ABSTRACT
Severe infection and its evolution to sepsis are becoming more prevalent every day and are among the leading causes of critical illness and mortality. Proper management is crucial to improve prognosis. This document addresses three essential points that have a significant impact on this objective: a) early recognition of patients with sepsis criteria, b) identification of those patients who suffer from an infection and have a high risk of progressing to sepsis, and c) adequate selection and optimization of the initial antimicrobial treatment.
Keywords: sepsis, septic shock, severe infection, selection and optimization of antimicrobial treatment, ceftazidime-avibactam, ceftolozane-tazobactam, meropenem
RESUMEN
La infección grave y su evolución a sepsis son cada vez más frecuentes y se encuentran entre las principales causas de enfermedad crítica y mortalidad. El manejo adecuado es crucial para mejorar el pronóstico. Este documento desarrolla tres puntos esenciales que tienen un impacto significativo en este objetivo: a) el reconocimiento temprano de los pacientes que cumplen los criterios de sepsis, b) la identificación de aquellos pacientes que sufren una infección y tienen un alto riesgo de progresar a sepsis, y c) realizar una correcta elección y optimización del tratamiento antimicrobiano inicial.
Keywords: Sepsis, shock séptico, infección grave, selección y optimización del tratamiento antimicrobiano, ceftazidima-avibactam, ceftolozanotazobactam, meropenem
INTRODUCTION
Infection meeting criteria for sepsis is one of the leading causes of critical illness and mortality worldwide [1, 2]. Improving the prognosis of sepsis requires: a) early recognition of patients with sepsis criteria, b) identifying those patients who suffer from an infection and have a high risk of progressing to sepsis, and c) adequate selection and optimization of the initial treatment. These three points are addressed in this document.
IDENTIFICATION OF THE PATIENT WITH SEPSIS OR AT HIGH RISK OF EVOLVING INTO A SITUATION OF SEPSIS
In the third international consensus of sepsis [3], the Society of Critical Care Medicine and the European Society of Intensive Care Medicine agreed to define sepsis as “a life-threatening organ dysfunction caused by an unregulated host response to infection;” defining organ dysfunction as an acute increase in the Sequential Organ Failure Assessment (SOFA) score of two or more points as a consequence of the infection. This abrupt change implies an organ or system failure (central nervous system, respiratory system, cardiac system, hepatic system, renal system, or coagulation system) or a minor failure in two of these organs or systems. In order to facilitate early identification of patients with sepsis outside the ICU, a reduced SOFA score (quick SOFA or qSOFA) was proposed [4] based on three criteria: altered mental status, systolic blood pressure ≤ 100 mm Hg, and respiratory rate ≥ 22 breaths/minute. Infected patients with at least 2 out these 3 criteria are included in sepsis category, but it should be confirmed with a complete SOFA score as soon as possible. Other early warning scoring systems to identify patients at risk of poor outcomes, such as the National Early Warning Score (NEWS) and the Modified Early Warning Score (MEWS), may be equal to or more sensitive than the qSOFA in predicting the need for transfer to the ICU because they consider all the parameters of the qSOFA score as well as additional parameters that help in the evaluation of patient’s status.
Septic shock is defined as the subset of patients with sepsis in which the underlying circulatory and cellular/metabolic abnormalities are significant enough to substantially increase the mortality. Septic shock is defined by the presence of sepsis criteria with (a) hypotension that requires the administration of vasopressors to maintain a mean arterial pressure of 65 mm Hg and (b) a serum lactate> 2 mmol/L (18 mg/dL), both despite adequate fluid replacement [3].
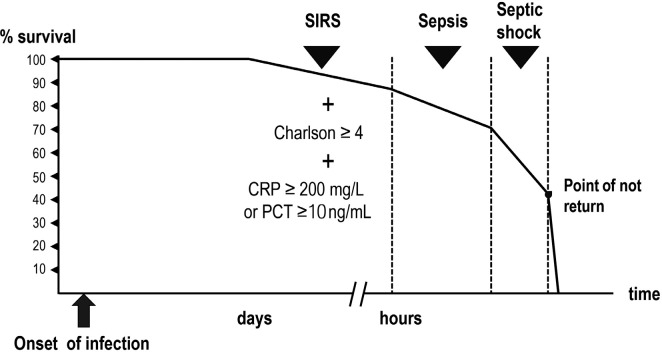
In the course of an infection, the progression into sepsis significantly reduce the probability of spontaneous reversion and without treatment, the patient inexorably will develop multi-organ failure and septic shock. From this point, the probability of success, even with appropriate treatment, rapidly decreases until a “no-return” point where the collapse of different organs aggravates other organs’ failure being even the best treatment futile (Figure 1).
Figure 1.
Potentially serious infection, sepsis, and septic shock. Probability of survival.
The closer the patient is to the point of no-return, the more urgent the initiation of adequate treatment is and the shorter the margin of error is for the choice of antibiotic. Even with treatment, mortality estimates for patients with two qSOFA criteria are 15-20% [5] and those for sepsis / septic shock in some series range between 27% and 40% [6–10]. In addition, even starting treatment within the window of potential benefit, in terms of mortality reduction, the injury inflicted to some organs could be only partially reversible.
Clinical experience confirms the existence of a direct relationship between a delay in the initiation of appropriate antibiotic treatment (according to the antibiogram) and mortality [11–20] or the risk of suffering acute lung [21] or kidney [22] damage. The relationship between treatment delay and mortality is particularly significant in infections due to Gram-negative organisms [23].
The organic dysfunction that defines sepsis starts hours before it can be recognized by clinical and/or laboratory tests. How fast a patient progress to organ failure mainly depends on his/her functional reserve. In patients older than 65 years with one or more co-morbidities (Charlson index ≥ 4) or some degree of immunesuppression, even starting from an apparent stability, the infection can progress in few hours. In most cases, it is impossible to predict how quickly the infection will evolve, the degree of reversibility of the organic damage, or the proximity to the no-return point.
In the first consensus of the American College of Chest Physicians/Society of Critical Care Medicine [24], systemic inflammatory response syndrome (SIRS) was defined by the presence of at least two of the following criteria: (a) fever > 38.5°C or hypothermia <35°C, (b) tachycardia> 90 beats/minute, (c) respiratory rate> 20 breaths/minute or PaCO2 <32 mmHg, and (d) leukocyte count >12,000 cells/mL or <4,000 cells/mL or more than 10% of immature forms. SIRS reflects the host’s response to infection, appropriate or not, and by itself is not necessarily a step before sepsis. However, patients who meet the criteria for SIRS in the course of infection without appropriate treatment have a significantly higher probability of presenting an unfavorable evolution. Indeed, a study [5] evaluating the mortality in ICU patients with a suspected infection and 2 or 3 SIRS criteria was 15 and 20%, respectively. It was similar to that found in patients with 2 qSOFA criteria (20%). The SIRS criteria are less specific but more sensitive than the qSOFA criteria for predicting the risk of unfavorable evolution of an infection and death due to sepsis [25–27]. From a clinical point of view, when a wrong diagnosis has severe consequences for the patient’s outcome and there is no or few space for correction, it is preferable to prioritize sensitivity over specificity.
Different studies have shown that in patients with bacteremia who meet the SIRS criteria, an appropriate initial empirical antibiotic treatment compared to an inappropriate one significantly reduces mortality, both in patients with and without sepsis criteria [28–35]. In a systematic review of 114 studies published from 2007 to 2019 in which the impact of appropriate vs. inappropriate initial antibiotic treatment was analyzed in patients with severe bacterial infections, significant differences were observed in favor of the former in terms of the reduction in mortality, length of hospital stay, and cost of treatment as well as the increased likelihood of clinical cure [23, 36].
Biological markers of infection include total neutrophil count, neutrophil/lymphocyte ratio, C-reactive protein (CRP), and procalcitonin (PCT). CRP slightly increases within the first 24h from infection onset and may remain low in patients with advanced liver failure (Child-Pugh C) and in those receiving corticosteroids in pharmacological doses or treated with monoclonal antibodies directed against IL-6 or its receptor. However, a high or very high value (≥ 200 mg/L) is indicative of the importance of the inflammatory response and the severity of the infection. Elevated CRP has been associated with mortality in adults with bacteremia [37, 38] or with severe sepsis or septic shock [39] and in patients with community-acquired pneumonia [40, 41]. Other studies have observed a positive correlation between the initial CRP value and the pneumonia severity index [42], mortality at 30 days [43, 44], and the need for Intensive Care Unit admission [42, 43]. PCT is detectable 3-4 hours after the infection onset and peaks between 6 and 12 hours later. PCT is processed to calcitonin in thyroid C cells and, to a lesser extent, in other neuroendocrine cells. In response to a bacterial infection, production is activated in all parenchymal tissues, mediated by IL-6, TNFα, and IL-1 β. These tissues cannot cleave PCT to its mature form, calcitonin, leading to the accumulation of PCT. Interferonγ, secreted primarily in response to viral infections, attenuates PCT production and makes it helpful in differentiating between viral and bacterial infections. In advanced renal failure, baseline PCT values of 0.1-1.8 ng/mL [45] can be observed. A PCT concentration ≥ 10 ng/mL in the course of an infection is associated with a high probability that the patient suffers from sepsis or septic shock [45]. Elevated PCT values have also been associated with higher mortality in patients with pneumonia [40, 46–48], sepsis [49–53] or intra-abdominal infection [54].
In conclusion, in the course of an infection, there are at least three clinical situations in which there is no margin of error regarding the choice of the initial empirical treatment:
The patient meets the criteria for sepsis (acute change in the SOFA score of 2 or more points from the baseline value).
The patient who meets at least 2 SIRS criteria, has a Charlson index ≥ 4 and a CRP ≥ 200 mg/L or a PCT ≥ 10 ng/mL.
A patient with an infection whose location carries a high and immediate risk of irreversible organ damage (e.g., endophthalmitis, bacterial meningitis). The treatment for these infections is not covered in this document.
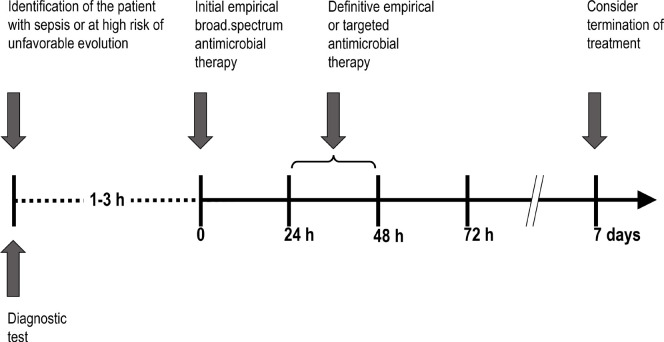
The diagnostic tests that must be performed before or at the same time treatment is started are discussed below and, subsequently, the bases for choosing the initial broad-spectrum empirical treatment regimen and the essential adjustment or “de-escalation” in 24-48h are thoroughly reviewed in the last section of the present document.
DIAGNOSTIC TESTS
The tests described below should be performed according to the most likely source of the infection.
Tests that provide information before the culture is available (≤24 hours) include a Gram staining on any biological sample (sputum, wound exudate, cerebrospinal fluid), detection of urinary pneumococcal and Legionella antigens, and of Clostridioides difficile toxin in an unformed stool sample.
Multiline molecular methods (multiplex panels), especially those used in respiratory samples (FilmArray pneumonia plus), are particularly useful for the etiological diagnosis of nosocomial pneumonia, but they are not usually available 24 hours a day in all microbiology laboratories. On the other hand, although the turnaround time is approximately one hour, in daily practice, the result is rarely available before 2 h from sample obtention, and in the patient with septic shock the result should not be awaited before administering the first dose of antibiotic.
Among the culture-based tests, the practice of two consecutive blood cultures in aerobic and anaerobic flasks obtained by venopuncture should be considered first. Blood samples must be obtained before administering the antibiotic [55] as long as the extraction does not delay the start of treatment. Blood cultures should be performed disregarding the presence of fever. If the patient has one or more vascular catheters, it is necessary to obtain blood cultures from venopuncture and from each lumen of the catheters in place for more than 48h or always in case of signs of phlebitis. It is essential to extract the same volume for each blood culture flask and to clearly identify the origin of the sample to interpret the differences in the time to positivity that could help to determine if the catheter is the most likely source of the infection. Situations that justify immediate removal of the catheter and culture of the tip, in the absence of local inflammatory signs, include (a) the absence of an apparent source of infection if the catheter has been in place for ≥ three days, (b) the appearance of fever after manipulation of the catheter, (c) the existence of a prosthetic valve or a recently placed arterial graft, and (d) the existence of septic shock with no apparent cause. Isolation in more than one blood culture bottle of coagulase-negative Staphylococcus, Corynebacterium jeikeium, Bacillus spp., Micrococcus, Mycobacteria (not M. tuberculosis), Candida, Rhodotorula, or Malassezia spp. suggests a cathether-related infection. The infused fluid or administered blood products should be cultured only if there is a clinical suspicion.
According to clinical findings is mandatory to culture urine, respiratory secretions, wound exudates, ascites, pleaural effusion, or cerebrospinal fluid. The presence of a small amount of pleural fluid is not uncommon in patients admitted to the ICU due to hypoalbuminemia, congestive heart failure, or abdominal surgery. It is not necessary to obtain samples of this liquid.
Most infections, particularly those acquired in the hospital, are endogenous and originate in the mucosal microbiota. Culture of nasopharingeal and rectal swabs, and tracheal secretions (in intubated patients) identifies the predominant aerobic bacterial colonizing the surface of different mucosa and if they have determinants of resistance. In many ICUs, surveillance cultures have been established upon admission and once or several times a week, with the aim of early detection and isolation of patients carrying resistant bacteria to minimize horizontal transmission. Additionally, the knowledge of colonizing microbiota and its susceptibility pattern plays a vital role in the selection of initial empirical antibiotic treatment and, in the subsequent adjustment or de-escalation in cases where the causative microorganism of the infection has not been identified.
In a recently published meta-analysis [56], the clinical utility of methicillin-resistant S. aureus (MRSA) nasal screening to rule out MRSA pneumonia (including community and nosocomially cases) was evaluated. The sensitivity and specificity were 70.9% and 90.3%, respectively, and assuming a prevalence of MRSA pneumonia of 10%, the positive predictive value of nasal swabs was 44.8%, and the negative predictive value 96.5%. Based on these data, a staphylococcal infection is highly unlikely to be caused by a methicillin-resistant strain if the methicillin-resistant strain is not isolated in the nasal swab. In such a situation, a specific antibiotic treatment against MRSA could be discontinued. In contrast, the isolation of MRSA from nasal swabs does not necessarily mean that it is the cause of the infection. However, until more data are available, it is advisable to start or maintain active antibiotic treatment.
Pharyngeal and rectal swabs are processed on chromogenic media to identify extended-spectrum β-lactamase- or carbapenemase-producing Enterobacteriaceae, P. aeruginosa, or other nonfermenting Gram-negative rods. A semi-quantitative estimate of the density of microorganisms in the sample can be made by successively seeding the sample in four quadrants of a plate with MacConkey’s medium. The growth in the first quadrant of the plate corresponds to a density of microorganisms in the sample ≥ 103 CFU/mL; if the growth also extends to the second quadrant, the number of microorganisms is ≥ 105 CFU/mL; ≥ 107 CFU/mL if the growth covers the third quadrant; and ≥ 109 CFU/mL if the growth covers the whole plate [57].
Endogenous infection is due to the translocation of the predominant aerobic microorganisms in the intestinal microbiota [58] or invasion of the lower respiratory tract from the oral microbiota [59]. The probability of translocation depends on the bacterial density on the mucosal surface and the area of the colonized surface. The presence of ≥ 105 CFU / mL of a Gram-negative bacilli (GNB) in the semi-quantitative culture of the rectal swab or the isolation of the same microorganism in ≥ 2 serial cultures reflects a bacterial overgrowth in the intestinal lumen. Isolation of the same bacteria in two different mucosa or locations (rectal, pharyngeal swab, tracheal secretion, urine culture) indicates that the colonized area is extensive. In neutropenic patients, it has been observed that ≥30% predominance of a certain GNB in the intestinal microbiota increases the risk of bacteremia caused by the same microorganism up to 5 times [60, 61]. Likewise, a relative abundance of Stenotrophomonas in the oral cavity of 36% (number of taxonomic units over the total) predicted infection by this microorganism with a sensitivity and specificity of around 95% [59].
Monitoring the presence and abundance of resistant Gram-negative microorganisms in mucosa allows, to a certain extent, the possible etiology of the infection and can improve the adequacy of empirical antimicrobial treatment [62]. In one study [63], colonization by GNB with resistance factors preceded bacteremia in 74.5% of cases. The relationship between intestinal colonization and the risk of bacteremia is particularly significant in the case of colonization by Klebsiella pneumoniae [64–66]. In ICU patients colonized by carbapenemase-producing K. pneumoniae, oral administration of an aminoglycoside decreased the density of K. pneumoniae in the colonic microbiota and was associated with a significant reduction in the number of infections during admission, despite not achieving a complete decolonization [67]. This fact suggests that the reduction in colonization density is sufficient to minimize the risk of infection. Additionally, colonization in differents sites by Candida spp. is strongly associated with the likelihood of invasive candidiasis among ICU patients with sepsis [68].
The composition of the intestinal microbiota can change within 72 hours after the arrival of a new microorganism or the start of antibiotic treatment. The practice of performing surveillance cultures once or twice a week does not rule out the possibility that a change in the microbiota composition has occurred in the days before the infection/sepsis episode. The authors of this document consider that it is convenient to perform a rectal swab and, whenever possible, to perform a semiquantitative culture of the sample. The result of this test, available in 24-36 hours, can be very useful when the causal microorganism is not identified, to decide what we designated as the definitive empirical antibiotic treatment (Figure 2). By performing cultures from mucosa at the moment of febrile episode onset can reduce the frequency of epidemiological surveillance cultures and, consequently, to reduce the microbiology laboratory’s workload.
Figure 2.
Management of antibiotic treatment in patients with serious infection of nosocomial origin.
The determination of (1-3)-β-D-glucan has a high negative predictive value for infection by Candida spp. and other yeasts (except Cryptococcus spp.). False positives can occur in the following situations: (a) exposure of serous membranes (pleura, peritoneum) to sponges or surgical gauze, (b) hemodialysis and continuous renal replacement techniques performed with cellulose membranes, (c) administration of intravenous immunoglobulin, albumin, plasma, coagulation factors (blood products processed using filters containing cellulose), (d) total parenteral nutrition, (e) intestinal translocation of β-glucan in processes involving increased gut mucosa permeability, and (f) infections by Nocardia spp., Rhizobium radiobacter, Pseudomonas spp., Enterococcus spp., and S. pneumoniae [69].
Regarding CRP or PCT determination, their values are not very representative of the severity of the infection in the first 12 h of evolution, particularly CRP. CRP begins to increase 6-8 hours after the onset of symptoms, its value doubles approximately every 8 hours, and the maximum concentration is reached at 36-48 hours. Corticosteroids administered at pharmacological doses, liver cirrhosis (Child B-C), and treatment with biologics that inhibit IL-6 activity can reduce the increase in CRP. Procalcitonin begins to increase from the first 3-4 hours after the onset of symptoms and reaches its maximum concentration between 6 and 24 hours. Unlike CRP, procalcitonin is not affected by corticosteroid treatment and is hardly increased in viral infections. Procalcitonin can be increased in candidemia, chronic renal failure and renal replacement techniques, lung cancer, and hematologic malignancies. The concentrations of CRP and PCT in blood correspond to the magnitude of the bacterial load in an infected patient and decrease with effective antimicrobial therapy. As already mentioned, CRP values > 200 mg/L or PCT ≥ 10 ng/mL, in infected patient is associated with a significant increase in mortality. Likewise, changes in CRP and PCT, after 48 hours of treatment, are a good indicator of treatment response [70, 71]. In contrast, an increase in CRP or PCT levels indicates that the antibiotic or source control are inappropriatted or insufficient. While a decreasing CRP and/or PCT indicate a favorable evolution of the disease.
A ratio of ≥ 10 between the neutrophil count and the lymphocyte count has been related to the severity and mortality of patients with community-acquired pneumonia [72] and with the probability of having bacteremia [73].
Imaging that can be performed without delay to identify the source of the infection, such as chest X-ray and abdominal ultrasound or computed tomography, should be considered based on clinical suspicion.
RECOMMENDATIONS FOR THE SELECTION OF ANTIBIOTIC TREATMENT
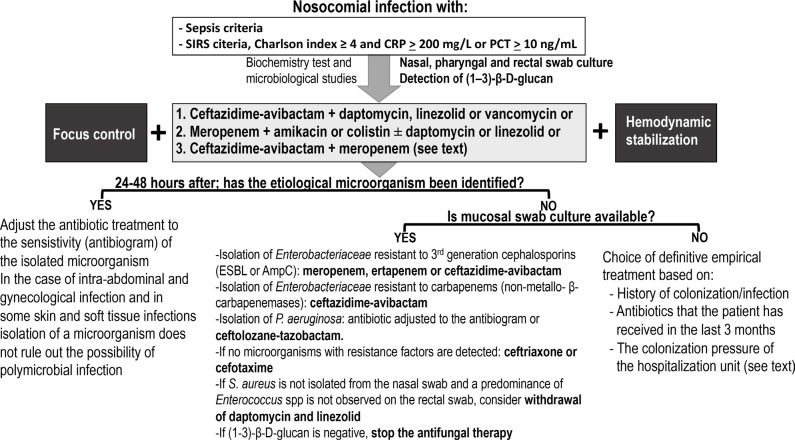
The following recommendations (Figure 3) apply to patients with suspected nosocomial infection who (a) meet the criteria for sepsis or septic shock or (b) meet two or more SIRS criteria, have a Charlson index ≥ 4 and a PCT > 10 ng/mL or a CRP ≥ 200 mg / L. In these situations, not making the right treatment selection is associated with a significantly higher risk of death.
Figure 3.
Antibiotic treatment guidelines for serious infection of nosocomial origin.
At the time of deciding the initial empirical treatment the patients’s situation is as follows:
The bacterial load in the infectious source is predictably high.
The microorganism(s) causing the infection and its susceptibility pattern are unknown.
We do not know the magnitude of the increase in the volume of distribution and the importance and direction of the variations in glomerular filtration rate.
We do not know how close the patient is to irreversible organ damage. Functional reserve is predictably low and may worsen rapidly if appropriate treatment is not promptly received.
The higher the bacterial load in the infectious source, the greater the concentration of antibiotic needed to inhibit the growth of the microorganism, and the higher the probability of selecting resistant mutants. Whenever feasible, the bacterial load must be reduced as soon as possible by physical procedures, such as drainage, deobstruction, debridement, and/or removal of the infected foreign material and, if appropriate, correction of the anatomical defect that originated the infection. If the collection is well defined and accessible, it should be drained percutaneously to avoid as much as possible the increase in the inflammatory response produced by surgical aggression. Endoscopic drainage of the bile duct or percutaneous nephrostomy is preferable to surgery. In patients with sepsis or septic shock, a delay from the time of diagnosis to source control greater than 6 hours [74, 75], greater than 12 hours [76, 77] or any degree of delay [78, 79] is associated with a significant increase in mortality. In a study that included 3,663 patients with sepsis or septic shock, mortality was lower in septic patients who underwent source control [80]. Infected pancreatic necrosis is an exception. In this case, if the patient remains stable, surgery can be delayed to allow adequate demarcation of the necrotic tissue [81].
The following refers to the initial antibiotic treatment regimen:
It must be active against as close as possible to 100% of the potentially implicated microorganisms.
Wherever feasible, it should contain a β-lactam antibiotic.
It must be administered as soon as possible at optimal doses, infusion times, and intervals according to the pharmacokinetic/pharmacodynamic (PK/PD) parameters of the antibiotics in patients with sepsis.
Several studies conducted in patients with sepsis or septic shock indicate that in 19% to 30% of cases, the initial empirical antibiotic treatment was not appropriate according to the in vitro susceptibility of the causative organism [11, 32, 82, 83]. Likewise, in a study carried out in patients with bacteremia, most of them without sepsis criteria, the percentage of inappropriate empirical treatment was 24.8% [31]. The error rate in the choice of initial antibiotic treatment was higher in infection by multiresistant microorganisms [32, 82]. As might be expected, treatment was most often appropriate when broad-spectrum antibiotic regimens or antibiotic combinations were used [32]. Inappropriate treatment was associated with a significant increase in mortality that was 3.8 [82] or 3.4 [11] times higher than that observed in patients receiving adequate antibiotic treatment. This difference was also observed in patients with bacteremia without sepsis [31, 32]
The study of colonized patients or with prior infection by resistant microorganisms and the comparison patients who are neither colonized nor infected has allowed the design of scales that stratify the individual risk of being infected or not by a multidrug-resistant microorganism. Predictive models of infection by carbapenemase-producing K. pneumoniae [66, 84], carbapenem-producing enterobacteria [85], and multi-drug resistant bacteria [86, 87] have been published. In general, the negative predictive value of these scales is high (> 90%), especially if resistant organisms are prevalent in the environment [88]. In contrast, the positive predictive value is low (18-38%) [66, 87, 88]. These scales are valid in the epidemiological setting in which they were established and for the group of patients studied, but they are not necessarily reproducible in different situations and, in any case, they do not ensure the necessary degree of certainty in the initial choice of antibiotic treatment for severe infections.
Among the currently available β-lactam antibiotics, in order from the highest to the lowest antibacterial spectrum and of the likelihood of achieving the optimal PK/PD target against Gram-negative bacilli, we can include: ceftazidime-avibactam, ceftolozane-tazobactam, and meropenem. The ceftazi-dime-avibactam association is active against 99% of non-class B carbapenemase-producing Enterobacteriaceae [95] and 94.2% of P. aeruginosa [96]. In the 1980s and 1990s, before the emergence of extended-spectrum β-lactamases (ESBLs), ceftazidime was used as monotherapy in empirical treatment regimens for patients with febrile neutropania [89]. In several prospective, and randomized studies, ceftazidime was compared with imipenem [90] or meropenem [91–94], and no significant differences were observed between the clinical efficacy of cephalosporin and carbapenems.
Ceftolozane-tazobactam is active against 85% of entero-bacteria (non-carbapenemase-producing) and 94.6% of P. aeruginosa, and meropenem is active against 99% of entero-bacteria (non-carbapenemase-producing) and 70.1% of P. aeruginosa [95, 96]. If meropenem is chosen, the association with amikacin or colistin should be considered since the percentage of meropenem-resistant P. aeruginosa is high, and it is not active against enterobacteria that produce carbapenemases. Ceftolozane-tazobactam should be reserved for the treatment of documented or highly likely P. aeruginosa infections.
Against Gram-positive microorganisms, daptomycin, line-zolid, tedizolid or vancomycin can be used. The choice depends on the location of the infection, renal function, and the need to use simultaneously other nephrotoxic drugs. The regimens that currently offer the best antibacterial spectrum for initial empirical treatment are the following associations:
ceftazidime-avibactam + daptomycin, linezolid, or vancomycin
meropenem + amikacin or colistin ± daptomycin or linezolid
ceftazidime-avibactam + meropenem. This association can reduce the selection of resistance in KPC-producing Klebsiella spp. and it has some synergistic activity against Klebsiella spp. and P. aeruginosa [97, 98]. Therefore, this regimen deserves to be considered if activity against methicillin-resistant S. aureus is not considered necessary.
Ceftazidime-avibactam can be substituted for ceftolozane-tazobactam if the patient has a history of infection / bronchial colonization by P. aeruginosa or when in the hospitalization unit there is a high colonization pressure by multidrug-resistant P. aeruginosa. Similarly, treatment with ceftolozane-tazobactam should be considered when the presence of P. aeruginosa is confirmed in the culture of respiratory secretions or rectal swab.
Ceftazidime-avibactam and ceftolozane-tazobactam lack activity against Bacteroides of the fragilis group. However, except in intra-abdominal infections, the participation of anaerobic microorganisms, in general, is not relevant and appropriate source control is sufficient. However, if needed, Gram-negative anaerobic microorganisms can be treated with the addition of metronidazole or, as a second-level alternative, daptomycin, linezolid, or vancomycin can be replaced by tigecycline, although its activity against B. fragilis is limited.
The choice between daptomycin and linezolid depends on the location of the infection. In case of pneumonia or infection of the central nervous system, linezolid is the first alternative. Vancomycin should be considered when daptomycin (pneumonia) or linezolid cannot be used (due to intolerance or thrombocytopenia) and its possible to measure serum concentration to adjust the dose in agreement with recent guidelines [99].
In certain circumstances, an antifungal treatment against Candida spp. is deemed necessary. A candin (micafungin, anidulafungin, or caspofungin) or alternatively a triazole (posaconazole, isavuconazole, or voriconazole) can be used. The risk of infection by Candida spp. arises, particularly, in patients who have received antibiotic treatment for more than seven days in an ICU, in case of severe pancreatitis, recent upper abdominal surgery, existence of multifocal colonization by Candida spp., parenteral nutrition, or renal failure requiring renal replacement techniques. A score ≥ 2.5 in the “Candida score” [100] supports the initiation of empirical treatment against Candida spp.
The strategy based on the initial use of broad-spectrum antibiotic therapy carries the risk of treating patients who do not have a bacterial infection or the risk of using antibiotics with a broader spectrum than necessary. In a study conducted in two Dutch ICUs of 2,579 patients admitted with a presumed diagnosis of sepsis, the existence of infection was not confirmed in 13% of them [101]. The experience was similar in another study, in which 18% of the patients admitted to the emergency service with a diagnosis of sepsis finally received a noninfectious disease diagnostic [102]. To achieve the highest cure rate, the risk of overtreatment must be assumed. This risk is higher the earlier the treatment is initiated in the course of a potential infection. The challenge is to provide early and appropriate therapy while limiting the side effects of antibiotics in patients who do not need it.
The undesirable effects of antibiotics include the possible toxicity, the risk of selecting resistant mutants, and the impact on the intestinal microbiota reflected in the risk of colitis due to Clostridioides difficile and the overgrowth of Enterococcus, multi-drug resistant GNB and Candida spp. In short treatments, the toxicity of β-lactams is irrelevant except for possible neurotoxicity when high doses are used, especially in patients with renal failure [103]. The effects on the microbiota are mainly dependent on the time of exposure to the antibiotic. Although they can be observed even with the administration of a single dose, they begin to be significant after 72 hours of treatment [104–106]. Therefore, after the first 24-36 hours of treatment, it is necessary and unavoidable to rethink the indication of an initial broad-spectrum therapy. In a study carried out in patients with sepsis or septic shock admitted to an ICU, a significant reduction in mortality was observed in the group of patients in whom antibiotic treatment was de-escalated within the first five days of treatment [107]. If microbio-logical tests have identified the causative organism, antibiotic treatment should be adjusted to its susceptibility as soon as the antibiogram is available. However, it must be taken into account that, in specific locations (intra-abdominal or gyneco-logical infection or in some skin and soft tissue infections), the isolation of a microorganism does not rule out the possibility of polymicrobial infection.
The risk of overtreatment arises, particularly, when the microbiological tests are negative, a fact that in some series is observed in more than half of the patients with sepsis [102]. In this scenario, after source control and 24-36 hours of broad-spectrum empirical treatment, the predictible situation is as follows:
The bacterial load will have decreased significantly.
Second, although we still do not know the etiological agent, the list of potential microorganisms can by resonarly reduced by evaluating the result of nasal, pharyngeal and / or rectal swab, tracheal aspirate and the determination of (1-3)-β-D-glucan. The absence of MRSA, ESBL- or carbapenemase-producing Enterobacteriaceae, and resistant P. aeruginosa, as well as a low value of β-D-glucan, permits to narrow the antibiotic spectrum.
Hemodynamic stability and organ function will have improved with normalization of blood pressure and a reduction in the requirement of other supporting measures. Under these conditions empirical treatment can be based on more objective parameters and even more important, the consequences of an error are less critical because the patient’s situation offers a greater margin for correction.
The new empirical treatment, probably definitive, must be adapted to the susceptibility of the colonizing microbiota identified in the rectal, pharyngeal, or nasal swabs or the tracheal aspirate. If third-generation cephalosporin-resistant Enterobacteriaceae is isolated (production of ESBLs or cephamycinases), treatment can be done with carbapenems or ceftazidime-avibactam. In the case of carbapenem-resistant Enterobacteriaceae (generally by the production of carbapenemases), treatment can be performed with ceftazidime-avibactam [108] or, in the case of metalloβlactamases, with the association of ceftazidime-avibactam with aztreonam [109–111] or with cefiderocol (if available) or with colistin as last option. If P. aeruginosa is isolated, the treatment is selected according to its susceptibility pattern, giving preference to the most active β-lactam in vitro (lower MIC) and with the lower risk of selection for resistant mutants. If the MIC value is not available and the bacterial load is predictably high (respiratory foci), it is preferable to use ceftolozane-tazobactam administered in high doses in extended infusion. If neither Enterobacteriaceae with resistance mechanisms nor P. aeruginosa are found, we can switch to a third-generation cephalosporin (cefotaxime or ceftriaxone). In any case, both amikacin and colistin can be withdrawn. Treatment with daptomycin, linezolid, or vancomycin should be reconsidered if MRSA is not isolated from the nasal swab and the risk of Enterococcus spp. infection is low or non-existent (skin and soft tissue infection or respiratory foci). If (1-3)-β-D-glucan is not elevated, the antifungal treatment should also be withdrawn.
Finally, if for any reason, cultures are not available, de-escalation and the choice of definitive empirical treatment should be based on: the past history of colonization/infection, the antibiotics that the patient has received in the past 3 months and colonization pressure from the hospitalization unit. In a prospective study carried out in a medical intensive care unit, a colonization pressure of P. aeruginosa (number of colonized patients/number of patients in the unit) > 0.43 was an independent predictor of acquisition of this microoganism [112].
DOSAGE, ADMINISTRATION GUIDELINES AND DURATION OF ANTIBIOTIC TREATMENT
Treatment should be started as soon as possible, and it is recommended to start within one hour in patients with septic shock [12] or even earlier (30 minutes) in patients with sepsis and neutropenia [113]. In all other situations, the start of treatment should not be delayed more than three hours after diagnosis.
The first β-lactam dose is 1-2 g iv administered as a bolus (5-10 minutes). The goal is to early reach a high serum concentration to (a) generate a high diffusion gradient into the tissues. In septic shock, the poor blood distribution into the microcirculation can decrease the antibiotic concentration at the infection site. (b) To neutralize the potential increase in the MIC produced by the inoculum effect, and (c) To exceed the concentration necessary to avoid the selection of resistant mutants. Additionally, the initial bolus compensates, at least partially, the increase in the volume of distribution (Vd) and/or in the renal clearance that is observed, in particular, in patients under 55 years of age who have hematological neoplasia or acute pancreatitis or have suffered extensive burns or multiple trauma. It is necessary to remark that the initial dose is independent of the renal function. After the bolus, the rest of the daily dose is administered in a continuous infusion. Another possibility is intermittent administration every 8 hours in a prolonged infusion of 3-4 hours. If this modality is chosen, it is preferable to administer at least 2 g per dose for the first 48 hours. The pharmacodynamic goal is to obtain a free plasma concentration of β-lactam that remains 4 to 8 times over the MIC during 100% of the dosing interval (fT ≥ 4-8 × MIC = 100%) for continuous infusion or a Cmin/MIC ≥ 4 for intermittent infusion. These targets are associated with a lower risk for resistant mutants selection in cases with documented GNB infection [114].
If the use of a renal replacement technique is required (continuous venous-venous hemofiltration, hemodiafiltration, or hemodialysis), it is advisable not to reduce the standard dose of β-lactam, at least during the first 24 hours, particularly for the treatment of multi-drug resistant bacteria. With extracorporeal membrane oxygenation (ECMO), the Vd can be increased by the circuit sequestration and the hemodilution of the priming solutions. However, studies carried out to date do not show a significant variation in the Vd or in the renal clearance. Modern ECMO circuits have minimal adsorption and impact on the pharmacokinetics of most antibiotics; therefore, pharmacokinetic changes reflect more the Vd and the renal clearance variations typical from critically ill patients and cannot be attributed to ECMO therapy by itself [115].
Amikacin is administered as a single daily dose of 25 mg/kg of total body weight [116]. The risk of renal toxicity is probably neglegible if treatment is reduced to a single day [117] and is low with regimens of ≤ 3 days [118], provided that other potentially nephrotoxic drugs are not used simultaneously such as vancomycin [119]. Serum concentration does not need to be determined if treatment is limited to less than three doses.
The duration of antibiotic treatment depends on (a) the possibility of carrying out adequate source control and (b) on two characteristics of the microorganism, how fast bacteria duplicates and the ability to generate quiescent forms or persistent bacteria, usually within biofilms. Microorganisms that grow in the cell cytoplasm usually have long duplication periods and, in general, require prolonged treatments. In contrast, micro-organisms that grow in the interstitial space have short duplication periods of approximately 1 hour, and optimal exposure (AUC0-24/MIC) to an active antibiotic can kill them at a similar rate to that observed in vitro. Most acute infections with sepsis are caused by planktonic extra-cellular microorganisms that rapidly duplicate in the interstitial space.
The duration of antibiotic treatment can be limited to a period of around seven days if the following conditions are met: (a) an appropriate source control has been carried out (including the elimination of any foreign material), (b) the patient is afebrile, hemodynamically stable and with improvement of the baseline symptoms, during the last 48-72 hours, and (c) the CRP is < 2.5 mg/L or has decreased > 50% with respect to the maximum value or the PCT is ≤ 0.5 ng/mL or has decreased ≥ 80% from the maximum value [120].
A meta-analysis of 5 studies that examined the duration of antibiotic treatment in patients with Enterobacteriaceae bacteremia did not find significant differences in the mortality of patients treated for more or less than ten days [121]. Several subsequent publications, also referring to hospitalized patients with Enterobacteriaceae bacteremia, confirmed that in patients who reach clinical stability before the seventh day, a 7-day course of antibiotics is not inferior to a 14-day course [122–125]. This recommendation does not apply to staphylococcal bacteremia or candidemia. These microorganisms are associated with a significant risk of metastasis and, even in case of uncomplicated infection, maintaining antibiotic treatment for two weeks after the first negative blood culture is mandatory.
Clinical cure is not necessarily accompanied by bacterial eradication. If the microorganism persists in the bronchial secretion, urine, or intestinal microbiota, it may cause a new infection. In patients with pneumonia, the persistence of MRSA in a nasal swab or a nonfermenting GNB in bronchial secretions is associated with a high risk of recurrence [126]. Extending the duration of treatment for ventilator-associated pneumonia can increase the eradication rate of non-fermenting GNB from bronchial secretions but it could increase the risk of selection of resistant microorganisms [127]. The potential solution lies in the addition of inhaled antibiotic treatment to reduce the density of bronchial colonization.
CONFLICT OF INTERESTS
RSG, JM, and RA have received honoraria for lectures and advisory boards from Pfizer. JP has received honoraria for lectures from Pfizer, MSD, Shionogi and Menarini and for advisory boards Pfizer, Shionogi, and Menarini. MS has received honoraria for lectures and advisory boards from Pfizer, MSD, Gilead, Angelini and Janssen. AE has received honoraria for lectures and advisory boards from Pfizer, Gilead, MSD and Shionogi. JP has received honoraria for lec tures from Pfizer, MSD, Angelini, Astellas, Advanz and Menarini. AS, has received honoraria for lectures and advisory boards from Pfizer, MSD, Angelini, Shionogi, Menarini and Gilead. Rest of authors declare no conflict of interest.
References
- 1.Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193:259–72. [DOI] [PubMed] [Google Scholar]
- 2.Vincent J-L, Marshall JC, Namendys-Silva SA, François B, Martin-Loeches I, Lipman J, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2:380–6. [DOI] [PubMed] [Google Scholar]
- 3.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, et al. Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults With Suspected Infection Admitted to the Intensive Care Unit. JAMA. 2017;317:290–300. [DOI] [PubMed] [Google Scholar]
- 6.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C-T, Tsai Y-J, Tsai P-R, Yu C-J, Ko W-J. Severe Sepsis and Septic Shock: Timing of Septic Shock Onset Matters. Shock. 2016;45:518–24. [DOI] [PubMed] [Google Scholar]
- 8.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304–77. [DOI] [PubMed] [Google Scholar]
- 9.Vincent J-L, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–53. [DOI] [PubMed] [Google Scholar]
- 10.Kumar G, Kumar N, Taneja A, Kaleekal T, Tarima S, McGinley E, et al. Nationwide trends of severe sepsis in the 21st century (2000-2007). Chest. 2011;140:1223–31. [DOI] [PubMed] [Google Scholar]
- 11.Vazquez-Guillamet C, Scolari M, Zilberberg MD, Shorr AF, Micek ST, Kollef M. Using the number needed to treat to assess appropriate antimicrobial therapy as a determinant of outcome in severe sepsis and septic shock. Crit Care Med. 2014;42:2342–9. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136:1237–48. [DOI] [PubMed] [Google Scholar]
- 14.Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Del-linger RP, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749–55. [DOI] [PubMed] [Google Scholar]
- 15.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med. 2017;376:2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiles BB, Deis AS, Simpson SQ. Increased Time to Initial Antimicrobial Administration Is Associated With Progression to Septic Shock in Severe Sepsis Patients. Crit Care Med. 2017;45:623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloos F, Rüddel H, Thomas-Rüddel D, Schwarzkopf D, Pausch C, Harbarth S, et al. Effect of a multifaceted educational intervention for anti-infectious measures on sepsis mortality: a cluster randomized trial. Intensive Care Med. 2017;43:1602–12. [DOI] [PubMed] [Google Scholar]
- 18.Pruinelli L, Westra BL, Yadav P, Hoff A, Steinbach M, Kumar V, et al. Delay Within the 3-Hour Surviving Sepsis Campaign Guideline on Mortality for Patients With Severe Sepsis and Septic Shock. Crit Care Med. 2018;46:500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peltan ID, Brown SM, Bledsoe JR, Sorensen J, Samore MH, Allen TL, et al. ED Door-to-Antibiotic Time and Long-term Mortality in Sepsis. Chest. 2019;155:938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falcone M, Bassetti M, Tiseo G, Giordano C, Nencini E, Russo A, et al. Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit Care. 2020;24:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iscimen R, Cartin-Ceba R, Yilmaz M, Khan H, Hubmayr RD, Afessa B, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36:1518–22. [DOI] [PubMed] [Google Scholar]
- 22.Bagshaw SM, Lapinsky S, Dial S, Arabi Y, Dodek P, Wood G, et al. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009;35:871–81. [DOI] [PubMed] [Google Scholar]
- 23.Zasowski EJ, Bassetti M, Blasi F, Goossens H, Rello J, Sotgiu G, et al. A Systematic Review of the Effect of Delayed Appropriate Antibiotic Treatment on the Outcomes of Patients With Severe Bacterial Infections. Chest. 2020;158:929–38. [DOI] [PubMed] [Google Scholar]
- 24.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. [DOI] [PubMed] [Google Scholar]
- 25.Serafim R, Gomes JA, Salluh J, Póvoa P. A Comparison of the Quick-SOFA and Systemic Inflammatory Response Syndrome Criteria for the Diagnosis of Sepsis and Prediction of Mortality: A Systematic Review and Meta-Analysis. Chest. 2018;153:646–55. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y-C, Luo Y-Y, Zhang X, Shou S-T, Gao Y-L, Lu B, et al. Quick Sequential Organ Failure Assessment as a prognostic factor for infected patients outside the intensive care unit: a systematic review and meta-analysis. Intern Emerg Med. 2019;14:603–15. [DOI] [PubMed] [Google Scholar]
- 27.Luo J, Jiang W, Weng L, Peng J, Hu X, Wang C, et al. Usefulness of qSOFA and SIRS scores for detection of incipient sepsis in general ward patients: A prospective cohort study. J Crit Care. 2019;51:13–8. [DOI] [PubMed] [Google Scholar]
- 28.Liu VX, Fielding-Singh V, Greene JD, Baker JM, Iwashyna TJ, Bhattacharya J, et al. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am J Respir Crit Care Med. 2017;196:856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garnacho-Montero J, Ortiz-Leyba C, Herrera-Melero I, Aldabó-Pallás T, Cayuela-Dominguez A, Marquez-Vacaro JA, et al. Mortality and morbidity attributable to inadequate empirical antimicrobial therapy in patients admitted to the ICU with sepsis: a matched cohort study. J Antimicrob Chemother. 2008;61:436–41. [DOI] [PubMed] [Google Scholar]
- 30.Bonine NG, Berger A, Altincatal A, Wang R, Bhagnani T, Gillard P, et al. Impact of Delayed Appropriate Antibiotic Therapy on Patient Outcomes by Antibiotic Resistance Status From Serious Gram-negative Bacterial Infections. Am J Med Sci. 2019;357:103–10. [DOI] [PubMed] [Google Scholar]
- 31.Retamar P, Portillo MM, López-Prieto MD, Rodríguez-López F, de Cueto M, García M V, et al. Impact of inadequate empirical therapy on the mortality of patients with bloodstream infections: a propensity score-based analysis. Antimicrob Agents Chemother. 2012;56:472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadri SS, Lai YL, Warner S, Strich JR, Babiker A, Ricotta EE, et al. Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect Dis. 2021;21:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H-C, Lin W-L, Lin C-C, Hsieh W-H, Hsieh C-H, Wu M-H, et al. Outcome of inadequate empirical antibiotic therapy in emergency department patients with community-onset bloodstream infections. J Antimicrob Chemother. 2013;68:947–53. [DOI] [PubMed] [Google Scholar]
- 34.Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother. 2010;54:4851–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adrie C, Garrouste-Orgeas M, Ibn Essaied W, Schwebel C, Darmon M, Mourvillier B, et al. Attributable mortality of ICU-acquired bloodstream infections: Impact of the source, causative micro-organism, resistance profile and antimicrobial therapy. J Infect. 2017;74:131–41. [DOI] [PubMed] [Google Scholar]
- 36.Bassetti M, Rello J, Blasi F, Goossens H, Sotgiu G, Tavoschi L, et al. Systematic review of the impact of appropriate versus inappropriate initial antibiotic therapy on outcomes of patients with severe bacterial infections. Int J Antimicrob Agents. 2020;56:106184. [DOI] [PubMed] [Google Scholar]
- 37.Devran O, Karakurt Z, Adıgüzel N, Güngör G, Moçin OY, Balcı MK, et al. C-reactive protein as a predictor of mortality in patients affected with severe sepsis in intensive care unit. Multidiscip Respir Med. 2012;7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burlaud A, Mathieu D, Falissard B, Trivalle C. Mortality and bloodstream infections in geriatrics units. Arch Gerontol Geriatr. 2010;51:e106-9. [DOI] [PubMed] [Google Scholar]
- 39.Robert Boter N, Mòdol Deltell JM, Casas Garcia I, Rocamora Blanch G, Lladós Beltran G, Carreres Molas A. Activation of a code sepsis in the emergency department is associated with a decrease in mortality. Med Clin (Barc). 2019;152:255–60. [DOI] [PubMed] [Google Scholar]
- 40.Viasus D, Del Rio-Pertuz G, Simonetti AF, Garcia-Vidal C, Acosta-Reyes J, Garavito A, et al. Biomarkers for predicting short-term mortality in community-acquired pneumonia: A systematic review and meta-analysis. J Infect. 2016;72:273–82. [DOI] [PubMed] [Google Scholar]
- 41.Arinzon Z, Peisakh A, Schrire S, Berner Y. C-reactive protein (CRP): an important diagnostic and prognostic tool in nursing-home-associated pneumonia. Arch Gerontol Geriatr. 2011;53:364–9. [DOI] [PubMed] [Google Scholar]
- 42.Hohenthal U, Hurme S, Helenius H, Heiro M, Meurman O, Nikoskelainen J, et al. Utility of C-reactive protein in assessing the disease severity and complications of community-acquired pneumonia. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2009;15:1026–32. [DOI] [PubMed] [Google Scholar]
- 43.Chalmers JD, Singanayagam A, Hill AT. C-reactive protein is an independent predictor of severity in community-acquired pneumonia. Am J Med. 2008;121:219–25. [DOI] [PubMed] [Google Scholar]
- 44.Menéndez R, Cavalcanti M, Reyes S, Mensa J, Martinez R, Marcos MA, et al. Markers of treatment failure in hospitalised community acquired pneumonia. Thorax. 2008;63:447–52. [DOI] [PubMed] [Google Scholar]
- 45.Samsudin I, Vasikaran SD. Clinical Utility and Measurement of Procalcitonin. Clin Biochem Rev. 2017;38:59–68. [PMC free article] [PubMed] [Google Scholar]
- 46.Krüger S, Ewig S, Marre R, Papassotiriou J, Richter K, von Baum H, et al. Procalcitonin predicts patients at low risk of death from community-acquired pneumonia across all CRB-65 classes. Eur Respir J. 2008;31:349–55. [DOI] [PubMed] [Google Scholar]
- 47.Huang DT, Weissfeld LA, Kellum JA, Yealy DM, Kong L, Martino M, et al. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52:48-58.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bloos F, Marshall JC, Dellinger RP, Vincent J-L, Gutierrez G, Rivers E, et al. Multinational, observational study of procalcitonin in ICU patients with pneumonia requiring mechanical ventilation: a multicenter observational study. Crit Care. 2011;15:R88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hillas G, Vassilakopoulos T, Plantza P, Rasidakis A, Bakakos P. C-reactive protein and procalcitonin as predictors of survival and septic shock in ventilator-associated pneumonia. Eur Respir J. 2010;35:805–11. [DOI] [PubMed] [Google Scholar]
- 50.Liu D, Su L, Han G, Yan P, Xie L. Prognostic Value of Procalcitonin in Adult Patients with Sepsis: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0129450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jain S, Sinha S, Sharma SK, Samantaray JC, Aggrawal P, Vikram NK, et al. Procalcitonin as a prognostic marker for sepsis: a prospective observational study. BMC Res Notes. 2014;7:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuetz P, Birkhahn R, Sherwin R, Jones AE, Singer A, Kline JA, et al. Serial Procalcitonin Predicts Mortality in Severe Sepsis Patients: Results From the Multicenter Procalcitonin MOnitoring SEpsis (MOSES) Study. Crit Care Med. 2017;45:781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sager R, Wirz Y, Amin D, Amin A, Hausfater P, Huber A, et al. Are admission procalcitonin levels universal mortality predictors across different medical emergency patient populations? Results from the multi-national, prospective, observational TRIAGE study. Clin Chem Lab Med. 2017;55:1873–80. [DOI] [PubMed] [Google Scholar]
- 54.Suarez-de-la-Rica A, Maseda E, Anillo V, Tamayo E, García-Bernedo CA, Ramasco F, et al. Biomarkers (Procalcitonin, C Reactive Protein, and Lactate) as Predictors of Mortality in Surgical Patients with Complicated Intra-Abdominal Infection. Surg Infect (Larchmt). 2015;16:346–51. [DOI] [PubMed] [Google Scholar]
- 55.Cheng MP, Stenstrom R, Paquette K, Stabler SN, Akhter M, Davidson AC, et al. Blood Culture Results Before and After Antimicrobial Administration in Patients With Severe Manifestations of Sepsis: A Diagnostic Study. Ann Intern Med. 2019;171:547–54. [DOI] [PubMed] [Google Scholar]
- 56.Parente DM, Cunha CB, Mylonakis E, Timbrook TT. The Clinical Utility of Methicillin-Resistant Staphylococcus aureus (MRSA) Nasal Screening to Rule Out MRSA Pneumonia: A Diagnostic Meta-analysis With Antimicrobial Stewardship Implications. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2018;67:1–7. [DOI] [PubMed] [Google Scholar]
- 57.van Saene HK, Damjanovic V, Murray AE, de la Cal MA. How to classify infections in intensive care units--the carrier state, a criterion whose time has come? J Hosp Infect. 1996;33:1–12. [DOI] [PubMed] [Google Scholar]
- 58.Ruppé E, Andremont A. Causes, consequences, and perspectives in the variations of intestinal density of colonization of multidrug-resistant enterobacteria. Front Microbiol. 2013;4:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aitken SL, Sahasrabhojane P V, Kontoyiannis DP, Savidge TC, Arias CA, Ajami NJ, et al. Alterations of the Oral Microbiome and Cumulative Carbapenem Exposure Are Associated With Stenotrophomonas maltophilia Infection in Patients With Acute Myeloid Leukemia Receiving Chemotherapy. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2021;72:1507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2012;55:905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stoma I, Littmann ER, Peled JU, Giralt S, van den Brink MRM, Pamer EG, et al. Compositional flux within the intestinal microbiota and risk for bloodstream infection with gram-negative bacteria. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2020. [DOI] [PMC free article] [PubMed]
- 62.Papadomichelakis E, Kontopidou F, Antoniadou A, Poulakou G, Koratzanis E, Kopterides P, et al. Screening for resistant gram-negative microorganisms to guide empiric therapy of subsequent infection. Intensive Care Med. 2008;34:2169–75. [DOI] [PubMed] [Google Scholar]
- 63.Blot S, Depuydt P, Vogelaers D, Decruyenaere J, De Waele J, Hoste E, et al. Colonization status and appropriate antibiotic therapy for nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in an intensive care unit. Infect Control Hosp Epidemiol. 2005;26:575–9. [DOI] [PubMed] [Google Scholar]
- 64.Hayden MK, Lin MY, Lolans K, Weiner S, Blom D, Moore NM, et al. Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2015;60:1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gorrie CL, Mirceta M, Wick RR, Edwards DJ, Thomson NR, Strugnell RA, et al. Gastrointestinal Carriage Is a Major Reservoir of Klebsiella pneumoniae Infection in Intensive Care Patients. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2017;65:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giannella M, Trecarichi EM, De Rosa FG, Del Bono V, Bassetti M, Lewis RE, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: a prospective observational multicentre study. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2014;20:1357–62. [DOI] [PubMed] [Google Scholar]
- 67.Machuca I, Gutiérrez-Gutiérrez B, Pérez Cortés S, Gracia-Ahufinger I, Serrano J, Madrigal MD, et al. Oral decontamination with aminoglycosides is associated with lower risk of mortality and infections in high-risk patients colonized with colistin-resistant, KPC-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2016;71:3242–9. [DOI] [PubMed] [Google Scholar]
- 68.Alenazy H, Alghamdi A, Pinto R, Daneman N. Candida colonization as a predictor of invasive candidiasis in non-neutropenic ICU patients with sepsis: A systematic review and meta-analysis. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2021;102:357–62. [DOI] [PubMed] [Google Scholar]
- 69.Finkelman MA. Specificity Influences in (1→3)-β-d-Glucan-Sup-ported Diagnosis of Invasive Fungal Disease. J fungi (Basel, Switzerland). 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Castelli GP, Pognani C, Cita M, Stuani A, Sgarbi L, Paladini R. Procalcitonin, C-reactive protein, white blood cells and SOFA score in ICU: diagnosis and monitoring of sepsis. Minerva Anestesiol. 2006;72:69–80. [PubMed] [Google Scholar]
- 71.Lobo SMA, Lobo FRM, Bota DP, Lopes-Ferreira F, Soliman HM, Mélot C, et al. C-reactive protein levels correlate with mortality and organ failure in critically ill patients. Chest. 2003;123:2043–9. [DOI] [PubMed] [Google Scholar]
- 72.de Jager CPC, Wever PC, Gemen EFA, Kusters R, van Gageldonk-Lafeber AB, van der Poll T, et al. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS One. 2012;7:e46561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Loonen AJM, de Jager CPC, Tosserams J, Kusters R, Hilbink M, Wever PC, et al. Biomarkers and molecular analysis to improve bloodstream infection diagnostics in an emergency care unit. PLoS One. 2014;9:e87315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Azuhata T, Kinoshita K, Kawano D, Komatsu T, Sakurai A, Chiba Y, et al. Time from admission to initiation of surgery for source control is a critical determinant of survival in patients with gastrointestinal perforation with associated septic shock. Crit Care. 2014;18:R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bloos F, Thomas-Rüddel D, Rüddel H, Engel C, Schwarzkopf D, Marshall JC, et al. Impact of compliance with infection management guidelines on outcome in patients with severe sepsis: a prospective observational multi-center study. Crit Care. 2014;18:R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karvellas CJ, Abraldes JG, Zepeda-Gomez S, Moffat DC, Mirzanejad Y, Vazquez-Grande G, et al. The impact of delayed biliary decompression and anti-microbial therapy in 260 patients with cholangitis-associated septic shock. Aliment Pharmacol Ther. 2016;44:755–66. [DOI] [PubMed] [Google Scholar]
- 77.Chao W-N, Tsai C-F, Chang H-R, Chan K-S, Su C-H, Lee Y-T, et al. Impact of timing of surgery on outcome of Vibrio vulnificus-related necrotizing fasciitis. Am J Surg. 2013;206:32–9. [DOI] [PubMed] [Google Scholar]
- 78.Wong C-H, Chang H-C, Pasupathy S, Khin L-W, Tan J-L, Low C-O. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am. 2003;85:1454–60. [PubMed] [Google Scholar]
- 79.Buck DL, Vester-Andersen M, Møller MH. Surgical delay is a critical determinant of survival in perforated peptic ulcer. Br J Surg. 2013;100:1045–9. [DOI] [PubMed] [Google Scholar]
- 80.Martínez ML, Ferrer R, Torrents E, Guillamat-Prats R, Gomà G, Suárez D, et al. Impact of Source Control in Patients With Severe Sepsis and Septic Shock. Crit Care Med. 2017;45:11–9. [DOI] [PubMed] [Google Scholar]
- 81.Jimenez MF, Marshall JC. Source control in the management of sepsis. Intensive Care Med. 2001;27 Suppl 1:S49-62. [DOI] [PubMed] [Google Scholar]
- 82.Zilberberg MD, Shorr AF, Micek ST, Vazquez-Guillamet C, Kollef MH. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit Care. 2014;18:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–55. [DOI] [PubMed] [Google Scholar]
- 84.Tumbarello M, Trecarichi EM, Tumietto F, Del Bono V, De Rosa FG, Bassetti M, et al. Predictive models for identification of hospitalized patients harboring KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2014;58:3514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller BM, Johnson SW. Demographic and infection characteristics of patients with carbapenem-resistant Enterobacteriaceae in a community hospital: Development of a bedside clinical score for risk assessment. Am J Infect Control. 2016;44:134–7. [DOI] [PubMed] [Google Scholar]
- 86.Vasudevan A, Mukhopadhyay A, Li J, Yuen EGY, Tambyah PA. A prediction tool for nosocomial multi-drug Resistant Gram-Negative Bacilli infections in critically ill patients-prospective observational study. BMC Infect Dis. 2014;14:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nseir S, Grailles G, Soury-Lavergne A, Minacori F, Alves I, Durocher A. Accuracy of American Thoracic Society/Infectious Diseases Society of America criteria in predicting infection or colonization with multidrug-resistant bacteria at intensive-care unit admission. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2010;16:902–8. [DOI] [PubMed] [Google Scholar]
- 88.Xie J, Ma X, Huang Y, Mo M, Guo F, Yang Y, et al. Value of American Thoracic Society guidelines in predicting infection or colonization with multidrug-resistant organisms in critically ill patients. PLoS One. 2014;9:e89687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pizzo PA, Hathorn JW, Hiemenz J, Browne M, Commers J, Cotton D, et al. A randomized trial comparing ceftazidime alone with combination antibiotic therapy in cancer patients with fever and neutropenia. N Engl J Med. 1986;315:552–8. [DOI] [PubMed] [Google Scholar]
- 90.Freifeld AG, Walsh T, Marshall D, Gress J, Steinberg SM, Hathorn J, et al. Monotherapy for fever and neutropenia in cancer patients: a randomized comparison of ceftazidime versus imipenem. J Clin Oncol Off J Am Soc Clin Oncol. 1995;13:165–76. [DOI] [PubMed] [Google Scholar]
- 91.Lindblad R, Rödjer S, Adriansson M, Andreasson B, Bäckström B, Johansson P, et al. Empiric monotherapy for febrile neutropenia--a randomized study comparing meropenem with ceftazidime. Scand J Infect Dis. 1998;30:237–43. [DOI] [PubMed] [Google Scholar]
- 92.Malik I, Shaharyar. Comparison of meropenem with ceftazidime as monotherapy of cancer patients with chemotherapy induced febrile neutropenia. J Pak Med Assoc. 2002;52:15–8. [PubMed] [Google Scholar]
- 93.Ferdosian F, Ghiliyan R, Hashemi A, Akhondzadeh B, Gholampoor E. Comparing the efficacy of ceftazidime and meropenem in treatment of febrile neutropenia in pediatric patients with cancer. Iran J Pediatr Hematol Oncol. 2013;3:103–7. [PMC free article] [PubMed] [Google Scholar]
- 94.Fleischhack G, Hartmann C, Simon A, Wulff B, Havers W, Marklein G, et al. Meropenem versus ceftazidime as empirical monotherapy in febrile neutropenia of paediatric patients with cancer. J Antimicrob Chemother. 2001;47:841–53. [DOI] [PubMed] [Google Scholar]
- 95.Sader HS, Flamm RK, Carvalhaes CG, Castanheira M. Comparison of ceftazidime-avibactam and ceftolozane-tazobactam in vitro activities when tested against gram-negative bacteria isolated from patients hospitalized with pneumonia in United States medical centers (2017-2018). Diagn Microbiol Infect Dis. 2020;96:114833. [DOI] [PubMed] [Google Scholar]
- 96.Del Barrio-Tofiño E, Zamorano L, Cortes-Lara S, López-Causapé C, Sánchez-Diener I, Cabot G, et al. Spanish nationwide survey on Pseudomonas aeruginosa antimicrobial resistance mechanisms and epidemiology. J Antimicrob Chemother. 2019;74:1825–35. [DOI] [PubMed] [Google Scholar]
- 97.Mikhail S, Singh NB, Kebriaei R, Rice SA, Stamper KC, Castanheira M, et al. Evaluation of the Synergy of Ceftazidime-Avibactam in Combination with Meropenem, Amikacin, Aztreonam, Colistin, or Fosfomycin against Well-Characterized Multidrug-Resistant Klebsiella pneumoniae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2019;63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nath S, Moussavi F, Abraham D, Landman D, Quale J. In vitro and in vivo activity of single and dual antimicrobial agents against KPC-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2018;73:431–6. [DOI] [PubMed] [Google Scholar]
- 99.Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediat. Am J Heal Pharm AJHP Off J Am Soc Heal Pharm. 2020;77:835–64. [DOI] [PubMed] [Google Scholar]
- 100.León C, Ruiz-Santana S, Saavedra P, Almirante B, Nolla-Salas J, Alvarez-Lerma F, et al. A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med. 2006;34:730–7. [DOI] [PubMed] [Google Scholar]
- 101.Klein Klouwenberg PMC, Cremer OL, van Vught LA, Ong DSY, Frencken JF, Schultz MJ, et al. Likelihood of infection in patients with presumed sepsis at the time of intensive care unit admission: a cohort study. Crit Care. 2015;19:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heffner AC, Horton JM, Marchick MR, Jones AE. Etiology of illness in patients with severe sepsis admitted to the hospital from the emergency department. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2010;50:814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beumier M, Casu GS, Hites M, Wolff F, Cotton F, Vincent JL, et al. Elevated β-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol. 2015;81:497–506. [PubMed] [Google Scholar]
- 104.Kritsotakis EI, Tsioutis C, Roumbelaki M, Christidou A, Gikas A. Antibiotic use and the risk of carbapenem-resistant extended-spectrum-{beta}-lactamase-producing Klebsiella pneumoniae infection in hospitalized patients: results of a double case-control study. J Antimicrob Chemother. 2011;66:1383–91. [DOI] [PubMed] [Google Scholar]
- 105.Drusano GL, Louie A, MacGowan A, Hope W. Suppression of Emergence of Resistance in Pathogenic Bacteria: Keeping Our Powder Dry, Part 1. Antimicrob Agents Chemother. 2015;60:1183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tam VH, Louie A, Fritsche TR, Deziel M, Liu W, Brown DL, et al. Impact of drug-exposure intensity and duration of therapy on the emergence of Staphylococcus aureus resistance to a quinolone antimicrobial. J Infect Dis. 2007;195:1818–27. [DOI] [PubMed] [Google Scholar]
- 107.Routsi C, Gkoufa A, Arvaniti K, Kokkoris S, Tourtoglou A, Theodorou V, et al. De-escalation of antimicrobial therapy in ICU settings with high prevalence of multidrug-resistant bacteria: a multicentre prospective observational cohort study in patients with sepsis or septic shock. J Antimicrob Chemother. 2020;75:3665–74. [DOI] [PubMed] [Google Scholar]
- 108.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeru. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2020.
- 109.Sieswerda E, van den Brand M, van den Berg RB, Sträter J, Schouls L, van Dijk K, et al. Successful rescue treatment of sepsis due to a pandrug-resistant, NDM-producing Klebsiella pneumoniae using aztreonam powder for nebulizer solution as intravenous therapy in combination with ceftazidime/avibactam. The Journal of antimicrobial chemotherapy. 2020;75:773–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Biagi M, Wu T, Lee M, Patel S, Butler D, Wenzler E. Searching for the Optimal Treatment for Metallo-and Serineβ-Lactamase Producing Enterobacteriaceae: Aztreonam in Combination with Ceftazidime-avibactam or Meropenem-vaborbactam. Antimicrob Agents Chemother. 2019;63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shaw E, Rombauts A, Tubau F, Padullés A, Càmara J, Lozano T, et al. Clinical outcomes after combination treatment with ceftazidime/ avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J Antimicrob Chemother. 2018;73:1104–6. [DOI] [PubMed] [Google Scholar]
- 112.Cobos-Trigueros N, Solé M, Castro P, Torres JL, Hernández C, Rinaudo M, et al. Acquisition of Pseudomonas aeruginosa and its resistance phenotypes in critically ill medical patients: role of colonization pressure and antibiotic exposure. Crit Care. 2015;19:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rosa RG, Goldani LZ. Cohort study of the impact of time to antibiotic administration on mortality in patients with febrile neutropenia. Antimicrob Agents Chemother. 2014;58:3799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sumi CD, Heffernan AJ, Lipman J, Roberts JA, Sime FB. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin Pharmacokinet. 2019;58:1407–43. [DOI] [PubMed] [Google Scholar]
- 115.Abdul-Aziz MH, Roberts JA. Antibiotic dosing during extracorporeal membrane oxygenation: does the system matter? Curr Opin Anaesthesiol. 2020;33:71–82. [DOI] [PubMed] [Google Scholar]
- 116.Taccone FS, Laterre P-F, Spapen H, Dugernier T, Delattre I, Layeux B, et al. Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit Care. 2010;14:R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cobussen M, de Kort JML, Dennert RM, Lowe SH, Stassen PM. No increased risk of acute kidney injury after a single dose of gentamicin in patients with sepsis. Infect Dis (London, England). 2016;48:274–80. [DOI] [PubMed] [Google Scholar]
- 118.Carlsen S, Boel J, Jarløv JO, Gjørup I, Søborg C, Arpi M. The effect of short-course gentamicin therapy on kidney function in patients with bacteraemia-a retrospective cohort study. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2018;37:2307–12. [DOI] [PubMed] [Google Scholar]
- 119.Ong DSY, Frencken JF, Klein Klouwenberg PMC, Juffermans N, van der Poll T, Bonten MJM, et al. Short-Course Adjunctive Gentamicin as Empirical Therapy in Patients With Severe Sepsis and Septic Shock: A Prospective Observational Cohort Study. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2017;64:1731–6. [DOI] [PubMed] [Google Scholar]
- 120.de Jong E, van Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16:819–27. [DOI] [PubMed] [Google Scholar]
- 121.Tansarli GS, Andreatos N, Pliakos EE, Mylonakis E. A Systematic Review and Meta-analysis of Antibiotic Treatment Duration for Bacteremia Due to Enterobacteriaceae. Antimicrob Agents Chemother. 2019;63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yahav D, Franceschini E, Koppel F, Turjeman A, Babich T, Bitterman R, et al. Seven Versus 14 Days of Antibiotic Therapy for Uncomplicated Gram-negative Bacteremia: A Noninferiority Randomized Controlled Trial. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2019;69:1091–8. [DOI] [PubMed] [Google Scholar]
- 123.Daneman N, Rishu AH, Pinto R, Aslanian P, Bagshaw SM, Carignan A, et al. 7 versus 14 days of antibiotic treatment for critically ill patients with bloodstream infection: a pilot randomized clinical trial. Trials. 2018;19:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hojat LS, Bessesen MT, Huang M, Reid M, Knepper BC, Miller MA, et al. Effectiveness of Shorter Versus Longer Durations of Therapy for Common Inpatient Infections Associated With Bacteremia: A Multicenter, Propensity-Weighted Cohort Study. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2020;71:3071–8. [DOI] [PubMed] [Google Scholar]
- 125.von Dach E, Albrich WC, Brunel A-S, Prendki V, Cuvelier C, Flury D, et al. Effect of C-Reactive Protein-Guided Antibiotic Treatment Duration, 7-Day Treatment, or 14-Day Treatment on 30-Day Clinical Failure Rate in Patients With Uncomplicated Gram-Negative Bacteremia: A Randomized Clinical Trial. JAMA. 2020;323:2160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Albin OR, Henig O, Patel TS, Valley TS, Pogue JM, Petty LA, et al. Clinical Implications of Microbiologic Treatment Failure in the Setting of Clinical Cure of Bacterial Pneumonia. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2020;71:3033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chastre J, Wolff M, Fagon J-Y, Chevret S, Thomas F, Wermert D, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588–98. [DOI] [PubMed] [Google Scholar]