the core component of the NLRP3 inflammasome, was considerably increased in LAMP-2A–deficient macrophages compared with WT macrophages (Figure 5A and 5B). Notably, NLRP3 mRNA levels in LAMP-2A–deficient macrophages and WT macrophages were comparable (Figure S5D in the Supplemental Material). In addition, there were no statistically significant differences in the intracellular levels of procaspase-1, ASC, or pro–IL-18 (Figure 5A, 5C, and 5D). However, the secretion of cleaved caspase-1 (p10) and mature IL-1β (p17) in the cellular supernatant was significantly elevated in LAMP-2A–deficient macrophages following lipopolysaccharide and ATP treatment (Figure 5A, 5F, and 5G), suggestive of enhanced caspase-1 activation and subsequent cleavage of pro–IL-18 into mature IL-1β. Consequently, decreased NLRP3 protein level and less cleaved caspase-1 were detected in LAMP-2A overexpressed macrophages (Figure S6A through S6G in the Supplemental Material). Furthermore, gain-of-function studies verified that restoration of LAMP-2A levels in LAMP-2A–deficient macrophages substantially attenuated expression levels of NLRP3 protein and secretion of activated caspase-1 (p10) and mature IL-1β (p17) in the cellular supernatant (Figure S6A through S6G in the Supplemental Material). The increased secretion of IL-1β and IL-18 were also reversed by LAMP-2A rescue (Figure S6H and S6I in the Supplemental Material). These data further supported the negative regulatory role of CMA in NLRP3 inflammation activation.
Figure 5.
LAMP-2A (lysosome-associated membrane protein type 2A) deficiency promoted NLRP3 (NLR [NOD-like receptor] family, pyrin domain containing 3) inflammasome activation. A, Representative western blot images and (B–G) quantitative analysis of protein expression of supernatants lysates (SLs) and cell lysates (CLs) of mouse peritoneal macrophages (MØs) from wild-type (WT) and L2A-mØKO mice (macrophage (MØ)-specific LAMP-2A (lysosome associated membrane protein type 2A; L2A)-knockout mice) (male) treated with PBS or lipopolysaccharide (LPS; 100 ng/mL) for 8 h with subsequent stimulation with ATP (5 mmol/L) for 30 min in the latter group. Five independent experiments were performed. Data were presented as medians and quartiles. Comparison was made only between WT and L2A-møKO groups. Statistical analysis was conducted using Mann-Whitney test. H, Representative immunofluorescence and (I and J) quantitative analysis of NLRP3/ASC (apoptosis-associated speck-like protein containing a CARD [C-terminal caspase-recruitment domain]) speck (yellow particles) formation induced by ATP (5 mmol/L) in 500 ng/mL LPS-primed peritoneal MØs from control WT and L2A-mØKO mice. Cells were stained with NLRP3 (green particles) and ASC (red particles) antibodies. Five independent experiments were performed. Data were presented as medians and quartiles. Statistical analysis was performed using Mann-Whitney test. Scale bar=10 μm. K, Representative western blot images and (L) quantitative analysis of NLRP3 and IL (interleukin)-1β expression in whole aortic lysates from ApoE−/− mice (n=5, male) and L2A-mØKO/ApoE−/− mice (n=5, male). Data were presented as medians and quartiles. Statistical analysis was done using Mann-Whitney test. Scale bar=100 μm. M, Representative double immunofluorescence analysis to detect NLRP3 (red particles) and IL-1β (green particles) in frozen aortic root sections from ApoE−/− mice (n=5, male) and L2A-mØKO/ApoE−/− mice (n=5, male). Scale bar=100 μm. DAPI indicates 4,6-diamidino-2-phenyiindolel and FC, fold change.
NLRP3/ASC speck formation induced by ATP in lipopolysaccharide-primed cells is another sign of NLRP3 inflammasome activation.30 We subsequently observed an increase in the number of cells with NLRP3/ASC specks as well as the number of specks per cell in LAMP-2A–deficient macrophages compared with WT macrophages (Figure 5H-J). Moreover, upon ATP treatment, the specks in LAMP-2A–deficient macrophages were larger than those in WT macrophages (Figure 5H).
To determine whether the NLRP3 inflammasome was also activated in CMA-deficient mice in vivo, we assessed NLRP3 and IL-1β protein levels in L2A-mØKO/ApoE−/− and ApoE−/− mice. Consistent with the in vitro results, LAMP-2A deficiency led to an obvious increase in NLRP3 and IL-1β expression in whole aortic lysates (Figure 5K-L) and in aortic root sections (Figure 5M). Altogether, these data suggested that LAMP-2A deficiency specifically promoted NLRP3 inflammasome activation in vitro and in vivo.
NLRP3 Interacts With HSC70 and LAMP-2A
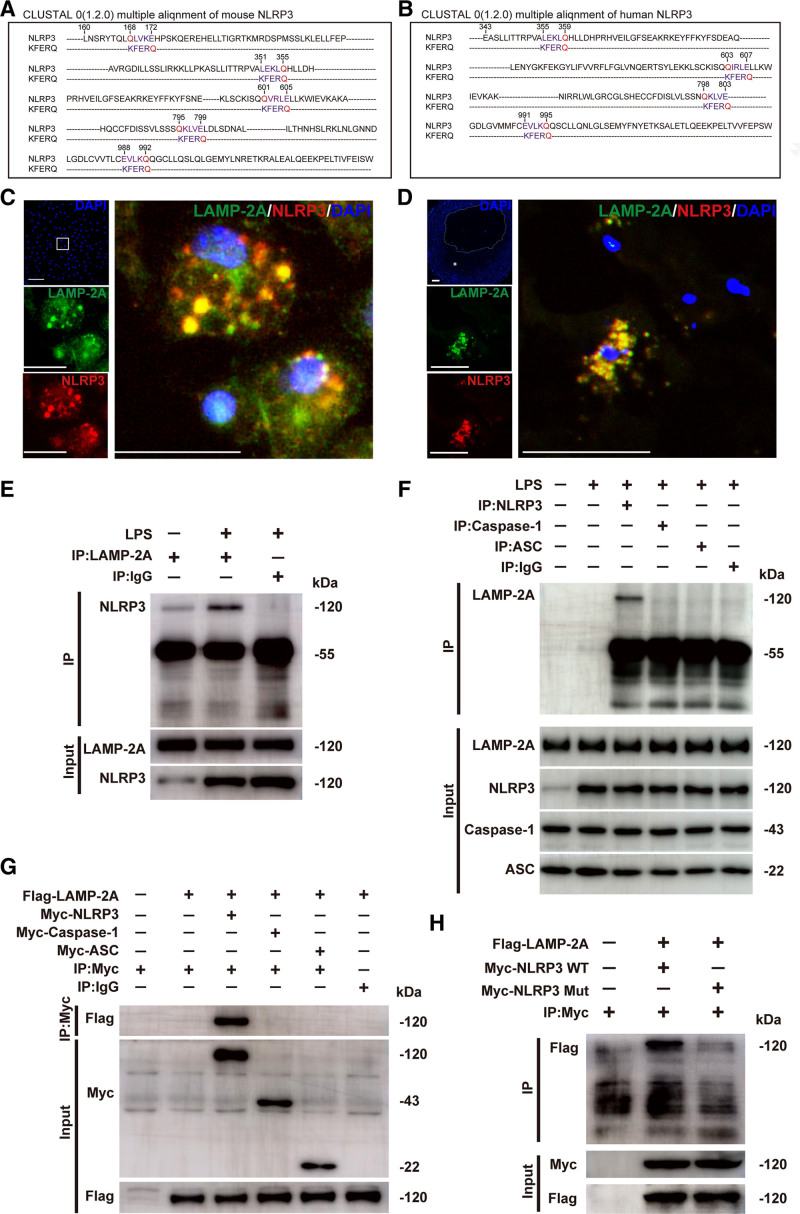
Next, we investigated the molecular mechanism by which CMA regulates NLRP3 inflammasome activation. Given that CMA serves as a catabolic pathway for the selective degradation of cytosolic proteins in lysosomes, combined with the finding that LAMP-2A deficiency had no effect on NLRP3 mRNA expression (Figure S5B in the Supplemental Material), we speculated that CMA deficiency promotes NLRP3 inflammasome activation by inhibiting the degradation of inflammasome protein components. CMA substrates are characterized by the presence of KFERQ-like motifs in their amino acid sequences.16 We first analyzed the amino acid sequences of NLRP3, Caspase-1, and ASC to determine which component may be a CMA substrate candidate. Four KFERQ-like motifs were found in the amino acid sequence of human NLRP3, but none was found in caspase-1 or ASC (Figure 6A, and Figure S7A and S7B in the Supplemental Material). Mouse NLRP3 contains 5 KFERQ-like motifs, caspase-1 contains 2, and ASC contains none (Figure 6B and Figure S7A and S7B in the Supplemental Material). Thus, among these proteins, NLRP3 is most likely a CMA substrate. This is consistent with our finding that LAMP-2A deficiency increased the protein level of intracellular NLRP3 but had no effect on that of procaspase-1 or ASC (Figure 5A).
Figure 6.
Identification of the NLRP3 (NLR [NOD-like receptor] family, pyrin domain containing 3) protein as a chaperone-mediated autophagy (CMA) substrate. A and B, Alignment of the amino acid sequences of mouse and human NLRP3 with the KFERQ motif. Within the KFERQ motifs, Q (in red) and the other 4 amino acids (in purple) were marked. C, Colocalization (yellow particles) of NLRP3 (red particles) with LAMP-2A (lysosome-associated membrane protein type 2A; green particles) in peritoneal macrophages treated with lipopolysaccharide (LPS; 100 ng/mL) for 8 h, followed by stimulation with ATP (5 mmol/L) for 30 min. Scale bar=10 μm. D, Colocalization (yellow particles) of NLRP3 (red particles) with LAMP-2A (green particles) in human coronary atherosclerotic plaques. Five independent experiments were performed. Scale bar=100 μm. E, Co-immunoprecipitation of endogenous LAMP-2A with NLRP3 from LPS and ATP-treated mouse peritoneal macrophages. The same amount of nonspecific antibody (Rabbit IgG) was used as a control. F, Co-immunoprecipitation of endogenous NLRP3, caspase-1, or ASC (apoptosis-associated speck-like protein containing a CARD [C-terminal caspase-recruitment domain]) with LAMP-2A from LPS and ATP-treated mouse peritoneal macrophages. The same amount of nonspecific antibody (Rabbit IgG) was used as a control. G, Co-immunoprecipitation of Flag-LAMP-2A with Myc (Myc epitope tag)-NLRP3, Myc-Caspase-1, or Myc-ASC in HEK293T cells. The same amount of nonspecific antibody (Rabbit IgG) was used as a control. H, Co-immunoprecipitation of Flag-LAMP-2A with wild-type (WT) Myc-NLRP3 (Myc-NLRP3 WT) or mutant Myc-NLRP3 (Myc-NLRP3 Mut) from HEK293T cells. All of the four KFERQ motifs in human NLRP3 were mutated to disrupt the interaction of the NLRP3 protein with the CMA receptor LAMP-2A. DAPI indicates 4,6-diamidino-2-phenyiindole; and mut, mutant.
The first step in CMA is the interaction between substrate and HSC70, forming HSC70-substrate complex, which then binds the 12-amino acid cytosolic tail of lysosomal LAMP-2A.16 We found that NLRP3 colocalized with both HSC70 (Figure S8A in the Supplemental Material) and LAMP-2A (Figure 6C) in macrophages treated with lipopolysaccharide and ATP. This colocalization was also observed in human coronary atherosclerotic plaques (Figure 6D), suggestive of an association between NLRP3 protein and CMA components. Co-immunoprecipitation further confirmed the direct interaction between endogenous NLRP3 and HSC70 (Figure S8B in the Supplemental Material) or LAMP-2A (Figure 6E and 6F). However, neither Caspase-1 nor ASC immunoprecipitated with LAMP-2A (Figure 6F). This result was also found in HEK293T cells transfected with plasmids for the expression of Flag-LAMP-2A and Myc (Myc epitope tag)-NLRP3, Myc-Caspase-1 or Myc-ASC (Figure 6G). These data demonstrated that NLRP3, but not Caspase-1 or ASC, interacted with CMA components.
To further confirm that NLRP3 is a substrate of CMA, we generated a mutant version of all putative motifs that might be recognized and targeted by HSC70. All of the 4 CMA motifs in human NLRP3 (355 LEKLQ 359, 603 QIRLE 607, 798 QKLVE 802, and 991 EVLKQ 995) were mutated by replacing Q and the residue next to it with AA, a method usually used to disrupt the interaction of a substrate with CMA components.21,31 Flag-LAMP-2A was cotransfected with WT Myc-NLRP3 or mutated Myc-NLRP3 into HEK293T cells. The NLRP3 mutant lost the ability to interact with LAMP-2A (Figure 6H). These data demonstrated that NLRP3 was a CMA substrate.
Deficient CMA Inhibited NLRP3 Inflammasome Degradation
To further determine whether the increased protein level of NLRP3 in CMA-deficient macrophages was due to impaired NLRP3 degradation by CMA, we analyzed the contribution of the CMA-lysosome pathway to NLRP3 degradation. We first detected the lysosomal content of NLRP3 inflammasome components in macrophages from normal chow-fed and starved WT mice. Starvation was found to activate macroautophagy and CMA, both of which led to an increased lysosomal degradation of the corresponding substrates.11,32 All 3 components of the NLRP3 inflammasome were enriched in lysosomes from starved mice (Figure 7A through 7G), suggesting that active lysosomes were able to take up more NLRP3 inflammasomes. Macroautophagy-dependent degradation of substrates requires the assistance of the ubiquitination system, which identifies and labels target proteins with specific ubiquitin chains, mainly lysine 63 (K63) and lysine 48 (K48) polyubiquitin chains.33 Similarly, strategies aimed at promoting NLRP3 inflammasome degradation through macroautophagy were also achieved by facilitating the ubiquitination of inflammasome constituent proteins.13,14 However, we found that NLRP3 ubiquitination was markedly decreased in both K48 and K63 chains in the presence of NLRP3 inflammasome activator ATP (Figure S9 in the Supplemental Material). This was consistent with previous studies that the NLRP3 protein is polyubiquitinated under physiological conditions but undergoes deubiquitination during NLRP3 inflammasome activation.34,35 Therefore, without drug stimulation or gene manipulation, the contribution of macroautophagy to the degradation of NLRP3 inflammasomes may be minimal.
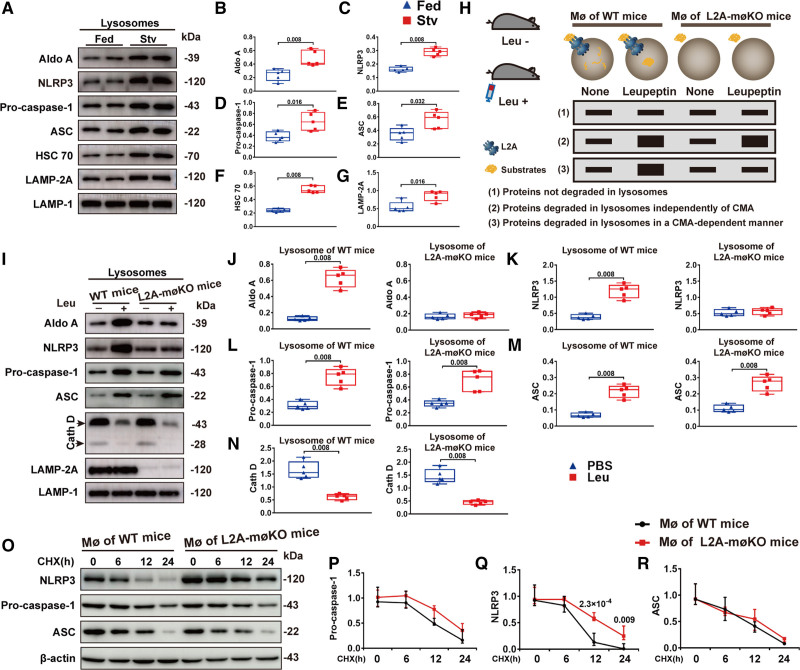
Figure 7.
Deficient chaperone-mediated autophagy (CMA) inhibited degradation of the NLRP3 (NLR [NOD-like receptor] family, pyrin domain containing 3) inflammasome. A–G, Representative immunoblot images and quantitative analysis to detect the indicated proteins in lysosomes isolated from the peritoneal macrophages (MØs) of fed or starved (Stv) C57BL/6 mice (n=5, male). C57BL/6 mice were fed or starved for 24 h and were intraperitoneally injected with lipopolysaccharide (LPS) 4 h before MØ isolation to activate NLRP3 inflammasome. Five independent experiments were performed. Data were presented as medians and quartiles. Statistical analysis was conducted using Mann-Whitney test. H, Schematic diagram showing the method used to determine whether a protein is degraded by CMA: (1) proteins not degraded in lysosomes, (2) proteins degraded in lysosomes independently of CMA, and (3) proteins degraded in lysosomes through CMA. I–N, Representative immunoblot images and quantitative analysis of lysosomes isolated from the peritoneal MØs of wild-type (WT) and L2A-mØKO mice (macrophage [MØ-specific] LAMP-2A [lysosome associated membrane protein type 2A; L2A]-knockout mice) (n=5, male) who were starved for 24 h and then injected with or without leupeptin (Leu) 2 h before isolation. Aldo A (aldolase), a well-characterized CMA substrate, was used as a positive control. Five independent experiments were performed. Data were presented as medians and quartiles. Comparison was made only between PBS and Leu groups. Statistical analysis was performed using Mann-Whitney test. O–R, Representative immunoblot images and quantitative analysis of protein expression in peritoneal MØ extracts from WT and L2A-mØKO mice (n=5, male). MØs were treated with LPS for 6 h, and after LPS was removed, cells were treated with cycloheximide (CHX, 5 μg/mL) for various durations. Five independent experiments were performed. Data were presented as medians and quartiles. Comparison was made only between WT and L2A-møKO groups. Statistical analysis was carried out using Multiple linear mixed-effects modeling. ASC indicates apoptosis-associated speck-like protein containing a CARD (C-terminal caspase-recruitment domain); HSC70, heat shock cognate 71 kDa protein; and LAMP-2A (lysosome-associated membrane protein type 2A.
An important finding of the present study was that lysosomes from starved mice contained more HSC70 and LAMP-2A (termed CMA-active lysosomes, in which Aldo A (aldolase), a CMA substrate, has been deemed as a positive control). As active lysosomes took up more NLRP3 inflammasomes, our results indicated an association between CMA and NLRP3 inflammasome. By comparative analysis of the protein content in the pool of CMA-active lysosomes isolated from starved WT and L2A-KO mice that remained untreated or were injected with leupeptin (to block lysosomal proteolysis; Figure 7H),21,28 we subsequently determined the pathways responsible for degradation of the three NLRP3 inflammasome components in vivo. Immunoblot analysis of these lysosomal fractions showed that only the NLRP3 protein in lysosomes had been degraded in a CMA-dependent manner (Figure 7I through 7N). In addition, immunoblot analysis of extracts from WT or L2A-KO mouse peritoneal macrophages treated with cycloheximide (to inhibit protein synthesis) for various durations further confirmed that LAMP-2A deficiency inhibited NLRP3 protein degradation but not the degradation of caspase-1 or ASC (Figure 7O through 7R). These findings suggested that activation of the NLRP3 inflammasome in CMA-deficient macrophages was due to compromised degradation of the NLRP3 protein by CMA.
Discussion
There were several important findings in the present study. First, atherosclerosis progression was accompanied by defective CMA. Second, deficient CMA of macrophages accelerated atherosclerosis in ApoE−/− mice, accompanied by increased serum levels of the proinflammatory factors IL-1β and IL-18 as well as elevated protein levels of NLRP3 and IL-1β in atherosclerotic plaques. Third, CMA deficiency promoted NLRP3 inflammasome activation and the subsequent cleavage of pro–IL-18 and pro–IL-18 into mature IL-1β and IL-18, respectively, in macrophages in vitro. Finally, these effects were not due to alterations at the transcriptional level but rather were the consequence of impaired degradation of the NLRP3 protein through the CMA-lysosome pathway (Figure 8).
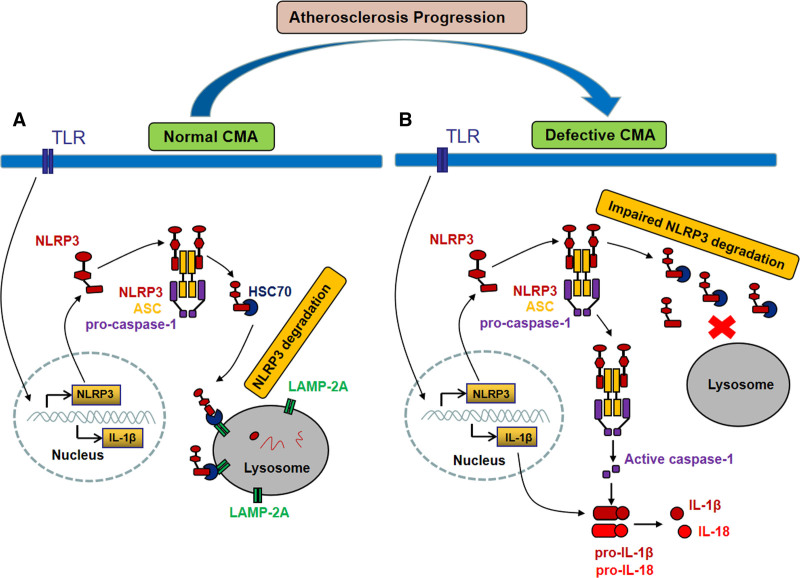
Figure 8.
Proposed mechanism of chaperone-mediated autophagy (CMA) deficiency promoting NLRP3 (NLR [NOD-like receptor] family, pyrin domain containing 3) inflammasome activation. A, In macrophages with normal CMA activity, the NLRP3 protein is degraded through the LAMP-2A (lysosome-associated membrane protein type 2A)–mediated CMA pathway, thus preventing excessive NLRP3 inflammasome activation. B, In macrophages with defective CMA, the NLRP3 protein cannot be effectively degraded and removed, resulting in excessive NLRP3 inflammasome activation and subsequent cleavage of pro–IL (interleukin)-1β and pro–IL-18 to mature IL-1β and IL-18, promoting vascular inflammation and atherosclerosis progression. ASC indicates apoptosis-associated speck-like protein containing a CARD (C-terminal caspase-recruitment domain); HSC70, heat shock cognate 71 kDa protein; and TLR, Toll-like receptor.
Serving as the main cell population in atherosclerotic plaques, macrophages are responsible for removing lipids and debris in the plaques.24 Macrophage dysfunction causes lipid accumulation and foam cell formation, cell apoptosis, and extensive inflammatory responses, which constitute the major events during atherogenesis. As lysosomes mediate the overall degradative capacity of cells,36 competent lysosomes are a prerequisite for macrophages to efficiently degrade exogenous and endogenous proatherogenic cargo. In mammalian cells, this process depends on three pathways: macroautophagy, microautophagy, and CMA. All 3 autophagic pathways fulfill special functions, together contributing to cellular quality control. Progressive dysfunction in the lysosomal apparatus underlies the hyperinflammatory state and abnormalities in lipid trafficking in atherosclerotic macrophages.36,37 Exploiting macrophage autophagy-lysosomal biogenesis as a potential therapy is fascinating. However, current studies in atherosclerosis mainly focus on macroautophagy, the role of CMA in atherosclerosis remains unclear. Our present work clearly demonstrated a decline in CMA during the progression of atherosclerosis and a causal link between CMA failure and atherogenesis. This is consistent with previous studies showing that CMA activity declines in age-related disorders, such as neurodegenerative diseases and metabolic disorders.27,38,39 The revelation that CMA is involved in the pathogenesis of atherosclerosis provides insight into the role of autophagy-lysosomal biogenesis in atherosclerosis. Because of the cooperation between macroautophagy and CMA in atherosclerosis, attempts to treat atherosclerosis by correcting the degradative capacity of macrophages should focus on not only macroautophagy but also CMA. The preservation of CMA activity is critical for normal lysosomal function, particularly in cases in which CMA serves as an upstream regulator of macroautophagy.40
Atherosclerosis is widely recognized as an inflammatory disease.1–3 NLRP3 inflammasome activation and the subsequent secretion of IL-1β and IL-18 contribute greatly to vascular inflammation and atherosclerosis progression. Therefore, therapies targeting the NLRP3 inflammasome have great potential and are attractive for the management of atherosclerosis and other inflammation-related diseases. Upstream regulators of the NLRP3 inflammasome have been well elucidated and include 2 independent signals: an initial priming signal that induces the transcriptional upregulation of NF-κB and a second stimulus that induces inflammasome assembly.4,8 However, the exact downstream mechanism that regulates NLRP3 is not completely understood. Macroautophagy13,14 and the ubiquitin-proteasome system15 have been reported to play a role in NLRP3 degradation, which occurs only when NLRP3 is ubiquitinated. By comparison, CMA is more specialized and more specific for protein degradation.16 In addition, CMA has proven to be involved in metabolic abnormalities by degrading lipid droplet-associated proteins (perilipin 2 and perilipin 3)40 and key enzymes in carbohydrate and lipid metabolism.28 Here, by focusing on CMA, we report a new mechanism by which the NLRP3 inflammasome is regulated in macrophages and atherosclerotic plaques. We first identified the NLRP3 protein as a CMA substrate and then demonstrated that deficient CMA in progressive atherosclerosis caused impaired NLRP3 protein degradation and excessive NLRP3 inflammasome activation.
As anti-inflammatory therapy via IL-1β-neutralizing antibodies has been shown to be effective in treating atherosclerosis,9 the activation of CMA might represent another new approach to alleviate the inflammatory response in atherosclerotic plaques. At present, inspiring results following attempts to improve CMA function have been observed in several mouse models of disease. For example, the restoration of CMA in T cells from old mice improved activation-induced responses through the targeted degradation of negative regulators of T cell activation.41 Furthermore, the restoration of CMA in the livers of aging mice improved cellular maintenance and hepatic function.42 Increasing CMA in mouse nigral dopaminergic neurons ameliorated α-synuclein–induced dopaminergic neurodegeneration, providing a novel therapeutic strategy in Parkinson disease.43 CMA is more selective and specific for protein degradation because of the unique mechanism that underlies lysosomal cargo binding and delivery.16 Aberrant activation of the NLRP3 inflammasome is the basis of inflammation-related disorders, such as type 2 diabetes, colitis, Alzheimer disease, and atherosclerosis.5,6 The timely and effective removal of overactivated inflammasomes is critical to control inflammation levels. In this work, we report for the first time the important role of CMA in the degradation of NLRP3 proteins, which opens new avenues for manipulation of the NLRP3 inflammasome degradation machinery. The activation of CMA may represent a potential therapy for the treatment of atherosclerosis as it promotes the degradation of NLRP3 inflammasomes as well as other proatherogenic proteins that may be CMA substrates.
Although our work has unveiled the important role of CMA in atherosclerosis, many additional questions need to be addressed. First, the reasons for the decline in CMA during the progression of atherosclerosis are not unclear, although previous studies suggested that compromised CMA might be secondary to aging27 or excessive lipid feeding.26 Understanding the mechanisms by which CMA becomes dysfunctional in atherosclerosis or the aging process will increase the possibility of manipulating CMA activity. Second, it is not clarified whether there are other pathways by which CMA affects atherosclerosis in addition to its ability to regulate NLRP3 inflammasome-dependent inflammation. Our previous study showed that CMA deficiency promoted lipid accumulation in macrophages,44 which indicates a possible link between CMA, lipids, and atherosclerosis. Unfortunately, we did not elucidate the mechanisms underlying lipid metabolism disorders in CMA-deficient macrophages. Third, many factors are involved in the activation of NLRP3 inflammasomes in atherosclerotic plaques and it is unknown to what extent CMA affects this process. Fourth, different proteolytic pathways (macroautophagy, UPS, and CMA) may coexist inside cells, and both macroautophagy and UPS have been reported to play a role in NLRP3 degradation,13–15 which occurs only when NLRP3 is ubiquitinated. However, NLRP3 protein undergoes deubiquitination during NLRP3 inflammasome activation.34,35 Thus, the contribution of macroautophagy and UPS to the degradation of NLRP3 inflammasomes may be minimal. In contrast, cargo recognition and binding in CMA does not rely on specific ubiquitin chains to label target proteins but requires substrates that contain a pentapeptide-targeting motif (KFERQ motifs).16 As a result, NLRP3 protein is degraded mainly through CMA pathway which may play a prominent role in atherosclerosis. Future studies are warranted to clarify the effect of CMA upregulation on NLRP3 inflammasomes and atherosclerosis progression. Finally, as in most murine experiments, we used only male mice to avoid a protective effect of estrogen on atherosclerosis.45 Thus, there might have been a sex difference in our results had the same experiments been performed in female mice. Further studies are needed to assess the reproducibility of our results in female mice.
In conclusion, the present study demonstrates that CMA plays an important role in the pathogenesis of atherosclerosis by dampening NLRP3 inflammasome activation through degradation of NLRP3 proteins. Our study unveils a new mechanism by which NLRP3 inflammasome is regulated in macrophages and atherosclerosis and provides a new insight into the role of autophagy-lysosomal pathway in atherosclerosis. Pharmacological activation of CMA may provide a novel therapeutic strategy for atherosclerosis and other NLRP3 inflammasome/IL-1β–driven diseases.
Article Information
Acknowledgments
We thank Mingxiang Zhang, Prof. Wencheng Zhang, Prof. Qunye Zhang and Prof. Shuangxi Wang for their suggestions in experimental design. We thank George and Qi Xiao for their company.
Sources of Funding
This work was supported by the grants of the National Natural Science Foundation of China (No. 81770436, 81920108003, 82030051, 81770442, 31770977, 81873516, 82000411, and 81970373), the National Key Project of Chronic Noncommunicable Diseases of China (No.2016 YFC1300403), the grants of the Shandong Natural Science Foundation (No. ZR2020YQ53, ZR2020QH023), Program of Introducing Talents of Discipline to Universities (No. BP0719033), and the Fundamental Research Funds of Shandong University (No. 2018JC001) and Taishan Scholar Project of Shandong Province of China (C. Zhang and M. Zhang).
Disclosures
None.
Supplemental Materials
Supplemental Methods
Tables S1 and S2
Figures S1–S9
Major Resources Table
References 22,46–50
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ASC
- apoptosis-associated speck-like protein containing a CARD (C-terminal caspase-recruitment domain)
- CMA
- chaperone-mediated autophagy
- HFD
- high-fat diet
- HSC70
- heat shock cognate 71 kDa protein
- IL
- interleukin
- LAMP
- lysosome-associated membrane protein
- NLRP3
- NOD-like receptor (NLR) family, pyrin domain containing 3
- SMCs
- smooth muscle cells
L.Q. and J.M. contributed equally.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.121.318908.
For Sources of Funding and Disclosures, see page 1156.
Contributor Information
Lei Qiao, Email: qiaolei900216@126.com.
Jing Ma, Email: sdumajing@163.com.
Zihao Zhang, Email: zhangyun@sdu.edu.cn.
Wenhai Sui, Email: suiwenhai@126.com.
Chungang Zhai, Email: zhaichungang87@sdu.edu.cn.
Dan Xu, Email: xudan660@sina.com.
Zunzhe Wang, Email: wangzz1007@163.com.
Huixia Lu, Email: luhuixia@sdu.edu.cn.
Meng Zhang, Email: zhangyun@sdu.edu.cn.
Cheng Zhang, Email: zhangyun@sdu.edu.cn.
Novelty and Significance
What Is Known?
The NLRP3 (NLR [NOD-like receptor] family, pyrin domain containing 3) inflammasome contributes to vascular inflammation that drives atherosclerosis initiation and progression.
Chaperone-mediated autophagy (CMA), one of the main lysosomal degradative processes, plays an essential role in the maintenance of cellular homeostasis.
CMA defects have been implicated in several human pathologies, including neurodegenerative diseases, metabolic disorders, and age-related diseases.
What New Information Does This Article Contribute?
CMA is gradually impaired with atherosclerosis progression and its dysfunction accelerates atherosclerosis.
CMA deficiency increases NLRP3 inflammasome activation in macrophages and atherosclerotic plaques, promoting vascular inflammation and atherosclerosis progression.
CMA regulates NLRP3 inflammasome activation by mediating NLRP3 protein degradation through the CMA-lysosomal pathway.
Pharmacological activation of CMA may provide a novel therapeutic strategy for atherosclerosis and other NLRP3 inflammasome/IL (interleukin)-1β–driven diseases.
The NLRP3 inflammasome and its products IL-1β and IL-18 contribute to vascular inflammation that drives atherosclerosis initiation and progression. Understanding the regulatory mechanisms of NLRP3 inflammasomes has important clinical significance for multiple inflammatory diseases, including atherosclerosis. In this study, we found that CMA became impaired during the progression of atherosclerosis and CMA dysfunction increased NLRP3 inflammasome activation and secretion of IL-1β and IL-18, promoting vascular inflammation and atherosclerosis progression. Our study unveils a new mechanism by which NLRP3 inflammasome is regulated in macrophages and atherosclerotic lesions and provides new insights into the role of autophagy-lysosomal pathway in atherosclerosis. Pharmacological activation of CMA may provide a novel therapeutic strategy for atherosclerosis and other NLRP3 inflammasome/IL-1β–driven diseases.
References
- 1.Aikawa M, Libby P. Atherosclerotic plaque inflammation: the final frontier? Can J Cardiol. 2004; 20:631–634 [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005; 352:1685–1695. doi: 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- 3.Christ A, Günther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Baßler K, et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell. 2018; 172:162, e14–175. doi: 10.1016/j.cell.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grebe A, Hoss F, Latz E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ Res. 2018; 122:1722–1740. doi: 10.1161/CIRCRESAHA.118.311362 [DOI] [PubMed] [Google Scholar]
- 5.Schroder K, Tschopp J. The inflammasomes. Cell. 2010; 140:821–832. doi: 10.1016/j.cell.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 6.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011; 29:707–735. doi: 10.1146/annurev-immunol-031210-101405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013; 13:397–411. doi: 10.1038/nri3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009; 183:787–791. doi: 10.4049/jimmunol.0901363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017; 377:1119–1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 10.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008; 451:1069–1075. doi: 10.1038/nature06639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshimori T. Autophagy: a regulated bulk degradation process inside cells. Biochem Biophys Res Commun. 2004; 313:453–458. doi: 10.1016/j.bbrc.2003.07.023 [DOI] [PubMed] [Google Scholar]
- 12.Majeski AE, Dice JF. Mechanisms of chaperone-mediated autophagy. Int J Biochem Cell Biol. 2004; 36:2435–2444. doi: 10.1016/j.biocel.2004.02.013 [DOI] [PubMed] [Google Scholar]
- 13.Chuang SY, Yang CH, Chou CC, Chiang YP, Chuang TH, Hsu LC. TLR-induced PAI-2 expression suppresses IL-1β processing via increasing autophagy and NLRP3 degradation. Proc Natl Acad Sci USA. 2013; 110:16079–16084. doi: 10.1073/pnas.1306556110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015; 160:62–73. doi: 10.1016/j.cell.2014.11.047 [DOI] [PubMed] [Google Scholar]
- 15.Song H, Liu B, Huai W, Yu Z, Wang W, Zhao J, Han L, Jiang G, Zhang L, Gao C, et al. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nat Commun. 2016; 7:13727. doi: 10.1038/ncomms13727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaushik S, Cuervo AM. The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol. 2018; 19:365–381. doi: 10.1038/s41580-018-0001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dice JF. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem Sci. 1990; 15:305–309. doi: 10.1016/0968-0004(90)90019-8 [DOI] [PubMed] [Google Scholar]
- 18.Dice JF. Altered degradation of proteins microinjected into senescent human fibroblasts. J Biol Chem. 1982; 257:14624–14627 [PubMed] [Google Scholar]
- 19.Chiang HL, Terlecky SR, Plant CP, Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989; 246:382–385. doi: 10.1126/science.2799391 [DOI] [PubMed] [Google Scholar]
- 20.Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996; 273:501–503. doi: 10.1126/science.273.5274.501 [DOI] [PubMed] [Google Scholar]
- 21.Patel B, Cuervo AM. Methods to study chaperone-mediated autophagy. Methods. 2015; 75:133–140. doi: 10.1016/j.ymeth.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Razani B, Feng C, Coleman T, Emanuel R, Wen H, Hwang S, Ting JP, Virgin HW, Kastan MB, Semenkovich CF. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 2012; 15:534–544. doi: 10.1016/j.cmet.2012.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011; 13:655–667. doi: 10.1016/j.cmet.2011.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013; 13:709–721. doi: 10.1038/nri3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci USA. 2003; 100:13531–13536. doi: 10.1073/pnas.1735526100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Navarro JA, Kaushik S, Koga H, Dall’Armi C, Shui G, Wenk MR, Di Paolo G, Cuervo AM. Inhibitory effect of dietary lipids on chaperone-mediated autophagy. Proc Natl Acad Sci USA. 2012; 109:E705–E714. doi: 10.1073/pnas.1113036109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000; 275:31505–31513. doi: 10.1074/jbc.M002102200 [DOI] [PubMed] [Google Scholar]
- 28.Schneider JL, Suh Y, Cuervo AM. Deficient chaperone-mediated autophagy in liver leads to metabolic dysregulation. Cell Metab. 2014; 20:417–432. doi: 10.1016/j.cmet.2014.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM. Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci USA. 2006; 103:5805–5810. doi: 10.1073/pnas.0507436103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martine P, Chevriaux A, Derangère V, Apetoh L, Garrido C, Ghiringhelli F, Rébé C. HSP70 is a negative regulator of NLRP3 inflammasome activation. Cell Death Dis. 2019; 10:256. doi: 10.1038/s41419-019-1491-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004; 305:1292–1295. doi: 10.1126/science.1101738 [DOI] [PubMed] [Google Scholar]
- 32.Cuervo AM, Knecht E, Terlecky SR, Dice JF. Activation of a selective pathway of lysosomal proteolysis in rat liver by prolonged starvation. Am J Physiol. 1995; 2695 Pt 1C1200–C1208. doi: 10.1152/ajpcell.1995.269.5.C1200 [DOI] [PubMed] [Google Scholar]
- 33.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Øvervatn A, Bjørkøy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007; 282:24131–24145. doi: 10.1074/jbc.M702824200 [DOI] [PubMed] [Google Scholar]
- 34.Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013; 49:331–338. doi: 10.1016/j.molcel.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 35.Han S, Lear TB, Jerome JA, Rajbhandari S, Snavely CA, Gulick DL, Gibson KF, Zou C, Chen BB, Mallampalli RK. Lipopolysaccharide primes the NALP3 inflammasome by inhibiting its ubiquitination and degradation mediated by the SCFFBXL2 E3 ligase. J Biol Chem. 2015; 290:18124–18133. doi: 10.1074/jbc.M115.645549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emanuel R, Sergin I, Bhattacharya S, Turner J, Epelman S, Settembre C, Diwan A, Ballabio A, Razani B. Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arterioscler Thromb Vasc Biol. 2014; 34:1942–1952. doi: 10.1161/ATVBAHA.114.303342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sergin I, Evans TD, Zhang X, Bhattacharya S, Stokes CJ, Song E, Ali S, Dehestani B, Holloway KB, Micevych PS, et al. Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis. Nat Commun. 2017; 8:15750. doi: 10.1038/ncomms15750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaushik S, Kiffin R, Cuervo AM. Chaperone-mediated autophagy and aging: a novel regulatory role of lipids revealed. Autophagy. 2007; 3:387–389. doi: 10.4161/auto.4246 [DOI] [PubMed] [Google Scholar]
- 39.Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AH. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol. 2010; 67:1464–1472. doi: 10.1001/archneurol.2010.198 [DOI] [PubMed] [Google Scholar]
- 40.Kaushik S, Cuervo AM. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol. 2015; 17:759–770. doi: 10.1038/ncb3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valdor R, Mocholi E, Botbol Y, Guerrero-Ros I, Chandra D, Koga H, Gravekamp C, Cuervo AM, Macian F. Chaperone-mediated autophagy regulates T cell responses through targeted degradation of negative regulators of T cell activation. Nat Immunol. 2014; 15:1046–1054. doi: 10.1038/ni.3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang C, Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med. 2008; 14:959–965. doi: 10.1038/nm.1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xilouri M, Brekk OR, Landeck N, Pitychoutis PM, Papasilekas T, Papadopoulou-Daifoti Z, Kirik D, Stefanis L. Boosting chaperone-mediated autophagy in vivo mitigates α-synuclein-induced neurodegeneration. Brain. 2013; 136pt 72130–2146. doi: 10.1093/brain/awt131 [DOI] [PubMed] [Google Scholar]
- 44.Qiao L, Zhang X, Liu M, Liu X, Dong M, Cheng J, Zhang X, Zhai C, Song Y, Lu H, Chen W. Ginsenoside Rb1 enhances atherosclerotic plaque stability by improving autophagy and lipid metabolism in macrophage foam cells. Front Pharmacol. 2017; 8:727. doi: 10.3389/fphar.2017.00727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res. 1995; 36:2320–2328 [PubMed] [Google Scholar]
- 46.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994; 14:133–140. doi: 10.1161/01.atv.14.1.133 [DOI] [PubMed] [Google Scholar]
- 47.Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler Thromb Vasc Biol. 2000; 20:2587–2592. doi: 10.1161/01.atv.20.12.2587 [DOI] [PubMed] [Google Scholar]
- 48.Dong M, Yang X, Lim S, Cao Z, Honek J, Lu H, Zhang C, Seki T, Hosaka K, Wahlberg E, et al. Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Cell Metab. 2013; 18:118–129. doi: 10.1016/j.cmet.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, Robbins J, Martinez J, Tabas I. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012; 15:545–553. doi: 10.1016/j.cmet.2012.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sergin I, Bhattacharya S, Emanuel R, Esen E, Stokes CJ, Evans TD, Arif B, Curci JA, Razani B. Inclusion bodies enriched for p62 and polyubiquitinated proteins in macrophages protect against atherosclerosis. Sci Signal. 2016; 9:ra2. doi: 10.1126/scisignal.aad5614 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article adheres to the Transparency and Openness Promotion Guidelines. The data that support the findings of this study are available from the corresponding authors upon reasonable request. Please see the Major Resources Table in the Supplemental Material.